Abstract
HSP70 is one of the main molecular chaperones involved in the cellular stress response. Besides its chaperone action, HSP70 also modulates the immune response. Increased susceptibility to toxic insults in intra- and extracellular environments has been associated with insufficient amounts of inducible HSP70 in adult neurons. On the other hand, exogenous HSP70 administration has demonstrated neuroprotective effects in experimental models of age-related disorders. In this regard, this study investigated the effects of exogenous HSP70 in an animal model of dopaminergic denervation of the nigrostriatal axis. After unilateral intrastriatal injection with 6-hydroxydopamine (6-OHDA), the animals received purified recombinant HSP70 through intranasal administration (2 μg/rat/day) for 15 days. Our results indicate a neuroprotective effect of intranasal HSP70 against dopaminergic denervation induced by 6-OHDA. Exogenous HSP70 improved motor impairment and reduced the loss of dopaminergic neurons caused by 6-OHDA. Moreover, HSP70 modulated neuroinflammatory response in the substantia nigra, an important event in Parkinson’s disease pathogenesis. Specifically, HSP70 treatment reduced microglial activation and astrogliosis induced by 6-OHDA, as well as IL-1β mRNA expression in this region. Also, recombinant HSP70 increased the protein content of HSP70 in the substantia nigra of rats that received 6-OHDA. These data suggest the neuroprotection of HSP70 against dopaminergic neurons damage after cellular stress. Finally, our results indicate that HSP70 neuroprotective action against 6-OHDA toxicity is related to inflammatory response modulation.
Keywords: Exogenous HSP70, Intranasal treatment, 6-OHDA, Neuroprotection, Neuroinflammation, Neurodegenerative diseases, Parkinson’s disease
Highlights
-
•
HSP70 intranasal treatment protected against behavior alterations caused by 6-OHDA.
-
•
Exogenous HSP70 intranasally administered prevented neuronal loss induced by 6-OHDA.
-
•
HSP70 treatment attenuated the neuroinflammation associated with 6-OHDA exposure.
-
•
HPS70 intranasal administration increased HSP70 content in the SNpc.
1. Introduction
Heat shock proteins (HSPs) or molecular chaperones constitute a heterogeneous group classified into different families based on their molecular weight. Molecular chaperones stimulated in response to cellular stress are critical for maintaining protein homeostasis (Hartl et al., 2011). The HSP70 family is one of the main molecular chaperones involved in cellular stress response, acting as a critical cellular protection machinery component. These proteins are highly conserved in function and structure, from prokaryotes through eukaryotes (Radons 2016). HSP70 acts on the folding process, binding to partially unfolded or misfolded proteins, and either assist their refolding or directs them to safe disposal (Mayer and Bukau 2005). Also, HSP70 has several non-chaperone functions that contribute to cellular protection, e.g., interaction with immune cells, reduction of oxidative stress, and inhibition of apoptotic cell death cascades (Dukay et al., 2019).
The activity of endogenous HSP70 appears insufficient in several pathological states, such as in conditions of inflammation and degeneration (Radons 2016; Calderwood and Murshid 2017; Murshid et al., 2013). During aging, there is a disbalance of cellular homeostasis with a predominance of pro-inflammatory state and increased proteotoxic stress load (Sparkman and Johnson 2008; Ebrahimi-Fakhari et al., 2011). Moreover, the expression and functionality of inducible HSP70 declines in the brain tissue with aging and neurodegenerative disorders (Magrané et al., 2004). In this context, the neuroprotective role of exogenous HSP70 administration has been demonstrated in several animal models of age-related neurodegenerative disorders (Bobkova et al., 2014; Gifondorwa et al., 2007; Pastukhov et al., 2014).
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by progressive loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc), leading to depletion of dopamine in the striatum (Dauer and Przedborski 2003). In PD, misfolded α-synuclein (α-syn) aggregation is observed, which accumulates in intraneuronal inclusions named Lewy bodies (Goedert 2001). In the context of PD, the depletion of HSP70 could exacerbate α-syn toxicity and neurodegeneration (Ebrahimi-Fakhari et al., 2011). The neuroprotective role for HSP70 had already been demonstrated in different animal models of PD (Klucken et al., 2004; Li et al., 2019; Nagel et al., 2008; Tunesi et al., 2019; Pastukhov et al., 2014). For instance, Klucken et al. demonstrated that overexpression of HSP70 is protective against abnormal α-syn aggregation in α-syn transgenic mice (Klucken et al., 2004). Li et al. demonstrated a protective effect of HSP70 against neuroinflammation induced by rotenone in SH-SY5Y, inhibiting NF-κB, and STAT3 (Li et al., 2019). HSP70 fused with the cell-penetrating peptide Tat (Tat-Hsp70) displayed a protective effect against DA neuron loss in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA) toxicity models (Nagel et al., 2008; Tunesi et al., 2019). HSP70 neuroprotective effects were observed in PD models with or without α-syn aggregation, indicating the involvement of non-chaperone functions in HSP70 protective action.
Despite its neuroprotective potential, HSP70 is too large to cross the blood-brain barrier (BBB) freely. An efficient approach to drug delivery needs to be adopted to explore the therapeutic potential of HSP70 in the central nervous system (CNS) disorders. Intranasal administration has been demonstrated as an effective non-invasive approach to deliver large-sized drugs directly to CNS (Ying 2007). Bobkova et al. demonstrated that fluorescently labeled HSP70 was detected in several brain structures 3 h after intranasal administration (Bobkova et al., 2014). Besides, the neuroprotective effect of intranasal HSP70 was previously demonstrated in animal models of aging and neurodegenerative disorders (Bobkova et al. 2014, 2015).
In the present work, we tested the neuroprotective effects of HSP70 intranasal administration against DA denervation induced by 6-OHDA injected in Wistar rats’ striatum. Fifteen days of HSP70 treatment attenuated DA neurons loss and improved behavioral impairments caused by 6-OHDA. Also, intranasal HSP70 reduced neuroinflammation. These protective effects might be related to the increase in HSP70 protein content in the SNpc after cellular stress. Therefore, HSP70 intranasal administration showed a neuroprotective activity in the nigrostriatal axis, indicating a potential application in PD and disorders related to dopaminergic denervation.
2. Materials and methods
2.1. Ethics statement
All experimental procedures were performed in accordance with the guidelines of the National Institutes of Health (National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory 2011) and Behavior recommendations for animal care. Our research protocol was approved by the Ethical Committee for Animal Experimentation of the Universidade Federal do Rio Grande do Sul - Brazil (CEUA-UFRGS) under the project number #35627.
2.2. Animals and experimental design
Fifty-day-old male Wistar rats were obtained from the CREAL-UFRGS breeding colony. Animals were maintained in a 12-h light-dark cycle in a temperature-controlled colony room (21 °C). The animals were caged in four animals with free access to water and standard commercial food (Chow Nuvilab CR-1 type; PR, BRA). The rats were randomly distributed into four experimental groups (n = 16 per group): Control (vehicle + saline), HSP70 (vehicle + HSP70), 6-OHDA (6-OHDA + saline), and 6-OHDA + HSP70. The animals were handled for seven days before the procedures to reduce stress caused by subsequent manipulation.
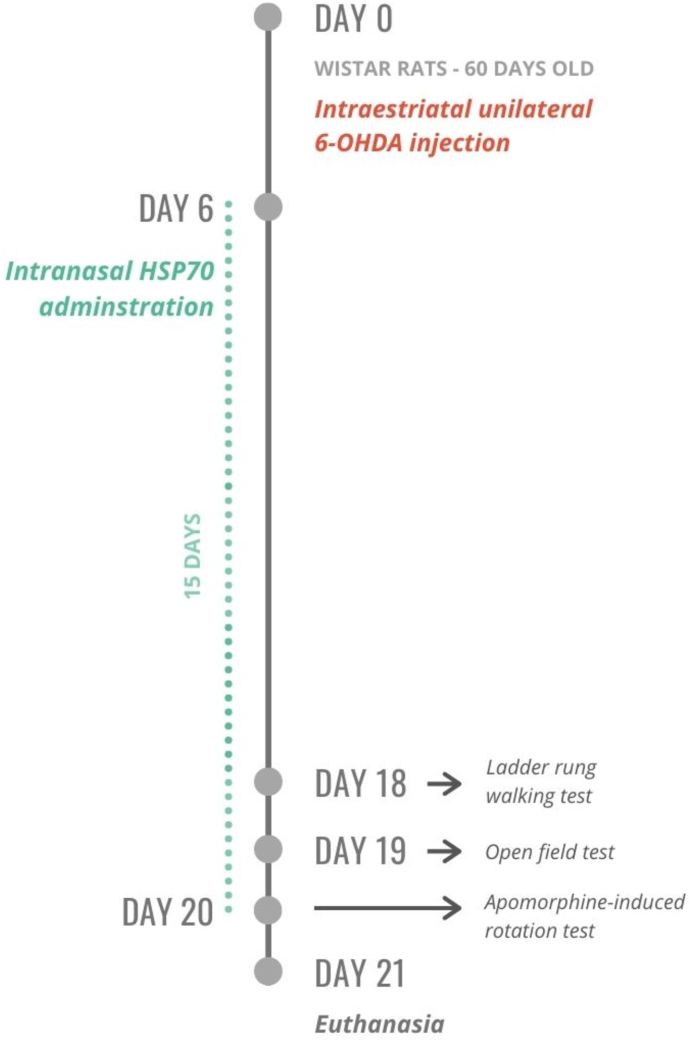
Fig. 1 summarizes the experimental design applied in this study. Each rat received an intrastriatal injection of 6-OHDA (20 μg/rat) or vehicle (saline with ascorbic acid) (Pang et al., 2016). The animals were habituated with a saline intranasal administration for five days after the surgical procedure. Then, each rat received a daily intranasal dose of HSP70 (2 μg/rat) or saline (Bobkova et al., 2014) for 15 days. Behavior tests were performed on the last three days of treatment. On the 21st day after the 6-OHDA lesion, all the animals were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) via intraperitoneal injection (i.p.). The animals were euthanized by decapitation (n = 8 per group), serum was collected for ELISA, and the brain was dissected for quantitative reverse-transcriptase PCR (RT-qPCR). The remaining rats were perfused via the vascular system for immunofluorescence microscopy assessment (n = 8 per group). The investigator was blinded to the groups during all experimental procedures. Only male animals were used in this study. The reason for this selection was that PD incidence and prevalence are higher in men (Haaxma et al., 2007). However, we acknowledge the absence of female animals as a limitation of the study. The findings presented here cannot be generalized for both sexes.
Fig. 1.
Experimental design. Wistar rats were distributed into four experimental groups (n = 16 per group): Control (vehicle + saline), HSP70 (vehicle + HSP70), 6-OHDA (6-OHDA + saline), and 6-OHDA + HSP70. On day 0, the animals had sixty days old. Each rat received an intrastriatal unilateral injection of 6-OHDA (20 μg/rat) or vehicle (saline with ascorbic acid). The animals were habituated with a saline intranasal administration for 5 days after the surgical procedure. Then, each rat received a daily intranasal dose of HSP70 (2 μg/rat) or saline for 15 days. Behavior tests – ladder rung walking test, open field test, apomorphine-induced rotation test – were performed on the last 3 days of treatment. On day 21, half of the animals were euthanized by decapitation, serum was collected for ELISA, and the brain was dissected to quantitative reverse transcription PCR (n = 8 per group). The remaining rats were perfused via the vascular system for immunofluorescence microscopy assessment (n = 8 per group).
2.3. Intrastriatal injection of 6-OHDA
6-OHDA was purchased from Sigma-Aldrich® (MO, USA) and prepared as a 10 μg/μL solution in 0.02% ascorbic acid dissolved in sterile saline, protected from heat and light. The animals were anesthetized with xylazine (10 mg/kg; i.p.) and ketamine (100 mg/kg; i.p.) for surgical procedure. The anesthetized rats were immobilized on a stereotaxic apparatus (Insight-EFF 338, SP, BRA) by securing via ear and nose bars. The fur was shaved with a pet clipper (SKU #: 09160-210 – Wahl; IL, USA), and a 10% povidone-iodine solution was applied to sterilize the incision site. The skulls were perforated at the appropriate location with a dental drill (3 mm). A single dose (2 μL) of 6-OHDA or vehicle was injected into striatum at the following stereotaxic coordinates from the Rat Brain Atlas in Stereotaxic Coordinates (Paxinos and Watson 2005): antero-posterior (AP): −0.2 mm from bregma; medio-lateral (ML): 2.5 mm from the midline; dorso-ventral (DV): 5.0 mm from the skull, using a 10-μL Hamilton® syringe 701SN, needle size 23s ga (Sigma-Aldrich®; MO, USA). A syringe was inserted into the brain at a rate of 2 mm/min, and the injection occurred at a rate of 0.5 μL/min. After the injection, the syringe was left in the place for 2 min and then removed at a rate of 2 mm/min. The incision was thoroughly cleaned with povidone-iodine solution and closed using three sutures. Lactated Ringer’s solution (1 mL) was injected subcutaneously to replenish electrolytes. Nebacetin® (5 mg/g neomycin sulfate and 250 UI/g of bacitracin zinc, Medley; RS, BRA) was applied topically on the incision to prevent infections. The animals were placed in a controlled temperature recovery cage (37 °C) until the recovery of consciousness.
2.4. HSP70 treatment
2.4.1. Recombinant HSP70 expression and purification
Recombinant HSP70 was expressed in E. coli BL21 and transformed with HSP70 plasmid resistant to ampicillin and chloramphenicol, using IPTG. Purification of the recombinant HSP70 was performed by affinity chromatography, followed by gel filtration chromatography. Endotoxins were removed from the purified HSP70, which was proven using the kit Pierce™ LAL Chromogenic Endotoxin Quantitation Kit (88282 - Thermo Fisher Scientific; MA, USA).
2.4.2. Intranasal administration
All intranasal administrations of purified recombinant HSP70 were unilateral and carried out with the aid of a micropipette on the ipsilateral side. Four μL of solution were placed in the left nostril and were inhaled by the animal in the process of normal breathing. All animals were habituated with intranasal administration, receiving a daily dose of saline 0.9% via intranasal for five days after the surgical procedure. It is essential to highlight that the animals did not show any signs of stress during inoculation. From day 6 to day 20, the groups HSP70 and 6-OHDA + HSP70 received a daily dose of HSP70 (2 μg/rat) via intranasal, whereas the other groups received an amount of saline solution 0.9% (Bobkova et al., 2014). The treatment was always performed in the evening, including during the days of behavior analysis. Therefore, on days 18, 19, and 20, the animals were subjected to behavioral tests and received the HSP70 intranasal treatment after the tests.
2.5. Behavior tests
2.5.1. Ladder rung walking test
The ladder rung walking test was performed on day 18 to analyze skilled movements and access motor performance. The procedure was performed as described by Metz and Whishaw (Metz and Whishaw 2002). The apparatus used was a horizontal ladder made of 2 transparent clear boards joined by metal rungs. A regular pattern of rungs (1 cm apart) was used in 3 training sessions. Then, 3 test trials were performed with an irregular pattern of rungs (1–3 cm apart). For each trial, the pattern of rungs changed, avoiding animals’ adaptation. All trials were video recorded and reviewed to evaluate each limb’s quality of steps and the error percentual. A qualitative evaluation was performed for each step using a foot fault scoring system. The system uses a 7-category scale: (0) total miss, (1) deep slip, (2) slight slip, (3) replacement, (4) correction, (5) partial placement, and (6) correct placement. Then, the average of scores per animal was measured. The percentage of errors was also calculated, counting scores of 0, 1, and 2 as errors.
2.5.2. Open field test
Open field test (OPF) was performed on day 19 to access general locomotor activity and anxiety behavior. The OPF apparatus consists of a square arena (50 × 50 × 50 cm) with black-painted walls. Each animal was placed in the center of the OPF apparatus and allowed to explore the arena for 5 min (Silva et al., 2016; Campos et al., 2013). The total distance traveled (m), average speed (m/s), total time immobile (s), total time mobile (s), total time freezing (s) and total time in the central zone (s), corner zone (s) and side zone (s) were determined using the video tracking system ANY-maze (Stoelting Co.; IL, USA).
2.5.3. Apomorphine-induced rotation test
The apomorphine-induced rotation test was performed on day 20 to assess the supersensitivity in the damaged side after DA denervation caused by the unilateral injection of 6-OHDA (Ungerstedt 1971). Apomorphine (A4393) was purchased from Sigma-Aldrich® (MO, USA). Rats received a subcutaneous injection of apomorphine 0.5 mg/kg (dissolved in a 0.2 mg/mL ascorbic acid in 0.9% saline solution) and were allowed to acclimate for 15 min. The animals were recorded over a 30 min session in a circular arena, and 360-degree contralateral rotations were quantified.
2.6. Immunofluorescence
On day 21, animals were perfused via the vascular system with descending aorta clamped. In this procedure, sterile saline was administered for 10 min, followed by more 10 min of 4% paraformaldehyde (PFA) solution in PBS pH 7.4. The brains were extracted and maintained into 4% PFA for 24 h at 4 °C, then transferred to 15% sucrose solution for 24 h at 4 °C followed by immersion in 30% sucrose for 24 h at 4 °C. After being lightly dried, brains were frozen at −20 °C. Using a cryostat (Jung Histoslide 2000R; Leica; Heidelberg, DEU) at −20 °C, the SNpc region was sectioned in slices of 20 μm thick coronal plane, which were collected in PBS containing 0.2% Triton X-100 (PBST). To block nonspecific binding, the sections were incubated with 3% albumin for 1 h at room temperature (21 ± 3 °C). Then, tissue slices were incubated with primary antibodies for 48 h at 4 °C. The details of the antibody source and dilutions are as follows: anti-TH (1:500; sc-25269) was from Santa Cruz Biotechnology, Inc. (TX, USA); anti-Iba1 (1:500; PTR2404) was from ©FUJIFILM Wako Pure Chemical; anti-GFAP (1:500; G6171) was from Sigma-Aldrich® (MO, USA); anti-HSP70 (1:50; sc1060) was from Santa Cruz Biotechnology, Inc. (TX, USA); all of them diluted in PBST containing 3% bovine serum albumin. Four adjacent slices per animal were stained with each primary antibody. Then, the tissue sections were washed four times in PBST and then incubated with secondary antibodies for 2 h at room temperature. The details of the antibody are as follows: anti-mouse Alexa 488, anti-mouse Alexa 555, anti-rabbit Alexa 488, anti-goat Alexa 555 from Invitrogen (CA, USA); all of them diluted 1:500 in PBST. The sections were washed four times in PBST. The tissue slices were then incubated for 5 min with DAPI for nucleic acid staining (1:1000; D9542 - Sigma-Aldrich®; MO, USA). The sections were washed several times in PBST transferred to gelatinized slides, mounted with FluorSave™ (345789 - Merck Millipore; MA, USA) and covered with coverslips. The images were obtained using a Microscopy EVOS FL Auto Imaging System (AMAFD1000 - Thermo Fisher Scientific; MA, USA). Quantifications were obtained using the software ImageJ, by measuring the pixels of binary images from the four adjacent slices of each animal. The average values of each animal were obtained and considered the value of each individual animal (average of four slices = one animal). The quantification of TH content was obtained from images with 10X of magnification, and the results were expressed as the ratio of TH staining in the ipsilateral side per contralateral side. Quantification of Iba1 and GFAP were obtained from images with 4X of magnification of ipsilateral SNpc, and the results were expressed as a percentage to control. Quantification of HSP70 was obtained from images with 10X of magnification of ipsilateral SNpc, and the results were expressed as a percentage to control.
2.7. Quantitative reverse transcriptase PCR (RT-qPCR)
Total RNA was extracted from SN with TRIzol® reagent (Invitrogen; CA, USA) according to the manufacturer’s instructions and quantified by spectrophotometry. The cDNA was synthesized with a High-Capacity cDNA Reverse Transcription® kit (Thermo Fisher Scientific, USA) using 3 μg of total RNA. RT-qPCR reactions were performed in 7300 Real-Time PCR System (Applied Biosystems; CA, USA) with PowerUp™ SYBR® Green Master Mix (Thermo Fisher Scientific, USA) following manufacturer’s instructions. Results were expressed in relation to constitutive normalization gene (ΔCt) and internal control group (ΔΔCt) using the formula 2−ΔΔCt. The primers used for amplification are available in Table 1.
Table 1.
Primer sequences for RT-qPCR. Forward and reverse primers used for amplification of cDNAs of interest.
| Gene | Primers |
|---|---|
| IL-1β | 5’ - ACCTGTTCTTTGAGGCTGAC |
| 5’ – AATGAGTGACACTGCCTTCC | |
| TNF-α | 5’ – CAGACCCTCACACTCAGATCAT |
| 5’ – ACCACCAGTTGGTTGTCTTTG | |
| IL-6 | 5’ - GCGATGATGCACTGTCAGAAA |
| 5’ – TCCAGAAGACCAGAGCAGATT |
2.8. Enzyme-linked immunosorbent assay (ELISA)
Interleukin-1beta (IL-1β), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) were quantified by indirect enzyme-linked immunosorbent assay (ELISA). According to protein content (Bradford, 1976), serum samples were normalized and incubated in an ELISA plate. After 24 h, the plates were washed three times with PBS +0.05% Tween 20 (PBSTw). The plates were incubated with 200 μL of 2,5% albumin for 2 h at room temperature to block nonspecific binding. Subsequently, the plates were washed three times with PBSTw and were incubated with 200 μL of primary antibodies for 3 h at room temperature. Antibodies utilized in this assay were anti-IL-1β (1:4000; ab9722), anti-TNF-α (1:4000; ab6671) and anti-IL-6 (1:4000; ab6672) from Abcam (Cambridge, UK). The plates were washed three times with PBSTw and were incubated with a rabbit IgG peroxidase-linked secondary antibody (1:4000) for 2 h. Anti-rabbit IgG peroxidase conjugated (#AP132P) was obtained from Merck Millipore (MA, USA). After washing the plate three times with PBSTw, 100 μL of substrate solution (TMB spectrophotometric ELISA detection kit) was added to each well and incubated for 15 min. The reaction was stopped with 50 μl per well of 12 M sulfuric acid, and the plate was read at 450 nm in SpectraMax i3 Multi-Mode Platform® (Molecular Devices, USA). We had a sample acquisition problem with the control group, so in this group we have n = 7 and in the other groups n = 8.
2.9. Statistical analysis
Statistical analysis was performed with GraphPad Prism version 7.00 (GraphPad Software Inc., San Diego, USA). Data was evaluated by two-way ANOVA, followed by Tukey’s Multiple Comparison post hoc test. Differences were considered significant when p < 0.05. Outliers were defined using the GraphPad Prism version 7.00 (GraphPad Software Inc., San Diego, USA) through ROUT method (Q = 5%) for behavior analysis and Grubb’s test (alpha 0.05) for the other analysis.
3. Results
3.1. Neuroprotective effect of HSP70 intranasal against 6-OHDA insult
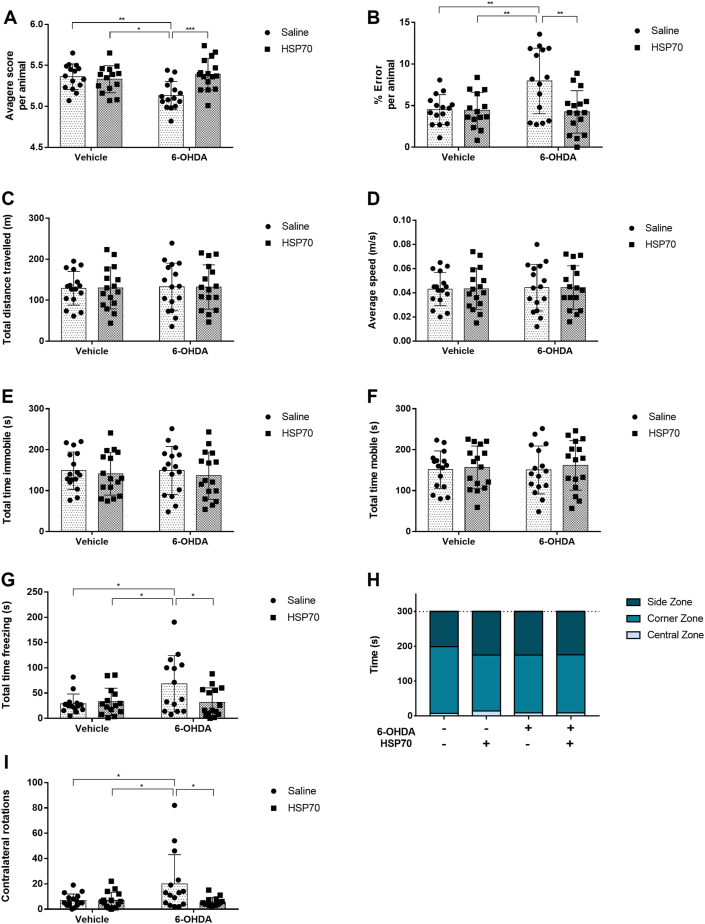
Firstly, we submitted the rats to behavioral tests to assess the effect of HSP70 treatment in motor impairment induced by unilateral dopaminergic denervation. At the ladder rung walking test, 6-OHDA treated animals presented a decreased index for quality of steps (Fig. 2A) and increased error percentage (Fig. 2B) compared with animals of all other groups. Notably, treatment with HSP70 significantly protected against the deleterious effect of 6-OHDA at both parameters at the ladder rung walking test (Fig. 2A and B). Also, animals of the 6-OHDA group had an increased freezing time at the OPF test compared with all other groups, which was ameliorated by HSP70 exposure (Fig. 2G). Nonetheless, experimental groups did not present significant differences in total distance traveled (Fig. 2C), average speed (Fig. 2D), total immobile time (Fig. 2E), total mobile time (Fig. 2F), and total time in the central zone, corners zone and sides zone (Fig. 2H). Animals injected with 6-OHDA performed more contralateral rotations than animals of other groups, including the 6-OHDA + HSP70 group (Fig. 2I), in the apomorphine-induced rotation test. The intranasal treatment with HSP70 also improved this behavior parameter that was impaired by 6-OHDA.
Fig. 2.
HSP70 treatment improves behavior impairments induced by 6-OHDA. On day 18, the ladder rung walking test was performed. A qualitative evaluation was performed for each step using a foot fault scoring system, using a 7-category scale: (0) total miss, (1) deep slip, (2) slight slip, (3) replacement, (4) correction, (5) partial placement and (6) correct placement. [A] The average of scores per animal was measured. [B] The percentual of errors was also measured, counting scores of 0, 1 and, 2 as errors. On day 19, the open field test was performed. [C] The total distance traveled (m); [D] average speed (m/s); [E] total time immobile (s); [F] total time mobile (s); [G] total time freezing (s); and [H] total time in the central zone, corners zone and sides zones (s) were determined in a 5 min session, using the video tracking system ANY-maze. On day 20, the apomorphine-induced rotation test was performed, and [I] 360-degree contralateral rotations were quantified in a 30 min session. Values represent mean ± SD. Two-way analysis of variance and Tukey’s Multiple Comparison posthoc test was applied to all data. The p values are represented as followed: ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05.
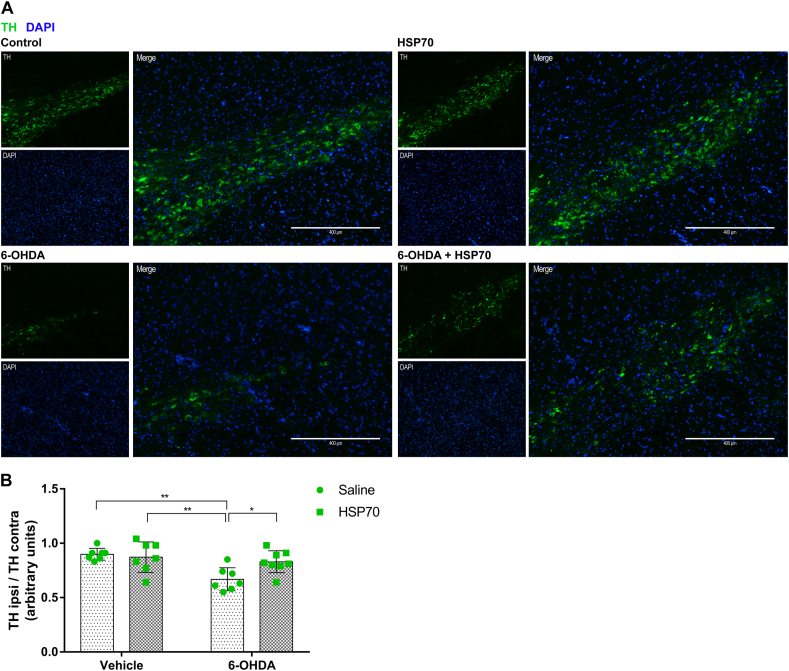
Next, immunostaining of tyrosine hydroxylase (TH - DA neuron marker) was performed to analyze the effect of HSP70 intranasal treatment in DA denervation induced by 6-OHDA. The unilateral injection of 6-OHDA caused a loss of DA neurons in the ipsilateral side without affect the contralateral side. The ratio of ipsilateral to contralateral TH staining was decreased in 6-OHDA in comparison with all other groups, while the treatment with HSP70 significantly protected against loss of TH staining caused by 6-OHDA (Fig. 3). It is important to mention that no differences in TH staining were observed between Control, HSP70, and 6-OHDA + HSP70 groups (Fig. 3B).
Fig. 3.
HSP70 treatment reduces 6-OHDA-induced dopaminergic denervation in SNpc. [A] Representative immunofluorescence images of SNpc co-immunostained for TH (green) and DAPI (blue). Images from ipsilateral sides of are Control (vehicle + saline), HSP70 (vehicle + HSP70), 6-OHDA (6-OHDA + saline), and 6-OHDA + HSP70 groups are shown. Guide bars represent 400 μm. Images are representative of independent experiments from eight animals per group. [B] The quantification of TH content was obtained using the software ImageJ measuring the pixels of immunofluorescence images with 10X of magnification, and results were expressed as the ratio of TH staining in ipsilateral side per contralateral side. Values represent mean ± SD. Two-way analysis of variance and Tukey’s Multiple Comparison posthoc test was applied to all data. The p values are represented as followed: ∗∗p < 0.01, ∗p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. HSP70 treatment reduced glial activation induced by 6-OHDA
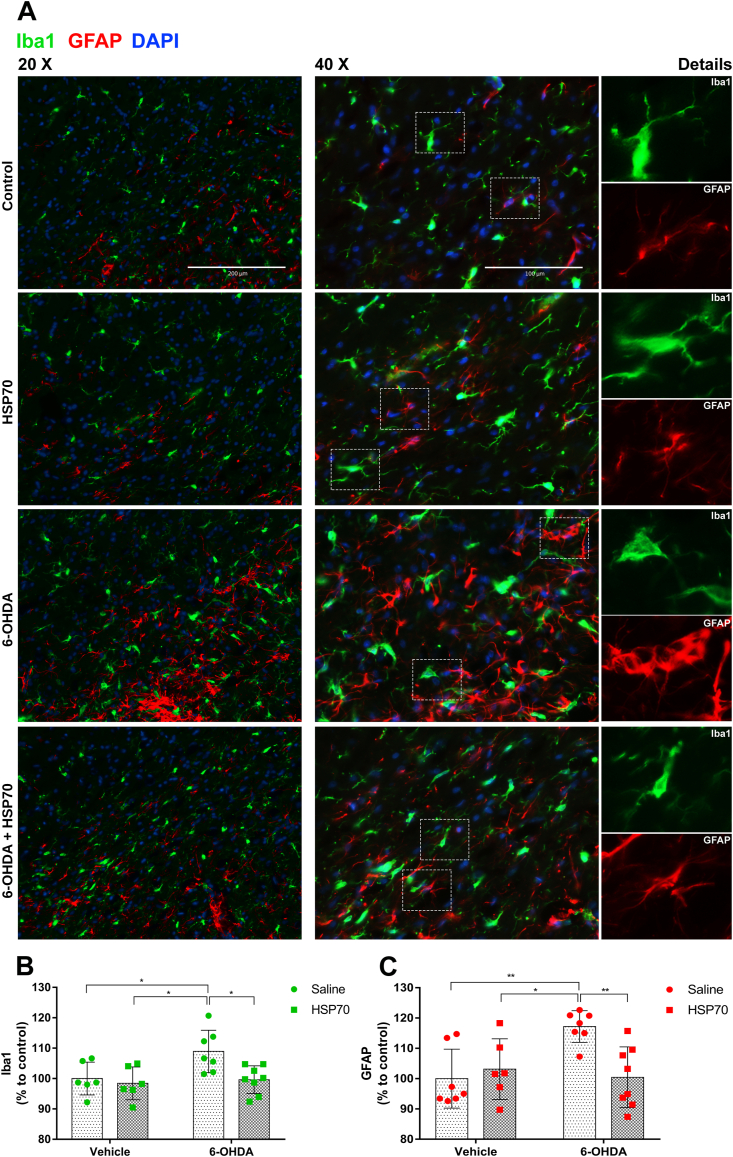
Co-immunostaining of ionized calcium-binding adapter molecule 1 (Iba1 – microglial marker) and glial fibrillary acidic protein (GFAP – astrocyte marker) was performed to analyze the effect of HSP70 intranasal treatment on glial activation in SNpc after 6-OHDA intrastriatal injection. As the 6-OHDA insult was unilateral, we compared GFAP and Iba1 staining between the groups on the ipsilateral side. As shown in Fig. 4A and B, an increase in Iba1 immunoreactivity was induced by 6-OHDA, and this effect was significantly decreased to control levels by intranasal treatment with HSP70. Moreover, through qualitative analysis, we observed ameboid morphology associated with Iba1 staining predominantly in the 6-OHDA group. It was replaced by a pattern of branched cell extensions in the 6-OHDA + HSP70 group, indicating that intranasal HSP70 administration could change the microglial phenotype from activated to resting state. Also, as shown in Fig. 4A and C, 6-OHDA group typically presented severe reactive astrogliosis with increased GFAP staining and formation of glial scars, which were significantly inhibited by intranasal exposure to HSP70. Therefore, these results indicate that HSP70 intranasal treatment reduces glial activation induced by 6-OHDA in the SNpc.
Fig. 4.
HSP70 treatment reduces 6-OHDA-induced glial activation in SNpc. [A] Representative immunofluorescence images of SNpc co-immunostained for Iba1 (green), GFAP (red), and DAPI (blue). Images from ipsilateral sides of Control (vehicle + saline), HSP70 (vehicle + HSP70), 6-OHDA (6-OHDA + saline), and 6-OHDA + HSP70 groups are shown. Merge images were obtained with 20X and 40X of magnification, guide bars represent 200 μm and 100 μm. Details of microglia and astrocytes morphology are shown in separated canals. Images are representative of independent experiments from eight animals per group. The quantification of Iba1 [B] and GFAP [C] content was obtained using the software ImageJ measuring the pixels from the ipsilateral SNpc region of immunofluorescence images with 4X of magnification, and results were expressed as a percentage to control. Values represent mean ± SD. Two-way analysis of variance and Tukey’s Multiple Comparison posthoc test was applied to all data. The p values are represented as followed: ∗∗p < 0.01, ∗p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Modulation of proinflammatory cytokines by intranasal HSP70 treatment
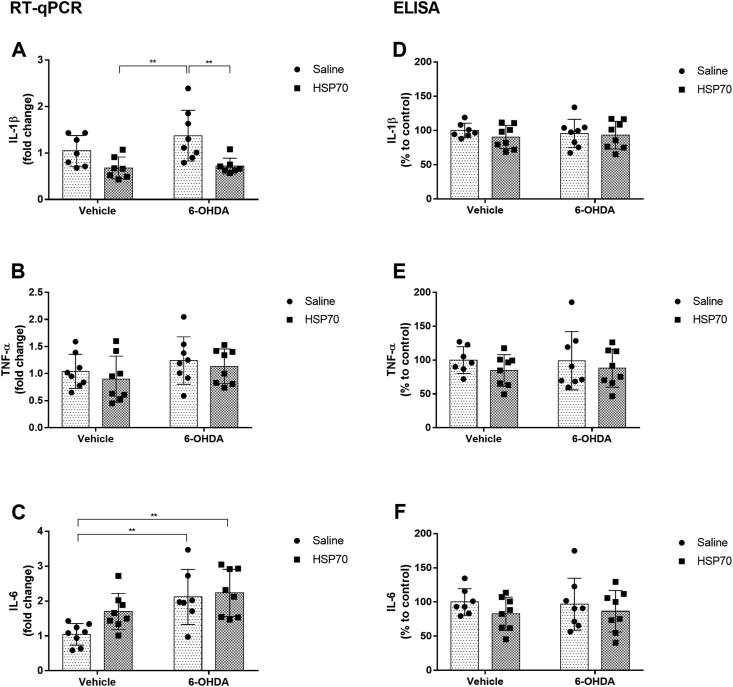
We analyzed mRNA expression of proinflammatory cytokines, such as IL-1β, TNF-α, and IL-6, in the substantia nigra through RT-qPCR analysis. Although a significant difference was not detected between control and 6-OHDA groups, we observed that both groups receiving intranasal HSP70 had a significant reduction in IL-1β gene expression compared with 6-OHDA treated animals (Fig. 5A). Besides, no significant differences in TNF-α mRNA levels were found between all groups (Fig. 5B). Regarding IL-6, the increase in mRNA expression induced by 6-OHDA was not affected by HSP70 intranasal treatment (Fig. 5C). We also analyzed the protein levels of these proinflammatory cytokines in serum through ELISA, and no significant differences between experimental groups on IL-1β, TNF-α, or IL-6 serum levels were found (Fig. 5D, E and F).
Fig. 5.
HSP70 treatment modulates proinflammatory cytokines induced by 6-OHDA. mRNA expression levels of [A] IL-1β, [B] TNF-α and [C] IL-6 in the SNpc were analyzed by RT-qPCR. β-actin was used as a constitutive normalization gene, and results were expressed in relation to β-actin (ΔCt) and internal control group (ΔΔCt) using the formula 2−ΔΔCt. Protein levels of [D] IL-1β, [E] TNF-α, and [F] IL-6 in the serum were analyzed by ELISA. The results of protein levels were expressed in percentage to control. Values represent mean ± SD. Two-way analysis of variance and Tukey’s Multiple Comparison posthoc test was applied to all data. The p values are represented as followed: ∗∗p < 0.01.
3.4. HSP70 treatment increased HSP70 protein content after 6-OHDA insult
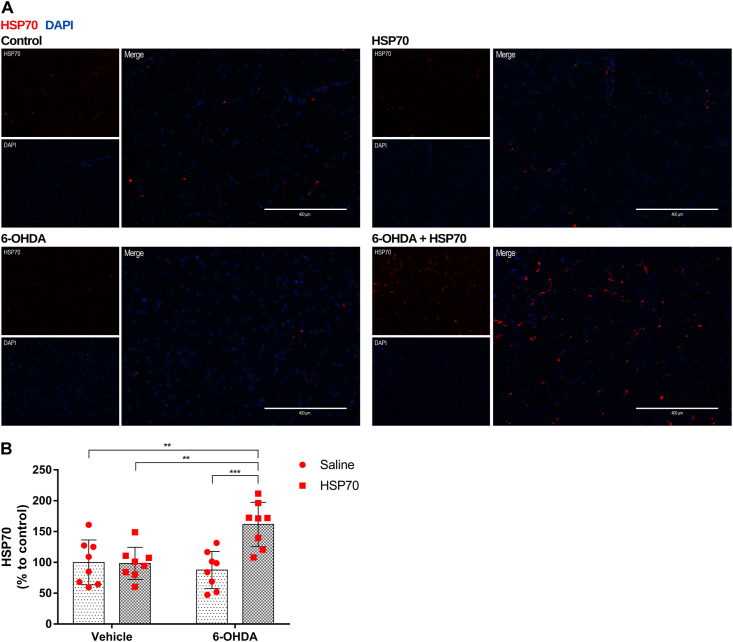
Immunostaining with anti-HSP70 was performed to analyze the modulation of HSP70 protein content by intranasal treatment with recombinant HSP70 in the SNpc. To access the effects of the intranasal treatment in the unilateral lesion induced by 6-OHDA, we evaluate the HSP70 staining in the ipsilateral side. Our results demonstrated that HSP70 treatment increased the HSP70 protein content in the SNpc from animals that received 6-OHDA (Fig. 6). This increase in HSP70 content is significant when compared with all other groups (Fig. 6B).
Fig. 6.
HSP70 treatment increases HSP70 protein content in the SNpc of rats induced by 6-OHDA. [A] Representative immunofluorescence images of SNpc co-immunostained for HSP70 (red) and DAPI (blue). Images from ipsilateral sides of are Control (vehicle + saline), HSP70 (vehicle + HSP70), 6-OHDA (6-OHDA + saline), and 6-OHDA + HSP70 groups are shown. Guide bars represent 400 μm. Images are representative of independent experiments from eight animals per group. [B] The quantification of HSP70 content was obtained using the software ImageJ measuring the pixels of immunofluorescence images with 10X of magnification, and results were expressed as a percentage to control. Values represent mean ± SD. Two-way analysis of variance and Tukey’s Multiple Comparison posthoc test was applied to all data. The p values are represented as followed: ∗∗∗p < 0.001, ∗∗p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
PD is characterized by motor symptoms, resulting from the progressive death of DA neurons in SNpc, leading to dopamine depletion in the striatum (Dauer and Przedborski 2003). Intrastriatal administration of 6-OHDA is widely used as an animal model of PD since it leads to DA denervation of the nigrostriatal system. After DA denervation, the supersensitivity of ipsilateral DA receptors leads to contralateral rotations when animals are stimulated by dopamine agonists, such as apomorphine (Ungerstedt 1971). In this study, we observed a significant increase of contralateral rotations induced by apomorphine in the 6-OHDA group; despite the significance, many animals in this group scored the same number of ipsilateral rotations than in other groups. Some works use the apomorphine induced-rotation test as a control for the success of 6-OHDA injection, excluding animals that did not present high number of contralateral rotations (Issy et al., 2015; Su et al., 2018). In our experimental design this control cannot be done, as the treatment with intranasal HSP70 starts at the 6th day after 6-OHDA injection and it is not possible to apply the apomorphine test or any other procedure to evaluate the efficacy of the 6-OHDA injection at this point. On the other hand, if we used the apomorphine test to exclude animals that did not present elevated rotation at the 20th day, this would lead to a bias error as it would be impossible to differ the animals in which 6-OHDA injection was not successful from animals that were protected by HSP70 in the 6-OHDA + HSP70 group. The injection of 6-OHDA in the striatum leads to retrograde degeneration of the SNpc DA neurons, which occurs progressively during the first weeks after the insult, differently from the abrupted degeneration observed with intranigral 6-OHDA injection (Tieu 2011; Schober 2004). Here, despite their performance in the apomorphine test, the reduction in TH levels in the SN is observed in most animals, confirming the efficacy of the model. We believe the high variability on the score of the animals in the apomorphine test was because we did not select animals, due to the reasons pointed above, and probably because the test was performed on the 20th day after surgery. The intranasal HSP70 treatment demonstrates to have neuroprotective effect against DA denervation induced by 6-OHDA since intranasal treatment reduced the loss of DA neurons and apomorphine-induced contralateral rotations. Moreover, HSP70 treatment also reduced the impairment in the ladder rung walking test. This test allows to discriminate slight impairments in motor function, combining qualitative and quantitative analysis (Metz and Whishaw 2002). The animals treated with 6-OHDA and HSP70 presented increased quality of steps and decreased error percentage compared to the 6-OHDA group.
The OPF test was performed to observe parameters related to anxiety behavior and general locomotor activity (Prut and Belzung 2003; Bové and Perier 2012). Depression and anxiety are common symptoms in PD (Fontoura et al., 2017), besides the characteristic motor symptoms of the disease. On the OPF apparatus, it is expected that animals prefer the peripheries than the central zone, being an increase of time in the central zone indicative of anxiety behavior (Prut and Belzung 2003). Our results demonstrated that all animals prefer the corner zone and side zone in relation to the central zone, without significant differences in total time in the central zone between the groups. The total time freezing was also measured because this parameter may reflect an anxiety profile, indicating fear and sensation of predatory risk on the OPF test (Roelofs 2017; Blanchard and Blanchard 1988). Our findings pointed out an increase of total time freezing in animals induced by 6-OHDA, which was reverted by intranasal treatment with HSP70. No alterations were observed on general locomotor activity, i.e., animals did not present differences between groups on total distance traveled, average speed, total immobile time, and total mobile time.
The critical point is that differentiated adult neurons have insufficient intracellular inducible HSP70 form, making them more susceptible to toxic changes in the intra- and extracellular environment (Lyon and Milligan 2019). In this regard, the neuroprotective effect of HSP70 was demonstrated in the 6-OHDA model by Tunesi and collaborators (Tunesi et al., 2019), using an intrastriatal cannula to administer Tat-Hsp70-loaded composites. Another study showed neuroprotective action of Tat-HSP70, which was administered intraperitoneally in the MPTP model. Tat, one of the most used penetrating cell peptides, have been used to facilitate the translocation of recombinant HSP70 across cell membranes and BBB (Nagel et al., 2008). The HSP70 is too large to cross the BBB freely; besides, it has a short half-life. Indeed, after intraperitoneal administration, it is impossible to detect HSP70 in the CNS (Lyon and Milligan 2019). Concerning the intravenous route, exogenous HSP70 is eliminated from the blood within 1 h after its administration (Evgen’ev et al., 2018).
Here, we used an intranasal approach to administrate HSP70. Intranasal administration is a non-invasive drug delivery approach that could directly deliver large-size drugs in CNS, bypassing the BBB, and minimizing potential systemic side effects (Ying 2007). HSP70 intranasal administration was already used in a PD model induced by repeated bilateral microinjections of lactacystin in SNpc. Intranasal HSP70 had reduced the loss of both DA neurons in SNpc and their terminals in the striatum. Besides, labeled HSP70 was found in DA neurons’ cytoplasm in the SNpc of rats (Pastukhov et al., 2014). Bobkova et al. also demonstrated intracellular fluorescently-labeled HSP70 in the olfactory bulbs, neocortex, hippocampus, n.raphe dorsalis, locus coeruleus, and cerebellum after intranasal administration (Bobkova et al., 2014). Therefore, exogenous HSP70 can penetrate different regions of the brain and its neuronal cells when delivered by intranasal administration.
In this work, we demonstrate an increase of HSP70 protein content in SNpc when animals received 6-OHDA insult and were treated with recombinant HSP70. In cellular stress conditions, the release of HSPs to the extracellular environment is exacerbated (Dukay et al., 2019). However, when animals only received 6-OHDA toxic insult, no alterations on HSP70 protein content were observed in SNpc. Also, no modifications in HSP70 protein content were observed when animals received HSP70 treatment without the toxic insult. This increase of HSP70 in the 6-OHDA + HSP70 group is not specific to the intracellular or the extracellular environments. In response to cellular stress, astrocytes could upregulate intracellular HSP70 expression and the release of HSP70 to the extracellular environment. This release of HSP70 may support neurons, which have insufficient amounts of HSP70 inducible form (Lyon and Milligan 2019). Moreover, extracellular HSPs act on modulating inflammatory response and expression of cytokines (Dukay et al., 2019). Besides its chaperone function, HSP70 is involved in modulating inflammatory response (Dukay et al., 2019).
Accumulation of misfolded proteins and unregulated inflammation contribute to the pathogenesis of age-related neurodegenerative disorders. DA neurons in SNpc are susceptible to sustained inflammation neurotoxic effects, whereas neuroinflammation is a crucial factor in PD pathogenesis (Wang et al., 2015). As resident innate immune cells of CNS, microglial activation is the first step of the inflammatory response (Perry and Teeling 2013; Gadani et al., 2015). Activated microglia assume an ameboid morphology, whereas microglia in the resting stage is characterized by a branched morphology (Nimmerjahn et al., 2005; Cho et al., 2006; Davalos et al., 2005). Uncontrolled activated microglia release pro-inflammatory mediators and reactive oxygen species, contributing to a neurotoxic environment (Halliwel and Gutteridge 2016). SNpc has a higher content of microglia compared with other brain structures. Whereas DA neurons have lower levels of antioxidants defenses, being more vulnerable to oxidative insults decurrent from microglial activation than most other types of neurons (Lawson et al., 1990; Kim et al., 2000; Sun et al., 2016; Loeffler et al., 1994). Reactive astrocytes also contribute to inflammatory responses against CNS insults. Pronounced upregulation of GFAP and loss of individual astrocytes domains were characteristics of severe reactive astrogliosis (Sofroniew and Vinters 2010; Sofroniew 2015). Herein, we demonstrated 6-OHDA insult increasing activation of microglia and astrocytes reactivity in SNpc, with a predominance of ameboid microglia and astrocyte scar formation. Animals treated with HSP70 presented less microglial ameboid morphology and more preserved astrocytes individual domains. Moreover, intranasal HSP70 decreased Iba1 and GFAP content in these animals. Therefore, HSP70 treatment reduced glial activation induced by 6-OHDA.
The release of proinflammatory mediators can be amplified by synergic activation of astrocytes and microglia, enhancing neurotoxic effects to DA neurons (Wang et al., 2015). IL-1β sustained expression in SNpc leads to pronounced DA neuron loss (Leal et al., 2013). The induction of this cytokine could lead to DA neurons’ loss, regardless of other inflammatory events. Previous work demonstrated chronic induction of IL-1β expression in SNpc inducing progressive DA neuronal loss, locomotor deficits, and glial activation, similar to other experimental models of PD (Ferrari et al., 2006). Importantly, here, we demonstrated that treatment with intranasal HSP70 decreased IL-1β mRNA expression in the SNpc of 6-OHDA rats.
On the other hand, HSP70 treatment did not reduce the increase of IL-6 mRNA expression induced by 6-OHDA. IL-6 is a typical pro-inflammatory cytokine involved in the degeneration and regeneration of neurons in the central and peripheral nervous system (Gruol and Nelson 1997; Gadient and Otten 1997). However, the role of IL-6 in PD neuropathology is not well established. Some studies indicated an inverse correlation between PD severity and increased IL-6 levels in the nigrostriatal region and the CSF (Mogi et al., 1996; Nagatsu 2002; Blum-Degen et al., 1995; Müller et al., 1998). On the other hand, another study suggested increased IL-6 levels as a risk marker for mortality in PD (Dufek et al., 2015).
TNF-α is another proinflammatory cytokine related to PD neuropathology (Yan et al., 2014). This cytokine is essential to the neuroinflammatory process, which many times occurs before BBB dysfunction, as occurs in the 6-OHDA model (Carvey et al., 2005). However, in our study, no differences in TNF-α mRNA expression were found in SNpc. Previous work has demonstrated TNF-α knockout, reducing microglial activation without decreasing DA neurons’ loss in the MPTP model (Zhao et al., 2007). Thus, TNF-α does not appear to be directly involved with DA neuron loss, only acting in the PD’s inflammatory modulation. Increased TNF- α is associated with BBB disruption (Tsao et al., 2001; Didier et al., 2003). By contrast, TNF-α knockout animals presented a reduction of BBB dysfunction in the MPTP model (Zhao et al., 2007). In our study, no alterations in serum protein levels of TNF-α, IL-1β, and IL-6 were found, which might be explained by the fact that serum samples were analyzed 21 days after 6-OHDA injection. Besides, no difference in TNF-α expression was observed in SNpc at the same time stage. Overall, our results indicate that HSP70 neuroprotective action might be related to its ability to modulate glial activation and reduce IL-1β mRNA expression in the SNpc. Therefore, our results suggest that the neuroprotective action of HSP70 treatment against 6-OHDA insult occurs by modulation of the inflammatory response, which might be related to the increase of HSP70 protein content in SNpc.
5. Conclusion
Intranasal administration of HSP70 shows neuroprotective actions in the rat model of 6-OHDA-induced DA denervation. The treatment with exogenous HSP70 reduced DA neurons’ loss and improved behavior impairments induced by 6-OHDA intrastriatal injection. Also, HSP70 has modulated neuroinflammatory response, which is an important feature of PD pathogenesis. HSP70 intranasal treatment reduced microglial activation and astrogliosis in the PD animal model, associated with a reduction in IL-1β mRNA expression after HSP70 administration. Moreover, the treatment with recombinant HSP70 increased the protein content of HSP70 in the SNpc of animals exposed to 6-OHDA. Our results suggest that HSP70 neuroprotective action against 6-OHDA toxicity is related to modulation of the inflammatory response, which might occur by the increase in HSP70 protein content in response to the injury. Finally, our findings demonstrated neuroprotective activity in the nigrostriatal axis using a noninvasive delivery approach to administer HSP70.
Funding sources
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) #408435/2018-6, FAPERGS #16/2551-0000499-4 and #17/2551-0000984-3, Propesq-UFRGS and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Blanchard D.C., Blanchard R.J. Ethoexperimental approaches to the biology of emotion. Annu. Rev. Psychol. 1988;39:43–68. doi: 10.1146/annurev.ps.39.020188.000355. [DOI] [PubMed] [Google Scholar]
- Blum-Degen D., Müller T., Kuhn W., Gerlach M., Przuntek H., Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of alzheimer’s and de novo Parkinson’s disease patients. Neurosci. Lett. 1995;202 doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Bobkova N.V., Evgen’ev M., Garbuz D.G. Exogenous Hsp70 delays senescence and improves cognitive function in aging mice. Proc. Natl. Acad. Sci. U. S. A. 2015;112:16006–16011. doi: 10.1073/pnas.1516131112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobkova N.V., Garbuz D.G., Nesterova I. Therapeutic effect of exogenous hsp70 in mouse models of Alzheimer’s disease. J. Alzheimers Dis. 2014;38:425–435. doi: 10.3233/JAD-130779. [DOI] [PubMed] [Google Scholar]
- Bové J., Perier C. Neurotoxin-based models of Parkinson’s disease. Neuroscience. 2012;211 doi: 10.1016/j.neuroscience.2011.10.057. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Calderwood S., Murshid A. Molecular chaperone accumulation in cancer and decrease in alzheimer’s disease: the potential roles of HSF1. Front. Neurosci. 2017;11 doi: 10.3389/fnins.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos F.L., Carvalho M.M., Cristovao A.C., Je G., Baltazar G., Salgado A.J., Kim Y.S., Sousa N. Rodent models of Parkinson’s disease: beyond the motor symptomatology. Front. Behav. Neurosci. 2013;7:175. doi: 10.3389/fnbeh.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvey P.M., Zhao C.H., Hendey B., Lum H., Trachtenberg J., Desai B.S., Snyder J., Zhu Y.G., Ling Z.D. 6-Hydroxydopamine-induced alterations in blood-brain barrier permeability. Eur. J. Neurosci. 2005;22:1158–1168. doi: 10.1111/j.1460-9568.2005.04281.x. [DOI] [PubMed] [Google Scholar]
- Cho B., Song D., Sugama S., Shin D., Shimizu Y., Kim S., Kim Y., Joh T. Pathological dynamics of activated microglia following medial forebrain bundle transection. Glia. 2006;53 doi: 10.1002/glia.20265. [DOI] [PubMed] [Google Scholar]
- Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Davalos D., Grutzendler J., Yang G., Kim J., Zuo Y., Jung S., Littman D., Dustin M., Gan W. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8 doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Didier N., Romero I.A., Creminon C., Wijkhuisen A., Grassi J., Mabondzo A. Secretion of interleukin-1beta by astrocytes mediates endothelin-1 and tumour necrosis factor-alpha effects on human brain microvascular endothelial cell permeability. J. Neurochem. 2003;86:246–254. doi: 10.1046/j.1471-4159.2003.01829.x. [DOI] [PubMed] [Google Scholar]
- Dufek M., Rektorova I., Thon V., Lokaj J., Rektor I. Interleukin-6 may contribute to mortality in Parkinson’s disease patients: a 4-year prospective study. Parkinsons Dis. 2015;2015:898192. doi: 10.1155/2015/898192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukay B., Csoboz B., Toth M.E. Heat-shock proteins in neuroinflammation. Front. Pharmacol. 2019;10:920. doi: 10.3389/fphar.2019.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Fakhari D., Wahlster L., McLean P.J. Molecular chaperones in Parkinson’s disease--present and future. J. Parkinsons Dis. 2011;1:299–320. [PMC free article] [PubMed] [Google Scholar]
- Evgen’ev M.B., Garbuz D.G., Morozov A.V., Bobkova N.V. In: Asea A., Kaur P., editors. vol. 14. Springer; Cham: 2018. Intranasal administration of Hsp70: molecular and therapeutic consequences. (HSP70 in Human Diseases and Disorders. Heat Shock Proteins). [Google Scholar]
- Ferrari C.C., Pott Godoy M.C., Tarelli R., Chertoff M., Depino A.M., Pitossi F.J. Progressive neurodegeneration and motor disabilities induced by chronic expression of IL-1beta in the substantia nigra. Neurobiol Dis. 2006;24:183–193. doi: 10.1016/j.nbd.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Fontoura J.L., Baptista C., Pedroso Fde B., Pochapski J.A., Miyoshi E., Ferro M.M. Depression in Parkinson’s disease: the contribution from animal studies. Parkinsons Dis. 2017;2017 doi: 10.1155/2017/9124160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani S.P., Walsh J.T., Lukens J.R., Kipnis J. Dealing with danger in the CNS: the response of the immune system to injury. Neuron. 2015;87:47–62. doi: 10.1016/j.neuron.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadient R., Otten U. Interleukin-6 (IL-6) --a molecule with both beneficial and destructive potentials. Prog. Neurobiol. 1997;52 doi: 10.1016/s0301-0082(97)00021-x. [DOI] [PubMed] [Google Scholar]
- Gifondorwa D.J., Robinson M.B., Hayes C.D., Taylor A.R., Prevette D.M., Oppenheim R.W., Caress J., Milligan C.E. Exogenous delivery of heat shock protein 70 increases lifespan in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2007;27:13173–13180. doi: 10.1523/JNEUROSCI.4057-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Gruol D., Nelson T. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol. Neurobiol. 1997;15 doi: 10.1007/BF02740665. [DOI] [PubMed] [Google Scholar]
- Haaxma C.A., Bloem B.R., Borm G.F., Oyen W.J.G., Leenders K.L., Eshuis S., Booij J., Dluzen D.E., Horstink M.W.I.M. Gender differences in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2007;78:819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwel B., Gutteridge J.M.C. Oxford University Press; USA: 2016. Free Radicals in Biology and Medicine. [Google Scholar]
- Hartl F.U., Bracher A., Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Issy A.C., Padovan-Neto F.E., Lazzarini M., Bortolanza M., Del-Bel E. Disturbance of sensorimotor filtering in the 6-OHDA rodent model of Parkinson’s disease. Life Sci. 2015;125:71–78. doi: 10.1016/j.lfs.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Kim W., Mohney R., Wilson B., Jeohn G., Liu B., Hong J. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J. Neurosci.: Off. J. Soc. Neurosci. 2000;20 doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken J., Shin Y., Masliah E., Hyman B.T., McLean P.J. Hsp70 reduces alpha-synuclein aggregation and toxicity. J. Biol. Chem. 2004;279:25497–25502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- Lawson L., Perry V., Dri P., Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39 doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Leal M.C., Casabona J.C., Puntel M., Pitossi F.J. Interleukin-1beta and tumor necrosis factor-alpha: reliable targets for protective therapies in Parkinson’s Disease? Front. Cell. Neurosci. 2013;7:53. doi: 10.3389/fncel.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Yang J., Wang Y., Liu Q., Cheng J., Wang F. Neuroprotective effects of increasing levels of HSP70 against neuroinflammation in Parkinson’s disease model by inhibition of NF-kappaB and STAT3. Life Sci. 2019;234:116747. doi: 10.1016/j.lfs.2019.116747. [DOI] [PubMed] [Google Scholar]
- Loeffler D., DeMaggio A., Juneau P., Havaich M., LeWitt P. Effects of enhanced striatal dopamine turnover in vivo on glutathione oxidation. Clin. Neuropharmacol. 1994;17 doi: 10.1097/00002826-199408000-00009. [DOI] [PubMed] [Google Scholar]
- Lyon M.S., Milligan C. Extracellular heat shock proteins in neurodegenerative diseases: new perspectives. Neurosci. Lett. 2019;711:134462. doi: 10.1016/j.neulet.2019.134462. [DOI] [PubMed] [Google Scholar]
- Magrané J., Smith R., Walsh K., Querfurth H. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J. Neurosci.: Off. J. Soc. Neurosci. 2004;24 doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M.P., Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz G.A., Whishaw I.Q. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and coordination. J. Neurosci. Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Mogi M., Harada M., Narabayashi H., Inagaki H., Minami M., Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci. Lett. 1996;211 doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- Müller T., Blum-Degen D., Przuntek H., Kuhn W. Interleukin-6 levels in cerebrospinal fluid inversely correlate to severity of Parkinson’s disease. Acta Neurol. Scand. 1998;98 doi: 10.1111/j.1600-0404.1998.tb01736.x. [DOI] [PubMed] [Google Scholar]
- Murshid A., Eguchi T., Calderwood S.K. Stress proteins in aging and life span. Int. J. Hyperther. 2013;29:442–447. doi: 10.3109/02656736.2013.798873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T. Parkinson’s disease: changes in apoptosis-related factors suggesting possible gene therapy. J. Neural. Transm. 2002:109. doi: 10.1007/s007020200061. Vienna, Austria : 1996. [DOI] [PubMed] [Google Scholar]
- Nagel F., Falkenburger B.H., Tonges L., Kowsky S., Poppelmeyer C., Schulz J.B., Bahr M., Dietz G.P. Tat-Hsp70 protects dopaminergic neurons in midbrain cultures and in the substantia nigra in models of Parkinson’s disease. J. Neurochem. 2008;105:853–864. doi: 10.1111/j.1471-4159.2007.05204.x. [DOI] [PubMed] [Google Scholar]
- National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory, A . Guide for the Care and Use of Laboratory Animals. National Academies Press (US)National Academy of Sciences.; Washington (DC): 2011. The national academies collection: reports funded by national Institutes of Health. [Google Scholar]
- Nimmerjahn A., Kirchhoff F., Helmchen F. Science; New York, N.Y.: 2005. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo; p. 308. [DOI] [PubMed] [Google Scholar]
- Pang Y., Lin S., Wright C., Shen J., Carter K., Bhatt A., Fan L.W. Intranasal insulin protects against substantia nigra dopaminergic neuronal loss and alleviates motor deficits induced by 6-OHDA in rats. Neuroscience. 2016;318:157–165. doi: 10.1016/j.neuroscience.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastukhov Y.F., Plaksina D.V., Lapshina K.V., Guzhova I.V., Ekimova I.V. Exogenous protein HSP70 blocks neurodegeneration in the rat model of the clinical stage of Parkinson’s disease. Dokl. Biol. Sci. 2014;457:225–227. doi: 10.1134/S0012496614040139. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. Elsevier Academic Press Amsterdam; Boston: 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Perry V., Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 2013;35 doi: 10.1007/s00281-013-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L., Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Radons J. The human HSP70 family of chaperones: where do we stand? Cell Stress Chaperones. 2016;21:379–404. doi: 10.1007/s12192-016-0676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs K. Freeze for action: neurobiological mechanisms in animal and human freezing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Silva T.P., Poli A., Hara D.B., Takahashi R.N. Time course study of microglial and behavioral alterations induced by 6-hydroxydopamine in rats. Neurosci. Lett. 2016;622:83–87. doi: 10.1016/j.neulet.2016.04.049. [DOI] [PubMed] [Google Scholar]
- Sofroniew M.V. vol. 7. 2015. Astrogliosis. (Cold Spring Harb Perspect Biol). [Google Scholar]
- Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman N., Johnson R. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15 doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R.J., Zhen J.L., Wang W., Zhang J.L., Zheng Y., Wang X.M. Time-course behavioral features are correlated with Parkinson’s disease-associated pathology in a 6-hydroxydopamine hemiparkinsonian rat model. Mol. Med. Rep. 2018;17:3356–3363. doi: 10.3892/mmr.2017.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Pham A., Waite T. Elucidation of the interplay between Fe(II), Fe(III), and dopamine with relevance to iron solubilization and reactive oxygen species generation by catecholamines. J. Neurochem. 2016;137 doi: 10.1111/jnc.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2011;1 doi: 10.1101/cshperspect.a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao N., Hsu H.P., Wu C.M., Liu C.C., Lei H.Y. Tumour necrosis factor-alpha causes an increase in blood-brain barrier permeability during sepsis. J. Med. Microbiol. 2001;50:812–821. doi: 10.1099/0022-1317-50-9-812. [DOI] [PubMed] [Google Scholar]
- Tunesi M., Raimondi I., Russo T. Hydrogel-based delivery of Tat-fused protein Hsp70 protects dopaminergic cells in vitro and in a mouse model of Parkinson’s disease. NPG Asia Mater. 2019;11:1–15. [Google Scholar]
- Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol. Scand. Suppl. 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- Wang Q., Liu Y., Zhou J. vol. 4. 2015. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. (Transl Neurodegener). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Fu Q., Cheng L., Zhai M., Wu W., Huang L., Du G. Inflammatory response in Parkinson’s disease (Review) Mol. Med. Rep. 2014;10:2223–2233. doi: 10.3892/mmr.2014.2563. [DOI] [PubMed] [Google Scholar]
- Ying W. The nose may help the brain: intranasal drug delivery for treating neurological diseases. Future Neurol. 2007;3 [Google Scholar]
- Zhao C., Ling Z., Newman M.B., Bhatia A., Carvey P.M. TNF-alpha knockout and minocycline treatment attenuates blood brain barrier leakage in MPTP-treated mice. Neurobiol. Dis. 2007;26:36–46. doi: 10.1016/j.nbd.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]