Abstract
Early life stress (ELS) adversely affects the brain and is commonly associated with the etiology of mental health disorders, like depression. In addition to the mood-related symptoms, patients with depression show dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, increased peripheral inflammation, and structural brain alterations. Although the underlying causes are unknown, polymorphisms in the FK506-binding protein 5 (FKBP5) gene, a regulator of glucocorticoid receptor (GR) activity, interact with childhood adversities to increase vulnerability to depressive disorders. We hypothesized that high FKBP5 protein levels combined with early life stress (ELS) would alter the HPA axis and brain, promoting depressive-like behaviors. To test this, we exposed males and females of a mouse model overexpressing FKBP5 in the brain (rTgFKBP5 mice), or littermate controls, to maternal separation for 14 days after birth. Then, we evaluated neuroendocrine, behavioral, and brain changes in young adult and aged mice. We observed lower basal corticosterone (CORT) levels in rTgFKBP5 mice, which was exacerbated in females. Aged, but not young, rTgFKBP5 mice showed increased depressive-like behaviors. Moreover, FKBP5 overexpression reduced hippocampal neuron density in aged mice, while promoting markers of microglia expression, but these effects were reversed by ELS. Together, these results demonstrate that high FKBP5 affects basal CORT levels, depressive-like symptoms, and numbers of neurons and microglia in the hippocampus in an age-dependent manner.
Keywords: Hippocampus, FKBP5, Early life stress, Maternal separation, Glucocorticoids, Stress, Aging, Microglia, Neurogenesis
Highlights
-
•
High FKBP5 reduces basal corticosterone levels in mice, especially in females.
-
•
ELS prevents FKBP5-induced susceptibility to depressive-like behavior in aged mice.
-
•
FKBP5 overexpression reduces hippocampal neuron density in aged mice, while increasing microglial markers.
Abbreviations
- ANOVA
Analysis of variance
- CamKII
Ca2+/calmodulin-dependent protein kinase II
- CORT
Cortisol, corticosterone
- DCX
Doublecortin
- ELISA
Enzyme-linked immunosorbent assay
- ELS
Early life stress
- FKBP5
FK506-binding protein 5/FK506-binding protein 51
- GFAP
Glial fibrillary acidic protein
- GLM
General linear model
- GR
Glucocorticoid receptor
- HPA
Hypothalamic-pituitary-adrenal
- HPC
Hippocampus
- Iba1
Ionized calcium binding adaptor molecule 1
- IHC
Immunohistochemistry
- LPS
Lipopolysaccharide
- OF
Open field
- P1
Postnatal Day 1
- P21
Postnatal Day 21
- PTSD
Post-traumatic stress disorder
- SEM
Standard error of the mean
- TST
Tail Suspension Test
1. Introduction
In recent years, mood and stress-related disorders have received more attention due to their prevalence and growing incidence worldwide (Kessler et al., 2010, 2005; Lim et al., 2018; Substance Abuse and Mental Health Services Administration, 2017). Despite each disorder having distinct features, there is a high overlap of behavioral and physiological symptoms among them. For example, individuals with anxiety and depressive disorders may experience persistent feelings of anxiety, difficulty concentrating, fatigue, and sleep problems (Uher et al., 2014). Likewise, these disorders are influenced by common genetic and environmental factors shaping an individual’s susceptibility, coping behavior, and neurobiological response (Blanco et al., 2014; Gillespie et al., 2009). The hypothalamic-pituitary-adrenal (HPA) axis is a key player that controls the stress response to environmental stimuli, preserving system homeostasis. This balance is mainly mediated by negative feedback from glucocorticoids, specifically cortisol in humans and corticosterone in rodents (CORT) (Sapolsky et al., 1984). CORT binds to glucocorticoid receptors (GR) located in the hypothalamus and hippocampus to inhibit neuroendocrine responses; this inhibition protects the body from prolonged stress effects (Herman et al., 2016; Kovacs and Makara, 1988).
HPA axis dysfunction has been linked to multiple mental health disorders, including depression (Juruena et al., 2018). Patients with depression have persistently high CORT levels and an exaggerated response in the combined dexamethasone suppression/corticotrophin-releasing hormone stimulation (DEX/CRH) test (Schüle et al., 2009; Spijker and van Rossum, 2012). Depressed individuals also show higher levels of cytoplasmic GR correlating with higher levels of the 51 kDa FK506-binding protein (FKBP51, FKBP5) (Lukic et al., 2015). FKBP5 is an Hsp90 co-chaperone that regulates GR sensitivity by reducing GR import into the nucleus (Davies et al., 2002; Wochnik et al., 2004). The use of antidepressants as well as FKBP5 knockdown or FKBP5 removal from the GR complex each restore the receptor’s affinity for CORT, facilitating GR-nuclear transactivation (Pariante et al., 2001; Sabbagh et al., 2018; Tatro et al., 2009). Thus, various studies have suggested that CORT resistance may be attributed to malfunction of GR itself or to the inhibitory action of FKBP5 on GR.
Notably, FKBP5 polymorphisms have been linked with increased susceptibility to stress, HPA axis function, GR resistance, and major depression, as well as to antidepressant treatment response (Binder et al., 2004; Ferrer et al., 2018; Menke et al., 2013; Roberts et al., 2015; Santarelli et al., 2017; Scheuer et al., 2016; Stamm et al., 2016). Homozygous carriers of the T allele in FKBP5 rs1360780 have shown higher expression of FKBP5 protein following stress, which implies a functional role of this variant that may contribute to greater GR inhibition through high protein levels (Binder et al., 2004). More recent findings support the role of FKBP5 polymorphisms and their interactions with the environment to increase the risk for mood- and stress-related disorders (Buchmann et al., 2014; Klengel et al., 2013; Lahti et al., 2016; Zimmermann et al., 2011). In addition to gene-stress interactions triggered during early life, mental and biological effects from these stressful experiences can also manifest throughout the lifespan (Binder et al., 2008; Klengel et al., 2013; Kohrt et al., 2015; Santarelli et al., 2017). For example, FKBP5 polymorphisms interact with early life stressors, such as childhood trauma, conferring higher risk for depression in adolescence and adulthood (Appel et al., 2011; Kohrt et al., 2015; Lahti et al., 2016; Zimmermann et al., 2011). In young adult mice, early life stress (ELS) and high FKBP5 interact to induce anxiety-like behavior and changes in the molecular pathways involved in cell survival and growth in the hippocampus (Criado-Marrero et al., 2019).
The hippocampus is highly susceptible to stress due to the high concentration of GRs (Ladd et al., 2004; Magariños et al., 1996; Sapolsky, 1985; Wei et al., 2015). This area is responsible for inhibiting the HPA axis after stress and for processing emotional memories and responses (Sapolsky et al., 1984; Snyder et al., 2011). Moreover, ELS can alter hippocampal development across the lifespan (Brunson et al., 2005; Réus et al., 2019; Wei et al., 2015). Some stress-related adverse effects in this area include atrophy of dendrites, reduced synaptic density and inhibition of neurogenesis (Brunson et al., 2001; Dioli et al., 2019; Lupien et al., 2018). ELS exposure can further affect hippocampal development by altering non-neuronal cells, like astrocytes and microglia (Delpech et al., 2016; Réus et al., 2019; Roque et al., 2016). Decreased astrocyte and increased microglia cell counts have been reported in multiple studies investigating depressive-like symptoms in animals and depression in humans (Rajkowska et al., 2001; Rajkowska and Stockmeier, 2013; Réus et al., 2019; Rohan Walker et al., 2013). These cells play an essential role in stress-induced pro-inflammatory and cytotoxic responses, which affect neuronal differentiation and neurogenesis (Aarum et al., 2003; Koo et al., 2010; de Lucia et al., 2016). Interestingly, FKBP5 mRNA and protein expression in the hippocampus are induced by chronic stress and exogenous CORT, supporting the relationship between FKBP5 and stress (Lee et al., 2010; Scharf et al., 2011; Wagner et al., 2012). High-risk FKBP5 variants have also been associated with reduced gray matter and hippocampal volume in patients with depression who were previously exposed to ELS (Mikolas et al., 2019; Tozzi et al., 2018; Zobel et al., 2010). Therefore, malfunction or imbalance of FKBP5 and stress-induced effects on the hippocampus may each contribute to cell atrophy and reduction in hippocampal volume in depression (Campbell et al., 2004; Han et al., 2017; Lucassen et al., 2001; Vythilingam et al., 2002).
We previously showed that the interaction of ELS and FKBP5 increases anxiety-like levels in young mice, inducing changes in the hippocampus (Criado-Marrero et al., 2019). Due the high comorbidity of anxiety and depression, here we investigated whether this interaction, ELS x FKBP5, promotes a depressive-like behavior and affects animals differently over time. To test this, transgenic mice overexpressing FKBP5 in the brain, rTgFKBP5, were exposed to ELS using the maternal separation paradigm. We compared behavioral, neuroendocrine and hippocampal changes across two ages, young and aged adulthood. These timepoints were chosen because of the high prevalence of depression during early and middle adulthood (Lim et al., 2018). Moreover, given the genetic association between FKBP5 variants and reduced hippocampal volume with depression (Zobel et al., 2010), we used immunohistochemical and stereological analyses to evaluate whether high FKBP5 x ELS promotes hippocampal neuronal loss and neuroinflammation.
2. Materials and methods
2.1. Animal subjects
All mice were bred and maintained in our institutional vivarium under standard conditions on a 12 h light/dark cycle (light cycle beginning at 06:00 and ending at 18:00) A maximum of 5 mice were housed per cage with free access to food and water. All animal experiments were carried out accordingly with the NIH Guide for the Care and Use of Laboratory Animals and approved by the USF Institutional Animal Care and Use Committee (IACUC).
Complete details about the construction of the rTgFKBP5 vector and generation of the rTgFKBP5 mice has been previously described (Blair et al., 2019). Experiments were carried out using 3 genotypes of mice: rTgFKBP5 (contains a single insertion of human FKBP5 gene and tTA on the CamKIIα promoter), tTA (expresses the tet-transactivator on the CamKIIα promoter), and wild-type (WT). We used tTA mice, in addition to WT, because they are a suitable control for rTgFKBP5 mice, since they express the tetracycline-off transactivator driven by the CamKIIα promoter but lack the FKBP5 insertion. Genotypes were confirmed by PCR amplifying DNA from ear tissue using Qiaxcel (Qiagen, Valencia, CA, USA) to analyze the PCR product.
2.2. Early life stress model
We used the maternal separation paradigm as an early life stressor (ELS) to study the interaction of the FKBP5 gene and the environment in both young and adult mice, as we have done previously (Criado-Marrero et al., 2019). The protocol consisted of relocating the dams to new cages in a separate room for 3 h daily during the morning. A heating pad under the cage was used to maintain ambient temperature during the separation time. This procedure was repeated for 14 consecutive days starting on postnatal day 1 (P1). The non-stress control group remained undisturbed with their dams until they were weaned (P21). Age groups at the time of behavioral testing corresponded to young (60 days-old) and aged (210 days-old) adulthood. These mice were divided among 12 groups (Fig. 1A and B) to examine behavioral and molecular changes induced by environmental factors (stress and non-stress), genotype (WT, tTA and rTgFKBP5), and age (young and aged). The interactions among these factors were also investigated. The young group consisted of non-stressed control (WT = 11, tTA = 10, rTgFKBP5 = 9) and ELS mice (WT = 10, tTA = 10, rTgFKBP5 = 10), while the aged group consisted of non-stressed control (WT = 10, tTA = 11, rTgFKBP5 = 10) and ELS mice (WT = 10, tTA = 11, rTgFKBP5 = 10).
Fig. 1.
Experimental procedure. (A) Timeline for early life stress protocol, behavioral experiment, and collection of specimens. Maternal separation was used as the early life stressor, ELS, were pups were relocated in a separate room from their dams for 3 h daily for 14 consecutive days. (B) Total number of animals per group for each genotype, stress condition, and age period. A balanced number of males and females were used per condition. WT = wild-type, tTA = CamKIIα-tTA, rTgFKBP5 = FKBP5 overexpressing mice, M = males, F = females.
2.3. Open field test (OF)
Over the course of forty days, mice were put through a behavioral battery to assess cognition and affective-like symptoms. Only those implicated in depressive and fear phenotypes are described here. The OF was used to measure locomotor activity. It consisted of placing a mouse in the center of a square gray Plexiglas box. Individually, mice were allowed to freely explore the arena for 10 min while being video recorded (ANY-Maze software; www.anymaze.com). The chamber was cleaned with 70% ethanol between trials. The total distance travelled was recorded to evaluate locomotor differences.
2.4. Tail suspension test (TST)
For TST, each mouse was suspended by its tail with adhesive tape from a lever and video recorded for 6-min using the ANY-Maze software. An experimenter blinded to animal assignments measured the total time each mouse spent immobile during each session.
2.5. Associative fear conditioning
For this experiment, we focused only on the working memory component of fear conditioning by examining behavior during training. Mice were placed in a closed chamber with Plexiglas walls and a metal grid floor and allowed 2 min of exploration (adaptation period). At 2 min, an auditory cue (tone) was presented at 70 dB for 10 s. After 1 min, a total of four paired tone-foot shock pairings (10 s tone, 70 dB; 1 s shock, 0.5 mA) were administered with 60 s inter-stimulus intervals. Animals were video recorded, and a blinded experimenter calculated the time animals spent freezing (immobile) for the first 30 s after each tone-shock presentation. Percent time freezing was used as an index of associative learning performance.
2.6. Tissue collection and immunohistochemical (IHC) staining
This procedure has been previously described (Criado-Marrero et al., 2019). Briefly, after behavioral training, mice were euthanized with a Somnasol overdose at 150 (young) or 300 (aged) days of age. Following transcardial perfusion with 0.9% saline, brains were collected and fixed in 4% paraformaldehyde. Following cryopreservation, tissue was sectioned into horizontal slices at 25 and 50 μm for IHC and stereological analyses, respectively. Free-floating sections were stained as previously described (Blair et al., 2013; Dickey et al., 2009) using the following primary antibodies: anti-Ki-67 (Cell Signaling, Danvers, Massachusetts, USA, CS-9449; 1:1000), anti-DCX (Cell Signaling, Danvers, Massachusetts, USA,CS-4604; 1:300), anti-GFAP (Millipore, Burlington, MA, USA, MAB360, 1:10,000), anti-Iba1 (WAKO, Richmond, VA, USA, 016-26461, 1:10,000). Images were taken at 20× using a Zeiss AxioScan.Z1 slide scanner (Zeiss, Oberkochen, Germany) and tissue segmentation was performed using the NearCYTE software (www.nearcyte.org).
2.7. CORT ELISA assay
We collected blood from the submandibular area ten days before behavioral testing. Blood samples were taken during the morning between 10:00 h and 12:00 h within a 3-min period to prevent disturbances (Vahl et al., 2005). Samples from five animals were excluded in the analysis because of noticeable stress during blood extraction. Samples were centrifuged for serum separation (SAI Infusion Technologies, Lake Villa, IL, #PMTS-SG-1.1) and stored at −80 °C until analyzed. CORT levels were quantified with an enzyme-linked immunosorbent assay (ELISA) kit (Enzo Life Sciences, Farmingdale NY, USA, # ADI-901-097) according to the manufacturer’s instructions using 10 μL of serum.
2.8. Statistical analysis
Total distance travelled in the OF test was measured using ANY-Maze. Data distributions were tested using Shapiro-Wilk’s test and equality of variances using Levene’s test. Behavioral data were analyzed by multifactorial analysis of variance (ANOVA) using the general linear model (GLM) procedure in the SPSS Statistics software (v25). GLM repeated measures was used for associative fear conditioning stimuli. When there was a group difference, GLM was followed by Bonferroni multiple comparison test. Three- and two-way ANOVA was used to analyze interactions between variables. Significant difference was considered if p < 0.05. Data are presented as mean ± SEM. Main and interactive effects by genotype, ELS and age on dependent variables (immobility, distance travelled, fear expression, and neuronal number) are described in Table 1. Generalized linear models with a Huber-White correction (robust error estimator) were used to analyze CORT and tissue expression (Ki-67, DCX, GFAP and Iba1) as equal variances were not achieved even after log transformation of data. When statistically significant, analysis was followed by Bonferroni post hoc test. Interactions and main effects are described in Table 1 (CORT) and Table 2 (tissue).
Table 1.
Summary of statistical analyses of CORT levels, behavioral tasks, and hippocampal stereology.
| Figure | Measured condition | Groups analyzed | Factor | W or F statistic and p-value |
|---|---|---|---|---|
| 2A | Serum CORT | All groups | Genotype | W(2, 113) = 33.47, p < 0.001 |
| ELS | W(1, 114) = 8.73, p = 0.003 | |||
| Sex | W(1, 114) = 17.02, p < 0.001 | |||
| Age | W(1, 114) = 6.43, p = 0.011 | |||
| Age/ELS | W(1, 112) = 15.29 p < 0.001 | |||
| Gen/ELS | W(2, 110) = 10.56, p = 0.005 | |||
| Gen/ELS/Age | W(2, 104) = 0.44, p = 0.799 | |||
| Gen/ELS/Sex | W(2, 104) = 9.017, p = 0.01 | |||
| Gen/ELS/Age/Sex | W(2, 92) = 1.33, p = 0.514 | |||
| 2B | Serum CORT | Young Males | Genotype | W(2, 27) = 11.37, p = 0.003 |
| ELS | W(1, 28) = 3.96, p = 0.046 | |||
| Gen/ELS | W(2, 24) = 1.411, p = 0.494 | |||
| Aged Males | Genotype | W(2, 27) = 1.707, p = 0.426 | ||
| ELS | W(1, 28) = 4.83 p = 0.028 | |||
| Gen/ELS | F(2, 24) = 0.282, p = 0.869 | |||
| 2C | Young Females | Genotype | F(2, 25) = 41.93, p < 0.001 | |
| ELS | F(1, 26) = 30.66, p < 0.001 | |||
| Gen/ELS | F(2, 22) = 12.24, p = 0.002 | |||
| Aged Females | Genotype | F(2, 25) = 1.613, p = 0.446 | ||
| ELS | F(1, 26) = 0.105, p = 0.745 | |||
| Gen/ELS | F(2, 22) = 6.53, p = 0.038 | |||
| 3A | TST: Total immobile time | All groups | Genotype | F(2, 120) = 15.5, p < 0.001 |
| ELS | F(1, 121) = 0.004, p = 0.952 | |||
| Sex | F(1, 121) = 2.50, p = 0.117 | |||
| Age | F(1, 121) = 129.7, p < 0.001 | |||
| Age/ELS | F(1, 119) = 4.03, p = 0.047 | |||
| Gen/ELS | F(2, 117) = 2.54, p = 0.08 | |||
| Gen/ELS/Age | F(2, 111) = 0.85, p = 0.429 | |||
| Gen/ELS/Sex | F(2, 111) = 1.75, p = 0.178 | |||
| Gen/ELS/Age/Sex | F(2, 99) = 0.78, p = 0.458 | |||
| 3B | OF: Total distance travelled | All groups | Genotype | F(2,120) = 2.59, p = 0.08 |
| ELS | F(1, 121) = 5.31, p = 0.023 | |||
| Sex | F(1, 121) = 11.40, p = 0.001 | |||
| Age | F(1, 121) = 14.07, p < 0.001 | |||
| Age/ELS | F(1, 119) = 2.43, p = 0.122 | |||
| Gen/ELS | F(2, 117) = 0.06, p = 0.934 | |||
| Gen/ELS/Age | F(2, 111) = 0.19, p = 0.820 | |||
| Gen/ELS/Sex | F(2, 111) = 0.08, p = 0.917 | |||
| Gen/ELS/Age/Sex | F(2, 99) = 1.75 p = 0.178 | |||
| Supporting data for 3B | OF: Total distance travelled | Young Males | Genotype | F(2, 28) = 0.49, p = 0.615 |
| ELS | F(1, 29) = 1.77, p = 0.196 | |||
| Gen/ELS | F(2, 25) = 0.13, p = 0.871 | |||
| Aged Males | Genotype | F(2, 27) = 1.28, p = 0.297 | ||
| ELS | F(1, 28) = 0.11, p = 0.916 | |||
| Gen/ELS | F(2, 24) = 0.76, p = 0.476 | |||
| Young Females | Genotype | F(2, 28) = 1.07, p = 0.359 | ||
| ELS | F(1, 29) = 2.83, p = 0.105 | |||
| Gen/ELS | F(2, 25) = 0.55, p = 0.583 | |||
| Aged Females | Genotype | F(2, 31) = 1.44, p = 0.253 | ||
| ELS | F(1, 22) = 1.97, p = 0.171 | |||
| Gen/ELS | F(2, 28) = 1.479, p = 0.246 | |||
| 3C | TST: Total immobile time | Young Males | Genotype | F(2, 28) = 6.09, p = 0.007 |
| ELS | F(1, 29) = 0.36, p = 0.552 | |||
| Gen/ELS | F(2, 25) = 0.32, p = 0.72 | |||
| Aged Males | Genotype | F(2, 27) = 3.51, p = 0.04 | ||
| ELS | F(1, 28) = 0.84, p = 0.36 | |||
| Gen/ELS | F(2, 24) = 1.26, p = 0.30 | |||
| 3D | Young Females | Genotype | F(2, 28) = 6.43, p = 0.006 | |
| ELS | F(1, 29) = 3.27, p = 0.08 | |||
| Gen/ELS | F(2, 25) = 2.98, p = 0.07 | |||
| Aged Females | Genotype | F(2, 31) = 5.32, p = 0.01 | ||
| ELS | F(1, 32) = 0.86, p = 0.35 | |||
| Gen/ELS | F(2, 28) = 1.02, p = 0.37 | |||
| 4A-D | Fear expression (freezing), 4 conditioning tones | Repeated measures, All groups | Genotype | F(2, 120) = 15.44, p < 0.001 |
| ELS | F(1, 121) = 0.021, p = 0.886 | |||
| Sex | F(1, 121) = 5.61, p = 0.02 | |||
| Age | F(1, 121) = 24.63, p < 0.001 | |||
| Age/ELS | F(1, 119) = 10.26, p = 0.018 | |||
| Gen/ELS | F(2, 117) = 0.44, p = 0.64 | |||
| Gen/ELS/Age | F(2, 111) = 1.25, p = 0.289 | |||
| Gen/ELS/Sex | F(2, 111) = 0.29, p = 0.74 | |||
| Gen/ELS/Age/Sex | F(2, 99) = 0.57, p = 0.565 | |||
| 4A-D | Fear expression | Young Males | Genotype | F(2, 28) = 8.029, p = 0.002 |
| ELS | F(1, 29) = 0.013, p = 0.91 | |||
| Gen/ELS | F(2, 25) = 0.181, p = 0.83 | |||
| Aged Males | Genotype | F(2, 27) = 1.413, p = 0.26 | ||
| ELS | F(1, 28) = 0.511, p = 0.48 | |||
| Gen/ELS | F(2, 24) = 2.665, p = 0.09 | |||
| Young females | Genotype | F(2, 28) = 3.94, p = 0.03 | ||
| ELS | F(1, 29) = 0.209, p = 0.65 | |||
| Gen/ELS | F(2, 25) = 0.008, p = 0.99 | |||
| Aged females | Genotype | F(2, 31) = 4.503, p = 0.02 | ||
| ELS | F(1, 32) = 0.678, p = 0.41 | |||
| Gen/ELS | F(2, 28) = 0.140, p = 0.87 | |||
| 6B | IHC: HPC NeuN/CV | All groups | Genotype | F(2, 55) = 2.72, p = 0.076 |
| ELS | F(1, 56) = 1.01, p = 0.320 | |||
| Age | F(1, 56) = 4.78, p = 0.034 | |||
| Age/ELS | F(1, 54) = 8.155, p = 0.006 | |||
| Gen/ELS | F(2, 52) = 0.09, p = 0.910 | |||
| Gen/ELS/Age | F(2, 46) = 1.61, p = 0.211 | |||
| 6C | IHC: HPC Volume | All groups | Genotype | F(2, 45) = 0.34, p = 0.713 |
| ELS | F(1, 56) = 0.002, p = 0.968 | |||
| Age | F(1, 56) = 0.34, p = 0.563 | |||
| Age/ELS | F(1, 54) = 0.002, p = 0.968 | |||
| Gen/ELS | F(2, 52) = 0.71, p = 0.497 | |||
| Gen/ELS/Age | F(2, 46) = 0.04, p = 0.959 |
CORT: corticosterone, ELS: Early life stress, GEN: Genotype, TST: tail suspension test, OF: open field, IHC: immunohistochemistry, HPC: hippocampus, NeuN/CV: neuronal nuclear antigen/cresyl violet, W: Wald statistic, F: F statistic. The CORT data followed a normal distribution with unequal variances. This was analyzed using linear regression with Huber-White correction followed by Bonferroni post hoc test as part of the generalized linear model in SPSS. All other group data was analyzed by factorial analysis of variance (ANOVA) using the general linear model (GLM) procedure in the SPSS Statistics software.
Table 2.
Summary of statistical analyses of neurogenesis and neuroinflammatory markers.
| Figure | Measured condition | Groups analyzed | Factor | Wald Chi-Square | N | df | p-value |
|---|---|---|---|---|---|---|---|
| 5A-B | Ki-67 | All groups | Genotype | 8.687 | 61 | 2 | .013 |
| ELS | 2.279 | 61 | 1 | .131 | |||
| Age | 54.058 | 61 | 1 | .000 | |||
| Age/ELS | 0.253 | 61 | 1 | .615 | |||
| Age/Genotype | 4.929 | 61 | 2 | .085 | |||
| Gen/ELS | 10.367 | 61 | 2 | .006 | |||
| Gen/ELS/Age | 4.527 | 61 | 2 | .104 | |||
| 5A-B | Ki-67 | Young | Genotype | 1.028 | 31 | 2 | .598 |
| ELS | 1.563 | 31 | 1 | .211 | |||
| Gen/ELS | 1.582 | 31 | 2 | .453 | |||
| Aged | Genotype | 8.261 | 30 | 2 | .016 | ||
| ELS | 1.208 | 30 | 1 | .272 | |||
| Gen/ELS | 8.857 | 30 | 2 | .012 | |||
| 5C-D | DCX | All groups | Genotype | 0.336 | 63 | 2 | .845 |
| ELS | 0.462 | 63 | 1 | .497 | |||
| Age | 58.069 | 63 | 1 | .000 | |||
| Age/ELS | 0.000 | 63 | 1 | .991 | |||
| Age/Genotype | 0.126 | 63 | 2 | .939 | |||
| Gen/ELS | 1.841 | 63 | 2 | .398 | |||
| Gen/ELS/Age | 0.580 | 63 | 2 | .748 | |||
| 5C-D | DCX | Young | Genotype | 10.981 | 32 | 2 | .004 |
| ELS | 5.184 | 32 | 1 | .023 | |||
| Gen/ELS | 4.426 | 32 | 2 | .109 | |||
| Aged | Genotype | 0.030 | 31 | 2 | .985 | ||
| ELS | 0.114 | 31 | 1 | .736 | |||
| Gen/ELS | 1.145 | 31 | 2 | .564 | |||
| 7A-B | GFAP | All groups | Genotype | 23.902 | 59 | 2 | .000 |
| ELS | 1.027 | 59 | 1 | .311 | |||
| Age | 106.628 | 59 | 1 | .000 | |||
| Age/ELS | 10.713 | 59 | 1 | .001 | |||
| Age/Genotype | 4.354 | 59 | 2 | .113 | |||
| Gen/ELS | 10.502 | 59 | 2 | .005 | |||
| Gen/ELS/Age | 2.487 | 59 | 2 | .288 | |||
| 7A-B | GFAP | Young | Genotype | 25.559 | 28 | 2 | .000 |
| ELS | 3.472 | 28 | 1 | .062 | |||
| Gen/ELS | 4.079 | 28 | 2 | .130 | |||
| Aged | Genotype | 7.341 | 31 | 2 | .025 | ||
| ELS | 7.265 | 31 | 1 | .007 | |||
| Gen/ELS | 8.849 | 31 | 2 | .012 | |||
| 7C-D | Iba1 | All groups | Genotype | 0.363 | 60 | 2 | .834 |
| ELS | 1.104 | 60 | 1 | .293 | |||
| Age | 14.402 | 60 | 1 | .000 | |||
| Age/ELS | 1.166 | 60 | 1 | .280 | |||
| Age/Genotype | 0.472 | 60 | 2 | .790 | |||
| Gen/ELS | 11.781 | 60 | 2 | .003 | |||
| Gen/ELS/Age | 11.883 | 60 | 2 | .003 | |||
| 7C-D | Iba1 | Young | Genotype | 4.552 | 30 | 2 | .103 |
| ELS | 0.724 | 30 | 1 | .395 | |||
| Gen/ELS | 0.276 | 30 | 2 | .871 | |||
| Aged | Genotype | 0.416 | 30 | 2 | .812 | ||
| ELS | 1.135 | 30 | 1 | .287 | |||
| Gen/ELS | 11.834 | 30 | 2 | .003 |
DCX: doublecortin, GFAP: Glial fibrillary acidic protein, Iba1: Ionized calcium binding adaptor molecule 1, ELS: early life stress, Gen: genotype, df: degrees of freedom, N: number of samples. The relative intensity expression of each protein (Ki-67, DCX, GFAP, Iba1) was analyzed by Generalized Linear Models with Huber-White correction (robust error estimator) using the SPSS Statistics software. When significant, this analysis was followed with a Bonferroni correction post hoc test.
3. Results
3.1. High FKBP5 lowers CORT levels
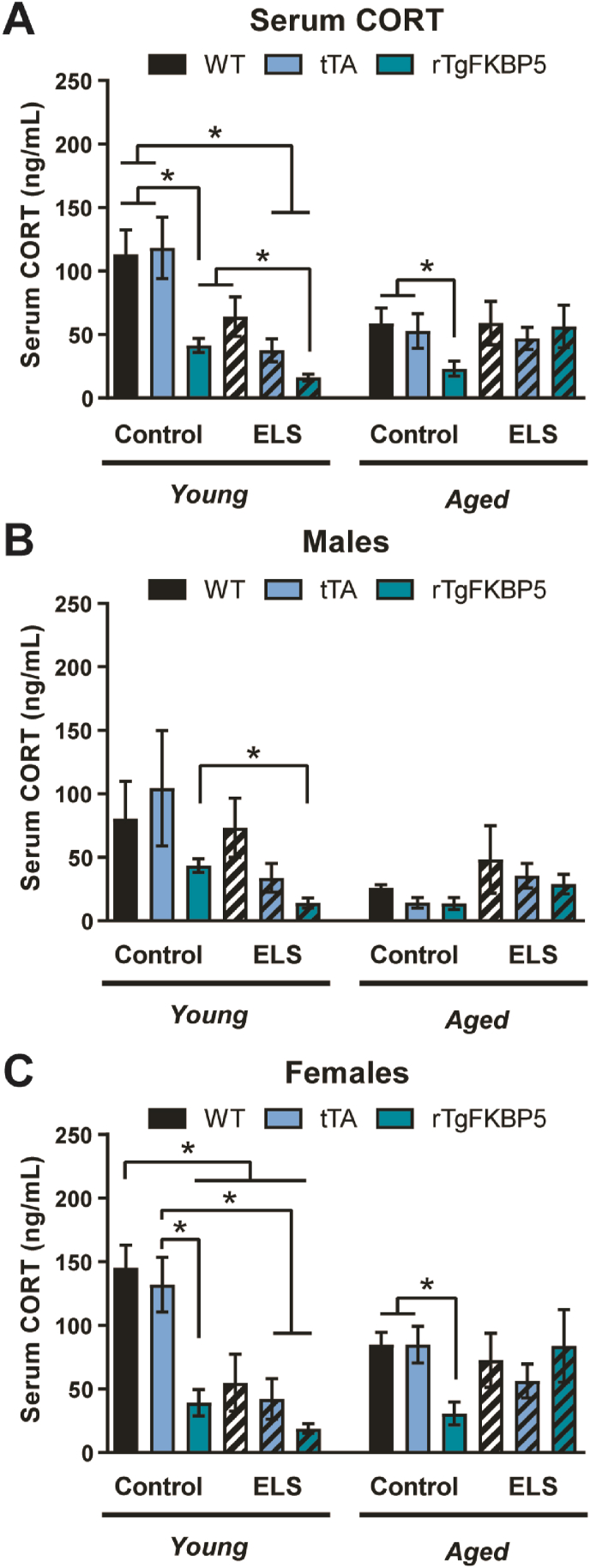
Because CORT secretion is tightly regulated by the HPA axis and dysfunction of this neuroendocrine system has been implicated in the pathophysiology of depression, we collected blood from mice prior to behavioral testing (see experimental timeline in Fig. 1A and animal groups in Fig. 1B). We evaluated whether basal serum CORT levels were affected by genotype, age and/or ELS. We found significant effects of genotype (p < 0.001), ELS (p = 0.003), sex (p < 0.001) and age (p = 0.011), as well as interactive effects of ELS x age (p < 0.001), genotype x ELS (p = 0.005), and genotype x ELS x sex (p = 0.01).
Post-hoc tests demonstrated that the rTgFKBP5 genotype was associated with significantly reduced CORT levels compared to both other genotypes overall, regardless of sex or age (Fig. 2A). ELS also reduced CORT levels, and FKBP5 and ELS produced a synergistic decrease in CORT levels, but only in young mice (p < 0.05). On the other hand, CORT levels were reduced in aged mice independent of any other factors (p < 0.05). These findings were all greater in female mice, due primarily to the greater levels of CORT at both ages in female vs. male control genotypes (Fig. 2B and C). This suggests that malfunction of FKBP5 alone at young ages can impact HPA function, and that this impact may be greater in females.
Fig. 2.
High FKBP5 reduces basal CORT levels. (A) Blood was collected from mice at 50 (young) or 200 (aged) postnatal days. After centrifugation, serum was collected, and total CORT levels were analyzed by ELISA. (B) Basal CORT levels in males and (C) females. WT = wild-type, tTA = CamKIIα-tTA, rTgFKBP5 = FKBP5 overexpressing mice, CORT, corticosterone, ng = nanogram, mL = milliliter, ELS = early life stress. Data are represented as standard error of the mean (SEM) and analyzed by linear regression followed by Bonferroni post hoc test (see Table 1). Significant results were considered when ∗p < 0.05.
3.2. ELS counteracts FKBP5-induced susceptibility to depressive-like symptoms in aged mice
We previously showed that the interaction of high FKBP5 and ELS increases anxiety levels in young mice (Criado-Marrero et al., 2019). Here, we investigated whether this interaction also drives depressive behavior, since depression symptoms are highly comorbid with anxiety. Using the tail suspension test (TST), we found that aged mice spent less time immobile than young mice (age effect, F1,98 = 129.7, p < 0.0005; Fig. 3A). While overexpression of FKBP5 in aged mice led to more time immobile, ELS prevented this effect (control x ELS, p < 0.05). This suggests that FKBP5 counteracts the effect of aging on depressive-like behavior in this model. In both age groups, WT and tTA were not affected by ELS. Using the open field test, we assessed whether animals had mobility differences that may have confounded the TST observations. Overall locomotion, as measured by total distance travelled, was not altered between genotypes, although both ELS and aging were associated with a significant decrease in locomotor activity. A follow-up analysis on these effects revealed that there were no differences in either sex between age groups (see supporting data for Fig. 3B in Table 1), confirming that sex differences in TST were not due locomotor variations (Fig. 3B). Although sex alone did not affect active escape-oriented behavior during TST monitoring, we found a significant interactive effect of sex with age and genotype on total time immobile (p < 0.006). Hence, we analyzed immobility differences in males (Fig. 3C) and females (Fig. 3D) separately. For the males, aging was associated with a significant decrease in time immobile independent of ELS or genotype, whereas ELS increased time immobile in FKBP5 mice. Time immobile was also reduced by age in WT and tTA, but not rTgFKBP5 female mice. In aged rTgFKBP5 females, ELS counteracted the FKBP5-induced susceptibility to depressive-like symptoms by reducing time immobile.
Fig. 3.
ELS prevents FKBP5-induced susceptibility to depressive-like behavior in aged mice. (A) Total immobile time in the tail suspension test and (B) distance travelled during the open field test as measure of locomotor activity. Immobile time spent in the tail suspension test by (C) males and (D) females. WT = wild-type, tTA = CamKIIα-tTA, rTgFKBP5 = FKBP5 overexpressing mice, s = seconds, m = meters, ELS = early life stress. Mice were 60-days-old (young) and 210-days-old (aged) when subjected to behavioral testing; N = 9-11 per group. Data are represented as standard error of the mean (SEM) and analyzed by three- and two-way Analysis of variance (ANOVA)s (see Table 1). Significant results were considered when ∗p < 0.05 and were followed by Bonferroni post hoc tests. #p = 0.08.
3.3. Age, but not FKBP5, influences fear learning
In addition to anxiety and depression, gene and protein changes in FKBP5 have been associated with enhanced fear responses, which is a common symptom in PTSD (Fani et al., 2016, 2012). Using a fear conditioning paradigm, we examined whether fear learning was affected by overexpression of FKBP5, age or exposure to ELS. Fear expression was higher in aged control mice when compared to young animals (p < 0.05) (Fig. 4A and B). This age-related increase in fear expression was independent of ELS (p < 0.05, young x aged) (Fig. 4C and D). Although there were main effects of genotype and sex on fear learning, the interplay of each factor with ELS exposure (two-way interaction) was not significant. Both aged female and male mice showed slightly higher fear expression compared to young mice (Supplementary Fig. 1 and Table 1), but no significant differences between the groups were found after following up with a Bonferroni multiple comparison analysis.
Fig. 4.
High FKBP5 does not impact auditory fear learning. Percentage of time freezing during the fear conditioning test by non-stressed (A) young and (B) aged animals. Freezing time for (C) young and (D) aged mice exposed to ELS. WT = wild-type, tTA = CamKIIα-tTA, rTgFKBP5 = FKBP5 overexpressing mice, ELS = early life stress; N = 9-11 per group. Data are represented as standard error of the mean (SEM) and analyzed by repeated measures three- and two-way Analysis of variance (ANOVA)s (see Table 1 for analysis).
3.4. Neuronal loss in rTgFKBP5 mice is age-dependent
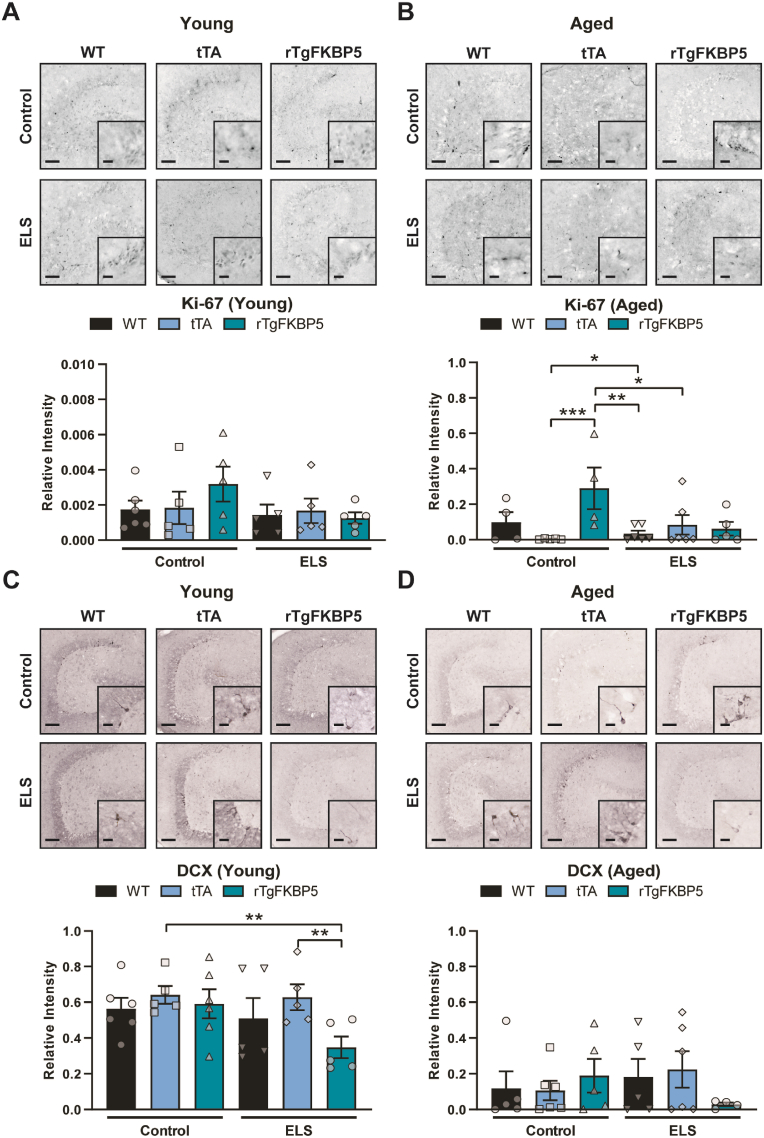
Prolonged exposure to stress or oral administration of CORT are known to increase FKBP5 expression and suppress hippocampal neurogenesis (Lee et al., 2010; Scharf et al., 2011; Zhang et al., 2016). Multiple studies have also shown that aging reduces hippocampal neurogenesis (Kuhn et al., 1996; Ben Abdallah et al., 2010; Lugert et al., 2010). Thus, we tested whether FKBP5 genotype and ELS interact to impact hippocampal neurogenesis and if this change is affected by aging. First, we measured the levels of Ki-67 (a proliferation cell marker). There was a statistically significant difference in Ki-67 among the groups (p < 0.0001; Fig. 5A and B), which was mainly driven by age, genotype and the interaction of ELS x genotype (Table 2). Specifically, aged rTgFKBP5 mice showed higher Ki-67 expression than aged tTA (Fig. 5A and B). Young WT and tTA animals did not show differences from FKBP5 mice in Ki-67 expression. Then, we measured levels of doublecortin (DCX, immature progenitor and migration cell marker). We found an overall group difference (p < 0.001) and a main effect of age (p < 0.0001). DCX was decreased in aged mice, suggesting an age-related decline in cell differentiation or migration. ELS interacted with FKBP5 significantly in young mice, but not in aged mice. Specifically, ELS reduced DCX expression in FKBP5 mice compared to tTA (p < 0.05) mice (Fig. 5C and D). In both age groups, ELS-rTgFKBP5 animals showed the greatest suppression in DCX expression, highlighting the importance of gene x environment in cellular processes. Since levels of these cell markers, Ki-67 and DCX, may vary based on the cell phase transition (Miller et al., 2018), we counted the total number of neurons to determine whether there were significant changes in neuronal counts. Different from what we expected, ELS did not cause significant loss of NeuN-positive neurons within young (Fig. 6A) or aged (Fig. 6B) groups. However, a Bonferroni multiple comparison analysis revealed a significant reduction of neurons in aged rTgFKBP5 mice when compared to young WT and rTgFKBP5 mice (Fig. 6C). The hippocampal volume was not affected by age, ELS or genotype (Fig. 6D).
Fig. 5.
FKBP5 x ELS reduces DCX-positive cells in the dentate gyrusof young mice. Representative images and relative expression of Ki-67 (cell proliferation marker) in the hippocampal dentate gyrus of (A) young and (B) aged animals. (C-D) Representative images and DCX expression in the same mice. Hippocampal sections were collected from mice at 150 (young) and 300 (aged) days of age; N = 5-6 per group. Interaction of stress x genotype was analyzed by linear regression followed by Bonferroni post hoc test (see Table 2). Data represented as standard error of the mean (SEM). Statistical significance was considered when ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bar represents 200 μm; inset scale represents 20 μm. DCX = doublecortin; ELS, early life stress.
Fig. 6.
FKBP5 overexpression x age interaction reduces neuronal number in the hippocampus. Representative images of hippocampal sections and analysis from (A) young and (B) animals. Analysis of total neurons in the hippocampus from (C) young and (D) aged mice. Hippocampal sections collected from mice at 150 (young) and 300 (aged) days of age were stained with NeuN (brown) and cresyl violet (violet). N = 4-6 animals per group. ELS = early life stress. Scale bar represents 200 μm. Values are represented as standard error of the mean (SEM). Significant results were considered when ∗p < 0.05 and were followed by Bonferroni post hoc tests (see Table 1). WT = wild-type, tTA = CamKIIα-tTA, rTgFKBP5 = FKBP5 overexpressing mice, ELS = early life stress.
3.5. Microglial expression is increased in aged rTgFKBP5 mice
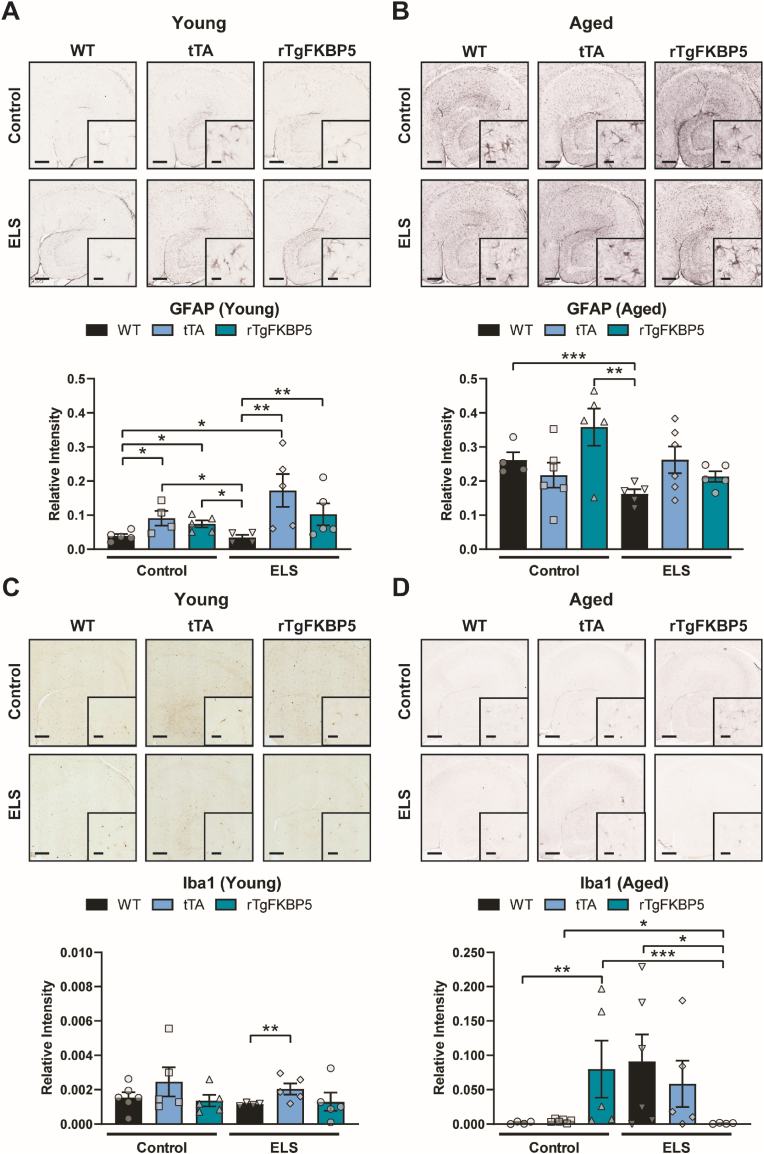
Astrocyte and microglia alterations are linked to early life adversities and depression (Abbink et al., 2019; Rajkowska and Stockmeier, 2013; Réus et al., 2019). Therefore, we investigated changes in astrocyte and microglial counts, since their numbers can be affected by stress and aging (Réus et al., 2019; Tynan et al., 2010). Chronic activation of these cells has been implicated in various brain diseases including major depressive disorder (Rajkowska and Stockmeier, 2013). We stained for the glial fibrillary acidic protein (GFAP) and ionized calcium binding adaptor molecule 1 (Iba1), which are highly expressed in astrocytes and microglia, respectively. In the young cohort, tTA-ELS animals had the highest levels of GFAP (Fig. 7A). GFAP levels were increased in tTA animals as well as in the rTFKBP5 group, but there was no difference between tTA and FKBP5 genotypes. Different from what we expected, GFAP was reduced by ELS in aged rTgFKBP5 and WT mice when compared to animals of the same genotype without stress exposure. Also, in the aged group, there was a significant genotype x ELS interaction due to significantly higher GFAP expression in rTgFKBP5 mice compared to WT-ELS (Fig. 7B). Like GFAP, we found significantly higher levels of Iba1 in aged mice and an interaction in genotype x ELS (Fig. 7C and D). Surprisingly, this microglial marker was highly induced in aged rTgFKBP5 mice. ELS induced lba1 in aged WT and tTA mice but blocked the age-induced increase in lba1 in FKBP5 mice (Fig. 7D). These results demonstrate an age-related increase in microglial markers in rTgFKBP5 mice and that hippocampal levels of these markers can be differentially affected by ELS.
Fig. 7.
FKBP5 induces microglial markers in aged mice. Representative images and relative fold change of GFAP (astrocyte marker) in the hippocampal dentate gyrus of (A) young and (B) aged animals. (C-D) Representative images and fold-change of Iba1 expression in the dentate gyrus of the hippocampus in the same mice. Hippocampal sections were collected from mice at 150 (young) and 300 (aged) days of age; N = 5-6 per group. Interaction of stress and genotype was analyzed by linear regression followed by Bonferroni post hoc test (see Table 2). Data represented as standard error of the mean (SEM). Statistical significance was considered when ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bar represents 200 μm; inset scale represents 20 μm. DCX = doublecortin; ELS, early life stress; GFAP, glial fibrillary acidic protein; Iba1, Ionized calcium binding adaptor molecule 1.
4. Discussion
4.1. Summary
Over the last decade, there have been numerous studies on how the environment fosters epigenetic, genetic and molecular changes in the brain, increasing predisposition to mental disorders (Daskalakis et al., 2013; Eid et al., 2019; Hui et al., 2011). This study adds to our previous finding demonstrating that an FKBP5 x ELS interaction increases anxiety levels (Criado-Marrero et al., 2019). Here, we tested whether this interaction, FKBP5 x ELS, may affect depressive-like symptoms. We tested this possibility using a mouse model overexpressing FKBP5 in the brain (rTgFKBP5) and two control groups, WT and tTA (similar to rTgFKBP5, tTA express the tetracycline-off transactivator driven by the CamKIIα promoter). Since ELS can have long-lasting effects in the brain, we also evaluated depressive-like symptoms and brain changes at two ages. First, we found that high FKBP5 affected neuroendocrine regulation by reducing basal CORT levels in rTgFKBP5 mice, especially in females. Second, we observed an age-dependent effect wherein young mice displayed higher depressive-like symptoms than older mice during the tail suspension test. Aged, but not young, rTgFKBP5 mice with prior exposure to ELS were resistant to these depressive-like symptoms. Third, ELS decreased Ki-67-positive cells in aged rTgFKBP5 mice, while affecting DCX expression in young animals. Lastly, FKBP5 overexpression increased microglial markers and promoted neuronal loss in the hippocampus of aged mice. This suggests that high expression of FKBP5 may accelerate an age-related activated microglia response to neuronal damage or neuroinflammation. Overall, our findings indicate that the interplay of FKBP5 with ELS impacts brain function and physiology throughout the lifecycle, affecting emotional responding. Importantly, our data demonstrate that key factors in the vulnerability for mental disorders, like age and sex, can influence behavioral and neurobiological outcomes.
4.2. FKBP5 affects the HPA axis in a sex-dependent manner
Clinical reports have documented an association between CORT levels and depression (see review (Sanjay Nandam et al., 2020)); however, we did not find a correlation between basal CORT levels and depressive-like symptoms in this study. Instead, we found that animals overexpressing FKBP5 showed reduced basal CORT levels. This reduction in basal CORT levels was most evident in females. This is interesting because similar observations have been reported in FKBP5 knockout mice where females, but not males, showed reduced basal CORT levels (Hoeijmakers et al., 2014; Touma et al., 2011). This indicates the negative feedback sensitivity of the HPA axis can be affected by levels of FKBP5 in a sex-dependent manner. Specifically, the FKBP5 SNP rs1360780 has been linked to increased risk for depression where TT carriers have higher basal FKBP5 and present with GR resistance. Notably, the susceptibility for depressive symptoms in individuals with these FKBP5 variants are sex-dependent and moderated by adverse experiences at early ages (Appel et al., 2011; VanZomeren-Dohm et al., 2015).
In contrast to our hypothesis, FKBP5 lowered CORT levels in young mice, implying a hypocortisolism phenotype. A different FKBP5 SNP, rs9296158, has been linked to childhood abuse and low levels of CORT in depressed patients (Kohrt et al., 2015). Moreover, hypocortisolism has been described in patients with atypical depression and in females with depression, suggesting that FKBP5 may affect specific endophenotypes in depression (Bremmer et al., 2007; Juruena et al., 2018; Maripuu et al., 2014; Penninx et al., 2007). Moreover, women with other mental illnesses, like PTSD, have shown lower CORT levels than healthy women (Freidenberg et al., 2009; Meewisse et al., 2007), which suggests that GR dysregulation in females is not exclusive to depression. In the present study, we found that young rTgFKBP5 females had lower CORT levels than young males as well as older animals. The exact mechanism underlying low basal CORT levels in females is still unknown. Based on previous studies, activity of steroid receptors and hormone levels (e.g. estrogen) may contribute to sex differences (see review (Bourke et al., 2012). Particularly, in an aged population, a greater CORT variability should be anticipated in women because of the impact exerted by menopausal status. Another possibility is that the half-life of cortisol is shorter in women as compared to men (Roelfsema et al., 2017). Investigators reported that these differences were found in individuals younger than 50 years old, and no differences were found in older adults. This is in line with our findings showing that sex differences were evident in young but not aged mice. Follow-up studies with additional timepoints throughout the day are needed to fully evaluate the CORT dynamics in these animals to determine if they have decreased CORT overall, if the circadian dynamics are shifted so that the peak and trough of CORT varies between groups, or if the amplitude of the CORT rhythm varies between groups.
4.3. FKBP5 reduces hippocampal neurons in aged mice
Multiple studies have reported the adverse impact of chronic and early life stress on brain maturation and development (Chocyk et al., 2013, 2011; Delpech et al., 2016; Dioli et al., 2019). Specifically, stress induces adverse effects in the hippocampus by impairing both neuron maturation and formation of synaptic circuits; these developmental deficits are associated with changes in mood and affect (Delpech et al., 2016; Wei et al., 2015, 2011). Here, we observed that high FKBP5 increases hippocampal Ki-67 proliferation in aged animals, while ELS prevents this effect. However, ELS reduced DCX in young animals without affecting aged mice. This suggests that stress exerts a stronger effect on hippocampal cell growth and differentiation. We previously showed that long-term potentiation is modestly increased in rTgFKBP5 mice (Blair et al., 2019). Thus, it is possible that the enhancement of long-term potentiation in these mice is secondary to an increase in cell proliferation and in brain-derived neurotrophic factor (BDNF) expression, as previously reported (Chun et al., 2006; Gassen et al., 2015). Also, rTgFKBP5 mice exhibited reversal spatial learning deficits in the Morris water maze (Blair et al., 2019). Reduced neurogenesis has been linked to cognitive impairments (Anacker and Hen, 2017), thus the spatial learning deficit in rTgFKBP5 mice may be due to loss of hippocampal neurons as reported in this study. Age was a determining factor in high FKBP5-expressing animals, as only aged control rTgFKBP5 mice demonstrated this reduction in neurons. Our observations are in line with prior reports showing that proliferation and neurogenesis do not necessarily correlate and that ELS induces its effects in an age- and sex-dependent manner (Hulshof et al., 2011; Loi et al., 2014). However, we cannot exclude possible ELS-induced changes in the structure of hippocampal neurons. Based on a previous study, ELS can promote expression of immature spines while reducing the number of mature spines in CA1 neurons (Wei et al., 2015). The authors also showed that ELS can impair synaptic maturation in the hippocampus, implying a direct effect by ELS on hippocampal-dependent processes like emotional responses.
4.4. Markers of microglia are increased by FKBP5 in aged mice
Lastly, we found that Iba1 levels were significantly increased by FKBP5 in control animals and by ELS in aged WT and tTA mice. This is interesting because Iba1 is upregulated in microglia in response to peripheral inflammation and brain damage as well as after CORT exposure (see review (Delpech et al., 2015)). As shown in previous studies, it is possible that high FKBP5 recruits more microglia by increasing proinflammatory cytokines like TNFa, promoting NF-κB–driven peripheral inflammation (Klengel et al., 2013; Lively and Schlichter, 2018; Zannas et al., 2019). However, ELS prevented upregulation of microglia in aged rTgFKBP5 mice, possibly via inhibiting GR. Depending on length of stress or CORT exposure, CORT is known to exert both suppressive and stimulatory effects on microglia (Rohan Walker et al., 2013; Tanaka et al., 1997). Since aging and stress can each induce the expression of FKBP5, it is possible that the enhanced overexpression of FKBP5 is enough to abolish the GR-induced inflammatory response to stress. As previously reported, this will inhibit microglial activation and proinflammatory cytokine release (Frank et al., 2012). Importantly, we must note that independent of FKBP5, aging also determined the levels of microglia, which may correlate with GR activation in the hippocampus and cytokine activation. For instance, Barrientos et al. showed that there is greater microglial activation in the hippocampus of middle-aged rats compared to young rats (Barrientos et al., 2015). They also showed that GR inhibition reduced the expression of lipopolysaccharide (LPS)-induced proinflammatory cytokines (IL-1β and TNF-α) in microglia cultures. In a separate study, peripheral LPS injection produced a greater induction of cytokines from isolated microglia in aged animals when compared to adults (Henry et al., 2009). Interestingly, FKBP5-deficient mice have reduced levels of IL-1β at 6 months, but no differences were detected at older timepoints (>10 month of age) (Sabbagh et al., 2014). These findings suggest that the influence of FKBP5 on inflammatory markers, and possibly on microglia, may depend on age and stress factors.
4.5. Limitations and future studies
We limited our study to measure basal CORT levels at a single time point. Although no association was found between CORT and depressive-like symptoms, more research is needed to examine whether FKBP5 alters CORT feedback regulation and dynamics after chronic stress. To test this, the DEX/CRH test should be used, since it is more suitable to detect abnormalities in the HPA axis in response to stress than measuring basal levels. Also, repeating stress later in life may reflect whether resiliency is altered in rTgFKBP5-ELS animals by dysregulating CORT levels and identifying behavioral disturbances. This is from the perspective of the previously described three-hit hypothesis where brain and HPA axis responses adapt to ongoing gene-environment interactions involving (1-hit) genetic predisposition [high FKBP5], (2-hit) early-life environment [maternal separation], and (3-hit) later-life stressful environment [e.g. chronic variable stress] (Daskalakis et al., 2013). Moreover, we did not observe an effect of FKBP5 x ELS on associative fear conditioning. Nevertheless, follow up studies should evaluate other types of memory that depend on extra-hippocampal brain areas, like prefrontal cortex-dependent working memory. Specifically, healthy aged individuals (50+ years old) carrying the T allele of the FKBP5 variant rs1360780 had poor working memory in two independent memory tests (Fujii et al., 2014). These individuals also presented with lower CORT reactivity in the DEX/CRH test. Thus, it is probable that FKBP5 and stress trigger other symptoms, like the cognitive deficits commonly reported in depression (Mcintyre et al., 2013).
Additionally, future research should seek to uncover epigenetic changes induced by FKBP5 x ELS in young and aged groups. In the ELS mice, we would expect a decrease in DNA methylation since corticosterone exposure is known to reduce FKBP5 methylation in the CpG4 of two intronic regions: intron 1 and 5 (Lee et al., 2010). In humans, lower methylation in two FKBP5 CpGs, cg20813374 and cg00130530, correlates with high FKBP5 mRNA levels and is associated with aging and stress-related phenotypes (Zannas et al., 2016). In a separate study, it was reported that the strongest DNA demethylation is in the CpG Intron 7 of FKBP5 after DEX-induction of GR in human hippocampal progenitor cells (Klengel et al., 2013). They also reported that early trauma and its interaction with this high-risk allele of FKBP5 were significant predictors for lifetime PTSD risk, supporting the link between epigenetics and environmental factors with mood disorders.
Another limitation of our study is the reduced power to establish sex differences in tissue analyses due the small sample size per sex/group. ELS is known to induce sex-specific differences in neurogenesis, thus we may expect variations between males and females. For example, one study showed that males exposed to ELS had higher DCX-positive neurons where females had significantly fewer (Oomen et al., 2009). In another study, adult male mice had fewer neurons after ELS, but no change was found in females (Naninck et al., 2015). Sex can also affect microglia in the hippocampus. Female rats possess more activated microglia during juvenile and early adulthood; this may help to explain FKBP5 and stress effects in microglia at different ages. Thus, due to the influence of sex and age in stress-related disorders, it will be important to further investigate whether FKBP5 differentially affects neurogenesis, microglia and cytokine population in both sexes.
Finally, we have focused this and our previous investigation on understanding the impact of stress on non-cognitive (affective) symptoms related to mood disorders. We have examined changes in the hippocampus, but other areas expressing CamKII in the limbic system like the amygdala and cortex may also contribute to our results. Additionally, considering that ELS can promote amyloid-beta plaque deposition (Lesuis et al., 2018) and FKBP5 has been shown to promote protein oligomerization (Blair et al., 2013), our findings are relevant to understand whether high FKBP5 and early exposure to stress may influence the age of onset and severity of age-related diseases like Alzheimer’s disease (AD). It would be highly valuable to investigate whether the interplay of FKBP5-environment plays a role in the progression and development of AD neuropathology.
5. Conclusions
Our findings, as an extension of previous studies, demonstrate that FKBP5 contributes to the susceptibility for stress-related disorders, which is highly influenced by age and sex. Like in patients with depression, we found endocrine and inflammatory alterations in mice overexpressing FKBP5. Specifically, we found that high levels of FKBP5 reduce basal CORT levels, reflecting its role in HPA axis function. Additionally, FKBP5 had a robust effect on the levels of microglial markers in the hippocampus, suggesting that it may promote neuronal loss by altering glial cells in the brain. Interestingly, we observed that ELS inhibits the effects induced by FKBP5 in microglia. Recent evidence indicates that childhood adversity increases levels of FKBP5 and leukocyte activity and reduces cortisol levels in patients with anxious depression (Menke et al., 2018). Therefore, together with our previous study showing that ELS promotes higher anxiety in mice overexpressing FKBP5, these findings are highly relevant to understanding this subtype of depression due to similarity of observations. It is important to highlight that age was a common factor affecting depressive-like behavior and cellular changes in the hippocampus (summarized in Table 3). Certainly, further investigation is needed to better understand age-related effects on the complex etiology and heterogeneity of depression. Overall, our findings provide important evidence of changes at the neuroendocrine, behavioral as well cellular and molecular levels in the mouse hippocampus that may underlie mechanisms of how risk genes and stress promote susceptibility to mental health disorders during older age.
Table 3.
Summary of ELS and FKBP5 interaction results.
| Measurements | Factors |
|||||
|---|---|---|---|---|---|---|
| Genotype | ELS | Sex | Age | Interactions | Key finding(s) | |
| CORT levels | Yes | Yes | Yes | Yes | Gen x ELS | Young mice are susceptible to high levels of FKBP5 |
| Depressive-like symptoms | Yes | No | No | Yes | None | ELS protects aged effect on rTgFKBP5 mice |
| Fear expression | Yes | No | Yes | Yes | None | Age modestly increases fear expression |
| Neural proliferation and differentiation | Yes (Ki-67) | No | – | Yes (Ki-67) | Gen x ELS (Ki-67) | Aged rTgFKBP5 mice showed increased proliferation. |
| HPC neurons | No | No | – | Yes | None | Age negatively impacts number of neurons in rTgFKBP5 mice |
| Neuroinflammation | Yes (GFAP) | No | – | Yes | Gen x ELS (GFAP, Iba1) | FKBP5 induces microglia expression in aged mice and ELS prevents this effect. |
DCX: doublecortin, GFAP: Glial fibrillary acidic protein, Iba1: Ionized calcium binding adaptor molecule 1, ELS: early life stress, Gen: genotype, CORT: corticosterone, HPC: hippocampus.
Author contributions
Conceptualization, original draft preparation, and writing, M.C.-M. and L.J.B.; Behavioral testing and analysis, M.C.-M. and T.M.S.; CORT testing and analysis, M.C.-M, D.G. and Z.S. Histopathological and immunohistochemistry analysis, M.C.-M., L.A.G., D.G. and S.K.; Stereological analyses, H.J.P.; Resources and funding acquisition, C.A.D. and L.J.B.; Supervision and project administration, L.J.B. All authors participated in reviewing the manuscript, except for Dr. Chad Dickey who is deceased.
Funding
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH103848 and National Institute of Neurological Disorders and Stroke under Award Number R01NS073899. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported in part by Merit Review Award #I01 BX004626 from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Service. The views expressed in this article are those of the authors and do not necessarily reflect the position, policy or views of the Department of Veterans Affairs or the United States Government.
Declaration of competing interest
The authors declare the following competing financial interest(s): #C.A.D. and L.J.B. are co-inventors of the following patent application: “Transgenic mouse model for conditional fkbp51 expression and related methods.” US Patent Application# US20150327523 A1. The other authors have no competing financial interest to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100143.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aarum J., Sandberg K., Budd Haeberlein S.L., Persson M.A.A. Migration and differentiation of neural precursor cells can be directed by microglia. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbink M.R., van Deijk A.-L.F., Heine V.M., Verheijen M.H. Korosi | Aniko. The involvement of astrocytes in early-life adversity induced programming of the brain. Glia. 2019;67:1637–1653. doi: 10.1002/glia.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C., Hen R. Adult hippocampal neurogenesis and cognitive flexibility — linking memory and mood. Nat. Rev. Neurosci. 2017;18:335. doi: 10.1038/NRN.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel K., Schwahn C., Mahler J., Schulz A., Spitzer C., Fenske K. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology. 2011;36:1982–1991. doi: 10.1038/npp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R.M., Thompson V.M., Kitt M.M., Amat J., Hale M.W., Frank M.G. Greater glucocorticoid receptor activation in hippocampus of aged rats sensitizes microglia. Neurobiol. Aging. 2015;36:1483–1495. doi: 10.1016/j.neurobiolaging.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Abdallah N.M.B., Slomianka L., Vyssotski A.L., Lipp H.P. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging. 2010;31(1):151–161. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. J. Am. Med. Assoc. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder E.B., Salyakina D., Lichtner P., Wochnik G.M., Ising M., Pütz B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Blair L.J., Criado-Marrero M., Zheng D., Wang X., Kamath S., Nordhues B.A. The disease-associated chaperone FKBP51 impairs cognitive function by accelerating AMPA receptor recycling. ENeuro. 2019;6 doi: 10.1523/ENEURO.0242-18.2019. eneuro.0242-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair L.J., Nordhues B.A., Hill S.E., Scaglione K.M., O’Leary J.C., Fontaine S.N. Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J. Clin. Invest. 2013;123:4158–4169. doi: 10.1172/JCI69003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C., Rubio J., Wall M., Wang S., Jiu C.J., Kendler K.S. Risk factors for anxiety disorders: common and specific effects in a national sample. Depress. Anxiety. 2014;31:756–764. doi: 10.1002/da.22247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke C.H., Harrell C.S., Neigh G.N. Stress-induced sex differences: adaptations mediated by the glucocorticoid receptor. Horm. Behav. 2012;62:210–218. doi: 10.1016/j.yhbeh.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer M.A., Deeg D.J.H., Beekman A.T.F., Penninx B.W.J.H., Lips P., Hoogendijk W.J.G. Major depression in late life is associated with both hypo-and hypercortisolemia. Biol. Psychiatr. 2007;62:479–486. doi: 10.1016/j.biopsych.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Brunson K.L., Eghbal-Ahmadi M., Bender R., Chen Y., Baram T.Z. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc. Natl. Acad. Sci. Unit. States Am. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson K.L., Kramár E., Lin B., Chen Y., Colgin L.L., Yanagihara T.K. Mechanisms of late-onset cognitive decline after early-life stress. J. Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A.F., Holz N., Boecker R., Blomeyer D., Rietschel M., Witt S.H. Moderating role of FKBP5 genotype in the impact of childhood adversity on cortisol stress response during adulthood. Eur. Neuropsychopharmacol. 2014;24:837–845. doi: 10.1016/j.euroneuro.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Campbell S., Marriott M., Nahmias C., Macqueen G.M. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am. J. Psychiatr. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Chocyk A., Bobula B., Dudys D., Przyborowska A., Majcher-Maślanka I., Hess G. Early-life stress affects the structural and functional plasticity of the medial prefrontal cortex in adolescent rats. Eur. J. Neurosci. 2013;38:2089–2107. doi: 10.1111/ejn.12208. [DOI] [PubMed] [Google Scholar]
- Chocyk A., Dudys D., Przyborowska A., Majcher I., Maćkowiak M., Wędzony K. Maternal separation affects the number, proliferation and apoptosis of glia cells in the substantia nigra and ventral tegmental area of juvenile rats. Neuroscience. 2011;173:1–18. doi: 10.1016/j.neuroscience.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Chun S.K., Sun W., Park J.J., Jung M.W. Enhanced proliferation of progenitor cells following long-term potentiation induction in the rat dentate gyrus. Neurobiol. Learn. Mem. 2006;86:322–329. doi: 10.1016/j.nlm.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Criado-Marrero M., Gebru N.T., Gould L.A., Smith T.M., Kim S., Blackburn R.J. Early life stress and high FKBP5 interact to increase anxiety-like symptoms through altered AKT signaling in the dorsal Hippocampus. Int. J. Mol. Sci. 2019;20:2738. doi: 10.3390/ijms20112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis N.P., Bagot R.C., Parker K.J., Vinkers C.H., de Kloet E.R., Peters J.J. The three-hit concept of vulnerability and resilience: towards understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology. 2013;38:1858–1873. doi: 10.1016/j.psyneuen.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T.H., Ning Y.-M., Sanchez E.R. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J. Biol. Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- Delpech J.-C., Madore C., Nadjar A., Joffre C., Wohleb E.S., Lay S. Microglia in neuronal plasticity: influence of stress. Neuropharmacology. 2015;96:19–28. doi: 10.1016/j.neuropharm.2014.12.034. [DOI] [PubMed] [Google Scholar]
- Delpech J.-C., Wei L., Hao J., Yu X., Madore C., Butovsky O. Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav. Immun. 2016;57:79–93. doi: 10.1016/j.bbi.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey C., Kraft C., Jinwal U., Koren J., Johnson A., Anderson L. Aging analysis reveals slowed tau turnover and enhanced stress response in a mouse model of tauopathy. Am. J. Pathol. 2009;174:228–238. doi: 10.2353/ajpath.2009.080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioli C., Patrício P., Sousa N., Kokras N., Dalla C., Guerreiro S. Chronic stress triggers divergent dendritic alterations in immature neurons of the adult hippocampus, depending on their ultimate terminal fields. Transl. Psychiatry. 2019;9:143. doi: 10.1038/s41398-019-0477-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid R.S., Chaiton J.A., Lieblich S.E., Bodnar T.S., Weinberg J., Galea L.A.M. Early and late effects of maternal experience on hippocampal neurogenesis, microglia, and the circulating cytokine milieu. Neurobiol. Aging. 2019;78:1–17. doi: 10.1016/j.neurobiolaging.2019.01.021. [DOI] [PubMed] [Google Scholar]
- Fani N., King T.Z., Shin J., Srivastava A., Brewster R.C., Jovanovic T. Structural and functional connectivity IN posttraumatic stress disorder: associations with FKBP5. Depress. Anxiety. 2016;33:300–307. doi: 10.1002/da.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N., Tone E.B., Phifer J., Norrholm S.D., Bradley B., Ressler K.J. Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol. Med. 2012;42:533–543. doi: 10.1017/S0033291711001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer A., Costas J., Labad J., Salvat-Pujol N., Segalàs C., Urretavizcaya M. FKBP5 polymorphisms and hypothalamic-pituitary-adrenal axis negative feedback in major depression and obsessive-compulsive disorder. J. Psychiatr. Res. 2018;104:227–234. doi: 10.1016/j.jpsychires.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Frank M.G., Thompson B.M., Watkins L.R., Maier S.F. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav. Immun. 2012;26:337–345. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidenberg B.M., Gusmano R., Hickling E.J., Blanchard E.B., Douglas Bremner J., Frye C. Women with PTSD have lower basal salivary cortisol levels later in the day than do men with PTSD: a preliminary study. Physiol. Behav. 2009;99:234–236. doi: 10.1016/j.physbeh.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T., Ota M., Hori H., Hattori K., Teraishi T., Matsuo J. The common functional FKBP5 variant rs1360780 is associated with altered cognitive function in aged individuals. Sci. Rep. 2014;4:6696. doi: 10.1038/srep06696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen N.C.N.C., Fries G.R., Zannas A.S., Hartmann J., Zschocke J., Hafner K. Chaperoning epigenetics: FKBP51 decreases the activity of DNMT1 and mediates epigenetic effects of the antidepressant paroxetine. Sci. Signal. 2015;8:119. doi: 10.1126/scisignal.aac7695. [DOI] [PubMed] [Google Scholar]
- Gillespie C.F., Phifer J., Bradley B., Ressler K.J. Risk and resilience: genetic and environmental influences on development of the stress response. Depress. Anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K.-M., Won E., Sim Y., Kang J., Han C., Kim Y.-K. Influence of FKBP5 polymorphism and DNA methylation on structural changes of the brain in major depressive disorder. Sci. Rep. 2017;7:42621. doi: 10.1038/srep42621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C.J., Huang Y., Wynne A.M., Godbout J.P. Peripheral Lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain Behav. Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Mcklveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comp. Physiol. 2016;6:603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers L., Harbich D., Schmid B., Lucassen P.J., Wagner K v, Schmidt M v. Depletion of FKBP51 in female mice shapes HPA axis activity. PloS One. 2014;9 doi: 10.1371/journal.pone.0095796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui J.-J., Zhang Z.-J., Liu S.-S., Xi G.-J., Zhang X.-R., Teng G.-J. Hippocampal neurochemistry is involved in the behavioural effects of neonatal maternal separation and their reversal by post-weaning environmental enrichment: a magnetic resonance study. Behav. Brain Res. 2011;217:122–127. doi: 10.1016/j.bbr.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Hulshof H.J., Novati A., Sgoifo A., Luiten P.G.M., den Boer J a, Meerlo P. Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behav. Brain Res. 2011;216:552–560. doi: 10.1016/j.bbr.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Juruena M.F., Bocharova M., Agustini B., Young A.H. Atypical depression and non-atypical depression: is HPA axis function a biomarker? A systematic review. J. Affect. Disord. 2018;233:45–67. doi: 10.1016/j.jad.2017.09.052. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatr. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M. Childhood adversities and adult psychopathology in the WHO world mental health surveys. Br. J. Psychiatry. 2010;197:378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T., Mehta D., Anacker C., Rex-haffner M., Jens C., Pariante C.M. Allele-specific FKBP5 DNA demethylation mediates gene– childhood trauma interactions. Nat. Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275.Allele-specific. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt B.A., Worthman C.M., Ressler K.J., Mercer K.B., Upadhaya N., Koirala S. Cross-cultural gene- environment interactions in depression, post-traumatic stress disorder, and the cortisol awakening response: FKBP5 polymorphisms and childhood trauma in South Asia. Int. Rev. Psychiatr. 2015;27:180–196. doi: 10.3109/09540261.2015.1020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo J.W., Russo S.J., Ferguson D., Nestler E.J., Duman R.S. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs K.J., Makara G.B. Corticosterone and dexamethasone act at different brain sites to inhibit adrenalectomy-induced adrenocorticotropin hypersecretion. Brain Res. 1988;474:205–210. doi: 10.1016/0006-8993(88)90435-0. [DOI] [PubMed] [Google Scholar]
- Kuhn H.G., Dickinson-Anson H., Gage F.H. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;76(6):2027–2033. doi: 10.1523/jneurosci.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd C.O., Huot R.L., Thrivikraman K v, Nemeroff C.B., Plotsky P.M. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biol. Psychiatr. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Lahti J., Ala-Mikkula H., Kajantie E., Haljas K., Eriksson J.G., Räikkönen K. Associations between self-reported and objectively recorded early life stress, FKBP5 polymorphisms, and depressive symptoms in midlife. Biol. Psychiatr. 2016;80:869–877. doi: 10.1016/j.biopsych.2015.10.022. [DOI] [PubMed] [Google Scholar]
- Lee R.S., Tamashiro K.L.K., Yang X., Purcell R.H., Harvey A., Willour V.L. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010;151:4332–4343. doi: 10.1210/en.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuis S.L., Weggen S., Baches S., Lucassen P.J., Krugers H.J. Targeting glucocorticoid receptors prevents the effects of early life stress on amyloid pathology and cognitive performance in APP/PS1 mice. Transl. Psychiatry. 2018;8:53. doi: 10.1038/s41398-018-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G.Y., Tam W.W., Lu Y., Ho C.S., Zhang M.W., Ho R.C. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-21243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively S., Schlichter L.C. Microglia responses to pro-inflammatory stimuli (LPS, IFNγ+TNFα) and reprogramming by resolving cytokines (IL-4, IL-10) Front. Cell. Neurosci. 2018;12:215. doi: 10.3389/fncel.2018.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi A., Koricka M., Lucassen S.J., Joels P.J. Age- and sex-dependent effects of early life stress on hippocampal neurogenesis. Front. Endocrinol. 2014;5 doi: 10.3389/fendo.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen P.J., Müller M.B., Holsboer F., Bauer J., Holtrop A., Wouda J. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am. J. Pathol. 2001;158:453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucia C., Rinchon A., Olmos-Alonso A., Riecken K., Fehse B., Boche D. Microglia regulate hippocampal neurogenesis during chronic neurodegeneration. Brain Behav. Immun. 2016;55:179–190. doi: 10.1016/j.bbi.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugert S., Basak O., Knuckles P., Haussler U., Fabel K., Götz M. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem. 2010;6(5):445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Lukic I., Mitic M., Soldatovic I., Jovicic M., Maric N., Radulovic J. Accumulation of cytoplasmic glucocorticoid receptor is related to elevation of FKBP5 in lymphocytes of depressed patients. J. Mol. Neurosci.: M. Inc. 2015;55:951–958. doi: 10.1007/s12031-014-0451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., Juster R.P., Raymond C., Marin M.F. The effects of chronic stress on the human brain: from neurotoxicity, to vulnerability, to opportunity. Front. Neuroendocrinol. 2018;49:91–105. doi: 10.1016/j.yfrne.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Magariños A.M., McEwen B.S., Flügge G., Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maripuu M., Wikgren M., Karling P., Adolfsson R., Norrback K.F. Relative hypo- and hypercortisolism are both associated with depression and lower quality of life in bipolar disorder: a cross-sectional study. PloS One. 2014;9 doi: 10.1371/journal.pone.0098682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcintyre R.S., Cha D.S., Soczynska J.K., Woldeyohannes H.O., Gallaugher L.A., Kudlow P. Cognitive deficits and functional outcomes IN major depressive disorder: determinants, substrates, and treatment interventions. Depress. Anxiety. 2013;30:515–527. doi: 10.1002/da.22063. [DOI] [PubMed] [Google Scholar]
- Meewisse M.-L., Reitsma J.B., de Vries G.-J., Gersons B.P.R., Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br. J. Psychiatry: J. Ment. Sci. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Menke A., Klengel T., Rubel J., Brückl T., Pfister H., Lucae S. Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Gene Brain Behav. 2013;12:289–296. doi: 10.1111/gbb.12026. [DOI] [PubMed] [Google Scholar]
- Menke A., Lehrieder D., Fietz J., Leistner C., Wurst C., Stonawski S. Childhood trauma dependent anxious depression sensitizes HPA axis function. Psychoneuroendocrinology. 2018;98:22–29. doi: 10.1016/j.psyneuen.2018.07.025. [DOI] [PubMed] [Google Scholar]
- Mikolas P., Tozzi L., Doolin K., Farrell C., O’keane V., Frodl T. Effects of early life adversity and FKBP5 genotype on hippocampal subfields volume in major depression. J. Affect. Disord. 2019;252:152–159. doi: 10.1016/j.jad.2019.04.054. [DOI] [PubMed] [Google Scholar]
- Miller I., Min M., Yang C., Tian C., Gookin S., Carter D. Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep. 2018;24:1105–1111. doi: 10.1016/j.celrep.2018.06.110. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naninck E.F.G., Hoeijmakers L., Kakava-Georgiadou N., Meesters A., Lazic S.E., Lucassen P.J. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 2015;25:309–328. doi: 10.1002/hipo.22374. [DOI] [PubMed] [Google Scholar]
- Oomen C.A., Girardi C.E.N., Cahyadi R., Verbeek E.C., Krugers H., Joëls M. Opposite effects of early maternal deprivation on neurogenesis in male versus female rats. PloS One. 2009;4:e3675. doi: 10.1371/journal.pone.0003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante C.M., Makoff A., Lovestone S., Feroli S., Heyden A., Miller A.H. Antidepressants enhance glucocorticoid receptor function in vitro by modulating the membrane steroid transporters. Br. J. Pharmacol. 2001;134:1335–1343. doi: 10.1038/sj.bjp.0704368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx B.W.J.H., Beekman A.T.F., Bandinelli S., Corsi A.M., Bremmer M., Hoogendijk W.J. Late-life depressive symptoms are associated with both hyperactivity and hypoactivity of the hypothalamo-pituitary-adrenal Axis NIH public access. Am. J. Geriatr. Psychiatr. 2007;15:522–529. doi: 10.1097/JGP.0b013e318033ed80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G., Halaris A., Selemon L.D. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol. Psychiatr. 2001;49:741–752. doi: 10.1016/S0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Rajkowska G., Stockmeier C.A. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr. Drug Targets. 2013;14:1225–1236. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réus G.Z., Silva R.H., de Moura A.B., Presa J.F., Abelaira H.M., Abatti M. Early maternal deprivation induces microglial activation, alters glial fibrillary acidic protein immunoreactivity and indoleamine 2,3-dioxygenase during the development of offspring rats. Mol. Neurobiol. 2019;56:1096–1108. doi: 10.1007/s12035-018-1161-2. [DOI] [PubMed] [Google Scholar]
- Roberts S., Keers R., Lester K.J., Coleman JR I., Breen G., Arendt K. Hpa axis related genes and response to psychological therapies: genetics and epigenetics. Depress. Anxiety. 2015;32:861–870. doi: 10.1002/da.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema F., van Heemst D., Iranmanesh A., Takahashi P., Yang R., Veldhuis J.D. Impact of age, sex and body mass index on cortisol secretion in 143 healthy adults. Endocr. Connect. 2017;6:500–509. doi: 10.1530/EC-17-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan Walker F., Nilsson M., Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr. Drug Targets. 2013;14:1262–1276. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque A., Ochoa-Zarzosa A., Torner L. Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain Behav. Immun. 2016;55:39–48. doi: 10.1016/j.bbi.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Sabbagh J.J., Cordova R.A., Zheng D., Criado-Marrero M., Lemus A., Li P. Targeting the FKBP51/GR/Hsp90 complex to identify functionally relevant treatments for depression and PTSD. ACS Chem. Biol. 2018;13:2288–2299. doi: 10.1021/acschembio.8b00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh J.J., O’Leary J.C., Blair L.J., Klengel T., Nordhues B.A., Fontaine S.N. Age-associated epigenetic upregulation of the FKBP5 gene selectively impairs stress resiliency. PloS One. 2014;9 doi: 10.1371/journal.pone.0107241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjay Nandam L., Brazel M., Zhou M., Jhaveri D.J. Cortisol and major depressive disorder-translating findings from humans to animal models and back. Front. Psychiatr. 2020;10:1–15. doi: 10.3389/fpsyt.2019.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli S., Zimmermann C., Kalideris G., Lesuis S.L., Arloth J., Uribe A. An adverse early life environment can enhance stress resilience in adulthood. Psychoneuroendocrinology. 2017;78:213–221. doi: 10.1016/j.psyneuen.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. A mechanism for glucocorticoid toxicity in the hippocampus: increased neuronal vulnerability to metabolic insults. J. Neurosci. 1985;5:1228–1232. doi: 10.1523/JNEUROSCI.05-05-01228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R.M., Krey L.C., McEwen B.S. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc. Natl. Acad. Sci. U. S. A. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf S.H., Liebl C., Binder E.B., Schmidt M v, Müller M.B. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PloS One. 2011;6 doi: 10.1371/journal.pone.0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer S., Ising M., Uhr M., Otto Y., von Klitzing K., Klein A.M. FKBP5 polymorphisms moderate the influence of adverse life events on the risk of anxiety and depressive disorders in preschool children. J. Psychiatr. Res. 2016;72:30–36. doi: 10.1016/j.jpsychires.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Schüle C., Baghai T.C., Eser D., Hä S., Born C., Herrmann S. The combined dexamethasone/CRH test (DEX/CRH test) and prediction of acute treatment response in major depression. PLoS One. 2009;4:e4324. doi: 10.1371/journal.pone.0004324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J.S., Soumier A., Brewer M., Pickel J., Cameron H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker a T., van Rossum E.F.C. Glucocorticoid sensitivity in mood disorders. Neuroendocrinology. 2012;95:179–186. doi: 10.1159/000329846. [DOI] [PubMed] [Google Scholar]
- Stamm T.J., Rampp C., Wiethoff K., Stingl J., Mössner R., O′Malley G. The FKBP5 polymorphism rs1360780 influences the effect of an algorithm-based antidepressant treatment and is associated with remission in patients with major depression. J. Psychopharmacol. 2016;30:40–47. doi: 10.1177/0269881115620459. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration Key substance use and mental health indicators in the United States: results from the 2016 national survey on drug use and health. HHS publication No SMA 17-5044, NSDUH series H-52. 2017. https://www.samhsa.gov/data/
- Tanaka J., Fujita H., Matsuda S., Toku K., Sakanaka M., Maeda N. Glucocorticoid- and mineralocorticoid receptors in microglial cells: the two receptors mediate differential effects of corticosteroids. Glia. 1997;20:23–37. doi: 10.1002/(SICI)1098-1136(199705)20:1<23::AID-GLIA3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tatro E.T., Everall I.P., Kaul M., Achim C.L. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: implications for major depressive disorder. Brain Res. 2009;1286:1–12. doi: 10.1016/j.brainres.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touma C., Gassen N.C., Herrmann L., Cheung-Flynn J., Bll D.R., Ionescu I.a. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol. Psychiatr. 2011;70:928–936. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Tozzi L., Farrell C., Booij L., Doolin K., Nemoda Z., Szyf M. Epigenetic changes of FKBP5 as a link connecting genetic and environmental risk factors with structural and functional brain changes in major depression. Neuropsychopharmacology. 2018;43:1138–1145. doi: 10.1038/npp.2017.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan R.J., Naicker S., Hinwood M., Nalivaiko E., Buller K.M., Pow D v. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav. Immun. 2010;24:1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Uher R., Payne J.L., Pavlova B., Perlis R.H. Major depressive disorder in DSM-5: implications for clinical practice and research of changes from DSM-IV. Depress. Anxiety. 2014;31:459–471. doi: 10.1002/da.22217. [DOI] [PubMed] [Google Scholar]
- Vahl T.P., Ulrich-lai Y.M., Ostrander M.M., Dolgas C.M., Elfers E.E., Seeley R.J. Comparative analysis of ACTH and corticosterone sampling methods in rats. 2005. 45237. 823-828. [DOI] [PubMed]
- VanZomeren-Dohm A.A., Pitula C.E., Koss K.J., Thomas K., Gunnar M.R. FKBP5 moderation of depressive symptoms in peer victimized, post-institutionalized children. Psychoneuroendocrinology. 2015;51:426–430. doi: 10.1016/j.psyneuen.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M., Heim C., Newport J., Miller A.H., Anderson E., Bronen R. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am. J. Psychiatr. 2002;159:2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K v, Marinescu D., Hartmann J., Wang X.-D., Labermaier C., Scharf S.H. Differences in FKBP51 regulation following chronic social defeat stress correlate with individual stress sensitivity: influence of paroxetine treatment. Neuropsychopharmacology. 2012;37:2797–2808. doi: 10.1038/npp.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Hao J., Lacher R.K., Abbott T., Chung L., Colangelo C.M. Early life stress perturbs key cellular programs in the developing mouse hippocampus. Dev. Neurosci. 2015;37:476–488. doi: 10.1159/000430861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Meaney M.J., Duman R.S., Kaffman A. Affiliative behavior requires juvenile, but not adult neurogenesis. J. Neurosci. 2011;31:14335–14345. doi: 10.1523/JNEUROSCI.1333-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wochnik G.M., Rüegg J., Abel G.A., Schmidt U., Holsboer F., Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J. Biol. Chem. 2004;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- Zannas A.S., Jia M., Hafner K., Baumert J., Wiechmann T., Pape J.C. Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-κB–driven inflammation and cardiovascular risk. Proc. Natl. Acad. Sci. Unit. States Am. 2019;116:11370–11379. doi: 10.1073/PNAS.1816847116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas A.S., Wiechmann T., Gassen N.C., Binder E.B. vol. 41. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology; 2016. pp. 261–274. (Gene-Stress-Epigenetic Regulation of FKBP5: Clinical and Translational Implications). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Pan X., Wang F., Ma J., Su G., Dong Y. Baicalin promotes hippocampal neurogenesis via SGK1- and FKBP5-mediated glucocorticoid receptor phosphorylation in a neuroendocrine mouse model of anxiety/depression. Sci. Rep. 2016;6:1–9. doi: 10.1038/srep30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Brückl T., Nocon A., Pfister H., Binder E.B., Uhr M. Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: results from a 10-year prospective community study. Am. J. Psychiatr. 2011;168:1–18. doi: 10.1176/appi.ajp.2011.10111577.Interaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel A., Schuhmacher A., Jessen F., Hö Fels S., von Widdern O., Metten M. DNA sequence variants of the FKBP5 gene are associated with unipolar depression. Int. J. Neuropsychopharmacol. 2010;13:649–660. doi: 10.1017/S1461145709991155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.