Abstract
BACKGROUND
Diabetes mellitus (DM) is associated with adverse clinical outcomes and high mortality in patients with coronavirus disease 2019 (COVID-19). The relationship between diabetes and COVID-19 is known to be bidirectional.
AIM
To analyze the rate of new-onset diabetes in COVID-19 patients and compare the clinical outcomes of new-onset diabetes, pre-existing diabetes, hyperglycemic, and non-diabetes among COVID-19 patients.
METHODS
We used the Meta-analysis of Observational Studies in Epidemiology statement for the present meta-analysis. Online databases were searched for all peer-reviewed articles published until November 6, 2020. Articles were screened using Covidence and data extracted. Further analysis was done using comprehensive meta-analysis. Among the 128 studies detected after thorough database searching, seven were included in the quantitative analysis. The proportion was reported with 95% confidence interval (CI) and heterogeneity was assessed using I2.
RESULTS
Analysis showed that 19.70% (CI: 10.93-32.91) of COVID-19 patients had associated DM, and 25.23% (CI: 19.07-32.58) had associated hyperglycemia. The overall mortality rate was 15.36% (CI: 12.57-18.68) of all COVID-19 cases, irrespective of their DM status. The mortality rate was 9.26% among non-diabetic patients, 10.59% among patients with COVID-19 associated hyperglycemia, 16.03% among known DM patients, and 24.96% among COVID-19 associated DM patients. The overall occurrence of adverse events was 20.52% (CI: 14.21-28.70) among COVID-19 patients in the included studies, 15.29% among non-diabetic patients, 20.41% among patients with COVID-19 associated hyperglycemia, 20.69% among known DM patients, and 45.85% among new-onset DM. Meta-regression showed an increasing rate of mortality among new hyperglycemic patients, known diabetics, and new-onset DM patients in comparison to those without diabetes.
CONCLUSION
A significantly higher rate of new onset DM and hyperglycemia was observed. Higher mortality rates and adverse events were seen in patients with new-onset DM and hyperglycemia than in the non-diabetic population.
Keywords: Acute respiratory distress syndrome, COVID-19, Diabetes mellitus, Hyperglycemia, Mortality
Core Tip: The relationship between diabetes and coronavirus disease 2019 (COVID-19) is known to be bidirectional. The rate of COVID-19 associated diabetes mellitus (DM) and hyperglycemia was significantly high. Higher mortality rates and adverse events were seen in patients with new-onset DM and hyperglycemia in comparison to the non-diabetic population.
INTRODUCTION
The ongoing coronavirus disease 2019 (COVID-19) has infected 93 million patients and claimed the lives of 2.02 million people as of January 19, 2021[1]. Extensive research has been conducted to study the comorbidities associated with increased severity of disease and worse clinical outcomes. Diabetes has consistently been associated with adverse clinical outcomes and high mortality in COVID-19 patients independent of or in association with other comorbidities[2-4]. Such findings have been linked to the alteration of immune and inflammatory responses caused by hyperglycemia among diabetic patients suffering from COVID-19[5]. However, it is now known that the relationship between diabetes and COVID-19 is bidirectional[6]. Not only does having diabetes increase the risk of severe COVID-19, but severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is also known to have diabetogenic effects.
Multiple theories have been postulated to explain the increasing rate of new-onset diabetes in COVID-19 patients. One of the proposed mechanisms is that SARS-CoV-2 binds to the angiotensin-converting enzyme-2 (ACE-2) receptors expressed on adipose tissue, lungs, small intestine, kidneys, and pancreas. After endocytosis of the virus, downregulation of ACE-2 occurs, leading to overexpression of angiotensin II, which may impede insulin secretion. Similarly, it has been suggested that the direct entry of SARS-CoV-2 into the islet cells of the pancreas damages the beta cells, which normally secrete insulin[7,8].
In the light of new evidence and theories suggesting that there is increased susceptibility of worsening pancreas function and glucose homeostatic mechanisms in COVID-19 patients, the objective of this study is to analyze the rate of new-onset diabetes in COVID-19 patients and compare their clinical outcomes with those of other COVID-19 patients who had normal or increased blood sugar levels or a pre-existing diagnosis of diabetes.
MATERIALS AND METHODS
This study was conducted according to the Meta-analysis of Observational Studies in Epidemiology statement[9]. Our protocol was registered in the PROSPERO International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42021219284).
Search strategy
Investigators independently searched databases such as PubMed, PubMed Central, Scopus, Embase, and Google Scholar for all peer-reviewed articles published until November 6, 2020. The terms “New onset diabetes mellitus (DM)”, “DM”, “hyperglycemia”, “SARS-Cov-2” and “COVID-19” connected with “OR” and “AND”. Boolean operators were searched under the medical subject headings terms. The reference section of each study shortlisted from this process was checked to identify further studies not found in the previous database searches. Additional studies collected from this method were included if they fulfilled the inclusion and exclusion criteria. Electronic search details are provided in Supplementary Material 1.
Selection of studies
The studies were selected based on the following criteria: Inclusion criteria: (1) Study type(s): Observational studies with a comparison of outcomes among individuals with new onset diabetes, pre-existing diabetes, hyperglycemic and non-diabetics with COVID-19 were included in this review; (2) Study participant(s): Individuals of any age, gender, or nationality diagnosed with COVID-19 and new-onset DM; and (3) Objective outcome(s): Mortality, mechanical ventilation/intubation, and intensive care unit (ICU) admission were defined as the primary outcomes of our study. Complications such as Acute Respiratory Distress Syndrome (ARDS), acute cardiac injury, acute liver injury, acute kidney injury, cerebrovascular accident, coagulopathy, and secondary infection were secondary outcomes. Exclusion criteria: (1) Inadequate or unclear descriptions; (2) Animal studies; (3) Review articles; (4) Full text unavailable; and (5) Studies published in a language other than English.
Data extraction
The titles and abstracts of studies retrieved in Covidence during the search were screened independently by two reviewers (PG and SR). The full-texts of potentially relevant studies were then reviewed by two reviewers (SA and SR) according to the eligibility criteria. Any conflict in the first phase of review was resolved by SA and in the second phase by PG. The included studies were then collated, and the three reviewers extracted the data using standardized data extraction formats. The extracted data included: First author, year of publication, country of study, study design, number of patients, age, sex, comorbidities, case definitions, inclusion and exclusion criteria, COVID-19 associated DM, COVID-19 associated hyperglycemia, outcomes, and follow-up duration. The outcomes were mortality and adverse events such as severe COVID-19, intubation, complications and ICU admission. All three reviewers matched their data with each other after extraction and revisited papers in case of disagreements. Discrepancies were resolved through consensus among the reviewers.
Data analysis: The data were analyzed using comprehensive meta-analysis, employing a random effect model. Proportions were presented appropriately using 95% confidence intervals (CI). Forest plots were derived for a visual representation of the analysis. Sensitivity analysis was performed, excluding individual studies to gauge the impact of those studies on the overall results. Meta-regression was undertaken for mortality, considering diabetes status as a moderator among patients with hyperglycemia, patients with new-onset DM, patients with known diabetes, and the non-diabetic population.
Risk of bias in individual studies: We assessed the risk of bias using the JBI tool to evaluate the quality of case reports, case series, and retrospective studies (Tables 1, 2, 3)[10]. Publication bias across the included studies was evaluated using funnel plot.
Table 1.
JBI bias assessment for observational studies
|
Questions (Yes/No/Unclear/Not applicable)
|
Smith et al [19], 2021
|
Zhou et al [16], 2020
|
Wang et al [20], 2020
|
Fadini et al [17], 2020
|
Wang et al [21], 2020
|
Li et al[14], 2020
|
| Were the two groups similar and recruited from the same population? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the exposures measured similarly to assign people to both exposed and unexposed groups? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the exposure measured in a valid and reliable way? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were confounding factors identified? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were strategies to deal with confounding factors stated? | Yes | No | No | Yes | No | Yes |
| Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the outcomes measured in a valid and reliable way? | Yes | Yes | Yes | Yes | Yes | Yes |
| Was the follow-up time reported and sufficient to be long enough for outcomes to occur? | No | No | No | No | Yes | Yes |
| Was follow-up complete, and if not, were the reasons for loss to follow-up described and explored? | Yes | Yes | Yes | Yes | Yes | Yes |
| Were strategies to address incomplete follow-up utilized? | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | Yes |
| Was appropriate statistical analysis used? | Yes | Yes | Yes | Yes | Yes | Yes |
| Overall appraisal | Include | Include | Include | Include | Include | Include |
Table 2.
JBI critical appraisal for case series
| Question |
Ref.
|
||
|
Suwanwongse and Shabarek[22], 2021
|
Kuchay et al[23], 2020
|
Yang et al[24], 2020
|
|
| Were there clear criteria for inclusion in the case series? | Yes | Yes | Yes |
| Was the condition measured in a standard, reliable way for all participants included in the case series? | Yes | Yes | Yes |
| Were valid methods used for the identification of the condition for all participants included in the case series? | Yes | Yes | Yes |
| Did the case series have consecutive inclusion of participants? | No | No | Yes |
| Did the case series have complete inclusion of participants? | No | No | Yes |
| Was there clear reporting of the demographics of the participants in the study? | Yes | Yes | Yes |
| Was there clear reporting of clinical information of the participants? | Yes | Yes | Yes |
| Were the outcomes or follow-up results of cases clearly reported? | Yes | Yes | Yes |
| Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | No | No | Yes |
| Was statistical analysis appropriate? | Unclear | Unclear | Yes |
| Overall: (Include/Exclude/Seek Further Info) | Include | Include | Include |
Table 3.
JBI critical appraisal checklist for case reports
|
Ref.
|
JBI critical appraisal checklist for case reports
|
Remarks
|
| Marchand et al[25], 2020 | Were the patient's demographic characteristics clearly described? | Yes |
| Was the patient’s history clearly described and presented as a timeline? | Yes | |
| Was the current clinical condition of the patient on presentation clearly described? | Yes | |
| Were diagnostic tests or assessment methods and the results clearly described? | Yes | |
| Was the intervention(s) or treatment procedure(s) clearly described? | No | |
| Was the post-intervention clinical condition clearly described? | No | |
| Were adverse events (harms) or unanticipated events identified and described? | Yes | |
| Does the case report provide takeaway lessons? | Yes | |
| Overall: (Include/Exclude/Seek Further Info) | Include |
RESULTS
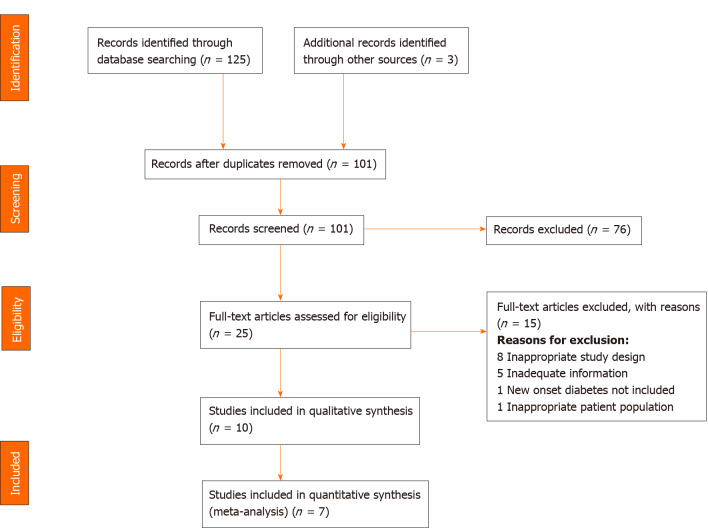
We imported 128 studies after a thorough database search and removed 27 duplicates. The title and abstract of 101 studies were screened, and we excluded 76 irrelevant studies. We assessed the full text of 25 studies and excluded 15 studies with definite reasons (Figure 1). Finally, ten studies were included in our qualitative analysis (Table 4) and seven in our quantitative analysis.
Figure 1.
PRISMA flow diagram.
Table 4.
Qualitative analysis of included studies
|
Ref.
|
Type of study
|
Country
|
Population
|
Outcome
|
| Smith et al[19], 2021 | Retrospective study, spanning over 7 wk | New Jersey, United States | n = 184, M/F = 98/86. Avg age = 64.4 yr (21-100). Below or equal to 60 yr = 75, Above 60 yr = 109. Mean BMI = 29.8 (17.5-61.4). COVID-19 diagnosis based on: 177 patients: Confirmed positive lab test for SARS-CoV-2. Remaining (7 patients): Clinical diagnosis. Case definitions used by the study: New-onset DM: Persistently elevated FBG > 125 mg/dL and requiring insulin therapy; Pre-DM: HbA1C of 5.7% to 6.4%; Non-diabetic patients: HbA1C < 5.7% and FBG ≤ 125 mg/dL | DM = 114/184 (New-onset DM= 29/184). Pre-DM = 44/184. Non-DM = 26/184. HbA1C levels: (1) ≥ 6.5% = 82/171; and (2) 5.7% to 6.4% = 64/171. Among intubated patients (44/184): (1) DM = 35/44 (Newly diagnosed DM = 7/44; New onset DM = 5/44); (2) Pre-DM with high FBG levels = 7/44; and (3) Non-DM = 1/44 (normal HbA1C and FBG levels at admission, but was clinically obese with a BMI > 30). Among intubated patients (44/184): (1) Mean BMI = 32.2 (vs 29.3 in non-intubated); (2) Mean HbA1C (%) = 8.0 (vs 7.2 in non-intubated); and (3) Mean FBG (mg/dL) = 238.0 (vs 163.7 in non-intubated). Death before intubation: 24/184: (1) DM = 17/24; (2) Pre-DM = 4/24; and (3) Non-DM = 3/24 |
| Zhou et al[16], 2020 | Retrospective study | Hefei, China | n = 80. Euglycemia group: (1) 44 (21 males and 23 females); and (2) Age range was 27-52 yr. Secondary hyperglycemia group: (1) 22 (17 males and 5 females); (2) Conditions of no past histories of diabetes, HbA1c < 6.5%, random blood glucose > 11.1 mmol/L during hospitalization, and normal blood glucose after discharge from the hospital; (3) Age range was 40-70 yr; and (4) 5 patients among them had elevated blood sugar after glucocorticoid therapy. Diabetes group: (1) 14 patients (10 males and 4 females); (2) All were T2DM patients; (3) Treated with oral antidiabetics or insulin before hospitalization and without glucocorticoid therapy during hospitalization; and (4) Ages ranged from 43 to 67 yr | Euglycemia group: 44/80. Secondary hyperglycemia group: 22/80. Diabetes group: 14/80. Non-severe COVID: (1) Euglycemia (n = 44): 34 (77.27); (2) Secondary hyperglycemia (n = 22): 15 (68.18); and (3) Diabetes (n = 14): 6 (42.86). Severe COVID: (1) Euglycemia (n = 44): 10 (22.73); (2) Secondary hyperglycemia (n = 22): 7 (31.82); and (3) Diabetes (n = 14): 8 (57.14). Evidence of pneumonia on CT = 78/80: (1) Euglycemia group = 42/44; (2) Secondary hyperglycemia group = 22/22; and (3) Diabetes group = 14/14 |
| Wang et al[20], 2020 | Retrospective study | Beijing, China | n = 132. Exclusion criteria: (1) If not tested positive for COVID-19; (2) Receiving glucocorticoids; (3) Hemolytic anemia; (4) Myelosuppression after leukemia chemotherapy; and (5) Median time from onset to admission was 14 (IQR 10.0–17.8) d. Three groups: A, B, and C-(1) Group A had no diabetes and their HbA1c level was 6.0; (2) Group B had no diabetes and their HbA1c level was > 6.0; (3) Group C were diabetic | 41/132 patients in group A. 44/132 patients in group B. 47/132 patients in group C: (1) 31/47 = History of type 2 diabetes; and (2) 16/47 = Newly diagnosed with diabetes. Death = 22/132: (1) Deaths in group A = 4/41; (2) Deaths in group B = 5/44; and (3) Deaths in group C = 13/47 |
| Suwanwongse and Shabarek[22], 2021 | Case series | United States | n = 3 (18/M, 51/M , 64/F) | New-onset diabetes was diagnosed after infection with COVID-19. 2 out of 3 cases were diagnosed as Diabetic Ketoacidosis. All were discharged home after successful management of blood glucose levels. None of the cases developed any pulmonary, renal, hepatic or cardiac complications due to COVID-19. Invasive Mechanical Ventilation, ICU Admission, or Death did not occur in any of the three cases |
| Marchand et al[25], 2020 | Short communication | France | n = 1 | New-onset type-I DM after COVID-19. No information on severity or outcome of COVID-19 |
| Kuchay et al[23], 2020 | Case series | Haryana, India | n = 3 (30/M, 60/M, 34/M). Follow up duration: 14 wk. Three patients with newly diagnosed Diabetes Mellitus and Diabetic Ketoacidosis with positive SARS-CoV-2 laboratory report. Case Definition: Diabetic Ketoacidosis: DKA was defined as plasma glucose > 250 mg/dL, a positive test for urine or serum ketones, and arterial pH < 7.35 and/or a bicarbonate level less than 18 mmol/L | All three patients responded well to intravenous fluids, antibiotics, and insulin and were discharged after the third week. All three patients were given oral antihyperglycemic drugs after their requirement for exogenous insulin diminished after 4-6 wk. No mortality |
| Fadini et al[17], 2020 | Retrospective study | Italy | COVID-19 positive hospitalized patients included: n (Total) = 413. Median observation time of 17 d | No diabetes = 306/413. Diabetes = 107/413 (Pre-existing diabetes = 86/413; Newly-diagnosed diabetes = 21/413). Primary Outcome (composite of ICU admission or death): 62/306 (20.3%); 7/86 (31.4%); 13/21 (61.9%). Death: 33/306 (10.8%); 12/86 (14.0%); 3/21 (14.3%). Discharged alive: 238/306 (77.8%); 51/86 (59.3%); 9/21 (42.9%). Mean time to discharge in alive pts: 10.1 ± 5.7 (n = 306); 11.6 ± 6.6 (n = 74); 17.4 ± 8.5 (n = 18). Mean days of hospitalization in survivors: 11.3 ± 7.1 (n = 306); 13.8 ± 8.0 (n = 74): 19.7 ± 9.3 (n = 18) |
| Wang et al[21], 2020 | Multicenter retrospective study | China | Without previous diagnosis of diabetes. n = 605 among 1258. Non-survivor = 114. Survivor = 491. Median age: 59.0 yr (IQR 47.0, 68.0). M/F = 322/283. Out of total patients included in analysis: (1) FBG < 6.1 mmol/L (n) = 329; (2) FBG 6.1-6.9 mmol/L (n) = 100; and (3) FBG ≥ 7.0 mmol/L (n) = 176 | Major outcome studied: 28-d mortality. Admission FBG (Total Non-survivor Survivor): (1) < 6.1 mmol/L = 329/605, 35/114, 294/491; (2) 6.1–6.9 mmol/L = 100/605, 21/114, 79/491; (3) ≥ 7.0 mmol/L = 176/605, 58/114, 118/491; and (4) Complications 237/605, 114/114, 123/491. With complications: (1) < 6.1 mmol/L = 86/605, 35/114, 51/491; (2) 6.1–6.9 mmol/SL = 48/608, 21/114, 27/491; and (3) ≥ 7.0 mmol/L = 103/605, 58/114, 45/489. Without complications: (1) < 6.1 mmol/L = 243/605, 0/114, 243/491; (2) 6.1–6.9 mmol/L = 52/605, 0/114, 52/491; and (3) ≥ 7.0 mmol/L = 73/603, 0/114, 73/490 |
| Yang et al[24], 2020 | Retrospective case series | China | n = 69 among 120 evaluated. Exclusion Criteria: (1) Previously diagnosed Diabetes Mellitus; (2) Patients treated with Glucocorticoids; (3) Patients with heart disease (myocardial infarction and heart failure); (4) Patients with kidney disease (maintenance dialysis or renal 20 transplantation); and (5) Patients with liver disease (liver cirrhosis). Median age = 61 (IQR 52-67). M/F = 34/35 | FBG ≥ 7.0 mmol/L for two times during hospitalization and without a history of diabetes in COVID-19 patients: 69/120. COVID-19 Severity: (1) Moderate = 23/69; (2) Severe = 20/69; and (3) Critical = 26/69. Mortality = 16/69 |
| Li et al[14], 2020 | Retrospective study | China | Inclusion: Laboratory confirmed SARS-CoV-2 Infection. Exclusion: Incomplete data available, cases without clinical results, patients with pneumonia due to other pathogens. n = 453. Non survivor (n) = 39. Recovered (n) = 414. Median age = 61 yr (IQR 49-68). Divided into four groups: (1) Normal glucose: FBG < 5.6 mmol/L, HBA1c: < 5.7% (n = 132); (2) Hyperglycemia: FBG 5.6-6.9 mmol/L HbA1c: 5.7%-6.4% (n = 129); (3) Newly diagnosed Diabetes: No history of previous Diabetes. FBG: ≥ 7 mmol/L and/or HbA1c ≥ 6.5% (n = 94); and (4) Known Diabetes: Previously diagnosed Diabetes Mellitus (n = 98) | Main clinical outcomes: (1) Invasive mechanical ventilation: 3/132; 6/129; 11/94; 9/98; (2) ICU admission: 2/132, 8/129, 11/94, 4/98; and (3) Death: 2/132, 6/129, 20/94, 11/98. Other outcomes: (1) ARDS: 1/132, 4/129, 10/94, 3/98; (2) Acute Cardiac Injury: 27/132, 26/129, 23/94, 32/98; (3) Coagulopathy: 12/132, 12/129, 15/94, 17/98; (4) Hypoalbuminemia: 14/132, 15/129, 37/94, 36/98; and (5) Length of hospital stay (days): 22.5 (1.19), 21.9 (1.16), 26.5 (1.37), 23.6 (1.37) |
ARDS: Acute Respiratory Distress Syndrome; BMI: Body mass index; COVID-19: Coronavirus disease 2019; CT: Computed tomography; DKA: Diabetic ketoacidosis; DM: Diabetes mellitus; F: Female; FBG: Fasting blood glucose; HbA1C: Hemoglobin A1C; ICU: Intensive care unit; IQR: Inter quartile range; M: Male; N: Total participants; Non-DM: Non-diabetes mellitus; Pre-DM: Pre-diabetes mellitus; T2DM: Type 2 diabetes mellitus; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Qualitative summary
A summary of the included studies including type of study, location, study population and the relevant outcomes is presented in Table 4.
Quantitative result
A total of 7 papers were included in the quantitative synthesis.
COVID-19 associated DM
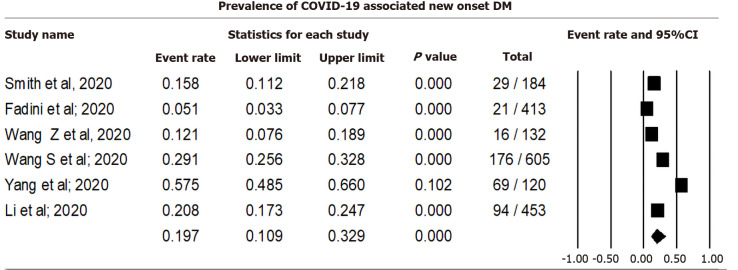
Pooling data from six studies that reported new-onset diabetes among COVID-19 cases using a random effect model showed that 19.70% (CI: 10.93-32.91, I2= 96.71) of COVID-19 cases were associated with DM (Figure 2). Sensitivity analysis after excluding individual studies is shown in Supplementary Material 2 and Figure 1.
Figure 2.
Prevalence of coronavirus disease 2019 associated new onset diabetes mellitus. COVID-19: Coronavirus disease 2019; DM: Diabetes mellitus.
COVID-19 associated hyperglycemia
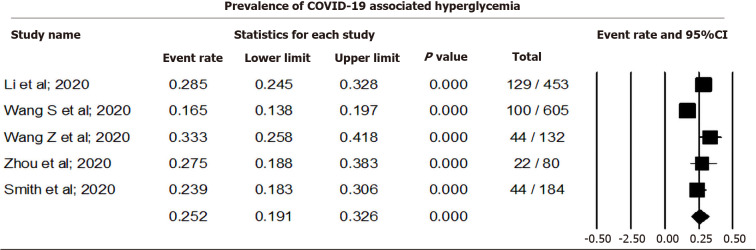
Pooling data from five studies that reported hyperglycemia among COVID-19 cases using a random effect model showed that 25.23% (CI: 19.07-32.58, I2= 86.6) of COVID-19 cases were associated with hyperglycemia (Figure 3). Sensitivity analysis after removing individual studies is shown in Supplementary Material 2, and Figures 2 and 3.
Figure 3.
Prevalence of coronavirus disease 2019 associated hyperglycemia. COVID-19: Coronavirus disease 2019.
Mortality outcome
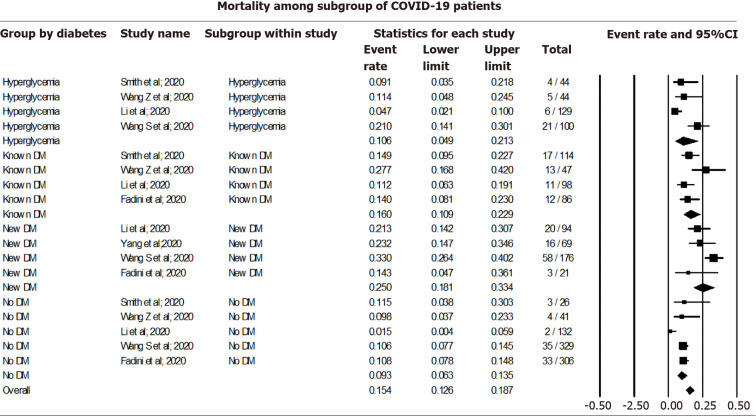
Pooling data among COVID-19 cases using a random effect model showed a 9.26% mortality rate among non-diabetic (CI: 6.28-13.46, I2= 50.69), 10.59% among those with COVID-19 associated hyperglycemia (CI: 4.92-21.33, I2 = 77.49), 16.03% among known DM patients (CI: 10.95-22.88, I2= 54.35), and 24.96% among new-onset DM (CI: 18.10-33.37, I2= 55.88). The overall mortality rate was 15.36% (CI: 12.57-18.68, I2= 81.75) among all COVID-19 cases, irrespective of their DM status (Figure 4).
Figure 4.
Mortality among coronavirus disease 2019 cases with subgroup analysis based on their diabetes status. COVID-19: Coronavirus disease 2019; DM: Diabetes mellitus.
Adverse events such as severe COVID-19, intubation, complications, and ICU admission
Pooling data for the occurrence of adverse events among COVID-19 cases using a random effect model showed 15.29% occurrence among non-diabetic patients (CI: 9.06-24.65, I2= 84.47), 20.41% among those with COVID-19 associated hyperglycemia (CI: 6.20-49.86, I2= 93.41), 20.69% among known DM patients (CI: 8.12-43.50, I2= 90.14), and 45.85% among those with new-onset DM (CI: 22.23-71.50, I2= 94.21). The overall occurrence of adverse events was 20.52% (CI: 14.21-28.70, I2= 93.53) among all COVID cases irrespective of their DM status (Figure 5).
Figure 5.
Occurrence of adverse events among coronavirus disease 2019 cases with subgroup analysis based on their diabetes status. COVID-19: Coronavirus disease 2019; DM: Diabetes mellitus; ICU: Intensive care unit.
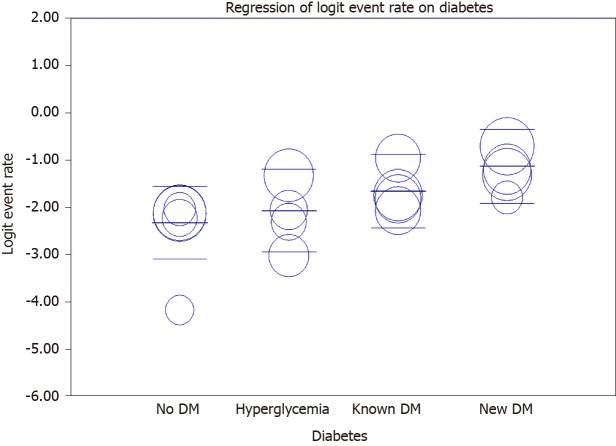
Meta-regression for mortality outcome
Meta-regression showed an increasing rate of mortality among newly hyperglycemic patients, known diabetic patients, and new-onset DM compared to non-diabetic patients (Figure 6 and Table 5).
Figure 6.
Meta regression of diabetes status and mortality.
Table 5.
Main results for meta-regression model, random effects, Z-distribution, logit event rate
|
Covariate
|
Coefficient
|
SE
|
95% lower
|
95% upper
|
Z value
|
P
value
|
| Intercept: No DM | -2.3183 | 0.2504 | -2.8091 | -1.8276 | -9.26 | 0 |
| Hyperglycemia | 0.2519 | 0.3788 | -0.4905 | 0.9944 | 0.67 | 0.506 |
| Known DM | 0.6642 | 0.3552 | -0.0319 | 1.3603 | 1.87 | 0.0615 |
| New DM | 1.1865 | 0.3552 | 0.4903 | 1.8827 | 3.34 | 0.0008 |
Test of the model: Simultaneous test that all coefficients (excluding intercept) are zero: Q = 12.51, df = 3, P = 0.0058. Goodness of fit: Test that unexplained variance is zero: Tau² = 0.1610, Tau = 0.4012, I² = 62.66%, Q = 34.81, df = 13, P = 0.0009. Total between-study variance (intercept only): Tau² = 0.3751, Tau = 0.6124, I² = 81.75%, Q = 87.66, df = 16, P = 0.0000. Proportion of total between-study variance explained by Model 1: R² analog = 0.57. DM: Diabetes mellitus.
Publication bias
Publication bias across the included studies was evaluated using Egger's test to evaluate funnel plot asymmetry. Publication bias reporting new-onset DM showed some publication bias depicted by the asymmetry of the funnel plot (Supplementary Material 2 and Figure 4). Similarly, publication bias for mortality outcome is shown in Supplementary Material 2 and Figure 5.
DISCUSSION
Our meta-analysis is the first to pool the prevalence of new-onset DM and compare mortality and adverse events among patients with new-onset DM vs patients with hyperglycemia, pre-existing DM, or no DM. Prior meta-analyses have shown DM to be associated with mortality, severe COVID-19, ARDS, and disease progression[11-13]. However, there was a paucity of data to compare the outcomes among infected patients with pre-existing diabetes compared to new-onset DM. We found the pooled prevalence of COVID-19 associated DM (new-onset) to be 19.7%, while the prevalence of COVID-19 associated hyperglycemia was 25.23%. Angiotensin II has been shown to increase hepatic glucose production and decrease insulin sensitivity. A multitude of explanations have been proposed for impaired blood glucose levels among patients infected with COVID-19, including downregulation of ACE-2 receptors leading to increased angiotensin II and defective insulin secretion as well as direct damage to beta cells of islets of the pancreas[7,8]. Infection with the virus itself leads to oxidative stress, resulting in hypoxia and inflammation, which aggravates glucose homeostasis[14]. Additionally, damage to key organs involved in glucose metabolism such as the kidney and the liver resulting in abnormal blood glucose levels, has been observed in cases of COVID-19 infection. The use of corticosteroids is common among COVID-19 patients, especially those with severe COVID-19[15]. However, in our meta-analysis, only one study[16] included patients receiving steroids, which eliminates steroid use as a possible cause of hyperglycemia. The mortality rate was highest among patients with new-onset DM (24.96%), followed by known DM patients (16.03%), patients with COVID-19 associated hyperglycemia (10.59%), and non-diabetic patients (9.26%). The higher prevalence in patients with new-onset DM could be explained by the masked presence of organ damage due to ongoing diabetes, which cannot be accounted for during statistical analysis in contrast to cases of pre-existing diabetes in which organ damage is accounted for statistically[17]. Similarly, metabolic inflammation caused by high blood sugar levels affects the body’s immune system and healing process prolonging recovery[14]. Hyperglycemia has been found to affect lung volume and diffusion capacity, causing respiratory deterioration and a decrease in PaO2/FiO2 ratio[17]. Chronic hyperglycemia causes down regulation of ACE-2, which has a protective effect against inflammation and in turn leads to inflammatory damage by the virus and potential cytokine storm. These are the reasons for increased mortality among patients with diabetes and hyperglycemia compared to non-diabetic patients. The pooled mortality of 16.03% among diabetic patients was lower than that shown in Shang’s meta-analysis (21.4%) and higher than that in Miller et al[11] (9.9%). Adverse events such as severe COVID-19, intubation, complications, and ICU admissions were highest among new-onset DM (45.85%), followed by known DM patients (20.69%), patients with COVID-19 associated hyperglycemia (20.41%), and non-diabetic patients (15.29%). Our findings concurred with previous studies that have shown a strong association between DM and severe COVID-19, leading to increased complications, including multi-organ dysfunction and ICU admissions[18]. The need for intubation can be explained by the respiratory deterioration noted among patients with hyperglycemia.
Our study has several limitations. Due to the inadequate number of existing studies, we could not include controlled studies, instead using only observational studies, case reports, and case series. The included studies had small sample sizes and low power. Each study had its own limitations, such as the absence of data on body mass index, Hemoglobin A1C in all patients, the possibility of stress hyperglycemia, single-center study, retrospective study design, etc.
CONCLUSION
The pooled prevalence of COVID-19 associated DM was 19.70%, and for COVID-19 associated hyperglycemia was 25.23%. Among COVID-19 patients, higher mortality rates and adverse events were seen in patients with new-onset DM compared to those with pre-existing diabetes, those with COVID-19 associated hyperglycemia, and those without diabetes.
ARTICLE HIGHLIGHTS
Research background
Diabetes has been shown to be associated with worsening severity of disease and poor prognosis in coronavirus disease 2019 (COVID-19). Interestingly, various cases of new onset diabetes mellitus (DM) were seen in patients with COVID-19. The virus is believed to bind to angiotensin-converting enzyme-2 receptors leading to increased angiotensin II and subsequent decreased insulin secretion.
Research motivation
In relation to various theories and proposed mechanisms of how COVID-19 may lead to abnormal glucose homeostasis, our study was conducted to evaluate new onset DM in COVID-19.
Research objectives
The study aimed to pool the prevalence of new onset DM and hyperglycemia in COVID-19 patients and compare various outcomes such as mortality, intubation and complications among infected patients who had hyperglycemia or preexisting DM or new onset DM or normal blood sugar levels.
Research methods
Meta-analysis of Observational Studies in Epidemiology was used for the meta-analysis. Studies were screened using Covidence after searching various databases including PubMed, PubMed Central, Embase and Scopus. Comprehensive meta-analysis software was used for data analysis.
Research results
The results showed that 19.70% and 25.23% of patients had COVID-19 associated DM and hyperglycemia, respectively. The mortality rate was highest among COVID-19 associated DM patients (24.96%) followed by patients with preexisting DM (16.03%), and was least in non-diabetic patients (9.29%). The occurrence of adverse events was highest among COVID-19 associated new-onset DM patients followed by patients with preexisting DM, COVID-19 associated hyperglycemia and non-diabetic patients.
Research conclusions
COVID-19 was associated with hyperglycemia and new-onset DM. Infected patients with new onset DM had worse prognosis in terms of mortality and adverse events.
Research perspectives
The findings of this study should alarm clinicians that new onset diabetes and hyperglycemia is a bad prognostic factor for COVID-19.
Footnotes
Conflict-of-interest statement: The authors declare that they have no competing interests.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Nepal Medical Association, No. 6859/L-6319; and Nepal Medical Council, No. 21732.
Peer-review started: February 20, 2021
First decision: May 14, 2021
Article in press: July 5, 2021
Specialty type: Virology
Country/Territory of origin: Nepal
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mobasher MA S-Editor: Fan JR L-Editor: Webster JR P-Editor: Ma YJ
Contributor Information
Dhan Bahadur Shrestha, Department of Internal Medicine, Mount Sinai Hospital, Chicago, IL 60608, United States. medhan75@gmail.com.
Pravash Budhathoki, Department of Internal Medicine, BronxCare Health System, Bronx, NY 10457, United States.
Sumit Raut, Department of Emergency Medicine, Kathmandu Medical College, Kathmandu 44600, Nepal.
Sugat Adhikari, Department of Internal Medicine, Nishtar Medical University, Multan 59330, Pakistan.
Prinska Ghimire, Department of Internal Medicine, Tribhuvan University, Kathmandu 44600, Nepal.
Sabin Thapaliya, Department of Internal Medicine, Tribhuvan University Teaching Hospital, Kathmandu 44600, Nepal.
Ali A Rabaan, Molecular Diagnostic Laboratory, Johns Hopkins Aramco Healthcare, Dhahran 34465, Saudi Arabia; Department of Public Health & Nutrition, The University of Haripur, Haripur 22620, Pakistan.
Bibodh Jung Karki, Division of Infectious Diseases, University of Louisville, Louisville, KY 40292, United States.
References
- 1.WHO WHO Coronavirus Disease (COVID-19) Dashboard. [cited 10 March 2021]. Available from: https://covid19.who.int/
- 2.Zhang Y, Cui Y, Shen M, Zhang J, Liu B, Dai M, Chen L, Han D, Fan Y, Zeng Y, Li W, Lin F, Li S, Chen X, Pan P medical team from Xiangya Hospital to support Hubei. China Association of diabetes mellitus with disease severity and prognosis in COVID-19: A retrospective cohort study. Diabetes Res Clin Pract. 2020;165:108227. doi: 10.1016/j.diabres.2020.108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.The Lancet Diabetes Endocrinology. COVID-19 and diabetes: a co-conspiracy? Lancet Diabetes Endocrinol. 2020;8:801. doi: 10.1016/S2213-8587(20)30315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Lancet. Redefining vulnerability in the era of COVID-19. Lancet. 2020;395:1089. doi: 10.1016/S0140-6736(20)30757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, Mingrone G, Boehm B, Cooper ME, Chai Z, Del Prato S, Ji L, Hopkins D, Herman WH, Khunti K, Mbanya JC, Renard E. New-Onset Diabetes in Covid-19. N Engl J Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.Critical Appraisal Tools. Joanna Briggs Institute. [cited 10 March 2021]. Available from: https://jbi.global/critical-appraisal-tools .
- 11.Miller LE, Bhattacharyya R, Miller AL. Diabetes mellitus increases the risk of hospital mortality in patients with Covid-19: Systematic review with meta-analysis. Medicine (Baltimore) 2020;99:e22439. doi: 10.1097/MD.0000000000022439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang L, Shao M, Guo Q, Shi J, Zhao Y, Xiaokereti J, Tang B. Diabetes Mellitus is Associated with Severe Infection and Mortality in Patients with COVID-19: A Systematic Review and Meta-analysis. Arch Med Res. 2020;51:700–709. doi: 10.1016/j.arcmed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Tian S, Chen T, Cui Z, Shi N, Zhong X, Qiu K, Zhang J, Zeng T, Chen L, Zheng J. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab. 2020;22:1897–1906. doi: 10.1111/dom.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? Diabetes Metab Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou W, Ye S, Wang W, Li S, Hu Q. Clinical Features of COVID-19 Patients with Diabetes and Secondary Hyperglycemia. J Diabetes Res. 2020;2020:3918723. doi: 10.1155/2020/3918723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadini GP, Morieri ML, Boscari F, Fioretto P, Maran A, Busetto L, Bonora BM, Selmin E, Arcidiacono G, Pinelli S, Farnia F, Falaguasta D, Russo L, Voltan G, Mazzocut S, Costantini G, Ghirardini F, Tresso S, Cattelan AM, Vianello A, Avogaro A, Vettor R. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020;168:108374. doi: 10.1016/j.diabres.2020.108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith SM, Boppana A, Traupman JA, Unson E, Maddock DA, Chao K, Dobesh DP, Brufsky A, Connor RI. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J Med Virol. 2021;93:409–415. doi: 10.1002/jmv.26227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Du Z, Zhu F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res Clin Pract. 2020;164:108214. doi: 10.1016/j.diabres.2020.108214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Ma P, Zhang S, Song S, Wang Z, Ma Y, Xu J, Wu F, Duan L, Yin Z, Luo H, Xiong N, Xu M, Zeng T, Jin Y. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia. 2020;63:2102–2111. doi: 10.1007/s00125-020-05209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suwanwongse K, Shabarek N. Newly diagnosed diabetes mellitus, DKA, and COVID-19: Causality or coincidence? J Med Virol. 2021;93:1150–1153. doi: 10.1002/jmv.26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuchay MS, Reddy PK, Gagneja S, Mathew A, Mishra SK. Short term follow-up of patients presenting with acute onset diabetes and diabetic ketoacidosis during an episode of COVID-19. Diabetes Metab Syndr. 2020;14:2039–2041. doi: 10.1016/j.dsx.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang JK, Jin JM, Liu S, Bai P, He W, Wu F, Liu XF, Han DM. Blood Glucose Is a Representative of the Clustered Indicators of Multi-Organ Injury for Predicting Mortality of COVID-19 in Wuhan, China. SSRN Electron J. 2020:2020.04.08.20058040. [Google Scholar]
- 25.Marchand L, Pecquet M, Luyton C. Type 1 diabetes onset triggered by COVID-19. Acta Diabetol Springer . 2020;57:1265–1266. doi: 10.1007/s00592-020-01570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]