Abstract
Thyroid-associated ophthalmopathy is a typical autoimmune disease of orbital tissues. Alternative splicing significantly influences many diseases progression, including cancer, age-related macular degeneration, and multiple sclerosis, by modulating the expression of transcripts. However, its role in thyroid-associated ophthalmopathy is still unclear. In this study, differential expression transcripts and differential alternative splicing genes in orbital adipose/connective tissues of thyroid-associated ophthalmopathy patients were detected using RNA sequencing, Cuffdiff, and replicate multivariate analysis of transcript splicing. Three thousand ninety six differential expression transcripts and 2355 differential alternative splicing genes were screened out, while functional enrichment analysis indicated that differential expression transcript and differential alternative splicing genes were associated with immune modulation, extracellular matrix remodeling, and adipogenesis. The expression of the SORBS1, SEPT2, COL12A1, and VCAN gene transcripts was verified by qRT-PCR. In conclusion, prevalent alternative splicing is involved in the disease development in thyroid-associated ophthalmopathy. More attention should be paid to the mechanism of alternative splicing to explore more potential therapeutic targets in thyroid-associated ophthalmopathy.
Keywords: Thyroid-associated ophthalmopathy, alternative splicing, rMATS, adipogenesis, adipose/connective tissue
Impact statement
Thyroid-associated ophthalmopathy (TAO) is a common ocular autoimmue complication of thyroid dysfunction, but its mechanism is poorly understood. To our best knowledge, we are the first to sequence total RNA from orbital adipose/connective tissue samples to analyze alternative splicing pattern in TAO. We found the prevalence of alternative splicing in transcriptome of TAO, which significantly influences immune response, extracellular matrix remodeling, and adipogenesis in orbit of patients. This manuscript shed light on the important role of alternative splicing mechanism in modulating the development of TAO, and it will also provide contribution to current understanding of TAO as well as the potential treatment strategy against autoimmune disease for further studies.
Introduction
Thyroid-associated ophthalmopathy (TAO) is a common ocular manifestation involved in thyroid dysfunction, which occurs most frequently in patients with Graves’ disease. 1 As an autoimmune disease, TAO is characterized by an abnormal autoimmune response against antigens, such as thyroid-stimulating hormone receptor (TSHR), calsequestrin, and collagen XIII, shared by the thyroid and the orbit.2,3 In the orbit, infiltrating T cells mainly provoke the activation of orbital fibroblasts and lead to extracellular matrix (ECM) remodeling.4,5 Orbital fibroblasts (OFs) are the critical effectors in TAO and are divided into two populations according to the expression of surface glycoprotein Thy-1. Thy-1-positive cells are prone to differentiating into myofibroblasts, whereas Thy-1-negative cells mainly undergo differentiation into adipocytes in response to elevated levels of peroxisome proliferator-activated receptor gamma (PPARγ), which is responsible for an increased volume of orbital adipose/connective tissue (OACT).5,6 Merging studies have revealed the close association of OFs with adipogenesis and TAO. For example, the expression of TSHR is increased during adipogenesis in TAO patients, while insulin-like growth factor-1 (IGF-1) boosts stromal cells to significantly proliferate in TAO periorbital adipose tissues.7,8 However, the modulatory mechanism underlying the activation of fibroblasts and adipogenesis in adipose/connective tissues in TAO has yet to be discovered.
Accumulating epidemiological evidence demonstrates the genetic etiology of TAO. More than 50 genes that contribute to TAO have been identified, including TSHR, CTLA-4, CD40, HLA-DR, HLA-DQ, and TNF-α.5,9 Moreover, differentially expressed genes associated with the cell cycle, pathways of ribosomes, and retinol metabolism have been identified as critical regulators of TAO, 10 providing new insights into the pathogenesis and therapeutic targets of the disease. The important role of post-transcriptional regulation in orbitopathy has recently been confirmed. In OFs in TAO, miR-21 is overexpressed and regulates orbital muscle fibrosis. 11 Our previous study revealed the potential regulatory function of the circRNA_14940/CCND1/Wnt signaling pathway in TAO using high-throughput RNA sequencing. 12
Alternative splicing (AS) is a regulated process that enables a single messenger RNA (mRNA) precursor (pre-mRNA) to produce different functional proteins by rearranging the pattern of intron and exon elements, thereby significantly increasing the complexity of gene expression. 13 AS has been proven to play a vital role in many biological processes over the entire life span and in the development of the brain, testes, and immune system. 13 Furthermore, AS-related factors, such as Sam68, SRSF10, and SRp40, participate in adipogenesis processes and regulate the AS events of the mammalian target of rapamycin (mTOR), Lipin1, and PPARγ. 14 The activation and differentiation of human monocytes under stimulation is also modulated by specific splicing events. 15 Merging evidence also reveals that alternative splicing is associated with the pathogenesis of macular and autoimmune diseases, such as age-related macular disease, multiple sclerosis, and cancer.16–18 However, the knowledge of the relationship between AS and TAO remains to be expanded.
In this study, to explore the potential relationship between AS and TAO, differential expression transcripts (DETs) and differential alternative splicing (DAS) genes were identified based on high-throughput RNA sequencing data of tissues from TAO and control subjects, using replicate multivariate analysis of transcript splicing (rMATS). Then, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis were obtained to screen out significantly enriched functions through the DAVID database. Furthermore, quantitative real-time polymerase chain reaction (qRT-PCR) was performed to validate DET and DAS genes.
Materials and methods
Patients and tissue samples
All patients included in this study were diagnosed with TAO based on the Bartley criteria and had normal thyroid functions after treatment with antithyroid drugs. 19 The following patients were excluded: (i) patients with other ocular or systemic inflammatory or autoimmune diseases, (ii) patients who had undergone radioiodine therapy or thyroid operations, and (iii) patients who had been treated with anti-inflammatory and/or immunosuppressive drugs (e.g. steroids) within six months prior to the study. Six TAO patients were screened out, and all of these cases were recognized as inactive TAO with a clinical activity score (CAS) of less than three for at least six months. 20 Additionally, six individuals who had not been diagnosed with thyroid or orbital diseases or any inflammatory/autoimmune diseases participated in the study as controls. OACT samples of TAO patients were collected from tissue removed during orbital decompression surgery. Besides, tissues of control individuals obtained in plastic surgery were collected as control samples.
Ethical approval
This study was approved by the Ethics Committee of Changzheng Hospital, Second Military Medical University. All patients signed informed consent forms based on a full understanding of the study’s aims and procedures before being enrolled. The research protocol complied with the provisions of the Declaration of Helsinki.
RNA isolation and sequencing
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The integrity of RNA was evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Its quality and quantity were assessed using a Nanodrop 2000 (Thermo Scientific, Waltham, MA, USA).
Using a TruSeq Stranded Total RNA kit and Ribo-Zero Gold treatment (Illumina, San Diego, CA, USA), strand-specific RNA sequencing libraries were established after ribosomal RNA was depleted according to the manufacturer’s protocol. For each sample, extracted RNA was used for double stranded cDNA synthesis, and then was purified and adenylated and ligated with adapters at the 3′ ends. The DNA fragments were enriched by PCR with 14 cycles and the main peak of the library was 350 bp. After evaluating the library quality using an Agilent 2100 Bioanalyzer (Agilent Technologies), high-throughput RNA sequencing was performed using a HiSeq 2500 System (Illumina). The RNA sequencing data of each sample was 97.96 M to 99.79 M reads.
Sequence assembly and analysis
The quality of raw reads was assessed using Trimmomatic. 21 Adapters, low-quality bases, and reads were filtered out. The remaining clean reads were aligned with the human genome (GRCh38.p12) for assembly using HISAT2. 22 Sequence segments were spliced and annotated, and the transcript expression counts were calculated using the htseq-count script. 23 Gene expression was quantified with Cufflinks with fragments per kilobase of exon per million mapped reads (FPKM), 24 and the read counts were normalized with DESeq. 25 PCA of samples was exhibited based on the expression of the transcripts.
AS allows one gene to produce multiple mRNA transcripts that may be translated into different proteins. Based on the mapping results (in BAM format), we detected the presence of AS events in the samples using rMATS. 26 Through rMATS, alternative splicing events that corresponded to known alternative splicing patterns was automatically detected and analyzed.
DET and DAS analysis
DETs were screened out based on the FPKM values according to the criteria of log2|fold change| > 1 and P < 0.05. To identify homogeneous groups of all samples, pheatmap package in R was used to make hierarchical clustering analysis according to the expression levels of the transcripts. 27
DAS genes in TAO patients and controls were identified using rMATS. 26 Five main alternative splicing events, A3SS, A5SS, MXE, RI, and SE, were analyzed. The DAS genes were calculated with a threshold of |IncLevelDifference| > 0.01 and FDR-adjusted P < 0.05.
Functional enrichment analysis
DET and DAS genes were selected and subjected to Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway analysis. In terms of the GO analysis, the related genes were annotated according to BP, CC, or MF. For KEGG analysis, the differentially enriched pathways were ranked by the enrichment scores.
Quantitative real-time PCR
Total RNA of orbital tissue from two groups was isolated using TRIzol reagent (Invitrogen), and was reverse-transcribed into complementary DNA using a HiScript II Q RT SuperMix IIa Kit (Vazyme Biotech, Shanghai, China). Quantitative RT-PCR was performed using a ChamQ SYBR qPCR Master Mix Kit (Vazyme) on a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). All samples were normalized to internal β-actin controls, and the relative expression levels of target genes were calculated using the 2–ΔΔCt method. The primer sequences of all target genes are shown in Table S1.
Results
DETs in OACT samples of TAO patients
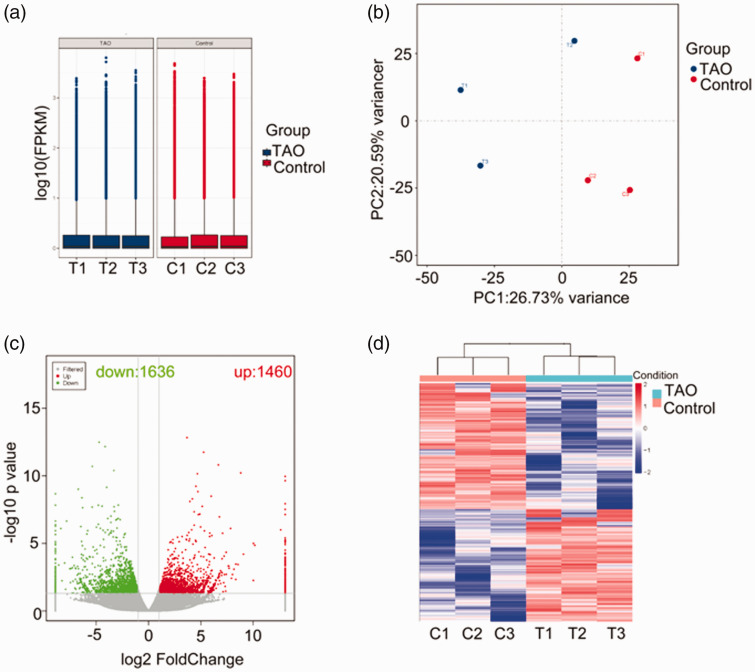
In total, 113,095 genes were identified in RNA sequencing profile derived from samples of TAO and control group. The unique mapped reads were assembled into transcripts using Cufflinks, and the expression of mRNAs was quantified by the FPKM values. The box plot of the FPKM values of mRNAs revealed no abnormal expression in three samples of each group (Figure 1(a)). Principal component analysis (PCA) revealed a clear separation between the samples of the two groups (Figure 1(b)). With a threshold of log2|fold change| >1 and a P value of <0.05, a total of 3096 DETs were screened, consisting of 1460 upregulated genes and 1636 downregulated transcripts (Figure 1(c)). Hierarchical clustering analysis showed two distinct separate clusters, suggesting that TAO and control samples could evidently be discriminated according to the expression of the 3096 DE genes (Figure 1(d)).
Figure 1.
Qualification and analysis of identified genes from RNA sequencing data. (a) Box plot of FPKM values of transcripts in TAO and control groups. (b) Scatter plot of the PCA analysis for each sample on the first (PC1) and second principal components (PC2). (c) Volcano plot of differential expression transcripts based on a threshold of log2|fold change| > 1 and P < 0.05. (d) Heatmap of hierarchical clustering analysis for all samples based on the expression of genes. (A color version of this figure is available in the online journal.)
DAS gene analysis
To investigate potential AS in TAO patients, five main types of AS events, including alternative 3′ splice sites (A3SS), alternative 5′ splice sites (A5SS), mutually exclusive exons (MXE), retained intron (RI), and skipped exon (SE), were analyzed using rMATS (Figure 2(a)). AS events were detected in 12,021 genes. A3SS events occurred in 4298 genes, A5SS events occurred in 3593 genes, MXE events occurred in 3193 genes, RI events occurred in 1196 genes, and SE events occurred in 11,767 genes (Figure 2(b)). Within them, 130 overlapping genes were regulated by all five main AS events. Furthermore, 2533 DAS genes were identified in TAO and control groups, with a threshold of |IncLevelDifference| >0.01 and a false recovery rate (FDR)-adjusted P value of <0.05 (Figure 2(c)). The numbers of A3SS, A5SS, MXE, RI, and SE events were 503, 386, 203, 140, and 2347, respectively. SE was the most prevalent AS event in TAO patients, whereas RI was the least prevalent.
Figure 2.
Analysis of differential alternative splicing (AS) genes and distribution of the five main AS events. (a) Schematic diagrams of the mechanisms of the five main AS events. (b) Venn diagram of the detected genes undergoing the five AS events and overlap of these genes. (c) Distribution of differential AS events based on a threshold of |IncLevelDifference| > 0.01 and FDR-adjusted P < 0.05. (A color version of this figure is available in the online journal.)
Enrichment analysis of DET genes
Aimed at investigating the functions of DET genes, the functional enrichment analysis was exhibited based on GO annotation and KEGG pathway databases. The top 30 most significant GO terms of upregulated and downregulated DET genes were identified (Figures 3 and 4). The upregulated mRNAs of TAO samples were mainly related to the T cell antigen processing and presentation (biological process [BP]) and prostaglandin F receptor activity (molecular function [MF]), which were involved in the immune response and inflammation in TAO, as well as microtubule anchoring (BP), proteinaceous extracellular matrix (cellular component [CC]), kinetochore microtubule (CC), and microtubule plus-end binding (MF), which were associated with fibroblast activation (Figure 3, Table 1). The downregulated mRNAs were mostly connected with to cell adhesion (BP), the collagen catabolic process (BP), collagen type XII trimer (CC), extracellular matrix (CC), integrin binding (MF), and extracellular matrix structural constituent conferring tensile strength (MF), which were mainly associated with ECM remodeling and the interaction between immune cells and the ECM (Figure 4, Table 1).
Figure 3.
Gene Ontology (GO) function enrichment analysis of upregulated DET genes. Top 30 most significant GO terms of upregulated DET genes associated with biological processes (BP), cellular components (CC), and molecular functions (MF). (A color version of this figure is available in the online journal.)
Figure 4.
Gene Ontology (GO) function enrichment analysis of downregulated DET genes. Top 30 most significant GO terms of downregulated DET genes associated with biological processes (BP), cellular components (CC), and molecular functions (MF). (A color version of this figure is available in the online journal.)
Table 1.
The top 30 GO terms of up- or down-regulated DET genes between TAO and control samples.
| DET | ID | Term | Category | Gene |
|---|---|---|---|---|
| Upregulated | GO:0002457 | T cell antigen processing and presentation | BP | KDM5D |
| GO:0060746 | Parental behavior | BP | ZFY | |
| GO:0072272 | Proximal/distal pattern formation involved in metanephric nephron development | BP | IRX1; IRX2 | |
| GO:0035116 | Embryonic hindlimb morphogenesis | BP | PITX2; PITX1; ALX3; RSPO2; MED1; OSR2 | |
| GO:0010634 | Positive regulation of epithelial cell migration | BP | GLIPR2; CLASP2; CLASP1; DOCK1; DOCK5; CLASP2 | |
| GO:0072086 | Specification of loop of Henle identity | BP | IRX1; IRX2 | |
| GO:0046889 | Positive regulation of lipid biosynthetic process | BP | TCF7L2; SORBS1; CREB1; MLXIPL | |
| GO:2000675 | Negative regulation of type b pancreatic cell apoptotic process | BP | CAST; TCF7L2 | |
| GO:0034453 | Microtubule anchoring | BP | PCM1; CLASP2; CLASP1 | |
| GO:0034720 | Histone h3-k4 demethylation | BP | KDM5D | |
| GO:0098983 | Symmetric, gaba-ergic, inhibitory synapse | CC | NLGN4Y | |
| GO:0098985 | Asymmetric, glutamatergic, excitatory synapse | CC | NLGN4Y | |
| GO:0044295 | Axonal growth cone | CC | PARD3; BOC; GPM6A; FLRT3; CLASP2 | |
| GO:0089717 | Spanning component of membrane | CC | NLGN4Y | |
| GO:1903754 | Cortical microtubule plus-end | CC | CLASP2 | |
| GO:0005578 | Proteinaceous extracellular matrix | CC | LTBP1; CPZ; LINGO2; ECM2; BGN; SPON1; LRRN1; WNT2B; PHOSPHO1; MATN2; FLRT3; ADAMTSL2; EPYC; PODN; COL24A1; COL19A1; FBLN5; FLRT2; GPLD1 | |
| GO:0005828 | Kinetochore microtubule | CC | CLASP2; CLASP1 | |
| GO:0005614 | Interstitial matrix | CC | VIT; ABI3BP; ECM2; KAZALD1; VWA1; ABI3BP; NAV2 | |
| GO:0034686 | Integrin alphav-beta8 complex | CC | ITGB8 | |
| GO:0045180 | Basal cortex | CC | CLASP2; CLASP1 | |
| GO:0051213 | Dioxygenase activity | MF | KDM5D; UTY; KDM6A; KDM3A | |
| GO:0032452 | Histone demethylase activity | MF | KDM5D; UTY; KDM6A; KDM4B; KDM3A | |
| GO:0071558 | Histone demethylase activity (H3-K27 specific) | MF | UTY; KDM6A | |
| GO:0050436 | Microfibril binding | MF | LTBP1; ADAMTSL2 | |
| GO:0004958 | Prostaglandin F receptor activity | MF | PTGFR | |
| GO:0032453 | Histone demethylase activity (H3-K4 specific) | MF | KDM5D | |
| GO:0010859 | Calcium-dependent cysteine-type endopeptidase inhibitor activity | MF | CAST | |
| GO:0002162 | Dystroglycan binding | MF | CLASP2; CLASP1 | |
| GO:1990782 | Protein tyrosine kinase binding | MF | CLASP2; HSP90AA1 | |
| GO:0051010 | Microtubule plus-end binding | MF | DST; CLASP2; CLASP1 | |
| Downregulated | GO:0030574 | Collagen catabolic process | BP | COL5A1; CTSK; COL6A6; COL4A6; COL12A1; ADAMTS2; COL18A1; COL6A3; COL4A4; PHYKPL; MMP27 |
| GO:0051252 | Regulation of RNA metabolic process | BP | NOVA1; RASA1 | |
| GO:0090037 | Positive regulation of protein kinase C signaling | BP | ADRA1A; VEGFA; WNT11 | |
| GO:0007155 | Cell adhesion | BP | COL5A1; ANOS1; LAMA2; CD44; COL6A6; CGREF1; EGFL6; ITGBL1; EDIL3; MAEA; NTM; COL4A6; COL12A1; FAP; THEMIS2; SPON2; SUSD5; TNXB; CD177; ADAM22; COL18A1; PCDHAC1; COL6A3; ERBIN; DST; ARHGAP5; MTSS1; EGFL7; PKN2; FER; EPHB4; PPFIBP1; NEO1; ADGRG1; MYH10; EMILIN2 | |
| GO:0018032 | Protein amidation | BP | PAM | |
| GO:0001519 | Peptide amidation | BP | PAM | |
| GO:0048570 | Notochord morphogenesis | BP | WNT11; KDM6A | |
| GO:0046688 | Response to copper ion | BP | PAM | |
| GO:0009404 | Toxin metabolic process | BP | PAM | |
| GO:0022602 | Ovulation cycle process | BP | PAM | |
| GO:0005595 | Collagen type XII trimer | CC | COL12A1 | |
| GO:0031012 | Extracellular matrix | CC | COL5A1; FBN1; LAMA2; FBLN2; COL6A6; PRG4; SSC5D; EDIL3; HMCN2; MFAP5; JUP; CMA1; CPA3; CTSG; WNT2; COL12A1; ASPN; TNXB; COL18A1; COL6A3; ABI3BP; EGFL7; MMP27; EMILIN2 | |
| GO:0005578 | Proteinaceous extracellular matrix | CC | FBN1; ANOS1; FBLN2; COL6A6; PTPRZ1; OLFML2A; MFAP5; WNT2; WNT11; SPON2; ADAMTS2; ADAMTS7; ASPN; TNXB; WNT4; COL6A3; WNT16; ADAMTS16; MAMDC2; OLFML2A; IL1RL1; LINGO2; ADAMTSL1; EMILIN2; WNT5B; ADAMTS5 | |
| GO:0005788 | Endoplasmic reticulum lumen | CC | COL5A1; FBN1; CES1; ERAP1; SUMF1; SHISA5; COL4A6; COL12A1; ADAMTS7; COLGALT2; COL18A1; WNT4; COL6A3; COL4A4; ADAMTSL1; COL27A1; COLGALT1; EOGT; ARSJ; WNT5B; ADAMTS5 | |
| GO:0005576 | Extracellular region | CC | COL5A1; FBN1; ANOS1; CTSK; LAMA2; FBLN2; PNLIPRP3; FAM3C; ERAP1; FAM19A5; COL6A6; MFAP3; VASH2; NELL2; CGREF1; IL34; GHR; ENTPD5; CYB5D2; IST1; GUSB; MFAP5; DNASE1L1; ANGPT1; GCA; CAP1; NTM; JUP; FAM49B; IGSF1; CMA1; COL4A6; CPA3; CTSG; PSG5; TPSAB1; WNT2; LBP; COL12A1; WNT11; VEGFC; NELL1; TPSD1; ADAMTS2; APOL3; TNXB; SCUBE2; C14orf93; TPSB2; COL18A1; WNT4; FNDC1; RSPO3; ITIH5; COL6A3; WNT16; ANGPTL5; HSD17B13; FAM131A; ABI3BP; PRRG3; NTNG1; IMPDH2; FAM180A; VWCE; RGN; COL4A4; AOAH; EGFL7; COL27A1; CD163L1; TENM1; PDGFC; ARSJ; EPHB4; PLIN2; HABP4; EMILIN2; ENTPD6; WNT5B; HHIPL2; ADAMTS5; ATG7 | |
| GO:1903561 | Extracellular vesicle | CC | FBLN2; EDIL3; TUBB2A; COL12A1; COL6A3 | |
| GO:0042383 | Sarcolemma | CC | LAMA2; SGCE; AHNAK2; SLC8A1; ANK2; AHNAK; COL6A3; SGCZ; SLMAP; ANK3; KCNJ8 | |
| GO:0098982 | GABA-ergic synapse | CC | GLRB | |
| GO:0005589 | Collagen type vi trimer | CC | COL6A3 | |
| GO:0005604 | Basement membrane | CC | COL5A1; FBN1; LAMA2; EGFL6; HMCN2; NTN4; COL18A1; CCDC80; ERBIN; DST; HMCN1 | |
| GO:0005109 | Frizzled binding | MF | WNT2; WNT11; WNT4; RSPO3; WNT16; WNT5B | |
| GO:0004504 | Peptidylglycine monooxygenase activity | MF | PAM | |
| GO:0004598 | Peptidylamidoglycolate lyase activity | MF | PAM | |
| GO:0030020 | Extracellular matrix structural constituent conferring tensile strength | MF | COL12A1 | |
| GO:0016933 | Extracellularly glycine-gated ion channel activity | MF | GLRB | |
| GO:0043175 | RNA polymerase core enzyme binding | MF | SCAF8 | |
| GO:0005178 | Integrin binding | MF | COL5A1; FBN1; TSPAN4; EGFL6; JAM3; EDIL3; FAP; TNXB; CD177; ADAM22; ITGB1BP1; ERBIN; DST; ADAMTS5 | |
| GO:0030247 | Polysaccharide binding | MF | PRG4 | |
| GO:0016167 | Glial cell-derived neurotrophic factor receptor activity | MF | GFRA1 | |
| GO:0019003 | GDP binding | MF | RAB28; RERG; RAP1B; RAB18; RRAGC; RAB27B; RALA; RAP2C |
The top 20 enrichment KEGG pathways were listed (Figures 5 and 6). The pathways associated with upregulated mRNAs included the PPAR signaling pathway, the transforming growth factor beta (TGF-β) signaling pathway, the insulin signaling pathway, the regulation of the actin cytoskeleton, and the interleukin 17 (IL-17) signaling pathway, which were involved in adipogenesis, inflammation, and immune response (Figure 5, Table 2). Enrichment pathways associated with downregulated mRNAs included protein digestion and absorption, ECM-receptor interaction, the PI3K/Akt signaling pathway, and the mTOR signaling pathway (Figure 6, Table 2). The results of KEGG analysis were consistent with the GO annotations.
Figure 5.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of upregulated DET genes. Top 20 enriched KEGG pathways of upregulated DET genes. (A color version of this figure is available in the online journal.)
Figure 6.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of downregulated DET genes. Top 20 enriched KEGG pathways of downregulated DET genes. (A color version of this figure is available in the online journal.)
Table 2.
The top 20 KEGG pathways of up- or down-regulated DET genes between TAO and control samples.
| DET | ID | Term | Gene |
|---|---|---|---|
| Upregulated | hsa03320 | PPAR signaling pathway | SLC27A6; ACOX3; PPARD; SORBS1; AQP7; PPARG; ACADL; FADS2; SCD; ACSL1; GK |
| hsa05202 | Transcriptional misregulation in cancer | EYA1; IL2RB; PLAT; UTY; KDM6A; ZEB1; PBX3; PTK2; PPARG; ATF1; FCGR1A; PBX1; NSD2; MDM2; RUNX1T1; ETV6; EWSR1; FUS; NCOR1 | |
| hsa04310 | Wnt signaling pathway | TCF7L2; PPARD; CCND3; CAMK2D; FZD2; SFRP2; WIF1; BAMBI; WNT2B; PRKACA; VANGL2; CTBP2; CAMK2G; PRICKLE2; MAPK10; PRICKLE1; TBL1Y | |
| hsa04380 | Osteoclast differentiation | FOSB; FCGR3A; IL1R1; PPARG; CREB1; FCGR1A; TGFBR1; CYLD; STAT2; STAT1; MAPK10; MAPK14; LILRB3 | |
| hsa04350 | TGF-beta signaling pathway | PITX2; LTBP1; SMAD9; BMP5; BAMBI; TGFBR1; BMPR1B; RPS6KB1 | |
| hsa04530 | Tight junction | PARD3; CGNL1; RAPGEF2; TIAM1; CLDN11; MYH14; PRKACA; AFDN; MPDZ; DLG1; TJAP1; PATJ; MAPK10; PRKAG2; DLG2; ACTN4; NF2 | |
| hsa03013 | RNA transport | EIF4G3; NUP155; MAGOHB; NCBP1; EIF1AY; TGS1; RANBP2; UPF3A; SEH1L; RGPD1; SRRM1; RGPD5; FXR1 | |
| hsa05150 | Staphylococcus aureus infection | FCGR3A; C2; FPR1 | |
| hsa00600 | Sphingolipid metabolism | SMPD4; CERS1; CERS2; DEGS2; SMPD1; B4GALT6 | |
| hsa04726 | Serotonergic synapse | HTR2A; GNG4; PLA2G4B; CACNA1C; ALOX15; CYP4X1; GNG7; GNAO1; APP; PRKACA; ADCY5; MAOB | |
| hsa04910 | Insulin signaling pathway | PHKA1; PYGL; SOCS2; SORBS1; PRKACA; MKNK1; PRKAR2B; ACACA; MAPK10; PRKAG2; RPS6KB1; SREBF1 | |
| hsa05322 | Systemic lupus erythematosus | FCGR3A; C2; HIST1H2BD; FCGR1A; ACTN4; TROVE2 | |
| hsa05216 | Thyroid cancer | TCF7L2; PPARG; TFG | |
| hsa05140 | Leishmaniasis | FCGR3A; FCGR1A; STAT1; TLR2; MAPK14 | |
| hsa04666 | Fc gamma R-mediated phagocytosis | PLA2G4B; FCGR3A; GSN; PTPRC; PIP5K1B; FCGR1A; BIN1; RPS6KB1 | |
| hsa01040 | Biosynthesis of unsaturated fatty acids | ACOX3; FADS2; SCD; ELOVL7 | |
| hsa04810 | Regulation of actin cytoskeleton | GSN; FGFR1; SPATA13; PTK2; TIAM1; PIP5K1B; TMSB4Y; FGFR2; MYH14; ABI2; ITGA7; GNA12; DOCK1; CHRM3; ITGB8; ACTN4 | |
| hsa05412 | Arrhythmogenic right ventricular cardiomyopathy (ARVC) | CACNA1C; TCF7L2; CACNB4; SLC8A1; CACNA2D1; ITGA7; ITGB8 | |
| hsa04657 | IL-17 signaling pathway | FOSB; S100A8; LCN2; USP25; IL17RE; HSP90AA1; MAPK10; MAPK14 | |
| hsa04217 | Necroptosis | PLA2G4B; ALOX15; MLKL; PYGL; DNM1L; CAMK2D; CAMK2G; SMPD1; CYLD; CHMP2B; HSP90AA1; STAT2; STAT1; MAPK10 | |
| Downregulated | hsa04974 | Protein digestion and absorption | COL5A1; COL6A6; SLC8A1; COL4A6; CPA3; DPP4; KCNN4; COL12A1; COL18A1; COL6A3; COL4A4; COL27A1; COL4A5 |
| hsa04512 | ECM-receptor interaction | LAMA2; CD44; ITGA11; SDC1; COL6A6; COL4A6; THBS2; TNXB; COL6A3; COL4A4; COL4A5; SV2C; FN1 | |
| hsa05217 | Basal cell carcinoma | FZD6; TCF7L2; CDKN1A; APC; WNT2; WNT11; WNT4; WNT16; WNT5B | |
| hsa05225 | Hepatocellular carcinoma | CDKN2A; NQO1; FZD6; TCF7L2; CDKN1A; NFE2L2; APC; WNT2; WNT11; SHC3; WNT4; WNT16; GSTM1; GAB1; PBRM1; MGST2; WNT5B; SMARCA4; PIK3CB | |
| hsa05416 | Viral myocarditis | LAMA2; CD55; SGCA; EIF4G3; HLA-DQB1; BID; CASP9; SGCD; FYN | |
| hsa04614 | Renin-angiotensin system | CMA1; CPA3; CTSG; AGTR1 | |
| hsa04934 | Cushing syndrome | CDKN2A; PDE11A; FZD6; TCF7L2; CDKN1A; RAP1B; APC; WNT2; WNT11; CDKN2B; AGTR1; RASD1; WNT4; WNT16; CAMK2G; CREB1; ARNT; WNT5B; PDE8A; PLCB4 | |
| hsa00740 | Riboflavin metabolism | ACP5; ENPP1 | |
| hsa04115 | p53 signaling pathway | CDKN2A; IGFBP3; RRM2B; CDKN1A; BID; SESN3; SHISA5; CASP9; MDM2; EI24; PERP | |
| hsa04151 | PI3K-Akt signaling pathway | LAMA2; IL6; ITGA11; COL6A6; ANGPT2; CDKN1A; GHR; CASP9; VEGFA; ANGPT1; LPAR1; PTK2; PPP2R5C; PRKAA1; COL4A6; ERBB3; THBS2; VEGFD; VEGFC; SGK1; TNXB; COL6A3; BDNF; PPP2R1B; MDM2; COL4A4; CREB1; MAGI2; COL4A5; FOXO3; PKN2; FN1; PDGFC; PCK1; PIK3CB | |
| hsa04510 | Focal adhesion | LAMA2; ITGA11; COL6A6; PARVB; RAP1B; VEGFA; MAPK8; PTK2; COL4A6; THBS2; VEGFD; VEGFC; SHC3; TNXB; COL6A3; ARHGAP5; COL4A4; BCAR1; COL4A5; FN1; MAPK10; PDGFC; FYN; PIK3CB | |
| hsa05165 | Human papillomavirus infection | LAMA2; ITGA11; COL6A6; FZD6; TCF7L2; CDKN1A; VEGFA; PTK2; PPP2R5C; APC; COL4A6; THBS2; WNT2; WNT11; TNXB; WNT4; COL6A3; WNT16; PPP2R1B; TBK1; MDM2; COL4A4; CREB1; HDAC4; COL4A5; FN1; DLG1; UBE3A; WNT5B; PIK3CB | |
| hsa05224 | Breast cancer | PGR; ESR1; FZD6; TCF7L2; CDKN1A; APC; WNT2; WNT11; SHC3; WNT4; WNT16; WNT5B; PIK3CB | |
| hsa04150 | mTOR signaling pathway | MAPKAP1; PRR5; FZD6; SLC38A9; RRAGC; PRKAA1; WNT2; RPS6KA3; WNT11; SGK1; WNT4; WNT16; RNF152; TTI1; GRB10; ULK2; WNT5B; PIK3CB | |
| hsa00982 | Drug metabolism - cytochrome P450 | FMO3; FMO5; GSTM1; HPGDS; MGST2 | |
| hsa02010 | ABC transporters | ABCB5; ABCC10; ABCG1; ABCD4; ABCB11; ABCA6 | |
| hsa05205 | Proteoglycans in cancer | CD44; SDC1; ESR1; PLAU; FZD6; CDKN1A; VEGFA; MMP2; PTK2; ANK2; ERBB3; WNT2; WNT11; ANK1; WNT4; WNT16; GAB1; MDM2; ANK3; CAMK2G; FN1; WNT5B; PIK3CB | |
| hsa04310 | Wnt signaling pathway | FZD6; TCF7L2; NFATC2; MAPK8; APC; WNT2; WNT11; DKK2; CTNNBIP1; WNT4; WNT16; PRICKLE2; CAMK2G; TBL1XR1; CTBP1; MAPK10; WNT5B; PLCB4 | |
| hsa05226 | Gastric cancer | FZD6; TCF7L2; CDKN1A; JUP; APC; WNT2; WNT11; CDKN2B; SHC3; WNT4; WNT16; GAB1; WNT5B; PIK3CB | |
| hsa05202 | Transcriptional misregulation in cancer | IGFBP3; IL6; RUNX2; HIST2H3D; PLAU; EWSR1; RUNX1T1; CDKN1A; EYA1; PTK2; JUP; ETV6; MMP3; GRIA3; KDM6A; WNT16; NR4A3; MDM2; MLF1; ATF1; FUT8; NCOR1; MAF |
Enrichment analysis of DAS genes
The function of DAS genes was also investigated using GO annotation and KEGG pathway analysis. As the most prevalent events in OACT of TAO, SE events mainly participated in the activation of Janus kinase activity (BP), protein localization to centrosome (BP), centrosome (CC), and actin binding (MF), which were associated with fibroblast migration, adipogenesis (cytoskeleton reorganization), and ECM remodeling (Figure 7). A3SS events were related to positive regulation of type I interferon production (BP), the centrosome cycle (BP), negative regulation of TOR signaling (BP), the microtubule cytoskeleton (CC), cytoskeletal adaptor activity (MF), and thyroid hormone receptor binding (MF) (Supplementary Figure 1(a)). A5SS events were related to cytoskeleton organization, negative regulation of type I interferon production (BP), the centrosome, the cytosol, the Z disc (CC), and the structural constituent of muscle (MF) (Supplementary Figure 2(a)). MXE events were related to the fatty acid metabolic process, smoothened signaling pathway, cilium assembly (BP), ECM (CC), metalloendopeptidase activity, and actin binding (MF) (Supplementary Figure 3(a)).
Figure 7.
Gene Ontology (GO) function enrichment analysis of DAS genes edited by SE events. Top 30 most significant GO terms in BP, CC, and MF of DAS genes edited by SE events. (A color version of this figure is available in the online journal.)
The top 20 KEGG enrichment pathways of DAS genes are shown in Figure 5. SE events of DAS genes were involved in fatty acid biosynthesis, the PPAR signaling pathway, glycerophospholipid metabolism, and adherens junction, which were related to adipogenesis in TAO (Figure 8). A3SS events were involved in fatty acid degradation, glycerophospholipid metabolism, the toll-like receptor signaling pathway, thyroid hormone synthesis, and the PPAR signaling pathway (Supplementary Figure 1(b)). A5SS events were involved in the regulation of lipolysis in adipocytes, the adipocytokine signaling pathway, and Th1/Th2 cell differentiation (Supplementary Figure 2(b)). MXE events were involved in the regulation of lipolysis in adipocytes, the PPAR signaling pathway, the JAK/STAT signaling pathway, and cytokine–cytokine receptor interaction (Supplementary Figure 3(b)).
Figure 8.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of DAS genes edited by SE events. Top 20 enriched KEGG pathways of DAS genes edited by SE events. (A color version of this figure is available in the online journal.)
In summary, the enriched GO terms and KEGG pathways in DAS genes were largely associated with adipogenesis and ECM remodeling, which is consistent with the results of the DET gene enrichment analysis.
Validation of the expression of DET genes
In accordance with the differential expression analysis, the samples of TAO patients exhibited differential splicing transcript expression of VCAN, SORBS1, SEPT2, and COL12A1. To validate the RNA sequencing results, we detected the expression levels of transcript variant 1 (NM_004385.4) and transcript variant 3 (NM_001164097.1) of VCAN, transcript variant 12 (NM_001290298.1) of SORBS1, transcript variant X2 (XM_024452921.1) of SEPT2, and transcript variant short (NM_080645.2) and transcript variant X2 (XM_017010252.2) of COL12A1.
Transcript NM_001164097.1 of VCAN lacked an exon in the coding region compared with transcript NM_004385.4 (Figure 9(a)). The RNA sequencing analysis revealed that the levels of exon inclusion reads and NM_004385.4 of VCAN were lower in TAO patients (Figure 9(b) and (c)), indicating that the occurrence of SE events in VCAN was more frequent in TAO patients’ samples than in those of controls. Moreover, transcript NM_001290298.1 of SORBS1 and transcript XM_024452921.1 of SEPT2 were upregulated, whereas transcripts NM_080645.2 and XM_017010252.2 of COL12A1 were downregulated (Figure 9(c)). The qRT-PCR results confirmed that the expression levels of NM_001290298.1 of SORBS1 and XM_024452921.1 of SEPT2 were elevated, whereas the levels of NM_080645.2 and XM_017010252.2 of COL12A1 and NM_004385.4 of VCAN were reduced (Figure 9(d)). Thus, the qRT-PCR results were consistent with the RNA sequencing results.
Figure 9.
Validation of the expression of DET genes in TAO and control samples. (a) Sequences and exon distribution in transcripts NM_004385.4 and NM_001164097.1 of the VCAN gene. (b) Level of exon inclusion reads, which represents the reads across the junction between exons, of VCAN in TAO and control samples. (c) Expression levels of six transcripts from four genes (SORBS1, SEPT2, COL12A1, and VCAN) based on the RNA sequencing data. (d) Expression levels of six transcripts from four genes based on the qRT-PCR results. (A color version of this figure is available in the online journal.)
Discussion
TAO is characterized by the remodeling and expansion of orbital tissue. Dysregulated metabolism of the ECM and increased orbital adipose/connective tissue are considered the two major elements associated with the pathogenesis of TAO. However, the underlying mechanism is not yet fully understood. In this study, we used RNA sequencing to analyze the DET and DAS genes in OACT of TAO patients in order to investigate the genetic regulation of TAO. Among 113,095 identified genes from RNA sequencing data, 3096 DETs were predicted, of which 1460 were upregulated and 1636 were downregulated. Moreover, 4278 DAS genes were compared between TAO patients and control subjects. Among five main AS events, SE events were the most prevalent, accounting for 66.48%, while RI events were the rarest, accounting for 2.59%. The results indicated the prevalence and importance of the mechanism of AS in the regulation of TAO. Moreover, SE events may play a critical role in the pathological changes of adipose/connective tissue.
The enriched GO terms and KEGG pathways provided more insights into the role of DET and DAS genes in TAO. Based on the results, we found that numerous DETs were associated with the autoimmune response, ECM remodeling, and adipogenesis in adipose/connective tissue. T cell infiltration is a major trigger of pathogenesis in the orbit. 28 In terms of GO analysis, DETs associated with the BP of T cell antigen presentation (GO:0002457) may influence the function of immune cells. The pathway analysis revealed the relationship between DETs and cytokine signaling pathways, including TGF-β (hsa04380) and IL-17 (hsa04657). Aberrant cytokine signaling plays a critical role in the pathogenesis of TAO. Growing evidence shows that the cytokine profile is derived not only from monocytes/macrophages and lymphocytes but also from thyrocytes, OFs, and even adipocytes, participating in inflammation and fibroblast activation in adipose/connective tissue. 28 IL-17A can significantly promote the proinflammatory and profibrotic functions of OFs. 29 Moreover, in the presence of proinflammatory signaling, TGF-β promotes the differentiation of naïve CD4+ T cells into Th17 cells, which mainly produces IL-17. 30 Hence, TGF-β and IL-17 cooperate in promoting TAO.
DETs were associated with the CC of the ECM (GO:0031012), the BPs of the collagen catabolic process (GO:0030574) and cell adhesion (GO:0007155), and the MF of integrin binding (GO:0005178). The ECM-receptor interaction pathway (hsa04512), associated with downregulated DETs, was also screened out. Profound ECM remodeling mediated by activated fibroblasts is a major process in TAO. 5 The accumulation of ECM components, such as collagen, is a hallmark of ECM remodeling and provides multiple potential autoantigens to exacerbate an aberrant autoimmune response. 31 Previous studies have demonstrated that integrin-dependent pathways mediate the recruitment of activated T lymphocytes in the retro-orbital space. 32 Impaired interaction between T cells and the ECM also stimulates this process. 33
The GO terms related to the microtubule cytoskeleton function (GO:0034453, GO:0005828, and GO:0051010) predicted that the related transcripts modulated the adipogenesis of OFs. 34 The KEGG analysis revealed that the DETs were associated with pathways such as the PPAR signaling pathway (hsa03320), the insulin signaling pathway (hsa04910), the PI3K/Akt signaling pathway (hsa04151), and the mTOR signaling pathway (hsa04150), which are also closely related to adipogenesis.7,35 Growing evidence shows that the increased volume of the orbital space is largely due to the adipogenesis process. OFs cultured from TAO mouse models with high TSHR and IGF-1R levels exhibited increased adipogenesis, indicating its important role in TAO. 36 PPARγ is the most important transcriptional modulator in adipogenesis. 37 It has been found that the level of PPARγ is positively correlated with adipogenesis and the level of TSHR in adipose/connective tissues of TAO patients. 38 Another member of the PPAR family, PPARα, also participates in modulating the secretion of CXCL8 and CXCL10 of OFs and preadipocytes, which regulate the inflammatory response. 39 IGF-1 and IGF-1R also play major roles in TAO. IGF-1R is an autoantigen on the cell surface that can form a physical and functional signaling complex with TSHR on fibrocytes, contributing to the transduction of downstream signaling. 40 A randomized placebo-controlled trial investigated human IGF-1R-inhibiting monoclonal antibody teprotumumab as a potential therapeutic strategy to attenuate pathogenesis, producing good outcomes compared with the placebo. 41 Consequently, the related DETs are also potential regulators of the pathogenesis of TAO.
Our DAS analysis showed that differential AS events were also significantly related to the above processes, especially adipogenesis. AS leads to the generation of differential splicing transcripts from one gene, which can be translated into differential functional isoforms. In the DAS analysis, we found that the enriched GO terms associated with AS events were related to centrosome, actin, and microtubule functions. Other GO terms included the BF of positive regulation of type I interferon production, the MF of thyroid hormone receptor binding, the CC of the ECM, and the MF of metalloendopeptidase activity, which were related to cytokine function and ECM remodeling. The KEGG pathway analysis revealed the most enriched pathways, including fatty acid biosynthesis, the PPAR signaling pathway, glycerophospholipid metabolism, and adherens junction. Microtubules and actin filaments are the two main cytoskeleton networks supporting the intracellular architecture and the centrosome, which are considered the organizing centers of both. 42 Cytoskeleton changes interacting with the ECM and influencing the shape and function of cells are observed during adipogenesis.43,44 However, their role in TAO has not yet been elucidated. On the other hand, several pathways have been integratedly analyzed. Fatty acid biosynthesis and glycerophospholipid metabolism are critical parts of adipogenesis, with close interactions with the PPAR family. 45
Our analysis showed a remarkable overlap between the functions of DET and DAS genes. Among them, SORBS1 encodes Cbl-associated protein (CAP), which plays a role in signaling transduction and cytoskeleton rearrangement. CAP is enriched in insulin-sensitive tissues and participates in the insulin signaling pathway and in adipocyte differentiation. 46 A PPAR response element in its promoter has been identified in the sequence of SORBS1, which can regulate the level of CAP. 47 Our analysis revealed one transcript of SORBS1 upregulated in TAO patients, suggesting its potential regulatory function in adipogenesis. The septin family is involved in cytoskeleton reorganization, cytokinesis, and membrane dynamics. 48 Little is known about the exact function of septin-2, encoded by the SEPT2 gene, in TAO. However, PPARγ can regulate the expression and function of SEPT2 in hepatoma cells, 49 suggesting the potential role of SEPT2 in cytoskeleton regulation and PPAR-mediated signaling. Our results showed opposite expression patterns in two transcripts of SEPT2. The importance of this difference remains to be studied.
Collagen XII is a critical component of the ECM, helping to maintain its structure and function. AS of COL12A1 results in a large isoform collagen (XIIA; 320 kD) and a short isoform collagen (XIIB; 220 kD). 50 Collagen XIIA has an NC3 domain carrying glycosaminoglycan chains, whereas collagen XIIB does not. We only discovered low levels of the short splicing transcript, which is involved in the collagen catabolic process according to the GO terms. VCAN encodes versican, an ECM proteoglycan associated with glycosaminoglycan metabolism, which potentially influences TAO. The protein domains encoded by exons 7 and 8 can attach to glycosaminoglycan residues, 51 while AS produces different transcripts either containing these exons or not. According to our analysis, SE events were more prevalent in VCAN genes of TAO patients, indicating high expression of transcripts without exon 7. This also suggests the modulatory role of AS in TAO.
Taken together, the integrated analysis of DET and DAS genes demonstrated that multiple pathways modulating the development of TAO are regulated by the mechanism of AS, resulting in the differential expression of diverse functional transcripts. In particular, the upregulation of components and pathways associated with adipogenesis in our analysis is notable. However, previous studies have reported contradictory results regarding the stimulation of adipogenesis-inhibiting pathways. A study showed that the TGF-β and IL-17 signaling pathways promote fibrosis of Thy+ orbital fibroblasts but inhibit the adipogenesis of Thy−OFs. 52 Moreover, PGF-2α suppresses the function of PPARγ and activates the MEK/ERK cascade, inhibiting the early phase of adipogenesis through prostaglandin F receptor. 53 In our study, PGF receptor activity and the TGF-β/IL-17 signaling pathway were related to upregulated DETs. It is assumed that they exert a potentially protective effect against adipogenesis, which is indicative of the complex modulatory network of adipogenesis and inflammation. Previous research has revealed increased inflammation and adipogenesis in active TAO, which can be attenuated in inactive TAO. 54 Thus, some pathways associated with the inhibition of adipogenesis and the activation of fibrosis may be triggered in response to a change in TAO activity. In our study, the TAO patient tissue samples were in the inactive phase. Hence, it is possible that some adipogenesis-inhibiting signaling pathways were significantly activated. More attention should be paid to AS events to explore new biomarkers and therapeutic targets for TAO and to study changes in AS events during the different phases of TAO.
Conclusions
In this study, we conducted an analysis of DET and DAS genes of adipose/connective tissues of TAO patients, predicting 3096 DETs and 4278 DAS genes. Using rMATS, we found that SE is the most prevalent of all AS events. GO and KEGG analysis showed that the most enriched functions are related to the immune response, ECM remodeling, and adipogenesis. Our results suggest a potentially important role of AS in the pathogenesis of TAO. AS of candidate genes may provide insights into the underlying mechanisms and new therapeutic targets for TAO in the near future.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211017292 for Differential expression and alternative splicing of transcripts in orbital adipose/connective tissue of thyroid-associated ophthalmopathy by Lianqun Wu, Yu Liang, Nan Song, Xiying Wang, Chao Jiang, Xinxin Chen, Bing Qin, Xiantao Sun, Guohua Liu and Chen Zhao in Experimental Biology and Medicine
ACKNOWLEDGEMENTS
The authors thank Professor Ruili Wei (Department of Ophthalmology, Changzheng Hospital, Second Military Medical University) for providing orbital adipose/connective tissue samples of TAO patients. For bioinformatics analysis, the authors appreciated the assistance of Dr. Yao Cheng (OE Biotech, Inc., Shanghai, China, http://www.oebiotech.com/).
AUTHORS’ CONTRIBUTIONS: Lianqun Wu conceived and designed the experiments. Lianqun Wu and Yu Liang participated in the paper writing. Xiying Wang and Chao Jiang performed the experiments. Nan Song and Xinxin Chen collected the samples. Bing Qin, Xiantao Sun, and Guohua Liu analyzed the data. Chen Zhao supervised the progress and revised the manuscript. All authors read and approved the final manuscript.
DECLARATION OF CONFLICTING INTERESTS: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ETHICAL APPROVAL: The study was approved in China by the Ethics Committee of Changzheng Hospital, Second Military Medical University (2018SL039A). All patients got written informed consent, and the research protocol followed the principle of the Declaration of Helsinki.
FUNDING: This study was supported by Shanghai Natural Science Foundation (20ZR1409800 to L.Q.W), National Natural Science Foundation of China (81600765 to L.Q.W., 81670864 and 81730025 to C.Z.), Excellent Academic Leaders of Shanghai (18XD1401000 to C.Z.), Shanghai Municipal Health and Family Planning Commission (201640120 to L.Q.W.).
ORCID iD: Chen Zhao https://orcid.org/0000-0003-1373-7637
SUPPLEMENTAL MATERIAL: Supplemental material for this article is available online.
References
- 1.Bahn RS. Graves' ophthalmopathy. N Engl J Med 2010; 362:726–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGregor AM. Has the target autoantigen for Graves' ophthalmopathy been found? Lancet 1998; 352:595–6 [DOI] [PubMed] [Google Scholar]
- 3.Wall JR, Lahooti H. Pathogenesis of thyroid eye disease – does autoimmunity against the TSH receptor explain all cases? Endokrynol Pol 2010; 61:222–7 [PubMed] [Google Scholar]
- 4.Feldon SE, Park DJ, O'Loughlin CW, Nguyen VT, Landskroner-Eiger S, Chang D, Thatcher TH, Phipps RP. Autologous T-lymphocytes stimulate proliferation of orbital fibroblasts derived from patients with Graves' ophthalmopathy. Invest Ophthalmol Vis Sci 2005; 46:3913–21 [DOI] [PubMed] [Google Scholar]
- 5.Eckstein AK, Johnson KT, Thanos M, Esser J, Ludgate M. Current insights into the pathogenesis of Graves' orbitopathy. Horm Metab Res 2009; 41:456–64 [DOI] [PubMed] [Google Scholar]
- 6.Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Stimulation of adipogenesis, peroxisome proliferator-activated receptor-gamma (PPARgamma), and thyrotropin receptor by PPARgamma agonist in human orbital preadipocyte fibroblasts. J Clin Endocrinol Metab 2002; 87:2352–8 [DOI] [PubMed] [Google Scholar]
- 7.Zhao P, Deng Y, Gu P, Wang Y, Zhou H, Hu Y, Chen P, Fan X. Insulin-like growth factor 1 promotes the proliferation and adipogenesis of orbital adipose-derived stromal cells in thyroid-associated ophthalmopathy. Exp Eye Res 2013; 107:65–73 [DOI] [PubMed] [Google Scholar]
- 8.Bahn RS. Thyrotropin receptor expression in orbital adipose/connective tissues from patients with thyroid-associated ophthalmopathy. Thyroid 2002; 12:193–5 [DOI] [PubMed] [Google Scholar]
- 9.Yang HW, Wang YX, Bao J, Wang SH, Lei P, Sun ZL. Correlation of HLA-DQ and TNF-α gene polymorphisms with ocular myasthenia gravis combined with thyroid-associated ophthalmopathy. Biosci Rep 2017; 37:BSR20160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao P, Yin H, Tao C, Chen P, Song Y, Yang W, Liu L. Latent pathways identification by microarray expression profiles in thyroid-associated ophthalmopathy patients. Endocr Pathol 2015; 26:200–10 [DOI] [PubMed] [Google Scholar]
- 11.Tong BD, Xiao MY, Zeng JX, Xiong W. MiRNA-21 promotes fibrosis in orbital fibroblasts from thyroid-associated ophthalmopathy. Mol Vis 2015; 21:324–34 [PMC free article] [PubMed] [Google Scholar]
- 12.Wu L, Zhou R, Diao J, Chen X, Huang J, Xu K, Ling L, Xia W, Liang Y, Liu G, Sun X, Qin B, Zhao C. Differentially expressed circular RNAs in orbital adipose/connective tissue from patients with thyroid-associated ophthalmopathy. Exp Eye Res 2020; 196:108036. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Liu J, Huang BO, Xu YM, Li J, Huang LF, Lin J, Zhang J, Min QH, Yang WM, Wang XZ. Mechanism of alternative splicing and its regulation. Biomed Rep 2015; 3:152–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JC. Impacts of alternative splicing events on the differentiation of adipocytes. Int J Mol Sci 2015; 16:22169–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Lorenzini PA, Zhang F, Xu S, Wong MSM, Zheng J, Roca X. Alternative splicing analysis in human monocytes and macrophages reveals MBNL1 as major regulator. Nucleic Acids Res 2018; 46:6069–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pujol-Lereis LM, Liebisch G, Schick T, Lin Y, Grassmann F, Uchida K, Zipfel PF, Fauser S, Skerka C, Weber BHF. Evaluation of serum sphingolipids and the influence of genetic risk factors in age-related macular degeneration. PLoS One 2018; 13:e0200739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardamone G, Paraboschi EM, Rimoldi V, Duga S, Soldà G, Asselta R. The characterization of GSDMB splicing and backsplicing profiles identifies novel isoforms and a circular RNA that are dysregulated in multiple sclerosis. Int J Mol Sci 2017; 18:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farina AR, Cappabianca L, Sebastiano M, Zelli V, Guadagni S, Mackay AR. Hypoxia-induced alternative splicing: the 11th hallmark of cancer. J Exp Clin Cancer Res 2020; 39:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartley GB, Gorman CA. Diagnostic criteria for Graves' ophthalmopathy. Am J Ophthalmol 1995; 119:792–5 [DOI] [PubMed] [Google Scholar]
- 20.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves' ophthalmopathy. Clin Endocrinol 1997; 47:9–14 [DOI] [PubMed] [Google Scholar]
- 21.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 2014; 30:2114–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 2015; 12:357–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anders S, Pyl PT, Huber W. HTSeq – a python framework to work with high-throughput sequencing data. Bioinformatics 2015; 31:166–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts A, Pimentel H, Trapnell C, Pachter L. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics 2011; 27:2325–9 [DOI] [PubMed] [Google Scholar]
- 25.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol 2010; 11:R106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen S, Park JW, Lu ZX, Lin L, Henry MD, Wu YN, Zhou Q, Xing Y. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci U S A 2014; 111:E5593–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Cao C, Ma Q, Zeng Q, Wang H, Cheng Z, Zhu G, Qi J, Ma H, Nian H, Wang Y. RNA-seq analyses of multiple meristems of soybean: novel and alternative transcripts, evolutionary and functional implications. BMC Plant Biol 2014; 14:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gianoukakis AG, Khadavi N, Smith TJ. Cytokines, Graves' disease, and thyroid-associated ophthalmopathy. Thyroid 2008; 18:953–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang S, Huang Y, Wang S, Zhang Y, Luo X, Liu L, Zhong S, Liu X, Li D, Liang R, Miranda P, Gu P, Zhou H, Fan X, Li B. IL-17A exacerbates fibrosis by promoting the proinflammatory and profibrotic function of orbital fibroblasts in TAO. J Clin Endocrinol Metab 2016; 101:2955–65 [DOI] [PubMed] [Google Scholar]
- 30.Lee GR. The balance of Th17 versus treg cells in autoimmunity. Int J Mol Sci 2018; 19:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bednarczuk T, Stolarski C, Pawlik E, Slon M, Rowinski M, Kubota S, Hiromatsu Y, Bartoszewicz Z, Wall JR, Nauman J. Autoantibodies reactive with extracellular matrix proteins in patients with thyroid-associated ophthalmopathy. Thyroid 1999; 9:289–95 [DOI] [PubMed] [Google Scholar]
- 32.Heufelder AE, Scriba PC. Characterization of adhesion receptors on cultured microvascular endothelial cells derived from the retroorbital connective tissue of patients with Grave's ophthalmopathy. Eur J Endocrinol 1996; 134:51–60 [DOI] [PubMed] [Google Scholar]
- 33.Bednarczuk T, Kiljanski J, Mrowiec T, Slon M, Ing E, Stolarski C, Kennerdell JS, Gorski A, Nauman J, Wall JR. T cell interactions with extracellular matrix proteins in patients with thyroid-associated ophthalmopathy. Autoimmunity 1998; 27:221–30 [DOI] [PubMed] [Google Scholar]
- 34.Padilla-Benavides T, Velez-delValle C, Marsch-Moreno M, Castro-Muñozledo F, Kuri-Harcuch W. Lipogenic enzymes complexes and cytoplasmic lipid droplet formation during adipogenesis. J Cell Biochem 2016; 117:2315–26 [DOI] [PubMed] [Google Scholar]
- 35.Cai H, Dong LQ, Liu F. Recent advances in adipose mTOR signaling and function: therapeutic prospects. Trends Pharmacol Sci 2016; 37:303–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Görtz GE, Moshkelgosha S, Jesenek C, Edelmann B, Horstmann M, Banga JP, Eckstein A, Berchner-Pfannschmidt U. Pathogenic phenotype of adipogenesis and hyaluronan in orbital fibroblasts from female Graves' orbitopathy mouse model. Endocrinology 2016; 157:3771–8 [DOI] [PubMed] [Google Scholar]
- 37.Mota de Sá P, Richard AJ, Hang H, Stephens JM. Transcriptional regulation of adipogenesis. Compr Physiol 2017; 7:635–74 [DOI] [PubMed] [Google Scholar]
- 38.Kumar S, Coenen MJ, Scherer PE, Bahn RS. Evidence for enhanced adipogenesis in the orbits of patients with Graves' ophthalmopathy. J Clin Endocrinol Metab 2004; 89:930–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrari SM, Ragusa F, Paparo SR, Nasini F, Nardi M, Franceschini SS, Fallahi P, Antonelli A. Differential modulation of CXCL8 versus CXCL10, by cytokines, PPAR-gamma, or PPAR-alpha agonists, in primary cells from Graves' disease and ophthalmopathy. Autoimmun Rev 2019; 18:673–8 [DOI] [PubMed] [Google Scholar]
- 40.Smith TJ, Janssen J. Insulin-like growth factor-I receptor and thyroid-associated ophthalmopathy. Endocr Rev 2019; 40:236–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, Harris GJ, Antonelli A, Salvi M, Goldberg RA, Gigantelli JW, Couch SM, Shriver EM, Hayek BR, Hink EM, Woodward RM, Gabriel K, Magni G, Douglas RS. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med 2017; 376:1748–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farina F, Gaillard J, Guérin C, Couté Y, Sillibourne J, Blanchoin L, Théry M. The centrosome is an actin-organizing Centre. Nat Cell Biol 2016; 18:65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mor-Yossef Moldovan L, Lustig M, Naftaly A, Mardamshina M, Geiger T, Gefen A, Benayahu D. Cell shape alteration during adipogenesis is associated with coordinated matrix cues. J Cell Physiol 2019; 234:3850–63 [DOI] [PubMed] [Google Scholar]
- 44.Trivanović D, Drvenica I, Kukolj T, Obradović H, Okić Djordjević I, Mojsilović S, Krstić J, Bugarski B, Jauković A, Bugarski D. Adipoinductive effect of extracellular matrix involves cytoskeleton changes and SIRT1 activity in adipose tissue stem/stromal cells. Artif Cells Nanomed Biotechnol 2018; 46:S370–82 [DOI] [PubMed] [Google Scholar]
- 45.Barquissau V, Ghandour RA, Ailhaud G, Klingenspor M, Langin D, Amri EZ, Pisani DF. Control of adipogenesis by oxylipins, GPCRs and PPARs. Biochimie 2017; 136:3–11 [DOI] [PubMed] [Google Scholar]
- 46.Zhang M, Kimura A, Saltiel AR. Cloning and characterization of cbl-associated protein splicing isoforms. Mol Med 2003; 9:18–25 [PMC free article] [PubMed] [Google Scholar]
- 47.Baumann CA, Chokshi N, Saltiel AR, Ribon V. Cloning and characterization of a functional peroxisome proliferator activator receptor-gamma-responsive element in the promoter of the CAP gene. J Biol Chem 2000; 275:9131–5 [DOI] [PubMed] [Google Scholar]
- 48.Neubauer K, Zieger B. The mammalian septin interactome. Front Cell Dev Biol 2017; 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao LQ, Shao ZL, Liang HH, Zhang DW, Yang XW, Jiang XF, Xue P. Activation of peroxisome proliferator-activated receptor-γ (PPARγ) inhibits hepatoma cell growth via downregulation of SEPT2 expression. Cancer Lett 2015; 359:127–35 [DOI] [PubMed] [Google Scholar]
- 50.Koch M, Bohrmann B, Matthison M, Hagios C, Trueb B, Chiquet M. Large and small splice variants of collagen XII: differential expression and ligand binding. J Cell Biol 1995; 130:1005–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kloeckener-Gruissem B, Neidhardt J, Magyar I, Plauchu H, Zech JC, Morlé L, Palmer-Smith SM, Macdonald MJ, Nas V, Fry AE, Berger W. Novel VCAN mutations and evidence for unbalanced alternative splicing in the pathogenesis of Wagner syndrome. Eur J Hum Genet 2013; 21:352–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang S, Huang Y, Zhong S, Li Y, Zhang Y, Li Y, Sun J, Liu X, Wang Y, Zhang S, Xu T, Sun X, Gu P, Li D, Zhou H, Li B, Fan X. Regulation of orbital fibrosis and adipogenesis by pathogenic Th17cCells in Graves orbitopathy. J Clin Endocrinol Metab 2017; 102:4273–83 [DOI] [PubMed] [Google Scholar]
- 53.Ueno T, Fujimori K. Novel suppression mechanism operating in early phase of adipogenesis by positive feedback loop for enhancement of cyclooxygenase-2 expression through prostaglandin F2α receptor mediated activation of MEK/ERK-CREB Cascade. FEBS J 2011; 278:2901–12 [DOI] [PubMed] [Google Scholar]
- 54.Khong JJ, Wang LY, Smyth GK, McNab AA, Hardy TG, Selva D, Llamas B, Jung CH, Sharma S, Burdon KP, Ebeling PR, Craig JE. Differential gene expression profiling of orbital adipose tissue in thyroid orbitopathy. Invest Ophthalmol Vis Sci 2015; 56:6438–47 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_15353702211017292 for Differential expression and alternative splicing of transcripts in orbital adipose/connective tissue of thyroid-associated ophthalmopathy by Lianqun Wu, Yu Liang, Nan Song, Xiying Wang, Chao Jiang, Xinxin Chen, Bing Qin, Xiantao Sun, Guohua Liu and Chen Zhao in Experimental Biology and Medicine