Abstract
Background
The neutrophil-lymphocyte ratio (NLR) is easily calculated blood test parameter, which can be used as marker to predict many inflammatory disorders. The aim of this study was to assess and compare the NLR in maternal blood with the white blood cell (WBC) count and C-reactive protein (CRP) concentration for the prediction of histological chorioamnionitis.
Methods
This was a case-control study of 137 woman with preterm premature rupture of membranes (PPROM) at a gestational age between 22+ 0 and 34+ 6 weeks. Blood samples, collected less than 48 h before delivery and at least 48 h after the administration of corticosteroids, were selected for the analysis. The NLR was calculated by dividing the number of neutrophils by the number of lymphocytes. Chorioamnionitis was diagnosed by the histopathological evaluation of placental membranes and chorionic plate.
Results
Patients with diagnosed histological chorioamnionitis (HCA) had significantly higher levels of WBC, CRP and NLR (p-value < 0.001). Levels of WBC, CRP and NLR predicted HCA with an area under the curve (AUC) of 0.81, 0.81 and 0.89, respectively. NLR had statistically significantly higher AUC than WBC, but no significant difference was found between AUCs of NLR and CRP. The cut-off level of NLR was found to be 5,97, which had a sensitivity of 77 % and a specificity of 95 %.
Conclusion
NLR has a good predictive value for HCA and could be used as an additional diagnostic marker for predicting histological chorioamnionitis in cases with preterm premature rupture of membranes before 34 weeks of gestation.
Keywords: chorioamnionitis, preterm premature rupture of membranes, neutrophil-lymphocyte ratio, white blood cell, C-reactive protein
Background
Preterm premature rupture of the membranes (PPROM) is the spontaneous rupture of foetal membranes during pregnancy prior to 37 weeks of gestation. One of the most serious complications of PPROM is chorioamnionitis, which complicates almost half of all PPROM cases [1] and is associated with increased risks of neonatal morbidity and mortality [2, 3]. Induction of labour is the most effective intervention for protecting the foetus against intrauterine infection, but preterm delivery, particularly before 32 weeks of gestation, is associated with high risks of neonatal morbidity and mortality because of foetal immaturity [3, 4]. Thus, when choosing the method of PPROM management, the benefits of pregnancy prolongation and the risk of chorioamnionitis must be balanced.
The white blood cells (WBC) and C-reactive protein (CRP) are inflammatory markers used worldwide for the early diagnosis of chorioamnionitis. A systemic review and meta-analysis performed by Sabogal with colleagues demonstrated the low sensitivity (51 %) and specificity (65 %) of leucocytosis for the diagnosis of histologic chorioamnionitis (HCA) [5]. Another systemic review by Trochez-Martinez and colleagues showed controversial results for the prediction value of CRP for chorioamnionitis [6]. Other serum inflammatory markers, such as soluble intercellular adhesion molecule-1, interleukin-6, matrix metalloproteinase-9, tissue inhibitor of metalloproteinases-1, angiopoietin-2, and insulin like growth factor binding protein-2 have also been found to be associated with chorioamnionitis, but these laboratory tests are not performed routinely [7–9].
The neutrophil–lymphocyte ratio (NLR) is a marker of systemic inflammation that is calculated by dividing the absolute neutrophilic count by the absolute lymphocyte count [10]. The NLR may be used to diagnose various diseases, such as congenital infections and COVID-19 infection and as the diagnostic and prognostic tool for sepsis [10–12]. It is an inexpensive, universal biomarker derived from a routinely performed complete blood count.
The aim of this study was to assess and compare the NLR in maternal blood with the WBC and CRP concentration to determine the best maternal blood inflammatory marker for predicting HCA.
Materials and methods
This case–control study included 185 patients who were diagnosed with PPROM prior to 34 weeks of gestation and were admitted to Vilnius University Hospital Santaros Klinikos between July 2017 and July 2019. The study was approved by the Vilnius Regional Biomedical Research Ethics Committee (2017-07-04 No. 158200-17-931-434), and all participants signed an informed consent form before enrolment.
The inclusion criteria were as follows: (1) maternal age ≥ 18 years; (2) singleton gestation; (3) gestational age 22+ 0–33+ 6 weeks; (4) diagnosed with PPROM; and (5) the absence of a maternal hypertensive disorder, gestation diabetes and intrahepatic cholestasis. Exclusion criteria included (1) multiple gestations; and (2) foetal malformations.
Clinical characteristics such as maternal age, gravidity, parity, gestational age, newborn birthweight, Apgar score, and umbilical cord arterial pH were retrieved from the patients’ medical records.
According to the institution’s standard protocol, all patients with diagnosed PPROM prior to 34 weeks of gestation were managed with antibiotics, a single course of antenatal corticosteroids, and tocolytics. Intravenous ampicillin 2 g and oral erythromycin 250 mg every 6 h were used for 48 h. The patients were then placed on oral amoxicillin 500 mg every 8 h and erythromycin 250 mg every 6 h to complete a 7-day course of antibiotic therapy. Two doses of 12 mg of dexamethasone given intramuscularly 12 h apart were used for accelerating fetal lung maturation. We analysed blood samples collected 24–48 h before delivery. Additionally, due to the presence of corticosteroid-induced leucocytosis, we only analysed blood samples that were collected at least 48 h after the administration of corticosteroids. Thus, 137 participants were included in the final analysis (Fig.1).
According to the institution’s standard protocol, all postpartum placentas were examined histologically after preterm delivery. Classification of placental lesions was based on Amsterdam Placental Workshop Group criteria [13], grading and staging of the inflammation was performed according to the diagnostic criteria proposed by the Perinatal Section of the Society for Pediatric Pathology [14].The occurrence of histological chorioamnionitis was determined based on the presence of maternal neutrophil infiltration in the amnion, chorion, and parietal decidua. Based on the histological analysis, patients were grouped into the histological chorioamnionitis group (Group I), when acute placental infectious inflammatory lesions were found, or the control group without these histological changes (Group II).
Data analysis was performed using R package (version 4.0.3) (R Core Team, 2020). The distribution of the data was determined by the Shapiro–Wilk test. All continuous variables were not normally distributed and are presented as the medians and interquartile ranges (IQRs). The categorical data are expressed as frequencies and percentages. Differences were compared between the two groups using the Mann–Whitney U test for continuous variables and the Pearson Chi-square test for categorical variables. Receiver operating characteristic (ROC) curves were constructed to estimate the ability of variables to differentiate between the groups. The area under the curve (AUC) was calculated to indicate the average sensitivity of a marker over the entire ROC curve. The DeLong test was used to compare the AUCs of the different models. The best cut-off values to predict the outcome were determined by the Youden index. A multivariate logistic regression analysis was performed to evaluate independent prognostic factors associated with HCA. p-Values of < 0.05 were considered statistically significant for all tests.
Results
The study included 137 patients with preterm premature rupture of the membranes prior to 34 weeks of gestation: 52 patients in Group I and 85 patients in Group II. The clinical characteristics of the patients and their distribution within the groups are listed in Table 1. Maternal age, gravidity, parity, gestational age, neonatal birthweight, and umbilical cord arterial pH were similar between groups and did not differ statistically. Newborns in Group I, where histological chorioamnionitis was diagnosed, had lower Apgar scores (p-value = 0.01). Also, the time from steroid administration to delivery was longer in Group I than in Group II (p-value = 0.008).
Table 1.
Characteristics of Patients According to the Groups. PPROM, preterm premature rupture of the membranes; Group I, patients with diagnosed histological chorioamnionitis; Group II, patients without diagnosed histological chorioamnionitis
| Characteristics | Group I (n = 52) | Group II (n = 85) | p-value |
|---|---|---|---|
| Age of mother (years) | 31 (28–34) | 31 (27–35) | 0.89 |
| Primigravida, n (%) | 18 (34.6) | 36 (42.4) | 0.34 |
| Multigravida, n (%) | 34 (65.4) | 49 (57.6) | 0.34 |
| Primiparous, n (%) | 23 (44.2) | 46 (54.1) | 0.26 |
| Multiparous, n (%) | 29 (55.8) | 39 (45.9) | 0.26 |
| Gestational age at birth (weeks) | 32 (27–34) | 33 (31–34) | 0.06 |
| Birthweight (g) | 1770 (1170–2203) | 1925 (1510–2310) | 0.12 |
| Apgar score < 7 at 5 min, n (%) | 6 (11.5) | 1 (1.2) | 0.01 |
| Umbilical cord arterial pH | 7.35 (7.29–7.4) | 7.32 (7.26–7.37) | 0.08 |
| Clinical chorioamnionitis, n (%) | 4 (7.7) | 1 (1.2) | 0.05 |
| Latency between PPROM and delivery (hours) | 148.3 (91.1-246.5) | 93.5 (65.1-166.05) | 0.007 |
| Time from steroid administration to delivery (hours) | 134.5 (78.05–233) | 89 (52.01–152) | 0.008 |
The white blood cell (WBC), neutrophil and lymphocyte counts, C-reactive protein (CRP) concentration, and the NLR are shown in Table 2. The WBC and neutrophil counts, and CRP concentration were statistically significantly higher in Group I than in Group II. The NLR was also higher in Group I, whereas the level of lymphocytes was lower in Group I than in Group II.
Table 2.
The levels of blood inflammatory markers. Data are presented as medians (interquartile ranges). Group I, patients with diagnosed histological chorioamnionitis; Group II, patients without diagnosed histological chorioamnionitis
| Blood inflammatory marker | Group I (n = 52) | Group II (n = 85) | p-value |
|---|---|---|---|
| White blood cells (cells x 109/L) | 14.58 (12.56–16.6) | 10.68 (8.78–12.57) | < 0.001 |
| Neutrophils (cells x 109/L) | 12 (9.75–13.9) | 7.5 (6.1–9.44) | < 0.001 |
| Lymphocytes (cells x 109/L) | 1.5 (1.13–1.9) | 2 (1.6–2.4) | < 0.001 |
| C-reactive protein (mg/L) | 15.5 (8.71–38.38) | 3.66 (2.23–6.73) | < 0.001 |
| Neutrophil–lymphocyte ratio | 8.01 (6.18–9.72) | 4.09 (3.3–4.93) | < 0.001 |
To assess the relative importance of blood inflammatory markers in the prediction of HCA, we performed a multiple logistic regression analysis. CRP and NLR were found to be the independent variables and were significantly associated with the occurrence of HCA (Table 3).
Table 3.
Adjusted risk factors for the occurrence of histological chorioamnionitis in patients with preterm rupture of the membranes prior to 34 weeks of gestation. CI, confidence interval
| Blood inflammatory markers | Adjusted odds ratio (95 % CI) | p-value |
|---|---|---|
| White blood cells | 1.84 (0.35–9.7) | 0.47 |
| Neutrophils | 1.69 (0.06–2.77) | 0.35 |
| Lymphocytes | 0.21 (0.41–61.49) | 0.21 |
| C-reactive protein | 1.07 (1.03–1.1) | < 0.001 |
| Neutrophil-lymphocyte ratio | 2.59 (1.85–3.62) | < 0.001 |
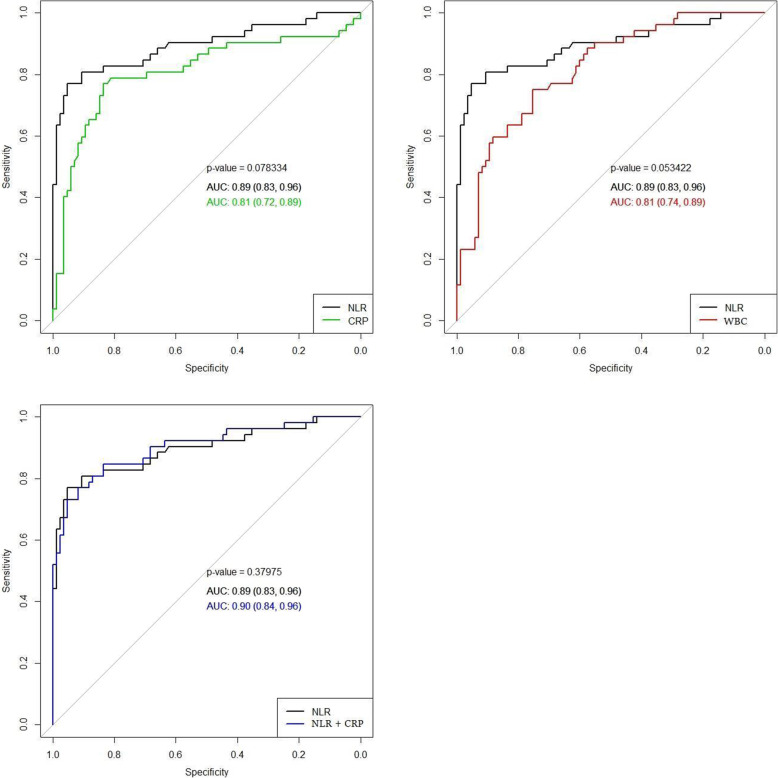
ROC curves were constructed to determine the ability of CRP, WBC and NLR to differentiate between groups. Figure 1 shows a comparison of the ROC curves for the prediction of histological chorioamnionitis. CRP and NLR predicted the occurrence of histological chorioamnionitis with AUCs of 0.81 and 0.89, respectively. The DeLong test demonstrated no statistically significant difference between the ROC curves of CRP and NLR (p-value = 0.08). Additionally, we constructed a ROC curve of the WBC and compared it with the ROC curve of the NLR. The results were approaching an acceptable significance level (p-value = 0.053). Moreover, we assessed the effectiveness of CRP as an additional diagnostic marker to NLR for predicting the occurrence of HCA. This model predicted the occurrence of HCA with an AUC of 0.9 but did not differ significantly from the AUC of NLR alone (p-value = 0.38).
Fig. 1.
Comparison of receiver operating characteristic (ROC) curves of blood inflammatory markers. NLR, neutrophil–lymphocyte ratio; CRP, C-reactive protein; WBC, white blood cells; AUC, area under the curve
The optimum cut-off values for CRP, WBC and NLR were identified using the Youden index. Regarding the prediction of histological chorioamnionitis, the cut-off value was found to be 8.5 mg/L for CRP, 12.62 × 109/L for WBC and 5.97 for NLR. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the markers are demonstrated in Table 4.
Table 4.
Diagnostic indices of C-reactive protein, white blood cell count, and the neutrophil–lymphocyte ratio in study subjects. CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value
| Blood inflammatory markers | Cut-off value | Sensitivity % (95 % CI) |
Specificity % (95 % CI) |
PPV % (95 % CI) | NPV % (95 % CI) |
|---|---|---|---|---|---|
| C-reactive protein | 8.5 | 84 (74–91) | 77 (63–87) | 74 (60–85) | 86 (76–92) |
| White blood cells | 12.62 × 109 | 75 (65–84) | 75 (61–86) | 65 (65–77) | 83 (73–91) |
| Neutrophil–lymphocyte ratio | 5.97 | 77 (63–97) | 95 (88–99) | 91 (78–97) | 87 (79–93) |
Discussion
The NLR has been proposed as an additional infection marker and a potential parameter for predicting bacterial infection. The NLR increases following the progression of inflammatory disease: chemokines release neutrophils from the bone marrow and increase their life span in the blood [15, 16], while increased levels of adrenocorticotropic hormone, cortisol, catecholamines, and corticosteroids reduce the lymphocyte count [17].
Our study demonstrates that an increased NLR is an appropriate indicator for the prenatal diagnosis of HCA. According to the ROC curve analysis, the prognostic value of NLR does not differ significantly from that of the CRP, but it has greater prognostic value than the WBC and may be used as additional marker to predict HCA. The optimal NLR cut-off value to predict HCA was found to be 5.97 with a sensitivity of 77 % and a specificity of 95 %.
To our knowledge, other published studies on the use of the NLR in the prediction of chorioamnionitis have been sporadic [18, 19]. Kim and colleagues evaluated the predictive value of the NLR in the placental inflammatory response and found that the NLR had a better diagnostic value than the maternal serum CRP or WBC counts. The optimal NLR cut-off value to predict the placental inflammatory response was found to be 6.48, similar to our result, but that study included all patients who underwent a preterm delivery between 24 and 37 weeks of gestation. Additionally, those authors showed that patients with a high NLR were at risk of impending preterm delivery in the context of a normal CRP level, and NLR together with CRP can help to predict poor pregnancy outcomes in patients with a placental inflammatory response [18].
Recent studies focused on the ability of the NLR to predict preterm delivery. Kim and colleagues found that a combined model consisting of cervical length and NLR had a higher predictive value for spontaneous preterm delivery than using cervical length alone or other inflammatory markers such as CRP and WBC [20]. A study by Akgun et al. demonstrated that an elevated NLR is associated with preterm delivery and lower birthweight. The authors hypothesized that an elevated NLR would be affected by the maternal hyperinflammatory state that led to foetal growth restriction and early initiation of delivery [21].
Patients with an imminent preterm delivery prior to 34 weeks of gestation receive corticosteroid therapy to accelerate foetal lung development. Corticosteroids are known to increase the WBC and predominantly the neutrophil count. The biological effects that contribute to the increase in neutrophils are the release of immature neutrophils from the bone marrow into the circulation, the demargination of neutrophils from the endovascular lining, the delayed migration of neutrophils into tissue, and the lower rate of apoptosis [22–24]. Leucocytosis is induced for up to 24–48 h after corticosteroid administration [25]. Thus, the NLR is a misleading predictive factor of infection during this period.
The NLR changes during some pregnancy-related disorders. The NLR may increase in preeclampsia, gestational diabetes mellitus, and intrahepatic cholestasis [26–28]. Furthermore, Orgul and colleagues evaluated how the NLR changes in pregnant women who are administrated magnesium sulfate for foetal neuroprotection and found that the NLR increased 6 h after starting magnesium sulfate [29]. Thus, the NLR should be evaluated carefully in the presence of pregnancy-related disorders, such as preeclampsia, gestational diabetes mellitus, and intrahepatic cholestasis and in cases where magnesium sulfate treatment occurs.
Practical recommendation
Our clinical expectation is that the NLR together with other maternal blood inflammatory markers will improve the prediction of histological chorioamnionitis. NLR analysis is simple, inexpensive, and easily obtained.
Strengths and limitations
To the best of our knowledge, this is the first study to evaluate the NLR for the prediction of HCA in patients with PPROM prior to 34 weeks of gestation. Additionally, this study excluded blood samples affected by the administration of corticosteroids and cases with preeclampsia, gestational diabetes mellitus, and intrahepatic cholestasis. The limitation of our study is its retrospective design. Further prospective cohort studies are required to evaluate our result and to produce stronger evidence. Moreover, chorioamnionitis was defined by histological examination of the placenta. Although histological chorioamnionitis is the gold standard for diagnosing intrauterine infections [30], there is controversy as to whether histological chorioamnionitis is correlated with higher rates of neonatal morbidity and mortality [30–32].
Conclusions
The NLR has a good predictive value for the occurrence of HCA and could be used as an additional diagnostic marker for predicting histological chorioamnionitis in cases with preterm premature rupture of the membranes prior to 34 weeks of gestation.
Acknowledgements
We thank the study participants for their support of the study.
Abbreviations
- NLR
neutrophil-lymphocyte ratio
- WBC
white blood cell
- CRP
C-reactive protein
- HCA
histological chorioamnionitis
- PPROM
preterm premature rupture of the membranes
- IQR
interquartile range
- ROC
receiver operating curve
- AUC
area under the curve
Authors' contributions
D.R. designed the study, together with G.S.D and I.D. G.B, V.G, and R.V. applied for ethical approval and collected data. G.B. analysed the data with support from G.K.B and V.G. G.B and G.K.B wrote the main manuscript text, prepared tables and figures. The article was revised and edited by I.P., G.S.D., I.D., and D.R. All authors approved the version to be published.
Funding
This study was funded by the Research Council of Lithuania under grant No. S-MIP-19-57.
Availability of data and materials
The data that support the findings of this study are available on request from corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Ethics approval and consent to participate
This study was approved by the Vilnius Regional Bio-medical Research Ethics Committee (2017-07-04 No. 158200-17-931-434), and all participants signed an informed consent form before enrolment. All methods were performed in accordance with the relevant guidelines and regulations (Declaration of Helsinki).
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittendorf R, Montag AG, MacMillan W, Janeczek S, Pryde PG, Besinger RE, et al. Components of the systemic fetal inflammatory response syndrome as predictors of impaired neurologic outcomes in children. Am J Obstet Gynecol. 2003;188:1438–46. doi: 10.1067/mob.2003.380. [DOI] [PubMed] [Google Scholar]
- 3.Zanardo V, Vedovato S, Cosmi E, Litta P, Cavallin F, Trevisanuto D, et al. Preterm premature rupture of membranes, chorioamnion inflammatory scores and neonatal respiratory outcome: PPROM, chorioamnionitis and neonatal respiratory outcome. BJOG Int J Obstet Gynaecol. 2010;117:94–8. doi: 10.1111/j.1471-0528.2009.02358.x. [DOI] [PubMed] [Google Scholar]
- 4.Rogers EE, Hintz SR. Early neurodevelopmental outcomes of extremely preterm infants. Semin Perinatol. 2016;40:497–509. doi: 10.1053/j.semperi.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Catano-Sabogal CP, Fonseca J, Garcia-Perdomo HA. Validation of Diagnostic Tests for Histologic Chorioamnionitis: Systematic Review and Meta-Analysis. Eur J Obstet Gynecol Reprod Biol. 2018;228:13–26. doi: 10.1016/j.ejogrb.2018.05.043. [DOI] [PubMed] [Google Scholar]
- 6.Trochez-Martinez RD, Smith P, Lamont RF. Use of C-reactive protein as a predictor of chorioamnionitis in preterm prelabour rupture of membranes: a systematic review. BJOG. 2007;114:796–801. doi: 10.1111/j.1471-0528.2007.01385.x. [DOI] [PubMed] [Google Scholar]
- 7.Zou L, Zhang H, Zhu J, Zhu J. The value of the soluable intercellular adhesion molecule-1 levels in matermal serum for determination of occult chorioamnionitis in premature rupture of membranes. J Huazhong Univ Sci Technolog Med Sci. 2004;24:154–7. doi: 10.1007/BF02885417. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Portilla RJ, Hawkins-Villarreal A, Alvarez-Ponce P, Chinolla-Arellano ZL, Moreno-Espinosa AL, Sandoval-Mejia AL, et al. Maternal Serum Interleukin-6: A Non-Invasive Predictor of Histological Chorioamnionitis in Women with Preterm-Prelabor Rupture of Membranes. Fetal Diagn Ther. 2019;45:168–75. doi: 10.1159/000488080. [DOI] [PubMed] [Google Scholar]
- 9.Park JW, Park KH, Kook SY, Jung YM, Kim YM. Immune biomarkers in maternal plasma to identify histologic chorioamnionitis in women with preterm labor. Arch Gynecol Obstet. 2019;299:725–32. doi: 10.1007/s00404-019-05061-8. [DOI] [PubMed] [Google Scholar]
- 10.Martins EC, Silveira L da F, Viegas K, Beck AD, Fioravantti-Junior G, Cremonese RV, et al. Neutrophil-lymphocyte ratio in the early diagnosis of sepsis in an intensive care unit: a case-control study. Rev Bras Ter Intensiva. 2019;31:63–70. [DOI] [PMC free article] [PubMed]
- 11.Ozel A, Davutoglu EA, Yurtkal A, Madazli R. How do platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio change in women with preterm premature rupture of membranes, and threaten preterm labour? J Obstet Gynaecol. 2020;40:195–9. doi: 10.1080/01443615.2019.1621807. [DOI] [PubMed] [Google Scholar]
- 12.Imran MM, Ahmed U, Usman U, Ali M, Shaukat A, Gul N. Neutrophil/Lymphocyte Ratio – A Marker of COVID-19 Pneumonia Severity. Int J Clin Pract. 2021;00:e13698. doi: 10.1111/ijcp.13698. [DOI] [PubMed] [Google Scholar]
- 13.Khong T, Mooney Y, Ariel EE, Balmus I, Boyd NCM, Brundler TK, et al. Sampling and Definitions of Placental Lesions Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016;140:698–713. [DOI] [PubMed]
- 14.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, Amniotic Fluid Infection Nosology Committee Society for Pediatric Pathology Perinatal Section. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–48. [DOI] [PubMed]
- 15.Davis JM, Albert JD, Tracy KJ, Calvano SE, Lowry SF, Shires GT, et al. Increased neutrophil mobilization and decreased chemotaxis during cortisol and epinephrine infusions. J Trauma. 1991;31:725–31. [PubMed]
- 16.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31:318–24. [DOI] [PMC free article] [PubMed]
- 17.Matalka KZ, Sidk A. Academic Stress — Influence on Leukocyte Distribution, Cortisol, and Prolactin. Lab Med. 1998;29:697–701.
- 18.Kim MA, Lee YS, Seo K. Assessment of Predictive Markers for Placental Inflammatory Response in Preterm Births. PLoS One. 2014;9:e107880. doi: 10.1371/journal.pone.0107880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singareddy A, Lee ASE, Sweeney PL, Finkle AE, Williams HL, Buchanan PM, et al. Elevated neutrophil-lymphocyte ratios in extremely preterm neonates with histologic chorioamnionitis. J Perinatol. 2021 doi: 10.1038/s41372-021-00964-4. [DOI] [PubMed] [Google Scholar]
- 20.Kim MA, Lee BS, Park YW, Seo K. Serum markers for prediction of spontaneous preterm delivery in preterm labour. European journal of clinical investigation. 2011;41:773–80. doi: 10.1111/j.1365-2362.2011.02469.x. [DOI] [PubMed] [Google Scholar]
- 21.Akgun N, Namli Kalem M, Yuce E, Kalem Z, Aktas H. Correlations of maternal neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) with birth weight. The Journal of Maternal-Fetal & Neonatal Medicine. 2017;30:2086–91. doi: 10.1080/14767058.2016.1237497. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa M, Terashima T, D’yachkova Y, Bondy GP, Hogg JC, van Eeden SF. Glucocorticoid-induced granulocytosis: contribution of marrow release and demargination of intravascular granulocytes. Circulation. 1998;98:2307–13. doi: 10.1161/01.CIR.98.21.2307. [DOI] [PubMed] [Google Scholar]
- 23.Weber PSD, Toelboell T, Chang L-C, Tirrell JD, Saama PM, Smith GW, et al. Mechanisms of glucocorticoid-induced down-regulation of neutrophil L-selectin in cattle: evidence for effects at the gene-expression level and primarily on blood neutrophils. J Leukoc Biol. 2004;75:815–27. doi: 10.1189/jlb.1003505. [DOI] [PubMed] [Google Scholar]
- 24.Liles WC, Dale DC, Klebanoff SJ. Glucocorticoids inhibit apoptosis of human neutrophils. Blood. 1995;86:3181–8. doi: 10.1182/blood.V86.8.3181.3181. [DOI] [PubMed] [Google Scholar]
- 25.Bauer ME, Price LK, MacEachern MP, Housey M, Langen ES, Bauer ST. Maternal leukocytosis after antenatal corticosteroid administration: a systematic review and meta-analysis. J Obstet Gynaecol. 2018;38:210–6. doi: 10.1080/01443615.2017.1342614. [DOI] [PubMed] [Google Scholar]
- 26.Kurtoglu E, Kokcu A, Celik H, Tosun M, Malatyalioglu E. May ratio of neutrophil to lymphocyte be useful in predicting the risk of developing preeclampsia? A pilot study. J Matern Fetal Neonatal Med. 2015;28:97–9. doi: 10.3109/14767058.2014.905910. [DOI] [PubMed] [Google Scholar]
- 27.Sahbaz A, Cicekler H, Aynioglu O, Isik H, Ozmen U. Comparison of the predictive value of plateletcrit with various other blood parameters in gestational diabetes development. J Obstet Gynaecol. 2016;36:589–93. doi: 10.3109/01443615.2015.1110127. [DOI] [PubMed] [Google Scholar]
- 28.Kirbas A, Biberoglu E, Daglar K, Iskender C, Erkaya S, Dede H, et al. Neutrophil-to-lymphocyte ratio as a diagnostic marker of intrahepatic cholestasis of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2014;180:12–5. doi: 10.1016/j.ejogrb.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 29.Orgul G, Agbal T, Celen S, Caglar AT. Neuroprotective magnesium sulfate administration increases maternal Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio and Systemic Immune-Inflammation Index. Arch Gynecol Obstet. 2020 doi: 10.1007/s00404-020-05866-y. [DOI] [PubMed] [Google Scholar]
- 30.Pugni L, Pietrasanta C, Acaia B, Merlo D, Ronchi A, Ossola MW, et al. Chorioamnionitis and neonatal outcome in preterm infants: A clinical overview. J. Matern Fetal Neonatal Med. 2016;29:1525–9. doi: 10.3109/14767058.2015.1053862. [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Kusanovic JP, Yoon BH, et al. Clinical chorioamnionitis at term VI: Acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J. Perinat Med. 2016;44:33–51. doi: 10.1515/jpm-2015-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ocheke N, Ocheke IE, Agaba PA, Imadde GE, Silas OA, Ajetunmobi OI, et al. Maternal and Neonatal Outcomes of Histological Chorioamnionitis. J West Afr Coll Surg. 2016;6:1–14. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from corresponding author. The data are not publicly available due to privacy or ethical restrictions.