Abstract
Brain diseases, including brain tumors, neurodegenerative disorders, cerebrovascular diseases, and traumatic brain injuries, are among the major disorders influencing human health, currently with no effective therapy. Due to the low regeneration capacity of neurons, insufficient secretion of neurotrophic factors, and the aggravation of ischemia and hypoxia after nerve injury, irreversible loss of functional neurons and nerve tissue damage occurs. This damage is difficult to repair and regenerate the central nervous system after injury. Neural stem cells (NSCs) are pluripotent stem cells that only exist in the central nervous system. They have good self-renewal potential and ability to differentiate into neurons, astrocytes, and oligodendrocytes and improve the cellular microenvironment. NSC transplantation approaches have been made for various neurodegenerative disorders based on their regenerative potential. This review summarizes and discusses the characteristics of NSCs, and the advantages and effects of NSCs in the treatment of brain diseases and limitations of NSC transplantation that need to be addressed for the treatment of brain diseases in the future.
Keywords: Neural stem cell, Brain disease, Therapy, Animal experiment, Clinical trial, Cellular therapy
Core Tip: In this review, we elaborate on the characteristics of neural stem cells (NSCs) and their effects on the treatment of traumatic brain injury, hypoxic-ischemic brain injury, Alzheimer’s disease and Parkinson’s disease. At the same time, we discuss the applications and limitations of NSCs to treat brain diseases.
INTRODUCTION
Brain diseases are among the major disorders influencing human health. The main types of brain diseases include brain tumors, neurodegenerative disorders, cerebrovascular diseases, and traumatic brain injury (TBI). Previous studies have suggested that repair and regeneration is a complex process and is challenging due to the following reasons: (1) Nerve cells, including neurons, are highly differentiated terminal cells, with very low regenerative capability; (2) Insufficient secretion of neurotrophic factors is unable to sustain the homeostasis of local environment results in the failure to repair damaged nerve system; and (3) Following injury, the secretion of inflammatory factors and various cytokines is upregulated, which inhibits synaptic regeneration and aggravates hypoxia and ischemia. The major cause of nerve regeneration disorders is the scar formation at the injuries, which may act as a physical and chemical barrier, suppress nerve regeneration, and dysregulate the extension and growth of synapses. Therefore, various physiological processes, including the supply of neurotrophic factors, regeneration of axons, plasticity of synapses, and the microenvironment, are involved in the repair and regeneration of the central nervous system (CNS) after injuries, and the underlying mechanisms are very complex.
Cellular therapy uses neurogenic or non-neurogenic cells to replace, repair, or improve the functions of the injured nerve system, which are implemented mainly through transplantation of cells into the system. Stem cell transplantation therapy has been widely applied in treating CNS diseases because of its ability of regeneration in nerve repair and tissue damage. The mechanisms underlying the treatment of brain diseases with stem cell transplantation are similar: facilitating the local microenvironment, promoting blood vessel development, supporting neuron regeneration, and reducing inflammatory responses. The commonly used stem cells include neural stem cells (NSCs), mesenchymal stem cells (MSCs), adipose mesenchymal stem cells, and human-derived umbilical cord blood stem cells, among which NSCs have been widely used and has unique advantages in the treatment of brain disease.
In this review, we discuss the role and generation of NSCs for various neurodegenerative disorders. Recent studies using different types of NSCs and transplantation approaches have been discussed in detail, and the limitations of NSCs for neurodegenerative disorders are also discussed.
BASIC CHARACTERISTICS OF NSCS
During development, the brain and spinal cord are generated from a small number of NSCs lining the neural tube. These cells are undifferentiated cells and can differentiate into different cells[1]. The subgranular zone (SGZ) of the dentate gyrus (DG) and subventricular zone (SVZ) in adult brains are two neurogenic regions for neurogenesis[2]. The neurogenic regions, especially the hippocampus, participate in cell renewal by developing new neurons from the neural progenitor cells[3]. Several sources can be used for NSCs. They can be collected from brain tissue, reprogrammed from somatic cells[4,5], or differentiated from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs)[6,7]. In addition, NSCs can differentiate into lineage-specific cells, such as neurons, oligodendrocytes and astrocytes[8]. They exist in highly-specific microenvironments, consisting of cell and extracellular components, such as ependymal cells, vasculature, extracellular matrix proteins, soluble factors, astrocytes, microglia, and pericytes[9,10]. Interaction of cells, transcription factors, neurotrophins, cytokines (such as growth factors, neurotransmitters, hormones and signaling molecules) have a crucial role in the proliferation and differentiation of NSCs. Cytokines (TNF-α) has been shown to induce proliferation of neural stem cells via IKK/NF-κB signaling. While BMP4/LIF has been shown to induce neuronal stem cells in monkeys, it was shown to induce astrocyte-like differentiation of monkey NSCs[11-14]. Neural stem cells are involved in various biological functions and continue to play their role throughout the lifespan of an organism. Both intra and extracellular signals regulate the functional properties of NSCs. Sox2 is one of the major regulators among transcription factors that serve as molecular switches[15]. The association of NSCs and migration in blood vessels were recently studied and shown that blood vessels play a significant role in neuronal migration during brain development. Moreover, NSCs can migrate to designated regions, such as injured regions, following injury[16].
Preclinical studies on treating brain diseases with NSCs have reported promising results, while clinical trials in patients are still ongoing. Nevertheless, experiments on animal models or in vitro studies have shown that NSCs may be induced and activated to differentiate into neurons, consequently replacing the lost neurons, improving the local microenvironment, promoting blood vessel development, regulating inflammatory responses, and restored homeostasis of the brain.
NSCS AND ALZHEIMER’S DISEASE
Alzheimer's disease (AD) is a progressive multifactorial brain disorder characterized by the amyloid-β (Aβ) deposition, as insoluble deposits or inclusions of proteins, accumulations of neurofibrillary tangles, and intracellular tau aggregation. It is the most common cause of dementia that slowly destroys memory and thinking skills. More than 26 million people are living with AD worldwide, and this number is expected to increase to 100 million over the next 35 years[17,18].
Targeting Aβ levels has been the central strategy to halt, retard, and reverse or cure AD pathology progression. Though great efforts have been made to cure AD symptoms and delay its progression, limited treatment options are available. Only four cholinesterase inhibitors (tacrine, donepezil, galantamine and rivastigmine are rarely prescribed due to its possible side effects) and NMDAR antagonists (memantine) have only been approved by United States Food and Drug Administration for AD. There is not a single drug approved in the last two decades. The available drugs (cholinesterase inhibitors), can only reduce the acetylcholinesterase activity to prevent the buildup of acetylcholine levels synaptic region. However, neither drug design to reverse the AD pathology nor immunotherapy that targets amyloid or Tau is the ultimate solution for Alzheimer's. Several lines of evidence have shown the successful approach of neural stem cells for the treatment of neurodegenerative disorders, including AD, amyotrophic lateral sclerosis and PD[19].
This approach of NSCs transplantation offers a tremendous therapeutic potential to cure neurodegenerative disorders based on its self-renewal ability and differentiate into neuronal, oligodendrocytes and astrocytes cells[20]. Tg2576 neural stem cells isolated from mice represent an Alzheimer disease model related to Aβ plaque. Tg2576 derived cells showed a disease model with reduced neuronal growth and MAP-2 expression. This model has been studied in various studies and offers to screen new molecules for the treatment of AD[21].
Ager et al[22] used NSCs derived from the fetal brain tissue and transplanted to the hippocampus of 3xTg-AD murine models and found that the transplanted NSCs improve the cognitive functions and enhanced synaptogenesis. The human neural stem cell population, HuCNS-SC, has been clinically tested before for different neurodegenerative disorders. Transplantation of HuCNS-SCs has been shown to improve cognition in two different models of neurodegeneration. Migration and differentiation of HuCNS-SC into immature neurons and glial cells were observed. Researchers have found the association of significant synaptic increase and other growth-associated markers were found in both 3xTg-AD and CaM/Tet-DTA mice models.
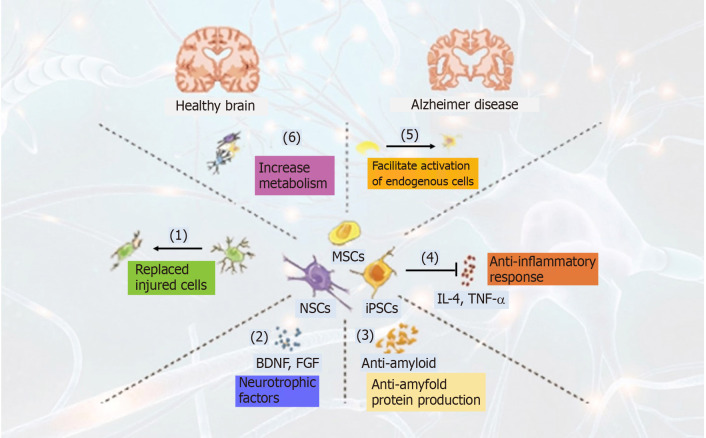
The hippocampus, which is critical for learning new memories, is normally affected at earlier stages of AD. Disruption of metabolic activities in hippocampal neurons has been demonstrated in earlier studies in AD[23]. The following diagram shows the different mechanisms of stem cells associated with AD (Figure 1).
Figure 1.
Mechanism of action of stem cells in Alzheimer disease. (1) Replaced injured or lost cells; (2) Enhanced secretion of neurotrophic factors (BDNF, GDNF, FGF, etc.); (3) Anti-amyloid protein production; (4) Inhibit inflammatory response; (5) Facilitate activation of endogenous cells; and (6) Enhanced metabolic activity of neuronal cells in the brain.
A study conducted by Li et al[24], 2016 showed that metabolic activity was increased in the frontal cortex and hippocampal neurons. The human brain-derived NSCs (hNSCs) were transplanted into the hippocampus transgenic mouse model of AD to assess the role of hNSC on behavior and Alzheimer’s pathology. Six weeks later, transplanted hNSCs migrated in different brain regions and slowly differentiated into neuronal cell types of CNS. These transplanted cells rescue AD symptoms, including cognitive defects, learning and memory impairment, by increasing neuronal connectivity and metabolic activity. This study suggests the role of hNSCs in modulating the metabolism of neuronal cells and validates the association between hippocampal neuronal metabolism and AD symptoms[24].
Chronic inflammation has a significant role and contributes to AD pathology in the brain. Transplantation of NSCs has been assessed to inhibit inflammatory processes. Researchers have shown that NSCs transplantation into the hippocampus attenuates inflammatory reactions and supports a neuroprotective role in beta-amyloid 42 (Aβ-42) peptide injected rat hippocampus, suggests an important role of NSCs in the inhibition of inflammatory reactions[25]. Neural stem cells are making a dominant appearance because of it neurogenic abilities, based on the recent findings that neurogenesis reduces significantly in AD patients compared to healthy subjects[26]. Progress is currently being made to differentiate the transplanted NSCs into cholinergic neurons, to compensate for the loss of injured neurons, the main research focuses on the treatment of AD.
A summary of preclinical studies of stem cells of different sources in rat and mice models of AD was showed in Table 1[27-37]. Ibotenic acid lesion or APP/PS1 transgenic mice were used in most of the AD model. Stem cells of different origin were used, which include rat, mouse and also from human. Genetically modified stem cells are also used in some studies, which have increased capacity to migrate from transplantation sites. Damage neuron replaced by transplanted stem cells. Stem cells migrate to the lesion site and differentiate to specific neurons e.g., cholinergic neuron, clear beta-amyloid, and produced anti-inflammatory effects. These studies showed that transplantation of stem cells (ECS-derived, NSCs, and MSCs) improved or restored learning and memory in AD-model rats.
Table 1.
Therapeutic potential of stem cell transplantation in Alzheimer’s disease models
|
|
Animal model
|
Transplanted cells
|
Density of transplanted cells
|
Transplantation site
|
Therapeutic effects
|
Unique features
|
Results
|
Ref.
|
| 1 | Mice (Transgenic 3 x Tg- AD and Thy1-APP) | NSCs | 100000 cells in 2 µL | Hippocampus | Aβ-clearance, increased synaptic density | Neprilysin gene transfer | Not assessed | Blurton-Jones et al[37] |
| 2 | Mouse (NBM lesion) | ESC-derived neurosphere | 400 µL/injection, 1-5 × 104 cells/µL | Prefrontal and parietal cortices | ChAT and serotonin-positive neurons | ChAT + cells↑ | Working memory ↑ | Wang et al[27] |
| 3 | Rat (Forebrain), Okadaic acid | NSC (rat) | 5 µL /injection site (2 injections) 2 × 104 cells/mL | Hippocampus and cerebral cortex | replace damaged or lost neuron | NGF(human), gene transfer | Memory ↑ | Wu et al[28] |
| 4 | Mice (Transgenic Tg2576) | MSCs from human UCB | 100000 cells/ Mouse (i.v.) | Systematic | Anti-inflammatory, anti-amyloidogenic | None | Not assessed | Nikolic et al[29] |
| 5 | Rat (NBM lesion) Ibotenic acid | ESC-derived NPC (mouse) | 2 × 105 cells in 2 µL | Forebrain specially NBM | Forming cholinergic cell phenotype | Shh-primed | Water maze↑; Spatial probe↑ | Moghadam et al[30] |
| 6 | Mouse (3X TG-AD) | NSC (mouse) | 100000 murine NSCs | Hippocampus | Neurotropic effects | BDNF-mediated effect | Working memory↑ | Blurton-Jones et al[31] |
| 7 | Rat (Hippocampus) Kainic acid | Immortalized NSC (human, HB1.F3) | 1 × 106 cells/rat | Hippocampal CA3 region | Migrate to injured site differentiate into neurons overexpressing ChAT | ChAT (human), gene transfer | Water maze↑; Spatial probe↑ | Park et al., 2012a[33] |
| 8 | Rat (NBM lesion) AF64A toxin | Immortalized NSC (human, HB1.F3) | 1 × 106 cells/rat | ICV | migrate to various brain regions including cerebral cortex and hippocampus | ChAT (human) gene transfer | Water maze↑; Spatial probe↑ | Park et al[32] |
| 9 | Mice (Transgenic APP/PS1) | MSCs from human UCB | 1 × 105 cells in 3 µL(3 injection once after 2 wk) | Hippocampus | Anti-inflammatory, anti-amyloidogenic, anti-phosphorylation of tau | None | Improved learning and memory | Lee et al[34] |
| 10 | Mouse (Hippocampus) Ibotenic acid | Immortalized NSC (human, HB1.F3) | 2 × 105 cell suspension 2 µL | Hippocampus | migrated to lesion sites and differentiated into neurons and astrocytes | NGF (human); Gene transfer | Water maze↑; Spatial probe↑ | Lee et al[35] |
| 11 | Mice (Transgenic APP/PS1) | MSCs from human UCB | 2 × 104 cells per head | Hippocampus, cortical region | Anti-inflammatory, Aβ-clearance | - | Not assessed | Kim et al[36] |
NBM: Nucleus basalis of Meynert; ESC: Embryonic stem cell; NGF: Nerve growth factor; 3XTG: Triple transgenic/APP-presenilin-tau; BDNF: Brain-derived growth factor; ChAT: Choline acetyltransferase; NPC: Neural precursor cell; NSC: Neural stem cell; SHH: Sonic hedgehog protein; UCB: Umbilical cord blood; Aβ: Beta-amyloid; MSCs: Mesenchymal stem cells; APP: Amyloid-β precursor protein; ICV: Intra-cerebro ventricular.
NSCS AND PARKINSON’S DISEASE
Parkinson’s disease (PD) is a complex neurodegenerative disease that result from the loss of dopaminergic neurons in the substantia nigra, pars compacta (SNc) and mesencephalon, and the formation of α-synuclein-containing Lewy bodies, which consequently induce motor disorders[38]. The stem cell approach offers a significant therapeutic output to a wide range of neurodegenerative disorders including PD, because of the regenerative potential to renew the cells and replace the affected cells. Several studies have reported using neural stem cell approach to find a cure and explore the disease mechanism.
Induced neural stem cells (iNSCs) exhibited different stem cell biomarkers with self-renewal properties and has shown the potential to differentiate into dopaminergic (DA) neurons. Researchers have shown the role of grafted cells for the neuronal network by assessing synaptic markers. Analysis of 4 wk of post-transplantation showed an extensive network of presynaptic neurons. hESC-derived neural cells has been reported to reduce the tumorigenicity and function of DA neurons in a prolonged mature culture. The transfer of such grafts in monkeys improved behavior for 12 mo period, reflecting the significance of matured hESCs that can act as a source for DA neurons[39].
Studies have shown that transplantation of iNSCs transformed from somatic cells into PD mice brains improves motor manifestation behavior. Wernig et al[40] shown that iPS cells efficiently differentiate into neural precursor cells, further giving rise to neuronal and glial cells. Transplantation of iNSCs into the brain of fetal mice shows the potential of stem cell migration into different brain regions and its differentiation into glia and neurons, including glutamatergic, catecholamines and GABaergic subtypes. Moreover, induced iPS cells were differentiated into DA neurons after transplantation into the adult brain.
Researchers have shown that steroli cells can be directly converted into iPS cells, which exhibit different stem cell biomarkers with self-renewal properties and can differentiate into DA neurons. These grafted cells were validated for a matured neuronal network by assessing synaptic markers. Analysis of 4 wk of post-transplantation showed an extensive network of presynaptic neurons, suggest a crucial role of steroli based iNSCs may provide a source of replacement of affected cells with new fresh cells[41]. iNSCs derived from fibroblasts have been shown to improve PD symptoms. Transplantation of iNSC into the 6-hydroxydopamine (6-OHDA)-injected mice striatum shows substantial reduction in apomorphine mediated rotational symmetry. The engrafted iNSCs show the differentiation pattern to all neuronal lineages and differentiate to DA neurons[42].
Yang et al[43] shows that neural stem cells transplantation into a 6-hydroxydopamine-lesioned rat, migrate to the striatum and express dopaminergic traits. Studies demonstrated the role of single factors, (Platelet-derived growth factor (DGF-AA), -AB, and –BB) which plays a role in the differentiation of primary stem cells derived from fetal and adult CNS, differentiate C17.2 cells in vitro, suggesting its significance that C17.2 NSCs lead to the development of dopaminergic neurons and a source for transplantation[44].
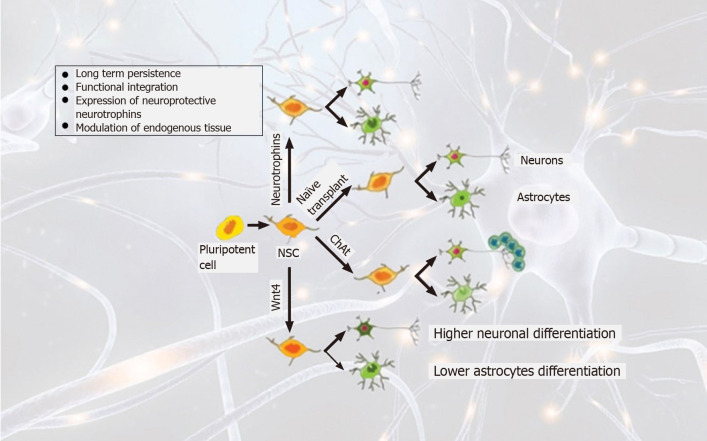
Nurr1 is a transcription factor and is specifically required to induce DA neurons in the midbrain region[45,46]. However, later in another separate study, Wagner et al[47] used the same stem cell line C17.2 and demonstrated that Nurr1 alone was unable to induce the differentiation of C17.2 cells into dopaminergic neurons. While, in a combination of other factors derived from local type 1 astrocytes, overexpression of Nurr1 in NSCs (C17.2) generates dopaminergic neurons (Figure 2).
Figure 2.
Overview of lineages of stem cells and transplantation strategies in Parkinson diseases. Pluripotent stem cells are directly converted to stem cells that can be further differentiated to long-term survival neurons by overexpressing neurotrophins. Wnt4 overexpression drives differentiation into neuronal cells while reducing glial scar formation.
A summary of preclinical studies of stem cells of different sources in rat, mice and monkey models of PD was showed in Table 2[42,48-54]. OHDA (rats and mice) and MPTP (monkey) drugs were used to create Parkinson’s model in these studies. Transplantation cells of different origin were used, which include rat, monkeys and from human. Genetically modified stem cells are also used in some studies, which had unique features. Results of these studies showed that transplantation of stem cells in different cell densities (ECS-derived, NSCs and MSCs) in striatum decreased rotation and improved motor function in PD model.
Table 2.
Therapeutic potentials of stem cell transplantation in Parkinson's disease models
|
|
Disease model
|
Source of transplanted cells
|
Transplantation location
|
Density of transplanted cells
|
Unique feature or treatment
|
Results
|
Ref.
|
| 1 | Rat, 6-OHDA | Immortalized NSC (mouse, C17-2) | Striatum | 106 cells | TH/GTPCH1; Gene transfer | Rotation↓ | Ryu et al[48] |
| 2 | Monkey, MPTP | ESC (monkey) | Bilateral putamen | 3 x 105-6 x 105 cells per side | Stromal cell (mouse) feeder | PFS-parkinsonian factor score↓ | Takagi et al[49] |
| 3 | Rat, 6-OHDA | Immortalized NSC (human, HB1.F3) | Striatum | 3 × 105 cells/3 μL | TH/GTPCH1 gene transfer | Rotation↓ | Kim et al[50] |
| 4 | Rat, 6-OHDA | Immortalized NSC (human, HB1.F3) | Striatum | 2 x 105/3µL | NSC migration | Rotation↓ | Yasuhara et al[51] |
| 5 | Rat, 6-OHDA | MSCs from human UCB | Striatum | 1 × 105 cells/10 µL | FGF8/SHH | Rotation↓ | Fu et al[52] |
| 6 | Rat, 6-OHDA | DA neurons from ESC (human) | Striatum | 5 x 105 cells | None | Rotation↓, beam walking↓ | Cho et al[53] |
| 7 | Mice, 6-OHDA | DA neurons from ESC (human) | Striatum | 1.5 × 105 cells /1.5 µL | Wnt signal; SHH | Rotation↓ | Kriks et al[54] |
| 8 | Mice, 6-OHDA | iNSCs (rat) | Striatum | 1 × 105 cells | Tripotential differentiation capacity | Rotation↓ | Choi et al[42] |
6-OHDA: 6-hydroxydopamine; MSC: Mesenchymal stem cell; ESC: Embryonic stem cell; FGF8: Fibroblast growth factor 8; GTPCH-1: GTP cyclohydrolyrase-1; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; iNSC: Induced neural stem cell; TH: Tyrosine hydrpxylase; NTN: Neurturin; UC: Umbilical cord blood; SHH: Sonic hedgehog protein; CN: Caudate nucleus; SN: Substantia nigra.
NSCS AND TBI
Traumatic brain injury (TBI) refers to a disruption of normal function of the brain and/or pathological injuries of brain tissues caused by external forces instead of disorders of brain tissues. TBI has a complex pathological condition, which includes breakage of the blood-brain barrier, massive neuroinflammation, axonal injury and lesions[55]. It has been estimated that about 50-60 million patients globally are newly diagnosed with TBI every year. In developing countries, TBI is mainly caused by traffic accidents, while in developed countries, by the falling of the elderly[56]. Based on the population census in 2013, TBI mortality rates in China were 13/100000, while in the 27 United States, TBI accounted for 30% of all trauma-induced deaths. In the United States, about 5.3 million individuals are living with TBI-related disabilities[57,58].
Despite having the higher frequency of TBI, a large proportion of molecular mechanisms and the basis of cognitive deficits and brain insults remain unknown.
Over the recent years, studies have demonstrated that neurogenesis in SVZ and SGZ was enhanced after TBI[59]. Endogenous NSCs get activated and migrate to regions of nerve injuries, which differentiate into neuroglial cells or oligodendrocytes and integrate into the injured local neurovascular network, promote the secretion of neurotrophic factors, and participate in nerve repair. Therefore, activating endogenous neurogenesis following TBI to contribute to post-injury functions may be a potential therapeutic approach[60,61]. On one hand, neurogenesis and nerve migration in human beings mainly exist in neonates younger than 18 mo but drastically decrease in adults, suggesting that neurogenesis following TBI in middle-aged and elderly people is substantially lower than in adolescents. While, glial scars have been reported to prevent the regeneration of axons and directly limit the repair of injuries in the late stage of TBI[62,63]. In addition, massive cell death and inflammatory responses in the late stage of TBI may disturb the local microenvironment, reduce the survival rate of new endogenous NSCs, and limit injury repair.
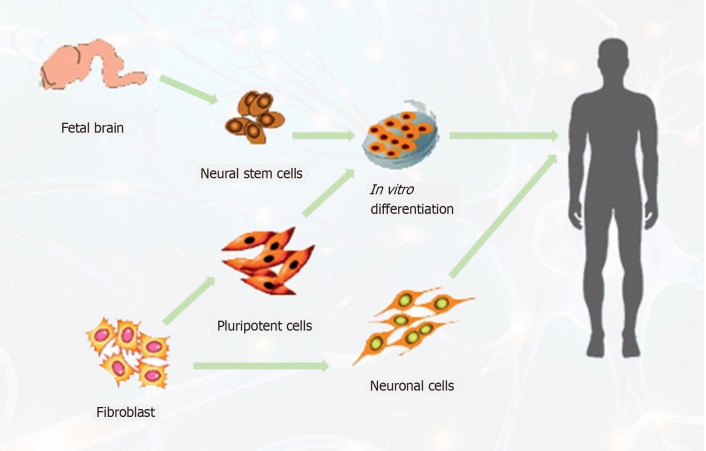
Transplantation of pre-differentiated human endogenous neural stem cells (ENSCs) has been reported to increase angiogenesis and neuronal survival in the lesion area and decrease astrogliosis, resulting in improved motor functions[64,65]. Moreover, researchers have shown that immediate transplantation of embryonic cortical neurons in the adult cortex after injury facilitates the restoration of injured motor pathways and supports the development of neuronal projections[66,67] (Figure 3).
Figure 3.
Schematic diagram of possible sources of neural stem cells to target stroke patients. (1) Neural stem cells from the fetal brain, differentiated to neuronal cells; (2) Neuronal cells directly generated from fibroblast cells, expanded to neuronal cells to replace the lost cells.
Exogenous NSC transplantation can compensate for the disadvantage of insufficient endogenous NSCs to a certain degree and has a significant impact on the treatment of TBI[68,69]. Experiments in mice and rats have been demonstrated that, upon NSCs transplantation, the transplanted stem cells survive in affected regions and differentiate into mature astrocytes, oligodendrocytes, and neurons, which can then be integrated into the neural circuit of the host to improve the injury-related cognitive and motor disorders[70,71]. When transferring human fetal NSCs to the hippocampus of TBI rats at 24 h post-injury, the transplanted cells survived. In addition, treating in vitro cultured NSCs with basic fibroblast growth factor, heparin, and laminin promote its differentiation into neurons at the injured area and the expression and secretion of glial-cell-line-derived neurotrophic factor in vivo from the transplanted cells, thus improving the internal environment of the brain, promoting the endogenous repair, and finally improving the cognitive functions of TBI rats[72]. The approach of cell therapy by transplanting ENSCs reduces neuroinflammation and supports neurogenesis in the adult injured cortex of the controlled cortical impact mouse model[69].
NSCS AND HYPOXIC ISHEMIC BRAIN INJURY
Cerebrovascular disease is a global health issue, where the incidence and mortality rate of ischemic stroke are high levels. Thrombolytic therapy is considered the best treatment procedure for ischemic stroke[73,74]. Though it is not safe and tissue damage is usually inevitable. It is a complex process, which involves oxidative damage and apoptosis of neurons[73,75].
The sub-ventricular zone and dentate gyrus are the primary sites of endogenous NSCs. Exogenous NSCs are mainly extracted from three main sources for therapeutic purposes: extraction from brain tissue, differentiation from IPSc, and trans-differentiation from somatic cells[76]. Studies have been reported the methods of generating different types of NSCs and its applications in neurodegenerative diseases[76,77]. The SVZ NSCs have been shown its association with glioma progression and its occurrence. Effect of conditioned medium derived from NSCs has confirmed its association with SVZ NSCs, and found that conditioned medium from NSCs promote the glioma proliferation and invasion[78]. Earlier studies reported the characteristics of exogenous NSCs that it can migrate into ischemic brain regions, and differentiate into neurons and glial cells and facilitate endogenous NSCs differentiation and proliferation[79-81]. Transplantation of human NSCs in a stroke model of rats showed neuroprotective effects by enhancing dendrites branching, increasing corticospinal tract projections and inhibited inflammation[82,83]. It has been demonstrated that NSCs improved the condition of stroke rats when transplanted, suggesting a role of NSCs mediated regulation of angiogenesis and formation of brain microvasculature because of increased activity of proangiogenic factors[84].
Researchers conducted a small Phase 1 translational study and demonstrate the role of CTCoE3 human NSCs in stroke patients. Upon implantation of human NSCs into the putamen, they found patients safe even for 2 years after transplantation and no side effects showed. However, a slight improvement showed in the NIH stroke scale[85]. The use of primary human tissue is limited because of the ethical and logistic complications to obtain large quantities of fetal neurons. Therefore, much effort is required to develop alternate sources of human cells for use in transplantation. One source is the NT2/D1 human embryonic carcinoma-derived cell line. These cells can proliferate and differentiate into human neuronal cells (LBS-Neurons) upon treatment with retinoic acid. These neuronal cells have been reported to survive, express neurotransmitters and regulate functional synapses.
Despite its significant role of NSCs in treating most neurodegenerative diseases, there are still some limitations. Modulation of cell dose is a critical factor, as low dose cannot provide therapeutic outcomes. While transplantation of high cell dose of tissue-derived NSCs can clot in vivo and may have a poor survival rate[2]. Furthermore, understanding molecular mechanisms of endogenous NSCs regulation largely remain unknown in patients with ischemic brain injury[86].
Due to the effectiveness of NSCs in animal models of cerebral stroke, clinical trials using NSCs have been conducted for the treatment of chronic ischemic cerebral stroke[87]. Although over 50 clinical trials have been registered for the treatment of cerebral stroke by stem cells, only human neural precursor cell line NT2/D1 and immortal human NSC line CTX have progressed to stage 1 and stage 2 phases. NT2/D1 cell, also known as NT2 cell, is a human teratoma-derived pluripotent embryonic carcinoma stem cell line, considered a neural precursor cell line. Treating NT2/D1 cells with tretinoin induces mitosis of anaphase neuron-like cell NT2N neuron (trade name: LBS-Neurons). A phase 1 clinical trial investigated the effects of NT2N neurons in basal ganglia stroke patients with severe motor disturbance. The 18-mo serum or imaging evaluations confirmed the safety and applicability of brain neuron transplantation in cerebral infarction patients with motor disturbance[88-90].
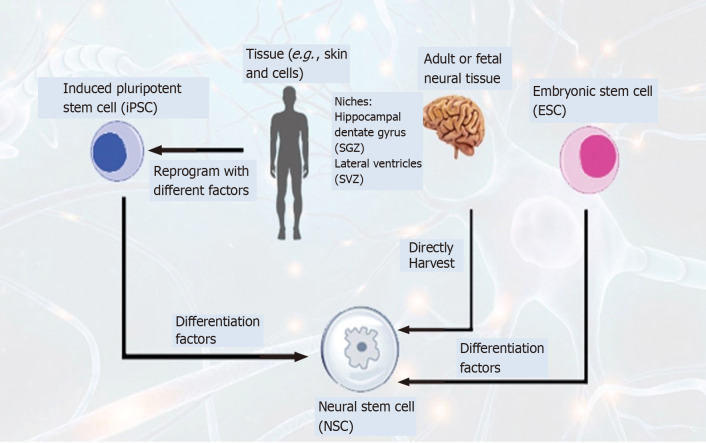
CTX0E03 is an immortalized human NSC line derived from human embryo brain tissues. CTX0E03 has been used as a clinical-grade NSC, based on which the commercial product CTX-DP was developed to treat chronic cerebral stroke (the ReNeuron PISCES trial)[90]. The 5-year follow up findings of phase 1 clinical trial of CTX0E03 in chronic cerebral stroke patients (PISCES I, NCT01151124) showed the following results: no immune or cell-related adverse events occurred, and only adverse influences from surgical procedures or complications were found; the overall NIHSS score improved by 2 points at 2 years after transplantation, which was associated with the improvement of neurological functions[85]. In another phase 2 clinical trial of CTX0E03 (PISCES II, NCT02117635), the 12-mo follow-up showed no cell-related safety events, while clinical related function improvement was found in 15 patients. CTX0E03 PISCESIII (NCT03629275), has already been approved, is a randomized, controlled, phase 2b clinical trial that aims to evaluate the safety and effectiveness of CTX cells in patients with chronic cerebral stroke (Figure 4).
Figure 4.
Schematic diagram of generation of neural stem cells via different methods to treat neurodegenerative disorders. Neural stem cells (NSCs) can be generated by extracting directly from the subgranular zone of the hippocampal dentate gyrus and subventricular zone of the lateral ventricles from fetal or adult brain. NSCs isolation from patients can be reprogrammed by using different factors such as transcription factors, small molecules, microRNAs, and other morphogens. NSCs can also be generated from blastocyst-derived embryonic stem cells by using differentiation factors. SGZ: Subgranular zone; SVZ: Subventricular zone.
CONCLUSION
The stem cells approach offers a significant output to a wide range of disorders, including neurodegenerative disorders, because of the regenerative potential to renew the cells and replace the affected cells. Neural stem cells are making a dominant appearance because of it neurogenic abilities, that neurogenesis reduces significantly in neurodegenerative patients compared to healthy subjects. Although studies on brain diseases with NSCs-based therapy are continuously increasing, and the NSC treatment strategy has provided an exciting and promising treatment method for brain diseases, there are still various uncertainties and potential risks involved in NSC transplantation, similar to the treatments with other stem cells: (1) Modulation of cell dose is a critical factor, as low dose is unable to provide the therapeutic outcomes. While transplantation of high cell dose of tissue derived NSCs can clot in vivo, and may have a poor survival rate; (2) Furthermore, understanding molecular mechanisms of endogenous NSCs regulation largely remain unknown in patients with neurodegenerative disorders; (3) Transplantation approaches can be improved by region specific regulation of local microenvironment in the brain: precise regulation of the microenvironment through genetic engineering techniques and combination transplantation may promote the proliferation and differentiation of transplanted NSCs, and greatly increase the treatment efficacy; and (4) Methods, timing, and doses of transplantation: strategies should be made to improve the transplantation methods to favor the aggregation of NSCs to the injured regions.
However, based on the shortcomings of various in vitro and in vivo neurodegenerative disease models, the translational effects of NSCs into human patients remains unknown. Thus, a more definite role of NSCs in various transplantation settings further needs to be explored. Many studies provided the evidence of the association of cognitive improvement with increase in synaptic activity, which is closely correlated with increase in neuronal and glial cells. NSCs transplantations supports behavioral and cognitive functions. Although specific cell types that associate with improvements, that NSCs need to differentiate into, remains unknown. The selection of the best time window for stem cell treatment is closely associated with the clinical prognosis of patients; however, thus far, no studies have reported the best treatment time window. The differentiation potential of NSCs derived from different sources may also vary, and how to determine the doses of transplanted cells is, therefore, an important issue for future research studies. There are still great challenges in preventing immunological rejection responses, improving the survival rate of transplanted NSCs, and consequently obtaining activated young stem cells with a clinically effective grade.
ACKNOWLEDGEMENTS
The authors would like to thank all the Tianjin Institution of Acupuncture and Moxibustion members who provided us with critical comments and assistance.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: February 27, 2021
First decision: May 5, 2021
Article in press: August 27, 2021
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lee YY, Li Y, Schmidt N S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Wang LYT
Contributor Information
Lan Zhao, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300381, China; National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300381, China. lanzhao69@163.com.
Jian-Wei Liu, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China.
Hui-Yan Shi, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300381, China; National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300381, China.
Ya-Min Ma, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin 300381, China; National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin 300381, China.
References
- 1.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang Y, Yu P, Cheng L. Current progress in the derivation and therapeutic application of neural stem cells. Cell Death Dis. 2017;8:e3108. doi: 10.1038/cddis.2017.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Boström E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisén J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahbazi E, Mirakhori F, Ezzatizadeh V, Baharvand H. Reprogramming of somatic cells to induced neural stem cells. Methods. 2018;133:21–28. doi: 10.1016/j.ymeth.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Hermann A, Storch A. Induced neural stem cells (iNSCs) in neurodegenerative diseases. J Neural Transm (Vienna) 2013;120 Suppl 1:S19–S25. doi: 10.1007/s00702-013-1042-9. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Morales PL, Revilla A, Ocaña I, González C, Sainz P, McGuire D, Liste I. Progress in stem cell therapy for major human neurological disorders. Stem Cell Rev Rep. 2013;9:685–699. doi: 10.1007/s12015-013-9443-6. [DOI] [PubMed] [Google Scholar]
- 7.Wen Y, Jin S. Production of neural stem cells from human pluripotent stem cells. J Biotechnol. 2014;188:122–129. doi: 10.1016/j.jbiotec.2014.07.453. [DOI] [PubMed] [Google Scholar]
- 8.Kahroba H, Ramezani B, Maadi H, Sadeghi MR, Jaberie H, Ramezani F. The role of Nrf2 in neural stem/progenitors cells: From maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing Res Rev. 2021;65:101211. doi: 10.1016/j.arr.2020.101211. [DOI] [PubMed] [Google Scholar]
- 9.Shabani Z, Ghadiri T, Karimipour M, Sadigh-Eteghad S, Mahmoudi J, Mehrad H, Farhoudi M. Modulatory properties of extracellular matrix glycosaminoglycans and proteoglycans on neural stem cells behavior: Highlights on regenerative potential and bioactivity. Int J Biol Macromol. 2021;171:366–381. doi: 10.1016/j.ijbiomac.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Marchetti B, Tirolo C, L'Episcopo F, Caniglia S, Testa N, Smith JA, Pluchino S, Serapide MF. Parkinson's disease, aging and adult neurogenesis: Wnt/β-catenin signalling as the key to unlock the mystery of endogenous brain repair. Aging Cell. 2020;19:e13101. doi: 10.1111/acel.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci. 2006;7:64. doi: 10.1186/1471-2202-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Yu L, Zhu LY, He H, Ren J, Pan J, Xie X, Cai C, Lu L, Tian H, Chen L, Zhang Y, Liu Y, Zhang C, Gao Z, Han XX. Cytokines Induce Monkey Neural Stem Cell Differentiation through Notch Signaling. Biomed Res Int. 2020;2020:1308526. doi: 10.1155/2020/1308526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 2013;23:935–942. doi: 10.1016/j.conb.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang GL, Zhu ZH, Wang YZ. Neural stem cell transplantation therapy for brain ischemic stroke: Review and perspectives. World J Stem Cells. 2019;11:817–830. doi: 10.4252/wjsc.v11.i10.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimozaki K. Sox2 transcription network acts as a molecular switch to regulate properties of neural stem cells. World J Stem Cells. 2014;6:485–490. doi: 10.4252/wjsc.v6.i4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujioka T, Kaneko N, Sawamoto K. Blood vessels as a scaffold for neuronal migration. Neurochem Int. 2019;126:69–73. doi: 10.1016/j.neuint.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 19.Anand R, Gill KD, Mahdi AA. Therapeutics of Alzheimer's disease: Past, present and future. Neuropharmacology. 2014;76 Pt A:27–50. doi: 10.1016/j.neuropharm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Rueger MA, Schroeter M. In vivo imaging of endogenous neural stem cells in the adult brain. World J Stem Cells. 2015;7:75–83. doi: 10.4252/wjsc.v7.i1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosacak MI, Bhattarai P, Kizil C. Alzheimer's disease, neural stem cells and neurogenesis: cellular phase at single-cell level. Neural Regen Res. 2020;15:824–827. doi: 10.4103/1673-5374.268896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ager RR, Davis JL, Agazaryan A, Benavente F, Poon WW, LaFerla FM, Blurton-Jones M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer's disease and neuronal loss. Hippocampus. 2015;25:813–826. doi: 10.1002/hipo.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salehi A, Swaab DF. Diminished neuronal metabolic activity in Alzheimer's disease. Review article. J Neural Transm (Vienna) 1999;106:955–986. doi: 10.1007/s007020050216. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Zhu H, Sun X, Zuo F, Lei J, Wang Z, Bao X, Wang R. Human Neural Stem Cell Transplantation Rescues Cognitive Defects in APP/PS1 Model of Alzheimer's Disease by Enhancing Neuronal Connectivity and Metabolic Activity. Front Aging Neurosci. 2016;8:282. doi: 10.3389/fnagi.2016.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu JK, Cho T, Wang YT, McLarnon JG. Neural progenitor cells attenuate inflammatory reactivity and neuronal loss in an animal model of inflamed AD brain. J Neuroinflammation. 2009;6:39. doi: 10.1186/1742-2094-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldassarro VA, Lizzo G, Paradisi M, Fernández M, Giardino L, Calzà L. Neural stem cells isolated from amyloid precursor protein-mutated mice for drug discovery. World J Stem Cells. 2013;5:229–237. doi: 10.4252/wjsc.v5.i4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Matsumoto Y, Shindo T, Miyake K, Shindo A, Kawanishi M, Kawai N, Tamiya T, Nagao S. Neural stem cells transplantation in cortex in a mouse model of Alzheimer's disease. J Med Invest. 2006;53:61–69. doi: 10.2152/jmi.53.61. [DOI] [PubMed] [Google Scholar]
- 28.Wu S, Sasaki A, Yoshimoto R, Kawahara Y, Manabe T, Kataoka K, Asashima M, Yuge L. Neural stem cells improve learning and memory in rats with Alzheimer's disease. Pathobiology. 2008;75:186–194. doi: 10.1159/000124979. [DOI] [PubMed] [Google Scholar]
- 29.Nikolic WV, Hou H, Town T, Zhu Y, Giunta B, Sanberg CD, Zeng J, Luo D, Ehrhart J, Mori T, Sanberg PR, Tan J. Peripherally administered human umbilical cord blood cells reduce parenchymal and vascular beta-amyloid deposits in Alzheimer mice. Stem Cells Dev. 2008;17:423–439. doi: 10.1089/scd.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moghadam FH, Alaie H, Karbalaie K, Tanhaei S, Nasr Esfahani MH, Baharvand H. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation. 2009;78:59–68. doi: 10.1016/j.diff.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park D, Joo SS, Kim TK, Lee SH, Kang H, Lee HJ, Lim I, Matsuo A, Tooyama I, Kim YB, Kim SU. Human neural stem cells overexpressing choline acetyltransferase restore cognitive function of kainic acid-induced learning and memory deficit animals. Cell Transplant. 2012;21:365–371. doi: 10.3727/096368911X586765. [DOI] [PubMed] [Google Scholar]
- 33.Park D, Lee HJ, Joo SS, Bae DK, Yang G, Yang YH, Lim I, Matsuo A, Tooyama I, Kim YB, Kim SU. Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Exp Neurol. 2012;234:521–526. doi: 10.1016/j.expneurol.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 34.Lee HJ, Lee JK, Lee H, Carter JE, Chang JW, Oh W, Yang YS, Suh JG, Lee BH, Jin HK, Bae JS. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer's disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33:588–602. doi: 10.1016/j.neurobiolaging.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Lee HJ, Lim IJ, Park SW, Kim YB, Ko Y, Kim SU. Human neural stem cells genetically modified to express human nerve growth factor (NGF) gene restore cognition in the mouse with ibotenic acid-induced cognitive dysfunction. Cell Transplant. 2012;21:2487–2496. doi: 10.3727/096368912X638964. [DOI] [PubMed] [Google Scholar]
- 36.Kim JY, Kim DH, Kim JH, Lee D, Jeon HB, Kwon SJ, Kim SM, Yoo YJ, Lee EH, Choi SJ, Seo SW, Lee JI, Na DL, Yang YS, Oh W, Chang JW. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell Death Differ. 2012;19:680–691. doi: 10.1038/cdd.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blurton-Jones M, Spencer B, Michael S, Castello NA, Agazaryan AA, Davis JL, Müller FJ, Loring JF, Masliah E, LaFerla FM. Neural stem cells genetically-modified to express neprilysin reduce pathology in Alzheimer transgenic models. Stem Cell Res Ther. 2014;5:46. doi: 10.1186/scrt440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burbulla LF, Krüger R. Converging environmental and genetic pathways in the pathogenesis of Parkinson's disease. J Neurol Sci. 2011;306:1–8. doi: 10.1016/j.jns.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Doi D, Morizane A, Kikuchi T, Onoe H, Hayashi T, Kawasaki T, Motono M, Sasai Y, Saiki H, Gomi M, Yoshikawa T, Hayashi H, Shinoyama M, Refaat MM, Suemori H, Miyamoto S, Takahashi J. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson's disease. Stem Cells. 2012;30:935–945. doi: 10.1002/stem.1060. [DOI] [PubMed] [Google Scholar]
- 40.Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheng C, Zheng Q, Wu J, Xu Z, Wang L, Li W, Zhang H, Zhao XY, Liu L, Wang Z, Guo C, Wu HJ, Liu Z, He S, Wang XJ, Chen Z, Zhou Q. Direct reprogramming of Sertoli cells into multipotent neural stem cells by defined factors. Cell Res. 2012;22:208–218. doi: 10.1038/cr.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi DH, Kim JH, Kim SM, Kang K, Han DW, Lee J. Therapeutic Potential of Induced Neural Stem Cells for Parkinson's Disease. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang M, Stull ND, Berk MA, Snyder EY, Iacovitti L. Neural stem cells spontaneously express dopaminergic traits after transplantation into the intact or 6-hydroxydopamine-lesioned rat. Exp Neurol. 2002;177:50–60. doi: 10.1006/exnr.2002.7989. [DOI] [PubMed] [Google Scholar]
- 44.Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 45.Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, Cox JJ, De Mayo F, Burbach JP, Conneely OM. Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci U S A. 1998;95:4013–4018. doi: 10.1073/pnas.95.7.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zetterström RH, Solomin L, Jansson L, Hoffer BJ, Olson L, Perlmann T. Dopamine neuron agenesis in Nurr1-deficient mice. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 47.Wagner J, Akerud P, Castro DS, Holm PC, Canals JM, Snyder EY, Perlmann T, Arenas E. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nat Biotechnol. 1999;17:653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- 48.Ryu MY, Lee MA, Ahn YH, Kim KS, Yoon SH, Snyder EY, Cho KG, Kim SU. Brain transplantation of neural stem cells cotransduced with tyrosine hydroxylase and GTP cyclohydrolase 1 in Parkinsonian rats. Cell Transplant. 2005;14:193–202. doi: 10.3727/000000005783983133. [DOI] [PubMed] [Google Scholar]
- 49.Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115:102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim SU, Park IH, Kim TH, Kim KS, Choi HB, Hong SH, Bang JH, Lee MA, Joo IS, Lee CS, Kim YS. Brain transplantation of human neural stem cells transduced with tyrosine hydroxylase and GTP cyclohydrolase 1 provides functional improvement in animal models of Parkinson disease. Neuropathology. 2006;26:129–140. doi: 10.1111/j.1440-1789.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 51.Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, Kim SU, Borlongan CV. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease. J Neurosci. 2006;26:12497–12511. doi: 10.1523/JNEUROSCI.3719-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 53.Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim MH, Kim JH, Leem JW, Oh SK, Choi YM, Hwang DY, Chang JW, Kim DW. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiong Y, Mahmood A, Lu D, Qu C, Kazmi H, Goussev A, Zhang ZG, Noguchi CT, Schallert T, Chopp M. Histological and functional outcomes after traumatic brain injury in mice null for the erythropoietin receptor in the central nervous system. Brain Res. 2008;1230:247–257. doi: 10.1016/j.brainres.2008.06.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Büki A, Chesnut RM, Citerio G, Coburn M, Cooper DJ, Crowder AT, Czeiter E, Czosnyka M, Diaz-Arrastia R, Dreier JP, Duhaime AC, Ercole A, van Essen TA, Feigin VL, Gao G, Giacino J, Gonzalez-Lara LE, Gruen RL, Gupta D, Hartings JA, Hill S, Jiang JY, Ketharanathan N, Kompanje EJO, Lanyon L, Laureys S, Lecky F, Levin H, Lingsma HF, Maegele M, Majdan M, Manley G, Marsteller J, Mascia L, McFadyen C, Mondello S, Newcombe V, Palotie A, Parizel PM, Peul W, Piercy J, Polinder S, Puybasset L, Rasmussen TE, Rossaint R, Smielewski P, Söderberg J, Stanworth SJ, Stein MB, von Steinbüchel N, Stewart W, Steyerberg EW, Stocchetti N, Synnot A, Te Ao B, Tenovuo O, Theadom A, Tibboel D, Videtta W, Wang KKW, Williams WH, Wilson L, Yaffe K InTBIR Participants and Investigators. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 57.Cheng P, Yin P, Ning P, Wang L, Cheng X, Liu Y, Schwebel DC, Liu J, Qi J, Hu G, Zhou M. Trends in traumatic brain injury mortality in China, 2006-2013: A population-based longitudinal study. PLoS Med. 2017;14:e1002332. doi: 10.1371/journal.pmed.1002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9:231–236. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- 59.Clark LR, Yun S, Acquah NK, Kumar PL, Metheny HE, Paixao RCC, Cohen AS, Eisch AJ. Mild Traumatic Brain Injury Induces Transient, Sequential Increases in Proliferation, Neuroblasts/Immature Neurons, and Cell Survival: A Time Course Study in the Male Mouse Dentate Gyrus. Front Neurosci. 2020;14:612749. doi: 10.3389/fnins.2020.612749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- 61.Goings GE, Sahni V, Szele FG. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 2004;996:213–226. doi: 10.1016/j.brainres.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 62.Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kernie SG, Erwin TM, Parada LF. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res. 2001;66:317–326. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- 64.Skardelly M, Gaber K, Burdack S, Scheidt F, Hilbig H, Boltze J, Förschler A, Schwarz S, Schwarz J, Meixensberger J, Schuhmann MU. Long-term benefit of human fetal neuronal progenitor cell transplantation in a clinically adapted model after traumatic brain injury. J Neurotrauma. 2011;28:401–414. doi: 10.1089/neu.2010.1526. [DOI] [PubMed] [Google Scholar]
- 65.Skardelly M, Gaber K, Burdack S, Scheidt F, Schuhmann MU, Hilbig H, Meixensberger J, Boltze J. Transient but not permanent benefit of neuronal progenitor cell therapy after traumatic brain injury: potential causes and translational consequences. Front Cell Neurosci. 2014;8:318. doi: 10.3389/fncel.2014.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gaillard A, Prestoz L, Dumartin B, Cantereau A, Morel F, Roger M, Jaber M. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat Neurosci. 2007;10:1294–1299. doi: 10.1038/nn1970. [DOI] [PubMed] [Google Scholar]
- 67.Singec I, Snyder EY. Quo vadis brain repair? Cell Stem Cell. 2007;1:355–356. doi: 10.1016/j.stem.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 68.Luo ML, Pan L, Wang L, Wang HY, Li S, Long ZY, Zeng L, Liu Y. Transplantation of NSCs Promotes the Recovery of Cognitive Functions by Regulating Neurotransmitters in Rats with Traumatic Brain Injury. Neurochem Res. 2019;44:2765–2775. doi: 10.1007/s11064-019-02897-z. [DOI] [PubMed] [Google Scholar]
- 69.Nasser M, Ballout N, Mantash S, Bejjani F, Najdi F, Ramadan N, Soueid J, Zibara K, Kobeissy F. Transplantation of Embryonic Neural Stem Cells and Differentiated Cells in a Controlled Cortical Impact (CCI) Model of Adult Mouse Somatosensory Cortex. Front Neurol. 2018;9:895. doi: 10.3389/fneur.2018.00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun D, Gugliotta M, Rolfe A, Reid W, McQuiston AR, Hu W, Young H. Sustained survival and maturation of adult neural stem/progenitor cells after transplantation into the injured brain. J Neurotrauma. 2011;28:961–972. doi: 10.1089/neu.2010.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin GQ, He XF, Liang FY, Guo Y, Sunnassee G, Chen J, Cao XM, Chen YY, Pan GJ, Pei Z, Tan S. Transplanted human neural precursor cells integrate into the host neural circuit and ameliorate neurological deficits in a mouse model of traumatic brain injury. Neurosci Lett. 2018;674:11–17. doi: 10.1016/j.neulet.2018.02.064. [DOI] [PubMed] [Google Scholar]
- 72.Gao J, Prough DS, McAdoo DJ, Grady JJ, Parsley MO, Ma L, Tarensenko YI, Wu P. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp Neurol. 2006;201:281–292. doi: 10.1016/j.expneurol.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 73.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/Reperfusion. Compr Physiol. 2016;7:113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thornton C, Baburamani AA, Kichev A, Hagberg H. Oxidative stress and endoplasmic reticulum (ER) stress in the development of neonatal hypoxic-ischaemic brain injury. Biochem Soc Trans. 2017;45:1067–1076. doi: 10.1042/BST20170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suksuphew S, Noisa P. Neural stem cells could serve as a therapeutic material for age-related neurodegenerative diseases. World J Stem Cells. 2015;7:502–511. doi: 10.4252/wjsc.v7.i2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otsu M, Nakayama T, Inoue N. Pluripotent stem cell-derived neural stem cells: From basic research to applications. World J Stem Cells. 2014;6:651–657. doi: 10.4252/wjsc.v6.i5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang GL, Qian C, Zhang SZ, Tuo YH, Zeng BY, Ji YX, Wang YZ. Effect of conditioned medium from neural stem cells on glioma progression and its protein expression profile analysis. World J Stem Cells. 2020;12:1396–1409. doi: 10.4252/wjsc.v12.i11.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng Y, Zhang J, Deng L, Johnson NR, Yu X, Zhang N, Lou T, Zhang Y, Wei X, Chen Z, He S, Li X, Xiao J. Intravenously delivered neural stem cells migrate into ischemic brain, differentiate and improve functional recovery after transient ischemic stroke in adult rats. Int J Clin Exp Pathol. 2015;8:2928–2936. [PMC free article] [PubMed] [Google Scholar]
- 80.Ryu S, Lee SH, Kim SU, Yoon BW. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural Regen Res. 2016;11:298–304. doi: 10.4103/1673-5374.177739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang L, Zhang L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog Neurobiol. 2019;173:1–17. doi: 10.1016/j.pneurobio.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, Schaar BT, Svendsen CN, Bliss TM, Steinberg GK. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134:1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- 84.Hicks C, Stevanato L, Stroemer RP, Tang E, Richardson S, Sinden JD. In vivo and in vitro characterization of the angiogenic effect of CTX0E03 human neural stem cells. Cell Transplant. 2013;22:1541–1552. doi: 10.3727/096368912X657936. [DOI] [PubMed] [Google Scholar]
- 85.Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388:787–796. doi: 10.1016/S0140-6736(16)30513-X. [DOI] [PubMed] [Google Scholar]
- 86.Zhang R, Zhang Z, Chopp M. Function of neural stem cells in ischemic brain repair processes. J Cereb Blood Flow Metab. 2016;36:2034–2043. doi: 10.1177/0271678X16674487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Othman FA, Tan SC. Preconditioning Strategies to Enhance Neural Stem Cell-Based Therapy for Ischemic Stroke. Brain Sci. 2020;10 doi: 10.3390/brainsci10110893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nelson PT, Kondziolka D, Wechsler L, Goldstein S, Gebel J, DeCesare S, Elder EM, Zhang PJ, Jacobs A, McGrogan M, Lee VM, Trojanowski JQ. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am J Pathol. 2002;160:1201–1206. doi: 10.1016/S0002-9440(10)62546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J, Decesare S, Jovin T, Zafonte R, Lebowitz J, Flickinger JC, Tong D, Marks MP, Jamieson C, Luu D, Bell-Stephens T, Teraoka J. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg. 2005;103:38–45. doi: 10.3171/jns.2005.103.1.0038. [DOI] [PubMed] [Google Scholar]
- 90.Wechsler LR, Bates D, Stroemer P, Andrews-Zwilling YS, Aizman I. Cell Therapy for Chronic Stroke. Stroke. 2018;49:1066–1074. doi: 10.1161/STROKEAHA.117.018290. [DOI] [PubMed] [Google Scholar]