Abstract
The coronavirus disease 2019 (COVID-19) pandemic has been challenging for healthcare professionals worldwide. One of the populations affected by the pandemic are patients on renal replacement therapy, as kidney disease is an independent risk factor for severe COVID-19 and maintenance dialysis (a life-sustaining therapy) cannot be interrupted in the vast majority of cases. Over the past months, several authors and medical societies have published recommendations and guidelines on the management of this population. This article is a comprehensive review regarding the measures to prevent, contain and deal with a COVID-19 pandemic in the dialysis setting. We recapitulate the epidemiology and pathophysiology of COVID-19 in kidney dysfunction and present the main recommendations concerning the screening of healthcare personnel, dialysis patients and visitors as well as measures to improve the safety of the dialysis facilities’ environments. In addition to preventive measures, this article briefly describes actions directed towards management of an outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) within a dialysis facility, the management of complications in dialysis patients with COVID-19 and overall data regarding the management of children with kidney disease.
Keywords: COVID-19; SARS-CoV-2; Renal dialysis; Renal replacement therapy; Hemodialysis units, Hospital
Core Tip: Dialysis patients are more vulnerable to develop severe coronavirus disease 2019 (COVID-19) infection. To minimize risks, some measures should be followed by dialysis units, healthcare personnel, patients and visitors. Until vaccination against COVID-19 is widely available to dialysis patients worldwide, an evidence-based approach is required to avoid the spread of the virus and consequently more death of patients.
INTRODUCTION
The outbreak of the coronavirus disease 2019 (COVID-19) pandemic in early 2020 proved to be a massive challenge for healthcare professionals all around the world. Clinically, its symptoms range from pulmonary (e.g., cough and dyspnea) to extrapulmonary manifestations (e.g., fever, myalgia, anosmia and ageusia), revealing the systemic nature of the aforementioned malady[1,2].
Due to the aforementioned variety of clinical manifestations attributed to the infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), different areas of the medical field became highly interested in the better comprehension of COVID-19, one of them being nephrology. The glomerular epithelium, the proximal tubular cells of the nephrons and endothelial cells have considerable levels of angiotensin-converting enzyme 2, which explains why COVID-19 patients may develop renal injury[3-6].
Such interest emerged as doctors recognized the necessity for guaranteeing the safety of patients treated with renal replacement therapy (RRT) during the pandemic, focusing on preventing an outbreak in dialysis units. The attentiveness to COVID-19 by nephrologists was reinforced when multiple studies from different countries suggested that patients who acquire the disease have a significant risk for developing acute kidney injury (AKI)[7-9].
In this article, we review the epidemiology of COVID-19 in dialysis centers as well as the main recommendations concerning the screening of healthcare personnel (HCP), dialysis patients and visitors, the safety of the dialysis facilities’ environments, the conduct regarding an outbreak of SARS-CoV-2 infection within a dialysis facility, the management of complications in dialysis patients with COVID-19 and the conduct directed towards children with kidney disease.
EPIDEMIOLOGY OF COVID-19 IN DIALYSIS UNITS
As of March 2021 there have been over 125 million confirmed cases of COVID-19 and over 2.7 million deaths, giving the disease a case fatality rate of 2.22%[10]. The currently available literature suggests that the frequency of COVID-19 among dialysis patients is approximately between 2% and 20%, a difference possibly explained by the region in which each study was conducted[11-14]. Meanwhile, the proportion of infected individuals appears to be lower in other health services, for both HCP and patients, and also in the general community[13-17].
Infection by SARS-CoV-2 in dialysis units does not seem to depend on sex, ethnicity, time of dialysis or presence of diabetes but is likely associated with in-center dialysis and older patient age; the higher risk of infection in healthcare facilities has been attributed to a higher rate of self-reported illness among the staff[11,18]. Chronic kidney disease (CKD) patients, especially those in dialysis, are more vulnerable to SARS-CoV-2 infection, given that a decrease in the estimated glomerular filtration rate has been associated with death by COVID-19 in one large cohort study that obtained data using OpenSAFELY[19]. The mortality of dialysis patients who contracted COVID-19 is approximately between 21% and 33%, being above both the general population’s death rate due to SARS-CoV-2 infection[12,14,20,21]. Some studies have also shown that hemodialysis (HD) patients are more likely to contract the disease than peritoneal dialysis patients, something that is at least partially explained by the fact that HD patients cannot perform dialysis at home, while peritoneal dialysis patients can[13,14].
COVID-19 can also cause AKI. It has been documented that about one fifth of patients with the disease end up developing AKI treated with RRT (AKI-RRT). CKD is associated with higher risk of developing AKI-RRT among COVID-19 patients as well as diabetes mellitus, hypertension, higher body mass index and high levels of D-dimer. The mortality is extremely high among AKI-RRT patients with COVID-19, even more than in the previously mentioned group, reaching levels above 60%[22].
HEALTHCARE PERSONNEL, PATIENTS AND VISITORS
It is known that AKI and RRT increase the risk of complications and death in COVID-19, so it is necessary to follow specific rules to avoid infection[23,24]. In addition, HD units are classified as high risk of contagion, hence the need to further tighten these measures in these environments[25]. It is possible to divide protective actions into measures for HCP, for patients and visitors.
The first group includes doctors at the HD unit, nurses, technicians and cleaning staff[26], and they must receive the following instructions: (1) The use of personal protective equipment (PPE: surgical or N95 masks, gloves, hair caps and clothing with waterproof insulation) must be mandatory and constant[26-28]; (2) Educational actions on how to properly use PPE, how to properly sanitize hands and how to dispose of contaminated items should be promoted[26]; (3) Updates and training on new knowledge related to the epidemic need to be encouraged[26,27]; (4) Nurses must be trained to collect the nasopharynx swab to perform the COVID-19 polymerase chain reaction (PCR)[26]; (5) Groups of face-to-face activities, including discussion groups, ought to be avoided and should be done digitally[27,29]; (6) Teams from different parts of the health unit must have meals at different times in order to avoid contact[27]; (7) The team should, if possible, avoid using public transport as well as participating in large agglomerations[27,29]; (8) The presence of COVID-19 symptoms in the team as well as in their family members should be monitored closely. Members with suspected infection should notify the unit, perform the PCR for COVID-19 and quarantine themselves in order to avoid contaminating patients[26,27]; and (9) HCP vaccination against SARS-CoV-2 should be implemented on a large scale as soon as possible[30,31].
Patients also need to take several protective measures in order to further mitigate the possibility of contagion, such as: (1) The use of surgical masks, N95 or similar should be mandatory and the use of homemade cloth masks should be discouraged. However, due to economic reasons and the low availability of surgical masks, N95 or similar, some emerging countries recommended universal use of cloth masks for dialysis patients[32]. Although these are a better option than not using masks, surgical masks are about three times more effective in blocking the transmission of the virus[26,27,29,33-35]; (2) Educational measures, such as avoiding the use of public transport, practicing social isolation, wearing appropriate face masks, not traveling, staying away from agglomerations, preventing contact with people outside your residence, must be promoted[26,27,29]; (3) It is necessary to instruct, even in the dialysis units, on proper hand hygiene, on the cough etiquette and on the main symptoms of COVID-19[26,27,29,33]; (4) The medicines previously prescribed must be continued, with due medical follow-up. This includes angiotensin-converting enzyme inhibitors, other medications for the treatment of hypertension, glucocorticoids, immunosuppressants, medications for diabetes and anemia and any other necessary for the patient[27,36]; (5) Vaccination against influenza should be encouraged in dialysis units[29]; (6) Measures of attention to psychosocial care must be taken, as dialysis patients are predisposed to problems such as anxiety, depression and insomnia during the pandemic[27]; (7) Vaccination against SARS-CoV-2 in patients with kidney disease should be implemented on a large scale as soon as possible. So far, this is the most effective measure in the prevention and containment of COVID-19[30,31]; and (8) If possible, the patient should be transferred to a home dialysis program[37].
Dialysis units should be encouraged to decrease the flow of people during the pandemic; therefore, it is not indicated that other individuals accompany patients on dialysis[26,29]. It can be allowed in situations of extreme need, judged on a case-by-case analysis. In this matter, it is recommended that the companions wear surgical masks, N95 or similar and obey the same basic rules as dialysis patients, e.g., social distancing[26,28,29,33].
SAFETY OF DIALYSIS FACILITIES
The pandemic reinforced the importance of a safe environment for dialysis. Although current recommendations advise prioritizing the use of telehealth whenever is deemed possible[38], dialysis patients’ demands are not always solved by those services alone. Thus, the ongoing scenario required that dialysis units adapted themselves to minimize SARS-CoV-2 infection rates within their installations.
General measures include the patient assessment for COVID-19 symptoms or exposure to diseased individuals in every dialysis session and planning for SARS-CoV-2 viral detection testing. In general, testing for COVID-19 (and other respiratory diseases) in outpatient HD facilities and home dialysis should be considered if the individual presents any signs or symptoms of the illness, even mild and atypical ones, or if there is suspicion of exposure to someone potentially infected with the virus.
It is also the role of the facility to ensure that the screening of staff, patients and visitors is being adequately done, including body temperature checking at entrance (and at both start and end of the dialysis session for patients) and that all rooms are well ventilated[26,29,39-41].
Also, safe patient placement is an important component of the strategy that dialysis facilities have been following. It is highly advisable that the minimum separation of six feet (approximately 180 cm) between patients, either in a waiting area or in the treatment area, is ensured in the whole facility. The same guidance applies to cohorting patients unless the individuals in question are confirmedly infected with the disease, in which case they can be cohorted together. Whenever possible, patients with suspected or confirmed SARS-CoV-2 infection should go through dialysis in a separate room. Also, single use of dialyzers is highly recommended in patients with confirmed or suspected cases of COVID-19; the once widespread (and still a reality in emerging countries) practice of reusing dialyzers should be avoided in patients with SARS-CoV-2 infection[29,42].
Given that the coronaviruses can persist on surfaces like glass, metal and plastic, cleaning and disinfection (C&D) has been frequently recommended to counteract SARS-CoV-2 transmission. The standard C&D course of action is considered satisfactory for COVID-19 cases, but the chemical product used for surface disinfection has to be capable of inactivating SARS-CoV-2, e.g., ethanol, sodium hypochlorite and hydrogen peroxide[42,43]. It has been recommended that bed linens get changed between shifts and that the used ones are correctly contained or laundered, that constantly touched surfaces within the dialysis units are cleaned and disinfected regularly and that the adequate PPE is equipped when C&D is being performed[42,44].
However, it is arguable that too much focus is being directed towards C&D. Studies have suggested that the risk of infection by fomites is low and often exaggerated due to the inapplicability of the circumstances obtained in an artificial lab environment in daily life situations[45-47]. The reasons why C&D remains a constant aspect of many guidelines despite its apparent low impact on the dissemination of SARS-CoV-2 vary from public expectation and reliance on C&D protocols, as seen in cases in which people fumigate and/or wash the streets and sidewalks, measures which have been deemed by health authorities as ineffective[48].
As previously mentioned, telehealth plays a pivotal role in the current pandemic and should be used wherever and whenever possible. Even though it does not satisfy every need a dialysis patient may have, given that it is a complementary practice and not a substitutive one. Its benefits must not be downplayed, especially on the subject of home dialysis. There have been reports regarding the benefits of telehealth in dialysis in patients, released both before and during the pandemic and especially concerning peritoneal dialysis[49-51]. However, the quality of the obtained evidence is disputed[52,53]. Special attention must be given to specificities of home dialysis care, such as the likelihood of shortages of PPE and peritoneal dialysis fluid and the higher possibility of developing a more severe form of COVID-19. Dialysis facilities should also provide useful guidance to patients who are dialyzed at home and update their HCP on the clinical knowledge of COVID-19[27,54].
DEALING WITH AN OUTBREAK
Despite all the protective measures being taken, it is still possible for a case of COVID-19 to appear in the dialysis units precisely because of the current pandemic. During an outbreak period, several patients and doctors visit dialysis units in general, which makes them a high-risk environment for nosocomial coronavirus infection[55]. In this scenario, it is possible to deal with two types of cases: (1) Suspected infection in patients or visitors; and (2) In HCP.
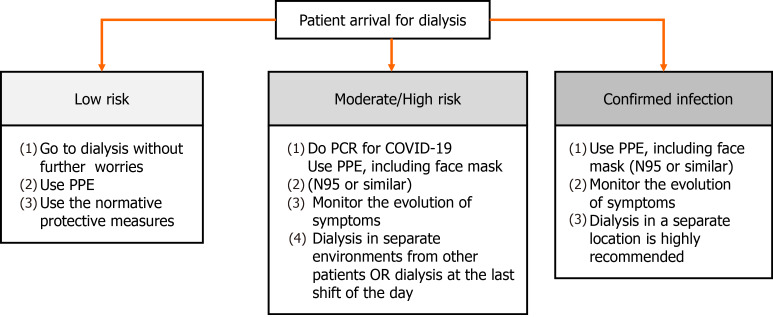
Still at the entrance to the dialysis unit, patients and visitors must undergo both symptomatic (e.g., presence of fever, dyspnea, myalgia, coughing and sneezing) and epidemiological screening (e.g., contact with people positive for COVID-19 in the last 14 d)[42,56-58]. If the patient is at low risk of infection, he must be referred to dialysis and must obey the protective measures already addressed in this article, e.g., wearing PPE and keeping a minimum distance of 6 feet from other people[42,58]. If the patient has symptoms of COVID-19 or has had contact with someone who is positive for the virus, he must do the PCR for the disease and has to be treated as moderate/high risk for infection. Also, the monitoring of the evolution of the symptoms is mandatory, even if it is absent. In such cases, as previously mentioned, patients have to wear PPE and must be dialyzed in separate environments from other patients, with the door closed. If this is not possible, treatment should be carried out at the end of the day, in places away from the main passage of personnel, such as at the end of the corridor or in a corner[42,56,58,59]. If the patient already has a positive PCR for COVID-19, care must be increased and dialysis in a separate location is highly recommended[42]. These recommendations can be seen in Figure 1.
Figure 1.
Conduct related to dialysis patients, stratified according to their risk or current status of being infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19: Coronavirus disease 2019; PCR: Polymerase chain reaction; PPE: Personal protective equipment.
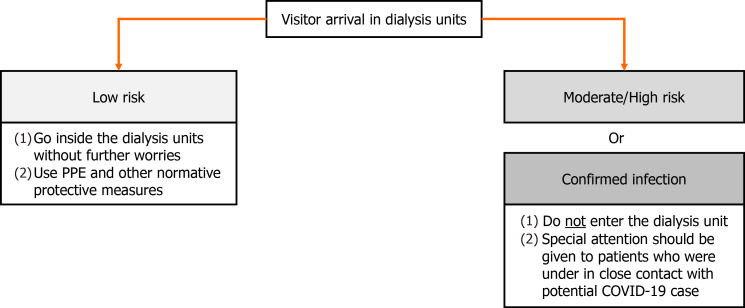
If the visitors are symptomatic or were in close contact with people with COVID-19 in the last 14 d, their entry should be prohibited. If they are classified as a low risk of infection, they should continue to follow protective measures against COVID-19 within healthcare centers. In addition, it is recommended that only patients confirmed for COVID-19 are dialyzed together whenever the facility’s infrastructure enables, thus patients with suspicion of SARS-CoV-2 infection (not yet confirmed) ought to be treated separately from them[42,56,59]. The healthcare team responsible for the treatment of patients suspected or confirmed disease should use N95 or equivalent or higher-level respirator, eye protection, glove and isolation gown[42]. Recommendations towards visitors are shown in Figure 2.
Figure 2.
Conduct in regard to visitors in a dialysis facility. COVID-19: Coronavirus disease 2019; PPE: Personal protective equipment.
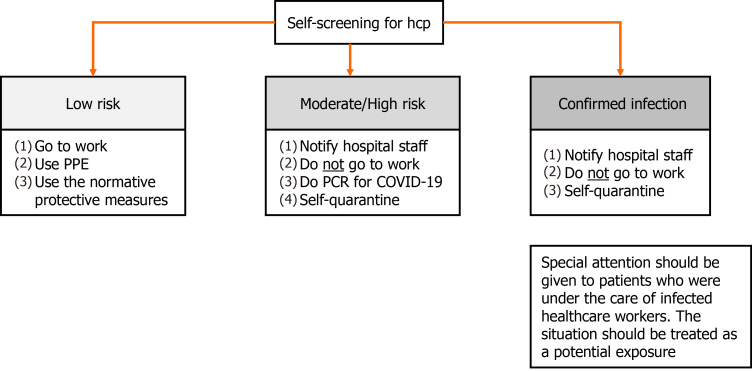
In a situation in which the outbreak originates from the healthcare team, two fronts of action should be adopted. First, the healthcare worker must be immediately and temporarily relieved from work and has to self-quarantine for 14 d or 10 d as long as remains asymptomatic for 3 consecutive days from the last exposure to a contaminated individual[42,60,61]. Furthermore, special attention should be given to patients who were under the care of this HCP. If the patient had contact with the infected individual at a distance of less than 6 feet for more than 15 min, the situation should be treated as a potential exposure. If the patient wears a surgical mask at the moment of contact, he will be considered as a low risk for infection and other symptoms should be monitored without further concern. However, if the mask used is homemade or even without a mask, the patient will be considered at high risk for infection and all the measures described previously must be taken into action (Figure 3)[42].
Figure 3.
Self-screening for healthcare personnel, stratified according to their risk or current status of being infected by severe acute respiratory syndrome coronavirus 2. COVID-19: Coronavirus disease 2019; HCP: Healthcare personnel; PCR: Polymerase chain reaction; PPE: Personal protective equipment.
Finally, it is important to note that the perception of a nosocomial transmission of COVID-19 is challenging due to the large circulation of people and the possibility that they have acquired the infection outside the units. Still, if this type of transmission is identified, the situation must be considered an outbreak and containment measures must be taken immediately[42,55].
MANAGEMENT OF COMPLICATIONS
It should be reiterated that dialysis patients cannot interrupt RRT. Therefore, aside from the previously mentioned conducts related to preventing the dissemination of the virus within the facility, HCP must be able to know how to deal with possible renal complications in COVID-19 patients. The nephrologist plays a crucial role in the correct management of aggravations such as AKI, electrolyte imbalance and acid-base disorders.
The physiopathology of AKI in COVID-19 is not thoroughly known, but it is believed that it originates from a multitude of factors. Some of the proposed pathophysiological mechanisms relate to both prerenal and renal AKI, such as direct viral-related injury, corporal fluid disbalance, cytokine release syndrome, overstimulation of the renin-angiotensin-aldosterone system, hypercoagulation, complement system dysregulation and multiple organ dysfunction syndrome[62,63].
Regarding RRT, the basic principle that guides all the others is that the entry of HCP in isolated areas must be limited and preference should be given to those who have already developed an effective immune response to SARS-CoV-2. When it comes to choosing the dialysis modality for AKI patients, continuous RRT offers some considerable benefits regarding less physical contact between HCP and patients. However, due to the variability of resources in each healthcare setting, continuous RRT might not be available for a wide population. Therefore, other modalities might be more logistically adequate to use in certain areas[64]. Vascular access for RRT is usually done in the right jugular vein. While the left jugular vein comes as a natural second option, the femoral access has been suggested for consideration in order to reduce the likelihood of HCP contamination[65]. Also, the intensity of RRT in AKI related to COVID-19 should not be any different compared to the usual one, unless proven different[66]. It has been suggested that early RRT intervention in COVID-19 patients may provide benefits[67], but that assumption is not yet scientifically supported since one previous study detected no significant differences between early and delayed RRT start in general dialysis patients[68].
COVID-19 IN THE NEPHROPEDIATRIC POPULATION
Although CKD is considerably more frequent in the adult population, children are also susceptible to the development of renal impairment. Data regarding infants and teenagers with CKD is scarce, therefore it is difficult to determine any reliable values for its incidence and prevalence in this population.
As a possible reflex of the rarity of severe COVID-19 cases in children, there are few studies related to the damages of the aforementioned disease in the lives of said individuals, and those who exist are not enough to build a solid evidence-based approach. One of them, an Italian national-scale study, attempted to determine the impact of the pandemic in children with CKD or immunosuppression related to kidney transplant but found no severe cases of COVID-19 among individuals under the age of 18. That same research, on the other hand, estimated that around 80% of children with CKD have a glomerular filtration rate ≤ 60 mL/min/1.73 m² and that 25% of this fraction are under dialysis treatment[69]. A Spanish retrospective study (n = 16) also concluded that there seems to be no difference in the actual clinical course of the disease between healthy children and children with CKD but reiterated that special attention should be brought upon fluid management and the adjustment of drug doses[70]. Other case reports have been encountered; however, due to the limited methodological design intrinsic to these types of studies, they do not provide any information that can be applied in a larger scenario[71,72].
There were no registries of COVID-related AKI cases among children without chronic renal pathologies. As a result of the relative absence of information or overall existence of clinically relevant COVID-19 cases in pediatric nephrological patients, the guidelines directed to them do not differ much when compared to the ones orientated towards the adult population[73].
CONCLUSION
In summary, dialysis patients are more vulnerable to develop severe COVID-19 and are at higher risk of a worst prognosis. Because of that, it is necessary to secure that the counteractive measures related to the pandemic are being thoroughly followed by dialysis units and HCP alike as well as ensuring that patients and visitors adhere to this public health commitment. However, even if all is correctly done, an outbreak can still occur in the dialysis unit setting. Until the vaccine against COVID-19 is widely available to dialysis patients worldwide, an evidence-based approach is required to avoid the spread of the virus and consequently the death of patients.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Manuscript source: Invited manuscript
Corresponding Author’s Membership in Professional Societies: Brazilian Society of Nephrology; International Society of Nephrology; American Society of Nephrology.
Peer-review started: April 21, 2021
First decision: June 7, 2021
Article in press: August 13, 2021
Specialty type: Urology and nephrology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bhatt KP, Wang MK, Yu L S-Editor: Wang JL L-Editor: Filipodia P-Editor: Xing YX
Contributor Information
Gabriel Martins Nogueira, Department of Medicine, Bahiana School of Medicine and Public Health, Salvador 40290-000, Brazil.
Moisés Santana Oliveira, Department of Medicine, Bahiana School of Medicine and Public Health, Salvador 40290-000, Brazil.
Ana Flávia Moura, Department of Internal Medicine, Bahiana School of Medicine and Public Health, Salvador 40290-000, Brazil.
Constança Margarida Sampaio Cruz, Department of Internal Medicine, Bahiana School of Medicine and Public Health, Salvador 40290-000, Brazil; Department of Internal Medicine, Hospital Santo Antônio, Salvador 40415-006, Brazil.
José A Moura-Neto, Department of Internal Medicine, Bahiana School of Medicine and Public Health, Salvador 40290-000, Brazil. mouraneto@bahiana.edu.br.
References
- 1.Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, Zhang HY, Sun W, Wang Y. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soler MJ, Wysocki J, Batlle D. ACE2 alterations in kidney disease. Nephrol Dial Transplant. 2013;28:2687–2697. doi: 10.1093/ndt/gft320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Stang MB, Desenclos J, Flamant M, Chousterman BG, Tabibzadeh N. The Good Treatment, the Bad Virus, and the Ugly Inflammation: Pathophysiology of Kidney Involvement During COVID-19. Front Physiol. 2021;12:613019. doi: 10.3389/fphys.2021.613019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YT, Shao SC, Hsu CK, Wu IW, Hung MJ, Chen YC. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24:346. doi: 10.1186/s13054-020-03009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available from: https://covid19.who.int/
- 11.Corbett RW, Blakey S, Nitsch D, Loucaidou M, McLean A, Duncan N, Ashby DR West London Renal and Transplant Centre. Epidemiology of COVID-19 in an Urban Dialysis Center. J Am Soc Nephrol. 2020;31:1815–1823. doi: 10.1681/ASN.2020040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creput C, Fumeron C, Toledano D, Diaconita M, Izzedine H. COVID-19 in Patients Undergoing Hemodialysis: Prevalence and Asymptomatic Screening During a Period of High Community Prevalence in a Large Paris Center. Kidney Med. 2020;2:716–723.e1. doi: 10.1016/j.xkme.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Meester J, De Bacquer D, Naesens M, Meijers B, Couttenye MM, De Vriese AS NBVN Kidney Registry Group. Incidence, Characteristics, and Outcome of COVID-19 in Adults on Kidney Replacement Therapy: A Regionwide Registry Study. J Am Soc Nephrol. 2021;32:385–396. doi: 10.1681/ASN.2020060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quintaliani G, Reboldi G, Di Napoli A, Nordio M, Limido A, Aucella F, Messa P, Brunori G Italian Society of Nephrology COVID-19 Research Group. Exposure to novel coronavirus in patients on renal replacement therapy during the exponential phase of COVID-19 pandemic: survey of the Italian Society of Nephrology. J Nephrol. 2020;33:725–736. doi: 10.1007/s40620-020-00794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee C, Baker M, Vaidya V, Tucker R, Resnick A, Morris CA, Klompas M CDC Prevention Epicenters Program. Incidence of Nosocomial COVID-19 in Patients Hospitalized at a Large US Academic Medical Center. JAMA Netw Open. 2020;3:e2020498. doi: 10.1001/jamanetworkopen.2020.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen LH, Drew DA, Joshi AD, Guo CG, Ma W, Mehta RS, Sikavi DR, Lo CH, Kwon S, Song M, Mucci LA, Stampfer MJ, Willett WC, Eliassen AH, Hart JE, Chavarro JE, Rich-Edwards JW, Davies R, Capdevila J, Lee KA, Lochlainn MN, Varsavsky T, Graham MS, Sudre CH, Cardoso MJ, Wolf J, Ourselin S, Steves CJ, Spector TD, Chan AT. Risk of COVID-19 among frontline healthcare workers and the general community: a prospective cohort study. medRxiv. 2020 doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alajmi J, Jeremijenko AM, Abraham JC, Alishaq M, Concepcion EG, Butt AA, Abou-Samra AB. COVID-19 infection among healthcare workers in a national healthcare system: The Qatar experience. Int J Infect Dis. 2020;100:386–389. doi: 10.1016/j.ijid.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu CM, Weiner DE, Aweh G, Miskulin DC, Manley HJ, Stewart C, Ladik V, Hosford J, Lacson EC, Johnson DS, Lacson E Jr. COVID-19 Among US Dialysis Patients: Risk Factors and Outcomes From a National Dialysis Provider. Am J Kidney Dis. 2021;77:748–756.e1. doi: 10.1053/j.ajkd.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Couchoud C, Bayer F, Ayav C, Béchade C, Brunet P, Chantrel F, Frimat L, Galland R, Hourmant M, Laurain E, Lobbedez T, Mercadal L, Moranne O French REIN registry. Low incidence of SARS-CoV-2, risk factors of mortality and the course of illness in the French national cohort of dialysis patients. Kidney Int. 2020;98:1519–1529. doi: 10.1016/j.kint.2020.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pio-Abreu A, do Nascimento MM, Vieira MA, de Menezes Neves PDM, Lugon JR, Sesso R. High mortality of CKD patients on hemodialysis with Covid-19 in Brazil. J Nephrol. 2020;33:875–877. doi: 10.1007/s40620-020-00823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, Sutherland A, Puri S, Srivastava A, Leonberg-Yoo A, Shehata AM, Flythe JE, Rashidi A, Schenck EJ, Goyal N, Hedayati SS, Dy R, Bansal A, Athavale A, Nguyen HB, Vijayan A, Charytan DM, Schulze CE, Joo MJ, Friedman AN, Zhang J, Sosa MA, Judd E, Velez JCQ, Mallappallil M, Redfern RE, Bansal AD, Neyra JA, Liu KD, Renaghan AD, Christov M, Molnar MZ, Sharma S, Kamal O, Boateng JO, Short SAP, Admon AJ, Sise ME, Wang W, Parikh CR, Leaf DE STOP-COVID Investigators. AKI Treated with Renal Replacement Therapy in Critically Ill Patients with COVID-19. J Am Soc Nephrol. 2021;32:161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52:1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunutsor SK, Laukkanen JA. Renal complications in COVID-19: a systematic review and meta-analysis. Ann Med. 2020;52:345–353. doi: 10.1080/07853890.2020.1790643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen G, Zhou Y, Zhang L, Wang Y, Hu RR, Zhao X, Song D, Xia JH, Qin Y, Chen LM, Li XM. Core principles for infection prevention in hemodialysis centers during the COVID-19 pandemic. Infect Control Hosp Epidemiol. 2020;41:865–866. doi: 10.1017/ice.2020.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basile C, Combe C, Pizzarelli F, Covic A, Davenport A, Kanbay M, Kirmizis D, Schneditz D, van der Sande F, Mitra S. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant. 2020;35:737–741. doi: 10.1093/ndt/gfaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Li SX, Zhao LF, Kong DL, Guo ZY. Management recommendations for patients with chronic kidney disease during the novel coronavirus disease 2019 (COVID-19) epidemic. Chronic Dis Transl Med. 2020;6:119–123. doi: 10.1016/j.cdtm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karkar A, Bouhaha BM, Dammang ML. Infection control in hemodialysis units: a quick access to essential elements. Saudi J Kidney Dis Transpl. 2014;25:496–519. doi: 10.4103/1319-2442.132150. [DOI] [PubMed] [Google Scholar]
- 29.Moura-Neto JA, Abreu AP, Delfino VDA, Misael AM, D'Avila R, Silva DRD, Andreoli MCC, Kraychete A, Bastos K, Nascimento MMD. Good Practice Recommendations from the Brazilian Society of Nephrology to Dialysis Units Concerning the Pandemic of the New Coronavirus (Covid-19) J Bras Nefrol. 2020;42:15–17. doi: 10.1590/2175-8239-JBN-2020-S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers of Disease Control and Prevention. Benefits of Getting a COVID-19 Vaccine. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html .
- 31.World Heath Organization. Evidence to recommendations for COVID-19 vaccines: Evidence framework. World Heath Organization, 2020: 1-9. [Google Scholar]
- 32.Abreu AP, Moura Neto JA, Delfino VDA, Palma LMP, Nascimento MMD. Recommendations from the Brazilian Society of Nephrology regarding the use of cloth face coverings, by chronic kidney patients in dialysis, during the new coronavirus pandemic (Covid-19) J Bras Nefrol. 2020;42:9–11. doi: 10.1590/2175-8239-JBN-2020-S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kliger AS, Cozzolino M, Jha V, Harbert G, Ikizler TA. Managing the COVID-19 pandemic: international comparisons in dialysis patients. Kidney Int. 2020;98:12–16. doi: 10.1016/j.kint.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies A, Thompson KA, Giri K, Kafatos G, Walker J, Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep. 2013;7:413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chughtai AA, Seale H, Macintyre CR. Effectiveness of Cloth Masks for Protection Against Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2610.200948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Yu J, Pan LY, Jiang HY. ACEI/ARB use and risk of infection or severity or mortality of COVID-19: A systematic review and meta-analysis. Pharmacol Res. 2020;158:104927. doi: 10.1016/j.phrs.2020.104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sociedad Latino Americana de Nefrologia e Hipertensión. Recomendaciones para el manejo de pacientes portadores de enfermedad renal frente a la epidemia de coronavirus (COVID-19). Jun 2020. [Google Scholar]
- 38.Centers of Disease Control and Prevention. Healthcare Facilities: Managing Operations During the COVID-19 Pandemic. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-hcf.html .
- 39.Centers of Disease Control and Prevention. Interim SARS-CoV-2 Testing Guidelines for Patients in Outpatient Hemodialysis Facilities. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/dialysis/testing-patients.html .
- 40.National Kidney Foundation. Dialysis & COVID-19. Available from: https://www.kidney.org/coronavirus/dialysis-covid-19 .
- 41.de Sequera Ortiz P, Quiroga B, de Arriba de la Fuente G, Macía Heras M, Salgueira Lazo M, Del Pino Y Pino MD en representación de la Sociedad Española de Nefrología. Protocol against coronavirus diseases in patients on renal replacement therapy: Dialysis and kidney transplant. Nefrologia (Engl Ed) 2020;40:253–257. doi: 10.1016/j.nefro.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centers of Disease Control and Prevention. Interim Additional Guidance for Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed COVID-19 in Outpatient Hemodialysis Facilities. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/dialysis.html .
- 43.Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lobo V, Khanna U, Rajapurkar M, Mahapatra HS, Verma H, Prasad N, Agarwal SK COVID-19 Working Group of Indian Society of Nephrology. Guidelines for Dialysis with Reference to COVID-19. Indian J Nephrol. 2020;30:166–170. doi: 10.4103/ijn.IJN_166_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect Dis. 2020;20:892–893. doi: 10.1016/S1473-3099(20)30561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ben-Shmuel A, Brosh-Nissimov T, Glinert I, Bar-David E, Sittner A, Poni R, Cohen R, Achdout H, Tamir H, Yahalom-Ronen Y, Politi B, Melamed S, Vitner E, Cherry L, Israeli O, Beth-Din A, Paran N, Israely T, Yitzhaki S, Levy H, Weiss S. Detection and infectivity potential of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) environmental contamination in isolation units and quarantine facilities. Clin Microbiol Infect. 2020;26:1658–1662. doi: 10.1016/j.cmi.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harvey AP, Fuhrmeister ER, Cantrell M, Pitol AK, Swarthout JM, Powers JE, Nadimpalli ML, Julian TR, Pickering AJ. Longitudinal monitoring of SARS-CoV-2 RNA on high-touch surfaces in a community setting. medRxiv. 2020 doi: 10.1021/acs.estlett.0c00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. Cleaning and Disinfection of Environmental Surfaces in the context of COVID-19: Interim guidance. World Health Organization, 2020: 7. [Google Scholar]
- 49.Deboni LM, Neermann EMV, Calice-Silva V, Hanauer MA, Moreira A, Ambrósio A, Guterres DB, Vieira MA, Nerbass FB. Development and implementation of telehealth for peritoneal dialysis and kidney transplant patients monitoring during the COVID-19 pandemic. J Bras Nefrol. 2020 doi: 10.1590/2175-8239-JBN-2020-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lew SQ, Sikka N. Operationalizing Telehealth for Home Dialysis Patients in the United States. Am J Kidney Dis. 2019;74:95–100. doi: 10.1053/j.ajkd.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 51.Magnus M, Sikka N, Cherian T, Lew SQ. Satisfaction and Improvements in Peritoneal Dialysis Outcomes Associated with Telehealth. Appl Clin Inform. 2017;8:214–225. doi: 10.4338/ACI-2016-09-RA-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cartwright EJ, Zs Goh Z, Foo M, Chan CM, Htay H, Griva K. eHealth interventions to support patients in delivering and managing peritoneal dialysis at home: A systematic review. Perit Dial Int. 2021;41:32–41. doi: 10.1177/0896860820918135. [DOI] [PubMed] [Google Scholar]
- 53.Lunney M, Lee R, Tang K, Wiebe N, Bello AK, Thomas C, Rabi D, Tonelli M, James MT. Impact of Telehealth Interventions on Processes and Quality of Care for Patients With ESRD. Am J Kidney Dis. 2018;72:592–600. doi: 10.1053/j.ajkd.2018.02.353. [DOI] [PubMed] [Google Scholar]
- 54.Centers of Disease Control and Prevention. Special Considerations for Patients on Home Dialysis. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/dialysis/home-dialysis.html .
- 55.Lim MA, Pranata R. The Importance of COVID-19 Prevention and Containment in Hemodialysis Unit. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420939256. doi: 10.1177/1179548420939256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ikizler TA, Kliger AS. Minimizing the risk of COVID-19 among patients on dialysis. Nat Rev Nephrol. 2020;16:311–313. doi: 10.1038/s41581-020-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kliger AS, Silberzweig J. Mitigating Risk of COVID-19 in Dialysis Facilities. Clin J Am Soc Nephrol. 2020;15:707–709. doi: 10.2215/CJN.03340320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watnick S, McNamara E. On the Frontline of the COVID-19 Outbreak: Keeping Patients on Long-Term Dialysis Safe. Clin J Am Soc Nephrol. 2020;15:710–713. doi: 10.2215/CJN.03540320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikizler TA. COVID-19 and Dialysis Units: What Do We Know Now and What Should We Do? Am J Kidney Dis. 2020;76:1–3. doi: 10.1053/j.ajkd.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wells CR, Townsend JP, Pandey A, Moghadas SM, Krieger G, Singer B, McDonald RH, Fitzpatrick MC, Galvani AP. Optimal COVID-19 quarantine and testing strategies. Nat Commun. 2021;12:356. doi: 10.1038/s41467-020-20742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Centers of Disease Control and Prevention. COVID-19: When to Quarantine. Available from: https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/quarantine.html .
- 62.Izzedine H, Jhaveri KD. Acute kidney injury in patients with COVID-19: an update on the pathophysiology. Nephrol Dial Transplant. 2021;36:224–226. doi: 10.1093/ndt/gfaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmadian E, Hosseiniyan Khatibi SM, Razi Soofiyani S, Abediazar S, Shoja MM, Ardalan M, Zununi Vahed S. Covid-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev Med Virol. 2021;31:e2176. doi: 10.1002/rmv.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suassuna JHR, Lima EQ, Rocha E, Castro A, Burdmann EA, Carmo LPFD, Yu L, Ibrahim MY, Betônico GN, Cuvello Neto AL, Ávila MON, Gonçalves ARR, Costa CBS, Bresolin NL, Abreu AP, Lobo SMA, Nascimento MMD. Technical note and clinical instructions for Acute Kidney Injury (AKI) in patients with Covid-19: Brazilian Society of Nephrology and Brazilian Association of Intensive Care Medicine. J Bras Nefrol. 2020;42:22–31. doi: 10.1590/2175-8239-JBN-2020-S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pittiruti M, Pinelli F GAVeCeLT Working Group for Vascular Access in COVID-19. Recommendations for the use of vascular access in the COVID-19 patients: an Italian perspective. Crit Care. 2020;24:269. doi: 10.1186/s13054-020-02997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8:738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, Boyer A, Chevrel G, Lerolle N, Carpentier D, de Prost N, Lautrette A, Bretagnol A, Mayaux J, Nseir S, Megarbane B, Thirion M, Forel JM, Maizel J, Yonis H, Markowicz P, Thiery G, Tubach F, Ricard JD, Dreyfuss D AKIKI Study Group. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med. 2016;375:122–133. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 69.Mastrangelo A, Morello W, Vidal E, Guzzo I, Annicchiarico Petruzzelli L, Benetti E, Materassi M, Giordano M, Pasini A, Corrado C, Puccio G, Chimenz R, Pecoraro C, Massella L, Peruzzi L, Montini G COVID-19 Task Force of the Italian Society of Pediatric Nephrology; COVID-19 TASK FORCE of the Italian Society of Pediatric Nephrology. Impact of COVID-19 Pandemic in Children with CKD or Immunosuppression. Clin J Am Soc Nephrol. 2021;16:449–451. doi: 10.2215/CJN.13120820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Melgosa M, Madrid A, Alvárez O, Lumbreras J, Nieto F, Parada E, Perez-Beltrán V Spanish Pediatric Nephrology Association. SARS-CoV-2 infection in Spanish children with chronic kidney pathologies. Pediatr Nephrol. 2020;35:1521–1524. doi: 10.1007/s00467-020-04597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rawson A, Wilson AC, Schwaderer AL, Spiwak E, Johnston B, Anderson S, Nailescu C, Gupta S, Christenson JC, Hains DS, Starr MC. Coronavirus disease 2019 (COVID-19) in two pediatric patients with kidney disease on chronic immunosuppression: A case series. Hemodial Int. 2021;25:E1–E5. doi: 10.1111/hdi.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Basalely A, Brathwaite K, Duong MD, Liu D, Mazo A, Xie Y, Del Rio M, Goilav B, Hayde N, Kaskel FJ, Zolotnitskaya A, Reidy KJ. COVID-19 in Children With Kidney Disease: A Report of 2 Cases. Kidney Med. 2021;3:120–123. doi: 10.1016/j.xkme.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tavares MS, Penido MGMG, Andrade OVB, Koch VHK, Bernardes RP, Garcia CD, Moura-Neto JA, Nascimento MM, Palma LMP. Recommendations Of The Brazilian Society Of Nephrology Regarding Pediatric Patients On Renal Replacement Therapy During The Covid-19 Pandemic. J Bras Nefrol. 2020;42:32–35. doi: 10.1590/2175-8239-JBN-2020-S108. [DOI] [PMC free article] [PubMed] [Google Scholar]