Abstract
Previous research has demonstrated that perfectionism is implicated in poorer health and earlier mortality. However, to our knowledge, research has not yet determined how individual differences in perfectionistic cognitions are related to intermediary health markers such as inflammation. Thus, within the theoretical frameworks of the perfectionism diathesis-stress model (Hewitt and Flett, 1993) and the cognitive theory of perfectionism (Flett et al., 2018; Flett et al., 2016) the aims of our study were to test whether individual differences in perfectionistic cognitions were associated with low-grade inflammation via c-reactive CRP and IL-6 biomarkers and whether these relationships varied as a function perceived stress. The sample included 248 Canadian young adults (52% female, Mage = 22.89, SD = 1.53) who completed surveys assessing key constructs such as perfectionistic cognitions and perceived stress along with providing assessments of body fat percentage and serum samples of IL-6 and CRP. Regression analyses indicated that perfectionistic cognitions were not related to IL-6 under any conditions of stress. However, under high levels of stress perfectionistic cognitions were associated with elevated levels of CRP and these findings held after accounting for the effects of smoking status, body fat percentage, and respondent sex. The present work adds to the growing body of evidence supporting links between personality and inflammation. These findings raise the possibility that experiencing more frequent thoughts centered on the need to be perfect when coupled with higher levels of stress may set the stage for greater vulnerability for chronic inflammation.
Keywords: C-reactive protein, Diathesis-stress model, Inflammation, Interleukin-6, Pro-inflammatory biomarkers, Health, Perfectionism, Perfectionistic cognitions, Personality, Stress
Highlights
-
•
Perfectionistic cognitions were related to elevated levels of CRP under high levels of perceived stress.
-
•
Perfectionistic cognitions were not related to CRP under low or moderate levels of perceived stress.
-
•
Perfectionistic cognitions were not related to IL-6 under any conditions of stress.
1. Introduction
The relentless and uncompromising need to be perfect appears to be prevalent in adults (Stoeber and Stoeber, 2009) and youth (Flett and Hewitt, 2014). Further, perfectionism has been increasing among young adults in Canada, the United States, and Britain over the course of the last three decades (Curran and Hill, 2019). This is an alarming trend in light of increasing evidence indicating that perfectionism is a core vulnerability factor for a vast array of mental and physical health problems (Sirois and Molnar, 2016). However, the psychobiological processes underlying links between perfectionism and health remain largely unknown. Chronic low grade inflammation may represent one potential biological mechanism, given established links between personality traits and inflammatory pathways (Luchetti et al., 2014), and substantial evidence demonstrating the public health relevance of inflammatory markers of chronic illness (e.g., Harris et al., 1999). Thus, this study examined whether individual differences in perfectionism were associated with immune dysfunction, particularly a pro-inflammatory state, within the context of the perfectionism diathesis-stress model (Flett et al., 1995; Hewitt and Flett, 1993).
Two intermediate inflammatory biomarkers of health identified that impact the development, maintenance, and exacerbation of disease are C-reactive protein (CRP) and interleukin-6 (IL-6). CRP is a marker of chronic inflammation that has garnered considerable attention because of its links to metabolic syndrome, cancers, coronary heart disease, and early mortality (Aggarwal and Gehlot, 2009; Casas et al., 2008; Harris et al., 1999; Park et al., 2009; Pradhan et al., 2001). IL-6, a cytokine that can be both pro- and anti-inflammatory, depending on context, generally stimulates the immune system in response to injury or infection in part by stimulating liver production of CRP (McCarty, 1999; Steptoe et al., 2007) and has been associated with a wide range of consequential health outcomes (Maggio et al., 2006).
A growing body of work has implicated personality traits in low grade chronic inflammation through analysis of the CRP-IL-6 axis. Within the widely accepted five factor model of personality, which posits that five broad personality traits (i.e., neuroticism, conscientiousness, extraversion, openness to experience, and agreeableness) summarizing individuals’ typical ways of feeling, behaving, and thinking, conscientiousness has been most consistently linked to inflammation. For example, lower levels of conscientiousness have been associated with elevated levels of IL-6 and CRP (Chapman et al., 2011; Luchetti et al., 2014; Mõttus et al., 2013; Sutin et al., 2010; Turiano et al., 2013); however, not all studies have found such associations (Armon et al., 2013). Other personality traits such as lower extraversion, higher neuroticism, and lower openness have also been linked to greater inflammation, albeit not as consistently as conscientiousness (Armon et al., 2013; Luchetti et al., 2014; Mõttus et al., 2013; Sutin et al., 2010; Turiano et al., 2013).
Finally, lower-order personality facets (i.e., specific personality traits that underlie broader personality traits) such as hostility, self-directedness, and dispositional depression also appear to be relevant for inflammatory processes (Brummett et al., 2010; Henningsson et al., 2008; Mwendwa et al., 2013), underscoring the importance of examining narrower personality traits with respect to biomarkers of health. Indeed, it may be the case that some inconsistencies in linking broader personality traits to inflammatory markers may be due to differences in measures that emphasize some facets more than others. Chapman et al. (2009), for instance, established that extraversion was not associated with inflammation at the higher-order level. Yet, they found that activity level (a facet of extraversion) was negatively linked with inflammation. Similarly, Sutin et al. (2010) found that facet level personality traits of neuroticism such as hostility, vulnerability, and impulsivity were related to greater inflammation whereas the depression and anxiety facets were not. Therefore, investigating narrower personality traits may provide more precision to better understand the association between personality and inflammation.
One lower-level personality trait that is gaining increased attention in physical health is perfectionism (Sirois and Molnar, 2016). Indeed, research has established that perfectionism is related to experiencing greater levels of both general and specific health symptoms in samples of postsecondary students (e.g., Bottos and Dewey, 2004; Martin et al., 1996; Molnar et al., 2012; 2019) as well as adults from the community (e.g., Molnar et al., 2006; Saboonchi and Lundh, 2003), with longitudinal studies showing that perfectionism contributes to increased health symptoms and compromised health over time (Bonvanie et al., 2015; Pritchard et al., 2007; Sumi and Kanda, 2002). Specifically, perfectionism has been implicated in a host of chronic physical health issues including cardiovascular disease (Corson et al., 2018), irritable bowel syndrome (Flett et al., 2011), chronic fatigue syndrome (Kempke et al., 2016)), headaches (Bottos and Dewey, 2004), fibromyalgia (Molnar et al., 2012; Sirois et al., 2019), and ultimately earlier mortality (Fry and Debats, 2009).
According to the cognitive theory of perfectionism (Flett et al., 2016), individual differences in the frequency with which people experience perfectionistic cognitions (i.e., automatic and repetitive thoughts reflecting the need for perfection) are particularly relevant for health problems (Flett et al., 2016, 2018). This premise is in keeping with the perseverative cognition hypothesis (i.e., the notion that rumination extends the stress response, therefore conferring increased risk for health problems). While individual differences in perfectionistic cognitions are relatively understudied with respect to health, early empirical research supports this premise showing that perfectionistic cognitions are linked to psychosomatic symptoms in young adults (Flett et al., 2012) and uniquely contribute to distress (Casale et al., 2020; Tyler et al., 2020). Therefore, to expand our understanding of how perfectionism contributes to health, the present study concentrated uniquely on individual differences in the frequency with which individuals experience ruminative and automatic thoughts centering on the necessity to be perfect and their abiding sense of failure.
Finally, it is important to acknowledge the key role of stress when examining links between perfectionism and health. According to Hewitt and Flett’s perfectionism diathesis-stress model, perfectionism serves as a vulnerability factor that becomes triggered during times of stress (Flett et al., 1995; Hewitt and Dyck, 1986; Hewitt and Flett, 1993). This notion is supported by several studies showing that individuals higher in perfectionism are especially vulnerable to poorer outcomes when under conditions of greater stress (e.g., (Dunkley, 2003; Dunkley et al., 2000; Enns and Cox, 2005). Consequently, it is critical to not only assess direct links between perfectionistic cognitions and inflammation, but also to test whether associations between perfectionistic cognitions and inflammation are exacerbated by higher levels of stress.
1.1. Objectives and hypotheses
Collectively, extant literature provides compelling evidence that perfectionism plays an important role in health and illness, and that the effects of perfectionism appear to be rather pervasive, cutting across multiple indices of health. However, it must be noted that the majority of studies to date have relied upon subjective measures (see Fry and Debats, 2009, for an exception) rather than biomarkers of health. Moreover, to our knowledge, research has yet to gauge how individual differences in perfectionism are related to inflammation. Thus, within the contexts of the perfectionism diathesis-stress model (Hewitt and Flett, 1993) and the cognitive theory of perfectionism (Flett et al., 2018; Flett et al., 2016) this study examined associations between individual differences in perfectionistic cognitions and low-grade inflammation via CRP and IL-6 biomarkers and whether these associations varied as a function of stress. We also tested whether these associations held after including potential confounds that were of theoretical interest (e.g., cigarette smoking, percentage body fat, biological sex, and clinical diagnosis of depression (Cartier et al., 2009; de Maat and Kluft, 2002; Ellulu, Patimah, Khaza’ai, Rahmat and Abed, 2017; Marques-Vidal et al., 2012; Mendall et al., 2000; Tracy et al., 1997; Valkanova et al., 2013) and were significantly related to IL-6 or CRP to assess the incremental predictive utility of perfectionistic cognitions with respect to inflammation. We hypothesized that perfectionistic cognitions would be associated with elevated levels of CRP and IL-6 over and above the covariates and, consistent with the perfectionism diathesis-stress model, that these links would be stronger under higher levels of stress.
2. Materials & methods
2.1. Participants
Participants were drawn from the Niagara Longitudinal Heart Study, an ongoing multidisciplinary study of how adverse childhood experiences impact cardiovascular health from childhood to early adulthood (Wade et al., 2019). This project received clearance by our university’s Research Ethics Board and all participants read and signed consent forms before completing the study protocol. The current study uses the midpoint study sample of 248 participants of which 175 participants had complete data for personality and stress as well as biological specimens to measure IL-6, whereas 173 had CRP data. The sample was comprised of young adults whose age ranged from 19 to 26 years of which 52% were female (Table 1).
Table 1.
Correlation matrix with means, standard deviations, and Cronbach’s alpha for all model variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. CRP | – | ||||||
| 2. IL-6 | .533∗ | – | |||||
| 3. PC | .023 | .018 | – | ||||
| 4. STRESS | .164∗ | .135 | .320∗ | – | |||
| 5. BFP | .530∗ | .320∗ | -.088 | .192∗ | – | ||
| 6. AGE | .021 | .006 | -.040 | -.005 | -.047 | – | |
| 7. SEX | -.290∗ | -.153∗ | .010 | -.250∗ | -.483∗ | .059 | – |
| Mean | 3.34 | .59 | 44.67 | 27.13 | 24.48 | 22.89 | .46 |
| SD | 4.25 | .52 | 17.95 | 5.74 | 12.14 | 1.53 | .50 |
| α | n/a | n/a | .93 | .83 | n/a | n/a | n/a |
Note. ∗p < .05; CRP = C-reactive protein; IL-6 = Interleukin 6; PC = perfectionistic cognitions; BFP = body fat percentage; SEX = respondent sex, 0 = female & 1 = male; n/a = not applicable because a single item.
2.2. Measures
Perfectionism. The Perfectionism Cognitions Inventory (PCI; Flett et al., 1998) was used to assess perfectionistic cognitions. The PCI is a 25-item measure of the frequency over the past week of automatic thoughts indicating the need to achieve perfection such as ‘‘Why can’t I be perfect?’‘, “I should be perfect”, and “My work should be flawless”. Higher scores indicate experiencing more frequent perfectionistic thoughts. Scores on the PCI can theoretically range from 0 to 100, with scores for the present sample ranging from 6 to 95. The PCI has demonstrated high internal consistency and validity in both student and clinical samples (Flett et al., 2004; Flett et al., 1998; Flett et al., 2007). Internal consistencies for study measures are in Table 1.
Perceived Stress. Perceived stress was measured using the Perceived Stress Scale (PSS; Cohen et al. (1983)). The PSS consists of 14 items that assess the extent to which events are appraised as being stressful (e.g., “In the last month, how often have you felt nervous and ‘stressed’?“; Cohen et al. (1983)). A 5-point Likert-type scale ranging from 0 (never) to 4 (very likely) is used to score each item. Cohen et al. (1983) reported Cronbach’s alphas between .84 and 0.86 across three different samples, and an adequate test-retest reliability (α = 0.85).
2.3. Biological samples
Participants were instructed to fast for at least 4 h prior to the start of testing, and to consume no caffeine or alcohol, and not perform any strenuous physical activity for at least 12 h prior to the start of testing. Participants were not tested if they were recently sick or identified any recent antibiotic use. Both IL-6 and CRP were measured in processed serum. Blood samples were collected from the antecubital fossa of participants by a registered nurse into serum separation tubes (SST) (BD biosciences, #367986, Franklin Lakes, New Jersey, USA). Blood was allowed to clot for 30 min, and centrifuged at 1500×g for 15 min at 4 °C. Following centrifugation, serum was aliquoted into single-use specimens and stored at −80 °C until use. IL-6 was assessed using a multiplex bead-based assay kit (Bio-Rad, California, USA) according to the manufacturer’s suggested protocol (assay range 0.7–12,000 pg/mL and sensitivity of 0.1 pg/mL). Samples were assayed in duplicate, and fluorescence results were collected and analyzed using the Magpix system (Luminex Corporation, Texas, USA). Final concentrations were calculated based on a standard curve specific to each assay plate, and results are expressed as pg/mL. Levels of CRP were analyzed using a Human CRP Quantikine ELISA kit (R&D Systems, #DCRP00, Minneapolis, Minnesota, USA) according to the manufacturer’s suggested protocol (assay range 0.8–50 ng/mL and sensitivity of 0.022 ng/mL). Samples were assayed in duplicate and absorbance was measured at 450 nm, with a correction wavelength of 540 nm. Final concentration was calculated from the respective standard curve on each plate and results were converted from ng/mL to mg/L.
2.4. Potential covariates
Body composition including body fat percentage was measured using air-displacement plethysmography (BODPOD, Life Measurement, Concord, California, USA). Information on smoking status, clinical diagnosis of depression, and steroidal and non-steroidal drug use were collected in the self-report questionnaire. Specifically, smoking was assessed by asking participants to report whether they currently smoked cigarettes daily, occasionally, or not at all. Self-reported clinical diagnosis of depression was measured by asking participants to respond to the following question “Have you ever been diagnosed by a medical professional as having depression?“. Finally, participants were asked to indicate all over the counter and prescribed medications that they had taken within the past month to determine steroidal and non-steroidal drug use.
3. Results
Overall, participants were missing information on 0.427 variables on average (SD = 0.711) (Little, 1988). MCAR test indicated that the data was missing completely at random (χ2(50) = 50.05, p < .471). The PROCESS MACRO (Hayes, 2018) was used to conduct multiple regression which employs listwise deletion for missing data resulting in a sample of 175 participants for analyses concerning IL-6 and 173 concerning CRP. Univariate outliers (i.e., scores that were more than |3.00| standard deviations from the mean) were Winsorized, such that the scores were adjusted to represent z-scores that would have been within 3 standard deviations from the mean, while maintaining rank order to one decimal place. Results indicated that 28.2% of participants in this sample had CRP values that were considered relatively high in North American samples (Pearson et al., 2003). Descriptive statistics and bivariate correlations for all model variables are presented in Table 1.
Analyses concerning potential confounds including cigarette smoking, biological sex, clinical depression diagnosis, steroid drug use, nonsteroid drug use, and percentage of body fat were conducted next using correlations, independent sample t tests, or one-way analysis of variance (ANOVA) as appropriate. Potential confounds with significant bivariate associations with IL-6 or CRP were included as covariates in all remaining analyses (if they were associated at p < .10). Results demonstrated that the only potential confounds related to CRP were respondent sex (t(159.43) = 4.066, p < .001), such that women (M = 4.43, SD = 4.78) had higher levels of CRP than men (M = 2.00, SD = 3.02); smoking status (Welch’s F (1,25.95) = 5.12, p = .013) in which daily smokers (M = 5.89, SD = 5.93) had higher levels of CRP than occasional smokers (M = 1.51, SD = 2.16); and body fat percentage (r = 0.53, p < .001), such that greater body fat was related to elevated CRP. Analyses concerning steroid drug use (t(171) = 0.23, p = .819), nonsteroid drug use (t(171) = 0.34, p = .905) and diagnosis of clinical depression or not (t(171) = −0.88, p = .382) showed that these variables were unrelated to CRP. Thus, the covariates included in analyses concerning CRP were respondent sex, smoking status, and body fat percentage. Results showed the only potential confounds related to IL-6 were respondent sex (t(173) = 1.98, p = .049) such that women (M = 0.66, SD = 0.54) had higher IL-6 levels than men (M = 0.50, SD = 0.47); body fat percentage (r = 0.32, p < .001), such that greater body fat was associated with elevated IL-6; and smoking status (Welch’s F (2, 31.18) = 2.61, p = .089). However, none of the pairwise comparisons for smoking status were significant at p < .10. Nonsteroidal drug use (t(173) = −1.34, p = .183), steroidal drug use (t(173) = −0.691, p = .490), and diagnosis of clinical depression or not (t(173) = −0.918, p = .360) shared no relations with IL-6. Thus, only body fat percentage, smoking status, and respondent sex were included as covariates in the subsequent analyses.
3.1. Regression analyses
Multiple regression was employed using the PROCESS MACRO (Hayes, 2018) with SPSS version 26 statistical software to test whether the links between perfectionistic cognitions and IL-6 or between perfectionistic cognitions and CRP were moderated by perceived stress after accounting for the effects of respondent sex, percentage of body fat, and smoking status. An interaction term for perfectionistic cognitions and perceived stress was computed by PROCESS. Because composite variables, such as interaction terms, are correlated with their component variables, to ease the interpretation and probing of significant interactions, each of the component variables (i.e., perfectionistic cognitions, perceived stress) was centered around their sample mean before the interaction term was computed (Aiken and West, 1991; Cohen et al., 2003). Significant interactions were further probed using a series of post hoc regression equations, referred to as simple slopes analysis (Aiken and West, 1991).
For both regressions examination of the residuals indicated that the assumptions of normality and homoscedasticity were not tenable. Consequently, the HC3 (Hayes and Cai, 2007) heteroskedasticity-consistent standard error estimator was employed along with bootstrapping with 5000 bootstrap samples to generate all estimates for both regressions. Overall, results of the first regression in which IL-6 was the outcome of interest indicated that the model was statistically significant (F7,167 = 3.14, p = .039), accounting for 13.02% of the variance in IL-6. Results indicated that percentage of body fat was positively associated with IL-6. None of the other associations were statistically significant (see Table 2).1
Table 2.
Bootstrapped Regressions in which CRP and IL-6 were regressed on perfectionistic cognitions, stress, and the interaction between perfectionistic cognitions and stress after accounting for the effects of respondent sex, smoking status, and body fat percentage.
|
IL-6 (pg/mL) R2 = .130 |
CRP (mg/L) R2 = .371 |
|||||||
|---|---|---|---|---|---|---|---|---|
| b | b (bootstrap mean) | Se(b) (bootstrap) | 95%CI(β) (bootstrap) | b | b (bootstrap mean) | Se(b) (bootstrap) | 95%CI(β) (bootstrap) | |
| Perfectionistic Cognitions | .001 | .001 | .002 | -.003,.005 | .017 | .016 | .016 | -.013, .048 |
| Perceived Stress | .004 | .003 | .007 | -.010, .018 | .004 | .001 | .049 | -.092, .102 |
| Perfectionistic Cognitions by Perceived Stress | .000 | .000 | .000 | -.001, .001 | .007∗ | .007 | .003 | .001, .013 |
| Sex | -.008 | -.009 | .088 | -.175, .171 | -.725 | -.712 | .545 | −1.80, .356 |
| Body Fat Percentage | .014∗ | .013 | .003 | .007, .020 | .169∗ | .169 | .028 | .115, .224 |
| Daily Smoker or Not | .277 | .276 | .197 | -.102, .663 | 3.34∗ | 3.34 | 1.33 | .709, 5.98 |
| Occasional Smoker or Not | -.051 | -.049 | .091 | -.227, .127 | −1.02 | −1.02 | .722 | −2.42, .442 |
Note. ∗p < .05; Sex = biological sex (1 = male); Daily smoker or not (1 = daily smoker); Occasional smoker or not (1 = occasional smoker).
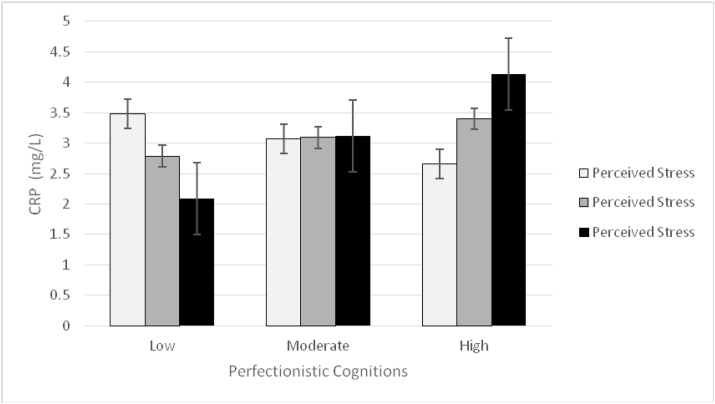
Results of the second regression in which CRP was the outcome of interest indicated that the model was statistically significant (F7,165 = 9.346, p < .001), accounting for 37.10% of the variability in CRP. With respect to main effects, results indicated that percentage of body fat and daily smoking were positively related to CRP (see Table 2). An interaction between perfectionistic cognitions and perceived stress was also observed after accounting for the main effects and covariates (see Fig. 1)1. As hypothesized, results of follow-up simple slopes analyses revealed that a significant positive relationship existed between perfectionistic cognitions and CRP at high levels (i.e., 1 SD above the mean) of perceived stress (b = 0.0573, p = .049, 95%CI [0.0003, 0.1143]), whereas, under low (i.e., 1 SD below the mean) and moderate levels (i.e., average levels) of perceived stress, the relationship between perfectionistic cognitions and CRP was not statistically significant (b = −.0230, p = .289, 95%CI [-0.0656, 0.0196]; b = 0.0171, p = .291, 95%CI[-0.0148, 0.0491], respectively).
Fig. 1.
Two-way interaction between perfectionistic cognitions and perceived stress on C-reactive protein.
4. Discussion
Consistent with the perfectionism diathesis-stress model (Flett et al., 1995; Hewitt and Dyck, 1986; Hewitt and Flett, 1993), individual differences in perfectionistic cognitions were associated with greater inflammation (i.e., elevated levels of CRP) under high levels of perceived stress. Perfectionistic cognitions were not related to IL-6 under any condition of stress. To our knowledge this is the first study to indicate a positive association between individual differences in perfectionistic cognitions and low grade inflammation, which is a risk factor for poorer health and earlier mortality (Harris et al., 1999; Maggio et al., 2006). These results are important from a construct validation perspective. Specifically, the current findings demonstrated that perfectionistic cognitions was associated with elevated levels of CRP, whereas other research has demonstrated that conscientiousness is consistently associated with lower levels of CRP (Luchetti et al., 2014; Mõttus et al., 2013). Taken together the findings support the notion that perfectionism reflects a motivational, cognitive, and behavioural proclivity toward a form of hyper-conscientiousness that can be differentiated from conscientiousness (Flett and Hewitt, 2015).
Importantly, the present results are in keeping with research demonstrating the negative health effects of perfectionism (Flett et al., 2012; Molnar et al., 2018). Indeed, research has implicated perfectionism in experiencing greater health symptoms, poorer self-rated health and a wide range of health conditions as well as difficulties adjusting to chronic illness (Kempke et al., 2016; Molnar et al., 2006, 2012; Sirois and Molnar, 2017). A particularly compelling display of the role of perfectionism in health was provided by Fry and Debats (2009) longitudinal study that investigated whether perfectionism conferred risk for all-cause mortality. Their results indicated that self-oriented perfectionism was predictive of earlier all-cause mortality over a time period of six and half years in a sample of older adults (i.e., 65 to over 80 years of age), such that people with high scores on self-oriented perfectionism (i.e., 70th to the 100th percentile) were at a 51% increased risk of earlier death compared to people with low scores on self-oriented perfectionism (i.e., 0 to the 30th percentile). Importantly, these results held after statistically accounting for the effects of other robust predictors of health problems.
The results from the present work extend these findings in several intriguing ways. First, research on perfectionism and health has predominantly concentrated on multidimensional trait perfectionism, typically measured by inventories such as the Multidimensional Perfectionism Scale (Frost et al., 1990; Hewitt and Flett, 1991). Unfortunately, other potentially relevant conceptualizations of perfectionism, such as individual differences in perfectionistic cognitions, have been overlooked with respect to health outcomes. Our results support the cognitive theory of perfectionism (Flett et al., 2016, 2018) and previous empirical research (Flett et al., 2012), by lending credence to the notion that perfectionists who engage in overthinking focused on their incessant need to be perfect and flaws are setting the stage for later health problems. Second, we were able to extend links between perfectionism and health, which have traditionally been based on self-reported health data, to intermediate biological markers of health. Thus, biases associated with self-report health data were mitigated. Importantly, the current findings are in line with the broader personality literature suggesting that low-grade chronic inflammation may be one pathway that connects personality (e.g., individual differences in perfectionistic cognitions) to broader health outcomes. For example, higher levels of CRP have been linked with earlier mortality within the context of cardiovascular disease (Man et al., 2006), and perfectionism has also been implicated in earlier mortality (Fry and Debats, 2009). Consequently, chronic low grade inflammation in individuals higher in perfectionism and under higher levels of stress may confer risk for poorer health outcomes and earlier mortality.
Current findings also underscore the importance of assessing how narrower personality traits, such as perfectionism, contribute to inflammatory processes, which may allow for more targeted prevention and intervention efforts. Finally, our research took a theoretically-driven approach to testing links between individual differences in perfectionistic cognitions and inflammation by specifically testing these associations within the context of the perfectionism diathesis-stress model. As such, it makes a significant contribution to the existing literature on perfectionistic cognitions and health by not simply testing whether perfectionism is related to intermediate inflammatory biomarkers of health, but also testing under what conditions this may be the case. Indeed, our findings support previous findings illustrating that perfectionism is a vulnerability for adverse outcomes under higher levels of stress (Dunkley et al., 2000; Flett et al., 1995; Hewitt and Dyck, 1986; Hewitt and Flett, 1993).
Contrary to expectations, there were no significant links between perfectionism and IL-6 at any level of stress. To our knowledge there are no other studies that have specifically assessed these associations. However, it appears that our results are somewhat at odds with findings within the context of the broader literature that has examined broader personality traits in relation to IL-6 that found significant associations for conscientiousness (Chapman et al., 2011; Luchetti et al., 2014; Mõttus et al., 2013; Sutin et al., 2010; Wagner et al., 2019), openness to experience (Mõttus et al., 2013), neuroticism (Sutin et al., 2010), and extraversion (Wagner et al., 2019). While IL-6 is generally considered a pro-inflammatory cytokine, its role in anti-inflammatory contexts is also well-established, pointing to context-specific complexity when considering IL-6 utility as a biomarker (Scheller et al., 2011). Thus, researchers are encouraged to replicate these findings in future studies.
The results of this study must be understood within the context of its strengths and limitations. First, the cross-sectional design of this study precludes inferences with respect to temporal precedence and causality concerning associations between perfectionistic cognitions and chronic inflammatory processes. Core vulnerability models of perfectionism (Hewitt et al., 1996) and the cognitive theory of perfectionism (Flett et al., 2016, 2018) posit that chronically engaging in perseverative thinking about the need to be perfect and past failure experiences can exact a toll on health (Flett et al., 2012, 2016). Indeed longitudinal studies have established that perfectionism confers risk for later health problems (Bonvanie et al., 2015; Pritchard et al., 2007; Sumi and Kanda, 2002). However, it is possible that environmental or genetic factors that give rise to inflammation similarly impact perfectionism. (Reichenberg et al., 2001), for example, found that experimentally increasing cytokines such as IL-6 resulted in poorer performance on memory tasks and elevated negative affect. Thus, multi-wave longitudinal studies of perfectionism and inflammation are encouraged to address issues concerning temporal precedence.
Second, the differential effects showing that perfectionistic cognitions were related to CRP under high levels of stress, but not associated with IL-6, point toward investigating other inflammatory markers. Such studies would be worthwhile in light of research demonstrating that relationships between psychosocial factors and biomarkers vary as a function of the biomarker under study (O’Donovan et al., 2010; Wagner et al., 2019). Third, whereas self-report measures of perfectionism are widely used and have been validated, it would be beneficial for future research to include informant reports given that previous studies have established that informant reports add to the incremental predictive utility of perfectionism (Sherry et al., 2013). Fourth, whereas we focused on individual differences in perfectionistic cognitions given theory and empirical research, which support that individual differences in perfectionistic cognitions uniquely set the stage for poorer outcomes over and above multidimensional trait measures of perfectionism (Casale et al., 2020), researchers are encouraged to test associations between perfectionism and pro-inflammatory markers with other measures of perfectionism such as Hewitt and Flett (1991) or Frost et al. (1990) Multidimensional Perfectionism Scale. Finally, our study focused on a community sample of emerging adults given that perfectionism is rising among this age group (Curran and Hill, 2019) and that research on personality and inflammatory markers in this age group is relatively sparse. Indeed, research in this area tends to focus on middle-aged or older individuals (Mõttus et al., 2013; Turiano et al., 2013; Wagner et al., 2019) though see (Luchetti et al., 2014) for an exception. However, future studies would benefit from studying links between perfectionism and biomarkers of health across a wider age range to better assess the generalizability of the findings.
5. Conclusions
In conclusion, these results suggest that individual differences in the frequency with which individuals experience automatic perfectionistic thoughts are implicated in inflammatory processes. In keeping with the perfectionism diathesis-stress model (Flett et al., 1995; Hewitt and Flett, 1993), the results of the present study support the notion that perceived stress exacerbates links between perfectionistic cognitions and inflammation. Future research could extend the present findings by incorporating a longitudinal design, including informant reports of perfectionism, assessing multidimensional trait perfectionism, and testing associations between perfectionism and pro-inflammatory processes with samples that cover a wider range of the lifespan. In sum, this study meaningfully builds on the extant literature to present a novel contribution to the growing body of literature on the role of perfectionism in health and to the role of personality in inflammatory processes. The present results support clinicians assessing inflammatory markers in those who appear to be struggling with higher levels of perfectionism as these markers may be indicative of poorer health outcomes and indicate an opportunity for intervention.
Declaration of competing interest
None.
Acknowledgements
The research in this article was funded by CIHR (grant # 399332) and an Ontario Government Early Researcher Award awarded to Dr. Molnar (Ministry of Research and Innovation, Government of Ontario).
Footnotes
Analyses are presently reported with the inclusion of covariates. However, analyses were also conducted without potential confounds and there were no meaningful differences in results when the effects of respondent sex, smoking status, and body fat percentage were not accounted for in the analyses.
References
- Aggarwal B.B., Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr. Opin. Pharmacol. 2009;9(4):351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken L.S., West S.G. Sage Publications, Inc; Thousand Oaks, CA, US: 1991. Multiple Regression: Testing and Interpreting Interactions. [Google Scholar]
- Armon G., Melamed S., Shirom A., Berliner S., Shapira I. The associations of the Five Factor Model of personality with inflammatory biomarkers: a four-year prospective study. Pers. Indiv. Differ. 2013;54(6):750–755. doi: 10.1016/j.paid.2012.11.035. [DOI] [Google Scholar]
- Bonvanie I.J., Rosmalen J.G.M., van Rhede van der Kloot C.M., Oldehinkel A.J., Janssens K.A.M. Short report: functional somatic symptoms are associated with perfectionism in adolescents. J. Psychosom. Res. 2015;79(4):328–330. doi: 10.1016/j.jpsychores.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Bottos S., Dewey D. Perfectionists’ appraisal of daily hassles and chronic headache. Headache. 2004;44(8):772–779. doi: 10.1111/j.1526-4610.2004.04144.x. [DOI] [PubMed] [Google Scholar]
- Brummett B.H., Boyle S.H., Ortel T.L., Becker R.C., Siegler I.C., Williams R.B. Associations of depressive symptoms, trait hostility, and gender with C-reactive protein and interleukin-6 response after emotion recall. Psychosom. Med. 2010;72(4):333–339. doi: 10.1097/PSY.0b013e3181d2f104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier A., Côté M., Lemieux I., Pérusse L., Tremblay A., Bouchard C., Després J.-P. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am. J. Clin. Nutr. 2009;89(5):1307–1314. doi: 10.3945/ajcn.2008.27030. [DOI] [PubMed] [Google Scholar]
- Casale S., Fioravanti G., Rugai L., Flett G.L., Hewitt P.L. What lies beyond the superordinate trait perfectionism factors? The perfectionistic self-presentation and perfectionism cognitions inventory versus the big three perfectionism scale in predicting depression and social anxiety. J. Pers. Assess. 2020;102(3):370–379. doi: 10.1080/00223891.2019.1573429. [DOI] [PubMed] [Google Scholar]
- Casas J.P., Shah T., Hingorani A.D., Danesh J., Pepys M.B. C-reactive protein and coronary heart disease: a critical review. J. Intern. Med. 2008;264(4):295–314. doi: 10.1111/j.1365-2796.2008.02015.x. [DOI] [PubMed] [Google Scholar]
- Chapman B.P., Khan A., Harper M., Stockman D., Fiscella K., Walton J.…Moynihan J. Gender, race/ethnicity, personality, and interleukin-6 in urban primary care patients. Brain Behav. Immun. 2009;23(5):636–642. doi: 10.1016/j.bbi.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B.P., van Wijngaarden E., Seplaki C.L., Talbot N., Duberstein P., Moynihan J. Openness and conscientiousness predict 34-week patterns of Interleukin-6 in older persons. Brain Behav. Immun. 2011;25(4):667–673. doi: 10.1016/j.bbi.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Cohen P., West S.G., Aiken L.S. third ed. Lawrence Erlbaum Associates Publishers; Mahwah, NJ, US: 2003. Applied Multiple Regression/correlation Analysis for the Behavioral Sciences. [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24(4):385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Corson A.T., Loveless J.P., Mochrie K.D., Whited M.C. Perfectionism in relation to stress and cardiovascular disease among gifted individuals and the need for affective interventions. Roeper Rev. 2018;40(1):46–55. doi: 10.1080/02783193.2017.1393711. [DOI] [Google Scholar]
- Curran T., Hill A.P. Perfectionism is increasing over time: a meta-analysis of birth cohort differences from 1989 to 2016. Psychol. Bull. 2019;145(4):410–429. doi: 10.1037/bul0000138. [DOI] [PubMed] [Google Scholar]
- de Maat M.P.M., Kluft C. The association between inflammation markers, coronary artery disease and smoking. Vasc. Pharmacol. 2002;39(3):137–139. doi: 10.1016/S1537-1891(02)00301-4. [DOI] [PubMed] [Google Scholar]
- Dunkley D.M. ProQuest Information & Learning; US: 2003. The Relation between Perfectionism and Distress: Daily Stress, Coping, and Perceived Social Support as Mediators and Moderators. [Google Scholar]
- Dunkley D.M., Blankstein K.R., Halsall J., Williams M., Winkworth G. The relation between perfectionism and distress: hassles, coping, and perceived social support as mediators and moderators. J. Counsel. Psychol. 2000;47(4):437–453. doi: 10.1037/0022-0167.47.4.437. [DOI] [Google Scholar]
- Ellulu M.S., Patimah I., Khaza’ai H., Rahmat A., Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci. : AMS. 2017;13(4):851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns M.W., Cox B.J. Perfectionism, stressful life events, and the 1-year outcome of depression. Cognit. Ther. Res. 2005;29(5):541–553. doi: 10.1007/s10608-005-2414-8. [DOI] [Google Scholar]
- Flett G., Greene A., Hewitt P. Dimensions of perfectionism and anxiety sensitivity. J. Ration. Emot. Cogn. Behav. Ther. 2004;22:39–57. doi: 10.1023/B:JORE.0000011576.18538.8e. [DOI] [Google Scholar]
- Flett G., Hewitt P., Nepon T., Besser A. 2018. Perfectionism Cognition Theory: the Cognitive Side of Perfectionism; pp. 89–110. [Google Scholar]
- Flett G.L., Baricza C., Gupta A., Hewitt P.L., Endler N.S. Perfectionism, psychosocial impact and coping with irritable bowel disease: a study of patients with Crohn’s disease and ulcerative colitis. J. Health Psychol. 2011;16(4):561–571. doi: 10.1177/1359105310383601. [DOI] [PubMed] [Google Scholar]
- Flett G.L., Hewitt P.L. A proposed framework for preventing perfectionism and promoting resilience and mental health among vulnerable children and adolescents. Psychol. Sch. 2014;51:899–912. doi: 10.1002/pits.21792. [DOI] [Google Scholar]
- Flett G.L., Hewitt P.L. In: Measures of Personality and Social Psychological Constructs. Boyle G.J., Saklofske D.H., Matthews G., editors. Elsevier Academic Press; San Diego, CA, US: 2015. Measures of Perfectionism; pp. 595–618. [Google Scholar]
- Flett G.L., Hewitt P.L., Blankstein K.R., Gray L. Psychological distress and the frequency of perfectionistic thinking. J. Pers. Soc. Psychol. 1998;75(5):1363–1381. doi: 10.1037//0022-3514.75.5.1363. [DOI] [PubMed] [Google Scholar]
- Flett G.L., Hewitt P.L., Blankstein K.R., Mosher S.W. Perfectionism, life events, and depressive symptoms: a test of a diathesis-stress model. Curr. Psychol. 1995;14(2):112–137. doi: 10.1007/BF02686885. [DOI] [Google Scholar]
- Flett G.L., Hewitt P.L., Whelan T., Martin T.R. The Perfectionism Cognitions Inventory: psychometric properties and associations with distress and deficits in cognitive self-management. J. Ration. Emot. Cogn. Behav. Ther. 2007;25(4):255–277. doi: 10.1007/s10942-007-0055-4. [DOI] [Google Scholar]
- Flett G.L., Molnar D.S., Nepon T., Hewitt P.L. A mediational model of perfectionistic automatic thoughts and psychosomatic symptoms: the roles of negative affect and daily hassles. Pers. Indiv. Differ. 2012;52(5):565–570. doi: 10.1016/j.paid.2011.09.010. [DOI] [Google Scholar]
- Flett G.L., Nepon T., Hewitt P.L. In: Perfectionism, Health, and Well-Being. Molnar F.M.S.D.S., editor. Springer International Publishing; Cham, Switzerland: 2016. Perfectionism, Worry, and Rumination in Health and Mental Health: A Review and a Conceptual Framework for a Cognitive Theory of Perfectionism; pp. 121–155. [Google Scholar]
- Frost R.O., Marten P., Lahart C., Rosenblate R. The dimensions of perfectionism. Cognit. Ther. Res. 1990;14(5):449–468. doi: 10.1007/BF01172967. [DOI] [Google Scholar]
- Fry P.S., Debats D.L. Perfectionism and the five-factor personality traits as predictors of mortality in older adults. J. Health Psychol. 2009;14(4):513–524. doi: 10.1177/1359105309103571. [DOI] [PubMed] [Google Scholar]
- Harris T.B., Ferrucci L., Tracy R.P., Corti M.C., Wacholder S., Ettinger W.H.…Wallace R. Associations of elevated Interleukin-6 and C-Reactive protein levels with mortality in the elderly. Am. J. Med. 1999;106(5):506–512. doi: 10.1016/S0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. second ed. The Guilford Press; New York, New York: 2018. Introduction to Mediation, Moderation, and Conditional Process Analysis: a Regression-Based Approach. [Google Scholar]
- Hayes A.F., Cai L. Using heteroskedasticity-consistent standard error estimators in OLS regression: an introduction and software implementation. Behav. Res. Methods. 2007;39(4):709–722. doi: 10.3758/BF03192961. [DOI] [PubMed] [Google Scholar]
- Henningsson S., Baghaei F., Rosmond R., Holm G., Landén M., Anckarsäter H., Ekman A. Association between serum levels of C-reactive protein and personality traits in women. Behav. Brain Funct. 2008;4 doi: 10.1186/1744-9081-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt P.L., Dyck D.G. Perfectionism, stress, and vulnerability to depression. Cognit. Ther. Res. 1986;10(1):137–142. doi: 10.1007/BF01173389. [DOI] [Google Scholar]
- Hewitt P.L., Flett G.L. Perfectionism in the self and social contexts: conceptualization, assessment, and association with psychopathology. J. Pers. Soc. Psychol. 1991;60(3):456–470. doi: 10.1037//0022-3514.60.3.456. [DOI] [PubMed] [Google Scholar]
- Hewitt P.L., Flett G.L. Dimensions of perfectionism, daily stress, and depression: a test of the specific vulnerability hypothesis. J. Abnorm. Psychol. 1993;102(1):58–65. doi: 10.1037//0021-843x.102.1.58. [DOI] [PubMed] [Google Scholar]
- Hewitt P.L., Flett G.L., Ediger E. Perfectionism and depression: longitudinal assessment of a specific vulnerability hypothesis. J. Abnorm. Psychol. 1996;105(2):276–280. doi: 10.1037//0021-843x.105.2.276. [DOI] [PubMed] [Google Scholar]
- Kempke S., Van Houdenhove B., Claes S., Luyten P. In: Perfectionism, Health, and Well-Being. Sirois F.M., Molnar D.S., Sirois F.M., Molnar D.S., editors. Springer International Publishing; Cham, Switzerland: 2016. The Role of Perfectionism in Chronic Fatigue Syndrome; pp. 101–118. [Google Scholar]
- Little R.J.A. A test of missing completely at random for multivariate data with missing values. J. Am. Stat. Assoc. 1988;83(404):1198–1202. doi: 10.1080/01621459.1988.10478722. [DOI] [Google Scholar]
- Luchetti M., Barkley J.M., Stephan Y., Terracciano A., Sutin A.R. Five-factor model personality traits and inflammatory markers: New data and a meta-analysis. Psychoneuroendocrinology. 2014;50:181–193. doi: 10.1016/j.psyneuen.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio M., Guralnik J.M., Longo D.L., Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61(6):575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S.F.P., Connett J.E., Anthonisen N.R., Wise R.A., Tashkin D.P., Sin D.D. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61(10):849–853. doi: 10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Vidal P., Bochud M., Bastardot F., Lüscher T., Ferrero F., Gaspoz J.M.…Vollenweider P. Association between inflammatory and obesity markers in a Swiss population-based sample (CoLaus study) Obesity Facts. 2012;5(5):734–744. doi: 10.1159/000345045. [DOI] [PubMed] [Google Scholar]
- Martin T.R., Flett G.L., Hewitt P.L., Krames L., Szanto G. Personality correlates of depression and health symptoms: A test of a self-regulation model. J. Res. Pers. 1996;30(2):264–277. doi: 10.1006/jrpe.1996.0017. [DOI] [Google Scholar]
- McCarty M.F. Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, diabetes, and visceral obesity: down-regulation with essential fatty acids, ethanol and pentoxifylline. Med. Hypotheses. 1999;52(5):465–477. doi: 10.1054/mehy.1997.0684. [DOI] [PubMed] [Google Scholar]
- Mendall M.A., Strachan D.P., Butland B.K., Ballam L., Morris J., Sweetnam P.M., Elwood P.C. C-reactive protein: relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. Eur. Heart J. 2000;21(19):1584–1590. doi: 10.1053/euhj.1999.1982. [DOI] [PubMed] [Google Scholar]
- Molnar D.S., Flett G.L., Sadava S.W., Colautti J. Perfectionism and health functioning in women with fibromyalgia. J. Psychosom. Res. 2012;73(4):295–300. doi: 10.1016/j.jpsychores.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Molnar D.S., Reker D.L., Culp N.A., Sadava S.W., DeCourville N.H. A mediated model of perfectionism, affect, and physical health. J. Res. Pers. 2006;40(5):482–500. doi: 10.1016/j.jrp.2005.04.002. [DOI] [Google Scholar]
- Molnar D.S., Sirois F.M., Flett G.L., Janssen W.F., Hewitt P.L. Routledge/Taylor & Francis Group; New York, NY, US: 2018. Perfectionism and Health: the Roles of Health Behaviors and Stress-Related Processes The Psychology of Perfectionism: Theory, Research, Applications; pp. 200–221. [Google Scholar]
- Mõttus R., Luciano M., Starr J.M., Pollard M.C., Deary I.J. Personality traits and inflammation in men and women in their early 70s: the lothian birth cohort 1936 study of healthy aging. Psychosom. Med. 2013;75(1) doi: 10.1097/PSY.0b013e31827576cc. [DOI] [PubMed] [Google Scholar]
- Mwendwa D.T., Ali M.K., Sims R.C., Cole A.P., Lipscomb M.W., Levy S.A.…Campbell A.L. Dispositional depression and hostility are associated with inflammatory markers of cardiovascular disease in African Americans. Brain Behav. Immun. 2013;28:72–82. doi: 10.1016/j.bbi.2012.10.019. [DOI] [PubMed] [Google Scholar]
- O’Donovan A., Hughes B.M., Slavich G.M., Lynch L., Cronin M.-T., O’Farrelly C., Malone K.M. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion–biology relationships. Brain Behav. Immun. 2010;24(7):1074–1077. doi: 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K., Steffes M., Lee D.H., Himes J.H., Jacobs D.R. Association of inflammation with worsening HOMA-insulin resistance. Diabetologia. 2009;52(11):2337–2344. doi: 10.1007/s00125-009-1486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T.A., Mensah G.A., Alexander R.W., Anderson J.L., Cannon R.O., 3rd, Criqui M., Fadl Y.Y., Fortmann S.P., Hong Y., Myers G.L., Rifai N., Smith S.C., Jr., Taubert K., Tracy R.P., Vinicor F., Centers for Disease Control and Prevention American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003 Jan 28;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. PMID: 12551878. [DOI] [PubMed] [Google Scholar]
- Pradhan A.D., Manson J.E., Rifai N., Buring J.E., Ridker P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. J. Am. Med. Assoc. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Pritchard M.E., Wilson G.S., Yamnitz B. What predicts adjustment among college students? A longitudinal panel study. J. Am. Coll. Health. 2007;56(1):15–21. doi: 10.3200/jach.56.1.15-22. [DOI] [PubMed] [Google Scholar]
- Reichenberg A., Yirmiya R., Schuld A., Kraus T., Haack M., Morag A., Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatr. 2001;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Saboonchi F., Lundh L.G. Perfectionism, anger, somatic health and positive affect. Pers. Indiv. Differ. 2003;35:1585–1599. [Google Scholar]
- Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Sherry S., Nealis L., Macneil M., Stewart S., Lee-Baggley D., Smith M. Informant reports add incrementally to the understanding of the perfectionism–depression connection: evidence from a prospective longitudinal study. Pers. Indiv. Differ. 2013;54:957–960. doi: 10.1016/j.paid.2013.01.002. [DOI] [Google Scholar]
- Sirois F.M., Molnar D.S. Springer International Publishing; Cham, Switzerland: 2016. Perfectionism, Health, and Well-Being. [Google Scholar]
- Sirois F.M., Molnar D.S. Perfectionistic strivings and concerns are differentially associated with self-rated health beyond negative affect. J. Res. Pers. 2017;70:73–83. doi: 10.1016/j.jrp.2017.06.003. [DOI] [Google Scholar]
- Sirois F.M., Toussaint L., Hirsch J.K., Kohls N., Weber A., Offenbächer M. Trying to be perfect in an imperfect world: a person-centred test of perfectionism and health in fibromyalgia patients versus healthy controls. Pers. Indiv. Differ. 2019;137:27–32. doi: 10.1016/j.paid.2018.08.005. [DOI] [Google Scholar]
- Steptoe A., Hamer M., Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 2007;21(7):901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Stoeber J., Stoeber F. Domains of perfectionism: Prevalence and relationships with perfectionism, gender, age, and satisfaction with life. Pers. Indiv. Differ. 2009;46(4):530–535. doi: 10.1016/j.paid.2008.12.006. [DOI] [Google Scholar]
- Sumi K., Kanda K. Relationship between neurotic perfectionism, depression, anxiety, and psychosomatic symptoms: a prospective study among Japanese men. Pers. Indiv. Differ. 2002;32(5):817–826. doi: 10.1016/S0191-8869(01)00088-5. [DOI] [Google Scholar]
- Sutin A.R., Terracciano A., Deiana B., Naitza S., Ferrucci L., Uda M.…Costa P.T., Jr. High neuroticism and low conscientiousness are associated with interleukin-6. Psychol. Med. 2010;40(9):1485–1493. doi: 10.1017/S0033291709992029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy R.P., Psaty B.M., Macy E., Bovill E.G., Cushman M., Cornell E.S., Kuller L.H. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler. Thromb. Vasc. Biol. 1997;17(10):2167–2176. doi: 10.1161/01.ATV.17.10.2167. [DOI] [PubMed] [Google Scholar]
- Turiano N.A., Mroczek D.K., Moynihan J., Chapman B.P. Big 5 personality traits and interleukin-6: evidence for "healthy Neuroticism" in a US population sample. Brain Behav. Immun. 2013;28:83–89. doi: 10.1016/j.bbi.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J., Mu W., McCann J., Belli G., Asnaani A. The unique contribution of perfectionistic cognitions to anxiety disorder symptoms in a treatment-seeking sample. Cognit. Behav. Ther. 2020:1–17. doi: 10.1080/16506073.2020.1798497. [DOI] [PubMed] [Google Scholar]
- Valkanova V., Ebmeier K.P., Allan C.L. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013;150(3):736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Wade T.J., O’Leary D.D., Dempster K.S., MacNeil A.J., Molnar D.S., McGrath J., Cairney J. Adverse childhood experiences (ACEs) and cardiovascular development from childhood to early adulthood: study protocol of the Niagara Longitudinal Heart Study. BMJ Open. 2019;9(7) doi: 10.1136/bmjopen-2019-030339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E.N., Ajdacic-Gross V., Strippoli M.F., Gholam-Rezaee M., Glaus J., Vandeleur C., von Känel R. Associations of personality traits with chronic low-grade inflammation in a Swiss community sample. Front. Psychiatr. 2019;10:819. doi: 10.3389/fpsyt.2019.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]