Abstract
Background:
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that delivers constant, low electrical current resulting in changes to cortical excitability. Prior work suggests it may enhance motor learning giving it the potential to augment surgical technical skill acquisition.
Objectives:
The aim of this study was to test the efficacy of tDCS, coupled with motor skill training, to accelerate laparoscopic skill acquisition in a pre-registered (NCT03083483), double-blind and placebo-controlled study. We hypothesized that relative to sham tDCS, active tDCS would accelerate the development of laparoscopic technical skills, as measured by the Fundamentals of Laparoscopic Surgery (FLS) Peg Transfer task quantitative metrics.
Methods:
In this study, sixty subjects (mean age 22.7 years with 42 females) were randomized into sham or active tDCS in either bilateral primary motor cortex (bM1) or supplementary motor area (SMA) electrode configurations. All subjects practiced the FLS Peg Transfer Task during six 20-min training blocks, which were preceded and followed by a single trial pre-test and post-test. The primary outcome was changes in laparoscopic skill performance over time, quantified by group differences in completion time from pre-test to post-test and learning curves developed from a calculated score accounting for errors.
Results:
Learning curves calculated over the six 20-min training blocks showed significantly greater improvement in performance for the bM1 group than the sham group (t = 2.07, p = 0.039), with the bM1 group achieving approximately the same amount of improvement in 4 blocks compared to the 6 blocks required of the sham group. The SMA group also showed greater mean improvement than sham, but exhibited more variable learning performance and differences relative to sham were not significant (t = 0.85, p = 0.400). A significant main effect was present for pre-test versus post-test times (F = 133.2, p < 0.001), with lower completion times at post-test, however these did not significantly differ for the training groups.
Conclusion:
Laparoscopic skill training with active bilateral tDCS exhibited significantly greater learning relative to sham. The potential for tDCS to enhance the training of surgical skills, therefore, merits further investigation to determine if these preliminary results may be replicated and extended.
Keywords: Transcranial direct current stimulation, FLS Peg transfer task, Bilateral motor cortex, Supplementary motor area, Visual-motor learning
Introduction
The field of surgery has continued to evolve over time with technological advances, work hour restrictions, and emphasis on patient safety [1]. This evolution has driven surgical residencies to focus on simulation and competency-based training to target technical skill development in a safe, controlled environment prior to patient interaction [2]. The importance of this training paradigm has been exemplified by the American Board of Surgery requiring that all general surgery residents pass the one-time, simulation-based certifications in Fundamentals of Laparoscopic Surgery (FLS) and Fundamentals of Endoscopic Surgery (FES™) [3,4]. The development of these basic technical skills requires the removal of residents from the bedside in order to achieve repetition of training within the simulation lab [5–8]. Therefore, it is imperative that new technologies and training techniques are developed symbiotically to expedite the time-consuming acquisition of basic technical skills in the simulation lab.
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that delivers constant, low electrical current via electrodes on the scalp to modify neuronal excitability in the underlying cortex [9–11]. Anodal stimulation has been shown to increase cortical excitability and facilitate spontaneous neuronal firing, whereas cathodal stimulation has been demonstrated to decrease excitability and reduce neuronal firing [12]. As such, tDCS is able to induce polarity-specific excitability shifts that depend on the location, size and spacing of the stimulating electrodes [13]. Numerous studies suggest that tDCS to motor cortex, particularly coupled with simultaneous motor skill training, may enhance motor learning in healthy individuals [14–18] as well as faster motor-system recovery following stroke in clinical populations [19,20], with limited, but growing, use in bimanual skill learning applications [21]. While a recent consensus paper on tDCS and motor learning described promising results, it also highlighted a pressing need for prospective, pre-registered, hypothesis-driven studies to improve the transparency and replicability of this approach so that the potential of these interventions may be rigorously established [22]. The ease with which tDCS can be implemented and tolerated, paired with the possibility for lasting changes in cortical excitability and associated motor learning, gives this approach the powerful potential to accelerate the development of surgical technical skills [23,24]. Additionally, the safety profile of tDCS has been well described with parameters feasible for implementation within a surgical skills training lab [25–27].
Three prior studies have been published on the application of tDCS within surgery [28–30]. In the first study, Ciechanski and colleagues [29] applied anodal or sham tDCS to 22 medical students performing a uni-manual neurosurgical tumor resection task (done with the dominant hand). Results demonstrated improved efficiency and effectiveness of performance with little to no decay at a 6-week retest. A second study from the same authors [28] tested the efficacy of anodal versus sham tDCS as 40 participants performed the FLS pattern-cutting and peg transfer tasks (predominantly uni-manual, and bi-manual tasks, respectively). Here it was observed that active tDCS produced significant gains in the pattern-cutting task that were retained at the 6-week retest, but no differences were found for the peg transfer task. A third study from these same authors [30] replicated the findings from their previous FLS task in 22 participants, while also identifying preliminary spectral electroencephalographic (EEG) markers that modulate selectively with tDCS-paired surgical training. While these studies provide important and compelling initial evidence that tDCS can positively affect surgical skill training, they present gains only for unimanual tasks, using a unilateral stimulation montage with anodal stimulation to the dominant primary motor cortex and cathodal stimulation to the contralateral supraorbital region (M1–SO montage), and provide no more than 24 total minutes of training. Given that most surgical tasks involve bimanual control and that greater gains may be achieved with longer training-paired stimulation, there remains an important need to test electrode configurations that will lead to the greatest generalizable performance gains for these skills.
Past research has investigated the neural mechanisms giving rise to fine bimanual motor control, such as that exerted in surgery. These studies have identified a network of brain areas including the primary motor cortex (M1), the supplemental motor cortex (SMA), and the premotor cortex, as well as the cerebellum, that communicate through highly reciprocal neuronal pathways to allow millisecond and millimeter motor control (reviewed in Ref. [31]). Within these networks, interhemispheric inhibition (IHI) has been proposed as an essential mechanism allowing for bimanual coordination for skills in which the two hands must interact to achieve movement goals [32,33]. Past empirical studies measuring IHI have found that individuals with greater transcallosal inhibition demonstrate reduced contralateral EMG activity during unimanual actions, indicating greater efficiency, but also produce the poorest performance during bimanual force production tasks [34]. As such, these authors suggest that a high capacity for IHI from one cortex to another can reduce “motor overflow” during unimanual tasks, but also limit interhemispheric cooperation during bimanual control. While such relationships appear to differ for highly trained individuals, such as experienced musicians [35,36], reduced IHI may present a specific mechanism contributing to the learning of bimanual skills that can be modulated by tDCS.
The current study, therefore, aimed to test the effectiveness of tDCS during laparoscopic skill training to determine if it has the capacity to accelerate technical skill acquisition. We selected tDCS amplitude and timing parameters to be in the range of those previously reported to affect motor skill training (e.g. 2 mA, 20 min) [17]. Since the optimal electrode configuration for bimanual skill learning is yet unknown, we evaluated two separate electrode configurations, each targeting the motor system. Based on past demonstrations that anode current to the dominant M1 cortex and cathode current to the nondominant M1 cortex can enhance motor skill acquisition [15,16], and the observation that this montage (bilateral M1 or bM1) decreases IHI in a manner that correlates positively with improvement in motor performance [37], this montage was selected as one configuration. Due to the functional role of SMA in motor sequence planning [38,39], and particularly its contribution to bimanual coordination [40], a second montage was tested with anodal current delivered over SMA and cathode current over position FPz on the 10–20 electrode system. We hypothesized that both of the active stimulation conditions would lead to faster and larger learning gains relative to sham tDCS.
Materials and methods
Participant enrollment
This double-blinded, randomized, and sham-controlled study was approved by the Duke University Institutional Review Board, pre-registered at ClinicalTrials.gov (NCT03083483), and conformed to the CONsolidated Standards of Reporting Trials (CONSORT) recommendations.
Healthy adults with the ability to provide consent and follow directions were included in the study. Exclusion criteria were specific to tDCS including past brain tumor, psychotropic medications, seizure disorder, substance abuse, and metal implants in the head. Females had to provide a negative urine pregnancy test at the initiation of the study, but were not screened based on hormonal profile. Subjects meeting inclusion criteria on a screening survey provided written consent and were enrolled into the study. Subjects were provided $20 per hour compensation for their participation. Prior to the start of the study, simple randomization was used to assign a tDCS code and electrode configuration to each participant. Each code was programmed as either active or sham stimulation by the device manufacturer, and this information remained blinded to the study team and subjects until after statistical analysis. Electrode configurations targeted either the bilateral primary motor cortex (bM1) or the supplementary motor area (SMA) and balanced randomization was performed to assign subjects equally to active bM1, active SMA, and sham groups (half with bM1 and half with SMA configurations) [41].
Experimental design and procedures
This study consisted of three visits that occurred over a 7-day window. As illustrated in Fig. 1, during the first visit, participants were screened, consented, familiarized with the FLS device and completed brief questionnaires. They then completed the pre-test, followed by performing two 20-min blocks of training, spread out by a short break, while receiving either active or sham tDCS. Visits two and three each also consisted of two 20-min practice blocks with concurrent active or sham stimulation and a post-test performed at the end of training on the third visit.
Fig. 1.

Illustration of the experimental design showing activities for each visit. The first row shows the preparation activities and approximate duration. The second row show two images of the pre-test peg transfer task, one for the movement of objects from right-to-left and one for movement of objects from left-to-right. The third row illustrates the two 20-min task blocks, separated by a 5-min break. The fourth row shows the post-test which is identical to the pre-test.
During the first visit, each subject viewed the same online instructional video on how to perform the FLS Task 1 Peg Transfer (https://www.youtube.com/watch?v=gAQPXHWqdXQ). This was followed by approximately 4-min of acclimation to the FLS box trainer (Limbs & Things Ltd., Savannah, GA) which included familiarization with the instruments and task goals of moving the triangle objects from side-to-side while receiving verbal feedback from a study team member to point out any violations of the procedure. Following familiarization, the tDCS device was placed on the participant’s head (as described in the tDCS Specifications section below). Participants then performed a pre-test, consisting of a single, timed trial moving the six triangle objects from their starting positions on the left, to the six pegs on the right, and back. No tDCS was delivered during this pre-test and timekeeping started with the touch of the first object and ended when the final object was placed. At this point, participants began skill training, which proceeded in 20-min blocks during which the subject repeatedly performed the FLS Peg Transfer Task as active or sham tDCS was delivered. Participants were encouraged to complete as many peg transfers in the 20-min, while minimizing errors or drops. Two 20-min blocks were done on each visit and separated by a break of no more than 5 min in between. On visits two and three, participants began the session with brief questionnaires and warm up prior to training. Following the end of training in visit three, participants performed another single timed trial post-test without any tDCS intervention regardless of randomization.
tDCS specifications
The Soterix Medical 1 × 1 tDCS device (Soterix Medical Inc., NewYork, NY) was used. Active stimulation consisted of a 30-s, gradual ramping up of electrical current to a maximum sustained level of 2 mA for the duration of the 20-min training block, followed by a 30-s down ramp to a 25 μA current, the minimum needed to measure contact quality. Sham tDCS consisted of the 30-s on ramp, followed immediately by the off ramp to create an initial cutaneous sensation of stimulation without the sustained current delivery [35]. The device provided another 30-s of stimulation at the end of the 20-min training block. Both active and sham stimulation were delivered during concurrent practice with the FLS task.
Electrodes were placed on each subject utilizing the 10–20 electroencephalography electrode system, with the two, 3 cm × 5 cm, saline-soaked sponges held in place using two Soterix Medical Elastic Fastener “Blue” bands (Soterix Medical Inc., New York, NY). For the bM1 configuration, the cathodal electrode was placed 20% to the right of vertex over C4 while the anodal electrode was placed 20% to the left of vertex over C3. SMA configuration placed the cathodal electrode 10% posterior from the nasion over Fpz and the anodal electrode 15% anterior to vertex over Cz, with the long edge of the sponge pads oriented left–right, leading to the greatest outward current flow over the SMA region. Electrode configurations and underlying cortical electric field illustrations are shown in Fig. 2. Electric field simulation was performed using SimNIBS 2.0.1 [36]. Briefly, the generic T1-weight MRI was segmented and meshed using the headreco command in SimNIBS. The diffusion weighted imaging data was converted to anisotropic conductivity data using the dwi2cond command. Standard conductivity values were assigned: 0.126 S/m for white matter, 0.275 S/m for grey matter, 1.654 S/m for cerebrospinal fluid, 0.01 S/m for bone, 0.465 S/m for scalp, 0.5 S/m for eyeballs. We used the volume normalized anisotropic conductivity with a maximum eigenvalue ratio of 10 and maximum eigenvalue of 2.
Fig. 2.

Electrode configurations and underlying cortical electric field illustrations for the A) bilateral primary motor cortex montage (bM1) and B) the supplementary motor area (SMA) montage. Images on the left show transparent tissue segmentations for the scalp, skull, and cortex with electrode placements for the anode (red) and cathode (blue) sponges. Images on the right show the underlying electric field distribution on a generic T1-weighted MRI with a color scale bar indicating the electric field magnitude.
Electrode conductivity was measured continuously throughout the session, and instances of increased contact impedance were noted and addressed by adding more saline to the sponges or increasing the amount of pressure applied by the elastic bands. If necessary, the session was stopped and restarted for repositioning of the electrodes. The occurrence and duration of any critical connectivity events were documented. No subject had to be excluded due to an inability to provide adequate tDCS.
Data acquisition and outcomes
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Duke University [42].
Subjects completed a questionnaire consisting of demographic characteristics, prior surgical skill experience, and personal behaviors. Demographic characteristics included age, sex, ethnicity, race, and handedness. Prior experience with either open or laparoscopic surgery was obtained. Personal behaviors included self-identification as an athlete, musician, video gamer, or artist in addition to servings of caffeine consumed and hours of sleep obtained the day of skills training. These consumption questions were repeated at the start of each session, but all other questions were only queried on the first visit.
Videos of each study visit were recorded using the Argus Science Frame Grabber (Argus Science, LLC, Tyngsborough, MA). This system digitizes the analog signal directly from the camera located within the FLS box trainer. Each video was reviewed and scored at a later date by a single study team member (MLC) with surgical experience to ensure internal consistency. Time-to-completion was recorded for both pre- and post-tests. Data obtained from each 20-min training block included the number of full repetitions completed, the number of individual objects moved, and errors committed. Errors were defined as 1) drops of the object outside the field of view and 2) improper transfers (transfer on a drop, transfer with the wrong hand, resting the object during transfer). These errors were penalized by deducting either 3 (drop out of field-of-view) or 1 (improper transfer) from the total number of objects moved. A drop out of the field-of-view was deemed a larger error as this would lead to disqualification during FLS testing, and the error had to be corrected by the study team for the participant to be able to continue training.
An overall score was calculated for each of the 20-min blocks by subtracting the penalties accrued from errors from the total number of objects moved according to the formula:
where, Total Error Deduction = 3 × drops outside the field-of-view + 1 × improper transfers.
Statistical analysis
Baseline characteristics, personal behaviors, and prior surgical skill data were compiled and analyzed. Comparisons of pre-test and post-test time-to-completion scores across the intervention groups were performed with repeated-measure analysis of variance (rANOVA) with effect sizes calculated as partial eta squared (). For three participants, pre- or post-test data was not acquired and these participants were not included in the rANOVA. Comparisons between categorical variables were made with chi-square test for association.
Learning curves were calculated to evaluate total change over the six 20-min training blocks. Overall scores were normalized relative to the first block and effects of the intervention group assignment were analyzed using linear mixed effects models (LME), with subjects as random factors and statistical results reported for the level comparisons between the training groups. A repeated effect of training block was used to model the covariance of the residuals with a compound symmetry structure and R2 values were calculated to determine the total variance explained by the model. In addition to the main effects of intervention and block, the intervention-by-block interaction was also included. LME was performed using the lme4 package in R v3.5.0.
Sub-analyses applied independent samples t-tests to determine differences in performance metrics based on sex, handedness, and prior surgical experience. Finally, performance metrics were correlated to the various baseline characteristics and personal behaviors obtained on the questionnaire. A p-value of <0.05 was considered statistically significant. Statistical analysis was completed using R v3.5.0 (The R Foundation for Statistical Computing, Vienna, Austria) and SPSS v25 (IBM Corp, Armonk, NY, USA).
Results
Demographics
Sixty subjects (84.5% of screened participants) completed all six training blocks, including 20 each in the active bM1, active SMA, and sham (10 bM1, 10 SMA) tDCS groups (Fig. 3). Among the three intervention groups, there was no difference in mean age (bM1 21.9 ± 5.2 vs SMA 23.5 ± 5.4 vs sham 22.7 ± 3.7 years), sex (males: bM1 8, SMA 4, sham 6), ethnicity (Caucasian: bM1 6, SMA 11, sham 8), and handedness (right: bM1 17, SMA 18, sham 18) (all p > 0.05).
Fig. 3.
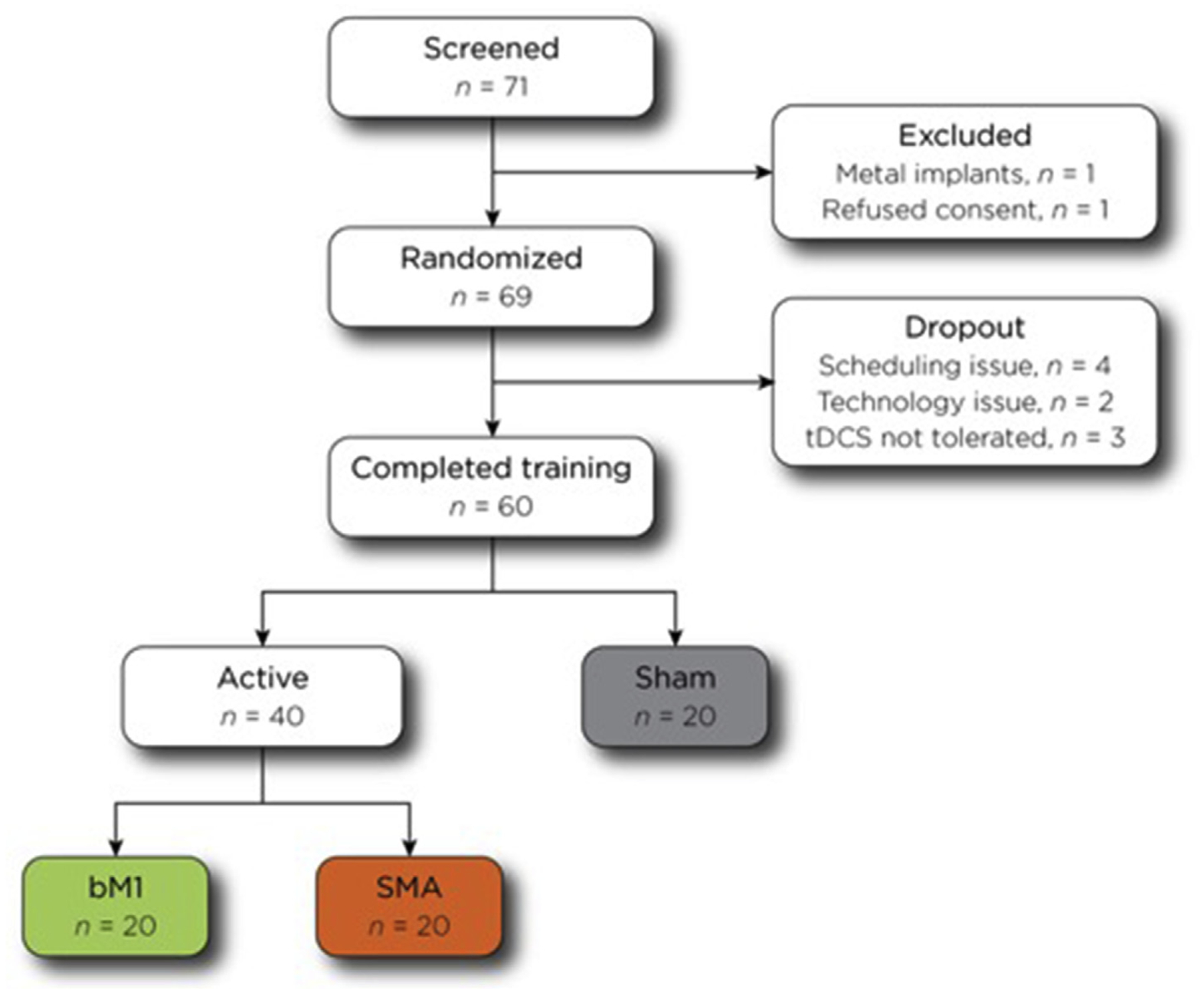
Consort diagram showing the steps in the study, including the numbers of participants who completed each stage as well as the numbers who were excluded or dropped out. Color coding for the bM1 (green), SMA (orange), and sham (grey) groups are preserved in Figs. 4 and 5.
All subjects were recruited from a large academic campus including undergraduate and graduate students across numerous different fields of study. Six subjects had prior open surgical experience, and 4 had prior laparoscopic surgical experience. These individuals were distributed evenly amongst the three groups (open p = 0.574 and laparoscopic p = 0.765). Self-identified athletes (n = 34, 56.7%), musicians (n = 22, 36.7%), and artists (n = 10, 16.7%) were also equally represented across groups (all p > 0.05). However, video gamers had a larger presence within the bM1 group compared to SMA and sham (χ2 = 9.412, p 0.009). There was no difference in the average amount of caffeine consumed (p = 0.15) or hours of sleep obtained (p = 0.24) prior to each training session across groups. Complete baseline characteristics are in Table 1.
Table 1.
Baseline characteristics.
| Total | Bilateral | SMA | Sham | p-valuea | |
|---|---|---|---|---|---|
| N = 60 | N = 20 | N = 20 | N = 20 | ||
| Age | 22.7 (4.8) | 21.9 (5.2) | 23.5 (5.4) | 22.7 (3.7) | p = 0.60 |
| Gender | p = 0.39 | ||||
| Male | 18 (30%) | 8 (40%) | 4 (20%) | 6 (30%) | |
| Female | 42 (70%) | 12 (60%) | 16 (80%) | 14 (70%) | |
| Ethnicity | p = 0.86 | ||||
| Hispanic | 7 (11.7%) | 2 (10%) | 3 (15%) | 2 (10%) | |
| Non-Hispanic | 53 (88.3%) | 18 (90%) | 17 (85%) | 18 (90%) | |
| Race | p = 0.70 | ||||
| White | 25 (42%) | 6 (30%) | 11 (52%) | 8 (42%) | |
| African American | 9 (15%) | 3 (15%) | 2 (10%) | 4 (21%) | |
| Asian | 20 (33%) | 9 (45%) | 6 (29%) | 5 (26%) | |
| Native American/Alaska Native | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Native Hawaiian/Pacific Islander | 1 (1%) | 0 (0%) | 0 (0%) | 1 (5%) | |
| More than one | 4 (7%) | 1 (5%) | 2 (10%) | 1 (5%) | |
| Not reported | 1 (1%) | 1 (5%) | 0 (0%) | 0 (0%) | |
| Handedness | p = 0.86 | ||||
| Right | 53 (88.3%) | 17 (85%) | 18 (90%) | 18 (90%) | |
| Left | 7 (11.7%) | 3 (15%) | 2 (10%) | 2 (10%) | |
| Prior Open Surgery Experience | 6 (10%) | 1 (5%) | 2 (10%) | 3 (15%) | p = 0.57 |
| Prior Laparoscopic Surgery Experience | 4 (6.7%) | 1 (5%) | 2 (10%) | 1 (5%) | p = 0.77 |
| Self-Identification | |||||
| Athlete | 34 (56.7%) | 11 (55%) | 12 (60%) | 11 (55%) | p = 0.94 |
| Musician | 22 (36.7%) | 5 (25%) | 9 (45%) | 8 (40%) | p = 0.39 |
| Gamer | 9 (15%) | 7 (35%) | 1 (5%) | 1 (5%) | p < 0.01 |
| Artist | 10 (16.7%) | 6 (30%) | 1 (5%) | 3 (15%) | p = 0.10 |
| Servings of Caffeine prior to Training | p = 0.15 | ||||
| Day 1 | 0.45 (0.8) | 0.55 (0.8) | 0.2 (0.4) | 0.6 (1.1) | |
| Day 2 | 0.45 (0.8) | 0.65 (0.9) | 0.2 (0.4) | 0.5 (0.9) | |
| Day 3 | 0.45 (0.8) | 0.60 (1.1) | 0.25 (0.4) | 0.5 (0.7) | |
| Hours of Sleep prior to Training | p = 0.24 | ||||
| Day 1 | 6.8 (1.1) | 7.0 (1.2) | 6.6 (0.9) | 6.8 (1.1) | |
| Day 2 | 6.9 (1.0) | 7.2 (1.1) | 6.7 (0.7) | 7.0 (1.0) | |
| Day 3 | 6.8 (1.4) | 6.5 (1.4) | 6.6 (1.5) | 7.3 (1.2) |
All data reported as count (percent) or mean (SD).
ANOVA for age, caffeine, and sleep. χ2 for remaining comparisons.
Training data and learning curves
All three groups had a gradual improvement in the average number of total repetitions completed (range:10.3 to 18.4) and average number of total objects moved (range: 128.7 to 225.1) as they progressed through the 6 training blocks. Errors made by dropping the object outside of the field-of-view remained fairly stable throughout training, ranging from 0.7 to 1.8 across all groups. The average number of improper transfers gradually decreased in the SMA group (3.1–0.7) and had greater variability in the bM1 and sham groups. The overall scores accounting for objects moved and errors committed also increased over all six training blocks for all training groups (bM1 123.4 to 220.1, SMA 124.7 to 212.5, sham 130.5 to 208.5) (see Table 2).
Table 2.
Average performance metrics by intervention group.
| Bilateral | SMA | Sham | |
|---|---|---|---|
| N = 20 | N = 20 | N = 20 | |
| Time-to-completion | |||
| Pre-test | 166.8 (59.5) | 159.2 (93.0) | 135.7 (31.3) |
| Post-test | 55.3 (15.4) | 54.5 (13.1) | 59.5 (18.3) |
| Completed Repetitions | |||
| Session 1 | 10.3 (3.1) | 10.7 (3.6) | 11.2 (3.0) |
| Session 2 | 13.0 (4.3) | 13.4 (3.3) | 13.6 (4.1) |
| Session 3 | 14.2 (4.0) | 14.1 (4.3) | 14.9 (3.8) |
| Session 4 | 16.6 (4.7) | 15.7 (4.5) | 15.7 (3.9) |
| Session 5 | 17.2 (4.8) | 15.9 (4.4) | 16.8 (3.5) |
| Session 6 | 18.4 (4.8) | 17.7 (4.6) | 17.2 (4.1) |
| Total Objects Moved | |||
| Session 1 | 128.7 (36.5) | 133.0 (40.9) | 137.7 (35.8) |
| Session 2 | 160.8 (51.9) | 164.8 (38.7) | 168.4 (49.8) |
| Session 3 | 176.1 (47.4) | 173.5 (50.5) | 183.1 (44.3) |
| Session 4 | 203.3 (55.4) | 194.5 (52.9) | 193.4 (47.0) |
| Session 5 | 211.3 (56.6) | 195.1 (53.8) | 206.1 (43.0) |
| Session 6 | 225.1 (56.7) | 216.9 (55.9) | 212.1 (48.4) |
| Errors - Drops Out of View | |||
| Session 1 | 1.3 (1.6) | 1.4 (1.6) | 1.2 (1.2) |
| Session 2 | 1.5 (1.4) | 1.6 (1.7) | 1.5 (1.7) |
| Session 3 | 1.2 (1.2) | 1.8 (1.3) | 1.3 (1.7) |
| Session 4 | 1.7 (2.0) | 0.8 (0.9) | 1.2 (1.3) |
| Session 5 | 1.3 (1.4) | 1.8 (1.9) | 0.7 (0.7) |
| Session 6 | 1.0 (1.1) | 1.2 (1.4) | 1.0 (1.7) |
| Errors - Improper Transfers a | |||
| Session 1 | 3.8 (12.9) | 3.1 (6.6) | 2.2 (3.3) |
| Session 2 | 4.8 (14.0) | 1.5 (2.4) | 1.2 (2.2) |
| Session 3 | 1.1 (2.1) | 1.0 (1.6) | 0.7 (1.4) |
| Session 4 | 0.7 (1.3) | 1.0 (1.5) | 1.4 (3.0) |
| Session 5 | 0.8 (2.6) | 0.7 (0.9) | 1.0 (2.4) |
| Session 6 | 2.0 (5.8) | 0.7 (0.9) | 1.1 (2.2) |
| Overall Scores | |||
| Session 1 | 123.4 (36.3) | 124.7 (45.0) | 130.5 (38.3) |
| Session 2 | 154.5 (54.2) | 158.6 (40.3) | 162.5 (54.1) |
| Session 3 | 171.1 (47.5) | 166.9 (51.7) | 178.1 (46.8) |
| Session 4 | 197.0 (57.1) | 190.9 (53.0) | 188.4 (51.6) |
| Session 5 | 206.4 (57.1) | 188.8 (55.2) | 202.6 (44.5) |
| Session 6 | 220.1 (58.7) | 212.5 (56.8) | 208.5 (52.3) |
All data reported as mean (SD).
Defined as transfers on a drop, with the wrong hand, or while resting the object.
As illustrated in Fig. 5a for the group means, and 5b for the individual subjects, scored performance improvements were generally monotonically over the blocks, with bM1 peaking at an average of 83% improvement, SMA at 80%, and sham at 64% on the sixth block. Linear mixed effects modeling on the overall score data demonstrated a main effect of learning across the six 20-min training blocks (t = 10.18, p < 0.001). A significant group by block interaction was observed: compared to the sham group, the bM1 group showed significantly greater improvement in overall scores over the six training blocks (t = 2.07, p = 0.039), whereas the difference between the SMA and sham groups did not differ significantly (t = 0.85, p = 0.400). In addition, the difference between the bM1 and SMA groups did not differ significantly (t = 1.22, p = 0.221). The conditional R2 of the model with the interaction term is 0.67, demonstrating that the model accounts for a substantial amount of the total variance.
Fig. 5.
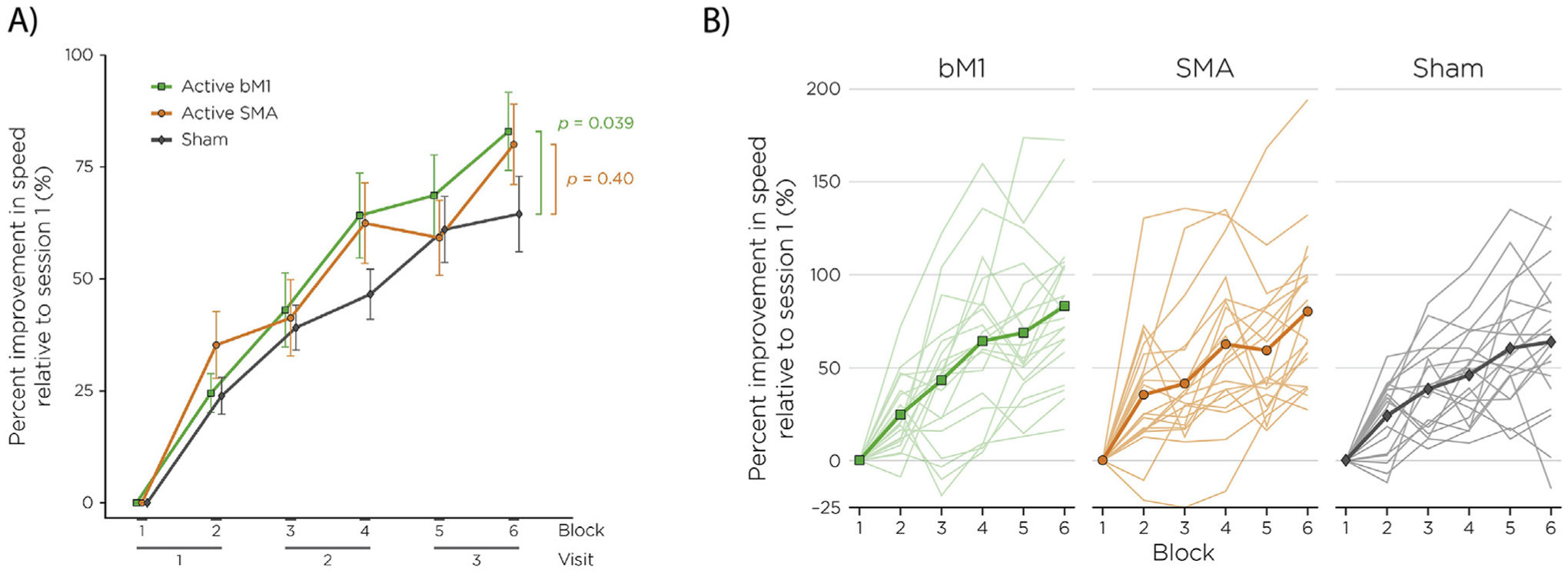
Learning curves for the bM1 (green), SMA (orange), and sham (grey) groups, reported as percent improvement in seconds normalized to block 1 performance. Whiskers denote standard error of the mean.
Pre- and post-test performance
Repeated-measures ANOVA calculated for the three groups revealed a significant difference between pre-test and post-test completion times (F (1,54) = 133.2, p < 0.001, ), with lower completion times at post-test. There was not, however, a significant main effect of training group (F (2,54) = 0.51, p = 0.6, ), and the group-by-block interaction was not statistically significant (F (2,54) = 1.40, p = 0.256; ) (Fig. 4). While differences were present in completion times at pre-test (bM1 166.8, SMA 159.2, sham 135.7 s), these were not statistically significant between the three groups (F (1,38) = 1.1, p = 0.29).
Fig. 4.
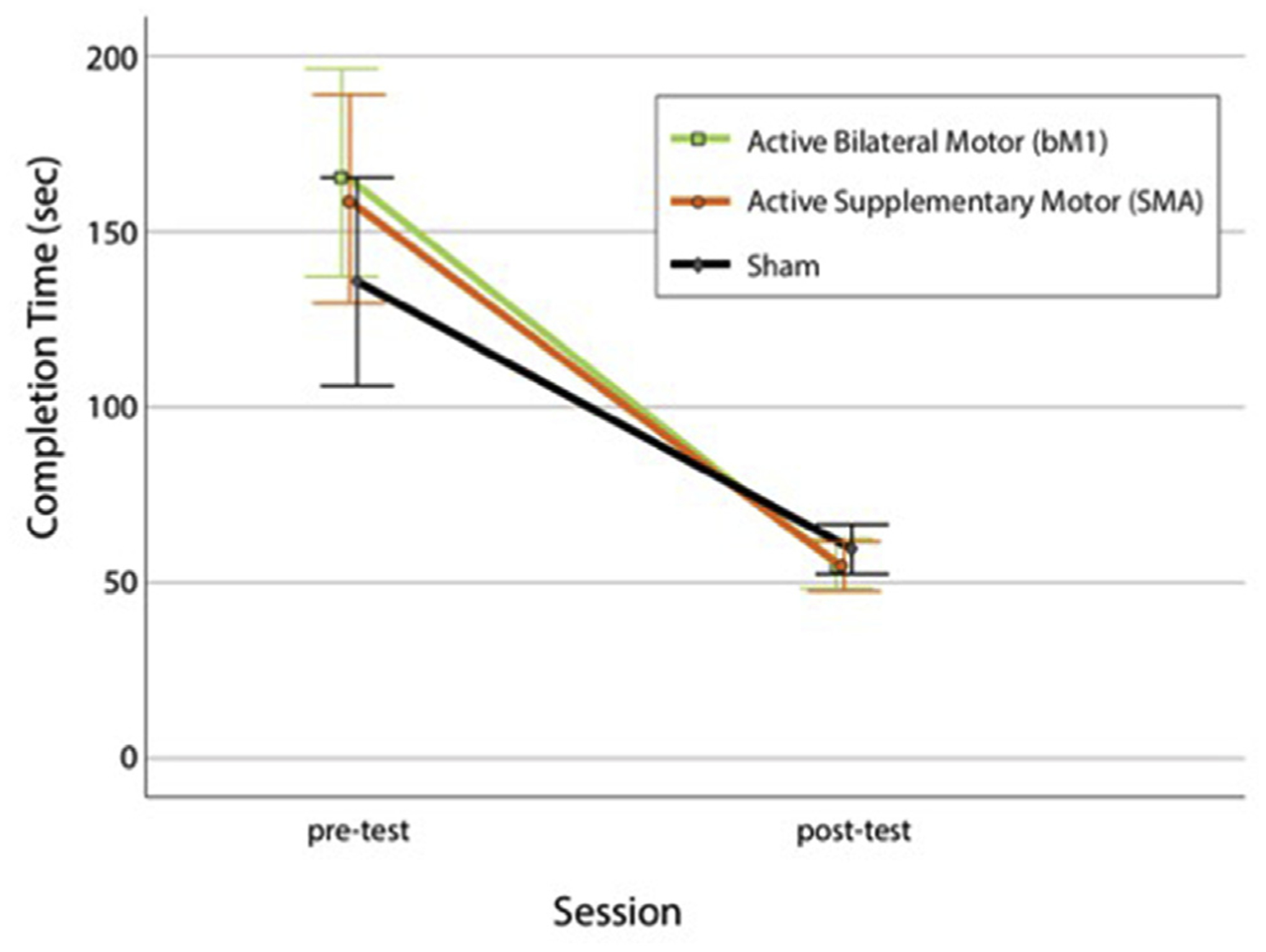
Mean completion times in seconds for the bM1 (green), SMA (orange), and sham (grey) groups at pre-test and post-test. Whiskers denote 95% confidence intervals.
Performance variability
There was no difference in performance metrics between male and female subjects. Similarly, no differences were observed in performance between individuals who self-reported as athletes, musicians, artists, or video gamers versus those reporting not to belong to these classifications (all p > 0.05). Age, servings of caffeine, and hours of sleep did not correlate with any of the task metrics (all p > 0.05).
Subjects with prior laparoscopic surgery experience had significantly better performance in the second (t = 4.1, p < 0.001) and third (t = 3.7, p = 0.001) 20-min training blocks compared to subjects without laparoscopic surgery experience. This analysis was limited by the small sample size with only four subjects endorsing previous laparoscopic surgical experience.
Compliance and adverse events
Eleven participants (15.5%) who were screened did not complete the entire study protocol. One participant did not consent while another was excluded at screening due to metal implants. Six participants withdrew due to scheduling conflicts. Three participants had intolerance of tDCS due to lightheadedness leading to early cessation of training and exclusion from analysis. Two of these three subjects were in an active tDCS group; one subject revealed she had nothing to eat throughout the day prior to the study session while the other subject disclosed that she ran 10 miles directly prior to the study session. The third intolerant subject was in the sham group and experienced dizziness during the initial ramp up and final ramp down during the second training block.
Discussion
We present results from a large, pre-registered, randomized, and sham-controlled study of the effectiveness of tDCS coupled with simultaneous surgical skills training, and the first study to contrast two different tDCS electrode configurations, bM1 and SMA, during bimanual surgical skill learning. Training coupled with active tDCS in the bM1 montage exhibited significantly greater improvements in the Fundaments of Laparoscopic Surgery Peg Transfer Task compared to sham tDCS. While the SMA configuration produced increased learning, these individuals also produced greater variability, and performance was not significantly different than for the sham group. The results from this pilot study demonstrate the potential of accelerating laparoscopic technical skill acquisition through the addition of bM1 tDCS. Given the safety and ease of administering tDCS along with these promising preliminary results, this novel technique could be a useful and practical augmentation to current technical skill training within surgical residency.
All general surgery residents utilize deliberate practice within the surgical simulation lab in order to pass the national, standardized FLS curriculum allowing for board eligibility. However, the amount of time allotted for training is restricted by the maximum 80-h work week. Our results suggest trainees could achieve faster and greater learning with the application of tDCS during practice. Subjects training with active tDCS reached approximately the same level of technical skill in roughly two thirds the time as compared with sham tDCS, based on performance levels following the fourth and sixth training blocks. This translates to about 40 min less total training time when using active tDCS, a savings that could likely be compounded when factoring in training on all five FLS tasks. In terms of absolute completion times, expert proficiency in this particular task is 48 s [43] underscoring the potential benefits of these improvements that result from relatively limited exposure. In addition to these improvements, the learning curves suggest there was increased skill acquisition from one training block to the next within the same study visit. Blocks were only separated by 5-min, whereas visits did not occur on the same day, suggesting that longer sessions and fewer training blocks might lead to optimal gains.
As discussed above, there are currently three prior studies utilizing tDCS within the field of surgery [28–30] and while these studies demonstrate positive gains due to active stimulation over sham, they are limited to unimanual tasks with unilateral montages that don’t reflect the breadth of manual skills needed for many surgical procedures. Therefore, to build upon this previous research, the current study tested two electrode configurations during a longer, three-visit protocol. We selected the SMA target due to its functional role in motor planning and execution, as well as bimanual coordination, while prior tDCS studies have also shown that stimulation of the SMA can increase performance on motor tasks [44]. Of note, while active anodal SMA stimulation did produce improvement on average, this condition produced considerable variability from participant-to-participant, contributing to the lack of group significance versus sham. The SMA electrode configuration includes cathodal placement over the frontal cortex which could theoretically modify subjects’ attention or cognitive control [45]. Therefore, future research may wish to test other cathodal placements that are less likely to activate cognitive processes that may interact with motor performance. For the bM1 configuration, the present results are consistent with the hypothesis that interhemispheric inhibition contributes to bimanual coordination [34], with the introduction of contralateral, opposite polarity stimulation leading to reduced IHI and greater bimanual coordination, as previous reported by Williams and colleagues [37]. A corollary explanation may be that the cathodal placement may have decreased neuronal excitability impairing performance, while anodal stimulation may have led to even greater excitation and increased performance improvements, leading to overall net positive behavioral gain. These possibilities, and the optimal electrode configuration, current intensity, duration of stimulation, and timing of stimulation relative to manual-skill training merits further investigation.
The current study, therefore, expands upon the findings of Ciechanski and colleagues to utilize the FLS Peg Transfer Task over multiple training sessions with substantially more accrued training time. Our methodology also included a training curriculum designed to prevent an early plateau in performance and allow enough training over time for the development of sufficient learning curves to determine the effect of our intervention. These studies together support the rationale to continue evaluation of tDCS as an augmentation to technical skill training within the surgical simulation lab. Additionally, this study utilized a tDCS device permitting complete double-blinding of subjects and investigators to active versus sham conditions. Most importantly, our methodology fulfills the majority of suggested criteria in a recent tDCS consensus article by Buch and colleagues [22] who presented a checklist of standards for tDCS studies promoting methodological reproducibility.
Limitations of this study include the presence of non-significant, but meaningful pre-test differences in performance between the active and sham groups. A baseline difference was not anticipated given the overall sample size of 60 individuals and randomized group assignments. While this baseline difference was unfortunate, it should be noted that it was based on only a single trial of the Peg Task for each participant. The promising results with greater improvement over time in the much longer six 20-min training blocks is stronger evidence for the application of active tDCS during laparoscopic skills training. However, to mitigate baseline differences, future studies may wish to adopt a stratified randomization approach. Moreover, future studies may wish to employ a retention test that when combined with stratified randomization may allow for inference as to whether the observed learning effects are limited to online behavioral modifications, or if they are retained over time.
As reported in the 2017 consensus review of tDCS on motor learning by Buch and colleagues [22], the average sample size in the 39 reviewed studies was 13.4 subjects per group. As such, the current study randomized 20 subjects into each group with the intent to substantially overshoot the typical sample size in the field. Moreover, using the effect size of the recent studies by Ciechanski and colleagues, which wasn’t available at time of this study design, intervention groups of 20 subjects are more than adequate to detect a difference in performance. Nonetheless, due to the common prevalence of reported (e.g. Ref. [46–48]) and unreported null results in tDCS studies, future research will want to continue to increase sample sizes and stress replication as an essential aspect of the experimental design.
The current study included both right handed (n = 53) and left handed (n = 7) subjects, but the anode electrode was always placed over the left hemisphere for all participants. Given the bimanual nature of the task future studies may wish to quantify the lateralized effects of anodal and cathodal stimulation or use unipolar motor system montages. Furthermore, it is possible that the presence of more self-reported video gamers in the bM1 group may have contributed to greater learning effects in this group, particularly in light of the advanced bimanual skills that are promoted in many video games. Additionally, participants were not queried after the study to determine if they realized that they were in the active or placebo groups, and therefore it is unclear if blinding was maintained throughout the study. Finally, the current study utilized a single bimanual task leaving the generalizability of this intervention yet to be determined. However, tDCS did provide a training advantage, and dexterity utilized in the Peg Transfer Task applies to other surgical skills including open, endoscopic, and robotic tasks.
Moving forward, future studies will be needed to replicate these findings and determine if similar benefits can be gained with surgical residents who have already achieved a higher baseline level of surgical knowledge and skill compared to the studied group. The utilization of individualized functional magnetic resonance imaging can also provide a more translational science approach to determining the optimal electrode placements for specific unimanual and bimanual tasks. Finally, translation of these findings to a commercially available tDCS device would also allow more feasible implementation into the surgical skills laboratory, allowing training without research supervision.
Conclusion
The results of this study suggest the acquisition of basic laparoscopic skill can be enhanced with the addition of active tDCS during training. This finding has the potential to accelerate general surgery training within the current climate of work hour restrictions and limited time for the development of technical skills within competency-based curricula.
Acknowledgements
The collaboration opportunity and funding for this research study was provided by Dr. Allan Kirk, Chair of the Duke University Department of Surgery. Additionally, our work would not have been possible without the Surgical Education and Activities Lab (SEAL) at Duke University and its staff including Jennie Phillips, Layla Triplett, and Sheila Peeler. We would also like to thank Dr. Ranjan Sudan for acting as our study physician.
Funding
MLC is supported by a National Institutes of Health T32 Training Grant with grant number T32HL069749. LGA is supported by United States Army Research Office award [W911NF-15-1-0390]. ZD and SHL are supported by the National Institute of Mental Health Intramural Research Program (ZIAMH002955).
Abbreviations
- bM1
bilateral primary motor cortex
- EMG
Electromyography
- FES
Fundamentals of Endoscopic Surgery
- FLS
Fundamentals of Laparoscopic Surgery
- IHI
Interhemispheric Inhibition
- SMA
supplementary motor area
- tDCS
transcranial direct current stimulation
Footnotes
Presentations
This study was presented in part as an oral presentation at the Innovative Techniques session at the 2018 Annual American College of Surgeons (ACS) Surgical Simulation Summit: An International Multi-Professional Meeting in Chicago, IL on March 16–17, 2018 as well as a poster presentation at the 2018 NYC Neuromodulation Conference and North American Neuromodulation Series (NANS) Summer Series in New York City, NY on August 24–26, 2018.
Disclosures
Drs. Cox, Deng, Beynel, Young, Lisanby, Migaly, and Appelbaum in addition to Ms. Palmer and Watts have no conflicts of interest or financial ties to disclose.
Dr. Sarah H. Lisanby, now at the National institute of Mental Health, contributed to this article while at Duke University, prior to joining NIMH. The views expressed are her own and do not necessarily represent the views of the National Institutes of Health or the United States Government.
Declaration of competing interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brs.2020.03.009.
References
- [1].Ahmed N, et al. A systematic review of the effects of resident duty hour restrictions in surgery: impact on resident wellness, training, and patient outcomes. Ann Surg 2014;259(6):1041–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lewis FR, Klingensmith ME. Issues in general surgery residency training–2012. Ann Surg 2012;256(4):553–9. [DOI] [PubMed] [Google Scholar]
- [3].Vassiliou MC, et al. FLS and FES: comprehensive models of training and assessment. Surg Clin 2010;90(3):535–58. [DOI] [PubMed] [Google Scholar]
- [4].Peters JH, et al. Development and validation of a comprehensive program of education and assessment of the basic fundamentals of laparoscopic surgery. Surgery 2004;135(1):21–7. [DOI] [PubMed] [Google Scholar]
- [5].Ritter EM, Scott DJ. Design of a proficiency-based skills training curriculum for the fundamentals of laparoscopic surgery. Surg Innovat 2007;14(2):107–12. [DOI] [PubMed] [Google Scholar]
- [6].Kirkman MA. Deliberate practice, domain-specific expertise, and implications for surgical education in current climes. J Surg Educ 2013;70(3):309–17. [DOI] [PubMed] [Google Scholar]
- [7].Stefanidis D, et al. Closing the gap in operative performance between novices and experts: does harder mean better for laparoscopic simulator training? J Am Coll Surg 2007;205(2):307–13. [DOI] [PubMed] [Google Scholar]
- [8].Nagendran M, et al. Laparoscopic surgical box model training for surgical trainees with no prior laparoscopic experience. Cochrane Database Syst Rev 2014;(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001;57(10): 1899–901. [DOI] [PubMed] [Google Scholar]
- [10].Rahman A, et al. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol 2013;591(10):2563–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fritsch B, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 2010;66(2): 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000;527(3): 633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nitsche MA, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 2007;97(4):3109–17. [DOI] [PubMed] [Google Scholar]
- [14].Goodwill AM, et al. Formation of cortical plasticity in older adults following tDCS and motor training. Front Aging Neurosci 2013;5:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hashemirad F, et al. The effect of anodal transcranial direct current stimulation on motor sequence learning in healthy individuals: a systematic review and meta-analysis. Brain Cognit 2016;102:1–12. [DOI] [PubMed] [Google Scholar]
- [16].Nitsche MA, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cognit Neurosci 2003;15(4):619–26. [DOI] [PubMed] [Google Scholar]
- [17].Reis J, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A 2009;106(5):1590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stagg CJ, et al. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 2011;49(5): 800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Madhavan S, Shah B. Enhancing motor skill learning with transcranial direct current stimulation–a concise review with applications to stroke. Front Psychiatr 2012;3:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Menezes IS, et al. Combined brain and peripheral nerve stimulation in chronic stroke patients with moderate to severe motor impairment. Neuromodulation: Technol Neural Interface 2018;21(2):176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pixa NH, Pollok B. Effects of tDCS on bimanual motor skills: a brief review. Front Behav Neurosci 2018;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Buch ER, et al. Effects of tDCS on motor learning and memory formation: a consensus and critical position paper. Clin Neurophysiol 2017;128(4): 589–603. [DOI] [PubMed] [Google Scholar]
- [23].Reis J, Fritsch B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr Opin Neurol 2011;24(6):590–6. [DOI] [PubMed] [Google Scholar]
- [24].Brunoni AR, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain stimulation 2012;5(3):175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bikson M, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul 2016;9(5):641–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Iyer MB, et al. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 2005;64(5):872–5. [DOI] [PubMed] [Google Scholar]
- [27].Poreisz C, et al. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull 2007;72(4–6):208–14. [DOI] [PubMed] [Google Scholar]
- [28].Ciechanski P, et al. Effects of transcranial direct-current stimulation on laparoscopic surgical skill acquisition. BJS open 2018;2(2):70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ciechanski P, et al. Effects of transcranial direct-current stimulation on neurosurgical skill acquisition: a randomized controlled trial. World Neurosurg 2017;108:876–84. [DOI] [PubMed] [Google Scholar]
- [30].Ciechanski P, et al. Electroencephalography correlates of transcranial direct-current stimulation enhanced surgical skill learning: a replication and extension study. Brain Res 2019;1725:146445. [DOI] [PubMed] [Google Scholar]
- [31].Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 2008;31:195–218. [DOI] [PubMed] [Google Scholar]
- [32].Morishita T, Uehara K, Funase K. Changes in interhemispheric inhibition from active to resting primary motor cortex during a fine-motor manipulation task. J Neurophysiol 2012;107(11):3086–94. [DOI] [PubMed] [Google Scholar]
- [33].Perez MA, Cohen LG. Interhemispheric inhibition between primary motor cortices: what have we learned? J Physiol 2009;587(Pt 4):725–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fling BW, Seidler RD. Task-dependent effects of interhemispheric inhibition on motor control. Behav Brain Res 2012;226(1):211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Furuya S, et al. Ceiling effects prevent further improvement of transcranial stimulation in skilled musicians. J Neurosci 2014;34(41):13834–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kuo YL, Kutch JJ, Fisher BE. Relationship between interhemispheric inhibition and dexterous hand performance in musicians and non-musicians. Sci Rep 2019;9(1):11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Williams JA, Pascual-Leone A, Fregni F. Interhemispheric modulation induced by cortical stimulation and motor training. Phys Ther 2010;90(3):398–410. [DOI] [PubMed] [Google Scholar]
- [38].Jenkins IH, et al. Motor sequence learning: a study with positron emission tomography. J Neurosci 1994;14(6):3775–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee D, Quessy S. Activity in the supplementary motor area related to learning and performance during a sequential visuomotor task. J Neurophysiol 2003;89(2):1039–56. [DOI] [PubMed] [Google Scholar]
- [40].Brinkman C Lesions in supplementary motor area interfere with a monkey’s performance of a bimanual coordination task. Neurosci Lett 1981;27(3): 267–70. [DOI] [PubMed] [Google Scholar]
- [41].Hupfeld KE, Ketcham CJ, Schneider HD. Transcranial direct current stimulation (tDCS) to the supplementary motor area (SMA) influences performance on motor tasks. Exp Brain Res 2017;235(3):851–9. [DOI] [PubMed] [Google Scholar]
- [42].Harris PA, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Scott DJ EM R. Fundamentals of Laparoscopic Surgery technical skills proficiency-based training curriculum. 2018-05-20]; Available from, https://www.flsprogram.org/wp-content/uploads/2014/02/Proficiency-Based-Curriculum-Word-File-updated-February-2014.pdf; 2014.
- [44].Vollmann H, et al. Anodal transcranial direct current stimulation (tDCS) over supplementary motor area (SMA) but not pre-SMA promotes short-term visuomotor learning. Brain Stimul 2013;6(2):101–7. [DOI] [PubMed] [Google Scholar]
- [45].Imburgio MJ, Orr JM. Effects of prefrontal tDCS on executive function: methodological considerations revealed by meta-analysis. Neuropsychologia 2018;117:156–66. [DOI] [PubMed] [Google Scholar]
- [46].Horvath JC, Forte JD, Carter O. Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial direct current stimulation (tDCS). Brain Stimul 2015;8(3):535–50. [DOI] [PubMed] [Google Scholar]
- [47].Medina J, Cason S. No evidential value in samples of transcranial direct current stimulation (tDCS) studies of cognition and working memory in healthy populations. Cortex 2017;94:131–41. [DOI] [PubMed] [Google Scholar]
- [48].Thibaut A, et al. Understanding negative results in tDCS research: the importance of neural targeting and cortical engagement. Front Neurosci 2017;11:707. [DOI] [PMC free article] [PubMed] [Google Scholar]


