Abstract
A strong body of evidence supports a role for immune dysregulation across many psychiatric disorders including depression, the leading cause of global disability. Recent progress in the search for genetic variants associated with depression provides the opportunity to strengthen our current understanding of etiological factors contributing to depression and generate novel hypotheses. Here, we provide an overview of the literature demonstrating a role for immune dysregulation in depression, followed by a detailed discussion of the immune-related genes identified by the most recent genome-wide meta-analysis of depression. These genes represent strong evidence-based targets for future basic and translational research which aims to understand the role of the immune system in depression pathology and identify novel points for therapeutic intervention.
Keywords: Depression, GWAS, Genetics, Neuroscience, Psychiatry, Mood disorders
Highlights
-
•
Dysregulated immune function has been strongly linked to depression.
-
•
Recent genome-wide association studies support this relationship.
-
•
These genes represent strong candidates for etiological and translational research.
1. Introduction
Depression, a complex heterogeneous disorder characterized by core symptoms of low mood and anhedonia, is relatively common and can lead to severe impairment in daily life, making it the world’s leading cause of disability (American Psychiatric Association, 2013; WHO, 2018, 2017). Despite its high prevalence and severe negative consequences, the most common pharmacological treatments are only moderately effective and have undesirable side-effects (Locher et al., 2017; Sugarman et al., 2014). Moreover, our understanding of underlying biological factors contributing to depression etiology is severely limited compared to non-psychiatric disorders. Further complications in mechanistic research arise from the high degree of etiological and symptomatic heterogeneity seen among depressed patients and high comorbidity with other psychiatric disorders (Kessler et al., 2003; Rush, 2007).
Although clinical depression represents a severe and disabling condition, depression in general may be conceptualized as a continuum of context-dependent cognitive, mood, and behavioral responses which have evolved to facilitate rest, recuperation, and re-evaluation following a perceived loss of a vital resource (Beck and Bredemeier, 2016; Bergstrom and Meacham, 2016; Nesse, 2000). These responses may have been beneficial for survival in our ancestors, who likely faced frequent and acute physical threats and stressors. However, in our modern environment, where risks and stressors are often sustained and psychosocially-mediated, these responses may have become maladaptive, leading to clinical depression in the most severe cases. An inherited biological basis of depression is supported by twin studies, which showed that additive genetic factors accounted for between 31% and 41% of variation in susceptibility (Sullivan et al., 2000). Recently, large-scale genome-wide association studies (GWASs) have implicated 269 genes for depression (Howard et al., 2019), which can be used to improve our understanding of the underlying biological contributors to depression and prioritize targets for drug development (King et al., 2019; Visscher et al., 2017).
A somewhat surprising biological contributor to depression is immune system dysregulation, as depression is usually conceptualized as a mental rather than physical disorder. However, given the inextricable link between physiology and psychology, such strict dichotomization may do more harm than good if our goal is a more thorough understanding of depression etiology. Immune dysregulation has in fact been consistently associated with depression and other psychiatric disorders (Miller and Raison, 2016). This review attempts to summarise epidemiological and neurobiological evidence supporting a role for immune dysregulation in depression psychopathology, and discuss the genes implicated from the most recent meta-analysis of genome-wide association studies (GWASs) in depression (Howard et al., 2019) in the context of their known immune-related functions.
2. Depression and systemic immune dysregulation
The innate branch of the immune system constitutes the primary line of defense against infection, and includes the complement system, Toll-like receptors, and phagocytic cells (Yatim and Lakkis, 2015). The adaptive branch evolved later and includes B and T lymphocytes, whose immune-receptor and immunoglobulin genes undergo somatic rearrangements to detect new antigens and produce corresponding antibodies. Regulatory lymphocytes control and terminate the immune response by the secretion of cytokines. As a defensive response to immediate threats such as infection or injury, the innate immune system initiates acute inflammation, whose cardinal signs include redness, pain, heat, and swelling (Kuprash and Nedospasov, 2016). Chronic inflammation occurs when the threat is recurrent or cannot be completely eliminated by physiological defenses, and is associated with both tissue damage and repair, accompanied by systemic symptoms such as tiredness and fever. The immune system sometimes malfunctions and attacks the body’s own cells, resulting in autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, or becomes hypersensitive to harmless stimuli, causing allergic reactions.
Infection and autoimmune disease, not only of the brain but also the rest of the body, has been associated with depression. Hospital contact for either autoimmune disease or infection has been associated with increased risk of subsequent mood disorders in a dose-dependent manner (Benros et al., 2013), and a number of specific autoimmune or inflammatory disorders, including rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, and asthma, have been associated with increased depression risk (Goodwin et al., 2004; Graff et al., 2009; Kurina et al., 2001; Marrie et al., 2017). Viral infections have also been associated with depressive symptoms and immunological changes in the brain (Coughlin et al., 2018; Prusty et al., 2018). These associations of depression with autoimmune disease and infection have led to the hypothesis that systemic or brain alterations of the immune system have a specific effect in increasing depression risk (Amodeo et al., 2018; Bullmore, 2018).
However, depression has been associated with many chronic physical diseases other than infections and autoimmune diseases, including coronary heart disease, stroke, cancer, and diabetes (Voinov et al., 2013), often in a bidirectional fashion. For example, while stroke is followed by an increased risk of depression, depressive symptoms are also a risk factor for stroke (Eurelings et al., 2018; Hackett et al., 2014; Villa et al., 2018). A similar bidirectional relationship has been reported between metabolic syndrome and depression (Pan et al., 2012). Indeed, results from the Whitehall II study indicate that any long-standing illness is associated with an almost 2-fold increase in risk of depression (Kivimäki et al., 2012).
Multiple mechanisms may contribute to the extensive associations between chronic physical diseases and depression. For example, the marked increase in depression risk for stroke patients (Towfighi et al., 2017) may partly be the direct result of hypoxic brain damage, and partly an emotional reaction to the loss of abilities such as mobility and communication. Immune dysfunction is another possible contributor, since hypoxic brain damage can activate inflammatory or immune responses (Ferrucci and Fabbri, 2018; Rea et al., 2018), and chronic inflammation has been implicated in many common diseases including coronary heart disease and diabetes (Tsalamandris et al., 2019; Willerson and Ridker, 2004).
Chronic fatigue syndrome (CFS), characterized by prolonged and debilitating fatigue, shares many symptoms in common with depression, but also maintains clear distinctive features (Deale and Wessely, 2000; Griffith and Zarrouf, 2008). Although the etiology of CFS is still unclear and there is no definitive diagnostic laboratory test, many cases are preceded by viral infection, and present with elevated levels of some immune markers and cell types. Whether the evidence for immune dysfunction in depression is derived entirely from “misdiagnosed” CFS patients, or from a more substantial and representative fraction of depressed patients, is at present unclear. CFS demonstrates not only that immunological events can trigger psychological and behavioral symptoms, but that perception of physical symptoms by patients and even physicians can significantly impact diagnosis and treatment (Wessely, 2012; Wessely and Powell, 1989).
A powerful clue that immune dysregulation contributes to the link between chronic diseases and depression is that medical treatment which directly alters inflammation also alters mood. Pro-inflammatory drugs used to treat hepatitis C and cancer are associated with an increase in depression, sometimes within days (Capuron et al., 2004, 2000; Udina et al., 2012). Conversely, immunosuppressant drugs used to treat psoriasis were shown to reduce symptoms of depression (Langley et al., 2010; Tyring et al., 2006). Further evidence comes from a meta-analysis of randomized clinical trials which found that non-steroidal anti-inflammatory drugs and cytokine inhibitors significantly reduced depressive symptoms compared to placebo (Köhler et al., 2014). Furthermore, in mice, regulatory T cell depletion induces depression-like behaviors (Kim et al., 2012), while knockout of a proinflammatory gene reduced such behaviors following chronic restraint stress (Alcocer-Gómez et al., 2016). Together, this evidence from human and animal studies suggests a specific positive association between systemic inflammation and depression independent of any psychological effects from loss of physical ability or function.
3. Behavior, mood, and the immune response
One evolutionary theory for why the immune system may directly influence brain function to alter behavior and mood is the “sickness behavior” hypothesis. In 1964, Neal Miller hypothesized that feeling sick during acute infection helps organisms conserve energy and prioritize behaviors needed for immediate survival (Miller, 1964). When we have a cold or flu, we often experience a loss of motivation, lack of energy, decreased appetite, reduced cognitive ability, and diminished desire for social interaction (Dantzer et al., 2008), responses which overlap with hallmark symptoms of depression. Indeed, the name “sickness behavior” suggests that depression-like symptoms may be one of the manifestations of body’s immune response to disease. Viewed in this way, sickness behavior may reduce the activity level of a sick animal, so that it can focus on healing and recovery, and avoid external dangers. In prehistoric humans, as in the poorer countries until recent times, injuries and infections were the most frequent causes of death (Institute for Health Metrics and Evaluation (IHME), 2017), which may have created strong positive selection pressure for sickness behavior (Miller and Raison, 2016). However, when sickness behavior is prolonged and dysregulated, it may become maladaptive and develop into a syndrome very similar to clinical depression.
The “hygiene hypothesis” suggests that the human immune system is mis-calibrated to be overaly active and vigilant in modern societies (Straub and Schradin, 2016), where infections have fallen by an order of magnitude as a proportion of causes of death (IHME, 2017). With the lack of exposure to co-evolved commensal microbes commonly experienced by our ancestors, the immune system reacts excessively to harmless dust and food (allergies), begins attacking our own cells (autoimmune diseases), or maintains chronic inflammation with harmful effects on multiple body systems.
Various evidence is consistent with the hygiene hypothesis in relation to allergies and autoimmune disorders. Children who sucked their thumbs or bit their nails have been shown to develop less atopic sensitization (Lynch et al., 2016). Children who were born vaginally or whose parents sucked their pacifiers to clean them have less allergy, asthma, and/or eczema (Hesselmar et al., 2013). Amish, who practice traditional farming with close exposure to animals, had much less asthma and allergic sensitization than Hutterites, who are similar to Amish in genetics and lifestyle, except that they practice modern farming with much less animal exposure (Stein et al., 2016). The reported increase in prevalence of autoimmune and other immune system related diseases associated with improvement in socio-economic conditions improved is also consistent with the “hygiene hypothesis”. Table 1 displays the shares of all causes of disability adjusted life-years lost (DALYs) in the poorest countries in 1990 and the richest countries in 2017 (IHME, 2017). The contributions of psoriasis, inflammatory bowel disease, and multiple sclerosis rose by about an order of magnitude, while that of asthma rose several fold, consistent with the hygiene hypothesis. The lone exception is asthma in ages 15–49, which fell slightly. This might be due to the enormous health impact of indoor air pollution in poor regions (Schraufnagel et al., 2019).
Table 1.
Comparison of contribution of common diseases to disability adjusted life years.
| Poor Regions, 1990 |
Rich Regions, 2017 |
|||||
|---|---|---|---|---|---|---|
| Age (y): | 0–5 | 5–14 | 15–49 | 0–5 | 5–14 | 15–49 |
| Disease | ||||||
| Asthma | 0.33% | 1.36% | 1.67% | 1.77% | 4.82% | 1.10% |
| Psoriasis | 0.00% | 0.09% | 0.12% | 0.28% | 1.64% | 0.95% |
| Inflammatory bowel disease | 0.00% | 0.01% | 0.03% | 0.07% | 0.16% | 0.28% |
| Multiple sclerosis | 0.00% | 0.00% | 0.02% | 0.00% | 0.01% | 0.23% |
| Depression | 0.00% | 0.43% | 1.89% | 0.00% | 3.40% | 4.94% |
Autoimmune diseases and depression have tended to become much more common in developed regions. Percentages indicate the contribution of each disease to all disability adjusted life years lost within each age range and region (Institute for Health Metrics and Evaluation (IHME). GBD Compare Data Visualization. Seattle, WA: IHME, University of Washington, 2017. Available fromhttp://vizhub.healthdata.org/gbd-compare.)
Consistent with a link between dysregulated immune function and increased sickness behavior is the increased rates of depression with improvements in socio-economic conditions. The proportion of DALYs due to depression is greater in developed than in developing regions where commensal microbes may be more prevalent (Table 1), and lifetime rates of depression are increasing, especially in developed countries (Twenge et al., 2019; WHO, 2017). However, these findings may be confounded by availability of mental healthcare and changes in diagnostic practice; it has been suggested that many patients currently diagnosed with depression would have been considered to have only “normal sadness” in the past (Horwitz and Wakefield, 2007).
An inherent difficulty with evolutionary explanations, such as sickness behavior or the hygiene hypothesis, is that they are difficult, if not impossible, to test experimentally. Observational studies used to support these hypotheses are easily influenced by confounders, and alternative explanations cannot be conclusively ruled out. However, while keeping these caveats in mind, evolutionary hypotheses can still provide useful frameworks with which to organize higher-order and long-term observational trends such as the increasing rates of depression across the world, which follow advancements in infection control and sanitation.
A mechanism for sickness behavior was first proposed by Smith (1991), who posited that cytokines affect the brain and can induce depression. In 1993, Maes reported an increase in the acute-phase protein haptoglobin in melancholic depression, which associated positively with a number of other inflammatory markers and cells, accompanied vegetative symptoms of depression including decreased appetite, weight loss, psychomotor retardation, sleep disorders, and lack of energy (Maes, 1993). Further preclinical studies have since uncovered other cellular and molecular systems underlying sickness behavior (Miller and Raison, 2016), while human neuroimaging studies of immune challenge response have identified specific brain regions associated with functional changes predictive of sickness behaviors, including the insula, substantia nigra, amygdala, and ventral striatum (Critchley and Harrison, 2013), some of which have also been implicated in depression (Price and Drevets, 2010; Satizabal et al., 2019; Schmaal et al., 2016).
Cytokines are likely to play a role in depression-like sickness behavior through a diverse set of mechanisms. Primary evidence comes from animal studies, which show that both peripheral and central injection of pro-inflammatory cytokines can induce a neuroinflammatory response and result in sickness behaviors which are reduced in immune-deficient or cytokine-knockout models (Poon et al., 2015). These results are corroborated by studies in humans undergoing treatment with IFN-α, a pro-inflammatory cytokine which causes neurovegetative symptoms of depression including decreased appetite, fatigue, psychomotor retardation, and sleep disturbance in most patients and also induces anxiety, pain, depressed mood, anhedonia, cognitive impairment, anger, hostility, and suicidal ideation in half of patients (Slavich and Irwin, 2014). IFN-α decreases dopamine release in the basal ganglia and is associated with decreased effort-based motivation and decreased activation of basal ganglia reward circuitry (Capuron et al., 2012; Miller and Raison, 2016). By contrast, IFN-α increases glutamate release in the basal ganglia and dorsal anterior cingulate cortex (Miller and Raison, 2016). Injection of other pro-inflammatory agents are also able to cause sickness behavior, with some having receptors available in brain neurons (Dantzer et al., 2008). Thus, this converging evidence points to the conclusion that systemic and neuroinflammation have direct effects on emotional and behavioral processes inherent to depression.
Chronic inflammation, which results from sustained elevation of pro-inflammatory cytokines, may also contribute to some aspects of sickness behavior by increasing prostaglandin E2 (PGE2) levels or inducing p38 mitogen-activated protein kinase (Miller and Raison, 2016; Stein et al., 2017; Zhu et al., 2010). These kinases can increase the expression and function of serotonin reuptake transporters, reducing the availability of serotonin, a key molecule in depression (Ressler and Nemeroff, 2000) which is targeted by first-line treatment with selective serotonin reuptake inhibitors (SSRIs).
Prostaglandins, with PGE2 being the most extensively studied, are also important mediators of inflammation and depression-like sickness behaviors. The rate-limiting enzymes in prostaglandin production are constitutively expressed in brain tissue and are increased in response to stress (Müller et al., 2011; Poon et al., 2015). Peripheral immune challenges have been shown to increase PGE2 levels in the brain, but as a lipid molecule, PGE2 produced in the periphery is also able to cross the blood-brain barrier, unlike most other inflammatory signaling molecules (Poon et al., 2015). Depending on the subtype, PGE2 receptors of microglia may elicit pro-inflammatory and anti-inflammatory responses, while receptors near synapses may alter the neurochemical properties of neurons. In addition to its role in systemic and brain inflammation, PGE2 has been shown to mediate sickness behavior including fever, reduced food intake, and cognitive deficits (Poon et al., 2015). Importantly, PGE2 levels are increased in depressed patients, while SSRIs and tricyclic antidepressants have been shown to decrease PGE2 production (Müller et al., 2011).
The vagus nerve is an important mediator of the parasympathetic nervous system which also modulates motivation and emotion by transmitting information about visceral states, including inflammation, to the central nervous system (CNS; Critchley and Harrison, 2013). Vagal afferents can respond to the proinflammatory cytokines in the abdominal cavity, relaying signals to the brain and resulting in an increased production of pro-inflammatory IL-1β in the hippocampus and hypothalamus (Poon et al., 2015). Rodent studies have shown that cytokine-induced activation of the vagus leads to depression-like sickness behavior, notably social withdrawal, reduced locomotion, and reduced food intake (Poon et al., 2015). Although the release of IL-1β in the hypothalamus may initially produce short-term neuroinflammation, it subsequently activates the hypothalamic-pituitary-adrenal (HPA) axis to release cortisol, which reduces inflammation (Bonaz et al., 2016; Tracey, 2009). Return to homeostasis may be further facilitated by vagal efferents which release acetylcholine, suppressing the release of pro-inflammatory cytokines by macrophages throughout the body.
On the other hand, some commensal bacteria can suppress inflammation and are associated with reduced anxious or depressive behavior in rats, or symptoms in patients (Frank et al., 2018; Valles-Colomer et al., 2019). Giving people protective bacteria as probiotics to prevent or treat depression has been considered for over a century, with George Porter Phillips delivering lactic acid bacteria to “melancholia” patients in 1910 and reporting decreased depressive symptoms (Herman, 2019). In recent years, some studies have shown that administering Lactobacillus or Bifidobacterium improved mood or reduced depressive symptoms for both healthy individuals and depressed patients (Herman, 2019). One possible mechanism for the role of gut bacteria in depression is that stress weakens tight junctions in the gut, allowing bacteria to escape, leading to inflammation. Probiotic Lactobacillus bind to gut epithelium, competing against and preventing pathogenic bacteria from doing so and leaking into the body. The vagus nerve may also play a role, as vagotomy in mice blocked Lactobacillus-associated reductions in depressive behavior (Herman, 2019).
Immune system activity is tightly linked with other biological systems strongly linked to depression including neurotransmitter availability and function, along with the stress response. By activating cytokines and glucocorticoid release, stress and inflammation shift the balance of tryptophan metabolism towards kynurenine, whose levels are elevated relative to serotonin in depressed patients compared to controls (Agudelo et al., 2014; Dantzer et al., 2008; Herbert and Lucassen, 2016; Kelly et al., 2016; Slavich and Irwin, 2014). Through various other effects of the kynurenine pathway, inflammation may impact depression symptoms including cognitive deficits, low mood, and sleep disturbances by promoting degradation of monoamines and melatonin, hippocampal atrophy, and production of endogenous NMDA-receptor agonists or antagonists (Caumo et al., 2019; Herman et al., 2019; Jeon and Kim, 2017; Miller and Raison, 2016; Schlittler et al., 2016). The gut microbiome likely affects kynurenine metabolism and depression, as evidenced by the observation that fecal transplants from depressed people to rats raise their ratio of kynurenine to tryptophan and cause them to exhibit depressive behaviors (Kelly et al., 2016), whereas probiotic treatment reduces expression of a kynurenine-synthesizing enzyme and depressive behavior (Marin et al., 2017).
During periods of stress, release of epinephrine and norepinephrine by the sympathetic nervous system raises levels of circulating lymphocytes, perhaps to enable a rapid immune response if a threat results in injury (Dhabhar, 2009). Normally, the slower subsequent release of cortisol as part of the HPA axis response leads to a decrease in circulating lymphocytes and generally suppresses immune activity (Dhabhar, 2009; Slavich and Irwin, 2014), which eventually helps to restore homeostasis (Russell et al., 2018). However, somewhat like the boy who cried wolf, persistent glucocorticoid secretion induces habituation to repeated or chronic stimuli (Slavich and Irwin, 2014). Such glucocorticoid resistance can arise from the effects of chronic mild stress or childhood trauma on glucocorticoid receptor availability, reduction of corticotropin releasing factor (CRF) receptors, or decreased sensitivity of immune cells to glucocorticoid inhibition (Miller and Raison, 2016; Nezi et al., 2015). Glucocorticoid resistance increases levels of pro-inflammatory molecules, lowers levels of anti-inflammatory molecules, and ultimately increases risk of depression (Russell et al., 2018). As discussed earlier, depression also increases risk for inflammation and other diseases, thus, these factors may form a positive feedback loop which maintains depressive symptoms (Slavich and Irwin, 2014).
Although they provide a helpful framework, there is relatively weak evidence to support evolutionary theories for the association between increased hygiene and dysregulated immune function as well as the potential evolutionary value of sickness behavior. Regardless of the validity of these evolutionary rationales, we have reviewed the strong evidence that chronic inflammation can occur in the absence of a real immune threat and that such mis-calibration of the immune system can lead to improper long-term activation of depression-like sickness behaviors through a number of distinct mechanisms.
4. Immune-related genes and depression
Having reviewed evidence supporting a role of immune dysregulation in depression, we synthesize immune-related genes identified in the most recent GWAS meta-analysis by Howard et al. (2019) with evidence of their known biological function from existing literature. Although Howard et al. (2019) found no significant evidence for enrichment of immune-related gene-sets, other gene-set and expression studies show support for cytokine and immune-related pathway enrichment in depressed patients (Elovainio et al., 2015; Jansen et al., 2016; Leday et al., 2018; Wray et al., 2018). Thus, more research will be necessary to firmly establish gene-set enrichment, but current experimental, epidemiological, and genetic evidence linking inflammation with depression warrants a systematic evaluation of the functions of immune-related genes identified by Howard et al. (2019) as candidates for deeper depression-focused study.
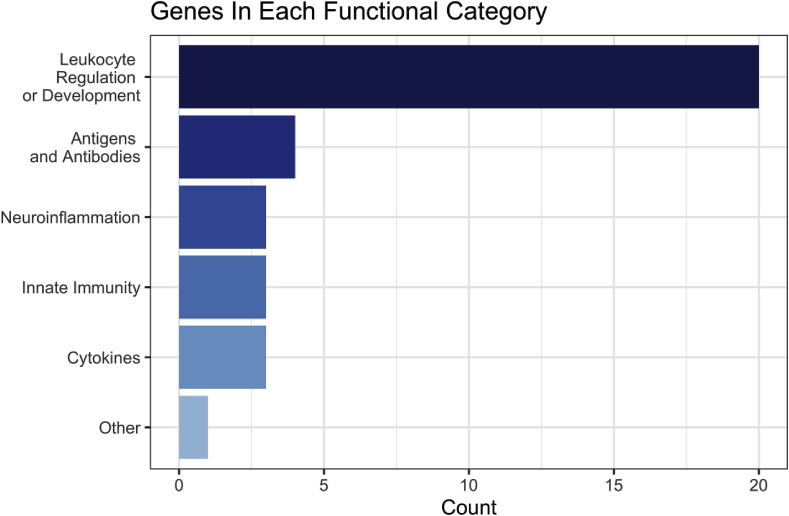
For each of the 269 genes identified by Howard et al. (2019), we conducted a manual search of the NCBI Gene database (National Library of Medicine (US) National Center for Biotechnology Information, n.d.) and PubMed for evidence linking the gene or its protein product with the development, production, trafficking, or function of immunological cells or markers. 34 of the examined genes were found to have evidence for immune-related functions, which are discussed in detail below. For ease of interpretation and discussion, we manually sorted these genes post-hoc into a few manageable sub-categories based on their major function: leukocyte regulation and development, antigens and antibodies, cytokines, innate immunity, and neuroinflammation. Fig. 1 shows the number of genes which fall into each of these categories.
Fig. 1.
This figure shows the number of genes manually categorized into each post-hoc functional category. Of the 34 total genes which had evidence for an immune-related function, a majority (20) of them appeared to have a major function related to leukocyte regulation and development. 4 showed involvement in antigen and antibody function. Neuroinflammation, innate immunity, and cytokine functional categories each contained 3 genes. TMEM258 did not clearly fit into any of these categories.
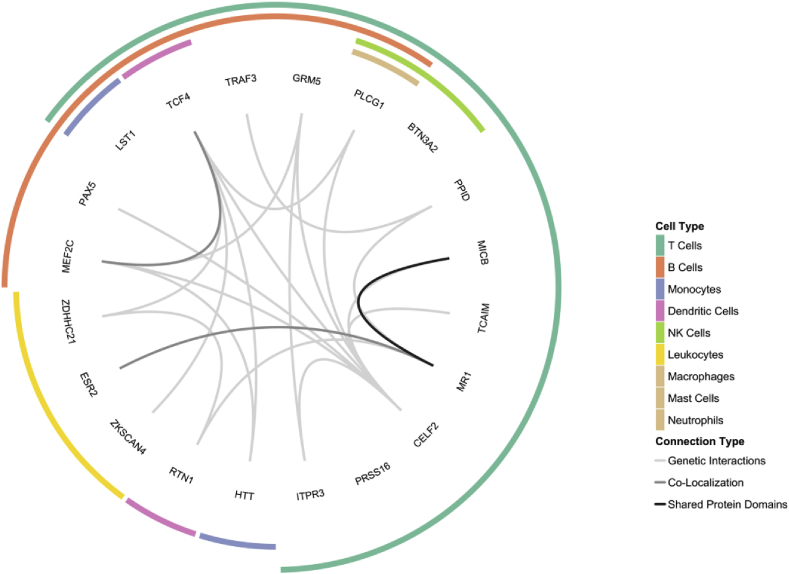
The largest of these sub-categories, with 20 genes, implicated regulatory and developmental processes of white blood cells. Fig. 2 visualizes likely connections between these genes, as curated by the GeneMANIA prediction server (Warde-Farley et al., 2010), and visualizes which immune cell types are implicated by the function of each gene, which are discussed in more detail below.
Fig. 2.
The interior of this circle diagram shows connections between the 20 genes which exhibited a function related to leukocyte regulation and development as curated through GeneMania (Warde-Farley et al., 2010). The outer portion of this diagram indicates which immune cell type(s) each gene affects directly through their function, which are referenced and discussed in more detail within the main text.
Some genes implicated multiple cell types, with TCF4 being involved in general lymphoid development (Forrest et al., 2014), LST1 negatively regulating lymphocyte proliferation (Rollinger-Holzinger et al., 2000), ZDHHC21 controlling lymphocyte adhesion (Beard et al., 2016), and estrogen receptors like ESR2 involved in lymphocyte development and function (Kovats, 2015). MICB (Raulet et al., 2013) and TRAF3 (Bishop and Xie, 2007) both regulate the activation of multiple lymphocyte types. Other genes seem to exhibit a cell-type specific function. TCAIM, MR1, and BTN3A2 each play a role in the activation of T-cells (Rhodes et al., 2015; Schumann et al., 2014; Vogel et al., 2015), while CELF2, PRSS16, and PLCG1 are involved in their development (Fu et al., 2010; Mallory et al., 2015; Viret et al., 2011). ITPR3 influences both the activation and differentiation of T-cells (Nagaleekar et al., 2008). PAX5 (Cobaleda et al., 2007) and MEF2C (Wilker et al., 2008) are necessary regulators of B-cell development and function whose expression has been observed in microglia of the rodent brain (Deczkowska et al., 2017; Maurya and Mishra, 2018). PPID can bind the immunosuppressant cyclosporin A and has been shown to regulate glucocorticoid signaling (Bourke et al., 2013; Ratajczak et al., 2003), which is an important mediator of HPA-induced inflammatory processes. GRM5 is involved in neurotransmitter, stress, and immune function through its mediation of glutamatergic effects on lymphocyte activity (Pacheco et al., 2004) and inflammation-induced microglia activation (Liu et al., 2014). ZKSCAN4 interacts with glucocorticoid receptors and is able to repress transcription initiated by glucocorticoids (Ecker et al., 2009). As glucocorticoids are pivotal to the HPA stress response, but also suppress inflammation, it is yet unclear by which pathway this gene could exert an effect on depression. Finally, RTN1 is a marker for dendritic cells, and HTT, known for its causal role in Huntington’s disease, also regulates monocyte migration (Kwan et al., 2012).
Genes of the second largest sub-category, HLA-B, BTN3A3, HLA-DQB1, and HLA-DQA1, all participate in the presentation of antigens to the immune system. Four genes, PLCG1, CHD6, SERPING1, and OLFM4, are also implicated in the innate immune response. PLCG1 has been shown to partially mediate the innate immune response (Bae et al., 2017), whereas CHD6 is able to hinder the replication of the influenza virus (Alfonso et al., 2011). SERPING1 regulates the complement cascade, an important component of the innate immune system (Davis, 2004). OLFM4, which contains the most significant SNP from the GWAS meta-analysis of Wray et al. (2018), is thought to be involved in innate immunity and gastrointestinal inflammation (Liu and Rodgers, 2016). The next sub-category, comprising cytokine-related genes, includes EMILIN3 and LTBP3, which are suggested to be involved in TGFβ signaling (Robertson et al., 2015; Schiavinato et al., 2012), along with ACVR1B, a receptor for activins, which have been shown to facilitate cytokine release (Xia and Schneyer, 2009). Neuroinflammation-related genes include LRFN5, which suppresses the immune response in the nervous system and regulates synaptic development (Choi et al., 2016; Zhu et al., 2016), CSMD1, which regulates inflammation in developing neurons (Kraus et al., 2006), and PLA2R1, which may inhibit phospholipase A2 (Murakami et al., 2015), an enzyme that mediates both general pro-inflammatory processes (Burke and Dennis, 2009) and neuroinflammation specifically (Sun et al., 2010). Two genes did not fit clearly into any of these categories, but had some evidence for an immune-related function. Finally, TMEM258, involved in intestinal inflammation and endoplasmic reticulum stress (Graham et al., 2016), was not categorized.
Only a few of these genes have been explicitly examined for a role in depression and other neuropsychiatric disorders. The enzymatic product of the PLCG1 gene, phospholipase-C γ1 (PLCγ1), catalyzes the formation of inositol phosphate 3 (IP3), which is an important secondary messenger throughout the body that regulates calcium signaling, including in the brain (Foskett, 2010) and immune system (Vig and Kinet, 2009). PLCG1 has been proposed as a mediating factor in schizophrenia, bipolar disorder, Alzheimer’s, Huntington’s, and epilepsy (Yang et al., 2016) and has been associated with lithium treatment response in bipolar patients (Løvlie et al., 2001; Turecki et al., 1998). Supportive of a role in depression, antidepressants have been shown to activate PLCγ in mice (Rantamäki et al., 2007), while loss of expression in mouse forebrain leads to manic-like behavior, including reduced depression-like behavior (Yang et al., 2017). Additionally, PLCG1 expression is absent in the postmortem brains of some suicide victims, while being present in control subjects (Lo Vasco et al., 2015).
Likewise, evidence also points to a specific role for HTT in depression apart from its most well-known role in Huntington’s disease. Repeat polymorphisms are associated with an increased lifetime depression risk in humans (Gardiner et al., 2017), and ablation in mice led to decreased depressive- and anxiety-like behaviors along with increased hippocampal neurogenesis (Ben M’Barek et al., 2013). Notably, depression is the most common comorbid psychiatric disorder in Huntington’s patients, with symptoms often emerging early in disease progression (Epping and Paulsen, 2011).
GRM5 expression in T cells has been linked to inflammation that results in fatigue in patients after cancer radiation therapy (Feng et al., 2018), suggesting an involvement in sickness behavior. Clinical trials assessing the effects of antagonists for the metabotropic glutamate receptor encoded by GRM5 on depression symptoms have been unsuccessful (Barnes et al., 2018), but administration of ketamine reduced this receptor’s availability in depressed patients and was associated with symptom improvement in one recent study (Esterlis et al., 2018). Studies in rodents have demonstrated a role for GRM5 in modulation of behavioral and immunological responses to both acute and chronic stress paradigms (Peterlik et al., 2017; Shin et al., 2015; Sun et al., 2017).
One recent study has shown that knockdown of SERPING1 in mice leads to neuroinflammation, leakage across the blood-brain barrier, cognitive deficits, and depressive-like behavior (Farfara et al., 2019). Additionally, reduced TCF4 serum expression has been observed in depression patients compared to healthy controls (Mossakowska-Wójcik et al., 2018) and is a known risk allele for schizophrenia (Forrest et al., 2018; Xia et al., 2018). CSMD1 was recently found to be significantly associated with depression in Han Chinese through a rare variant burden test (Zhang et al., 2019). Finally, variants within both HLA-DQB1, and HLA-DQA1 have been strongly associated with narcolepsy through candidate gene studies and GWAS (Hor et al., 2010; Ollila et al., 2014). Importantly, sleep disturbances often co-occur with symptoms of depression and can be an important predictor of future episodes of depression (Nutt et al., 2008).
5. Discussion
In the first half of this review, we discuss evidence supporting immune system involvement in depression symptoms. Initial evidence comes from the observed bidirectional temporal association between immune disorders and depression. It is possible that the immune to depression association could be explained as a psychological response to pain or perceived loss of health and ability, rather than a consequence of underlying immune dysregulation. However, studies showing antidepressant effects of anti-inflammatory medications and depressogenic effects of pro-inflammatory drugs in patients and rodent suggest a specific role for the immune system. Some specific biological mechanisms linking immune function to depression symptoms have been identified including neuroinflammation, vagus nerve signaling, gut microbiota, brain regions which respond to immune challenges, and interactions with neurotransmitters and the stress response.
One parsimonious explanation for both the rise in autoimmune disease and depression in developed countries comes from evolutionary theory. The hygiene hypothesis suggests that decreased exposure to environmental microbes and immune challenges during development increases the sensitivity of our immune system. Such an overactive or tonic immune response could cause chronic “sickness behaviors”, which overlap with the hallmark symptoms of depression, contributing to the increase of rates of depression in developed countries. Of course, evolutionary theories can often be post-hoc rationalizations of observational trends. They are difficult to test and observational support can be severely biased by confounding factors. Nevertheless, while acknowledging these limitations, such theories can still be helpful as a framework for interpreting findings and generating novel testable hypotheses.
The second half of this review synthesizes gene-based results from the most recent GWAS meta-analysis of depression with their known functions in the immune system, supporting existing evidence for a role of dysregulated immune function in depression. Such support from GWAS shows the value of hypothesis-free methods for identifying disease-relevant genes and prioritizing risk-associated biological systems. However, there are currently few clues about which of the previously discussed mechanisms might ultimately mediate the effects of these genes on depression risk. For example, whether LST1, by mis-regulating lymphocyte proliferation, causes tonic systemic inflammation and elicits depression-like behavior via vagal nerve signaling, or whether there is a specific effect on inflammation in the gut which causes protective microbes to be eliminated, will require targeted hypothesis-driven experiments and observational studies.
Although many genes identified by Howard et al. (2019) show an immune-related role, the remaining majority of genes currently have no supporting evidence for an immune function. It is likely that some of these genes have an immune-related function which we have yet to discover, but more than likely these genes are evidence of the highly polygenic and multifactorial nature of depression etiology. Furthermore, a large majority (23) of the immune-related genes also have known functions across other neurobiological systems, which may be difficult to tease apart. For example, ESR2 not only affects lymphocyte development and function, but also can raise the brain levels of tryptophan hydroxylase and serotonin, and is expressed in neurons in the paraventricular nucleus that release CRF, leading to higher blood glucocorticoid levels in response to stress (Borrow and Handa, 2017). One recent study has shown this gene to interact with negative life events in menopausal Chinese women to predict depression (Zhang et al., 2017). A discussion of all such overlapping functions for each gene is beyond the scope of this review, but it is critical to note their potential existence.
A high degree of pleiotropy is expected for these genes, given the strong links between the immune system, neurotransmitter regulation, and the biobehavioral stress response as well as the tendency for “moonlighting proteins” to serve multiple biological functions (Jeffery, 2018). Even the immune-related genes which currently have no evidence for cross-system effects may ultimately prove to harbor another neurobiological role. Statistical geneticists can work towards disentangling the effects of multi-function genes by using multi-level omics data across the transcriptome, proteome, and epigenome across relevant cell types. Preclinical research will ultimately be necessary to clarify which mechanism(s) truly underlie the link between these genes and depression. Our overview of these functions gives lab-based researchers a strong, evidence-based starting point to identify the most promising candidates within their area of expertise for follow-up experimentation.
The present review has three other major limitations. First, results from a gene-based test were used as the basis for our function categorization (Howard et al., 2019; Leeuw et al., 2015). Although these are powerful tools for identifying risk loci (Neale and Sham, 2004), due to linkage disequilibrium (LD) there is no guarantee that a true causal risk locus has been identified. This is especially problematic for loci within the highly conserved, gene-dense major histocompatibility (MHC) region (Horton et al., 2004), where high LD makes identifying the source of an observed association difficult. We note that nine of the 34 genes discussed here lie within the extended MHC. Notably, the MHC region had the strongest association signal for schizophrenia in patients of European ancestry (Ripke et al., 2014), which has been attributed to loci in the Complement 4 (C4) genes (Sekar et al., 2016). Follow-up analyses have begun to narrow down potentially relevant loci for depression in the MHC (Glanville et al., 2020; H. Li et al., 2019a), but further study will be necessary to pinpoint risk loci with more certainty. Due to the limitations of the applied method in handling genes in regions of high LD, some genes discussed here may not represent true causal loci. Recently, a conditional gene-based test has been introduced to address this issue (M. Li et al., 2019b).
Secondly, our review was designed as a qualitative stock-taking of the most up-to-date GWAS findings. Conducting and synthesizing results from manual searches of the literature may mean that relevant findings have been overlooked and the number of immune-related genes undercounted. Finally, as we relied on the findings from Howard et al. (2019) as the basis for our immune-related gene identification, we inherit any limitations of their study design and analysis. Notably, their findings come from a sample which is exclusively of European ancestry and contains a higher proportion of wealthy and highly educated individuals than the general population. It is also important to acknowledge that although there is some evidence for sex-specific genetic factors in depression, Howard and colleagues (2019) did not perform a genome-wide test for such effects.
Although there is strong sex discordance between rates of depression (Kessler et al., 2012), and evidence of sex-specific genetic factors from family studies (Flint and Kendler, 2014; Kendler et al., 2006), GWAS have so far failed to identify any specific loci (Hall et al., 2018; Trzaskowski et al., 2019). Notably, some evidence supports the existence of sex differences in the depressogenic effects of inflammation (for a review, see Bekhbat and Neigh, 2018). Thus, future studies examining the contributions of inflammatory genes on depression should consider testing for sex-specific effects when possible.
While we hope that this review serves as a platform for future inquiry to better understand the immunological mechanisms contributing to depression pathology, research should integrate measurements across multiple systems and consider environmental and genetic interactions when possible. Eventually, we must find ways to move beyond single-gene or omnigenic studies in cells and model organisms to a truly polygenic model, but these genes currently represent the most promising starting point for basic and translations researchers when designing studies with the aim of better understanding the underlying biology and improving treatment options for depression.
Declaration of competing interest
The authors declare no conflicts of interest.
References
- Agudelo L.Z., Femenía T., Orhan F., Porsmyr-Palmertz M., Goiny M., Martinez-Redondo V., Correia J.C., Izadi M., Bhat M., Schuppe-Koistinen I., Pettersson A.T., Ferreira D.M.S., Krook A., Barres R., Zierath J.R., Erhardt S., Lindskog M., Ruas J.L. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Alcocer-Gómez E., Ulecia-Morón C., Marín-Aguilar F., Rybkina T., Casas-Barquero N., Ruiz-Cabello J., Ryffel B., Apetoh L., Ghiringhelli F., Bullón P., Sánchez-Alcazar J.A., Carrión A.M., Cordero M.D. Stress-induced depressive behaviors require a functional NLRP3 inflammasome. Mol. Neurobiol. 2016;53:4874–4882. doi: 10.1007/s12035-015-9408-7. [DOI] [PubMed] [Google Scholar]
- Alfonso R., Lutz T., Rodriguez A., Chavez J.P., Rodriguez P., Gutierrez S., Nieto A. CHD6 chromatin remodeler is a negative modulator of influenza virus replication that relocates to inactive chromatin upon infection. Cell Microbiol. 2011;13:1894–1906. doi: 10.1111/j.1462-5822.2011.01679.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 2013. Depressive disorders. [DOI] [Google Scholar]
- Amodeo G., Allegra Trusso M., Fagiolini A. Depression and inflammation: disentangling a clear yet complex and multifaceted link. Neuropsychiatry. 2018;7:448–457. doi: 10.4172/neuropsychiatry.1000236. [DOI] [Google Scholar]
- Bae Y.S., Lee H.Y., Jung Y.S., Lee M., Suh P.G. Phospholipase Cγ in Toll-like receptor-mediated inflammation and innate immunity. Adv. Biol. Regul. 2017;63:92–97. doi: 10.1016/j.jbior.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Barnes S.A., Sheffler D.J., Semenova S., Cosford N.D.P., Bespalov A. Metabotropic glutamate receptor 5 as a target for the treatment of depression and smoking: robust preclinical data but inconclusive clinical efficacy. Biol. Psychiatr. 2018;83:955–962. doi: 10.1016/j.biopsych.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard R.S., Yang X., Meegan J.E., Overstreet J.W., Yang C.G.Y., Elliott J.A., Reynolds J.J., Cha B.J., Pivetti C.D., Mitchell D.A., Wu M.H., Deschenes R.J., Yuan S.Y. Palmitoyl acyltransferase DHHC21 mediates endothelial dysfunction in systemic inflammatory response syndrome. Nat. Commun. 2016;7:12823. doi: 10.1038/ncomms12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Bredemeier K. A unified model of depression: integrating clinical, cognitive, biological, and evolutionary perspectives. Clin. Psychol. Sci. 2016;4:596–619. doi: 10.1177/2167702616628523. [DOI] [Google Scholar]
- Bekhbat M., Neigh G.N. Sex differences in the neuro-immune consequences of stress: focus on depression and anxiety. Brain Behav. Immun. 2018;67:1–12. doi: 10.1016/j.bbi.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben M’Barek K., Pla P., Orvoen S., Benstaali C., Godin J.D., Gardier A.M., Saudou F., David D.J., Humbert S. Huntingtin mediates anxiety/depression-related behaviors and hippocampal neurogenesis. J. Neurosci. 2013;33:8608–8620. doi: 10.1523/jneurosci.5110-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benros M.E., Waltoft B.L., Nordentoft M., Ostergaard S.D., Eaton W.W., Krogh J., Mortensen P.B. Autoimmune diseases and severe infections as risk factors for mood disorders a nationwide study. JAMA Psychiatr. 2013;70:812–820. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- Bergstrom C.T., Meacham F. Depression and anxiety: maladaptive byproducts of adaptive mechanisms. Evol. Med. Publ. Health. 2016;2016:214–218. doi: 10.1093/EMPH/EOW019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G.A., Xie P. Multiple roles of TRAF3 signaling in lymphocyte function. Immunol. Res. 2007;39:22–32. doi: 10.1007/s12026-007-0068-1. [DOI] [PubMed] [Google Scholar]
- Bonaz B., Sinniger V., Pellissier S. Anti-inflammatory properties of the vagus nerve: potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016;594:5781–5790. doi: 10.1113/JP271539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow A.P., Handa R.J. Vitamins and Hormones. Academic Press Inc.; 2017. Estrogen receptors modulation of anxiety-like behavior; pp. 27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke C.H., Raees M.Q., Malviya S., Bradburn C.A., Binder E.B., Neigh G.N. Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology. 2013;38:84–93. doi: 10.1016/j.psyneuen.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E. Inflamed depression. Lancet. 2018;392:1189–1190. doi: 10.1016/S0140-6736(18)32356-0. [DOI] [PubMed] [Google Scholar]
- Burke J.E., Dennis E.A. Phospholipase A 2 structure/function, mechanism, and signaling. J. Lipid Res. 2009;50:S237–S242. doi: 10.1194/jlr.r800033-jlr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L., Pagnoni G., Drake D.F., Woolwine B.J., Spivey J.R., Crowe R.J., Votaw J.R., Goodman M.M., Miller A.H. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch. Gen. Psychiatr. 2012;69:1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L., Ravaud A., Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J. Clin. Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- Capuron L., Ravaud A., Miller A.H., Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav. Immun. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Caumo W., Hidalgo M.P., Souza A., Torres I.L.S., Antunes L.C. Melatonin is a biomarker of circadian dysregulation and is correlated with major depression and fibromyalgia symptom severity. J. Pain Res. 2019;12:545–556. doi: 10.2147/JPR.S176857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Nam J., Whitcomb D.J., Song Y.S., Kim D., Jeon S., Um J.W., Lee S.G., Woo J., Kwon S.K., Li Y., Mah W., Kim H.M., Ko J., Cho K., Kim E. SALM5 trans-synaptically interacts with LAR-RPTPs in a splicing-dependent manner to regulate synapse development. Sci. Rep. 2016;6:26676. doi: 10.1038/srep26676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobaleda C., Schebesta A., Delogu A., Busslinger M. Pax5: the guardian of B cell identity and function. Nat. Immunol. 2007;8:463–470. doi: 10.1038/ni1454. [DOI] [PubMed] [Google Scholar]
- Coughlin J.M., Yang T., Rebman A.W., Bechtold K.T., Du Y., Mathews W.B., Lesniak W.G., Mihm E.A., Frey S.M., Marshall E.S., Rosenthal H.B., Reekie T.A., Kassiou M., Dannals R.F., Soloski M.J., Aucott J.N., Pomper M.G. Imaging glial activation in patients with post-treatment Lyme disease symptoms: a pilot study using [ 11 C]DPA-713 PET. J. Neuroinflammation. 2018;15:1–7. doi: 10.1186/s12974-018-1381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Harrison N.A. Visceral influences on brain and behavior. Neuron. 2013;77:624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A.E. Biological effects of C1 inhibitor. Drug News Perspect. 2004;17:439–446. doi: 10.1358/dnp.2004.17.7.863703. [DOI] [PubMed] [Google Scholar]
- Deale A., Wessely S. Diagnosis of psychiatric disorder in clinical evaluation of chronic fatigue syndrome. J. R. Soc. Med. 2000;93:310–312. doi: 10.1177/014107680009300608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deczkowska A., Matcovitch-Natan O., Tsitsou-Kampeli A., Ben-Hamo S., Dvir-Szternfeld R., Spinrad A., Singer O., David E., Winter D.R., Smith L.K., Kertser A., Baruch K., Rosenzweig N., Terem A., Prinz M., Villeda S., Citri A., Amit I., Schwartz M. Mef2C restrains microglial inflammatory response and is lost in brain ageing in an IFN-I-dependent manner. Nat. Commun. 2017;8:717. doi: 10.1038/s41467-017-00769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar F.S. Neuroimmunomodulation. 2009. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology; pp. 300–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker K., Lorenz A., Wolf F., Ploner C., Böck G., Duncan T., Geley S., Helmberg A. A RAS recruitment screen identifies ZKSCAN4 as a glucocorticoid receptor-interacting protein. J. Mol. Endocrinol. 2009;42:105–117. doi: 10.1677/JME-08-0087. [DOI] [PubMed] [Google Scholar]
- Elovainio M., Taipale T., Sepp I., Raitoharju E., Jokela M., Pulkki-råback L., Illig T. Activated immune e in fl ammatory pathways are associated with long- standing depressive symptoms : evidence from gene-set enrichment analyses in the Young. Finns Study € l a. 2015;71:120–125. doi: 10.1016/j.jpsychires.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Epping E.A., Paulsen J.S. Depression in the early stages of Huntington disease. Neurodegener. Dis. Manag. 2011;1:407–414. doi: 10.2217/nmt.11.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I., DellaGioia N., Pietrzak R.H., Matuskey D., Nabulsi N., Abdallah C.G., Yang J., Pittenger C., Sanacora G., Krystal J.H., Parsey R.V., Carson R.E., DeLorenzo C., Parsey V.R., Carson R.E., DeLorenzo C. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [11C]ABP688 and PET imaging study in depression. Mol. Psychiatr. 2018;23:824–832. doi: 10.1038/mp.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eurelings L.S.M., van Dalen J.W., Riet T.G., van Charante E.P.M., Richard E., van Gool W.A. Apathy and depressive symptoms in older people and incident myocardial infarction, stroke, and mortality: a systematic review and meta-analysis of individual participant data. Clin. Epidemiol. 2018;10:363–379. doi: 10.2147/CLEP.S150915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfara D., Feierman E., Richards A., Revenko A.S., MacLeod R.A., Norris E.H., Strickland S. Knockdown of circulating C1 inhibitor induces neurovascular impairment, glial cell activation, neuroinflammation, and behavioral deficits. Glia. 2019;67:1359–1373. doi: 10.1002/glia.23611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L.R., Fernández-Martínez J.L., Zaal K.J.M., deAndrés-Galiana E.J., Wolff B.S., Saligan L.N. mGluR5 mediates post-radiotherapy fatigue development in cancer patients. Transl. Psychiatry. 2018;8:110. doi: 10.1038/s41398-018-0161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J., Kendler K.S. The genetics of major depression. Neuron. 2014;81:484–503. doi: 10.1016/j.neuron.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest M.P., Hill M.J., Kavanagh D.H., Tansey K.E., Waite A.J., Blake D.J. The psychiatric risk gene transcription factor 4 (TCF4) regulates neurodevelopmental pathways associated with schizophrenia, autism, and intellectual disability. Schizophr. Bull. 2018;44:1100–1110. doi: 10.1093/schbul/sbx164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest M.P., Hill M.J., Quantock A.J., Martin-Rendon E., Blake D.J. The emerging roles of TCF4 in disease and development. Trends Mol. Med. 2014;20:322–331. doi: 10.1016/j.molmed.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Foskett J.K. Inositol trisphosphate receptor Ca2+ release channels in neurological diseases. Pflügers Arch. - Eur. J. Physiol. 2010;460:481–494. doi: 10.1007/s00424-010-0826-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.G., Fonken L.K., Dolzani S.D., Annis J.L., Siebler P.H., Schmidt D., Watkins L.R., Maier S.F., Lowry C.A. Immunization with Mycobacterium vaccae induces an anti-inflammatory milieu in the CNS: attenuation of stress-induced microglial priming, alarmins and anxiety-like behavior. Brain Behav. Immun. 2018;73:352–363. doi: 10.1016/J.BBI.2018.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G., Chen Y., Yu M., Podd A., Schuman J., He Y., Di L., Yassai M., Haribhai D., North P.E., Gorski J., Williams C.B., Wang D., Wen R. Phospholipase C{gamma}1 is essential for T cell development, activation, and tolerance. J. Exp. Med. 2010;207:309–318. doi: 10.1084/jem.20090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S.L., van Belzen M.J., Boogaard M.W., van Roon-Mom W.M.C., Rozing M.P., van Hemert A.M., Smit J.H., Beekman A.T.F., van Grootheest G., Schoevers R.A., Oude Voshaar R.C., Roos R.A.C., Comijs H.C., Penninx B.W.J.H., van der Mast R.C., Aziz N.A. Huntingtin gene repeat size variations affect risk of lifetime depression. Transl. Psychiatry. 2017;7(1277) doi: 10.1038/s41398-017-0042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville K.P., Coleman J.R.I., Hanscombe K.B., Euesden J., Choi S.W., Purves K.L., Breen G., Air T.M., Andlauer T.F.M., Baune B.T., Binder E.B., Blackwood D.H.R., Boomsma D.I., Buttenschøn H.N., Colodro-Conde L., Dannlowski U., Direk N., Dunn E.C., Forstner A.J., de Geus Eco J.C., Grabe H.J., Hamilton S.P., Jones I., Jones L.A., Knowles J.A., Kutalik Z., Levinson D.F., Lewis G., Lind P.A., Lucae S., Magnusson P.K., McGuffin P., McIntosh A.M., Milaneschi Y., Mors O., Mostafavi S., Müller-Myhsok B., Pedersen N.L., Penninx B.W.J.H., Potash J.B., Preisig M., Ripke S., Shi J., Shyn S.I., Smoller J.W., Streit F., Sullivan P.F., Tiemeier H., Uher R., Van der Auwera S., Weissman M.M., Wray N.R., Mattheisen M., Trzaskowski M., Byrne E.M., Abdellaoui A., Adams M.J., Agerbo E., Bacanu S.A., Bækvad-Hansen M., Beekman A.T.F., Bigdeli T.B., Bryois J., Bybjerg-Grauholm J., Cai N., Castelao E., Christensen J.H., Clarke T.K., Couvy-Duchesne B., Craddock N., Crawford G.E., Davies G., Deary I.J., Degenhardt F., Derks E.M., Dolan C.V., Eley T.C., Escott-Price V., Hassan Kiadeh F.F., Finucane H.K., Foo J.C., Frank J., Gaspar H.A., Gill M., Goes F.S., Gordon S.D., Grove J., Hall L.S., Hansen C.S., Hansen T.F., Herms S., Hickie I.B., Hoffmann P., Homuth G., Horn C., Hottenga J.J., Hougaard D.M., Howard D.M., Ising M., Jansen R., Jorgenson E., Kohane I.S., Kraft J., Kretzschmar W.W., Li Y., MacIntyre D.J., MacKinnon D.F., Maier R.M., Maier W., Marchini J., Mbarek H., McGrath P., Medland S.E., Mehta D., Middeldorp C.M., Mihailov E., Milani L., Mondimore F.M., Montgomery G.W., Mullins N., Nauck M., Ng B., Nivard M.G., Nyholt D.R., O’Reilly P.F., Oskarsson H., Owen M.J., Painter J.N., Pedersen C.B., Pedersen M.G., Peterson R.E., Pettersson E., Peyrot W.J., Pistis G., Posthuma D., Quiroz J.A., Qvist P., Rice J.P., Riley B.P., Rivera M., Mirza S.S., Schoevers R., Schulte E.C., Shen L., Sigurdsson E., Sinnamon G.C.B., Smit J.H., Smith D.J., Stefansson H., Steinberg S., Strohmaier J., Tansey K.E., Teismann H., Teumer A., Thompson W., Thomson P.A., Thorgeirsson T.E., Traylor M., Treutlein J., Trubetskoy V., Uitterlinden A.G., Umbricht D., van Hemert A.M., Viktorin A., Visscher P.M., Wang Y., Webb B.T., Weinsheimer S.M., Wellmann J., Willemsen G., Witt S.H., Wu Y., Xi H.S., Yang J., Zhang F., Arolt V., Berger K., Cichon S., de Geus E.J.C., DePaulo J.R., Domenici E., Domschke K., Esko T., Hayward C., Heath A.C., Kendler K.S., Kloiber S., Li Q.S., Madden P.A., Martin N.G., Metspalu A., Mortensen P.B., Nordentoft M., Nöthen M.M., O’Donovan M.C., Paciga S.A., Perlis R.H., Porteous D.J., Rietschel M., Schaefer C., Schulze T.G., Stefansson K., Völzke H., Werge T., Lewis C.M., Børglum A.D. Classical human leukocyte antigen alleles and C4 haplotypes are not significantly associated with depression. Biol. Psychiatr. 2020;87:419–430. doi: 10.1016/j.biopsych.2019.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin R.D., Fergusson D.M., Horwood L.J. Asthma and depressive and anxiety disorders among young persons in the community. Psychol. Med. 2004;34:1465–1474. doi: 10.1017/S0033291704002739. [DOI] [PubMed] [Google Scholar]
- Graff L.A., Walker J.R., Bernstein C.N. Depression and anxiety in iflammatory bowel disease: a review of comorbidity and management. Inflamm. Bowel Dis. 2009;15:1105–1118. doi: 10.1002/ibd.20873. [DOI] [PubMed] [Google Scholar]
- Graham D.B., Lefkovith A., Deelen P., de Klein N., Varma M., Boroughs A., Desch A.N., Ng A.C.Y., Guzman G., Schenone M., Petersen C.P., Bhan A.K., Rivas M.A., Daly M.J., Carr S.A., Wijmenga C., Xavier R.J. TMEM258 is a component of the oligosaccharyltransferase complex controlling ER stress and intestinal inflammation. Cell Rep. 2016;17:2955–2965. doi: 10.1016/j.celrep.2016.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J.P., Zarrouf F.A. A systematic review of chronic fatigue syndrome: don’t assume it’s depression. Prim. Care Companion J. Clin. Psychiatry. 2008 doi: 10.4088/PCC.v10n0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett M.L., Köhler S., O’Brien J.T., Mead G.E. Neuropsychiatric outcomes of stroke. Lancet Neurol. 2014;13:525–534. doi: 10.1016/S1474-4422(14)70016-X. [DOI] [PubMed] [Google Scholar]
- Hall L.S., Adams M.J., Arnau-Soler A., Clarke T.K., Howard D.M., Zeng Y., Davies G., Hagenaars S.P., Fernandez-Pujals A.M., Gibson J., Wigmore E.M., Boutin T.S., Hayward C., Scotland G., Porteous D.J., Deary I.J., Thomson P.A., Haley C.S., McIntosh A.M. Genome-wide meta-analyses of stratified depression in generation scotland and UK biobank. Transl. Psychiatry. 2018;8 doi: 10.1038/s41398-017-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert J., Lucassen P.J. Depression as a risk factor for Alzheimer’s disease: genes, steroids, cytokines and neurogenesis – what do we need to know? Front. Neuroendocrinol. 2016;41:153–171. doi: 10.1016/j.yfrne.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Herman A. Probiotics supplementation in prophylaxis and treatment of depressive and anxiety disorders - review of current research. Psychiatr. Pol. 2019;53:459–473. doi: 10.12740/PP/92392. [DOI] [PubMed] [Google Scholar]
- Herman F.J., Simkovic S., Pasinetti G.M. Neuroimmune nexus of depression and dementia: shared mechanisms and therapeutic targets. Br. J. Pharmacol. 2019 doi: 10.1111/bph.14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmar B., Sjöberg F., Saalman R., Åberg N., Adlerberth I., Wold A.E. Pacifier cleaning practices and risk of allergy development. Pediatrics. 2013;131 doi: 10.1542/peds.2012-3345. [DOI] [PubMed] [Google Scholar]
- Hor H., Kutalik Z., Dauvilliers Y., Valsesia A., Lammers G.J., Donjacour C.E.H.M., Iranzo A., Santamaria J., Peraita Adrados R., Vicario J.L., Overeem S., Arnulf I., Theodorou I., Jennum P., Knudsen S., Bassetti C., Mathis J., Lecendreux M., Mayer G., Geisler P., Benetó A., Petit B., Pfister C., Bürki J.V., Didelot G., Billiard M., Ercilla G., Verduijn W., Claas F.H.J., Vollenweider P., Waeber G., Waterworth D.M., Mooser V., Heinzer R., Beckmann J.S., Bergmann S., Tafti M. Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Nat. Genet. 2010;42:786–789. doi: 10.1038/ng.647. [DOI] [PubMed] [Google Scholar]
- Horton R., Wilming L., Rand V., Lovering R.C., Bruford E.A., Khodiyar V.K., Lush M.J., Povey S., Talbot C.C., Wright M.W., Wain H.M., Trowsdale J., Ziegler A., Beck S. Gene map of the extended human MHC. Nat. Rev. Genet. 2004 doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- Horwitz A.V., Wakefield J.C. Oxford University Press; 2007. The Loss of Sadness : How Psychiatry Transformed Normal Sorrow into Depressive Disorder. [DOI] [PubMed] [Google Scholar]
- Howard D.M., Adams M.J., Clarke T.-K.K., Hafferty J.D., Gibson J., Shirali M., Coleman J.R.I.I., Hagenaars S.P., Ward J., Wigmore E.M., Alloza C., Shen X., Barbu M.C., Xu E.Y., Whalley H.C., Marioni R.E., Porteous D.J., Davies G., Deary I.J., Hemani G., Berger K., Teismann H., Rawal R., Arolt V., Baune B.T., Dannlowski U., Domschke K., Tian C., Hinds D.A., Trzaskowski M., Byrne E.M., Ripke S., Smith D.J., Sullivan P.F., Wray N.R., Breen G., Lewis C.M., McIntosh A.M. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019;22(1) doi: 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Health Metrics and Evaluation (IHME) GBD Compare data visualization: Mozambique [WWW document] 2017. https://vizhub.healthdata.org/gbd-compare/ Univ. Washingt. accessed 7.15.19.
- Jansen R., Penninx B.W.J.H., Madar V., Xia K., Milaneschi Y., Hottenga J.J., Hammerschlag A.R., Beekman A., Van Der Wee N., Smit J.H., Brooks A.I., Tischfield J., Posthuma D., Schoevers R., Van Grootheest G., Willemsen G., De Geus E.J., Boomsma D.I., Wright F.A., Zou F., Sun W., Sullivan P.F. Gene expression in major depressive disorder. Mol. Psychiatr. 2016;21:339–347. doi: 10.1038/mp.2015.57. [DOI] [PubMed] [Google Scholar]
- Jeffery C.J. Protein moonlighting: what is it, and why is it important? Philos. Trans. Roy. Soc. B Biol. Sci. 2018 doi: 10.1098/rstb.2016.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S.W., Kim Y.-K. Inflammation-induced depression: its pathophysiology and therapeutic implications. J. Neuroimmunol. 2017;313:92–98. doi: 10.1016/j.jneuroim.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Kelly J.R., Borre Y., O’Brien C., Patterson E., Aidy S. El, Deane J., Kennedy P.J., Beers S., Scott K., Moloney G., Hoban A.E., Scott L., Fitzgerald P., Ross P., Stanton C., Cryan J.F., Dinan T.G., Clarke G. Towards an understanding of depression-associated gut microbiota induced neurobehavioural changes in the rat. Eur. Neuropsychopharmacol. 2016;26:S416–S417. doi: 10.1016/s0924-977x(16)31385-2. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Gatz M., Gardner C.O., Pedersen N.L. A Swedish national twin study of lifetime major depression. Am. J. Psychiatr. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Koretz D., Merikangas K.R., Rush A.J., Walters E.E., Wang P.S., National Comorbidity Survey Replication The epidemiology of major depressive disorder. J. Am. Med. Assoc. 2003;289:3095. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Petukhova M., Sampson N.A., Zaslavsky A.M., Wittchen H.U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int. J. Methods Psychiatr. Res. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.J., Lee H., Lee G., Oh S.J., Shin M.K., Shim I., Bae H. Cd4+cd25+ regulatory t cell depletion modulates anxiety and depression-like behaviors in mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E.A., Wade Davis J., Degner J.F. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet. 2019;15 doi: 10.1371/journal.pgen.1008489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimäki M., Shipley M.J., Allan C.L., Sexton C.E., Jokela M., Virtanen M., Tiemeier H., Ebmeier K.P., Singh-Manoux A. Vascular risk status as a predictor of later-life depressive symptoms: a cohort study. Biol. Psychiatr. 2012;72:324–330. doi: 10.1016/j.biopsych.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler O., Benros E., Nordentoft M., Farkouh M.E., Iyengar R.L., Mors O., Krogh J. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatr. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015;294:63–69. doi: 10.1016/J.CELLIMM.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus D.M., Elliott G.S., Chute H., Horan T., Pfenninger K.H., Sanford S.D., Foster S., Scully S., Welcher A.A., Holers V.M. CSMD1 is a novel multiple domain complement-regulatory protein highly expressed in the central nervous system and epithelial tissues. J. Immunol. 2006;176:4419–4430. doi: 10.4049/jimmunol.176.7.4419. [DOI] [PubMed] [Google Scholar]
- Kuprash D.V., Nedospasov S.A. Molecular and cellular mechanisms of inflammation. Biochemistry. 2016;81:1237–1239. doi: 10.1134/s0006297916110018. [DOI] [PubMed] [Google Scholar]
- Kurina L.M., Goldacre M.J., Yeates D., Gill L.E. Depression and anxiety in people with inflammatory bowel disease. J. Epidemiol. Community Health. 2001;55:716–720. doi: 10.1136/jech.55.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan W., Träger U., Davalos D., Chou A., Bouchard J., Andre R., Miller A., Weiss A., Giorgini F., Cheah C., Möller T., Stella N., Akassoglou K., Tabrizi S.J., Muchowski P.J. Mutant huntingtin impairs immune cell migration in Huntington disease. J. Clin. Invest. 2012;122:4737–4747. doi: 10.1172/JCI64484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley R.G., Feldman S.R., Han C., Schenkel B., Szapary P., Hsu M.C., Ortonne J.P., Gordon K.B., Kimball A.B. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: results from a randomized, double-blind, placebo-controlled phase III trial. J. Am. Acad. Dermatol. 2010;63:457–465. doi: 10.1016/j.jaad.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Leday G.G.R., Vértes P.E., Richardson S., Greene J.R., Regan T., Khan S., Henderson R., Freeman T.C., Pariante C.M., Harrison N.A., Vertes P., Cardinal R., Richardson S., Leday G., Freeman T., Regan T., Hume D., Wu Z., Pariante C., Cattaneo A., Zunszain P., Borsini A., Stewart R., Chandran D., Carvalho L., Bell J., Souza-Teodoro L., Perry H., Harrison N., Drevets W., Wittenberg G., Jones D., Khan S., Stylianou A., Henderson R., Perry V.H., Drevets W.C., Wittenberg G.M., Bullmore E.T. Replicable and coupled changes in innate and adaptive immune gene expression in two case-control studies of blood microarrays in major depressive disorder. Biol. Psychiatr. 2018;83:70–80. doi: 10.1016/j.biopsych.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuw C.A. de, Mooij J.M., Heskes T., Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 2015;11 doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Chang H., Song X., Liu W., Li L., Wang L., Yang Y., Zhang L., Li W., Zhang Y., Zhou D.S., Li X., Zhang C., Fang Y., Sun Y., Dai J.P., Luo X.J., Yao Y.G., Xiao X., Lv L., Li M. Integrative analyses of major histocompatibility complex loci in the genome-wide association studies of major depressive disorder. Neuropsychopharmacology. 2019;44:1552–1561. doi: 10.1038/s41386-019-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Jiang L., Mak T.S.H., Kwan J.S.H., Xue C., Chen P., Leung H.C.-M., Cui L., Li T., Sham P.C. A powerful conditional gene-based association approach implicated functionally important genes for schizophrenia. Bioinformatics. 2019;35:628–635. doi: 10.1093/bioinformatics/bty682. [DOI] [PubMed] [Google Scholar]
- Liu F., Zhou R., Yan H., Yin H., Wu X., Tan Y., Li L. Metabotropic glutamate receptor 5 modulates calcium oscillation and innate immune response induced by lipopolysaccharide in microglial cell. Neuroscience. 2014;281:24–34. doi: 10.1016/j.neuroscience.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Liu W., Rodgers G.P. Olfactomedin 4 expression and functions in innate immunity, inflammation, and cancer. Canc. Metastasis Rev. 2016;35:201–212. doi: 10.1007/s10555-016-9624-2. [DOI] [PubMed] [Google Scholar]
- Lo Vasco V.R., Leopizzi M., DellaRocca C., Fais P., Montisci M., Cecchetto G. Impairment and reorganization of the phosphoinositide-specific phospholipase C enzymes in suicide brains. J. Affect. Disord. 2015;174:324–328. doi: 10.1016/j.jad.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Locher C., Koechlin H., Zion S.R., Werner C., Pine D.S., Kirsch I., Kessler R.C., Kossowsky J. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta-analysis. JAMA Psychiatr. 2017;74:1011–1020. doi: 10.1001/jamapsychiatry.2017.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løvlie R., Berle J.O., Stordal E., Steen V.M. The phospholipase C-gamma1 gene (PLCG1) and lithium-responsive bipolar disorder: re-examination of an intronic dinucleotide repeat polymorphism. Psychiatr. Genet. 2001;11:41–43. doi: 10.1097/00041444-200103000-00008. [DOI] [PubMed] [Google Scholar]
- Lynch S.J., Sears M.R., Hancox R.J. Thumb-sucking, nail-biting, and atopic sensitization, asthma, and hay fever. Pediatrics. 2016;138 doi: 10.1542/peds.2016-0443. [DOI] [PubMed] [Google Scholar]
- Maes M. Α review on the acute phase response in major depression. Rev. Neurosci. 1993;4 doi: 10.1515/REVNEURO.1993.4.4.407. [DOI] [PubMed] [Google Scholar]
- Mallory M.J., Allon S.J., Qiu J., Gazzara M.R., Tapescu I., Martinez N.M., Fu X.-D., Lynch K.W. Induced transcription and stability of CELF2 mRNA drives widespread alternative splicing during T-cell signaling. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112:E2139–E2148. doi: 10.1073/pnas.1423695112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin I.A., Goertz J.E., Ren T., Rich S.S., Onengut-Gumuscu S., Farber E., Wu M., Overall C.C., Kipnis J., Gaultier A. Microbiota alteration is associated with the development of stress-induced despair behavior. Sci. Rep. 2017;7:1–10. doi: 10.1038/srep43859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie R.A., Walld R., Bolton J.M., Sareen J., Walker J.R., Patten S.B., Singer A., Lix L.M., Hitchon C.A., El-Gabalawy R., Katz A., Fisk J.D., Bernstein C.N. Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. J. Psychosom. Res. 2017;101:17–23. doi: 10.1016/j.jpsychores.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Maurya S.K., Mishra R. Co-localization and interaction of Pax5 with Iba1 in brain of mice. Cell. Mol. Neurobiol. 2018;38:919–927. doi: 10.1007/s10571-017-0566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N.E. Some psychophysiological studies of the motivation and of the behavioral effects of illness. Bull. Br. Psychol. Soc. 1964;17:1–20. [Google Scholar]
- Mossakowska-Wójcik J., Orzechowska A., Talarowska M., Szemraj J., Gałecki P. The importance of TCF4 gene in the etiology of recurrent depressive disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;80:304–308. doi: 10.1016/j.pnpbp.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Müller N., Myint A.-M., Schwarz M.J. Inflammatory biomarkers and depression. Neurotox. Res. 2011;19:308–318. doi: 10.1007/s12640-010-9210-2. [DOI] [PubMed] [Google Scholar]
- Murakami M., Sato H., Miki Y., Yamamoto K., Taketomi Y. A new era of secreted phospholipase A₂. J. Lipid Res. 2015;56:1248–1261. doi: 10.1194/jlr.R058123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaleekar V.K., Diehl S.A., Juncadella I., Charland C., Muthusamy N., Eaton S., Haynes L., Garrett-Sinha L.A., Anguita J., Rincón M. IP 3 receptor-mediated Ca 2+ release in naive CD4 T cells dictates their cytokine program. J. Immunol. 2008;181:8315–8322. doi: 10.4049/jimmunol.181.12.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Library of Medicine (US) National center for Biotechnology information, n.d. Gene [WWW document] https://www.ncbi.nlm.nih.gov/gene accessed 7.10.19.
- Neale B.M., Sham P.C. The future of association studies: gene-based analysis and replication. Am. J. Hum. Genet. 2004;75:353–362. doi: 10.1086/423901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse R.M. Is depression an adaptation? Arch. Gen. Psychiatr. 2000;57:14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- Nezi M., Mastorakos G., Mouslech Z. In: Endotext. MDText.Com, Inc., South Dartmouth (MA) Feingold K.R., Anawalt B., Boyce A., Chrousos G., Dungan K., Grossman A., Hershman J.M., Kaltsas G., Koch C., Kopp P., Korbonits M., McLachlan R., Morley J.E., New M., Perreault L., Purnell J., Rebar R., Singer F., Trence D.L., Vinik A., Wilson D.P., editors. 2015. Corticotropin releasing hormone and the immune/inflammatory response. [Google Scholar]
- Nutt D., Wilson S., Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin. Neurosci. 2008;10:329. doi: 10.31887/DCNS.2008.10.3/dnutt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollila H.M., Ravel J.-M., Han F., Faraco J., Lin L., Zheng X., Plazzi G., Dauvilliers Y., Pizza F., Hong S.-C., Jennum P., Knudsen S., Kornum B.R., Dong X.S., Yan H., Hong H., Coquillard C., Mahlios J., Jolanki O., Einen M., Arnulf I., Högl B., Frauscher B., Crowe C., Partinen M., Huang Y.S., Bourgin P., Vaarala O., Désautels A., Montplaisir J., Mack S.J., Mindrinos M., Fernandez-Vina M., Mignot E. HLA-DPB1 and HLA class I confer risk of and protection from narcolepsy. Am. J. Hum. Genet. 2015;96:136–146. doi: 10.1016/J.AJHG.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco R., Ciruela F., Casadó V., Mallol J., Gallart T., Lluis C., Franco R. Group I metabotropic glutamate receptors mediate a dual role of glutamate in T cell activation. J. Biol. Chem. 2004;279:33352–33358. doi: 10.1074/jbc.M401761200. [DOI] [PubMed] [Google Scholar]
- Pan A., Keum N., Okereke O.I., Sun Q., Kivimaki M., Rubin R.R., Hu F.B. Association between anxiety and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35:1171–1180. doi: 10.1016/j.psyneuen.2016.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlik D., Stangl C., Bauer A., Bludau A., Keller J., Grabski D., Killian T., Schmidt D., Zajicek F., Jaeschke G., Lindemann L., Reber S.O., Flor P.J., Uschold-Schmidt N. Blocking metabotropic glutamate receptor subtype 5 relieves maladaptive chronic stress consequences. Brain Behav. Immun. 2017;59:79–92. doi: 10.1016/j.bbi.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Poon D.C.H., Ho Y.S., Chiu K., Wong H.L., Chang R.C.C. Sickness: from the focus on cytokines, prostaglandins, and complement factors to the perspectives of neurons. Neurosci. Biobehav. Rev. 2015;57:30–45. doi: 10.1016/j.neubiorev.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusty B.K., Gulve N., Govind S., Krueger G.R.F., Feichtinger J., Larcombe L., Aspinall R., Ablashi D.V., Toro C.T. Active HHV-6 infection of cerebellar purkinje cells in mood disorders. Front. Microbiol. 2018;9:1955. doi: 10.3389/fmicb.2018.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantamäki T., Hendolin P., Kankaanpää A., Mijatovic J., Piepponen P., Domenici E., Chao M.V., Männistö P.T., Castrén E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-cγ signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32:2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- Ratajczak T., Ward B.K., Minchin R.F. Immunophilin chaperones in steroid receptor signalling. Curr. Top. Med. Chem. 2003;3:1348–1357. doi: 10.2174/1568026033451934. [DOI] [PubMed] [Google Scholar]
- Raulet D.H., Gasser S., Gowen B.G., Deng W., Jung H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013;31:413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea I.M., Gibson D.S., McGilligan V., McNerlan S.E., Denis Alexander H., Ross O.A. Age and age-related diseases: role of inflammation triggers and cytokines. Front. Immunol. 2018;9:1–28. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler K.J., Nemeroff C.B. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress. Anxiety. 2000 doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rhodes D.A., Chen H.-C., Price A.J., Keeble A.H., Davey M.S., James L.C., Eberl M., Trowsdale J. Activation of human γδ T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin. J. Immunol. 2015;194:2390–2398. doi: 10.4049/jimmunol.1401064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S., Neale B.M., Corvin A., Walters J.T.R., Farh K.H., Holmans P.A., Lee P., Bulik-Sullivan B., Collier D.A., Huang H., Pers T.H., Agartz I., Agerbo E., Albus M., Alexander M., Amin F., Bacanu S.A., Begemann M., Belliveau R.A., Bene J., Bergen S.E., Bevilacqua E., Bigdeli T.B., Black D.W., Bruggeman R., Buccola N.G., Buckner R.L., Byerley W., Cahn W., Cai G., Campion D., Cantor R.M., Carr V.J., Carrera N., Catts S.V., Chambert K.D., Chan R.C.K., Chen R.Y.L., Chen E.Y.H., Cheng W., Cheung E.F.C., Chong S.A., Cloninger C.R., Cohen D., Cohen N., Cormican P., Craddock N., Crowley J.J., Curtis D., Davidson M., Davis K.L., Degenhardt F., Del Favero J., Demontis D., Dikeos D., Dinan T., Djurovic S., Donohoe G., Drapeau E., Duan J., Dudbridge F., Durmishi N., Eichhammer P., Eriksson J., Escott-Price V., Essioux L., Fanous A.H., Farrell M.S., Frank J., Franke L., Freedman R., Freimer N.B., Friedl M., Friedman J.I., Fromer M., Genovese G., Georgieva L., Giegling I., Giusti-Rodríguez P., Godard S., Goldstein J.I., Golimbet V., Gopal S., Gratten J., De Haan L., Hammer C., Hamshere M.L., Hansen M., Hansen T., Haroutunian V., Hartmann A.M., Henskens F.A., Herms S., Hirschhorn J.N., Hoffmann P., Hofman A., Hollegaard M.V., Hougaard D.M., Ikeda M., Joa I., Julià A., Kahn R.S., Kalaydjieva L., Karachanak-Yankova S., Karjalainen J., Kavanagh D., Keller M.C., Kennedy J.L., Khrunin A., Kim Y., Klovins J., Knowles J.A., Konte B., Kucinskas V., Kucinskiene Z.A., Kuzelova-Ptackova H., Kähler A.K., Laurent C., Keong J.L.C., Lee S.H., Legge S.E., Lerer B., Li M., Li T., Liang K.Y., Lieberman J., Limborska S., Loughland C.M., Lubinski J., Lönnqvist J., Macek M., Magnusson P.K.E., Maher B.S., Maier W., Mallet J., Marsal S., Mattheisen M., Mattingsdal M., McCarley R.W., McDonald C., McIntosh A.M., Meier S., Meijer C.J., Melegh B., Melle I., Mesholam-Gately R.I., Metspalu A., Michie P.T., Milani L., Milanova V., Mokrab Y., Morris D.W., Mors O., Murphy K.C., Murray R.M., Myin-Germeys I., Müller-Myhsok B., Nelis M., Nenadic I., Nertney D.A., Nestadt G., Nicodemus K.K., Nikitina-Zake L., Nisenbaum L., Nordin A., O’Callaghan E., O’Dushlaine C., O’Neill F.A., Oh S.Y., Olincy A., Olsen L., Van Os J., Pantelis C., Papadimitriou G.N., Papiol S., Parkhomenko E., Pato M.T., Paunio T., Pejovic-Milovancevic M., Perkins D.O., Pietiläinen O., Pimm J., Pocklington A.J., Powell J., Price A., Pulver A.E., Purcell S.M., Quested D., Rasmussen H.B., Reichenberg A., Reimers M.A., Richards A.L., Roffman J.L., Roussos P., Ruderfer D.M., Salomaa V., Sanders A.R., Schall U., Schubert C.R., Schulze T.G., Schwab S.G., Scolnick E.M., Scott R.J., Seidman L.J., Shi J., Sigurdsson E., Silagadze T., Silverman J.M., Sim K., Slominsky P., Smoller J.W., So H.C., Spencer C.C.A., Stahl E.A., Stefansson H., Steinberg S., Stogmann E., Straub R.E., Strengman E., Strohmaier J., Stroup T.S., Subramaniam M., Suvisaari J., Svrakic D.M., Szatkiewicz J.P., Söderman E., Thirumalai S., Toncheva D., Tosato S., Veijola J., Waddington J., Walsh D., Wang D., Wang Q., Webb B.T., Weiser M., Wildenauer D.B., Williams N.M., Williams S., Witt S.H., Wolen A.R., Wong E.H.M., Wormley B.K., Xi H.S., Zai C.C., Zheng X., Zimprich F., Wray N.R., Stefansson K., Visscher P.M., Adolfsson R., Andreassen O.A., Blackwood D.H.R., Bramon E., Buxbaum J.D., Børglum A.D., Cichon S., Darvasi A., Domenici E., Ehrenreich H., Esko T., Gejman P.V., Gill M., Gurling H., Hultman C.M., Iwata N., Jablensky A.V., Jönsson E.G., Kendler K.S., Kirov G., Knight J., Lencz T., Levinson D.F., Li Q.S., Liu J., Malhotra A.K., McCarroll S.A., McQuillin A., Moran J.L., Mortensen P.B., Mowry B.J., Nöthen M.M., Ophoff R.A., Owen M.J., Palotie A., Pato C.N., Petryshen T.L., Posthuma D., Rietschel M., Riley B.P., Rujescu D., Sham P.C., Sklar P., St Clair D., Weinberger D.R., Wendland J.R., Werge T., Daly M.J., Sullivan P.F., O’Donovan M.C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I.B., Horiguchi M., Zilberberg L., Dabovic B., Hadjiolova K., Rifkin D.B. Latent TGF-β-binding proteins. Matrix Biol. 2015;47:44–53. doi: 10.1016/J.MATBIO.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollinger-Holzinger I., Eibl B., Pauly M., Griesser U., Hentges F., Auer B., Pall G., Schratzberger P., Niederwieser D., Weiss E.H., Zwierzina H. LST1: a gene with extensive alternative splicing and immunomodulatory function. J. Immunol. 2000;164:3169–3176. doi: 10.4049/jimmunol.164.6.3169. [DOI] [PubMed] [Google Scholar]
- Rush A.J. The varied clinical presentations of major depressive disorder. J. Clin. Psychiatr. 2007 [PubMed] [Google Scholar]
- Russell A.L., Tasker J.G., Lucion A.B., Fiedler J., Munhoz C.D., Wu T., yiao J., Deak T. Factors promoting vulnerability to dysregulated stress reactivity and stress-related disease. J. Neuroendocrinol. 2018;30:1–13. doi: 10.1111/jne.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satizabal C.L., Adams H.H.H., Hibar D.P., White C.C., Knol M.J., Stein J.L., Scholz M., Sargurupremraj M., Jahanshad N., Roshchupkin G.V., Smith A.V., Bis J.C., Jian X., Luciano M., Hofer E., Teumer A., van der Lee S.J., Yang J., Yanek L.R., Lee T.V., Li S., Hu Y., Koh J.Y., Eicher J.D., Desrivières S., Arias-Vasquez A., Chauhan G., Athanasiu L., Rentería M.E., Kim S., Hoehn D., Armstrong N.J., Chen Q., Holmes A.J., den Braber A., Kloszewska I., Andersson M., Espeseth T., Grimm O., Abramovic L., Alhusaini S., Milaneschi Y., Papmeyer M., Axelsson T., Ehrlich S., Roiz-Santiañez R., Kraemer B., Håberg A.K., Jones H.J., Pike G.B., Stein D.J., Stevens A., Bralten J., Vernooij M.W., Harris T.B., Filippi I., Witte A.V., Guadalupe T., Wittfeld K., Mosley T.H., Becker J.T., Doan N.T., Hagenaars S.P., Saba Y., Cuellar-Partida G., Amin N., Hilal S., Nho K., Mirza-Schreiber N., Arfanakis K., Becker D.M., Ames D., Goldman A.L., Lee P.H., Boomsma D.I., Lovestone S., Giddaluru S., Le Hellard S., Mattheisen M., Bohlken M.M., Kasperaviciute D., Schmaal L., Lawrie S.M., Agartz I., Walton E., Tordesillas-Gutierrez D., Davies G.E., Shin J., Ipser J.C., Vinke L.N., Hoogman M., Jia T., Burkhardt R., Klein M., Crivello F., Janowitz D., Carmichael O., Haukvik U.K., Aribisala B.S., Schmidt H., Strike L.T., Cheng C.Y., Risacher S.L., Pütz B., Fleischman D.A., Assareh A.A., Mattay V.S., Buckner R.L., Mecocci P., Dale A.M., Cichon S., Boks M.P., Matarin M., Penninx B.W.J.H., Calhoun V.D., Chakravarty M.M., Marquand A.F., Macare C., Kharabian Masouleh S., Oosterlaan J., Amouyel P., Hegenscheid K., Rotter J.I., Schork A.J., Liewald D.C.M., de Zubicaray G.I., Wong T.Y., Shen L., Sämann P.G., Brodaty H., Roffman J.L., de Geus E.J.C., Tsolaki M., Erk S., van Eijk K.R., Cavalleri G.L., van der Wee N.J.A., McIntosh A.M., Gollub R.L., Bulayeva K.B., Bernard M., Richards J.S., Himali J.J., Loeffler M., Rommelse N., Hoffmann W., Westlye L.T., Valdés Hernández M.C., Hansell N.K., van Erp T.G.M., Wolf C., Kwok J.B.J., Vellas B., Heinz A., Olde Loohuis L.M., Delanty N., Ho B.C., Ching C.R.K., Shumskaya E., Singh B., Hofman A., van der Meer D., Homuth G., Psaty B.M., Bastin M.E., Montgomery G.W., Foroud T.M., Reppermund S., Hottenga J.J., Simmons A., Meyer-Lindenberg A., Cahn W., Whelan C.D., van Donkelaar M.M.J., Yang Q., Hosten N., Green R.C., Thalamuthu A., Mohnke S., Hulshoff Pol H.E., Lin H., Jack C.R., Schofield P.R., Mühleisen T.W., Maillard P., Potkin S.G., Wen W., Fletcher E., Toga A.W., Gruber O., Huentelman M., Davey Smith G., Launer L.J., Nyberg L., Jönsson E.G., Crespo-Facorro B., Koen N., Greve D.N., Uitterlinden A.G., Weinberger D.R., Steen V.M., Fedko I.O., Groenewold N.A., Niessen W.J., Toro R., Tzourio C., Longstreth W.T., Ikram M.K., Smoller J.W., van Tol M.J., Sussmann J.E., Paus T., Lemaître H., Schroeter M.L., Mazoyer B., Andreassen O.A., Holsboer F., Depondt C., Veltman D.J., Turner J.A., Pausova Z., Schumann G., van Rooij D., Djurovic S., Deary I.J., McMahon K.L., Müller-Myhsok B., Brouwer R.M., Soininen H., Pandolfo M., Wassink T.H., Cheung J.W., Wolfers T., Martinot J.L., Zwiers M.P., Nauck M., Melle I., Martin N.G., Kanai R., Westman E., Kahn R.S., Sisodiya S.M., White T., Saremi A., van Bokhoven H., Brunner H.G., Völzke H., Wright M.J., van ‘t Ent D., Nöthen M.M., Ophoff R.A., Buitelaar J.K., Fernández G., Sachdev P.S., Rietschel M., van Haren N.E.M., Fisher S.E., Beiser A.S., Francks C., Saykin A.J., Mather K.A., Romanczuk-Seiferth N., Hartman C.A., DeStefano A.L., Heslenfeld D.J., Weiner M.W., Walter H., Hoekstra P.J., Nyquist P.A., Franke B., Bennett D.A., Grabe H.J., Johnson A.D., Chen C., van Duijn C.M., Lopez O.L., Fornage M., Wardlaw J.M., Schmidt R., DeCarli C., De Jager P.L., Villringer A., Debette S., Gudnason V., Medland S.E., Shulman J.M., Thompson P.M., Seshadri S., Ikram M.A. Genetic architecture of subcortical brain structures in 38,851 individuals. Nat. Genet. 2019;51:1624–1636. doi: 10.1038/s41588-019-0511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavinato A., Becker A.-K.A., Zanetti M., Corallo D., Milanetto M., Bizzotto D., Bressan G., Guljelmovic M., Paulsson M., Wagener R., Braghetta P., Bonaldo P. EMILIN-3, peculiar member of elastin microfibril interface-located protein (EMILIN) family, has distinct expression pattern, forms oligomeric assemblies, and serves as transforming growth factor β (TGF-β) antagonist. J. Biol. Chem. 2012;287:11498–11515. doi: 10.1074/jbc.M111.303578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlittler M., Goiny M., Agudelo L.Z., Venckunas T., Brazaitis M., Skurvydas A., Kamandulis S., Ruas J.L., Erhardt S., Westerblad H., Andersson D.C. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am. J. Physiol. 2016;310:C836–C840. doi: 10.1152/ajpcell.00053.2016. [DOI] [PubMed] [Google Scholar]
- Schmaal L., Veltman D.J., van Erp T.G.M., Sämann P.G., Frodl T., Jahanshad N., Loehrer E., Tiemeier H., Hofman A., Niessen W.J., Vernooij M.W., Ikram M.A., Wittfeld K., Grabe H.J., Block A., Hegenscheid K., Völzke H., Hoehn D., Czisch M., Lagopoulos J., Hatton S.N., Hickie I.B., Goya-Maldonado R., Krämer B., Gruber O., Couvy-Duchesne B., Rentería M.E., Strike L.T., Mills N.T., de Zubicaray G.I., McMahon K.L., Medland S.E., Martin N.G., Gillespie N.A., Wright M.J., Hall G.B., MacQueen G.M., Frey E.M., Carballedo A., van Velzen L.S., van Tol M.J., van der Wee N.J., Veer I.M., Walter H., Schnell K., Schramm E., Normann C., Schoepf D., Konrad C., Zurowski B., Nickson T., McIntosh A.M., Papmeyer M., Whalley H.C., Sussmann J.E., Godlewska B.R., Cowen P.J., Fischer F.H., Rose M., Penninx B.W.J.H., Thompson P.M., Hibar D.P., Erp T.G.M., van Sämann P.G., Frodl T., Jahanshad N., Loehrer E., Tiemeier H., Hofman A., Niessen W.J., Vernooij M.W., Ikram M.A., Wittfeld K., Grabe H.J., Block A., Hegenscheid K., Völzke H., Hoehn D., Czisch M., Lagopoulos J., Hatton S.N., Hickie I.B., Goya-Maldonado R., Krämer B., Gruber O., Couvy-Duchesne B., Rentería M.E., Strike L.T., Mills N.T., Zubicaray G.I., de McMahon K.L., Medland S.E., Martin N.G., Gillespie N.A., Wright M.J., Hall G.B., MacQueen G.M., Frey E.M., Carballedo A., Velzen L.S., van Tol M.J., van Wee N.J., van der Veer I.M., Walter H., Schnell K., Schramm E., Normann C., Schoepf D., Konrad C., Zurowski B., Nickson T., McIntosh A.M., Papmeyer M., Whalley H.C., Sussmann J.E., Godlewska B.R., Cowen P.J., Fischer F.H., Rose M., Penninx B.W.J.H., Thompson P.M., Hibar D.P., van Erp T.G.M., Sämann P.G., Frodl T., Jahanshad N., Loehrer E., Tiemeier H., Hofman A., Niessen W.J., Vernooij M.W., Ikram M.A., Wittfeld K., Grabe H.J., Block A., Hegenscheid K., Völzke H., Hoehn D., Czisch M., Lagopoulos J., Hatton S.N., Hickie I.B., Goya-Maldonado R., Krämer B., Gruber O., Couvy-Duchesne B., Rentería M.E., Strike L.T., Mills N.T., de Zubicaray G.I., McMahon K.L., Medland S.E., Martin N.G., Gillespie N.A., Wright M.J., Hall G.B., MacQueen G.M., Frey E.M., Carballedo A., van Velzen L.S., van Tol M.J., van der Wee N.J., Veer I.M., Walter H., Schnell K., Schramm E., Normann C., Schoepf D., Konrad C., Zurowski B., Nickson T., McIntosh A.M., Papmeyer M., Whalley H.C., Sussmann J.E., Godlewska B.R., Cowen P.J., Fischer F.H., Rose M., Penninx B.W.J.H., Thompson P.M., Hibar D.P. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatr. 2016;21:806–812. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufnagel D.E., Balmes J.R., Cowl C.T., De Matteis S., Jung S.H., Mortimer K., Perez-Padilla R., Rice M.B., Riojas-Rodriguez H., Sood A., Thurston G.D., To T., Vanker A., Wuebbles D.J. Air pollution and noncommunicable diseases: a review by the forum of international respiratory societies’ environmental committee, Part 1: the damaging effects of air pollution. Chest. 2019;155:409–416. doi: 10.1016/j.chest.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J., Stanko K., Woertge S., Appelt C., Schumann M., Kühl A.A., Panov I., Schliesser U., Vogel S., Ahrlich S., Vaeth M., Berberich-Siebelt F., Waisman A., Sawitzki B. The mitochondrial protein TCAIM regulates activation of T cells and thereby promotes tolerance induction of allogeneic transplants. Am. J. Transplant. 2014;14:2723–2735. doi: 10.1111/ajt.12941. [DOI] [PubMed] [Google Scholar]
- Sekar A., Bialas A.R., De Rivera H., Davis A., Hammond T.R., Kamitaki N., Tooley K., Presumey J., Baum M., Van Doren V., Genovese G., Rose S.A., Handsaker R.E., Daly M.J., Carroll M.C., Stevens B., McCarroll S.A. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S., Kwon O., Kang J.I., Kwon S., Oh S., Choi J., Kim C.H., Kim D.G. mGluR5 in the nucleus accumbens is critical for promoting resilience to chronic stress. Nat. Neurosci. 2015;18:1017–1024. doi: 10.1038/nn.4028. [DOI] [PubMed] [Google Scholar]
- Slavich G.M., Irwin M.R. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.S. Vol. 1. 1991. Smith (1991)_macrophage theory.pdf; pp. 298–306. [Google Scholar]
- Stein D.J., Vasconcelos M.F., Albrechet-Souza L., Ceresér K.M.M., de Almeida R.M.M. Microglial over-activation by social defeat stress contributes to anxiety- and depressive-like behaviors. Front. Behav. Neurosci. 2017;11 doi: 10.3389/fnbeh.2017.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M.M., Hrusch C.L., Gozdz J., Igartua C., Pivniouk V., Murray S.E., Ledford J.G., Marques dos Santos M., Anderson R.L., Metwali N., Neilson J.W., Maier R.M., Gilbert J.A., Holbreich M., Thorne P.S., Martinez F.D., von Mutius E., Vercelli D., Ober C., Sperling A.I. Innate immunity and asthma risk in amish and hutterite farm children. N. Engl. J. Med. 2016;375:411–421. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub R.H., Schradin C. Chronic inflammatory systemic diseases – an evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol. Med. Publ. Health. 2016:eow001. doi: 10.1093/emph/eow001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman M.A., Loree A.M., Baltes B.B., Grekin E.R., Kirsch I. The efficacy of paroxetine and placebo in treating anxiety and depression: a meta-analysis of change on the Hamilton Rating Scales. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P.F., Neale M.C., Kendler K.S. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatr. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Sun G.Y., Shelat P.B., Jensen M.B., He Y., Sun A.Y., Simonyi A. Phospholipases A2 and inflammatory responses in the central nervous system. NeuroMolecular Med. 2010;12:133–148. doi: 10.1007/s12017-009-8092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Su R., Zhang X., Wen J., Yao D., Gao X., Zhu Z., Li H. Hippocampal GR- and CB1-mediated mGluR5 differentially produces susceptibility and resilience to acute and chronic mild stress in rats. Neuroscience. 2017;357:295–302. doi: 10.1016/J.NEUROSCIENCE.2017.06.017. [DOI] [PubMed] [Google Scholar]
- Towfighi A., Ovbiagele B., El Husseini N., Hackett M.L., Jorge R.E., Kissela B.M., Mitchell P.H., Skolarus L.E., Whooley M.A., Williams L.S. Poststroke depression: a scientific statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2017;48:e30–e43. doi: 10.1161/STR.0000000000000113. [DOI] [PubMed] [Google Scholar]
- Tracey K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzaskowski M., Mehta D., Peyrot W.J., Hawkes D., Davies D., Howard D.M., Kemper K.E., Sidorenko J., Maier R., Ripke S., Mattheisen M., Baune B.T., Grabe H.J., Heath A.C., Jones L., Jones I., Madden P.A.F., McIntosh A.M., Breen G., Lewis C.M., Børglum A.D., Sullivan P.F., Martin N.G., Kendler K.S., Levinson D.F., Wray N.R. Quantifying between-cohort and between-sex genetic heterogeneity in major depressive disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2019;180:439–447. doi: 10.1002/ajmg.b.32713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalamandris S., Antonopoulos A.S., Oikonomou E., Papamikroulis G.A., Vogiatzi G., Papaioannou S., Deftereos S., Tousoulis D. The role of inflammation in diabetes: current concepts and future perspectives. Eur. Cardiol. Rev. 2019 doi: 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecki G., Grof P., Cavazzoni P., Duffy A., Grof E., Ahrens B., Berghöfer A., Müller-Oerlinghausen B., Dvoráková M., Libigerová E., Vojtechovský M., Zvolský P., Joober R., Nilsson A., Prochazka H., Licht R.W., Rasmussen N.A., Schou M., Vestergaard P., Holzinger A., Schumann C., Thau K., Rouleau G.A., Alda M. Evidence for a role of phospholipase C-γ1 in the pathogenesis of bipolar disorder. Mol. Psychiatr. 1998;3:534–538. doi: 10.1038/sj.mp.4000447. [DOI] [PubMed] [Google Scholar]
- Twenge J.M., Cooper A.B., Joiner T.E., Duffy M.E., Binau S.G. 2019. Age, Period, and Cohort Trends in Mood Disorder Indicators and Suicide-Related Outcomes in a Nationally Representative Dataset. 2005-2017. [DOI] [PubMed] [Google Scholar]
- Tyring S., Gottlieb A., Papp K., Gordon K., Leonardi C., Wang A., Lalla D., Woolley M., Jahreis A., Zitnik R., Cella D., Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Udina M., Castellví P., Moreno-España J., Navinés R., Valdés M., Forns X., Langohr K., Solà R., Vieta E., Martín-Santos R. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J. Clin. Psychiatr. 2012;73:1128–1138. doi: 10.4088/JCP.12r07694. [DOI] [PubMed] [Google Scholar]
- Valles-Colomer M., Falony G., Darzi Y., Tigchelaar E.F., Wang J., Tito R.Y., Schiweck C., Kurilshikov A., Joossens M., Wijmenga C., Claes S., Van Oudenhove L., Zhernakova A., Vieira-Silva S., Raes J. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019;4:623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- Vig M., Kinet J.-P. Calcium signaling in immune cells. Nat. Immunol. 2009;10:21–27. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa R.F., Ferrari F., Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol. Ther. 2018;184:131–144. doi: 10.1016/j.pharmthera.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Viret C., Leung-Theung-Long S., Serre L., Lamare C., Vignali D.A.A., Malissen B., Carrier A., Guerder S. Thymus-specific serine protease controls autoreactive CD4 T cell development and autoimmune diabetes in mice. J. Clin. Invest. 2011;121:1810–1821. doi: 10.1172/JCI43314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher P.M., Wray N.R., Zhang Q., Sklar P., McCarthy M.I., Brown M.A., Yang J. 10 Years of GWAS discovery: biology, function, and translation. Am. J. Hum. Genet. 2017;101:5–22. doi: 10.1016/J.AJHG.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S.Z., Schlickeiser S., Jürchott K., Akyuez L., Schumann J., Appelt C., Vogt K., Schröder M., Vaeth M., Berberich-Siebelt F., Lutz M.B., Grütz G., Sawitzki B. TCAIM decreases T cell priming capacity of dendritic cells by inhibiting TLR-induced Ca 2+ influx and IL-2 production. J. Immunol. 2015;194:3136–3146. doi: 10.4049/jimmunol.1400713. [DOI] [PubMed] [Google Scholar]
- Voinov B., Richie W.D., Bailey R.K. Depression and chronic diseases: it is time for a synergistic mental health and primary care approach. Prim. Care Companion CNS Disord. 2013;15 doi: 10.4088/PCC.12r01468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warde-Farley D., Donaldson S.L., Comes O., Zuberi K., Badrawi R., Chao P., Franz M., Grouios C., Kazi F., Lopes C.T., Maitland A., Mostafavi S., Montojo J., Shao Q., Wright G., Bader G.D., Morris Q. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010. [DOI] [PMC free article] [PubMed]
- Wessely S. A modern perspective on some of the most highly cited JNNP papers of all time: the nature of fatigue: a comparison of chronic “postviral” fatigue with neuromuscular and affective disorders. J. Neurol. Neurosurg. Psychiatry. 2012 doi: 10.1136/jnnp-2011-301216. [DOI] [PubMed] [Google Scholar]
- Wessely S., Powell R. Fatigue syndromes: a comparison of chronic “postviral” fatigue with neuromuscular and affective disorders. J. Neurol. Neurosurg. Psychiatry. 1989;52:940–948. doi: 10.1136/jnnp.52.8.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2018. International Statistical Classification of Diseases and Related Health Problems.https://icd.who.int/browse11/l-m/en (11th Revision) [WWW Document] [Google Scholar]
- WHO . World Health Organization; 2017. Depression and Other Common Mental Disorders: Global Health Estimates. [Google Scholar]
- Wilker P.R., Kohyama M., Sandau M.M., Albring J.C., Nakagawa O., Schwarz J.J., Murphy K.M. Transcription factor Mef2c is required for B cell proliferation and survival after antigen receptor stimulation. Nat. Immunol. 2008;9:603–612. doi: 10.1038/ni.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerson J.T., Ridker P.M. Inflammation as a cardiovascular risk factor. Circulation. 2004 doi: 10.1161/01.cir.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- Wray N.R., Ripke S., Mattheisen M., Trzaskowski M., Byrne E.M., Abdellaoui A., Adams M.J., Agerbo E., Air T.M., Andlauer T.M.F., Bacanu S.A., Bækvad-Hansen M., Beekman A.F.T., Bigdeli T.B., Binder E.B., Blackwood D.R.H., Bryois J., Buttenschøn H.N., Bybjerg-Grauholm J., Cai N., Castelao E., Christensen J.H., Clarke T.K., Coleman J.I.R., Colodro-Conde L., Couvy-Duchesne B., Craddock N., Crawford G.E., Crowley C.A., Dashti H.S., Davies G., Deary I.J., Degenhardt F., Derks E.M., DIrek N., Dolan C.V., Dunn E.C., Eley T.C., Eriksson N., Escott-Price V., Kiadeh F.H.F., Finucane H.K., Forstner A.J., Frank J., Gaspar H.A., Gill M., Giusti-Rodríguez P., Goes F.S., Gordon S.D., Grove J., Hall L.S., Hannon E., Hansen C.S., Hansen T.F., Herms S., Hickie I.B., Hoffmann P., Homuth G., Horn C., Hottenga J.J., Hougaard D.M., Hu M., Hyde C.L., Ising M., Jansen R., Jin F., Jorgenson E., Knowles J.A., Kohane I.S., Kraft J., Kretzschmar W.W., Krogh J., Kutalik Z., Lane J.M., Li Yihan, Li Yun, Lind P.A., Liu X., Lu L., MacIntyre D.J., MacKinnon D.F., Maier R.M., Maier W., Marchini J., Mbarek H., McGrath P., McGuffin P., Medland S.E., Mehta Di, Middeldorp C.M., Mihailov E., Milaneschi Y., Milani L., Mill J., Mondimore F.M., Montgomery G.W., Mostafavi S., Mullins N., Nauck M., Ng B., Nivard M.G., Nyholt D.R., O’Reilly P.F., Oskarsson H., Owen M.J., Painter J.N., Pedersen C.B., Pedersen M.G., Peterson R.E., Pettersson E., Peyrot W.J., Pistis G., Posthuma D., Purcell S.M., Quiroz J.A., Qvist P., Rice J.P., Riley B.P., Rivera M., Saeed Mirza S., Saxena R., Schoevers R., Schulte E.C., Shen L., Shi J., Shyn S.I., Sigurdsson E., Sinnamon G.B.C., Smit J.H., Smith D.J., Stefansson H., Steinberg S., Stockmeier C.A., Streit F., Strohmaier J., Tansey K.E., Teismann H., Teumer A., Thompson W., Thomson P.A., Thorgeirsson T.E., Tian C., Traylor M., Treutlein J., Trubetskoy V., Uitterlinden A.G., Umbricht D., Van Der Auwera S., Van Hemert A.M., Viktorin A., Visscher P.M., Wang Y., Webb B.T., Weinsheimer S.M., Wellmann J., Willemsen G., Witt S.H., Wu Y., Xi H.S., Yang J., Zhang F., Arolt V., Baune B.T., Berger K., Boomsma D.I., Cichon S., Dannlowski U., De Geus E.C.J., Depaulo J.R., Domenici E., Domschke K., Esko T., Grabe H.J., Hamilton S.P., Hayward C., Heath A.C., Hinds D.A., Kendler K.S., Kloiber S., Lewis G., Li Q.S., Lucae S., Madden P.F.A., Magnusson P.K., Martin N.G., McIntosh A.M., Metspalu A., Mors O., Mortensen P.B., Müller-Myhsok B., Nordentoft M., Nöthen M.M., O’Donovan M.C., Paciga S.A., Pedersen N.L., Penninx B.W.J.H., Perlis R.H., Porteous D.J., Potash J.B., Preisig M., Rietschel M., Schaefer C., Schulze T.G., Smoller J.W., Stefansson K., Tiemeier H., Uher R., Völzke H., Weissman M.M., Werge T., Winslow A.R., Lewis C.M., Levinson D.F., Breen G., Børglum A.D., Sullivan P.F. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018;50:668–681. doi: 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H., Jahr F.M., Kim N.-K., Xie L., Shabalin A.A., Bryois J., Sweet D.H., Kronfol M.M., Palasuberniam P., McRae M., Riley B.P., Sullivan P.F., van den Oord E.J., McClay J.L. Building a schizophrenia genetic network: transcription factor 4 regulates genes involved in neuronal development and schizophrenia risk. Hum. Mol. Genet. 2018;27:3246–3256. doi: 10.1093/hmg/ddy222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Schneyer A.L. The biology of activin: recent advances in structure, regulation and function. J. Endocrinol. 2009;202:1–12. doi: 10.1677/JOE-08-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.R., Jung J.H., Kim S.-J., Hamada K., Suzuki A., Kim H.J., Lee J.H., Kwon O.-B., Lee Y.K., Kim J., Kim E.-K., Jang H.-J., Kang D.-S., Choi J.-S., Lee C.J., Marshall J., Koh H.-Y., Kim C.-J., Seok H., Kim S.H., Choi J.H., Choi Y.-B., Cocco L., Ryu S.H., Kim J.-H., Suh P.-G. Forebrain-specific ablation of phospholipase Cγ1 causes manic-like behavior. Mol. Psychiatr. 2017;22:1473–1482. doi: 10.1038/mp.2016.261. [DOI] [PubMed] [Google Scholar]
- Yang Y.R., Kang D.S., Lee C., Seok H., Follo M.Y., Cocco L., Suh P.G. Primary phospholipase C and brain disorders. Adv. Biol. Regul. 2016;61:80–85. doi: 10.1016/j.jbior.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Yatim K.M., Lakkis F.G. A brief journey through the immune system. Clin. J. Am. Soc. Nephrol. 2015;10:1274–1281. doi: 10.2215/CJN.10031014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Chen L., Ma J., Qiao Z., Zhao M., Qi D., Zhao Y., Ban B., Zhu X., He J., Yang Y., Pan H. Interaction of estrogen receptor β and negative life events in susceptibility to major depressive disorder in a Chinese Han female population. J. Affect. Disord. 2017;208:628–633. doi: 10.1016/J.JAD.2016.08.083. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li M., Wang Q., Hsu J.S., Deng W., Ma X., Ni P., Zhao L., Tian Y., Sham P.C., Li T. A joint study of whole exome sequencing and structural MRI analysis in major depressive disorder. Psychol. Med. 2019;1–12 doi: 10.1017/S0033291719000072. [DOI] [PubMed] [Google Scholar]
- Zhu C. Bin, Lindler K.M., Owens A.W., Daws L.C., Blakely R.D., Hewlett W.A. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010;35:2510–2520. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Yao S., Augustine M.M., Xu H., Wang J., Sun J., Broadwater M., Ruff W., Luo L., Zhu G., Tamada K., Chen L. Neuron-specific SALM5 limits inflammation in the CNS via its interaction with HVEM. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1500637. [DOI] [PMC free article] [PubMed] [Google Scholar]