Abstract
Tendinopathy is a challenging complication observed in patients with diabetes mellitus. Tendinopathy usually leads to chronic pain, limited joint motion, and even ruptured tendons. Imaging and histological analyses have revealed pathological changes in various tendons of patients with diabetes, including disorganized arrangement of collagen fibers, microtears, calcium nodules, and advanced glycation end product (AGE) deposition. Tendon-derived stem/ progenitor cells (TSPCs) were found to maintain hemostasis and to participate in the reversal of tendinopathy. We also discovered the aberrant osteochondrogenesis of TSPCs in vitro. However, the relationship between AGEs and TSPCs in diabetic tendinopathy and the underlying mechanism remain unclear. In this review, we summarize the current findings in this field and hypothesize that AGEs could alter the properties of tendons in patients with diabetes by regulating the proliferation and differentiation of TSPCs in vivo.
Keywords: Tendinopathy, Diabetes mellitus, Tendon stem/progenitor cells, Advanced glycation end products
Core Tip: Patients with diabetic tendinopathy usually suffer from chronic pain, restricted joint motion, calcium deposition, and even tendon rupture. Advanced glycation end products (AGEs) have been shown to affect tendon biology and biomechanical properties. In addition, tendon-derived stem/progenitor cells (TSPCs) play an important role in tendon hemostasis, regeneration, and repair. However, the relationships between diabetic tendinopathy, AGEs, and TSPCs remain unclear. Thus, in this review, we summarize the current findings and discuss the possible relationships between AGEs and TSPCs. This might provide new guidance for the development of effective treatments for diabetic tendinopathy.
INTRODUCTION
Tendinopathy is a common musculoskeletal complication of diabetes mellitus (DM)[1,2]. Patients with DM have a higher incidence of tendinopathy than healthy patients of the same age[3]. The Achilles tendon, patellar tendon, and rotator cuffs are the most frequently affected[4]. The classic symptoms of diabetic tendinopathy usually manifest as chronic pain, limited range of joint motion (ROM), and tendon rupture[2,4,5]. With the progression of diabetic tendinopathy, increased stiffness and thickness and decreased biomechanical properties of Achilles tendons have been reported, and these symptoms usually result in an altered gait and accelerated plantar ulcer formation in DM patients with poor glucose control[2]. Disordered arrangements of excessive collagen fibers and even calcified sites were observed by ultrasound at the entheses of the Achilles tendons[6]. In their electron microscopy study, Grant et al[7] also reported that the collagen fibers of the Achilles tendon presented in twisted, curved, and overlapping arrangements in DM patients.
Histological analysis has revealed prominent fibrochondral metaplasia and granulation tissue hyperplasia in DM patients with stenosing flexor tenosynovitis[8]. We also observed microtears in disorganized collagen fibers, blood vessels, and rounded changing cells in the patellar tendons of rats with experimental DM[9]. Moreover, as the characteristic products of DM, advanced glycation end products (AGEs) were discovered in DM tendons[10,11]. Once irreversible AGEs accumulate, they can modify proteins and ultimately damage tendon tissues. Among the various types of cells in tendon tissue, tendon-derived stem/progenitor cells (TSPCs) show multidifferentiation potential and exhibit the ability to maintain hemostasis and reverse tendinopathy[12-16]. Our previous study reported that the impaired functions of diabetic tendon-derived TSPCs showed abnormal osteochondrogenic differentiation in vitro, which might also account for the dysfunctions of DM tendons[9]. The histopathological alterations in the tendons of diabetic subjects could partially explain the weakened tension, decreased biomechanical properties, limited ROM, and even the ease of rupture in DM patients. However, the underlying pathological mechanism of diabetic tendinopathy remains unclear. In this review, we summarize the current findings in the fields of diabetic tendinopathy, AGEs, and TSPCs and hypothesize that AGEs could alter the fates of TSPCs to exacerbate tendinopathy in DM patients.
HISTOPATHOLOGICAL FEATURES OF DIABETIC TENDINOPATHY
Many efforts have been made to investigate the histopathological changes associated with diabetic tendinopathy. Ji et al[17] observed blood vessel hyperplasia and excessive collagen fibers in leptin-deficient mice. In some subjects, microtears were found to have red blood cell (RBC) deposition and chondrocyte-like cells surrounding the sites of the microtears[17]. In streptozotocin (STZ)-induced DM rats, we also found characteristic histopathological features in DM tendons, such as RBC deposition and microtears, by hematoxylin and eosin staining[9]. By immunohistochemical staining, the expression of vascular endothelial growth factor was found to be significantly increased in the experimental tendons of patients with diabetes, which may contribute to vascularization changes[18]. As characteristic products of DM, AGEs have been reported to be deposited in various organs and tissues[19,20]. In tendons of patients with diabetes, AGEs accumulate in the extracellular matrix (ECM) of tendon cells. During the early stage of STZ-induced type I DM, we also found the deposition of AGEs in the ECM of rat patellar tendons. Moreover, we discovered decreased expression levels of type I collagen (Col I), tenomodulin (TNMD), and decorin (DCN) in tendons of patients with diabetes. Nevertheless, these tendon cells express higher levels of osteochondrogenesis-associated proteins [osteopontin (OPN), osteocalcin (OCN), SOX9, and collagen type II (Col II)] in the ECM[9]. These results suggested that the pathologic manifestations of chondrification and ossification observed in tendons of patients with diabetes might be ascribed to the aberrant differentiation of these autologous TSPCs in tendon tissue into chondrocytes and osteocytes. However, current studies cannot fully explain the alterations, especially heterotopic calcification and chondrogenesis, in tendons of patients with diabetes at the cellular and histological levels[1,9].
FORMATION AND ACCUMULATION OF AGES IN TENDONS OF PATIENTS WITH DIABETES
The niche of TSPCs in tendon tissue is complicated. Numerous studies have demonstrated the importance of niches in mediating the proliferation and differentiation of stem cells[21-23]. Many factors, such as ECM, biomechanical stimulation, biologically active factors, and pH, could affect the functions of TSPCs in vivo[21].
As a distinctive product of DM, AGEs can excessively deposit in connective tissues[10,24-27]. AGEs are derived from nonenzymatic products of the interactions of long-lived proteins with glucose[19,28]. The formation of AGEs is quite slow and spontaneous in healthy subjects[29]. In low metabolic tissues, such as tendons and ligaments, AGEs can accumulate with aging. In addition to aging, the base level of glucose can also affect the formation and accumulation of AGEs in vivo[11]. The main component of tendon ECM is collagen type I (Col I), whose half-life ranges from 1 to 2 years; due to this longevity, it is sensitive to the glycoxidation process, which in turn highlights the accumulation of AGEs in tendons of patients with diabetes, which further alters the qualities of tendon ECM[11,26,30,31]. AGEs are mainly deposited in the outer layer or the most distal and proximal regions of the tendons instead of in the core regions in aged tendon samples[32]. However, to date, no studies have focused on region-specific histological analysis of AGE deposition in tendons of patients with diabetes.
Among the subtypes of AGEs, AGE-2 (glyceraldehyde-derived AGEs) and AGE-3 (glycolaldehyde-derived AGEs) are the main subtypes that can be detected in the sera of diabetic patients and they exhibit toxic bioactivities in various cells[33]. In osteoarthritis patients with DM, AGE deposition could lead to increased skeletal fragility and a higher fracture risk in aged people[34,35]. The main reason for this might be the cross-links formed by AGEs between the collagen strands[36]. The formation of these cross-links could result in increased stiffness and decreased biomechanical properties of diabetic cartilage and tendons.
In addition to the cross-links among the collagen fibers in DM tendons, the expression of the receptor for AGEs (RAGE) was also evaluated. Activation of AGE-RAGE could mediate many downstream signaling pathways in many kinds of cells and lead to many functional responses[37,38]. For instance, it induces cell death[39], regulates the expression of the inflammatory response[39], and degrades the ECM[40]. The study by Yokosuka et al[27] demonstrated the accumulation of AGEs in the ossified spinal ligament and suggested that the interaction of AGEs with RAGE is an important factor for the progression of spinal ligament ossification. In osteoblast-like cells, AGEs can regulate the differentiation stages via specific receptors[41]. Moreover, the latest research revealed that AGEs inhibited the osteogenic differentiation of mouse adipose-derived stem cells (ASCs) in vitro[42]. These studies demonstrated that the chronic accumulation of AGEs has negative impacts on these tissues and organs. Therefore, more attention has been given to determining the influences or underlying mechanisms of AGEs on musculoskeletal systems.
AGES ALTER THE BIOMECHANICAL PROPERTIES OF TENDONS
It has been documented that tendon tissue exhibits an inherent triple helix structure[43]. Accumulated AGEs could cross-link neighboring collagen molecules within the tendons[20]. The intermolecular cross-links between neighboring collagen molecules may connect lysine to arginine residues or lysine to lysine[44]. In DM patients, the arrangement of collagen fibers in the Achilles tendon exhibited a highly disorganized structure under electron microscopy, and these structural abnormalities might be ascribed to the deposition of AGEs[7].
Various studies have demonstrated that cross-links between collagen fibers could affect the biomechanical properties of the musculoskeletal system. Currently, few studies have investigated the biomechanical effects of AGEs on human tendon tissues. In osteoarthritis, cross-links caused by AGEs increased the stiffness of the collagen network in human articular cartilage[36].
However, the conclusions about the effects of AGEs on tendon mechanics are contradictory. Sell and Monnier[45] reported that the cross-links formed by AGEs could increase the C57BL/6 mouse tendon strain. In isolated rabbit Achilles tendons, after glycation in vitro, the maximum load, stress, strain, and Young’s modulus of elasticity were increased compared with those of the nonglycated tendons[46]. Biochemical analysis revealed significantly increased expression of pentosidine, which is recognized as a marker of AGEs, in glycated rabbit Achilles tendons. The cross-links formed by AGEs between collagens increased the stiffness of the matrix[47]. Thus, the authors concluded that cross-links could directly affect the matrix stiffness and stimulate the biomechanical properties of tendons. In addition, AGEs have been reported to damage the biomechanical properties of tendon collagen in various species by diminishing tendon fiber sliding[11,48,49]. In rat tail tendons, Fessel et al[48] discovered that lateral molecular interconnectivity by AGEs could reduce the side-by-side sliding of collagen fibers, thus leading to increased collagen fiber failure resistance in vivo. An in vitro study also revealed dramatically decreased tendon fiber sliding and viscoelastic behavior by tissue glycation[11]. In bovine tail tendons, Lee and Veres[20] found that the cross-links formed by AGEs could significantly inhibit biomechanical plasticity in vitro. Some other researchers considered that the cross-links could affect the biomechanical properties by taking up space in the ECM[50]. In both aged tendons and glycated tendons in vitro, the molecular spacing was linearly increased[11], which might be ascribed to the formation of cross-links by AGEs between collagen fibers. Another argument was that AGEs primarily affect the mechanical properties at the failure regions of tendons of patients with diabetes[51,52].
RELATIONSHIPS BETWEEN AGES AND THE ECM OF TENDONS OF PATIENTS WITH DIABETES
In addition to the cross-links formed between collagen fibers, the deposited AGEs in the ECM could also interact with various kinds of cytokines and proteins, cause biological effects, and subsequently impair their material properties[44,45,48]. It has been reported that Fe2+ in tendons of patients with diabetes could promote the accumulation of AGEs in collagens, which in turn stimulated the glycosylation of Col I and other matrix proteins in vivo[53]. Once deposited in the ECM, these AGEs could suppress the function of the mitochondria of Achilles tendon-derived fibroblasts and impair their proliferation, further leading to reduced remodeling of the ECM[54]. In porcine patellar tendons, the proteoglycan level was decreased after sustained hyperglycemia caused the production of AGEs in vitro[55]. Nevertheless, only a few studies have focused on the interactions of AGEs and factors in the ECM of tendon cells, especially TSPCs. In other tissues or cell types, such as ligaments and fibroblasts, AGEs have been demonstrated to affect the expression levels of matrix metalloproteinases (MMPs), bone morphogenetic proteins (BMPs), and other factors. Accumulated AGEs in the ossified spinal ligament could elevate the expression levels of BMP-7, BMP-2, alkaline phosphatase (ALP), and OCN, an osteoblast-specific transcription factor 1[27]. In human fibroblasts, AGEs could decrease Col I and increase MMP-1 levels in vitro[56]. In osteoblast-like cells, AGEs could promote the degradation of Col I by stimulating the secretion of MMP-2 and MMP-9 in vitro[57] and stimulate the mRNA expression and serum levels of fibroblast growth factor 23 in chronic disease[58]. The expression of MMP-1 in human gingival fibroblasts was also significantly increased at both the mRNA and protein levels in vitro after treatment with AGEs[59].
AGES INDUCE CELLULAR EVENTS IN TENDON CELLS AND THE UNDERLYING MECHANISM
AGEs induce cellular effects on various kinds of cells mainly by activating the RAGE in vivo. Many studies have reported that the AGE-mediated events of various kinds of cells are activated through the interactions of AGE-RAGE[60,61]. RAGE is a receptor that can activate many kinds of ligands and it exists in normal tendon tissues. It is expressed at low levels under normal blood glucose levels, and its expression could be increased while AGEs accumulate under sustained hyperglycemia[21,62]. In addition to RAGE, many other molecules have been shown to act as receptors of AGEs, such as scavenger receptor class AI/AII[63], scavenger receptor class B type I[64], and CD36[65]. In our unpublished research, we also observed the expression levels of AGEs and RAGE in the ECM of diabetic tendon cells in vivo and in isolated TSPCs in vitro. After the receptors for AGEs are activated, a variety of downstream cellular signaling pathways can be excited and they subsequently alter cell functions, such as proliferation, migration, apoptosis, and differentiation.
Proliferation
Generally, AGEs have been demonstrated to attenuate the proliferation abilities of various kinds of cells, such as bone mesenchymal stem cells (MSCs) and retinal pericytes[29,66]. In human MSCs, Kume et al[29] found that higher concentrations of AGE-2 and AGE-3 (1-100 μg/mL) could inhibit their proliferation ability and stimulate apoptosis in vitro, probably by upregulating intracellular reactive oxygen species (ROS). The generation of ROS has been reported to regulate these AGE-RAGE-induced cellular events[61,67]. Yang et al[68] reported that AGEs inhibited bone MSC proliferation and migration by inducing chemokine/cytokine secretion via the p38 pathway in vitro. Moreover, AGE-2 could suppress the proliferation of cultured bovine retinal pericytes through downregulation of the expression ratio of BCL-2/BAX[66]. In addition, AGEs could stimulate the proliferation abilities of several other kinds of cells. In osteoblastic cell lines, the effects of AGEs on cell proliferation were reported to depend on their stage of differentiation[69]. Low concentrations of AGEs could stimulate mesangial cell proliferation[70]. AGEs enhance vascularization in diabetic retinopathy by interacting with RAGE and promoting vascular endothelial cell proliferation[71]. However, few studies have investigated the impacts of AGEs on TSPCs, and further research is required.
Apoptosis
In addition to their influence on proliferation, AGEs also induce the apoptosis of many kinds of cells, including TSPCs, retinal pericytes, myoblastic cell lines, mononuclear cells, and endothelial progenitor cells[67,72-75]. Xu et al[72] reported that AGEs could induce TSPC apoptosis, and pioglitazone showed the ability to rescue AGE-induced apoptosis and other abnormal alterations both in vitro and in vivo. In bovine retinal pericytes, AGE-initiated apoptosis was reported to be ascribed to the activation of the caspase-10 pathway[67]. AGEs could induce the apoptosis of mouse myoblastic C2C12 cells and inhibit myogenic differentiation, while insulin-like growth factor-I exhibited therapeutic potential to attenuate the detrimental effects of AGEs on C2C12 cells[73]. In human mononuclear cells isolated from the peripheral blood of patients with type II DM, increased cellular apoptosis and decreased osteoblastic differentiation ability were highly correlated with RAGE expression[74]. The activation of ROS, Akt/eNOS, MAP kinases, and the FOXO1 transcription factor have all been reported to participate in AGE-induced apoptosis progression[54,75].
Differentiation
Several studies have illustrated that accumulated AGEs could affect the differentiation properties of stem cells in the musculoskeletal system. In TSPCs, AGEs have been reported to exacerbate osteogenic differentiation potential in vitro[72]. For other kinds of cells, AGEs could inhibit the osteogenic differentiation potential of mouse ASCs by suppressing the expression of OPN and runt-related transcription factor 2 (Runx2) through activating the Wnt/β-catenin signaling pathway[42]. In human periodontal ligament stem cells, AGEs attenuate osteogenesis in vitro, and the canonical Wnt/β-catenin and JNK signaling pathways might be involved[76-78]. RAGE in MSCs could be activated by AGE-2 and AGE-3; thus, the AGE-RAGE interaction was found to participate in the osteogenic and chondrogenic differentiation processes of MSCs[29]. AGE-3 was reported to inhibit the osteogenic differentiation and bone nodule formation of MSCs by activating RAGE and upregulating the expression of TGF-β in vitro[29,79]. The expression levels of ALP and intracellular calcium in MSCs were upregulated by AGEs, while mineralization and bone nodule formation were both decreased in vitro. The chondrogenic and adipogenic differentiation potentials of the MSCs were also attenuated by AGEs in vitro[29].
AGEs and TSPCs
To date, only a few studies have focused on the influence of AGEs on TSPCs. Xu et al[72] reported that AGEs could reduce cell viability and increase apoptosis and autophagy of TSPCs in vitro. In that study, they found that AGEs induced senescence and enhanced the ossification of TSPCs in vitro. However, the researchers did not further investigate the underlying mechanisms of AGE-induced ossification of TSPCs. In MSCs, AGE-2 and AGE-3 showed the ability to enhance ALP activity and intracellular calcium content by activating RAGE in vitro[29]. Therefore, we speculate that the activation of RAGE in TSPCs could also lead to apoptosis, senescence, and aberrant differentiation by activating several signaling pathways, such as the Wnt/β-catenin, P38/MAPK, Notch, ROS, and Akt/eNOS pathways.
TSPCS IN DIABETIC TENDINOPATHY
The progression of diabetic tendinopathy is complicated and involves various kinds of factors and types of cells. Previously, we have summarized the current findings of diabetic tendinopathy, especially the cellular and underlying mechanisms[80]. In addition to tenocytes, there are many other types of cells inside the tendons. Bi et al[12] and Rui et al[13] proved the existence of stem/progenitor cells in the tendons of mice and rats. TSPCs exhibit self-colony ability and multidifferentiation properties in vitro[14-16]. In the patellar tendon of a collagenase-induced rat tendinopathy model, TSPCs presented lower proliferation capacity and higher osteogenic and chondrogenic differentiation potentials[16]. In an injury-induced rat tendinopathy model, TSPCs showed increased proliferation ability and higher type III collagen (Col III) and α-SMA expression than in collagenase-induced rats[15]. These findings indicate the involvement of TSPCs in maintaining tendon tissue homeostasis and mediating the pathological process of chronic tendinopathy[81]. During the development of diabetic tendinopathy, as tissue-specific cells are contained in tendon tissue, TSPCs are the most likely cells to participate in the early response. TSPCs are thought to differentiate into tenocytes and play key roles in maintaining, regenerating, and replacing differentiated tenocytes in tendon tissues. In rats with experimental DM, we found that the fate of TSPCs isolated from patellar tendons was altered, and these cells exhibited decreased proliferation properties and enhanced osteochondrogenic potential[9]. High glucose (11.1 mmol/L) could stimulate an inflammatory response of TSPCs in the human patellar tendon in vitro[82]. Our previous study found that high glucose (15 mmol/L and 25 mmol/L) could inhibit rat TSPC proliferation and induce apoptosis in vitro[83]. Moreover, insulin has been reported to increase ALP activity and the expression levels of osteogenesis-associated markers in TSPCs isolated from horse superficial digital flexor tendons[84]. Taken together, these studies indicate that the aberrant proliferation and differentiation of TSPCs are possible underlying mechanisms of diabetic tendinopathy. AGEs have been shown to induce apoptosis and to exacerbate the osteogenic differentiation potential of TSPCs in vitro[72]. However, the mediating mechanisms of AGEs on diabetic TSPC multidifferentiation potential are still unclear, and future studies are required to investigate the underlying processes.
CONCLUSION
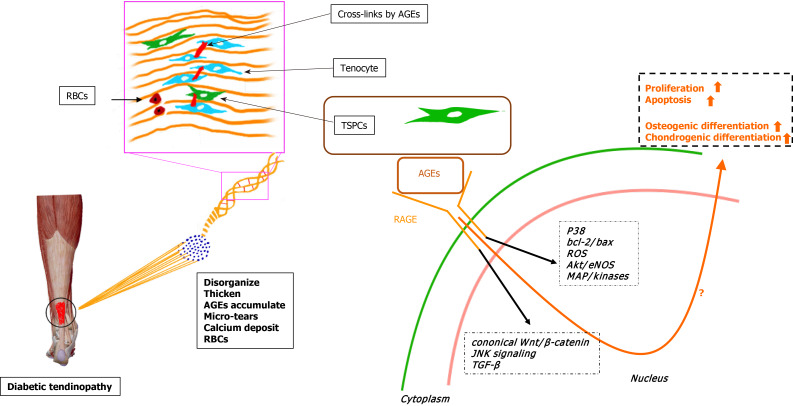
In summary, we have described the enhanced osteochondrogenic differentiation ability of TSPCs from experimental diabetic rats cultured in induction medium[9]. Additionally, the outstanding expression of osteochondrogenic-associated markers and AGE accumulation were also noted. In vitro studies revealed that AGEs could affect the proliferative capacity, apoptosis, and multidifferentiation potential of TSPCs and other kinds of stem cells under certain pathological conditions. Taken together, we hypothesize that the accumulated AGEs in the ECM of diabetic TSPCs lead to aberrant differentiation fates and futures, contributing to the development of chronic tendinopathy in DM subjects (Figure 1). Understanding the relationships among diabetic tendinopathy, TSPCs, and AGEs will be crucial for developing new treatments for diabetic tendinopathy therapy.
Figure 1.
Hypothesis of the molecular mechanism by which advanced glycation end products regulate the fate of tendon-derived stem/progenitor cells in diabetic tendinopathy. RBCs: Red blood cells; AGEs: Advanced glycation end products; TSPCs: Tendon-derived stem/progenitor cells; RAGE: Receptor for advanced glycation end product; ROS: Reactive oxygen species; TGF-β: Transforming growth factor β.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest for this manuscript.
Manuscript source: Invited manuscript
Peer-review started: April 6, 2021
First decision: May 12, 2021
Article in press: August 17, 2021
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cigrovski Berkovic M, Saengboonmee C S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Xing YX
Contributor Information
Liu Shi, Department of Orthopaedics, Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Pan-Pan Lu, Department of Orthopaedics, Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Guang-Chun Dai, Department of Orthopaedics, Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Ying-Juan Li, Department of Geriatrics, Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China.
Yun-Feng Rui, Department of Orthopaedics, Zhongda Hospital, School of Medicine, Southeast University, Nanjing 210009, Jiangsu Province, China. ruiyunfeng@126.com.
References
- 1.de Oliveira RR, Lemos A, de Castro Silveira PV, da Silva RJ, de Moraes SR. Alterations of tendons in patients with diabetes mellitus: a systematic review. Diabet Med. 2011;28:886–895. doi: 10.1111/j.1464-5491.2010.03197.x. [DOI] [PubMed] [Google Scholar]
- 2.Shi L, Rui YF, Li G, Wang C. Alterations of tendons in diabetes mellitus: what are the current findings? Int Orthop. 2015;39:1465–1473. doi: 10.1007/s00264-015-2775-x. [DOI] [PubMed] [Google Scholar]
- 3.Aydeniz A, Gursoy S, Guney E. Which musculoskeletal complications are most frequently seen in type 2 diabetes mellitus? J Int Med Res. 2008;36:505–511. doi: 10.1177/147323000803600315. [DOI] [PubMed] [Google Scholar]
- 4.Abate M, Schiavone C, Salini V, Andia I. Management of limited joint mobility in diabetic patients. Diabetes Metab Syndr Obes. 2013;6:197–207. doi: 10.2147/DMSO.S33943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lui PPY. Tendinopathy in diabetes mellitus patients-Epidemiology, pathogenesis, and management. Scand J Med Sci Sports. 2017;27:776–787. doi: 10.1111/sms.12824. [DOI] [PubMed] [Google Scholar]
- 6.Abate M, Salini V, Antinolfi P, Schiavone C. Ultrasound morphology of the Achilles in asymptomatic patients with and without diabetes. Foot Ankle Int. 2014;35:44–49. doi: 10.1177/1071100713510496. [DOI] [PubMed] [Google Scholar]
- 7.Grant WP, Sullivan R, Sonenshine DE, Adam M, Slusser JH, Carson KA, Vinik AI. Electron microscopic investigation of the effects of diabetes mellitus on the Achilles tendon. J Foot Ankle Surg. 1997;36:272–8; discussion 330. doi: 10.1016/s1067-2516(97)80072-5. [DOI] [PubMed] [Google Scholar]
- 8.Kameyama M, Chen KR, Mukai K, Shimada A, Atsumi Y, Yanagimoto S. Histopathological characteristics of stenosing flexor tenosynovitis in diabetic patients and possible associations with diabetes-related variables. J Hand Surg Am. 2013;38:1331–1339. doi: 10.1016/j.jhsa.2013.03.049. [DOI] [PubMed] [Google Scholar]
- 9.Shi L, Li YJ, Dai GC, Lin YC, Li G, Wang C, Chen H, Rui YF. Impaired function of tendon-derived stem cells in experimental diabetes mellitus rat tendons: implications for cellular mechanism of diabetic tendon disorder. Stem Cell Res Ther. 2019;10:27. doi: 10.1186/s13287-018-1108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abate M, Schiavone C, Salini V, Andia I. Occurrence of tendon pathologies in metabolic disorders. Rheumatology (Oxford) 2013;52:599–608. doi: 10.1093/rheumatology/kes395. [DOI] [PubMed] [Google Scholar]
- 11.Gautieri A, Passini FS, Silván U, Guizar-Sicairos M, Carimati G, Volpi P, Moretti M, Schoenhuber H, Redaelli A, Berli M, Snedeker JG. Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue. Matrix Biol. 2017;59:95–108. doi: 10.1016/j.matbio.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 13.Rui YF, Lui PP, Li G, Fu SC, Lee YW, Chan KM. Isolation and characterization of multipotent rat tendon-derived stem cells. Tissue Eng Part A. 2010;16:1549–1558. doi: 10.1089/ten.TEA.2009.0529. [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Luo JW, Zhang KK, Lin LX, Liang T, Luo ZP, Zhuang YQ, Sun YL. Tendon-Derived Stem Cell Differentiation in the Degenerative Tendon Microenvironment. Stem Cells Int. 2018;2018:2613821. doi: 10.1155/2018/2613821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SJ, Song DH, Kim SJ. Characteristics of tendon derived stem cells according to different factors to induce the tendinopathy. J Cell Physiol. 2018;233:6196–6206. doi: 10.1002/jcp.26475. [DOI] [PubMed] [Google Scholar]
- 16.Rui YF, Lui PP, Wong YM, Tan Q, Chan KM. Altered fate of tendon-derived stem cells isolated from a failed tendon-healing animal model of tendinopathy. Stem Cells Dev. 2013;22:1076–1085. doi: 10.1089/scd.2012.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji J, wang Z, Shi D, Gao X, Jiang Q. Pathologic changes of Achilles tendon in leptin-deficient mice. Rheumatol Int. 2010;30:489–493. doi: 10.1007/s00296-009-1001-9. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira RR, Martins CS, Rocha YR, Braga AB, Mattos RM, Hecht F, Brito GA, Nasciutti LE. Experimental diabetes induces structural, inflammatory and vascular changes of Achilles tendons. PLoS One. 2013;8:e74942. doi: 10.1371/journal.pone.0074942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyer DG, Dunn JA, Thorpe SR, Lyons TJ, McCance DR, Baynes JW. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. Ann N Y Acad Sci. 1992;663:421–422. doi: 10.1111/j.1749-6632.1992.tb38687.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee JM, Veres SP. Advanced glycation end-product cross-linking inhibits biomechanical plasticity and characteristic failure morphology of native tendon. J Appl Physiol (1985) 2019;126:832–841. doi: 10.1152/japplphysiol.00430.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6:103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal AK, Gohr CM, Mitton E, Monnier V, Burner T. Advanced glycation end products increase transglutaminase activity in primary porcine tenocytes. J Investig Med. 2009;57:460–466. doi: 10.2310/JIM.0b013e3181954ac6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Yokosuka K, Park JS, Jimbo K, Yoshida T, Yamada K, Sato K, Takeuchi M, Yamagishi S, Nagata K. Immunohistochemical demonstration of advanced glycation end products and the effects of advanced glycation end products in ossified ligament tissues in vitro. Spine (Phila Pa 1976) 2007;32:E337–E339. doi: 10.1097/01.brs.0000263417.17526.35. [DOI] [PubMed] [Google Scholar]
- 28.Ansari NA, Dash D. Amadori glycated proteins: role in production of autoantibodies in diabetes mellitus and effect of inhibitors on non-enzymatic glycation. Aging Dis. 2013;4:50–56. [PMC free article] [PubMed] [Google Scholar]
- 29.Kume S, Kato S, Yamagishi S, Inagaki Y, Ueda S, Arima N, Okawa T, Kojiro M, Nagata K. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res. 2005;20:1647–1658. doi: 10.1359/JBMR.050514. [DOI] [PubMed] [Google Scholar]
- 30.Thorpe CT, Screen HR. Tendon Structure and Composition. Adv Exp Med Biol. 2016;920:3–10. doi: 10.1007/978-3-319-33943-6_1. [DOI] [PubMed] [Google Scholar]
- 31.Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 32.Eriksen CS, Svensson RB, Gylling AT, Couppé C, Magnusson SP, Kjaer M. Load magnitude affects patellar tendon mechanical properties but not collagen or collagen cross-linking after long-term strength training in older adults. BMC Geriatr. 2019;19:30. doi: 10.1186/s12877-019-1043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi M, Yamagishi S. TAGE (toxic AGEs) hypothesis in various chronic diseases. Med Hypotheses. 2004;63:449–452. doi: 10.1016/j.mehy.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 34.Saudek DM, Kay J. Advanced glycation endproducts and osteoarthritis. Curr Rheumatol Rep. 2003;5:33–40. doi: 10.1007/s11926-003-0081-x. [DOI] [PubMed] [Google Scholar]
- 35.Verzijl N, Bank RA, TeKoppele JM, DeGroot J. AGEing and osteoarthritis: a different perspective. Curr Opin Rheumatol. 2003;15:616–622. doi: 10.1097/00002281-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Verzijl N, DeGroot J, Ben ZC, Brau-Benjamin O, Maroudas A, Bank RA, Mizrahi J, Schalkwijk CG, Thorpe SR, Baynes JW, Bijlsma JW, Lafeber FP, TeKoppele JM. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 37.Xie J, Méndez JD, Méndez-Valenzuela V, Aguilar-Hernández MM. Cellular signalling of the receptor for advanced glycation end products (RAGE) Cell Signal. 2013;25:2185–2197. doi: 10.1016/j.cellsig.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterszegi G, Molinari J, Ravelojaona V, Robert L. Effect of advanced glycation end-products on cell proliferation and cell death. Pathol Biol (Paris) 2006;54:396–404. doi: 10.1016/j.patbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Strieder-Barboza C, Baker NA, Flesher CG, Karmakar M, Neeley CK, Polsinelli D, Dimick JB, Finks JF, Ghaferi AA, Varban OA, Lumeng CN, O'Rourke RW. Advanced glycation end-products regulate extracellular matrix-adipocyte metabolic crosstalk in diabetes. Sci Rep. 2019;9:19748. doi: 10.1038/s41598-019-56242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarthy AD, Etcheverry SB, Cortizo AM. Advanced glycation endproduct-specific receptors in rat and mouse osteoblast-like cells: regulation with stages of differentiation. Acta Diabetol. 1999;36:45–52. doi: 10.1007/s005920050144. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Wang L, Zhang M, Huang K, Yao Z, Rao P, Cai X, Xiao J. Advanced glycation end products inhibit the osteogenic differentiation potential of adipose-derived stem cells by modulating Wnt/β-catenin signalling pathway via DNA methylation. Cell Prolif. 2020;53:e12834. doi: 10.1111/cpr.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Millar NL, Murrell GA, McInnes IB. Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol. 2017;13:110–122. doi: 10.1038/nrrheum.2016.213. [DOI] [PubMed] [Google Scholar]
- 44.Avery NC, Bailey AJ. The effects of the Maillard reaction on the physical properties and cell interactions of collagen. Pathol Biol (Paris) 2006;54:387–395. doi: 10.1016/j.patbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Sell DR, Monnier VM. Age-related association of tail tendon break time with tissue pentosidine in DBA/2 vs C57BL/6 mice: the effect of dietary restriction. J Gerontol A Biol Sci Med Sci. 1997;52:B277–B284. doi: 10.1093/gerona/52a.5.b277. [DOI] [PubMed] [Google Scholar]
- 46.Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit achilles tendon. Exp Diabesity Res. 2004;5:143–153. doi: 10.1080/15438600490277860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reddy GK, Stehno-Bittel L, Enwemeka CS. Glycation-induced matrix stability in the rabbit achilles tendon. Arch Biochem Biophys. 2002;399:174–180. doi: 10.1006/abbi.2001.2747. [DOI] [PubMed] [Google Scholar]
- 48.Fessel G, Li Y, Diederich V, Guizar-Sicairos M, Schneider P, Sell DR, Monnier VM, Snedeker JG. Advanced glycation end-products reduce collagen molecular sliding to affect collagen fibril damage mechanisms but not stiffness. PLoS One. 2014;9:e110948. doi: 10.1371/journal.pone.0110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Fessel G, Georgiadis M, Snedeker JG. Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol. 2013;32:169–177. doi: 10.1016/j.matbio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Naresh MD, Brodsky B. X-ray diffraction studies on human tendon show age-related changes in collagen packing. Biochim Biophys Acta. 1992;1122:161–166. doi: 10.1016/0167-4838(92)90319-9. [DOI] [PubMed] [Google Scholar]
- 51.Svensson RB, Smith ST, Moyer PJ, Magnusson SP. Effects of maturation and advanced glycation on tensile mechanics of collagen fibrils from rat tail and Achilles tendons. Acta Biomater. 2018;70:270–280. doi: 10.1016/j.actbio.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 52.Eriksen C, Svensson RB, Scheijen J, Hag AM, Schalkwijk C, Praet SF, Schjerling P, Kjær M, Magnusson SP, Couppé C. Systemic stiffening of mouse tail tendon is related to dietary advanced glycation end products but not high-fat diet or cholesterol. J Appl Physiol (1985) 2014;117:840–847. doi: 10.1152/japplphysiol.00584.2014. [DOI] [PubMed] [Google Scholar]
- 53.Xiao H, Cai G, Liu M. Fe2+-catalyzed non-enzymatic glycosylation alters collagen conformation during AGE-collagen formation in vitro. Arch Biochem Biophys. 2007;468:183–192. doi: 10.1016/j.abb.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 54.Liang C, Ren Y, Tan H, He Z, Jiang Q, Wu J, Zhen Y, Fan M, Wu Z. Rosiglitazone via upregulation of Akt/eNOS pathways attenuates dysfunction of endothelial progenitor cells, induced by advanced glycation end products. Br J Pharmacol. 2009;158:1865–1873. doi: 10.1111/j.1476-5381.2009.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burner T, Gohr C, Mitton-Fitzgerald E, Rosenthal AK. Hyperglycemia reduces proteoglycan levels in tendons. Connect Tissue Res. 2012;53:535–541. doi: 10.3109/03008207.2012.710670. [DOI] [PubMed] [Google Scholar]
- 56.Chen YS, Wang XJ, Feng W, Hua KQ. Advanced glycation end products decrease collagen I levels in fibroblasts from the vaginal wall of patients with POP via the RAGE, MAPK and NF-κB pathways. Int J Mol Med. 2017;40:987–998. doi: 10.3892/ijmm.2017.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W, Ling W, Teng X, Quan C, Cai S, Hu S. Effect of advanced glycation end products, extracellular matrix metalloproteinase inducer and matrix metalloproteinases on type-I collagen metabolism. Biomed Rep. 2016;4:691–693. doi: 10.3892/br.2016.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bär L, Wächter K, Wege N, Navarrete Santos A, Simm A, Föller M. Advanced glycation end products stimulate gene expression of fibroblast growth factor 23. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201601019. [DOI] [PubMed] [Google Scholar]
- 59.Yu S, Li H, Ma Y, Fu Y. Matrix metalloproteinase-1 of gingival fibroblasts influenced by advanced glycation end products (AGEs) and their association with receptor for AGEs and nuclear factor-κB in gingival connective tissue. J Periodontol. 2012;83:119–126. doi: 10.1902/jop.2011.100754. [DOI] [PubMed] [Google Scholar]
- 60.Abe R, Shimizu T, Sugawara H, Watanabe H, Nakamura H, Choei H, Sasaki N, Yamagishi S, Takeuchi M, Shimizu H. Regulation of human melanoma growth and metastasis by AGE-AGE receptor interactions. J Invest Dermatol. 2004;122:461–467. doi: 10.1046/j.0022-202X.2004.22218.x. [DOI] [PubMed] [Google Scholar]
- 61.Fukami K, Ueda S, Yamagishi S, Kato S, Inagaki Y, Takeuchi M, Motomiya Y, Bucala R, Iida S, Tamaki K, Imaizumi T, Cooper ME, Okuda S. AGEs activate mesangial TGF-beta-Smad signaling via an angiotensin II type I receptor interaction. Kidney Int. 2004;66:2137–2147. doi: 10.1111/j.1523-1755.2004.66004.x. [DOI] [PubMed] [Google Scholar]
- 62.Nedić O, Rattan SI, Grune T, Trougakos IP. Molecular effects of advanced glycation end products on cell signalling pathways, ageing and pathophysiology. Free Radic Res. 2013;47 Suppl 1:28–38. doi: 10.3109/10715762.2013.806798. [DOI] [PubMed] [Google Scholar]
- 63.Araki N, Higashi T, Mori T, Shibayama R, Kawabe Y, Kodama T, Takahashi K, Shichiri M, Horiuchi S. Macrophage scavenger receptor mediates the endocytic uptake and degradation of advanced glycation end products of the Maillard reaction. Eur J Biochem. 1995;230:408–415. doi: 10.1111/j.1432-1033.1995.0408h.x. [DOI] [PubMed] [Google Scholar]
- 64.Ohgami N, Miyazaki A, Sakai M, Kuniyasu A, Nakayama H, Horiuchi S. Advanced glycation end products (AGE) inhibit scavenger receptor class B type I-mediated reverse cholesterol transport: a new crossroad of AGE to cholesterol metabolism. J Atheroscler Thromb. 2003;10:1–6. doi: 10.5551/jat.10.1. [DOI] [PubMed] [Google Scholar]
- 65.Ohgami N, Nagai R, Ikemoto M, Arai H, Kuniyasu A, Horiuchi S, Nakayama H. CD36, a member of class B scavenger receptor family, is a receptor for advanced glycation end products. Ann N Y Acad Sci. 2001;947:350–355. doi: 10.1111/j.1749-6632.2001.tb03961.x. [DOI] [PubMed] [Google Scholar]
- 66.Yamagishi S, Hsu CC, Taniguchi M, Harada S, Yamamoto Y, Ohsawa K, Kobayashi K, Yamamoto H. Receptor-mediated toxicity to pericytes of advanced glycosylation end products: a possible mechanism of pericyte loss in diabetic microangiopathy. Biochem Biophys Res Commun. 1995;213:681–687. doi: 10.1006/bbrc.1995.2185. [DOI] [PubMed] [Google Scholar]
- 67.Lecomte M, Denis U, Ruggiero D, Lagarde M, Wiernsperger N. Involvement of caspase-10 in advanced glycation end-product-induced apoptosis of bovine retinal pericytes in culture. Biochim Biophys Acta. 2004;1689:202–211. doi: 10.1016/j.bbadis.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Yang K, Wang XQ, He YS, Lu L, Chen QJ, Liu J, Shen WF. Advanced glycation end products induce chemokine/cytokine production via activation of p38 pathway and inhibit proliferation and migration of bone marrow mesenchymal stem cells. Cardiovasc Diabetol. 2010;9:66. doi: 10.1186/1475-2840-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCarthy AD, Etcheverry SB, Cortizo AM. Effect of advanced glycation endproducts on the secretion of insulin-like growth factor-I and its binding proteins: role in osteoblast development. Acta Diabetol. 2001;38:113–122. doi: 10.1007/s005920170007. [DOI] [PubMed] [Google Scholar]
- 70.Geoffroy K, Wiernsperger N, Lagarde M, El Bawab S. Bimodal effect of advanced glycation end products on mesangial cell proliferation is mediated by neutral ceramidase regulation and endogenous sphingolipids. J Biol Chem. 2004;279:34343–34352. doi: 10.1074/jbc.M403273200. [DOI] [PubMed] [Google Scholar]
- 71.Mamputu JC, Renier G. Advanced glycation end products increase, through a protein kinase C-dependent pathway, vascular endothelial growth factor expression in retinal endothelial cells. Inhibitory effect of gliclazide. J Diabetes Complications. 2002;16:284–293. doi: 10.1016/s1056-8727(01)00229-x. [DOI] [PubMed] [Google Scholar]
- 72.Xu L, Xu K, Wu Z, Chen Z, He Y, Ma C, Moqbel SAA, Ran J, Zhang C, Wu L, Xiong Y. Pioglitazone attenuates advanced glycation end products-induced apoptosis and calcification by modulating autophagy in tendon-derived stem cells. J Cell Mol Med. 2020;24:2240–2251. doi: 10.1111/jcmm.14901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adachi N, Kanazawa I, Tanaka KI, Takeno A, Notsu M, Tanaka S, Sugimoto T. Insulin-Like Growth Factor-I Protects Against the Detrimental Effects of Advanced Glycation End Products and High Glucose in Myoblastic C2C12 Cells. Calcif Tissue Int. 2019;105:89–96. doi: 10.1007/s00223-019-00537-w. [DOI] [PubMed] [Google Scholar]
- 74.Phimphilai M, Pothacharoen P, Kongtawelert P, Chattipakorn N. Impaired osteogenic differentiation and enhanced cellular receptor of advanced glycation end products sensitivity in patients with type 2 diabetes. J Bone Miner Metab. 2017;35:631–641. doi: 10.1007/s00774-016-0800-9. [DOI] [PubMed] [Google Scholar]
- 75.Alikhani M, Maclellan CM, Raptis M, Vora S, Trackman PC, Graves DT. Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor. Am J Physiol Cell Physiol. 2007;292:C850–C856. doi: 10.1152/ajpcell.00356.2006. [DOI] [PubMed] [Google Scholar]
- 76.Zhang LN, Wang XX, Wang Z, Li KY, Xu BH, Zhang J. Berberine improves advanced glycation end productsinduced osteogenic differentiation responses in human periodontal ligament stem cells through the canonical Wnt/βcatenin pathway. Mol Med Rep. 2019;19:5440–5452. doi: 10.3892/mmr.2019.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fang H, Yang K, Tang P, Zhao N, Ma R, Luo X, Liu Q. Glycosylation end products mediate damage and apoptosis of periodontal ligament stem cells induced by the JNK-mitochondrial pathway. Aging (Albany NY) 2020;12:12850–12868. doi: 10.18632/aging.103304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yan W, Chao D, Kun Y, Xiaoxia C, Qi L, Yan J. [Canonical Wnt signaling pathway of the osteogenic differentiation of human periodontal ligament stem cells induced by advanced glycation end products] Hua Xi Kou Qiang Yi Xue Za Zhi. 2015;33:627–632. doi: 10.7518/hxkq.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Notsu M, Yamaguchi T, Okazaki K, Tanaka K, Ogawa N, Kanazawa I, Sugimoto T. Advanced glycation end product 3 (AGE3) suppresses the mineralization of mouse stromal ST2 cells and human mesenchymal stem cells by increasing TGF-β expression and secretion. Endocrinology. 2014;155:2402–2410. doi: 10.1210/en.2013-1818. [DOI] [PubMed] [Google Scholar]
- 80.Lu PP, Chen MH, Dai GC, Li YJ, Shi L, Rui YF. Understanding cellular and molecular mechanisms of pathogenesis of diabetic tendinopathy. World J Stem Cells. 2020;12:1255–1275. doi: 10.4252/wjsc.v12.i11.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ni M, Lui PP, Rui YF, Lee YW, Tan Q, Wong YM, Kong SK, Lau PM, Li G, Chan KM. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. J Orthop Res. 2012;30:613–619. doi: 10.1002/jor.21559. [DOI] [PubMed] [Google Scholar]
- 82.Kwan CK, Fu SC, Yung PS. A high glucose level stimulate inflammation and weaken pro-resolving response in tendon cells - A possible factor contributing to tendinopathy in diabetic patients. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2020;19:1–6. doi: 10.1016/j.asmart.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin YC, Li YJ, Rui YF, Dai GC, Shi L, Xu HL, Ni M, Zhao S, Chen H, Wang C, Li G, Teng GJ. The effects of high glucose on tendon-derived stem cells: implications of the pathogenesis of diabetic tendon disorders. Oncotarget. 2017;8:17518–17528. doi: 10.18632/oncotarget.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Durgam SS, Altmann NN, Coughlin HE, Rollins A, Hostnik LD. Insulin Enhances the In Vitro Osteogenic Capacity of Flexor Tendon-Derived Progenitor Cells. Stem Cells Int. 2019;2019:1602751. doi: 10.1155/2019/1602751. [DOI] [PMC free article] [PubMed] [Google Scholar]