Abstract
Disruption of neurodevelopmental trajectories can alter brain circuitry and increase the risk of psychopathology later in life. While preclinical studies have demonstrated that the immune system and cytokines influence neurodevelopment, whether immune activity and in particular which cytokines at birth are associated with psychopathology remains poorly explored in children. We used data and biological samples from 869 mother-child pairs participating in the French mother-child cohort EDEN. As proxies for immune activity at birth, we measured the levels of 27 cytokines in umbilical cord blood sera (CBS). We then explored the association between CBS cytokine levels and five psychopathological dimensions assessed in 5-year-old children using the Strengths and Difficulties Questionnaire (SDQ). Five cytokines were positively associated with psychopathology: C-X-C motif chemokine Ligand (CXCL)10, interleukin (IL)-10 and IL-12p40 with emotional symptoms, C–C motif chemokine Ligand (CCL)11 with conduct problems, and CCL11, and IL-17A with peer relationships problems. In contrast, seven cytokines were negatively associated with psychopathology: IL-7, IL-15 and Tumor Necrosis Factor (TNF)-β with emotional symptoms, CCL4 and IL-6 with conduct problems, CCL26 and IL-15 with peer relationships problems, and CCL26, IL-7, IL-15, and TNF-α with abnormal prosocial behavior. Without implying causation, these associations support the notion that cytokines influence neurodevelopment in humans and the risk of psychopathology later in life.
Keywords: Cytokines, Inflammation, Mother-child cohort, Psychopathology, Behavior, Neurodevelopment
Highlights
-
•
Twelve cytokines at birth are associated with psychopathology in 5-year-old children.
-
•
IL-7, IL-10, IL-12p40, IL-15, TNF-β and CXCL10 are associated with emotional symptoms.
-
•
IL-6, CCL4 and CCL11 are associated with conduct problems.
-
•
IL-15, IL-17A, CCL11 and CCL26 are associated with peer relationship problems.
-
•
IL-7, IL-15, TNF-α and CCL26 are associated with prosocial behavior.
Nomenclature
- ASD
autism spectrum disorders
- AUROC
area under the receiver operating curve
- BBB
blood-brain barrier
- BMI
body mass index
- CBS
cord blood serum
- CCL
C-C motif chemokine ligand
- CI
confidence interval
- CV
coefficient of variation
- CXCL
C-X-C motif chemokine ligand
- DBS
dried blood spot
- IFN
interferon
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IL
interleukin
- LLOD
lower limit of detection
- MIA
maternal immune activation
- NDD
neurodevelopmental disorders
- NPC
neural progenitor cells
- OR
odds ratio
- SDQ
strengths and difficulties questionnaire
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
- VIP
variable inclusion probability
1. Introduction
Brain ontogeny is a finely tuned developmental process that begins during the third week of gestation in humans and extends up to late adolescence and arguably throughout the lifespan (Stiles and Jernigan, 2010). Disruption of developmental trajectories can alter the formation of brain circuits and contribute to neurodevelopmental disorders (NDD) such as attention deficit and hyperactivity disorder, intellectual disability, and Autism Spectrum Disorder (ASD) (Thapar et al., 2017). NDD result from complex interactions between genetic susceptibility and exposure to environmental risks during the perinatal period. NDD risk increases in children exposed in utero either to Maternal Immune Activation (MIA) due to gestational infection or to conditions accompanied with immune activation (e.g depression, obesity, gestational diabetes, exposure to environmental toxins like alcohol, tobacco, and pollutants) (Grandjean and Landrigan, 2014; Knuesel et al., 2014; Kong et al., 2018; Melchior et al., 2015; Polańska et al., 2015). More generally, immune activity during the perinatal period, and most importantly the levels of proinflammatory cytokines could contribute to psychopathology later in life (Cattane et al., 2018).
Animal studies have demonstrated that at least two cytokines, IL-6 and IL-17a, are the mediators of MIA effects on brain development and behavior (Estes and McAllister, 2015). In pregnant dams, MIA causes a transient elevation of these proinflammatory cytokines, which translate into elevated IL-6 and IL-17a signaling in the fetal brain that interferes with neurodevelopment (Choi et al., 2016; Smith et al., 2007). In support of this, injection of IL-6 or IL-17a to pregnant dams or direct injection of IL-17a in the fetal brain was sufficient to recapitulate MIA-induced behavioral alterations in the offspring, while blocking either IL-6 or IL-17a function using antibodies prevented MIA effects (Choi et al., 2016; Smith et al., 2007). Cytokines also regulate neurodevelopment and brain function in homeostatic conditions (Deverman and Patterson, 2009). In brain parenchyma, neural progenitor cells (NPC), neurons, astrocytes and microglia express cytokines and/or cytokine receptors in spatiotemporally-defined patterns (Deverman and Patterson, 2009). IL-6 neonatal overexpression in mice regulates the balance between excitatory/inhibitory synapses formation (Wei et al., 2012). Low doses of TNF promote the survival, proliferation, and neuronal differentiation of murine NPC (Bernardino et al., 2008). Young Tnf-KO mice exhibit accelerated hippocampal maturation (Golan et al., 2004) and synaptic scaling defects (Stellwagen and Malenka, 2006). These early neurodevelopmental changes, dependent on cytokine signaling, can influence behavioral outcome. For example, maternal Tnf-deletion reduced fear response in the offspring (Zupan et al., 2017), and deletion of TNF receptors reduced anxio-depressive-like behaviors (Simen et al., 2006). Deletion of Ifn-γ had a detrimental impact on social behavior (Filiano et al., 2016), while ablation of IL-15 receptor (IL15R) induced depressive-like behavior and cognitive deficits (Wu et al., 2010).
Human studies relied on in vitro NPC models treated with cytokines and on epidemiological studies addressing the associations between perinatal cytokine levels and NDD diagnosis. In vitro, IL-7 is able to shift the differentiation of human NPC towards the glial cell lineage (Moors et al., 2010), while CCL5 exerts profound effects on the growth and survival of human first-trimester forebrain astrocytes (Bakhiet et al., 2001). CCL3 promotes NPC proliferation and CX3CL1 and TNF-α improve their survival, while CCL11 and CXCL12 inhibit their proliferation (Kim et al., 2020; Krathwohl and Kaiser, 2004). In addition, several studies support a relationship between higher proinflammatory cytokines levels during pregnancy and later development of NDD (Cattane et al., 2018). Increased maternal serum levels of Granulocyte Macrophage Colony Stimulating Factor (GM-CSF), IFN-γ, IL-1α, IL-4, IL-5 and IL-6 during gestation were associated with later diagnosis of ASD in the offspring (Goines et al., 2011; Jones et al., 2017). Also, higher maternal serum levels of IL-8 were associated with lower odds of neurological abnormalities in childhood (Gilman et al., 2017). Finally, associations between cytokines levels measured in dried blood spots (DBS) from newborns and NDD diagnosis or psychopathology were identified in several studies (Abdallah et al., 2013; Ghassabian et al., 2018; Heuer et al., 2019; Krakowiak et al., 2017; Skogstrand et al., 2019; Zerbo et al., 2014). Notably, higher DBS levels of IL-8 and IL-6 levels were associated with increased odds of ASD diagnosis between 4.5 and 9-year-old in a large case-control study involving 370 ASD patients and 378 neurotypical controls (Heuer et al., 2019).
Hence, studies linking cytokine levels at birth to later psychopathology in a population sample of children remain scarce. Of note, most association studies have relied on categorical approaches for NDD diagnosis and not on dimensional constructs of psychopathology which could better capture inter-individual heterogeneity at the population level, as supported by the Research Domain Criteria (RDoC) initiative (Morris and Cuthbert, 2012). To fill this gap, in this exploratory study, we have investigated associations between cytokine levels measured in umbilical cord blood serum (CBS) and five psychopathological dimensions assessed in a large population sample of 5-year-old children.
2. Materials and methods
Extended Materials and Methods are available in Supplementary Information.
2.1. Study sample
The present study is nested within the EDEN mother-child cohort (Barbosa et al., 2020; Heude et al., 2016). Pregnant women were recruited before 24 weeks of gestation in the French University Hospitals of Nancy and Poitiers. Exclusion criteria included multiple pregnancies, a known history of diabetes, the inability to speak and read French or plans to move out of the study region in the following 3 years. Clinical and psychosocial data were gathered from medical records, interviews with the mother and auto-questionnaires. Our study sample consisted of the 869 mother-child pairs for which both CBS and the behavioral outcome at 5-year-old were available.
2.2. Covariates and confounding factors
Previous studies have identified maternal, perinatal and psychosocial variables associated with behavioral abnormalities in children (De La Rochebrochard and Joshi, 2013; Gumusoglu and Stevens, 2019; Melchior et al., 2015; Noonan, 2018; Polańska et al., 2015; van der Waerden et al., 2015). Based on these studies, and considering that many of these variables can also influence cytokine production (O’Connor et al., 2009), we selected the following variables in our models: maternal age at delivery (years), maternal pre-pregnancy body mass index (BMI in kg/m2), smoking during pregnancy (number of cigarettes/day), caffeine intake during pregnancy (mg caffeine/day), alcohol drinking during pregnancy (mean number of glasses/week), gestational age (weeks of amenorrhea), delivery mode (vaginal, C-section), birth weight (g), birth trimester and sex (female/male), maternal and paternal education duration (years), multiparity (number of older siblings), record of depression during pregnancy (yes/no) and symptoms of prenatal anxiety. Maternal depression was assessed at 24–28 weeks of amenorrhea, using the Center for Epidemiological Studies Depression questionnaire (CES-D (Radloff, 1991)) and women presenting a CES-D score above a cutoff of 17 were considered as depressed. Symptoms of maternal anxiety were assessed at 24–28 weeks of amenorrhea, using the State-Trait Anxiety Inventory (STAI (Spielberger and Vagg, 1984)) and the continuous STAI score was used to assess the severity of anxiety symptoms. Information regarding maternal diagnosis of autoimmune disorder and undergoing treatment were collected. However, the proportion of pregnant women with autoimmune disorders and/or undergoing treatment was very low in our study sample. These variables were therefore not included in our models.
2.3. Outcome assessment: child’s psychopathology at age 5
Child’s behavioral outcome was assessed using the Strengths and Difficulties Questionnaire (SDQ) completed by the mother when the child was 5–6 years of age (Goodman, 2001). The SDQ is a broadly used psychometric instrument and entails five psychopathological subscales measured by five items each: emotional symptoms, conduct problems, hyperactivity/inattention, peer relationship problems and prosocial behavior. To stratify children at high-risk and low-risk for psychopathology, we dichotomized the SDQ difficulties subscales and the prosocial subscale at the 85th upper percentile and 15th lower percentile respectively (Supplementary Figure 1). Such a dichotomization was used in previous studies (Barbosa et al., 2020; Melchior et al., 2015; Philippat et al., 2017) and in studies using similar psychometric scales (Amone-P’Olak et al., 2009; Huisman et al., 2010; Schneiders et al., 2003). For conduct problems, hyperactivity/attention disorder, peer relationship problems and prosocial behavior 4 for emotional symptoms, we used cutoffs of 4, 5, 6, 3 and 6, respectively, yielding a high-risk class accounting for 20.6%, 14.8%, 14.5%, 14.8% and 14.9% of all children respectively (Supplementary Figure 1).
2.4. Cytokine measurements in CBS
Venous cord blood was sampled immediately after birth and allowed to clot. Blood samples were centrifuged within 24 h post collection, CBS were collected and stored at −80 °C. CBS were assessed for 27 cytokines (CCL2, CCL3, CCL4, CCL11, CCL17, CCL26, CXCL10, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12/IL-23 p40, IL-12 p70, IL-13, IL-15, IL-16, IL-17A, Vascular Endothelial Growth Factor (VEGF)-A, TNF-α and TNF-β) using a multiplex electrochemiluminescence (ECL)-based immunoassay (Meso Scale Diagnostics, Rockville, USA). For cytokine concentrations below the lower limit of detection (LLOD), half the LLOD value was imputed as recommended for immunological measurements constrained by detection limits (Uh et al., 2008). Cytokines whose levels were below the LLOD in more than 15% of the samples were not used in downstream analysis. Inter-runs coefficients of variations (CV) were below 20%, therefore complying with the recommendations of the US Food and Drugs Administration (FDA) regarding ligand binding assays (Supplementary Table 1).
2.5. Descriptive statistics
Comparison analyzes of variables in the low-versus high-risk group based on each SDQ subscore were performed using the Wilcoxon-Mann & Whitney U test for numerical variables or with the Chi-square test for categorical variables, without and with Benjamini & Hochberg’s multiple testing correction (False Discovery Rate). Correlations between concentrations of cytokine pairs were performed using Spearman’s rho correlation coefficient rank test with Benjamini & Hochberg’s multiple testing correction. Statistical significance was set at a p-value < 0.05.
2.6. Association studies
The methodological workflow is summarized in Supplementary Figure 2. We used an Elastic Net penalized regression to model the associations between cytokine levels in CBS at birth, clinical and psychosocial variables collected until birth and increased odds of developing behavioral difficulties at 5-year-old, with each of the SDQ subscores considered independently. Missing data (corresponding to NAs in Table 1) were imputed using the Multivariate Imputation (MI) by Chained Equations (MICE) procedure in R (Buuren and Groothuis-Oudshoorn, 2010) and non-parametric bootstrap was used for statistical inference (Abram et al., 2016). The CARET (Kuhn, 2008a) and GLMNET (Friedman et al., 2010) R packages were used to implement Elastic Net penalized logistic regression models. For each variable we computed the variable inclusion probability (VIP, % runs in which the variable was selected by the penalized regression), the median odd ratios (OR) and the percentile bootstrap confidence intervals (CI). The predictive capabilities of each model were estimated by computing the overall predicted accuracy using the Area Under the Receiver Operating Characteristics (AUROC), sensitivity, specificity, and positive and negative predictive values using the CARET, rms and pROC packages (Harrell, 2001; Kuhn, 2008b; Robin et al., 2011).
Table 1.
Study population variables. N, available values; SD, standard deviation; NAs, non-available values; BMI, body mass index; STAI, State-Trait Anxiety Inventory.
| N | Mean (SD) | NAs (%) | |||
|---|---|---|---|---|---|
| Maternal variables | Age at delivery (years) | 869 | 30.2 (4.7) | 0.0 | |
| Pre-pregnancy BMI (kg.m-2) | 855 | 23.2 (4.3) | 1.6 | ||
| Smoking during pregnancy (cigarettes/day) | 849 | 1 (2.7) | 2.3 | ||
| Caffeine consumption during pregnancy (mg/day) | 856 | 90.4 (108.3) | 1.5 | ||
| Alcohol drinking during pregnancy (mean glasses/week) |
565 |
0,4 (1.6) |
35.0 |
||
| Perinatal variables | Gestational age (weeks of amennorhea) | 869 | 39.3 (1.6) | 0.0 | |
| Delivery mode | Vaginal | 759 | 0.1 | ||
| C-section | 109 | ||||
| Birth weight (g) | 869 | 3314.9 (489.6) | 0.0 | ||
| Birth trimester | 1 (Jan.–March) | 219 | 0.0 | ||
| 2 (April–June) | 250 | ||||
| 3 (July–Sept.) | 219 | ||||
| 4 (Oct.–Dec.) | 181 | ||||
| Sex | Male | 461 | 0.0 | ||
| Female |
408 |
||||
| Psychosocial variables | Maternal prenatal anxiety (STAI scale) | 867 | 9.9 (9.6) | 0.2 | |
| Maternal depression during pregnancy (no) | 674 | 0.5 | |||
| Maternal depression during pregnancy (yes) | 191 | ||||
| Maternal education (years) | 867 | 14.1 (2.6) | 0.2 | ||
| Paternal education (years) | 805 | 13.4 (2.7) | 7.4 | ||
| Multiparity (number of older siblings) | 798 | 0.9 (1) | 8.2 | ||
2.7. Dataset availability
Our dataset will be made available by the corresponding author after request and acceptance by the EDEN birth cohort steering committee.
2.8. Ethics
The EDEN cohort received approval from the Ethical Research Committee (CCPPRB) of Bicêtre Hospital and from the French National Data Protection Agency (CNIL). Informed written consent was obtained from parents at the time of enrollment and after delivery.
3. RESULTS
3.1. Study sample characteristics
Our study sample consisted of 869 mother-child pairs for which both CBS samples and SDQ psychometric scores at the age of 5 were available. The distributions of socioeconomic variables in this sample were comparable to national estimates (Blondel et al., 2012; Heude et al., 2016) with the exception of a higher level of maternal education (Blondel et al., 2012; Heude et al., 2016) (Table 1). The high-risk group consisted of children presenting SDQ subscores within the 15th upper percentile for the emotions, conduct, hyperactivity/inattention and peer relationships dimensions, and within the 15th lowest percentile for the prosocial behavior dimension (Supplementary Figure 1), consistent with previous studies (Barbosa et al., 2020; Melchior et al., 2015; Philippat et al., 2017). After correction for multiple testing, comparison analysis between the low- and high-risk groups showed that they only differed in the number of older siblings for emotional symptoms; maternal education and depression during pregnancy for conduct problems; maternal age, smoking during pregnancy, sex, maternal and paternal education and number of older siblings for hyperactivity; paternal and maternal education for peer relationship problems (Supplementary Table 2).
3.2. Cytokine levels at birth
We assessed CBS samples for 27 cytokines including 7 chemokines (CCL2, CCL3, CCL4, CCL11, CCL17, CCL26, CXCL10), one member of the Platelet-Derived Growth factor family (GM-CSF), one member of the type I interferon family (IFN-γ), 15 interleukins (IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-16, IL-17A), VEGF-A, and two members of the TNF family (TNF-α and TNF-β). We selected these cytokines for three main reasons. First, their role as effector or regulatory molecules of the immune system has been well documented. Second, several of these cytokines have been previously demonstrated to modulate neuronal and glial functions. Third, low levels of these cytokines can be detected using the multiplexing MSD platform that offers access to high-performance electro-chemiluminescence immunoassays. Compared to enzyme-linked immunosorbent assay (ELISA) or multiplexed bead-based immunoassays, the MSD multi-array technology offers ultra-low detection limits, easily handles complex matrices such as serum and plasma, and provides up to five logs of linear dynamic range. IL-1α, IL-2, IL-4, IL-5, IL-12p70, IL-13, VEGF-A and GM-CSF were excluded because their concentration was below the LLOD in more than 15% of the samples (Table 2). All other 19 cytokines displayed inter-assay CV lower than 20% and were included in downstream analysis (Supplementary Table 1). None of the cytokines were differentially expressed between the low- and high-risk groups after correction for multiple testing (Supplementary Table 2). Moderate to strong correlations were observed between cytokine pairs (Supplementary Figure 3), indicating multicollinearity among cytokines.
Table 2.
Serum cytokine concentrations in umbilical cord blood. LLOD, the number (Nb < LLOD) and proportion (% <LLOD) of serum samples in which cytokine concentrations were below LLOD, minimum (Min.), median, maximum (Max.), mean concentrations, and standard deviation (SD) are indicated for the 27 biomarkers assessed. Filled circles: variable included.
| Cytokines | LLOD | Nb < LLOD | % <LLOD | Min. (pg/ml) | Median (pg/ml) | Max. (pg/ml) | Mean (pg/ml) | SD (pg/ml) | Included |
|---|---|---|---|---|---|---|---|---|---|
| CCL2 | 0.09 | 2 | 0.23 | 0.05 | 291.97 | 4945.75 | 330.33 | 249.38 | ● |
| CCL3 | 3.02 | 7 | 0.80 | 1.51 | 20.84 | 4364.65 | 34.26 | 159.91 | ● |
| CCL4 | 0.17 | 0 | 0.00 | 1.15 | 247.44 | 2382.74 | 265.52 | 132.57 | ● |
| CCL11 | 3.26 | 1 | 0.11 | 1.63 | 208.60 | 656.03 | 223.03 | 93.52 | ● |
| CCL17 | 0.22 | 1 | 0.11 | 0.11 | 1222.50 | 9638.18 | 1474.60 | 1092.40 | ● |
| CCL26 | 1.77 | 33 | 3.79 | 0.89 | 9.83 | 247.05 | 13.09 | 16.27 | ● |
| CXCL10 | 0.37 | 2 | 0.23 | 0.19 | 127.50 | 9733.82 | 203.61 | 445.22 | ● |
| GM-CSF | 0.16 | 274 | 31.46 | 0.07 | 0.21 | 0.34 | 0.31 | 0.56 | |
| IFN-γ | 0.37 | 3 | 0.34 | 0.10 | 2.23 | 69.91 | 2.77 | 3.26 | ● |
| IL-1α | 0.09 | 375 | 43.05 | 0.04 | 0.19 | 51.01 | 0.90 | 3.46 | |
| IL-1β | 0.05 | 73 | 8.38 | 0.02 | 0.23 | 99.04 | 1.05 | 5.19 | ● |
| IL-2 | 0.09 | 347 | 39.84 | 0.04 | 0.11 | 12.17 | 0.18 | 0.61 | |
| IL-4 | 0.02 | 327 | 37.54 | 0.01 | 0.03 | 8.15 | 0.09 | 0.38 | |
| IL-5 | 0.14 | 393 | 45.12 | 0.11 | 0.26 | 91.50 | 0.51 | 3.12 | |
| IL-6 | 0.06 | 0 | 0.00 | 0.16 | 2.66 | 3415.47 | 24.76 | 177.42 | ● |
| IL-7 | 0.12 | 1 | 0.11 | 0.08 | 8.51 | 34.87 | 9.27 | 3.83 | ● |
| IL-8 | 0.07 | 0 | 0.00 | 0.13 | 11.72 | 5084.48 | 58.73 | 330.18 | ● |
| IL-10 | 0.04 | 0 | 0.00 | 0.04 | 0.59 | 109.88 | 1.18 | 4.55 | ● |
| IL-12/IL-23 p40 | 0.33 | 1 | 0.11 | 0.20 | 615.85 | 1928.67 | 656.45 | 249.82 | ● |
| IL-12 p70 | 0.11 | 604 | 69.35 | 0.06 | 0.06 | 15.43 | 0.25 | 1.07 | |
| IL-13 | 0.24 | 423 | 48.56 | 0.12 | 0.27 | 50.32 | 0.80 | 2.42 | |
| IL-15 | 0.15 | 1 | 0.11 | 0.09 | 2.12 | 184.03 | 2.80 | 7.21 | ● |
| IL-16 | 2.83 | 1 | 0.11 | 1.42 | 294.33 | 10104.90 | 448.53 | 576.33 | ● |
| IL-17A | 0.10 | 3 | 0.34 | 0.37 | 8.14 | 47.75 | 9.60 | 6.55 | ● |
| VEGF-A | 1.12 | 178 | 20.44 | 0.56 | 190.84 | 25142863.00 | 153775.50 | 1449508.00 | |
| TNF-α | 0.34 | 4 | 0.46 | 0.02 | 3.23 | 45.77 | 3.59 | 2.50 | ● |
| TNF-β | 0.08 | 1 | 0.11 | 0.03 | 0.65 | 6.09 | 0.79 | 0.55 | ● |
3.3. Association between serum cytokine levels at birth and psychopathology
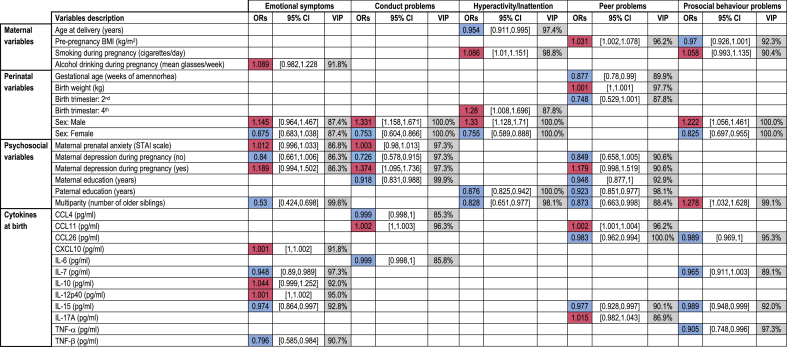
To explore the associations between cytokine levels at birth and psychopathology, we used an Elastic Net penalized regression framework that computes, for each variable, the VIP, median OR and percentile bootstrap CI (cf 2.6.). According to a seminal paper, the VIP can be interpreted as the posterior probability of including a variable in the model (Bunea et al., 2011). Buena et al. recommended a “conservative VIP threshold of 50%” for variable selection, because their goal was “not to miss any possibly relevant predictors”. However, this 50% threshold dramatically increases the risk of false positives. To define a stringent VIP threshold, we relied on the VIP computed for the clinical and psychosocial variables commonly described as associated with psychopathology in children: maternal variables (age at delivery, pre-pregnancy BMI, smoking, alcohol consumption), birth variables (gestational age, birth weight, sex) and psychosocial variables (maternal anxiety and depression during pregnancy, parental education, number of older siblings), consistent with a previous study (Barbosa et al., 2020). Because VIP for these variables ranged from 86.3% to 100% (Table 3, Supplementary Table 3), we selected a VIP threshold of 85% to identify cytokines stably associated with each behavioral outcome. Based on this threshold, no cytokine was associated with hyperactivity/inattention, while 12 cytokines were associated with at least one of the four other SDQ dimensions. Five cytokines were positively associated with psychopathology at the age of 5: CXCL10, IL-10 and IL-12/IL-23 p40 with emotional symptoms, CCL11 with conduct problems, and CCL11 and IL-17A with peer problems. In contrast, seven cytokines were negatively associated with psychopathology: IL-7, IL-15 and TNF-β with emotional symptoms, CCL4 and IL-6 with conduct problems, CCL26 and IL-15 with peer problems, and CCL26, IL-7, IL-15 and TNF-α with abnormal prosocial behavior.
Table 3.
Adjusted associations between selected variables and high-risk of behavioral problems in 5-year-old children. Median Odd Ratios (ORs), 95% Confidence Interval (95% CI) and Variable Inclusion Probability (VIP) for each of the variables selected by the Elastic Net. Only selected variables with VIPs above 85% and OR distinct from 1 are presented. In the ORs column, positive (shaded red) and negative (shaded blue) associations are indicated. See also Supplementary Table 3 detailing the results for all 34 variables included.
3.4. Predictive capabilities
We then determined the abilities of our models to predict the risk of belonging to the group at high-risk for adverse behavioral outcome at 5, based on the 19 cytokines measured at birth and the 15 clinical and psychosocial variables collected until birth (Table 4). The predictive performance of the models, assessed by computing AUROC, ranged from 64.6% to 71.7% depending on the SDQ dimension. Sensitivity ranged from 58.9% to 70.1%, specificity from 58% to 69.2%. Positive predictive from 21.9% to 26.5% and negative predictive value from 90.2% to 93.9%. These results indicated the overall poor predictive performance of our models.
Table 4.
Predictive abilities of the immune signatures at birth. Mean and 95% Confidence Interval (95% CI) for AUROC, sensitivity, specificity, positive and negative predictive values and optimal threshold of the different models used to predict the risk of belonging to the high-risk group for each of the five dimensions.
| Emotional symptoms |
Conduct problems |
Hyperactivity/Inattention |
Peer problems |
Prosocial behavior problems |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Performance metrics | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI |
| AUROC | 0.716 | [0.685,0.738] | 0.649 | [0.632,0.667] | 0.717 | [0.694,0.732] | 0.659 | [0.625,0.677] | 0.646 | [0.622,0.672] |
| Sensitivity | 0.701 | [0.533,0.85] | 0.612 | [0.367,0.814] | 0.659 | [0.476,0.802] | 0.589 | [0.38,0.829] | 0.667 | [0.434,0.853] |
| Specificity | 0.660 | [0.495,0.807] | 0.622 | [0.416,0.861] | 0.692 | [0.539,0.852] | 0.673 | [0.422,0.853] | 0.580 | [0.374,0.795] |
| Positive Predictive Value | 0.223 | [0.187,0.285] | 0.221 | [0.193,0.327] | 0.265 | [0.224,0.357] | 0.239 | [0.198,0.319] | 0.219 | [0.188,0.271] |
| Negative Predictive Value | 0.939 | [0.923,0.96] | 0.902 | [0.885,0.928] | 0.923 | [0.905,0.942] | 0.903 | [0.886,0.934] | 0.909 | [0.887,0.938] |
| Optimal threshold | 0.125 | [0.079,0.179] | 0.150 | [0.101,0.229] | 0.155 | [0.107,0.231] | 0.159 | [0.107,0.232] | 0.138 | [0.085,0.2] |
4. DISCUSSION
Here, we have identified six interleukins, two members of the TNF family and four chemokines whose levels in umbilical cord blood are associated with altered odds of psychopathology in 5-year-old children. Some of these associations are positive indicative of a detrimental effect, while others are negative suggestive of a protective effect.
While association does not imply causation, it is tempting to speculate that changes in cytokine levels at birth could influence perinatal neurodevelopment and eventually behavior in children. While IL-6, IL-15, TNF-α and CCL11 were shown to cross the Brain Blood Barrier (BBB) in rodents (Banks, 2015), it is not known for IL-7, IL-10, IL-12/IL-23 p40, IL-17A, CCL4, CCL26 and CXCL10. Further, nothing is known on the ability of these peripheral cytokines to cross the BBB in humans. Would this be the case, cytokines could reach the brain parenchyma and act on NPC, differentiating neurons, astrocytes that regulate neuron survival and synaptic plasticity (Ben Achour and Pascual, 2010) or microglia that shape neuronal connections by pruning supernumerary synapses and axons (Mosser et al., 2017). In this respect, several cytokine receptors are expressed in human NPC or fetal brain, including those for CCL4 (CCR5 and CCR8) (Kalev et al., 2006), CCL11 and CCL26 (CCR3) (van der Meer et al., 2001), CXCL10 (CXCR3) (Goldberg et al., 2001; van der Meer et al., 2001), IL-6 (IL-6R) (Dame and Juul, 2000; Ulfig and Friese, 1999), IL-15 (IL-15R) (Kurowska et al., 2002), IL-17A (IL-17RA and IL-17RC) (Elain et al., 2014), and for both TNF-α and TNF-β (TNFR-1 and TNFR-2) (Sheng et al., 2005). The ability of these cytokines to act on different cell types in the human brain is well documented. For example, IL-1β and TNF-α can activate fetal astrocytes to secrete other cytokines (Choi et al., 2014). Moreover, several cytokines can affect the proliferation, survival and differentiation of human NPC. IL-7 promotes the survival of NPCs (Araujo and Cotman, 1993), while IL-6 promotes their differentiation (Johansson et al., 2008). In differentiating NPC, TNF-α induces a shift from the neuronal to the astroglial lineage (Johansson et al., 2008), likely by increasing the apoptosis of NPC-derived neurons (Wang et al., 2011). Thus, by influencing the development/survival of the main brain cell types, the cytokines that we have identified could modulate the development of yet-to-be identified brain structures.
While some cytokines could cross the BBB and target NPC, neuronal or glial cells expressing their receptors, others could act indirectly on the developing brain by changing BBB permeability. For example, IL-17A was found to open the BBB, allowing the passage of other cytokines and immune cells (Cipollini et al., 2019). Alternatively, cytokines could influence the activation, differentiation or survival of immune cells. In this respect, it is noteworthy that both IL-7 and IL-15 promote the proliferation and survival of T lymphocytes (Boyman et al., 2007), the latter cells being critical for normal emotional and social behavior in rodents (Filiano et al., 2016; Rattazzi et al., 2013). Finally, altered cytokine levels at birth could also reflect changes in immune activity and condition an increased susceptibility to early childhood infections, an acknowledged risk factor for later development of neuropsychiatric disorders (Blomström et al., 2014; Dalman et al., 2008; Köhler et al., 2017).
Out of the 11 cytokines measured at birth associated with psychopathology, seven (IL-7, IL-10, IL-15, IL-16, IL-17A, TNF-α, CXCL10) were previously cross-sectionally associated with psychopathology at age 5 on the same population sample (Barbosa et al., 2020). However, these cytokines were associated with distinct pathopsychological domains and sometimes in opposite manner. We found here that higher levels of IL-17A at birth increase the odds of exhibiting peer problems at 5, while we previously reported that increased levels of IL-17A decreased the odds of emotional problems at 5 years (Barbosa et al., 2020). Studies in mice have shown that, in wildtype mice, acute intracerebroventricular injection of IL-17a in the embryonic brain impairs later social behavior in adulthood (Choi et al., 2016), while acute intracerebral injection of IL-17a rescues sociability deficits in the MIA and genetic models of ASD but not in unmanipulated mice (Reed et al., 2020). This supports the notion that some cytokines can exert antagonistic effects on behavior depending on the developmental stage considered and preexistent environmental or genetic susceptibility.
Neonatal immune activity is influenced by the immune environment during pregnancy, including maternal cytokines (Yu et al., 2018). In this context, the type of biological matrix elected to measure cytokine concentrations as proxies for immune activity at birth is critical. Most studies have relied on DBS collected from the neonate several hours or days after birth (Abdallah et al., 2013; Ghassabian et al., 2018; Heuer et al., 2019; Krakowiak et al., 2017; Mizejewski et al., 2013; Nelson et al., 2006; Skogstrand et al., 2019; Thürmann et al., 2019; Zerbo et al., 2014). In contrast, we have measured cytokine levels in CBS, i.e. blood from the fetoplacental unit. Compared to DBS, we believe that the CBS better reflect the immune milieu to which the fetus is exposed at birth, without immune interferences from postnatal pathogen exposure or breastfeeding.
Regarding the possible use of CBS cytokines as biomarkers of psychopathology later in life, we investigated the predictive abilities of our models to decipher whether an immune signature at birth could predict behavioral outcome at 5. The performances of our models were at best borderline-good for the emotional (AUROC = 0.716) and hyperactivity (AUROC = 0.717) dimensions and suboptimal for the other dimensions (AUROC range: 0.646–0.659), and they are likely to be inflated due to the unbalance in the outcome classes (Luque et al., 2019). Our models display suboptimal sensitivity and specificity and predict only 21.9–26.5% of the children at risk for behavioral problems. These data suggest that an immune signature at birth bears very limited potential for prediction of later psychopathology and confirm a previous study showing the limited utility of cytokine signature to predict ASD risk (Heuer et al., 2019). Even though we have introduced in our model 34 variables (19 cytokines and 15 clinical/psychosocial variables), our study either calls for the identification of additional explanatory variables to improve the prediction capacities of our models or merely suggests that postnatal events are determinant to precipitate the development of psychopathological traits later in childhood.
While we believe that the present study provides new insight into the role of cytokines in brain neurodevelopment during the perinatal period, we are aware of its limitations. First, our results have not been replicated in an independent sample. Second, we have not considered a few confounding factors that could impact both behavioral outcomes and immune responses, such as in utero exposure to environmental pollutants, maternal diet, maternal diagnosis of autoimmune disorder or treatment with drugs. Third, our socially advantaged population sample may preclude generalization of our findings. Finally, the penalized regression that we have used to analyze our datasets is known to shrink the coefficient resulting in OR which are underestimated. This may limit their interpretation in terms of effect size.
5. CONCLUSION
To our knowledge, this study is the first to thoroughly investigate the associations between cytokine levels in umbilical cord blood and child’s psychopathology. While association does not imply causation, our data are compatible with a model in which changes in cytokine levels early in life impact brain development in humans. On a related topic, several maternal variables are associated with an increased risk of psychopathology in children, including smoking, alcohol consumption, depression and obesity during pregnancy ((Kong et al., 2018; Melchior et al., 2015; Polańska et al., 2015; van der Waerden et al., 2015) and this study). As these variables also influence peripheral cytokine levels (O’Connor et al., 2009), their effect on the child’s psychopathology may be mediated by cytokines. Further studies set in a mediation framework should address the possible role of cytokines as mediators of maternal determinants on child’s psychopathology.
Declaration of competing interest
None.
Acknowledgements
We thank the families who participated to this study. We thank the midwife research assistants for data collection, the psychologists and the data entry operators. We thank the EDEN Mother-Child Cohort Study Group which includes the following members: I. Annesi-Maesano, J.Y. Bernard, J. Botton, M.A. Charles, P. Dargent-Molina, B. de Lauzon-Guillain, P. Ducimetière, M. de Agostini, B. Foliguet, X. Fritel, A. Germa, V. Goua, R. Hankard, M. Kaminski, B. Larroque, N. Lelong, J. Lepeule, G. Magnin, L. Marchand, C. Nabet, F. Pierre, R. Slama, M.J. Saurel-Cubizolles, M. Schweitzer, and O. Thiebaugeorges. We thank the Center de Calculs Interactifs de l’Université Côte d’Azur for access to the computing facility. LD, CD and BH acknowledge the generous support of the Fondation de France (AAP Autisme et neurodéveloppement).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100141.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abdallah M.W., Larsen N., Grove J., Bonefeld-Jørgensen E.C., Nørgaard-Pedersen B., Hougaard D.M., Mortensen E.L. Neonatal chemokine levels and risk of autism spectrum disorders: findings from a Danish historic birth cohort follow-up study. Cytokine. 2013;61:370–376. doi: 10.1016/j.cyto.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Abram S.V., Helwig N.E., Moodie C.A., DeYoung C.G., MacDonald A.W., 3rd, Waller N.G. Bootstrap enhanced penalized regression for variable selection with neuroimaging data. Front. Neurosci. 2016;10:344. doi: 10.3389/fnins.2016.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amone-P’Olak K., Burger H., Ormel J., Huisman M., Verhulst F.C., Oldehinkel A.J. Socioeconomic position and mental health problems in pre- and early-adolescents: the TRAILS study. Soc. Psychiatr. Psychiatr. Epidemiol. 2009;44:231–238. doi: 10.1007/s00127-008-0424-z. [DOI] [PubMed] [Google Scholar]
- Araujo D.M., Cotman C.W. Trophic effects of interleukin-4, -7 and -8 on hippocampal neuronal cultures: potential involvement of glial-derived factors. Brain Res. 1993;600:49–55. doi: 10.1016/0006-8993(93)90400-h. [DOI] [PubMed] [Google Scholar]
- Bakhiet M., Tjernlund A., Mousa A., Gad A., Strömblad S., Kuziel W.A., Seiger A., Andersson J. RANTES promotes growth and survival of human first-trimester forebrain astrocytes. Nat. Cell Biol. 2001;3:150–157. doi: 10.1038/35055057. [DOI] [PubMed] [Google Scholar]
- Banks W.A. The blood-brain barrier in neuroimmunology: tales of separation and assimilation. Brain Behav. Immun. 2015;44:1–8. doi: 10.1016/j.bbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa S., Khalfallah O., Forhan A., Galera C., Heude B., Glaichenhaus N., Davidovic L. Serum cytokines associated with behavior: a cross-sectional study in 5-year-old children. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.01.005. [DOI] [PubMed] [Google Scholar]
- Ben Achour S., Pascual O. Glia: the many ways to modulate synaptic plasticity. Neurochem. Int. 2010;57:440–445. doi: 10.1016/j.neuint.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Bernardino L., Agasse F., Silva B., Ferreira R., Grade S., Malva J.O. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cell. 2008;26:2361–2371. doi: 10.1634/stemcells.2007-0914. [DOI] [PubMed] [Google Scholar]
- Blomström Å., Karlsson H., Svensson A., Frisell T., Lee B.K., Dal H., Magnusson C., Dalman C. Hospital admission with infection during childhood and risk for psychotic illness–a population-based cohort study. Schizophr. Bull. 2014;40:1518–1525. doi: 10.1093/schbul/sbt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel B., Lelong N., Kermarrec M., Goffinet F. Trends in perinatal health in France from 1995 to 2010. Results from the French national perinatal surveys. J. Gynecol. Obstet. Biol. Reprod. 2012;41:e1–e15. doi: 10.1016/j.jgyn.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Boyman O., Purton J.F., Surh C.D., Sprent J. Cytokines and T-cell homeostasis. Curr. Opin. Immunol. 2007;19:320–326. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Bunea F., She Y., Ombao H., Gongvatana A., Devlin K., Cohen R. Penalized least squares regression methods and applications to neuroimaging. Neuroimage. 2011;55:1519–1527. doi: 10.1016/j.neuroimage.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buuren S.v., Groothuis-Oudshoorn K. MICE: multivariate imputation by chained Equations in R. Journal of statistical software in press. 2010 1-null. [Google Scholar]
- Cattane N., Richetto J., Cattaneo A. Prenatal exposure to environmental insults and enhanced risk of developing Schizophrenia and Autism Spectrum Disorder: focus on biological pathways and epigenetic mechanisms. Neurosci. Biobehav. Rev. 2018 doi: 10.1016/j.neubiorev.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Choi G.B., Yim Y.S., Wong H., Kim S., Kim H., Kim S.V., Hoeffer C.A., Littman D.R., Huh J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.S., Lee H.J., Lim I., Satoh J., Kim S.U. Human astrocytes: secretome profiles of cytokines and chemokines. PloS One. 2014;9:e92325. doi: 10.1371/journal.pone.0092325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipollini V., Anrather J., Orzi F., Iadecola C. Th17 and cognitive impairment: possible mechanisms of action. Front. Neuroanat. 2019;13:95. doi: 10.3389/fnana.2019.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalman C., Allebeck P., Gunnell D., Harrison G., Kristensson K., Lewis G., Lofving S., Rasmussen F., Wicks S., Karlsson H. Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. Am. J. Psychiatr. 2008;165:59–65. doi: 10.1176/appi.ajp.2007.07050740. [DOI] [PubMed] [Google Scholar]
- Dame J.B., Juul S.E. The distribution of receptors for the pro-inflammatory cytokines interleukin (IL)-6 and IL-8 in the developing human fetus. Early Hum. Dev. 2000;58:25–39. doi: 10.1016/s0378-3782(00)00064-5. [DOI] [PubMed] [Google Scholar]
- De La Rochebrochard E., Joshi H. Siblings and child development. Longitudinal and Life Course Studies. 2013;4:276–287. [Google Scholar]
- Deverman B.E., Patterson P.H. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Elain G., Jeanneau K., Rutkowska A., Mir A.K., Dev K.K. The selective anti-IL17A monoclonal antibody secukinumab (AIN457) attenuates IL17A-induced levels of IL6 in human astrocytes. Glia. 2014;62:725–735. doi: 10.1002/glia.22637. [DOI] [PubMed] [Google Scholar]
- Estes M.L., McAllister A.K. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat. Rev. Neurosci. 2015;16:469–486. doi: 10.1038/nrn3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano A.J., Xu Y., Tustison N.J., Marsh R.L., Baker W., Smirnov I., Overall C.C., Gadani S.P., Turner S.D., Weng Z., Peerzade S.N., Chen H., Lee K.S., Scott M.M., Beenhakker M.P., Litvak V., Kipnis J. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature. 2016;535:425–429. doi: 10.1038/nature18626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Software. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A., Sundaram R., Chahal N., McLain A.C., Bell E.M., Lawrence D.A., Gilman S.E., Yeung E.H. Concentrations of immune marker in newborn dried blood spots and early childhood development: results from the Upstate KIDS Study. Paediatr. Perinat. Epidemiol. 2018;32:337–345. doi: 10.1111/ppe.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S.E., Hornig M., Ghassabian A., Hahn J., Cherkerzian S., Albert P.S., Buka S.L., Goldstein J.M. Socioeconomic disadvantage, gestational immune activity, and neurodevelopment in early childhood. Proc. Natl. Acad. Sci. U. S. A. 2017;114:6728–6733. doi: 10.1073/pnas.1617698114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P.E., Croen L.A., Braunschweig D., Yoshida C.K., Grether J., Hansen R., Kharrazi M., Ashwood P., Van de Water J. Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: a case-control study. Mol. Autism. 2011;2 doi: 10.1186/2040-2392-2-13. 13–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan H., Levav T., Mendelsohn A., Huleihel M. Involvement of tumor necrosis factor alpha in hippocampal development and function. Cerebr. Cortex. 2004;14:97–105. doi: 10.1093/cercor/bhg108. [DOI] [PubMed] [Google Scholar]
- Goldberg S.H., van der Meer P., Hesselgesser J., Jaffer S., Kolson D.L., Albright A.V., González-Scarano F., Lavi E. CXCR3 expression in human central nervous system diseases. Neuropathol. Appl. Neurobiol. 2001;27:127–138. doi: 10.1046/j.1365-2990.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Landrigan P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumusoglu S.B., Stevens H.E. Maternal inflammation and neurodevelopmental programming: a review of preclinical outcomes and implications for translational psychiatry. Biol. Psychiatr. 2019;85:107–121. doi: 10.1016/j.biopsych.2018.08.008. [DOI] [PubMed] [Google Scholar]
- Harrell F. Springer-Verlag; New York: 2001. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis. [Google Scholar]
- Heude B., Forhan A., Slama R., Douhaud L., Bedel S., Saurel-Cubizolles M.J., Hankard R., Thiebaugeorges O., De Agostini M., Annesi-Maesano I., Kaminski M., Charles M.A. Cohort Profile: the EDEN mother-child cohort on the prenatal and early postnatal determinants of child health and development. Int. J. Epidemiol. 2016;45:353–363. doi: 10.1093/ije/dyv151. [DOI] [PubMed] [Google Scholar]
- Heuer L.S., Croen L.A., Jones K.L., Yoshida C.K., Hansen R.L., Yolken R., Zerbo O., DeLorenze G., Kharrazi M., Ashwood P., Van de Water J. An exploratory examination of neonatal cytokines and chemokines as predictors of autism risk: the early markers for autism study. Biol. Psychiatr. 2019;86:255–264. doi: 10.1016/j.biopsych.2019.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman M., Araya R., Lawlor D.A., Ormel J., Verhulst F.C., Oldehinkel A.J. Cognitive ability, parental socioeconomic position and internalising and externalising problems in adolescence: findings from two European cohort studies. Eur. J. Epidemiol. 2010;25:569–580. doi: 10.1007/s10654-010-9473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S., Price J., Modo M. Effect of inflammatory cytokines on major histocompatibility complex expression and differentiation of human neural stem/progenitor cells. Stem Cell. 2008;26:2444–2454. doi: 10.1634/stemcells.2008-0116. [DOI] [PubMed] [Google Scholar]
- Jones K.L., Croen L.A., Yoshida C.K., Heuer L., Hansen R., Zerbo O., DeLorenze G.N., Kharrazi M., Yolken R., Ashwood P., Van de Water J. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol. Psychiatr. 2017;22:273–279. doi: 10.1038/mp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalev I., Kaasik A., Zarkovski A., Mikelsaar A.V. Chemokine receptor CCR5 expression in in vitro differentiating human fetal neural stem/progenitor and glioblastoma cells. Neurosci. Lett. 2006;394:22–27. doi: 10.1016/j.neulet.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Kim M., Jung K., Ko Y., Kim I.S., Hwang K., Jang J.H., Shin J.E., Park K.I. TNF-α pretreatment improves the survival and function of transplanted human neural progenitor cells following hypoxic-ischemic brain injury. Cells. 2020;9 doi: 10.3390/cells9051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I., Chicha L., Britschgi M., Schobel S.A., Bodmer M., Hellings J.A., Toovey S., Prinssen E.P. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- Köhler O., Petersen L., Mors O., Mortensen P.B., Yolken R.H., Gasse C., Benros M.E. Infections and exposure to anti-infective agents and the risk of severe mental disorders: a nationwide study. Acta Psychiatr. Scand. 2017;135:97–105. doi: 10.1111/acps.12671. [DOI] [PubMed] [Google Scholar]
- Kong L., Norstedt G., Schalling M., Gissler M., Lavebratt C. The risk of offspring psychiatric disorders in the setting of maternal obesity and diabetes. Pediatrics. 2018;142:e20180776. doi: 10.1542/peds.2018-0776. [DOI] [PubMed] [Google Scholar]
- Krakowiak P., Goines P.E., Tancredi D.J., Ashwood P., Hansen R.L., Hertz-Picciotto I., Van de Water J. Neonatal cytokine profiles associated with autism spectrum disorder. Biol. Psychiatr. 2017;81:442–451. doi: 10.1016/j.biopsych.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krathwohl M.D., Kaiser J.L. Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cell. 2004;22:109–118. doi: 10.1634/stemcells.22-1-109. [DOI] [PubMed] [Google Scholar]
- Kuhn M. Building predictive models in R using the caret package. J. Stat. Software. 2008 028. [Google Scholar]
- Kuhn M. Caret package. J. Stat. Software. 2008;28 [Google Scholar]
- Kurowska M., Rudnicka W., Maślińska D., Maśliński W. Expression of IL-15 and IL-15 receptor isoforms in select structures of human fetal brain. Ann. N. Y. Acad. Sci. 2002;966:441–445. doi: 10.1111/j.1749-6632.2002.tb04245.x. [DOI] [PubMed] [Google Scholar]
- Luque A., Carrascob A., Martína A., de las Herasa A. The impact of class imbalance in classification performance metrics based on the binary confusion matrix. Pattern Recogn. 2019;91:216–231. [Google Scholar]
- Melchior M., Hersi R., van der Waerden J., Larroque B., Saurel-Cubizolles M.J., Chollet A., Galéra C. Maternal tobacco smoking in pregnancy and children’s socio-emotional development at age 5: the EDEN mother-child birth cohort study. Eur. Psychiatr. 2015;30:562–568. doi: 10.1016/j.eurpsy.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Mizejewski G.J., Lindau-Shepard B., Pass K.A. Newborn screening for autism: in search of candidate biomarkers. Biomarkers Med. 2013;7:247–260. doi: 10.2217/bmm.12.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moors M., Vudattu N.K., Abel J., Krämer U., Rane L., Ulfig N., Ceccatelli S., Seyfert-Margolies V., Fritsche E., Maeurer M.J. Interleukin-7 (IL-7) and IL-7 splice variants affect differentiation of human neural progenitor cells. Gene Immun. 2010;11:11–20. doi: 10.1038/gene.2009.77. [DOI] [PubMed] [Google Scholar]
- Morris S.E., Cuthbert B.N. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin. Neurosci. 2012;14:29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser C.A., Baptista S., Arnoux I., Audinat E. Microglia in CNS development: shaping the brain for the future. Prog. Neurobiol. 2017;149–150:1–20. doi: 10.1016/j.pneurobio.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Nelson P.G., Kuddo T., Song E.Y., Dambrosia J.M., Kohler S., Satyanarayana G., Vandunk C., Grether J.K., Nelson K.B. Selected neurotrophins, neuropeptides, and cytokines: developmental trajectory and concentrations in neonatal blood of children with autism or Down syndrome. Int. J. Dev. Neurosci. 2006;24:73–80. doi: 10.1016/j.ijdevneu.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Noonan R.J. Poverty, weight status, and dietary intake among UK adolescents. Int. J. Environ. Res. Publ. Health. 2018;15 doi: 10.3390/ijerph15061224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M.F., Bower J.E., Cho H.J., Creswell J.D., Dimitrov S., Hamby M.E., Hoyt M.A., Martin J.L., Robles T.F., Sloan E.K., Thomas K.S., Irwin M.R. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C., Nakiwala D., Calafat A.M., Botton J., De Agostini M., Heude B., Slama R. 2017. Prenatal exposure to nonpersistent endocrine disruptors and behavior in boys at 3 and 5 Years. Environ health perspect 125, 097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polańska K., Jurewicz J., Hanke W. Smoking and alcohol drinking during pregnancy as the risk factors for poor child neurodevelopment - a review of epidemiological studies. Int. J. Occup. Med. Environ. Health. 2015;28:419–443. doi: 10.13075/ijomeh.1896.00424. [DOI] [PubMed] [Google Scholar]
- Radloff L.S. The use of the center for epidemiologic studies depression scale in adolescents and young adults. J. Youth Adolesc. 1991;20:149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- Rattazzi L., Piras G., Ono M., Deacon R., Pariante C.M., D’Acquisto F. CD4⁺ but not CD8⁺ T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Transl. Psychiatry. 2013;3:e280. doi: 10.1038/tp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M.D., Yim Y.S., Wimmer R.D., Kim H., Ryu C., Welch G.M., Andina M., King H.O., Waisman A., Halassa M.M., Huh J.R., Choi G.B. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature. 2020;577:249–253. doi: 10.1038/s41586-019-1843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.C., Muller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinf. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiders J., Drukker M., van der Ende J., Verhulst F.C., van Os J., Nicolson N.A. Neighbourhood socioeconomic disadvantage and behavioural problems from late childhood into early adolescence. J. Epidemiol. Community Health. 2003;57:699–703. doi: 10.1136/jech.57.9.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng W.S., Hu S., Ni H.T., Rowen T.N., Lokensgard J.R., Peterson P.K. TNF-alpha-induced chemokine production and apoptosis in human neural precursor cells. J. Leukoc. Biol. 2005;78:1233–1241. doi: 10.1189/jlb.0405221. [DOI] [PubMed] [Google Scholar]
- Simen B.B., Duman C.H., Simen A.A., Duman R.S. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol. Psychiatr. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Skogstrand K., Hagen C.M., Borbye-Lorenzen N., Christiansen M., Bybjerg-Grauholm J., Bækvad-Hansen M., Werge T., Børglum A., Mors O., Nordentoft M., Mortensen P.B., Hougaard D.M. Reduced neonatal brain-derived neurotrophic factor is associated with autism spectrum disorders. Transl. Psychiatry. 2019;9:252. doi: 10.1038/s41398-019-0587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.E., Li J., Garbett K., Mirnics K., Patterson P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D., Vagg P.R. Psychometric properties of the STAI: a reply to ramanaiah, franzen, and schill. J. Pers. Assess. 1984;48:95–97. doi: 10.1207/s15327752jpa4801_16. [DOI] [PubMed] [Google Scholar]
- Stellwagen D., Malenka R.C. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stiles J., Jernigan T.L. The basics of brain development. Neuropsychol. Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A., Cooper M., Rutter M. Neurodevelopmental disorders. Lancet Psychiatry. 2017;4:339–346. doi: 10.1016/S2215-0366(16)30376-5. [DOI] [PubMed] [Google Scholar]
- Thürmann L., Herberth G., Rolle-Kampczyk U., Röder S., Borte M., von Bergen M., Lehmann I., Trump S. Elevated gestational IL-13 during fetal development is associated with hyperactivity and inattention in eight-year-old children. Front. Immunol. 2019;10:1658. doi: 10.3389/fimmu.2019.01658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uh H.W., Hartgers F.C., Yazdanbakhsh M., Houwing-Duistermaat J.J. Evaluation of regression methods when immunological measurements are constrained by detection limits. BMC Immunol. 2008;9:59. doi: 10.1186/1471-2172-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N., Friese K. Interleukin-6 receptor is highly expressed in the ganglionic eminence of the human fetal brain. Biol. Neonate. 1999;76:320–324. doi: 10.1159/000014174. [DOI] [PubMed] [Google Scholar]
- van der Meer P., Goldberg S.H., Fung K.M., Sharer L.R., González-Scarano F., Lavi E. Expression pattern of CXCR3, CXCR4, and CCR3 chemokine receptors in the developing human brain. J. Neuropathol. Exp. Neurol. 2001;60:25–32. doi: 10.1093/jnen/60.1.25. [DOI] [PubMed] [Google Scholar]
- van der Waerden J., Galéra C., Larroque B., Saurel-Cubizolles M.J., Sutter-Dallay A.L., Melchior M. Maternal depression trajectories and children’s behavior at age 5 years. J. Pediatr. 2015;166:1440–1448. doi: 10.1016/j.jpeds.2015.03.002. e1441. [DOI] [PubMed] [Google Scholar]
- Wang C., Luan Z., Yang Y., Wang Z., Cui Y., Gu G. Valproic acid induces apoptosis in differentiating hippocampal neurons by the release of tumor necrosis factor-α from activated astrocytes. Neurosci. Lett. 2011;497:122–127. doi: 10.1016/j.neulet.2011.04.044. [DOI] [PubMed] [Google Scholar]
- Wei H., Chadman K.K., McCloskey D.P., Sheikh A.M., Malik M., Brown W.T., Li X. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim. Biophys. Acta. 2012;1822:831–842. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Wu X., He Y., Hsuchou H., Kastin A.J., Rood J.C., Pan W. Essential role of interleukin-15 receptor in normal anxiety behavior. Brain Behav. Immun. 2010;24:1340–1346. doi: 10.1016/j.bbi.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.C., Khodadadi H., Malik A., Davidson B., Salles É D.S.L., Bhatia J., Hale V.L., Baban B. Innate immunity of neonates and infants. Front. Immunol. 2018;9:1759. doi: 10.3389/fimmu.2018.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O., Yoshida C., Grether J.K., Van de Water J., Ashwood P., Delorenze G.N., Hansen R.L., Kharrazi M., Croen L.A. Neonatal cytokines and chemokines and risk of autism spectrum disorder: the early markers for autism (EMA) study: a case-control study. J. Neuroinflammation. 2014;11:113. doi: 10.1186/1742-2094-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupan B., Liu B., Taki F., Toth J.G., Toth M. Maternal brain TNF-α programs innate fear in the offspring. Curr. Biol. 2017;27:3859–3863. doi: 10.1016/j.cub.2017.10.071. e3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.