Abstract
Background
Autoimmune-mediated encephalitis is a disease that often encompasses psychiatric symptoms as its first clinical manifestation’s predominant and isolated characteristic. Novel guidelines even distinguish autoimmune psychosis from autoimmune encephalitis. The aim of this review is thus to explore whether a wide range of psychiatric symptoms and syndromes are associated or correlate with autoantibodies.
Methods
We conducted a PubMed search to identify appropriate articles concerning serum and/or cerebrospinal fluid (CSF) autoantibodies associated with psychiatric symptoms and syndromes between 2000 and 2020. Relying on this data, we developed a diagnostic approach to optimize the detection of autoantibodies in psychiatric patients, potentially leading to the approval of an immunotherapy.
Results
We detected 10 major psychiatric symptoms and syndromes often reported to be associated with serum and/or CSF autoantibodies comprising altered consciousness, disorientation, memory impairment, obsessive-compulsive behavior, psychosis, catatonia, mood dysfunction, anxiety, behavioral abnormalities (autism, hyperkinetic), and sleeping dysfunction. The following psychiatric diagnoses were associated with serum and/or CSF autoantibodies: psychosis and schizophrenia spectrum disorders, mood disorders, minor and major neurocognitive impairment, obsessive-compulsive disorder, autism spectrum disorders (ASD), attention deficit hyperactivity disorder (ADHD), anxiety disorders, eating disorders and addiction. By relying on these symptom clusters and diagnoses in terms of onset and their duration, we classified a subacute or subchronic psychiatric syndrome in patients that should be screened for autoantibodies. We propose further diagnostics entailing CSF analysis, electroencephalography and magnetic resonance imaging of the brain. Exploiting these technologies enables standardized and accurate diagnosis of autoantibody-associated psychiatric symptoms and syndromes to deliver early immunotherapy.
Conclusions
We have developed a clinical diagnostic pathway for classifying subgroups of psychiatric patients whose psychiatric symptoms indicate a suspected autoimmune origin.
Keywords: Autoimmunity, Psychiatric symptoms, Autoantibodies, Psychiatric syndrome, Autoimmune psychosis
Highlights
-
•
Autoantibodies are associated with a broad spectrum of psychiatric syndromes.
-
•
More systematic studies are needed to elucidate the significance of autoantibodies.
-
•
We developed a pathway to identify autoantibody-associated psychiatric syndromes.
1. Introduction
Currently, psychiatric syndromes such as depression, psychosis, and cognitive dysfunction are often the first or only clinical appearance of autoimmune encephalitis in addition to seizures (Herken and Prüss, 2017; Graus et al., 2016). In this review, we thus aim to characterize the spectrum of psychiatric symptoms and syndromes found to be associated with serum or cerebrospinal fluid (CSF) autoantibodies in the literature in order to develop a diagnostic pathway for these patients. Isolated psychiatric symptoms can be associated with synaptic and neuronal autoantibodies in serum and/or CSF termed SNAps (synaptic and neuronal autoantibodies associated with psychiatric syndromes) according to Al-Diwani (2017). Recent guidelines for classifying autoimmune psychosis have coined the term autoimmune psychosis (Pollak et al., 2020; Endres et al., 2020a). In addition, autoantibody-associated psychiatric symptoms may result from systemic inflammatory disorders such as systemic lupus erythematosus (Kanapathy et al., 2019) or Hashimoto encephalopathy (Churilov et al., 2019). However, autoantibodies are not an exclusive parameter by which to diagnose autoimmune-mediated encephalitis with psychiatric manifestations, since autoimmune encephalitis might occur without detection of known antibodies (Graus et al., 2018). The aim of our review is to describe the literature on the psychiatric symptoms and syndromes associated with autoantibodies. Based on the existing knowledge about autoantibody-associated psychiatric symptoms and syndromes, we propose a diagnostic pathway to help to detect those psychiatric patients with associated autoantibodies systematically, and to determine the significance of these autoantibodies.
2. Approximation of the relationship between autoantibodies and psychiatric symptoms
We conducted a PubMed search for the terms antibody in conjunction with psychiatry, mood, affective disorder, depression, bipolar disorder, schizophrenia, psychotic disorder, obsessive compulsive disorder, catatonia, anxiety, impulse control disorder, aggressive behavior, tic disorder, Gilles de la Tourette syndrome, ADHD, autism, sleep disorder, addiction and eating disorder between 2000 and February 2020 to identify appropriate articles written in English and describing psychiatric disorders and symptoms associated with autoantibodies from 2000 to 2020 within the article limit of references. We classified the relationship between autoantibodies and psychiatric symptoms as an association if serum or CSF autoantibodies and psychiatric symptoms coincided in the same patient. The associations were in most cases either identified by analyzing reports from patient cohorts presenting autoantibodies in whom psychiatric symptoms had been assessed, but also from psychiatric patient cohorts whose autoantibodies had been tested. The second approach is usually taken for patients experiencing their first episode of psychosis or those with psychotic symptoms admitted to acute psychiatric care. Our review is a narrative review and does not cover studies on children.
3. Immune dysregulation and autoimmunity in major psychiatric disease
Autoantibody-associated psychosis emerge as a distinct disease entity that can be distinguished from autoimmune encephalitis. We do not yet know whether other autoantibody-mediated psychiatric symptoms and syndromes represent subgroups of psychiatric disorders, or depict an independent disease entity. Our review first outlines the indications for immune dysregulation in psychiatric disorders to provide further insights into how autoimmunity is related to psychiatric disease and whether it plays a role in psychiatric disease. Genome-wide association studies have recently foregrounded the concept of immune dysregulation in psychiatric disorders as in schizophrenia (Ripke et al., 2014). Schizophrenia is a severe mental disorder hallmarked by altered cognition and behavior leading to social and working disability. Recent genetic studies revealed specific polymorphisms in the human leukocyte antigen (HLA) complex involved in psychiatric disorders such as schizophrenia (Tamouza et al., 2019b) and in specific forms of autism (Tamouza et al., 2019a) as developmental mental disorders accompanied by impaired social and communication skills. A recent study suggested that specific protein-encoding genes might contribute to the development of autoimmunity in psychiatric disorders (Pouget et al., 2019). Furthermore, several autoimmune diseases share gene variants with schizophrenia, and are genetically interrelated (Pouget et al., 2019) suggesting an association between autoimmunity and schizophrenia. Besides the interaction of the immune system and genes, the interface between the human brain and immune system assessable via CSF is an important step in diagnosing immunopsychiatric diseases. CSF analysis is especially relevant, as CSF abnormalities are frequently detected in conjunction with psychiatric disorders (Endres et al., 2015; Maxeiner et al., 2009; Baumgaertel et al., 2019). An impaired blood-CSF barrier function might be a prerequisite for the development of autoimmune-mediated psychiatric symptoms such as subgroups of patients with schizophrenia (Melkersson et al., 2018; Orlovska-Waast et al., 2019), psychosis (Melkersson et al., 2018, Orlovska-Waast et al., 2019) or affective disorders (Orlovska-Waast et al., 2019). Specific, elevated inflammatory proteins such as interleukin-6 (IL-6) and interleukin-8 (IL-8) in the CSF, part of the innate immune system, have been ascertained in patients with schizophrenia, or with psychotic (Orlovska-Waast et al., 2019; Wang and Miller et al., 2018) or affective disorders (Wang and Miller, 2018), supporting the likelihood of CNS inflammation playing a role in these disorders. Innate immune cells like the microglia are often activated in patients with schizophrenia and bipolar disorders (Wang et al., 2018), priming CNS autoimmunity in some subtypes of these disorders. The presented examples of autoimmune-related phenomena in major psychiatric disorders point towards an underestimated relationship between autoimmunity and psychiatric disease.
4. Antibody-associated psychiatric disease and link to autoimmunity
The concept and relevance of autoimmune-mediated symptoms and syndromes in psychiatric disease based on autoantibody-mediated effects has been emphasized in catatonic patients with NMDAR (N-methyl-D-aspartate receptor) antibodies (Rogers et al., 2019). However, the significance of autoantibodies in serum remains incompletely understood. There are other criteria involving specific diagnostic measures in these patients that must be met to prove an autoimmune origin of symptoms. Furthermore, many human-to-rodents transfer studies with different human autoantibodies against synaptic proteins such as NMDAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), glutamic acid decarboxylase 65 (GAD65), Glycin or leucine-rich glioma-inactivated 1 (LGI1) derived from patients have demonstrated altered synaptic receptor physiology and its downstream signal cascades leading to impaired synaptic plasticity, brain pathology and abnormalities in behavior, emotional regulation, perception and cognition in rodents (Rauschenberger et al., 2020; Jurek et al., 2019; Haselmann et al., 2018; Petit Pedrol et al., 2018; Planaguma et al., 2015; Geis et al., 2012). These cause-effect studies in rodents provide a link as to how autoantibodies might trigger psychiatric disease in animals, and depict in analogy to rodent studies a potential basis for autoimmunity in antibody-associated psychiatric syndromes in humans. Through this review, we aim to give an overview on psychiatric symptoms associated with autoantibodies and establish criteria by which a possible/probable or definitive autoimmune-mediation of psychiatric symptoms is conceivable.
5. Classification of autoantibodies
Autoantibodies are classified into categories depending on the site of their target antigen (for review, see Tanaka et al., 2020) or on their association with inflammatory disorders (Nikolopoulos et al., 2020; Barbero et al., 2019; Barbuti et al., 2017). The target antigen might be located in the intracellular space or on the membrane surface. Antibodies targeting intracellular antigens are mostly paraneoplastic, as a precursor or consequence of a tumor (Gultekin et al., 2000). Intracellular antibodies comprise, for example, CV2 (cronveinten 2)/CRMP5 (collapsin response mediator protein 5), Hu, Ma1/2, SOX1, Yo and Zic4 antibodies (Tanaka et al., 2020; Endres et al., 2020b). Another group of antibodies is directed against intracellular antigens such as the GAD65 (glutamic acid decarboxylase 65) antibodies that are often non-paraneoplastic (Hansen et al., 2016, 2018). Similar to GAD65 antibodies, membrane-surface antibodies are sometimes non-paraneoplastic, but can also be paraneoplastic and consist of antibodies targeting membrane receptors and proteins such AMPAR, CASPR2 (contactin-associated protein like 2), DPPX (dipeptidyl-peptidase-like protein 6), GABAA/BR (γ-amino butyric acid A/B receptor), IgLON5, LGI1, mGluR 1/5 (metabotropic glutamate receptor 1/5), Neurexin3alpha, NMDAR and Synapsin (Al-Diwani et al., 2019; Saether et al., 2019; Dalmau and Graus, 2018; Spatola et al., 2018; Coutino et al., 2017; Honorat et al., 2017; Pettingill et al., 2015; Boronat et al., 2013). The type of antibodies associated with neuropsychiatric symptoms might be significant for the underlying disease pathomechanism. T-cell cytotoxicity is suggested to mediate paraneoplastic autoimmune-encephalitis (Bien et al., 2012), whereas the autoantibodies themselves drive the pathogenic effects in membrane-bound antibody-associated encephalitis (Malviya et al., 2017).
A third group of antibodies may arise due to systemic inflammatory disorders accompanying CNS inflammation such as neuropsychiatric lupus erythematosus or Sjögren’s syndrome [anti-ds DNA (double-stranded desoxyribonucleic acid) antibodies, anti-antiphospholipid (APL) antibodies, anti-β2- glycoprotein-I (β2-GPI) antibodies, anti-cardiolipin (CL) antibodies, lupus anticoagulans antibodies, anti- Sjögren’s-syndrome-related antigen A/B antibodies (SSA (Ro)/B(La)), smith antibody (sm), anti-ribonucleoprotein antibodies (anti-RNP)] (Nikolopoulos et al., 2020) and antiphospholipid syndrome (APL antibodies) (Aneja et al., 2020). In anti-thyroid autoimmunity associated with psychiatric symptoms, antibodies against thyroid peroxidase (TPO) and thyreoglobulin (TG) can be detected in serum (Barbero et al., 2019; Barbuti et al., 2017).
6. Autoimmune encephalitis with psychiatric symptomatology
According to Graus et al. (2016), autoimmune encephalitis is a conglomerate of subacute symptoms ranging from seizures, memory disturbances to psychiatric abnormalities in addition to proof of an encephalitis entailing signal alterations in temporal MRI, a pleocytosis in CSF and/or temporal abnormalities in electroencephalography (EEG) (slowing or epileptic potentials). Our literature review revealed disorientation, confusion, memory impairment, behavioral dysfunction, psychosis, mood dysfunction, anxiety, catatonia, and sleep dysfunction as the main psychiatric symptoms in autoimmune encephalitis associated with antibodies against membrane surface and intracellular antigens (Bien et al., 2019; Boronat et al., 2013; Dalmau et al., 2008; Do et al., 2017; Finke et al., 2012; Höftberger et al., 2015; Si et al., 2019; Spatola et al., 2018; Tsukamato et al., 1993). Autoantibody-negative limbic encephalitis is a further clinical entity of autoimmune-mediated psychiatric symptomatology that comprises autoimmune encephalitis lacking proof of known autoantibodies in CSF or serum (Graus et al., 2018; von Rhein et al., 2017).
7. Psychiatric symptoms and syndromes associated with autoantibodies
Psychiatric symptoms may appear even though autoimmune encephalitis criteria have not been met. Our focus in this review is to characterize the spectrum, but not the mechanisms of psychiatric symptoms that might be based on autoimmunity. Below, we outline the main results (Table 1, proposed simplified clinical pathway see Fig. 1, main psychiatric symptoms and syndromes see Fig. 2) of our literature research encompassing each psychiatric disease category and symptoms.
Table 1.
Psychiatric disorders and symptoms associated with autoantibodies in adults.
| DISORDERS/SYMTPOMS | ABS ASSCOCIATED | REFERENCES |
|---|---|---|
| PSYCHIATRIC DISORDERS | ||
| A. CSF | ||
| Psychosis | CV2/CRMP5, HuD, NMDAR,VGKC, Yo | Endres et al., 2015, Endres et al., 2020b |
| B. Serum | ||
| First Episode Psychosis, Psychosis, Post partum Psychosis, induced Psychosis, Schizophrenia | α7nAChR, AMPAR, ANA, APL, CASPR2, CLβ2GPI, CV2, dsDNA, GABAAR, GAD65, HuD, LGI1, Ma1/2, MOG, NMDAR, Recoverin, P (2 B, 2, 5), RNP, sm, SOX1,SSA/,SSB, P (2 B, 2, 5), RNP, sm, SOX1,SSA/,SSB, Synapsin, TPO, TG,VGKC, Yo, Zic4 | Ando et al., 2016; Barbero et al., 2019; Chandle et al., 2009; Dahm et al., 2014; Endres et al., 2020b; Grain et al., 2017; Jézéquel et al., 2017b, Lennox et al., 2017; Mantere et al., 2018; Nikolopoulos et al., 2020, Oviedo-Salcedo et al., 2018; Saether et al., 2017, 2019; Steiner et al., 2013; Shiwaku et al., 2020; Tanaka et al., 2003; Tong et al., 2019; Yarlagadda et al., 2008; Zandian et al., 2017; Zandi et al., 2011 |
| Bipolar disorder/Mania | ATPA, AMPAR, CASPR2, GAD65, NMDAR, Ma1, TPO, TG, VGKC | Barbuti et al., 2017; Endres et al., 2020b; Padmos et al., 2004; Schou et al., 2016; Steiner et al., 2013 |
| Depressive disorder | CASPR2, CRHM1, GAD65, HuD, NMDAR, SOX1, Synapsin | Endres et al., 2020b; Saether et al., 2019; Schou et al., 2016, 2018; Steiner et al., 2013; Tanaka et al., 2003 |
| Obsessive-Compulsive disorder | ABGA | Nicholson et al., 2012; Pearlman et al., 2014 |
| ADHD | PC | Haukanes et al., 2015. Mazon-Cabrera et al., 2019 |
| CASPR2, CRMP1/2, 5-HTR, NAFP, GFAP | ||
| Autism spectrum disorder | GAD65, GM | Mazon-Cabrera et al. (2019) |
| Gilles de la Tourette syndrome | ABGA | Martino et al. (2008) |
| Eating disorder | Ghrelin, α-MSH | Fetissov et al., 2005; Terashi et al., 2011 |
| Addiction | Glutamate, GABA | Vetrile et al. (2015) |
| PSYCHIATRIC SYMPTOMS | ||
| A. CSF | ||
| Psychosis as syndrome (see A) | ||
| Sleep dysfunction | IgLON5 | Honorat et al. (2017) |
| B.Serum | ||
| Psychosis as syndrome (see A) | ||
| Mood dysfunction | ANA, AMPAR, APL, CASPR2, CLβ2GPI, dsDNA, GABAAR, GAD65, NMDAR, RNP, sm, SSA/SSB, VGKC | Ando et al., 2016; Samra et al., 2019; Nikolopoulos et al., 2020; Schou et al., 2016, 2018; Somers et al., 2011 |
| Cognitive dysfunction | ARHGAP26, AMPAR, CASPR2, CL, GABAAR, Hu, LGI1, MOG, NMDAR, PRE-GLRA1 | Bartels et al., 2020; Escudero et al., 2017; Kanapathy et al., 2019; Pettingill et al., 2015; Somers et al., 2011 |
| Catatonia | GABAAR, NMDAR | Ando et al. (2016) |
| Anxiety | APL, GABAAR, GAD65, NMDAR, VGKC | Ando et al., 2016; Henningsen and Meinck, 2003; Pettingill et al., 2015; Somers et al., 2011; Sadetski et al., 2018 |
| Orientation, confusion | AMPAR, CASPR2, GABABR, LGI1, NMDAR, VGKC, GABAAR, DR1, lysoganglioside | Escudero et al., 2017; Somers et al., 2011; Pettingill et al., 2015; Cox et al., 2015 |
| Behavioral dysfunction | CASPR2, GAD65, GABAAR, NMDAR | Pettingill et al., 2015; Schou et al., 2018 |
| Sleep dysfunction | IgLON5 | Honorat et al. (2017) |
Abbreviations: ABGA = anti basal ganglia antibodies, ADHD = attention deficit hyperactive disorder, ARHGAP26 = Rho GTPase-activating protein 26, α-MSH = alpha melanocyte-stimulating hormone, AMPAR = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, ANA = antinuclear antibodies, ATPA = H/K adenosine triphosphate, CASPR2 = contactin-associated protein 2, CL = cardiolipin, β2GPI = β2 glycoprotein, CRHM1 = cholinergic receptor muscarinic 1, CRMP1/2 = collapsing response mediator protein 1/2, CV2/CRMP5 = cronveinten 2/collapsin response mediator protein 5, dsDNA = double strand DNA, DPPX = dipeptidyl-peptidase-like-protein 6, GABAA/BR = Gamma-aminobutyric acid A/B receptor, GAD65 = glutamic acid decarboxylase 65, GM1 = ganglioside-monosialic acid, LGI1 = leucine-rich glioma inactivated 1, MOG = myelin oligodendrocytic glycoprotein, NAFP = human neuron axon filament protein, NMDAR = N-methyl-D-aspartate receptor, PAGE (2, 2 B, E5) = P antigen (2, 2 B, E5), PC = Purkinje cell, PRE-GLRA1 = pre glycine receptor alpha 1, RNP = ribonucleoprotein, sm = smith antibody, SSA/B = Sjögren’s-syndrome-related antigen A/B, VGKC = voltage gated potassium channel, 5-HTR = serotonin receptor.
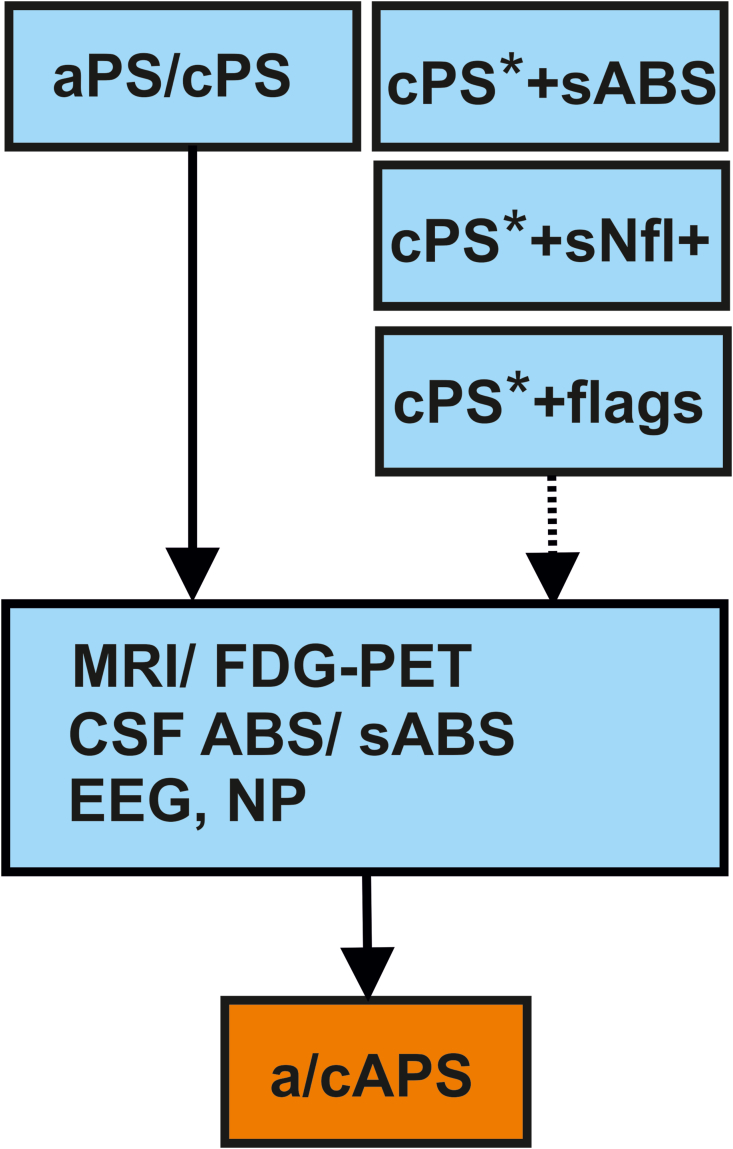
Fig. 1.
Clinical pathway for diagnostics of autoantibody-associated psychiatric symptoms and syndromes in adults.
Fig. 1 demonstrates a simplified clinical pathway. Consider in particular subacute (aPS) or subchronic (cPS) psychiatric syndrome with the following suspected diagnosis subgroups [ = psychosis and schizophrenia spectrum disorders, mood disorders, minor and major neurocognitive impairment, obsessive-compulsive disorder, autism spectrum disorders, attention deficit hyperactivity disorder, anxiety disorders, eating disorders and addiction] and one symptom of the following symptom cluster [# = altered conciousness, disorientation, memory dysfunction, mood dysfunction, psychosis, catatonia, anxiety, obsessive-compulsive, behavioral abnormalities (autism, hyperkinetic, impulsive) and sleep dysfunction]. If prior diagnostics are already performed in cPS (cPS∗), serum antibodies (sABS) or serum elevations of neurofilaments (sNfl) or flags justify additional diagnostics (EEG, MRI/FDG-PET or CSF).
Abbreviations: ABS = auto-antibodies, a/c APS = subacute or subchronic autoimmune psychiatric syndrome, a/c PS = subacute or subchronic psychiatric syndrome, EEG = electroencephalography, NP = neuropsychological testing, FDG-PET = fluorodesoxyglucose positron emissions tomography, MRI = magnetic resonance imaging. ∗means cPS with prior diagnostics (EEG, MRI, CSF). cPS∗ + sABS/sNfl+/flags = cPS with prior diagnostics and presence of serum ABS/or serum Nfl elevations or flags.
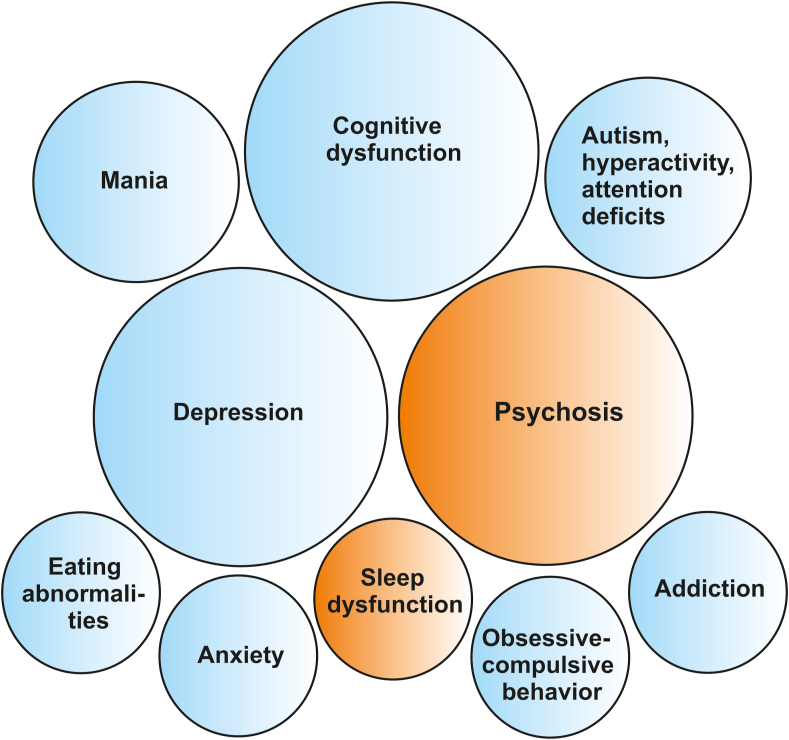
Fig. 2.
Main psychiatric symptoms and syndromes relevant for screening of autoantibodies.
Blue colour indicates associated serum antibodies, whereas the orange colour implies associated serum and/or CSF antibodies. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
7.1. Cognitive dysfunction - minor and major cognitive impairment
Memory loss in conjunction with autoantibodies is often reported in patients with dementia-like or amnestic syndrome associated with major cognitive impairment and dementia. Serum AMPAR, NMDAR, GABAR, CASPR2, IgLON5 and LGI1 antibodies are known to be associated with memory loss in patients aged over 60 years without an autoimmune-encephalitis diagnosis. Furthermore, CASPR2 and IgLON5 antibodies in those patients are associated with a cognitive dysfunction of progressive nature (Escudero et al., 2017). Memory impairment is reported mainly in patients presenting membrane-surface antibodies such as AMPAR, CASPR2, GABAAR, LGI1, myelin oligodendrocyte glycoprotein (MOG), NMDAR, pre glycine receptor alpha 1 (pre-GLRA1) and Rho GTPase-activating protein 26 (ARHGAP26) serum antibodies (Bartels et al., 2020; Escudero et al., 2017; Pettingill et al., 2015; Somers et al., 2011) (Table 1). Minor or mild cognitive impairment is a symptom often reported in patients with systemic lupus erythematosus associated with cardiolipin (CL) immunoglobulin M (IgM) antibodies (Kanapathy et al., 2019). Our findings reveal that memory decline is a frequently observed phenomenon in psychiatric patients presenting with associated membrane-surface autoantibodies, although their biological significance remains unclear and yields low to moderate evidence for underlying autoimmunity (see Table 1, Fig. 2).
7.2. Schizophrenia spectrum disorders and psychosis
In presumed psychosis and schizophrenia spectrum disorders associated with psychotic symptoms, various antibodies in serum have been reported: α7nAChR (alpha 7 subunit nicotinergic acethylcholine receptor), CV2/CRMP5-, CRHM1 (cholinergic receptor, muscarinergic 1), DPPX, GABAAR, HuD, LGI1, NMDAR, Recoverin, Synapsin and VGKC (Chandley et al., 2009; Dahm et al., 2014; Endres et al., 2015; Grain et al., 2017; Lennox et al., 2017; Mantere et al., 2018; Oviedo-Salcedo et al., 2018; Sæther et al., 2017, 2019; Shiwaku et al., 2020, Steiner et al., 2013;Tanaka et al., 2003; Tong et al., 2019; Yarlagadda, 2008; Zandi et al., 2011; Zandian et al., 2017) (Table 1). A much smaller group of CSF antibodies (CV2/CRMP5, DPPX, Hu, NMDAR, VGKC and Yo-antibodies, Table 1) is known to be associated with psychotic disorders (Endres et al., 2015) suggesting a high probability of this disorder’s CNS autoimmune origin (Table 1, Table 2). NMDAR antibodies have frequently been detected in schizophrenic patients (Tong et al., 2019; Steiner et al., 2013). There is research evidence that psychotic symptoms are associated with the levels of antibodies in patients compared to controls, ie, those of NMDAR (Tong et al., 2019) or GAD65 antibodies (Yarlagadda et al., 2008). Assessing antibodies, neuroimaging and CSF diagnostics are all relevant to diagnose a probable or definitive autoimmune-mediated psychosis (Pollack et al., 2020). EEG and MRI are important diagnostic tools in these patients, as two-thirds of all patients in large 180-patient cohort with psychotic disorders had pathological MRIs and EEGs. However, an autoimmune psychosis was not diagnosed in all of those patients with EEG or MRI abnormalities (Endres et al., 2015). If CSF abnormalities are analyzed in detail, not only CSF pleocytosis, but also intrathecal IgG synthesis (e.g. oligoclonal bands) should be considered as relevant indicators for a CNS inflammation in patients presenting with severe psychiatric syndromes. This observation is strengthened by recent data demonstrating that intrathecal IgG synthesis (IgG synthesis by using Reiber graphs, elevated IgG index, oligoclonal bands) is quite often present in patients exhibiting psychotic symptoms and with detectable autoantibodies (Blinder and Lewerenz, 2019; Endres et al., 2015; Melkersson et al., 2018). We thus recommend incorporating these parameters in the diagnostic procedure to determine an autoimmune origin for psychiatric symptoms (Fig. 1, Table 2).
Table 2.
Definitions of autoimmune based psychiatric syndrome.
| A. POSSIBLE AUTOIMMUNE PSYCHIATRIC SYNDROME |
| a) a/cAPS: |
|
| b) a/cPS with one of the following items: |
|
| B. PROBABLE AUTOIMMUNE PSYCHIATRIC SYNDROME |
| a) a/cPS with one the following nine items: |
|
| b) a/cPS with one of the following items: |
|
| C. DEFINITIVE AUTOIMMUNE PSYCHIATRIC SYNDROME |
|
Abbreviations:a/cAPS = subacute or subchronic autoimmune based psychiatric syndrome, a/cPS = subacute or subchronic psychiatric syndrome, DD = differential diagnosis, EEG = electroencephalography, IgG = immunoglobulin G, MRI = magnetic resonance imaging, Nfl = neurofilament light chain.
The proposed definitions have been developed in consideration of the criteria for autoimmune psychosis from Pollak (2020) and autoimmune encephalitis from Graus (2016).
A current hypothesis for inducing psychosis proposes (Jézéquel et al., 2017a) a glutamatergic hypofunction resulting from altered NMDAR transmission due to NMDAR autoantibodies. Furthermore, altered dopaminergic transmission could be caused by NMDAR-antibodies altering the dopamine D1-receptor and trafficking on the membrane surface (Grea et al., 2019). These assumptions are based on animal data describing glutamatergic hypofunction and dopaminergic hyperfunction reinforcing the “glutamate” (Kim et al., 1980) and “dopamine” hypotheses (Meltzer and Stahl, 1976) that describe the origin of psychotic (positive) symptoms in schizophrenia. The crucial role of NMDAR antibodies in subgroups of schizophrenic patients are supported by the frequently reported observation of associated NMDAR antibodies in patients suffering their initial episode of psychosis (Lennox et al., 2017; Jézéquel et al., 2017b). This potential relationship is further supported by the correlation between the antibody serum status and psychotic and catatonic symptoms in the acute psychotic phase (Lennox et al., 2017), unless the mechanisms are incompletely understood.
TPO antibodies seem to correlate with symptom expression in psychotic patients in addition to NMDAR (Barbero et al., 2019). Nevertheless, the mechanistic foundation of these findings is unclear. Besides NMDAR-dependent hypofunction, a cholinergic deficit driven by autoantibody-mediated effects might also contribute to psychotic symptoms, as anti-muscarinic acetylcholine-receptor antibodies have been found in subgroups of schizophrenic patients (for review see Ryan et al., 2019). Psychotic symptoms are also frequently observed as the neuropsychiatric manifestation of an underlying systemic lupus erythematosus (SLE) associated with diverse autoantibodies such as antinuclear antibodies (ANA), anti-APL, anti-dsDNA, anti-CL/β2-GPI complex, anti-RNP, anti-sm and/or anti-SSA (Ro)/SSB(La) or in Hashimoto’s encephalopathy (Nikolopoulos et al., 2020; Menon et al., 2011). Furthermore, in antiphospholipid antibody syndrome, psychosis with acoustic hallucinations and/or delusions is the predominant psychiatric manifestation and responsive to treatment (Hallab et al., 2017).
7.3. Mood dysfunction
7.3.1. Depressive syndrome
Depressive disorders are known to be associated with neuronal serum antibodies to a lesser extent than psychotic disorders (Schou et al., 2016). Depressive symptoms were reported in a large cohort to be associated with neuronal serum antibodies against CASPR2, GAD65 and NMDAR (Schou, 2018). Serum AMPAR, CASPR2, GABAAR, NMDAR and VGKC antibodies have often (Ando et al., 2016; Schou et al., 2016; Somers et al., 2011) been associated with depressive symptoms. NMDAR-antibody encephalitis can also present with unipolar depressive episodes (Al-Diwani et al., 2019). Likewise, neuropsychiatric systemic lupus erythematosus and Hashimoto’s encephalopathy might present with depressive symptoms accompanied by the typical antibody spectrum (Table 1) (Menon et al., 2011; Nikolopoulos et al., 2020). The autoantibody spectrum in serum is mildly distinct when patients with diagnosed depressive disorders are examined [CRHM1 (CRHM1 = cholinergic receptor, muscarinergic 1), CASPR2, GAD65, NMDAR and Synapsin antibodies] (Schou et al., 2016, 2018; Steiner et al., 2013; Tanaka et al., 2003; Saether et al., 2019). The diversity and frequency of serum antibodies associated with depressive symptoms suggest depressive symptoms as a major component of antibody-associated psychiatric symptomatology.
However, there are no studies to date involving cohorts of patients with depressive disorders and systematic CSF. Thus, the significance of autoantibodies for subgroups of depression remains vague and requires further investigation.
7.3.2. Depressive and manic syndrome
Bipolar affective disorder (BD) patients suffer from alternating mood states encompassing a gradual spectrum over time ranging from depression to mania. Patients with bipolar disorder frequently present with antibodies against AMPAR, ATPA (H/K Adenosin Triphosphat), CASPR2, GAD65, NMDAR and VGKC complex in serum (Padmos et al., 2004; Schou et al., 2016; Steiner et al., 2013). The serum antibody frequency seems to resemble those with psychotic disorders, as GAD65 antibodies have been reported in 11–12% of patients with BD compared to 3–6% in controls (Padmos et al., 2004), indicating that BD is a disease accompanied relatively often by serum autoantibodies like psychosis in comparison with other psychiatric diseases. Moreover, in addition to GAD65 antibodies, patients with suspected BD should be screened for systemic inflammatory disorder antibodies such as thyroid autoantibodies because they are an independent risk factor for BD (Barbuti et al., 2017). In particular, the rapid cycling BD subtype is associated with thyroidperoxidase-antibodies (Snijders et al., 2019). Serum levels of antibodies such as NMDAR antibodies also play a key role in the development of an acute manic, untreated mood state (Ferensztajn-Rochowiak et al., 2019). Surprisingly, mania-like symptoms such as euphoria may often be the first symptom in patients with NMDAR encephalitis, as indicated in a recent study by Restrepo-Martinez (2019), thus screening for NMDAR-antibodies is clinically relevant in patients presenting an acute onset of mania combined with a psychopathology characterized by a strong sense of delight and a lack of inhibition. This study further highlights the importance of obtaining CSF in patients presenting acute manic syndrome (Fig. 1). As in individuals suffering from depression, the role of autoantibodies in those affected by bipolar disorder is unclear, as CSF antibodies have not been detected in a small study and other systematic studies are missing (Endres et al., 2016).
7.3.3. Anxiety symptoms
Serum antibody-distribution identified in patients with diverse anxiety symptoms comprises APL, GABAR, GAD65, NMDAR antibodies (Ando et al., 2016; Henningsen and Meinck, 2003; Pettingill et al., 2015; Somers et al., 2011) (Table 1). In addition, the symptom anxiety is apparently associated with GABAA receptor serum antibodies (Table 1, Pettingil et al., 2015). The anxiety symptomatology could be explained by alterations in the GABAergic inhibitory neurotransmission due to CSF, but not serum GABAAR antibodies. Although less frequent than depressive or psychotic symptoms, serum antibodies have also been detected in patients with anxiety symptoms and disorders (Table 1).
Of note, anxiety is a widespread psychiatric symptom in patients with VGKC complex autoimmunity and Hashimoto’s encephalopathy (Somers et al., 2011; Menon et al., 2011). CSF antibodies have not been studied in anxiety disorders so far.
7.3.4. Phobic symptoms
Phobias are characterized by anxiety to specific objects or situations, and are rarely associated with autoantibodies. However, some autoimmune disorders such as comorbid autoimmune disorders like GAD65 antibody-positive stiff person syndrome often have a phobic phenotype - usually a task-specific phobia (Henningsen and Meinck, 2003). Although the mechanism is not fully understood, in passive man-to-rat transfer experiments, we attributed GAD65 antibody-mediated anxiety to immunoglobulin deposits in the basolateral amygdala (Hansen et al., 2013). In addition to intracellularly-located antibodies, plasma membrane-bound APL antibodies are interesting to look for in phobic patients also, as they have been identified in phobic patients (Sadetski et al., 2018). Screening for autoantibodies should be considered in phobic patients presenting with a degree of phobia corresponding to a psychiatric syndrome coinciding with red or yellow flags (Table 2, Table 3).
Table 3.
Important clinical features as indicators for autoimmune involvement in adults.
| RED FLAGS |
| Aphasia, mutism, or dysarthria+ |
| Autonomic disturbance |
| Central hypoventilation |
| Decreased level of consciousness+ |
| Epileptic Seizures+ |
| Faciobrachial dystonic seizures |
| Focal neurological disease |
| Hyponatraemia (not explained by medication) |
| Infectious prodrome with fever+ |
| Movement disorder (eg, catatonia, hypo-or hyperkinetic movements)+ |
| New-onset severe headache or clinically significant change in headache pattern Adverse response to antipsychotics or antidepressants or other psychopharmacologic drugs+ |
| Optic hallucinations+ |
| Other autoimmune disorders |
| Paresthesia |
| Presence of a tumor, or history of a recent tumor+ |
| Presence of neuroleptic malignant syndrome |
| Severe otherwise not explained cognitive dysfunction+ |
| YELLOW FLAGS |
| Confusion |
| Dynamic course |
| Early resistance to therapy |
| Fluctuating psychopathology |
| Psychomotor symptoms |
Red and yellow flags are modified from Herken and Prüss (2017); DGPPN e.V. (Hrsg.) 2019, Pollak et al. (2020). Symbol: + = item classifies into probable subacute or subchronic autoimmune based psychiatric syndrome.
7.4. Obsessive-compulsive symptoms
Obsessive-compulsive disorder (OCD) is characterized by compulsory actions and/or thoughts. Neuronal serum antibodies such as dopamine D1 receptor (DR1) antibodies (Cox et al., 2015), lysoganglioside antibodies (Cox et al., 2015) and GABAAR (Pettingil et al., 2015) antibodies are associated with OCD symptoms in affected patients. Purkinje cells (PC) CSF antibodies would suggest a dysfunction in dopaminergic synaptic transmission within the cortico-striatal-thalamic-cortical circuit known to be affected in OCD (Xing et al., 2020). According to a review of 844 patients from case-control studies, OCD was diagnosed more frequently in those patients with serum anti-basal ganglia antibodies (ABGA) than in those without those antibodies (Pearlman et al., 2014). In another study of 96 patients with OCD, ABGA antibodies were detected in 20% of the patients versus 4% of controls (Nicholson, 2012) suggesting possible neuroinflammatory reactions within the basal ganglia system; the cortico-striatal-thalamic-cortical circuit plays a relevant role in inducing OCD symptoms in patients when other signs of CNS inflammation coincide. Studies on the prevalence of antibodies in CSF of OCD patients are lacking in adults.
7.5. Behavioral abnormalities
7.5.1. Autism spectrum disorder
Autism spectrum disorders (ASD) encompass a condition of deficits in social skills in conjunction with repetitive sensory-motor behaviors (Lord et al., 2018). In patients with ASD, autoantibodies against diverse antigens have been identified such as serotonin 5 (5-HT) receptor, human neuron axon filament protein (NAFP), glial fibrillic acid protein (GFAP), GAD65 and ganglioside-monosialic acid (GM1) antibodies (Mazon-Cabrera et al., 2019). This supports the heterogeneity of autism’s spectrum immunity mechanisms that underlie a specific immune mechanism responsible for symptom induction. Furthermore, other serum autoantibodies such as CASPR2, CRMP1 (collapsin response mediator protein 1), and CRMP2 (collapsin response mediator protein 2) have been identified (Mazon-Cabrera et al., 2019) in both autistic patients and the mothers of ASD patients – a finding that supports a potential transfer of maternal antibodies during birth. It is conceivable that these autoantibodies might have an impact on brain development. Serum GAD65 antibodies were detected in 15% of 20 patients with autism. ASD serum immunoreactivity with PC in mouse cerebellum might support the cerebellum as a crucial structure for ASD immunopathology, as shown in functional neuroimaging studies (Rout et al., 2012).
7.5.2. Attention deficit hyperactivity disorder
Attention deficit hyperactivity disorder is characterized by hyperactivity, impulsivity, and attentional deficits. In 21% of 48 patients with ADHD, serum PC-antibodies (Haukanes et al., 2015) possibly associated with these patients’ clinical features (ie, hyperactivity and impulse control deficits) and may be further evidence of CNS inflammation. Studies on the prevalence of antibodies in CSF of ADHD patients are lacking in adults.
7.5.3. Gilles de la Tourette syndrome
Gilles de la Tourette syndrome (TS) is a disorder characterized by combined vocal or motor tics. Oligoclonal bands were detected in the CSF of 5% of 20 patients (Baumgärtel et al., 2019) suggestive of CNS inflammation in a minority of Gilles de la Tourette syndrome patients. Serum ABGA (Martino, 2008), but no other neuronal antibodies (Baumgärtel et al., 2019) have been identified so far in TS. However, no CSF ABGA antibodies have yet been proven. As a possible pathophysiological basis of TS, neuroinflammation in the cortico-striatal-thalamic-cortical circuit similar to that diagnosed in OCD patients is conceivable - indicating this circuit as a common pathogenic pathway in subtypes of these disorders.
7.6. Eating abnormalities
Patients with anorexia nervosa and bulimia nervosa reveal abnormal eating behavior, and they present evidence of increased IgM antibodies levels against alpha melanocyte-stimulating hormone (α-MSH) compared to controls (Fetissov et al., 2005). These IgM α-MSH antibodies correlate with the main clinical psychopathological features of these eating disorders (Fettissov et al., 2005), supporting potentially acute immune involvement of the melanocortin system in generating the psychopathological features of eating disorders. Further studies are required to gain deeper insights into the immunopathology of these disorders. Other autoantibodies in patients with anorexia nervosa might indicate renutrition in patients suffering acute weight loss, such as IgM autoantibodies against acylated ghrelin (Terashi et al., 2011). Studies on the prevalence of antibodies in serum/CSF of patients with eating disorders are lacking in adults.
7.7. Addictive symptoms
There is limited evidence for autoimmunity in subgroups of addicted patients, as only serum autoantibodies against glutamate and GABA were detected in a large series of 129 opium-addicted patients (Vetrile et al., 2015).
7.8. Sleep dysfunction
Sleep dysfunction incorporates insomnia and in particular parasomnia, a disorder characterized by abnormal behaviors or movements during rapid eye movement (REM) or non-REM sleep. These disorders and sleep apnea are known to be closely associated with serum and CSF IgLON5 antibodies (Honorat et al., 2017). How IgLON5 antibodies are associated with sleep disorders is incompletely understood, but we suspect that the thalamus or formatio reticularis are targets of IgLON5 antibodies interfering with sleep-relevant rhythmic generators. IgLON5 antibodies should be screened in patients suffering from severe sleep dysfunction (Table 3). Interestingly, severe insomnia is also a cardinal symptom of Morvan syndrome and thus often present in patients with VGKC complex autoimmunity (Cornelius et al., 2011).
8. Proposal of diagnostic approach
8.1. Clinical spectrum of psychiatric symptoms
Although most studies and clinical pathways have focused on psychosis so far, our literature review underlines the broad variability of psychiatric disorders and symptoms that are implicated in autoantibody-mediated disorders. Thus, we suggest that serum and CSF autoantibodies should be assessed in all patients presenting aPS [subacute (≤3 months) psychiatric syndrome] or cPS [subchronic (≥3 months) psychiatric syndrome] with no prior diagnostic tests (EEG, MRI, CSF) in conjunction with CSF analysis, EEG and MRI (Fig. 1, Table 2). For cPS including prior to diagnostic tests, additional red or yellow flags should be present to warrant a serum autoantibody investigation (Fig. 1, Table 2, Table 3). We recommend autoantibody screening in the patient groups we depict (patients need to be given detailed facts and information, and should provide informed consent prior to diagnostic lumbar puncture, see ethical aspects) so that the clinician can decipher the psychiatric syndromes associated with autoantibodies (assuming a potential pathogenic role of these autoantibodies in psychiatric symptomatology). This presumption is based on the aforementioned studies and the known cause-effect human autoantibody-to-rodent transfer investigations (Rauschenberger et al., 2020; Jurek et al., 2019; Haselmann et al., 2018; Petit Pedrol et al., 2018; Planaguma et al., 2015; Hansen et al., 2013; Geis et al., 2012). According to our literature review findings and the above-mentioned associations between psychiatric symptoms and serum autoantibodies, we suggest to screen for antibodies in serum or CSF. Antibody screening is advisable when an a/cPS is in agreement with one of the following assumed diagnoses, e.g., psychosis and schizophrenia spectrum disorders, mood disorders, minor and major cognitive impairment, obsessive-compulsive disorder, autism spectrum disorders (ASD), attention deficit hyperactivity disorder (ADHD), anxiety disorders, eating disorders and addiction or symptom clusters such as an altered consciousness, disorientation, memory dysfunction, obsessive-compulsive, psychosis, catatonia, mood dysfunction, anxiety, behavioral abnormalities (autism, hyperkinetic) and sleep are present (Fig. 1, Fig. 2, Table 2, Table 3). The CSF analysis should include cell count, lactate, and the complete CSF protein chemistry including blood–CSF–barrier function analysis (albumin quotient, total protein) and evaluation of intrathecal synthesis of immunoglobulins (IgA, IgG, IgM) according to quantitative measurement and Reiber calculation as well as a qualitative demonstration of the intrathecal IgG synthesis (oligoclonal bands).
8.2. Autoantibody panel
We suggest to test for the following antibody panel comprising the following serum antibodies: (1) paraneoplastic antibodies such as anti-Amiphiphysin, -CV2/CRMP5, -Hu, -Ma1/Ma2/Ta, -Ri, -Yo, -SOX1,-Tr/-DNER, -Zic4, (2) anti-GAD65 antibodies and (3) antibodies against membrane-surface antigens such as anti-AMPAR-, -CASPR2,- DPPX,- GABAA/BR, -LGI1, -NMDAR, -VGKC and (4) the serum antibodies TPO, TG, TRAK and ANA. Furthermore, if the patient agrees to CSF analysis (see ethical aspects), we advise testing CSF antibodies in groups (2) and (3) (Fig. 1). Furthermore, we recommend testing for additional antibodies [Serum + CSF: ABGA, H/K adenosine triphosphate (ATPA), αChR7, CRHM1, glia fibrillic acid protein (GFAP), IgLON5, lysoganglioside (LG), mGluR5, neurexin3α and synapsin; serum: ghrelin, α-MSH, CRMP1/2, NAFP or PC] if standard panel values are negative (Table 1, Fig. 1). If the analysis of serum antibodies reveals ANA antibodies, further specific tests for systemic inflammatory disorders should be done [APL, complement factors, dsDNA antibodies and extractable nuclear antigen (ENA)]. Furthermore, if certain clinical features such as lupus erythematosus with a depressive or psychotic appearance is present (Table 1), the following serum autoantibodies could be searched for (anti-CLß2GPI, anti-sm, anti-RNP, anti-SSA (Ro)/SSB(La)).
For CSF or serum-antibody testing in a/cPS patients, we recommend using cell-based assays (CBA) for antibodies against membrane-surface antigens, immunoblots (IB) to test for paraneoplastic antibodies by recombinant target antigens, on test stripes and enzyme-linked immunosorbent assays (ELISA) combined with absorption-spectrometry to detect GAD65 antibodies. These testing procedures should be followed by testing tissue-based systems via indirect immunofluorescence and immunohistochemistry to confirm previous specific antibodies (Schwenkenbecher et al., 2016) or to detect hints for novel antibodies such as unknown Purkinje cell antibodies (Schwenkenbecher et al., 2018). These methods can help us differentiate between neuropil and non-neuropil antibodies observed as a reaction by the serum/CSF from patients to cerebellar or hippocampal monkey, rat or mouse brain tissue. Non-neuropil antibodies may indicate paraneoplastic antibodies. Thus, we recommend tumor screening in those patients (Fig. 1). If neuropil antibodies are detected, further antibody tests for antibodies against membrane-surface antigens in CSF should be undertaken [groups (2) + (3)]. If non-neuropil antibodies are positive in serum, testing should be considered for non-neuropil antibodies in the CSF too. If serum antibodies are present in patients with cPS with prior diagnostics, further diagnostics are essential, such as CSF, EEG and MRI (Fig. 1).
8.3. Clinical indicators of possible autoimmunity
Additional clinical and laboratory features (called red and yellow flags (Table 3) by Herken and Prüss (2017) and others (Pollak et al., 2020; DGPPN e.V. 2019) may help to identify patients with autoimmune encephalitis or those with a subacute/subchronic autoimmune based psychiatric syndrome (a/cAPS). Specific red flags (one of 9 items: Table 2, Table 3) mean a possible a/cAPS, leading to more elaborate diagnostics via EEG, MRI and CSF in patients with cPS with prior performed diagnostics (EEG, MRI, CSF) (Fig. 1, Table 2). 9 red flags have been identified as crucial for an autoimmune involvement. To identify these 9 red flags, the 6 red flags critical for autoimmune psychosis have been selected according to Pollak et al. (2020) (tumor, movement disorder, seizures, adverse response to psychopharmacologic drugs, severe cognitive dysfunction, altered consciousness level), and an additional three novel red flags have been supplemented suggesting a possible organic cause such as (1) aphasia, dysarthria, (2) optic hallucinations or (3) infectious prodrome with fever. However, autonomic dysfunction has not served as a red flag of high relevance as there might be substantial differential diagnostic overlap with anxiety disorders. If the serum reveals no antibodies, but yellow flags are present, more specific testing is called for in patients with a/cPS (Fig. 1). However, if no antibodies and no yellow flags are present, markers of neuronal degeneration such neurofilament light chains (Nfl) (see neurodegenerative markers) should be investigated at different time points. Transient elevated serum Nfl not attributable to other factors such as a CNS disorder should necessitate EEG, MRI and CSF examinations in cPS with prior diagnostics (EEG, MRI, CSF) (Fig. 1). If Nfl is further increasing a differential diagnosis such as a neurodegenerative disorder has to be considered. In addition to antibodies’ testing in patients with a/cPS, routine laboratory diagnostics covering common infections should be carried out.
8.4. Cerebrospinal fluid
CSF pleocytosis is a crucial criterion for probable or definitive autoimmune encephalitis (Graus et al., 2016). Accumulating evidence has shown that such pleocytosis varies widely among autoimmune encephalitis subsyndromes such as AMPAR-, GABAB-, DPPX- and the NMDAR-positive encephalitis often associated with pleocytosis, whereas other antibodies such as CASPR2-, GlyR-, IgLON5-antibody-positive encephalitis appear often less frequently in conjunction with pleocytosis (Blinder and Lewerenz, 2019). Thus, other CSF indices revealing a potential CNS inflammation such as an intrathecal IgG synthesis (oligoclonal bands) are often present in AMPAR-, GABABR-, GAD65-, NMDAR-positive encephalitides, but seldom in GABAAR-, GlycinR-, IgLON5- and LGI1-positive encephalitis (Blinder and Lewerenz, 2019). Thus, intrathecal IgG synthesis should be integrated in the diagnostic protocol for aPS and cPS patients as depicted recently to be important to differentiate neuropsychiatric syndromes (Schwenkenbecher et al., 2016).
8.5. EEG
We recommend searching for different EEG patterns to support autoimmunity in a/cPS. The classical EEG pattern typically found in autoimmune encephalitis patients (such as focal slowing or epileptic potentials, or specific patterns such as generalized rhythmic delta activity called extreme delta brush associated with NMDA receptor-positive encephalitis (Graus et al., 2016) might also be present in patients presenting isolated symptoms supporting an autoimmune origin. To identify further grapho-elements possibly indicating functional disturbances in specific or various brain regions, EEG recordings should be screened in a/cPS patients for the EEG patterns found occasionally in autoimmune encephalitis such as frontal irregular delta activity in EEG (Baysal Kirac et al., 2016) (Table 2).
8.6. Neuroimaging
Neuroimaging is essential to providing a basis for an autoimmune-mediated origin of psychiatric symptoms. In patients with a probable a/cPS, multisequence brain MRI should be performed including the following pulse sequences [similar to GENERATE MRI recommendations, e.g. axial FLAIR (fluid attenuated reversion recovery), optional 3D-FLAIR, FLAIR coronal hippocampus, coronal T2-weighted coronar, axial DWI (diffusion weighted imaging) axial, axial T2∗-weighted or susceptibility-weighted imaging (SWI), T1-weighted (preferably 3D) before and after the i. v. administration of gadolinium-based contrast media. Typical limbic-encephalitis findings are the high signal-intensity or volume changes in the mesiotemporal region typically found in AMPAR-, CASPR2-, GABAB- and LGI1-positive encephalitis (Kelley et al., 2017). Unilateral temporal-lobe signal abnormalities should already be regarded as a substantial hint to prove a probable or definitive autoimmune mediated a/cPS if no autoimmune encephalitis criteria are fulfilled. If D2R- or GABAAR-antibodies are present, we recommend screening for hyperintense lesions outside the limbic system such as in D2R- and GABAAR-positive encephalitis (Kelley et al., 2017). If GlycinR-, IgLON5-, Neurexin-3 antibodies are detected, no MRI abnormalities are likely, as shown in encephalitis patients with these antibodies (Gresa-Arribas et al., 2016; Honorat et al., 2017; Piquet et al., 2019). If brain MRI is negative in a/cPS patients, 18 F-FDG-PET should be done to detect brain abnormalities. Similar to cases of autoimmune encephalitis, one can expect to observe lobar hypometabolism in FDG-PET exams (following negative MRI results). Overall, neuroimaging is a crucial step for precise diagnosis in psychiatric patients with an illness of suspected autoimmune origin.
8.7. Neurodegeneration markers
Nfl are cytoskeleton proteins and markers of axonal degeneration within the CNS. Recent studies indicate that Nfl in PB and CSF are elevated compared to controls in the acute, but not the chronic phase of NMDA receptor-positive encephalitis (Constantinescu et al., 2016; Mariotto et al., 2019) - indicating Nfl as a transient marker of neuroaxonal degeneration. This transient Nfl elevation might function in future studies as a marker indicating an organic origin of psychiatric symptoms. Furthermore, the Nfl in peripheral blood correlate closely with CSF Nfl (Hendricks et al., 2019) indicating blood Nfl as a promising biomarker for unspecific microstructural brain damage in patients with a/cPS. Furthermore, Nfl should be determined in serum, as studies indicate that symptoms due to frontotemporal dementia are accompanied by higher Nfl compared with various psychiatric syndromes (Katisko et al., 2020; Al Shweiki et al., 2019). These studies indicate that Nfl might be a biomarker for distinguishing between a psychiatric disorder with neuronal damage versus psychiatric symptoms without brain damage. Tau-protein in CSF could likewise be used to identify neuroaxonal degeneration, as total tau-protein (T-tau) is known to be elevated in the acute phase (dropping over time) in patients with autoimmune encephalitis (Constantinescu et al., 2016). In conclusion: serum Nfl and CSF T-tau should be investigated in those PSa/c patients without PB antibodies and no red or yellow flags in order to seek some indication of unspecific brain damage to prove the organic basis of symptoms. It would be useful diagnostically to differentiate organic (eg: autoimmune-mediated psychosis) from psychiatric disorders not associated with brain damage (eg: schizophrenia).
9. Synopsis: autoimmune-mediated psychiatric symptoms and syndromes
Taken together, we suggest that isolated psychiatric features might be based on autoimmunity provided the criteria below are fulfilled (see Table 2). To diagnose possible autoimmune-mediated psychiatric symptoms, a/cPS must be accompanied by one of the nine red flags (Table 2, Table 3; Fig. 1). If additional features are diagnosed via special diagnostics (such as a pleocytosis or intrathecal IgG synthesis) in the CSF, MRI T2 or FLAIR abnormalities suggestive of encephalitis, EEG focal epileptic potentials or slowing, as well as serum antibodies), a probable a/cAPS can be assumed (Fig. 1). Moreover, T-tau or Nfl changes over time might be a critical factor and potential marker of neuroaxonal degeneration that might enable a possible-to-probable a/cAPS diagnosis. If the psychiatric symptomatology is accompanied by IgG antibodies in the CSF in probable a/cAPS, you can assume it is a definitive a/cAPS (Table 2). If a probable or definitive a/cAPS is detected, our latest knowledge suggests immunotherapy for such patients after a tumor screen in line with autoimmune-encephalitis guidelines (Rössling and Prüss, 2020; Graus et al., 2016) or autoimmune psychosis (Pollak et al., 2020; Endres et al., 2020a) for which immunotherapy is known to be effective. As immunotherapeutic options in autoantibody-associated syndromes are beyond the scope of this review, we only summarize the main facts on immunotherapy as an individual experimental treatment trial for patients. In short, the main evidence level for such potential immunotherapy is moderate, as there have been no randomized, placebo-controlled trials on autoantibody-associated psychiatric syndromes, but retrospective cohort studies do exist. However, the evidence level is moderate to high for autoimmune encephalitis and autoimmune psychosis, as two randomized placebo-controlled trials with small cohorts exist for LG1-positive encephalitis (Dubey et al., 2020) with an immunoglobulin effect in 75% of patients and involving antibody-positive psychosis [study of immunotherapy in antibody-positive psychosis: feasibility and acceptability (SINAPPS1)] with a relevant reduction of psychotic symptoms (Lennox et al., 2017). Observational, retrospective studies indicate that immunotherapy is beneficial in NMDAR-positive psychosis in 43–80% (Zandi et al., 2014; Scott et al., 2018) or in autoimmune dementia in 64% of patients (Flanagan et al., 2010). A recent systematic review supports these results described an immunotherapeutic effect in 94% of 145 patients with autoantibody-associated psychiatric syndromes (Endres et al., 2020c).
10. Ethical aspects
CSF analysis is optional in patients with psychiatric symptoms other than acute psychotic syndromes or unexplained sleep dysfunction, as the evidence level for the autoimmune mediation of these symptoms is low to moderate. However, we recommend CSF analysis provided the patient agrees and had given written consent after a going through a thorough consent procedure and consultation clarifying its major benefits and risks. Furthermore, immunotherapy in autoimmune-based psychiatric syndrome is an optional therapy whose evidence level is low to moderate (evidence level IV through case control and cohort studies). Thus, the patient should be informed and agree to undergo the treatment on an individual therapeutic trial basis.
10.1. Limitations
The limitations of our narrative review concern the small sample size of the majority of the studies we discuss. Furthermore, case-control studies are often not performed, so that the significance of autoantibodies in psychiatric disease cannot be determined. A few case-control studies exist that show more autoantibodies in patients (0–9%) than in controls (0–4.9%) (Lennox et al., 2017; Saether et al., 2017). Further investigations with control subjects, a deep phenotyping of patients and longitudinal investigations are needed to thoroughly elucidate the role of autoantibodies in psychiatric patients. As mentioned earlier, the evidence level of the presented studies is often low to moderate as mostly case series are depicted. Another issue is that most of the studies presented only depict an association or correlation between autoantibodies and psychiatric symptoms. Only a few cause-effect studies in rodents exist reporting on the transfer of human autoantibodies to animals (Rauschenberger et al., 2020; Jurek et al., 2019; Haselmann et al., 2018; Petit Pedrol et al., 2018; Planaguma et al., 2015; Geis et al., 2012) emphasizing the potential pathogenic role of autoantibodies in generating psychiatric symptoms. In addition, note that we do not postulate that psychiatric disorders are autoimmune in nature, but demonstrate evidence that it is worthwhile selecting psychiatric patients for autoantibody screening, and to investigate systematically the role of autoantibodies over a broad range of psychiatric syndromes so that we may better understand the function of autoantibodies in psychiatric disease. Autoantibodies against synaptic proteins are often diagnosed in conjunction with many psychiatric syndromes, such as GAD65, GABAR and NMDAR (Table 1). It is questionable how these autoantibodies might play a pathogenic role in such a wide range of psychiatric symptoms. To answer the question how autoantibodies mechanistically have an impact on psychiatric phenotype, more studies have to be conducted to investigate in greater detail the distribution of autoantibodies and their subtypes, the occurrence of blood brain-barrier dysfunction, the coexistence of other autoantibodies, and the amount and accumulation of autoantibodies in strategic structures as potential contributing factors to phenotypology.
11. Conclusions
We present a diagnostic algorithm for specific subgroups of patients presenting psychiatric symptoms that might be based on CNS inflammation directed toward self-antigens. Our literature review revealed a broad spectrum of psychiatric symptoms to be associated with different antibodies. Thus, not only psychosis but other acute psychiatric syndromes in general warrants for a thorough immunological differential diagnosis. Nevertheless, caution has to be taken when causally linking these symptoms to detected antibodies, especially in serum, due to the lack of systematic studies.
CSF antibodies have been found in a few patients with psychosis and sleep dysfunction. CSF antibodies have not been detected or searched for in other subgroups of patients with psychiatric symptoms and disorders, ie, those with anxiety, depression, bipolar mood dysfunction, behavioral or eating abnormalities and addiction. The pathogenic role of associated serum antibodies remains unclear in those patients, when no other diagnostic signs of possible CNS inflammation have been detected. More investigations of this unknown factor are warranted to enable us to better guide our patients to adequate therapy.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowlegdements
Prof. Dr. J. Wiltfang is supported by an Ilídio Pinho professorship, iBiMED (UIDB/04501/2020) at the University of Aveiro, Portugal.
References
- Al Shweiki M.R., Steinacker P., Oeckl P., Hengerer B., Danek A., Fassbender K. MR Neurofilament light chain as a blood biomarker to differentiate psychiatric disorders from behavioural variant frontotemporal dementia. J. Psychol. Res. 2019;113:137–140. doi: 10.1016/j.jpsychires.2019.03.019. [DOI] [PubMed] [Google Scholar]
- Al-Diwani A., Pollak T.A., Langford A.E., Lennox B.R. Synaptic and neuronal autoantibody-associated psychiatric syndromes: controversies and hypotheses. Front. Psychol. 2017;8:13. doi: 10.3389/fpsyt.2017.00013. https://doi:10.3389/fpsyt.2017.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Diwani A., Handel A., Townsend L., Pollak T., Leite M.I., Harrison P.J. The psychopathology of NMDAR-antibody encephalitis in adults: a systematic review and phenotypic analysis of individual patient data. Lancet Psychiatr. 2019;6:235–246. doi: 10.1016/S2215-0366(19)30001-X. https://doi:10.1016/S2215-0366(19)30001-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando Y., Shimazaki H., Shiota K., Tetsuka S., Nakao K., Shimada T. Prevalence of elevated serum anti-N-methyl-D-aspartate receptor antibody titers in patients presenting exclusively with psychiatric symptoms: a comparative follow-up study. BMC Psychiatr. 2016;16:226. doi: 10.1186/s12888-016-0948-9. https://doi:10.1186/s12888-016-0948-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aneja J., Kuppili P.P., Paul K., Panda S., Purohit A. Antiphospholipid syndromes presenting as treatment resistant bipolar disorder and thrombocytopenia in a young male. J. Neuroimmunol. 2020;343:577238. doi: 10.1016/j.jneuroim.2020.577238. [DOI] [PubMed] [Google Scholar]
- Barbero J.D., Palacín A., Serra P., Solé M., Ortega L., Cabezas Á. Association between anti-thyroid antibodies and negative symptoms in early psychosis. Early Interv Psych. 2019;14:470–475. doi: 10.1111/eip.12873. https://doi:10.1111/eip.12873 [DOI] [PubMed] [Google Scholar]
- Barbuti M., Carvalho A.F., Köhler C.A., Murru A., Verdolini N., Guiso G. Thyroid autoimmunity in bipolar disorder: a systematic review. J. Affect. Disord. 2017;221:97–106. doi: 10.1016/j.jad.2017.06.019. https://doi:10.1016/j.jad.2017.06.019 [DOI] [PubMed] [Google Scholar]
- Bartels F., Strönisch T., Farmer K., Rentzsch K., Kiecker F., Finke C. Neuronal autoantibodies associated with cognitive impairment in melanoma patients. Ann. Oncol. 2020;30:823–829. doi: 10.1093/annonc/mdz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgaertel C., Skripuletz T., Kronenberg J., Stangel M., Schwenkenbecher P., Sinke C. Immunity in Gilles de la Tourette-Syndrome: results from a cerebrospinal fluid study. Front. Neurol. 2019;10:732. doi: 10.3389/fneur.2019.00732. https://doi:10.3389/fneur.2019.00732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysal-Kirac L., Tuzun E., Altindag E., Ekizoglu E., Kinay D., Bilgic B. Are there any specific EEG findings in autoimmune epilepsies? Clin. EEG Neurosci. 2016;47:224–234. doi: 10.1177/1550059415595907. https://doi:10.1177/1550059415595907 [DOI] [PubMed] [Google Scholar]
- Bien C.G., Vincent A., Barnett M.H., Becker A.J., Blümcke I., Graus F. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135:1622–1638. doi: 10.1093/brain/aws082. https://doi:10.1093/brain/aws082 [DOI] [PubMed] [Google Scholar]
- Bien C.I., Nehls F., Kollmar R., Weis M., Steinke W., Woermann F., Dalmau J. Identification of adenylate kinase 5 antibodies during routine diagnostics in a tissue-based assay: three new cases and a review of the literature. J. Neuroimmunol. 2019;334:576975. doi: 10.1016/j.jneuroim.2019.576975. https://doi:10.1016/j.jneuroim.2019.576975 [DOI] [PubMed] [Google Scholar]
- Blinder T., Lewerenz J. Cerebrospinal fluid findings in patients with autoimmune encephalitis- a systematic analysis. Front. Neurol. 2019;10:804. doi: 10.3389/fneur.2019.00804. https://doi:10.3389/fneur.2019.00804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronat A., Gelfand J.M., Gresa-Arribas N., Jeong H.Y., Walsh M., Roberts K. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann. Neurol. 2013;73:120–128. doi: 10.1002/ana.23756. https://doi:10.1002/ana.23756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandley M.J., Miller M.N., Kwasigroch C.N., Wilson T.D., Miller B.E. Increased antibodies for the alpha 7 subunit of the nicotinic receptor in schizophrenia. Schizophr. Res. 2009;109:98–101. doi: 10.1016/j.schres.2009.01.023. https://doi:10.1016/j.schres.2009.01.023 [DOI] [PubMed] [Google Scholar]
- Churilov L.P., Sobolevskaia P.A., Stroev Y.I. Thyroid gland and brain: enigma of Hashimoto’s encephalopathy. Best Pract. Res. Clin. Endocrinol. Metabol. 2019 doi: 10.1016/j.beem.2019.101364. https://doi:10.1016/j.beem.2019.101364 101364. [DOI] [PubMed] [Google Scholar]
- Constantinescu R., Krýsl D., Bergquist F., Andrén K., Malmeström C., Asztély F. Cerebrospinal fluid markers of neuronal and glial cell damage to monitor disease activity and predict long-term outcome in patients with autoimmune encephalitis. Eur. J. Neurol. 2016;23:796–806. doi: 10.1111/ene.12942. https://doi:10.1111/ene.12942 [DOI] [PubMed] [Google Scholar]
- Cornelius J.R., Pittcok S.J., McKeon A., Lennon V.A., Aston P.A., Josephs K.A. Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch. Neurol. 2011;68:733–738. doi: 10.1001/archneurol.2011.106. https://doi:10.1001/archneurol.2011.106 [DOI] [PubMed] [Google Scholar]
- Coutinho E., Jacobson L., Pedersen M.G., Benros M.E., Nørgaard-Pedersen B., Mortensen P.B. CASPR2 autoantibodies are raised during pregnancy in mothers of children with mental retardation and disorders of psychological development but not autism. J. Neurol. Neurosurg. Psychiatr. 2017;88:718–721. doi: 10.1136/jnnp-2016-315251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.J., Zuccolo A.J., Edwards E.V., Mascaro-Blanco A., Alvarez K., Stoner J., Chang K. Antineuronal antibodies in a heterogeneous group of youth and young adults with tics and obsessive-compulsive disorder. J. Child Adolesc. Psychopharmacol. 2015;25:76–85. doi: 10.1089/cap.2014.0048. https://doi:10.1089/cap.2014.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm L., Ott C., Steiner J., Stepniak B., Teegen B., Saschenbrecker S. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann. Neurol. 2014;76:82–94. doi: 10.1002/ana.24189. https://doi:10.1002/ana.24189 [DOI] [PubMed] [Google Scholar]
- Dalmau J., Graus F. Antibody-mediated encephalitis. N. Engl. J. Med. 2018;378:840–851. doi: 10.1056/NEJMra1708712. [DOI] [PubMed] [Google Scholar]
- Dalmau J., Gleichman A.J., Hughes E.G., Rossi J.E., Peng X., Lai M. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. https://doi:10.1016/S1474-4422(08)70224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DGPPN e.V. Langfassung; 2019. (Hrsg.) für die Leitliniengruppe: S3-Leitlinie Schizophrenie. Version 1.0, zuletzt geändert am 15. März 2019. [Google Scholar]
- Do L.D., Chanson E., Desestret V., Joubert B., Ducray F., Brugière S. Characteristics in limbic encephalitis with anti-adenylate kinase 5 autoantibodies. Neurology. 2017;88:514–524. doi: 10.1212/WNL.0000000000003586. https://doi:10.1212/WNL.0000000000003586 [DOI] [PubMed] [Google Scholar]
- Dubey D., Britton J., McKeon A., Gadoth A., Zekeridou A., Lopez Chiriboga S.A. Randomized placebo-controlled trial of intravenous immunoglobulin in autoimmune LGI1/CASPR2 epilepsy. Ann. Neurol. 2020;87:313–323. doi: 10.1002/ana.25655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D., Perlov E., Baumgartner A., Hottenrott T., Dersch R., Stich O. Immunological findings in psychotic syndromes: a tertiary care hospital’s CSF sample of 180 patients. Front. Hum. Neurosci. 2015;9:476. doi: 10.3389/fnhum.2015.00476. https://doi:10.3389/fnhum.2015.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D., Dersch R., Hottenrott T., Perlov E., Maier S. Alterations in cerebrospinal fluid in patients with bipolar syndromes. Front. Psychiatr. 2016;7:194. doi: 10.3389/fpsyt.2016.00194. https://doi:10.3389/fpsyt.2016.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D., Leypoldt F., Bechter K., Hasan A., Steiner J., Domschke K. Autoimmune encephalitis as a differential diagnosis of schizophreniform psychosis: clinical symptomatology, pathophysiology, diagnostic approach, and therapeutic considerations. Eur. Arch. Psychiatr. Clin. Neurosci. 2020 doi: 10.1007/s00406-020-01113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D., Meixensberger S., Dersch R., Feige B., Stich O., Venhoff N. Cerebrospinal fluid, antineuronal autoantibody, EEG, and MRI findings from 992 patients with schizophreniform and affective psychosis. Transl. Psychiatry. 2020;10:279. doi: 10.1038/s41398-020-00967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D., Maier V., Leypoldt F., Wandinger K.P., Lennox B., Pollak T.A. Autoantibody-associated psychiatric syndromes: a systematic literature review resulting in 145 cases. Psychol. Med. 2020;7:1–12. doi: 10.1017/S0033291720002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero D., Guasp M., Ariño H., Gaig C., Martínez-Hernández E., Dalmau J. Antibody-associated CNS syndromes without signs of inflammation in the elderly. Neurology. 2017;89:1471–1475. doi: 10.1212/WNL.0000000000004541. https://doi:10.1212/WNL.0000000000004541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferensztajn-Rochowiak E., Kaczmarek M., Wójcicka M., Kaufman-Szukalska E., Dziuda S., Remlinger-Molenda A. Glutamate-related antibodies and peripheral insulin-like growth factor in bipolar disorder and lithium prophylaxis. Neuropsychobiology. 2019;77:49–56. doi: 10.1159/000493740. https://doi:10.1159/000493740 [DOI] [PubMed] [Google Scholar]
- Fetissov S.O., Harro J., Jaanisk M., Järv A., Podar I., Allik J. Autoantibodies against neuropeptides are associated with psychological traits in eating disorders. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14865–14870. doi: 10.1073/pnas.0507204102. https://doi:10.1073/pnas.0507204102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke C., Kopp H.U., Prüss H., Dalmau J., Wandinger K.P., Ploner C.J. Cognitive deficits following anti-NMDA receptor encephalitis. J. Neurol. Neurosurg. Psychiatr. 2012;83:195–198. doi: 10.1136/jnnp-2011-300411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan E.P., McKeon A., Lennon V.A., Bradley F.B., Trennery M.R., Tan M.K. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin. Proc. 2010;85:881–897. doi: 10.4065/mcp.2010.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis C., Grünewald B., Weishaupt A., Wultsch T., Toyka K.V., Reif A. Human IgG directed against amphiphysin induces anxiety behaviour in a rat model after intrathecal passive transfer. J. Neural. Transm. 2012;119:981–985. doi: 10.1007/s00702-012-0773-3. [DOI] [PubMed] [Google Scholar]
- Grain R., Lally J., Stubbs B., Malik S., LeMince A., Nicholson T.R. Autoantibodies against voltage-gated potassium channel and glutamic acid decarboxylase in psychosis: a systematic review, meta-analysis, and case series. Psychiatr. Clin. Neurosci. 2017;71:678–689. doi: 10.1111/pcn.12543. https://doi:10.1111/pcn.12543 [DOI] [PubMed] [Google Scholar]
- Graus F., Titulaer M.J., Balu R., Benseler S., Bien C.G., Cellucci T. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. https://doi:10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus F., Escudero D., Oleaga L., Bruna J., Villarejo-Galende A., Ballabriga J. Syndrome and outcome of antibody-negative limbic encephalitis. Eur. J. Neurol. 2018;25:1011–1016. doi: 10.1111/ene.13661. https://doi:10.1111/ene.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gréa H., Bouchet D., Rogemond V., Hamdani N., Le Guen E., Tamouza R. Human autoantibodies against N-Methyl-D-Aspartate receptor modestly alter dopamine D1 receptor surface dynamics. Front. Psychiatr. 2019;10:670. doi: 10.3389/fpsyt.2019.00670. https://doi:10.3389/fpsyt.2019.00670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresa Arribas N. Human neurexin-3-alpha- antibodies associate with encephalitis and alter synapse development. Neurology. 2016;86:2235–2242. doi: 10.1212/WNL.0000000000002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gultekin S.H., Rosenfeld M.R., Voltz R., Eichen J., Posner J.B., Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2020;123:1481–1494. doi: 10.1093/brain/123.7.1481. [DOI] [PubMed] [Google Scholar]
- Hallab A., Naveed S., Altibi A., Abdelkhalek M., Ngo H.T., Le T.P. Vol. 8. 2017. pp. 261–267.https://doi:10.1016/j.genhosppsych.2017.11.005 (Association of psychosis with antiphospholipid antibody syndrome: A Systematic Review of Clinical Studies). [DOI] [PubMed] [Google Scholar]
- Hansen N., Grünewald B., Weishaupt A., Colaço M.N., Toyka K.V., Sommer C. Human Stiff person syndrome IgG-containing high-titer anti-GAD65 autoantibodies induce motor dysfunction in rats. Exp. Neurol. 2013;239:202–209. doi: 10.1016/j.expneurol.2012.10.013. https://doi:10.1016/j.expneurol.2012.10.013 [DOI] [PubMed] [Google Scholar]
- Hansen N., Widman G., Witt J.A., Wagner J., Becker A.J., Elger C.E. Seizure control and cognitive improvement via immunotherapy in late onset epilepsy patients with paraneoplastic versus GAD65 autoantibody-associated limbic encephalitis. Epilepsy Behav. 2016;65:18–24. doi: 10.1016/j.yebeh.2016. [DOI] [PubMed] [Google Scholar]
- Hansen N., Widman G., Stuff S., Becker A.J., Witt J.A., Ahmadzadehfar H. Cancer frequency detected by positron emission tomography-computed tomography in limbic encephalitis. Epilepsy Behav. 2018;89:105–111. doi: 10.1016/j.yebeh.2018.09.043. [DOI] [PubMed] [Google Scholar]
- Haselmann H., Mannara F., Werner C., Planagumà J., Miguez-Cabello F., Schmidl L. Human autoantibodies against the AMPA receptor subunit GluA2 induce receptor reorganization and memory dysfunction. Neuron. 2018;100:91–105. doi: 10.1016/j.neuron.2018.07.048. [DOI] [PubMed] [Google Scholar]
- Haukanes B.I., Hegvik T.A., Eichler T., Haavik J., Vedeler C. Paraneoplastic syndrome-associated neuronal antibodies in adult ADHD. J. Neuroimmunol. 2015;288:87–91. doi: 10.1016/j.jneuroim.2015.08.018. https://doi:10.1016/j.jneuroim.2015.08.018 [DOI] [PubMed] [Google Scholar]
- Hendricks R., Baker D., Brumm J., Davancaze T., Harp C., Herman A. Establishment of neurofilament light chain simoa assay in cerebrospinal fluid and blood. Bioanalysis. 2019;11:1405–1418. doi: 10.4155/bio-2019-0163. https://doi:10.4155/bio-2019-0163 [DOI] [PubMed] [Google Scholar]
- Henningsen P., Meinck H.M. Specific phobia is a frequent non-motor feature in stiff man syndrome. J. Neurol. Neurosurg. Psychiatr. 2003;74 doi: 10.1136/jnnp.74.4.462. https://doi:10.1136/jnnp.74.4.462 462-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herken J., Prüss H. Red flags: clinical signs for identifying autoimmune encephalitis in psychiatric patients. Front. Psychiatr. 2017;8:25. doi: 10.3389/fpsyt.2017.00025. https://doi:10.3389/fpsyt.2017.00025.%20eCollection%202017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höftberger R., van Sonderen A., Leypoldt F., Houghton D., Geschwind M., Gelfand J. Encephalitis and AMPA receptor antibodies: novel findings in a case series of 22 patients. Neurology. 2015;84:2403–2412. doi: 10.1212/WNL.0000000000001682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honorat J.A., Komorowski L., Josephs K.A., Fechner K., St Louis E.K. IgLON5 antibody: neurological accompaniments and outcomes in 20 patients. Neurol Neuroimmunol Neuroinflamm. 2017;4:e385. doi: 10.1212/NXI.0000000000000385. https://doi:10.1212/NXI.0000000000000385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jézéquel J., Johansson E.M., Dupuis J.P., Rogemond V., Gréa H., Kellermayer B. Dynamic disorganization of synaptic NMDA receptors triggered by autoantibodies from psychotic patients. Nat. Commun. 2017;8:1791. doi: 10.1038/s41467-017-01700-3. https://doi:10.1038/s41467-017-01700-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jézéquel J., Rogemond V., Pollak T., Lepleux M., Jacobson L., Gréa H. Cell- and single molecule-based methods to detect anti-N-Methyl-D-Aspartate receptor autoantibodies in patients with first-episode psychosis from the OPTiMiSE project. Biol. Psychiatr. 2017;82:766–772. doi: 10.1016/j.biopsych.2017.06.015. https://doi:10.1016/j.biopsych.2017.06.015 [DOI] [PubMed] [Google Scholar]
- Jurek B., Chayka M., Kreye J., Lang K., Kraus L., Fidzinski P. Human gestational N-methyl-d-aspartate receptor autoantibodies impair neonatal murine brain function. Ann. Neurol. 2019;86:656–670. doi: 10.1002/ana.25552. [DOI] [PubMed] [Google Scholar]
- Kanapathy A., Nik Jaafar N.R., Shaharir S.S., Chan L.F., Rozita M., Ch’ng S.S. Prevalence of cognitive impairment using the Montreal Cognitive Assessment questionnaire among patients with systemic lupus erythematosus: a cross-sectional study at two tertiary centres in Malaysia. Lupus. 2019;28:854–861. doi: 10.1177/0961203319852153. https://doi:10.1177/0961203319852153 [DOI] [PubMed] [Google Scholar]
- Katisko K., Cajanus A., Jääskeläinen O., Kontkanen A., Hartikainen P., Korhonen V.E. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J. Neurol. 2020;267:162–167. doi: 10.1007/s00415-019-09567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley B.P., Patel S.C., Marin H.L., Corrigan J.J., Mitsias P.D., Griffith B. Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am. J. Neuroradiol. 2017;38:1070–1078. doi: 10.3174/ajnr.A5086. https://doi:10.3174/ajnr.A5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.S., Kornhuber H.H., Schmid-Burgk W., Holzmüller B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci. Lett. 1980;20:379–382. doi: 10.1016/0304-3940(80)90178-0. https://doi:10.1016/0304-3940(80)90178-0 [DOI] [PubMed] [Google Scholar]
- Lennox B.R., Palmer-Cooper E.C., Pollak T., Hainsworth J., Marks J., Jacobson L. Prevalence and clinical characteristics of serum neuronal cell-surface antibodies in first-episode psychosis: a case control study. Lancet Psychiatry. 2017;4:42–48. doi: 10.1016/S2215-0366(16)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Elsabbagh M., Baird G., Veenstra-Vanderweele J. Autism spectrum disorder. Lancet. 2018;392:508–520. doi: 10.1016/S0140-6736(18)31129-2. https://doi:10.1016/S0140-6736(18)31129-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malviya M., Barman S., Golombeck K.S., Planagumà J., Mannara F., Strutz-Seebohm N. NMDAR encephalitis: passive transfer from man to mouse by a recombinant antibody ann. Clin. Transl. Neurol. 2017;4:768–783. doi: 10.1002/acn3.444. https://doi:10.1002/acn3.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantere O., Saarela M., Kieseppä T., Raij T., Mäntylä T., Lindgren M. Anti-neuronal anti-bodies in patients with early psychosis. Schizophr. Res. 2018;192:404–407. doi: 10.1016/j.schres.2017.04.027. https://doi:10.1016/j.schres.2017.04.027 [DOI] [PubMed] [Google Scholar]
- Mariotto S., Gajofatto A., Zuliani L., Zoccarato M., Gastaldi M., Franciotta D. Serum and CSF neurofilament light chain levels in antibody-mediated encephalitis. J. Neurol. 2019;266:1643–1648. doi: 10.1007/s00415-019-09306-z. https://doi:10.1007/s00415-019-09306-z [DOI] [PubMed] [Google Scholar]
- Martino D., Draganski B., Cavanna A., Church A., Defazio G., Robertson M.M. Anti-basal ganglia antibodies and Tourette’s syndrome: a voxel-based morphometry and diffusion tensor imaging study in an adult population. J. Neurol. Neurosurg. Psychiatr. 2008;79:820–822. doi: 10.1136/jnnp.2007.136689. https://doi:10.1136/jnnp.2007.136689 [DOI] [PubMed] [Google Scholar]
- Maxeiner H.G., Rojewski M.T., Tumani T., Herzog S., Fuchs D., Schmitt A. Immunological and histochemical analyses of cerebrospinal fluid and peripheral blood from patients with neurological and psychiatric disorders. Acta Neuropsychiatr. 2009;21:51–57. doi: 10.1017/S0924270800032737. [DOI] [PubMed] [Google Scholar]
- Mazón-Cabrera R., Vandormael P., Somers V. Antigenic targets of patient and maternal autoantibodies in autism spectrum disorder. Front. Immunol. 2019;10:1474. doi: 10.3389/fimmu.2019.01474. https://doi:10.3389/fimmu.2019.01474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkersson K., Bensing S. Signs of impaired blood-brain barrier function and lower IgG synthesis within the central nervous system in patients with schizophrenia or related psychosis, compared to that in controls. Neuroendocrinol. Lett. 2018;39:33–42. PMID: 29803205. [PubMed] [Google Scholar]
- Meltzer H.Y., Stahl S.M. The dopamine hypothesis of schizophrenia: a review. Schizophr. Bull. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. https://doi:10.1093/schbul/2.1.19 [DOI] [PubMed] [Google Scholar]
- Menon V., Subramanian K., Thamzih J.S. Psychiatric presentations heralding hashimoto’s encephalopathy: a systematic review and analysis of cases reported in literature. J. Neurosci. Rural Pract. 2011;68:733–738. doi: 10.4103/jnrp.jnrp_440_16. https://doi:10.4103/jnrp.jnrp_440_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson T.R., Ferdinando S., Krishnaiah R.B., Anhoury S., Lennox B.R., Mataix-Cols D. Prevalence of anti-basal ganglia antibodies in adult obsessive-compulsive disorder: cross-sectional study. Br. J. Psychiatr. 2012;200:381–386. doi: 10.1192/bjp.bp.111.092007. https://doi:10.1192/bjp.bp.111.092007 [DOI] [PubMed] [Google Scholar]
- Nikolopoulos D., Kostopoulou M., Pieta A., Karageorgas T., Tseronis D., Chavatza K. Evolving phenotype of systemic lupus erythematosus in Caucasians: low incidence of lupus nephritis, high burden of neuropsychiatric disease and increased rates of late-onset lupus in the ’Attikon’ cohort. Lupus. 2020;29:514–520. doi: 10.1177/0961203320908932. https://doi:10.1177/0961203320908932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovska-Waast S., Köhler-Forsberg O., Brix S.W., Nordentoft M., Kondziella D., Krogh J., Benros M.E. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: a systematic review and meta-analysis. Mol. Psychiatr. 2019;24:869–887. doi: 10.1038/s41380-018-0220-4. https://doi:10.1038/s41380-018-0220-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo-Salcedo T., de Witte L., Kümpfel T., Kahn R.S., Falkai P., Eichhorn P. Absence of cerebrospinal fluid antineuronal antibodies in schizophrenia spectrum disorders. Br. J. Psychiatry. 2018;212:318–320. doi: 10.1192/bjp.2018.24. https://doi:10.1192/bjp.2018.24 [DOI] [PubMed] [Google Scholar]
- Padmos R.C., Bekris L., Knijff E.M., Tiemeier H., Kupka R.W., Cohen D. A high prevalence of organ-specific autoimmunity in patients with bipolar disorder. Biol. Psychiatr. 2004;56:476–482. doi: 10.1016/j.biopsych.2004.07.003. https://doi:10.1016/j.biopsych.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Pearlman D.M., Vora H.S., Marquis B.G., Najjar S., Dudley L.A. Anti-basal ganglia antibodies in primary obsessive-compulsive disorder: systematic review and meta-analysis. Br. J. Psychiatry. 2014;205:8–16. doi: 10.1192/bjp.bp.113.137018. https://doi:10.1192/bjp.bp.113.137018 [DOI] [PubMed] [Google Scholar]
- Petit-Pedrol M., Sell J., Planagumà J., Mannara F., Radosevic M., Haselmann H. LGI1 antibodies alter Kv1.1. and AMPA receptors changing synaptic excitability, plasticity and memory. Brain. 2018;141:3144–3159. doi: 10.1093/brain/awy253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettingill P., Kramer H.B., Coebergh J.A., Pettingill R., Maxwell S., Nibber A. Antibodies to GABAA receptor α1 and γ2 subunits: clinical and serologic characterization. Neurology. 2015;84:1233–1241. doi: 10.1212/WNL.0000000000001326. https://doi:10.1212/WNL.0000000000001326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquet A.L., Khan M., Warner J.E.A., Wicklund M.P., Bennett J.L., Leehey M.A. Novel clinical features of glycine receptor antibody syndrome: a series of 17 cases. Neurol Neuroimmunol Neuroinflamm. 2019;6 doi: 10.1212/NXI.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planaguma J., Leypoldt F., Mannara F., Gutiérrez-Cuesta J., Martín-García E., Aguilar E. Human N-methyl D aspartate receptor antibodies alter memory and behaviour in mice. Brain. 2015;138:94–109. doi: 10.1093/brain/awu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak T.A., Lennox B.R., Müller S., Benros M.E., Prüss H., Tebartz van Elst L. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7:93–108. doi: 10.1016/S2215-0366(19)30290-1. [DOI] [PubMed] [Google Scholar]
- Pouget J.G., Schizophrenia Working Group of the Psychiatric Genomics Consortium Cross-disorder analysis of schizophrenia and 19 immune-mediated diseases identifies shared genetic risk. Hum. Mol. Genet. 2019;28:3498–3513. doi: 10.1093/hmg/ddz145. https://doi:10.1093/hmg/ddz145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschenberger V., von Wardenburg N., Schaefer N., Ogino K., Hirata H., Lillesaar C. Glycine receptor autoantibodies impair receptor function and induce motor dysfunction. Ann. Neurol. 2020 doi: 10.1002/ana.25832. [DOI] [PubMed] [Google Scholar]
- Ripke S., Neale B.M., Corvin A., Walters J.T., Farh K.H., Holmans P.A., Lee P., Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. https://doi:10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.P., Pollak T.A., Blackman G., David A.S. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6:620–630. doi: 10.1016/S2215-0366(19)30190-7. https://doi:10.1016/S2215-0366(19)30190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössling R., Prüss H. Vol. 2. 2020. pp. 1–7. (SOP: antibody-associated autoimmune encephalitis. Neurological research and practice). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout U.K., Mungan N.K., Dhossche D.M. Presence of GAD65 autoantibodies in the serum of children with autism or ADHD. Eur. Child Adolesc. Psychiatr. 2012;21:141–147. doi: 10.1007/s00787-012-0245-1. https://doi:10.1007/s00787-012-0245-1 [DOI] [PubMed] [Google Scholar]
- Ryan A.E., Mowry B.J., Kesby J.P., Scott J.G., Greer J.M. Is there a role for antibodies targeting muscarinic acetylcholinereceptors in the pathogenesis of schizophrenia? Aust. N. Z. J. Psychiatr. https://doi:10.1177/0004867419864438 53, 1059-1069. [DOI] [PubMed]
- Sadetski M., Tourinho Moretto M.L., Correia de Araujo R.P., de Carvalho J.F. Frequency of psychological alterations in primary antiphospholipid syndrome: preliminary study. Lupus. 2018;27:837–840. doi: 10.1177/0961203317751063. https://doi:10.1177/0961203317751063 [DOI] [PubMed] [Google Scholar]
- Samra K., Rogers J., Mahdi-Rogers M., Biba Stanton B. Catatonia with GABA A receptor antibodies pract. Neurol. 2019;20:139–143. doi: 10.1136/practneurol-2019-002388. https://doi:10.1136/practneurol-2019-002388 [DOI] [PubMed] [Google Scholar]
- Sæther S.G., Schou M., Stoecker W., Teegen B., Borowski K., Vaaler A. Onconeural antibodies in acute psychiatric inpatient care. J. Neuropsychiatry Clin. Neurosci. 2017;29:74–76. doi: 10.1176/appi.neuropsych.16050110. https://doi:10.1176/appi.neuropsych.16050110 [DOI] [PubMed] [Google Scholar]
- Sæther S.G., Vaaler A., Evjenth A., Aune T., Höltje M., Ruprecht K. Subtle phenotype differences in psychiatric patients with and without serum immunoglobulin G antibodies to synapsin. Front. Psychiatr. 2019;401 doi: 10.3389/fpsyt.2019.00401. https://doi:10.3389/fpsyt.2019.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou M., Sæther S.G., Borowski K., Teegen B., Kondziella D., Stoecker W. Prevalence of serum anti-neuronal autoantibodies in patients admitted to acute psychiatric care. Psychol. Med. 2016;46:3303–3313. doi: 10.1017/S0033291716002038. https://doi:10.1017/S0033291716002038 [DOI] [PubMed] [Google Scholar]
- Schou M.B., Sæther S.G., Drange O.K., Krane-Gartiser K., Reitan S.K., Vaaler A.E. The significance of anti-neuronal antibodies for acute psychiatric disorders: a retrospective case-controlled study. BMC Neurosci. 2018;19:68. doi: 10.1186/s12868-018-0471-7. https://doi:10.1186/s12868-018-0471-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenkenbecher P., Chacko L.P., Wurster U., Pars K., Pul R., Sühs K.W. Intrathecal synthesis of anti-Hu antibodies distinguishes patients with paraneoplastic peripheral neuropathy and encephalitis. BMC Neurol. 2016;16:136. doi: 10.1186/s12883-016-0657-5. https://doi:10.1186/s12883-016-0657-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenkenbecher P., Chacko L., Pul R., Sühs K.W., Wegner F., Wurster U. Paraneoplastic cerebellar syndromes associated with antibodies against Purkinje cells. Int. J. Neurosci. 2018;128:721–728. doi: 10.1080/00207454.2017.1412967. https://doi:10.1080/00207454.2017.1412967 [DOI] [PubMed] [Google Scholar]
- Scott J.G., Gilles J., Ryan A.E., Hargovan H., Gundarpi N., McKeon G., Hatherill S. The prevalence and treatment outcomes of antineuronal antibody-positive patients admitted with first episode of psychosis. BJPsych Open. 2018;4:69–74. doi: 10.1192/bjo.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiwaku H. Detection of autoantibodies against GABAARalpha1 in patients with schizophrenia. Schizophr. Res. 2020;216:543–546. doi: 10.1016/j.schres.2019.10.007. [DOI] [PubMed] [Google Scholar]
- Si Z., Wang A., Liu J., Zhang Z., Hu K. Typical clinical and imaging manifestations of encephalitis with anti-γ-aminobutyric acid B receptor antibodies: clinical experience and a literature review. Neurol. Sci. 2019;40:769–777. doi: 10.1007/s10072-018-3679-5. https://doi:10.1007/s10072-018-3679-5 [DOI] [PubMed] [Google Scholar]
- Snijders G.J.L.J., van Mierlo H.C., Boks M.P., Begemann M.J.H., Sutterland A.L., Litjens M. The association between antibodies to neurotropic pathogens and bipolar disorder : a study in the Dutch Bipolar (DB) Cohort and meta-analysis. Transl. Psychiatry. 2019;9:311. doi: 10.1038/s41398-019-0636-x. https://doi:10.1038/s41398-019-0636-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers K.J., Lennon V.A., Rundell J.R., Pittock S.J., Drubach D.A., Trenerry M.R. Psychiatric manifestations of voltage-gated potassium-channel complex autoimmunity. J. Neuropsychiatry Clin. Neurosci. 2011;23:425–433. doi: 10.1176/jnp.23.4.jnp425. https://doi:10.1176/jnp.23.4.jnp425 PMID: 22231314. [DOI] [PubMed] [Google Scholar]
- Spatola M., Sabater L., Planagumà J., Martínez-Hernandez E., Armangué T., Prüss H. Encephalitis with mGluR5 antibodies: symptoms and antibody effects. Neurology. 2018;90 doi: 10.1212/WNL.0000000000005614. https://doi:10.1212/WNL.0000000000005614 e1964-e1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J., Walter M., Glanz W., Sarnyai Z., Bernstein H.G., Vielhaber S. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry. 2013;70:271–278. doi: 10.1001/2013.jamapsychiatry.86. https://doi:10.1001/2013.jamapsychiatry.86 [DOI] [PubMed] [Google Scholar]
- Tamouza R., Fernell E., Eriksson M.A., Anderlid B.M., Manier C., Mariaselvam C.M., Boukouaci W., Leboyer M., Gillberg C. HLA polymorphism in regressive and non-regressive autism: a preliminary study. Autism Res. 2019;13(2):182–186. doi: 10.1002/aur.2217. https://doi:10.1002/aur.2217 [DOI] [PubMed] [Google Scholar]
- Tamouza R., Krishnamoorthy R., Giegling I., Leboyer M., Rujescu D. The HLA 8.1 Ancestral Haplotype in schizophrenia: dual implication in neuro-synaptic pruning and autoimmunity? Acta Psychiatr. Scand. 2019;141:169–171. doi: 10.1111/acps.13125. https://doi:10.1111/acps.13125 [DOI] [PubMed] [Google Scholar]
- Tanaka S., Matsunaga H., Kimura M., Tatsumi Ki, Hidaka Y., Takano T., Uema T., Takeda M., Amino N. Autoantibodies against four kinds of neurotransmitter receptors in psychiatric disorders. J. Neuroimmunol. 2003;141:155–164. doi: 10.1016/s0165-5728(03)00252-2. https://doi:10.1016/s0165-5728(03)00252-2 [DOI] [PubMed] [Google Scholar]
- Tanaka K. Significance of autoantibodies in autoimmune encephalitis in relation to antigen localization: an outline of frequently reported autoantibodies with a non-systematic review. Int. J. Mol. Sci. 2020 doi: 10.3390/ijms21144941. PMID: 32668637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashi M., Asakawa A., Harada T., Ushikai M., Coquerel Q., Sinno M.H. Ghrelin reactive autoantibodies in restrictive anorexia nervosa. Nutrition. 2011;27:407–413. doi: 10.1016/j.nut.2011.01.002. https://doi:10.1016/j.nut.2011.01.002.PMID:%2021392704 [DOI] [PubMed] [Google Scholar]
- Tong J., Huang J., Luo X., Chen S., Cui Y., An H., Xiu M. Elevated serum anti-NMDA receptor antibody levels in first-episode patients with schizophrenia. Brain Behav. Immun. 2019;81:213–219. doi: 10.1016/j.bbi.2019.06.017. https://doi:10.1016/j.bbi.2019.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T., Mochizuki R., Mochizuki H., Noguchi M., Kayama H., Hiwatashi M. Paraneoplastic cerebellar degeneration and limbic encephalitis in a patient with adenocarcinoma of the colon. J. Neurol. Neurosurg. Psychiatry. 1993;56:713–716. doi: 10.1136/jnnp.56.6.713. https://doi:10.1136/jnnp.56.6.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrile L.A., Fomina V.G., Nevidimova T.I., Vetlugina T.P., Batukhtina E.L., Savochkina E.L. Autoantibodies to glutamate and GABA in opiate addiction. Patol. Fiziol. Eksp. Ter. 2015:38–43. [PubMed] [Google Scholar]
- von Rhein B., Wagner J., Widman G., Malter M.P., Elger C.E., Helmstaedter C. Suspected antibody negative autoimmune limbic encephalitis: outcome of immunotherapy. Acta Neurol. Scand. 2017;135:134–141. doi: 10.1111/ane.12575. https://doi:10.1111/ane.12575 [DOI] [PubMed] [Google Scholar]
- Wang D., Hao Q., He L., Wang Q. LGI1 antibody and Psychosis. Australas. Psychiatr. 2018;26:612–614. doi: 10.1177/1039856218771513. [DOI] [PubMed] [Google Scholar]
- Wang A.K., Miller B.J. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr. Bull. 2018;13(44):75–83. doi: 10.1093/schbul/sbx035. https://doi:10.1093/schbul/sbx035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing X., Jin L., Li Q., Yang Q., Han H., Xu C., Wei Z. Modeling essential connections in obsessive-compulsive disorder patients using functional MRI. Brain Behav. 2020;10 doi: 10.1002/brb3.1499. https://doi:10.1002/brb3.1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarlagadda A., Helvink B., Chou C., Gladieux K., Keller A., Clayton A. Glutamic acid decarboxylase (GAD) antibodies in tardive dyskinesia (TD) as compared to patients with schizophrenia without TD and normal controls. Schizophr. Res. 2008;105:287–288. doi: 10.1016/j.schres.2008.06.019. https://doi:10.1016/j.schres.2008.06.019 [DOI] [PubMed] [Google Scholar]
- Zandi M.S., Irani S.R., Lang B., Waters P., Jones P.B., McKenna P., Coles A.J., Vincent A., Lennox B.R. Disease-relevant autoantibodies in first episode schizophrenia. J. Neurol. 2011;258:686–688. doi: 10.1007/s00415-010-5788-9. https://doi:10.1007/s00415-010-5788-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi M.S., Deakin J.B., Morris K., Buckley c., Jacobson L., Scoriels L. Immunotherapy for patients with acute psychosis and serum N-Methyl-D-aspartate receptor (NMDAR) antibodies: a description of a treated case series. Schizophr. Res. 2014;160:193–195. doi: 10.1016/j.schres.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Zandian A., Wingård L., Nilsson H., Sjöstedt E., Johansson D.X., Just D. Untargeted screening for novel autoantibodies with prognostic value in first-episode psychosis. Transl. Psychiatry. 2017;7:e1177. doi: 10.1038/tp.2017.160. https://doi:10.1038/tp.2017.160 [DOI] [PMC free article] [PubMed] [Google Scholar]