Abstract
Disturbances of the immune system and immune responses after activation are a common finding in neuropsychiatric disorders. Psychotic and affective disorders such as major depressive disorder (MDD), schizophrenia (SCZ) and bipolar disorder (BD) also share high rates of comorbidity with inflammatory and metabolic disorders. Evidence of elevated circulating inflammatory cytokines, altered numbers and function of immune cells, and evidence of neuroinflammation including activation of microglia in the brain have been found in patients with SCZ, BD and MDD. Often these findings correlate to psychological state at the time of measurement. However, significant variation exists across these studies in many aspects, creating challenges in identifying a specific signature of immune dysfunction in these disorders. Innate immune dysfunction, and alterations in monocytes, the critical sentinel cells of the innate immune system, have been seen repeatedly in all three of these disorders, with frequent overlap in findings. In this review, dysfunction specific to the innate arm of the immune system is compared for overlapping evidence across three major psychotic and affective disorders.
Keywords: Inflammation, Monocytes, Microglia, Neuroinflammation, Immune dysfunction, Schizophrenia, Major depressive disorder, Bipolar disorder, Psychosis
1. Introduction
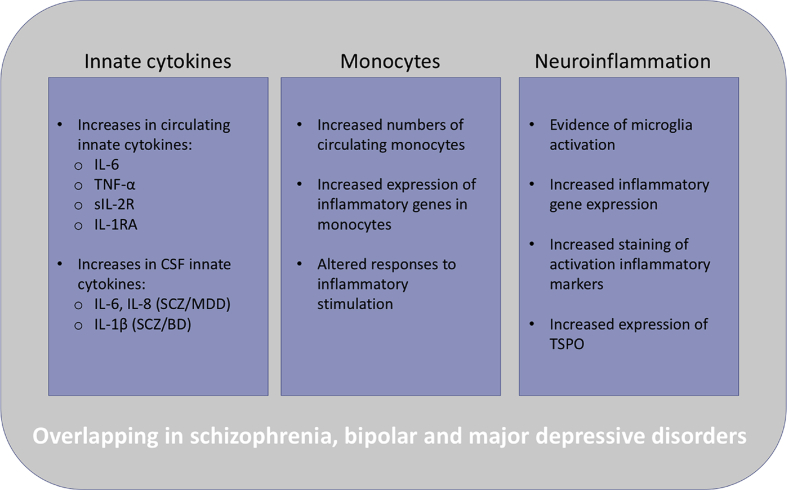
The etiologies of neuropsychiatric disorders are largely unknown, however immune dysfunction and abnormalities have been noted for decades in these disorders and continue to be vigorously investigated as more evidence reveals the interplay of the immune and nervous systems. Evidence that the immune system plays a role in psychotic and affective disorders includes pathway analyses of data from genome-wide association studies (GWAS) that link genes involved in immune system signaling with schizophrenia (SCZ), bipolar disorder (BD) and major depressive disorder (MDD) (Network and Pathway Analysis Subgroup of Psychiatric Genomics, 2015). Prenatal infections confer an increased risk for psychiatric disorders in offspring (Brown, 2012; Canetta et al., 2014; Miller et al., 2013; Parboosing et al., 2013) and these findings are supported by preclinical models of maternal infection (Meyer and Feldon, 2010; Smith et al., 2007). Results from cytokine studies indicate that immune dysfunction may occur in psychiatric patients. Within these studies, increases in inflammatory cytokines associated with monocytes and macrophages are seen with consistency across these disorders [reviewed in (Goldsmith et al., 2016)]. These findings have led to the hypothesis that myeloid cell dysfunction may be participating in the pathogenesis of these disorders. This review provides an overview and comparison of abnormal innate immune findings in SCZ, BD and MDD, including similarities across all three disorders (Fig. 1).
Fig. 1.
Overview of the innate immune findings that overlap in schizophrenia and psychotic disorder, bipolar disorder, and major depressive disorder. Inflammatory immune findings are common in neuropsychiatric disorders and have been under investigation for decades. This review discusses findings specific to innate immunity and monocyte/microglia activation and dysfunction. Specific alterations of the innate arm of the immune system overlap in psychotic and affective disorders, and may suggest common pathophysiological pathways. However, these findings are not always consistent and state of the disorder (i.e. acute episodes versus chronic illness) may be influencing immunological outcomes. Inconsistencies may also be due to treatments such as antidepressant and antipsychotic medications.
Activation of the immune system is a highly regulated process involving two separate arms: the innate and adaptive arms. The innate immune system is the first line of defense intended to confer rapid and broad protection against invading pathogens by recognition of pathogen-associated molecular patterns (PAMPS) via toll-like receptors (TLRs) and other pattern recognition receptors (Mogensen, 2009). Cells of the innate immune system include cells of myeloid lineage such as neutrophils, monocytes, macrophages and dendritic cells, which play important roles in phagocytosis of pathogens, debris or dead and dying cells, and in antigen-presenting to the adaptive immune T cells (Chaplin, 2010). The adaptive arm of the immune system often develops unique specificity to pathogenic antigens and confers protective memory, however this process takes time and considerable energy/resources to develop after initial exposure to pathogens and coordination with cells of the innate arm. The adaptive immune system mainly consists of T and B cells, derived from lymphoid progenitors (Chaplin, 2010). Other components of the immune system include acute phase proteins, complement, antibodies, and cytokines which are signaling proteins produced in response to activation of both innate and adaptive immune cells, and exposure of other cells (such as epithelial cells) to PAMPs (Chaplin, 2010).
2. Inflammatory cytokines
Cytokines are the chemical messengers of the immune system, many with plieotropic functions to enhance or inhibit the inflammatory processes of various immune cells. Identifying differences in circulating cytokines can offer a comparative snapshot of inflammatory and anti-inflammatory profiles between groups as well as during different states of disease. Over the past few decades, there have been dozens of studies measuring cytokines in plasma, serum and cerebral spinal fluid (CSF) in psychiatric disorders, with significant heterogeneity in results. Meta-analyses can help clarify which inflammatory patterns are most often seen across studies, with several recent meta-analyses coming to the consensus that elevations in circulating inflammatory cytokines associated with innate immune activation are seen in psychotic and affective disorders. In non-medicated first episode psychosis (FEP) patients, interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α and soluble IL-2 receptor (sIL-2R) were all significantly elevated (Upthegrove et al., 2014). Pillinger et al. also found increases in IL-6, IL-17, TNF-α, and interferon (IFN)-γ in FEP patients (Pillinger et al., 2019). Increased transforming growth factor (TGF)-β was noted, however there was significant heterogeneity within the patient population for this cytokine (Pillinger et al., 2019). In BD, significant elevations of inflammatory cytokines were found in manic patients compared to healthy controls, including TNF-α, soluble tumor necrosis factor receptor type 1 (sTNF-R1) and sIL-2R. This meta-analysis also found that manic patients had these elevations compared to the euthymic BD patients, indicating differences in inflammatory profiles based on clinical status (Munkholm et al., 2013). A separate meta-analysis from the same year found increased IL-4, TNF-α, and several soluble inflammatory receptors in both manic and euthymic BD patients compared to HC, along with a trend in increased IL-6 (Modabbernia et al., 2013). Increased IL-6 was often seen across studies, including a meta-analysis of cytokines measured in unmedicated MDD patients that found increased TNF-α and IL-6 in depressed patients compared to controls (Dowlati et al., 2010). Our own research recently identified increased plasma IL-6 in SCZ compared to healthy controls (Lesh et al., 2018), and expression of IL-6 mRNA in peripheral blood mononuclear cells (PBMC) has been proposed as a biomarker in SCZ (Chase et al., 2016).
Goldsmith et al. performed a meta-analysis of blood cytokines across SCZ, BD and MDD, and included differences between acutely versus chronically ill patients. Their findings suggest a common dysfunction in inflammatory pathways in all three disorders, which potentially mediates aspects of their pathophysiology. Significant elevations of IL-6, TNF-α, sIL-2R and IL-1RA were found during acute phases of all three disorders compared to healthy controls. IL-6 was elevated in chronically ill patients across disorders, and IL-1β and sIL-2R was increased in chronic SCZ and euthymic BD. They also identified reductions in inflammatory cytokines including IL-6 after treatment of acute episodes (Goldsmith et al., 2016). In addition to blood levels of cytokines across these disorders, a meta-analysis of CSF cytokines found similar results, with increases in IL-1β in SCZ and BD, and both IL-6 and IL-8 in SCZ and MDD, however correlations to clinical status were not included in this meta-analysis (Wang and Miller, 2018). The role of these inflammatory cytokines in these disorders is yet unknown, however, it is noteworthy that cytokine-based immunotherapy for unrelated disorders such as hepatitis C have been associated with episodes of psychosis and depression, and adjunctive treatment with anti-inflammatory medications such as NSAIDS is associated with improved clinical scores in SCZ (Miller and Buckley, 2016; Nitta et al., 2013), therefore, elevations in inflammatory cytokines may be contributing to psychopathology of these disorders.
3. Myeloid cell dysfunction
Inflammatory cytokines such as IL-1β, TNF-α, and IL-6 are associated with innate immune activation. These cytokines are known to induce fever and IL-6 is a major mediator of acute phase protein production in the liver. Large amounts of these cytokines are produced by activated innate immune cells including monocytes/macrophages during acute phase responses to pathogens. The consistent elevations of these cytokines seen in these disorders may suggest chronic activation or dysfunction in this subset of cells (Arango Duque and Descoteaux, 2014).
Derived from myeloid precursors in bone marrow, circulating monocytes are sentinels tasked with patrolling their environment and during inflammatory events they are recruited to tissues where they join tissue-resident macrophages (Dey et al., 2015). Macrophages have high phenotypic plasticity, and can be polarized in vitro to classical inflammatory “M1” phenotype through stimulation with LPS plus IFN-γ or TNF-α. Through stimulation with IL-4 and IL-13, the M1 phenotype is inhibited and cells skew to the alternatively activated M2 phenotype, of which there are several sub-phenotypes. These cells can also lie somewhere along the spectrum between phenotypes, and depending on the local milieu, macrophages can interconvert between phenotypes. When activated, M1 macrophages are IL-12hiIL-23hiIL-10lo and produce high levels of proinflammatory cytokines including IL-1β, IL-6, IL-8 and TNF-α, and participate in Th1 cell polarization. M2 macrophages are IL-12loIL-23loIL-10hiTGF-βhi and produce anti-inflammatory IL-10 which can suppress an M1 phenotype (Italiani and Boraschi, 2014).
Altered cell concentrations and responses attributed to innate immune cells have often been seen in neuropsychiatric disorders (summarized in Table 1). For example, increased circulating monocytes have been seen in SCZ and FEP (Drexhage et al., 2011a; Orhan et al., 2018; Rothermundt et al., 1998; Zorrilla et al., 1996), and recent meta-analysis identified significantly elevated neutrophil/lymphocyte and monocyte/lymphocyte ratios in non-affective psychosis patients compared to health controls, indicating the presence of systemic inflammation (Mazza et al., 2019). During acute psychotic episodes, monocytes were increased in CSF and found to occasionally aggregate with lymphocytes, indicating upregulation of adhesion molecules or functional costimulatory interactions occurring between cells (Nikkilä et al., 2001). Increased monocytes have also been seen in MDD (Seidel et al., 1996) and BD (Barbosa et al., 2014), however, results have not always been as consistent and some studies found increases in only non-classical monocytes (Hasselmann et al., 2018), decreases in monocytes or no differences compared to healthy controls (Drexhage et al., 2011b; Torres et al., 2009).
Table 1.
Summary of myeloid cell findings in SCZ, BD and MDD.
| Numbers of myeloid cells | |||
|---|---|---|---|
| Author/Year | Subjects | Methods | Findings |
| Zorrilla et al. (1996) | 92 SCZ 94 HC |
Automated cell counter | ↑ total leukocytes ↑ total numbers of monocytes and granulocytes |
| Seidel et al. (1996) | 33 MDD 44 HC |
Automated cell counter | ↑ total leukocytes ↑ total numbers of monocytes and granulocytes (mainly neutrophils). Monocytes remained elevated over time in patients with more severe MDD |
| Rothermundt et al. (1998) | 44 acutely ill SCZ 44 HC |
Flow cytometry on whole blood | ↑ absolute and relative monocyte numbers |
| Nikkilä et al. (2001) | 30 SCZ with acute psychosis 46 HC |
Papanicolaou stain of fixed CSF slides | ↑ percentage of macrophages/monocytes, with occasional aggregation with lymphocytes consistent with increased activation |
| Torres et al. (2009) | 8 SCZ 7 BD 7 HC |
Flow cytometry on PBMC | ↓ percentage of CD14+ monocyte in SCZ. No relative difference in CD14+ monocytes numbers in BD |
| Drexhage et al. (2011a) | 26 SCZ with acute psychosis 26 HC |
Flow cytometry on PBMC | ↑ percentage of monocytes |
| Drexhage et al. (2011b) | 38 BD 22 HC |
Flow cytometry on PBMC | No differences in percentage of monocytes |
| Barbosa et al. (2014) | 21 euthymic BD 21 HC |
Flow cytometry on PBMC | ↑ percentage of CD14+ monocytes |
| Orhan et al. (2018) | 42 FEP 22 HC |
Automated cell counter | ↑ blood monocyte numbers |
| Hasselmann et al. (2018) | 35 MDD 35 HC |
Flow cytometry on PBMC | ↓ frequency of classical CD14++/CD16- monocytes ↑ frequency and absolute numbers of non-classical CD14+/CD16++ monocytes |
| Mazza et al. (2019) | 8 observational studies involving FEP and SCZ patients | Meta-analysis that identified differences in WBC ratios in patients compared to HC | ↑ neutrophil/lymphocyte ratio (NLR) ↑ monocyte/lymphocyte ratios (MLR) |
|
| |||
| Gene and protein expression in monocytes/macrophages | |||
| Author/Year | Subjects | Methods | Findings |
| Padmos et al. (2008) | 42 BD 25 HC 54 BD offspring 70 HC |
Affymetrix microarray and q-PCR on mRNA from pooled patient CD14+ monocytes | ↑ inflammatory and activated immune signature in monocytes of BD compared to HC. Positive signature was predictive of later onset of mood disorder in unrelated offspring cohort. Downregulation of inflammatory genes seen in monocytes from lithium and antipsychotic treated patients. |
| Torres et al. (2009) | 8 SCZ 7 BD 7 HC |
Flow cytometry on PBMC | ↓ DARPP-32 expression in CD14+ monocytes of BD |
| Drexhage et al. (2010) | 27 SCZ 56 BD |
Affymetrix microarray and q-PCR on mRNA from pooled patient CD14+ monocytes | Strong overlap in inflammatory gene expression in monocytes of SCZ and BD. Cluster analysis identified three subsets of inflammatory genes in monocytes, two of which were shared between SCZ and BD, and one in which was upregulated in BD but downregulated in SCZ. |
| Carvalho et al. (2014) | 47 MDD 42 HC |
RTq-PCR of 47 genes on mRNA from purified CD14+ monocytes | ↑ inflammatory and chemotaxic gene expression in MDD monocytes. Extensive overlap with clusters previously identified in BD and SCZ. |
| Brambilla et al. (2014) | 20 SCZ 20 BD 20 HC |
RT-qPCR of 25 immune genes on PBMC mRNA | ↑ expression of M1 markers ↓ expression of M2 markers in PBMC |
| Becking et al. (2015) | 82 euthymic BD 8 BD with mood episode 69 HC |
RTq-PCR of 35 inflammatory genes on mRNA from purified CD14+ monocytes | ↑ inflammatory gene expression in monocytes during mood episodes. Study limited by small sample size of episodic patients. |
| Grosse et al. (2015) | 56 MDD 57 HC |
RTq-PCR of 38 inflammatory/immune activation genes on mRNA from purified CD14+ monocytes | Inflammatory gene expression in monocytes only ↑ in patients ≥ 28 years. |
| Ormel et al. (2017) | 15 SCZ 15 HC |
RT-qPCR of 24 genes on mRNA from 7-day cultured then stimulated CD14+ monocytes isolated from PBMC. | No differences in inflammatory gene expression of monocyte-derived macrophages, however P2RX7 expression involved in purinergic signaling was significantly reduced after LPS stimulation. |
| Ferrari et al. (2018) | 5 manic BD 5 depressed BD 8 euthymic BD 5 HC |
Monocyte cell line cultured with serum from BD patients and HC | ↑ monocyte production of IL-1β and TNF-α when exposed to serum from patients experiencing manic or depressive episodes. |
| Hasselmann et al. (2018) | 35 MDD 35 HC |
RT q-PCR on mRNA from CD14+ monocytes | ↓ expression of steroid signaling genes GR and GILZ in CD14+ monocytes |
| Ascoli et al. (2019) | 9 early-stage BD 9 late-stage BD 10 HC |
Stages of BD determined by FAST scoring. MDM obtained from PBMC adherence isolation and 7-day culture with M-CSF. Skewed to M1/M2 with IFNγ + LPS or IL-4 respectively. | ↓ inflammatory responses in M1 and M2 MDM in late-stage BD compared to early-stage BD and HC, suggesting progressive dysfunction of innate immunity in BD. |
SCZ = schizophrenia; HC = healthy control; MDD = major depressive disorder; CSF = cerebral spinal fluid; BD = bipolar disorder; FEP = first episode psychosis; WBC = white blood cells; q-PCR = quantitative polymerase chain reaction; RT = reverse transcriptase; mRNA = messenger RNA; M1 = classically activated phenotype; M2 = alternatively activated phenotype; LPS = lipopolysaccharide; IL-1β = interleukin 1-beta; TNF-α = tumor necrosis factor - alpha; IFNγ = interferon gamma; IL-4 = interleukin 4; FAST = Functioning Assessment Short Test; M-CSF = macrophage colony-stimulating factor; MDM = monocyte-derived macrophages.
Altered expression of inflammatory genes have been seen in monocytes from patients with BD (Padmos et al., 2008), and in a later study that included SCZ patients they found a high “inflammatory set point” established in monocytes from both SCZ and BD patients, with elevated expression of subsets of genes shared across disorders. BD monocytes also had dysregulation in motility/adhesion factors (Drexhage et al., 2010). These researchers later identified increases in inflammatory genes during mood episodes compared to euthymic states, suggesting that inflammation in BD may be dependent on state (Becking et al., 2015). Gene expression of monocytes from MDD patients were found to have enrichment for several inflammatory and chemotaxis mediators (Carvalho et al., 2014), and more recent investigations of MDD monocytes found that inflammatory gene expression increased with age and were associated with a decrease in T regulatory cells, which are critical for immune regulation after activation (Grosse et al., 2015, 2016). Brambilla et al. measured gene expression in PBMC and found elevated M1 markers il6 and ccl3 in BD compared to HC and SCZ, indicative of inflammatory monocytes in the bipolar group only. Markers of M2 or alternatively activated monocytes were reduced (Brambilla et al., 2014). A pre-clinical model of psychotic disorders that found differences in cytokine production of bone marrow derived macrophages (BMDM) from offspring of dams treated with polyI:C during gestation. BMDM were stimulated to skew to either an M1 or M2 phenotype, and produced increased inflammatory cytokines and reduced anti-inflammatory cytokines under each condition compared to macrophages from control animals (Onore et al., 2014). Similar activated M1 phenotypes in PBMC stimulated with LPS were seen in a non-human primate model of maternal immune activation (MIA) (Rose et al., 2017).
Not all studies investigating monocytes/macrophages in these disorders had elevated inflammatory phenotypes or responses. When characterizing gene expression specifically from monocyte-derived macrophages (MDMs) in SCZ patients, Ormel et al. did not find differences in phenotype or function of these cells. They found only one gene involved in purinergic signaling with reduced expression in SCZ (Ormel et al., 2017). A significant reduction in inflammatory responses in stimulated MDMs was seen in late stage BD compared to early stage BD and healthy controls, specifically a reduction in IL-1β, IL-6 and IL-10 (Ascoli et al., 2019). Previous investigations by this group found increased production of IL-1β and TNF-α in monocytes from the human monocyte cell line U-937 when exposed to serum from manic and depressive BD patients compared to controls and euthymic BD (Ferrari et al., 2018). They also identified increased circulating molecules that signal cell stress and tissue damage (damage-associated molecular patterns, DAMPs) in serum of BD patients (Stertz et al., 2015). High levels of DAMPs are associated with inflammatory diseases and can augment monocyte responses in vivo (Piccinini and Midwood, 2010) however, some DAMPs are capable of signaling through TLRs and activating NFkB, and exposure to circulating DAMPs could also be leading to the dampened responses seen in monocytes when cultured with TLR agonists away from the endogenous milieu. For example, mitochondrial DAMPs have been found to induce endotoxin tolerance, a state of impaired inflammatory responses to LPS in monocytes (Ascoli et al., 2019; Fernández-Ruiz et al., 2014; Hernandez-Jimenez et al., 2017; Piccinini and Midwood, 2010). Due to the variable results seen in monocytes/macrophages across these disorders, more studies examining the phenotype and function of these cells are needed.
4. Neuroinflammation
Considered the innate phagocytic immune cells of the brain, the microglia are the guardians and “gardeners” of the brain, essential for the developing architecture of the brain and responsible for responding quickly to danger signals and invaders (reviewed in (Hughes, 2012) (Li and Barres, 2018):]. These cells share functional similarities with monocytes, however, they are sourced differently. Colonized early during development, several fate-mapping studies show that microglia are derived from yolk sac progenitors (Ginhoux and Guilliams, 2016; Gomez Perdiguero et al., 2015). As neurogenesis nears completion, the microglia remove neuronal precursor cells through phagocytosis, regulating the size of the neuronal precursor pool (Cunningham et al., 2013). Microglia activation is required to some degree during brain development (Cunningham et al., 2013), however, excessive activation can reduce neuronal connectivity and chronic activation may affect brain volume and is associated with neurodegenerative diseases (Smith et al., 2012).
Microglia also regulate numbers of developing neurons by inducing cell death through respiratory bursts (Marin-Teva et al., 2004). Conversely, these cells also produce trophic factors such as microglia-derived insulin-like growth factor (IGF-) 1 and brain-derived neurotrophic factor (BDNF) that are important for brain homeostasis, neuronal function and survival (Ferrini and De Koninck, 2013). They are critically involved in the maturation of synapses and in remodeling/pruning of immature synapses through constant monitoring of the local environment and synapses by extending and retracting their ramified processes and making contact with synapses and neighboring astrocytes and neurons (Nimmerjahn et al., 2005; Wake et al., 2009). Similar to their peripheral macrophage counterparts, microglia display phenotypic plasticity and when activated under inflammatory conditions can produce inflammatory cytokines, including IL-1β, IL-6 and TNF-α (Smith et al., 2012). However, new insights into the heterogeneity of microglia from transcriptomic studies indicate they may have increased diversity and do not necessarily follow the phenotypic skewing of M1/M2 macrophages, both during homeostatic and activated conditions compared to their peripheral counterparts (Ransohoff, 2016). In fact, both M1 and M2 markers are present on microglia during neurodevelopment and throughout adulthood, indicating they are not fully committed to either pro versus anti-inflammatory, or “resting” versus “activated” phenotypes (Crain et al., 2013; Ransohoff, 2016).
Neuroinflammation has been proposed to be involved in the pathogenesis of psychotic and affective disorders, however the evidence of microglia activation among these disorders is somewhat inconclusive. Increased inflammatory gene expression and staining of activation and neuroinflammatory markers such as HLA-DR, c-FOS, iNOS, IL-1β, IL-6, IL-1R, TNF-α, and NF-κB expression have been seen in the frontal and prefrontal cortex of psychotic and affective disorders (Bayer et al., 1999; Dean et al., 2010; Fillman et al., 2013; Rao et al., 2010). Increased density of MHC-II+ microglia were also identified in post-mortem prefrontal cortex of brains from SCZ (Fillman et al., 2013), and a recent meta-analysis of 41 studies identifying alterations in cell density found a significant increase in microglia density in schizophrenia (van Kesteren et al., 2017). Increased expression of SERPINA3, known to be produced by macrophages and reactive astroglia, and immune-associated IFITM were consistently seen across SCZ studies (Trepanier et al., 2016). However, patterns of reduced numbers and density of glial cells and neurons have also been identified in schizophrenia and mood disorder post-mortem tissue, with differences in density dependent on the region examined (Ongür et al., 1998; Rajkowska et al., 1999, 2001) and were sometimes specific to type of disorder (Hamidi et al., 2004). Other studies found no differences in microglia density or activation (Hercher et al., 2014; Sneeboer et al., 2019). As a whole, these studies are inconclusive as to whether neuroinflammation is occurring consistently across these disorders, and could, in part, be due to heterogeneity across disorders and variability in study designs, including disparate brain regions and cortical layers studied, differing methods of measurement, medications and/or stage of disorder. In vivo studies using positron emission tomography (PET) imaging can further help elucidate activation of the immune system in the CNS.
Activated microglia upregulate expression of the 18 kDa translocator protein (TSPO) previously known as the peripheral benzodiazepine receptor. TSPO is normally expressed in low levels in the healthy brain, therefore identifying increased expression of TSPO via PET imaging can correlate to the extent of microglial activation in the living brain. TSPO radioligands developed for in vivo visualization via PET scan determine if this protein is upregulated in glial cells and is potentially an important biomarker of neuroinflammation (Venneti et al., 2013), however, consistency in regions of interest studies, radiotracers used, and outcome measures have been lacking thus far in psychotic and affective disorders (Marques et al., 2019). Although some studies have indicated that TSPO expression is increased in various brain regions of SCZ, BD and MDD patients (Bloomfield et al., 2016; Doorduin et al., 2009; Haarman et al., 2014; Holmes et al., 2018; Richards et al., 2018; Setiawan et al., 2015, 2018; van Berckel et al., 2008), other studies have not seen this same phenomenon (summarized in Table 2) (Collste et al., 2017; Coughlin et al., 2016; Di Biase et al., 2017; Hafizi et al., 2017; Kenk et al., 2015; Notter et al., 2017; van der Doef et al., 2016). Different outcome measures such as binding potential (BP) versus total distribution volume (VT) could explain inconsistencies seen across studies. A recent meta-analysis looked at TSPO studies solely in SCZ/psychosis patients. They chose to exclude any studies not specifically reporting VT values as outcome measure. This resulted in reporting five studies in which they concluded that total distribution volume of TSPO is reduced in this disorder, and medication does not appear to be contributing to this reduction (Plavén-Sigray et al., 2018). A separate meta-analysis came to the conclusion that elevations in TSPO were clearly evident when outcome measure was binding potential (BP), but not so when VT was the outcome measure. VT also resulted in more variability across studies, and the authors suggest that future research follow both measurement approaches for more accurate comparison (Marques et al., 2019). These inconsistencies reported across studies may also be due to variations in the type of radiotracer used. First generation TSPO radiotracers exhibit high levels of non-specific binding and low signal-to-noise ratio. Newer radiotracers are promising, however they can have variable binding based on the polymorphism in the gene that encodes TSPO, therefore some patients with the low-affinity polymorphism should be omitted from studies otherwise there is a risk for false negative readings (Venneti et al., 2013). Timing of study after diagnosis could contribute to variability, for example some studies measured TSPO at “early onset” while others measured chronic disease in patients years after diagnosis. Medication use may also influence outcomes, as antipsychotics are known to influence expression of TSPO (Danovich et al., 2008).
Table 2.
Summary of TSPO PET studies in SCZ, BD and MDD.
| Reference | N= | Clinical details | Ligand | OM | Regions of Interest (ROI) | Findings |
|---|---|---|---|---|---|---|
| van Berckel et al. (2008) | 10 SCZ 10 HC |
Recent onset | [11C](R)-PK11195 | BPp | Whole brain (total gray area) | ↑BPp in total gray area |
| Doorduin et al. (2009) | 7 SCZ 8 HC |
Current psychotic episode | [11C](R)-PK11195 | BP | frontal, occipital, temporal and parietal CTX, basal ganglia, HC, CB, thalamus, pons, brain stem | ↑BP in HC |
| Takano et al. (2010) | 14 SCZ 14 HC |
Chronically ill | [11C]DAA1106 | BPND | mPFC, DLPFC, lateral temporal CTX, parietal and occipical CTX, thalamus, striatum, CB, ACC and PCC | No differences in BPND. Medial, frontal, temporal and occipital CTX BPND correlated with positive PANSS scores. BPND in total cortical regions correlated with duration of illness. |
| Hannestad et al. (2013) | 10 MDD 10 HC |
Mild-to moderately depressed patients in current MDE | [11C]PBR28 | VT | Frontal, temporal, parietal, and occipital CTX, caudate, putamen, thalamus, CB and white matter | No differences in VT, no correlations with depression severity. |
| Haarman et al. (2014) | 14 BD 11 HC |
Medicated, euthymic BD (except one) | [11C](R)-PK11195 | BP | HC, DLPFC, temporal parietal, frontal and occipital CTX, ACC, PCC, CB, basal ganglia. | ↑ BP in right HC |
| Setiawan et al. (2015) | 20 MDD 20 HC |
Moderate to severe MDD in current MDE, unmedicated | [18F]FEPPA | VT | PFC, ACC, insula | ↑ VT in all ROIs examined, correlated with severity of MDE (especially in ACC) |
| Kenk et al. (2015) | 16 SCZ 27 HC |
Chronically ill and on antipsychotics | [18F]FEPPA | VT | HC, mPFC, DLPFC, temporal CTX, striatum, corpus callosum, cingulum, superior longitudinal fasciculus, posterior limb of the internal capsule | No differences seen, no correlations with clinical measures, length of illness, or MRI volumes |
| Bloomfield et al. (2016) | 14 UHR 14 HC 14 SCZ 14 HC |
Two separate cohorts: 1) unmedicated UHR; 2) SCZ taking antipsychotics | [11C]PBR28 | VT and DVR | total gray matter, frontal and temporal lobes, CB, brain stem | ↑ DVR in total gray matter, frontal and temporal gray matter in both. UHR DVR in total gray matter correlated with clinical severity scores on CAARMS. |
| Coughlin et al. (2016) | 12 SCZ 14 HC |
Recent onset | [11C]DPA-713 | VT | HC, amygdala and 6 cortical regions: insula, cingulate, parietal, frontal, temporal and occipital CTX | No differences in VT. ↑ IL-6 in CSF and plasma, CSF IL-6 levels correlated with plasma levels. |
| van der Doef et al. (2016) | 19 PD 17 HC |
Recent onset | [11C](R)-PK11195 | BPND | frontal, temporal and parietal CTX, striatum, and thalamus | No differences in BPND |
| Hafizi et al. (2017) | 19 FEP 20 HC |
Unmedicated FEP, 14 anti-psychotic naïve | [18F]FEPPA | VT and DVR | DLPFC, HC, mPFC, temporal CTX, total gray matter and whole brain | No differences in VT or DVR |
| Collste et al. (2017) | 16 FEP 16 HC |
Drug naïve FEP | [11C]PBR28 | VT | Gray matter, frontal and temporal CTX, HC, white matter | ↓ Vt in all ROIs except white matter |
| Di Biase et al. (2017) | 10 UHR 33 SCZ 27 HC |
UHR, 15 recent onset and 18 chronically ill SCZ. Medicated vs unmedicated |
[11C](R)-PK11195 | BP | Dorsal frontal, orbital frontal, and medial temporal CTX, thalamus, insula, ACC | No differences in BP across groups |
| Holmes et al. (2018) | 14 MDD 13 HC |
Unmedicated, 9 MDD were suicidal | [11C](R)-PK11195 | BPND | ACC, PFC and insula | ↑BPND in ACC |
| Setiawan et al. (2018) | 50 MDD 30 HC |
Current MDE. 25 were untreated greater than 10 years. | [18F]FEPPA | VT | ACC, PFC and insula | ↑ VT in ACC, PFC and insula associated with duration of untreated illness |
| Richards et al. (2018) | 18 MDD 20 HC |
Current MDE, 12 un-medicated | [11C]PBR28 | VT | ACC and sgPFC | Trend for ↑ VT in unmedicated ACC and sgPFC |
| Notter et al. (2017) | 12 SCZ 14 HC |
Recent onset | [11C]DPA-713 | VT | MFG | ↓ VT in MFG |
OM = outcome measure; HC = Healthy control; SCZ = schizophrenia; MDD = major depressive disorder; MDE = major depressive episode; BD = bipolar disorder; UHR = ultra-high risk of psychosis; PD = psychotic disorder; FEP = first episode psychosis; CHR = clinical high risk; CTX = cortex; HC = hippocampus; CB = cerebellum; PFC = prefrontal cortex; mPFC = medial prefrontal cortex; DLPFC = dorsolateral prefrontal cortex; ACC = anterior cingulate cortex; BP = binding potential (-p plasma, ND; BPP = specific binding over plasma; BPND = specific binding over non-displaceable binding; VT = total volume of distribution; DVR = distribution volume ratio; ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; sgPFC = subgenual prefrontal cortex; MFG = mid frontal gyrus; PANSS = The Positive and Negative Syndrome Scale; CAARMS = Comprehensive Assessment of the At-Risk Mental States.
A theme has emerged that neuroinflammation may be associated with acute psychotic or affective episodes, and the significant variability seen across studies might be due in part to differences in disease states at time of study. For example, in post-mortem studies identifying microglia activation in schizophrenia and affective disorder, increased microglial cell density was seen only in those who had committed suicide (Steiner et al., 2008b), and ionized calcium-binding adapter molecule 1 (IBA-1) staining showed increased numbers of primed microglia with upregulated gene expression of IBA-1 and MCP-1 compared to non-suicidal subjects (Torres-Platas et al., 2014). Suicide was also associated with increased density of IBA-1 positive activated microglia in the ventral portion of the prefrontal cortex, and density was greater at or near blood vessels in this region of the brain in those who died from suicide (Schnieder et al., 2014). S100B, a protein that acts as a DAMP involved in inflammatory processes, was seen elevated in post-mortem SCZ brain tissue depending on paranoid versus residual state (Steiner et al., 2008a). Higher density of HLA-DR+ microglia was also seen in paranoid versus residual states of SCZ (Busse et al., 2012). A recent case-control study compared TSPO BP in unmedicated MDD patients experiencing a major depressive episode (MDE) compared to healthy controls. They found increased TSPO binding in patients with suicidal ideations, compared to healthy controls and patients without suicidal thoughts. This increase was found to be significant within the anterior cingulate cortex (ACC), with BP in this region and the insula correlating with severity of depression (Holmes et al., 2018). Setiawan et al. also found that unmedicated MDD patients experiencing MDE had elevated gray matter TSPO density in the prefrontal cortex (PFC), ACC and insula, and scores from the Hamilton Depression rating Scale (HDRS) correlated significantly with TSPO VT in the ACC (Setiawan et al., 2015). TSPO VT was also increased in two additional studies of untreated MDD patients, compared to treated MDD patients and healthy controls (Richards et al., 2018) with longer durations of unmedicated illness showing greater increases in TSPO VT (Setiawan et al., 2018). TSPO VT was not significantly different in any brain regions of interest when evaluating patients with milder symptoms of depression (Hannestad et al., 2013). In one study of SCZ patients that saw no significant differences in TSPO, cortical binding was still found to be correlated to positive symptoms of psychosis and duration of illness (Takano et al., 2010). Most of these studies are limited by the small patient population and may have variability due to non-specific binding however they do provide some evidence that alterations in immune function are taking place in these disorders and further evaluation of TSPO expression in various brain regions, especially when correlated to clinical features, is warranted.
5. Conclusion
Decades of research have made clear that some level of immune dysfunction likely plays a role in neuropsychiatric disorders. Unfortunately, due to the high variability across studies, there is little consensus as to the nature and origin of this dysfunction. We summarized recent research indicating evidence of innate immune dysfunction, as the inflammatory cytokine network most often seen in SCZ, BD and MDD reflect activation of innate immune cells such as monocytes and macrophages. There is some evidence, both in post-mortem brain tissue as well as in living PET studies that neuroinflammation and activated microglia may be present during active states of these disorders. However, many of these studies had conflicting or inconclusive results, complicating the overall picture of the degree of immune dysfunction present in SCZ, BD and MDD.
There are many potential confounders to take into account when comparing across these studies, including methodological variation and small sample sizes. Patients groups in these studies were highly heterogenous and age and gender differences could also account for differing immune responses. The immune system differs in males versus females, for example, women are more prone to certain autoimmune disorders (Klein and Flanagan, 2016) and age can be a significant confounder in immune studies, as immune responses are more robust in younger persons and diminish with age (Montecino-Rodriguez et al., 2013). Substance use and addiction are highly comorbid in these populations (Ross and Peselow, 2012; Sheidow et al., 2012) and may influence immunological results. For example, serum from schizophrenic smokers increased T cell proliferation and expression of activation markers and costimulatory molecules compared to serum from schizophrenic non-smokers (Herberth et al., 2010). Disease comorbidity need also be taken into account as metabolic disturbances are higher in psychiatric disorders, including higher prevalence of obesity, diabetes and metabolic disorders. These increases in inflammatory disorders could be due to medication or inherent to condition (SayuriYamagata et al., 2017; Ventriglio et al., 2015). Overall, studies with larger yet less heterogenous cohorts, ideally with medication-naïve patients and that follow similar methodology across studies can help clarify the role of immune dysfunction in these disorders.
Funding
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. 1650042; NIH grants #R21HD086669, R21ES025560, R21MH116383, RO1HD090214, R01MH118209, R01ES015359, P30ES23513, U54HD079125, and P01ES011269; Jane Botsford Johnson Foundation, Jonty Foundation and NARSAD Foundation.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Arango Duque G., Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 2014;5:491. doi: 10.3389/fimmu.2014.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli B.M., Parisi M.M., Bristot G., Antqueviezc B., Gea L.P., Colombo R., Kapczinski F., Guma F., Brietzke E., Barbe-Tuana F.M., Rosa A.R. Attenuated inflammatory response of monocyte-derived macrophage from patients with BD: a preliminary report. Int J Bipolar Disord. 2019;7:13. doi: 10.1186/s40345-019-0148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa I.G., Rocha N.P., Assis F., Vieira É.L.M., Soares J.C., Bauer M.E., Teixeira A.L. Monocyte and lymphocyte activation in bipolar disorder: a new piece in the puzzle of immune dysfunction in mood disorders. Int. J. Neuropsychopharmacol. 2014;18:pyu021. doi: 10.1093/ijnp/pyu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer T.A., Buslei R., Havas L., Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci. Lett. 1999;271:126–128. doi: 10.1016/s0304-3940(99)00545-5. [DOI] [PubMed] [Google Scholar]
- Becking K., Haarman B.C.M., van der Lek R.F.R., Grosse L., Nolen W.A., Claes S., Drexhage H.A., Schoevers R.A. Inflammatory monocyte gene expression: trait or state marker in bipolar disorder? Int. J. Behav. Dev. 2015;3:20. doi: 10.1186/s40345-015-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield P.S., Selvaraj S., Veronese M., Rizzo G., Bertoldo A., Owen D.R., Bloomfield M.A., Bonoldi I., Kalk N., Turkheimer F., McGuire P., de Paola V., Howes O.D. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am. J. Psychiatr. 2016;173:44–52. doi: 10.1176/appi.ajp.2015.14101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P., Bellani M., Isola M., Bergami A., Marinelli V., Dusi N., Rambaldelli G., Tansella M., Finardi A.M., Martino G., Perlini C., Furlan R. Increased M1/decreased M2 signature and signs of Th1/Th2 shift in chronic patients with bipolar disorder, but not in those with schizophrenia. Transl. Psychiatry. 2014;4 doi: 10.1038/tp.2014.46. e406-e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.S. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. 2012;72:1272–1276. doi: 10.1002/dneu.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse S., Busse M., Schiltz K., Bielau H., Gos T., Brisch R., Mawrin C., Schmitt A., Jordan W., Muller U.J., Bernstein H.G., Bogerts B., Steiner J. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav. Immun. 2012;26:1273–1279. doi: 10.1016/j.bbi.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Canetta S.E., Bao Y., Co M.D., Ennis F.A., Cruz J., Terajima M., Shen L., Kellendonk C., Schaefer C.A., Brown A.S. Serological documentation of maternal influenza exposure and bipolar disorder in adult offspring. Am. J. Psychiatr. 2014;171:557–563. doi: 10.1176/appi.ajp.2013.13070943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho L.A., Bergink V., Sumaski L., Wijkhuijs J., Hoogendijk W.J., Birkenhager T.K., Drexhage H.A. Inflammatory activation is associated with a reduced glucocorticoid receptor alpha/beta expression ratio in monocytes of inpatients with melancholic major depressive disorder. Transl. Psychiatry. 2014;4:e344. doi: 10.1038/tp.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010;125:S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase K.A., Cone J.J., Rosen C., Sharma R.P. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatr. 2016;16:152. doi: 10.1186/s12888-016-0866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collste K., Plaven-Sigray P., Fatouros-Bergman H., Victorsson P., Schain M., Forsberg A., Amini N., Aeinehband S., Erhardt S., Halldin C., Flyckt L., Farde L., Cervenka S. Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [(11)C]PBR28. Mol. Psychiatr. 2017;22:850–856. doi: 10.1038/mp.2016.247. [DOI] [PubMed] [Google Scholar]
- Coughlin J.M., Wang Y., Ambinder E.B., Ward R.E., Minn I., Vranesic M., Kim P.K., Ford C.N., Higgs C., Hayes L.N., Schretlen D.J., Dannals R.F., Kassiou M., Sawa A., Pomper M.G. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [11C]DPA-713 PET and analysis of CSF and plasma. Transl. Psychiatry. 2016;6:e777. doi: 10.1038/tp.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain J.M., Nikodemova M., Watters J.J. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J. Neurosci. Res. 2013;91:1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C.L., Martinez-Cerdeno V., Noctor S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. : Off. J. Soc. Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danovich L., Veenman L., Leschiner S., Lahav M., Shuster V., Weizman A., Gavish M. The influence of clozapine treatment and other antipsychotics on the 18 kDa translocator protein, formerly named the peripheral-type benzodiazepine receptor, and steroid production. Eur. Neuropsychopharmacol : J. Eur. Coll. Neuropsychopharmacol. 2008;18:24–33. doi: 10.1016/j.euroneuro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Dean B., Tawadros N., Scarr E., Gibbons A.S. Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. J. Affect. Disord. 2010;120:245–248. doi: 10.1016/j.jad.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Dey A., Allen J., Hankey-Giblin P.A. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front. Immunol. 2015;5:683. doi: 10.3389/fimmu.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase M.A., Zalesky A., O’Keefe G., Laskaris L., Baune B.T., Weickert C.S., Olver J., McGorry P.D., Amminger G.P., Nelson B., Scott A.M., Hickie I., Banati R., Turkheimer F., Yaqub M., Everall I.P., Pantelis C., Cropley V. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.193. e1225-e1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J., de Vries E.F., Willemsen A.T., de Groot J.C., Dierckx R.A., Klein H.C. Neuroinflammation in schizophrenia-related psychosis: a PET study. J. Nucl. Med. : Off. Publ. Soc. Nucl. Med. 2009;50:1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctot K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatr. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Drexhage R.C., Hoogenboezem T.A., Cohen D., Versnel M.A., Nolen W.A., van Beveren N.J., Drexhage H.A. An activated set point of T-cell and monocyte inflammatory networks in recent-onset schizophrenia patients involves both pro- and anti-inflammatory forces. Int. J. Neuropsychopharmacol. 2011;14:746–755. doi: 10.1017/S1461145710001653. [DOI] [PubMed] [Google Scholar]
- Drexhage R.C., Hoogenboezem T.H., Versnel M.A., Berghout A., Nolen W.A., Drexhage H.A. The activation of monocyte and T cell networks in patients with bipolar disorder. Brain Behav. Immun. 2011;25:1206–1213. doi: 10.1016/j.bbi.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Drexhage R.C., van der Heul-Nieuwenhuijsen L., Padmos R.C., van Beveren N., Cohen D., Versnel M.A., Nolen W.A., Drexhage H.A. Inflammatory gene expression in monocytes of patients with schizophrenia: overlap and difference with bipolar disorder. A study in naturalistically treated patients. Int. J. Neuropsychopharmacol. 2010;13:1369–1381. doi: 10.1017/S1461145710000799. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz I., Arnalich F., Cubillos-Zapata C., Hernández-Jiménez E., Moreno-González R., Toledano V., Fernández-Velasco M., Vallejo-Cremades M.T., Esteban-Burgos L., de Diego R.P., Llamas-Matias M.A., García-Arumi E., Martí R., Boscá L., Andreu A.L., López-Sendón J.L., López-Collazo E. Mitochondrial DAMPs induce endotoxin tolerance in human monocytes: an observation in patients with myocardial infarction. PloS One. 2014;9 doi: 10.1371/journal.pone.0095073. e95073-e95073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari P., Parisi M.M., Colombo R., Becker M., Fries G., Ascoli B.M., Gea L.P., Anna M.K., Kapczinski F., Klamt F., Guma F., Rosa A.R., Barbe-Tuana F.M. Depression and Mania induce pro-inflammatory activation of macrophages following application of serum from individuals with bipolar disorder. Clin. Psychopharmacol. Neurosci. : Off. Sci. J. Korean Coll. Neuropsychopharmacol. 2018;16:103–108. doi: 10.9758/cpn.2018.16.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini F., De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013;2013 doi: 10.1155/2013/429815. 429815-429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman S.G., Cloonan N., Catts V.S., Miller L.C., Wong J., McCrossin T., Cairns M., Weickert C.S. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol. Psychiatr. 2013;18:206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Ginhoux F., Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Goldsmith D.R., Rapaport M.H., Miller B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatr. 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Perdiguero E., Klapproth K., Schulz C., Busch K., Azzoni E., Crozet L., Garner H., Trouillet C., de Bruijn M.F., Geissmann F., Rodewald H.R. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse L., Carvalho L.A., Wijkhuijs A.J.M., Bellingrath S., Ruland T., Ambrée O., Alferink J., Ehring T., Drexhage H.A., Arolt V. Clinical characteristics of inflammation-associated depression: monocyte gene expression is age-related in major depressive disorder. Brain Behav. Immun. 2015;44:48–56. doi: 10.1016/j.bbi.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Grosse L., Hoogenboezem T., Ambrée O., Bellingrath S., Jörgens S., de Wit H.J., Wijkhuijs A.M., Arolt V., Drexhage H.A. Deficiencies of the T and natural killer cell system in major depressive disorder. T regulatory cell defects are associated with inflammatory monocyte activation. Brain Behav. Immun. 2016;54:38–44. doi: 10.1016/j.bbi.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Haarman B.C., Riemersma-Van der Lek R.F., de Groot J.C., Ruhe H.G., Klein H.C., Zandstra T.E., Burger H., Schoevers R.A., de Vries E.F., Drexhage H.A., Nolen W.A., Doorduin J. Neuroinflammation in bipolar disorder - a [(11)C]-(R)-PK11195 positron emission tomography study. Brain Behav. Immun. 2014;40:219–225. doi: 10.1016/j.bbi.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Hafizi S., Tseng H.H., Rao N., Selvanathan T., Kenk M., Bazinet R.P., Suridjan I., Wilson A.A., Meyer J.H., Remington G., Houle S., Rusjan P.M., Mizrahi R. Imaging microglial activation in untreated first-episode psychosis: a PET study with [(18)F]FEPPA. Am. J. Psychiatr. 2017;174:118–124. doi: 10.1176/appi.ajp.2016.16020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M., Drevets W.C., Price J.L. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol. Psychiatr. 2004;55:563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Hannestad J., DellaGioia N., Gallezot J.D., Lim K., Nabulsi N., Esterlis I., Pittman B., Lee J.Y., O’Connor K.C., Pelletier D., Carson R.E. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [(1)(1)C]PBR28 PET study. Brain Behav. Immun. 2013;33:131–138. doi: 10.1016/j.bbi.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmann H., Gamradt S., Taenzer A., Nowacki J., Zain R., Patas K., Ramien C., Paul F., Wingenfeld K., Piber D., Gold S.M., Otte C. Pro-inflammatory monocyte phenotype and cell-specific steroid signaling alterations in unmedicated patients with major depressive disorder. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.02693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberth M., Krzyszton D.N., Koethe D., Craddock M.R., Bulger E., Schwarz E., Guest P., Leweke F.M., Bahn S. Differential effects on T-cell function following exposure to serum from schizophrenia smokers. Mol. Psychiatr. 2010;15:364–371. doi: 10.1038/mp.2008.120. [DOI] [PubMed] [Google Scholar]
- Hercher C., Chopra V., Beasley C.L. Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J. Psychiatry Neurosci. : JPN. 2014;39:376–385. doi: 10.1503/jpn.130277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Jimenez E., Gutierrez-Fernandez M., Cubillos-Zapata C., Otero-Ortega L., Rodriguez-Frutos B., Toledano V., Martinez-Sanchez P., Fuentes B., Varela-Serrano A., Avendano-Ortiz J., Blazquez A., Mangas-Guijarro M.A., Diez-Tejedor E., Lopez-Collazo E. Circulating monocytes exhibit an endotoxin tolerance status after acute ischemic stroke: mitochondrial DNA as a putative explanation for poststroke infections. J. Immunol. 2017;198:2038–2046. doi: 10.4049/jimmunol.1601594. [DOI] [PubMed] [Google Scholar]
- Holmes S.E., Hinz R., Conen S., Gregory C.J., Matthews J.C., Anton-Rodriguez J.M., Gerhard A., Talbot P.S. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol. Psychiatr. 2018;83:61–69. doi: 10.1016/j.biopsych.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Hughes V. Microglia: the constant gardeners. Nature. 2012;485:570–572. doi: 10.1038/485570a. [DOI] [PubMed] [Google Scholar]
- Italiani P., Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. Functional differentiation. Front. Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenk M., Selvanathan T., Rao N., Suridjan I., Rusjan P., Remington G., Meyer J.H., Wilson A.A., Houle S., Mizrahi R. Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr. Bull. 2015;41:85–93. doi: 10.1093/schbul/sbu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Lesh T.A., Careaga M., Rose D.R., McAllister A.K., Van de Water J., Carter C.S., Ashwood P. Cytokine alterations in first-episode schizophrenia and bipolar disorder: relationships to brain structure and symptoms. J. Neuroinflammation. 2018;15:165. doi: 10.1186/s12974-018-1197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Barres B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018;18:225–242. doi: 10.1038/nri.2017.125. [DOI] [PubMed] [Google Scholar]
- Marin-Teva J.L., Dusart I., Colin C., Gervais A., van Rooijen N., Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Marques T.R., Ashok A.H., Pillinger T., Veronese M., Turkheimer F.E., Dazzan P., Sommer I.E.C., Howes O.D. Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychol. Med. 2019;49:2186–2196. doi: 10.1017/S0033291718003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Lucchi S., Rossetti A., Clerici M. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio and platelet-lymphocyte ratio in non-affective psychosis: a meta-analysis and systematic review. World J. Biol. Psychiatr. : Off. J. World Fed. Soc. Biol. Psychiatr. 2019:1–13. doi: 10.1080/15622975.2019.1583371. [DOI] [PubMed] [Google Scholar]
- Meyer U., Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog. Neurobiol. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Miller B.J., Buckley P.F. The case for adjunctive monoclonal antibody immunotherapy in schizophrenia. Psychiatr. Clin. 2016;39:187–198. doi: 10.1016/j.psc.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Miller B.J., Culpepper N., Rapaport M.H., Buckley P. Prenatal inflammation and neurodevelopment in schizophrenia: a review of human studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;42:92–100. doi: 10.1016/j.pnpbp.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Modabbernia A., Taslimi S., Brietzke E., Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol. Psychiatr. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22 doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecino-Rodriguez E., Berent-Maoz B., Dorshkind K. Causes, consequences, and reversal of immune system aging. J. Clin. Invest. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkholm K., Vinberg M., Vedel Kessing L. Cytokines in bipolar disorder: a systematic review and meta-analysis. J. Affect. Disord. 2013;144:16–27. doi: 10.1016/j.jad.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Network, Pathway Analysis Subgroup of Psychiatric Genomics, C. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat. Neurosci. 2015;18:199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkilä H.V., Müller K., Ahokas A., Rimón R., Andersson L.C. Increased frequency of activated lymphocytes in the cerebrospinal fluid of patients with acute schizophrenia. Schizophr. Res. 2001;49:99–105. doi: 10.1016/s0920-9964(99)00218-2. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain Parenchyma in vivo. Science. 2005;308:1314. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nitta M., Kishimoto T., Müller N., Weiser M., Davidson M., Kane J.M., Correll C.U. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr. Bull. 2013;39:1230–1241. doi: 10.1093/schbul/sbt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter T., Coughlin J.M., Gschwind T., Weber-Stadlbauer U., Wang Y., Kassiou M., Vernon A.C., Benke D., Pomper M.G., Sawa A., Meyer U. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol. Psychiatr. 2017;23:323. doi: 10.1038/mp.2016.248. [DOI] [PubMed] [Google Scholar]
- Ongür D., Drevets W.C., Price J.L. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore C.E., Schwartzer J.J., Careaga M., Berman R.F., Ashwood P. Maternal immune activation leads to activated inflammatory macrophages in offspring. Brain Behav. Immun. 2014;38:220–226. doi: 10.1016/j.bbi.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhan F., Schwieler L., Fatouros-Bergman H., Malmqvist A., Cervenka S., Collste K., Flyckt L., Farde L., Sellgren C.M., Piehl F., Engberg G., Erhardt S. Increased number of monocytes and plasma levels of MCP-1 and YKL-40 in first-episode psychosis. Acta Psychiatr. Scand. 2018;138:432–440. doi: 10.1111/acps.12944. [DOI] [PubMed] [Google Scholar]
- Ormel P.R., van Mierlo H.C., Litjens M., Strien M.E.v., Hol E.M., Kahn R.S., de Witte L.D. Characterization of macrophages from schizophrenia patients. NPJ Schizophr. 2017;3:41. doi: 10.1038/s41537-017-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmos R.C., Hillegers M.H.J., Knijff E.M., Vonk R., Bouvy A., Staal F.J.T., de Ridder D., Kupka R.W., Nolen W.A., Drexhage H.A. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. JAMA Psychiatry. 2008;65:395–407. doi: 10.1001/archpsyc.65.4.395. [DOI] [PubMed] [Google Scholar]
- Parboosing R., Bao Y., Shen L., Schaefer C.A., Brown A.S. Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry. 2013;70:677–685. doi: 10.1001/jamapsychiatry.2013.896. [DOI] [PubMed] [Google Scholar]
- Piccinini A.M., Midwood K.S. DAMPening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010;2010:672395. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillinger T., Osimo E.F., Brugger S., Mondelli V., McCutcheon R.A., Howes O.D. A meta-analysis of immune parameters, variability, and assessment of modal distribution in psychosis and test of the immune Subgroup hypothesis. Schizophr. Bull. 2019;45(5):1120–1133. doi: 10.1093/schbul/sby160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavén-Sigray P., Matheson G.J., Collste K., Ashok A.H., Coughlin J.M., Howes O.D., Mizrahi R., Pomper M.G., Rusjan P., Veronese M., Wang Y., Cervenka S. Positron emission tomography studies of the glial cell marker translocator protein in patients with psychosis: a meta-analysis using individual participant data. Biol. Psychiatr. 2018;84:433–442. doi: 10.1016/j.biopsych.2018.02.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G., Halaris A., Selemon L.D. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol. Psychiatr. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Rajkowska G., Miguel-Hidalgo J.J., Wei J., Dilley G., Pittman S.D., Meltzer H.Y., Overholser J.C., Roth B.L., Stockmeier C.A. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression∗∗See accompanying Editorial, in this issue. Biol. Psychiatr. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Ransohoff R.M. A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- Rao J.S., Harry G.J., Rapoport S.I., Kim H.W. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol. Psychiatr. 2010;15:384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards E.M., Zanotti-Fregonara P., Fujita M., Newman L., Farmer C., Ballard E.D., Machado-Vieira R., Yuan P., Niciu M.J., Lyoo C.H., Henter I.D., Salvadore G., Drevets W.C., Kolb H., Innis R.B., Zarate C.A., Jr. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 2018;8:57. doi: 10.1186/s13550-018-0401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D.R., Careaga M., Van de Water J., McAllister K., Bauman M.D., Ashwood P. Long-term altered immune responses following fetal priming in a non-human primate model of maternal immune activation. Brain Behav. Immun. 2017;63:60–70. doi: 10.1016/j.bbi.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S., Peselow E. Co-occurring psychotic and addictive disorders: neurobiology and diagnosis. Clin. Neuropharmacol. 2012;35:235–243. doi: 10.1097/WNF.0b013e318261e193. [DOI] [PubMed] [Google Scholar]
- Rothermundt M., Arolt V., Weitzsch C., Eckhoff D., Kirchner H. Immunological dysfunction in schizophrenia: a systematic approach. Neuropsychobiology. 1998;37:186–193. doi: 10.1159/000026501. [DOI] [PubMed] [Google Scholar]
- SayuriYamagata A., Brietzke E., Rosenblat J.D., Kakar R., McIntyre R.S. Medical comorbidity in bipolar disorder: the link with metabolic-inflammatory systems. J. Affect. Disord. 2017;211:99–106. doi: 10.1016/j.jad.2016.12.059. [DOI] [PubMed] [Google Scholar]
- Schnieder T.P., Trencevska I., Rosoklija G., Stankov A., Mann J.J., Smiley J., Dwork A.J. Microglia of prefrontal white matter in suicide. J. Neuropathol. Exp. Neurol. 2014;73:880–890. doi: 10.1097/NEN.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel A., Arolt V., Hunstiger M., Rink L., Behnisch A., Kirchner H. Major depressive disorder is associated with elevated monocyte counts. Acta Psychiatr. Scand. 1996;94:198–204. doi: 10.1111/j.1600-0447.1996.tb09849.x. [DOI] [PubMed] [Google Scholar]
- Setiawan E., Attwells S., Wilson A.A., Mizrahi R., Rusjan P.M., Miler L., Xu C., Sharma S., Kish S., Houle S., Meyer J.H. Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psychiatr. 2018;5:339–347. doi: 10.1016/S2215-0366(18)30048-8. [DOI] [PubMed] [Google Scholar]
- Setiawan E., Wilson A.A., Mizrahi R., Rusjan P.M., Miler L., Rajkowska G., Suridjan I., Kennedy J.L., Rekkas P.V., Houle S., Meyer J.H. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatr. 2015;72:268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheidow A.J., McCart M., Zajac K., Davis M. Prevalence and impact of substance use among emerging adults with serious mental health conditions. Psychiatr. Rehabil. J. 2012;35:235–243. doi: 10.2975/35.3.2012.235.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.A., Das A., Ray S.K., Banik N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012;87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.E., Li J., Garbett K., Mirnics K., Patterson P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. : Off. J. Soc. Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneeboer M.A.M., Snijders G.J.L.J., Berdowski W.M., Fernández-Andreu A., Psychiatric Donor Program of the Netherlands Brain B., van Mierlo H.C., Berdenis van Berlekom A., Litjens M., Kahn R.S., Hol E.M., de Witte L.D. Microglia in post-mortem brain tissue of patients with bipolar disorder are not immune activated. Transl. Psychiatry. 2019;9:153. doi: 10.1038/s41398-019-0490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J., Bernstein H.G., Bielau H., Farkas N., Winter J., Dobrowolny H., Brisch R., Gos T., Mawrin C., Myint A.M., Bogerts B. S100B-immunopositive glia is elevated in paranoid as compared to residual schizophrenia: a morphometric study. J. Psychiatr. Res. 2008;42:868–876. doi: 10.1016/j.jpsychires.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Steiner J., Bielau H., Brisch R., Danos P., Ullrich O., Mawrin C., Bernstein H.-G., Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Stertz L., Fries G.R., Rosa A.R., Kauer-Sant’anna M., Ferrari P., Paz A.V., Green C., Cunha A.B., Dal-Pizzol F., Gottfried C., Kapczinski F. Damage-associated molecular patterns and immune activation in bipolar disorder. Acta Psychiatr. Scand. 2015;132:211–217. doi: 10.1111/acps.12417. [DOI] [PubMed] [Google Scholar]
- Takano A., Arakawa R., Ito H., Tateno A., Takahashi H., Matsumoto R., Okubo Y., Suhara T. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int. J. Neuropsychopharmacol. 2010;13:943–950. doi: 10.1017/S1461145710000313. [DOI] [PubMed] [Google Scholar]
- Torres K.C.L., Souza B.R., Miranda D.M., Nicolato R., Neves F.S., Barros A.G.A., Dutra W.O., Gollob K.J., Correa H., Romano-Silva M.A. The leukocytes expressing DARPP-32 are reduced in patients with schizophrenia and bipolar disorder. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2009;33:214–219. doi: 10.1016/j.pnpbp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Torres-Platas S.G., Cruceanu C., Chen G.G., Turecki G., Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Trepanier M.O., Hopperton K.E., Mizrahi R., Mechawar N., Bazinet R.P. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol. Psychiatr. 2016;21:1009–1026. doi: 10.1038/mp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upthegrove R., Manzanares-Teson N., Barnes N.M. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr. Res. 2014;155:101–108. doi: 10.1016/j.schres.2014.03.005. [DOI] [PubMed] [Google Scholar]
- van Berckel B.N., Bossong M.G., Boellaard R., Kloet R., Schuitemaker A., Caspers E., Luurtsema G., Windhorst A.D., Cahn W., Lammertsma A.A., Kahn R.S. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol. Psychiatr. 2008;64:820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- van der Doef T.F., de Witte L.D., Sutterland A.L., Jobse E., Yaqub M., Boellaard R., de Haan L., Eriksson J., Lammertsma A.A., Kahn R.S., van Berckel B.N.M. In vivo (R)-[11C]PK11195 PET imaging of 18kDa translocator protein in recent onset psychosis. NPJ Schizophr. 2016;2:16031. doi: 10.1038/npjschz.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren C.F.M.G., Gremmels H., de Witte L.D., Hol E.M., Van Gool A.R., Falkai P.G., Kahn R.S., Sommer I.E.C. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl. Psychiatry. 2017;7 doi: 10.1038/tp.2017.4. e1075-e1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S., Lopresti B.J., Wiley C.A. Molecular imaging of microglia/macrophages in the brain. Glia. 2013;61:10–23. doi: 10.1002/glia.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventriglio A., Gentile A., Stella E., Bellomo A. Metabolic issues in patients affected by schizophrenia: clinical characteristics and medical management. Front. Neurosci. 2015;9:297. doi: 10.3389/fnins.2015.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H., Moorhouse A.J., Jinno S., Kohsaka S., Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. : Off. J. Soc. Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.K., Miller B.J. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr. Bull. 2018;44:75–83. doi: 10.1093/schbul/sbx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla E.P., Cannon T.D., Gur R.E., Kessler J. Leukocytes and organ-nonspecific autoantibodies in schizophrenics and their siblings: markers of vulnerability or disease? Biol. Psychiatr. 1996;40:825–833. doi: 10.1016/0006-3223(95)00598-6. [DOI] [PubMed] [Google Scholar]