Abstract
Cerebral amyloid angiopathy (CAA) is one of the common causes of lobar intracerebral hemorrhage and vascular cognitive impairment (VCI) in the aging population. Increased amyloid plaque deposition within cerebral blood vessels, specifically the smooth muscle layer, is linked to increased cerebral microbleeds (CMBs) and impaired cognition in CAA. Studies in Alzheimer’s disease (AD) have shown that amyloid plaque pathology is more prevalent in the brains of elderly women (2/3rd of the dementia population) compared with men, however, there is a paucity of studies on sex differences in CAA. The objective of this study was to discern the sexual dichotomies in CAA. We utilized male and female Tg-SwDI mice (mouse model of CAA) at 12–14 months of age for this study. We evaluated sex differences in CMBs, cognitive function and inflammation. Cognition was assessed using Y-maze (spatial working memory) and Fear Conditioning (contextual memory). CMBs were quantified by ex vivo brain MRI scans. Inflammatory cytokines in brain were quantified using ELISA. Our results demonstrated that aging Tg-SwDI female mice had a significantly higher burden of CMBs on MRI as compared to males. Interestingly, these aging Tg-SwDI female mice also had significantly impaired spatial and contextual memory on Y maze and Fear Conditioning respectively. Furthermore, female mice had significantly lower circulating inflammatory cytokines, IL-1α, IL-2, IL-9, and IFN-γ, as compared to males. Our results demonstrate that aging female Tg-SwDI mice are more cognitively impaired and have higher number of CMBs, as compared to males at 12–14 months of age. This may be secondary to reduced levels of neural repair cytokines (IL-1α, IL-2, IL-9 and IFN-γ) involved in sex specific inflammatory signaling in CAA.
Keywords: Cerebral amyloid angiopathy, MRI, Cerebral microbleeds, Cognition
Highlights
-
•
Sex Difference exist in Cerebral Amyloid Angiopathy (CAA).
-
•
Aging Female Tg SwDI mice (CAA mouse model) have more cerebral microbleeds (CMBs) as compared to males on MRI.
-
•
Aging Female Tg SwDI mice have more impaired spatial and contextual memory as compared to males onbehavioral tasks.
-
•
IL-1α, IL-2, IL-9, INF-γ are lower in female Tg SwDI mice as compared to males.
1. Introduction
As the population ages, the prevalence of dementia will continue to increase. In 2010, there were an estimated 36 million people suffering from dementia with this number expected to increase to 115 million by 2050 (van der Flier et al., 2018; Dichgans and Leys, 2017). Vascular cognitive impairment (VCI) is the second most common cause of dementia (Smith, 2017; Jiwa et al., 2010). It encompasses a constellation of changes in the cerebral vasculature that lead to impairments in multiple domains of executive function, memory, and planning (Dichgans and Leys, 2017; Khan et al., 2016). Cerebral amyloid angiopathy (CAA), or the deposition of amyloid beta (Aβ) within cerebral blood vessels (Iadecola, 2013), is being increasingly recognized as a common cause of VCI. CAA is a neurodegenerative disease characterized by depositions of aggregated Aβ in the media and adventitia of small and medium sized arteries of the cerebral cortex (Charidimou et al., 2017). This accumulation of Aβ causes impairment of the brain microvasculature via inflammation, leading to cerebral microbleeds (CMBs) (Freeze et al., 2019). Using the Boston Criteria for diagnosis of CAA, CMBs (detected on brain MRI) are the major hallmarks of CAA in patients (Smith and Greenberg, 2003; Greenberg and Charidimou, 2018). These CMBs can extend to lobar hemorrhages and further contribute to morbidity and mortality in the elderly (Smith and Greenberg, 2003).
CAA often co-exists with Alzheimer’s disease (AD) (Farid et al., 2017) but a significant number of CAA patients lack AD pathology. The estimated general prevalence of CAA is in the range of 10 - 50% in the elderly population and around 80% in patients with pathological features of AD at autopsy (Auriel and Greenberg, 2012). One pathological difference is that the longer Aβ42 protein is associated with AD, while the shorter protein, Aβ40 is the dominant misfolded protein in CAA (Zipfel et al., 2009). Sex differences exist in dementia and AD, with women being affected more than men (Mazure and Swendsen, 2016; Gannon et al., 2019). In individuals with AD, men outperform women in episodic, semantic, non-semantic, visuo-spatial and verbal memory tasks (Sundermann et al., 2016; Irvine et al., 2012). This is attributed to women having enhanced and earlier progression to cognitive impairment as compared to men (Laws et al., 2016). In late-onset familial AD, which is associated with the apolipoprotein E (APOE)-epsilon4 allele, women demonstrate a higher risk of developing AD at younger ages than men (Payami et al., 1996; Neu et al., 2017). Since AD and CAA share some pathological features (Aβ deposition), sex differences may also exist in CAA, however, this has been understudied. The aim of this study was to determine if sex differences exist in CAA using a transgenic mouse model that harbors the Swedish, Dutch, and Iowa mutation (Tg-SwDI mice) (Davis et al., 2004). We used male and female Tg-SwDI (12–14 months old) mice that are symptomatic for CAA to study cognition, CMB’s and inflammatory cytokines.
2. Materials & methods
2.1. Animal model
Transgenic mice (Tg-Thy1-APPSwedish/Dutch/Iowa) BWevn/Mmjax-SwDI (Davis et al., 2004) purchased from Jackson Laboratories were bred and aged in house. Aged (12–14 month) Tg-SwDI male and female mice were used for all experiments.
2.2. Animal housing
Animals were housed in the Center for Laboratory Animal Medicine and Care at the University Of Texas Health Science Center. Housing conditions conformed to Association for Assessment and Accreditation of Laboratory Animal Care International, IACUC, and protocol standards (Animal Welfare Committee-18-0128). Mice were maintained and aged at an ambient temperature in a humidity controlled vivarium and had access to food and water ad libitum. Vivarium rooms were on a 12-h light-dark cycle (6:30-18:30).
2.3. Behavior
Behavioral experiments (Y-maze and Fear Conditioning) were performed by two experimenters. Experimenter 1 performed all behavioral experiments and experimenter 2 analyzed the data blinded to sex. All behavioral experiments were video-recorded using Noldus EthoVisionXT Video Tracking Software (Noldus et al., 2001) and saved to a dedicated UT Health network drive. For Y-maze, experimenter 1 recorded arm entries over a 5 minute trial and experimenter 2 used the exported data for calculation and analysis. For Fear Conditioning, experimenter 1 performed three trials (Training, Shock, and Test) using Noldus EthoVision XT. Fear Conditioning videos were analyzed using Noldus EthoVisionXT software; exported data was provided to experimenter 2 for analysis. Behavior experiments were consistently performed at the beginning of animals’ light cycle, at 06:30 each day.
2.3.1. Y-maze
Cognitive deficits in spatial working memory were assessed as changes in percent (%) alternation and total arm entries using Y-maze (Holter et al., 2015; Wang et al., 2017; Knowles et al., 2013). Briefly, mice were placed in the start arm of Y-maze and allowed to explore for 5 minutes. Arms were labeled A, B, and C; all mice started in arm A. EthoVisionXT tracking software and experimenter 1 recorded the animal’s arm entries for all trials. An alternation was counted when each arm was visited consecutively without repeats (eg. ABC, BCA, CAB). Mice with intact working memory visit all three arms in alternation (choose the novel arm) and do not return to the recently explored (familiar) arm immediately. Total arm entry included each arm visited regardless of order. Percent alternation (% Alternation) was calculated as (Total number of alternations)/(Total arm entries - 2) ∗ 100, as described previously (Tucker et al., 2016). A decreased % Alternation indicates impaired spatial working memory (Tucker et al., 2016).
2.3.2. Fear Conditioning
Contextual memory was assessed as Cumulative Duration (seconds) of Inactive State (inactivity/freezing) using fear conditioning through shock habituation (Bergstrom, 2020; Lugo et al., 2014). For all tests (Training, Shock, and Testing), mice were placed inside a 12”W by 10”D by 12”H rectangular box. Mice underwent three phases for hippocampal learning and memory, Phase 1 Training/Acclimation, Phase 2 Shock, and Phase 3 Memory Testing. For Phase 1 Training/Acclimation, mice were placed inside the box with the chamber light on to acclimate for 2 min. Mice were then returned to their home cage for 60 minutes. Following 60-minutes, mice were returned to the chamber for Phase 2. For Phase 2, mice underwent the same conditions as Phase 1, but at the 2-min mark, a 1000 μA shock was administered for 2 milliseconds. Mice were then returned to their home cage for 24 hours. For Phase 3 memory testing, mice were placed in the chamber with the light on for 3 minutes under the same conditions as Phase 1 and 2. EthoVisionXT tracking software recorded Mean Inactive State (MIS), the freezing behavior, in seconds for all phases in 60-second intervals. The first 60 seconds of MIS for Training and Testing phases was analyzed to determine difference in inactivity (Testing-Training) or the Delta Inactive State (DIS) (Pham et al., 2009). Decreased freezing behavior (DIS) after the shock is an index of impaired contextual memory.
2.4. Imaging
2.4.1. Magnetic resonance imaging (MRI)
Following behavioral testing, mice were euthanized, as described previously (Manwani et al., 2011). Briefly, mice were anesthetized through an intraperitoneal injection of 2,2,2-tribromoethanol (Avertin). Anesthesia was confirmed through loss of reflex response via toe pinch. Mice were then perfused with cold heparinized 1X PBS and 4% paraformaldehyde, and brains harvested for ex-vivo MRI. MRI study was conducted using a 9.4 T system (Bruker BioSpec 94/20, Bruker BioSpin, Billerica, MA USA) equipped with a BGA6 microgradient with a maximum strength of 1000 mT/m and interfaced with ParaVision 5.1. Images were acquired using a 30 mm mouse volume coil to transmit and receive. A 2D multi-echo gradient echo, T2∗ weighted sequence was used for CMB detection with the following imaging parameters: TR/TE: 1500/4 10 16 ms, FA: 30°, NA: 4, voxel size was 90 × 90 × 750 μm3. Total imaging time was 9 min and 36 s. We also acquired a T2 weighted RARE, anatomical dataset with the following parameters: TR/TE: 2500/24 ms; FA 1800, voxel size was 90 × 90 × 750 μm3, 10 slices and RARE factor = 4, NA = 2. Total imaging time was 10 min and 40 s.
2.5. ELISA
An additional cohort of mice was sacrificed (n = 8 males and 10 females) for brain cytokine analysis. A blinded investigator performed brain cytokines level assessment using an ELISA (Bio-Plex Pro Mouse Cytokine 23-plex assay, Bio-Rad Laboratories, M60009RDPD). Briefly, brain hemispheres from 12-14 months old male and female Tg-SwDI were collected and homogenized in ice-cold phosphate-buffered saline (PBS) with protease inhibitors as described previously (Ritzel et al., 2018). 50 μl of 10 μg/μl of whole brain lysate was loaded in each well. Each sample was run in duplicate. Samples were assayed according to the manufacturer’s instructions using a Bio-Plex® 200 system. The observed concentrations on the calibration curve were in the following ranges; IL-1α (2.28 – 8543.87 pg/ml), IL-2 (5.23 – 18263.05 pg/ml), IL-9 (15.4 – 15955.63 pg/ml), and IFN-γ (2.75 – 11801.31 pg/ml).
2.6. Statistical analysis
Descriptive statistics and scatter dot plots were presented for different variables. Normality of the data for each variable was checked and confirmed. Unpaired two-sample t-test was used to compare variables between male and female. P value less than 0.05 was considered as significant. Graph Pad Prism 7.03 was used for statistical analysis and graphing.
3. Results
3.1. Behavior
3.1.1. Y-maze
To determine differences in spatial working memory, aging female and male mice underwent Y-maze testing. Measurements included Total Arm Entry and % Alternation. There was no difference in Total Arm Entry between Tg-SwDI female (26.11 ± 3.19, n = 9) and male (25.78 ± 3.475, n = 9), (t (16) = 0.070, p = 0.944) mice (Fig. 1A). Interestingly, female mice exhibited a significant decrease in % Alternation (48.23 ± 4.077, n = 9) compared to male mice (62.49 ± 4.439, n = 9), (t (16) = 2.367, p = 0.031) (Fig. 1B), indicating a significantly impaired spatial working memory in females compared to males.
Fig. 1.
Spatial working memory test using Y-maze for Tg-SwDI female (n = 9) vs Tg-SwDI male (n = 9) at 12–14 months of age, A) Total Arm Entry and B) % Alternation. Total testing time per trial of 5 minutes demonstrated no difference in Total Arm Entry between female and male mice (A), but female mice demonstrated a significant reduction in % Alternations, or inability to distinguish the novel arm versus familiar arm, compared to male mice. This suggests a more impaired spatial working memory in females, ∗ indicates p = 0.03.
3.1.2. Fear conditioning
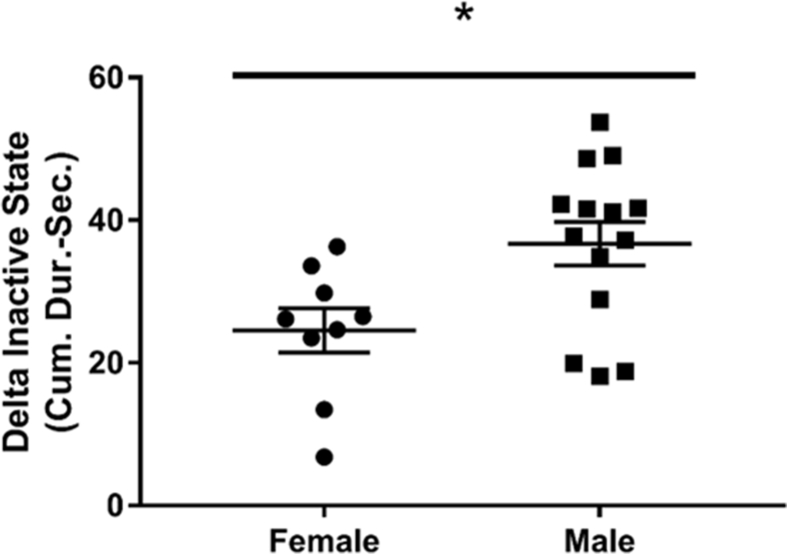
Differences in contextual memory were assessed using Fear Conditioning. There was a significant difference in the Delta Inactive State (difference between Testing and Training) or freezing behavior in female (24.56 ± 3.1, n = 9) versus male mice (36.73 ± 3.05, n = 14), (t (21) = 2.668, p = 0.014) (Fig. 2). The significant reduction in Delta Inactive State (freezing behavior) in females demonstrates their decreased cognitive ability to associate the environment with a prior negative stimulus (shock).
Fig. 2.
Fear Conditioning to test contextual memory for Tg-SwDI female (n = 9) vs Tg-SwDI male (n = 14) at 12–14 months of age. Delta Inactive State was calculated using first 60 seconds of Training (pre-shock) – Testing (post-shock) to determine “freezing” behavior. Female mice demonstrated a significant reduction in Delta Inactive State or decreased freezing time compared to male mice, suggesting deficits in contextual memory. ∗ indicates p < 0.01.
3.2. Imaging
3.2.1. MRI
Ex-vivo brains were imaged to determine sex differences in total CMBs for female and male Tg-SwDI mice. At 12–14 months of age, females (16.75 ± 3.4, n = 4) had significantly more CMBs as compared to males (3.25 ± 1.25, n = 8), (t (10) = 4 0.635, p = 0.009) (Fig. 3A and B).
Fig. 3.
A. MRI for cerebral microbleeds (CMBs) in Tg-SwDI female (n = 4) vs Tg-SwDI male (n = 8) at 12–14 months age. Female mice had significantly more total CMBs compared to male mice. ∗∗∗ indicates p = 0.009. Fig. 3 B. Top panel- Females, Bottom panel-males. Left panel: T2 anatomy scan (A and C), Right panel showing T2 star sequence on MRI (B and D). Arrows demonstrate the cerebral microbleeds (CMBs).
3.3. ELISA
Inflammatory cytokine markers were measured using ELISA cytokine assay in brain tissue lysate of 12–14 month old Tg-SwDI female (n = 8) and male (n = 10) mice. Female mice had significantly lower concentrations of Interleukin-1α (IL-1α) (18.89 ± 1.604 pg/ml) vs. males (25.24 ± 1.235 pg/ml), (t (13.94) = 3.134, p = 0.007). Interleukin-2 (IL-2) levels were also significantly lower in females (27.31 ± 2.452 pg/ml) vs. males (35.43 ± 1.817 pg/ml), (t (13.61) = 2.662, p = 0.018). Similarly, Interleukin-9 (IL-9) was lower in females (30.64 ± 4.549 pg/ml) vs. males (44.88 ± 2.462 pg/ml), (t (9.415) = 2.309, p = 0.018). and, Interferon-gamma (IFN-γ) was also found to be lower in females (36.97 ± 3.76 pg/ml) vs. males (50.84 ± 3.011 pg/ml), (t (14.29) = 2.878, p = 0.012) (Fig. 4).
Fig. 4.
Inflammatory cytokines identified by ELISA from whole brain tissue lysate in Tg-SwDI female (n = 8) vs male (n = 10) mice at 12–14 months of age. Concentrations are represented in pg/ml. Sex differences were identified in four inflammatory cytokine markers with male Tg-SwDI mice having significantly higher concentrations of A) IL-1α, B) IL-2, C) IL-9, and D) IFN-γ, when compared to female Tg-SwDI mice. ∗ IL-1α, p = 0.007∗∗; IL-2, p = 0.01; IL-9, p = 0.01, and IFN-γ, p = 0.012.
4. Discussion
Our study demonstrates that sex differences exist in Tg-SwDI, a murine model of CAA. We found that females have higher number of CMBs (on MRI) and poor spatial and contextual memory (on Y-maze and Fear Conditioning behavioral tasks) as compared to males. This is the first study to demonstrate sex differences in a murine model of CAA using symptomatic Tg-SwDI mice. These transgenic mice express the human APP gene (isoform 770) containing the Swedish (K670N/M671L), Dutch (E693Q), and Iowa (D694N) mutations under the mouse Thy1 promotor and develop Aβ deposits primarily in the cerebral microvasculature, simulating CAA pathology (Davis et al., 2004). They develop VCI which has been detected on the Barnes Maze task in homozygotes at 3, 9, and 12 months of age. Tg-SwDI recapitulate CAA type 1 that has mostly capillary CAA, but unlike other mouse models, it also models the vascular neuroinflammation associated with CAA (Jakel et al., 2017). Perivascular and vascular Aβ deposits can be detected starting at three months of age and the pathology is extensive by 12 months of age (Van Vickle et al., 2008). Therefore, we used 12–14 month old mice to study sex differences.
Sex differences have been described in AD. However, sexual dichotomies in CAA, that shares some pathological features with AD, are less well defined. With the advancement in small animal MRI technology, it is now possible to visualize CMBs, the hallmark of a possible or probable CAA diagnosis in humans (Greenberg and Charidimou, 2018). In our study, MRI T2 star imaging demonstrated that Tg-SwDI female mice had significantly more CMBs than age matched males. This is consistent with studies in APP/PS1 and transgenic mice expressing human APOE, where females had greater CMBs, as detected by immunohistochemistry (Jiao et al., 2016; Cacciottolo et al., 2016). There is a correlation of CMBs with the levels of Aβ (Cacciottolo et al., 2016) and female mice have been shown to have a greater Aβ burden than males (Callahan et al., 2001) in Tg human amyloid precursor protein (hAPP), Tg-2576, and human APP with the Swedish & presenilin-1 A246E mutation-APP/PS1 mice (Wang et al., 2003). In contrast to these studies in animal models, studies in human autopsy samples from AD patients found increased CAA severity in men, as suggested by levels of Aβ (Shinohara et al., 2016; Nelson et al., 2013). One study found more CMBs on MRI in men with AD in association with APOE4 allele than women (Cacciottolo et al., 2016). This disconnect in mouse and human studies may be secondary to the presence of CMBs related to hypertension, a condition that is more commonly seen in men. In addition, studies on CMB load are difficult in humans as there are multiple other co-morbidities (like hypertension, which also causes CMBs), different ages and even different disease stages when autopsies or MRIs are done. Moreover, the autopsy studies have been done in patients with AD/other forms of dementia and when comorbid with CAA, the prevalence of CMBs and the cause of CAA (hereditary, sporadic or AD-related) may become difficult to distinguish.
In line with sex difference in CMBs on MRI, we observed sexual dimorphism in both spatial and contextual working memory behavioral tasks. Alternation/exploration of different arms of the Y maze was used as a gauge of spatial working memory (Tucker et al., 2016). Aging females chose the familiar arm significantly more than the novel arm, demonstrating impaired spatial working memory. Contextual memory was tested using fear conditioning (Pham et al., 2009). When introduced into an environment where an aversive stimulus (shock) is previously administered, a freezing behavior/inactivity is expected as the mouse associates the environment with the aversive stimulus (Curzon et al., 2009). Aging female mice had significant lack of freezing behavior, suggesting deficits in associative learning or impaired memory of the negative stimulus (Curzon et al., 2009). This is consistent with prior studies in AD mouse models, APP/PS1/tau triple-transgenic and Tg-2576 mice where female mice demonstrated a more impaired cognition on behavioral tasks as compared to males (Yang et al., 2018; Schmid et al., 2019). Sexual dichotomy in cognitive deficits has also been observed in clinical population. In AD patients, women perform poorly on verbal memory tasks and have a faster cognitive decline compared to men (Sundermann et al., 2016; Laws et al., 2016). Women also have more AD pathology vs. men (Oveisgharan et al., 2018), for each additional unit of pathology, there is a threefold increase in the odds of clinical AD in men and 20 fold increase in women (Barnes et al., 2005). However, the etiology of these sex differences in AD and CAA is not well understood.
There appears to be an inevitable role of inflammation in the formation of CMBs and cognitive decline. Aβ accumulation and CMBs cause an innate inflammatory response characterized by an increase and activation of microglia, macrophages and astrocytes (Rosidi et al., 2011). This inflammation leads to further neuronal damage and cognitive decline (Rosidi et al., 2011; Ungvari et al., 2017). Using a series of in vivo two-photon imaging and histological studies, a recent study found recruitment of microglia and monocytes/macrophages around an induced 100 μm sized hemorrhage (Ahn et al., 2018). The increase in these microglia is only found in AD patients who also had CAA and was not seen in patients with no CAA, suggesting a role of microglia in pathogenesis of CAA (Zabel et al., 2013). Microglia are known to secrete both pro and anti-inflammatory cytokines (Smith et al., 2012), that may have both positive (Rea, 2018; DiSabato, 2016) and negative effects (Michaud, 2013; Newcombe, 2018; Wyss-Coray, 2016) on cognition in aging and neurodegenerative diseases. We measured cytokine concentrations in brain lysates from Tg-SwDI mice and found a sex difference in brain concentrations of IL-1α, IL-2, IL-9 and IFN-γ in symptomatic Tg-SwDI mice. Female mice were found to have significantly lower concentration of these cytokines as compared to males. Sexual dichotomies in cytokine levels in CAA have not been reported previously. Overexpression of IL-1α causes increased microglial activation (Shaftel et al., 2008), and decreases concentrations of APP and Aβ1-40 (Domingues et al., 2017). With females having decreased IL-1α, their Aβ load maybe increased correlating with increased CMBs as compared to males. IL-2 has anti-inflammatory effects (Saadoun et al., 2011) and IL-2 knockout mice are known to have impairments in learning and memory (Petitto et al., 2015). In an AD mouse model, IL-2 administration leads to astrocytic recruitment and reduction of amyloid plaques (Alves et al., 2017). IL-9 is upregulated in AD (Taipa et al., 2019) and is known to exert its anti-inflammatory properties by decreasing the activation state of macrophages in other disease models (Donninelli et al., 2020). Interferon gamma (IFN-γ) has pleiotropic effects and in a murine model of AD, ten months of IFN-γ expression has been shown to cause microglial activation and neurogenesis (Domingues et al., 2017; Monteiro et al., 2017; Mastrangelo et al., 2009). Moreover, interferon gamma (IFN-γ) has been also shown to improve spatial working memory in wild type aging mice (Monteiro et al., 2017; Baron et al., 2008). Our results suggest that a sexually dichotomized immune dysregulation contributes to CAA pathogenesis. A paucity of neural repair (IFN- γ) and anti-inflammatory cytokines (IL-1α, IL-2, IL-9) may contribute to the worst cognitive deficits seen in female sex in our study. There may be a role of endogenous sex hormones (Christensen and Pike, 2015, 2017), genetics or epigenetic factors in the CAA induced differential inflammatory pathways in males and females. Future studies on immune cell types and downstream signaling pathways using both sexes may further decipher the mechanisms of this sexual dimorphism.
Our study does elucidate the importance of using sex as a biological variable in neurodegenerative diseases like CAA. However, our study validated this in only one mouse model of CAA, the Tg-SwDI mice. It has been speculated that an active estrogen response element exists on the Thy 1 promotor of Tg-SwDI mice (using prediction analysis), but this has not been established in-vivo (Sadleir et al., 2015). Since our findings are in aging females (when estrogen levels are low) and consistent with prior studies of female excess of CMBs in AD mouse models (APP/PS1) (Jiao et al., 2016), this phenotype is less likely to be an effect of the estrogen response element or restricted to this mouse model. Future research in humans and in different mouse models of CAA is needed to confirm these findings.
In summary, we report that females have more CMBs and a more impaired cognition as compared to males in a mouse model of CAA. This may be secondary to differences in neuroinflammatory pathways of repair and regeneration in males and females. Future studies are needed to understand the sexual dimorphism in CAA across the lifespan, in terms of pathological and functional outcomes.
Funding
TARCC (Texas Alzheimer’s Research and Care Consortium) to BM and NIH-NIA (National Institutes of Health-National Institute On Aging) RF1 AG058463-01 to LDM.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahn S.J., Anrather J., Nishimura N., Schaffer C.B. Diverse inflammatory response after cerebral microbleeds includes coordinated microglial migration and proliferation. Stroke. 2018;49(7):1719–1726. doi: 10.1161/STROKEAHA.117.020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves S., Churlaud G., Audrain M., Michaelsen-Preusse K., Fol R., Souchet B. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer’s disease mice. Brain. 2017;140(3):826–842. doi: 10.1093/brain/aww330. [DOI] [PubMed] [Google Scholar]
- Auriel E., Greenberg S.M. The pathophysiology and clinical presentation of cerebral amyloid angiopathy. Curr. Atherosclerosis Rep. 2012;14(4):343–350. doi: 10.1007/s11883-012-0254-z. [DOI] [PubMed] [Google Scholar]
- Barnes L.L., Wilson R.S., Bienias J.L., Schneider J.A., Evans D.A., Bennett D.A. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch. Gen. Psychiatr. 2005;62(6):685–691. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- Baron R., Nemirovsky A., Harpaz I., Cohen H., Owens T., Monsonego A. IFN-gamma enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer’s disease. Faseb. J. 2008;22(8):2843–2852. doi: 10.1096/fj.08-105866. [DOI] [PubMed] [Google Scholar]
- Bergstrom H.C. Assaying fear memory discrimination and generalization: methods and concepts. Curr Protoc Neurosci. 2020;91(1):e89. doi: 10.1002/cpns.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciottolo M., Christensen A., Moser A., Liu J., Pike C.J., Smith C. The APOE4 allele shows opposite sex bias in microbleeds and Alzheimer’s disease of humans and mice. Neurobiol. Aging. 2016;37:47–57. doi: 10.1016/j.neurobiolaging.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan M.J., Lipinski W.J., Bian F., Durham R.A., Pack A., Walker L.C. Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am. J. Pathol. 2001;158(3):1173–1177. doi: 10.1016/s0002-9440(10)64064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charidimou A., Boulouis G., Gurol M.E., Ayata C., Bacskai B.J., Frosch M.P. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017;140(7):1829–1850. doi: 10.1093/brain/awx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A., Pike C.J. Menopause, obesity and inflammation: interactive risk factors for Alzheimer’s disease. Front. Aging Neurosci. 2015;7:130. doi: 10.3389/fnagi.2015.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A., Pike C.J. Age-dependent regulation of obesity and Alzheimer-related outcomes by hormone therapy in female 3xTg-AD mice. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0178490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon P., Rustay N.R., Browman K.E. In: Methods of Behavior Analysis in Neuroscience. Frontiers in Neuroscience. Boca Raton (FL) Buccafusco J.J., editor. 2009. Cued and contextual fear conditioning for rodents. nd. [Google Scholar]
- Davis J., Xu F., Deane R., Romanov G., Previti M.L., Zeigler K. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J. Biol. Chem. 2004;279(19):20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- Dichgans M., Leys D. Vascular cognitive impairment. Circ. Res. 2017;120(3):573–591. doi: 10.1161/CIRCRESAHA.116.308426. [DOI] [PubMed] [Google Scholar]
- DiSabato D. Neuroinflammation: the devil is in the details. J. Neurochem. 2016;139:136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues C., da Cruz E.S.O.A.B., Henriques A.G. Impact of cytokines and chemokines on Alzheimer’s disease neuropathological hallmarks. Curr. Alzheimer Res. 2017;14(8):870–882. doi: 10.2174/1567205014666170317113606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donninelli G., Saraf-Sinik I., Mazziotti V., Capone A., Grasso M.G., Battistini L. Interleukin-9 regulates macrophage activation in the progressive multiple sclerosis brain. J. Neuroinflammation. 2020;17(1):149. doi: 10.1186/s12974-020-01770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid K., Charidimou A., Baron J.C. Amyloid positron emission tomography in sporadic cerebral amyloid angiopathy: a systematic critical update. Neuroimage Clin. 2017;15:247–263. doi: 10.1016/j.nicl.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze W.M., Bacskai B.J., Frosch M.P., Jacobs H.I.L., Backes W.H., Greenberg S.M. Blood-brain barrier leakage and microvascular lesions in cerebral amyloid angiopathy. Stroke. 2019;50(2):328–335. doi: 10.1161/STROKEAHA.118.023788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon O.J., Robison L.S., Custozzo A.J., Zuloaga K.L. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem. Int. 2019;127:38–55. doi: 10.1016/j.neuint.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Greenberg S.M., Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the Boston criteria. Stroke. 2018;49(2):491–497. doi: 10.1161/STROKEAHA.117.016990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter S.M., Garrett L., Einicke J., Sperling B., Dirscherl P., Zimprich A. Assessing cognition in mice. Curr Protoc Mouse Biol. 2015;5(4):331–358. doi: 10.1002/9780470942390.mo150068. [DOI] [PubMed] [Google Scholar]
- Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine K., Laws K.R., Gale T.M., Kondel T.K. Greater cognitive deterioration in women than men with Alzheimer’s disease: a meta analysis. J. Clin. Exp. Neuropsychol. 2012;34(9):989–998. doi: 10.1080/13803395.2012.712676. [DOI] [PubMed] [Google Scholar]
- Jakel L., Van Nostrand W.E., Nicoll J.A.R., Werring D.J., Verbeek M.M. Animal models of cerebral amyloid angiopathy. Clin. Sci. (Lond.) 2017;131(19):2469–2488. doi: 10.1042/CS20170033. [DOI] [PubMed] [Google Scholar]
- Jiao S.S., Bu X.L., Liu Y.H., Zhu C., Wang Q.H., Shen L.L. Sex dimorphism profile of Alzheimer’s disease-type pathologies in an APP/PS1 mouse model. Neurotox. Res. 2016;29(2):256–266. doi: 10.1007/s12640-015-9589-x. [DOI] [PubMed] [Google Scholar]
- Jiwa N.S., Garrard P., Hainsworth A.H. Experimental models of vascular dementia and vascular cognitive impairment: a systematic review. J. Neurochem. 2010;115(4):814–828. doi: 10.1111/j.1471-4159.2010.06958.x. [DOI] [PubMed] [Google Scholar]
- Khan A., Kalaria R.N., Corbett A., Ballard C. Update on vascular dementia. J. Geriatr. Psychiatr. Neurol. 2016;29(5):281–301. doi: 10.1177/0891988716654987. [DOI] [PubMed] [Google Scholar]
- Knowles J.K., Simmons D.A., Nguyen T.V., Vander Griend L., Xie Y., Zhang H. Small molecule p75NTR ligand prevents cognitive deficits and neurite degeneration in an Alzheimer’s mouse model. Neurobiol. Aging. 2013;34(8):2052–2063. doi: 10.1016/j.neurobiolaging.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws K.R., Irvine K., Gale T.M. Sex differences in cognitive impairment in Alzheimer’s disease. World J. Psychiatr. 2016;6(1):54–65. doi: 10.5498/wjp.v6.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo J.N., Smith G.D., Holley A.J. Trace fear conditioning in mice. JoVE. 2014;85 doi: 10.3791/51180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B., Liu F., Xu Y., Persky R., Li J., McCullough L.D. Functional recovery in aging mice after experimental stroke. Brain Behav. Immun. 2011;25(8):1689–1700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo M.A., Sudol K.L., Narrow W.C., Bowers W.J. Interferon-{gamma} differentially affects Alzheimer’s disease pathologies and induces neurogenesis in triple transgenic-AD mice. Am. J. Pathol. 2009;175(5):2076–2088. doi: 10.2353/ajpath.2009.090059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure C.M., Swendsen J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016;15(5):451–452. doi: 10.1016/S1474-4422(16)00067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M. Proinflammatory cytokines, aging, and age-related diseases. J. Am. Med. Dir. Assoc. 2013;14(12):877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Monteiro S., Roque S., Marques F., Correia-Neves M., Cerqueira J.J. Brain interference: revisiting the role of IFNgamma in the central nervous system. Prog. Neurobiol. 2017;156:149–163. doi: 10.1016/j.pneurobio.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Nelson P.T., Pious N.M., Jicha G.A., Wilcock D.M., Fardo D.W., Estus S. APOE-epsilon2 and APOE-epsilon4 correlate with increased amyloid accumulation in cerebral vasculature. J. Neuropathol. Exp. Neurol. 2013;72(7):708–715. doi: 10.1097/NEN.0b013e31829a25b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu S.C., Pa J., Kukull W., Beekly D., Kuzma A., Gangadharan P. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 2017;74(10):1178–1189. doi: 10.1001/jamaneurol.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe E.A. Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflammation. 2018;15:26. doi: 10.1186/s12974-018-1313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noldus L.P., Spink A.J., Tegelenbosch R.A. EthoVision: a versatile video tracking system for automation of behavioral experiments. Behav. Res. Methods Instrum. Comput. 2001;33(3):398–414. doi: 10.3758/bf03195394. [DOI] [PubMed] [Google Scholar]
- Oveisgharan S., Arvanitakis Z., Yu L., Farfel J., Schneider J.A., Bennett D.A. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol. 2018;136(6):887–900. doi: 10.1007/s00401-018-1920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payami H., Zareparsi S., Montee K.R., Sexton G.J., Kaye J.A., Bird T.D. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am. J. Hum. Genet. 1996;58(4):803–811. [PMC free article] [PubMed] [Google Scholar]
- Petitto J.M., Cushman J.D., Huang Z. Effects of brain-derived IL-2 deficiency and the development of Autoimmunity on spatial learning and fear conditioning. J Neurol Disord. 2015;3(1):196. doi: 10.4172/2329-6895.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham J., Cabrera S.M., Sanchis-Segura C., Wood M.A. Automated scoring of fear-related behavior using EthoVision software. J. Neurosci. Methods. 2009;178(2):323–326. doi: 10.1016/j.jneumeth.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea I.M. Age and age-related diseases: role of inflammation triggers and cytokines. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel R.M., Lai Y.J., Crapser J.D., Patel A.R., Schrecengost A., Grenier J.M. Aging alters the immunological response to ischemic stroke. Acta Neuropathol. 2018;136(1):89–110. doi: 10.1007/s00401-018-1859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosidi N.L., Zhou J., Pattanaik S., Wang P., Jin W., Brophy M. Cortical microhemorrhages cause local inflammation but do not trigger widespread dendrite degeneration. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun D., Rosenzwajg M., Joly F., Six A., Carrat F., Thibault V. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 2011;365(22):2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- Sadleir K.R., Eimer W.A., Cole S.L., Vassar R. Abeta reduction in BACE1 heterozygous null 5XFAD mice is associated with transgenic APP level. Mol. Neurodegener. 2015;10:1. doi: 10.1186/1750-1326-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S., Rammes G., Blobner M., Kellermann K., Bratke S., Fendl D. Cognitive decline in Tg2576 mice shows sex-specific differences and correlates with cerebral amyloid-beta. Behav. Brain Res. 2019;359:408–417. doi: 10.1016/j.bbr.2018.11.022. [DOI] [PubMed] [Google Scholar]
- Shaftel S.S., Griffin W.S., O’Banion M.K. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J. Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M., Murray M.E., Frank R.D., Shinohara M., DeTure M., Yamazaki Y. Impact of sex and APOE4 on cerebral amyloid angiopathy in Alzheimer’s disease. Acta Neuropathol. 2016;132(2):225–234. doi: 10.1007/s00401-016-1580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.E. Clinical presentations and epidemiology of vascular dementia. Clin. Sci. (Lond.) 2017;131(11):1059–1068. doi: 10.1042/CS20160607. [DOI] [PubMed] [Google Scholar]
- Smith E.E., Greenberg S.M. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Curr. Atherosclerosis Rep. 2003;5(4):260–266. doi: 10.1007/s11883-003-0048-4. [DOI] [PubMed] [Google Scholar]
- Smith J.A., Das A., Ray S.K., Banik N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012;87(1):10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann E.E., Maki P.M., Rubin L.H., Lipton R.B., Landau S., Biegon A. Female advantage in verbal memory: evidence of sex-specific cognitive reserve. Neurology. 2016;87(18):1916–1924. doi: 10.1212/WNL.0000000000003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipa R., das Neves S.P., Sousa A.L., Fernandes J., Pinto C., Correia A.P. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol. Aging. 2019;76:125–132. doi: 10.1016/j.neurobiolaging.2018.12.019. [DOI] [PubMed] [Google Scholar]
- Tucker L.B., Fu A.H., McCabe J.T. Performance of male and female C57BL/6J mice on motor and cognitive tasks commonly used in pre-clinical traumatic brain injury research. J. Neurotrauma. 2016;33(9):880–894. doi: 10.1089/neu.2015.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z., Tarantini S., Kirkpatrick A.C., Csiszar A., Prodan C.I. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am. J. Physiol. Heart Circ. Physiol. 2017;312(6):H1128–H1143. doi: 10.1152/ajpheart.00780.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier W.M., Skoog I., Schneider J.A., Pantoni L., Mok V., Chen C.L.H. Vascular cognitive impairment. Nat Rev Dis Primers. 2018;4:18003. doi: 10.1038/nrdp.2018.3. [DOI] [PubMed] [Google Scholar]
- Van Vickle G.D., Esh C.L., Daugs I.D., Kokjohn T.A., Kalback W.M., Patton R.L. Tg-SwDI transgenic mice exhibit novel alterations in AbetaPP processing, Abeta degradation, and resilient amyloid angiopathy. Am. J. Pathol. 2008;173(2):483–493. doi: 10.2353/ajpath.2008.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Tanila H., Puolivali J., Kadish I., van Groen T. Gender differences in the amount and deposition of amyloidbeta in APPswe and PS1 double transgenic mice. Neurobiol. Dis. 2003;14(3):318–327. doi: 10.1016/j.nbd.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Wang X., Song R., Lu W., Liu Z., Wang L., Zhu X. YXQN reduces Alzheimer’s disease-like pathology and cognitive decline in APPswePS1dE9 transgenic mice. Front. Aging Neurosci. 2017;9:157. doi: 10.3389/fnagi.2017.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016:18. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.T., Wang Z.J., Cai H.Y., Yuan L., Hu M.M., Wu M.N. Sex differences in neuropathology and cognitive behavior in APP/PS1/tau triple-transgenic mouse model of Alzheimer’s disease. Neurosci Bull. 2018;34(5):736–746. doi: 10.1007/s12264-018-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel M., Schrag M., Crofton A., Tung S., Beaufond P., Van Ornam J. A shift in microglial beta-amyloid binding in Alzheimer’s disease is associated with cerebral amyloid angiopathy. Brain Pathol. 2013;23(4):390–401. doi: 10.1111/bpa.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel G.J., Han H., Ford A.L., Lee J.M. Cerebral amyloid angiopathy: progressive disruption of the neurovascular unit. Stroke. 2009;40(3 Suppl. l):S16–S19. doi: 10.1161/STROKEAHA.108.533174. [DOI] [PMC free article] [PubMed] [Google Scholar]