Abstract
Background & aims:
Nutrition therapy for Intensive Care Unit (ICU) patients involves complex decision-making, especially during the COVID-19 pandemic. We investigated the use of nutrition therapy in ICU patients with and without COVID-19 infections.
Methods
Nutrition therapy was evaluated during a world-wide one-day prevalence study focused on implementation of the ABCDEF bundle (A: regular pain assessment, B: both spontaneous awakening and breathing trials, C: regular sedation assessment, D: regular delirium assessment, E: early mobility and exercise, and F: family engagement and empowerment) during the COVID-19 pandemic. Basic ICU and patient demographics including nutrition therapy delivery were collected on the survey day. Physical activity for patients with and without COVID infections was categorized using the ICU mobility scale (IMS). Multivariable regression analysis of nutrition was conducted using ICU parameters.
Results
The survey included 627 non-COVID and 602 COVID patients. A higher proportion of COVID-19 patients received energy ≥20 kcal/kg/day (55% vs. 45%; p = 0.0007) and protein ≥1.2 g/kg/day (45% vs. 35%; p = 0.0011) compared to non-COVID patients. Enteral nutrition was provided to most COVID patients even with prone positioning (91%). Despite nutrition therapy, IMS was extremely low in both groups; median IMS was 1 in non-COVID patients and 0 in COVID patients. The rate of energy delivery ≥20 kcal/kg/day was significantly higher in patients with COVID-19 infections in the subgroup of ICU days ≤5 days and IMS ≤2. Having a dedicated ICU nutritionist/dietitian was significantly associated with appropriate energy delivery in patients both with and without COVID-19 infections, but not with protein delivery.
Conclusion
During the COVID-19 pandemic, patients with COVID-19 infections received higher energy and protein delivery. Generally low mobility levels highlight the need to optimize early mobilization with nutrition therapy in all ICU patients.
Keywords: COVID-19, Critical care, Energy, Mobility, Nutrition, Protein
1. Introduction
Nutrition therapy is an important component of critical care for the maintenance of patients’ life, immune system and body composition [1,2]. To avoid overfeeding, target energy delivery is currently recommended at about 70% of calculated energy expenditures or 20 kcal/kg/day in the early acute phase (Day 1–2), while adequate energy at about 30 kcal/kg/day or more, should be provided for recovery after the acute phase [1,2]. The importance of protein dose has also been evaluated. More than 1.2 g/kg/day [1] or 1.3 g/kg/day protein [2] should be provided according to available guidelines. Focusing on protein delivery as part of nutrition therapy, it is needed more in the recovery phase to prevent post-intensive care syndrome (PICS) and intensive care unit (ICU) acquired weakness (ICU-AW) [[3], [4], [5]]. However, previous observational studies including worldwide surveys [6,7] showed that nutrition targets, especially for protein, were often never achieved and with a number of barriers limiting adequate nutrition therapy [8,9]. Some approaches related to ICU administrative structures, such as dietitian participation [10] or nutrition protocols [11], may contribute to achieving nutrition targets.
The recent COVID-19 pandemic has contributed to more complexity surrounding ICU nutrition practices. PICS and ICU-AW after ARDS has been reported to persist for a long time [12], and PICS after severe COVID-19 infections has also been reported [13,14]. Statements from professional societies have urged provision of nutrition therapy similar to routine ICU guidelines to manage patients with COVID-19 [15,16]. There were many suggestions to provide enhanced nutritional support especially to elderly or malnourished patients receiving COVID-19 treatment [17,18]. Since the COVID-19 pandemic affects ICU care of patients with and without COVID-19 infections [19], nutrition practices may be negatively impacted for all patients due to intensified focus on resuscitation and stabilization. Coupled with challenges of early mobilization and exercise during the pandemic [20], there is an urgent need to optimize both nutrition and provision of early mobility to optimize outcomes for all ICU patients. Along with hyper-catabolism in patients with COVID-19 or acute respiratory distress syndrome, an imbalance between nutrition provided and physical activity could be potentially harmful. There have been no large-scale studies of nutrition therapy during the COVID-19 pandemic to the best of our knowledge [[21], [22], [23]].
We recently conducted a worldwide one-day prevalence study of ICU care including nutrition therapy. In this analysis, we specifically investigate nutrition therapy for patients with/without COVID-19 infections and examine whether nutrition practices changed during the COVID-19 pandemic. We simultaneously analyzed patient physical activity on that day. We also evaluated what ICU administrative structures significantly influenced nutrition therapy, to consider ways of overcoming some of the barriers limiting adequate nutrition support.
2. Materials and Methods
This was an international one-day point prevalence study conducted on 27 th January, 2021, with approval by the ethics committee of the Saiseikai Utsunomiya Hospital (2020–69) and pre-registration in UMIN (UMIN000040405). The study outline is described in detail in the previous report. Briefly, participants were recruited by sending an invitation letter to the members of Indian Society of Critical Care Medicine, the Korean Society of Critical Care Medicine, and other local or national networks in collaboration with regional/national coordinators. According to the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan, ethical approval at each participating institution was waived because of the anonymous nature of this study which did not collect specific data that could identify ICUs or individual patients. This ethical policy was explained to all clinicians, and ICUs participated only if they agreed after referring to the ethics policies in their regions and countries. If participation presented difficulty on the ethical policy, clinicians could decide to participate after obtaining ethical approval. Participants who agreed with study policies could register their ICU and there were no exclusion criteria.
All registration and questionnaires were conducted using Google Forms (Google. LLC. California. US.). Using the registration URL, the name of one representative for each participating ICU, the name of the hospital, and the location were collected. Basic hospital and ICU information were collected on line, including type of hospital, type of ICU, total number of ICU beds and beds dedicated to patients with COVID-19, nurse-to-patient ratio, presence of a nutritionist/dietitian dedicated to the ICU and the existence/absence of written protocols for nutrition therapy, provided by the registered representatives. Each representative who completed this questionnaire received a different Facility Registration Number. On the survey date, 27 January 2021, the URL for the survey was sent to the registered representatives. Only representatives who had their specific Facility Registration Number could continue to complete the survey.
The implementation rate for elements of the ABCDEF bundle (A: regular pain assessment, B: both spontaneous awakening and breathing trials, C: regular sedation assessment, D: regular delirium assessment, E: early mobility and exercise, and F: family engagement and empowerment) was collected for patients with COVID-19 infections (COVID) and patients without COVID-19 infections (non-COVID), with age, gender, body mass index (BMI), ICU length of stay at the time of the survey, use of medical devices, continuous use of neuromuscular blockade, vasoactive agents, analgesia and sedation agents, prone positioning and its duration, ICU mobility scale (IMS) [24] on the survey date, and nutrition therapy on that day. The route of nutrition therapy used within the preceding 24 h was collected, including enteral nutrition (EN), oral or parenteral routes. Total energy and protein provided in the 24 h until the morning on the survey date were evaluated and categorized; energy delivery; x < 10, 10 ≤ x < 20, 20 ≤ x < 30 or 30 ≤ x kcal/kg/day and protein delivery; <1.2 g/kg/day or ≥1.2 g/kg/day. In the case of oral nutrition, total estimated energy and protein is calculated, based on the actual amount of intake. The representatives completed one questionnaire for each patient, except for patients who were terminally ill and receiving palliative care. Data obtained from the survey were linked to the data of the hospital and ICU based on the Facility Registration Number.
After categorizing patients into COVID and non-COVID groups, we evaluated the delivery of nutrition categorizing by ICU day and analyzed it according to IMS. Subgroup analyses of patients undergoing mechanical ventilation, with a BMI ≥25 [25], and undergoing prone positioning (hours/day) among patients with COVID-19 infections were conducted. Finally, we conducted multivariable regression analysis for the nutrition achievement; energy delivery ≥20 kcal/kg/day and protein delivery ≥1.2 g/kg/day, with the ICU administrative structures evaluated in this study and possibly related to nutrition therapy.
2.1. Statistical analysis
Continuous variables are expressed as the mean ± standard deviation and are compared using Student's t-test or one-way analysis of variance when the null hypothesis is not rejected by the Shapiro–Wilk test. Continuous variables are expressed as the median (interquartile range) and are compared using the Mann–Whitney U test or Kruskal–Wallis test when the null hypothesis is rejected by the Shapiro–Wilk test. For categorical variables, the proportions of patients in each category were calculated. Then the groups were compared using chi-squared tests. Categorizing patients into COVID and non-COVID groups, multivariable logistic regression analysis for the energy delivery ≥20 kcal/kg/day and protein delivery ≥1.2 g/kg/day was performed including ICU administrative structural factors. To check multicollinearity between independent variables, the variance inflation factor was calculated before performing multivariate logistic regression. Multicollinearity was regarded as present when the variance inflation factor was >10. All statistical analyses were conducted using software (JMP 14; SAS Institute Inc.).
3. Results
A total of 1229 patients (627 non-COVID and 602 COVID) were registered and analyzed from 135 worldwide ICUs. Hospital information and ICU administrative structures for participating institutions are shown in Table .1 . Most patients were accrued from Asia (52.6%) and Africa (28.4%), followed by Europe (15.3%). The type of hospitals and ICUs, and nurse-to-patient ratios differed somewhat between the non-COVID and COVID groups (Table 1). The rates of nutritionists/dietitians dedicated to the ICU and existence of written protocols for nutrition therapy were 35.0% and 50.8%, respectively, with no significant differences between the non-COVID and COVID groups.
Table 1.
Study patients: Hospital and ICU information.
| n | non-COVID 627 | COVID 602 | p value |
|---|---|---|---|
| Continent, n (%) | <0.0001∗ | ||
| Asia | 361 (57.6) | 286 (47.5) | |
| Africa | 193 (30.8) | 156 (25.9) | |
| Europe | 35 (5.6) | 153 (25.4) | |
| South-America | 16 (2.6) | 6 (1) | |
| Others | 22 (3.5) | 1 (0.2) | |
| Type of hospital, n (%) | |||
| University hospital | 290 (46.3) | 231 (38.4) | 0.0099∗ |
| University affiliated hospital | 123 (19.6) | 121 (20.1) | |
| Community hospital | 164 (26.2) | 206 (34.2) | |
| Others | 50 (8.0) | 44 (7.3) | |
| Type of ICU, n (%) | |||
| Medical-Surgical mixed ICU | 479 (76.4) | 394 (65.5) | <0.0001∗ |
| Medical ICU | 51 (8.1) | 153 (25.4) | |
| Surgical ICU including cardiac surgery | 64 (10.2) | 24 (4.0) | |
| Pediatric ICU | 14 (2.2) | 12 (2.0) | |
| other types of ICU | 19 (3.0) | 19 (3.2) | |
| Tele ICU, n (%) | 17 (2.7) | 35 (5.8) | 0.0064∗ |
| Total number of ICU beds | 14 (10, 25) | 13 (8, 27) | 0.0246∗ |
| ICU beds for COVID-19 | 4 (2,8) | 4 (4, 18) | <0.0001∗ |
| Nurse-to-patient ratio | <0.0001∗ | ||
| 1:1 | 172 (27.4) | 254 (42.2) | |
| 1:2 | 396 (63.2) | 296 (49.2) | |
| 1:4 | 45 (7.2) | 36 (6.0) | |
| ≥4 | 14 (2.1) | 16 (2.7) | |
| Nutritionist/dietitian dedicated to the ICU, n (%) | 217 (34.6) | 213 (35.4) | 0.78 |
| Written protocol for nutrition therapy, n (%) | 305 (48.6) | 319 (53.0) | 0.13 |
| Multidisciplinary rounds more than once a week, n (%) | 234 (37.3) | 188 (31.2) | 0.029∗ |
ICU, intensive care unit; COVID, coronavirus disease. ∗ infers a significant difference with p <0.05.
Patient baseline and treatments are shown in Table 2 . Variable distributions in age, gender (male), and BMI were seen between two groups. The ICU length of stay was significantly longer in the COVID group (5 vs 9 days, p < 0.001). Continuous neuromuscular blockade and analgesia/sedation agents were more frequently given to the COVID group. A higher introduction rate and longer duration of prone positioning was seen in the COVID group (Table 2). These differences were similarly found in patients undergoing mechanical ventilation.
Table 2.
Patient characteristics and basic treatment.
| n | Overall |
Mechanically ventilated |
||||
|---|---|---|---|---|---|---|
| non-COVID 627 | COVID 602 | p value | non-COVID 339 | COVID 458 | p value | |
| Age (years), n(%) | <0.0001∗ | <0.0001∗ | ||||
| x <20 | 54 (8.6) | 4 (0.7) | 30 (8.9) | 1 (0.2) | ||
| 20≤ x <30 | 28 (4.5) | 8 (1.3) | 13 (3.8) | 6 (1.3) | ||
| 30≤ x <40 | 51 (8.1) | 30 (5.0) | 26 (7.7) | 23 (5.0) | ||
| 40≤ x <50 | 57 (9.1) | 65 (10.8) | 28 (8.3) | 47 (10.3) | ||
| 50≤ x <60 | 90 (14.4) | 132 (21.9) | 50 (14.8) | 105 (22.9) | ||
| 60≤ x <70 | 120 (19.1) | 193 (32.1) | 77 (22.7) | 152 (33.2) | ||
| 70≤ x <80 | 136 (21.7) | 146 (24.3) | 64 (18.9) | 109 (23.8) | ||
| x ≥80 | 91 (14.5) | 24 (4.0) | 51 (15.0) | 15 (3.3) | ||
| Male, n (%) | 391 (62.4) | 425 (70.6) | 0.0022∗ | 209 (61.7) | 329 (71.8) | 0.0025∗ |
| BMI (kg/m2), n(%) | <0.0001∗ | <0.0001∗ | ||||
| x <18.5 | 84 (13.4) | 10 (1.7) | 49 (14.5) | 4 (0.9) | ||
| 18.5≤ x <25 | 310 (49.4) | 150 (24.9) | 147 (43.4) | 104 (22.7) | ||
| 25≤ x <30 | 155 (24.7) | 218 (36.2) | 99 (29.2) | 168 (36.7) | ||
| 30≤ x <35 | 54 (8.6) | 140 (23.3) | 27 (8.0) | 114 (24.9) | ||
| x ≥35 | 24 (3.8) | 84 (14.0) | 17 (5.0) | 68 (14.9) | ||
| ICU length of stay (days) | 5 (2, 10) | 9 (4.5, 16) | <0.0001∗ | 6 (2, 12) | 9 (5, 16) | <0.0001∗ |
| Use of support devices, n(%) | ||||||
| Extracorporeal membrane oxygenation | 18 (2.9) | 30 (5.0) | 0.055 | 12 (3.5) | 24 (5.2) | 0.25 |
| Renal replacement therapy | 66 (10.59) | 56 (9.3) | 0.47 | 41 (12.1) | 54 (11.8) | 0.90 |
| Left ventricular unloading device (ImpellaⓇ, IABP) | 10 (1.6) | 1 (0.2) | 0.0041∗ | 7 (2.1) | 1 (0.2) | 0.0076∗ |
| Continuous use of neuromuscular blockage, n(%) | 19 (3.0) | 159 (26.4) | <0.0001∗ | 15 (4.4) | 159 (34.7) | <0.0001∗ |
| Continuous use of vasoactive drugs, n(%) | 208 (33.2) | 186 (30.9) | 0.39 | 155 (45.7) | 180 (39.3) | 0.070 |
| Continuous use of analgesia and sedation agents, n(%) | 329 (52.5) | 394 (65.5) | <0.0001∗ | 242 (71.4) | 370 (80.8) | 0.0020 |
| Prone positioning (hours), n(%) | <0.0001∗ | <0.0001∗ | ||||
| 0 (not done) | 191 (30.5) | 333 (55.3) | 143 (42.2) | 266 (58.1) | ||
| 0≤ x <6 | 11 (1.8) | 58 (9.6) | 6 (1.8) | 42 (9.2) | ||
| 6≤ x <12 | 2 (0.3) | 38 (6.3) | 2 (0.6) | 25 (5.5) | ||
| 12≤ x <18 | 4 (0.6) | 57 (9.5) | 4 (1.2) | 51 (11.1) | ||
| 18≤ x ≤24 | 0 (0) | 56 (9.3) | 0 (0) | 53 (17.3) | ||
| Not a candidate (no respiratory failure) | 419 (66.8) | 60 (10.0) | 184 (54.3) | 21 (4.6) | ||
COVID, coronavirus disease; BMI, body mass index; IABP, intra-aortic balloon pump. ∗ infers a significant difference with p <0.05.
Nutrition support for all ICU patients is shown in Table 3 . Enteral nutrition (EN) was used more often in the COVID group regardless of the presence of mechanical ventilation, while PN was less frequent. The rate of energy delivery ≥20 kcal/kg/day and the protein delivery ≥1.2 g/kg/day was significantly higher in the COVID group (44.8 vs 54.5%, p = 0.0007 and 35.4 vs 45%, p = 0.0011, respectively) with the same low level of activity in both groups as described by the IMS. Similar tendencies and significant differences between the non-COVID and COVID groups were observed in the subgroup with a BMI ≥25 kg/m2 (Supplementary Table 1). IMS was lower in these populations, and was significantly lower in the COVID group (0 (0, 2) vs 0 (0, 1), p = 0.029). Given the duration of prone positioning in the COVID group (Supplementary Table 2), EN was predominantly used for patients without prone positioning or 12–24–h prone positioning, while the oral route was used for patients with 0-12-h of prone positioning. Over 90% of patients received nutrition therapy as EN or orally regardless of the duration. Energy and protein delivery were similar levels regardless of the duration of the prone positioning.
Table 3.
Nutrition Therapy and physical activity.
| n | Overall |
Mechanically ventilated |
||||
|---|---|---|---|---|---|---|
| non-COVID 627 | COVID 602 | p value | non-COVID 339 | COVID 458 | p value | |
| Route of Nutrition therapy, n (%) | ||||||
| Enteral (not oral) | 313 (49.9) | 416 (69.1) | <0.0001∗ | 228 (67.3) | 374 (81.7) | <0.0001∗ |
| Oral | 155 (24.7) | 165 (27.4) | 0.28 | 28 (8.3) | 63 (13.8) | 0.014∗ |
| Parenteral | 121 (19.3) | 37 (6.2) | <0.0001∗ | 71 (20.9) | 31 (6.8) | <0.0001∗ |
| Total energy delivery (kcal/kg/day), n (%) | <0.0001∗ | <0.0001∗ | ||||
| x <10 | 155 (24.7) | 53 (4.3) | 86 (25.4) | 44 (9.6) | ||
| 10≤ x <20 | 191 (30.5) | 221 (36.7) | 105 (31.0) | 173 (37.8) | ||
| 20≤ x <30 | 220 (35.1) | 286 (47.5) | 118 (34.8) | 206 (45.0) | ||
| x ≥30 | 61 (9.7) | 42 (7.0) | 30 (8.9) | 35 (7.6) | ||
| Protein delivery ≥1.2 g/kg/day, n (%) | 216 (35.4) | 232 (45.0) | 0.0011∗ | 121 (36.0) | 179 (47.0) | 0.0029∗ |
| IMS on the survey day | 1 (0, 2) | 0 (0, 3) | 0.067 | 0 (0, 1) | 0 (0, 1) | 0.82 |
COVID, coronavirus disease; IMS, intensive care unit mobility scale. ∗ infers a significant difference with p <0.05.
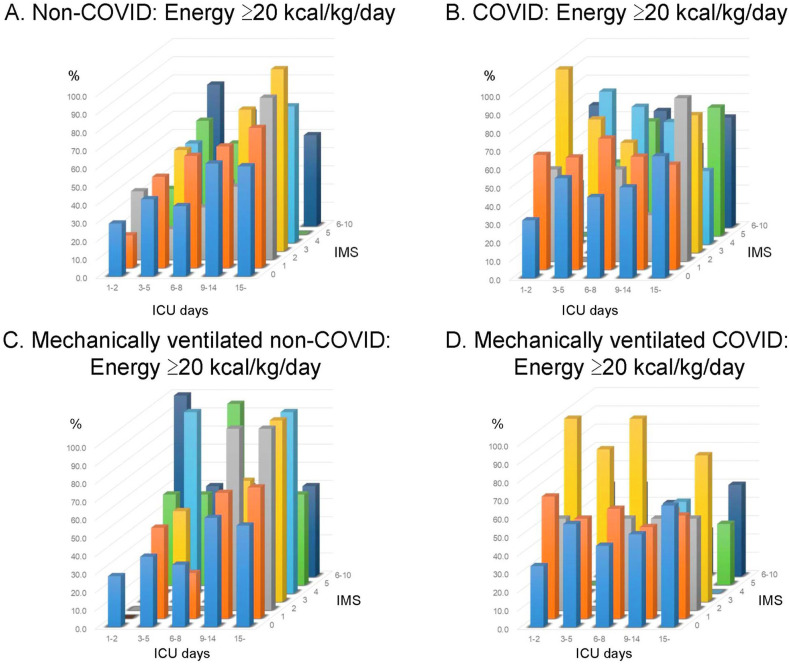
Considering the percentage of patients who were given adequate energy with the ICU length of stay and physical activity level, the rate of energy delivery ≥20 kcal/kg/day in contrast with ICU days and IMS is shown in Fig. 1 . As the COVID bars were tended to be high in the left (earlier ICU days) and front (lower IMS) side, the energy was delivered more in patients for whom the physical activity was low in the acute phase (Fig. 1A, B). A similar trend was also seen when it was limited to those who were mechanically ventilated (Fig. 1C, D). Accordingly, in the subgroup of ICU days ≤ 5 days and IMS ≤2, the rate of energy delivery ≥20 kcal/kg/day was 32.9% in non-COVID group and 48.1% in COVID group, with a significant difference (p = 0.0037). Detailed energy provision is described in Supplementary Table 3.
Fig. 1.
Rate of energy delivery ≥20 kcal/kg/day according to intensive care unit (ICU) days and ICU Mobility scale. The rate of energy delivery ≥20 kcal/kg/day contrasting to ICU days and IMS is shown. The bars in front side represent the nutrition therapy in whom physical activity was low. The patients with COVID-19 infections received adequate energy delivery more frequently with lower physical activity in early acute phase than those without COVID-19 infections, both in overall and in mechanically ventilated. A: energy delivery in the non-COVID group, B: energy delivery in the COVID group, C: energy delivery in mechanically ventilated non-COVID, D: energy delivery in mechanically ventilated COVID. ICU, intensive care units; IMS, ICU mobility scale; COVID, coronavirus disease.
ICU administrative structures which influenced the achievement of energy delivery ≥20 kcal/kg/day and protein delivery ≥1.2 g/kg/day were evaluated with multivariable logistic regression analysis (Table 4 ). The associated independent factors were variable and not consistent between the non-COVID and COVID groups. The presence of a nutritionist/dietitian dedicated to the ICU was a significant independent factor associated with achieving energy delivery ≥20 kcal/kg/day in both groups. After excluding patients admitted for <3 days, the odds of >20 kcal/kg/day achievement was higher in both groups with a dedicated dietitian (2.13 (95% Confidence Interval: 1.22 to 3.75, p = 0.0081) in non-COVID and 2.98 (95% Confidence Interval: 1.47–6.04, p = 0.0024) in COVID. The presence of a nutritionist/dietitian was not significantly associated with protein delivery even for patients on ICU days 1 and 2 were excluded. Written protocols for nutrition therapy and multidisciplinary rounds were not independently associated with the achieving energy and protein delivery goals.
Table 4.
Multivariable Logistic Regression Analysis for energy delivery ≥20 kcal/kg/day and protein delivery ≥1.2 g/kg/day.
| Energy delivery ≥20 kcal/kg/day |
Protein delivery ≥1.2 g/kg/day |
|||||||
|---|---|---|---|---|---|---|---|---|
| non-COVID |
COVID |
non-COVID |
COVID |
|||||
| odds ratio (95% CI) | p value | odds ratio (95% CI) | p value | odds ratio (95% CI) | p value | odds ratio (95% CI) | p value | |
| Continent | ||||||||
| Asia | (reference) | (reference) | (reference) | (reference) | ||||
| Africa | 6.60 (3.75–11.6) | <0.0001 | 0.46 (0.22–0.95) | 0.035∗ | 6.84 (3.77–112.4) | <0.0001∗ | 0.75 (0.38–1.49) | 0.41 |
| Europe | 2.29 (0.86–6.07) | 0.096 | 0.56 (0.27–1.14) | 0.11 | 1.28 (0.49–3.36) | 0.61 | 0.19 (0.094–0.40) | <0.0001∗ |
| South-America | 3.68 (0.81–16.6) | 0.091 | 1.58 (0.11–21.4) | 0.61 | 1.31 (0.26–6.61) | 0.75 | 0.75 (0.098–5.69) | 0.78 |
| Others | 11.8 (3.36–41.8) | 0.0001∗ | 0 | 1 | 17.2 (4.79–61.4) | <0.0001∗ | 0 | 1 |
| Total hospital beds | ||||||||
| x <200 | (reference) | (reference) | (reference) | (reference) | ||||
| 200≤ x <400 | 0.54 (0.19–1.51) | 0.39 | 0.35 (0.12–1.03) | 0.056 | 0.68 (0.23–2.01) | 0.48 | 1.06 (0.37–1.39) | 0.27 |
| 400≤ x <600 | 0.40 (0.17–0.94) | 0.035∗ | 0.85 (0.31–2.31) | 0.75 | 0.42 (0.18–1.01) | 0.94 | 2.88 (1.10–7.56) | 0.031∗ |
| 600≤ x <800 | 0.62 (0.29–1.31) | 0.21 | 1.79 (0.63–5.13) | 0.28 | 0.54 (0.26–1.14) | 0.11 | 1.10 (0.40–3.04) | 0.85 |
| x ≥800 | 0.72 (0.33–1.53) | 0.39 | 1.00 (0.33–3.00) | 0.99 | 0.70 (0.33–1.52) | 0.37 | 1.59 (0.56–4.55) | 0.38 |
| Type of hospital | ||||||||
| University hospital | 1.07 (0.59–1.93) | 0.83 | 1.56 (0.83–2.97) | 0.17 | 0.38 (0.70–2.70) | 0.35 | 1.43 (0.74–2.75) | 0.28 |
| University affiliated hospital | 0.90 (0.42–1.93) | 0.79 | 1.18 (0.59–2.36) | 0.64 | 3.54 (1.61–7.80) | 0.0017∗ | 1.29 (0.6302.63) | 0.49 |
| Community hospital | (reference) | (reference) | (reference) | (reference) | ||||
| Others | 0.54 (0.21–1.41) | 0.21 | 3.88 (1.26–11.9) | 0.018∗ | 2.61 (0.95–7.19) | 0.064 | 7.48 (2.56–21.8) | 0.0002∗ |
| Type of ICU | ||||||||
| Medical-Surgical mixed ICU | (reference) | (reference) | (reference) | (reference) | ||||
| Medical ICU | 1.08 (0.49–2.37) | 0.85 | 2.91 (1.42–5.96) | 0.0035∗ | 0.27 (0.12–0.65) | 0.0031∗ | 1.19 (0.58–2.41) | 0.64 |
| Surgical ICU including cardiac surgery | 0.54 (0.25–1.18) | 0.12 | 0.52 (0.18–1.51) | 0.23 | 0.37 (0.16–0.83) | 0.016∗ | 0.84 (0.30–2.34) | 0.74 |
| Pediatric ICU | 0.11 (0.02–0.52) | 0,0053∗ | 2.76 (0.38–20.0) | 0.31 | 0.89 (0.11–7.30) | 0.91 | 1 | |
| Other types of ICU | 0,44 (0.14–1.42) | 0.17 | 1.02 (0.32–3.21) | 0.98 | 0.17 (0.04–0.74) | 0.017∗ | 0.57 (0.19–1.72) | 0.32 |
| Tele-ICU | 11.6 (1.28–105.4) | 0.029∗ | 1.42 (0.43–4.71) | 0.57 | 1.24 (0.35–4.33) | 0.74 | 1.42 (0.41–4.89) | 0.58 |
| Total of ICU beds | 1.00 (0.97–1.02) | 0.76 | 0.99 (0.98–1.01) | 0.46 | 0.98 (0.05–1.02) | 0.32 | 0.99 (0.98–1.01) | 0.44 |
| ICU beds for COVID-19 | 1.00 (0.96–1.05) | 0.78 | 0.99 (0.98–1.01) | 0.32 | 1.02 (0.97–1.06) | 0.42 | 1.01 (0.99–1.02) | 0.38 |
| Nurse-to-patient ratio | ||||||||
| 1:1 | 0.62 (0.34–1.11) | 0.11 | 0.62 (0.32–1.18) | 0.15 | 0.87 (0.47–1.61) | 0.67 | 0.96 (0.53–1.74) | 0.89 |
| 1:2 | (reference) | (reference) | (reference) | (reference) | ||||
| 1:3 | 0.74 (0.30–1.82) | 0.51 | 0.90 (0.36–2.24) | 0.83 | 1.17 (0.44–3.13) | 0.75 | 0.41 (0.16–1.04) | 0.059 |
| ≥4 | 0.45 (0.11–1.87) | 0.27 | 3.78 (0.79–18.1) | 0.096 | 0.24 (0.037–1.54) | 0.13 | 1.66 (0.43–6.42) | 0.061 |
| Nutritionist/dietitian dedicated to the ICU | 1.68 (1.07–2.66) | 0.025∗ | 3.21 (1.64–6.29) | 0.0006∗ | 1.19 (0.71–1.97) | 0.51 | 1.26 (0.68–2.37) | 0.46 |
| Written protocol for nutrition therapy | 1.27 (0.82–1.96) | 0.29 | 0.53 (0.30–0.95) | 0.032∗ | 1.15 (0.71–1.86) | 0.57 | 0.72 (0.41–1.27) | 0.26 |
| Multidisciplinary rounds | ||||||||
| More than once a week | 1.46 (0.81–2.63) | 0.20 | 1.11 (0.61–2.03) | 0.74 | 1.48 (0.78–2.80) | 0.23 | 0.87 (0.48–1.59) | 0.66 |
| None or less above | (reference) | (reference) | (reference) | (reference) | ||||
COVID, coronavirus disease; ICU, intensive care units. ∗ infers a significant difference with p <0.05.
4. Discussion
In this worldwide one-day point prevalence study which characterized ICU care for more than 1000 ICU patients with and without COVID-19 infections, the provision of nutrition therapy, including both achievements of energy ≥20 kcal/kg/day and protein >1.2 g/kg/day, was higher in patients with COVID-19 infections. However, nutrition was provided with very low physical activity in the early acute phase in the patients with COVID-19 infections. The presence of a nutritionist/dietitian dedicated to the ICU was associated with improved energy delivery both in patients with COVID and those without COVID, but not protein delivery.
This is the most recent investigation of critical care nutrition, and to the best of our knowledge represents the largest such study during the COVID-19 pandemic era. Although nutrition management differs among countries and this study did not include the United States of America or Australia, energy delivery goals tended to be achieved using current critical care nutrition including patients with COVID-19 infections. This may be due to implementation of guidelines and nutrition recommendations for patients with COVID-19 infections [1,2,15,16,18]. Protein delivery goals tended not to be achieved both in patients with and without COVID-19 infections. As reported in previous studies [6,8,9], there were a number of barriers to achieve protein delivery targets and it remains an important challenge in critical care.
Although it was reported that digestive complications occasionally occur in patients with COVID-19 infections [26,27], EN might be easier to use because patients who are severely ill with COVID-19 infections suffered mainly from respiratory failure comparing with other ICU-related diseases. In a small observational study of patients severely ill with COVID-19 infections also showed that energy goals were generally met [28,29]. Furthermore, this study showed that EN could be given even to patients in the prone position.
However, providing ICU care was affected in patients with COVID-19 infections, and early mobilization was especially affected despite it being a key part of the ABCDEF bundle [20]. Physical activity was extremely low in patients who were severely ill with COVID-19 infections in this study. Although energy ≥20 kcal/kg/day was not overfeeding, a positive correlation between energy expenditure and physical activity was reported in critically ill patients [30]. In general, physical activity and exercise should be progressively increased with the provision of nutrition therapy [22]. We should optimize early mobilization in all ICU patients including those with COVID-19 infections.
As obesity definitely contributes to illness severity in COVID-19 infections [31,32], many patients with COVID-19 and higher BMI were included in this study. These results showed a similar tendency in obese patients, and furthermore, physical activity level was even lower in obese patients with COVID-19. Active early exercise and rehabilitation would be difficult to introduce to such patients [33]. As careful nutrition therapy has been recommended for patients with COVID-19 infections and obesity [34], we should pay attention to their physical activity simultaneously.
The presence of a nutritionist/dietitian dedicated to the ICU was significantly associated with achieving energy delivery goals for both the COVID and non-COVID groups. It has been reported that active intervention by a nutritionist/dietitian would be meaningful for patients in the ICU [35], and they might contribute directly to patients’ nutrition care or to influence nutrition therapy provided by critical care teams. Their importance has been also reported in less severely ill patients and for prevention of COVID-19 [36,37]. Specialists for nutrition therapy might be needed for active critical care nutrition support. Other ICU administrative structures, including written protocols and multidisciplinary rounds were not significantly associated with providing nutrition therapy. Participation by a nutritionist/dietitian may contribute more to adequate provision of nutrition therapy than protocols. If a nutritionist/dietitian typically participates in multidisciplinary round team, their contribution to nutrition therapy might be greater in the future. However, protein delivery was not affected by the presence of a dedicated ICU nutritionist/dietitian. Protein delivery is limited by cost, the need for EN products with high protein/energy ratio and/or amino acids used in PN [8,9]. Since nutrition therapy was supplied via enteral route in many of the patients both with and without COVID-19 infections, the lack of variety in high protein EN products might be related to the variability of protein delivery practice by nutritionists/dietitians despite achieving energy targets. We may have to consider using specific EN products with high protein or whey protein powder content [11]. There was an opinion that PN should be considered because PN could reduce the contact risk with patients who have COVID-19 infections [38].
This study has several limitations. First, the limited number of patients and participating countries could cause selection bias and limit the generalizability to other ICUs and countries. Some of the hotspots in the COVID-19 pandemic are under-represented. Second, the nature of a point prevalence study does not define a causal relationship and reflects the overwhelming situation at participating sites. Third, we could collect only estimates of energy and protein delivery which did not conflict with individual information, not necessarily accurate energy and protein delivery. Fourth, we could not collect nutrition assessment data, and were not able to establish the diagnosis of malnutrition. Fifth, we did not investigate the use of indirect calorimetry. Greater than 20 kcal/kg/day might be appropriate (not overfeeding) even based on individual physical activity if it was determined with indirect calorimetry monitoring. In the COVID-19 pandemic era, however, indirect calorimetry might be less frequently used, especially in patients with COVID-19 infections, to protect against infection. Finally, potential confounding factors, such as conditions related to EN, ileus or abdominal surgery, were not investigated. It was not possible to assess all of the conditions in patients without COVID-19 infections, and further research might lead to different results regarding the provision of nutrition therapy in patients without COVID-19 infections.
5. Conclusions
During the Covid-19 pandemic, patients with COVID-19 infections are receiving more adequate energy and protein delivery than those without COVID-19 infections beginning in the acute phase of illness. However, physical activity level was extremely low which highlights the need to optimize early mobilization in all ICU patients. The presence of a nutritionist/dietitian dedicated to the ICU was associated with higher energy delivery to both patients with and without COVID-19 infections, but not with protein delivery.
Acknowledgements
We wish to thank the Indian Society of Critical Care Medicine and the Korean Society of Critical Care Medicine. We thank all the investigators from the countries listed in Appendix.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnu.2021.09.033.
Author’s contributions
KN, KL, HK contributed equally to this paper. Study conception and design: KN, KL, HK, PN, EWE, SRK. Statistical analysis, or interpretation of data: KN, KL, HK, PN, EWE, SRK. Drafting the manuscript: KN, KL, HK, PN, EWE, SRK. Critical review and revision of the manuscript for important intellectual insight: KL, HK, PN, EWE, SRK, SI, AKL, and ON. Study supervision: PN, EWE, SRK. Recruitment the participating ICUs in overseas countries: KN, KL, HK, PN, EWE, SRK, SI, and ON. All authors drafted the manuscript for important intellectual content, contributed to revision of the final version of the manuscript, approved the final version submitted, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. KN and ON are the guarantors of the study. The corresponding author confirmed that all authors meet authorship criteria according to ICMJE.
Ethical approval
The study protocol were approved by the ethics committee of the Saiseikai Utsunomiya Hospital (No 2020–69). The informed consent from the patient was waived in this study.
Consent for publication
All authors have approved the manuscript.
Availability of data and material
Dataset available upon reasonable request.
Funding and source of funding
KN reports personal fees from Abbott Laboratory, Nestle, TERUMO, GETINGE, Asahi Kasei Pharma, Ono Pharmaceutical, Japan Blood Products Organization, Nihon Pharmaceutical, Otsuka Pharmaceutical, Pfizer, Toray, and Baxter, and grants from Asahi Kasei Pharma outside the submitted work. KL reports personal fees from MERA and receives a salary from TXP Medical completely outside the submitted work. HK receives a salary from the Japanese Society for Early Mobilization (non-profit society) as a chair (full time) outside the submitted work. EWE reports grants from the VA/NIH; personal fees from Pfizer, Orion, and Lilly; personal fees from Masimo; and grants from Kohler outside the submitted work. SI reports personal fees from MERA, Abbott Laboratory, Teijin Pharma, Nestle, and Nihon Pharmaceutical. ON reports grants from Asahi Kasei Pharma, Ono Pharmaceutical, Baxter, Maruishi Pharmaceutical, Torii Pharmaceutical, Teijin Pharma, Shionogi Pharmaceutical, and Fuso Pharmaceutical outside the submitted work.
Conflict of interest
The authors declare that they have no competing interests for the submitted work. Some authors report potential conflicts of interest outside of this submitted study.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.McClave S.A., Taylor B.E., Martindale R.G., Warren M.M., Johnson D.R., Braunschweig C., et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American society for parenteral and enteral nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. Feb 2016;40(2):159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 2.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 3.Inoue S., Hatakeyama J., Kondo Y., Hifumi T., Sakuramoto H., Kawasaki T., et al. Post-intensive care syndrome: its pathophysiology, prevention, and future directions. Acute Med Surg. 2019;6(3):233–246. doi: 10.1002/ams2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landi F., Camprubi-Robles M., Bear D.E., Cederholm T., Malafarina V., Welch A.A., et al. Muscle loss: the new malnutrition challenge in clinical practice. Clin Nutr. 2019;38(5):2113–2120. doi: 10.1016/j.clnu.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Wischmeyer P.E., Puthucheary Z., San Millán I., Butz D., Grocott M.P.W. Muscle mass and physical recovery in ICU: innovations for targeting of nutrition and exercise. Curr Opin Crit Care. 2017;23(4):269–278. doi: 10.1097/MCC.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Compher C., Chittams J., Sammarco T., Nicolo M., Heyland D.K. Greater protein and energy intake may Be associated with improved mortality in higher risk critically ill patients: a multicenter, multinational observational study. Crit Care Med. 2017;45(2):156–163. doi: 10.1097/CCM.0000000000002083. [DOI] [PubMed] [Google Scholar]
- 7.Abi Saleh W., Bou Khalil P., Ouaijan K., Abillama F., Akiki S., Ahmad N., et al. Evaluation of nutrition support practices: results from a nationwide survey. Clin Nutr. 2018;37(6 Pt A):1976–1979. doi: 10.1016/j.clnu.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Lee Z.Y., Noor Airini I., Barakatun-Nisak M.Y. Relationship of energy and protein adequacy with 60-day mortality in mechanically ventilated critically ill patients: a prospective observational study. Clin Nutr. 2018;37(4):1264–1270. doi: 10.1016/j.clnu.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Yatabe T., Egi M., Sakaguchi M., Ito T., Inagaki N., Kato H., et al. Influence of nutritional management and rehabilitation on physical outcome in Japanese intensive care unit patients: a multicenter observational study. Ann Nutr Metab. 2019;74(1):35–43. doi: 10.1159/000495213. [DOI] [PubMed] [Google Scholar]
- 10.Arney B.D., Senter S.A., Schwartz A.C., Meily T., Pelekhaty S. Effect of registered dietitian nutritionist order-writing privileges on enteral nutrition administration in selected intensive care units. Nutr Clin Pract. 2019;34(6):899–905. doi: 10.1002/ncp.10259. [DOI] [PubMed] [Google Scholar]
- 11.Yeh D.D., Ortiz L.A., Lee J.M., Chan J., Mckenzie K., Young B., et al. PEP uP (enhanced protein-energy provision via the enteral route feeding protocol) in surgical patients-A multicenter pilot randomized controlled trial. JPEN J Parenter Enteral Nutr. 2020;44(2):197–204. doi: 10.1002/jpen.1521. [DOI] [PubMed] [Google Scholar]
- 12.Herridge M.S., Cheung A.M., Tansey C.M., Matte-Martyn A., Diaz-Granados N., Al-Saidi F., et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 13.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morin L., Savale L., Pham T., Colle R., Figueiredo S., Harrois A., et al. Writing Committee for the COMEBAC Study Group Four-Month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021;325(15):1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martindale R., Patel J.J., Taylor B., Arabi Y.M., Warren M., McClave S.A. Nutrition therapy in critically ill patients with coronavirus disease 2019. JPEN J Parenter Enteral Nutr. 2020;44(7):1174–1184. doi: 10.1002/jpen.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arkin N., Krishnan K., Chang M.G., Bittner E.A. Nutrition in critically ill patients with COVID-19: challenges and special considerations. Clin Nutr. 2020;39(7):2327–2328. doi: 10.1016/j.clnu.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapple L.S., Fetterplace K., Asrani V., Burrell A., Cheng A.C., Collins P., et al. Nutrition management for critically and acutely unwell hospitalised patients with coronavirus disease 2019 (COVID-19) in Australia and New Zealand. Nutr Diet. 2020;77(4):426–436. doi: 10.1111/1747-0080.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chanques G., Constantin J.M., Devlin J.W., Ely W.W., Fraser G.L., Gélinas C., et al. Analgesia and sedation in patients with ARDS. Intensive Care Med. 2020;46(12):2342–2356. doi: 10.1007/s00134-020-06307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu K., Nakamura K., Katsukawa H., Elhadi M., Nydahl P., Ely E.W., et al. ABCDEF bundle and supportive ICU practices for patients with coronavirus disease 2019 infection: an international point prevalence study. Crit Care Explor. 2021;3(3) doi: 10.1097/CCE.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyland D.K., Stapleton R.D., Mourtzakis M., Hough C.L., Morris P., Deutz N.E., et al. Combining nutrition and exercise to optimize survival and recovery from critical illness: conceptual and methodological issues. Clin Nutr. 2016;35(5):1196–1206. doi: 10.1016/j.clnu.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 22.van Zanten A.R.H., De Waele E., Wischmeyer P.E. Nutrition therapy and critical illness: practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit Care. 2019;23(1):368. doi: 10.1186/s13054-019-2657-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura K., Nakano H., Naraba H., Mochizuki M., Takahashi Y., Sonoo T., et al. High protein versus medium protein delivery under equal total energy delivery in critical care: a randomized controlled trial. Clin Nutr. 2021;40(3):796–803. doi: 10.1016/j.clnu.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 24.Hodgson C., Needham D., Haines K., Bailey M., Ward A., Harrold M., et al. Feasibility and inter-rater reliability of the ICU mobility scale. Heart Lung. 2014;43(1):19–24. doi: 10.1016/j.hrtlng.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Kanazawa M., Yoshiike N., Osaka T., Numba Y., Zimmet P., Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. World Rev Nutr Diet. 2005;94:1–12. doi: 10.1159/000088200. [DOI] [PubMed] [Google Scholar]
- 26.Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J., et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Formenti P., Bichi F., Castagna V., Pozzi T., Chiumello D. Nutrition support in patients with acute respiratory distress syndrome COVID-19. Nutr Clin Pract. 2021;36(2):500–501. doi: 10.1002/ncp.10645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farina N., Nordbeck S., Montgomery M., Cordwin L., Blair F., Cherry-Bukowiec J., et al. Early enteral nutrition in mechanically ventilated patients with COVID-19 infection. Nutr Clin Pract. 2021;36(2):440–448. doi: 10.1002/ncp.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beach L.J., Fetterplace K., Edbrooke L., Parry S.M., Curtis R., Rechnitzer T., et al. Measurement of physical activity levels in the Intensive Care Unit and functional outcomes: an observational study. J Crit Care. 2017;40:189–196. doi: 10.1016/j.jcrc.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Hendren N.S., de Lemos J.A., Ayers C., Das S.R., Rao A., Carter S., et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American heart association COVID-19 cardiovascular disease registry. Circulation. 2021;143(2):135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- 32.Eastment M.C., Berry K., Locke E., Green P., O’Hare A., Crothers K., et al. BMI and outcomes of SARS-CoV-2 among US Veterans. Obesity. 2021;29(5):900–908. doi: 10.1002/oby.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura K., Nakano H., Naraba H., Mochizuki M., Hashimoto H. Early rehabilitation with dedicated use of belt-type electrical muscle stimulation for severe COVID-19 patients. Crit Care. 2020;24(1):342. doi: 10.1186/s13054-020-03080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barazzoni R., Bischoff S.C., Busetto L., Cederholm T., Chourdakis M., Cuerda C., et al. Nutritional management of individuals with obesity and COVID-19: ESPEN expert statements and practical guidance. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fadeur M., Preiser J.C., Verbrugge A.M., Misset B., Rousseau A.F. Oral nutrition during and after critical illness: SPICES for quality of care! Nutrients. 2020;12(11) doi: 10.3390/nu12113509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunton C., Arensberg M.B., Drawert S., Badaracco C., Everett W., McCauley S.M. Perspectives of registered dietitian nutritionists on adoption of telehealth for nutrition care during the COVID-19 pandemic. Healthcare (Basel) 2021;9(2) doi: 10.3390/healthcare9020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ansu V., Papoutsakis C., Gletsu-Miller N., Spence L.A., Kelley K., Woodcock L., et al. Nutrition care practice patterns for patients with COVID-19 - A preliminary report. JPEN J Parenter Enteral Nutr. 2021 doi: 10.1002/jpen.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salazar E., Yen P.B., Ye C.L., Boo T.L., Wen Y.P., Chuen M.C.C. A modified minimal contact COVID-19 workflow allows for safe, remote, parenteral nutrition prescribing in non-critically ill patients. JPEN J Parenter Enteral Nutr. 2021;45(6):1364–1368. doi: 10.1002/jpen.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.