Abstract
Background and objectives
People with HIV (PWH) often suffer from depressive symptoms which have a deleterious impact on numerous domains including antiretroviral adherence and quality of life. In the general population, a treatment-resistant phenotype of depression is associated with systemic inflammation, which is of considerable importance as it responds favorably to anti-inflammatory medications. Aging PWH experience increasing inflammation. We sought to evaluate the impact of chronic inflammation in aging PWH on depressed mood.
Methods
PWH were recruited at 6 U.S. academic medical centers. Depressed mood was assessed using the Beck Depression Inventory (BDI)-II. Inflammatory biomarkers measured at the 12-year follow-up visit in blood plasma using immunoassays were neopterin, sTNFRII, d-dimer, IL-6, CRP, MCP-1, sCD14 and sCD40L. Factor analyses with oblique Equamax rotation were employed to reduce the dimensionality of the biomarkers.
Results
Participants were 78 PWH, 14 (17.9%) women, 40 (51.3%) non-White, mean age 55.3 (±SD 8.29), with a nadir and current CD4 of 134 (IQR 36, 204) and 567 (316, 797), respectively. 80.5% were virally suppressed. A factor analysis of the eight inflammatory biomarkers in plasma at the 12-year follow-up visit yielded 3 Factors, with Factor 1 loading on neopterin and sTNFRII, Factor 2 loading on d-dimer, IL-6 and CRP, and Factor 3 loading on sCD40L (MCP-1 and sCD14 did not appear in any of the factors). Univariate regressions of each factor vs BDI-II scores yielded significance only for Factor 2 (r = 0.295; p = 0.0083 (Bonferroni-adjusted p = 0.0261). Of the Factor 2 component biomarkers, BDI-II scores correlated significantly with d-dimer and IL-6, but not CRP. Women had worse BDI-II scores (p = 0.0127). In a logistic regression with sex and Factor 2, both variables were significant (sex p = 0.0246, Factor 2 p = 0.0168). The relationship between Factor 2 and BDI was significant for men (r = 0.348 [95% CI 0.111, 0.547]; p = 0.0049), but not women (r = 0.0580 95% CI -0.488, 0.571]; p = 0.844). Viral suppression was not significant in the multivariate model.
Conclusions
Some PWH with depressed mood have elevated markers of inflammation in blood. Men showed this relationship, while women did not. Together with previous findings that an inflammatory depression phenotype responds to treatment with anti-inflammatory medications, our findings suggest that treatment with anti-inflammatory medications might benefit at least a subset of depressed PWH who have a high inflammatory biomarker profile, as well as poor response to antidepressant medications alone, and that the pathophysiology of depression in men and women with HIV may differ.
Keywords: Depression, Inflammation, Sex differences, HIV infection, Quality of life
1. Introduction
Despite viral suppression on combination antiretroviral therapy (ART), people with HIV (PWH) suffer from depressed mood and chronic inflammation. Depression is the most common psychiatric comorbidity in HIV (Antelman et al., 2007; Bing et al., 2001; Ciesla and Roberts, 2001). Depressed PWH show poorer medication adherence (Gonzalez et al., 2011), lower rates of viral suppression (Ickovics et al., 2001; Ironson et al., 2005), greater polypharmacy (refs), poorer quality of life (Bengtson et al., 2015; Zimpel and Fleck, 2014) and shorter survival (French et al., 2009; Villes et al., 2007).
A subtype of treatment-resistant depression in the general population is associated with chronic inflammation (Haroon et al., 2018; Jha, 2019). The potential clinical significance of this is high, since the anti-inflammatory TNF-alpha blocker tocilizumab and other drugs such as the antibiotic minocycline, the interleukin 17 (IL-17) receptor antibody, brodalumab, and the monoclonal antibody, sirukumab, have been shown to be effective treatment for this depression subtype (Khandaker et al., 2018), but these have not been studied in the context of HIV. Inflammation is associated with greater symptom severity, differential response to treatment, and greater odds of hospitalization in patients with major depressive disorder (MDD).
Chronic inflammation persists in virally suppressed PWH and predicts morbidity and mortality (Tenorio et al., 2014). There also is an extensive literature on showing that depression correlates with markers of inflammation and immune activation in PWH (e.g., elevated levels of Il-6 and TNF-α) (Norcini Pala et al., 2016; Hellmuth et al., 2017; Musinguzi et al., 2018; Rivera-Rivera et al., 2014), but most of these studies were performed in individuals who were not virally suppressed. We hypothesized that inflammation in virally suppressed PWH would be associated with poorer mood.
2. Methods
Participants underwent standardized clinical and laboratory evaluations at 6 U.S. academic centers in the CHARTER study at baseline and 12 years later at follow-up. Inclusion criteria included HIV seropositivity by Western blot. Exclusion criteria were active neurological illnesses other than HIV, visible intoxication as determined by clinicians trained to recognize signs of this and active psychiatric or substance use disorder (eg, psychosis) that might interfere with completing study evaluations. All participants signed an IRB-approved informed consent document.
2.1. Laboratory evaluations
HIV disease was diagnosed by enzyme-linked immunosorbent assay with Western blot confirmation. HIV viral load in plasma was measured using commercial assays and deemed undetectable at a lower limit of quantitation (LLQ) of 50 copies/ml. CD4 T cells were measured by flow cytometry and nadir CD4 was assessed by self-report. Inflammatory biomarkers measured in blood plasma at the 12-year follow-up visit using immunoassays were neopterin, soluble tumor necrosis factor alpha type II (sTNFRII), d-dimer, interleukin-6 (IL-6), C-reactive protein (CRP), monocyte chemoattractant protein type I (MCP-1), soluble CD14 (sCD14) and soluble CD40 ligand (sCD40L). We selected these markers based on previous studies linking them to depressed mood (Celik et al., 2010; Grassi-Oliveira et al., 2009; Anderson et al., 2013; von Kanel et al., 2009; Kiecolt-Glaser et al., 2018; Leo et al., 2006). Biomarkers were measured only at the 12-year follow-up visit, and correlations were assessed with BDI-II at the same visit, and secondary at the initial visit.
2.2. Clinical evaluations
Current mood at baseline and 12-year follow-up was assessed using the Beck Depression Inventory (BDI)-II (Beck et al., 1996). Lifetime major depressive disorder (MDD) and substance use disorders were assessed using the computer-assisted Composite International Diagnostic Interview (CIDI) (Nelson, 1999), a structured instrument widely used in psychiatric research. The CIDI classifies current and lifetime diagnoses of mood disorders and substance use disorders, as well as other mental disorders. Additional assessments measured activities of daily living, disability, employment and quality of life. Quality of life was assessed using the Medical Outcomes Study HIV Health Survey Short Form 36 (MOS-HIV SF-36), a reliable and valid tool for assessing overall quality of life, daily functioning, and physical health (Wachtel et al., 1992; Wu et al., 1997). The MOS-HIV contains 36 questions that assess various physical and mental dimensions of health. Items are grouped into two overall categories (Physical and Mental Health), with 11 subcategories (Physical functioning, Role functioning, Pain, Social functioning, Emotional well-being, Energy/fatigue, Cognitive functioning, General health, Health distress, Overall QoL, Health transition). These are scored as summary percentile scales ranging from 0 to 100, with higher scores indicating better health. Disability was assessed using the Karnofsy Scale (Mor et al., 1984). Dependence in instrumental activities of daily living (IADLs) was assessed with a modified version of the Lawton and Brody Scale (Lawton and Brody, 1969) that asks participants to rate their current and best lifetime levels of independence for 13 major IADLs such as shopping, financial management, transportation, and medication management (Heaton et al., 2004). An employment questionnaire asked about job loss, decreases in work productivity, accuracy, and quality; increased effort required to do one’s usual job; and increased fatigue with the usual workload. Neurocognitive function was assessed using a comprehensive, standardized battery described in detail previously (Heaton et al., 2010). The battery covered 7 cognitive domains known to be commonly affected by HIV-associated CNS dysfunction. The best available normative standards were used, which correct for effects of age, education, sex, and ethnicity, as appropriate. Test scores were automatically converted to demographically corrected standard scores (t scores) using available computer programs.
2.3. Statistical analyses
Demographic and clinical characteristics were summarized using means and standard deviations, median and interquartile ranges or percentages, as appropriate. Log10 transformation was used to normalize the biomarker distributions for parametric analysis. Factor analyses with oblique Equamax rotation were employed to reduce the dimensionality of the biomarkers. Factor analysis is a statistical method used to describe variability among observed, correlated variables in terms of a potentially lower number of unobserved variables called factors. Thus, factor analysis is a method for dimensionality reduction and can help control false discovery. It is important to check the identified factors against known physiological relationships. To validate the factors, we examined intercorrelations between the biomarkers assigned to each factor. Pearson’s r and Spearman’s rho were calculated to compare factors with BDI-II scores. Secondary analyses evaluated correlations with quality of life (MOS-HIV), neurocognitive function, and employment status. We used multivariable linear regression models to test interaction effects. In the absence of an interaction, additive effects were tested. Analyses were conducted using JMP Pro® version 15.0.0 (SAS Institute Inc., Cary, NC, 2018).
3. Results
Participants were 78 PWH, 14 (17.9%) women, 40 (51.3%) non-White, mean age 55.3 (8.29), with a nadir and current CD4 of 134 (IQR 36, 204) and 567 (316, 797), respectively. 80.5% were virally suppressed on ART. The median interval between baseline and follow-up was 12.3 years [IQR 12.1, 12.7]. 26 participants 33.3% took antidepressant medications, and those on antidepressants had marginally higher BDI-II scores (12.7 ± 8.54 versus 8.23 ± 10.3; p = 0.0607). No participants met criteria for current DSM substance use disorder based on structured psychodiagnostic interview (Composite International Diagnostic Interview) and none were visibly intoxicated during evaluations.
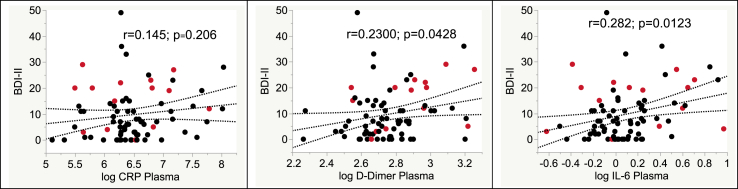
A factor analysis with Equamax rotation and a loading value cutoff of 0.4 for the eight inflammatory biomarkers in plasma at the 12-year follow-up visit yielded 3 Factors, with Factor 1 loading on neopterin and sTNFRII, Factor 2 loading on d-dimer, IL-6 and CRP, and Factor 3 loading on sCD40L (MCP-1 and sCD14 did not appear in any of the factors). Univariate regressions of each factor vs BDI-II scores yielded significance only for Factor 2 (r = 0.295; p = 0.0083 (Bonferroni-adjusted p = 0.0261; Spearman rho 0.309; p = 0.003). Of the Factor 2 component biomarkers, BDI-II scores correlated significantly with d-dimer and IL-6, but not CRP (Fig. 1), though the direction of the latter correlation was consistent with those of the other biomarkers. Higher Factor 2 scores correlated with worse scores on the Apathy (r = 0.355; p = 0.0014), Somatic (r = 0.302; p = 0.0071) and Affective (r = 0.341; p = 0.0023) subscales of the BDI-II, but not with the Cognitive subscale (r = 0.18; p = 0.121). BDI-II scores at baseline and 12-year follow-up were highly correlated (r = 0.653; p < 0.0001). Factor 2 (at 12-years) was related to the total BDI-II at the baseline visit 12 years earlier as well (r = 0.257; p = 0.0234).
Fig. 1.
Correlations of individual biomarker components of Factor 2 with BDI-II scores. Red points, women; black points, men. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
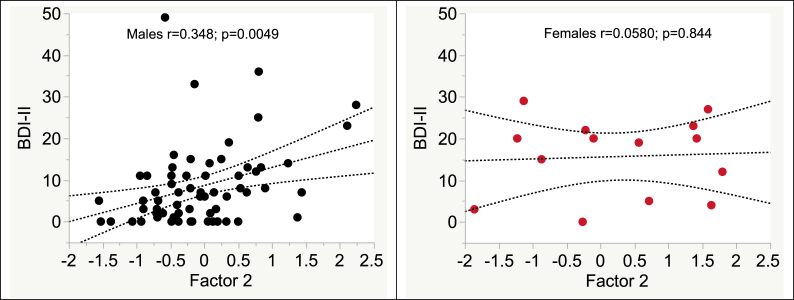
Women had worse BDI-II scores (p = 0.0127) and non-significantly higher values on Factor 2 than men (0.241 ± 1.22 vs −0.0527 ± 0.771; p = 0.253). In a multivariable regression with sex and Factor 2 predicting BDI-II scores, both variables were significant (sex p = 0.0246, Factor 2 p = 0.0168). As shown in Fig. 2 The relationship between Factor 2 and BDI was significant for men (r = 0.348 [95% CI 0.111, 0.547]; p = 0.0049), but not women (r = 0.0580 95% CI -0.488, 0.571]; p = 0.844). Factor 2 scores were not influenced by age (p = 0.097), sex (p = 0.253) or ethnicity (p = 0.115), and did not associate with lifetime MDD. Non-Hispanic whites had higher BDI-II scores than other ethnicities combined (12.5 ± 11.2 vs 7.1 ± 7.85; p = 0.0158). In a multivariable regression, both ethnicity and Factor 2 values remained significant (p = 0.00202 and 0.00114, respectively). No interaction between ethnicity and Factor 2 values was found. Plasma viral suppression was not related to Factor 2 (detectable vs undetectable, mean ± SD, 0.00585 ± 0.696 vs 0.0158 ± 0.905; p = 0.9685) and viral suppression was not significant in the multivariate model. BDI-II scores were not influenced by viral suppression (p = 0.674), nadir (p = 0.539) or current CD4 (p = 0.337) or ART use (p = 0.995). BDI-II scores were not related to age (p = 0.516).
Fig. 2.
Higher Factor 2 scores, loading on d-dimer, IL-6 and CRP, were associated with worse depression in men (panel A), but not women (panel B). Red points, women; black points, men. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.1. Impact of depressed mood and factor 2 scores on quality of life, disability, activities of daily living and cognitive complaints
Higher BDI-II scores were strongly associated with poorer overall physical and mental quality of life as measured by the HIV-MOS (r = −0.535, p < 0.0001; r = −0.839, p < 0.0001, respectively). Among the MOS subscales, higher Factor 2 scores were significantly associated with worse Physical functioning, Role functioning, Pain, Social functioning, General health, Health distress and Overall QoL, but not with Cognitive function, Mental health, Energy/fatigue, Quality of life and Health transition. For every 10-point increase in BDI-II, the odds of being dependent in instrumental activities of daily living increased by 2.85 (95% CI 1.62, 5.62). Worse disability as measured by the Karnofsy index were correlated with both Factor 2 scores were (r = −0.300; p = 0.0077) and worse BD-II scores (r = −0.50866; p < 0.0001). Individuals who used opiates as assessed by urine toxicology had marginally higher Factor 2 scores than those with negative urine toxicology (0.478 ± 0.824 versus −0.0859 ± 0.832 p = 0.0738). Inclusion of urine toxicology in a multivariable model did not alter the significance of the relationship between Factor 2 scores and BDI-II (p = 0.0321). On neurocognitive testing, higher Factor 2 scores were significantly associated with worse motor mean T score, but not with the following mean T scores: Global, Verbal, Executive, Speeded information processing, Learning, Recall and Working Memory.
3.2. Substance use disorders
Rates of lifetime substance use disorders were as follows: alcohol abuse, 29.5%; alcohol dependence, 35.9%; cannabis abuse, 35.9%, cannabis dependence, 17.9%; cocaine abuse, 5.13%; cocaine dependence, 29.5%; methamphetamine abuse, 5.13%; methamphetamine dependence, 15.4%; opioid abuse, 12.8%; opioid dependence, 11.5%; any substance disorder, 73.1%. None of the substance use disorders was associated with higher Factor 2 levels; lifetime cannabis abuse was associated with lower Factor 2 levels (0.392 ± 1.032 - versus 0.101 ± 0.797 p = 0.0416). Participants with a lifetime history methamphetamine abuse ever had higher BDI-II scores than those without (14.3 ± 14.7 versus 8.879 ± 8.68); none of the other substance use disorders was associated with BDI-II.
4. Discussion
We found that higher concentrations of a specific panel of markers of inflammation (see below) in blood were seen in PWH with worse depression. Additionally, PWH with depressed mood had markedly reduced quality of life and were more dependent in IADLs. In addition, higher inflammation associated with worse scores on numerous life quality indicators. Chronic HIV-associated inflammation and immune dysfunction have emerged as key factors that are strongly linked to non-AIDS complications (Hsue et al., 2012; Deeks, 2011). Our findings confirm those of previous investigations (Norcini Pala et al., 2016; Hellmuth et al., 2017; Musinguzi et al., 2018; McIntosh et al., 2018; Bekhbat et al., 2018), and extend them by evaluating a more comprehensive panel of biomarkers and more extensive evaluation of impact on daily functioning and quality of life. If the link between inflammation and depression is causal, our results suggest that treatment with selected anti-inflammatory medications might benefit mood and life quality in some PWH.
Depressed mood was specifically associated with a factor loading on d-dimer, IL-6 and CRP. Factor analysis is a statistical method used to describe variability among observed, correlated variables in terms of a potentially lower number of unobserved variables called factors. Thus, factor analysis is a method for dimensionality reduction and can help control false discovery. Additionally, however, it is important to check the identified factors against known physiological relationships. Several prior reports link these specific markers with each other, particularly in the context of HIV, suggesting that they represent a physiologically congruent aspect of the inflammatory cascade. For example, the pro-inflammatory cytokine IL-6 stimulates the production of C-reactive protein in the liver (Gabay and Kushner, 1999). In one study, higher pre-ART CRP, D-dimer, and IL-6 levels were associated with new AIDS events or death (Boulware et al., 2011). Also, in HIV patients, IL-6, hsCRP and D-dimer were intercorrelated and each was associated with an increased risk of cardiovascular disease (CVD) independent of other CVD risk factors (Reinfuss, 1987). In another report, baseline IL-6 and D-dimer were strong predictors of coronary risk in non-HIV-infected individuals and were associated with each other and with CRP (Lowe et al., 2004). Additional support for the coherence of Factor 2 is that its components in this dataset demonstrate robust and statistically significant intercorrelations, while their correlations with other biomarkers are typically weaker and not statistically significant (Table 1).
Table 1.
Biomarker intercorrelations showing that components of Factor 2 (d-dimer, IL-6, CRP; bold typeface) demonstrate robust and statistically significant correlations with each other. Similarly, components of Factor 1 (sTNFR-II, italicized) are highly intercorrelated.
| r | p | ||
|---|---|---|---|
| d-dimer | CRP | 0.3336 | 0.0028 |
| IL-6 | CRP | 0.5163 | <.0001 |
| IL-6 | d-dimer | 0.3472 | 0.0018 |
| MCP-1 | CRP | 0.0934 | 0.4161 |
| MCP-1 | d-dimer | 0.0990 | 0.3884 |
| MCP-1 | IL-6 | 0.1002 | 0.3826 |
| neopterin | CRP | 0.3593 | 0.0012 |
| neopterin | d-dimer | 0.4986 | <.0001 |
| neopterin | IL-6 | 0.1836 | 0.1075 |
| neopterin | MCP-1 | 0.2966 | 0.0084 |
| sCD14 | CRP | 0.0073 | 0.9491 |
| sCD14 | d-dimer | −0.0389 | 0.7351 |
| sCD14 | IL-6 | 0.0266 | 0.8171 |
| sCD14 | MCP-1 | −0.1086 | 0.3439 |
| sCD14 | neopterin | −0.0976 | 0.3952 |
| sCD40L | CRP | 0.1058 | 0.3564 |
| sCD40L | d-dimer | 0.2376 | 0.0362 |
| sCD40L | IL-6 | −0.0022 | 0.9846 |
| sCD40L | MCP-1 | 0.2816 | 0.0125 |
| sCD40L | neopterin | 0.0117 | 0.9192 |
| sCD40L | sCD14 | −0.0783 | 0.4958 |
| sTNFR-II | CRP | 0.3542 | 0.0015 |
| sTNFR-II | d-dimer | 0.4197 | 0.0001 |
| sTNFR-II | IL-6 | 0.2637 | 0.0197 |
| sTNFR-II | MCP-1 | 0.1284 | 0.2625 |
| sTNFR-II | neopterin | 0.6706 | <.0001 |
| sTNFR-II | sCD14 | 0.0422 | 0.7140 |
| sTNFR-II | sCD40L | −0.1111 | 0.3328 |
Previous studies have demonstrated the importance for depression of the specific biomarkers identified in Factor 2. Thus, cognitive symptoms of depression at follow-up were associated with higher baseline plasma levels of CRP and IL-6 at baseline (Gimeno et al., 2009; Dinan, 2009). In depressed patients, increased d-dimer, has been documented{Geiser, 2008 #39}{von Kanel et al., 2009 #26}. In two studies, individuals with a CRP >1 mg/L, the cut off for moderate inflammation, were less likely to respond to SSRIs (Jha et al., 2017; Uher et al., 2014). In a clinical, high CRP was also shown to predict response to the anti-inflammatory drug infliximab, an inhibitor of TNF (Raison et al., 2013).
BDI-II scores at baseline and follow-up were highly correlated. Together with the finding that higher inflammatory markers at 12-year follow-up also were associated with depressed mood at baseline, these findings suggest that depressed mood is an enduring phenotype.
A novel finding in this study was that although women had worse depressive symptoms, the association with inflammatory markers was seen only in men. While perhaps reflecting limited power due to the small number of women, this suggests that the underlying pathophysiology of depression is different in men and women with HIV. Of note, women tended to have higher markers of inflammation than men, consistent with a previous report (Wachtel et al., 1992).
We found worse depression in non-Hispanic whites than in other ethnicities. This is consonant with higher rates of depressive disorders in whites in previous studies (Watkins et al., 2015; Assari and Moazen-Zadeh, 2016). The relationship between inflammation and depressive mood remained after accounting for ethnicity. Inflammation was not related to CD4 or viral load in this cohort of mostly virally suppressed PWH. Unlike other studies, elevations in inflammatory biomarkers were not associated with substance use disorders.
Higher inflammatory biomarkers also were associated with greater disability (Karnofsky), motor impairment, poor physical health (MOS), poorer general health, physical function, role function, social function, pain function, and worse health distress, emphasizing the importance of this phenotype.
Inferences are limited by several factors in this study. There were relatively few women, However, inspection of the scatterplot reveals that there was no suggestion of a trend for an association between inflammation and depression in women. The panel of soluble biomarkers studied was limited, and important associations may have been missed. We did not characterize cellular markers of inflammation in these participants. The absence of a control group precludes consideration of whether effects of inflammation on depression are mediated or otherwise influenced by HIV infection itself.
As noted previously, anti-inflammatory medications have shown promise for treatment-resistance depression. Future studies might evaluate the effectiveness of anti-inflammatory medications for the treatment of depression in PWH selected for the presence of inflammation and treatment resistance.
Declaration of competing interest
The authors report no conflicts of interest
Acknowledgements
The CNS HIV Anti-Retroviral Therapy Effects Research was supported by awards N01 MH22005, HHSN271201000036C, HHSN271201000030C and R01 MH107345 from the National Institutes of Health.
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with Johns Hopkins University; the Icahn School of Medicine at Mount Sinai; University of California, San Diego; University of Texas, Galveston; University of Washington, Seattle; Washington University, St. Louis; and is headquartered at the University of California, San Diego and includes: Directors: Robert K. Heaton, Ph.D., Scott L. Letendre, M.D.; Center Manager: Donald Franklin, Jr.; Coordinating Center: Brookie Best, Pharm.D., Debra Cookson, M.P.H, Clint Cushman, Matthew Dawson, Ronald J. Ellis, M.D., Ph.D., Christine Fennema Notestine, Ph.D., Sara Gianella Weibel, M.D., Igor Grant, M.D., Thomas D. Marcotte, Ph.D. Jennifer Marquie-Beck, M.P.H., Florin Vaida, Ph.D.; Johns Hopkins University Site: Ned Sacktor, M.D. (P.I.), Vincent Rogalski; Icahn School of Medicine at Mount Sinai Site: Susan Morgello, M.D. (P.I.), Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.); University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Sher Storey, PA-C.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Mengesha Teshome, M.D.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
References
- Anderson G. Increased IL-6 trans-signaling in depression: focus on the tryptophan catabolite pathway, melatonin and neuroprogression. Pharmacol. Rep. 2013;65(6):1647–1654. doi: 10.1016/s1734-1140(13)71526-3. [DOI] [PubMed] [Google Scholar]
- Antelman G. Depressive symptoms increase risk of HIV disease progression and mortality among women in Tanzania. J. Acquir. Immune Defic. Syndr. 2007;44(4):470–477. doi: 10.1097/QAI.0b013e31802f1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assari S., Moazen-Zadeh E. Ethnic variation in the cross-sectional association between domains of depressive symptoms and clinical depression. Front. Psychiatr. 2016;7:53. doi: 10.3389/fpsyt.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T. Comparison of Beck depression inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bekhbat M. HIV and symptoms of depression are independently associated with impaired glucocorticoid signaling. Psychoneuroendocrinology. 2018;96:118–125. doi: 10.1016/j.psyneuen.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson A.M. Improvements in depression and changes in quality of life among HIV-infected adults. AIDS Care. 2015;27(1):47–53. doi: 10.1080/09540121.2014.946386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing E.G. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch. Gen. Psychiatr. 2001;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Boulware D.R. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J. Infect. Dis. 2011;203(11):1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik C. The association between serum levels of neopterin and number of depressive episodes of major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34(2):372–375. doi: 10.1016/j.pnpbp.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Ciesla J.A., Roberts J.E. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am. J. Psychiatr. 2001;158(5):725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Deeks S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan T.G. Inflammatory markers in depression. Curr. Opin. Psychiatr. 2009;22(1):32–36. doi: 10.1097/YCO.0b013e328315a561. [DOI] [PubMed] [Google Scholar]
- French A.L. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J. Acquir. Immune Defic. Syndr. 2009;51(4):399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Gimeno D. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 2009;39(3):413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J.S. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J. Acquir. Immune Defic. Syndr. 2011;58(2):181–187. doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R. Increased soluble tumor necrosis factor-alpha receptors in patients with major depressive disorder. Psychiatr. Clin. Neurosci. 2009;63(2):202–208. doi: 10.1111/j.1440-1819.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- Haroon E. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology. 2018;95:43–49. doi: 10.1016/j.psyneuen.2018.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.K. The impact of HIV-associated neuropsychological impairment on everyday functioning. J. Int. Neuropsychol. Soc. 2004;10(3):317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- Heaton R.K. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmuth J. Depression and anxiety are common in acute HIV infection and associate with plasma immune activation. AIDS Behav. 2017;21(11):3238–3246. doi: 10.1007/s10461-017-1788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsue P.Y., Deeks S.G., Hunt P.W. Immunologic basis of cardiovascular disease in HIV-infected adults. J. Infect. Dis. 2012;205(Suppl 3):S375–S382. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickovics J.R. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. J. Am. Med. Assoc. 2001;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Ironson G. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom. Med. 2005;67(6):1013–1021. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha M.K. Anti-inflammatory treatments for major depressive disorder: what’s on the horizon? J. Clin. Psychiatr. 2019;80(6) doi: 10.4088/JCP.18ac12630. [DOI] [PubMed] [Google Scholar]
- Jha M.K. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology. 2017;78:105–113. doi: 10.1016/j.psyneuen.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M. Protocol for the insight study: a randomised controlled trial of single-dose tocilizumab in patients with depression and low-grade inflammation. BMJ Open. 2018;8(9) doi: 10.1136/bmjopen-2018-025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J.K. Marital distress, depression, and a leaky gut: translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology. 2018;98:52–60. doi: 10.1016/j.psyneuen.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontol. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Leo R. Association between enhanced soluble CD40 ligand and proinflammatory and prothrombotic states in major depressive disorder: pilot observations on the effects of selective serotonin reuptake inhibitor therapy. J. Clin. Psychiatr. 2006;67(11):1760–1766. doi: 10.4088/jcp.v67n1114. [DOI] [PubMed] [Google Scholar]
- Lowe G.D. Interleukin-6, fibrin D-dimer, and coagulation factors VII and XIIa in prediction of coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2004;24(8):1529–1534. doi: 10.1161/01.ATV.0000135995.39488.6c. [DOI] [PubMed] [Google Scholar]
- McIntosh R.C. Resting-state connectivity and spontaneous activity of ventromedial prefrontal cortex predict depressive symptomology and peripheral inflammation in HIV. J. Neurovirol. 2018;24(5):616–628. doi: 10.1007/s13365-018-0658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor V. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53(9):2002–2007. doi: 10.1002/1097-0142(19840501)53:9<2002::aid-cncr2820530933>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Musinguzi K. Association between major depressive disorder and pro-inflammatory cytokines and acute phase proteins among HIV-1 positive patients in Uganda. BMC Immunol. 2018;19(1):1. doi: 10.1186/s12865-017-0239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. The composite international diagnostic interview (CIDI) web site. Bull. World Health Organ. 1999;77(7) 614-614. [Google Scholar]
- Norcini Pala A. Subtypes of depressive symptoms and inflammatory biomarkers: an exploratory study on a sample of HIV-positive patients. Brain Behav. Immun. 2016;56:105–113. doi: 10.1016/j.bbi.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinfuss M. [Kaposi’s sarcoma. Clinical picture, treatment and prognosis. I. Clinical forms of Kaposi’s sarcoma] Nowotwory. 1987;37(3):195–206. [PubMed] [Google Scholar]
- Rivera-Rivera Y. Depression correlates with increased plasma levels of inflammatory cytokines and a dysregulated oxidant/antioxidant balance in HIV-1-Infected subjects undergoing antiretroviral therapy. J. Clin. Cell. Immunol. 2014;5(6) doi: 10.4172/2155-9899.1000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenorio A.R. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J. Infect. Dis. 2014;210(8):1248–1259. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am. J. Psychiatr. 2014;171(12):1278–1286. doi: 10.1176/appi.ajp.2014.14010094. [DOI] [PubMed] [Google Scholar]
- Villes V. The effect of depressive symptoms at ART initiation on HIV clinical progression and mortality: implications in clinical practice. Antivir. Ther. 2007;12(7):1067–1074. [PubMed] [Google Scholar]
- von Kanel R., Bellingrath S., Kudielka B.M. Association of vital exhaustion and depressive symptoms with changes in fibrin D-dimer to acute psychosocial stress. J. Psychosom. Res. 2009;67(1):93–101. doi: 10.1016/j.jpsychores.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Wachtel T. Quality of life in persons with human immunodeficiency virus infection: measurement by the Medical Outcomes Study instrument. Ann. Intern. Med. 1992;116(2):129–137. doi: 10.7326/0003-4819-116-2-129. [DOI] [PubMed] [Google Scholar]
- Watkins D.C., Assari S., Johnson-Lawrence V. Race and ethnic group differences in comorbid major depressive disorder, generalized anxiety disorder, and chronic medical conditions. J Racial Ethn Health Disparities. 2015;2(3):385–394. doi: 10.1007/s40615-015-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A.W. Evidence for reliability, validity and usefulness of the medical Outcomes study HIV health Survey (MOS-HIV) Qual. Life Res. 1997;6(6):481–493. doi: 10.1023/a:1018451930750. [DOI] [PubMed] [Google Scholar]
- Zimpel R.R., Fleck M.P. Depression as a major impact on the quality of life of HIV-positive Brazilians. Psychol. Health Med. 2014;19(1):47–58. doi: 10.1080/13548506.2013.772302. [DOI] [PubMed] [Google Scholar]