Abstract
Growing evidence suggests that galectin-3 (Gal-3) is instrumental in orchestrating innate immune response and microglia activation following different brain pathologies. However, its role remains controversial. We recently showed that a readily available natural product glucosamine may act as a strong modulator of Gal-3. Glucosamine is a naturally occurring sugar and a precursor in the synthesis of glycosylated proteins. It is often used as a supplement to treat symptoms of various inflammatory conditions. Our recent work suggests that by increasing the synthesis and availability of Gal-3 ligands and/or by regulating its expression levels, glucosamine may significantly modulate Gal-3 signaling. Because evidence suggests that Gal-3 might be differentially regulated after ischemic injury in the brains of female mice, here we examined and compared the immunomodulatory potential of glucosamine in male and female stroke. The mice were subjected to transient middle cerebral artery occlusion (MCAO), followed by different reperfusion periods. The short-term 5 days treatment with glucosamine (150 mg/kg i.p.) was initiated 2 hrs after stroke. To visualize the effects of glucosamine treatment on post-stroke inflammation, we took advantage of a transgenic mouse model bearing the dual reporter system luciferase/GFP under transcriptional control of a murine TLR2 promoter (TLR2-luc-GFP) allowing in vivo bioluminescence imaging of innate immune response and microglial activation. We report that after stroke, both, male and female mice strongly up-regulate the TLR2 bioluminescence signals from activated microglia, however, the observed in vivo immunomodulatory effects of glucosamine after stroke were sex-dependent. Analysis of cytokine profiles at protein level, in glucosamine-treated male mice 72hsr after stroke, revealed down regulation of pro-inflammatory cytokines, an increase in levels of anti-inflammatory cytokines including IL-4, IL13 and colony stimulating factors MCFC and GM-CSF and a significant decrease in the size of ischemic lesion in male mice. Conversely, in female mice glucosamine markedly increases the pro-inflammatory signaling and exacerbates ischemic injury. Analysis of the downstream signaling target of glucosamine/Gal-3 revealed that glucosamine administration restored PPAR-γ activity in male but not in female mice 3 days following MCAO. Together, our results suggest that glucosamine acts as a fine tuner of post-ischemic inflammation in a sex dependent-manner and may have therapeutic potential after stroke in males. Based on our results propose that targeting immune system after stroke may require adapted sex-specific therapeutic approaches.
Keywords: Glucosamine, Sexual dimorphism, Galectin-3, Biophotonic/bioluminescence imaging, PPAR-Gamma, Stroke
Highlights
-
•
Immunomodulatory effects of glucosamine are sex dependent.
-
•
Glucosamine differentially modulates galectin-3/IL4R signaling in male and female mice.
-
•
Glucosamine restores PPAR-gamma transcriptional activity in male mice and protects against stroke in male mice.
-
•
Glucosamine increases inflammatory signaling and exacerbates ischemic injury in female mice.
1. Introduction
Stroke is a leading cause of death and a major cause of a long-term disability worldwide (Dirnagl et al., 1999; Lo et al., 2003). However, in spite of intensive research efforts, effective therapies remain elusive. Evidence suggests that inflammation represents a key element of the pathobiology of stroke (Iadecola and Anrather, 2011; Kamel and Iadecola, 2012). Indeed, experimentally and clinically, stroke is followed by acute and prolonged inflammatory response characterized by activation of peripheral and resident immune cells such as microglia, production of pro- and anti-inflammatory cytokines and leukocyte infiltration in the brain, events that may contribute to ischemic brain injury (d’Avila et al., 2012; Kawabori et al., 2015; Kriz and Lalancette-Hebert, 2009; Rahimian et al., 2018; Rahimian et al., 2019a). Microglia are the principal immune cells of the brain and its activation is a hallmark of the brain response to ischemic injury in male as well as in female brains. Growing evidence however, suggests a marked sexual dimorphism in the processes associated with microglial activation after stroke (Rahimian et al., 2019b).
We recently demonstrated that a delayed treatment with immunomodulatory molecule galectin-3 (Gal-3) shifts microglial activation towards alternative phenotypes and confers neuroprotection after stroke (Rahimian et al., 2019b). Gal-3 is a glycoprotein involved in fine-tuning of inflammatory responses at periphery and in the brain (Lalancette-Hebert et al., 2007, 2012). Undetectable under physiological conditions, Gal-3 expression is robustly increased in the brain, in particular in ischemic-injury activated microglia (Lalancette-Hebert et al., 2007, 2012). Interestingly, comparative analysis of Gal-3 expression patterns after stroke revealed significantly higher expression levels of Gal-3 in female ischemic brains (Rahimian et al., 2019b). A current view is that microglial cells after stroke in female brains acquire more protective phenotypes (Acaz-Fonseca et al., 2015; Bodhankar et al., 2015; Caplan et al., 2017). Indeed, recent work by Villa and colleagues revealed a marked neuroprotective potential of female microglia once transplanted after stroke into the ischemic brains of male mice (Villa et al., 2018). Based on our previous work and recent findings, we hypothesized that pharmacological modulation of Gal-3 by either increasing its expression levels and/or potentiating its downstream signaling may therapeutically modulate immune response and/or microglial activation profiles after stroke.
Glucosamine is a naturally occurring sugar and an essential component of glycoproteins and proteoglycans. Evidence suggests that glucosamine may act as a pharmacological Gal-3 modulator by enhancing both, the availability of Gal-3 ligands and Gal-3 expression. Therefore we hypothesized that administration of glucosamine after stroke may therapeutically modulate microglia polarization and immune responses after stroke (Fluri et al., 2015; He et al., 2017; Hwang et al., 2010; Rahimian et al., 2019b). Here, we examined the immune-modulatory effects of glucosamine in female and male stroke. We show that glucosamine modulates microglia activation, morphology and secretory profile in a sex-dependent manner, revealing a therapeutic potential in male but not in female mice. As expected, the glucosamine increases Gal-3 levels and confers neuroprotection after stroke in male mice. Conversely, the Gal-3 levels were decreased in glucosamine treated female mice which was further associated with an increase in pro-inflammatory signaling and larger infarctions. Next, we show that the downstream signaling cascade including peroxisome proliferator-activated receptor gamma (PPARγ)-p65 pathway might underlie the sex specific effects of glucosamine following middle cerebral artery occlusion (MCAO).
2. Methods
2.1. Experimental animals
Experimental procedures were performed on Toll-like receptor 2 (TLR2)-luc-GFP transgenic mice, Gal-3 KO mice and WT littermates as previously described (Lalancette-Hebert et al., 2009). In this mouse model, luciferase and GFP reporters are driven under the transcriptional control of the murine Toll-like receptor 2 gene promoter. Transgenic animals were identified by polymerase chain reaction (PCR) detection of the luciferase transgene with the following primers: 5′-CAG-CAG-GAT-GCT-CTC-CAG-TTC-3′ AND 5′-GGC-GCA-GTA-GGC-AAG-GTG-GT-3’. Genotyping was performed as previously described (Lalancette-Hebert et al., 2009). All experimental animals used in this study were provided with water and healthy diet and monitored during the entire experimental protocol. The animals were held in the pathogen free animal facility of the CERVO Brain Research Institute (Université Laval), 3–5 mice per cage in the controlled environment having the 12hrs day and night cycles. Male and female mice (age 2–3 months) were used for experiments. All experimental procedures were approved (protocol no. 017–133) by the Université Laval Animal Care Ethics Committee and are in accordance with The Guide to the Care and Use of Experimental Animals of the Canadian Council on Animal Care.
2.2. Surgical procedures: transient middle cerebral artery occlusion (MCAO)
As previously described, unilateral transient focal cerebral ischemia was induced by MCAO during 1 h followed by different reperfusion time (Lalancette-Hebert et al., 2007, 2009, 2011, 2012; Weng and Kriz, 2007). Transient focal cerebral ischemia was induced by intraluminal filament occlusion of the left middle cerebral artery (MCA) with a 6-0 silicone coated monofilament suture for 1 h followed by different perfusion times. The MCAO was performed on 2–3-month-old male and female mice (20–25 g). Briefly, the animals were anesthetized with 2% isoflurane in 100% oxygen at a flow rate of 1.8 L/min. To avoid cooling, the body temperature was monitored and maintained at 37 °C with a heating pad. The common carotid artery (CCA) was isolated and carefully separated from the adjacent tissue and a 12-mm-long 6–0 silicon-coated monofilament suture was inserted via the proximal external carotid artery (ECA) into the ICA and then into the circle of Willis to occlude the MCA. During the surgery, the correct placement of the filament was confirmed by Laser Doppler measurements PF5001, Perimed Sweden). As previously described, 24 hrs after initial surgery the animals were examined for early neurological deficits (Cordeau et al., 2008; Weng and Kriz, 2007). After surgery, all animals were allowed ad libitum access to water and food before and after surgery.
2.3. Treatment protocol
D-(+)-Glucosamine hydrochloride (≥99%, Sigma-Aldrich) was first administered (150 mg/kg/day, i.p. in saline 0.9%) starting 2 h after stroke. The animals received treatment 1x day for 5 consecutive days. Saline 0.9% has been administered as vehicle for the control group. Four experimental groups were considered: 1) male mice following MCAO treated with glucosamine (150 mg/kg/day, i.p.) 2) female, cycled-match mice following MCAO treated with glucosamine (150 mg/kg/day, i.p. in saline 0.9%) 3) male mice underwent MCAO and received saline 0.9% (i.p. for 5 days), 4) female cycle-match mice underwent MCAO and received Saline 0.9% (i.p. for 5 days) as vehicle for the control group.
2.4. Vaginal smears and hormone detection
As previously described, to avoid the effects of physiological fluctuation of estrogen levels on immune response, the vaginal epithelial cells were obtained from mice by a vaginal wash with saline 0.9% and smeared onto cleaned slides (Cordeau et al., 2008). Slides were then evaluated for the presence of white blood cells and the morphology of epithelial cells to determine the stage of the estrus cycle. The female control group underwent MCAO at the beginning of the pro-estrus period (Cordeau et al., 2008; Cordeau et al., 2016).
2.5. Tissue collection
The animals were anesthetized via an intraperitoneal injection of ketamine (10 mg/ml)/xylazine (1 mg/ml) and transcardially perfused with cold PBS 1X, followed by PBS-buffered 4% paraformaldehyde (PFA) at pH 7.4. Tissue samples were then fixed overnight in 4% PFA and equilibrated in phosphate-buffered 20% sucrose for 48 hrs. Brains were embedded into Tissue-Tek (O.C.T. compound; Sakura), frozen at −80 °C overnight, and cut into coronal sections (35 μm thick) with a Cryostat and stored at −20 °C. In order to prepare samples for Western blot and cytokine array analysis, animals were anesthetized with ketamine/xylazine and transcardially perfused with ice-cold 0.9%. The brain samples were surgically removed and immediately frozen in liquid nitrogen and then transferred to −80 °C freezer (Bohacek et al., 2012).
2.6. Histological evaluation of the size of infarction
The size of infraction was evaluated in different time points after glucosamine treatment in both female and male mice. Tissue sections were stained with cresyl-violet and were digitized. The area of infarction, direct stroke area, was quantified with ImageJ program (NIH), and infarct area was calculated as percentage of control, non-stroke area in the contralateral hemisphere with appropriate corrections for brain edema as previously described (Bohacek et al., 2012; Lalancette-Hebert et al., 2011, 2012).
2.7. In vivo bioluminescence imaging
As previously described (Cordeau et al., 2008; Gravel et al., 2011; Lalancette-Hebert et al., 2009), bioluminescence/biophotonic images were captured using IVIS Spectrum Imaging System (PerkinElmer, Hopkinton MA). Prior to imaging session, mice received intraperitoneal (i.p.) injection of D-luciferine, a luciferase substrate (150 mg/kg, PerkinElmer, Hopkinton MA) dissolved in 0.9% saline. Bioluminescence emission was normalized and displayed in physical units of surface radiance, photons per second per centimeter squared per steradian (photons/sec/cm2/sr). The light output has been quantified by determining the total number of photons emitted per second using the Living Image 4.1 acquisition and imaging software (PerkinElmer, USA).
2.8. Immunofluorescence analysis
To validate in vivo imaging data mice from each experimental group were euthanized by overdose of anesthetic. For the double-immunofluorescence analysis, mice were perfused with PBS (pH 7.5) followed by 40 mg/ml paraformaldehyde and incubated overnight in 200 mg/ml phosphate-buffered sucrose. The sections were blocked in 10% goat serum and then incubated overnight at room temperature using primary antibodies diluted in 5% goat serum/PBS1x+0.25% Triton X-100 (1:500 rabbit polyclonal anti-Iba1 (Wako), 1:500 anti-Gal-3 (ATCC), 1:250 rabbit anti IL-4 receptor (Santa Cruz), 1:500 rabbit anti CD11b (Serotech), 1:500 rabbit polyclonal anti-Ym1 (Stem Cell technologies) and 1:500 rabbit anti actin (Invitrogen). After washes in PBS, the sections were then incubated in corresponding fluorescent goat secondary antiserum for 2 h (1:500, Invitrogen), washed and mounted with Flouromount G (Thermofisher) (Rahimian et al., 2019b). Alexa Fluor® 488 phalloidin (Thermo Fisher Scientific) was used to study microglia morphology (Leica CTR 500 microscope).
2.9. Cytokine array analyses
As described (Lalancette-Hebert et al., 2012), a mouse antibody array (Raybio®Mouse Inflammation Antibody Array 1.1, Ray Biotech) was used to identify change in pro-and anti-inflammatory cytokine levels. Protein lysate was obtained by homogenization of mouse brains in 1X Cell Lysis Buffer (included in the Ray Biotech kit) with Protease and phosphatase inhibitor cocktail (Sigma). The protein concentration was determined for each sample. Samples for each group (3–4 mice/group) were pooled and incubated with the array membrane (total concentration of 500 μg for each membrane) overnight at 4 °C. The samples were run as biological duplicates. After washes, the membranes were incubated with the biotin-conjugated antibodies overnight at 4 °C. The membranes were then processed according to Raybiotech protocol. Membrane were exposed to x-ray film (Kodak film Biomax MR1, #8701302) and analyzed by ImageJ software.
2.10. Peroxisome proliferator-activated receptor (PPAR)-γ transcription factor activity
Three days after stroke induction PPAR-γ transcription factor activity was measured in ipsilateral stroked brain nuclear extract of glucosamine treated and non-treated male and female mice using an ELISA kit (Abcam). Nuclear extracts were incubated in a multi-well plate coated with specific peroxisome proliferator response element (PPRE) probes, and PPAR-γ bound to the PPRE was detected using a specific antibody against the γ isoform. Following addition of HRP-conjugated secondary antibody, binding was measured by reading the absorbance at 450 nm (Fakhfouri et al., 2012; Rahimian et al., 2016).
2.11. Western blotting
Total protein extracts were obtained from the ipsilateral hemisphere of the brain 3 day after cerebral ischemia by homogenization in a 1X Cell Lysis Buffer (included in the Ray Biotech kit) with Protease inhibitor cocktail (Sigma). Non-specific binding was blocked by pre-incubation of the nitrocellulose membrane in PBS containing 0.1% tween 20 (PBS-T) and 5% skimmed milk for 1 h. The nitrocellulose was then incubated overnight at 4 °C with antibodies against the targeted proteins as follows: 1:500 rabbit anti Iba1 (Wako), 1:500 rat anti Gal- 3 (Cell Signaling), 1:500 rabbit anti Ym1 (Stem Cell technologies), 1:250 rabbit anti IL-4 receptor (Santa Cruz), 1:500 mouse anti phospho-p65 (Cell Signaling) and 1:20 000 rabbit anti actin (Santa Cruz). Primary antibody was detected with HRP-conjugated anti-rabbit or anti-mouse antibody (1:2000–1:5000) and blots were developed using an enhanced chemiluminescence detection system (ECL kit; Thermo Fisher Scientific). The density of the specific bands was quantified with Image J software and normalized to β-actin as a housekeeping protein (Lalancette-Hebert et al., 2011).
2.12. Primary adult microglia culture
As previously described (Lalancette-Hebert et al., 2012; Rahimian et al., 2019b), the primary cell culture was performed on the brains of the adult, 8–9 weeks old C57BL/6 wild-type mice. The mice were anesthetized by isoflurane 2% and transcardially perfused with ice-cold saline (Hospira) supplemented with 2 units/ml heparine (Sigma-Aldrich). The brains were collected and placed in ice-cold Hibernate medium [Hibernate A medium (BrainBits LLC) supplemented with B-27 (1X) and 0.5 mM GLUTAMAX-I, L-alanyl-L-glutamine (Gibco, Invitrogen)]. After mechanical dissociation, 5–6 brains were incubated in a 0.25% Trypsin-EDTA solution (Sigma) containing 250 K U/ml of DNase I (Sigma). After mechanical dissociation, 5–6 brains were incubated in 0.25% Trypsin-EDTA solution (Sigma) containing 250 K U/ml of DNase I (Sigma). After centrifugation, the pellet was resuspended in 2 ml of 37% Percoll that was overlaid 2 ml of 70% Percoll in a 5-ml centrifuge tube and centrifuged at 600×g for 40 min at room temperature with slow acceleration and no stop-brake. Cells were collected from the interphase, washed with dPBS and kept in culture medium. Cells were plated on culture-treated glass slides (Becton Dickinson) as 1,250,000 cell/ml and incubated at 37 °C in 95% air/5% CO2 (Lalancette-Hebert et al., 2012; Rahimian et al., 2019b).
2.13. Statistical analysis
All data are presented as mean ± SEM. Statistical analysis was performed by one-way ANOVA followed by post hoc comparison test (Tukey-Kramer test) or unpaired t-test. ***p ≤ 0.001 **p ≤ 0.01 and *p ≤ 0.05. Statistical analyses were performed using the GraphPad Prism 6 software (GraphPad, La Jolla, CA). N for each experiment has been indicated under the respective section.
3. Results
3.1. Glucosamine alters morphology and phenotype of primary microglia via Gal-3/IL-4 dependent mechanism
Evidence suggests that Gal-3 plays an important role in regulation of microglia morphology and its activation profiles (Lalancette et al., 2012; Rahimian et al., 2019b). We recently showed that expression levels and/or activity of the endogenous Gal-3 can be additionally modulated by glucosamine. Namely, glucosamine increases availability of the Gal-3 ligand which, in turn, may increase bioavailability of endogenous Gal-3 and thus potentiate its downstream effects including previously reported changes in microglia morphology (Rahimian et al., 2019b). To assess whether glucosamine acts as Gal-3 modulator and increases Gal-3 expression and/or downstream signaling, we first investigated its effects using our well established adult primary microglia culture model-system. Mimicking the Gal-3/IL4 effects on microglia morphology, glucosamine significantly increases the number and length of filopodia (visualized by actin expression) in Gal-3 positive cells (Fig. 1A). Interestingly, administration of CD124 (an IL-4 receptor blocker) reduced microglia arborization, suggesting the involvement of IL-4 receptor signaling on microglia morphology after glucosamine treatment (Fig. 1A, n = 6, ***P < 0.001; **P < 0.01). Next, as shown in Fig. 1B and C, immunofluorescence and Western blot analysis revealed a significantly higher expression levels of Gal-3 and IL-4 receptor in glucosamine treated primary microglia cultures as compared to controls (n = 4, ***P < 0.001; *P < 0.05). Importnatly, effects of glucosamine on microglia morphology and/or IL4R expression levels have been abolished in the context of Gal-3 deficiency in vitro and in vivo following ischemic injury (Fig. 1D and E), further suggesting that glucosamine exerts its effects by potentiating Gal-3/IL4 downstream effects and/or signaling in microglia cells (Rahimian et al., 2019b). Next, to assess glucosamine immune-modulatory potential in vivo, we examined its therapeutic following brain ischemic injury in male and female mice.
Fig. 1.
Glucosamine changes morphology and phenotype of primary cultured microglia. A, Primary microglia cell cultures, when treated for 24 h with glucosamine (1 mM), revealed more ramification. Glucosamine treatment increased both the number and the length of filopodia. Administration of IL-4 receptor blocker CD124 (50μg/ml) starting 2 h prior to glucosamine addition, diminished the effect of Glucosamine on microglia morphology (n = 6, ***P < 0.001; **P < 0.01). Significance was determined by one-way ANOVA and Tukey’s post hoc test. B and C, Immunofluorescence and Western blot experiments indicated that Glucosamine treatment induces IL-4 receptor and Gal-3 expression in primary microglia (n = 4, ***P < 0.001; *P < 0.05). Significance was determined by unpaired t-test. D, we examined the effects of glucosamine (1 mM) treatment on morphology of primary microglia obtained from Gal-3 knockout mice. In the presence of glucosamine (1 mM), microglia morphology did not change indicating the importance of Gal-3 for glucosamine-induced microglia ramification (n = 6, P>0.05). Significance was determined by unpaired t-test. E, Glucosamine (1 mM) treatment did not change the expression of IL-4 receptor in microglia obtained from Gal-3 knockout mice. Moreover, Western blot analysis of Gal-3 knockout mice brain lysates showed glucosamine treatment did not exert any significant effects on the protein levels of IL-4 receptor 72 h after MCAO (n = 4, P>0.05) indicating the crucial role of Gal-3 for glucosamine-induced anti-inflammatory phenotype in vivo. Significance was determined by unpaired t-test.
3.2. In vivo imaging reveals a significant temporal shift in TLR2 activation patterns after glucosamine treatment
Evidence suggests that inflammatory response may have differential role in acute and more chronic phases of brain ischemia (Bohacek et al., 2012; Kim et al., 2014b). Indeed, our recent work suggests that enhancing a delayed inflammatory response, by delivery of recombinant Gal-3 confers neuroprotection (Rahimian et al., 2019b). Because our initial results suggest that glucosamine may act as Gal-3 modulator by increasing its expression in vivo, we tested its therapeutic potential in the context of ischemic injury. We designed a short-term post-stroke immunomodulatory protocol. Treatment with glucosamine was initiated 2 hrs after stroke for 5 consecutive days (1 x day, 150 mg/kg i.p.). To assess innate immune response and microglial activation in vivo we took advantage of the TLR2 reporter mouse, a mouse model previously generated in our laboratory (Lalancette-Hebert et al., 2009, 2011). In this in vivo model-system, the TLR2 induction (luciferase expression detectable as bioluminescence photon emission) can be visualized longitudinally from the brain of the living mice using bioluminescence/biophotonic imaging and a high sensitivity/high resolution CCD camera (Lalancette-Hebert et al., 2009). Our previous analyses revealed that after MCAO, over 90% of the TLR2 signal arises from the Iba1 positive microglial cell (Lalancette-Hebert et al., 2009, 2011). As shown in Fig. 2A the mice were imaged over the period of 3 weeks following MCAO. As expected, at the baseline conditions the low intensity bioluminescence signal was restricted to olfactory bub area. After stroke, a robust signal arising from the ischemic part of the brain was detected in all animals. The signal peaked 24 hrs after stroke and started to decline between 72 h and 5 days following ischemic injury. The quantitative analysis of total photon emission arising from the ischemic brains revealed a significant difference in the TLR2 signal induction patterns in glucosamine treated animals when compared to controls (Fig. 2A, n = 6–11, *P < 0.05). Treatment with glucosamine initiated 2hrs after stroke significantly decreases induction of the TLR2 signal at 24 h after MCAO (Fig. 2A). However, as further revealed in Fig. 2A, the TLR2 signal, in the glucosamine treated group, increases over time to become significantly higher than the vehicle treated group at 5 and 7 days after stroke (respectively, 1.7 and 1.66 fold that of vehicle group). This suggests that glucosamine may exert a dual immunomodulatory action by decreasing an early phase innate immune response and stimulation the later phase of the post -stroke inflammatory response. Our previous work (Lalancette-Hebert et al., 2007, 2011) demonstrated that Gal-3 upregulation in ischemic-injury activated microglia normally peaks 24–72 h after stroke. Because glucosamine has been shown to act as Gal-3 modulator by increasing its expression levels in vitro, we next investigated whether treatment with glucosamine increases Gal-3 expression levels after stroke in vivo. Double immunofluorescence analysis revealed a marked difference in microglia activation patterns between 2 groups revealing an increase in the expression levels of Gal-3/Iba1 positive cells in glucosamine treated animals (72 h). The increase in Gal-3 levels in glucosamine treated mice were further confirmed by Western blot analysis (Fig. 2B, n = 6, **P < 0.01). As shown in Fig. 2C, this effect was accompanied by an increase in expression level of Ym1, an alternative microglia/macrophage’s marker.
Fig. 2.
Glucosamine enhances microglia activation/TLR2 responses following MCAO in male mice. A, Real-time imaging of the TLR2 response following MCAO in TLR2-luc-GFP mice revealed higher expression of TLR2 5 and 7 days after glucosamine treatment. Images were longitudinally recorded from the same experimental animal revealing the dynamics of microglial activation/TLR2 response at 1–21 days after MCAO. The scales on the right are the color maps for photon counts. Luciferase signals were quantified using Living Image software (CaliperLS, Alameda, CA, USA) (n = 6–11, *P < 0.05). B, three days after MCAO higher levels of Gal-3 and iba-1 co-localization was detected in Glucosamine treated animals, indicative of microglial activation. Western blot analysis showed 72 h after MCAO Glucosamine treatment increases the protein level of Gal-3 (n = 6, **P < 0.01). C, Glucosamine treatment was found to increase the co-localization Gal-3 and ym1 three days after stroke induction. Western blot analysis showed 72 h after MCAO glucosamine treatment increases the protein level of Gal-3 and Ym1 (n = 6, *P < 0.05). Significance was determined by unpaired t-test for all experiments. All graphs are mean ± SEM. Scale bar represent 100 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Glucosamine treatment reverses pro-and anti-inflammatory cytokine response after stroke and confers neuroprotection in male mice
To further assess the effects of glucosamine on post-ischemic inflammation, as previously described, we performed a comprehensive analysis of several pro- and anti-inflammatory mediators (Lalancette et al., 2012; Doll et al., 2014; Rahimian et al., 2019b). The multiplex cytokine array analysis (at protein levels) was performed 72 h following glucosamine administration, the time point associated with a significant increase in Gal-3 levels in glucosamine treated animals. As shown in Fig. 3A, a marked shift in the expression levels of pro- and anti-inflammatory cytokine and colony stimulating factors was observed in glucosamine treated animals after stroke. The expression levels of pro-inflammatory cytokines (TNF-α, IL-1β, INF-γ and IL-17; n = 6, ***P < 0.001; **P < 0.01) were significantly decreased, while the levels of anti-inflammatory cytokines (IL-4 and IL-13; n = 6) were significantly increased in glucosamine treated animals when compared to control (Fig. 3A). In addition, treatment with glucosamine significantly increased the expression levels of monocyte/macrophages colony stimulating factors M-CSF and GM-SCF known to be associated with protective effects after stroke (Lalancette-Hebert et al., 2007). Next, we examined to what extent the observed shift in cytokine expression patterns affect the evolution of ischemic injury after MCAO. As previously described (Bohacek et al., 2012), we measured stroke area at 1, 3 and 7 days after MCAO. Quantitative analysis of the stroke area revealed a significant decrease in the size of the ischemic lesion in glucosamine-treated mice at 3 and 7 days post MCAO when compared to controls (Fig. 3B and C, n = 9, **P < 0.01). Together, our data suggests that in males, treatment with glucosamine, initiated after stroke, modulates endogenous Gal-3 levels, shift microglia activation towards more alternative phenotypes and confers neuroprotection.
Fig. 3.
Glucosamine treatment decreases expression levels of pro-inflammatory cytokines and ischemic size after MCAO in male mice. A, Three days after MCAO Glucosamine decreased the content of pro-inflammatory cytokines (such as TNF-α, IL-1β, IL-17, INF-gamma), increased anti-inflammatory cytokine IL-4, IL-13 and also M-CSF and GM-CSF in ipsilateral brain. Furthermore, glucosamine administration did not affect the levels of pro-inflammatory cytokine Il-6 anti-inflammatory cytokines including IL-10 (n = 3, ***P < 0.001; **P < 0.01). B and C, Stroke area was measured 1, 3 and 7 days after MCAO. The size of ischemic lesions decreased after glucosamine treatment when compared to the control 3 days and 7 days but not after 1 day (n = 9, **P < 0.01). Significance was determined by unpaired t-test for all experiments. All graphs are mean ± SEM.
3.4. Glucosamine increases inflammatory signaling and exacerbates ischemic injury in female mice
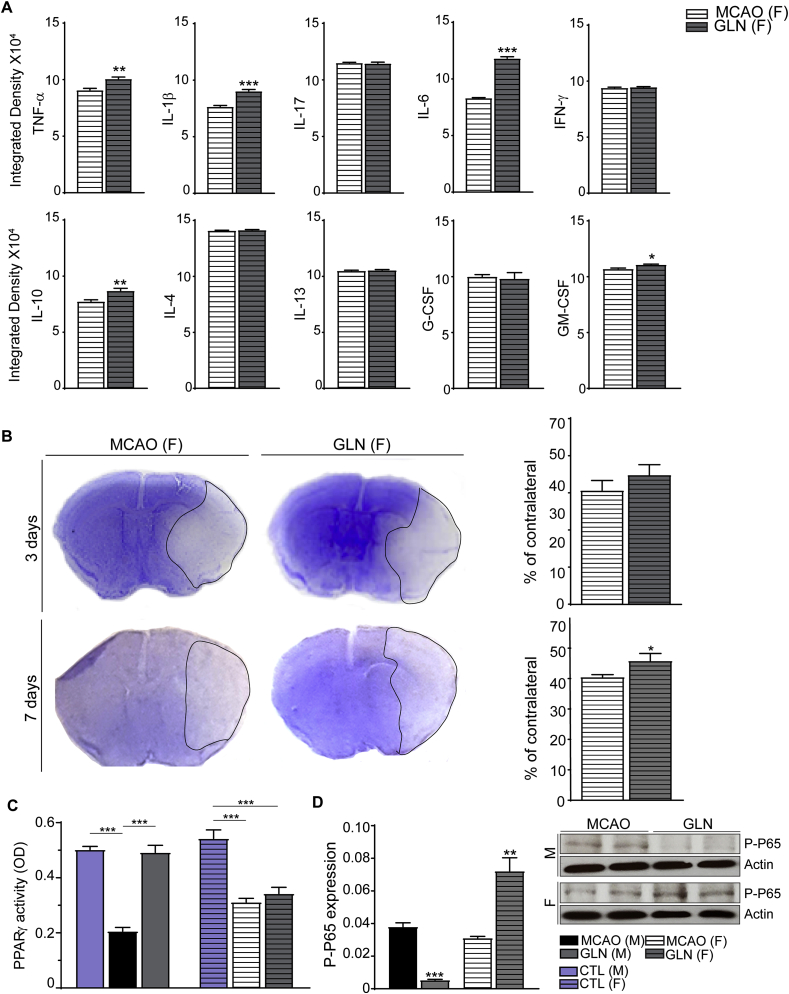
Next, we asked whether treatment with glucosamine exerts immune-modulatory effects after stroke in female mice. Namely, Gal-3 is more robustly upregulated in female stroke (Rahimian et al., 2019a) and evidence suggests that after MCAO, microglial cells from female mice develop less inflammatory phenotype compared to males (Bodhankar et al., 2015) (Acaz-Fonseca et al., 2015; Caplan et al., 2017). Therefore, we hypothesized that treatment with glucosamine may further increase Gal-3 levels in female stroke and confer additional protection. To avoid physiological fluctuations in estrogen levels that occur during estrus cycle, as previously described, all female mice underwent MCAO surgery at the beginning of the pro-estrus period (Cordeau et al., 2008, 2016). As shown in Fig. 4A and B, brain response to ischemic injury was associated with a robust increase in the TLR2 bioluminescence signals in both experimental groups. To our surprise, (and unlike the results obtained after glucosamine treatment in male mice) treatment with glucosamine did not significantly affect temporal dynamics and induction patterns of the TLR2 signal after stroke in female mice (Fig. 4A). Next, at the cellular level, double immunofluorescence analysis did not reveal a measurable difference in the Gal-3/Iba1+ cell population in the ischemic brains of female mice treated with glucosamine as compared to controls (Fig. 4B). However, Western blot analysis performed 3 days after stroke revealed a significant decrease in Gal-3 expression levels after glucosamine treatment in female stroke when compared to controls (Fig. 4C, n = 6, *P < 0.05). To further assess the effects of glucosamine in female stroke, we performed a comprehensive analysis of pro- and anti-inflammatory cytokines after stroke. As shown in Fig. 5 A, (and contrary to the results obtained in male stroke), treatment with glucosamine significantly increased expression levels of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 (n = 3, ***P < 0.001; **P < 0.01; *P < 0.05) while the level of the anti-inflammatory cytokine IL-4 remained unchanged in female mice when compared to controls (72 h). The levels of IL-10 and GM-CSF were slightly but significantly increased in glucosamine treated females (Fig. 5A). Next, we examined the effects of glucosamine on the size of ischemic lesion following MCAO in female mice. The stroke area was measured at 3 and 7 days after MCAO. As shown in Fig. 3, Fig. 5 days following MCAO, administration of glucosamine did not have significant effect on size of ischemic lesion in female mice. Importantly, 7 days after MCAO, quantitative analysis of the stroke area revealed a significant increase in the size of infarction in glucosamine treated female mice as compared to controls (Fig. 5B, n = 9, *P < 0.05). Hence, contrary to neuroprotection observed in male stroke, treatment with glucosamine exacerbated ischemic injury in females.
Fig. 4.
Glucosamine did affect the TLR2 responses after MCAO in female mice. A, Real-time imaging of the TLR2 response following stroke in TLR2-luc-GFP mice showed no significant difference between glucosamine treated and non-treated female subjects. Images were longitudinally recorded from the same experimental animal revealing the dynamics of microglial activation/TLR2 response at 1–7 days after stroke (n = 7, P>0.05). B, Three days after stroke the levels of Gal-3 and iba-1 co-localization was almost the same in glucosamine treated and non-treated female mice. C, Western blot analysis showed 72 h after MCAO glucosamine diminished the protein level of Gal-3 (n = 6, *P < 0.05). Significance was determined by unpaired t-test for all experiments. All graphs are mean ± SEM. Scale bar represent 100 μm.
Fig. 5.
Glucosamine treatment enhances pro-inflammatory cytokine levels and exacerbate ischemic injury after MCAO in female mice. A, Three days after MCAO glucosamine increased the content of pro-inflammatory cytokines (such as TNF-α, IL-1β and IL-6) in stroked female mice. The levels of anti-inflammatory cytokine IL-10 and the growth factor GM-CSF were also augmented in ipsilateral brain following Glucosamine treatment. Furthermore, glucosamine administration did not affect the levels of anti-inflammatory cytokine IL-4. Significance was determined by unpaired t-test. Values indicate as mean ± SEM (n = 3, ***P < 0.001; **P < 0.01; *P < 0.05). B, Stroke area was measured 3 and 7 days after MCAO in female subjects. The size of ischemic lesions after glucosamine treatment was increased significantly 7 days after MCAO when compared to the control non-treated group (n = 9, *P < 0.05). Significance was determined by unpaired t-test. C, the PPAR- γ activity was decreased in ipsilateral brains of both male and female stroked mice (72 h), however, treatment with glucosamine after stroke restored transcriptional activity of this protein only in male mice. Significance was determined by one-way ANOVA and Tukey’s post hoc test. Values indicate as mean ± SEM (n = 4, ***P < 0.001). D, three days following MCAO, glucosamine treatment upregulated the levels of phospho-p65 in female subjects and downregulates the expression of phospho-p65 in male mice (n = 6, ***P < 0.001; **P < 0.01). Significance was determined by unpaired t-test. All graphs are mean ± SEM.
To search for potential sex-dependent mechanisms in response to glucosamine treatment, we first compared the expression levels of Gal-3 and IL-4 in the ischemic brains of glucosamine-treated male and female mice. As shown in Fig. 2, Fig. 3 Gal-3/IL4 expression levels were significantly increased after stroke (72 h) in glucosamine-treated male mice. On the other side, the expression levels of Gal-3 were significantly decreased and IL-4 not affected (see Figs. 4 and 5) in the ischemic brains of glucosamine -treated females. Next, as a downstream target of the Gal-3/IL4 receptor signaling we analyzed transcriptional activity of PPAR-γ. It has been shown that IL4 receptor-mediated PPAR-γ activation elicits anti-inflammatory properties in different cell types by interfering with transcriptional regulation of inflammatory responses such as nuclear factor kappa-light-chain-enhancer (NF-κB) (Remels et al., 2009; Victor et al., 2006). Evidence suggests that increase in PPAR-γ activity may induce anti-inflammatory and neuroprotective phenotype in microglia after stroke (Xia et al., 2015). As shown in Fig. 3, Fig. 5 days following MCAO the PPAR-γ activity was impaired in ischemic, ipsilateral hemisphere of the brains in both male and female mice. Importantly, treatment with glucosamine after stroke increased/restored transcriptional activity of this protein in male but not in female mice (Fig. 5C, n = 4, ***P < 0.001). In keeping with a role of PPAR-γ as a regulator of NF-κB (Scirpo et al., 2015) we analyzed p-p65 levels in glucosamine treated male and female mice and controls. As further shown in Fig. 5C, glucosamine treatment is associated with decreased phospho-p65 expression levels in males and increased phospho-p65 in female mice (Fig. 5D, n = 6, ***P < 0.001; **P < 0.01), suggesting a sex-dependent and diverging effects of glucosamine on Gal-3/IL4 downstream signaling targets. Taken together, our results suggest that diverging Gal-3/IL4 receptor/PPAR-γ/NF-kB immune signaling after stroke may underlie sexual dimorphism in response to glucosamine treatment after stroke.
4. Discussion
Here, we provide in vivo evidence of the divergent effects of glucosamine on the post-stroke inflammation in male and female mice. We show that glucosamine increases Gal-3 expression levels, induces alternative inflammation and exerts marked neuroprotection in male stroke. Conversely, in female mice, glucosamine treatment after stroke decreases Gal-3 expression, enhances post-ischemic inflammation and exacerbates ischemic injury. Furthermore, we showed that glucosamine differentially regulate PPAR-γ/NF-kB immune signaling in female and male stroke. Taken together, our results suggest that diverging Gal-3/IL4 receptor/PPAR-γ/NF-kB immune signaling after stroke may underlie sexual dimorphism in response to glucosamine treatment after stroke.
Glucosamine is a naturally occurring sugar commonly used for treatment of osteoarthritis; however, its anti-inflammatory and/or immunomodulatory potential has been also demonstrated in different disease models including hypoxia, ischemia, and in sepsis condition (Fluri et al., 2015; Hwang et al., 2010, 2019; Largo et al., 2003; Lee et al., 2018). As revealed in our study, in male mice, glucosamine increases the endogenous Gal-3 levels and regulates the TLR2 signaling in early and sub-chronic post-stroke inflammatory response. Importantly, we and others have shown that, enhancing delayed immune responses and/or shifting the immune balance towards alternative inflammation may represent an important strategy to promote repair after stroke (Hwang et al., 2010; Bohacek et al., 2012; Kim et al., 2014a; Rahimian et al., 2018). The therapeutic potential of glucosamine has been also investigated in animal model of multiple sclerosis (EAE). Different route of glucosamine administration significantly attenuated EAE symptoms, CNS inflammation and demyelination, further supporting its immune-modulatory potential (Zhang et al., 2005). In addition, glucosamine have been shown to possess immunosuppressive properties and was effective in prolonging graft survival in mice (Ma et al., 2002). As a mechanism, it has been shown that O-Linked β-N-acetylglucosamine products of glucosamine are potent post-translational regulator of genes involved in the general inflammation process. NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and nuclear factor of activated T-cells (NFAT) are two important inflammatory transcriptional factors that their activation is negatively affected by O-glycosylation (Ma et al., 2002). At present, however, sexual dimorphism in response to glucosamine treatment has not been reported and/or investigated.
Over the past years, the observed differences between male and female immune responses have been a growing topic of interest. In fact, the intrinsic dissimilarities have been demonstrated in normal condition (Guneykaya et al., 2018) and following brain injuries including MCAO (Villa et al., 2018). In addition, several in vitro studies also suggested male/female differences in response to acute inflammatory challenge. For example, in vitro, male neonatal microglia show higher pro-inflammatory response to LPS, and, intriguingly estrogen has an anti-inflammatory impact on male neonatal microglial cultures but pro-inflammatory effect on female cells (Caplan et al., 2017; Gunther et al., 2015; Loram et al., 2012). Sex dimorphism has been also revelaed in TLR-mediated pro-inflammatory response in microglia (Loram et al., 2012; Cordeau et al., 2016) thus suggesting that sex -dependent microglia activation patterns after stroke may have significant impact on the evolution of the ischemic brain injury and potential treatments (Manwani and McCullough, 2011; Rahimian et al., 2019a).
Our recent work revealed marked sex differences in the expression patterns of the microglial protein Gal-3 after stroke (Rahimian et al., 2019a). To our surprise, and as demonstrated in the current study, glucosamine differentially regulated Gal-3 expression in the ischemic brains of male and female mice after MCAO. Contrary to robust increase Gal-3 levels in males, Gal-3 levels were significantly decreased after stroke in the brains of female mice. The observed decrease in Gal-3 levels may, in part, explain a shift towards pro-inflammatory phenotypes in female microglia following brain ischemia. Namely, evidence suggests that Gal-3 may directly induce activation of PPAR-γ (Baek et al., 2015). This transcriptional factor has important role in polarizing microglia and inhibiting of inflammatory signaling pathways by decreasing nuclear translocation of the NF-kB subunit p65 in neuron-glia cells in neurodegenerative conditions (Baek et al., 2015; Dehmer et al., 2004; Yenari et al., 2010). It has been shown that the expression and activity of this transcriptional factor is sex dependent (Kadowaki et al., 2007; Rahimian et al., 2019a) and its reduction was more prominent in male mice following brain ischemia (Victor et al., 2006). Interestingly, glucosamine treatment restored PPAR-γ activity in male but not in female mice.
In conclusion, our results revealed that glucosamine regulates innate immune response after stroke (including inflammatory canonical pathways such as NF-κB in macrophage/microglia) in a sex specific manner. In male mice, it potentiates Gal-3-mediated microglia/macrophages alternative activation and confers neuroprotection. On the other side, in female stroke, glucosamine increases pro-inflammatory signaling and exacerbates ischemic injury. Together, our results strongly suggest that targeting immune system after stroke may require adapted sex-specific therapeutic strategies. At present, however, our understanding of the molecular mechanisms involved in sexual dimorphism in post-stroke microglial activation and responsiveness to therapy remains inadequate. Therefore, an important question that arises here is to what extent and/or whether glucosamine therapeutic effects are sexually dimorphic in humans as well? Glucosamine is readily available drug, and as a dietary supplement, is commonly used in the treatment of osteoarthritis and other inflammatory conditions. It is noteworthy that although glucosamine seems to be well-tolerated and appear to be safe for use in humans, the number of long-term studies to confirm its long-term safety and effectiveness is rather limited (Zhu et al., 2018). Initially prescribed as a cartilage preserving drug, growing evidence suggests that glucosamine may exert (additional) marked immune-modulatory properties. Importantly, beyond the mechanistically interesting biological sex differences observed in our pre-clinical studies, our data suggest that glucosamine may exacerbate inflammation and post-ischemic injury in females. Therefore, further studies may be needed to examine its effectiveness and/or investigate the potential sexual dimorphism in responsiveness to glucosamine treatment of human disease.
Funding
This work was supported by the Heart and Stroke Foundation of Canada, Grant in Aid no G-17-0018372 (J.K.)
Declaration of competing interest
All coauthors have seen and agreed with the content of the manuscript. The material in the manuscript has not been published and is not being considered for publication elsewhere in whole or in part in any language except as an abstract. The Authors disclose no financial and/or conflict of interest.
References
- Acaz-Fonseca E., Duran J.C., Carrero P., Garcia-Segura L.M., Arevalo M.A. Sex differences in glia reactivity after cortical brain injury. Glia. 2015;63:1966–1981. doi: 10.1002/glia.22867. [DOI] [PubMed] [Google Scholar]
- Baek J.H., Kim S.J., Kang H.G., Lee H.W., Kim J.H., Hwang K.A., Song J., Chun K.H. Galectin-3 activates PPARgamma and supports white adipose tissue formation and high-fat diet-induced obesity. Endocrinology. 2015;156:147–156. doi: 10.1210/en.2014-1374. [DOI] [PubMed] [Google Scholar]
- Bodhankar S., Lapato A., Chen Y., Vandenbark A.A., Saugstad J.A., Offner H. Role for microglia in sex differences after ischemic stroke: importance of M2. Metab. Brain Dis. 2015;30:1515–1529. doi: 10.1007/s11011-015-9714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohacek I., Cordeau P., Lalancette-Hebert M., Gorup D., Weng Y.C., Gajovic S., Kriz J. Toll-like receptor 2 deficiency leads to delayed exacerbation of ischemic injury. J. Neuroinflammation. 2012;9:191. doi: 10.1186/1742-2094-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan H.W., Cox C.S., Bedi S.S. Do microglia play a role in sex differences in TBI? J. Neurosci. Res. 2017;95:509–517. doi: 10.1002/jnr.23854. [DOI] [PubMed] [Google Scholar]
- Cordeau P., Jr., Lalancette-Hebert M., Weng Y.C., Kriz J. Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke. 2008;39:935–942. doi: 10.1161/STROKEAHA.107.501460. [DOI] [PubMed] [Google Scholar]
- Cordeau P., Jr., Lalancette-Hebert M., Weng Y.C., Kriz J. Estrogen receptors alpha mediates postischemic inflammation in chronically estrogen-deprived mice. Neurobiol. Aging. 2016;40:50–60. doi: 10.1016/j.neurobiolaging.2016.01.002. [DOI] [PubMed] [Google Scholar]
- d’Avila J.C., Lam T.I., Bingham D., Shi J., Won S.J., Kauppinen T.M., Massa S., Liu J., Swanson R.A. Microglial activation induced by brain trauma is suppressed by post-injury treatment with a PARP inhibitor. J. Neuroinflammation. 2012;9:31. doi: 10.1186/1742-2094-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmer T., Heneka M.T., Sastre M., Dichgans J., Schulz J.B. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J. Neurochem. 2004;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Doll D.N., Barr T.L., Simpkins J.W. Cytokines: their role in stroke and potential use as biomarkers and therapeutic targets. Aging Dis. 2014;5:294–306. doi: 10.14336/AD.2014.0500294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhfouri G., Ahmadiani A., Rahimian R., Grolla A.A., Moradi F., Haeri A. WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-gamma pathway. Neuropharmacology. 2012;63:653–666. doi: 10.1016/j.neuropharm.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Fluri F., Grunstein D., Cam E., Ungethuem U., Hatz F., Schafer J., Samnick S., Israel I., Kleinschnitz C., Orts-Gil G., Moch H., Zeis T., Schaeren-Wiemers N., Seeberger P. Fullerenols and glucosamine fullerenes reduce infarct volume and cerebral inflammation after ischemic stroke in normotensive and hypertensive rats. Exp. Neurol. 2015;265:142–151. doi: 10.1016/j.expneurol.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Gravel M., Weng Y.C., Kriz J. Model system for live imaging of neuronal responses to injury and repair. Mol. Imag. 2011;10:434–445. [PubMed] [Google Scholar]
- Guneykaya D., Ivanov A., Hernandez D.P., Haage V., Wojtas B., Meyer N., Maricos M., Jordan P., Buonfiglioli A., Gielniewski B., Ochocka N., Comert C., Friedrich C., Artiles L.S., Kaminska B., Mertins P., Beule D., Kettenmann H., Wolf S.A. Transcriptional and translational differences of microglia from male and female brains. Cell Rep. 2018;24:2773–2783. doi: 10.1016/j.celrep.2018.08.001. e2776. [DOI] [PubMed] [Google Scholar]
- Gunther M., Davidsson J., Plantman S., Norgren S., Mathiesen T., Risling M. Neuroprotective effects of N-acetylcysteine amide on experimental focal penetrating brain injury in rats. J. Clin. Neurosci. 2015;22:1477–1483. doi: 10.1016/j.jocn.2015.03.025. [DOI] [PubMed] [Google Scholar]
- He Y., Ma X., Li D., Hao J. Thiamet G mediates neuroprotection in experimental stroke by modulating microglia/macrophage polarization and inhibiting NF-kappaB p65 signaling. J. Cerebr. Blood Flow Metabol. 2017;37:2938–2951. doi: 10.1177/0271678X16679671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.S., Kim K.H., Park J., Kim S.M., Cho H., Lee Y., Han I.O. Glucosamine improves survival in a mouse model of sepsis and attenuates sepsis-induced lung injury and inflammation. J. Biol. Chem. 2019;294:608–622. doi: 10.1074/jbc.RA118.004638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.Y., Shin J.H., Hwang J.S., Kim S.Y., Shin J.A., Oh E.S., Oh S., Kim J.B., Lee J.K., Han I.O. Glucosamine exerts a neuroprotective effect via suppression of inflammation in rat brain ischemia/reperfusion injury. Glia. 2010;58:1881–1892. doi: 10.1002/glia.21058. [DOI] [PubMed] [Google Scholar]
- Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nat. Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki K., Fukino K., Negishi E., Ueno K. Sex differences in PPARgamma expressions in rat adipose tissues. Biol. Pharm. Bull. 2007;30:818–820. doi: 10.1248/bpb.30.818. [DOI] [PubMed] [Google Scholar]
- Kamel H., Iadecola C. Brain-immune interactions and ischemic stroke: clinical implications. Arch. Neurol. 2012;69:576–581. doi: 10.1001/archneurol.2011.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabori M., Kacimi R., Kauppinen T., Calosing C., Kim J.Y., Hsieh C.L., Nakamura M.C., Yenari M.A. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J. Neurosci. 2015;35:3384–3396. doi: 10.1523/JNEUROSCI.2620-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Park K.W., Lee E.W., Jang W.S., Seo J., Shin S., Hwang K.A., Song J. Suppression of PPARgamma through MKRN1-mediated ubiquitination and degradation prevents adipocyte differentiation. Cell Death Differ. 2014;21:594–603. doi: 10.1038/cdd.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Kawabori M., Yenari M.A. Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr. Med. Chem. 2014;21:2076–2097. doi: 10.2174/0929867321666131228205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz J., Lalancette-Hebert M. Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol. 2009;117:497–509. doi: 10.1007/s00401-009-0496-1. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M., Gowing G., Simard A., Weng Y.C., Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette-Hebert M., Julien C., Cordeau P., Bohacek I., Weng Y.C., Calon F., Kriz J. Accumulation of dietary docosahexaenoic acid in the brain attenuates acute immune response and development of postischemic neuronal damage. Stroke. 2011;42:2903–2909. doi: 10.1161/STROKEAHA.111.620856. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M., Phaneuf D., Soucy G., Weng Y.C., Kriz J. Live imaging of Toll-like receptor 2 response in cerebral ischaemia reveals a role of olfactory bulb microglia as modulators of inflammation. Brain. 2009;132:940–954. doi: 10.1093/brain/awn345. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M., Swarup V., Beaulieu J.M., Bohacek I., Abdelhamid E., Weng Y.C., Sato S., Kriz J. Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J. Neurosci. 2012;32:10383–10395. doi: 10.1523/JNEUROSCI.1498-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largo R., Alvarez-Soria M.A., Diez-Ortego I., Calvo E., Sanchez-Pernaute O., Egido J., Herrero-Beaumont G. Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2003;11:290–298. doi: 10.1016/s1063-4584(03)00028-1. [DOI] [PubMed] [Google Scholar]
- Lee Y., Lee S., Park J.W., Hwang J.S., Kim S.M., Lyoo I.K., Lee C.J., Han I.O. Hypoxia-induced neuroinflammation and learning-memory impairments in adult zebrafish are suppressed by glucosamine. Mol. Neurobiol. 2018;55:8738–8753. doi: 10.1007/s12035-018-1017-9. [DOI] [PubMed] [Google Scholar]
- Lo E.H., Dalkara T., Moskowitz M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Loram L.C., Sholar P.W., Taylor F.R., Wiesler J.L., Babb J.A., Strand K.A., Berkelhammer D., Day H.E., Maier S.F., Watkins L.R. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–1699. doi: 10.1016/j.psyneuen.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Rudert W.A., Harnaha J., Wright M., Machen J., Lakomy R., Qian S., Lu L., Robbins P.D., Trucco M., Giannoukakis N. Immunosuppressive effects of glucosamine. J. Biol. Chem. 2002;277:39343–39349. doi: 10.1074/jbc.M204924200. [DOI] [PubMed] [Google Scholar]
- Manwani B., McCullough L.D. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Womens Health (Lond) 2011;7:319–339. doi: 10.2217/whe.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimian R., Beland L.C., Kriz J. Galectin-3: mediator of microglia responses in injured brain. Drug Discov. Today. 2018;23:375–381. doi: 10.1016/j.drudis.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Rahimian R., Cordeau P., Jr., Kriz J. Brain response to injuries: when microglia go sexist. Neuroscience. 2019;405:14–23. doi: 10.1016/j.neuroscience.2018.02.048. [DOI] [PubMed] [Google Scholar]
- Rahimian R., Lively S., Abdelhamid E., Lalancette-Hebert M., Schlichter L., Sato S., Kriz J. Delayed Galectin-3-mediated reprogramming of microglia after stroke is protective. Mol. Neurobiol. 2019;56:6371–6385. doi: 10.1007/s12035-019-1527-0. [DOI] [PubMed] [Google Scholar]
- Rahimian R., Zirak M.R., Keshavarz M., Fakhraei N., Mohammadi-Farani A., Hamdi H., Mousavizadeh K. Involvement of PPARgamma in the protective action of tropisetron in an experimental model of ulcerative colitis. Immunopharmacol. Immunotoxicol. 2016;38:1–9. doi: 10.1080/08923973.2016.1231202. [DOI] [PubMed] [Google Scholar]
- Remels A.H., Langen R.C., Gosker H.R., Russell A.P., Spaapen F., Voncken J.W., Schrauwen P., Schols A.M. PPARgamma inhibits NF-kappaB-dependent transcriptional activation in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009;297:E174–E183. doi: 10.1152/ajpendo.90632.2008. [DOI] [PubMed] [Google Scholar]
- Scirpo R., Fiorotto R., Villani A., Amenduni M., Spirli C., Strazzabosco M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-gamma limits NF-kappaB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology. 2015;62:1551–1562. doi: 10.1002/hep.28000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor N.A., Wanderi E.W., Gamboa J., Zhao X., Aronowski J., Deininger K., Lust W.D., Landreth G.E., Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. Eur. J. Neurosci. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Villa A., Gelosa P., Castiglioni L., Cimino M., Rizzi N., Pepe G., Lolli F., Marcello E., Sironi L., Vegeto E., Maggi A. Sex-specific features of microglia from adult mice. Cell Rep. 2018;23:3501–3511. doi: 10.1016/j.celrep.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y.C., Kriz J. Differential neuroprotective effects of a minocycline-based drug cocktail in transient and permanent focal cerebral ischemia. Exp. Neurol. 2007;204:433–442. doi: 10.1016/j.expneurol.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Xia C.Y., Zhang S., Gao Y., Wang Z.Z., Chen N.H. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int. Immunopharm. 2015;25:377–382. doi: 10.1016/j.intimp.2015.02.019. [DOI] [PubMed] [Google Scholar]
- Yenari M.A., Kauppinen T.M., Swanson R.A. Microglial activation in stroke: therapeutic targets. Neurotherapeutics. 2010;7:378–391. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.X., Yu S., Gran B., Rostami A. Glucosamine abrogates the acute phase of experimental autoimmune encephalomyelitis by induction of Th2 response. J. Immunol. 2005;175:7202–7208. doi: 10.4049/jimmunol.175.11.7202. [DOI] [PubMed] [Google Scholar]
- Zhu X., Sang L., Wu D., Rong J., Jiang L. Effectivness and safety of glucosamine and chondroitin for the treatment of osteoarthritis: a meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2018;13:170. doi: 10.1186/s13018-018-0871-5. [DOI] [PMC free article] [PubMed] [Google Scholar]