Abstract
Background
Inflammation plays an important role in postoperative cognitive dysfunction (POCD), particularly in elderly patients. Enteral enriched nutrition was shown to inhibit the response on inflammatory stimuli. Aim of the present study was to explore the therapeutic potential of enteral enriched nutrition in our rat model for POCD. The anticipated mechanism of action was examined in young rats, while responses in the target group of elderly patients were evaluated in old rats.
Methods
Male 3 and 23 months old Wistar rats received a bolus of enteral fat/protein-enriched nutrition 2 h and 30 min before surgery. The inflammatory response was evaluated by systemic inflammation markers and brain microglia activity. Additionally, in old rats, the role of the gut-brain axis was studied by microbiome analyses of faecal samples. Days 9–14 after surgery, rats were subjected to cognitive testing. Day 16, rats were sacrificed and brains were collected for immunohistochemistry.
Results
In young rats, enriched nutrition improved long-term spatial learning and memory in the Morris Water Maze, reduced plasma IL1-β and VEGF levels, but left microglia activity and neurogenesis unaffected. In contrast, in old rats, enriched nutrition improved short-term memory in the novel object- and novel location recognition tests, but impaired development of long-term memory in the Morris Water Maze. Systemic inflammation was not affected, but microglia activity seemed even increased. Gut integrity and microbiome were not affected.
Conclusion
Enteral enriched nutrition before surgery in young rats indeed reduced systemic inflammation and improved cognitive performance after surgery, whereas old rats showed a mixed favorable/unfavorable cognitive response, without effect on systemic inflammation. Anti-inflammatory effects of enriched nutrition were not reflected in decreased microglia activity. Neither was an important role for the gut-brain axis observed. Since the relatively straight forward effects of enriched nutrition in young rats could not be shown in old rats, as indicated by a mixed beneficial/detrimental cognitive outcome in the latter, caution is advised by translating effects seen in younger patients to older ones.
Keywords: Enriched nutrition, Postoperative cognitive dysfunction, Cognition, Inflammation, Neuroinflammation, Microbiome
Highlights
-
•
Enriched nutrition reduced inflammation after surgery in young rats.
-
•
Enriched nutrition improved postoperative cognitive outcome in young rats.
-
•
Enteral enriched nutrition did not inhibit neuroinflammation.
-
•
Effects in young rats do not predict effects in old rats.
-
•
Enteral enriched nutrition caused mixed improved/declined cognition in old rats.
1. Introduction
Postoperative cognitive dysfunction (POCD) has become recognized as a major complication of surgery, particularly in elderly patients. Extensive literature (Alam et al., 2018; Hovens et al., 2012; Plas et al., 2017; Skvarc et al., 2018) supports a major role for surgery-associated inflammatory responses in the pathophysiology of POCD. Interference with this inflammatory response has been indicated to improve cognitive outcome after surgery. Non-specific attenuation of innate immunity with minocycline and functional inhibition of interleukin (IL)-1β (IL-1 receptor antagonist or IL-1R−/− mice) prevented surgery-induced neuroinflammation and memory dysfunction in mice (Cibelli et al., 2010). Intracisternal administration of the IL6 antagonist tocilizumab at the time of surgery attenuated the inflammatory response and improved cognitive outcome in rats (Jiang et al., 2015). However, this has not led to effective therapeutic interventions in the clinic yet.
A rational approach for anti-inflammatory intervention is interference with endogenous regulator systems. One such system is the vagal anti-inflammatory reflex (Borovikova et al., 2000; Pavlov and Tracey, 2005; Schweighofer et al., 2016; Williamson et al., 2010). A promising way of activating this reflex was applied by enteral administration of boluses of nutrition, enriched with lipids and specific proteins (Lubbers, de Haan et al., Lubbers et al., 2010a, Lubbers et al., 2010b, Lubbers et al., 2010c). These nutrients stimulated cholecystokinin (CCK)-mediated CCK-1 receptors in the gut, thereby activating the afferent vagal nerves. Consequently, vagal efferent nerves in the central nervous system are stimulated, resulting in inhibition of the release of pro-inflammatory cytokines via nicotinergic acetylcholine receptors (de Haan et al., 2010; de Haan et al., 2010a, de Haan et al., 2010b; de Haan et al., 2014; Lubbers et al., 2010a, Lubbers et al., 2010b, Lubbers et al., 2010c; Luyer et al., 2013). In mice, lipid-rich nutrition before Lipopolysaccharide (LPS) administration attenuated the inflammatory response and intestinal damage through the nutritional vagal anti-inflammatory pathway (Lubbers et al., 2010). Similarly, in humans, postpyloric administration of lipid and protein enriched nutrition attenuated the LPS-evoked response of proinflammatory cytokines, tumor necrosis factor-α and interleukins 1 and-6, and increased the level of anti-inflammation-associated IL-10 (Lubbers et al., 2013).
Over the last decade, we developed a clinically relevant rat model for POCD. This model supported the role of (neuro)inflammation in the development of POCD (Hovens et al., 2014a, Hovens et al., 2014b). Moreover, known risk factors for POCD in patients, such as older age, type of surgery, or inflammation history, exacerbated and extended POCD from limited hippocampus-related tasks and associated neuroinflammation, to more wide-spread neuroinflammation and associated cognitive domains (Hovens et al., 2013a; Hovens et al., 2015b; Hovens et al., 2016). In addition to the hippocampus-associated spatial learning and memory, these increased risk conditions shared hippocampus-unrelated object recognition- and cognitive flexibility impairment, associated with higher circulating levels of proinflammatory cytokines and microglia activation in the prefrontal cortex. Interfering with the surgery-induced inflammatory responses then may prevent POCD. Indeed, studies in animal models indicate that anti-inflammatory treatment can attenuate POCD development (Barrientos et al., 2012; Cibelli et al., 2010; Jiang et al., 2015; Terrando et al., 2010)]. That these studies did not have resulted in clinical effective treatment yet may be supported by results obtained in a bile duct ligation model of POCD, indicating that inhibition of peripheral inflammation would be insufficient to recover cognitive impairment (Mohammadian et al., 2019). Gut microbiome changes (Guyton and Alverdy, 2017), intestinal permeability (Obrenovich, 2018), blood brain barrier (BBB) integrity (Mohammadian et al., 2019), as part of the gut-brain axis (Cryan, O'Riordan, Sandhu, Peterson and ) may contribute to (neuro)inflammation and POCD as well.
Aim of the present study was to explore the therapeutic potential of enteral enriched nutrition before surgery to prevent/inhibit POCD in our rat model. In young rats, the anticipated mechanism, inhibition of surgery-induced inflammation and consequently improved postoperative cognitive performance was evaluated. Next, as older age is the main risk factor for POCD, in old rats, effects of presurgical enteral enriched nutrition were studied regarding (neuro)inflammation, aspects of the gut-brain axis, and cognitive performance.
2. Materials & methods
2.1. Animals
Young (3 months) and old (23 months) male Wistar (RjHAN:WI) rats were obtained from Janvier Labs (Saint-Isle, France) and kept under standard conditions (temperature of ±21 °C; humidity of ±55%; 12:12 reversed light: dark cycle and ad libitum access to water and standard rat chow) for 14–30 days before entering the study. The rats were housed in pairs until the start of the study. All experiments were approved by the local animal experiment and welfare committee (Dier Experiment Commissie, Groningen, the Netherlands).
2.2. Experimental design
2.2.1. Studies
The experiment was designed as 2 separate studies. Study 1 was performed to examine whether the anti-inflammatory effects of the acutely applied enriched food, as previously shown by the group of de Haan, Lubbers and co-workers, would also work in our model of surgery-induced inflammation and cognitive decline. For that, 3 experimental groups were compared; rats receiving enteral enriched nutrition (n = 13) or equicaloric control nutrition (n = 13) or were just control handled (n = 13) before surgery. As results turned out positive, and age is the main risk factor for developing POCD, next old rats were included, potentially reflecting a clinically more relevant condition. Therefore, in study 2 the effects of enriched nutrition were studied in old rats. As in study 1 control nutrition did not have major effects, in order to limit the number of rats, in study 2 enriched nutrition treated old rats (n = 12) were only compared to control fasted rats (n = 12). Additionally, as the gut microbiome may play a role in development of POCD via the gut-brain axis, faecal samples were collected for gut microbiome analyses.
2.2.2. Design
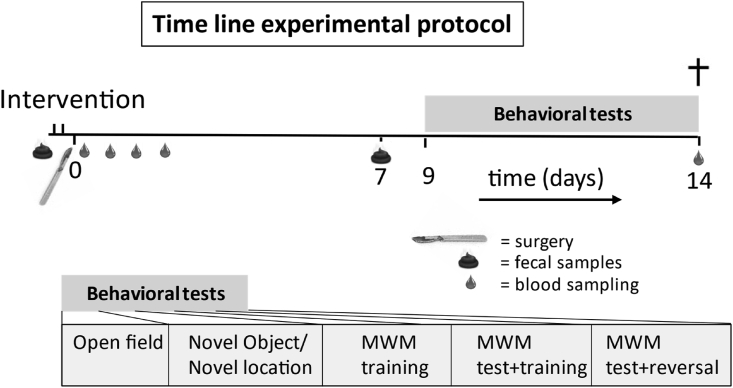
The study design is presented in Fig. 1. Rats were habituated to daily handling the week before surgery, and weighted daily. At the day of surgery, rats were fasted from 2 h before lights off and randomly assigned to the experimental groups, and housed individually 2 h before surgery. Enteral nutrition, control nutrition or control handling were provided twice: 2 h and 30 min before surgery. During this 2-h period, baseline fecal samples were collected from spontaneous releases (study 2). After surgery rats were weighted daily. The maximal weight loss after surgery was determined and represented as a percentage of the two last measurements before surgery. Timed blood samples were collected after surgery in order to measure circulating markers of inflammation. At day 7 after surgery fecal samples were collected during a 2-h period to evaluate long-term changes in the microbiome (study 2). From day 9 to day 14 after surgery, behavioral tests were performed. Rats were sacrificed 16 days after surgery. Blood samples were collected, and brain tissue was processed for immunohistochemical examination of neuroinflammation and neurogenesis.
Fig. 1.
Time line and experimental design. Interventions: enteral enriched nutrition, control nutrition (only study 1) or control handling, 2 h and 30 min before surgery. Fecal samples were only collected in study 2. MWM = Morris Water Maze.
2.3. Nutrition
On the day of surgery, standard rat chow was taken away 2 h before lights off. Control nutrition or enriched nutrition was administered to the respective experimental groups twice, 2 h and 30 min before surgery, by means of oral droplets (2,5 ml/kg). Rats liked the nutrition and sipped it in from a syringe spontaneously. Control nutrition consisted of 16% fat, 9% protein, and 75% carbohydrate. Enriched nutrition consisted of 46% fat, 24% protein, and 30% carbohydrate, similar to what was used in humans (Lubbers et al., 2013). Both the control and enriched nutrition had a caloric value of 1.0 kcal/ml. The bolus is not considered to affect daily nutrition intake as it consists of approximately 2% of daily caloric intake (60–100 kcal/day). Control and enriched nutrition were kindly provided by Danone Research (Utrecht, the Netherlands). To control for the handling that occurred during the administration of the oral droplets, the rats from the fasted group were handled as well.
2.4. Surgery
Abdominal surgery was performed as previously described (Hovens et al., 2014a, Hovens et al., 2014b). Briefly, under sevoflurane anesthesia (±3% in O2 at 0,8 L/min) and buprenorphine as intraoperative analgesic (3,0 μg/kg s.c.), a midline incision was made, the intestines were exteriorized and ischaemia was induced by clamping of the superior mesenteric artery for 30 min. A permanent jugular vein catheter was placed for continuous blood sampling. Blood was sampled at 0.5, 1, 2, 6 and 24 h after mesenteric reperfusion.
2.5. Behavioral tests
Behavioral testing was performed in the first half of the dark phase under dim light conditions, in a room adjacent to the animal room, as described previously in detail (Hovens et al., 2014a, Hovens et al., 2014b).
2.5.1. Open field
On postoperative day 9, an open field test (OF) was performed to assess exploratory activity and anxiety. The rats were placed in a square arena (100 × 100 cm) and their behavior was recorded for 5 min. The arena was divided into 4 corners (20 × 20 cm), 4 wall areas (20 × 60 cm) and a center area (60 × 60 cm). Total distance moved was used as measure for exploration, while time spent in the center area was analyzed as reciprocal for anxiety (Ethovision, Noldus, Wageningen, the Netherlands).
2.5.2. Novel object and novel location recognition
To determine short-term object and spatial memory, a novel object- (NOR) and a novel location recognition test (NLR) were performed. The rats were habituated to the test arena (60 × 50 cm) on postoperative day 9. Testing was performed on postoperative day 10, and consisted of 3 phases. In between phases, the objects were removed from the arena for approximately 40 s and cleaned with 70% alcohol. Two objects (bottles) were placed into the arena in opposite corners and the rats were allowed to explore these objects for 3 min (habituation phase). After that, a novel object replaced one of the objects and rats were allowed to explore the objects for 3 min (novel object phase). The novel object was then relocated to an opposite corner and exploration was recorded for another 3 min (novel location phase). All behavior was recorded and the exploration time of each object was later analyzed using Observer (Noldus, Wageningen, the Netherlands). Trials that included a total object exploration time of less than 3% were regarded as no interest in the objects, and excluded from analysis. Novel object- or location recognition were determined as time spent on exploration of the novel/relocated object minus time spent on the familiar object, divided by the time exploring both objects (index).
2.5.3. Morris water maze
Assessment of long-term spatial learning, spatial memory, and cognitive flexibility occurred on postoperative days 11, 12 and 13 in the Morris Water Maze (MWM). For the test, a round pool (160 cm diameter) was used with a water temperature of 26 ± 1 °C. The pool was virtually divided into 4 quadrants with a platform present 1 cm below the water surface in the target quadrant. A total of 5 training sessions were carried out, each training session consisting of three 60-s trials. When the platform was not reached within 60 s, the rats were guided to the platform. The average escape latencies for each training session were calculated, and the area under the curve (AUC) of the 5 sessions was obtained as measure for spatial learning. The first three training sessions were carried out on postoperative day 11, followed by a 1-min probe trial and two more training sessions on postoperative day 12. During the probe trial, the platform was removed and behavior was recorded for 1 min. Pilot studies indicated that this interim probe trial did not disturb the learning process, but give us insight in the development speed of the memory. A second probe trial was performed on postoperative day 13. Each probe trial was followed by a retraining trial. The time spent in the target quadrant, as well as the number of platform crossings and the total distance moved, were used to assess spatial memory. A reversal training, involving the relocation of the platform to the opposite quadrant, was executed to determine cognitive flexibility. The reversal training consisted of three 60-s trials, divided over two training sessions. The average escape latencies were again calculated for each training session.
2.6. Blood sampling and ELISA
Blood was collected via the jugular vein catheter at 30 min, 1 h, 2 h (study 1), 6 h and 24 h after clamp removal. Blood was immediately transferred to sampling tubes containing 20 μl/ml saturated EDTA solution, stored on ice, and centrifuged for 10 min at 2600 g at 4 °C. Plasma was stored at −80 °C. Concentrations of tumor necrosis factor-α (TNF), interleukins (IL) 1β, 6 and 10, and vascular endothelial growth factor (VEGF), were determined in plasma, using the Bio-Plex Pro Rat 5-plex cytokine assay (Bio-Rad Laboratories BV, Veenendaal, the Netherlands).
Additionally, plasma levels of intestinal fatty acid binding protein (IFABP) were determined as measure for loss of intestinal integrity (Pelsers et al., 2003), using a rat I-FABP ELISA kit (R&D systems Inc, MN) following the manufacturer's instructions.
Hippocampi, collected at sacrifice were homogenized by sonification. The supernatant was collected, and further processed for ELISA (Hovens et al., 2014a, Hovens et al., 2014b) to measure IL1-β levels, according to manufacturer's instructions (rat IL1-β ELISA, Invitrogen, Vienna, Austria). The hippocampal IL1-β levels were expressed per milligram of hippocampal tissue.
2.7. Immunohistochemistry
At sacrifice, rats were anesthetized with pentobarbital (60 mg/kg, ip) and transcardially perfused with saline. The brains were dissected, fixed with 4% paraformaldehyde, dehydrated with 30% sucrose, stored at −80 °C, and then sliced into sections (20 μm thickness). Microscopic sections were stained to visualize microglia or immature neurons as published before (Hovens et al., 2014).
2.7.1. Microglia staining with IBA-1
Coronal sections of the prefrontal cortex (PFC) and the dorsal hippocampus (DH) (25 μm thickness) were cut. Sections were stained for ionized-binding adaptor protein (IBA)-1 staining to visualize microglia. Sections were mounted on glass. Microscopic pictures (200× magnification) were taken of the CA1 region, the dentate gyrus inner blade (DG), the hilus, and the CA3 region of the hippocampus. For the CA1 region pictures were taken of the stratum radiatum and for the CA3 region of the stratum lucidum. For the PFC, the Zilles's Cg1 was chosen as an area of interest. Microglia density, coverage and cell body areas were obtained using Image-Pro Plus, and averaged from 3 to 4 photographs per are per rat, blinded for experimental procedures. To assess microglial activation, the cell body to cell size ratio was calculated (Hovens et al., 2014a, Hovens et al., 2014b).
2.7.2. Immature neurons staining with DCX
To indicate neurogenesis, immature neurons were stained with double cortin (DCX), as described in detail previously (Hovens et al., 2014a, Hovens et al., 2014b). Briefly, sections were incubated with 1:1000 goat-anti-DCX (Santa Cruz, Dallas, USA), followed by 1:500 rabbit-anti-goat secondary antibody (Jackson, Wet Grove, USA). Labeling was visualized by DAB. Sections were transferred to glass. Microscopic pictures (40× magnification) were taken of the dentate gyrus. To assess neurogenesis, the DCX-positive area in young rats, or the number of positive cells in old rats, were divided by the length of the total dentate gyrus, using Image-Pro Plus (6.0).
2.8. Microbiome extraction and analyzes from fecal samples
Fecal samples were collected just before surgery to evaluate acute changes due to the enteral nutrition administration, and 7 days after surgery to study more long-term changes that may be associated with the outcome of the subsequent behavioral testing. Fecal samples were collected from spontaneous releases within 2 h after individual housing at baseline (before surgery) and on postoperative day 7, and processes as previously described (El Aidy et al., 2017) (IBU). For microbiome analyzes, the bacterial 16 S ribosomal RNA (rRNA) gene (V4–V5 region) was amplified using PCR reaction in triplicates. Amplicons of each sample were pooled, purified and quantified using a NanoDrop 2000 (Thermo Scientific, Waltham, MA). Samples were sequenced at GENEWIZ (South Plainfield, NJ) on an Illumina MiSeq platform in a 2 × 300bp paired-end (PE) configuration and analyzed using the Quantitative Insights Into Microbial Ecology (QIIME) (Kuczynski et al., 2012). The QIIME toolkit was also used to calculate the weighted and unweighted UniFrac distance matrices for community comparisons, as well as α-diversity measurements, including bacterial richness, based on the number of species per sample (number of observed OTUs) and bacterial diversity (Shannon H’), considering both the number of species as well as their frequencies (equitability). The overall microbial communities were compared (β-diversity) using principal coordinate analysis, which provide indication of the distribution of the taxa and relative abundance of detected species among age and treatment.
2.9. Statistical analysis
Results are presented as mean ± Standard Error of the Mean (SEM). Statistical analyses were performed using SPSS. Results that exceeded twice the standard deviation of its group were regarded as outlier and omitted from further analyses (maximal 1 per group). Study 1 was analyzed using one-way ANOVA with post-hoc LSD test. For study 2, means were compared using an independent sample t-test. A one-sample t-test was performed for the NOR/NLR test as well as the MWM test to identify differences from chance level.
For microbiome analyses, β-diversity (differences between community composition) between different age and treatment groups was assessed, using permutational multivariate analysis of variance (PerMANOVA Primer 6 version 6.1.16 & PERMANOVA + version 1.0.6) (van der Goot, van Spronsen, Falcao Salles, & van der Zee, E A, 2020). Changes in β-diversity were calculated as the difference, in percentage, between the average values per time point within treatment based on the weighted UniFrac (Lozupone et al., 2006). Results were regarded statistically significant when p ≤ 0.05. Relevant trends were presented for p ≤ 0.10.
Regression analysis was performed using SPSS to examine relevant correlations between variables.
3. Results study 1; young rats
3.1. General
One rat died because of technical failure during surgery. No further surgery-associated complications were observed. Baseline body weights for the different groups were 460±5 g, 468±5 g and 465±6 g for fasted, control nutrition and enriched nutrition-treated rats, respectively, and did not differ between groups. On average body weight loss due to surgery was 5.7 ± 0.7% in fasted control rats, which was not significantly affected by either control nutrition (4.4 ± 0.4%) or enriched nutrition (5.2 ± 0.4%).
3.2. Behavioral tests
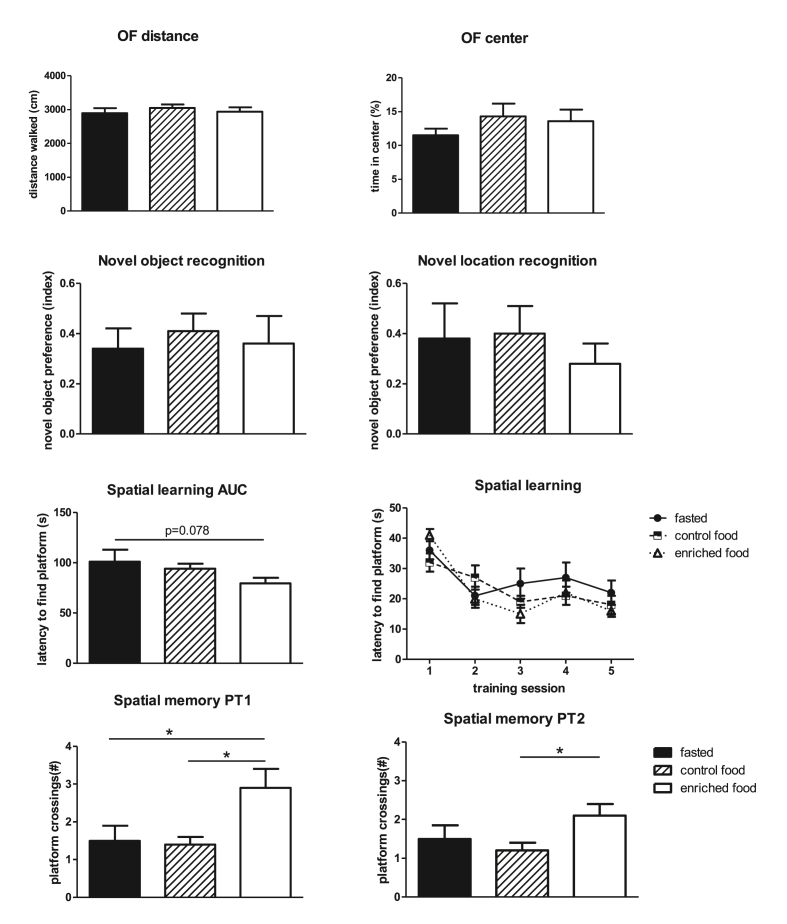
Results of behavioral tests are shown in Fig. 2. Behavior in the open field was not altered by either pretreatment. Fasted rats undergoing surgery performed well in the NOR and NLR tests: significant preference when compared to random level (zero) for all groups. This performance was not affected by enteral administration of control- or enriched nutrition. In contrast, long-term spatial learning and memory seemed affected by presurgical enteral enriched nutrition administration, as indicated by a significantly improved spatial memory and a tendency to improved spatial learning. Learning was not significantly affected by the interim probe trial.
Fig. 2.
Results of behavioral tests in control fasted (fasted), control nutrition pretreated (control nutrition) or enriched nutrition pretreated (enriched nutrition) young rats. AUC = area under the curve of latencies to find the platform in the Morris Water Maze (MWM); PT1 = probe trial after 3 trainings sessions; PT2 = probe trial after 5 trainings sessions; ∗ = significant difference (p < 0.05).
3.3. Systemic inflammation
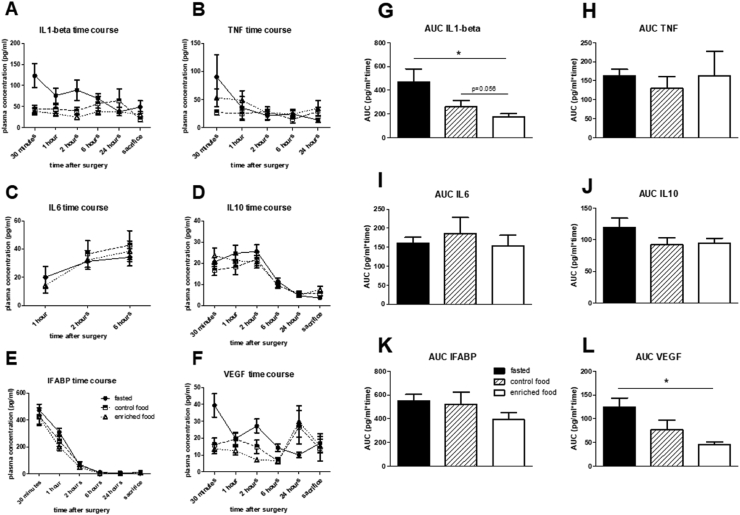
Plasma levels of inflammation markers could not be obtained at all time points, because values were below the detection levels. Average values from 3 or more data per timepoint/group are summarized in Fig. 3. Whereas time courses of IL10, IL6, TNF and IFABP were not affected by either pretreatment, results indicated an early suppression of levels of IL1-β and VEGF after enriched nutrition pretreatment. This was supported by the calculated area under the curve (AUC) over the first 6 h after surgery; significantly lower AUC for IL1-β and VEGF. However, these altered responses did not correlate to any of the cognitive parameters. VEGF levels in control and enriched nutrition pretreated, but not in control fasted rats, seemed to rise again at 24 h after surgery. These 24-h levels correlated significantly with the AUC in the reversal learning trial; as measure for cognitive flexibility (r = −0.43; p = 0.040), but not with any other cognitive parameter. Moreover, tendencies for correlations with this cognitive flexibility parameter were also observed for AUC IL1-β (r = −0.35; p = 0.056) and AUC VEGF (r = −0.44; p = 0.061).
Fig. 3.
Time course (A–F) and area under the curve (AUC; for the first 6 h) (G–L) for the different plasma markers in young rats. IL = interleukin; VEGF = vascular endothelial growth factor; IFABP = intestinal fatty acid-binding protein; TNF = tumor necrosis factor alpha. ∗ = p < 0.05.
3.4. Neuroinflammation and neurogenesis
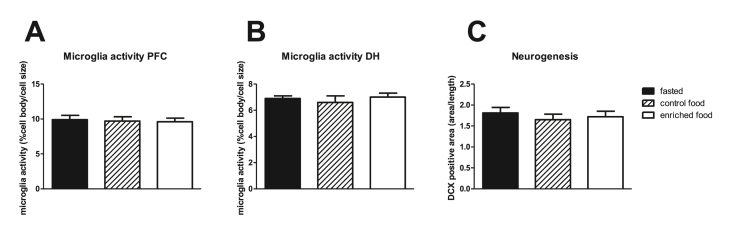
Neuroinflammation was measured as microglia activity obtained from morphological changes. Microglia activity in the prefrontal cortex and the dorsal hippocampus were not affected by either treatment (Fig. 4 A and B, respect.). Similarly, microglia activity in the different hippocampal areas did not show changes due to pretreatment (Table 1). Moreover, IL1-β in the hippocampus was not different in the experimental groups (fasted: 7.68 ± 2.86 pg/mg; control nutrition: 11.05 ± 2.19 pg/mg and enriched nutrition: 6.07 ± 1.30 pg/mg). Neurogenesis, measured as DCX positive area per DG length, was not affected by either pretreatment (Fig. 4C). Average hippocampal microglia activity correlated significantly with AUC of reversal learning, as measure for cognitive flexibility (r = −0.389; p = 0.014), but not with the other cognitive parameters, nor with circulating IL1- β or VEGF. Hippocampal IL1-β did not correlated with microglia activity nor with behavior, but correlated significantly with plasma IL1-β levels at sacrifice (r = 0.712; p = 0.009).
Fig. 4.
A: Microglia activity in the prefrontal cortex (PFC) and B: dorsal hippocampus (DH). C: neurogenesis in dentate gyrus of the hippocampus in young rats.
Table 1.
Microglia activity, measured as cell body/cell size, in the different areas of the hippocampus, in fasted, and control- or enriched nutrition pretreated rats.
| Experimental group | n | CA1 | CA3 | DG | Hilus |
|---|---|---|---|---|---|
| fasted | 13 | 5.60 ± 0.24 | 5.76 ± 0.34 | 4.78 ± 0.22 | 11.38 ± 0.31 |
| control | 13 | 6.01 ± 0.61 | 5.18 ± 0.45 | 4.71 ± 0.30 | 10.67 ± 0.75 |
| enriched | 12 | 6.15 ± 0.38 | 5.56 ± 0.23 | 4.61 ± 0.21 | 11.57 ± 0.51 |
4. Results study 2; old rats
4.1. General
All rats survived surgery. No surgery-associated complications were observed. Body weight at baseline was 673 ± 28 g in fasted rats and 626 ± 20 g in the enriched nutrition group. On average maximal surgery-induced body weight loss was 8.2 ± 0.7% in fasted rats and tended to be less, 6.3 ± 0.7% (p = 0.062) in enriched nutrition pretreated rats. Liver and spleen weight were not significantly altered by enriched nutrition pretreatment.
4.2. Behavior
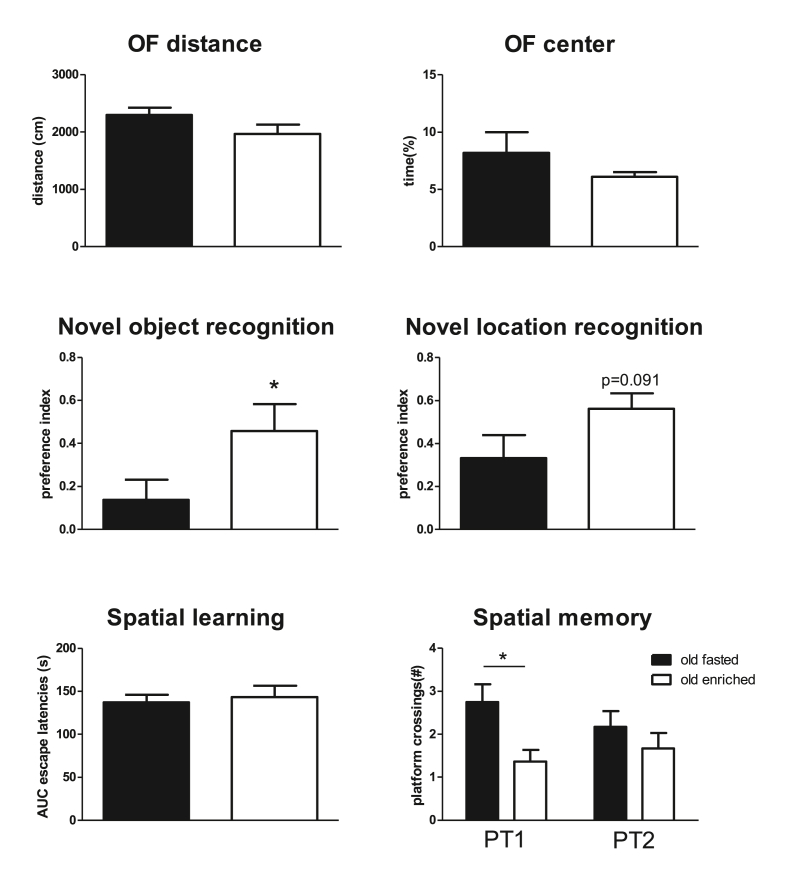
Results from the behavioral tests in old rats are summarized in Fig. 5. Most of the behavioral aspects showed age-related impairment, when compared to the results in young rats in study 1. Enriched nutrition before surgery significantly improved short-term object memory (NOR) after surgery and a strong tendency to improve short-term spatial memory (NLR). Whereas long-term spatial learning and reversal learning were not affected by enriched nutrition, long-term memory development seemed delayed and suppressed in enriched nutrition pretreated old rats. This observation was demonstrated by significantly lower platform crossings in the first probe trial (Fig. 5), but also by significantly lower time in the target quadrant (24.1 ± 1.8 versus 31.7 ± 2.0% in control fasted rats) in the first probe trial. Although time in target quadrant was slightly increased in the second probe trial (27.2 ± 1.8%), values still did not differ from random (=25%). Control fasted old rats performed significantly above random in both trials.
Fig. 5.
Overview of outcomes of behavioral tests in control fasted and enriched nutrition pretreated old rats. OF = open field test; AUC = area under the curve of latencies to find the platform in the Morris Water Maze (MWM); PT1 = probe trial after 3 trainings sessions; PT2 = probe trial after 5 trainings sessions; ∗ = significant difference (p < 0.05).
4.3. Systemic inflammation
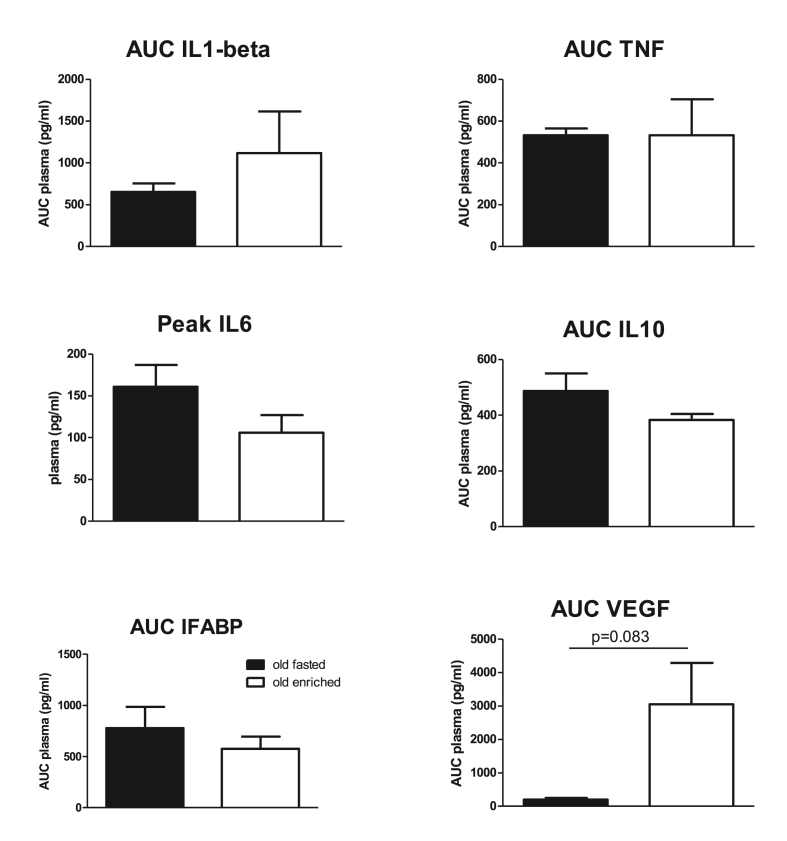
The area under the curve (AUC) of plasma levels of inflammation markers for the first 6 h after surgery are summarized in Fig. 6. For IL6 only peak values at 6 h are presented since at the other time points values could not be obtained in 3 or more rats. None of the circulating markers was significantly affected by enriched nutrition before surgery; only VEGF levels suggested an increase, but with high variability. Accordingly, none of the markers differed when measured at sacrifice (Table 2), but levels of proinflammatory markers IL1-β, TNF, IFABP and VEGF may show consistently higher levels after enriched nutrition. Moreover, early VEGF levels (AUC over first 6 h after surgery) showed significant correlations with cognitive performance (r = 0.878 with NOR; r = −0.896 with time in target quadrant). Furthermore, IL6 levels correlated significantly with spatial learning (AUC learning curve; r = −0.926). No significant correlations of IL1-β, or IFABP with cognitive performance were observed.
Fig. 6.
Area under the curve (AUC) over the first 6 h after surgery (or peak levels in case of insufficient data per time point) for plasma levels of inflammatory markers in control fasted and enriched nutrition pretreated old rats. IL = interleukin; VEGF = vascular endothelial growth factor; IFABP = intestinal fatty acid-binding protein; TNF = tumor necrosis factor alpha.
Table 2.
Concentration of inflammation markers (pg/ml) in plasma samples collected at sacrifice, in control fasted and enriched nutrition pretreated old rats. Number of rats between brackets. X: p = 0.061.
| IL1-β | TNF | IL6 | IL10 | IFABP | VEGF | |
|---|---|---|---|---|---|---|
| fasted | 125 ± 21 (11) | 51 ± 25 (4) | 58 ± 5 (8) | 32 ± 6 (5) | 27 ± 5 (10) | 36 ± 9 (11) |
| enriched | 151 ± 28 (11) | 98 ± 37 (8) | 54 ± 11 (9) | 20 ± 4 (5) | 273 ± 137 (8) × | 46 ± 12 (9) |
4.4. Neuroinflammation and neurogenesis
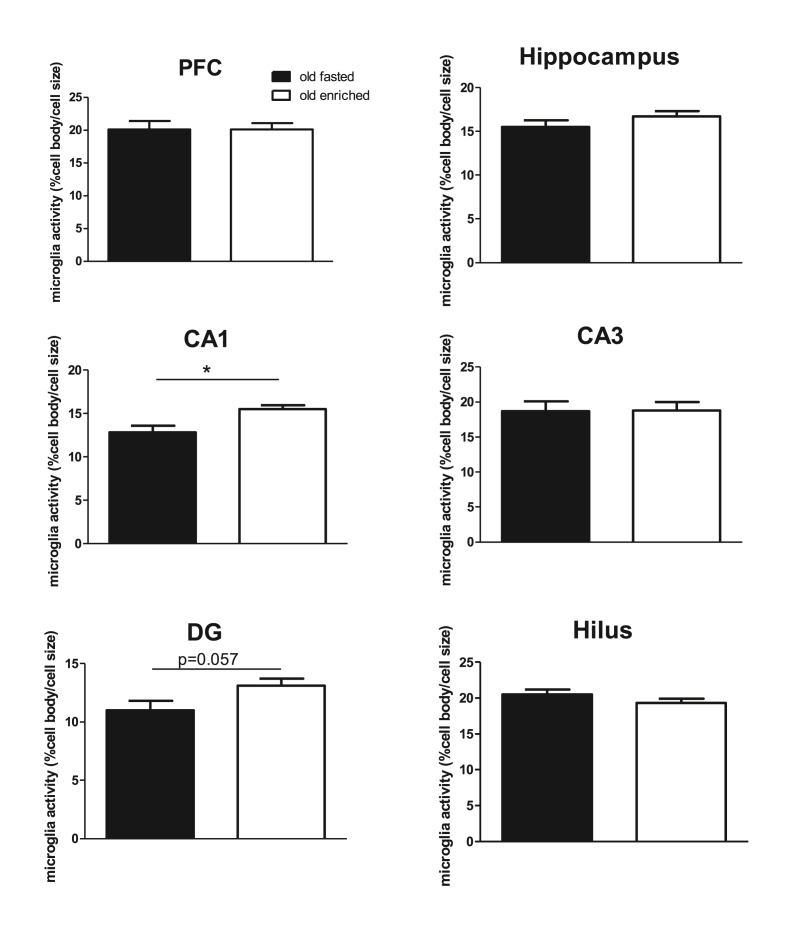
Neuroinflammation in the prefrontal cortex (PFC) and dorsal hippocampus, measured as microglia activity, was not affected by enriched nutrition pretreatment. However, when the dorsal hippocampus was studied in detail, microglia activity was significantly increased in the CA1 area, and a similar tendency was observed in the dentate gyrus (DG) (see Fig. 7). Hippocampal microglia activity significantly positively correlated with VEGF levels (r = 0.974) and negatively with spatial memory (r = −0.596 with time in target quadrant). IL1-β Levels in the hippocampus were not affected (6.41 ± 0.57 pg/mg in fasted rats versus 6.48 ± 0.81 pg/mg in enriched nutrition treated rats).Neurogenesis was not affected by pretreatment (0.51 ± 0.24 in fasted and 0.62 ± 0.15 cells per DG length in enriched nutrition pretreated rats), and did not correlate with systemic inflammation marker, microglia activity or cognitive performance.
Fig. 7.
Microglia activity in the prefrontal cortex (PFC) and Hippocampus overall, as well as in the different areas of the hippocampus in old rats. ∗: p < 0.05.
4.5. Microbiome
The gut microbiome could contribute to neuroinflammation via the gut-brain axis. Population diversity parameters of the gut microbiome before and 7 days after surgery are presented in Table 3. Diversity parameters indicated that the gut microbiome did not undergo major changes during pretreatment (before surgery), nor seemed affected by surgery or pretreatment at 7 days after surgery. Regression analysis revealed a significant correlation between Shannon's index at day 7 and time in the center of the open field (r = 0.487; p = 0.047) with a similar tendency for number of observed species (r = 0.465; p = 0.060). Although not statistically significant, Firmicutes/Bacteroidetes consistently declined after surgery, similar for treated and non-treated groups. Except for a significant correlation between Firmicutes/Bacteroidetes and the AUC of reversal learning (r = −0.562; p = 0.015), no significant correlations were observed between microbiome parameters and behavioral or (neuro)inflammation parameters.
Table 3.
Diversity parameters of the gut microbiome, as number of observed species and Shannon's index, and firmicutes/Bacteroidetes before and 7 days after surgery, in fasted and enriched nutrition pretreated old rats.
| Treatment | N | Before surgery |
7 days after surgery |
||||
|---|---|---|---|---|---|---|---|
| # species | Shannon's | Firm/Bact | # species | Shannon's | Firm/Bact | ||
| fasted | 8 | 619 ± 29 | 8.5 ± 0.2 | 1.16 ± 0.11 | 634 ± 37 | 8.6 ± 0.2 | 0.95 ± 0.06 |
| enriched | 9–10 | 598 ± 23 | 8.3 ± 0.2 | 1.19 ± 0.12 | 601 ± 21 | 8.5 ± 0.1 | 0.94 ± 0.06 |
5. Discussion
5.1. General
Surgery-induced (neuro)inflammation is considered to play an important role in the pathophysiology of POCD. Enteral administered enriched nutrition shortly before surgery was anticipated to activate the vagal anti-inflammatory reflex during surgery, thereby limiting the inflammatory response and consequently inhibiting POCD. Accordingly, in young rats, enriched nutrition reduced circulating IL1-β and VEGF levels, and improved long-term spatial learning and memory. However, neuroinflammation (microglia activation and hippocampal IL1-β) and neurogenesis were not affected. In contrast, in old rats enriched nutrition did not affect systemic inflammation; plasma IL1-β and VEGF levels may even slightly increase. Microglia activity as measure for neuroinflammation was significantly increased. This was associated with improved short-term memory, but impaired development of long-term memory. In conclusion, enteral enriched nutrition before surgery can improve cognitive performance after surgery in young rats, but in old rats, cognitive effects appeared mixed. The lack of decreased microglia activity in either age group suggests involvement of mechanisms other than neuroinflammation as well. However, unaltered gut microbiome and IFABP levels in old rats suggested no major role for the gut-brain axis either. Since the target population for POCD treatment consists of predominantly elderly individuals, the different response in old rats should specifically be addressed in follow-up studies. Caution is advised by translating effects seen in younger patients to older ones.
5.2. Enteral enriched nutrition
5.2.1. Effects in young rats
Enteral enriched nutrition before surgery improved the outcome for long-term spatial learning and memory after surgery. Short-term memory was not improved. However, since control rats already recognized the novel or relocated objects, there was not much to gain from the interventions.
As anticipated (Lubbers et al., 2010a, Lubbers et al., 2010b, Lubbers et al., 2010c; Lubbers et al., 2010a, Lubbers et al., 2010b, Lubbers et al., 2010c), enteral enriched nutrition inhibited the systemic inflammatory response shortly after surgery. The most pronounced effects were observed on circulating levels of IL1-β and VEGF. IL1-β is regarded a pro-inflammatory cytokine that mediates part of the inflammatory process in infection and injury (Dinarello, 1996). It exerts effects on memory function and neural plasticity of the hippocampus (Avital et al., 2003), mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression (Goshen et al., 2008), and is well recognized to play a role in the development of POCD (Barrientos et al., 2009, Barrientos et al., 2012; Cibelli et al., 2010). Accordingly, reduced circulating IL1-β levels coincided with improved POCD. However, neuroinflammation, measured as microglia activation and hippocampal IL1-β levels, and neurogenesis were not affected. Moreover, the reduced circulating IL1-β levels did not correlate with any cognitive parameter, nor with microglia activity or neurogenesis, indicating no direct (causal) relationship.
Astrocyte-derived VEGF drives blood-brain barrier disruption in inflammatory disease in the central nervous system (Argaw et al., 2012). The observation that IL-1beta regulated blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program (Argaw et al., 2006), would support interaction between the most pronouncedly affected factors with enteral enriched nutrition. VEGF levels were associated with blood brain barrier disruption and consequently cognitive impairment (Cao et al., 2018), but complete reversal of post-surgical blood brain barrier leakage was not associated with cognitive improvement (Mohammadian et al., 2019). Still, reduced VEGF levels in the present study may reflect preserved BBB integrity.
Hippocampal microglia activity and the somewhat unexpected second increase in VEGF levels at 24 h significantly correlated with cognitive flexibility (AUC reversal learning). Furthermore, strong tendencies for correlations of AUC IL1-β and AUC VEGF with cognitive flexibility were observed, supporting an association between (neuro)inflammation and cognitive flexibility. However, the above-mentioned associations appeared to be the only correlations between parameters of (neuro)inflammation and cognition in this study.
Thus, although there is ample evidence for a role of (neuro)inflammation in the pathophysiology of POCD, effects of anti-inflammatory intervention with enriched nutrition seemed to lack consistency. This observation suggests complex mechanisms, rather than a straight forward systemic inflammation-neuroinflammation-neuronal damage-cognitive dysfunction process.
5.2.2. Effects on old rats
The main risk factor for POCD is older age. Therefore, effects of enteral enriched nutrition were also investigated in old rats. Compared to young rats, values for behavior, including open field behavior, learning and memory, as well as values for (neuro)inflammation and neurogenesis in old control rats, represented age-related changes as described before (Hovens et al., 2013b, Hovens et al., 2015a). However, in these old rats, effects of enteral enriched nutrition before surgery appeared substantially different from those in young rats. Whereas short-term memory was significantly improved by enriched nutrition, development of long-term memory appeared delayed. This observation showed striking resemblance with results from a recent study in old Alzheimer mice, vaccinated against amyloid beta (Oberman et al., 2020); improved short-term memory with declined long-term memory. These opposite cognitive effects may refer to the competitive short-term and long-term spatial memory processes described by Sanderson and Bannerman (Sanderson and Bannerman, 2011), showing improved α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) gluA1 receptor dependent non-associative short-term memory in the NOR and NLR tests, coincided with a deteriorated gluA1-independent associative MWM memory. According to Wagner's dual-process memory model (Sanderson et al., 2010), indicating short- and long-term memory mechanisms exist in parallel and, under certain circumstances, compete with each other, this would suggest a role for AMPA gluA1 receptors in the effects on cognition in our old rats. However, to our knowledge, no data are available regarding effects of enteral enriched nutrition on AMPA GluA1 signaling.
Enteral enriched nutrition in old rats did not reduce systemic inflammation. Even, the 2 markers that were found reduced in young rats, IL1-β and VEGF, showed a tendency to increase in old rats. Moreover, though not statistically significant, at sacrifice still most of pro-inflammatory marker levels seemed consistently higher in enriched nutrition-pretreated rats. Accordingly, microglia activity (measured at sacrifice) was increased in CA1 and DG areas of the hippocampus, suggesting locally increased neuroinflammation. Striking finding was the correlation of VEGF levels with cognitive performance as well as with hippocampal neuroinflammation. As VEGF levels may indicate blood brain barrier integrity (Cao et al., 2018), higher levels would indicate more leakage, which would be consistent with the positive correlation with hippocampal neuroinflammation, and the subsequent negative correlation with hippocampus-associated spatial memory (time in target quadrant in probe trial of the MWM). The positive correlation with the hippocampus independent NOR test outcome may then relate to the competitive relationship between short and long-term memory as described before (Sanderson and Bannerman, 2011), as well as the different brain areas involved in these tests (Hovens et al., 2015).
General diversity parameters of the gut microbiome were not affected by surgery nor by enriched nutrition pre-treatment. Regarding relative abundance of species within the microbiome composition, higher Firmicutes Bacteroidetes ratios and higher relative abundance of Verrumomicrobia were associated with cognitive health in aged people (Manderino et al., 2017). Our results of consistently reduced Firmicutes/Bacteroidetes after surgery (compared to baseline) would fit with development of POCD in the old rats, but no effect of enriched nutrition could be distinguished. Besides the correlation between the Firmicutes Bacteroidetes ratio and reversal learning, no correlations with parameters of cognition and (neuro) inflammation were observed. Since IFABP levels did not change after enriched nutrition, indicating unaltered gut permeability (Bingold et al., 2015), and no major effects on the gut microbiome were observed, these results may indicate no major contribution of the gut-brain axis to the observed effects.
5.2.3. Clinical implications
The vagal anti-inflammatory reflex (Tracey, 2002, 2007) contributes to the regulation (inhibition/termination) of inflammatory responses. In different animal models (de Haan et al., 2010a, de Haan et al., 2010b; de Haan et al., 2010a, de Haan et al., 2010b; de Haan et al., 2013; Lubbers et al., 2010a, Lubbers et al., 2010b, Lubbers et al., 2010c; Lubbers et al., 2010a, Lubbers et al., 2010b, Lubbers et al., 2010c; Luyer et al., 2013), activation of this reflex by lipid-enriched nutrition inhibited the response on inflammatory stimuli. These effects were abrogated by vagotomy, CCK-1 receptor antagonists or nicotinergic acetylcholine receptor antagonists (Lubbers et al., 2010; Matteoli and Boeckxstaens, 2013). Therefore, this lipid-enriched nutrition could provide a promising therapeutic route to suppress inflammation after surgery, and hence inflammation-associated complications. Accordingly, in mice (Lubbers et al., 2010a, Lubbers et al., 2010b, Lubbers et al., 2010c), as well as in rats (Lubbers et al., 2009), postoperative ileus was reduced by enteral enriched nutrition. Translated into the human situation (Lubbers et al., 2013), enteral enriched nutrition from 1 h before to 6 h after lipopolysaccharide injection in healthy volunteers reduced the response of proinflammatory cytokines TNFα and IL6, and enhanced the response of the anti-inflammatory associated cytokine IL10. A meta-analysis on fatty-acid enriched nutrition studies in hospitalized patients indeed indicated reduced risk of infection and hospital stay, though no reduction in mortality rate (Pradelli et al., 2020). In most clinical studies, enriched nutrition has been applied continuously and for a prolonged period; from 3 h before to 6 h after surgery (Peters et al., 2018), or even from 3 days before to 14 days after surgery (Ma et al., 2018). Unfortunately, these interventions were not associated with postoperative cognitive improvement. In our previous rat study, effects of an anti-inflammatory diet improved cognitive outcome only when the diet was started before surgery, but not when started after surgery (Kurtys et al., 2017). Timing seemed crucial (Smeets and Luyer, 2018), as lipid enriched nutrition around the surgical procedure seemed most effective (Peters et al., 2015). However, in the clinical setting, anesthesia guidelines prohibit the use of enteral nutrition just before surgery, to reduce the risk of aspiration. This could be overcome by the use of a naso-jejunal tube for administration (Peters et al., 2018). As rodents cannot vomit, the details of timing pretreatment with enteral nutrition can be investigated in preclinical studies.
In addition to timing, the character of administration may indicate another potential important aspect. Firstly. the enteral enriched nutrition in the present study is not considered to affect daily nutrition; twice a bolus with low caloric value; 1.6 kcal per administration in 630 g rats versus a daily caloric intake 60–100 kcal. Secondly, by definition a reflex mechanism is a short-lasting response. Continuous stimulation as applied in the above clinical studies (Ma et al., 2018; Peters et al., 2015, Peters et al., 2018; Smeets and Luyer, 2018) may have extinguished the response. By activating the vagal anti-inflammatory reflex with bolus applications shortly before surgery, we anticipated that the response during the surgery process was affected, while leaving the subsequent healing process unaffected. Accordingly, the most pronounced effects on cytokine levels were observed in the first half hour after surgery.
Finally, as advanced age is the major risk factor for POCD, in the present study effects in old rats were studied as well. Since the relatively straight forward effects of enriched nutrition in young rats could not be shown in old rats, as indicated by a mixed beneficial/detrimental cognitive outcome in the latter, caution is advised by translating effects seen in younger patients to older ones. Studies focusing on effects in aged individuals are warranted.
5.3. Limitations
In our previous studies, consistent effects of surgery in young and aged rats were shown. In order to limit the number of rats and focus on the potential effects of intervention, non-surgery rats (treated or non-treated) were left out in the present study. In the present study, control rats were fasted before surgery, whereas in our previous studies rats had free access to food all the time. In hind sight, this could have affected the outcome after surgery. However, the fasted condition may better mimic the clinical setting and therefore enhance the relevance of the effects of the intervention.
The effects of enteral enriched nutrition pretreatment were studied after 2 bolus administrations, as the treatment was aimed at activation of the vagal anti-inflammatory reflex during the surgical procedure, while leaving the subsequent wound healing unaffected. However, the study protocol did not allow the actual confirmation of this aim.
Effects of pretreatment on neuroinflammation and cognition were measured at 1 time point. However, we are well aware to interfere with processes that each follow its own time course (wound healing, (neuro)inflammation and POCD) (Hovens et al., 2014a, Hovens et al., 2014b). By carefully choosing our parameters, clinically relevant data could be obtained. The observed differences in young versus old rats justify careful translation of effects in young individuals to older ones.
The gut microbiome was investigated from spontaneously produced fecal samples within a 2-h period. Unfortunately, this resulted in incomplete sample sets, potentially limiting conclusions. Active collection by massage of the rectum could have solved this issue, but inherently included extra discomfort to the rats.
Finally, plasma levels of inflammation markers were measured using a multiplex cytokine assay. The advantage is that more cytokines could be measured from the same sample. Disadvantage is that for all measured cytokines the same dilution was used, resulting in not reaching detection levels for all cytokines/time points.
6. Conclusions
The aim was to investigate whether enteral enriched nutrition before surgery could inhibit the inflammatory response on a surgical procedure, and hence decrease neuroinflammation and subsequent POCD. The first study in young rats confirmed the anticipated mechanism of inhibition of surgery-associated inflammation and subsequent improved cognitive outcome after enteral enriched nutrition. However, this effect seemed not mediated by inhibition of neuroinflammation. Effects obtained in old rats, in the second study, were substantially different; no anti-inflammatory effects and a mixed improved/impaired cognitive outcome. Locally, neuroinflammation seemed increased, excluding an anti-inflammatory effect within the brain. Moreover, as the gut microbiome and IFABP levels were not altered, no major role for the gut-brain axis was indicated here either.
In conclusion, although (neuro)inflammation may play an important role in the pathophysiology of POCD, intervention with enteral enriched nutrition aimed at anti-inflammatory effects, showed mixed and age-dependent effects. Since these observations may point to a different mechanism in young versus old rats, translating the effects seen in young individuals to older ones should be done carefully.
Ethics approval and consent to participate
Experimental protocols were approved by the national experimental animal committee (CCD) and the local animal experiment and welfare committee (Dier Experiment Commissie, Groningen, the Netherlands).
Availability of data and materials
Data and material are available upon request.
Funding
No external funding was obtained for this study. DANONE the Netherlands financially supported the measurements of plasma markers.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
We would like to thank Wanda Douwenga, Jan Keijser, Jan Bruggink and Kunja Slopsema and for their technical support. Master students Vlad Constantinescu and Francien Bouw are thanked for her support in behavioral testing and immunohistochemical analyses, while Franscisco Dini-Andreote and Tim Miedema have helped with the analyses of the gut microbiome. Ardy van Helvoort and Karen Knipping (DANONE, the Netherlands) were greatly appreciated for their (financial) support in measuring plasma markers.
References
- Alam A., Hana Z., Jin Z., Suen K.C., Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–556. doi: 10.1016/j.ebiom.2018.10.021. S2352-3964(18)30432-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw A.T., Asp L., Zhang J., Navrazhina K., Pham T., Mariani J.N. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Invest. 2012;122(7):2454–2468. doi: 10.1172/JCI60842. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw A.T., Zhang Y., Snyder B.J., Zhao M.L., Kopp N., Lee S.C. IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J. Immunol. 2006;177(8):5574–5584. doi: 10.4049/jimmunol.177.8.5574. 177/8/5574 [pii] [DOI] [PubMed] [Google Scholar]
- Avital A., Goshen I., Kamsler A., Segal M., Iverfeldt K., Richter-Levin G. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13(7):826–834. doi: 10.1002/hipo.10135. ([doi]) [DOI] [PubMed] [Google Scholar]
- Barrientos R.M., Frank M.G., Hein A.M., Higgins E.A., Watkins L.R., Rudy J.W. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav. Immun. 2009;23(1):46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R.M., Hein A.M., Frank M.G., Watkins L.R., Maier S.F. Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J. Neurosci. : The Official Journal of the Society for Neuroscience. 2012;32(42):14641–14648. doi: 10.1523/JNEUROSCI.2173-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingold T.M., Franck K., Holzer K., Zacharowski K., Bechstein W.O., Wissing H. Intestinal fatty acid binding protein: a sensitive marker in abdominal surgery and abdominal infection. Surg. Infect. 2015;16(3):247–253. doi: 10.1089/sur.2014.073. ([doi]) [DOI] [PubMed] [Google Scholar]
- Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Cao Y., Li Z., Li H., Ni C., Li L., Yang N. Hypoxia-inducible factor-1alpha is involved in isoflurane-induced blood-brain barrier disruption in aged rats model of POCD. Behav. Brain Res. 2018;339:39–46. doi: 10.1016/j.bbr.2017.09.004. S0166-4328(17)31022-7 [pii] [DOI] [PubMed] [Google Scholar]
- Cibelli M., Fidalgo A.R., Terrando N., Ma D., Monaco C., Feldmann M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann. Neurol. 2010;68(3):360–368. doi: 10.1002/ana.22082. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., O'Riordan K.J., Sandhu K., Peterson V., Dinan T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179–194. doi: 10.1016/S1474-4422(19)30356-4. S1474-4422(19)30356-4 [pii] [DOI] [PubMed] [Google Scholar]
- de Haan J.J., Lubbers T., Greve J.W., Buurman W.A. Exploring the link between inflammation and the vagus nerve. J. Intern. Med. 2010;267(1):130–131. doi: 10.1111/j.1365-2796.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- de Haan J.J., Pastille E., Wirsdorfer F., Lubbers T., Greve J.W., Zhang Y. Lipid-rich enteral nutrition improves the defense against an opportunistic infection during polymicrobial sepsis. Shock. 2014;41(2):109–114. doi: 10.1097/SHK.0000000000000062. [DOI] [PubMed] [Google Scholar]
- de Haan J.J., Thuijls G., Lubbers T., Hadfoune M., Reisinger K., Heineman E. Protection against early intestinal compromise by lipid-rich enteral nutrition through cholecystokinin receptors. Crit. Care Med. 2010;38(7):1592–1597. doi: 10.1097/CCM.0b013e3181e2cd4d. [DOI] [PubMed] [Google Scholar]
- de Haan J.J., Windsant I.V., Lubbers T., Hanssen S.J., Hadfoune M., Prinzen F.W. Prevention of hemolysis-induced organ damage by nutritional activation of the vagal anti-inflammatory reflex∗. Crit. Care Med. 2013;41(11):361. doi: 10.1097/CCM.0b013e31828e9262. [DOI] [PubMed] [Google Scholar]
- Dinarello C.A. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–2147. S0006-4971(20)65207-7 [pii] [PubMed] [Google Scholar]
- El Aidy S., Ramsteijn A.S., Dini-Andreote F., van Eijk R., Houwing D.J., Salles J.F. Serotonin transporter genotype modulates the gut microbiota composition in young rats, an effect augmented by early life stress. Front. Cell. Neurosci. 2017;11:222. doi: 10.3389/fncel.2017.00222. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I., Kreisel T., Ben-Menachem-Zidon O., Licht T., Weidenfeld J., Ben-Hur T. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatr. 2008;13(7):717–728. doi: 10.1038/sj.mp.4002055. 4002055 [pii] [DOI] [PubMed] [Google Scholar]
- Guyton K., Alverdy J.C. The gut microbiota and gastrointestinal surgery. Nat. Rev. Gastroenterol. Hepatol. 2017;14(1):43–54. doi: 10.1038/nrgastro.2016.139. ([doi]) [DOI] [PubMed] [Google Scholar]
- Hovens I.B., Nyakas C., Schoemaker R.G. A novel method for evaluating microglial activation using ionized calcium-binding adaptor protein-1 staining: cewll body to cell size ratio. [A novel method for evaluating microglial activation using ionized calcium-binding adaptor protein-1 staining. cewll body to cell size ratio] Neuroimmunology Neuroinflammation. 2014;1(2):82–88. [Google Scholar]
- Hovens I.B., Schoemaker R.G., van der Zee E.A., Absalom A.R., Heineman E., van Leeuwen, L B. Postoperative cognitive dysfunction: involvement of neuroinflammation and neuronal functioning. Brain Behav. Immun. 2014;38:202–210. doi: 10.1016/j.bbi.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Hovens I.B., Schoemaker R.G., van der Zee E.A., Heineman E., Izaks G.J., van Leeuwen B.L. Thinking through postoperative cognitive dysfunction: how to bridge the gap between clinical and pre-clinical perspectives. Brain Behav. Immun. 2012;26(7):1169–1179. doi: 10.1016/j.bbi.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Hovens I.B., Schoemaker R.G., van der Zee E.A., Heineman E., Nyakas C., van Leeuwen B.L. Surgery-induced behavioral changes in aged rats. Exp. Gerontol. 2013;48(11):1204–1211. doi: 10.1016/j.exger.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Hovens I.B., Schoemaker R.G., van der Zee E.A., Heineman E., Nyakas C., van Leeuwen B.L. Surgery-induced behavioral changes in aged rats. Exp. Gerontol. 2013;48(11):1204–1211. doi: 10.1016/j.exger.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Hovens I.B., van Leeuwen B.L., Mariani M.A., Kraneveld A.D., Schoemaker R.G. Postoperative cognitive dysfunction and neuroinflammation; cardiac surgery and abdominal surgery are not the same. Brain Behav. Immun. 2016;54:178–193. doi: 10.1016/j.bbi.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Hovens I.B., van Leeuwen B.L., Nyakas C., Heineman E., van der Zee E.A., Schoemaker R.G. Postoperative cognitive dysfunction and microglial activation in associated brain regions in old rats. Neurobiol. Learn. Mem. 2015;118:74–79. doi: 10.1016/j.nlm.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Hovens I.B., van Leeuwen B.L., Nyakas C., Heineman E., van der Zee E.A., Schoemaker R.G. Prior infection exacerbates postoperative cognitive dysfunction in aged rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309(2):148. doi: 10.1152/ajpregu.00002.2015. [DOI] [PubMed] [Google Scholar]
- Jiang P., Ling Q., Liu H., Tu W. Intracisternal administration of an interleukin-6 receptor antagonist attenuates surgery-induced cognitive impairment by inhibition of neuroinflammatory responses in aged rats. Experimental and Therapeutic Medicine. 2015;9(3):982–986. doi: 10.3892/etm.2014.2149. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kuczynski J., Stombaugh J., Walters W.A., Gonzalez A., Caporaso J.G., Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Current Protocols in Microbiology, Chapter. 2012;1 doi: 10.1002/9780471729259.mc01e05s27. Unit 1E.5, ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtys E., Hovens I., Cristiano Real C., Kopschina Feltes P., Vállez García D., Eisel U. Brain [11C]PK11195 and [18F]FDG PET imaging in a rat model of postoperative cognitive dysfunction. Eur. J. Nucl. Med. Mol. Imag. 2017;44(Suppl. 2) https://www.narcis.nl/publication/RecordID/oai:pure.rug.nl:publications%2F17d07cb1-525b-4065-9c67-1fa2ebb1ec8e Retrieved from. [Google Scholar]
- Lozupone C., Hamady M., Knight R. UniFrac–an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinf. 2006;7 doi: 10.1186/1471-2105-7-371. 371–371, 1471-2105-7-371 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbers T., De Haan J.J., Hadfoune M., Zhang Y., Luyer M.D., Grundy D. Lipid-enriched enteral nutrition controls the inflammatory response in murine gram-negative sepsis. Crit. Care Med. 2010;38(10):1996–2002. doi: 10.1097/CCM.0b013e3181eb90d7. [DOI] [PubMed] [Google Scholar]
- Lubbers T., de Haan J.J., Luyer M.D., Verbaeys I., Hadfoune M., Dejong C.H. Cholecystokinin/cholecystokinin-1 receptor-mediated peripheral activation of the afferent vagus by enteral nutrients attenuates inflammation in rats. Ann. Surg. 2010;252(2):376–382. doi: 10.1097/SLA.0b013e3181dae411. [DOI] [PubMed] [Google Scholar]
- Lubbers T., Kox M., de Haan J.J., Greve J.W., Pompe J.C., Ramakers B.P. Continuous administration of enteral lipid- and protein-rich nutrition limits inflammation in a human endotoxemia model. Crit. Care Med. 2013;41(5):1258–1265. doi: 10.1097/CCM.0b013e31827c0a17. [DOI] [PubMed] [Google Scholar]
- Lubbers T., Buurman W., Luyer M. Controlling postoperative ileus by vagal activation. World J. Gastroenterol. 2010;16(14):1683–1687. doi: 10.3748/wjg.v16.i14.1683. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbers T., Luyer M.D., de Haan J.J., Hadfoune M., Buurman W.A., Greve J.W. Lipid-rich enteral nutrition reduces postoperative ileus in rats via activation of cholecystokinin-receptors. Ann. Surg. 2009;249(3):481–487. doi: 10.1097/SLA.0b013e318194d187. ([doi]) [DOI] [PubMed] [Google Scholar]
- Luyer M.D., de Haan J.J., Lubbers T., Greve J.W., Buurman W.A. Parasympathetic stimulation via the vagus nerve prevents systemic organ dysfunction by abrogating gut injury and lymph toxicity in trauma and hemorrhagic shock. Shock. 2013;39(5):460–461. doi: 10.1097/SHK.0b013e31828def5a. [DOI] [PubMed] [Google Scholar]
- Ma C., Tsai H., Su W., Sun L., Shih Y., Wang J. Combination of arginine, glutamine, and omega-3 fatty acid supplements for perioperative enteral nutrition in surgical patients with gastric adenocarcinoma or gastrointestinal stromal tumor (GIST): a prospective, randomized, double-blind study. J. Postgrad. Med. 2018;64(3):155–163. doi: 10.4103/jpgm.JPGM_693_17. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manderino L., Carroll I., Azcarate-Peril M.A., Rochette A., Heinberg L., Peat C. Preliminary evidence for an association between the composition of the gut microbiome and cognitive function in neurologically healthy older adults. J. Int. Neuropsychol. Soc. : JINS. 2017;23(8):700–705. doi: 10.1017/S1355617717000492. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteoli G., Boeckxstaens G.E. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62(8):1214–1222. doi: 10.1136/gutjnl-2012-302550. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadian F., Firouzjaei M.A., Haghani M., Shabani M., Moosavi Shid, S M., Mohammadi F. Inhibition of inflammation is not enough for recovery of cognitive impairment in hepatic encephalopathy: effects of minocycline and ibuprofen. Brain Res. Bull. 2019;149:96–105. doi: 10.1016/j.brainresbull.2019.04.015. S0361-9230(19)30226-6 [pii] [DOI] [PubMed] [Google Scholar]
- Oberman K., Gouweleeuw L., Hoogerhout P., Eisel U.L.M., van Riet E., Schoemaker R.G. Vaccination prevented short-term memory loss, but deteriorated long-term spatial memory in alzheimer's disease mice, independent of amyloid-beta pathology. Journal of Alzheimer's Disease Reports. 2020;4(1):261–280. doi: 10.3233/ADR-200213. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrenovich M.E.M. Leaky gut, leaky brain? Microorganisms. 2018;6(4) doi: 10.3390/microorganisms6040107. E107 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V.A., Tracey K.J. The cholinergic anti-inflammatory pathway. Brain Behav. Immun. 2005;19(6):493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pelsers M.M., Namiot Z., Kisielewski W., Namiot A., Januszkiewicz M., Hermens W.T. Intestinal-type and liver-type fatty acid-binding protein in the intestine. tissue distribution and clinical utility. Clin. Biochem. 2003;36(7):529–535. doi: 10.1016/s0009-9120(03)00096-1. S0009912003000961 [pii] [DOI] [PubMed] [Google Scholar]
- Peters E.G., Smeets B.J.J., Nors J., Back C.M., Funder J.A., Sommer T. Perioperative lipid-enriched enteral nutrition versus standard care in patients undergoing elective colorectal surgery (SANICS II): a multicentre, double-blind, randomised controlled trial. The Lancet. Gastroenterology & Hepatology. 2018;3(4):242–251. doi: 10.1016/S2468-1253(18)30031-1. S2468-1253(18)30031-1 [pii] [DOI] [PubMed] [Google Scholar]
- Peters E.G., Smeets B.J., Dekkers M., Buise M.D., de Jonge W.J., Slooter G.D. The effects of stimulation of the autonomic nervous system via perioperative nutrition on postoperative ileus and anastomotic leakage following colorectal surgery (SANICS II trial): a study protocol for a double-blind randomized controlled trial. Trials. 2015;16:20-x. doi: 10.1186/s13063-014-0532-x. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plas M., Rotteveel E., Izaks G.J., Spikman J.M., van der Wal-Huisman H., van Etten B. Cognitive decline after major oncological surgery in the elderly. European Journal of Cancer (Oxford, England : 1990) 2017;86:394–402. doi: 10.1016/j.ejca.2017.09.024. [DOI] [PubMed] [Google Scholar]
- Pradelli L., Mayer K., Klek S., Alsaleh Omar, A J., Clark R.A.C., Rosenthal M.D. Omega-3 fatty-acid enriched parenteral nutrition in hospitalized patients: systematic review with meta-analysis and trial sequential analysis. JPEN - J. Parenter. Enter. Nutr. 2020;44(1):44–57. doi: 10.1002/jpen.1672. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson D.J., Bannerman D.M. Competitive short-term and long-term memory processes in spatial habituation. J. Exp. Psychol. Anim. Behav. Process. 2011;37(2):189–199. doi: 10.1037/a0021461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson D.J., McHugh S.B., Good M.A., Sprengel R., Seeburg P.H., Rawlins J.N. Spatial working memory deficits in GluA1 AMPA receptor subunit knockout mice reflect impaired short-term habituation: evidence for wagner's dual-process memory model. Neuropsychologia. 2010;48(8):2303–2315. doi: 10.1016/j.neuropsychologia.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer H., Rummel C., Roth J., Rosengarten B. Modulatory effects of vagal stimulation on neurophysiological parameters and the cellular immune response in the rat brain during systemic inflammation. Intensive Care Medicine Experimental. 2016;4(1) doi: 10.1186/s40635-016-0091-4. 19–4. Epub 2016 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skvarc D.R., Berk M., Byrne L.K., Dean O.M., Dodd S., Lewis M. Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci. Biobehav. Rev. 2018;84:116–133. doi: 10.1016/j.neubiorev.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Smeets B.J.J., Luyer M.D.P. Nutritional interventions to improve recovery from postoperative ileus. Curr. Opin. Clin. Nutr. Metab. Care. 2018;21(5):394–398. doi: 10.1097/MCO.0000000000000494. ([doi]) [DOI] [PubMed] [Google Scholar]
- Terrando N., Monaco C., Ma D., Foxwell B.M., Feldmann M., Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc. Natl. Acad. Sci. U. S. A. 2010;107(47):20518–20522. doi: 10.1073/pnas.1014557107. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K.J. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tracey K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 2007;117(2):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goot E., van Spronsen F.J., Falcao Salles J., van der Zee E.A. A microbial community ecology perspective on the gut-microbiome-brain axis. Front. Endocrinol. 2020;11:611. doi: 10.3389/fendo.2020.00611. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J.B., Lewis G., Grippo A.J., Lamb D., Harden E., Handleman M. Autonomic predictors of recovery following surgery: a comparative study. Auton. Neurosci. : Basic & Clinical. 2010;156(1–2):60–66. doi: 10.1016/j.autneu.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material are available upon request.