Abstract
Background
Immune dysregulation may play a role in the development of Rett syndrome (RTT), a neurodevelopmental disorder caused by mutations of the MECP2 gene. Abnormal cytokine concentrations have been documented in the serum of individuals with RTT. Measurement of salivary cytokines has been investigated as a potential alternative approach to measurement in blood and serum, but it is unclear whether salivary cytokine concentrations can provide valid information about systemic immune function in neurodevelopmental disorders. The goal of this study was to evaluate the potential validity of salivary cytokines as biomarkers of immune dysregulation in RTT.
Methods
Saliva samples from 16 individuals with RTT (all female; age range 2–40 years) and 16 healthy control females (age range 2–40 years) were analyzed for concentrations of 12 cytokines. Between-group differences in concentrations, and correlations with clinical severity in the RTT group were evaluated.
Results
Concentrations of several salivary cytokines (IL-1β, IL-6, IL-8, IL-10, GM-CSF, TNF-α, and VEGF) were increased in RTT compared to controls. The same cytokines showed significant positive correlations with clinical severity scores. There were no differences in concentrations of IL-2, IL-4, IL-5, IL-12p70, and IFN-γ.
Conclusion
The results suggest that salivary cytokines may be a possible indicator of immune dysregulation in RTT. Future research should investigate whether these results can be applied to other neurodevelopmental disorders.
Keywords: Rett syndrome, MECP2, Cytokines, Saliva, Biomarkers
Highlights
-
•
Immune dysregulation may play a role in the development of Rett syndrome (RTT), a disorder caused by mutations of the MECP2 gene.
-
•
Concentrations of several salivary cytokines were increased in RTT compared to controls.
-
•
These same cytokines showed significant positive correlations with clinical severity scores.
-
•
Results suggest that salivary cytokines may be a possible indicator of immune dysregulation in RTT.
Rett syndrome (RTT) is a neurodevelopmental genetic disorder caused, in most cases, by loss-of-function mutations to the X-linked methyl-CpG-binding protein (MECP2) gene (Amir et al., 1999). It is characterized by apparently normal early development followed by a loss of acquired communication and motor skills. Following this regression period, affected individuals typically develop stereotyped hand movements, as well as comorbid health problems such as epilepsy, gastrointestinal dysfunction, and autonomic dysfunction (Neul et al., 2010). There is increasing evidence that mitochondrial dysfunction and immune dysregulation may play a role in the development of RTT (Cortelazzo et al., 2014; Derecki et al., 2013; Cronk et al., 2015; Leoncini et al., 2015; Pecorelli et al., 2016), with evidence of altered cytokine levels in blood/plasma (Leoncini et al., 2015), and significant correlations between levels of several specific cytokines and clinical severity.
There has been increasing interest in the use of salivary biomarkers for evaluation of disease processes, as saliva is non-invasive and painless to collect, and does not require highly trained collection staff (e.g., Shields et al., 2019). It has been suggested that, among the general population, salivary cytokine dysregulation more accurately reflects an individual’s oral health environment than their overall systemic immune function (e.g., Riis et al., 2014). However, in Down syndrome, another neurodevelopmental disorder characterized by immune dysregulation, oral cytokine concentrations do not appear to be related to oral health, suggesting that different mechanisms may underlie oral cytokine productions among at least some populations with genetic neurodevelopmental disorders (Tsilingaridis et al., 2012). This suggests that different mechanisms may underlie oral cytokine production among at least some populations with genetic neurodevelopmental disorders. Together with the practical advantages of saliva as a diagnostic biofluid, the evidence that oral cytokines may reflect systemic processes within certain clinical groups warrants further investigation. The purpose of the current study was to evaluate the potential validity of salivary cytokines as biomarkers of immune dysregulation in RTT. To this end, we sought to answer two specific research questions: a) Are there significant differences in concentrations of salivary cytokines between a sample of individuals with RTT and a group of age-matched healthy females? and b) Are there relationships between concentrations of salivary cytokines and overall clinical severity within the RTT sample?
1. Materials and methods
1.1. Participants
A total of 18 females with confirmed pathogenic mutations of MECP2 and clinical diagnoses of classic RTT were recruited through Gillette Children’s Specialty Healthcare. Control participants were selected from among a larger set of female participants recruited at the University of Minnesota Driven to Discover research building at the Minnesota State Fair matched as closely as possible (e.g., within 12 months) to the participants with RTT. Potential participants with known chronic health conditions were excluded from participating in the control group. All participants (or their parent/legal guardian, as applicable) provided informed consent prior to participation in the study. All study activities were approved by the University of Minnesota Institutional Review Board.
Two samples from each of the two groups were excluded due to below detectable levels of all cytokines, resulting in a final sample of 32 participants: 16 in the RTT group (mean age: 18.5 years, range: 2–40), and 16 in the comparison group (mean age: 17.1 years, range: 2–40). Both groups consisted of 15 (94%) white and non-Hispanic/Latina participants, and one (6%) Asian participant. Among those in the control group, 3 (19%) were prescribed some form of hormonal birth control, and one (6%) an over the counter allergy medication for seasonal allergies. The remaining control participants did not report use of any medications daily. Demographic characteristics and medication status of each of the participants in the RTT sample are reported in Table 1. Although all participants had received formal genetic testing documenting pathogenic mutations of MECP2, two parents no longer had access to the original testing documentation and could not recall the specific mutation information.
Table 1.
Clinical characteristics of participants with RTT.
| ID | Age | Mutation | Medications |
Eats orally | Kerr CSS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seizures | Laxatives/acid reducers | Anxiety/depression | Hormonal contraceptives | Anticholinergics | Steroids | |||||
| 1 | 2 | P156G | + | + | – | – | – | – | Y | 24 |
| 2 | 3 | P152R | – | – | – | – | – | – | Y | 10 |
| 3 | 6 | T158M | + | – | – | – | – | – | N | 25 |
| 4 | 8 | G128P | + | + | – | – | – | – | Y | 13 |
| 5 | 9 | R270X | – | + | – | – | – | – | Y | 21 |
| 6 | 10 | Q152P | + | – | – | – | + | + | N | 26 |
| 7 | 14 | Deletion | + | + | – | – | – | – | Y | 15 |
| 8 | 16 | Ins/del | + | + | – | – | – | – | Y | 28 |
| 9 | 21 | K144X | + | + | + | – | – | – | Y | 20 |
| 10 | 22 | R294X | + | + | – | – | – | – | N | 33 |
| 11 | 23 | R294X | + | + | – | – | + | + | N | 31 |
| 12 | 23 | R133C | – | + | + | + | + | – | Y | 15 |
| 13 | 30 | R280X | – | + | + | – | – | – | Y | 18 |
| 14 | 30 | R133C | + | + | + | + | – | – | Y | 24 |
| 15 | 39 | MEPC2* | + | – | – | – | – | + | Y | 32 |
| 16 | 40 | MEPC2* | – | + | – | – | – | – | Y | 14 |
Note: *All participants had genetic testing that identified a pathogenic mutation of MECP2, but not all parents knew the specific mutation information.
1.2. Saliva collection and preparation
Due to the significant communication and motor impairments among the RTT group, whole saliva samples could not be obtained using the passive drool approach. Instead, approximately 3 mL of unstimulated saliva was collected from all participants using Toothette® oral swabs (Sage products, Cary IL, USA). To do so, the inside of the participant’s mouth was swabbed using 3–4 swabs, which were then placed into centrifuge tubes, and spun at 3000 rpm for 5 min to extract the saliva from the sponges. Samples were aliquoted into 100 μL cryovials and frozen at −80C.
1.3. Saliva assays
All assays were completed at the University of Minnesota Department of Pediatrics Cytokine Reference Laboratory, a CLIA-88 licensed facility. A commercially-available 12-plex human cytokine Luminex high performance assay (LBHS000; R&D Systems, Minneapolis, MN, USA) was used to evaluate levels of salivary cytokines: for IL-1 β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, GM-CSF, IFN-γ, TNF-α, and VEGF. Dilution series and standard curves were run for all samples. All assays were performed in duplicate. All cytokine levels are reported in pg/mL. The inter-assay and intra-assay CVs for these analytes are below 15% and 10%, respectively, according to the manufacturer.
1.4. Clinical information
For the participants with RTT, clinical severity was assessed using the Kerr clinical severity scale (Kerr et al., 2001). This scale includes 20 items reflecting common symptoms of RTT scored on a 3-point scale, ranging from 0 to 2, with higher scores reflecting greater symptom severity. Items were scored based on a combination of parent/caregiver reported survey items, review of medical records, and in-person assessment.
1.5. Data management and statistical analyses
All variable distributions were evaluated for potential outliers prior to analysis. For cytokine levels that were below the detection range for the assay, the value for each cell was replaced by half of the lower limit of detection (e.g., Zhang et al., 2004). Because most of the distributions of the cytokine levels were highly skewed, variables that deviated significantly from normality were log-transformed prior to statistical analysis. Descriptive statistics are reported as untransformed means and inter-quartile ranges. Independent samples T-tests were used to compare differences in mean cytokine levels by medication status, and between the RTT and control groups. Because clinical severity generally increases with age among individuals with RTT (Cuddapah et al., 2014), partial correlations controlling for age were used to evaluate relationships between cytokine levels and clinical severity scores within the RTT group. P-values associated with the T-tests and correlations were corrected for multiple comparisons using the Benjamini-Hochberg correction (Benjamini and Hochberg, 1995).
2. Results
Detectable levels of each analyte were found in at least 80% of the samples in both groups for all analytes except IFN- γ, which was detectable in only about half the samples in both groups (see Table 2). None of the analytes were significantly associated with age in either group. There were no differences in concentrations of any of the analytes associated with the use of anticonvulsants, laxatives, acid reducers, medications prescribed anxiety or depression, antihistamines, or anticholinergics in the RTT group (no members of the control group reported any of these medications at the time of participation). When participants from both groups were analyzed together, there were no significant differences in concentrations associated with the use of hormonal contraceptives. When the groups were analyzed separately, however, individuals in the control group taking hormonal contraceptives had significantly lower levels of IL-1β and IL-8 compared to the rest of the control group (see supplemental materials for additional details). There were no significant differences in concentrations by hormonal contraceptive status in the RTT group. Within the RTT group, differences were observed among participants taking oral or inhaled steroids for respiratory issues, which was associated with higher concentrations of IL-1β, IL-6, IL-8, IL-10, GMCSF, TNF-α, and lower concentrations of IL-4. Participants taking steroids also had significantly higher clinical severity scores compared to the rest of the group. Because it was unclear whether hormonal contraceptives and/or steroids affected concentrations of the cytokines, all subsequent analyses were conducted with the full sample, then replicated using a reduced sample excluding participants taking either of those medication classes, as a conservative test of the patterns of results. There were few differences between the two sets of analyses and thus only the results of the full sample are reported (results of these analyses are available in supplemental materials).
Table 2.
Untransformed means and interquartile ranges (IQR) for all cytokines (in pg/mL) for both groups, and correlations between concentrations and clinical severity in the RTT group.
| Analyte | LLOD (pg/mL) | Control |
Rett |
Group diff. p-value | Correlation with CSSb | Correlation p-value | ||
|---|---|---|---|---|---|---|---|---|
| % detect | Median (IQR) | % detect | Median (IQR) | |||||
| IL-1βa | 0.10 | 100 | 38.8 (27.6–66.7) | 100 | 142.9 (74.8–479.3) | .002 | .815 | .001 |
| IL-2 | 0.03 | 81 | 0.42 (0.17–1.39) | 94 | 0.65 (0.25–1.34) | .488 | -.197 | .598 |
| IL-4 | 1.00 | 100 | 23.1 (20.0–41.2) | 100 | 22.4 (18.7–35.8) | .431 | -.190 | .598 |
| IL-5 | 0.06 | 100 | 0.38 (0.21–0.94) | 100 | 0.42 (0.22–0.65) | .383 | -.073 | .868 |
| IL-6a | 0.09 | 100 | 1.65 (0.91–2.38) | 100 | 3.96 (2.03–10.65) | .006 | .817 | .001 |
| IL-8a | 0.08 | 100 | 189.0 (115.6–348.9) | 100 | 671.9 (271.5–3763.8) | .002 | .824 | .001 |
| IL-10a | 0.10 | 94 | 0.69 (0.41–1.14) | 100 | 1.58 (0.96–3.06) | .051 | .788 | .001 |
| IL-12p70a | 0.24 | 63 | 0.48 (0.12–6.69) | 81 | 1.31 (0.24–4.37) | .765 | -.214 | .598 |
| GM-CSF | 0.30 | 100 | 0.79 (0.56–1.33) | 100 | 2.05 (0.24–4.37) | .004 | .725 | .004 |
| IFN-γa | 0.10 | 50 | 0.36 (0.05–1.42) | 56 | 0.34 (0.05–1.36) | .983 | .044 | .875 |
| TNF-αa | 0.10 | 100 | 7.72 (4.93–10.44) | 100 | 11.01 (6.57–24.25) | .051 | .781 | .001 |
| VEGFa | 0.10 | 100 | 209.4 (156.8–346.0) | 100 | 533.3 (356.9–710.1) | .006 | .512 | .087 |
Note: LLOD: Lower limit of detection for each assay.
Denotes variable was log-transformed for all statistical analyses.
CSS: Clinical severity score. Correlations between concentrations and clinical severity score within RTT only.
2.1. Differences between the RTT and control groups
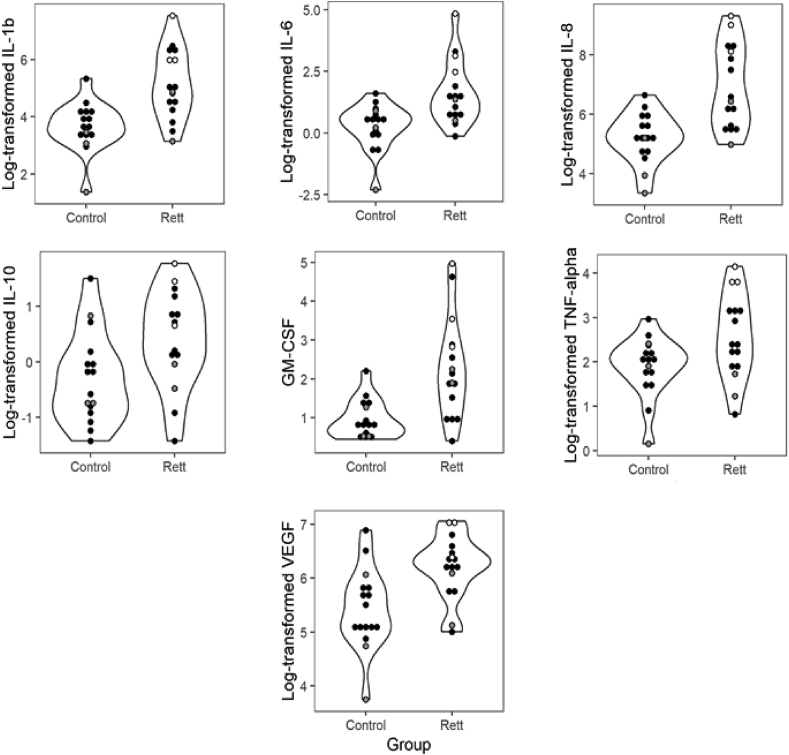
Elevated levels of IL-1β, IL-6, IL8, IL-10, GM-CSF, TNF- α and VEGF were observed in the RTT group compared to the control group (see Fig. 1). None of the cytokines analyzed were significantly lower in the RTT group compared to controls. There were also discrepancies in the distributions of the concentrations across the two groups, with the RTT group showing a much wider range of values for IL-1 β, IL-6, IL-8, GM-CSF, and TNF-α compared to controls, but IL-2, IL-4, and IL-5 showing less variability in the RTT group compared to controls.
Fig. 1.
Distributions of concentrations of the salivary analytes that differed between the control and Rett syndrome groups. White centers represent participants who were taking steroids and gray circles represent participants taking hormonal contraceptives at the time of collection.
2.2. Correlations with clinical severity
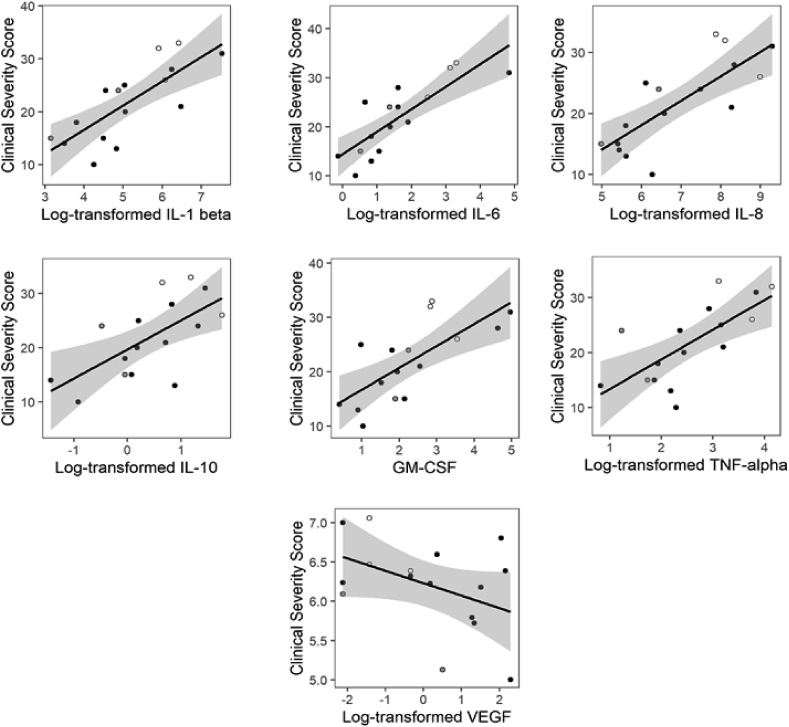
Among the cytokines that were elevated in the RTT group, IL-1β, IL-6, IL8, IL-10, GM-CSF, and TNF-α showed showing very strong associations (r > 0.70) with clinical severity scores, however VEGF was not significantly correlated with clinical severity (see Fig. 2).
Fig. 2.
Relationships between concentrations of salivary analytes and clinical severity scores in the Rett syndrome group. White centers represent participants who were taking steroids, and gray circles represent participants taking hormonal contraceptives at the time of collection.
3. Discussion
To the best of our knowledge, this is the first study examining levels of salivary cytokines among individuals with RTT, and evaluating relationships between concentrations of these analytes and clinical severity. The results suggest that several cytokines are increased in the saliva of individuals with RTT compared to healthy, age-matched females, providing further evidence for a role of inflammation or immune dysregulation in RTT. Specifically, concentrations of IL-1β, IL-6, IL8, IL-10, and TNF-α were all elevated in the RTT samples compared to the control group and were strongly associated with clinical severity scores. These findings appear to be relatively robust in that the associations remained even after removing several participants who were taking medications that may have affected the results.
Two studies have previously examined cytokine levels in the blood/serum of individuals with RTT, with some divergent results. Pecorelli et al. reported significantly elevated concentrations of IL-8 in the serum of individuals with RTT compared to healthy controls. They did not find any significant differences between the groups in concentrations of IL-1β, IL-4, IL-5, IL,6, IL-10, IL-12, GM-CSF, IFN-γ, TNF-α, or VEGF. It should be noted, however, that the study only had 10 participants with RTT and 8 controls, and that these participants were not matched for age or sex, which may account for some of the null findings. In contrast, Leoncini et al. (2015) reported significant elevations in IL-4, IL-5, IL-6, IL-8, and TNF-α, significant decreases in IFN-γ and IL-12p70, and no significant differences in IL-1β and IL-10 in the blood of 16 individuals with RTT compared to a sample of 24 healthy females of comparable age. The results of the current study are somewhat consistent with the results of Leoncini and colleagues in that we identified significant increases in IL-6, IL-8, and TNF-α. Notably, all of these cytokines are produced by macrophages, which have been shown to be direct targets of MeCP2 (Cronk et al., 2015; Schafer and Stevens, 2015).
On the other hand, we saw increased concentrations of analytes that were unchanged in previous studies examining blood/serum concentrations. Some of these (i.e., IL-1β, GM-CSF) are produced by macrophages, whereas IL-10 and VEGF are not. This saliva/blood discrepancy is consistent with previous work in Down syndrome, as Tsilingaridis et al. (2012) found that concentrations of eight of nine cytokines evaluated were elevated in the gingival crevicular fluid of individuals with Down syndrome compared to healthy controls (the ninth analyte was not significantly different between the groups), whereas Cetiner et al. (2010) found elevated levels of IL-4 and IL-10, but decreased levels of IL-6 and TNF-α in the serum of individuals with Down syndrome. These discrepancies suggest that, although production of cytokines in the blood and oral environments are both altered in at least some neurogenetic disorders, the specific mechanisms underlying these changes may be distinct.
A limitation of the current study is that we did not collect information on the oral health of the participants in either group, and factors such as periodontal disease are known to affect cytokine levels among individuals without systemic diseases. As noted previously, however, this relationship was not found in a sample of individuals with Down syndrome. Additionally, a study of salivary cytokines in children with cerebral palsy (Santos et al., 2017) found a similar dissociation between cytokine concentrations and oral health measures among children with more severe motor impairments, suggesting that the dissociation may not be specific to genetic neurodevelopmental syndromes. Although individuals with RTT do present with oral health problems (Fuertes-González and Silvestre, 2014), considering the strength of the relationships between clinical severity and cytokine concentrations, it seems unlikely that oral health alone would account for the findings. Nevertheless, future research should address the relationships between oral health and salivary cytokine concentrations in RTT and other neurodevelopmental syndromes.
Another limitation of the study is that the sample size, although relatively large for a rare disorder, was not large enough to allow for more fine-grained analyses of the relationships between specific clinical features and cytokine levels. Previous studies have documented relationships between cytokine levels and epilepsy (Yu et al., 2012), which may have played a role in the current findings. There were no significant differences in concentrations within this sample with regards to epilepsy treatment status, but the relatively small sample may have obscured potential differences. We did observe differences in cytokine concentrations within the control group with regard to hormonal contraceptives, but we did not see the same pattern within the RTT group, and removing participants who were using hormonal contraceptives did not change any of the patterns observed. The number of participants in both groups using birth control was very small, however, resulting in two potential explanations for the results: a) the effect was not observed in the RTT group due to inadequate statistical power, or b) the effect observed in the control group was spurious due to the small samples. Nevertheless, future studies should examine this factor, as, to our knowledge, there is no research specifically evaluating the impact of hormonal contraceptives on salivary cytokine concentrations. We also observed differences in cytokine concentrations among the three participants with RTT who were using steroids during the study. Although it is likely that steroids would have an impact on immune and inflammatory processes, thereby resulting in changes in cytokine concentrations, it remains unclear whether the differences observed in the study were attributable to steroid use, or whether the relationship would be explained primarily by the relationship between cytokine concentrations and clinical severity. As the relationships remained statistically significant when these participants were excluded from analyses, it seems likely that the steroids did not have a large enough impact to alter the general patterns of results, but additional research is needed to fully elucidate these relationships.
4. Conclusions
This study is the first to examine salivary cytokine concentrations among individuals with RTT and provides evidence of disruption to the oral immune environment associated with MeCP2 deficiency. The strong correlations between the cytokine concentrations and clinical severity provide additional support for the potential role of immune dysregulation in the etiology of RTT symptoms and suggest that measurement of salivary cytokines may provide a useful non-invasive, and less resource-intensive alternative to blood/serum collection.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Funding for this study was provided by grant #3116 from Rettsyndrome.org (grant #3116; PI: Symons), the Mayday Fund (grant #11211; PI: Symons), and the Eunice Kennedy Shriver National Institute for Child Health and Human Development (grants #44764; PI: Symons, and #101075; PI: Byiers).
Acknowledgements
Funding for this study was provided by grant #3116 from Rettsyndrome.org (grant #3116), the Mayday Fund (grant #11211), and the Eunice Kennedy Shriver National Institute for Child Health and Human Development (grant #44764). The authors gratefully acknowledge all of the families who participated in the study, as well the Gillette Children’s Specialty Healthcare Rett Syndrome Clinic, the Midwest Rett Syndrome Foundation, RettSyndrome.org, and the University of Minnesota Driven 2 Discover program for their assistance. The authors would also like to thank Michael Gleaves and John Blaeser for their assistance with data collection and processing for the healthy control samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2019.100008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Ser. Soc. B Stat. Methodol. 1995 doi: 10.1111/j.2517-6161.1995.tb02031.x. [WWW document] [DOI] [Google Scholar]
- Cetiner S., Demirhan O., Inal T.C., Tastemir D., Sertdemir Y. Analysis of peripheral blood T-cell subsets, natural killer cells and serum levels of cytokines in children with Down syndrome. Int. J. Immunogenet. 2010;37:233–237. doi: 10.1111/j.1744-313X.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- Cortelazzo A., De Felice C., Guerranti R., Signorini C., Leoncini S., Pecorelli A., Zollo G., Landi C., Valacchi G., Ciccoli L. Subclinical inflammatory status in Rett syndrome. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/480980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk J.C., Derecki N.C., Ji E., Xu Y., Lampano A.E., Smirnov I., Baker W., Norris G.T., Marin I., Coddington N., Wolf Y., Turner S.D., Aderem A., Klibanov A.L., Harris T.H., Jung S., Litvak V., Kipnis J. Methyl-CpG binding protein 2 regulates microglia and macrophage gene expression in response to inflammatory stimuli. Immunity. 2015;42:679–691. doi: 10.1016/j.immuni.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddapah V.A., Pillai R.B., Shekar K.V., Lane J.B., Motil K.J., Skinner S.A., Tarquinio D.C., Glaze D.G., McGwin G., Kaufmann W.E., Percy A.K., Neul J.L., Olsen M.L. Methyl-CpG-binding protein 2 (MEPC2) mutation type is associated with disease severity in Rett Syndrome. J. Med. Genet. 2014;51:152–158. doi: 10.1136/jmedgenet-2013-102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki N.C., Cronk J.C., Kipnis J. The role of microglia in brain maintenance: implications for Rett syndrome. Trends Immunol. 2013;34:144–150. doi: 10.1016/j.it.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes-González M.C., Silvestre F.J. Oral health in a group of patients with Rett syndrome in the regions of Valencia and Murcia (Spain): a case-control study. Med. Oral Patol. Oral Cir. Bucal. 2014;19:e598–e604. doi: 10.4317/medoral.19743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr A.M., Nomura Y., Armstrong D., Anvret M., Belichenko P.V., Budden S., Cass H., Christodoulou J., Clarke A., Ellaway C. Guidelines for reporting clinical features in cases with MECP2 mutations. Brain Dev. 2001;23:208–211. doi: 10.1016/s0387-7604(01)00193-0. [DOI] [PubMed] [Google Scholar]
- Leoncini S., De Felice C., Signorini C., Zollo G., Cortelazzo A., Durand T., Galano J.-M., Guerranti R., Rossi M., Ciccoli L. Cytokine dysregulation in MECP2-and CDKL5-related Rett syndrome: relationships with aberrant redox homeostasis, inflammation, and $ømega$-3 PUFAs. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/421624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neul J.L., Kaufmann W.E., Glaze D.G., Christodoulou J., Clarke A.J., Bahi-Buisson N., Leonard H., Bailey M.E., Schanen N.C., Zappella M. Rett syndrome: revised diagnostic criteria and nomenclature. Ann. Neurol. 2010;68:944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecorelli A., Cervellati F., Belmonte G., Montagner G., Waldon P., Hayek J., Gambari R., Valacchi G. Cytokines profile and peripheral blood mononuclear cells morphology in Rett and autistic patients. Cytokine. 2016;77:180–188. doi: 10.1016/j.cyto.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Riis J.L., Out D., Dorn L.D., Beal S.J., Denson L.A., Pabst S., Jaedicke K., Granger D.A. Salivary cytokines in healthy adolescent girls: intercorrelations, stability, and associations with serum cytokines, age, and pubertal stage. Dev. Psychobiol. 2014;56:797–811. doi: 10.1002/dev.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M.T.B.R., Diniz M.B., Guaré R.O., Ferreira M.C.D., Gutierrez G.M., Gorjão R. Inflammatory markers in saliva as indicators of gingival inflammation in cerebral palsy children with and without cervical motor control. Int. J. Paediatr. Dent. 2017;27:364–371. doi: 10.1111/ipd.12270. [DOI] [PubMed] [Google Scholar]
- Schafer D.P., Stevens B. Brains, blood, and guts: MeCP2 regulates microglia, monocytes, and peripheral macrophages. Immunity. 2015;42:600–602. doi: 10.1016/j.immuni.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Shields G.S., Slavich G.M., Perlman G., Klein D.N., Kotov R. The short-term reliability and long-term stability of salivary immune markers. Brain Behav. Immun. 2019 doi: 10.1016/j.bbi.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilingaridis G., Yucel-Lindberg T., Modéer T. T-helper-related cytokines in gingival crevicular fluid from adolescents with Down syndrome. Clin. Oral Investig. 2012;16:267–273. doi: 10.1007/s00784-010-0495-6. [DOI] [PubMed] [Google Scholar]
- Yu N., Di Q., Hu Y., Zhang Y., Su L., Liu X., Li L. A meta-analysis of pro-inflammatory cytokines in the plasma of epileptic patients with recent seizure. Neurosci. Lett. 2012;514:110–115. doi: 10.1016/j.neulet.2012.02.070. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lennox W.C., Panu U.S. Effect of percent non-detects on estimation bias in censored distributions. J. Hydrol. 2004;297:74–94. doi: 10.1016/j.jhydrol.2004.04.017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.