Abstract
Background
Conversion disorder/functional neurological disorder (CD/FND) occurs often in neurological settings and can lead to long-term distress, disability and demand on health care services. Systemic low-grade inflammation might play a role, however, the pathogenic mechanism is still unknown.
Aim
1) To explore the feasibility to establish and assess a cohort of CD/FND with motor symptoms, involving persons with lived experience (PPI). 2) To generate proof of concept regarding a possible role for cytokines, microRNA, cortisol levels and neurocognitive symptoms in patients with motor CD/FND.
Method
Feasibility study.
Results
The study showed active involvement of patients despite high clinical illness burden and disability, neurocognitive symptoms, childhood adverse experiences (ACE) and current life events. The study provided valuable knowledge regarding the feasibility of conducting a study in these patients that will inform future study phases. In the sample there were elevated levels of IL6, IL12, IL17A, IFNg, TNFa and VEGF-a, suggesting systemic low-grade inflammation. Also, microRNAs involved in inflammation and vascular inflammation were correlated with TNFa and VEGFa respectively, suggesting proof of concept for an epigenetic mechanism. Owing to the COVID-19 outbreak, the patient sample was limited to 15 patients.
Conclusion
It is a novelty that this study is conducted in the clinical setting. This innovative, translational study explores stress-related SLI in CD/FND patients and the feasibility of a larger project aiming to develop new treatments for this vulnerable population. Given the positive findings, there is scope to conduct further research into the mechanism of disease in CD/FND.
Keywords: Conversion disorder/functional neurological disorder, Functional movement disorder, Cytokines, Systemic low-grade inflammation, microRNA, Neurocognitive symptoms, Childhood adverse experiences (ACE), Epigenetic mechanism, Feasibility study
Highlights
-
•
It is feasible to establish and assess a cohort of CD/FND with motor symptoms, involving persons with lived experience (PPI).
-
•
IL6, TNFa, IFNg, TNFa, IL12 and IL17A are significantly higher than normal in CD/FND patients.
-
•
VEGF-a is significantly lower, suggesting a lack of vascular and neuronal support.
-
•
TNFa correlated with microRNA associated with inflammation (miR-146a and miR-155). This suggests an epigenetic mechanism.
-
•
VEGFa correlated with miR-21 and miR-132, suggesting an epigenetic mechanism associated with vascular inflammation.
1. Introduction
Conversion disorder/functional neurological disorder (CD/FND) as described in the DSM-5 (American psychiatric Association, 2013) can lead to long-term distress, disability, diminished quality of life and demand on health care services.(Gelauff et al., 2014; Mace and Trimble, 1996).
Treatment options for patients with CD/FND are limited and typically have a small effect, leading to a high unmet clinical need (Goldstein, 2020; Kroenke, 2007; Ruddy, 2005). Depending on the presenting symptoms, patients may receive psychosocial interventions (such as cognitive behavioural therapy; Hubschmid et al., 2015; Kompoliti, 2015) to explore the underlying causes (such as historical trauma), occupational therapy, or physiotherapy (Nielsen et al., 2013) to help with any physical symptoms (Goldstein, 2020; Kroenke, 2007; Ruddy, 2005).
Although several possible mechanisms for the pathogenesis of CD/FND are under consideration, its specific origin is unknown (Ratcliff and van der Feltz-Cornelis, 2020). Mechanisms that have been proposed are systemic low-grade inflammation (SLI) (Del Grande da Silva et al., 2016; Kovacs et al., 2016a, Kovacs et al., 2016b; Tiyekli et al., 2013; Viljoen and Panzer, 2005; Vogelzangs et al., 2016). Elevated CRP was found in some, but not all, children with CD/FND (Kozlowska et al., 2019); this may indicate that the relationship is not straightforward. There might be multiple strains with CD/FND, that each have different causes. (Ratcliff and van der Feltz-Cornelis, 2020). For example, another study found that hsCRP is elevated especially in case of motor function CD/FND (Tak et al., 2009). It might also be that an inflammatory process might occur at a certain pathological phase (Ratcliff and van der Feltz-Cornelis, 2020). For example, autonomous dysregulation could interfere with downregulation by corticosteroid production in the adrenal glands, resulting in peripheral inflammation that cannot be ‘switched off’ (Pongratz and Straub, 2014). Suggestions of such a mechanism have been found in CD/FND (Kozlowska et al., 2015; Maurer et al., 2015) and they may seem to hang together with adverse childhood events (ACE) (Baumeister et al., 2016). Although current stress levels and ACE have been explored in CD/FND (Ludwig et al., 2018), so far their association with systemic low-grade inflammation in CD/FND has not yet been explored. In terms of a possible underlying mechanism, childhood trauma has been suggested to prime microglia and this might be associated with adult mental disorder (Calcia et al., 2016), however whether this is the case in CD/FND has not been the subject of research so far. Nevertheless, subjective neurocognitive problems such as information processing speed occur in CD/FND (American Psychiatric Association, 2013; De Vroege et al., 2020) (Baumeister et al., 2016), and this might be an indication of neuroinflammation affecting brain activity i.e. neurocognitive function. Additionally, these neurocognitive symptoms have been shown relevant to treatment outcome (De Vroege et al., 2017).
Exposure to early-life adversity is known to predict DNA methylation patterns that may be related to risk for development of mental disorders (Dunn et al., 2019) and gene-environment interactions and epigenetics have been studied in anxiety disorders (Lin et al., 2020). However, so far, the links between epigenetic and post-transcriptional regulatory mechanisms and CD/FND have been poorly explored. For example microRNAs (miRNAs) are critical post-transcriptional and epigenetic regulators, yet their role in CD/FND has not been explored. In this study, we focus on plasma miRNAs as they have been studied extensively as biomarkers of inflammation in a variety of contexts including specifically neuroinflammation (Slota and Booth, 2019) and neurodegenerative diseases (Sheinermann et al., 2017) and studies investigating circulating miRNA levels in individuals with CD/FND and their relationship to circulating cytokine levels have not been conducted so far.
The objectives of this feasibility study were to inform further research steps by enabling us to generate a comprehensive testing procedure and evaluation of the proposed processes of recruitment, retention and data collection. A further objective was to generate proof of concept regarding a possible role for cytokines, microRNA, cortisol levels and neurocognitive symptoms in patients with motor CD/FND that may warrant a subsequent larger study exploring the underlying mechanism and possible treatment options related to it (Van der Feltz-Cornelis et al., 2020b). Lastly, we aimed to establish a patient and public involvement (PPI) advisory group to determine the acceptability of the study processes for patients and carers.
2. Material and methods
This was a feasibility study sponsored by the University of York. Study preparation started in March 2019. It was registered at the Research Registry (UIN: researchregistry5289). The study protocol was published (Van der Feltz-Cornelis et al., 2020b); study methods are summarised in the current article.
Participants were recruited through clinics and services within the Tees, Esk and Wear Valleys NHS Foundation Trust (TEWV), and Humber Teaching NHS Foundation Trust. Participants could also self-refer to the study via the study website (Conversion, 2019). The target population was adult patients, aged 18 and above with diagnosed CD/FND with motor symptoms. Both incident and prevalent cases could be included. Patients with any inflammatory condition, such as for example a diagnosis of Cushing’s syndrome; any blood-clotting disorders (e.g. haemophilia) or transfusion transmissible infections (e.g. HIV); on steroidal or other anti-inflammatory medication at the point of recruitment to the study, as taken from patient files or self-reported, were excluded. Patients with non-motor CD/FND, pseudo-seizures or epileptic seizures, as well as patients with brain injury or neuro-degenerative conditions (such as dementia or Parkinson’s disease) were excluded. Recruitment started August 2019 and was planned to end June 1st, 2020. All participants continued to receive their usual care throughout the study, which could consist of a combination of Cognitive Behavioral Treatment (CBT) and physiotherapy; it was out of scope of this feasibility study to record details about usual care provision. To confirm the diagnosis, psychiatric classification (PSE) following DSM-5 classification criteria (American Psychiatric Association, 2013) and Mini Mental State Examination (MMSE) (Schmand et al., 1995) were performed according to a predetermined format that included checking the medical history regarding diagnosis of CD/FND and establishing the main physical symptom.
2.1. Outcome measures
Outcome measures are summarised in Table 1.
Table 1.
Outcome measures.
| Instrument | Information |
|---|---|
| Physical symptoms: Physical Symptom Questionnaire (PSQ-51) |
Additional to the mean score, the following dimensions were discerned: CD/FND symptoms; postural orthostatic tachycardia syndrome (POTS); digestive symptoms; pain; fatigue; sleep score and sexual symptoms. (Allen et al., 2020; Van Hemert et al., 2004) |
| Pain: Brief Pain Inventory (BPI) |
Average severity of pain in the last week was assessed. An average score of 3 or more on the BPI in the last week is the cut score for clinically significant pain. |
| General functioning: RAND 36-Items | The RAND 36-Item Health Survey taps eight health concepts: physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health perceptions. The higher the score, the better the functioning. These RAND 36 items are identical to the MOS SF-36.(Ware and Sherbourne, 1992) They were adapted from longer instruments completed by patients participating in the Medical Outcomes Study (MOS), (Tarlov et al., 1989) an observational study of variations in physician practice styles and patient outcomes in different systems of health care delivery, for HIV patients (Hays and Shapiro, 1992) and patients with chronic medical conditions (Stewart et al., 1989) and depressive disorders. (Wells et al., 1989) |
| Quality of life: EuroQol EQ-5D-5L |
EQ-5D-5L percentages of patients scoring on the subscales mobility, selfcare, activities, pain and depression or anxiety were established. NICE does not recommend using UK EQ-5D-5L norm scores as there are issues with the scoring system, (NICE, 2020) hence we compared the mean score for the general health item with similar scores from other countries. |
| Childhood trauma: Adverse Childhood Experiences scale (WHO-ACE-IQ) | The WHO-ACE-IQ (WHO, 2018) provides a total score and differentiates between 9 domains: problems with marriage or parenthood, such as arranged marriage and unwanted pregnancies; parental neglect, parental illness, witnessing domestic abuse, being a victim of domestic abuse, being a victim of sexual abuse, experiencing bullying, external violence, and war experiences, all during childhood. |
| Current life events: Holmes and Rahe Life Events Stress Scale | The Holmes and Rahe Life Events Stress Scale assesses for 54 life events that can cause stress as they require a role transition that could be positive or negative in nature, whether they occurred over the last 12 months. A total score (Life Change Units) is provided based upon validated scores per life event (LCU).(Holmes and Rahe, 1967) |
| Chronic medical conditions | CBS checklist. Assesses presence and number of chronic medical conditions.(CBS, 2005) |
| Anxiety: GAD7 |
General Anxiety Disorder questionnaire (GAD-7) (Löwe et al., 2008) GAD-7 scores of 5 or higher indicate mild anxiety symptoms; scores of 10 or higher indicate a probability for an anxiety disorder.(Kroenke et al., 2010) |
| Depression: PHQ9 |
Patient Health Questionnaire-9 (PHQ-9) (Kroenke et al., 2001) PHQ-9 scores of 5 or higher indicate mild depressive symptoms; scores of 10 indicate a probability for a depressive disorder. (Kroenke et al., 2010) |
| Clinimetric measures: | Height, Weight, BMI, Waist circumference and Blood pressure recorded during first interview and Body Mass Index (BMI) calculated. |
| Inflammation markers: Cytokines TNFα; IL1β; IL6; IFNɣ; VEGF-a; ANG2 hSCRP | Tumor Necrosis Factor alpha (TNFα), (Baumeister et al., 2016; Zelová et al., 2013) Interleukin 1 beta (IL1β), (Kovacs et al., 2016a, Kovacs et al., 2016b) Interleukin 6 (IL6), (Money et al., 2016; Tanaka et al., 2014) Interferon gamma (IFNɣ), (Flaishon et al., 2000) Vascular endothelial growth factor A (VEGF-a) (Kut et al., 2007) and Angiopoietin-2 (ANG2) (Nataraj et al., 1999; Bai et al., 2014) hsCRP. (Seo et al., 2012) Before blood samples were taken, it was ascertained that the patient did not feel unwell, have an infectious condition, or needed anti-inflammatory medication in the week before the samples were taken. |
| Inflammation-associated microRNAs: | Targeted assessment of circulating (cell-free) inflammation-associated microRNAs miR-146a, miR-155, miR-21, miR-223, and miR-132.(Tahamtan et al., 2018) miR-146a and miR-155 are linked to inflammation and miR-21, miR-132, (Hewitson et al., 2019) miR-155 (Yee et al., 2017) are all linked to angiogenesis and vascular inflammatory responses |
| Chronic accumulative stress: Hair cortisol levels |
Cortisol hair test was used as biomarker for chronic accumulative stress.(Staufenbiel et al., 2013) Hair samples were chosen as levels of the hormone in the blood and saliva can rapidly fluctuate, giving a poor picture of long-term concentrations. |
2.2. Neurocognitive functioning
Assessment of neurocognitive functioning was performed using selected, standardised tests of information processing, executive functioning and memory. First the Test of Memory Malingering (TOMM test) (Tombaugh and Tombaugh, 1996; Teichner and Wagner, 1997) was performed to rule out malingering, because of the relevance of potential malingering in the clinical setting. For similar reasons, for effort the Digit Span sub-test of the Wechsler Adult Intelligence Scale (Wechsler, 2008) was used to assess effort (i.e. anomalous discrepancies between forwards and backwards components of the test) as well as working memory (see below). Sub-tests from the Delis-Kaplan Executive Functioning System (DKEFS) (Delis et al., 2004), were used to assess information processing, attention, motor speed and key aspects of executive functioning. This domain was assessed using the Digit Symbol Substitution Test (Coding) from the Wechsler Adult Intelligence Scale-IV (Wechsler, 2008). This is a timed task that examines information processing and psychomotor speed by asking respondents to match and complete corresponding symbols to the numbers presented. The Trail Making Test (TMT) from the DKEFS was also used to assess information processing, visual attention, switching and motor speed (Reitan, 1992). The Stroop Colour–Word Interference Test (CWIT) from the DKEFS was used to assess for verbal inhibition and switching (Delis et al., 2004; Stroop, 1938). Executive functioning was further explored using the Tower of London test, which assesses visual problem solving, rule following and visual working memory (Shallice, 1982). Working memory was assessed using the Wechsler Adult Intelligence Scale-IV Digit Span Test (Wechsler, 2008). This test consists of three conditions, in which participants are asked to verbally repeat numbers given in the same order, in reverse order, and in ascending order, respectively.
To compare the scores of patients with CD/FND with normative values, relevant scaled scores on each test were used. Across the WAIS IV and DKEFS sub-tests, the mean normative scales scores were 10 (SD = 3). It is acknowledged that there is no universal agreement regarding the threshold used to define cognitive impairment in neuropsychological research (Brooks, 2009; Crawford, 2011; Jak, 2009). Existing research studies that have utilised similar neuropsychological tests to the ones used in the current study have defined cognitive impairment as scores that are one SD or more (16th percentile or less) below the mean (Dozier, 2016; Holst, 2020; Karr, 2017; Keifer, 2013; Wexler, 2009). Given the purpose of this study was to assess feasibility, as opposed to a full-scale evaluation study, this criterion is appropriate to define cognitive impairment. Hence, mean scaled scores 1SD or more below the population mean for each neurocognitive test (e.g. less than or equal to a scaled score of 7) were considered to represent impairment in this study.
2.3. Biomarkers
Details of cytokine and hsCRP assays, microRNA plasma extraction and analysis and hair cortisol analysis (D’Anna-Hernandez et al., 2011) are provided in Supplement 1.
2.4. Statistical analysis
RAND SF 36 scores (Ware& Sherbourne, 1992) versus Medical Outcome Studies (MOS) (Tarlov et al., 1989) norm scores were compared and p-values presented for the differences by one sample t-test. Characteristics of the proposed outcome measures hsCRP (Seo, 2012) and cytokines including standard deviation were taken for future power calculation. The lab provided median and range scores from a commercial normal sample (N = 27) for IFNγ, IL1-b, IL-6, IL-12, IL 17a, TNFα, and VEGF-a. Range was calculated of the samples with concentrations above the Lowest Level Of Detection (LLOD). Significance level for the difference between our sample and the normal sample was estimated by Wilcoxon Signed Rank test. Significance level for the difference between hsCRP in the sample and cut-off score was estimated by One-Sample t-test. Outcomes for cytokines were then plotted against miRNA, calculating Pearson P values and slopes, analysed with GraphPad Prism. The hair sample outcomes were compared with hair sample outcomes from healthy volunteers with t-test.
3. Results
3.1. Feasibility
The feasibility of establishing the cohort and performing psycho-diagnostic assessment for description of the cohort was explored. This included feasibility of obtaining ethical approval and approval of local R&D departments at the different recruiting sites. The preparation phase of the study started in March 2019. The protocol for HRA approval was submitted on April 8, 2019 and approval granted on 10th June by the NHS North West 11 Research Ethics Committee, Preston (IRAS nr. 261252). TEWV confirmed capacity and capability on July 11, 2019 and recruited the first participant in the first week of August.
3.1.1. Lab infrastructure
We built an infrastructure for venepunctures, transport to lab and analysis of the blood. Arrangements were made with four different laboratories for cytokines and microRNA. All sites were able to provide phlebotomy services and research nurses for data collection. Blood from venepunctures was transported to the biochemistry laboratory based at James Cook University Hospital on the same day. Blood was centrifuged and divided over two vials, one for the Core Biochemical Assay Laboratory (CBAL), Cambridge University, assessing cytokines and the other for the University of York laboratory assessing microRNA. This was stored at the James Cook University Hospital biochemistry laboratory until the sample was complete and then transported to the other involved laboratories for analysis, in one procedure for all patients for standardisation purposes. This was feasible in the short timeframe of this study because colleagues in TEWV had existing contractual arrangements with the hospital. With a longer lead-in time it would be feasible to set up a similar infrastructure where existing arrangements are currently unavailable. As control measurements, the CBAL provided median and range data from a commercial sample of 27 healthy subjects for comparison of the cytokine levels, age and gender of whom were unavailable for analysis. Standardisation and analysis method was the same in both samples. All hair samples were analysed at the ARU Biomarker lab, Cambridge UK.
3.1.2. Recruitment
Recruitment and assessment was supported by the TEWV R&D department for different sites, recruitment was planned until June 1st, 2020. Setting up sites was feasible. Access to medical history data to decide on eligibility of patients was mostly provided by referring psychiatrists and from GP practices, but not always straightforward as the records were not always complete regarding letters of neurologists detailing their diagnostic assessments. An amendment to the consent process to allow access to pertinent information from participants’ GP records was submitted in February 2020. Research nurses and study team members were trained in the study procedures and administration and the scoring of the questionnaires in the case report form (CRF). Many participants were not able to travel so it was important to involve colleagues who were willing to visit participants in their own homes or their local hospital/clinic. Disclosure and Barring Service (DBS) checks and research passports had to be obtained. Four psychiatrists were trained and supported by a format in the CRF for the procedures for the psychiatric assessment. Following the introduction of the COVID-19 restrictions the assessments were carried out by video link. After ending the recruitment phase, the CRF format was audited and found feasible to complete. Its layout was improved based upon the audit. Three people were trained and supervised by a clinical psychologist in the administration of the neuro-cognitive tests. Testing and supervision went as planned.
3.1.3. COVID-19
Owing to the COVID-19 outbreak recruitment was suspended from March 2020 for the full further planned recruitment period, shortening it by 4 months. Because of this, seven patients identified as eligible were unable to consent and to be included in the study. Some face-to-face assessments, clinimetric assessments and one blood sampling in patients already recruited could not take place. At the time of the first UK COVID-19 lockdown (March 2020) the Humber NHS Teaching Foundation Trust had begun recruiting and the research team were in the process of setting up sites at York Teaching Hospital and a stroke unit in South Tees, as well as exploring how searches of GP records might be constructed for identifying this patient group. Although we don’t have a full data set on some variables, it is reasonable to suppose on the evidence from this study and conversations with participants and sites that collection of these data is feasible and acceptable.
3.1.4. Participants
Between August 2019 and March 2020, 95 potential participants had been identified through clinics at TEWV and Humber Teaching NHS Trusts before the study had to close prematurely. Of these, 13 met the criteria for recruitment and gave consent to join the study. Five further participants heard about the study and contacted the study team directly. Of these five, one was ineligible and four consented of whom two were prevented from meeting with the study team due to COVID-19 restrictions. As the flowchart in Fig. 1 shows, the closure of all non-COVID-19 related research had a considerable impact on study recruitment as Humber Teaching NHS Foundation Trust was starting recruitment and the TEWV medically unexplained physical symptoms (MUPS) clinic, closed from December 2019 until March 2020, was re-opening. From March 2020, the team started with data entry, lab analysis and statistical analysis, and the reporting phase of the study.
Fig. 1.
Flowchart CANDO study.
3.1.5. Sites
During the set-up period the study team was contacted by several mental health NHS trusts through the Clinical Research Network (CRN) portfolio, expressing an interest in taking part. We have also had interest from, and spoken to, neurologists, stroke units, pain clinics and physiotherapists, and more recently from individuals and charities. All of the six GP practices we consulted in and around York were also keen to be involved. Expanding the network of collaborators within the NHS and establishing a larger cohort is unlikely to be too difficult given appropriate time and resources. Collaboration and knowledge exchange within and outside the interdisciplinary team of researchers has been key to the conduct of and learning from this feasibility study. In view of this the indications are that recruiting a much larger cohort would be feasible.
3.2. PPI involvement and feasibility from the participant perspective
The number of patients identified through each of the recruitment methods and the proportion of those who were willing and eligible to take part were recorded. Willingness to complete assessment processes was monitored including attrition rates and reasons, to identify any barriers to completion. The majority of participants were able and willing to complete every part of the study. All participants recruited to the study completed all the questionnaires. Five participants preferred to have their responses written by the researcher because writing was difficult. Three participants did not complete any of the neuro-cognitive tests and the psychiatric assessments. One found the tests too difficult because of speech problems, one was too busy and one who was recruited shortly before the COVID-19 restrictions were put in place and the test had to be cancelled. A further four participants completed the psychiatric assessment by video link following the lockdown but have not been able to do the face-to-face neuro-cognitive tests.
The processes associated with the study were assessed for acceptability to participants and HCPs, canvassing views of both. All participants in the study were invited to provide feedback on study procedures and patient materials as part of the study. Additionally, we recruited interested people with lived experience of the condition to advise on and contribute to dissemination of the study findings and the design and management of future research projects. Participants were consulted individually and during study PPI group meetings. There was general agreement that the study procedures and the questionnaires were acceptable to participants despite some challenging content. The questionnaires took between 20 and 95 min to complete which was longer than anticipated in some cases, but participants appeared to be enlivened by taking part rather than tired by it. This was confirmed in subsequent discussion with participants. They liked the patient information booklet and found all the study documents easy to use.
3.3. Patient characteristics
Fifteen people participated in the study, 8 female. Demographic and clinical characteristics are shown in Table 2.
Table 2.
Patient characteristics.
| Demographic variables | |
|---|---|
| Age | Mean: 49.7 years Range: 23–63 years |
| Gender | Female (n = 8; 53.3%) Male (n = 7; 46.7%) |
| Ethnicity | White British (n = 15; 100%) |
| Education level | Secondary/High School (n = 6; 40%) College/University (n = 7; 46.7%) Postgraduate Degree (n = 1; 6.7%) Missing (n = 1; 6.7%) |
| Work status | Employed (n = 3; 20%) Student (n = 1; 6.7%) Homemaker (n = 1; 6.7%) Retired (n = 1; 6.7%) Unemployed/unable to work (n = 8; 53.3%) Missing (n = 1; 6.7%) |
| Relationship status | Single (n = 2; 13.3%) In a relationship (n = 1; 6.7%) Married (n = 7; 46.7%) Divorced/Separated (n = 3; 20%) Widowed (n = 1; 6.7%) Missing (n = 1; 6.7%) |
| Height (cm) | N = 8; mean = 167.7 cm (SD = 9.6; range 153–185) |
| Weight (kg) | N = 7; mean = 85.7 kg (SD = 21.1; range 57–125) |
| BMI | N = 7; mean = 30.15 (SD = 7.0; range 20–41). |
| Waist circumference (cm) | N = 5; mean 88.73 (SD = 13.99; range 71–107) |
| Systolic blood pressure (mm Hg) | N = 5; mean 149.0 (SD 29.1), range 120–188. |
| Diastolic blood pressure (mm Hg) | N = 5; mean 81.2 (SD 6.6), range 74–92 |
| Severity of main symptom | Mean = 8.73 (SD = 1.831) |
| Mean duration of symptoms | 69.4 months |
| Main reported symptoms | Difficulty walking (n = 12; 80%) Muscle weakness or paralysis to some extent (n = 12; 80%) Tremor or shaking (n = 11; 73%) Sensory symptoms (numbness or tingling) (n = 11; 73.3%) Blurred vision (n = 9; 60%) Temporary blindness (n = 1; 6.7%) Loss of voice (n = 8; 53%) Deafness (n = 2; 13.3%) |
| CD/FND Type | Mixed CD/FND (n = 12; 80%) Motor CD/FND (3 = 20%) |
| Clinical variables | |
| Mini-mental state examination (MMSE) score | n = 8; mean = 28.9 (SD = 1.4; range 27–30) |
| Clinical Global Impression (CGI) score | n = 9; mean = 4.7 (SD = 1.3; range 3–7) |
| Adverse Childhood Experience (ACE) score | N = 15; mean = 4 (SD = 2.4; range 0–7). ACE domains: Married without their consent = n = 1 Victim of childhood sexual abuse = n = 8 Parental neglect = n = 8 External violence = n = 9 Victim of domestic abuse = n = 9 Parental illness = n = 10 Witnessed domestic abuse = n = 10 Victim of bullying = n = 11 War experiences during childhood = n = 0. |
| Life Change Unit (LCU) score | N = 15; mean = 234 (SD = 134; range 0–483). (4 participants scored <150, 6 participants scored between 150 and 299, 5 scored >300). |
Participants were aged between 23 and 63 (mean 49.7) years; mean 54 years in males, 46 years in females. All described themselves as white British. Educational level was secondary school for 6, college or university for 7 and postgraduate for 1 participant Five were employed, in education or a homemaker, 8 were unable to work and 1 was retired. Mean main symptom severity was 8.73 out of 10 and mean duration of symptoms was 69 months; more than 5 years.
All participants had CD/FND according to DSM-5 criteria. The main reported symptoms were difficulty walking (12, 80%); muscle weakness or paralysis to some extent (12, 80%); and tremor or shaking (11, 73%). Sensory symptoms such as numbness or tingling were reported in 11 (73.3%) cases. 9 (60%) have blurred vision, 1 patient (6.7%) was sometimes blind. Eight (53%) suffered some kind of loss of voice, and 2 (13.3%) suffered from deafness. One participant had experienced seizures in the past but not during the episode with CD/FND. Three participants had only motor CD/FND; the rest of the cases were mixed CD/FND.
Mean MMSE scores (n = 8) were 28.9 (SD1.4) range 27–30. CGI scores (n = 9) were mean 4.7 (SD 1.3) with a range between 3 and 7.
3.4. Physical symptoms and quality of life
Mean PSQ-51 score was 60 (SD = 20) out of 88. Regarding the symptom domains, the mean CD/FND symptom load was 8.5 (SD = 3.5) out of 15. The mean digestive symptom load was 14.8 (5.6) out of 26; mean pain score was 13.1 (SD 6.7) out of 24 and only one patient reported no pain. Average fatigue scores were 4.5 (1.7) out of 6. All patients reported sleeping problems with mean 3.2 (SD = 1.9) out of 6. The average for sexual problems was 1.7 (SD = 1.6) out of 4. Pain assessed with the BPI showed that 11 of the 15 patients (73%) had an average score of 3 or more on the BPI in the last week and the whole sample had as mean score 5.2 (SD 2.7).
Only one participant had no comorbid medical conditions. The range was 0–16 chronic medical conditions and the average was 6.1. They had an average of 2.9 comorbid somatic comorbid medical conditions such as diabetes, cardiovascular disorder or COPD; and 3.3 comorbid functional disorders like burnout, depression, anxiety disorders or digestive tract problems like Irritable Bowel Syndrome, or unexplained pain.
On the EQ-5D-5L, 67% reported moderate to severe mobility problems. 53% reported moderate to severe self-care problems, 60% had moderate to severe problems with usual activities, 60% had moderate to severe pain/discomfort and 60% had moderate to severe anxiety or depression. The mean score for the general health item was 45.5 (SD = 21.4).
RAND SF-36 scores are shown in Table 3. Norm scores of the MOS are shown for comparison and p-values presented for the differences by one sample t-test.
Table 3.
RAND SF-36 domains sample and comparison MOS study.
| Variable | Mean (SD) sample | Mean (SD) MOS study | Mean difference (95% CI) | One sample T-test |
|---|---|---|---|---|
| Physical functioning | 33.00 (32.06) | 70.61 (27.42) | −37.610 (−55.36; −19.86) | −4.543; df 14 p = .000 |
| Role limitations physical | 15.00 (32.46) | 52.97 (40.78) | −37.970 (−55.95; −19.99) | −4.531; df 14 p = .000 |
| Role limitations emotional | 37.78 (37.52) | 65.78 (40.71) | −28.002 (−48.78; −7.23) | −2.891; df 14 p = .012 |
| Energy Fatigue | 23.67 (15.52) | 52.15 (22.39) | −28.483 (−37.08; −19.89) | −7.107; df 14 p = .000 |
| Emotional wellbeing | 49.33 (15.90) | 70.38 (21.97) | −21.047 (−29.85; −12.24) | −5.125 df 14 p = .000 |
| Social Functioning | 34.17 (23.84) | 78.77 (25.43) | −44.603 (−57.81; −31.40) | −7.245; df 14 P = .000 |
| Pain | 36.17 (30.89) | 70.77 (25.46) | −34.603 (−51.70; −17.50) | −4.340 df 14 p = .001 |
| General Health | 27.67 (16.02) | 56.99 (21.11) | −29.323 (−38.20; −20.45) | −7.089 df 14 p = .000 |
All sample scores are significantly lower and the mean differences are large, indicating that the participants in this sample had much worse functioning in all domains than clinical patients with chronic medical conditions in the MOS study.
3.5. Comorbid depression and anxiety
All patients scored above the level of mild depressive symptoms (PHQ-9 scores above 5). Only 2 were below 10 indicating a probability for a depressive disorder (mean14.4, SD = 4.6).
On the GAD-7, two participants (13%) did not report anxiety levels. Three (20%) scored mild anxiety and 10 (67%) score 10 or higher, indicating a probable anxiety disorder (mean = 10.9, SD = 4.8).
3.6. Trauma and stress
Findings are shown in Table 2. Regarding childhood trauma, two participants (13%) experienced no Adverse Childhood Experience (ACE). One participant (7%) experienced 1 ACE. 80% experienced multiple ACE, 60% experienced 3 or more ACE and the maximum number of ACE was 7. The average ACE score was 4.0 (SD = 2.4). The scores on the 10 ACE domains indicated that during childhood, one person was married without her consent; three (20%) were a victim of childhood sexual abuse; eight patients (53%) reported parental neglect; eight (53%) reported external violence such as being beaten up, 9 (60%) were a victim of domestic abuse; 10 (67%) reported parental illness; 10 (67%) witnessed domestic abuse; and 11 (73%) experienced bullying. Nobody reported war experiences during childhood.
Regarding current stressful life events, the average LCU score in the sample was 234 (SD = 134). 27% of the participants scored less than 150, 40% scored between 150 and 299 and 33% scored 300 or higher. These scores indicate very high stress levels requiring role adaptations in daily life to a great extent.
3.7. Neurocognitive functioning
TOMM Trial 1 test mean score was 47.0 (SD 2.4), median = 46 (SD 2.4), range = 6 (44–50). TOMM Trial 2 test mean score was 50.0 (SD 0.0) median = 50 (SD 0.0), range = 0 (50). No malingering was suspected in any study participant. Table 4 presents the neurocognitive tests with raw scores and corresponding norm-referenced scaled scores. All scaled scores have a normative standardised population mean of 10 (SD 3.0). Scores below 7 would indicate a difference of >1 SD from the population mean and are interpreted as impaired for the purpose of this study.
Table 4.
Outcome of neurocognitive testing (N = 8).
| Neurocognitive domain | Raw scores M(SD) | Scaled scores M(SD) | Interpretation |
|---|---|---|---|
| Colour Word Interference Test (Stroop) | |||
| Condition 1 Colour naming | 43,61 (SD 14,62) | 5,50 (SD 4,28) | Impaired |
| Condition 2 Word reading | 34,03 (SD 11,12) | 5,25 (SD 4,23) | Impaired |
| Condition 3 Inhibition | 84,08 (SD 28,67) | 6,38 (SD 4,87) | Impaired |
| Condition 4 Inhibition switching | 109,01 (SD 32,64) | 5,00 (SD 3,85) | Impaired |
| Trail Making Test (TMT) | |||
| TMT Visual scanning | 40,46 (SD 16,52) | 5,00 (SD 4,57) | Impaired |
| TMT Number sequencing | 55,4063 (SD 25,97) | 7,50 (SD 4,41) | Normal |
| TMT Letter sequencing | 59,06 (SD 25,68) | 7,25 (SD 4,20) | Normal |
| TMT Number-letter switching | 150,18 (SD 59,20) | 6,13 (SD 3,80) | Impaired |
| TMT Motor speed | 77,70 (SD 58,80) | 6,63 (SD 4,69) | Impaired |
| WAIS Digit Symbol Substitution Test | |||
| Coding score | 53,38 (SD 16,03) | 8,63 SD (2,50) | Normal |
| WAIS Digit Span Test | |||
| Total score | 28,13 (SD 4,52) | 11,13 (SD 1,89) | Normal |
| Tower of London Test | |||
| Total score | 17,88 (SD 4,12) | 10,86 (SD 2,54) | Normal |
Average scores on the Colour Word Interference Test (a timed test of cognitive flexibility, inhibition and switching) indicated impairment across conditions. Impaired scores on Conditions 1 and 2, relative to the population norm, suggests slowed information processing overall. Impaired mean scores on Conditions 3 and 4 indicates that participants had difficulties with inhibition and switching in relation to visual attention but it is possible that this was subject to slowed information processing overall given evident impairment on Conditions 1 and 2.
TMT visual scanning mean scores appeared impaired whereas number sequencing and letter sequencing were normal. The number letter switching condition is a key test of executive functioning, and mean scores here appeared impaired. However, this might be subject to impaired motor speed (Condition 5).
Scores on the WAIS digit symbol substitution test mean scores appeared to be in the normal range overall but some individuals showed impairment.
Total scores on the WAIS Digit Span Score appeared normal and there were no anomalous disparities between digits forwards and backwards conditions, indicating effort was not impaired overall.
Scores on the Tower of London Test (a measure of executive functioning in terms of visual memory and problem solving and rule following) were in the normal range; some participants performed particularly well on this test (scaled scores ranged from 9 to 16).
3.8. Biomarkers
3.8.1. Clinimetric measures
These measures and the number of patients that could be assessed are shown in Table 2.
3.8.2. Cytokines and hsCRP
Table 5 shows the cytokine scores compared with the normal sample for the study sample (N = 14) compared with cut-off score.
Table 5.
Cytokines and hsCRP scores.
| Variable | Mean (SD) sample N = 14 |
Median | Median [range] normal sample N = 27 | Wilcoxon Signed Rank test |
|---|---|---|---|---|
| IFNγ | 14.814 (7.465) | 13.2 | 3.8 [0.46–22.8] | p = .001 |
| IL-6 | .964 (.571) | .85 | 0.29 [0.12–0.99] | p = .001 |
| TNFα | 2.486 (.783) | 2.50 | 0.74 [0.31–2.32] | p = .001 |
| IL-1b | .214 (0.535) | .200 | 0.2 [0.11–0.94] | p = .317 |
| VEGF-A | 29.58 (18.84) | 24.900 | 54.5 [17.7–347] | p = .005 |
| ANG2 | 1729.14 (915.190) | 1377.00 | – | – |
| IL17A | 3.350 (1.497) | 3.350 | 0.59 [0.22–8.51] | p = .001 |
| IL12 | 220.221 (164.085) | 183.5 | 62.5 [15.1–395] | p = .001 |
Based upon this, for IL1B, 13 (93%) was normal, 1 (7%) was elevated. The median score of IL1B in this sample did not differ significantly from the median in the normal sample (p = .317).
Scores were elevated (100%) compared to the median from the normal sample and a Wilcoxon Signed Rank Test showed that this was significant (p = .001) for IL-6, IFNγ, IL17A, IL12 and TNFα. The VEGF-a score was significantly lower compared to the median of 54.5 in the normal sample (p = .005).
Angiopoietin-2 (ANG2) is involved with inflammation-induced vascular remodelling. Healthy plasma concentrations for ANG2 range between 1800 ± 874 pg/ml) (Li, 2015), or between 1-3μg/ml(Thurston, 2012). From that perspective, the ANG2 levels in our sample score in the normal range. To summarise, our sample shows significantly elevated IL-6, IFNγ and TNFα and significantly lower VEGF-a.
For hsCRP, 11 (79%) were normal, 3 (21%) were elevated (Ridker et al., 1998). A one-sample t-test showed that the mean hsCRP score was significantly lower than the cut-off score of 3.0 mg/ml; mean difference −1.35 (95% CI: 2.53;-0.18); p = .027). However, it was not undetectable. Although hsCRP can be detected in systemic low grade inflammation patients, in this sample it does not seem to be a useful biomarker.
3.8.3. microRNA
There is extensive literature linking several miRNAs to inflammation and neuroinflammation as well as pain (Andersen et al., 2014; Mi et al., 2013). We supplemented our analysis of circulating cytokines in the cohort by measuring levels of 5 miRNAs associated with inflammation (miR-146a, miR-223 (Wang et al., 2010) miR-155 (Alivernini et al., 2018; Singh et al., 2019)) and to angiogenesis and vascular inflammatory responses (miR-21 (Liu et al., 2011; Sheedy et al., 2010; Du et al., 2019), miR-132 (Kumarswamy et al., 2014) and miR-155 (Singh et al., 2019)) and miR-16, a highly abundant miRNA in circulation.
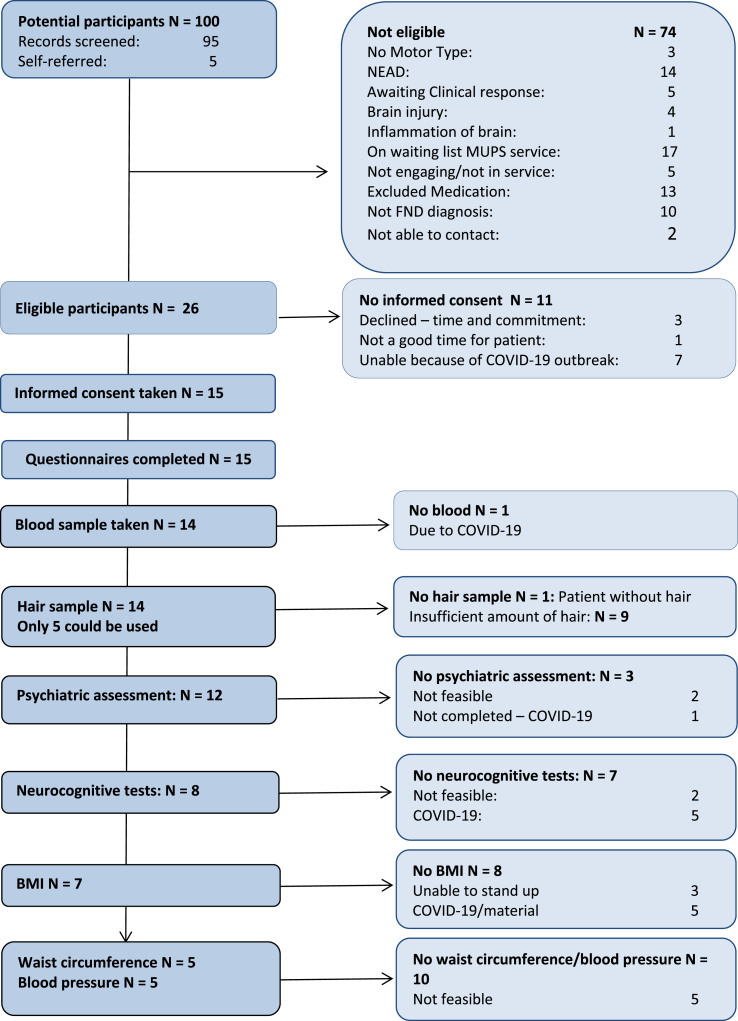
We observed a range of miRNA blood plasm levels within the cohort as shown in Fig. 2.
Fig. 2.
Plasma miRNA levels within the CANDO cohort (N = 14). For each miRNA, levels were normalised to spike-in control and average levels within the cohort (LEFT panel), or spike-in control, miR-16, and cohort average levels (RIGHT panel).
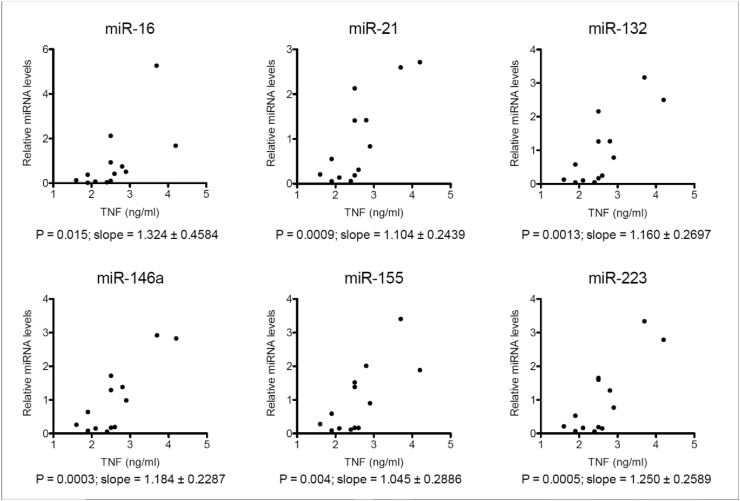
We also explored if levels of miRNAs correlated with levels of TNF in the cohort. We found that this was the case for all tested miRNAs. However, more significant Pearson P correlations (p < .005) were observed for each miRNA against TNF (analysed with GraphPad Prism). Fig. 3
Fig. 3.
Correlations between levels of tested miRNAs (normalised to spike-in control) against levels of TNF for each participant (N=13).
We specifically explored the correlation of VEGF with miR-132 and miR-21 as both of these miRNAs have been extensively linked to angiogenesis and endothelial cell function (Anand et al., 2010; Lagos et al., 2010; Kumarswamy et al., 2014; Liu et al., 2011; Luo et al., 2017; Du et al., 2019), as shown in Fig. 4.
Fig. 4.
Correlations between levels of tested miR-132 and miR-21 (normalised to spike-in control) against levels of VEGF-a for each participant (N = 14).
These correlation plots show a split of our patient group into two distinct groups: one with good correlation between microRNAs and VEGF and one with very low microRNA levels and no correlation.
3.8.4. Cortisol
Hair cortisol findings in both samples are shown in Table 6.
Table 6.
Hair cortisol levels in both samples.
| Sample |
CANDO N = 5 |
p-value |
Healthy N = 5 |
p-value |
||
|---|---|---|---|---|---|---|
| Hair cortisol (last month) | Hair cortisol (earlier) | Hair cortisol (last month) | Hair cortisol (earlier) | |||
| Mean (SD) | 9.99 (4.32) | 11.26 (5.74) | .429 | 13.38 (9.50) | 9.64 (6.78) | .548 |
| Median | 9.94 | 9.86 | 7.64 | 6.53 | ||
| Range | 11.85 | 14.65 | 21.54 | 14.94 | ||
| Min | 3.90 | 6.48 | 6.42 | 3.74 | ||
| Max | 15.75 | 21.13 | 27.96 | 18.68 | ||
| p-value comparing CANDO sample with healthy sample | .864 | |||||
Hair samples were taken in 14 participants, however hair samples need to weigh at least 25 g to assess, and in nine patients this was impossible to obtain because of hairstyle; hence only 5 samples were eligible to be assessed. They were compared with 5 healthy samples obtained and assessed in the same manner. The CANDO healthy sample was taken at the beginning of the lockdown. The last month levels in the healthy sample indicate levels at the beginning of lockdown (March 2020). The earlier healthy samples indicate levels approximately 1 month before the COVID-19 outbreak and lockdown. None of the levels differed significantly within the samples and there was no significant difference between the samples. The means were in the normal score ranges (Herane-Vives et al., 2018).
4. Discussion
4.1. Feasibility
We found that 85% of people approached could be recruited. Because of COVID-19, recruitment had to be stopped in 7 potential participants (27%). A variety of sites were interested in participating including sites outside mental health NHS trusts, such as neurology departments, physiotherapy practices, and primary care sites. Data were complete with an attrition rate of 13% before COVID-19 halted the study. We were also able to develop a reliable network for the collection, appropriate storage and transport to laboratories of samples ensuring valid test outcomes. Study procedures were acceptable for participants, in terms of tolerance for the duration of interviews, blood sampling and potentially distressing revisiting of past events, as well as willingness to attend psychiatric assessment. Participants were positively engaged and other members of the public engaged spontaneously. There was a general consensus that this condition is understudied and the PPI group supported this initiative wholeheartedly, which underscores the high clinical unmet need, the acceptability of the study procedures to patients and carers, and the wish of patients and carers to support research in this domain. As with most research studies, getting the design right, study set-up, obtaining approvals and recruiting to time and target are major challenges. This feasibility study has given the study team and collaborators the opportunity to refine the design and establish the networks needed for a full-scale trial. We now know a lot more about how and where to recruit participants other than through mental health NHS organisations. Also we know that patients may self-refer. A greater challenge than anticipated was the determination of potential eligibility from secondary care medical records. A two-part consent procedure might also be considered for a larger cohort. There is a need to include a mechanism for obtaining GP data which we would have if we were recruiting through primary care. We would then also possibly get a more representative sample. As each of the criteria that we set when we designed the study (Van der Feltz-Cornelis et al., 2020b) have been met, the evidence suggests that setting up a larger cohort would be feasible and valuable.
4.2. Characteristics of the patient group
Not only have the assessments proven to be mostly feasible, the outcomes also give an indication that this is a highly vulnerable patient group. Both physical and psychological symptom levels are high, comorbidity with other mental disorders such as depression and anxiety is high and physical symptoms not only occur in the domain of CD/FND, but also in other physical domains, even though we took great care to exclude patients with clear-cut inflammatory diseases or brain pathology. Quality of life was much worse than at population level in a developing country such as Indonesia (Purba et al., 2018). Levels of functioning in all domains of the RAND SF-36 were much worse than in clinical patients with chronic medical conditions in an USA sample (Tarlov et al., 1989). A similar finding has been reported in Somatic Symptom Disorders and Related Disorders, of which CD/FND is a subsection, where patients with SSRD without known chronic medical condition reported worse functioning levels than patients with SSRD with known chronic medical condition(Van der Feltz-Cornelis et al., 2020a). An explanation of this finding might be that levels of distress interfere with quality of life and general functioning and are worse in case of unexplained, very disabling physical symptoms, compared to disabling physical symptoms due to a known chronic medical condition. Actual stress levels as measured by the LCU are strikingly elevated with only 27% of the patients scoring less than 150, which is associated with higher levels of depression (Cornelis et al., 1989), or being in a crisis (Holmes and Rahe, 1967). 30% scored 300 or higher, which is associated with elevated risk for an accident or physical disease (Rahe et al., 1970; Rahe and Arthur, 1978). Such high symptom load and low functioning has also been described in patients with a different kind of CD/FND, namely patients with psychogenic non-epileptic seizures. They showed high comorbidity, and also higher mortality than the general population of a similar level as patients with epileptic seizures (Nightscales, 2020), indicating that CD/FND may not be a harmless condition to suffer from.
4.3. Adverse childhood Experience(ACE)
Regarding childhood trauma, 86% experienced at least one ACE which is substantially more than the 64% in the original N = 17,000 ACE study conducted in the USA general population (Centre of Disease Control and Prevention, 2016). It is also more than the 77% reported in mental health outpatients with anxiety or depression (Van der Feltz-Cornelis et al., 2019b), and more than 61.8% reported in outpatients with SSRD (Van der Feltz-Cornelis et al., 2020a). 80% experienced multiple ACE and the mean ACE score was 4 which is high compared to these studies as well. Childhood sexual abuse occurred in this sample and this is clinically relevant as this has been found to negatively influence treatment outcome in CD/FND. (Van der Feltz-Cornelis et al., 2019a) A recent study found that childhood physical neglect is associated with exaggerated systemic responses to short-term exposure to psychosocial distress in the Trier Social Stress Test, in which reactive gene expression might play a role (Schreier et al., 2020). As mean IL-6 scores in that study are much lower than in our sample, elevated cytokines in CD/FND may have other drivers than ACE as well. The hair cortisol levels in our sample were in the normal range,(Meyer and Novak, 2012) suggesting that HPA axis pathology did not play a role in this. In future studies, exploring the link between ACE, current stress, cytokines and microRNA in CD/FND should therefor certainly be pursued.
4.4. SLI, microRNA and epigenetics
Our study sample showed elevated IL-6, IFNγ, IL17A, IL12 and TNFα scores, normal IL1B scores, and VEGF-a scores significantly lower compared to the normal sample. Another study in acute CD/FND patients found elevated IL-6, TNFα and IL1B scores (Tiyekli, 2013), which is different from our findings as in our study, IL1B was normal. Dysregulated continual synthesis of IL-6 plays a role in pathologic chronic inflammation and autoimmunity (Tanaka, 2014) so our study supports the idea that IL-6 may play a role in CD/FND of longer duration. IFNγ is produced by CD4+ T helper 1 cells (Th1 cells). It plays a key role in B cell maturation, as it works to inhibit migration into lymph nodes while the B cells are still immature (Flaishon, 2000) and inhibits proliferation of T cells (Chu, 2000). Tumour Necrosis Factor Alpha (TNFα) is produced by macrophages/monocytes during inflammation and has widespread cellular effects, although still a great deal is unknown about its function and complex interplay with other cytokines (Zelova, 2013). IL-12 plays an important role in the activities of natural killer cells and T lymphocytes and has anti-angiogenic activity, which means it can block the formation of new blood vessels by increasing production of IFNγ and chemokines. Also, regarding vascular endothelial growth factor A or VEGF-a, we found significantly lower scores than in a healthy sample. VEGF-a was named after its role in angiogenesis. The VEGF-a serum level is elevated in coronary artery disease (CAD) and low serum VEGF-a has been found to indicate severe coronary artery lesions and a higher GRACE score, indicating poor clinical outcomes (Huang et al., 2020). Too low VEGF can lead to decreased angiogenesis and lack of oxygen to the tissues. It would be worthwhile to explore if this might also pertain to brain ischemia and neurodegeneration. There is emerging evidence that VEGF-a has effects on neural development, and VEGF-a changes could be linked to neural changes in adulthood, for astroglia and Schwann cells, supportive cells to neurones providing metabolic support and recycling neurotransmitters released into synapses (Mackenzie, 2012). It could be assumed that changes in VEGF-a could result in disruption in neurone nourishment, health and regulation. To summarise, our findings show an elevation of cytokines across the board, indicating peripheral inflammation; links to T cell activity; and to reduced angiogenesis and support to neuronal cells.
Our study suggests epigenetic processes as TNFα was correlated with miR-16, miR-132, miR-146a, miR-155, miR-223 and miR-21. miR-146a is known to be associated with inflammation and so is miR-155 (Yee, 2017). Also, regarding the association between microRNA and VEGF-a, we found two subgroups in our sample. One with a correlation between miRNA-21 and miRNA-132, both microRNAs implicated in vascular inflammation, and VEGF-a levels, and one with very low microRNA levels and no correlation. This suggests that there may be an epigenetic mechanism for a subgroup of patients with motor CD/FND. It might be indicative of an important role for the vascular and circulatory system in a subset of individuals with FND/CD. Suggestions for some vascular link have been made in a case study (Gürses et al., 2008) and in a recent review considering that a proportion of patients entering the care pathway present with functional symptoms mimicking stroke or have functional symptoms in addition to vascular stroke (Jones et al., 2020). The possibility that VEGFa and microRNA might play a role in this should be investigated in future studies.
4.5. Neurocognitive impairments
Neurocognitive tests showed no indications of impaired effort according to the WAIS Digit Span Scores and no evidence of malingering in the TOMM test. There was evidence of impairment in verbal and visual components of executive functioning (inhibition/switching on the CWIT and TMT). Equally, there was evidence of slowed information processing and motor speed overall, which could influence higher executive functions involving cognitive flexibility under timed conditions, such as inhibition and switching. Conversely, executive functions assessed by the WAIS digit symbol substitution test and the Tower of London Test (also a timed task) were in the normal range. If we compare these findings with findings with a sample of 29 patients with CD/FND, raw scores on both the (Stroop) CWIT test and TMT (visual scanning) were substantially lower in the current sample (De Vroege et al., 2020). Working memory scores were slightly higher in the current sample although still impaired. This warrants further, larger scale research in order to more fully establish a profile of neurocognitive impairment in this group and its association with neuro-inflammation markers as well as self-reported stress and adverse childhood experiences.
4.6. Implications for research
This feasibility study shows that there is scope to further explore an underlying pathogenic mechanism related to an inflammatory process and vascular inflammatory process in CD/FND with motor problems, cytokines, and the role of miRNA. Our results provide support for further studies investigating the role of inflammation in CD/FND pathogenesis. Understanding the links between inflammation and CD/FND pathogenesis can potentially lead to inclusion of anti-inflammatory agents in future research evaluation of new treatments for CD/FND. VEGF-a might be explored further. The role of microRNA needs further exploration, as mental disorders have been suggested to result from impairments in cellular plasticity cascades, disrupting cellular networks, rather than disturbances in any particular monoamine system. Such impairments may lead to aberrant information processing in the brain circuits that regulate mood, cognition, and neurovegetative functions. MicroRNAs are non-coding RNAs that can repress the gene translation of hundreds of their targets and are therefore well-positioned to target and impair a multitude of cellular mechanisms. They are altered by stress, glucocorticoids, and mood stabilizers (Hunsberger, 2009). The contribution of brain miRNAs to the regulation of behaviour may play a key role in the epigenetic hypothesis for mental disorders (Issler, 2015). miRNA levels may potentially be used in the diagnosis of mental disorders and might become novel treatment targets for mental disorders (Hanna et al., 2019). Another avenue for future research may be developing and evaluating a cognitive remediation training aimed at maintaining, switching and focusing attention, such as in brain injury patients. It is already common practice to work on attention before starting CBT in dementia patients in order to compensate for age-related changes in executive functioning and attentional skills, and this might be a path to explore in CD/FND patients as well, taking different cytokine and miRNA profiles into account towards the intervention.
4.7. Limitations of the study
This is a feasibility study that has obvious limitations. Firstly, although the study was getting set up to include patients from several NHS settings, due to the COVID-19 outbreak, most patients were recruited from a single NHS trust, limiting our knowledge of how the processes may work in other settings. Nevertheless, as other settings were starting up to contribute, we have no reason to doubt the feasibility of having multiple sites and settings contribute. Second, patient characteristics provide an indication of the potential relevance of further research to explore the hypothesis that systemic low-grade inflammation and microRNA might play a role in CD/FND; further research would be needed to explore this. Third, regarding the cytokines and microRNA, we only had one sample, taken at a random sample timepoint and not related to an intervention. Although we made an effort to not include patients with evidence of a concurring inflammatory condition, two baseline measures to check if an elevated score would not be temporary and related to a passing infection, but an indication of systemic low-grade inflammation, would have been a good addition. Fourth, although we found some statistically significant findings, and we compared with results from a healthy sample following the same methods at the same lab, the generalisability of the results is limited because of the small number and the lack of a control group that was matched for age and gender and examined at the same time as the patients. Also, in a future study we will need procedures to assess BMI and weight circumference in patients unable to stand, as well as smoking, (Yudkin et al., 1999) to enable us to correct for confounding factors.
4.8. Strengths of the study
This study has several strengths. We evaluated the feasibility to assess stress-related SLI in CD/FND patients with prevalent motor function FND, which is a very vulnerable population. The key strengths of this study are the use of both objective and subjective observational methods to assess stress related SLI, psychological and cognitive symptoms in this sample population. This is the first study exploring not only clinical characteristics regarding the condition itself, but also cytokines, microRNA, hair cortisol, and several variables exploring psychiatric comorbidity, current stress and trauma, and neurocognitive functioning. The finding that it is feasible to do such a comprehensive assessment in so vulnerable patients in a limited amount of time is important for the development of work in CD/FND. Furthermore, the findings suggest that there are indications for SLI in this condition as participants demonstrated elevated levels of inflammatory cytokines which correlated with levels of inflammation-associated and vascular inflammation associated miRNAs within the cohort. It is a strength of the study that the selection of a homogeneous group and the combination of cytokines and microRNA enabled us to find two subgroups regarding VEGF-a, indicating possible different mechanisms that may be helpful in future profiling studies (Van der Feltz-Cornelis, 2020c). Also, the finding that the participants evaluated the study positively and that patients were eager to engage is a strength of the study. These findings support the further development of a larger project aiming to explore this further and to develop new treatments for this population in the future.
5. Conclusion
This innovative, translational study explores stress-related SLI in CD/FND patients and the feasibility of a larger project aiming to develop new treatments for this vulnerable population. It provides knowledge about levels of SLI, psychological and neurocognitive symptoms in patients with CD/FND that is so far largely unknown and warrants further research. Furthermore it provides valuable knowledge regarding the feasibility of conducting a study in these patients that will inform future study phases. Given the positive findings, there is scope to conduct further research.
Funding
This work was part-funded by the Wellcome Trust UK [ref: 204829] through the Centre for Future Health (CFH) at the University of York (UK)
Declaration of competing interest
The authors have no conflicts of interest to report.
Acknowledgements
Dr. Lars de Vroege, Prof. Jon Stone and Prof. Roland von Kanel provided valuable insights as advisors to the study. Connie Newcombe provided advice on interpretation of neurocognitive tests. Dr. Sally James performed and described the microRNA analyses. The cytokine assays were performed by the NIHR Cambridge Biomedical Research Centre, Core Biochemistry Assay Laboratory. The ARU Biomarker Lab in Cambridge, UK performed the hair cortisol analyses. The Pathology Clinical Trials at James Cook University Hospital lab prepared and stored the blood samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2021.100228.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alivernini S., Gremese E., McSharry C., Tolusso B., Ferraccioli G., McInnes I.B., Kurowska-Stolarska M. MicroRNA-155—at the critical interface of innate and adaptive immunity in arthritis. Front. Immunol. 2018;8:1932. doi: 10.3389/fimmu.2017.01932.ISSN=1664-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S.F., van Hemert A.M., van der Feltz-Cornelis C.M. 2020. English Translation of the Physical Symptoms Questionnaire (PSQ-51) and Validation in a UK Sample. (submitted for publication) [Google Scholar]
- American Psychiatric Association . fifth ed. American Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. [Google Scholar]
- Anand S., Majeti B., Acevedo L. MicroRNA-132–mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat. Med. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen H.H., Duroux M., Gazerani P. MicroRNAs as modulators and biomarkers of inflammatory and neuropathic pain conditions. Neurobiol. Dis. 2014;71:159–168. doi: 10.1016/j.nbd.2014.08.003. ISSN 0969-9961. [DOI] [PubMed] [Google Scholar]
- Bai Y.M., Chiou W.F., Su T.P., Li C.T., Chen M.H. Pro-inflammatory cytokine associated with somatic and pain symptoms in depression. J. Affect. Disord. 2014;155:28–34. doi: 10.1016/j.jad.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Baumeister D., Akhtar R., Ciufolini S., Pariante C.M., Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol. Psychiatr. 2016;21(5):642–649. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B.L., Strauss E., Sherman E.M.S., Iverson G.L., Slick D.J. Developments in neuropsychological assessment: refining psychometric and clinical interpretive methods. Can. Psychol. 2009;50(3):196–209. doi: 10.1037/a0016066. [DOI] [Google Scholar]
- Calcia M.A., Bonsall D.R., Bloomfield P.S., Selvaraj S., Barichello T., Howes O.D. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology. 2016;233(9):1637–1650. doi: 10.1007/s00213-016-4218-9. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CBS . CBS vademecum; Den Haag: 2005. Netherlands Central Bureau of Statistics (CBS) List. (CBS) [Google Scholar]
- Centre for Disease Control and Prevention Prevalence of individual adverse childhood experiences. 2016. https://www.cdc.gov/violenceprevention/aces/about.html
- Chu C.Q., Wittmer S., Dalton D.K. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 2000;192(1):123–128. doi: 10.1084/jem.192.1.123. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conversion Neuro-inflammation disorder observational (CANDO) study. 2019. https://www.york.ac.uk/healthsciences/research/mental-health/projects/cando/ Accessed at Nov 6th 2020.
- Cornelis C.M., Ameling E.H., de Jonghe F. Life events and social network in relation to the onset of depression. A controlled study. Acta Psychiatr. Scand. 1989;80(2):174–179. doi: 10.1111/j.1600-0447.1989.tb01323.x. [DOI] [PubMed] [Google Scholar]
- Crawford J.R., Garthwaite P.H., Sutherland D., Borland N. Some supplementary methods for the analysis of the delis-kaplan executive function system. Psychol. Assess. 2011;23:888–898. doi: 10.1037/a0023712. [DOI] [PubMed] [Google Scholar]
- De Vroege L., Khasho D., Foruz A., van der Feltz-Cornelis C.M. Cognitive rehabilitation treatment for mental slowness in Conversion disorder. A case report. Cogent Psychology. 2017;4 doi: 10.1080/.23311908.2017.1348328. 1348328. [DOI] [Google Scholar]
- De Vroege L., Koppenol I., Kop W.J., Riem M.M.E., van der Feltz-Cornelis C.M. Neurocognitive functioning in patients with conversion disorder/functional neurological disorder. J. Neuropsychol. 2020 doi: 10.1111/jnp.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Grande da Silva G., Wiener C.D., Barbosa L.P., Gonçalves Araujo J.M., Molina M.L., San Martin P., Oses J.P., Jansen K., Dia de Mattos Souza L., Azevedo da Silva R. Pro-inflammatory cytokines and psychotherapy in depression: results from a randomized clinical trial. J. Psychiatr. Res. 2016;75:57–64. doi: 10.1016/j.jpsychires.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Delis D.C., Kramer J.H., Kaplan E., Holdnack J. Reliability and validity of the delis-kaplan executive function system: an update. J. Int. Neuropsychol. Soc.: JINS. 2004;10(2) doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Dozier M.E., Wetherell J.L., Twamley E.W., Schiehser D.M., Ayers C.R. The Relationship between age and neurocognitive and daily functioning in adults with hoarding disorder. Int. J. Geriatr. Psychiatr. 2016;31(12):1329–1336. doi: 10.1002/gps.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Hong L., Sun L. miR-21 induces endothelial progenitor cells proliferation and angiogenesis via targeting FASLG and is a potential prognostic marker in deep venous thrombosis. J. Transl. Med. 2019;17:270. doi: 10.1186/s12967-019-2015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn E.C., Soare T.W., Zhu Y., Simpkin A.J., Suderman M.J., Klengel T., Smith A.D.A.C., Ressler K.J., Relton C.L. Sensitive periods for the effect of childhood adversity on DNA methylation: results from a prospective, longitudinal study. Biol. Psychiatr. 2019;85(10):838–849. doi: 10.1016/j.biopsych.2018.12.023. Epub 2019 Jan 21. Erratum in: Biol Psychiatry. 2019 Jul 1;86(1):76. PMID: 30905381; PMCID: PMC6552666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna-Hernandez K.L., Ross R.G., Natvig C.L., Laudenslager M.L. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol. Behav. 2011;104(2):348–353. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaishon L., Hershkoviz R., Lantner F., Lider O., Alon R., Levo Y., Flavell R.C., Shachar I. Autocrine secretion of interferon gamma negatively regulates homing of immature B cells. J. Exp. Med. 2000;192(9):1381–1388. doi: 10.1084/jem.192.9.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelauff J., Stone J., Edwards M.J., Carson A. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J. Neurol. Neurosurg. Psychiatry. 2014;85(2):220–226. doi: 10.1136/jnnp-2013-305321. [DOI] [PubMed] [Google Scholar]
- Goldstein L.H., Robinson E., Mellers J.D.C., Stone J., Carson A., Reuber M., Medford N., McCrone P., Murray J., Richardson M.P., Pilecka I.P., Eastwood C., Moore M., Mosweu I., Perdue I., Landau S., Chalder T. Cognitive behavioural therapy for adults with dissociative seizures (CODES): a pragmatic, multicentre, randomised controlled trial. Lancet Psychiatry. 2020;7(6):491–505. doi: 10.1016/S2215-0366(20)30128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürses N., Temuçin C.M., Lay Ergün E., Ertuğrul A., Ozer S., Demir B. Konversiyon bozukluğunda uyarilmiş potansiyeller ve beyin kan akimi değişiklikleri: olgu sunumu ve gözden geçirme [Evoked potentials and regional cerebral blood flow changes in conversion disorder: a case report and review] Turk Psikiyatri Derg. Spring. 2008;19(1):101–107. Turkish. PMID: 18330748. [PubMed] [Google Scholar]
- Hanna J., Hossain G.S., Kocerha J. The potential for microRNA therapeutics and clinical research. Front. Genet. 2019;10(478) doi: 10.3389/fgene.2019.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays R.D., Shapiro M.F. An overview of generic health-related quality of life measures for HIV research. Qual. Life Res. 1992;1:91–97. doi: 10.1007/BF00439716. [DOI] [PubMed] [Google Scholar]
- Herane-Vives A., de Angel V., Papadopoulos A., Wise T., Chua K.C., Strawbridge R., Castillo D., Arnone D., Young A.H., Cleare A.J. Short-term and long-term measures of cortisol in saliva and hair in atypical and non-atypical depression. Acta Psychiatr. Scand. 2018;137(3):216–230. doi: 10.1111/acps.12852. [DOI] [PubMed] [Google Scholar]
- Hewitson J.P., Shah K.M., Brown N., Grevitt P., Hain S., Newling K., Sharp T.V., Kaye P.M., Lagos D. miR-132 suppresses transcription of ribosomal proteins to promote protective Th1 immunity. EMBO Rep. 2019;20(4) doi: 10.15252/embr.201846620. e46620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes T.H., Rahe R.H. The social readjustment rating scale. J. Psychosom. Res. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Holst Y., Thorell L.B. Functional impairments among adults with ADHD: a comparison with adults with other psychiatric disorders and links to executive deficits. Appl Neuropsychol Adult. 2020;27(3):243–255. doi: 10.1080/23279095.2018.1532429. [DOI] [PubMed] [Google Scholar]
- Huang A., Qi X., Cui Y., Wu Y., Zhou S., Zhang M. Serum VEGF: diagnostic value of acute coronary syndrome from stable Angina pectoris and prognostic value of coronary artery disease. Cardiol. Res. Pract. 2020;2020 doi: 10.1155/2020/6786302. 6786302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubschmid M., Aybek S., Maccaferri G.E., Chocron O., Gholamrezaee M.M., Rossetti A.O. Efficacy of brief interdisciplinary psychotherapeutic intervention for motor conversion disorder and nonepileptic attacks. Gen. Hosp. Psychiatr. 2015;37(5):448–455. doi: 10.1016/j.genhosppsych.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Hunsberger J.G., Austin D.R., Chen G., Manji H.K. MicroRNAs in mental health: from biological underpinnings to potential therapies. NeuroMolecular Med. 2009;11(3):173–182. doi: 10.1007/s12017-009-8070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issler O., Chen A. Determining the role of microRNAs in psychiatric disorders. Nat. Rev. Neurosci. 2015;16:201–212. doi: 10.1038/nrn3879. [DOI] [PubMed] [Google Scholar]
- Jak A.J., Bondi M.W., Delano-Wood L., Wierenga C., Corey-Bloom J., Salmon D.P., Delis D.C. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am. J. Geriatr. Psychiatr. 2009;17(5):368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A., O’Connell N., David A.S., Chalder T. Functional stroke symptoms: a narrative review and conceptual model. J. Neuropsychiatry Clin. Neurosci. 2020;32(1):14–23. doi: 10.1176/appi.neuropsych.19030075. Epub 2019 Nov 15. PMID: 31726918, 2020 Winter. [DOI] [PubMed] [Google Scholar]
- Karr J.E., Garcia-Barrera M.A., Holdnack J.A., Iverson G.L. Using multivariate base rates to interpret low scores on an abbreviated battery of the delis-kaplan executive function system. Arch. Clin. Neuropsychol. 2017;32(3):297–305. doi: 10.1093/arclin/acw105. [DOI] [PubMed] [Google Scholar]
- Keifer E., Tranel D. A neuropsychological investigation of the delis-kaplan executive function system. J. Clin. Exp. Neuropsychol. 2013;35(10):1048–1059. doi: 10.1080/13803395.2013.854319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompoliti K. Cognitive therapies for psychogenic movement disorders. J. Neurol. Sci. 2015;1:e479. [Google Scholar]
- Kovacs D., Eszlari N., Petschner P., Pap D., Vas S., Kovacs P., Gonda X., Juhasz G., Bagdy G. Effects of IL1B single nucleotide polymorphisms on depressive and anxiety symptoms are determined by severity and type of life stress. Brain Behav. Immun. 2016;56:96–104. doi: 10.1016/j.bbi.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Kovacs D., Eszlari N., Petschner P., Pap D., Vas S., Kovacs P., Gonda X., Bagdy G., Juhasz G. Interleukin-6 promoter polymorphism interacts with pain and life stress influencing depression phenotypes. J. Neural. Transm. 2016;123(5):541–548. doi: 10.1007/s00702-016-1506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowska K., Palmer D.M., Brown K.J., McLean L., Scher S., Gevirtz R. Reduction of autonomic regulation in children and adolescents with conversion disorders. Psychosom. Med. 2015;77(4):356–370. doi: 10.1097/PSY.0000000000000184. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., Chung J., Cruickshank B., McLean L., Scher S., Dale R.C. Blood CRP levels are elevated in children and adolescents with functional neurological symptom disorder. Eur. Child Adolesc. Psychiatr. 2019;28(4):491–504. doi: 10.1007/s00787-018-1212-2. 2019. [DOI] [PubMed] [Google Scholar]
- Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom. Med. 2007;69(9):881–888. doi: 10.1097/PSY.0b013e31815b00c4. 2007 Dec. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B.W., Löwe B. The Patient Health Questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen. Hosp. Psychiatr. 2010;32:345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Kumarswamy R., Volkmann I., Beermann J., Napp L.C., Jabs O., Bhayadia R., Melk A., Ucar A., Chowdhury K., Lorenzen J.M., Gupta S.K., Batkai S., Thum T. Vascular importance of the miR-212/132 cluster. Eur. Heart J. 2014;35(45):3224–3231. doi: 10.1093/eurheartj/ehu344. 1 December 2014. [DOI] [PubMed] [Google Scholar]
- Kut C., MacGaghann F., Popel A.S. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br. J. Canc. 2007;97(7):978–985. doi: 10.1038/sj.bjc.6603923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos D., Pollara G., Henderson S. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat. Cell Biol. 2010;12:513–519. doi: 10.1038/ncb2054. [DOI] [PubMed] [Google Scholar]
- Li P., He Q., Luo C., Qian L. Diagnostic and prognostic potential of serum angiopoietin-2 expression in human breast cancer. Int. J. Clin. Exp. Pathol. 2015;8(1):660–664. [PMC free article] [PubMed] [Google Scholar]
- Lin E., Tsai S.J. Gene-environment interactions and role of epigenetics in anxiety disorders. Adv. Exp. Med. Biol. 2020;1191:93–102. doi: 10.1007/978-981-32-9705-0_6. PMID: 32002924. [DOI] [PubMed] [Google Scholar]
- Liu L.-Z., Li C., Chen Q., Jing Y., Carpenter R., Jiang Y. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PloS One. 2011;6(4) doi: 10.1371/journal.pone.0019139. e19139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe B., Decker O., Muller S., Brahler E., Schellberg D., Herzog W., Herzberg P.Y. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med. Care. 2008;46(3):266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- Ludwig L., Pasman J., Nicholson T., Aybek S., David A.S., Tuck S., Kanaan R.A., Roelofs K., Carson A., Stone J. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry. 2018;5(4):307–320. doi: 10.1016/S2215-0366(18)30051-8. [DOI] [PubMed] [Google Scholar]
- Luo M., Tan X., Mu L. MiRNA-21 mediates the antiangiogenic activity of metformin through targeting PTEN and SMAD7 expression and PI3K/AKT pathway. Sci. Rep. 2017;7 doi: 10.1038/srep43427. 43427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace C.J., Trimble M.R. Ten-year prognosis of conversion disorder. Br. J. Psychiatry. 1996;169:282–288. doi: 10.1192/bjp.169.3.282. [DOI] [PubMed] [Google Scholar]
- Mackenzie F., Ruhrberg C. vol. 139. The Company of Biologists Ltd; 2012. (Diverse Roles for VEGF-A in the Nervous System). (2012)): Published by. [DOI] [PubMed] [Google Scholar]
- Maurer C.W., LaFaver K., Ameli R., Toledo R., Hallett M. A biological measure of stress levels in patients with functional movement disorders. Park. Relat. Disord. 2015;21(9):1072–1075. doi: 10.1016/j.parkreldis.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J.S., Novak M. Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 2012;153(9):4120–4127. doi: 10.1210/en.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S., Zhang J., Zhang W., Huang R.S. Circulating microRNAs as biomarkers for inflammatory diseases. MicroRNA. 2013;2(1):63–71. doi: 10.2174/2211536611302010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money K.M., Olah Z., Korade Z., Garbett K.A., Shelton R.C., Mirnics K. An altered peripheral IL6 response in major depressive disorder. Neurobiol. Dis. 2016;89:46–54. doi: 10.1016/j.nbd.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataraj C., Oliverio M., Mannon R.B., Mannon P.J., Audoly L.P., Amuchastegui C.S., Smithies O., Coffman T.M. Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J. Clin. Invest. 1999;104(12):1693–1701. doi: 10.1172/JCI7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE Technology appraisal guidance EQ-5D-5L. 2020. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l 2019. Retrieved from. 6th 2020.
- Nielsen G, Stone J, Edwards MJ. Physiotherapy for functional (psychogenic) motor symptoms: a systematic review. J Psychosom Res. 2013;75(2):93–102. doi: 10.1016/j.jpsychores.2013.05.006. PMID: 23915764. [DOI] [PubMed] [Google Scholar]
- Nightscales R., McCartney L., Auvrez C., Tao G., Barnard S., Malpas C.B., Perucca P., McIntosh A.M., Chen Z., Sivathamboo S., Ignatiadis S., Jones S., Adams S., Cook M.J., Kwan P., Velakoulis D., D’Souza W., Berkovic S.F., O’Brien T.J. Mortality in patients with psychogenic nonepileptic seizures. Neurology. 2020;95:1–e10. doi: 10.1212/WNL.0000000000009855. [DOI] [PubMed] [Google Scholar]
- Pongratz G., Straub R.H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 2014;16(6):504. doi: 10.1186/s13075-014-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purba F.D., Hunfeld J., Iskandarsyah A., Fitriana T.S., Sadarjoen S.S., Passchier J., Busschbach J.J.V. Quality of life of the Indonesian general population: test-retest reliability and population norms of the EQ-5D-5L and WHOQOL-BREF. PloS One. 2018;13(5) doi: 10.1371/journal.pone.0197098. e0197098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahe R.H., Arthur R. Life changes and illness studies: past history and future directions. J. Hum. Stress. 1978;4(1):3–15. doi: 10.1080/0097840X.1978.9934972. [DOI] [PubMed] [Google Scholar]
- Rahe R.H., Mahan J.L., Jr., Arthur R.J. Predicton of near-future health changes from subjects’ preceding life changes. J. Psychosom. Res. 1970;14(4):401–406. doi: 10.1016/0022-3999(70)90008-5. [DOI] [PubMed] [Google Scholar]
- Ratcliff J., Van der Feltz-Cornelis C. Conversion Disorder/Functional Neurological Disorder – a narrative review on current research into its pathological mechanism. Eur. J. Psychiatr. 2020;Volume 34(Issue 3):143–152. doi: 10.1016/j.ejpsy.2020.03.004. ISSN 0213-6163. [DOI] [Google Scholar]
- Reitan R.M. Reitan; Tucson, AZ: 1992. Trail Making Test: Manual for Administration and Scoring. [Google Scholar]
- Ridker P.M., Cushman M., Stampfer M.J., Tracy R.P., Hennekens C.H. Plasma concentration of C Reactive Protein and risk of developing peripheral vascular Disease. Circulation. 1998;97:425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- Ruddy R., House A. Psychosocial interventions for conversion disorder. Review. Cochrane Database Syst. Rev. 2005;4 doi: 10.1002/14651858.CD005331.pub2. CD005331. [DOI] [PubMed] [Google Scholar]
- Schmand B., Linderboom J., Launer L., Dinkgreve M., Hooijer C., Jonker C. What is a significant score change on the Mini-Mental State Examination? Int. J. Geriatr. Psychiatr. 1995;10(5):411–414. [Google Scholar]
- Schreier H.M.C., Kuras Y., McInnis C.M., Thoma M.V., St Pierre D.G., Hanlin L., Chen X., Wang D., Goldblatt D., Rohleder N. Childhood physical neglect is associated with exaggerated systemic and intracellular inflammatory responses to repeated psychosocial stress in adulthood. Front. Psychiatr. 2020;11 doi: 10.3389/fpsyt.2020.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H.S. The role and clinical significance of high-sensitivity C-reactive protein in cardiovascular disease. Korean Circ J. 2012;42(3):151–153. doi: 10.4070/kcj.2012.42.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Phil. Trans. Roy. Soc. Lond. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Sheedy F., Palsson-McDermott E., Hennessy E. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- Sheinerman K.S., Toledo J.B., Tsivinsky V.G. Circulating brain-enriched microRNAs as novel biomarkers for detection and differentiation of neuro-degenerative diseases. Alz Res Therapy. 2017;9:89. doi: 10.1186/s13195-017-0316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Patro P.S., Aggarwal A. MicroRNA-132, miR-146a, and miR-155 as potential biomarkers of methotrexate response in patients with rheumatoid arthritis. Clin. Rheumatol. 2019;38:877–884. doi: 10.1007/s10067-018-4380-z. [DOI] [PubMed] [Google Scholar]
- Slota J.A., Booth S.A. MicroRNAs in neuroinflammation: implications in disease pathogenesis, biomarker discovery and therapeutic applications. Non-coding RNA. 2019;5(2):35. doi: 10.3390/ncrna5020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staufenbiel S.M., Penninx B.W.J.H., Spijker A.T., Elzinga B.M., van Rossum E.F. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38(8) doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Stewart A.L., Greenfield S., Hays R.D., Wells K., Rogers W.H., Berry S.D., McGlynn E.A., Ware J.E., Jr. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. Jama. 1989;262(7):907–913. [PubMed] [Google Scholar]
- Stroop J.R. Factors affecting speed in serial verbal reactions. Psychol. Monogr. 1938;50(5):38. [Google Scholar]
- Tahamtan A., Teymoori-Rad M., Nakstad B., Salimi V. Anti-inflammatory MicroRNAs and their potential for inflammatory diseases treatment. Front. Immunol. 2018;9:1377. doi: 10.3389/fimmu.2018.01377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak L.M., Bakker S.J.L., Slaets J.P.J., Rosmalen J.G.M. Is high-sensitive C-reactive protein a biomarker for functional somatic symptoms? A population-based study. Brain Behav. Immun. 2009;23(7):1014–1019. doi: 10.1016/j.bbi.2009.05.059. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10) doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlov A.R., Ware J.E., Jr., Greenfield S., Nelson E.C., Perrin E., Zubkoff M. The Medical Outcomes Study. An application of methods for monitoring the results of medical care. Jama. 1989;262(7):925–930. doi: 10.1001/jama.262.7.925. [DOI] [PubMed] [Google Scholar]
- Teichner G., Wagner M.T. The Test of Memory Malingering (TOMM): normative data from cognitively intact and cognitively impaired individuals. Psychol. Assess. 1997;9:260–268. doi: 10.1016/S0887-6177(03)00078-7. [DOI] [PubMed] [Google Scholar]
- Thurston G., Daly C. The complex role of angiopoietin-2 in the angiopoietin–tie signaling pathway. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006650. a006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiyekli U., Caliyurt O., Tiyekli N.D. Proinflammatory cytokine levels in patients with conversion disorder. Acta Neuropsychiatr. 2013;25(3):137–143. doi: 10.1111/j.1601-5215.2012.00676.x. [DOI] [PubMed] [Google Scholar]
- Tombaugh T.N., Tombaugh P.W. Multi-Health Systems North Tonawanda; NY: 1996. Test of Memory Malingering: TOMM. [Google Scholar]
- Van der Feltz-Cornelis C.M. Diagnostic profiling in clinical translational research — a European perspective. Eur. J. Psychiatr. 2020;34(2 April–June):57–62. doi: 10.1016/j.ejpsy.2019.11.003. [DOI] [Google Scholar]
- Van der Feltz-Cornelis C.M., Allen S., van Eck van der Sluijs J.F. Childhood sexual abuse predicts treatment outcome in conversion disorder/functional neurological disorder. An observational longitudinal study. Brain and Behavior. 2019;10(3) doi: 10.1002/brb3.1558. e01558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Feltz-Cornelis C.M., Potters E.C., van Dam A., Koorndijk R.P.M., Elfeddali I., van Eck van der Sluijs J.F. Adverse Childhood Experiences (ACE) in outpatients with anxiety and depressive disorders and their association with psychiatric and somatic comorbidity and revictimization. Cross-sectional observational study. J. Affect. Disord. 2019;246:458–464. doi: 10.1016/j.jad.2018.12.096. [DOI] [PubMed] [Google Scholar]
- Van der Feltz-Cornelis C.M., Bakker M., Kaul A., Kuijpers T., Von Känel R., Van Eck van der Sluijs J.F. IL-6 and hsCRP in somatic symptom disorders and related disorders. Brain, Behavior and Immunity - Health. 2020;9 doi: 10.1016/j.bbih.2020.100176. 100176:ISSN 2666-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Feltz-Cornelis C.M., Brabyn S., Allen S.F., Reilly J., Clarke C., de Vroege L., Gilbody S., Wittington M., Lagos D. Conversion and neuro-inflammation disorder observational study (CANDO). Protocol of a feasibility study. Eur. J. Psychiatr. 2020;34(3):164–172. doi: 10.1016/j.ejpsy.2020.04.002. ISSN 0213-6163. [DOI] [Google Scholar]
- Van Hemert A.M., de Waal M.W.M., Van Rood Y.R. Meetinstrumenten bij somatoforme stoornissen. Tijdschr Psychiatr. 2004;46(10):693–696. [Google Scholar]
- Viljoen M., Panzer A. Non-termination of sickness behavior as precipitating factor for mental disorders. Med. Hypotheses. 2005;65(2):316–329. doi: 10.1016/j.mehy.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N., de Jonge P., Smit J.H., Bahn S., Penninx B.W.P. Cytokine production capacity in depression and anxiety. Transl. Psychiatry. 2016;6:e825. doi: 10.1038/tp.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.F., Yu M.-L., Yu G., Bian J.-J., Deng X.-M., Wan X.-J., Zhu K.-M. Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem. Biophys. Res. Commun. 2010;394(1):184–188. doi: 10.1016/j.bbrc.2010.02.145. ISSN 0006-291X. [DOI] [PubMed] [Google Scholar]
- Ware J.E., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36) Med. Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- Wechsler D. NCS Pearson; San Antonio, TX: 2008. Wechsler Adult Intelligence Scale – Fourth Edition (WAIS-IV) [Google Scholar]
- Wells K.B., Stewart A., Hays R.D., Burnam M.A., Rogers W., Daniels M., Berry S., Ware J. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. Jama. 1989;262(7):914–919. [PubMed] [Google Scholar]
- Wexler B.E., Zhu H., Bell M.D., Nicholls S.S., Fulbright R.K., Gore J.C., Colibazzi T., Amat J., Bansal R., Peterson B.S. Neuropsychological near normality and brain structure abnormality in schizophrenia. Am. J. Psychiatr. 2009;166(2):189–195. doi: 10.1176/appi.ajp.2008.08020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Adverse childhood experiences international questionnaire (ACE-IQ) 2018. http://www.who.int/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/
- Yee D., Shah K., Coles M.C., Sharp T.V., Lagos D. MicroRNA-155 induction via TNF-α and IFN-γ suppresses expression of programmed death ligand-1 (PD-L1) in human primary cells. J. Biol. Chem. 2017;292(50):20683–20693. doi: 10.1074/jbc.M117.809053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin J.S., Stehouwer C., Emeis J.J., Coppack S.W. C-reactive protein in healthy subjects. Association with obesity, insulinresistance, and endothelial dysfunction. A potential role for cytokinesoriginating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- Zelová H., Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm. Res. 2013;62:641–651. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.