Abstract
BACKGROUND
Gastrointestinal hemorrhage (GIH) is a common complication with gastrointestinal cancers (GIC). There is no comprehensive research that examines GIH in different types of GIC.
AIM
To study the prevalence, predictors, and interventions of GIH based on the anatomical location of GIC.
METHODS
This is a retrospective analysis of the 2016-2018 National Inpatient Sample database, the largest inpatient care database in the United States. All adult inpatients (≥ 18-year-old) were included. ICD-10-CM codes were used to identify patients with GIH and GIC. Prevalence of GIH was obtained based on the anatomical location of GIC. Predictors of GIH in the GIC population were studied using multivariate analysis. Interventions including endoscopy were compared to the non-intervention group to determine the differences in inpatient mortality.
RESULTS
Out of a total of 18173885 inpatients, 321622 (1.77%) cases had a diagnosis of GIC. Within GIC patients, 30507 (9.5%) inpatients had GIH, which was significantly (P < 0.001) more than the prevalence of GIH in patients without GIC (3.4%). The highest to lowest GIH rates are listed in the following order: Stomach cancer (15.7%), liver cancer (13.0%), small bowel cancer (12.7%), esophageal cancer (9.1%), colorectal cancer (9.1%), pancreatic cancer (7.2%), bile duct cancer (6.0%), and gallbladder cancer (5.1%). Within gastric cancer, the GIH rate ranged from 14.8% in cardia cancer to 25.5% in fundus cancer. Within small bowel cancers, duodenal cancers had a higher GIH rate (15.6%) than jejunal (11.1%) and ileal cancers (5.7%). Within esophageal cancers, lower third cancers had higher GIH (10.7%) than the middle third (8.0%) or upper third cancers (6.2%). When studying the predictors of GIH in GIC, socioeconomic factors such as minority race and less favorable insurances (Medicaid and self-pay) were associated with significantly higher GIH on multivariate analysis (P < 0.01). Chemotherapy and immunotherapy were also identified to have a lower risk for GIH [odds ratios (OR) = 0.74 (0.72-0.77), P < 0.001]. Out of 30507 GIC inpatients who also had GIH, 16267 (53.3%) underwent an endoscopic procedure, i.e., upper endoscopy or colonoscopy. Inpatient mortality was significantly lower in patients who underwent endoscopy compared to no endoscopy [5.5% vs 14.9%, OR = 0.42 (0.38-0.46), P < 0.001].
CONCLUSION
The prevalence of GIH in patients with GIC varies significantly based on the tumor’s anatomical location. Endoscopy, which appears to be associated with a substantial reduction in inpatient mortality, should be offered to GIC patients with GIH. Nevertheless, the decision on intervention in the GIC population should be tailored to individual patient's goals of care, the benefit on overall care, and long-term survival.
Keywords: Gastrointestinal hemorrhage, Gastrointestinal cancer, Anatomy, Risk factors, Gastrointestinal endoscopy
Core Tip: This is a retrospective analysis of the National Inpatient Sample database aiming to study the prevalence, predictors, and interventions of gastrointestinal hemorrhage (GIH) in the setting of gastrointestinal cancer (GIC). The prevalence of GIH varies based on the anatomical location of cancer, ranging between 15.7% in gastric cancer and 5.1% in gallbladder cancer. Many risk factors, including socioeconomic factors such as insurance and race, can affect the rates of GIH. Endoscopy is significantly associated with lower inpatient mortality in bleeding patients with GIC.
INTRODUCTION
Gastrointestinal hemorrhage (GIH) is a common complication in patients with gastrointestinal cancers (GIC). In terms of incidence and mortality, GICs are among the highest globally[1]; and thus remain an ongoing challenge as to management and treatment. GIH often serves as the initial symptom for GIC, locally invasive, and metastatic disease[2]. It can also carry a high mortality rate, as in the case of upper GIH[3]. An earlier study documented that bleeding gastrointestinal (GI) tumors accounted for roughly 12 percent of cases involving GIH[4]. Another analysis of studies purported that neoplasia constituted between 3%-11% of lower GIH[5]. On the other hand, in 5% of patients with upper GI bleeds, biopsy-proven tumors were the source of bleeding[6]. While existing literature studied the prevalence of GIC in GIH, and some assess GIH as a clinical symptom of a specific type of tumor[2,4,7,8], there are no inclusive studies that assess GIH in different types of GIC. Therefore, a more comprehensive and large sample size analysis is warranted to study GIH in all types of GIC.
Bleeding in GIC patients could be the result of many causes and risk factors. One study revealed that bleeding from the tumor site is the predominant source of upper GI bleeds in patients with cancer[9]. Another study found GIH common after chemoradiotherapy in patients with locally advanced pancreatic cancer[10]. Some existing literature examines the risk factors behind GIH in specific tumors, such as gastrointestinal stromal tumors[11]. In one study, risk factors implicated in GIH included initial tumor stage, smoking, and carbohydrate antigen 19-9 Levels at the time of pancreatic cancer diagnosis[8]. This current retrospective analysis assesses predictors of GIH in the setting of GIC. Another study found that GIH rate can vary based on pancreatic cancer location; however, the study was limited by the small sample size[8]. Therefore, further analysis on the prevalence of GIH regarding the anatomical location of neoplasm would assist in future clinical management of GIH in these patients.
Most importantly, investigating different interventions for GIH in the setting of GIC would provide vital information in developing treatment plans for these patients and preventing mortality. For example, literature reviews endoscopic hemostasis of GIH in both cancer and non-cancer settings, but data remains limited in specifically the setting of tumor bleeding[2,6,12,13]. Endoscopic therapy is often recommended for non-cancer related GIH, as it may decrease overall morbidity and the need for invasive surgery[14,15]. However, while hemostasis is often successfully achieved by endoscopic therapy for bleeding GIC, rebleeding rates, unfortunately, remain common[6,13].
This study’s goals involve estimating the prevalence of GIH in patients with GIC based on the anatomical location of tumors, evaluating the predictors of GIH in GIC, and the outcomes of different procedure modalities used in bleeding GIC patients.
MATERIALS AND METHODS
Study setting
This study is a retrospective analysis of the 2016 to 2018 National (Nationwide) Inpatient Sample (NIS) database, the largest national inpatient database. NIS is drawn from 48 states and includes more than 97% of the United States population. The NIS does not contain any patient identifier; therefore, it does not require review by the institutional review board.
Inclusion/exclusion criteria
All adult inpatients (≥ 18-year-old) were included.
Outcomes
(1) Estimate GIH prevalence in patients with GIC based on the anatomical location of cancer; (2) Study the predictors of GIH in patients with GIC; and (3) Study the mortality outcome of various procedural modalities used in GIH patients with GIC: (a) Endoscopy; (b) Surgery; (c) Trans-arterial embolization; and (d) Radiation therapy.
Exposure
(1) In all adult inpatients, the prevalence of GIH was compared between patients with and without GIC; (2) In inpatients with GIC, the prevalence of GIH was determined according to the anatomic location of GIC; (3) In inpatients with GIC, demographics, socioeconomic factors, comorbidities, and other disease-related factors were compared based on GIH status; and (4) In inpatients with GIC and GIH, mortality outcome was compared between patients who underwent or did not undergo interventions such as endoscopy, surgery, embolization, and radiation therapy.
Definitions
All diagnoses and procedures were reported based on ICD-10-CM and PCS coding listed in Table 1. GIH was defined as the presence of upper or lower GIH or the presence of hematemesis, melena, hematochezia, or unspecified source of GIH.
Table 1.
ICD-10-CM and PCS codes for diagnoses and procedures
|
Diagnosis
|
ICD-10-CM
|
| GI hemorrhage | Upper: I85.x1; (K25-K28).0,2,4,6; K29.x1; K318.11 K31.82 |
| Lower: K50.x11; K51.x11; K55.21; K57.x1; K57.x3 | |
| Total = upper + lower + K62.5; K92.0-2 | |
| GI cancer | |
| Esophageal cancer | C15; C49.A1; D00.1 |
| Upper third | C15.3 |
| Middle third | C15.4 |
| Lower third | C15.5 |
| Other/unspecified | C15.8-9; C49.A1; D00.1 |
| Gastric cancer | C16; C49.A2; D00.2 |
| Cardia | C16.0 |
| Fundus | C16.1 |
| Body | C16.2 |
| Pyloric antrum | C16.3 |
| Pylorus | C16.4 |
| GIST | C49.A2 |
| Other/unspecified | C16.5-9; D00.2 |
| Small bowel cancer | C17; C49.A3; D01.49 |
| Duodenum | C17.0 |
| Jejunum | C17.1 |
| Ileum | C17.2 |
| GIST | C49.A3 |
| Other/unspecified | C17.3-9; D01.49 |
| Liver cancer | C22; D01.5 |
| Hepatocellular carcinoma | C22.0 |
| Other primary liver | C22.2-8; D01.5 |
| Biliary cancer | C22.1; C24 |
| Intrahepatic | C22.1 |
| Extrahepatic | C24.0 |
| Ampulla of Vater | C24.1 |
| Other/unspecified | C24.8-9 |
| Gallbladder cancer | C23 |
| Pancreatic cancer | C25 |
| Head | C25.0 |
| Body | C25.1 |
| Tail | C25.2 |
| Duct | C25.3 |
| Endocrine | C25.4 |
| Other/unspecified | C25.7-9 |
| Colorectal cancer | C18; C19; C20; C26.0; C49.A4-5; D01.0-4 |
| Cecum | C18.0 |
| Appendix | C18.1 |
| Ascending colon | C18.2 |
| Hepatic flexure | C18.3 |
| Transverse colon | C18.4 |
| Splenic flexure | C18.5 |
| Descending colon | C18.6 |
| Sigmoid | C18.7 |
| Rectosigmoid junction | C19 |
| Rectum | C20 |
| Other/unspecified | C188.9-9; C26.0; C49.A4-5; D01.0-4 |
| Acute kidney injury | N17; N19; N99.0; O90.4 |
| Chronic kidney disease | D63.1; (E08-E13).22; I12.0,9; I13.10,11,20; N18; R88.0; Z49 |
| Congestive heart failure | I50; I97.13x; O29.12x; Z95.812; I09.81; I11.0; I13.0,2 |
| Cirrhosis and liver failure | K70.4; K70.3; K72; K91.82; K71.7; K74; K76.(6,7); K65.2; I85 |
| Radiation gastroenteritis/proctitis | K52.0; K62.7 |
| Metastasis | C77; C78; C79; C80.0 |
| Chemotherapy and immunotherapy | Z92.21; Z51.11-12; T45.1X; K12.31; D61.81; D64.81 |
| Severe malnutrition and cachexia | E40-43; R64 |
| Obesity | E66.01; E66.09; E66.(1,2,8,9); Z68.3-4 |
| Palliative care | Z521.5 |
| Aspirin/antiplatelets | Z79.82; Z79.02 |
| Anticoagulants | Z79.01 |
| Intestinal infection | A00-09; A18.32; A21.3; A22.2; B37.82; B25.8-9 |
| Hypovolemic shock | R57.1 |
| Procedures | ICD-10-PCS |
| Upper endoscopy | 06L34CZ; 0D5(1-9)8ZZ; 0DB(1-9)8ZX; 0DB(1-9)8ZZ; 0DBA8ZX; 0DJ08ZZ; 0DQ(6,7,9)8ZZ; 3E0G8TZ |
| Colonoscopy | 06LY4CC; 0D5(E-Q)8ZZ; 0DB(B-Q)8ZZ; 0DB(B-Q)8ZX; 0DJD8ZZ |
| Surgery | 0D(1,5,B,J,T); 0F(5,B,T); OW(J,3) excluding endoscopic approach |
| Trans-arterial embolization | 04(L,V)(1,2,3,5,6,7,9,B)3DZ |
| Radiation therapy | D(D,F,W)0(0-7)(0-6)Z(0,Z) |
GI: Gastrointestinal; GIST: Gastrointestinal stromal tumor.
Statistical analysis
Continuous variables were presented as mean and standard deviation. Categorical variables were presented as frequencies and percentages (%). Student t-test was used for the comparison of continuous variables, and Pearson’s χ2 test was used for categorical variables. P values were adjusted according to the Bonferroni method when pairwise comparisons were used. In a few instances, analysis was not performed due to lack of enough sample size (≤ 10 patients in a table cell), and the affected cells were left unfilled in the table.
Binary multiple logistic regression was performed for the following outcomes: (1) GIH (to assess the predictors of GIH in patients with GIC); and (2) Inpatient mortality (to assess the association between mortality and interventions such as endoscopy, surgery, embolization, and radiation therapy).
Multivariate analysis was used in the backward stepwise regression to select statistically significant variables. The binary logistic regression results were represented with adjusted OR and 95% confidence interval. Statistical significance was set at the 5% level. Statistical analysis was performed using IBM SPSS, version 27 (IBM Inc., Armonk, NY, United States).
RESULTS
Prevalence of GIH in the setting of GIC
The prevalence of GIH in adult inpatients was compared based on GIC (Table 2). Out of a total of 18173885 inpatients, 321622 (1.77%) cases had a diagnosis of GIC. Within patients with GIC, 30507 (9.5%) inpatients had GIH, which was significantly (P < 0.001) more than the prevalence of GIH in patients without GIC (3.4%).
Table 2.
Comparison of gastrointestinal hemorrhage between inpatients who have and do not have gastrointestinal cancer
|
GI cancer
|
Total
|
||||||
|
No
|
Yes
|
||||||
|
Count
|
Within GI cancer (%)
|
Count
|
Within GI cancer (%)
|
Count
|
Within total (%)
|
||
| GI bleeding | No | 17242568 | 96.6 | 291115 | 90.5 | 17533683 | 96.5 |
| Yes | 609695 | 3.4 | 30507 | 9.5 | 640202 | 3.5 | |
| Total | 17852263 | 100 | 321622 | 100 | 18173885 | 100 | |
P < 0.001. GI: Gastrointestinal.
Prevalence of GIH based on the anatomical location of GIC
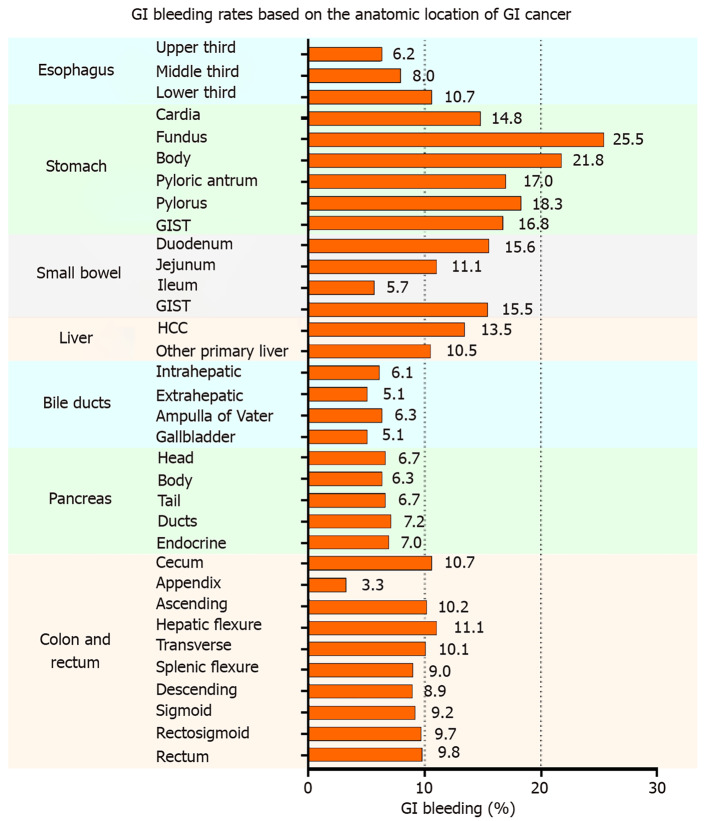
The highest to lowest GIH rates are listed in the following order: stomach cancer (15.7%), liver cancer (13.0%), small bowel cancer (12.7%), esophageal cancer (9.1%), colorectal cancer (9.1%), pancreatic cancer (7.2%), bile duct cancer (6.0%), and gallbladder cancer (5.1%). The prevalence of GIH was dissected more in detail by the anatomical location of GIC, as displayed in Figure 1. In esophageal cancer, GIH appears to become more prevalent in lower esophageal lesions (GIH in upper third esophageal cancer: 6.2% < middle third: 8.0% < lower third: 10.7%). Patients with stomach cancer have the highest GIH rates compared to other locations. The highest GIH rate occurs in patients with cancer of the stomach fundus (25.5%), and the lowest rate occurs in the cancer of the stomach cardia (14.8%). In the small bowel, cancer of the duodenum had the highest rate of GIH (15.6%), followed by jejunum (11.1%) and ileum (5.7%). Hepatocellular carcinoma was associated with a GIH rate of 13.5%, whereas biliary and gallbladder cancers had a GIH rate approximately 5%-6%, slightly differing by location. Patients with pancreatic cancers had GIH of approximately 6%-7%, slightly differing by location. Patients with cancers of the colon and rectum had comparable GIH rates (approximately 9%-11%) except for appendiceal cancer with a low bleeding rate (3.3%). The highest GIH rate in colorectal cancer patients belonged to hepatic flexure tumors (11.1%), and the lowest GIH (after appendiceal cancer) was for descending colon cancer (8.9%). Detailed data showing the patient counts determining the percentages mentioned above are available in Table 3. No statistical comparison was performed between different anatomical locations due to the numerous possibilities for comparisons and combinations; however, assessing the clinical significance of percentages and their differences is still valuable in making comparisons.
Figure 1.
The proportion of gastrointestinal bleeding in inpatients according to the anatomical location of gastrointestinal cancer. GI: Gastrointestinal; GIST: Gastrointestinal stromal tumor; HCC: Hepatocellular carcinoma.
Table 3.
Tabulated representation of data of Figure 1 which shows to the prevalence of gastrointestinal hemorrhage according to the anatomic location of gastrointestinal cancer
| Anatomic location of cancer |
GI hemorrhage
|
||||
|
|
No
|
Yes
|
|||
|
n
|
Count
|
Row (%)
|
Count
|
Row (%)
|
|
| Esophagus | 23674 | 21508 | 90.90 | 2166 | 9.10 |
| Upper third | 773 | 725 | 93.80 | 48 | 6.20 |
| Middle third | 1467 | 1349 | 92.00 | 118 | 8.00 |
| Lower third | 6540 | 5843 | 89.30 | 697 | 10.70 |
| Other/unspecified | 15161 | 13842 | 91.30 | 1319 | 8.70 |
| Stomach | 27409 | 23103 | 84.30 | 4306 | 15.70 |
| Cardia | 6829 | 5815 | 85.20 | 1014 | 14.80 |
| Fundus | 471 | 351 | 74.50 | 120 | 25.50 |
| Body | 1284 | 1004 | 78.20 | 280 | 21.80 |
| Pyloric antrum | 1881 | 1561 | 83.00 | 320 | 17.00 |
| Pylorus | 398 | 325 | 81.70 | 73 | 18.30 |
| GIST | 2477 | 2060 | 83.20 | 417 | 16.80 |
| Other/unspecified | 14410 | 12256 | 85.10 | 2154 | 14.90 |
| Small bowel | 6469 | 5646 | 87.30 | 823 | 12.70 |
| Duodenum | 3270 | 2760 | 84.40 | 510 | 15.60 |
| Jejunum | 513 | 456 | 88.90 | 57 | 11.10 |
| Ileum | 540 | 509 | 94.30 | 31 | 5.70 |
| GIST | 872 | 737 | 84.50 | 135 | 15.50 |
| Other/unspecified | 1322 | 1228 | 92.90 | 94 | 7.10 |
| Liver | 33452 | 29111 | 87.00 | 4341 | 13.00 |
| HCC | 27601 | 23877 | 86.50 | 3724 | 13.50 |
| Other primary liver | 5988 | 5357 | 89.50 | 631 | 10.50 |
| Bile ducts | 18706 | 17577 | 94.00 | 1129 | 6.00 |
| Intrahepatic | 12515 | 11749 | 93.90 | 766 | 6.10 |
| Extrahepatic | 2749 | 2608 | 94.90 | 141 | 5.10 |
| Ampulla of Vater | 2143 | 2008 | 93.70 | 135 | 6.30 |
| Other/unspecified | 1464 | 1368 | 93.40 | 96 | 6.60 |
| Gallbladder | 4268 | 4049 | 94.90 | 219 | 5.10 |
| Pancreas | 63636 | 59063 | 92.80 | 4573 | 7.20 |
| Head | 17643 | 16469 | 93.30 | 1174 | 6.70 |
| Body | 3077 | 2882 | 93.70 | 195 | 6.30 |
| Tail | 3892 | 3630 | 93.30 | 262 | 6.70 |
| Ducts | 774 | 718 | 92.80 | 56 | 7.20 |
| Endocrine | 589 | 548 | 93.00 | 41 | 7.00 |
| Other/unspecified | 38379 | 35489 | 92.50 | 2890 | 7.50 |
| Colon and rectum | 148943 | 135410 | 90.90 | 13533 | 9.10 |
| Cecum | 12171 | 10863 | 89.30 | 1308 | 10.70 |
| Appendix | 3967 | 3835 | 96.70 | 132 | 3.30 |
| Ascending | 16104 | 14458 | 89.80 | 1646 | 10.20 |
| Hepatic flexure | 3280 | 2916 | 88.90 | 364 | 11.10 |
| Transverse | 7439 | 6687 | 89.90 | 752 | 10.10 |
| Splenic flexure | 2033 | 1851 | 91.00 | 182 | 9.00 |
| Descending | 4239 | 3862 | 91.10 | 377 | 8.90 |
| Sigmoid | 17602 | 15976 | 90.80 | 1626 | 9.20 |
| Rectosigmoid | 17199 | 15527 | 90.30 | 1672 | 9.70 |
| Rectum | 29634 | 26730 | 90.20 | 2904 | 9.80 |
| Other/unspecified | 40531 | 37341 | 91.50 | 3190 | 8.50 |
GI: Gastrointestinal; GIST: Gastrointestinal stromal tumor; HCC: Hepatocellular carcinoma.
Predictors of GIH in patients with GIC
In this section, the predictors of GIH were studied in the population of patients with GIC. Table 4 shows a comparison of various demographic, socioeconomic, and other disease-related factors based on GIH status. Patients with GIH were slightly older compared to patients without GIH (68.2 ± 13.2 vs 66.2 ± 12.8 years old, P < 0.001). Patients with GIH were less likely to be females (37.8% vs 43.3%, P < 0.001). While minority races, including Black, Hispanic, Asian, and Native American, were more prevalent in patients with GIH, White race was less common in GIH patients (63.0% vs 68.3%, P < 0.001). Socioeconomic factors also were associated with varying GIH rates. Patients with GIH were more likely to be Medicare (60.3% vs 55.5%, P < 0.001), Medicaid, or self-pay patients, and they were less likely to have private insurance (21.3% vs 28.1%, P < 0.001). Likewise, GIH patients had a lower median household income compared to patients without GIH. Comorbidities such as acute kidney injury, chronic kidney disease, heart failure, cirrhosis, and liver failure were more common in patients with GIH. For cancer-related variables, patients with GIH had less metastatic disease (39.7% vs 43.1%, P < 0.001), were less treated with chemotherapy or immunotherapy (14.1% vs 19.6%, P < 0.001), and had more radiation gastroenteritis or proctitis (0.6% vs 0.3%, P < 0.001). GIH patients were also less obese and were more diagnosed with severe malnutrition and cachexia compared to non-GIH patients.
Table 4.
Bivariate analysis comparing various factors based on gastrointestinal hemorrhage status in a population of inpatients with gastrointestinal cancer
|
Inpatients with GI cancer
|
No GI hemorrhage
|
GI hemorrhage
|
P
value
|
|||
|
n
= 291115
|
n
= 30507
|
|
||||
|
Count/mean
|
Column%/SD
|
Count/mean
|
Column%/SD
|
|
||
| Demographic factors | ||||||
| Age (yr) | 66.2 | ± 12.8 | 68.2 | ± 13.2 | < 0.001 | |
| Female | 125898 | 43.30 | 11543 | 37.80 | < 0.001 | |
| Race | White | 192544 | 68.30 | 18633 | 63.00 | < 0.001 |
| Black | 37986 | 13.50 | 4727 | 16.00 | < 0.001 | |
| Hispanic | 29010 | 10.30 | 3462 | 11.70 | < 0.001 | |
| Asian or Pacific Islander | 11482 | 4.10 | 1562 | 5.30 | < 0.001 | |
| Native American | 1494 | 0.50 | 189 | 0.60 | 0.015 | |
| Other | 9345 | 3.30 | 999 | 3.40 | 0.543 | |
| Socioeconomic factors | ||||||
| Insurance | Medicare | 161272 | 55.50 | 18371 | 60.30 | < 0.001 |
| Medicaid | 33523 | 11.50 | 3859 | 12.70 | < 0.001 | |
| Private | 81599 | 28.10 | 6483 | 21.30 | < 0.001 | |
| Self-pay | 6348 | 2.20 | 894 | 2.90 | < 0.001 | |
| No charge | 628 | 0.20 | 71 | 0.20 | 0.544 | |
| Other | 7379 | 2.50 | 799 | 2.60 | 0.373 | |
| Median household income for patient ZIP Code | 1st quartile | 78840 | 27.60 | 8905 | 29.70 | < 0.001 |
| 2nd quartile | 73759 | 25.80 | 7733 | 25.80 | 0.965 | |
| 3rd quartile | 69806 | 24.40 | 7072 | 23.60 | 0.003 | |
| 4th quartile | 63693 | 22.30 | 6241 | 20.80 | < 0.001 | |
| Comorbidities | ||||||
| Acute kidney injury | 55007 | 18.90 | 7849 | 25.70 | < 0.001 | |
| Chronic kidney disease | 38425 | 13.20 | 5766 | 18.90 | < 0.001 | |
| Heart failure | 8704 | 3.00 | 1289 | 4.20 | < 0.001 | |
| Cirrhosis and liver failure | 32194 | 11.10 | 6154 | 20.20 | < 0.001 | |
| Intestinal infection | 6694 | 2.30 | 753 | 2.50 | 0.06 | |
| Cancer related | ||||||
| Metastasis | 125345 | 43.10 | 12120 | 39.70 | < 0.001 | |
| Chemo and Immunotherapy | 57005 | 19.60 | 4314 | 14.10 | < 0.001 | |
| Radiation gastroenteritis/proctitis | 849 | 0.30 | 189 | 0.60 | < 0.001 | |
| Palliative care | 38129 | 13.10 | 5318 | 17.40 | < 0.001 | |
| Nutritional status | ||||||
| Severe malnutrition and cachexia | 41008 | 14.10 | 4952 | 16.20 | < 0.001 | |
| Obesity | 32691 | 11.20 | 3127 | 10.30 | < 0.001 | |
| Use of antithrombotic/anticoagulants | ||||||
| Aspirin/antiplatelets | 30778 | 10.60 | 3605 | 11.80 | < 0.001 | |
| Anticoagulants | 22753 | 7.80 | 3345 | 11.00 | < 0.001 | |
Bold values represent a statistically significant higher column proportion. GI: Gastrointestinal.
Table 5 shows the multivariate analysis results, which validates the results of the bivariate analysis discussed above. In summary, predictors (in favor) of GIH were age, minority races (Black, Hispanic, Asian, Native American compared to White race), Insurance (Medicaid and Self-pay compared to Medicare), acute kidney injury, chronic kidney disease, heart failure, cirrhosis, and liver failure, radiation gastroenteritis or proctitis, severe malnutrition and cachexia, use of aspirin, antithrombotic and anticoagulants. Predictors against having GIH were female gender, private insurance (compared to Medicare), higher median household income, presence of metastatic disease, patient on chemotherapy or immunotherapy, and obesity. The factor with the highest OR for GIH was radiation gastroenteritis and proctitis [OR = 2.39 (2.02-2.81)]. The factor with the lowest OR for GIH was chemotherapy or immunotherapy [OR = 0.74 (0.72-0.77)].
Table 5.
The results of multivariate analysis showing the predictors of gastrointestinal hemorrhage in a population of patients with gastrointestinal cancer
|
Predictors of GI hemorrhage
| ||||
|
|
aOR
|
95%CI
|
P
value
|
|
| Demographic factors | ||||
| Age (yr) | 1.01 | (1.01-1.02) | < 0.001 | |
| Female | 0.84 | (0.81-0.86) | < 0.001 | |
| Race | White- Reference | 1.00 | - | - |
| Black | 1.27 | (1.22-1.31) | < 0.001 | |
| Hispanic | 1.19 | (1.14-1.24) | < 0.001 | |
| Asian or Pacific Islander | 1.42 | (1.34-1.50) | < 0.001 | |
| Native American | 1.24 | (1.06-1.46) | 0.007 | |
| Other | 1.13 | (1.05-1.21) | 0.001 | |
| Socioeconomic factors | ||||
| Insurance | Medicare- Reference | 1.00 | - | - |
| Medicaid | 1.17 | (1.12-1.22) | < 0.001 | |
| Private | 0.91 | (0.88-0.94) | < 0.001 | |
| Self-pay | 1.44 | (1.34-1.56) | < 0.001 | |
| No charge | 1.21 | (0.94-1.56) | 0.148 | |
| Other | 1.03 | (0.95-1.12) | 0.468 | |
| Median household income for patient ZIP Code | 1st quartile- Reference | 1.00 | - | - |
| 2nd quartile | 0.98 | (0.95-1.01) | 0.246 | |
| 3rd quartile | 0.96 | (0.93-0.99) | 0.022 | |
| 4th quartile | 0.94 | (0.90-0.97) | < 0.001 | |
| Comorbidities | ||||
| Acute kidney injury | 1.17 | (1.13-1.20) | < 0.001 | |
| Chronic kidney disease | 1.22 | (1.18-1.26) | < 0.001 | |
| Heart failure | 1.19 | (1.12-1.27) | < 0.001 | |
| Cirrhosis and liver failure | 1.84 | (1.78-1.90) | < 0.001 | |
| Cancer related | ||||
| Metastasis | 0.93 | (0.90-0.95) | < 0.001 | |
| Chemo and Immunotherapy | 0.74 | (0.72-0.77) | < 0.001 | |
| Radiation gastroenteritis/proctitis | 2.39 | (2.02-2.81) | < 0.001 | |
| Palliative care | 1.21 | (1.17-1.26) | < 0.001 | |
| Nutritional status | ||||
| Severe malnutrition and cachexia | 1.12 | (1.08-1.15) | < 0.001 | |
| Obesity | 0.94 | (0.90-0.98) | 0.001 | |
| Use of antithrombotic/anticoagulants | ||||
| Aspirin/antiplatelets | 1.09 | (1.05-1.13) | < 0.001 | |
| Anticoagulants | 1.48 | (1.42-1.54) | < 0.001 | |
Bold values represent a statistically significant odds ratio > 1 [in favor of gastrointestinal hemorrhage (GIH)]; multivariate logistic regression of outcome (GIH) was performed using the backward stepwise method to determine statistically significant factors; variables included in the analysis: Age, female, race, insurance, income, acute kidney injury, chronic kidney disease, heart failure, cirrhosis and liver failure, intestinal infection, metastasis, chemotherapy and immunotherapy, radiation gastroenteritis, palliative care, severe malnutrition and cachexia, obesity, aspirin/antiplatelet, and anticoagulant; intestinal infection was a statistically non-significant factor; GI: Gastrointestinal; CI: Confidence interval; OR: Odds ratio.
Interventions for GIH
Interventions that have been proposed and utilized in GIH patients with GIC were studied. Inpatient mortality was the outcome of interest. The four studied interventions were endoscopy, surgery, trans-arterial embolization, and radiation therapy. Multivariate analysis, using stepwise binary logistic regression, accounted for the following factors: Age, female, race, income, acute kidney injury, chronic kidney disease, heart failure, cirrhosis and liver failure, intestinal infection, metastasis, chemotherapy and immunotherapy, radiation gastroenteritis, palliative care, hypovolemic shock, endoscopy, surgery, embolization, and radiation therapy.
Endoscopy
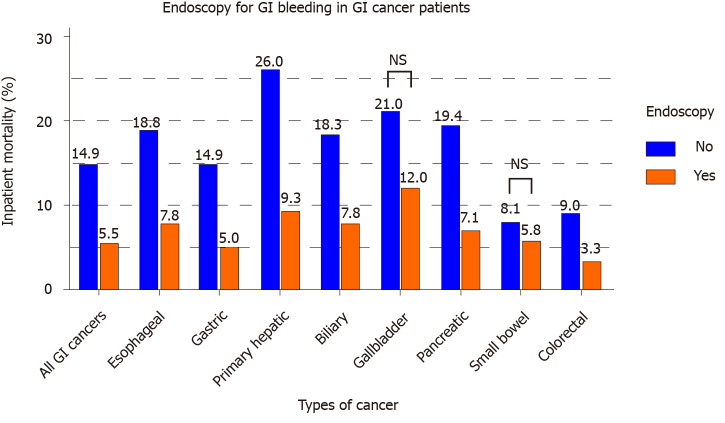
Out of 30507 inpatients with GIC who also had GIH, 16267 (53.3%) underwent an endoscopic procedure, i.e., upper endoscopy or colonoscopy. Figure 2 displays a significant decrease in mortality associated with endoscopy performance in patients with GIH and GIC (mortality with endoscopy: 5.5% vs no endoscopy: 14.9%, P < 0.001). Multivariate adjusted analysis (Table 6) shows a mortality reduction associated with endoscopy [OR = 0.42 (0.38-0.46)]. This association also applied to cancer subtypes, particularly esophageal, gastric, primary hepatic, biliary, pancreatic, and colorectal cancer. Gallbladder and small bowel cancer patients did not show a statistically significant association between mortality and endoscopy.
Figure 2.
The mortality outcomes of endoscopy in gastrointestinal cancer patients who have gastrointestinal hemorrhage. GI: Gastrointestinal; NS: Not significant.
Table 6.
The results of multivariate analysis showing the odds ratio of inpatient mortality associated with different interventions (endoscopy, surgery, embolization, radiation)
|
|
GI bleeding patients with cancer
|
|||||||||
|
|
All GI Ca
|
Esophageal Ca
|
Gastric Ca
|
Hepatic Ca
|
Biliary Ca
|
Gallbladder Ca
|
Pancreatic Ca
|
Small bowel Ca
|
Colorectal Ca
|
|
| Mortality aOR (95%CI) | Endoscopy | 0.42 (0.38-0.46) | 0.42 (0.31-0.57) | 0.42 (0.32-0.54) | 0.36 (0.29-0.43) | 0.43 (0.28-0.66) | 0.71 (0.24-2.11) | 0.36 (0.29-0.44) | 1.19 (0.59-2.43) | 0.45 (0.38-0.54) |
| Surgery | 0.97 (0.84-1.13) | - | 1.73 (1.00-3.00) | 1.30 (0.67-2.53) | - | - | 0.85 (0.49-1.48) | 2.26 (0.95-5.36) | 1.33 (1.09-1.62) | |
| Trans-arterial embolization | 1.35 (1.02-1.80) | - | 1.46 (0.81-2.62) | 1.12 (0.55-2.30) | - | - | 0.98 (0.56-1.69) | - | 2.52 (1.23-5.15) | |
| Radiation therapy | 0.55 (0.29-1.05) | - | - | - | - | - | - | - | - | |
Bold values: Statistically significant (P < 0.05). Adjusted odds ratio with 95% confidence interval; empty cells indicate that analysis for the corresponding intervention was not performed due to the insufficient sample size; multivariate logistic regression of outcome (mortality) was performed using the backward stepwise method to determine statistically significant factors; variables included in the analysis: Age, female, race, income, acute kidney injury, chronic kidney disease, heart failure, cirrhosis and liver failure, intestinal infection, metastasis, chemotherapy and immunotherapy, radiation gastroenteritis, palliative care, hypovolemic shock, endoscopy, surgery, embolization, and radiation therapy. GI: Gastrointestinal. CI: Confidence interval; Ca: Cancer; OR: Odds ratio.
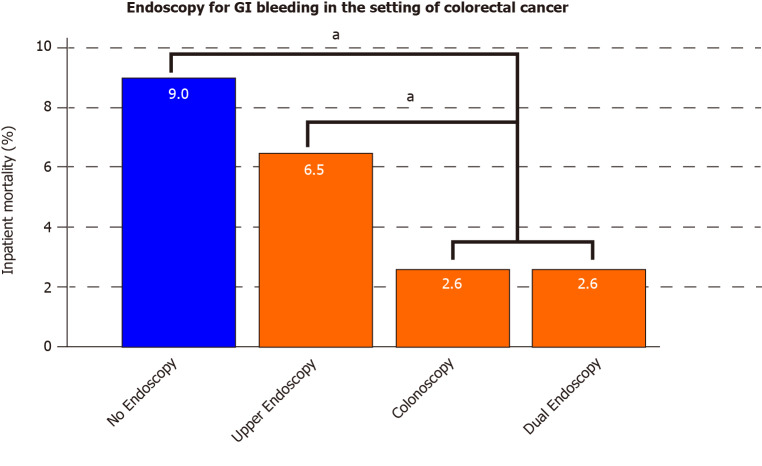
Colorectal cancer had a sufficient patient population to study the types of endoscopy performed and their association with inpatient mortality. Figure 3 shows that, in colorectal cancer patients with GIH, the lowest mortality was reported in patients who underwent either colonoscopy (2.6%) or dual (upper and lower) endoscopy (2.6%). This was significantly lower compared to mortality in patients who underwent upper endoscopy (6.5%) or no endoscopy (9.0%) (P < 0.001 for colonoscopy or dual endoscopy vs upper endoscopy or non-endoscopy group). Eight percent of all GIH causes in colorectal cancer patients were attributed to upper GIH, including 4.1% peptic ulcer disease and 0.9% esophageal varices.
Figure 3.
The mortality outcomes of different endoscopic approaches (upper, colonoscopy, or dual) in colorectal cancer patients who have gastrointestinal hemorrhage. aP < 0.05. GI: Gastrointestinal.
Surgery
Out of 30507 inpatients with GIC who also had GIH, 4568 (15.0%) underwent surgical exploration with or without bowel resection during hospitalization. Unadjusted analysis displays a significant decrease in mortality associated with the performance of surgery in GIH patients with GIC (total) (5.6% vs 10.6%, P < 0.001) and colorectal cancer (4.6% vs 6.5%, P < 0.001). On multivariate (adjusted) analysis shown in Table 6, results were different from unadjusted analysis. Surgery was not associated with any statistical difference decrease in mortality in GIC (total) but had increased odds of mortality in patients with gastric [OR = 1.73 (1.00-3.00)] and colorectal cancer [OR = 1.33 (1.09-1.62)]. Small bowel, hepatic, and pancreatic cancer patients did not show a statistical difference between surgery and non-surgery groups.
Trans-arterial embolization
Out of 30507 inpatients with GIC who also had GIH, 516 (1.7%) underwent trans-arterial embolization. Unadjusted analysis displays a significant increase in mortality associated with the performance of trans-arterial embolization in GIH patients with GIC (total) (14.7% vs 9.8%, P < 0.001). Gastric cancer (15.1% vs 8.7%, P = 0.01) and colorectal cancer (21.9% vs 5.9%, P < 0.001) were also associated with increased mortality in patients who underwent embolization. Similarly, on multivariate (adjusted) analysis in Table 6, embolization was associated with increased odds of mortality in GIC (total) [OR = 1.35 (1.02-1.80)] and colorectal cancer [OR = 2.52 (1.23-5.15)]. Gastric, hepatic, and pancreatic cancer patients did not show a statistical association between embolization and mortality on multivariate analysis.
Radiation therapy
Out of 30507 inpatients with GIC who also had GIH, radiation therapy was performed in 210 (0.7%) patients during the hospitalization. On bivariate analysis, the inpatient mortality of patients who underwent inpatient radiation therapy was lower than those who did not undergo radiation therapy (5.7% vs 9.9%, P = 0.04). On multivariate analysis (Table 6), inpatient radiation therapy for GI bleeding patients with GIC was not significantly associated with any inpatient mortality difference. Analysis was not performed on individual GIC types (esophageal, gastric, small bowel, …) due to insufficient sample in the radiation group.
DISCUSSION
This was a retrospective review of the 2016-2018 NIS database, which is one of the largest national inpatient databases. Our results, as presented in Table 2, our results showed that hospitalized patients with GIC have a significantly higher prevalence of GIH (9.5%) compared to that of the general inpatient population (3.4%). This estimate underscores that GIH is a common complication of GIC and corroborates this study’s importance.
Our study showed that GIH is note common in GIC patients and varies significantly based on the anatomical location of cancer. The highest to lowest GIH rates are listed in the following order: stomach cancer (15.7%), liver cancer (13.0%), small bowel cancer (12.7%), esophageal cancer (9.1%), colorectal cancer (9.1%), pancreatic cancer (7.2%), bile duct cancer (6.0%), and gallbladder cancer (5.1%). Figure 1 shows a more detailed representation of GIH rates based on the anatomical location of GIC. The rate of GIH can significantly vary with different tumor locations, even for locations within the same organ. The pattern of bleeding, displayed in Figure 1, shows the highest GIH rate in gastric cancers (ranging between 14.8% in the cardia and 25.5% in cancers of the fundus) followed by cancers adjacent to the stomach, such as cancer of the duodenum (15.6%) and lower third of the esophagus (10.7%). This could be related to the effect of the stomach’s acidic medium that can cause erosion and ulceration of the friable intraluminal cancerous tissue and subsequently bleeding. Thus, the further the cancerous tissue from the stomach, the less risk of GIH. Following the same logic, jejunal (11.1%) and ileal cancers (5.7%) have lower GIH rate than duodenal cancers (15.6%), and cancers of the upper (6.2%) and middle third (8.0%) of the esophagus have lower GIH than lower third cancers (10.7%). The correlation between the high incidence of GIH in hepatocellular carcinoma and underlying severe liver cirrhosis with resultant variceal hemorrhage has been demonstrated in previous studies.[16] Colorectal cancer’s GIH rates based on different anatomical locations were relatively comparable in the range between 9% to 11%. Appendiceal cancer was an exception with 3.3% GIH, which is similar to the general inpatient population (3.4%).
While our study reports the prevalence of GIH among GIC patients, prior studies have reported the reciprocal prevalence of GIC among patients with GIH[3,17,18]. For example, Sheibani et al[6] stated that tumor bleeding comprised 5% (106 cases) of all upper GIH with gastric cancer representing 73%, esophageal cancer 16%, and duodenal cancer 11%. The aforementioned study serves another purpose and cannot estimate the rates of GIH as it examines another parameter. In addition, the large sample size of our patients (30507 bleeding GIC) robustly increases the power of our GIH estimates and analysis.
Notable findings were also reported in the study of the predictors of GIH in GIC. Multivariate analysis results are shown in Table 5. A closer look at the prevalence of GIH in GIC, stratified by race, raises concerning questions on healthcare disparities. Compared to the White race, certain minority races (Black, Hispanic, Asian, and Native American) were predictors of GIH. Lower median household income was also a concerning predictor of GIH. GIH outcomes, stratified by race, have been studied before in various contexts. One study of patients hospitalized for upper GIH found that rebleeding rates were significantly lower in White patients than in Hispanic or Black patients[19]. In the instance of cancer, healthcare disparities also play a significant role in disease onset and outcome. Black patients are observed to have the highest incidence and mortality of many GI tract malignancies, including esophageal, gastric, small bowel, pancreas, colorectal, and anal cancer[20]. Despite the decline in colorectal cancer mortality rates in the past years, the reduction is not as prominent in Black patients. The causes of this are likely multifactorial, many of which are modifiable risk factors such as socioeconomic status, insurance coverage, education level, and consistent access to medical care[21]. The results of this study potentially reinforce these conclusions, as Medicaid patients and non-White patients with GIC experienced higher rates of GIH. Future studies should continue to examine outcomes of GIH in cancer patients, stratified by factors that would affect access to quality healthcare. Such data would be important in driving targeted screening and prevention efforts to high-risk populations. Our analysis also found other significant predictors of GIH, including cancer-related factors. Chemotherapy and immunotherapy were associated with lower risk for GIH [OR = 0.74 (0.72-0.77), P < 0.001]. We speculate that the associated decreased risk is related to tumor involution in response to chemotherapy. Radiation gastroenteritis and proctitis was the strongest predictor of GIH [OR = 2.39 (2.02-2.81), P < 0.001]. The presence of metastasis was associated with a lower risk of GIH [OR = 0.93 (0.90-0.95), P < 0.001]. This could be confounded by other factors that are not retrospectively available for analysis in this database, such as patients’ prior surgical history related to the malignancy.
In examining interventions for GIH in the setting of GIC, our data support that endoscopic therapy is associated with a substantial reduction in mortality. Figure 2 highlights the marked difference in mortality between endoscopy and non-endoscopy groups in various GICs (esophageal, gastric, liver, biliary, pancreatic, and colorectal cancer). There was no statistical difference in the subset of gallbladder and small bowel cancers. The type of endoscopy was studied particularly in our cohort of bleeding colorectal cancer patients. Performing either dual endoscopy or colonoscopy resulted in a statistically significant reduction in mortality compared to no endoscopy or upper endoscopy alone (Figure 3). We also have reported that eight percent of all GIH causes in colorectal cancer patients were attributed to upper GIH, including 4.1% peptic ulcer disease and 0.9% esophageal varices. From this standpoint, we can argue in favor of performing dual endoscopy, as upper endoscopy is a fast procedure that can generally be performed with ease along with colonoscopy. As discussed before, endoscopic therapy for GIH may decrease overall morbidity and the need for surgical intervention[14]. Multiple endoscopic methods such as injection, mechanical, and ablative therapies were suggested to stop bleeding from GI tumors; however, literature is mainly based on limited small sample size (10-100 patients) studies[22,23]. Based on our current knowledge, this current study has the largest analysis of endoscopy in bleeding GIC patients. Future studies should examine the different modalities of endoscopic therapy for the treatment of hemorrhage in the specific setting of cancer.
Trans-arterial embolization for GIH in GIC patients was associated with increased inpatient mortality, particularly for colorectal cancers. Surgical exploration with or without resection was not associated with mortality difference in bleeding GIC total population. However, it was associated with increased gastric and colorectal cancer mortality on multivariate analyses (Table 6). Surgery is usually reserved as a last resort for rebleeding or hemorrhage refractory to endoscopic therapy, and these cancer patients usually have an initial poor prognosis or advanced disease[12]. Radiation therapy was not associated with mortality difference in patients with GIH and GIC. The limitations are mainly due to the retrospective nature of the study. Important factors, such as the severity of GIH, intensive care admission, rebleeding rates, tumor’s size, and the stage and grade of cancer, were also not available for analysis in this database. Therefore, prospectively studying this patient population in the future would instead decrease potential information bias and would be able to fill in the gaps of the current research. However, our study’s strength is numerous and related to its uniqueness, novelty, and robust analysis. The current study provides a detailed and comprehensive examination of the subject of GIH in GIC and provides evidence to support the use of endoscopy in this patient population.
CONCLUSION
The prevalence of GIH in patients with GIC varies significantly based on the anatomical location of the tumor. GICs with the highest to the lowest likelihood of GIH are stomach cancer, liver cancer, small bowel cancer, esophageal cancer, colorectal cancer, pancreatic cancer, bile duct cancer, and lastly, gallbladder cancer. Endoscopy is associated with a substantial reduction in inpatient mortality and therefore should be offered to GIH patients with GIC. Nevertheless, the decision on intervention in the GIC population should be tailored to individual patient's goals of care, the benefit on overall care, and long-term survival.
ARTICLE HIGHLIGHTS
Research background
Gastrointestinal hemorrhage (GIH) is a common complication with gastrointestinal cancers (GIC).
Research motivation
There is no comprehensive research that examines GIH in different types of GIC. Furthermore, endoscopic therapy is insufficiently studied in this setting.
Research objectives
We aim to study the prevalence, predictors, and interventions of GIH based on the anatomical location of GIC.
Research methods
This is a retrospective analysis of the 2016-2018 National Inpatient Sample database, the largest inpatient care database in the United States. Adult inpatients were evaluated for the prevalence and predictors of GIH in the setting of GIC. In addition, inpatient mortality was compared between patients who underwent or did not undergo endoscopy.
Research results
The highest to lowest GIH rates are listed in the following order: stomach cancer (15.7%), liver cancer (13.0%), small bowel cancer (12.7%), esophageal cancer (9.1%), colorectal cancer (9.1%), pancreatic cancer (7.2%), bile duct cancer (6.0%), and gallbladder cancer (5.1%). Inpatient mortality was significantly lower in patients who underwent endoscopy compared to no endoscopy [5.5% vs 14.9%, OR = 0.42 (0.38-0.46)], P < 0.001).
Research conclusions
The prevalence of GIH in patients with GIC varies significantly based on the tumor’s anatomical location. Endoscopy appears to be associated with a substantial reduction in inpatient mortality and should be offered to GIC patients with GIH.
Research perspectives
Future studies, prospective and randomized trials, would help confirm the effectiveness of endoscopic therapy for GIH in patients with GIC.
Footnotes
Institutional review board statement: Not required because study involves only de-identified data.
Informed consent statement: Not required because study involves only de-identified data.
Conflict-of-interest statement: All authors have no relevant disclosures.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; American Gastroenterological Association.
Peer-review started: April 21, 2021
First decision: June 23, 2021
Article in press: August 6, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yu X S-Editor: Yan JP L-Editor: A P-Editor: Guo X
Contributor Information
Mohamad A Minhem, Internal Medicine, Loyola University Medical Center, Maywood, IL 60153, United States.
Ahmad Nakshabandi, Department of Gastroenterology, Medstar Georgetown University Hospital, Washington, DC 20007, United States.
Rabia Mirza, School of Medicine, Georgetown University, Washington, DC 20007, United States.
Mohd Amer Alsamman, Department of Gastroenterology, Medstar Georgetown University Hospital, Washington, DC 20007, United States.
Mark C Mattar, Department of Gastroenterology, Medstar Georgetown University Hospital, Washington, DC 20007, United States; School of Medicine, Georgetown University, Washington, DC 20007, United States. mark.c.mattar@medstar.net.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Schatz RA, Rockey DC. Gastrointestinal Bleeding Due to Gastrointestinal Tract Malignancy: Natural History, Management, and Outcomes. Dig Dis Sci. 2017;62:491–501. doi: 10.1007/s10620-016-4368-y. [DOI] [PubMed] [Google Scholar]
- 3.Roberts SE, Button LA, Williams JG. Prognosis following upper gastrointestinal bleeding. PLoS One. 2012;7:e49507. doi: 10.1371/journal.pone.0049507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lightdale CJ, Kurtz RC, Boyle CC, Sherlock P, Winawer SJ. Cancer and upper gastrointestinal tract hemorrhage. Benign causes of bleeding demonstrated by endoscopy. JAMA. 1973;226:139–141. [PubMed] [Google Scholar]
- 5.Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34:643–664. doi: 10.1016/j.gtc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Sheibani S, Kim JJ, Chen B, Park S, Saberi B, Keyashian K, Buxbaum J, Laine L. Natural history of acute upper GI bleeding due to tumours: short-term success and long-term recurrence with or without endoscopic therapy. Aliment Pharmacol Ther. 2013;38:144–150. doi: 10.1111/apt.12347. [DOI] [PubMed] [Google Scholar]
- 7.Wan W, Xiong Z, Zeng X, Yang W, Li C, Tang Y, Lin Y, Gao J, Zhang P, Tao K. The prognostic value of gastrointestinal bleeding in gastrointestinal stromal tumor: A propensity score matching analysis. Cancer Med. 2019;8:4149–4158. doi: 10.1002/cam4.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang YU, Yuan C, Liu X. Characteristics of gastrointestinal hemorrhage associated with pancreatic cancer: A retrospective review of 246 cases. Mol Clin Oncol. 2015;3:902–908. doi: 10.3892/mco.2015.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maluf-Filho F, Martins BC, de Lima MS, Leonardo DV, Retes FA, Kawaguti FS, Sato CF, Hondo FY, Safatle-Ribeiro AV, Ribeiro U Jr. Etiology, endoscopic management and mortality of upper gastrointestinal bleeding in patients with cancer. United European Gastroenterol J. 2013;1:60–67. doi: 10.1177/2050640612474652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KJ, Kim HM, Jung JW, Chung MJ, Park JY, Bang S, Park SW, Lee WJ, Seong JS, Song SY. Gastrointestinal hemorrhage after concurrent chemoradiotherapy in locally advanced pancreatic cancer. Gut Liver. 2013;7:106–111. doi: 10.5009/gnl.2013.7.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin Z, Gao J, Liu W, Huang C, Shuai X, Wang G, Tao K, Zhang P. Clinicopathological and Prognostic Analysis of Primary Gastrointestinal Stromal Tumor Presenting with Gastrointestinal Bleeding: a 10-Year Retrospective Study. J Gastrointest Surg. 2017;21:792–800. doi: 10.1007/s11605-017-3385-2. [DOI] [PubMed] [Google Scholar]
- 12.Heller SJ, Tokar JL, Nguyen MT, Haluszka O, Weinberg DS. Management of bleeding GI tumors. Gastrointest Endosc. 2010;72:817–824. doi: 10.1016/j.gie.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 13.Ofosu A, Ramai D, Latson W, Adler DG. Endoscopic management of bleeding gastrointestinal tumors. Ann Gastroenterol. 2019;32:346–351. doi: 10.20524/aog.2019.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cappell MS, Friedel D. Acute nonvariceal upper gastrointestinal bleeding: endoscopic diagnosis and therapy. Med Clin North Am. 2008;92:511–550, vii. doi: 10.1016/j.mcna.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Barnert J, Messmann H. Management of lower gastrointestinal tract bleeding. Best Pract Res Clin Gastroenterol. 2008;22:295–312. doi: 10.1016/j.bpg.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Lang BH, Poon RT, Fan ST, Wong J. Outcomes of patients with hepatocellular carcinoma presenting with variceal bleeding. Am J Gastroenterol. 2004;99:2158–2165. doi: 10.1111/j.1572-0241.2004.40336.x. [DOI] [PubMed] [Google Scholar]
- 17.Savides TJ, Jensen DM, Cohen J, Randall GM, Kovacs TO, Pelayo E, Cheng S, Jensen ME, Hsieh HY. Severe upper gastrointestinal tumor bleeding: endoscopic findings, treatment, and outcome. Endoscopy. 1996;28:244–248. doi: 10.1055/s-2007-1005436. [DOI] [PubMed] [Google Scholar]
- 18.Loftus EV, Alexander GL, Ahlquist DA, Balm RK. Endoscopic treatment of major bleeding from advanced gastroduodenal malignant lesions. Mayo Clin Proc. 1994;69:736–740. doi: 10.1016/s0025-6196(12)61090-8. [DOI] [PubMed] [Google Scholar]
- 19.Wollenman CS, Chason R, Reisch JS, Rockey DC. Impact of ethnicity in upper gastrointestinal hemorrhage. J Clin Gastroenterol. 2014;48:343–350. doi: 10.1097/MCG.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashktorab H, Kupfer SS, Brim H, Carethers JM. Racial Disparity in Gastrointestinal Cancer Risk. Gastroenterology. 2017;153:910–923. doi: 10.1053/j.gastro.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci. 2015;60:711–721. doi: 10.1007/s10620-014-3443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thosani N, Rao B, Ghouri Y, Batra S, Raju G, Shafi M, Guha S. Role of argon plasma coagulation in management of bleeding GI tumors: evaluating outcomes and survival. Turk J Gastroenterol. 2014;25 Suppl 1:38–42. doi: 10.5152/tjg.2014.4867. [DOI] [PubMed] [Google Scholar]
- 23.Kim YI, Choi IJ. Endoscopic management of tumor bleeding from inoperable gastric cancer. Clin Endosc. 2015;48:121–127. doi: 10.5946/ce.2015.48.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]