Abstract
Mesenchymal stromal cells (MSCs) are multipotent and self-renewing stem cells that have great potential as cell therapy for autoimmune and inflammatory disorders, as well as for other clinical conditions, due to their immunoregulatory and regenerative properties. MSCs modulate the inflammatory milieu by releasing soluble factors and acting through cell-to-cell mechanisms. MSCs switch the classical inflammatory status of monocytes and macrophages towards a non-classical and anti-inflammatory phenotype. This is characterized by an increased secretion of anti-inflammatory cytokines, a decreased release of pro-inflammatory cytokines, and changes in the expression of cell membrane molecules and in metabolic pathways. The MSC modulation of monocyte and macrophage phenotypes seems to be critical for therapy effectiveness in several disease models, since when these cells are depleted, no immunoregulatory effects are observed. Here, we review the effects of living MSCs (metabolically active cells) and metabolically inactive MSCs (dead cells that lost metabolic activity by induced inactivation) and their derivatives (extracellular vesicles, soluble factors, extracts, and microparticles) on the profile of macrophages and monocytes and the implications for immunoregulatory and reparative processes. This review includes mechanisms of action exhibited in these different therapeutic approaches, which induce the anti-inflammatory properties of monocytes and macrophages. Finally, we overview several possibilities of therapeutic applications of these cells and their derivatives, with results regarding monocytes and macrophages in animal model studies and some clinical trials.
Keywords: Mesenchymal stromal cells, Macrophage, Monocyte, Immunomodulation, Cell therapy, Immunoregulation
Core Tip: Mesenchymal stromal cells (MSCs) and their derivatives possess immunoregulatory and regenerative properties that involve the classical activation of monocytes and macrophages towards an anti-inflammatory profile, marked by the secretion of anti-inflammatory and reparative factors that guide the inflammation resolution and healing processes. This review will comprise the effects of living and metabolically inactive MSCs, MSC extracellular vesicles, subcellular microparticles, and cell extracts on monocytes and macrophages, as well as several possibilities of therapeutic applications.
INTRODUCTION
Mesenchymal stromal cells (MSCs) are multipotent, self-renewing stem cells with immunoregulatory and regenerative properties. Found in several tissues, these non-hematopoietic progenitor cells have the ability to differentiate into cells of mesenchymal origin, such as chondrocytes, osteoblasts, and adipocytes, as well as muscle, tendon, endothelial, stromal, and neural cells[1,2]. This way, MSCs can be easily isolated from several sources such as the bone marrow, adipose and muscle tissues, the trabecular bone, articular cartilage, deciduous teeth, and the umbilical cord[1,3]. In these tissues, MSCs maintain homeostasis by offering support to other resident cells[4].
MSCs have been targeted by several investigations due to their immunoregulatory and regenerative abilities. These cells secrete soluble factors including cytokines, chemokines, growth factors, and extracellular vesicles (including exosomes and microvesicles) that modulate immune cells such as T cells, B cells, and monocytic cells for orchestrating inflammatory resolution and regenerative processes[2,5-7]. Several published findings have also demonstrated that MSCs support immune suppression through cell-to-cell contact[8-11]. Moreover, MSCs express low levels of the class I major histocompatibility complex (MHC-I) and do not express MHC-II, which makes them cells with a low immunogenicity and hence low rejection risk[12].
Owing to these features, MSCs are great candidates for cell therapy in inflammatory and autoimmune disorders, as well as in other clinical conditions. The therapeutic potential of MSCs and their secreted extracellular vesicles has been demonstrated in several in vitro studies, animal models, and clinical trials[5,12]. Successful treatment with MSCs has been observed in experimental models of lupus[13], colitis[14], diabetes[15], graft-versus-host disease (GvHD)[16], cardiovascular malignancies[17], and pulmonary diseases[18].
Currently, there are many MSC clinical trials at different phases registered on US National Institutes of Health database (https://clinicaltrials.gov), demonstrating advances in MSC therapy for GvHD, amyotrophic lateral sclerosis, rheumatoid arthritis, liver cirrhosis, acute respiratory distress syndrome, diabetes, acute myocardial infarction, lupus erythematosus, Crohn’s disease, osteoarthritis, fibrosis, Parkinson’s disease, cystic fibrosis, multiple sclerosis, ulcerative colitis, organ transplant rejection, and the recent coronavirus disease 2019 (COVID-19) pandemic.
Since previous investigations have shown that MSCs are modulated by the inflammatory milieu and respond specifically to different stimuli, greater therapeutic potential is achieved through MSC priming[19]. MSC activation, for improving their anti-inflammatory capacities, happens through exposure to conditions that are commonly encountered in the inflammatory microenvironment, such as hypoxia, which enhances the angiogenic properties of MSC extracellular vesicles[20]. Moreover, nutrient deprivation also improves MSCs’ immunoregulatory properties[21]. This MSC priming step can be performed in vitro before cell administration, with the inflammatory cytokines interferon (IFN)-γ and tumor necrosis factor (TNF)-α or with Toll-like receptor 3 (TLR-3) agonists[19,22,23]. This stimulation enhances the secretion of bioactive factors tumor necrosis factor-inducible gene (TSG)-6, interleukin (IL)-6, and prostaglandin E2 (PGE2) by MSCs[23,24].
MSCs can also be activated by interaction with immune cells. The immunoregulatory potential of MSCs is enhanced in response to the macrophage secretome, which was demonstrated by an attenuation of macrophage pro-inflammatory activity[25,26]. In addition, since macrophages and monocytes are present at the inflammatory milieu and assume either a pro- or anti-inflammatory profile, thereby orchestrating inflammation progression or resolution[27-29], studies investigating the crosstalk between these cells and MSCs are needed to elucidate the mechanisms of action of MSC therapy.
Indeed, macrophages and monocyte-derived macrophages show wide heterogeneity in their responses to the microenvironment. The range of microenvironment stimuli induces different functional states in macrophages, which are usually classified in 2 distinct groups: the classically activated (M1) and alternatively activated (M2) macrophages. M1 macrophages are characterized by a cytotoxic phenotype and by the production of reactive species and pro-inflammatory mediators such as IL-1, IL-6, IL-12, IL-23 and TNF-α[27,30]. Meanwhile, M2 macrophages have a healing profile, pronounced by production of anti-inflammatory and angiogenic molecules, such as transforming growth factor (TGF)-β, IL-10, vascular endothelial growth factor (VEGF) and EGF, which support reparative processes[27,30]. However, new investigations have demonstrated that macrophage activation is more complex than previously thought, and a spectrum of intermediate phenotypes is defined by different transcriptional patterns. In this regard, M2 macrophages can be subdivided in different subsets: M2a, M2b, M2c, and M2d; these activation profiles are induced by distinct stimuli combinations[30]. Nonetheless, such classifications are still being elucidated, and this article will refer to M1 and M2 macrophages for simplification.
Furthermore, monocytes also present broad heterogeneity as recent investigations are uncovering different peripheral blood cell populations. In humans, these are represented by 3 subsets based on the expression of surface markers: classical monocytes are CD14+CD16- and account for almost 90% of the human monocyte population. The remaining cells are subdivided in 2 populations: intermediate (CD14+CD16+) and non-classical (CD14lowCD16+) monocytes[28]. Classical and intermediate monocytes correspond to murine Ly6C+ inflammatory monocytes, whilst non-classical monocytes resemble Ly6C- or alternative monocytes[28,31]. The physiological role, as well as the origin and development of monocyte subsets, is still unclear; however, initial evidence in mice indicates that there is a sequential differentiation of classical monocytes into non-classical monocytes, and these might be considered blood-resident macrophages. Therefore, since monocytes can differentiate into macrophages in conditions of altered homeostasis when there is a need for effector cells, monocytes can be recruited to assume either a pro-inflammatory or anti-inflammatory functional phenotype depending on the microenvironment stimuli, which is similar to the concept of macrophage plasticity[31].
The interaction of macrophages and monocytes with MSCs occurs right after intravenous infusion. Németh et al[32] demonstrated that MSCs and macrophages colocalize in the lungs after 10 minutes of cell administration. Biodistribution data further showed that MSCs are cleared through phagocytosis by the host’s monocytes and macrophages[33,34]. However, the fact that a significant part of infused MSCs get trapped in the lungs raises concerns about the deleterious effects of obstructive events[35-37]. In an attempt to improve therapy efficacy and safety, several studies have thus explored the immunoregulatory features of MSC-derived extracellular vesicles and microparticles, as well as metabolically inactive MSCs, as an alternative to living MSCs[6,38-40]. Their results have demonstrated that these substitutes maintain the immunomodulatory properties that induce a regulatory phenotype in monocytes and macrophages.
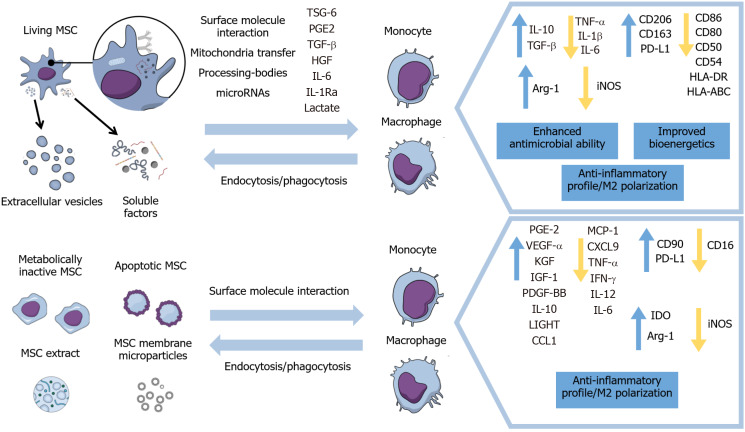
Therefore, this review will focus on the modulation of macrophages’ and monocytes’ immunophenotypes, activation status, and migration by living and metabolically inactive MSCs and their derivatives, as well as the implications on inflammation resolution and healing processes in different disease models. Furthermore, this paper will include the mechanisms of action exhibited in these different approaches for inducing anti-inflammatory properties in monocytes and macrophages (Figure 1) and results of therapeutic evidence presented in animal models and some clinical trials.
Figure 1.
Main effects of viable, inactivated, and apoptotic mesenchymal stromal cells and of mesenchymal stromal cells’ secretome and subcellular particles on monocytes and macrophages. MSC: Mesenchymal stromal cell; TSG-6: Tumor necrosis factor-inducible gene 6; PGE2: Prostaglandin E2; TGF-β: Transforming growth factor β; IL: Interleukin; HGF: Hepatocyte growth factor; TNF-α: tumor necrosis factor α; PD-L1: Programmed death-ligand 1; IGF-1: Insulin-like growth factor 1; VEGF: Vascular endothelial growth factor; iNOS: Inducible nitric oxide synthase.
MODULATION OF MACROPHAGES AND MONOCYTES BY LIVING MSCS AND EXTRACELLULAR VESICLES
Cytokine profile in monocytes and macrophages
MSCs and their extracellular vesicles can induce classic inflammatory monocytes towards a non-classic anti-inflammatory profile, as well as classically activated or M1 macrophages into alternatively activated or M2 macrophages[41-44]. This monocytic tolerogenic phenotype is characterized by changes in cytokine expression, represented by an increase in anti-inflammatory IL-10 and TGF-β in monocytes and macrophages[45-47] and a decrease in levels of TNF-α, IL-1β, and IL-6 inflammatory cytokines in macrophages[43,44,47,48].
This shift in the cytokine production pattern of macrophages and monocytes, mainly marked by regulatory IL-10 upregulation, drives inflammation resolution and alleviates injury in experimental models of allergic processes[49], colitis[47], and eye autoimmune and inflammatory disorders[45,50]. Indeed, the IL-10 derived from macrophages preconditioned with MSC exosomes has an inhibitory effect on the proliferation of CD4+ T cells, indicating different ways in which MSCs exert immunosuppressive effects that include macrophage functions[49].
Furthermore, reducing the production of inflammatory mediators like TNF-α and IL-1β has beneficial effects, since these cytokines promote inflammation maintenance. These bioactive factors are involved in the recruitment of inflammatory cells, apoptosis induction, and release of destructive enzymes (such as metalloproteinases) that lead to tissue degeneration. In addition, TNF-α facilitates autoimmunity by inhibiting T regulatory cells[51,52]. Therefore, the immunoregulatory action of MSCs on monocytic cells contributes to the resolution of inflammatory processes and reduction of tissue damage.
Expression of membrane molecules in monocytes and macrophages
The immunoregulatory effect of MSCs on macrophages is also demonstrated by the modulation of membrane protein expression. Murine and human macrophages in co-culture with MSCs were able to reduce the expression of the co-stimulatory molecule CD86 and increase that of mannose receptor CD206, which are well-known markers of M1 and M2 polarization, respectively[53,54]. These changes were also observed in in vivo mouse models of cutaneous wound healing, myocardial infarction, and diabetic cornea, since MSC transplantation decreased the number of CD86+ macrophages while increasing CD163+ and CD206+ anti-inflammatory macrophages[43,55,56]. Similarly, MSC exosomes and extracellular vesicles induce the same marker expression pattern of the M2 phenotype, both in vitro and in vivo[20,43,47,57].
M1 activated macrophages cultured with MSCs decreased the expression of CD80, CD86, CD50, CD54, HLA-DR, and HLA-ABC cell surface molecules. This indicates that MSC-conditioned macrophages acquire an immunosuppressive profile through the reduction of their antigen presentation functions, since these membrane proteins are involved in this process[58].
Regarding monocytes, de Witte et al[33] cultured human monocytes with MSCs and demonstrated that the predominant population of CD14++CD16- classical monocytes shifted to CD14++CD16+ regulatory intermediate monocytes. Moreover, co-cultured monocytes increased CD163, CD206, and programmed death-ligand 1 (PD-L1) expression.
It is also noteworthy that the co-culture of MSCs with the 3 human monocyte subsets (classical, non-classical, and intermediate) reduced the expression of the class II antigen presentation complex (HLA-DR) while upregulating MRC1, CD163, CD163L1, CD226, CD93, LILRB1 and PTGER2 membrane receptor genes[42]. MRC1 encodes CD206, which, along with CD163 and CD163L1, belongs to the scavenger receptors family, which mediates the remodeling function after tissue damage[42]. CD93 is important to phagocytosis and clearance of apoptotic cells, while CD226 is involved in monocyte migration[59,60]. Further, LILRB1 is an immunoglobulin-like receptor involved in MHC-I mediated immunosuppression[61]. PTGER2 encodes the EP2 receptor, which is activated through PGE2, one of MSCs’ bioactive factors. Meanwhile, researchers observed an upregulation of monocyte cytokines and growth factor genes, such as IL-10, IGF1, and VEGF-A[42]. Not coincidentally, IL-10 production is induced through MSC-derived PGE2, which results in reduced inflammation[32]. This expression profile, along with the CD14 upregulation, shows that MSCs altered the maturation of these monocyte subsets towards an M2 macrophage anti-inflammatory phenotype[42].
Metabolic changes
The MSC-induced M1-M2 phenotype switch is also accompanied by metabolic alterations. MSCs impair monocyte differentiation into antigen-presenting dendritic cells through metabolic reprogramming. Monocytes, instead of assuming an antigen presentation profile, show a transcriptional and phenotypic profile of M2 macrophages that induces a Th2 regulatory cytokine pattern in CD4+ T cells. In addition, these cells acquire higher spare respiratory capacity and more polarized mitochondrial membrane potential, resulting in a better capacity of stimuli response in case of high energy demand[41]. In the same way, monocyte-derived macrophages co-cultured with MSCs had increased mitochondrial function and ATP turnover, which resulted in greater macrophage phagocytosis and antimicrobial ability. These results were demonstrated both in vitro and in vivo[18].
Importantly, macrophages conditioned with MSCs or MSC exosomes increased their oxygen consumption rate while decreasing proton leak, indicating enhanced bioenergetics and mitochondrial coupling efficiency. In the same work, macrophages challenged with silica particles demonstrated homeostasis alterations highlighted by the mitochondrial production of reactive oxygen species, which was reverted by MSC exosomes[62]. On the other hand, Salmonella-infected macrophages co-cultured with MSCs had respiratory burst improvements. This was demonstrated by the enhanced expression of NADPH oxidase subunits, concomitantly with the activation of antioxidant protection mechanisms such as superoxide dismutase 2 (SOD2). These data, along with faster microbial clearance by macrophages promoted in the MSC co-culture, indicate that these metabolic changes enhance the macrophages’ ability to respond to pathogens[58].
In addition to improving the antimicrobial ability of macrophages and monocytes, MSC-induced metabolic changes modify macrophage energy generation pathways while promoting their transition towards the M2 phenotype. Since M1 activated macrophages have a high energy demand, they have an augmented expression of glucose transporter 1 (GLUT1), hexokinase 2 (HK2), and mTOR, which are proteins needed in the glycolytic pathway[58]. On the other hand, M2 macrophages exhibit a preference for mitochondrial fatty acid β-oxidation, demonstrated by a higher expression of carnitine palmitoyl trasferase 1α (CPT1α) and phosphorylated AMPKα (p-AMPKα)[58]. This way, the co-culture of M1 macrophages and MSCs reduced GLUT1 and HK2 expression and p-mTOR levels while increasing CPT1α expression and p-AMPKα levels; this indicated changes in energy metabolism underlying the MSC-induced M2 phenotype[58].
Regarding the amino acid metabolism, macrophages presented an augmented expression of arginase-1 and reduced expression of inducible nitric oxide (NO) synthase (iNOS) in response to MSCs or MSC exosomes[43,56,63,64]. These enzymes are responsible for the L-arginine metabolism, with arginase and iNOS enzymes competing for this substrate to convert it into urea and ornithine or NO, respectively. NO participates in the macrophage microbicidal and effector functions, while ornithine is a polyamine that is necessary for the cell proliferation and tissue remodeling functions of M2 macrophages[27]. The balance between their activities indicates M1 or M2 polarization, and macrophages co-cultured with MSCs exhibit decreased NO production in addition to increased urea levels, which indicates the regenerative and resolutive phenotype typical of M2 polarization[64].
Migration and recruitment
MSCs also modulate the migratory behavior of macrophages and monocytes. In vitro, macrophages and monocytes actively migrate towards MSCs[33]; in vivo, they are recruited to the lungs where they encounter MSCs after intravenous infusion[65]. In a murine model of myocarditis, MSCs recruited anti-inflammatory LyC6low monocytes to the inflammation site whilst decreasing pro-inflammatory LyC6high and LyC6middle monocyte levels. This regulation occurred through the modulation of local chemokines, reducing levels of MCP-1 (CCL2), MCP-3 (CCL7), and CCL5; abrogating the expression of ICAM-1 and VCAM-1 adhesion molecules; and increasing SDF-1α and CX3CL1 Levels. The migration of the anti-inflammatory monocyte subset helped with tissue repair and led to a reduction in myocarditis severity[66]. Notably, the intravenous infusion of MSC exosomes in experimental mouse models of pulmonary fibrosis also diminished the recruitment of pro-inflammatory Ly6Chigh monocytes whereas it increased the alveolar macrophages and the infiltration of anti-inflammatory monocytes. These changes were accompanied by a reduction in fibrosis measurements, in agreement with the monocyte reparative profile[62,67].
Moreover, the administration of MSCs and MSC-conditioned medium in mice with angiotensin II-induced aortic aneurysm increased CD206+ M2 macrophage infiltration and diminished iNOS+ M1 cells at the injured site, which was concomitant with decreased levels of CCL5, CCL2, CCL3, and CXCL10[48].
On the other hand, previous reports showed that MSC administration in mice increased MCP-1 (CCL2) levels, which recruited monocytic cells to the lungs via the CCL2-CCR2 axis. After migration, monocytes and macrophages were consistently modulated by MSCs and assumed an IL-10-producing phenotype[49,50]. Similarly, in a model of skeletal muscle injury, treatment with hypoxia-subjected MSC extracellular vesicles increased the expression of MCP-1 (CCL2) and the CD206/Ly6c cell ratio when compared to normoxia-derived extracellular vesicles and control groups, indicating M2 polarization[20].
Furthermore, type 2 diabetic mice showed augmented M2 macrophage counts in the liver, adipose tissue, skeletal muscle, pancreatic islands, and spleen after the intravenous infusion of MSCs. Concomitantly with a greater engraftment of administered MSCs in the spleen, this brings up the possibility that MSCs may directly modulate macrophage and monocyte populations in immune organs, which could lead to systemic effects[68]. In fact, mice with myocarditis treated with MSCs retained more pro-inflammatory monocytes in the spleen when compared to the control group, and recruited more anti-inflammatory monocytes to the heart, which improved healing processes and reduced inflammation[66].
In summary, despite the different triggered pathways and chemokine regulation involved in monocyte and macrophage recruitment, several investigations indicate that MSC treatment induces monocyte and macrophage migration to the inflammation site or to immune organs. Once at these sites, MSCs modulate the cell activation status and profile, promoting a monocytic anti-inflammatory phenotype and hence a reparative milieu.
Mechanisms of action
The mechanisms underlying MSC immunoregulatory capacities are still under investigation, but one of the most well-known processes for inducing a suppressive and anti-inflammatory phenotype in monocytes and macrophages is the secretion of soluble factors such as TSG-6, TGF-β, HGF (hepatocyte growth factor), IL-6, and the IL-1 receptor antagonist[45,53,69,70]. Moreover, lactate and PGE2 were also shown to reprogram macrophage metabolism to promote an M2 profile[41,58]. The abrogation of several of these bioactive factors prevented MSC-induced M2 macrophage polarization and immunoregulatory effects[24,25,41,58].
In addition to their paracrine action, MSCs are phagocytized by monocytic cells in an active process. After in vitro phagocytosis, monocytes acquire phenotypic and functional changes of CD14++CD116+ immune regulatory intermediate monocytes, such as upregulated expression of PD-L1 and CD90 surface molecules and IL-1b, IL-6, IL-8, IL-10, and TGF-β cytokines, whilst expression of pro-inflammatory TNF-α decreases[33]. In vivo, monocytes which phagocytized MSCs assume the same anti-inflammatory profile and migrate to other body sites, mainly to the liver, carrying the regulatory properties of MSCs[33]. Further, macrophages also phagocyte MSCs and acquire an anti-inflammatory M2 phenotype, characterized by increased IL-10 and TGF-β expression[33,34].
Organelle transfer is another mechanism triggered by MSCs that enhances macrophage functions. In vitro and in vivo assays have evidenced that MSCs transfer mitochondria to macrophages through exosomes and cytoplasmic bridges named tunneling nanotubes, which improves the macrophages’ phagocytic ability and bioenergetics[18,62]. Min et al[71] reported that monocytes and macrophages engulfed MSCs’ cytoplasmic processing bodies, which are membrane less organelles that store mRNA, miRNA, and proteins. This mechanism was mediated by lipoprotein receptor-related proteins (LPRs) and was critical to the reprogramming of monocytes and macrophages towards a transcriptional profile of reduced antigen presentation, as well as for the inhibition of T cell activation. Moreover, MSC processing bodies were required to prevent the infiltration of CD11b+ inflammatory monocytes and macrophages in lung tissue in a mouse model of lung inflammation[71].
Furthermore, MSCs can exert immunomodulatory effects through microRNA transfer[62]. The M2 macrophage phenotype promoted by treatment with MSC exosomes is, at least in part, dependent on the post transcriptional control (by miR-182 and miR-181) of TLR-4 and the subsequent downregulation of its downstream nuclear factor-κB (NF-κB) inflammatory pathway[43,72]. The inhibition of TLR-4/NF-κB activation is also triggered by the let-7b miRNA from MSC exosomes while this molecule induces signal transducer and activator of transcription 3 (STAT3) signaling, which in turn participates in M2 conversion[73]. The reduced expression of TLR-4 and enhanced levels of p-STAT3 in the healing wound site demonstrates that the regulation of these signaling pathways in macrophages promotes the M2 phenotype, with reparative properties[74].
miR147, derived from MSC extracellular vesicles, was also found to decrease macrophage activation via diminishing HMBG-1 secretion[75]. Moreover, He et al[76] reported that M2 macrophage polarization was associated with MSC exosome-derived miR-223 and a consequent decrease in Pknox1 levels, a homeobox protein associated with the regulation of M1 macrophage polarization[77,78]. Interestingly, miR-223 was also shown to reduce NLRP3 Levels; this is a protein of the inflammasome complex whose activation leads to inflammatory cytokine release and to the exacerbation of inflammation in cases of inflammatory bowel disease[79].
Similarly, MSCs induce the association of the yes-associated protein (YAP) and β-catenin in the macrophage nucleus. These are components of protein kinase cascades in the Hippo and Wnt signaling pathways, respectively, and the assembled protein complex operates to negatively control the target gene XBP1, which mediates NLRP3 activation. Data demonstrate that MSCs also regulate M2 polarization through Hippo signaling and subsequent repression of inflammasome activation[63]. Finally, MSCs suppress NLRP3 inflammasome-mediated IL-1β production by macrophages through a feedback mechanism where IL-1β may induce COX-2 signaling in MSCs[54].
MODULATION OF MACROPHAGES AND MONOCYTES BY METABOLICALLY INACTIVE, APOPTOTIC MSCS AND SUBCELLULAR PARTICLES
The modulation of macrophages and monocytes by non-viable MSCs or MSC subcellular particles is an emerging issue of interest in research, since investigations can contribute to understanding the immunomodulatory mechanisms of MSCs independently of their soluble secreted factors. In addition, although some studies have shown that MSCs display homing to the injured site[68,80], other experimental models of MSC infusion demonstrate that a great portion of these cells get trapped in the lung capillaries and lose viability after 24 h[35,36,80]. Nevertheless, the immunoregulatory effect of MSCs is maintained, raising questions on how these cells are still able to reduce local and systemic inflammation.
These questions bring up the hypothesis that MSCs transfer their immunomodulatory properties to other host cells, which can then act to decrease inflammatory parameters. In addition, tracking studies have demonstrated that the MSC signal found in the inflammation site, organs, and blood after intravenous administration derived from MSC debris phagocytized by immune cells (such as monocytes) instead of viable MSCs[33,36]. Therefore, inactivated and dead MSCs or even MSC extracts could trigger this immunoregulation without the need for metabolically active cells.
To overcome the low homing efficiency of systemically administered MSCs, studies have demonstrated the therapeutic potential of MSC extract instead of whole cells[81-83]. Song et al[81] infused the MSC extract in a chemically induced mouse model of colitis. They found that the extract inhibited inflammatory cytokines, recovered the damaged epithelial barrier, and polarized the macrophages’ functional phenotyping from M1 to M2 by reducing the expression of genes encoding for MCP1, CXCL9, and iNOS (M1 markers) and increasing that of genes corresponding to IL-10, LIGHT, CCL1, and Arg-1 (M2 markers).
Studies observed that MSC membrane nanoparticles without any cargo and heat-inactivated MSCs decreased the proportion of pro-inflammatory CD16+ monocytes by inducing apoptosis[40,84]. The MSC membrane nanoparticles were generated from unstimulated and IFN-γ-stimulated MSCs, and this difference seems to be important for the ultimate purpose. For instance, unstimulated and IFN-γ-stimulated nanoparticles were capable of increasing CD90+ monocyte population, (a natural MSC marker), but only IFN-γ-stimulated nanoparticles augmented the PD-L1+ monocyte subset[40]. Moreover, monocytes conditioned with IFN-γ-stimulated nanoparticles, but not with the unstimulated type, had enhanced indoleamine 2,3-dioxygenase (IDO) expression[40]. The possibility of changing stimuli to generate nanoparticles with different features and membrane compositions provides the opportunity of creating specific therapies according to distinct inflammatory disorders[40]. Importantly, PD-L1 is an immune checkpoint protein that inhibits the activation and function of its target PD-1-expressing immune cells, suppressing immune reactivity[85]. In addition, IDO is an enzyme that depletes the essential amino acid tryptophan and generates kynurenine pathway metabolites; these metabolic changes thus contribute to immune regulation[86]. Therefore, IFN-γ-stimulated MSC membrane particles with the ability to induce PD-L1 and IDO expression could be used in the treatment of severe inflammatory conditions that present inflammatory monocytes[40]. These studies also observed that MSC membrane nanoparticles bind and fuse to the monocyte membrane, demonstrating that physical interaction between cell surfaces is important for MSC-induced immunosuppression[40]. Furthermore, MSC membrane nanoparticles maintain ATPase and CD73 enzymatic activities at their surface, converting ATP to ADP and AMP to adenosine, respectively[40]. Adenosine, the last molecule of these reactions, has immunoregulatory functions via P1 receptor activation[87]. It is important to note that the activation of monocyte P1 receptors such as A2A and A2B inhibited TNF-α production[87].
Additionally, just as living cells, secretome-deficient heat-inactivated MSCs also disappear after 24 h of infusion in healthy mice and in an experimental model of kidney ischemia/reperfusion injury[39]. Despite their fast clearance, the administration of heat-inactivated MSCs still altered the expression levels of several cytokines and chemokines in the serum and lung tissue and reduced LPS-induced sepsis[39]. In vitro assays demonstrated that secretome-deficient heat-inactivated MSCs modulate monocytes through reducing TNF-α production[39,84]. This modulation occurs through phagocytosis of heat-inactivated MSCs, and the recognition of heat-inactivated MSCs by monocytes was even more efficient than that of intact MSCs[84]. Moreover, the supernatant of LPS-stimulated macrophages that phagocytized dead MSCs improved the survival of hypoxic cardiomyocytes[88]. After phagocytosis, macrophages augmented the production of PGE2, VEGF-α, KGF, IGF-1, and PDGF-BB reparative molecules while decreasing that of TNF-α, IFN-γ, IL-12, and IL-6[88]. Together, these data suggest that, at least in some sepsis models, monocytes that had phagocytized inactivated MSCs acquired their immunoregulatory properties and reduced inflammation[39,84].
Another therapeutic approach consists in the administration of apoptotic MSCs. Galleu et al[89] demonstrated that mice with GvHD lacking the cytotoxic activity of GvHD effector cells did not respond to MSC therapy due to the need for inducing MSC apoptosis. Therefore, the administration of in vitro-produced apoptotic MSCs in GvHD mice eliminated the requirement for promoting MSC apoptosis in vivo and induced IDO expression in recipient mice macrophages that had phagocytized the infused cells, which incited immunosuppression[89].
These new MSC-derived alternative therapies bring some advantages. Using non-viable MSCs ensures that the administered product is not altered after infusion, since once inside the target organism, they do not proliferate or secrete any molecules in response to nonspecific host signals. Owing to their small size, MSC membrane nanoparticles could pass through the lung capillaries and reach other areas of the body, avoiding problems such as emboli formation induced by the administration of intact MSCs[40,90].
The effects of non-viable and apoptotic MSCs on macrophages and monocytes are still under investigation. The mechanisms of action exhibited by these cells are still not fully understood, but MSC phagocytosis by monocytes and macrophages seems to be essential for the systemic effects of inactivated and apoptotic MSC therapy[89]. The interaction between cell membranes may also have an important role[40]. Future studies will be necessary to reveal the possible interactions between non-viable MSCs and macrophages or monocytes in vivo, as well as their implications in treatment results.
THERAPEUTIC APPLICATION POSSIBILITIES — IN VIVO STUDIES
The use of MSCs with the proven participation of monocytes and macrophages has been described as having therapeutic potential in several local and systemic disorders studied in animal models. Regarding lung injuries, MSC extracellular vesicles were able to alleviate induced acute lung injury in a murine model: researchers observed alterations in macrophage phenotypes and a decrease in macrophage recruitment[91]. In addition, preconditioned MSC exosomes prevented and reverted experimental pulmonary fibrosis and lung inflammation through the modulation of monocyte phenotypes in adult C57BL/6 mice[67]. Through the modulation of lung macrophage phenotypes, treatment using MSC exosomes alleviated bronchopulmonary dysplasia in a mouse model, resulting in improvement of lung function, decreased fibrosis, remodeling of pulmonary vasculature, and amelioration of pulmonary hypertension[92]. In a mouse model of acute respiratory distress syndrome, an improvement of lung injury was observed when using murine alveolar macrophages previously cultured with MSC extracellular vesicles and through the transference of MSC mitochondria to macrophages, resulting in an enhancement of macrophage phagocytosis. The enhanced host macrophage phagocytosis could promote a clearance of invading microorganism, which, combined with suppressive pro-inflammatory cytokine secretion, may improve clinical outcomes, since lung injury is associated with high inflammatory response and bacterial burden[18,93]. Moreover, the administration of MSCs increased CCL2 expression and monocyte recruitment in the lungs, suppressing allergic airway inflammation[49].
In relation to cardiac disorders, MSC application in Coxsackievirus B3-induced myocarditis in mice attenuated myocardial inflammation by suppressing the cardiac infiltration of pro-inflammatory monocytes while promoting the cardiac influx of anti-inflammatory monocytes, representing a promising strategy for the resolution of cardiac inflammation and prevention of disease progression[66]. MSC exosomes attenuate myocardial ischemia/reperfusion injury in mice via shuttling miR-182, which modifies the macrophages’ polarization status[43]. MSCs and their exosomes may also mediate the decrease in pro-inflammatory and increase in anti-inflammatory monocytes/macrophages after acute myocardial infarction[94,95]. Furthermore, a mouse model of dilated cardiomyopathy that received MSC exosomes showed cardiac function improvement, cardiac dilation attenuation, and cardiomyocyte apoptosis reduction due to the decrease in pro-inflammatory macrophages in both the blood and heart[57].
The use of MSCs and their derivatives can also be considered for other organ injuries. The injection of MSCs or their exosomes ameliorated dextran sulfate sodium-induced colitis in mice, and part of the associated mechanism includes a macrophage-dependent phenomenon[47,96]. Previous coculture of MSCs and macrophages induced the M2 phenotype, which combined with host cells, improved liver fibrosis in mice[97]. The internalization of MSC extracellular vesicles by macrophages, with an increasing number of reparative macrophages, was accompanied by a reduction in renal inflammation in a porcine model, suggesting that anti-inflammatory properties underpin the protective effects of MSC extracellular vesicles on the stenotic kidney[98]. In mice secondary lupus disease, MSCs ameliorated lupus nephritis, preventing podocyte injury, possibly through a reduction in macrophage infiltration and polarization into an anti-inflammatory phenotype[13]. MSC exosomes prevented cerebral injury in rat acute ischemic stroke by inhibiting autophagy-mediated microglial polarization to M1[99]. Therapy with MSC exosomes promoted M2 macrophage polarization and accelerated cutaneous wound healing in skin-defective mice[76]. Mice were protected against a subsequent immune challenge in corneal allotransplantation and experimental autoimmune uveitis after the intravenous infusion of MSC-preconditioned lung monocytes/macrophages[45]. The local administration of MSCs promoted diabetic corneal wound healing by modulating the immune response, inducing alternative activation of infiltrating macrophages towards M2 polarization[56].
In some other conditions, the study of the effects of MSCs on monocytes and macrophages is useful for future therapeutic applications. Regarding sepsis reports, the intravenous infusion of MSCs reduced mortality and bacteremia in gram-negative peritoneal sepsis in mice, partially by enhancing the phagocytic activity of blood monocytes[100]. MSC nanovesicles had protective immunomodulatory effects in a mouse model of sepsis owing to the reduction of pro-inflammatory cytokine production by macrophages and of monocyte infiltration in the peritoneum[101]. Exosomes of pretreated MSCs induced M2 macrophage polarization, increased survival, and effectively ameliorated symptoms in a mouse model of sepsis[102]. Apoptotic MSCs induced immunosuppression in a murine model of GvHD, engulfing recipient phagocytes[89]. MSC treatment prevented and alleviated atherosclerosis in mice, partly by decreasing monocytosis and modulating macrophage activation and differentiation. Plaque size and lipidic deposition in mice that received MSCs in both prevention and treatment groups were significantly smaller than those in the control group[103]. MSC exosomes repaired and regenerated critical osteochondral defects in a rat model of osteoarthritis through coordinated mobilization of multiple cell types and activation of several cellular processes, such as a regenerative immune phenotype characterized by a higher infiltration of CD163+ regenerative M2 macrophages over CD86+ M1 macrophages[104]. M2 macrophage polarization was also the target of the intravenous MSC exosomes studied in rats’ spinal cord injury recovery[105,106]. MSC infusion exerted anti-diabetic effects and significantly promoted islet repair in a type 2 diabetes mouse model, and this effect was partially attributed to a suppression of inflammation and induction of M2 macrophage polarization[25]. In diabetes complications, MSC exosomes alleviated neurovascular dysfunction and improved functional recovery in mice with diabetic peripheral neuropathy, including a mechanism of macrophage M1 decrease and M2 increase[107].
In clinical trials, performing some cellular analysis is difficult and may not be possible. Moreover, clinical improvements are the main evaluated outcomes. We will briefly introduce some reports that showed descriptions of clinical improvement using MSCs with a possible involvement of monocytes and macrophages.
MSC infusion in treatment of patients with knee osteoarthritis resulted in overall improvement of pain and symptoms and reduced synovial inflammation. Scores of clinical outcomes showed clinical efficacy and decreased levels of pro-inflammatory monocytes, macrophages, and IL-12 in the synovial fluid after MSC injection. Taken together, the decreases in IL-12 Levels along with pro-inflammatory monocytes/macrophages after MSC injection are supportive of an anti-inflammatory and immunomodulatory mechanism of action of MSCs, which is clinical evidence of the mechanism of these cells in osteoarthritis[108].
Regarding the use of MSCs for immunomodulation after solid organ transplantation, a phase I trial has demonstrated the downregulation of HLA-DR expression by CD14+ monocytes relative to pre liver transplant levels, which can be associated with a decrease in immunological reactivity[109]. MSCs can modulate the maturation and function of monocyte-derived dendritic cells via soluble factors, contributing to the improvement of liver allograft histology and suppression of acute rejection in liver transplant recipients[110].
Biopsies of ulcerative colitis showed improved histological results after MSC treatment. Inflammatory cell infiltration at histological evaluation showed that the score of the MSC-treated group was significantly lower when compared to the untreated group[111].
Considering the treatment of infectious diseases, authors have described that the intravenous injection of MSCs significantly improved the inflammation situation in COVID-19; serum levels of pro-inflammatory cytokines and chemokines were dramatically reduced, which attracted less mononuclear cells/macrophages to the fragile lung[112]. Several studies focused on the reduction of the general inflammatory cytokine profile after MSC infusion, and some of them included the specific macrophage inflammatory protein-1 alpha (MIP-1)[113]. Still considering the cytokine profile, the reduction of systemic immune activation after MSC treatment contradictorily improved immune reconstitution in HIV-1-infected immunological nonresponders and decreased specific cytokines such as MCP-1 and MIP-1β[114].
CONCLUSION
The interaction of macrophages and monocytes with either viable or non-viable MSCs seems to be critical for therapy effectiveness, since when these cells are depleted in several models of inflammatory diseases or are prevented from migrating to the inflammation site, no immunoregulatory effects or benefits occur[43,49,50,89,94]. As discussed, these immunoregulatory effects are mainly due to the induced shift towards the anti-inflammatory phenotype of monocytes and macrophages, induced by viable, non-viable, and apoptotic MSCs, as well as their subcellular particles.
This modulation of monocytes and macrophages by MSCs occurs through different complex mechanisms such as secreted soluble factors, mitochondria and micro-RNA transfer, and phagocytosis of MSCs. In addition, the emergence of different therapeutic approaches using non-viable MSCs and MSC membrane particles brings up the need for investigating their immunomodulatory mechanisms. The phagocytosis of MSCs by monocytes and macrophages was also observed, and the interaction between surface molecules of MSC membrane particles and these monocytic cells seems to be important.
Here, we discussed the effects of viable, non-viable, and apoptotic MSCs, as well as their secretome and subcellular particles on monocytes and macrophages (Figure 1). In summary, monocytes and macrophages can acquire the immunomodulatory features of MSCs, and this regulatory action seems to be crucial for therapy success in several clinical conditions.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: February 23, 2021
First decision: April 20, 2021
Article in press: September 3, 2021
Specialty type: Cell and tissue engineering
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tsui YP S-Editor: Gao CC L-Editor: A P-Editor: Wu RR
Contributor Information
Alexia Nedel Sant'Ana, Laboratório de Células Tecidos e Genes, Hospital de Clínicas de Porto Alegre, Porto Alegre 90035-903, RS, Brazil.
Anelise Bergmann Araújo, Centro de Processamento Celular, Hospital de Clínicas de Porto Alegre, Porto Alegre 90035-903, RS, Brazil. anelise_araujo@yahoo.com.br.
Fabiany da Costa Gonçalves, Department of Internal Medicine, Erasmus Medical Center, Rotterdam 3015 GD, Netherlands.
Ana Helena Paz, Laboratório de Células Tecidos e Genes, Hospital de Clínicas de Porto Alegre, Porto Alegre 90035-903, RS, Brazil; Departamento de Ciências Morfológicas, Instituto de Ciências Básicas da Saúde, Universidade Federal do Rio Grande do Sul, Porto Alegre 90035-903, RS, Brazil.
References
- 1.Chen FH, Rousche KT, Tuan RS. Technology Insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2:373–382. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 2.Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl Med. 2017;6:2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493–507. doi: 10.1038/s41581-018-0023-5. [DOI] [PubMed] [Google Scholar]
- 5.Li N, Hua J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 2017;74:2345–2360. doi: 10.1007/s00018-017-2473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carreras-Planella L, Monguió-Tortajada M, Borràs FE, Franquesa M. Immunomodulatory Effect of MSC on B Cells Is Independent of Secreted Extracellular Vesicles. Front Immunol. 2019;10:1288. doi: 10.3389/fimmu.2019.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luz-Crawford P, Noël D, Fernandez X, Khoury M, Figueroa F, Carrión F, Jorgensen C, Djouad F. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS One. 2012;7:e45272. doi: 10.1371/journal.pone.0045272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, Sweeney EM, English K, Shaw G, Murphy JM, Barry FP, Mahon BP, Belton O, Ceredig R, Griffin MD. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011;41:2840–2851. doi: 10.1002/eji.201141499. [DOI] [PubMed] [Google Scholar]
- 10.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Zhang D, Xu L, Dong L, Zheng J, Lin Y, Huang J, Zhang Y, Tao Y, Zang X, Li D, Du M. Cell-cell contact with proinflammatory macrophages enhances the immunotherapeutic effect of mesenchymal stem cells in two abortion models. Cell Mol Immunol. 2019;16:908–920. doi: 10.1038/s41423-019-0204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Niu L, Tang X, Feng R, Yao G, Chen W, Li W, Feng X, Chen H, Sun L. Mesenchymal stem cells prevent podocyte injury in lupus-prone B6.MRL-Faslpr mice via polarizing macrophage into an anti-inflammatory phenotype. Nephrol Dial Transplant. 2019;34:597–605. doi: 10.1093/ndt/gfy195. [DOI] [PubMed] [Google Scholar]
- 14.Alves VBF, de Sousa BC, Fonseca MTC, Ogata H, Caliári-Oliveira C, Yaochite JNU, Rodrigues Júnior V, Chica JEL, da Silva JS, Malmegrim KCR, Pernomian L, Cardoso CR. A single administration of human adipose tissue-derived mesenchymal stromal cells (MSC) induces durable and sustained long-term regulation of inflammatory response in experimental colitis. Clin Exp Immunol. 2019;196:139–154. doi: 10.1111/cei.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X, Hao H, Han Q, Song X, Liu J, Dong L, Han W, Mu Y. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res Ther. 2017;8:241. doi: 10.1186/s13287-017-0668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii S, Miura Y, Fujishiro A, Shindo T, Shimazu Y, Hirai H, Tahara H, Takaori-Kondo A, Ichinohe T, Maekawa T. Graft-Versus-Host Disease Amelioration by Human Bone Marrow Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Is Associated with Peripheral Preservation of Naive T Cell Populations. Stem Cells. 2018;36:434–445. doi: 10.1002/stem.2759. [DOI] [PubMed] [Google Scholar]
- 17.Huang P, Wang L, Li Q, Xu J, Xiong Y, Chen G, Qian H, Jin C, Yu Y, Liu J, Qian L, Yang Y. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res Ther. 2019;10:300. doi: 10.1186/s13287-019-1353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, O'Kane CM, Krasnodembskaya AD. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells. 2016;34:2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez LA 2nd, Mohammadipoor A, Alvarado L, Kamucheka RM, Asher AM, Cancio LC, Antebi B. Preconditioning in an Inflammatory Milieu Augments the Immunotherapeutic Function of Mesenchymal Stromal Cells. Cells. 2019;8 doi: 10.3390/cells8050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, Becherini P, Bosco MC, Varesio L, Franzin C, Pozzobon M, Cancedda R, Tasso R. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl Med. 2017;6:1018–1028. doi: 10.1002/sctm.16-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuhashi K, Tsuboi N, Shimizu A, Katsuno T, Kim H, Saka Y, Ozaki T, Sado Y, Imai E, Matsuo S, Maruyama S. Serum-starved adipose-derived stromal cells ameliorate crescentic GN by promoting immunoregulatory macrophages. J Am Soc Nephrol. 2013;24:587–603. doi: 10.1681/ASN.2012030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, Rosenzwajg M, Matthay MA, Lee JW. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am J Respir Crit Care Med. 2015;192:324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philipp D, Suhr L, Wahlers T, Choi YH, Paunel-Görgülü A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res Ther. 2018;9:286. doi: 10.1186/s13287-018-1039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song WJ, Li Q, Ryu MO, Ahn JO, Ha Bhang D, Chan Jung Y, Youn HY. TSG-6 Secreted by Human Adipose Tissue-derived Mesenchymal Stem Cells Ameliorates DSS-induced colitis by Inducing M2 Macrophage Polarization in Mice. Sci Rep. 2017;7:5187. doi: 10.1038/s41598-017-04766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin Y, Hao H, Cheng Y, Zang L, Liu J, Gao J, Xue J, Xie Z, Zhang Q, Han W, Mu Y. Human umbilical cord-derived mesenchymal stem cells direct macrophage polarization to alleviate pancreatic islets dysfunction in type 2 diabetic mice. Cell Death Dis. 2018;9:760. doi: 10.1038/s41419-018-0801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saldaña L, Bensiamar F, Vallés G, Mancebo FJ, García-Rey E, Vilaboa N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res Ther. 2019;10:58. doi: 10.1186/s13287-019-1156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 28.Guilliams M, Mildner A, Yona S. Developmental and Functional Heterogeneity of Monocytes. Immunity. 2018;49:595–613. doi: 10.1016/j.immuni.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 30.Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154:186–195. doi: 10.1111/imm.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A, Korevaar SS, Shankar AS, O'Flynn L, Elliman SJ, Roy D, Betjes MGH, Newsome PN, Baan CC, Hoogduijn MJ. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells. 2018;36:602–615. doi: 10.1002/stem.2779. [DOI] [PubMed] [Google Scholar]
- 34.Braza F, Dirou S, Forest V, Sauzeau V, Hassoun D, Chesné J, Cheminant-Muller MA, Sagan C, Magnan A, Lemarchand P. Mesenchymal Stem Cells Induce Suppressive Macrophages Through Phagocytosis in a Mouse Model of Asthma. Stem Cells. 2016;34:1836–1845. doi: 10.1002/stem.2344. [DOI] [PubMed] [Google Scholar]
- 35.Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. doi: 10.1186/s13287-015-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. doi: 10.3389/fimmu.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deak E, Rüster B, Keller L, Eckert K, Fichtner I, Seifried E, Henschler R. Suspension medium influences interaction of mesenchymal stromal cells with endothelium and pulmonary toxicity after transplantation in mice. Cytotherapy. 2010;12:260–264. doi: 10.3109/14653240903401840. [DOI] [PubMed] [Google Scholar]
- 38.Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lässer C, Segaliny AI, McIntyre LL, Shelke GV, Hutchins E, Hamamoto A, Calle EN, Crescitelli R, Liao W, Pham V, Yin Y, Jayaraman J, Lakey JRT, Walsh CM, Van Keuren-Jensen K, Lotvall J, Zhao W. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano. 2019;13:6670–6688. doi: 10.1021/acsnano.9b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luk F, de Witte SF, Korevaar SS, Roemeling-van Rhijn M, Franquesa M, Strini T, van den Engel S, Gargesha M, Roy D, Dor FJ, Horwitz EM, de Bruin RW, Betjes MG, Baan CC, Hoogduijn MJ. Inactivated Mesenchymal Stem Cells Maintain Immunomodulatory Capacity. Stem Cells Dev. 2016;25:1342–1354. doi: 10.1089/scd.2016.0068. [DOI] [PubMed] [Google Scholar]
- 40.Gonçalves FDC, Luk F, Korevaar SS, Bouzid R, Paz AH, López-Iglesias C, Baan CC, Merino A, Hoogduijn MJ. Membrane particles generated from mesenchymal stromal cells modulate immune responses by selective targeting of pro-inflammatory monocytes. Sci Rep. 2017;7:12100. doi: 10.1038/s41598-017-12121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selleri S, Bifsha P, Civini S, Pacelli C, Dieng MM, Lemieux W, Jin P, Bazin R, Patey N, Marincola FM, Moldovan F, Zaouter C, Trudeau LE, Benabdhalla B, Louis I, Beauséjour C, Stroncek D, Le Deist F, Haddad E. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget. 2016;7:30193–30210. doi: 10.18632/oncotarget.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wise AF, Williams TM, Rudd S, Wells CA, Kerr PG, Ricardo SD. Human mesenchymal stem cells alter the gene profile of monocytes from patients with Type 2 diabetes and end-stage renal disease. Regen Med. 2016;11:145–158. doi: 10.2217/rme.15.74. [DOI] [PubMed] [Google Scholar]
- 43.Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115:1205–1216. doi: 10.1093/cvr/cvz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin L, Deng Z, Zhang J, Yang C, Liu J, Han W, Ye P, Si Y, Chen G. Mesenchymal stem cells promote type 2 macrophage polarization to ameliorate the myocardial injury caused by diabetic cardiomyopathy. J Transl Med. 2019;17:251. doi: 10.1186/s12967-019-1999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ko JH, Lee HJ, Jeong HJ, Kim MK, Wee WR, Yoon SO, Choi H, Prockop DJ, Oh JY. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc Natl Acad Sci U S A. 2016;113:158–163. doi: 10.1073/pnas.1522905113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lohan P, Murphy N, Treacy O, Lynch K, Morcos M, Chen B, Ryan AE, Griffin MD, Ritter T. Third-Party Allogeneic Mesenchymal Stromal Cells Prevent Rejection in a Pre-sensitized High-Risk Model of Corneal Transplantation. Front Immunol. 2018;9:2666. doi: 10.3389/fimmu.2018.02666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H, Liang Z, Wang F, Zhou C, Zheng X, Hu T, He X, Wu X, Lan P. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 2019;4 doi: 10.1172/jci.insight.131273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou YZ, Cheng Z, Wu Y, Wu QY, Liao XB, Zhao Y, Li JM, Zhou XM, Fu XM. Mesenchymal stem cell-derived conditioned medium attenuate angiotensin II-induced aortic aneurysm growth by modulating macrophage polarization. J Cell Mol Med. 2019;23:8233–8245. doi: 10.1111/jcmm.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeda K, Webb TL, Ning F, Shiraishi Y, Regan DP, Chow L, Smith MJ, Ashino S, Guth AM, Hopkins S, Gelfand EW, Dow S. Mesenchymal Stem Cells Recruit CCR2+ Monocytes To Suppress Allergic Airway Inflammation. J Immunol. 2018;200:1261–1269. doi: 10.4049/jimmunol.1700562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ko JH, Lee HJ, Jeong HJ, Oh JY. Ly6Chi monocytes are required for mesenchymal stem/stromal cell-induced immune tolerance in mice with experimental autoimmune uveitis. Biochem Biophys Res Commun. 2017;494:6–12. doi: 10.1016/j.bbrc.2017.10.097. [DOI] [PubMed] [Google Scholar]
- 51.Kalliolias GD, Ivashkiv LB. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 53.Liu F, Qiu H, Xue M, Zhang S, Zhang X, Xu J, Chen J, Yang Y, Xie J. MSC-secreted TGF-β regulates lipopolysaccharide-stimulated macrophage M2-like polarization via the Akt/FoxO1 pathway. Stem Cell Res Ther. 2019;10:345. doi: 10.1186/s13287-019-1447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin TH, Kim HS, Kang TW, Lee BC, Lee HY, Kim YJ, Shin JH, Seo Y, Won Choi S, Lee S, Shin K, Seo KW, Kang KS. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis. 2016;7:e2524. doi: 10.1038/cddis.2016.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang B, Zhao N, Zhang J, Liu Y, Zhu D, Kong Y. Mesenchymal stem cells rejuvenate cardiac muscle through regulating macrophage polarization. Aging (Albany NY) 2019;11:3900–3908. doi: 10.18632/aging.102009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di G, Du X, Qi X, Zhao X, Duan H, Li S, Xie L, Zhou Q. Mesenchymal Stem Cells Promote Diabetic Corneal Epithelial Wound Healing Through TSG-6-Dependent Stem Cell Activation and Macrophage Switch. Invest Ophthalmol Vis Sci. 2017;58:4344–4354. doi: 10.1167/iovs.17-21506. [DOI] [PubMed] [Google Scholar]
- 57.Sun X, Shan A, Wei Z, Xu B. Intravenous mesenchymal stem cell-derived exosomes ameliorate myocardial inflammation in the dilated cardiomyopathy. Biochem Biophys Res Commun. 2018;503:2611–2618. doi: 10.1016/j.bbrc.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 58.Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci Rep. 2016;6:38308. doi: 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greenlee MC, Sullivan SA, Bohlson SS. CD93 and related family members: their role in innate immunity. Curr Drug Targets. 2008;9:130–138. doi: 10.2174/138945008783502421. [DOI] [PubMed] [Google Scholar]
- 60.Reymond N, Imbert AM, Devilard E, Fabre S, Chabannon C, Xerri L, Farnarier C, Cantoni C, Bottino C, Moretta A, Dubreuil P, Lopez M. DNAM-1 and PVR regulate monocyte migration through endothelial junctions. J Exp Med. 2004;199:1331–1341. doi: 10.1084/jem.20032206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colonna M, Navarro F, Bellón T, Llano M, García P, Samaridis J, Angman L, Cella M, López-Botet M. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186:1809–1818. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DW, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li C, Jin Y, Wei S, Sun Y, Jiang L, Zhu Q, Farmer DG, Busuttil RW, Kupiec-Weglinski JW, Ke B. Hippo Signaling Controls NLR Family Pyrin Domain Containing 3 Activation and Governs Immunoregulation of Mesenchymal Stem Cells in Mouse Liver Injury. Hepatology. 2019;70:1714–1731. doi: 10.1002/hep.30700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi: 10.1038/emm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, Korevaar SS, Mensah FK, Franquesa M, de Bruin RW, Betjes MG, Weimar W, Baan CC. Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev. 2013;22:2825–2835. doi: 10.1089/scd.2013.0193. [DOI] [PubMed] [Google Scholar]
- 66.Miteva K, Pappritz K, El-Shafeey M, Dong F, Ringe J, Tschöpe C, Van Linthout S. Mesenchymal Stromal Cells Modulate Monocytes Trafficking in Coxsackievirus B3-Induced Myocarditis. Stem Cells Transl Med. 2017;6:1249–1261. doi: 10.1002/sctm.16-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mansouri N, Willis GR, Fernandez-Gonzalez A, Reis M, Nassiri S, Mitsialis SA, Kourembanas S. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. 2019;4 doi: 10.1172/jci.insight.128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin Y, Hao H, Cheng Y, Gao J, Liu J, Xie Z, Zhang Q, Zang L, Han W, Mu Y. The homing of human umbilical cord-derived mesenchymal stem cells and the subsequent modulation of macrophage polarization in type 2 diabetic mice. Int Immunopharmacol. 2018;60:235–245. doi: 10.1016/j.intimp.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 69.Deng Y, Zhang Y, Ye L, Zhang T, Cheng J, Chen G, Zhang Q, Yang Y. Umbilical Cord-derived Mesenchymal Stem Cells Instruct Monocytes Towards an IL10-producing Phenotype by Secreting IL6 and HGF. Sci Rep. 2016;6:37566. doi: 10.1038/srep37566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luz-Crawford P, Djouad F, Toupet K, Bony C, Franquesa M, Hoogduijn MJ, Jorgensen C, Noël D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells. 2016;34:483–492. doi: 10.1002/stem.2254. [DOI] [PubMed] [Google Scholar]
- 71.Min H, Xu L, Parrott R, Overall CC, Lillich M, Rabjohns EM, Rampersad RR, Tarrant TK, Meadows N, Fernandez-Castaneda A, Gaultier A, Kurtzberg J, Filiano AJ. Mesenchymal stromal cells reprogram monocytes and macrophages with processing bodies. Stem Cells. 2021;39:115–128. doi: 10.1002/stem.3292. [DOI] [PubMed] [Google Scholar]
- 72.Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, Ma L, Yin H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine. 2016;8:72–82. doi: 10.1016/j.ebiom.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao S, Mao F, Zhang B, Zhang L, Zhang X, Wang M, Yan Y, Yang T, Zhang J, Zhu W, Qian H, Xu W. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-κB and signal transducer and activator of transcription 3 pathways. Exp Biol Med (Maywood) 2014;239:366–375. doi: 10.1177/1535370213518169. [DOI] [PubMed] [Google Scholar]
- 74.Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, Zhao Y, Liu H, Fu X, Han W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spinosa M, Lu G, Su G, Bontha SV, Gehrau R, Salmon MD, Smith JR, Weiss ML, Mas VR, Upchurch GR Jr, Sharma AK. Human mesenchymal stromal cell-derived extracellular vesicles attenuate aortic aneurysm formation and macrophage activation via microRNA-147. FASEB J. 2018:fj201701138RR. doi: 10.1096/fj.201701138RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He X, Dong Z, Cao Y, Wang H, Liu S, Liao L, Jin Y, Yuan L, Li B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019;2019:7132708. doi: 10.1155/2019/7132708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gou W, Zhang Z, Yang C, Li Y. MiR-223/Pknox1 axis protects mice from CVB3-induced viral myocarditis by modulating macrophage polarization. Exp Cell Res. 2018;366:41–48. doi: 10.1016/j.yexcr.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, Li H, Wang G, Evans AR, Safe S, Wu C, Zhou B. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125:2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 79.Neudecker V, Haneklaus M, Jensen O, Khailova L, Masterson JC, Tye H, Biette K, Jedlicka P, Brodsky KS, Gerich ME, Mack M, Robertson AAB, Cooper MA, Furuta GT, Dinarello CA, O'Neill LA, Eltzschig HK, Masters SL, McNamee EN. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J Exp Med. 2017;214:1737–1752. doi: 10.1084/jem.20160462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song JY, Kang HJ, Hong JS, Kim CJ, Shim JY, Lee CW, Choi J. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci Rep. 2017;7:9412. doi: 10.1038/s41598-017-09827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abughanam G, Elkashty OA, Liu Y, Bakkar MO, Tran SD. Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren's Syndrome-Like Disease. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20194750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su X, Fang D, Liu Y, Ruan G, Seuntjens J, Kinsella JM, Tran SD. Lyophilized bone marrow cell extract functionally restores irradiation-injured salivary glands. Oral Dis. 2018;24:202–206. doi: 10.1111/odi.12728. [DOI] [PubMed] [Google Scholar]
- 84.Weiss ARR, Lee O, Eggenhofer E, Geissler E, Korevaar SS, Soeder Y, Schlitt HJ, Geissler EK, Hoogduijn MJ, Dahlke MH. Differential effects of heat-inactivated, secretome-deficient MSC and metabolically active MSC in sepsis and allogenic heart transplantation. Stem Cells. 2020;38:797–807. doi: 10.1002/stem.3165. [DOI] [PubMed] [Google Scholar]
- 85.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:529–564. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu W, Fu C, Song L, Yao Y, Zhang X, Chen Z, Li Y, Ma G, Shen C. Exposure to supernatants of macrophages that phagocytized dead mesenchymal stem cells improves hypoxic cardiomyocytes survival. Int J Cardiol. 2013;165:333–340. doi: 10.1016/j.ijcard.2012.03.088. [DOI] [PubMed] [Google Scholar]
- 89.Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, von Bonin M, Barbieri L, Halai K, Ward S, Weng L, Chakraverty R, Lombardi G, Watt FM, Orchard K, Marks DI, Apperley J, Bornhauser M, Walczak H, Bennett C, Dazzi F. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 90.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 91.Huang R, Qin C, Wang J, Hu Y, Zheng G, Qiu G, Ge M, Tao H, Shu Q, Xu J. Differential effects of extracellular vesicles from aging and young mesenchymal stem cells in acute lung injury. Aging (Albany NY) 2019;11:7996–8014. doi: 10.18632/aging.102314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, Kwong A, Mitsialis SA, Kourembanas S. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med. 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O'Kane CM, Krasnodembskaya AD. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med. 2017;196:1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, Keating A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299–1310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 95.Xu R, Zhang F, Chai R, Zhou W, Hu M, Liu B, Chen X, Liu M, Xu Q, Liu N, Liu S. Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J Cell Mol Med. 2019;23:7617–7631. doi: 10.1111/jcmm.14635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawata Y, Tsuchiya A, Seino S, Watanabe Y, Kojima Y, Ikarashi S, Tominaga K, Yokoyama J, Yamagiwa S, Terai S. Early injection of human adipose tissue-derived mesenchymal stem cell after inflammation ameliorates dextran sulfate sodium-induced colitis in mice through the induction of M2 macrophages and regulatory T cells. Cell Tissue Res. 2019;376:257–271. doi: 10.1007/s00441-018-02981-w. [DOI] [PubMed] [Google Scholar]
- 97.Watanabe Y, Tsuchiya A, Seino S, Kawata Y, Kojima Y, Ikarashi S, Starkey Lewis PJ, Lu WY, Kikuta J, Kawai H, Yamagiwa S, Forbes SJ, Ishii M, Terai S. Mesenchymal Stem Cells and Induced Bone Marrow-Derived Macrophages Synergistically Improve Liver Fibrosis in Mice. Stem Cells Transl Med. 2019;8:271–284. doi: 10.1002/sctm.18-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eirin A, Zhu XY, Puranik AS, Tang H, McGurren KA, van Wijnen AJ, Lerman A, Lerman LO. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017;92:114–124. doi: 10.1016/j.kint.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L. Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell Physiol Biochem. 2018;47:864–878. doi: 10.1159/000490078. [DOI] [PubMed] [Google Scholar]
- 100.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1003–L1013. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park KS, Svennerholm K, Shelke GV, Bandeira E, Lässer C, Jang SC, Chandode R, Gribonika I, Lötvall J. Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via IL-10. Stem Cell Res Ther. 2019;10:231. doi: 10.1186/s13287-019-1352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, Ji J, Liu F, Ding L, Ni Y, Hou Y. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells. 2017;35:1208–1221. doi: 10.1002/stem.2564. [DOI] [PubMed] [Google Scholar]
- 103.Zhang X, Huang F, Li W, Dang JL, Yuan J, Wang J, Zeng DL, Sun CX, Liu YY, Ao Q, Tan H, Su W, Qian X, Olsen N, Zheng SG. Human Gingiva-Derived Mesenchymal Stem Cells Modulate Monocytes/Macrophages and Alleviate Atherosclerosis. Front Immunol. 2018;9:878. doi: 10.3389/fimmu.2018.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. doi: 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 105.Lankford KL, Arroyo EJ, Nazimek K, Bryniarski K, Askenase PW, Kocsis JD. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS One. 2018;13:e0190358. doi: 10.1371/journal.pone.0190358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun G, Li G, Li D, Huang W, Zhang R, Zhang H, Duan Y, Wang B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194–204. doi: 10.1016/j.msec.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 107.Fan B, Li C, Szalad A, Wang L, Pan W, Zhang R, Chopp M, Zhang ZG, Liu XS. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia. 2020;63:431–443. doi: 10.1007/s00125-019-05043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chahal J, Gómez-Aristizábal A, Shestopaloff K, Bhatt S, Chaboureau A, Fazio A, Chisholm J, Weston A, Chiovitti J, Keating A, Kapoor M, Ogilvie-Harris DJ, Syed KA, Gandhi R, Mahomed NN, Marshall KW, Sussman MS, Naraghi AM, Viswanathan S. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. Stem Cells Transl Med. 2019;8:746–757. doi: 10.1002/sctm.18-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soeder Y, Loss M, Johnson CL, Hutchinson JA, Haarer J, Ahrens N, Offner R, Deans RJ, Van Bokkelen G, Geissler EK, Schlitt HJ, Dahlke MH. First-in-Human Case Study: Multipotent Adult Progenitor Cells for Immunomodulation After Liver Transplantation. Stem Cells Transl Med. 2015;4:899–904. doi: 10.5966/sctm.2015-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shi M, Liu Z, Wang Y, Xu R, Sun Y, Zhang M, Yu X, Wang H, Meng L, Su H, Jin L, Wang FS. A Pilot Study of Mesenchymal Stem Cell Therapy for Acute Liver Allograft Rejection. Stem Cells Transl Med. 2017;6:2053–2061. doi: 10.1002/sctm.17-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu J, Zhao G, Zhang L, Qiao C, Di A, Gao H, Xu H. Safety and therapeutic effect of mesenchymal stem cell infusion on moderate to severe ulcerative colitis. Exp Ther Med. 2016;12:2983–2989. doi: 10.3892/etm.2016.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meng F, Xu R, Wang S, Xu Z, Zhang C, Li Y, Yang T, Shi L, Fu J, Jiang T, Huang L, Zhao P, Yuan X, Fan X, Zhang JY, Song J, Zhang D, Jiao Y, Liu L, Zhou C, Maeurer M, Zumla A, Shi M, Wang FS. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5:172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Z, Fu J, Xu X, Wang S, Xu R, Zhao M, Nie W, Wang X, Zhang J, Li T, Su L, Wang FS. Safety and immunological responses to human mesenchymal stem cell therapy in difficult-to-treat HIV-1-infected patients. AIDS. 2013;27:1283–1293. doi: 10.1097/QAD.0b013e32835fab77. [DOI] [PMC free article] [PubMed] [Google Scholar]