- PwMS
people with multiple sclerosis
- DMT
disease modifying therapy
- CD19+%
CD19+ B-cell population as percentage of total lymphocyte population
- EID
extended interval dosing
- SID
standard interval dosing
- EDSS
expanded disability status scale
Summary.
In this retrospective clinical audit, 136 patients with MS received Ocrelizumab during the COVID-19 pandemic. There was no significant difference in clinical relapse rate or radiological activity between 67 patients who received extended interval dosing (EID) (≥30 weeks, mean 48.3 ± 5.9 weeks) compared to standard interval dosing (<30 weeks) with average follow-up of over 4 months. CD19+ B-cell repopulation occurred in 94% (p<0.001) of EID patients at re-infusion and correlated strongly with re-dosing interval (rs=0.738, p=<0.0001) but was not associated with inflammatory disease activity. EID did not impact short-term disease activity, despite significant CD19+ B-cell repopulation and warrants long-term prospective study.
Alt-text: Unlabelled box
1. Introduction
The COVID-19 pandemic has significantly impacted the healthcare of many people with multiple sclerosis (PwMS) causing cancelled or delayed appointments, investigations and disease modifying therapy (DMT) (Vogel et al., 2020).
Anti-CD20 therapies were posited to increase risk of hospitalisation or intensive care admission with COVID-19 infection (Simpson-Yap et al., 2021). The Association of British Neurologists suggested delaying Ocrelizumab re-infusion during highly infectious periods using CD19+ B-cell repopulation (1% of total lymphocyte population) (Coles et al., 2020). From 15th July 2020, PwMS on Ocrelizumab at the Royal Stoke MS centre were routinely offered blood sampling for CD19+ B-cell population of total lymphocytes (CD19+%). With increasing use of telemedicine and hospital avoidance behaviours (Portaccio et al., 2021), practically CD19+% was obtained when patients attended hospital for infusions.
In this retrospective audit, we reviewed the clinical impact of Ocrelizumab extended interval dosing (EID) resulting from the COVID-19 pandemic at our tertiary neuroscience centre and explore associations of CD19+% with EID, clinical relapses and radiological activity.
2. Methods
We retrospectively reviewed electronic records of all PwMS on Ocrelizumab and identified patients who received standard interval dosing (SID) (<30 weeks) versus EID (≥30 weeks) during the COVID-19 pandemic.
Inclusion criteria were prior completion of first Ocrelizumab cycle (two 300 mg infusions 14 days apart), subsequent 600 mg infusion during the COVID-19 pandemic and CD19+% sampling. Data was extracted for age, sex, MS phenotype, disease duration, Expanded Disability Status Scale (EDSS) score, previous DMTs, past ocrelizumab cycles and dates of previous and subsequent ocrelizumab cycles. We reviewed clinical notes for relapses and MRI reports since previous ocrelizumab cycle for evidence of radiological activity (gadolinium-enhancing or new/enlarging T2 lesions).
The University Hospitals of North Midlands audit office approved this study. Ethical approval and consent was not sought, as this audit did not constitute research according to NHS Health Research Authority guidance.
2.1. Statistical analysis
We used IBM SPSS 23 for statistical analysis. Mann-Whitney U, Chi-square and Fisher's Exact tests were performed to compare continuous and categorical variables between groups. Results were considered significant at p<0.05 level. Spearman's rank-order correlation was performed to assess relationship between re-dosing interval and CD19+%. Binary logistic regression analyses were performed to assess whether CD19+% (>1%) or re-dosing interval (as independent variable) could predict binary (yes/no) outcomes for clinical relapses or radiological activity (dependant variables) with demographic variables as covariates.
3. Results
From 152 patients who previously received a full cycle of Ocrelizumab, 16 patients were excluded; 4 patients who did not have a subsequent infusion and 12 patients who did not have CD19+% sampling. Of 136 patients included, 69 patients had SID and 67 patients had EID (see Table 1 ).
Table 1.
Baseline characteristics and results.
| Extended Interval dosing (EID) | Standard interval dosing (SID) | p-value | |||||
|---|---|---|---|---|---|---|---|
| Baseline characteristics | n = 67 | n = 69 | |||||
| Age | 46.0 ±+ 10.0 | 43.4 ± 13.8 | 0.310a | ||||
| Disease duration | 9.9 ± 7.2 | 9.6 ± 7.1 | 0.828a | ||||
| Female, n (%) | 54 (81%) | 51 (74%) | 0.353b | ||||
| EDSS | 3.4 ± 1.6 | 3.1 ± 1.7 | 0.282a | ||||
| RRMS, n (%) | 63 (94%) | 63 (91%) | 0.745c | ||||
| PPMS, n (%) | 4 (6%) | 6 (9%) | |||||
| Previous DMTs, n (%) | 0 | 32 (48%) | 33 (48%) | 0.536b | |||
| 1 | 22 (33%) | 16 (23%) | |||||
| 2 | 9 (13%) | 14 (20%) | |||||
| 3 | 4 (6%) | 5 (7%) | |||||
| 4 | 0 | 1 (1%) | |||||
| First line injectables, n | 20 | 24 | 0.539b | ||||
| First line orals, n | 8 | 12 | 0.370b | ||||
| Fingolimod, n | 2 | 6 | |||||
| Alemtuzumab, n | 1 | 0 | |||||
| Natalizumab, n | 19 | 12 | |||||
| Prior infusions n (%) | 1 | 41 (61%) | 32 (46%) | 0.001*b | |||
| 2 | 26 (39%) | 24 (35%) | |||||
| 3+ | 0 | 13 (19%) | |||||
| Results | |||||||
| Re-dosing interval, weeks (range) | 48.3 ± 5.9 (32.4–56.6) | 25.4 ± 1.5 (22.7–29.7) | |||||
| Follow-up, weeks | 19.1 ± 5.7 | 20.8 ± 11.7 | |||||
| Total time observed, weeks | 67.3 ± 4.9 | 46.2 ± 12.1 | |||||
| CD19+ cells/μL | 99.8 ± 77.8 | 14.3 ± 41.3 | <0.001*a | ||||
| CD19+ count >100 cells/μL, n (%) | 26 (39%) | 3 (4%) | <0.001*b | ||||
| CD19+% | 5.51 ± 3.71 | 0.75 ± 1.89 | <0.001*a | ||||
| CD19+ level >1%, n (%) | 63 (94%) | 12 (17%) | <0.001*b | ||||
| Clinical Relapse (yes/no) | 2/67 (3.0%) | 3/69 (4.3%) | 1.000c | ||||
| Radiological Activity (yes/no) | 5/44(11%) | 6/40 (15%) | 0.622b | ||||
| New/enlarging T2 lesions | 5 | 6 | |||||
| Gadolinium enhancement | 0 | 3 | |||||
EID and SID patients did not differ significantly in terms of age, gender, disease duration, clinical phenotype, DMT use or baseline EDSS. However, EID appeared more common in patients with one prior Ocrelizumab infusion whilst patients with 3 or more prior infusions had no delays (p<0.001). The mean re-dosing interval was 48.3 weeks for EID versus 25.4 weeks for SID (mean difference 22.9 weeks). Retrospective follow-up was 19.1 weeks after EID and 20.8 weeks after SID. There were no significant differences in clinical relapses or radiological activity between EID and SID groups.
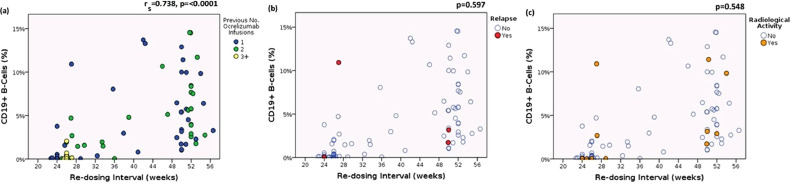
EID patients had significantly higher CD19+% (p<0.001) and CD19+ B-cell counts (p<0.001), with 94% of EID patients CD19+ replete (>1%) by next ocrelizumab cycle compared with 17% of SID patients (p<0.001). CD19+% correlated strongly with re-dosing interval (rs=0.738, p=<0.0001) but was not associated with clinical relapses or radiological activity (see Fig. 1 ).
Fig. 1.
Association of CD19±% with re-dosing interval, number of previous infusions, relapses and radiological activity
CD19+%: CD19+ B-cell population expressed as percentage of total lymphocytes
(a) Association of CD19+% with re-dosing interval and number of previous infusions. CD19+% is strongly correlated with re-dosing interval in all patients. Note that there were no patients with ≥3 infusions who had delayed treatment (b) Association of CD19+% with re-dosing interval and relapses (c) Association of CD19+% with re-dosing interval and radiological activity.
p-values shown in (b) and (c) refer to the association of CD19+% repletion (>1%) with either clinically documented relapse or radiological activity.
Binary logistic regression analyses found neither re-dosing interval nor CD19+% could predict clinical relapses of radiological activity.
4. Discussion
In this retrospective study, 48.3 week mean EID showed no effect on inflammatory disease activity over 19.1 weeks follow-up, despite CD19+ repopulation in almost all (94%) EID patients.
Our real-world data mirrors results from the Ocrelizumab phase II extension trial, where annualised relapse rates and 6-month disability progression remained low during 12–18 months treatment-free with fewer adverse effects and infections (Baker et al., 2020). The small number of patients imaged had no radiological activity. CD19+ B-cells took median 15 months to return to baseline or lower limit of normal. Although, we did not have baseline CD19+counts, 39% of EID patients had CD19+counts above the lower limit of normal (100 cells/μL).
Similar observations of EID have been reported during the COVID-19 pandemic, albeit with shorter EID intervals and follow-up duration. Personalised Ocrelizumab dosing using CD19+ B-cell counts led to EID in most patients, with low disease activity (van Lierop et al., 2021). Recently, a small retrospective analysis of 33 patients, showed no clinical consequences from mean EID of 33 weeks, although lacked radiological data (Barun et al., 2021). Moreover, a retrospective multicentre cohort study showed stable disease activity 3 months following EID in 116 patients with median delay of nearly 9 weeks. CD19+ B-cell counts were similarly not associated with re-emergent disease activity, although the majority of EID patients remained CD19+ deplete (Rolfes et al., 2021).
Tailoring EID to memory B-cell (CD19+ CD27+ co-expression) repopulation may be better, as early CD19+ repopulation produces immature and immune naïve B-cells from the bone marrow rather than pathogenic memory B-cells (Baker et al., 2020). This has been shown to reduce reinfusion requirements, whilst maintaining reduction of disease activity in PwMS on Rituximab. (Novi et al., 2020) EID to memory B-cells may also allow improved vaccine responses through naïve B-cell repopulation (Baker et al., 2020).
This study has limitations. Relatively low relapse rates in both groups suggest treatment efficacy, but limit statistical power to detect differences between dosing groups. Radiological activity may be missed as patients were not scanned regularly and not all patients were imaged, although >60% of our patients had MRI scans. Similarly, disability progression was not measured, as EDSS follow-up data was not acquired during the pandemic. We also cannot determine the longer-term consequences of EID, either from a single delayed dose or continued EID regimen.
In conclusion, our real-world observational data suggests that Ocrelizumab EID does not impact short-term disease activity, despite CD19+ B-cell repopulation and offers practical reassurance if delaying Ocrelizumab re-dosing to mitigate risk of adverse events. However, prospective studies of continued EID versus SID are necessary to address long-term efficacy, safety, and cost-effectiveness, as well as to identify optimum dosage interval and monitoring.
Declaration of Competing Interest
None.
Funding
No targeted funding was received for this study.
References
- Baker D., Roberts C.A.K., Pryce G., et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin. Exp. Immunol. 2020;202:149–161. doi: 10.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Pryce G., James L.K., et al. The ocrelizumab phase II extension trial suggests the potential to improve the risk: benefit balance in multiple sclerosis. Mult. Scler. Relat. Disord. 2020;44 doi: 10.1016/j.msard.2020.102279. [DOI] [PubMed] [Google Scholar]
- Barun B., Gabelić T., Adamec I., et al. Influence of delaying ocrelizumab dosing in multiple sclerosis due to COVID-19 pandemics on clinical and laboratory effectiveness. Mult. Scler. Relat. Disord. 2021;48 doi: 10.1016/j.msard.2020.102704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles A., Lim M., Giovannoni G., et al., 2020. ABN guidance on the use of disease-modifying therapies in multiple sclerosis in response to the threat of a coronavirus epidemic. Available from: https://www.theabn.org/resource/collection/65C334C7-30FA-45DB-93AA-74B3A3A20293/ABN_Guidance_on_DMTs_for_MS_and_COVID_19_VERSION_18_May_FINAL.pdf [Accessed 20 September 2021].
- Novi G., Bovis F., Fabbri S., et al. Tailoring B cell depletion therapy in MS according to memory B cell monitoring. Neurol. Neuroimmunol. Neuroinflamm. 2020;7:e845. doi: 10.1212/NXI.0000000000000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaccio E., Fonderico M., Hemmer B., et al. Impact of COVID-19 on multiple sclerosis care and management: results from the European Committee for Treatment and Research in Multiple Sclerosis survey. Mult. Scler. J. 2021 doi: 10.1177/13524585211005339. Epub ahead of print. PMID: 33764197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfes L., Pawlitzki M., Pfeuffer S., et al. Ocrelizumab extended interval dosing in multiple sclerosis in times of COVID-19. Neurol. Neuroimmunol. Neuroinflamm. 2021;8:e1035. doi: 10.1212/NXI.0000000000001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Yap S., De Brouwer E., Kalincik T., et al., 2021. Associations of DMT therapies with COVID-19 severity in multiple sclerosis. medRxiv. 02.08.21251316. Doi: 10.1101/2021.02.08.21251316. [DOI] [PMC free article] [PubMed]
- Vogel A.C., Schmidt H., Loud S., et al. Impact of the COVID-19 pandemic on the health care of >1,000 People living with multiple sclerosis: a cross-sectional study. Mult. Scler. Relat. Disord. 2020;46 doi: 10.1016/j.msard.2020.102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lierop Z.Y., Toorop A.A., van Ballegoij W.J., et al. Personalized B-cell tailored dosing of ocrelizumab in patients with multiple sclerosis during the COVID-19 pandemic. Mult. Scler. 2021 doi: 10.1177/13524585211028833. Epub ahead of print. PMID: 33764197. [DOI] [PMC free article] [PubMed] [Google Scholar]