Abstract
Background
Neuropsychiatric lupus (NPSLE) refers to the neurological and psychiatric manifestations that are commonly observed in patients with systemic lupus erythematosus (SLE). An important question regarding the pathogenesis of NPSLE is whether the symptoms are caused primarily by CNS-intrinsic mechanisms or develop as a consequence of systemic autoimmunity. Currently used spontaneous mouse models for SLE have already contributed significantly to unraveling how systemic immunity affects the CNS. However, they are less suited when interested in CNS primary mechanisms. In addition, none of these models are based on genes that are associated with SLE. In this study, we evaluate the influence of A20, a well-known susceptibility locus for SLE, on behavior and CNS-associated changes in inflammatory markers. Furthermore, given the importance of environmental triggers for disease onset and progression, the influence of an acute immunological challenge was evaluated.
Methods
Female and male A20 heterozygous mice (A20+/−) and wildtype littermates were tested in an extensive behavioral battery. This was done at the age of 10±2weeks and 24 ± 2 weeks to evaluate the impact of aging. To investigate the contribution of an acute immunological challenge, LPS was injected intracerebroventricularly at the age of 10±2weeks followed by behavioral analysis. Underlying molecular mechanisms were evaluated in gene expression assays on hippocampus and cortex. White blood cell count and blood-brain barrier permeability were analyzed to determine whether peripheral inflammation is a relevant factor.
Results
A20 heterozygosity predisposes to cognitive symptoms that were observed at the age of 10 ± 2 weeks and 24 ± 2 weeks. Young A20+/− males and females showed a subtle cognitive phenotype (10±2weeks) with distinct neuroinflammatory phenotypes. Aging was associated with clear neuroinflammation in female A20+/− mice only. The genetic predisposition in combination with an environmental stimulus exacerbates the behavioral impairments related to anxiety, cognitive dysfunction and sensorimotor gating. This was predominantly observed in females. Furthermore, signs of neuroinflammation were solely observed in female A20+/− mice. All above observations were made in the absence of peripheral inflammation and of changes in blood-brain barrier permeability, thus consistent with the CNS-primary hypothesis.
Conclusions
We show that A20 heterozygosity is a predisposing factor for NPSLE. Further mechanistic insight and possible therapeutic interventions can be studied in this mouse model that recapitulates several key hallmarks of the disease.
Keywords: Neuropsychiatric lupus, A20/TNFAIP3, Behavior, Neuroinflammation, Genetic predisposition, Environmental trigger, Female predominance
Highlights
-
•
A novel mouse model for neuropsychiatric lupus was established.
-
•
Loss of 1 copy of A20/TNFAIP3 leads to neuroinflammation and cognitive symptoms.
-
•
Female-specific neuroinflammation was seen with age.
-
•
An environmental stimulus worsens the behavioral impairments.
-
•
The results show that the brain is the primary affected site.
1. Background
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder with a high female to male ratio (9:1) in which the innate and adaptive immune systems inappropriately generate an immune response against a broad range of macromolecules that are primarily nucleus associated (Pisetsky, 2019). SLE is typically characterized by the production of autoantibodies that form immune complexes upon binding these nucleus-associated antigens and trigger abnormal inflammatory responses. Since any organ system can be affected, SLE is associated with a striking heterogeneity of clinical presentations (Kaul et al., 2016).
Neuropsychiatric systemic lupus erythematosus (NPSLE) refers to manifestations that involve the central and peripheral nervous system (ACR, 1999). Symptoms associated with the central nervous system (CNS) are more common and are subdivided in focal and diffuse (Hanly et al., 2010). Focal symptoms, such as cerebrovascular disease and seizures, are caused by vascular events that are often triggered by anti-phospholipid autoantibodies (Love and Santoro, 1990). They are associated with structural brain abnormalities and respond well to therapy (Hanly, 2014). Diffuse symptoms remain poorly understood but are believed to be caused by neuroinflammatory mechanisms (Gelb et al., 2018; Schwartz et al., 2019). Diffuse symptoms include depression, anxiety and mood disorders, psychosis, acute confusional state and cognitive impairment, and are not associated with structural brain abnormalities (ACR, 1999; Jeltsch-David and Muller, 2014).
The etiology of neuropsychiatric symptoms in lupus patients remains unresolved (Bortoluzzi et al., 2018). In humans, neuropsychiatric symptoms can be observed before or at the time of SLE diagnosis, indicating that they can appear in absence of peripheral organ damage (Hanly et al., 2007; Petri et al., 2010; van Dam et al., 1994). Furthermore, it is repeatedly observed that symptoms arise independent from systemic disease activity as measured by clinical and laboratory analysis (Magro-Checa et al., 2019; Shimojima et al., 2005). This evidence supports the ‘CNS primary hypothesis’, where neuropsychiatric symptoms are induced by CNS intrinsic mechanisms. On the other hand, neuropsychiatric symptoms can also arise as a secondary consequence of systemic disease activity and they can be triggered by therapy, infections and metabolic disorders. The latter is referred to as the ‘CNS secondary hypothesis’. Research using mouse models of NPSLE has provided supportive evidence for both hypotheses (Bendorius et al., 2018; Stock et al., 2015; Wen et al., 2016). Most spontaneous mouse models for SLE are characterized by key clinical manifestations associated with the autoimmune character of the disorder and allow to study how systemic immunity is influencing the CNS. However, these models are less appropriate to analyze how CNS intrinsic mechanisms contribute to the etiology of NPSLE. A further important drawback of these spontaneous mouse models for lupus is that none of them carry mutations in genes that are associated with SLE (Schwartz et al., 2019).
TNFAIP3 (tumor necrosis factor alpha induced protein 3) is a well-known susceptibility locus for SLE and recently, a mutation was discovered in a patient with NPSLE (Duan et al., 2019; Ma and Malynn, 2012). TNFAIP3 encodes the ubiquitin-editing enzyme A20, a strong negative regulator of the family of nuclear factor-kappa B (NF-κB) transcription factors and a prominent cytoprotective protein (Adrianto et al., 2011; Graham et al., 2008; Musone et al., 2008; S. Wang et al., 2013). The NF-κB pathway regulates the expression of a large number of genes that are critical for innate and adaptive immunity. This signaling pathway needs to be tightly regulated as uncontrolled activity is associated with several autoimmune disorders, chronic inflammatory diseases, neurological disorders and cancer (Zhang et al., 2017). Consistent with this notion, it has been shown in vitro for a pair of SLE-associated functional variants (TT > A enhancer) that they significantly reduce the expression of A20 which is associated with an increased activity of the NF-κB pathway (Wang et al., 2016).
The function of A20 is complex and still incompletely understood. Originally, A20 was thought to control NF-κB activity in a dual manner. Biochemical assays identified two motifs in the A20 protein that have ubiquitinating and de-ubiquitinating activity (Evans et al., 2004; Wertz et al., 2004). The ovarian tumor domain (OTU) located N-terminally harbors deubiquitinating activity resulting in the destabilization of protein-protein interactions (Evans et al., 2004; Wertz et al., 2004). On the other hand, Znf4, one of the seven zinc finger motifs located at the C-terminus of the A20 protein, was shown to have ubiquitin ligase activity that targets proteins for proteasomal degradation (Bosanac et al., 2010; Wertz et al., 2004). In vivo evaluation of protein variants in which the enzymatic function of either the OTU domain and ZnF4 was inactivated by mutation, questions the importance of these findings since the phenotypes observed in these mice did not resemble the phenotype observed upon A20 deficiency (De et al., 2014; Lu et al., 2013; Wertz et al., 2015). Currently, it is believed that A20 uses mainly non-catalytic ubiquitin binding mechanisms to control NF-κB activity (Skaug et al., 2011; Verhelst et al., 2012; Wertz et al., 2015).
The essential anti-inflammatory function of A20 was demonstrated in a knockout mouse model, where animals died prematurely due to severe organ inflammation (Lee et al., 2000). Furthermore, A20 knockout and heterozygous mouse models develop spontaneous neuroinflammation without changes to blood brain barrier (BBB) permeability (Guedes et al., 2014). The latter already indicates that the contribution of systemic immunity to the development of neuroinflammation is limited. Consequently, A20 heterozygous mice could represent a good model to disentangle the role of systemic immune response from CNS intrinsic mechanisms in the development of neuropsychiatric symptoms.
In this study, the behavioral phenotype of A20 heterozygous mice was evaluated to test the ‘CNS primary hypothesis’. This was done in young (3 months old) and more mature (6 months old) mice to evaluate the impact of aging. The latter is of importance since the peak age of incidence for SLE in humans ranges from 30 to 70 years old (Rees et al., 2017). Furthermore, since it is known that genetic as well as environmental factors contribute to the pathogenesis of NPSLE, the relative impact of A20 heterozygosity in the context of an acute immunological challenge was evaluated. This study identifies A20 heterozygosity as a predisposing factor and provides more insight in the mechanisms by which genetic predisposition can contribute to the establishment of neuropsychiatric symptoms and how environmental triggers can aggravate the disease symptomatology.
2. Methods
2.1. Animals
The conditional A20 allele was created by targeting exons 4 and 5 as described (Vereecke et al., 2010). A20 heterozygous knockout (A20+/−) mice were generated by breeding A20 heterozygous floxed (A20+/FL) females with PGK-Cre transgenic male mice. This resulted in four different genotypes: A20 heterozygous knockout (A20+/−), A20 heterozygous floxed (A20+/FL), PGK-Cre recombinase mice (PGK-Cre) and wildtype (WT) mice (Table 1). All experiments were performed on mice backcrossed into the C57Bl/6 background for at least 10 generations. Sex-mixed groups of animals aged 8–12 weeks were used for behavioral experiments on young mice (referred to as 3 months old) and mature mice at the age of 24 ± 2 weeks old (referred to as 6 months old). The test battery evaluated motor, emotional, social, cognitive and disease-related behavior. All experiments were conducted during the light phase of their active cycle. For the LPS dose-response study, 8-weeks old C57Bl/6J female mice were purchased from Elevage Janvier (Le-Genest Saint-Isle, France). After determination of the optimal LPS dose, wildtype and A20+/− mice were injected with LPS at the age between 8 and 11 weeks old (average age 9.5 weeks) and tested in a behavioral battery. This behavioral battery focused specifically on motor function, emotional, cognitive and disease-related phenotypes. All animals were group-housed under conventional laboratory conditions (12h light/dark, 22 °C) with ad libitum access to food and water. All protocols and experiments were approved by the KU Leuven Ethical Committee and in accordance with the European Community Council Directive 2010/63/EU.
Table 1.
Number of animals used for each behavioral phenotyping.
| BASELINE (3m old) |
BASELINE (6m old) |
LPS |
||||||
|---|---|---|---|---|---|---|---|---|
| WT | A20 ± | CRE | FLOX | WT | A20 ± | WT | A20 ± | |
| ♀ | 9 | 11 | 9 | 10 | 16 | 14 | 6 | 10 |
| ♂ | 12 | 7 | 7 | 8 | 10 | 11 | ||
2.2. Behavioral test battery
General neuromotor assessment: Cage activity and neuromotor coordination were assessed by various tests including 23h spontaneous cage activity, motor coordination and balance on the accelerating rotarod and grip strength measurement. Detailed protocols are described in (D’Hooge et al., 2005). 23h spontaneous cage activity was measured using a lab-built activity logger connected to three infrared photo beams. Mice were individually placed in 20 × 30 cm2 transparent home cages and their activity, measured by the number of beam crossings, was recorded over a period of 23 h. Protocols regarding neuromotor coordination and grip strength are described in Supplementary Methods.
Evaluation of exploratory, anxiety-related and social behavior: Exploration in open field and on elevated plus maze (Supplementary Methods) allows evaluation of anxiety-related behavior (Callaerts-Végh et al., 2006; D’Hooge et al., 2005). Before introducing the animals in the open field arena (50 × 50 cm2), animals were habituated in the dark for 30 min. Exploration in the arena was measured for 11 min (including 1 min habituation) using ANY-maze™ video tracking system software (Stoelting Co, IL, US). Total path length and the time spent in center and periphery were used as read-outs for exploratory behavior and anxiolysis, respectively. Assessment of social behavior was done using sociability and preference for social novelty (see Supplementary Methods) (Nadler et al., 2004; Naert et al., 2011a).
Cognitive assessment: Spatial learning, memory and cognitive flexibility were evaluated in the hidden-platform Morris water maze using acquisition and reversal learning protocols (Callaerts-Végh et al., 2012; D’Hooge et al., 2001; Stewart and Morris, 1993). A circular pool (diameter 150 cm) was filled with opacified water (nontoxic white paint) and maintained around 26 ± 1 °C. The platform (diameter 15 cm) was hidden 1 cm underneath the water surface. Its location was the same throughout the 10 days of spatial acquisition, but moved to the opposite quadrant during the additional 5 days of reversal learning. Probe trials (platform was removed from the pool) were performed on day 6 and 11 of acquisition and at the end of reversal learning. Animals were trained daily in four sequential sessions (15–30 min interval) starting from each of the four starting positions. Animals that were not able to find the platform within 120 s, were gently guided to the platform where they remained for 15 s before being transferred to their home cage. EthoVision video tracking equipment and software (Noldus, Wageningen, The Netherlands) were used to quantify and extract swim path parameters. The search strategy of each mouse during the probe trials was scored and classified using a 3-class scoring method (Brody and Holtzman, 2006; Callaerts-Végh et al., 2012; Lo et al., 2013). The 3-class scoring method distinguishes spatial memory-dependent strategies and less proficient, or non-spatial and repetitive strategies. Protocols regarding T-maze, Y-maze and cued- and context-dependent fear conditioning can be found in Supplementary Methods (Paradee et al., 1999).
Disease-associated phenotypes: Startle reactivity and prepulse inhibition (PPI) of the acoustic startle reflex were measured using a Med Associates acoustic startle box (St. Albans, VT, USA) as described previously (Naert et al., 2011b). Mice were restrained in a cubicle (ENV-406SM-8) that was mounted on a motion-sensitive platform located inside a sound-attenuating box and connected to Med Associates Startle Reflex software (v5.01). After a habituation period of 5 min, two startle pulse trial blocks were presented. The first phase consisted of five basic startle pulses (115 dB). In the second phase startle pulses, prepulses (70, 75 or 80 dB) and prepulse-startle pulses were presented at random. Percentage PPI (%PPI) was calculated according to the standard formula: %PPI = [1- (startle peak of prepulse-startle pulse)/(startle peak of startle pulse)] * 100. Depression-like behavior was analyzed using the forced swim test protocol (Supplementary Methods).
2.3. LPS administration
Animals were anesthetized using Iso-Vet (1000 mg/g Isofluran, Dechra Veterinary Products NV,Belgium) and placed in the robot stereotactic frame (Neurostar, Germany). Mice were placed on a heat pad (36 °C) to prevent hypothermia, and eyes were protected using Lacrinorm (Bausch&Lomb Pharma, Belgium). Before the onset of surgery, a local anesthetic, Xylocaine® (AstraZeneca, Belguim), was injected subcutaneously at the location of the incision. LPS from Escherichia coli 0111:B4 (Sigma-Aldrich, US) was dissolved in saline (Braun Vet care, Germany) to obtain the following concentrations: 10 ng/μl, 30 ng/μl, 100 ng/μl, 300 ng/μl and 1000 ng/μl. Bilateral intracerebroventricular (icv) injections (total volume 1 μl) were performed using a 10 μl syringe with a 26s gauge needle (Hamilton, Nevada US). Stereotactic injections were done at a constant rate of 0.1 μl/min, using the following coordinates (AP: 0.57 mm, Lat: ±1.18 mm, Z: 1.93 mm). After injection, the needle remained in position for an additional 5 min and was slowly retracted (100 μm per 30s) afterwards to avoid backflow. Intraperitoneal injection using 0.3 mg/ml Vetergesic® (20%BW, Ecuphar®, Breda, The Netherlands) was applied to limit postoperative pain and Triple antibiotic ointment (Walgreen Co, USA) was applied to prevent infections. The weight of all animals was monitored over the course of the experiment as an indicator for their general wellbeing.
2.4. Blood cell analysis and tissue isolation
Dolethal® (60 mg/kg, Vétequinol, Belgium) was injected intraperitoneally to deeply anesthetize the animals before blood collection and consecutive isolation of brain tissue. Blood samples were obtained from the retro-orbital sinus using Brand™ Micro-haematocrit capillary tubes (Fisher Scientific). White blood cell count was measured at least 24h after collection using the scil Vet ABC Plus™ (scil animal care company GmbH). After blood collection, hippocampus and cortex were isolated on ice and flash frozen for further gene expression analysis. At the time of tissue isolation, the group of animals used had the following age: 3-month old animals were approximately 19.5 weeks old, 6-month old animals were 36.5 weeks old and the animals injected at the age of 3-month old were approximately 14 weeks old.
2.5. Blood-brain barrier (BBB) permeability assay
To evaluate the permeability of the blood-brain barrier, fluorescein (FITC) labeled dextran (3000 MW, ThermoFisher Scientific) was injected intraperitoneally. The protocol was largely adopted from Devraj et al. (2018). FITC-dextran was injected either in mice at the age of 6 months or in 3 months old mice that received an LPS injection. After 5 min they received a lethal dose of Dolethal® (60 mg/kg, Vétequinol, Belgium). 15–20 min after the intraperitoneal injection of FITC-dextran, the animals were perfused and serum as well as the hemicerebrum were collected. Tissue was homogenized in 200 μl PBS and centrifuged at 15000 g, 4 °C for 20 min. Afterwards the 50 μl of the supernatant and serum (20 μl serum + 30 μl PBS) were measured using Infinite® 200 PRO (Tecan, Switserland). Raw fluorescence units were used to calculate the permeability index as described (Devraj et al., 2018).
2.6. qPCR analysis
Total RNA from hippocampal tissue and cortex was isolated using TRI reagent (Invitrogen). Reverse transcription on 1 μg RNA was performed using SensiFAST™ cDNA Synthesis Kit (Bioline) according to manufacturers’ instructions. Primers were designed to span an exon-exon junction and sequences are listed in (Table S1). qPCR was performed on a Viia 7 Real-Time PCR machine (ThermoFisher Scientific) using SYBR green detection (Bioline). Transcript levels were normalized to the geometric means of the reference genes Pgk1 (phosphoglycerate kinase 1) and Wdr33 (WD repeat domain 33). Candidate reference genes were obtained using the RefGenes tool (Genevestigator®, Nebion) and validated against commonly used reference genes. The geNorm algorithm (qbase+, Biogazelle) was used to select two reference genes with the most stable expression in the tissue of interest (Hellemans et al., 2007; Willems et al., 2008). The comparative Ct method was used to calculate relative gene expression (Schmittgen and Livak, 2008).
2.7. Statistical analysis
Results are expressed as mean ± SEMs unless stated otherwise. To analyze behavioral readouts, differences between mean values were determined using t-test, one-way analysis of variance (ANOVA) and two-way repeated measures analysis of variance (RM-ANOVA). Tukey tests were used for post hoc analysis. Log transformation of the data and non-parametric tests were performed when data did not meet the assumption of normality. Analysis of qPCR results was done using unpaired t-tests for pairwise comparisons or one-way ANOVA tests for multiple comparisons using Tukey’s multiple comparisons post hoc. All statistical analyses were performed using GraphPad Prism software and tests were performed assuming a significance level of 0.05.
3. Results
3.1. A20 heterozygosity leads to subtle cognitive impairments
NPSLE is characterized by a diversity of psychiatric symptoms. To identify the influence of A20 heterozygosity on behavioral phenotypes, A20+/− mice and wildtype control littermates were subjected to an extensive behavioral battery to analyze motor function, emotional function, social behavior, cognitive function and disease-related phenotypes. A20 heterozygous floxed (A20+/FL) and PGK-Cre littermates served as additional controls in the behavioral battery. Since no behavioral differences were found between A20+/FL, PGK-Cre and wildtype mice, further comparisons were limited to WT and A20+/− mice (Table S2).
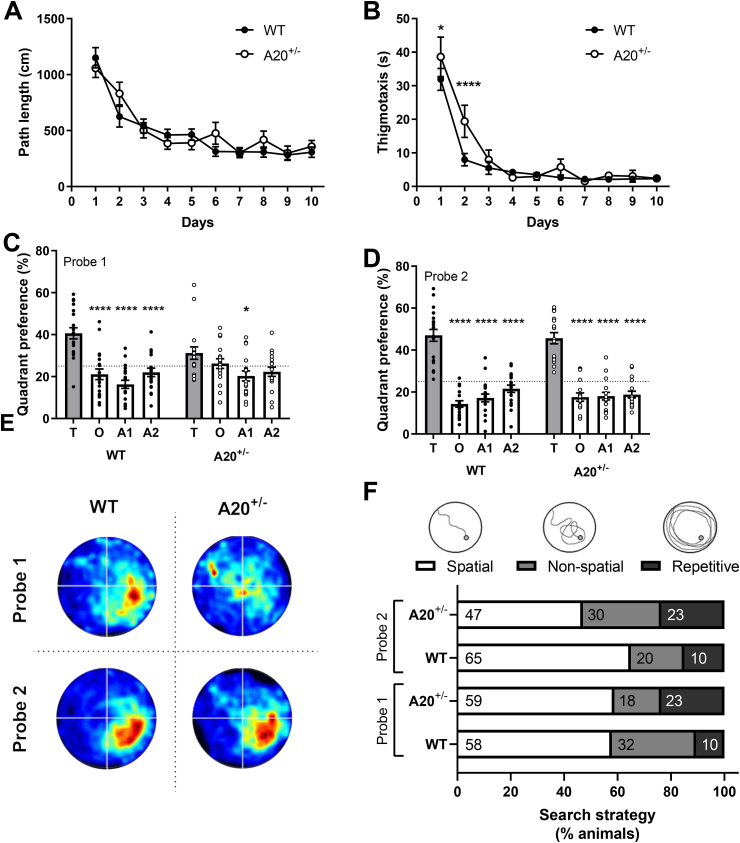
For most behavioral assays, no phenotypic differences between A20+/− and WT mice were found (Table S3). Only a subtle cognitive defect was detected in the Morris water maze (MWM) (Fig. 1). During the acquisition phase, overall performance of WT and A20+/− mice was similar. Only on the first two days of acquisition, thigmotaxis was increased in A20+/− mice compared to WT littermates (genotype x time: F27,585 = 1.80, P = 0.008, Fig. 1B). A memory-related defect was found in the first probe trial where wildtype animals spent significantly more time in the target quadrant (T) over the other quadrants (quadrant preference: F3,198 = 27.07, P < 0.0001; Fig. 1C), whereas A20+/− mice did not show this preference. However, they did show a significant preference for the target quadrant after ten days of training (quadrant preference: F3,198 = 134.8, P < 0.001; Fig. 1D). This indicates that A20+/− mice need more time to form a robust reference memory of the platform’s location. A visual representation, plotting the time spent in the different quadrants during the probe trials, confirms the preference for the target quadrant in WT animals after 5 days of training (Fig. 1E). The performance during the probe trials was further evaluated to identify the search strategies that were used by each animal individually (Brody and Holtzman, 2006). Spatial strategies are observed when spatial cues are used to swim directly to the location of the platform, whereas non-spatial strategies do not rely on cognitive abilities. The latter can be subdivided into non-spatial and repetitive search strategies. Here, search strategies were evaluated until the animal crossed the platform’s location for the first time (Fig. 1F). No significant differences were found between A20+/− and WT mice within the same probe trial, as well as between probe trial 1 and 2.
Fig. 1.
A subtle memory deficit was observed in A20 heterozygous mice in comparison to wildtype littermates.
(A) No difference in path length was observed between A20 heterozygous mice (A20+/−, white circles) and wildtype mice (WT; black circles) during acquisition; (B) A20+/− mice spent significantly more time in the periphery in comparison to WT littermates. This was most pronounced on the first and second day of acquisition; (C) After five days of training, A20+/− mice showed less preference for the target quadrant in comparison to the other three quadrants (T: Target, O: Opposite, A1: Adjacent 1 and A2: Adjacent 2 quadrants); (D) Preference for the target quadrant after ten days of training is seen for both genotypes; (E) Visual representation of the time spent in different quadrants for WT and A20+/− mice during both probe trials; (F) Search strategies used during the acquisition probe trials by A20+/− and WT littermates. No significant differences were found between A20+/− and WT littermates within as well as between probe trials. Data are represented as mean±SEMs (A-D) and as ratios (F). Statistical differences were determined using repeated-measures two-way ANOVA using Dunnett’s multiple comparisons test for post hoc analysis (A-D) and Fisher’s exact test (F); (*P < 0.05, ****P < 0.0001).
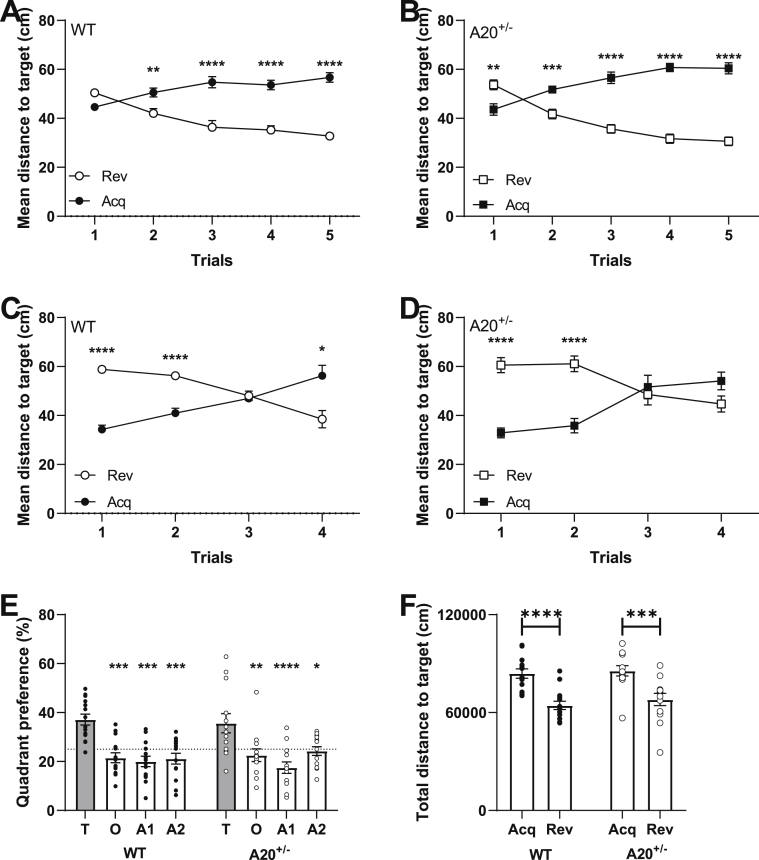
A recent systematic review of epidemiological studies on SLE has estimated the peak age of incidence for females between 30 and 70 years old (Rees et al., 2017). Thus far, the animals used for behavioral characterization were 3 months old, which corresponds to a human age of approximately 20 years (Flurkey et al., 2007). Since the peak age of incidence occurred mainly in middle-aged adults, we decided to evaluate the cognitive behavior of female A20+/− and WT littermates at the age of 6 months (≈30 years in humans) (Flurkey et al., 2007). Cognitive function was only evaluated in female A20+/− mice because of the female predominance that is observed in SLE (Table 1). During acquisition learning in the Morris water maze experiment, similar learning curves for both path length (Figs. S1–A) and time spent in periphery (Figs. S1–B) were observed for female A20+/− and WT littermates. The first probe trial, conducted after five days of training, revealed that none of the genotypes showed a significant preference for the target quadrant and that they needed more training to form a robust memory of the platforms location (Figs. S1–C). After ten days of training, all genotypes were spending significantly more time in the target quadrant (Figs. S1–D). Subsequently, we investigated cognitive flexibility by means of reversal learning in which the platform is moved to the opposite quadrant. Over the five days of training, a difference in mean distance to target was observed between WT and A20+/− mice (Fig. 2). On the first day of reversal learning wildtype animals did not show a significant difference in the distance towards the acquisition (acq) and reversal target (rev) (time x target: F4, 96 = 29.60, p < 0.0001; target: F4,96 = 46.18, p < 0.0001; post hoc day 1: t120 = 2.15, p = 0.15, Fig. 2A). In contrast, A20+/− mice were still swimming significantly closer to the acquisition target (time x target: F4,88 = 56.84, p < 0.0001; time: F1,22 = 64.71, p < 0.0001; post hoc day 1: t110 = 3.61, p = 0.002; Fig. 2B). This implies a difference in performance on the first day of reversal learning. This day is of particular interest as it gives an indication of how fast the animals are able to update their initial swim strategy. Further evaluation of the mean distance to target for A20+/− and control littermates confirms the previously observed difference in learning. Wildtype animals show a significant preference for the new location of the platform at the fourth trial on the first day of reversal learning (time x target: F3,72 = 28.81, p < 0.0001, time: F1,24 = 9.47, p = 0.005; post hoc trial 4: t23 = 3.19, p = 0.01; Fig. 2C), which was not observed for A20+/− (time x target: F3,66 = 18.22, p < 0.0001; time: F1,22 = 10.69, p = 0.0035; post hoc trial 4: t21 = 1.96, p = 0.24; Fig. 2D). After five days of reversal learning, a probe trial was conducted during which both wildtype and A20+/− mice showed a significant preference for the target quadrant (Fig. 2E) and are swimming significantly closer to the new location of the platform in comparison to the previous location (Fig. 2F). Thus, A20+/− females at the age of 6 months show a defect in reversal learning that corresponds to a deficit in cognitive flexibility.
Fig. 2.
A20+/− female mice show cognitive flexibility deficits at the age of 6 months.
(A) Mean distance to the acquisition (dark circles) and reversal (white circles) platforms for wildtype animals during reversal learning. On the first day, no significant preference for either the new nor the old location of the platform is seen; (B) In contrast, A20+/− mice are swimming significantly closer to the old location (acq, black squares) of the platform; (C) Detailed analysis of the first day of reversal learning, shows that wildtype animals show a significant preference for the new location of the platform after four training sessions; (D) whereas A20+/− mice fail to do so. (E) After five days of training, both WT and A20+/− mice show a significant preference for the target quadrant and; (F) are swimming significantly closer to the reversal platform. All data are represented as mean±SEMs and statistical significance was determined by using two-way repeated measures ANOVA (A-E) or two-way ANOVA (F) using Sidak’s multiple comparisons test as post hoc analysis; (**P < 0.01, ***P < 0.001).
In summary, at the age of 3 months, A20+/− animals show a subtle cognitive impairment compared to control littermates. The impairment includes the need for more time to form a robust reference memory. All other behavioral tests showed similar behavior in A20+/− mice compared to WT littermates. With age, female A20+/− mice show a learning defect in reversal learning and thus cognitive flexibility.
3.2. Immune challenge in A20+/− mice results in more behavioral phenotypes
The pathogenesis of neuropsychiatric lupus is characterized by a complex interplay of genetic, hormonal and environmental factors. In order to mimic the disease pathogenesis more closely, A20+/− mice were exposed to an immune challenge. Lipopolysaccharide (LPS), an endotoxin derived from the outer membrane of Gram-negative bacteria, is known to be a powerful trigger of inflammation by activating the NF-κB pathway (Bryant et al., 2010). To avoid systemic inflammation, intracerebroventricular (ICV) injections were performed.
LPS concentration study: In the literature, a wide range of LPS doses are used for ICV injections but a systematic analysis of optimal dosing is lacking. We performed a dose-response analysis with five different doses, ranging from 10 ng/μl to 1000 ng/μl, in wildtype female mice (Fig. S2). The effect of a single injection was followed over time (6h, 24h, 3d and 7d) and parameters related to neuroinflammation, peripheral inflammation and the general wellbeing of mice were evaluated (Table S4). Based on these results, 100 ng/μl LPS was selected for injection in A20+/− mice. This concentration was associated with a significant decrease in weight for at least three days after surgery, limited signs of peripheral inflammation and a strong pro-inflammatory response in the hippocampus that was counteracted by anti-inflammatory mediators. The effect of 100 ng/μl ICV LPS in comparison to saline ICV injections was evaluated in female A20+/− mice prior to the behavioral analysis (Fig. S3). A strong inflammatory response was observed in the animals injected with LPS. This response was characterized by the upregulation of pro-inflammatory cytokines (Tumor necrosis factor, Tnf 6h: t4 = 3.81, P = 0.01; 24h: t4 = 2.63, P = 0.058; Interleukin 1b, Il1b 24h: t4 = 2.81, P = 0.04), activation of microglia (Toll-like receptor 4, Tlr4 6h: t4 = 5.89, P = 0.004), oxidative stress (NADPH oxidase 2, Nox2 6h: t4 = 11.46, P = 0.0003) and apoptosis (Caspase 3, Casp3 6h: t4 = 9.63, P = 0.0006). Whereas the pro-inflammatory mediators were mainly upregulated for the first 2 days, the anti-inflammatory cytokine Transforming Growth Factor beta (Tgfb) was upregulated until 7 days after the injection (6h: t4 = 3.47, P = 0.02, 24h: t4 = 4.32, P = 0.01, 3d: t4 = 2.48, P = 0.06 and 7d: t4 = 5.20,P = 0.006). Evaluating peripheral inflammation using white blood cell markers showed no difference in absolute leukocyte levels in comparison to saline injected A20+/− female mice. This indicates that the neuroinflammatory reaction occurred in absence of peripheral inflammation.
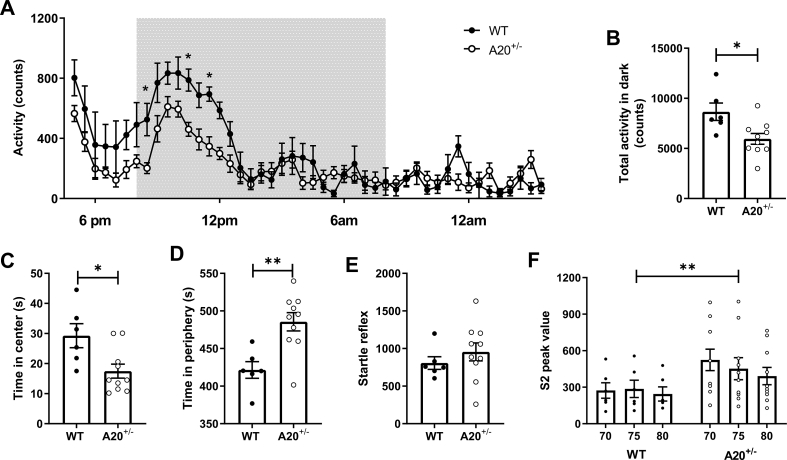
Neuromotor behavior: Two days after surgery, spontaneous activity levels over 23 h were measured in LPS injected wildtype and A20+/− littermates (both sexes, see Table 1). In general, A20+/− mice were hypoactive. This effect was observed in both A20+/− male and female mice. However, the effect was more pronounced in female mice where a significant decrease in overall spontaneous activity (genotype x time F14, 644 = 2.57, P < 0.0001; time: F46,644 = 18.17, P < 0.0001 and genotype: F1,14 = 5.81, P = 0.03; Fig. 3A) and decreased activity during the dark period (t14 = 2.8, P = 0.01; Fig. 3B) was observed. In males, only a decrease in overall activity was observed (time: F46,828 = 21.70, P < 0.0001; genotype x time F46,828 = 1.39, P = 0.045; Figs. S4–A) but no difference was seen during the dark period (t18 = 1.13; P = 0.27, Figs. S4–B). Besides hypoactivity, a significant reduction in weight was found specifically for female A20+/− mice compared to WT mice. This decrease was especially present on the second day after surgery (weight x genotype: F7,89 = 3.86, P = 0.001 and weight: F7,89 = 81.71, P < 0.0001; Fig. S5). The hypoactivity that is especially present in A20+/− female mice, is probably influenced by general malaise due to the LPS injections. The increased weight reduction in A20+/− female mice suggests a stronger impact from the acute injection compared to males.
Fig. 3.
Behavioral phenotypes observed in A20+/− female mice aggravate after immune challenge.
(A) Overall spontaneous activity and; (B) activity during the dark period of female A20+/− mice are significantly lower in comparison to wildtype littermates; (C) A20+/− female mice display an anxiety-related phenotype as is evidenced from the open field experiment where they spent significantly less time in the center and; (D) more time in the periphery in comparison to wildtype littermates; (E) A normal startle response was observed in both genotypes, however; (F) A20+/− female animals showed significantly higher startle reactivity when a prepulse was preceding the original startle tone. This is indicative for a sensorimotor gating impairment. Data are represented as mean±SEMs and statistical differences were determined using repeated-measures two-way ANOVA using Dunnett’s multiple comparisons test for post hoc analysis (A, F), unpaired t-test (B, C, E), Mann Whitney test (D) and two-way ANOVA using Sidak’s multiple comparisons test for post hoc analysis (F); (*P < 0.05, **P < 0.01).
Disease-associated behavior: The presence of an anxiety-like phenotype was assessed one week after surgery using the open field experiment. A20+/− female mice showed increased anxiety-related behavior since they spent less time in the center (Mann-Whitney U = 8, n1 = 6, n2 = 10, P = 0.01; Fig. 3C) and more in the periphery (t14 = 3.576, P = 0.003; Fig. 3D). No differences in exploratory behavior were observed, indicating that the anxiety-related phenotype is not influenced by motor impairment (Path length: Mann-Whitney U = 22, n1 = 59, n2 = 77, P = 0.47, data not shown). This effect was only observed in female A20+/− mice, as male A20+/− mice did not show any difference in time spent in the center (Mann-Whitney U = 54, n1 = 10, n2 = 11, P = 0.97; Figs. S4–C) or periphery (t19 = 1.06, P = 0.29; Figs. S4–D).
Startle reflex and prepulse inhibition to an acoustic startle were measured approximately 26 days after LPS injection. The acoustic startle reflex is a primitive response to a sudden and intense sound. The strong motor response induced by the startle tone can be modulated when animals are pre-exposed to a mild stimulus or prepulse. This process is called prepulse inhibition (PPI) and is a measure of sensorimotor gating. LPS injected A20+/− female mice showed a normal startle response (Fig. 3E) but a disrupted prepulse inhibition (genotype: F1,42 = 8.31, P = 0.006; Fig. 3F). This indicates that female mice have a deficit in their sensorimotor gating, meaning that they are less capable of processing and integrating sensory and cognitive/motor information. Again, this effect was found to be female-specific, since no differences in startle reflex (t19 = 0.57, P = 0.57; Figs. S4–E) and prepulse inhibition (genotype: F1,56 = 0.73; P = 0.39; Figs. S4–F) were found between A20+/− and WT male littermates. Furthermore, none of the above described behavioral deficits, related to activity, anxiety and prepulse inhibition, were found to show any difference between females A20+/− and WT littermates at the age of 3 months, which indicates that they were triggered by the presence of an immune challenge (Fig. S6).
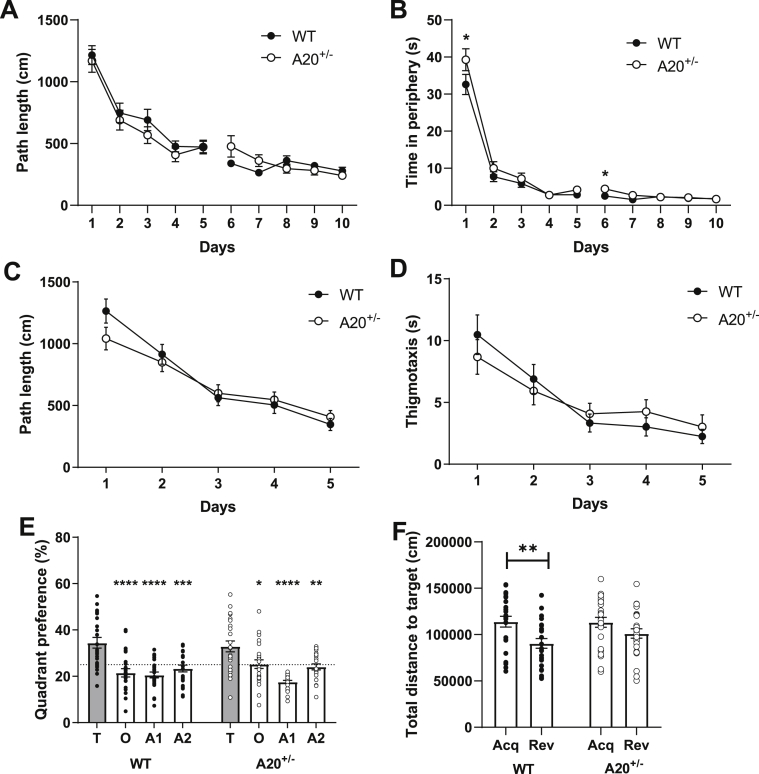
Cognitive behavior: The Morris water maze experiment was started ten days after the LPS challenge to evaluate spatial learning and memory. In the second phase of acquisition (days 6–10), a subtle learning defect was observed in A20+/− mice upon evaluating path length (genotype x time: F4,172 = 2.94, P = 0.02; time: F4,172 = 4.15, P = 0.003; Fig. 4A) and time in periphery (genotype x time: F4,172 = 3.25, P = 0.01; time: F4,172 = 6.94; Fig. 4B). These effects were most pronounced on the sixth day of training and were not caused by motor impairment as no difference in velocity was observed (t48 = 0.21, P = 0.83, data not shown). Cognitive flexibility, as measured during reversal learning, was similar for A20+/− and WT littermates (Fig. 4C and D). Despite A20+/− mice showing a significant preference for the target quadrant during the probe trial of reversal learning (Fig. 4E), only WT animals showed a significant preference for the location of the reversal platform in comparison to its previous location during acquisition (Fig. 4F). This was measured by comparing the total distance towards the location of both platforms. It indicates that the memory formation regarding the new location of the platform was not as robust in A20+/− mice in comparison to the controls. Taken together, A20+/− mice show learning deficits during the second phase of acquisition and a subtle memory impairment during reversal learning.
Fig. 4.
Learning and memory impairment detected in A20+/− mice after acute LPS challenge.
(A) During the second phase of acquisition learning, A20+/− mice had a longer path length in comparison to WT littermates. This was most pronounced on day 6; (B) A20+/− mice spent more time in the periphery, that was most pronounced on the day 1 and 6; (C) A20+/− and WT littermates showed a similar performance during reversal learning as evidenced by the similar pathlength; and (D) time spent in the periphery; (E) Both genotypes had a preference for the target quadrant over the other quadrants during the probe trial of reversal learning (T: Target, O: Opposite, A1: Adjacent; A2: Adjacent 2 quadrants); (F) For the reversal probe trial, the total distance towards the location of the platform during acquisition and reversal learning revealed that only WT mice show a significant preference for the new location of the platform; (Acq: Acquisition, Rev: Reversal). Data are represented as mean±SEMs and statistical differences were determined using repeated-measures two-way ANOVA using Dunnett’s multiple comparisons test for post hoc analysis (A-E) and two-way ANOVA using Sidak’s multiple comparisons test for post hoc analysis (F); (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
In summary, an acute endotoxin challenge in A20 heterozygous animals leads to the development of behavioral symptoms are observed up to 30 days after the injection. These phenotypes were predominantly present in female A20+/− mice and can be further characterized as hypoactivity, anxiety-related behavior, cognitive impairment and deficits in sensorimotor gating. It can be concluded that the presence of an environmental stimulus is a crucial component in disease pathogenesis and triggers more behavioral symptoms in a female-specific manner.
3.3. Sex-specific and context-dependent neuroinflammatory profile in A20+/− and WT mice
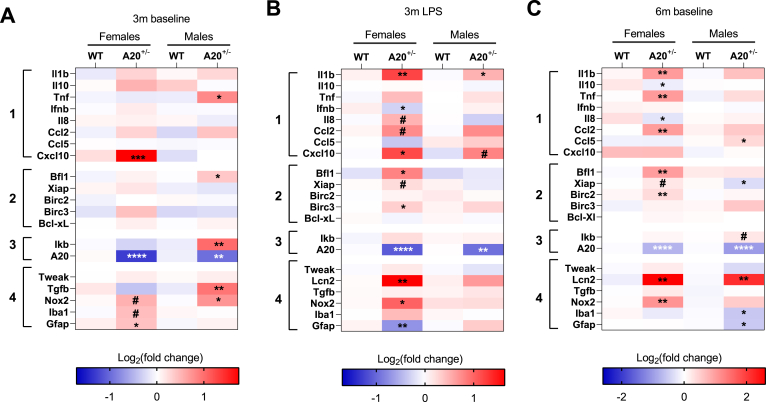
To investigate whether neuroinflammatory mechanisms underly the observed behavioral phenotypes in A20+/− mice, a gene expression analysis was conducted using qRT-PCR. Since A20 is a crucial regulator of the NF-κB pathway, the expression of several NF-κB target genes and markers related to neuroinflammation were evaluated in hippocampal and cortical brain tissue. The NF-κB target genes can be subdivided into three groups encompassing (1) cytokines and chemokines, (2) regulators of apoptosis and (3) regulators of the NF-κB pathway. The fourth group of markers consists of genes that were previously found to be associated with NPSLE in mice namely TWEAK (TNF related weak inducer of apoptosis) and Lcn2 (Lipocalin 2) (Mike et al., 2019; Stock et al., 2013). Furthermore, this group also contains markers including the anti-inflammatory Tgfb, glial associated markers Iba1 (ionized calcium binding adaptor molecule 1) and Gfap (glial fibrillary acidic protein), as well as Nox2, a marker for oxidative stress.
Under baseline conditions at the age of 3 months, a subtle neuroinflammatory phenotype is observed in the hippocampus of A20+/− male mice (Fig. 5A). In comparison to WT littermates, NF-κB activity is induced as observed by the significant upregulation of Ikb (inhibitor of NF-κB), (t10 = 3.46, p = 0.006). The pro-inflammatory cytokine Tnf (t10 = 2.26, p = 0.04) and the anti-apoptotic protein Bcl-2-related protein A1 (Bfl1) (t10 = 2.29, p = 0.04) are the only NF-κB target genes that show a significant upregulation. Looking at neuroinflammation in general, increased expression of the anti-inflammatory cytokine Tgfb (Mann-Whitney U = 1, p = 0.004), and the marker for oxidative stress (Nox2: t9 = 3.17, p = 0.01) were observed. Heterozygosity was confirmed by the 50% reduction in A20 expression in heterozygous animals (male A20: t10 = 3.34, p = 0.0075; female A20: t9 = 4.16, p = 0.002).
Fig. 5.
Sex-specific and context-dependent neuroinflammatory profile observed in A20+/− and WT littermates.
The neuroinflammatory profile in the hippocampus of female and male A20+/− and WT mice was determined using NF-κB target genes (group 1–3) as markers as well as markers for general neuroinflammation (group 4). The NF-κB target genes can be subdivided into cytokines and chemokines (group 1), anti-apoptotic mediators (group 2) and regulators of NF-κB activity (group 3); (A) Subtle neuroinflammation was observed in female and male A20+/− mice. For every sample n = 5; (B). A female predominant neuroinflammatory phenotype was observed in A20+/− and WT mice 30 days after LPS challenge. For every sample n = 6; (C) At the age of 6 months neuroinflammation was predominantly observed in A20+/− female mice. For every sample, n = 6. Statistical differences were determined using the unpaired t-test or Mann Whitney test (A-C) (*P < 0.05, **P < 0.01, ****P < 0.0001, # borderline significance 0.05 < P < 0.08).
In female A20+/− mice, the only NF-κB target gene that shows a significant upregulation is C-X-C motif chemokine 10 (Cxcl10, t9 = 6.44, p = 0.0001; Fig. 5A). Evaluating general markers for neuroinflammation indicates a significant upregulation of the astrocyte marker Gfap (t9 = 2.57, p = 0.03) and borderline significance for oxidative stress (Nox2: t7 = 2.11, p = 0.07) and microglial activity (Iba1: t9 = 1.94, p = 0.08). Overall, it can be concluded that female A20+/− mice show little activation of the NF-κB pathway and subtle increase in markers related to neuroinflammation.
At the age of 6 months, a different neuroinflammatory phenotype is observed in females with increased expression of several NF-κB regulated cytokines, chemokines and regulators of apoptosis (Fig. 5C). An increased expression of the pro-inflammatory cytokines Il1b (t10 = 3.92, p = 0.002) and Tnf was observed (t10 = 3.29, p = 0.008), as well as a significant downregulation of the anti-inflammatory cytokine Interleukin 10 (Il10: t7 = 3.30; p = 0.01). C–C-Motif Chemokine ligand 2 (Ccl2) showed a significant upregulation (t9 = 4.51, p = 0.001), whereas a significant downregulation was found for Interleukin 8 (Il8 Mann Whitney U = 0, p = 0.01). Both are important pro-inflammatory chemokines in which Ccl2 attracts monocytes, T-lymphocytes and natural killer cells, whereas Il8 is mainly a chemotactic for neutrophils. Several regulators of apoptosis were also significantly upregulated including Bfl1 (t10 = 4.39, p = 0.001) and Baculoviral IAP repeat-containing protein 2, Birc2 (Mann Whitney U = 1, p = 0.004). Borderline significance was observed for the X-linked inhibitor of apoptosis protein, Xiap (t10 = 2.17, p = 0.055). All three genes encode for anti-apoptotic proteins of which Birc2 and Xiap belong to the inhibitor of apoptosis (IAP) family that exert their function by interacting with caspases (Yi Li and Ming Li, 2000). Bfl1, on the other hand, belongs to the Bcl2 family that controls cell death by regulating mitochondrial outer membrane permeabilization (Kale et al., 2018). Furthermore, a significantly upregulated expression was observed for Nox2 (t10 = 4.08, p = 0.002) and Lcn2 (Mann Whitney U = 0, p = 0.004). Lcn2 protein has pleiotropic functions that involve regulation of innate immunity, cellular differentiation and survival (Mike et al., 2019). This protein is also found to be upregulated in SLE and a knockout of this gene in a lupus mouse model improved depression-like behavior and cognitive defects (Mike et al., 2019).
Even though A20+/− and WT male mice were not included in the cognitive phenotyping, hippocampal samples were isolated to evaluate their neuroinflammatory profile at the age of 6 months. Surprisingly, we did not observe the same pro-inflammatory response as seen in females. Borderline significance was found for Ikb (t10 = 1.98, p = 0.07). Besides an upregulation for Lcn2 (t10 = 3.44, p = 0.006) and C–C motif chemokine ligand 5 (Ccl5) (t10 = 2.98, p = 0.01), significant downregulation was observed for Xiap (t10 = 2.60, p = 0.02) and Iba1 and Gfap, markers for microglial and astrocytes, respectively (Iba1: Mann Whitney test: U = 3, p = 0.03; Gfap: Mann Whitney test: U = 3, p = 0.01).
In summary, aging has sex-specific effects on the neuroinflammatory phenotype in A20+/− mice. In male A20+/− mice, a subtle increase in NF-κB pathway activity and general inflammation was seen at the age of 3 and 6 months. For females, low-grade neuroinflammation was observed at the age of 3 months that became more pronounced with age.
Besides the effect of A20 heterozygosity on neuroinflammation, we also evaluated the influence of an environmental challenge (Fig. 5B). In female A20+/− mice, the prominent neuroinflammatory phenotype included the upregulation of multiple NF-κB target genes. Increased expression of pro-inflammatory cytokines Il1b (t10 = 3.68, p = 0.004) and Tnf (t10 = 2.49, p = 0.03) as well as the upregulation of pro-inflammatory chemokines Il8, Ccl2 and Cxcl10 (Il8: t7 = 2.27, p = 0.057; Ccl2: t9 = 2.11, p = 0.06; Cxcl10: t10 = 2.75, p = 0.02). Several genes encoding anti-apoptotic proteins also showed an increased expression, including Bfl1, Xiap and Birc3 (Baculoviral IAP Repeat Containing 3) (Bfl1: t8 = 2.93, p = 0.01; Xiap: t10 = 1.96, p = 0.07; Birc3: t10 = 2.25, p = 0.04). Furthermore, increased expression of oxidative stress marker Nox2 (t9 = 3.65, p = 0.005), Lcn2 (t7 = 4.94, p = 0.001) and a significant downregulation of astrocyte marker Gfap (t10 = 4.31, p = 0.015) were observed. In male A20+/− mice, the LPS challenge only resulted in an upregulation of the pro-inflammatory cytokine Il1b (t10 = 2.76, p = 0.02) and a borderline significance for Cxcl10 (t9 = 2.06, p = 0.06). For both A20+/− females as well as A20+/− males, heterozygosity was confirmed (female A20: t10 = 6.54, p < 0.0001; male A20: Mann Whitney U = 0, p = 0.002).
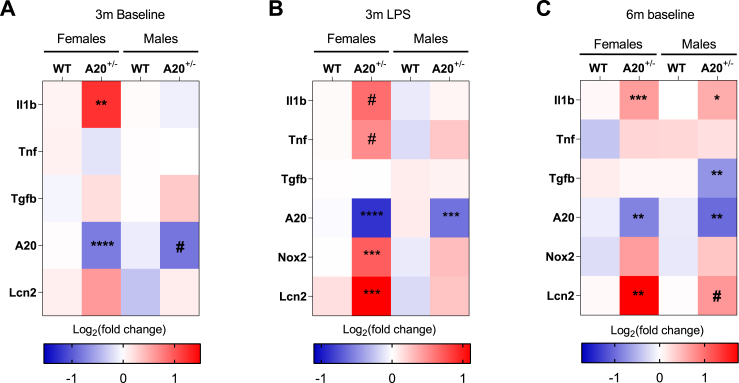
To estimate the extent of the inflammatory response, the expression levels of a limited set of genes was evaluated in the cortex (Fig. 6). At the age of 3 months, only females showed a significant upregulation of the inflammatory cytokine Il1b (t6 = 4.04, p = 0.006; Fig. 6A). Even though A20+/− male mice developed spontaneous neuroinflammation in the hippocampus, no increased expression of inflammatory cytokines was observed in the cortex. At the age of 6 months, an increased expression of Il1b (t10 = 4.91; p = 0.0006) and Lcn2 (Mann Whitney U = 0, p = 0.004) was observed specifically in females (Fig. 6C). A20+/− male mice also showed a significant upregulation of Il1b (t9 = 2.56, p = 0.03) and borderline significance for Lcn2 (t10 = 2.08; p = 0.06). Furthermore, a significant downregulation of Tgfb was observed (t10 = 3.74, p = 0.003). This indicates that in females at the age of 6 months the observed neuroinflammation is not restricted to the hippocampus, while also in male A20+/− mice, a subtle increase in neuroinflammation is observed in cortical samples.
Fig. 6.
Cortical inflammatory profile of A20+/− and WT littermates.
A limited set of neuroinflammatory markers was evaluated on cortical samples. (A) At the age of 3 months, only a significant upregulation was observed for the pro-inflammatory cytokine interleukin 1b (Il1b) in female A20+/− mice; (B) 30 days after injection of LPS, inflammation was only observed in female A20+/− mice in comparison to WT littermates. The inflammatory response was characterized by a significant increase in Nox2 (oxidative stress) and lipocalin 2 (Lcn2) as well as borderline significance for pro-inflammatory cytokines Il1b and Tnf; (C) At the age of 6 months, females showed a significant increase in the pro-inflammatory cytokine Il1b and Lcn2. In male A20+/− mice, upregulation for Il1b and downregulation of Tgfb were observed. Statistical differences were determined using unpaired t-test or Mann Whitney U test (A-C) (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, # borderline significance 0.05 < P < 0.08).
Analysis of cortical inflammation of LPS-injected mice showed a strong neuroinflammatory response in female A20+/− mice, whereas almost no sign of inflammation was detected in male A20+/− mice. Significant increases in Nox2 (t10 = 5.84, p = 0.0002) and Lcn2 (t10 = 4.68, p = 0.009; Fig. 6B) were observed as well as borderline significance for the pro-inflammatory cytokines Il1b (t9 = 2.06, p = 0.06) and Tnf (t10 = 2.01, p = 0.07) (Fig. 6B). Together, these results indicate that one single immune challenge results in widespread, chronic neuroinflammation in A20+/− female mice, but not in A20+/− male mice.
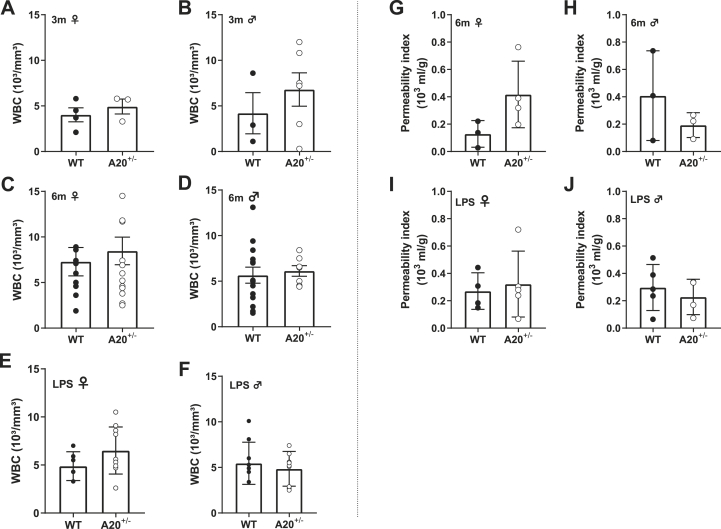
3.4. Normal white blood cell count and an intact blood-brain barrier were observed in A20 heterozygous mice
White blood cell related markers were evaluated to determine the presence of peripheral inflammation. Under baseline conditions, no increase was observed in the total white blood cell count for females (t5 = 0.79, p = 0.46; Fig. 7A) or males (t7 = 0.84, p = 0.42; Fig. 7B) at the age of 3 months. Similar results were observed upon analyzing samples obtained from 6 months old female mice (Mann Whitney U = 65.50, p = 0.80; Fig. 7C) and males (t19 = 0.35, p = 0.72; Fig. 7D). The same analyses were performed to exclude peripheral inflammation after LPS immune challenge (Fig. 7E and F). This analysis was performed 30 days post-LPS injection after completion of the behavioral protocol. No difference was observed in absolute leukocyte count for A20+/− female and male mice in comparison to WT littermates (females: t14 = 1.46, p = 0.17; males: t18 = 0.64, p = 0.53). Together, these results show that the behavioral and inflammatory phenotypes at baseline conditions and after an immune challenge occur in the absence of peripheral inflammation.
Fig. 7.
Evaluation of white blood cell count and blood-brain barrier permeability.
The presence of peripheral inflammation was measured by the total white blood cell count (WBC). For all conditions (baseline 3m
(A-B), baseline 6m (C-D) and LPS injection (E-F) no differences between A20+/− mice and wildtype littermates was observed. No differences were found in BBB permeability when evaluating 6 months old (G-H) and LPS injected mice (I-J). Statistical differences were determined using unpaired t-test or Mann Whitney test (A-J).
The blood-brain barrier (BBB) is a tightly regulated neurovascular unit that controls access of molecules and cells between plasma and brain (Sweeney et al., 2019). For long, the disruption of the BBB was considered a central event in the pathogenesis of neuropsychiatric lupus. However, the question remains whether an increased BBB permeability contributes to the development of diffuse symptoms or whether it occurs in later stages of the disease. We found no difference in BBB permeability in 6 months old and LPS injected A20+/− and WT mice (Fig. 7G–J). This observation is consistent with the previously observed absence of BBB disruption in 4–5 weeks old A20 heterozygous and knockout mice (Guedes et al., 2014). These findings further support the ‘CNS primary hypothesis’ and indicate that BBB disruption is probably not important in the early stages of disease development.
4. Discussion
4.1. Behavioral phenotypes in young A20 heterozygous mice show no sex bias, originate in the CNS and have distinct inflammatory profiles
The pathogenesis of neuropsychiatric symptoms is incompletely understood and controversy consists regarding their etiology. In this paper, we have evaluated the contribution of CNS-intrinsic mechanisms to the development of neuropsychiatric symptoms using the A20 heterozygous mouse model.
First, we observed subtle cognitive deficits in A20 heterozygous mice 3 months of age. This was accompanied by a subtle neuroinflammatory phenotype in hippocampus and cortex. Neuroinflammation was observed in females and males alike but was characterized by the upregulation of different markers. A20+/− female mice mainly showed upregulation of the chemokine Cxcl10 in hippocampus and pro-inflammatory cytokine Il1b in cortex. A20+/− male mice, on the other hand, showed increased activation of the NF-κB pathway in the hippocampus accompanied by increased expression of pro- and anti-inflammatory as well as anti-apoptotic genes. Previously, the neuroinflammatory profile of A20 heterozygous mice was evaluated at the age of 4–5 weeks, and revealed a trend towards increased basal inflammation in comparison to WT controls, albeit not statistically significant (Guedes et al., 2014). Here, the use of a more extensive set of markers revealed subtle neuroinflammation in male and female mice at the age of 19 weeks.
Neuropsychiatric symptoms can develop before the onset of SLE or very early after the diagnosis and they are not associated with markers of peripheral disease activity (Hanly et al., 2007; Magro-Checa et al., 2019; Petri et al., 2010; Shimojima et al., 2005; van Dam et al., 1994). Therefore, we investigated whether peripheral inflammation may have contributed to the development of spontaneous neuroinflammation and behavioral phenotypes. White blood cells comprise granulocytes, lymphocytes and monocytes that exert important roles in the defense against infection (Blumenreich, 1990). An increase in total white blood cell count is an indication for an increased inflammatory response. In SLE, a decrease in total white blood cell count is commonly observed (Fayyaz et al., 2015). Similar to neuropsychiatric symptoms, it is important to make a distinction between leukopenia caused by SLE itself or as a secondary consequence by immunosuppressive treatment (Fayyaz et al., 2015). In A20 heterozygous mice, we did not observe a difference in total white blood cell count, indicating there is no peripheral inflammation. This is supported by findings from Studer et al. in which it was seen that A20 heterozygosity did not result in hyperactivation of the innate and adaptive immune system (Studer et al., 2015). Furthermore, no increased BBB permeability was observed in A20 heterozygous mice (Guedes et al., 2014).
Overall, the neuroinflammatory profiles in males and females, despite the different composition, tend towards a moderate proinflammatory state that correlates with the subtle cognitive phenotype.
4.2. Female-biased behavioral phenotypes in A20 heterozygotes arise with age
Systemic lupus erythematosus occurs predominantly in females with a peak incidence at the age of 30–70 years old (Rees et al., 2017). Therefore, we evaluated the behavior of A20+/− female mice at the age of 6 months, equivalent to 30 years of age in humans and observed a subtle impairment in cognitive flexibility. The neuroinflammatory profile of female A20+/− mice showed a significant upregulation of several NF-κB target genes including pro-inflammatory cytokines, chemokines and anti-apoptotic mediators. In contrast, only a subtle neuroinflammatory profile was observed in male A20+/− mice. Since no significant differences were observed in the total white blood cell count and BBB permeability, we conclude that the neuroinflammation was initiated by CNS intrinsic mechanisms.
Aging is accompanied by a decrease in cognitive abilities (Barrientos et al., 2015; Di Benedetto et al., 2017). The underlying mechanisms are not fully understood, but neuroinflammation plays an important role (Berchtold et al., 2008; Niraula et al., 2017). An upregulation of inflammatory mediators was observed when comparing human brain samples from young (20–59 years) and old (60–99 years) females and males, but it was proportionally greater in females (Berchtold et al., 2008). This indicates that the magnitude of neuroinflammation with aging is sex-specific. Age-associated neuroinflammation can largely be attributed to changes in microglia, resident immune cells of the CNS with important functions in maintaining CNS homeostasis (Li and Barres, 2017; Mangold et al., 2017; Niraula et al., 2017). With age, microglia acquire a ‘primed’ phenotype, which is characterized by a slower initiation, but more sustained and uncontrolled inflammatory response (Damani et al., 2011; Perry and Holmes, 2014; Streit et al., 2004). A20 is mainly expressed in microglia and deletion of A20 in microglia results in microglial activation and synaptic dysfunction and cognitive defects (Zhang et al., 2014; Voet et al., 2018), while targeted knockout of A20 in neurons, astrocytes and oligodendrocytes did not lead to spontaneous CNS inflammation (Guedes et al., 2014; X. Wang et al., 2013). Therefore, it is likely that microglia have an important contribution to the sex-specific neuroinflammatory phenotype observed in A20 heterozygous mice at the age of 6 months.
Taken together, we propose that CNS-intrinsic mechanisms underly the behavioral phenotype observed in A20+/− female mice at the age of 6 months. Furthermore, the sex-specific neuroinflammatory profile can probably be explained by an increased activation of microglial cells that occurs with age.
4.3. Immune challenge triggers neurobehavioral phenotypes in A20 heterozygous females
NPSLE is characterized by a complex interplay between genetic, hormonal and environmental factors. Infections are well known environmental stimuli that can trigger or worsen SLE (Ribeiro and Signorelli, 2017). To mimic the disease pathology more closely, an acute immune challenge using a single dose of LPS, resembling an infection, was delivered in the intraventricular system of A20+/− and wildtype mice. Behavioral outcomes were followed up until one month after LPS administration. This resulted in several behavioral impairments and chronic inflammation that were predominantly observed in female A20+/− mice. The observed behavioral phenotypes included hypoactivity, anxiety-like behavior, cognitive impairment and prepulse inhibition and were found in the absence of peripheral inflammation. Except for hypoactivity, which reflects a decrease in general well-being in the first days after the injection, all other behavioral impairments can be translated to NPSLE patients. According to a recently performed meta-analysis, anxiety disorders are found in up to 40% of SLE patients and similar percentages have been reported for NPSLE (Jeltsch-David and Muller, 2014; Rees et al., 2017). Mild-to-moderate cognitive impairments are found in up to 80% of patients and have been shown to be unrelated to systemic disease activity and duration (Hanly and Harrison, 2005; Mak et al., 2009). In contrast, it was shown that cognitive impairment is more related to the presence of depression, anxiety and stress (Monastero et al., 2001; Montero-Lopéz et al., 2016). Prepulse inhibition is a very sensitive and robust measure for sensorimotor gating, a mechanism that filters irrelevant sensory stimuli in order to avoid an overload of sensory information (Swerdlow et al., 2008). PPI impairments are found in several neuropsychiatric disorders and are related to both cognitive and functional deficits, like psychosis (Kohl et al., 2013; Mena et al., 2016; Swerdlow et al., 2008). Despite the fact that cognitive deficits as well as primary acute psychosis have been reported in NPSLE patients and that the presence of psychosis is regarded as one of the classification criteria for systemic lupus erythematosus, no studies investigating prepulse inhibition in patients have been reported up until now (Appenzeller et al., 2008; ACR, 1999).
Thirty days after LPS challenge, a strong and widespread neuroinflammatory response was observed predominantly in 3-month old A20+/− female mice. This profile is comparable to what is seen in 6-month old mice without immune challenge. The response was also characterized by a strong upregulation of NF-κB target genes encoding pro-inflammatory cytokines, chemokines and anti-apoptotic proteins as well as general markers of neuroinflammation. In contrast, A20+/− male mice displayed a low-grade spontaneous neuroinflammatory phenotype. Again, no differences in total white blood cell count and BBB permeability were observed between A20 heterozygous mice and WT littermates indicating that peripheral inflammation was not an important factor in the inflammatory phenotypes.
Sex-related differences in immune and autoimmune responses are well described and conserved in different species (Klein and Flanagan, 2016). However, this strong immune activity is a double-edged sword as it also makes females more prone to the development of inflammatory and autoimmune disorders (Rubtsova et al., 2015). For systemic lupus erythematosus, a bias towards females with a 9:1 or even 15:1 female/male ratio has been observed (Petri, 2002; Rubtsov et al., 2010; Zandman-Goddard et al., 2012). The A20 heterozygous mouse model recapitulates a similar bias since behavioral impairments and neuroinflammation were mostly found in female mice at the age of 6 months and after LPS injection. Various factors have been proposed to explain this sex-specific bias, including sex hormones, X chromosome-linked effects and environmental factors such as diet (Rubtsova et al., 2015). Since A20 is not X chromosome-linked (chromosomes 10A3 in mice, 6q23.3 in humans) and the diet of A20 heterozygous male and female mice was the same, the most likely explanation for the differences are sex hormones. It has been described that 17-β-estradiol (E2) is an inhibitor of TNFAIP3 expression in breast carcinoma cells from postmenopausal women as seen in a significant downregulation of A20 expression (Vendrell et al., 2007). Furthermore, estrogen was found to increase the susceptibility for SLE and had an effect on the development of SLE flares (Bernier et al., 2009; Buyon et al., 2005; Costenbader et al., 2007; Hughes and Choubey, 2014).
Several cytokines and chemokines, that were upregulated in A20+/− female mice after LPS injection and at the age of 6 months, are also proposed as potential biomarkers for NPSLE (Jeltsch-David and Muller, 2014). This was based on the presence of cytokines (Il1, IL10, Tnf) and chemokines (Il8, Ccl2, Cxcl10) in cerebrospinal fluid of patients (Jeltsch-David and Muller, 2014). Furthermore, we also observed upregulation of Lcn2. This lipophilic protein was originally shown to play an important role in preventing bacterial outgrowth by sequestration of iron-loaded siderophores (Flo et al., 2004). It is a marker for neuroinflammation with a strong and rapid induction by activated microglia and astrocytes after LPS challenge (Bennett et al., 2016; Zamanian et al., 2012). Recently, Lcn2 was discovered to be a key mediator in the pathogenesis of neuropsychiatric lupus using a Sle1,3 mouse model (Mike et al., 2019). Lcn2 deficiency improved behavioral readouts related to depression, motor coordination and spatial memory as well as neuroinflammation (Mike et al., 2019). Importantly, Lcn2 deficiency had no influence on peripheral inflammation, indicating that the effect was primarily achieved via brain-intrinsic pathways. Lcn2 is a known target gene of the Il17 signaling pathway (Shen et al., 2006). Il17 is an important key player in autoimmune inflammation and increased serum levels are also found in NPSLE (Vincent et al., 2013). Il17 can induce activation of the NF-κB pathway (Shen et al., 2006). Previously, it was shown that A20 is a negative regulator of the Il17 mediated signaling pathway and that A20 deficiency in vitro is associated with a strong upregulation of Lcn2 (Grag et al., 2013). These results connect A20 heterozygosity with neuropsychiatric lupus at the molecular level.
4.4. A mouse model for neuropsychiatric lupus
For several reasons, the A20 heterozygous mouse model can contribute to future research investigating the pathophysiology of NPSLE. First of all, this model identifies a genetically predisposing factor that can lead to the development of neuropsychiatric symptoms via brain-intrinsic mechanisms. Furthermore, in the pathogenesis of NPSLE the contribution of environmental stimuli are considered to be important triggers in genetically predisposed persons. This was confirmed by the observations that behavioral phenotypes were exacerbated in the A20 heterozygous mouse model after exposing them to an immune challenge. Moreover, these phenotypes were predominantly found in females, a third important feature observed in NPSLE. Another advantage of this mouse model, is that the influence of A20 heterozygosity was evaluated in C57BL/6, a genetic background known to be permissive for lupus development but not spontaneously inducing it (Crampton et al., 2014). This is in sharp contrast with the spontaneous mouse models that are currently used for NPSLE research (Pikman et al., 2017; Schwartz et al., 2019). Even though these spontaneous models of SLE are invaluable, their main drawback is that none of them have mutations in genes that are associated with SLE (Schwartz et al., 2019). For example, the MRL/Lpr model, which is most extensively investigated in NPSLE-related research, is characterized by abnormal expression of the Fas gene (Watson et al., 1992). Fas-deficiency in humans is associated with lymphadenopathies, but they do not develop a lupus-like phenotype (Rensing-ehl et al., 2014). By contrast, A20 haploinsufficiency in humans was associated with the development of an early-onset autoimmune disorder, of which at least one patient was diagnosed with a condition resembling SLE (Zhou et al., 2015). The recent identification of a frameshift mutation in TNFAIP3 in a patient with NPSLE (Duan et al., 2019) provides further support for the validity of our model.
5. Conclusion
We have shown that heterozygosity for A20 defines a genetic mouse model that recapitulates numerous hallmark features of neuropsychiatric lupus including high prevalence in females, worsening of phenotypes with age and upon exposure to an environmental stimulus that mimics infection. Our results are consistent with the CNS primary hypothesis that posits that initial neuroinflammatory changes in the CNS are responsible for behavioral phenotypes observed in patients with neuropsychiatric lupus. We propose that this mouse model provides novel opportunities to gain mechanistic insight in pathological mechanisms of neuropsychiatric lupus, in disease progression, and conceivably into therapeutic targets.
Ethics approval
Not applicable, only for human participants, human data or human tissue.
Consent for publication
Not applicable.
Availability of data and material
All data generated and analyzed in the course of this study are included in the main manuscript and in the supplementary information files.
Funding
Carmen Daems was supported by a fellowship of the Flemish Research Foundation.
Authors’ contributions
CD, ZCV and PC designed the study. CD performed the majority of experiments with the help of MS for behavioral testing and of VV for gene expression analysis. RD provided the behavioral setups for performing all analyses and the stereotactic frame for ICV injections. GvL generated the A20FL mice used for breeding. CD and ZCV performed statistical analyses and CD, ZCV and PC analyzed and interpreted results. CD, RD, GvL, ZCV and PC wrote the manuscript.
Declaration of competing interest
None.
Acknowledgements
This study was supported by a fellowship from FWO to CD. Research in the GvL lab is supported by research grants from the FWO, the “Geneeskundige Stichting Koningin Elisabeth” (GSKE), the CBC Banque Prize, the Charcot Foundation, the “Belgian Foundation against Cancer”, “Kom op tegen Kanker”, and the GOA of the Ghent University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2019.100018.
Contributor Information
Z. Callaerts-Végh, Email: zsuzsanna.vegh@kuleuven.be.
P. Callaerts, Email: patrick.callaerts@kuleuven.be.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- ACR The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Adrianto I., Wen F., Templeton A., Wiley G., King J.B., Lessard C.J., Bates J.S., Hu Y., Kelly J.A., Kaufman K.M., Guthridge J.M., Alarcón-Riquelme M.E., Anaya J.M., Bae S.-C., Bang S.-Y., Boackle S.A., Brown E.E., Petri M.A., Gallant C., Ramsey-Goldman R., Reveille J.D., Vila L.M., Criswell L.A., Edberg J.C., Freedman B.I., Gregersen P.K., Gilkeson G.S., Jacob C.O., James J.A., Kamen D.L., Kimberly R.P., Martin J., Merrill J.T., Niewold T.B., Park S.-Y., Pons-Estel B.A., Scofield R.H., Stevens A.M., Tsao B.P., Vyse T.J., Langefeld C.D., Harley J.B., Moser K.L., Webb C.F., Humphrey M.B., Montgomery C.G., Gaffney P.M. Association of a functional variant downstream of TNFAIP3 with systemic lupus erythematosus. Nat. Genet. 2011;43:253–258. doi: 10.1038/ng.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenzeller S., Cendes F., Costallat L.T.L. Acute psychosis in systemic lupus erythematosus. Rheumatol. Int. 2008;28:237–243. doi: 10.1007/s00296-007-0410-x. [DOI] [PubMed] [Google Scholar]
- Barrientos R., Kitt M., Watkins L., Maier S. Neuroinflammation in the normal aging hippocampus. Neuroscience. 2015;309:84–99. doi: 10.1016/j.neuroscience.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendorius M., Po C., Muller S., Jeltsch-David H. From systemic inflammation to neuroinflammation: the case of neurolupus. Int. J. Mol. Sci. 2018;19:3588. doi: 10.3390/ijms19113588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M.L., Bennett F.C., Liddelow S.A., Ajami B., Zamanian J.L., Fernhoff N.B., Mulinyawe S.B., Bohlen C.J., Adil A., Tucker A., Weissman I.L., Chang E.F., Li G., Grant G.A., Gephart M.G.H., Barres B.A. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. 2016;113:E1738–E1746. doi: 10.1073/pnas.1525528113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold N.C., Cribbs D.H., Coleman P.D., Rogers J., Head E., Kim R., Beach T., Miller C., Troncoso J., Trojanowski J.Q., Zielke H.R., Cotman C.W. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. 2008;105:15605–15610. doi: 10.1073/pnas.0806883105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier M., Mikaeloff Y., Hudson M., Suissa S. Combined oral contraceptive use and the risk of systemic lupus erythematosus. Arthritis Rheum. 2009;61:476–481. doi: 10.1002/art.24398. [DOI] [PubMed] [Google Scholar]
- Blumenreich M.S. Clinical Methods: the History, Physical, and Laboratory Examinations. third ed. 1990. The white blood cell and differential count; pp. 724–727. [Google Scholar]
- Bortoluzzi A., Scirè C.A., Govoni M. Attribution of neuropsychiatric manifestations to systemic lupus erythematosus. Front. Med. 2018;5:1–5. doi: 10.3389/fmed.2018.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosanac I., Wertz I.E., Pan B., Yu C., Kusam S., Lam C., Phu L., Phung Q., Maurer B., Arnott D., Kirkpatrick D.S., Dixit V.M., Hymowitz S.G. Ubiquitin binding to A20 ZnF4 is required for modulation of NF-kB signaling. Mol. Cell. 2010;40:548–557. doi: 10.1016/j.molcel.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Brody D.L., Holtzman D.M. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp. Neurol. 2006;197:330–340. doi: 10.1016/j.expneurol.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant C.E., Spring D.R., Gangloff M., Gay N.J. The molecular basis of the host response to lipopolysaccharide. Nat. Rev. Microbiol. 2010;8:8–14. doi: 10.1038/nrmicro2266. [DOI] [PubMed] [Google Scholar]
- Buyon J.P., Petri M.A., Kim M.Y., Kalunian K.C., Grossman J., Hahn B.H. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in. Systemic Lupus. Ann. Intern. Med. 2005;142:953–962. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- Callaerts-Végh Z., Beckers T., Ball S.M., Baeyens F., Callaerts P.F., Cryan J.F., Molnar E., D’Hooge R., Hooge R.D., D’Hooge R. Concomitant deficits in working memory and fear extinction are functionally dissociated from reduced anxiety in metabotropic glutamate receptor 7-deficient mice. J. Neurosci. 2006;26:6573–6582. doi: 10.1523/JNEUROSCI.1497-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaerts-Végh Z., Leo S., Vermaercke B., Meert T., D’Hooge R. LPA 5 receptor plays a role in pain sensitivity , emotional. Genes Brain Behav. 2012;11:1009–1019. doi: 10.1111/j.1601-183X.2012.00840.x. [DOI] [PubMed] [Google Scholar]
- Costenbader K.H., Feskanich D., Stampfer M.J., Karlson E.W. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 2007;56:1251–1262. doi: 10.1002/art.22510. [DOI] [PubMed] [Google Scholar]
- Crampton S.P., Morawski P.A., Bolland S. Linking susceptibility genes and pathogenesis mechanisms using mouse models of systemic lupus erythematosus. Dis. Model. Mech. 2014;7:1033–1046. doi: 10.1242/dmm.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R., De Deyn P.P., Hooge R.D., Deyn P.P. De. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- D’Hooge R., Lüllmann-Rauch R., Beckers T., Balschun D., Schwake M., Reiss K., von Figura K., Saftig P. Neurocognitive and psychotiform behavioral alterations and enhanced hippocampal long-term potentiation in transgenic mice displaying neuropathological features of human alpha-mannosidosis. Neurobiol. Dis. 2005;25:6539–6549. doi: 10.1523/JNEUROSCI.0283-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damani M.R., Zhao L., Fontainhas A.M., Amaral J., Fariss R.N., Wong W.T. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10:263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De A., Dainichi T., Rathinam C.V., Ghosh S. The deubiquitinase activity of A20 is dispensable for NF-kB signaling. EMBO Rep. 2014;15:775–783. doi: 10.15252/embr.201338305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devraj K., Guérit S., Macas J., Reiss Y. An in vivo blood-brain barrier permeability assay in mice using fluorescently labeled tracers. J. Vis. Exp. 2018;132 doi: 10.3791/57038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto S., Müller L., Wenger E., Düzel S., Pawelec G. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci. Biobehav. Rev. 2017;75:114–128. doi: 10.1016/j.neubiorev.2017.01.044. [DOI] [PubMed] [Google Scholar]
- Duan R., Liu Q., Li J., Bian X., Yuan Q., Li Y., Long F., Gao S. A de novo frameshift mutation in TNFAIP3 impairs A20 deubiquitination function to cause neuropsychiatric systemic lupus erythematosus. J. Clin. Immunol. 2019;39:795–804. doi: 10.1007/s10875-019-00695-4. [DOI] [PubMed] [Google Scholar]
- Evans P.C., Ovaa H., Hamon M., Kilshaw P.J., Hamm S., Bauer S., Ploegh H.L., Smith T.S. Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. Biochem. J. 2004;378:727–734. doi: 10.1042/BJ20031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyaz A., Igoe A., Kurien B.T., Danda D., James J.A., Stafford H.A., Scofield R.H. Haematological manifestations of lupus. Lupus Sci. Med. 2015;2 doi: 10.1136/lupus-2014-000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flo T.H., Smith K.D., Sato S., Rodriguez D.J., Holmes M.A., Strong R.K., Akira S., Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Flurkey K., Currer J., Harrison D. The Mouse in Biomedical Research. 2007. The mouse in aging research; pp. 637–672. [Google Scholar]
- Gelb S., Stock A.D., Anzi S., Putterman C., Ben-zvi A. Mechanisms of neuropsychiatric lupus : the relative roles of the blood- cerebrospinal fl uid barrier versus blood-brain barrier. J. Autoimmun. 2018;91:34–44. doi: 10.1016/j.jaut.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grag A.V., Ahmed M., Vallejo A.N., Ma A., Gaffen S.L. The deubiquitinase A20 mediates feedback inhibition of interleukin-17 receptor signaling. Sci. Signal. 2013;6:ra44. doi: 10.1126/scisignal.2003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R.R., Cotsapas C., Davies L., Hackett R., Lessard C.J., Leon J.M., Burtt N.P., Guiducci C., Parkin M., Gates C., Plenge R.M., Behrens T.W., Wither J.E., Rioux J.D., Fortin P.R., Cunninghame D., Wong A.K., Vyse T.J., Daly M.J., Altshuler D., Moser K.L., Gaffney P.M., Graham D.C., Wong A.K., Vyse T.J., Daly M.J., Altshuler D., Moser K.L., Gaffney P.M. Genetic variants near TNFAIP3 on 6q23 are associated with systemic lupus erythematosus. Nat. Genet. 2008;40:1059–1061. doi: 10.1038/ng.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes R.P., Csizmadia E., Moll H.P., Ma A., Ferran C., da Silva C.G., Gonçalves C. A20 deficiency causes spontaneous neuroinflammation in mice. J. Neuroinflammation. 2014;11:1–16. doi: 10.1186/1742-2094-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanly J.G. Diagnosis and management of neuropsychiatric SLE. Nat. Rev. Rheumatol. 2014;10:338–347. doi: 10.1038/nrrheum.2014.15. [DOI] [PubMed] [Google Scholar]
- Hanly J.G., Harrison M.J. Management of neuropsychiatric lupus. Best Pract. Res. Clin. Rheumatol. 2005;19:799–821. doi: 10.1016/j.berh.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Hanly J.G., Urowitz M.B., Bae S.C., Gordon C., Wallace D.J., Isenberg D., Alarco G.S., Clarke A., Bernatsky S., Merrill J.T., Petri M., Dooley M.A., Gladman D., Fortin P.R., Steinsson K., Bruce I., Manzi S., Khamashta M., Zoma A., Aranow C., Ginzler E., Vollenhoven R. Van, Font J. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus an international inception cohort study. Arthritis Rheum. 2007;56:265–273. doi: 10.1002/art.22305. [DOI] [PubMed] [Google Scholar]
- Hanly J.G., Urowitz M.B., Su L., Bae S.C., Gordon C., Wallace D.J., Clarke A., Bernatsky S., Isenberg D., Rahman A., Alarcón G.S., Gladman D.D., Fortin P.R., Sanches-Guerrero J., Romero-Diaz J., Merrill J.T., Ginzler E., Bruce I.N., Steinsson K., Khamashta M., Petri M., Manzi S., Dooley M.A., Van Vollenhoven R., Nived O., Sturfelt G., Aranow C., Kalunian K., Ramos-Casals M., Zoma A., Douglas J., Thompson K., Farewell V., Vollenhoven R. Van, Nived O., Sturfelt G., Aranow C., Kalunian K., Zoma A., Douglas J., Thompson K., Farewell V., International L. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2010;69:529–535. doi: 10.1136/ard.2008.106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans J., Mortier G., De Paepe A., Speleman F., Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G.C., Choubey D. Modulation of autoimmune rheumatic diseases by oestrogen and progesterone. Nat. Rev. Rheumatol. 2014;10:740–751. doi: 10.1038/nrrheum.2014.144. [DOI] [PubMed] [Google Scholar]
- Jeltsch-David H., Muller S. Neuropsychiatric systemic lupus erythematosus : pathogenesis and biomarkers. Nat. Rev. Neurol. 2014;10:579–596. doi: 10.1038/nrneurol.2014.148. [DOI] [PubMed] [Google Scholar]
- Kale J., Osterlund E.J., Andrews D.W. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80. doi: 10.1038/cdd.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul A., Gordon C., Crow M.K., Touma Z., Urowitz M.B., Vollenhoven R., Van, Ruiz-irastorza G., Hughes G., van Vollenhoven R., Ruiz-irastorza G., Hughes G. Systemic lupus erythematosus. Nat. Rev. Dis. Primers. 2016;2:16039. doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Kohl S., Heekeren K., Klosterkötter J., Kuhn J. Prepulse inhibition in psychiatric disorders - apart from schizophrenia. J. Psychiatr. Res. 2013;47:445–452. doi: 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Lee E., Boone D., Chai S., Libby S., Chien M., Lodolce J., Ma A. Failure to regulate TNF-induced NF-kB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Barres B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2017;18:225–242. doi: 10.1038/nri.2017.125. [DOI] [PubMed] [Google Scholar]
- Lo A.C., Callaerts-vegh Z., Nunes A.F., Rodrigues C.M.P., Hooge R.D. Neurobiology of Disease Tauroursodeoxycholic acid ( TUDCA ) supplementation prevents cognitive impairment and amyloid deposition in APP/PS1 mice. Neurobiol. Dis. 2013;50:21–29. doi: 10.1016/j.nbd.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Love P.E., Santoro S.A. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann. Intern. Med. 1990;112:682–698. doi: 10.7326/0003-4819-112-9-682. [DOI] [PubMed] [Google Scholar]
- Lu T.T., Onizawa M., Hammer G.E., Turer E.E., Yin Q., Damko E., Agelidis A., Shifrin N., Advincula R., Barrera J., Malynn B.A., Wu H., Ma A. Article dimerization and ubiquitin mediated recruitment of A20 , a complex deubiquitinating enzyme. Immunity. 2013;38:896–905. doi: 10.1016/j.immuni.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A., Malynn B.A. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat. Rev. Genet. 2012;12:774–785. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro-Checa C., Kumar S., Ramiro S., Voorde L.J.B. De, Eikenboom J., Ronen I., Bresser J. De. Are serum autoantibodies associated with brain changes in systemic lupus erythematosus? MRI data from the Leiden NP-SLE cohort. Lupus. 2019;28:94–103. doi: 10.1177/0961203318816819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak A., Chun Man Ho R., Lau C.S. Clinical implications of neuropsychiatric systemic lupus erythematosus. Adv. Psychiatr. Treat. 2009;15:451–458. [Google Scholar]
- Mangold C.A., Wronowski B., Du M., Masser D.R., Hadad N., Bixler G.V., Brucklacher R.M., Ford M.M., Sonntag W.E., Freeman W.M. Sexually divergent induction of microglial- associated neuroinflammation with hippocampal aging. J. Neuroinflammation. 2017;14:1–19. doi: 10.1186/s12974-017-0920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena A., Ruiz-Salas J.C., Puentes A., Dorado I., Ruiz-Veguilla M., De la Casa L.G. Reduced prepulse inhibition as a biomarker of schizophrenia. Front. Behav. Neurosci. 2016;10:202. doi: 10.3389/fnbeh.2016.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mike E.V., Makinde H.M., Gulinello M., Vanarsa K., Herlitz L., Gadhvi G., Winter D.R., Mohan C., Hanly J.G., Mok C.C., Cuda C.M., Putterman C. Lipocalin-2 is a pathogenic determinant and biomarker of neuropsychiatric lupus. J. Autoimmun. 2019;96:59–73. doi: 10.1016/j.jaut.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastero R., Bettini P., Del Zotto E., Cottini E., Tincani A., Balestrieri G., Cattaneo R., Camarda R., Vignolo L.A., Padovani A. Prevalence and pattern of cognitive impairment in systemic lupus erythematosus patients with and without overt neuropsychiatric manifestations. J. Neurol. Sci. 2001;184:33–39. doi: 10.1016/s0022-510x(00)00492-5. [DOI] [PubMed] [Google Scholar]
- Montero-Lopéz E., Santos-Ruiz A., Navarrette-Navarette N., Ortego-Centeno N., Pérez-Garcia M., Peralta-Ramirez M. The effects of corticosteroids on cognitive flexibility and decision-making in women with lupus. Lupus. 2016;25:1470–1478. doi: 10.1177/0961203316642313. [DOI] [PubMed] [Google Scholar]
- Musone S.L., Taylor K.E., Lu T.T., Nititham J., Ferreira R.C., Ortmann W., Shifrin N., Petri M. a, Kamboh M.I., Manzi S., Seldin M.F., Gregersen P.K., Behrens T.W., Ma A., Kwok P., Criswell L. a. Multiple polymorphisms in the TNFAIP3 region are independently associated with systemic lupus erythematosus. Nat. Genet. 2008;40:1062–1064. doi: 10.1038/ng.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler J., Moy S., Dold G., Trang D., Simmons N., Perez A., Young N., Barbaro R., Piven J., Magnuson T., Crawley J. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Naert A., Callaerts-Vegh Z., D’Hooge R. Nocturnal hyperactivity , increased social novelty preference and delayed extinction of fear responses in post-weaning socially isolated mice. Brain Res. Bull. 2011;85:354–362. doi: 10.1016/j.brainresbull.2011.03.027. [DOI] [PubMed] [Google Scholar]
- Naert A., Callaerts-Vegh Z., Moechars D., Meert T., D’Hooge R. Vglut2 haploinsufficiency enhances behavioral sensitivity to MK-801 and amphetamine in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:1316–1321. doi: 10.1016/j.pnpbp.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Niraula A., Sheridan J.F., Godbout J.P. Microglia priming with aging and stress. Neuropsychopharmacol. Rev. 2017;42:318–333. doi: 10.1038/npp.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradee W., Melikian H., Rasmussen D., Kenneson A., Conn P., Warren S. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Perry V.H., Holmes C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014;10:217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- Petri M. Epidemiology of systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol. 2002;16:847–858. doi: 10.1053/berh.2002.0259. [DOI] [PubMed] [Google Scholar]
- Petri M., Naqibuddin M., Carson K.A., Daniel J., Weisman M.H., Holliday S.L., Padilla P.A., Brey R.L. Depression and cognitive impairment in newly diagnosed systemic lupus erythematosus. J. Rheumatol. 2010;37:2032–2038. doi: 10.3899/jrheum.091366. [DOI] [PubMed] [Google Scholar]
- Pikman R., Kivity S., Levy Y., Arango M.-T., Chapman J., Yonath H., Shoenfeld Y., Gofrit S. Neuropsychiatric SLE: from animal model to human. Lupus. 2017;26:470–477. doi: 10.1177/0961203317694261. [DOI] [PubMed] [Google Scholar]
- Pisetsky D.S. 2019. The Central Role of Nucleic Acids in the Pathogenesis of Systemic Lupus Erythematosus. F1000 Res. 8. pii: F1000 Faculty Rev-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees F., Doherty M., Grainge M.J., Lanyon P., Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology. 2017;56:1945–1961. doi: 10.1093/rheumatology/kex260. [DOI] [PubMed] [Google Scholar]
- Rensing-ehl A., Simon V., Speckmann C., Lorenz M.R., Ritter J., Janda A., Abinun M., Pircher H., Bengsch B., Thimme R., Fuchs I., Ammann S., Allg A., Huetker S., Ingrid K., Schwarz K., Ehl S., Völkl S., Speckmann C., Lorenz M.R., Ritter J., Janda A., Abinun M., Pircher H., Bengsch B., Thimme R., Fuchs I., Ammann S., Allgäuer A., Kentouche K., Cant A., Hambleton S., Bettoni de Cunha C., Huetker S., Kühnle I., Pekrun A., Seidel M.G., Hummel M., Mackensen A., Schwarz K., Ehl S. Abnormally differentiated CD4+ or CD8+ T cells with phenotypic and genetic features of double negative T cells in human Fas deficiency. Blood. 2014;124:851–861. doi: 10.1182/blood-2014-03-564286. [DOI] [PubMed] [Google Scholar]
- Ribeiro F., Signorelli F. The role of infections in neuropsychiatric lupus. Lupus. 2017;26:490–496. doi: 10.1177/0961203317691375. [DOI] [PubMed] [Google Scholar]
- Rubtsov A.V., Rubtsova K., Kappler J.W., Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmun. Rev. 2010;9:494–498. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsova K., Marrack P., Rubtsov A.V. Sexual dimorphism in autoimmunity. J. Clin. Investig. 2015;125:2187–2193. doi: 10.1172/JCI78082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schwartz N., Stock A.D., Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat. Rev. Rheumatol. 2019;15:137–152. doi: 10.1038/s41584-018-0156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F., Hu Z., Goswami J., Gaffen S.L. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J. Biol. Chem. 2006;281:24138–24148. doi: 10.1074/jbc.M604597200. [DOI] [PubMed] [Google Scholar]
- Shimojima Y., Matsuda M., Gono T., Ishii W., Ikeda S. Relationship between clinical factors and neuropsychiatric manifestations in systemic lupus erythematosus. Clin. Rheumatol. 2005;24:469–475. doi: 10.1007/s10067-004-1060-y. [DOI] [PubMed] [Google Scholar]
- Skaug B., Chen J., Du F., He J., Ma A., Chen Z.J. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol. Cell. 2011;44:559–571. doi: 10.1016/j.molcel.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C., Morris R. The watermaze. Behav. Neurosci. 1993;1:107–122. [Google Scholar]
- Stock A.D., Wen J., Doerner J., Herlitz L.C., Gulinello M., Putterman C. Neuropsychiatric systemic lupus erythematosus persists despite attenuation of systemic disease in MRL/lpr mice. J. Neuroinflammation. 2015;12 doi: 10.1186/s12974-015-0423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A.D., Wen J., Putterman C. Neuropsychiatric lupus, the blood brain barrier, and the TWEAK/Fn14 pathway. Front. Immunol. 2013;4:1–9. doi: 10.3389/fimmu.2013.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W.J., Sammons N.W., Kuhns A.J., Sparks L.D. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Studer P., Da Silva C., Revuelta Cervantes J., Mele A., Csizmadia E., Siracuse J., Damrauer S., Peterson C., Candinas D., Stroka D., Ma A., Bhasin M., Ferran C. Significant lethality following liver resection in A20 heterozygous knockout mice uncovers a key role for A20 in liver regeneration. Cell Death Differ. 2015;22:2068–2077. doi: 10.1038/cdd.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M.D., Zhao Zhen, Montagne Axel, Nelson A.R., Zlokovic B.V., Zhao Z., Montagne A., Ar N., Barrier Z.B.V.B. Blood-Brain barrier: from physiology to disease and back. Physiol. Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow N.R., Weber M., Qu Y., Light G.A., Braff D.L. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam A., Wekking E., Callewaert J., Schipperijn A., Oomen H., de Jong J., Swaak A., Smeenk R., Feltkamp T. Psychiatric symptoms before systemic lupus erythematosus is diagnosed. Rheumatol. Int. 1994;14:57–62. doi: 10.1007/BF00300248. [DOI] [PubMed] [Google Scholar]
- Vendrell J.A., Ghayad S., Ben-Larbi S., Dumontet C., Mechti N., Cohen P.A. A20/TNFAIP3, a new estrogen-regulated gene that confers tamoxifen resistance in breast cancer cells. Oncogene. 2007;26:4656–4667. doi: 10.1038/sj.onc.1210269. [DOI] [PubMed] [Google Scholar]
- Vereecke L., Sze M., Mc Guire C., Rogiers B., Chu Y., Schmidt-Supprian M., Pasparakis M., Beyaert R., van Loo G. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J. Exp. Med. 2010;207:1513–1523. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhelst K., Carpentier I., Kreike M., Meloni L., Verstrepen L., Kensche T., Dikic I., Beyaert R. A20 inhibits LUBAC-mediated NF-kB activation by binding linear polyubiquitin chains via its zinc finger 7. EMBO J. 2012;31:3845–3855. doi: 10.1038/emboj.2012.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent F.B., Northcott M., Hoi A., Mackay F., Morand E.F. Clinical associations of serum interleukin-17 in systemic lupus erythematosus. Arthritis Res. Ther. 2013;15:R97. doi: 10.1186/ar4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voet S., Mc Guire C., Hagemeyer N., Martens A., Schroeder A., Wieghofer P., Daems C., Staszewski O., Vande Walle L., Costa Jordao M.J., Sze M., Vikkula H., Demeestere D., Van Imschoot G., Scott C.L., Hoste E., Gonçalves A., Guilliams M., Lippens S., Libert C., Vandenbroucke R.E., Kim K., Jung S., Callaerts-vegh Z., Callaerts P., de Wit J., Lamkanfi M., Prinz M., van Loo G. A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat. Commun. 2018;9:2036. doi: 10.1038/s41467-018-04376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wen F., Tessneer K.L., Gaffney P.M. TALEN-mediated enhancer knockout in fluences TNFAIP3 gene expression and mimics a molecular phenotype associated with systemic lupus erythematosus. Nat. Genet. 2016;17:165–170. doi: 10.1038/gene.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wen F., Wiley G.B., Kinter M.T., Gaffney P.M. An enhancer element harboring variants associated with systemic lupus erythematosus engages the TNFAIP3 promoter to influence A20 expression. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Deckert M., Xuan N.T., Nishanth G., Just S., Waisman A., Naumann M., Schlüter D. Astrocytic A20 ameliorates experimental autoimmune encephalomyelitis by inhibiting NF-κB- and STAT1-dependent chemokine production in astrocytes. Acta Neuropathol. 2013;126:711–724. doi: 10.1007/s00401-013-1183-9. [DOI] [PubMed] [Google Scholar]
- Watson M.L., Rao J.K., Gilkeson G.S., Ruiz P., Eicher E.M., Pisetsky D.S., Matsuzawa A., Rochelle J.M., Seldin M.F. Genetic analysis of MRL/lpr mice: relationship of the Fas apoptotis gene to disease manifestations and renal disease-modifying loci. J. Exp. Med. 1992;176:1645–1656. doi: 10.1084/jem.176.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J., Doerner J., Chalmers S., Stock A., Wang H., Gullinello M., Shlomchik M.J., Putterman C. B cell and/or autoantibody deficiency do not prevent neuropsychiatric disease in murine systemic lupus erythematosus. J. Neuroinflammation. 2016;13:1–14. doi: 10.1186/s12974-016-0537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz I.E., Newton K., Seshasayee D., Kusam S., Lam C., Zhang J., Popovych N., Helgason E., Schoeffler A., Jeet S., Ramamoorthi N., Kategaya L., Newman R.J., Horikawa K., Dugger D., Sandoval W., Mukund S., Zindal A., Martin F., Quan C., Tom J., Fairbrother W.J., Townsend M., Warming S., Devoss J., Liu J., Dueber E., Goodnow C.C., Balazs M., Yu K., Kolumam G., Dixit V.M. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature. 2015;528:370–375. doi: 10.1038/nature16165. [DOI] [PubMed] [Google Scholar]
- Wertz I.E., O’Rourke K.M., Zhou H., Eby M., Aravind L., Seshagiri S., Wu P., Wiesmann C., Baker R., Boone D.L., Ma A., Koonin E.V., Dixit V.M. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kB signaling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- Willems E., Leyns L., Vandesompele J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2008;379:127–129. doi: 10.1016/j.ab.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Yi Li Y., Ming Li X. The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res. 2000;10:169–177. doi: 10.1038/sj.cr.7290046. [DOI] [PubMed] [Google Scholar]
- Zamanian J.L., Xu L., Foo L.C., Nouri N., Zhou L.L., Giffard R.G., Barres B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandman-Goddard G., Solomon M., Rosman Z., Peeva E., Shoenfeld Y. Environment and lupus-related diseases. Lupus. 2012;21:241–250. doi: 10.1177/0961203311426568. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Lenardo M.J., Baltimore D. 30 Years of NF-κB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O’Keeffe S., Phatnani H.P., Guarnieri P., Caneda C., Ruderisch N., Deng S., Liddelow S.A., Zhang C., Daneman R., Maniatis T., Barres B.A., Wu J.Q. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Wang H., Schwartz D.M., Stoffels M., Park Y.H., Zhang Y., Yang D., Demirkaya E., Takeuchi M., Tsai W.L., Lyons J.J., Yu X., Ouyang C., Chen C., Chin D.T., Zaal K., Chandrasekharappa S.C., Hanson E.P., Yu Z., Mullikin J.C., Hasni S.A., Wertz I.E., Ombrello A.K., Stone D.L., Hoffmann P., Jones A., Barham B.K., Leavis H.L., Royen-kerkof A. Van, Sibley C., Batu E.D., Gül A., Siegel R.M., Boehm M., Milner J.D., Ozen S., Gadina M., Chae J., Laxer R.M., Kastner D.L., Aksentijevich I. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat. Genet. 2015;48:67–73. doi: 10.1038/ng.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed in the course of this study are included in the main manuscript and in the supplementary information files.