Abstract
Background
Our Cardiac High Acuity Monitoring Program (CHAMP) uses home video telemetry (HVT) as an adjunct to monitor infants with single ventricle during the interstage period. This study describes the development of an objective early warning score using HVT, for identification of infants with single ventricle at risk for clinical deterioration and unplanned hospital admissions (UHA).
Methods and Results
Six candidate scoring parameters were selected to develop a pragmatic score for routine evaluation of HVT during the interstage period. We evaluated the individual and combined ability of these parameters to predict UHA. All infants with single ventricle monitored at home by CHAMP between March 2014 and March 2018 were included. Videos obtained within 48 hours before UHA were compared with videos obtained at baseline. We used binary logistic regression models and receiver operating characteristic curves to evaluate the parameters' performance in discriminating the outcome of interest.
Thirty‐nine subjects with 64 UHA were included. We compared 64 pre‐admission videos to 64 paired baseline videos. Scoring was feasible for a mean of 91.6% (83.6%–98%) of all observations. Three different HVT score models were proposed, and a final model composed of respiratory rate, respiratory effort, color, and behavior exhibited an excellent discriminatory capability with an area under the receiver operating characteristic curve of 93% (89%–98%). HVT score of 5 was associated with specificity of 93.8% and sensitivity of 88.7% in predicting UHA.
Conclusions
We developed a feasible and reproducible HVT score that can serve as a tool to predict UHA in infants with single ventricle. Future directions involve prospective, multicenter validation of this tool.
Keywords: early warning score, interstage, monitoring, single ventricle, telemedicine, univentricular heart
Subject Categories: Congenital Heart Disease
Nonstandard Abbreviations and Acronyms
- AUROC
area under the receiver operating characteristics
- BT
Blalock Taussig
- CHAMP
Cardiac High Acuity Monitoring Program
- EWS
early warning score
- HVT
home video telemetry
- SV
single ventricle
- UHA
unplanned hospital admission
Clinical Perspective
What Is New?
Cardiac High Acuity Monitoring Program is an advanced form of interstage home monitoring that incorporates symptoms, vital signs, and video telemetry.
The additive role of video telemetry to home monitoring has not been previously studied.
What Are the Clinical Implications?
This study provides a highly accurate, feasible, and reproducible early warning scoring system, utilizing home video telemetry, for prediction of unplanned hospital admissions in single ventricle infants.
Having a structured approach to video telemetry can increase healthcare providers’ confidence and comfort level with this type of virtual care.
Patients with single ventricle (SV) congenital heart disease remain at increased mortality risk, particularly during the interstage period; between the first and second palliative surgical stages. Despite the steadily improving survival to hospital discharge after the initial neonatal surgery, the incidence of death before stage II palliation remains at ≈10% to 12%.1, 2, 3, 4 Interstage home monitoring programs involving daily parental surveillance of parameters such as daily weight and oxygen saturations, have significantly improved interstage survival.5, 6, 7, 8, 9, 10, 11, 12, 13, 14
Cardiac High Acuity Monitoring Program (CHAMP) is an advanced form of interstage home monitoring which we developed at our institution, with the goal of standardizing management during this critical period. CHAMP is a tablet/smart phone‐based application that incorporates symptoms, vital signs, and video telemetry.15 It provides secure, and nearly instantaneous transfer of patient information to a cloud‐based server for immediate analysis and communication by the clinical management team. Since the implementation of this program in 2014, we have witnessed a significant reduction in interstage mortality from 17%14 to ~3%.16 The additive role of video telemetry to home monitoring has not been previously studied. This is particularly relevant as the COVID‐19 pandemic has forced all healthcare systems to rapidly implement telehealth services. In the midst of “stay at home” orders and physical distancing measures, a structured approach to video telemetry can increase healthcare providers’ confidence and comfort level with this type of virtual care, while minimizing out‐of‐home exposures for at‐risk populations.
While several early warning scores (EWS) are currently available for bedside prediction of cardiac arrest and/or unplanned intensive care unit transfer of general and cardiac pediatric patients, 17, 18, 19 data on the early identification of sick infants at home by video telemetry are still lacking. The primary aim of this study was to develop a systematic EWS system using home video telemetry (HVT) to identify SV interstage infants at risk for clinical deterioration and predict unplanned hospital admission (UHA).
Methods
Study Design and Participants
The data that support the findings of this study are available from the corresponding author upon reasonable request. A single center, cohort study, with retrospective analysis of CHAMP HVT was conducted. Infants enrolled in the CHAMP from March 2014 to March 2018 were considered eligible for the study. As reported in an earlier study,16 these included all infants with shunt‐dependent SV physiology, patients with aorto‐pulmonary shunts in anticipation of biventricular repair, patients with pulmonary artery banding and anticipated single ventricle palliation, and patients with stable neonatal physiology, with undetermined candidacy for biventricular repair. Patients had to have at least 5 videos during the entire period of monitoring and at least 1 UHA were included. Ethics approval was granted by the Institutional Review Board for the Protection of Human Subjects. Waiver of informed consent/assent was provided because of the retrospective nature of this study.
Home Video Telemetry
As a special feature of CHAMP, parents are instructed to record 15‐second‐long video clips of their infant at least once a day for routine surveillance, using the CHAMP device camera. These clips are transferred via cellular network to the CHAMP team through the CHAMP app, for later viewing (asynchronous HVT). Daily review and subjective assessment of the videos is performed by the clinical management team, which at our institution, includes dedicated registered nurse coordinators, advance practice nurses, and cardiologists.
HVT Score Development
To test the additive value of HVT in detecting imminent clinical deterioration among SV interstage infants, we developed an HVT based EWS that can be used to trigger critical evaluation of those patients. A multidisciplinary planning panel, including pediatric cardiologists, nurse practitioners, and software architects with significant involvement in CHAMP was set for development of the HVT score.
Since no similar video scoring systems exist, we modeled the HVT score after the previously established Pediatric Early Warning Score.17 Pediatric Early Warning Score is a validated in‐hospital bedside tool for early detection of clinical deterioration and prevention of cardiopulmonary arrests or unplanned transfers to the intensive care unit.17 Our planning panel identified the risk factors unique to infants with SV as well as markers of severity of clinical derangement.
A special challenge we faced during the development of our HVT score was that all score parameters of interest had to be visible to be remotely assessed. Therefore, variables such as heart rate, rhythm, and capillary refill had to be replaced with visible parameters. Also, and unlike the Children’s Early Warning Score; a modification to the Pediatric Early Warning Score by Children’s Hospital of Boston,20 we did not incorporate “families’ concerns” since we aimed for our clinical score to be based on objective assessment of the videos rather than on familiarity with the patient’s history. This specific parameter is separately evaluated within CHAMP as a “red flag warning”.
Score Parameters
As with Pediatric Early Warning Score, our HVT scorecard includes objective assessment of 3 domains: respiratory, cardiovascular, and neurologic. Our initial score included every possible visible parameter belonging to each of these domains (Table 1). Additionally, skin, specifically surgical wound status, was included as it was felt to be of clinical relevance to postoperative cardiac patients. Finally, appearance was included to indicate staff’s overall subjective clinical impression of the patient’s condition compared to the typical, healthy single ventricle patient.
Table 1.
The Home Video Telemetry Score for Single Ventricle Interstage Infants
| Score | Respiratory Rate | Respiratory Effort | Color | Behavior | Skin | Appearance |
|---|---|---|---|---|---|---|
| 0 | 20 to 39 | No accessory muscle use, no retractions | Pink or mild cyanosis | Playing/sleeping appropriately, baseline activity, smiling, and tracking parents | No wound concern/rash | Expected appearance |
| 1 | 40 to 49 | Mild subcostal retractions | Pale | Sleepy, somnolent when not disturbed | Wound infection concerns (erythema/swelling/discharge) or rash | Mild concern |
| 2 | 50 to 59 | Moderate subcostal retractions, flaring | Gray | Irritable, difficult to console | Significant concern | |
| 3 | 60 to 69 | Suprasternal/intercostal retractions | Gray and mottled | Lethargic, floppy, reduced response to stimulation or pain | ||
| 4 | <20 or ≥70 | Severe subcostal retractions, head bobbing, paradoxical breathing |
For individual parameters, observations were scored from 0 to 4, with higher scores indicating increasing level of concern (Table 1). Skin was dichotomous for the presence or absence of rash and/or infection and appearance was graded as expected, mild concern or significant concern. The initial HVT score ranged from 0 to 17.
Unplanned hospital admission (UHA) was considered an objective indicator of clinical deterioration and was used as the primary outcome. Hospital admissions were considered unplanned when unrelated to planned procedural or surgical intervention or diagnostic imaging.
Before implementation, 2 pediatric cardiologists (D.A., H.H.) received extensive education and training on how to apply the HVT score to CHAMP videos. Training was provided by a front‐line advanced practice nurse (L.E.) who uses CHAMP videos daily for triaging and patient care. Inter‐rater reproducibility of the HVT score was established between both physicians in a sample of 30 videos. Both raters were masked to the date and time of the videos.
To assess the HVT score’s ability to discriminate the outcome of interest, we compared HVT scores of videos obtained within 48 hours before the UHA (pre‐admission) to those obtained at baseline. Baseline status was defined as the period within 48 hours post‐discharge from a UHA or post‐neonatal (stage I palliation) discharge.
Randomization and Masking of Raters
An independent member of the planning panel (L.E.), who was not involved in scoring the videos, used chart review to identify patients eligible for inclusion and dates of their UHAs. Computer‐generated random selection of the videos was performed within the predefined timeframes. Selected videos were deidentified and subsequently inserted into a password encrypted database. Videos were scored by the primary investigator (D.A.) who was masked to date of the videos and admission status attached to the videos (baseline versus pre‐admission).
Statistical Analysis
Statistical analysis was performed using SPSS for Windows version 26.0 (SPSS, Inc., Chicago, IL, USA). Data were presented as means±SD and percentages, as appropriate. We compared the individual HVT score parameters as well as total composite scores for the same UHA, between pre‐admission and baseline. Kolmogorov‒Smirnov test was used to test for normality, and outliers were eliminated after being detected by box plots.
Given the dependent nature of data, we used paired samples t‐tests to compare means among continuous data and Wilcoxon signed‐rank test for non‐parametric data. A P value of <0.05 was considered significant. We used the receiver operating curves to evaluate the discriminant capability of the total score and individual parameters independently to identify UHA. An area under the receiver operating curves (AUROC) of one indicated perfect discrimination, and 0.5 reflected a discriminating capability no better than chance.
We then used binary logistic regression to assess the individual contribution of each candidate score parameter to predict UHA. The results were presented as odds ratios and 95% CI. Multicollinearity between the individual scoring parameters was assessed using Pearson correlation coefficient (r), variance inflation factor and tolerance values. The final HVT score was generated through forced entry regression. Inter‐rater reproducibility was assessed using intra‐class coefficients.
Results
Patient Characteristics and Unplanned Hospital Admissions
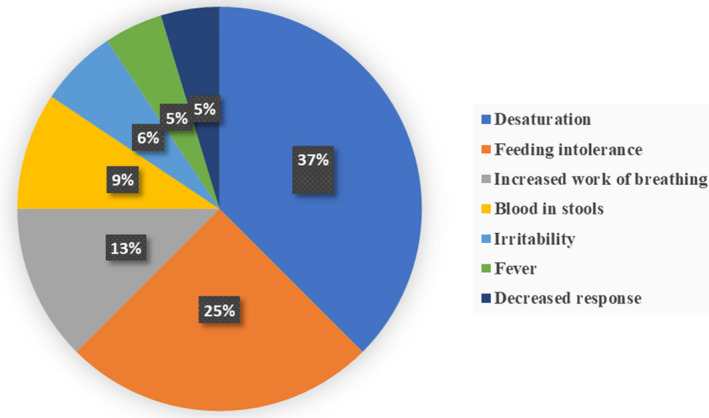
One hundred and two patients were enrolled in CHAMP during the study period. Thirty‐nine patients (38% of the CHAMP cohort) with 64 UHAs were included (Table 2). Forty‐one patients who had UHAs were excluded because of lack of videos within 48 hours of a readmission (n=35), or submission of <5 videos during the entire period of monitoring (n=6). Twenty‐two patients were excluded because of lack of UHA. Forty‐three percent of the study cohort had Norwood stage 1 palliation. Thirty‐six of the 64 UHAs were preceded by red flags. The most common indication for UHA was desaturation (n=24, 37%) followed by feeding intolerance (n=16, 25%) (Figure 1). Nearly 50% of UHAs included admissions to the intensive care unit, either directly or as a transfer from the regular care cardiac unit. Eight unplanned cardiac surgeries and 7 unplanned cardiac catheterizations were performed during these UHAs. There was a single mortality after experiencing cardiac arrest in an outside hospital; this patient died after 21 days of veno‐arterial extracorporeal membrane oxygenation.
Table 2.
Demographic and Unplanned Hospital Admission Characteristics
| Patients, n=39 | n (%) |
|---|---|
| Sex | |
| Women | 17 (43.6) |
| Men | 22 (56.4) |
| Anatomy | |
| HLHS | 16 (41.0) |
| Other* | 23 (59.0) |
| Type of palliation | |
| Norwood RV/PA shunt | 10 (25.6) |
| Norwood BT shunt | 7 (18) |
| Hybrid | 3 (7.7) |
| BT shunt | 9 (23) |
| PA band | 5 (13) |
| Others† | 5 (13) |
| Unplanned hospital admissions, n=64 | |
| Age at admission (days) | 133.2±85 |
| ICU admissions | 34 (53) |
| No. of ICU days | 7.4±10.0 |
| Unplanned cardiac surgery | 8 (12.5) |
| Glenn palliation | 2 |
| PA band | 3 |
| DKS/shunt | 1 |
| Sternal wound debridement | 1 |
| BT shunt revision | 1 |
| Unplanned cardiac catheterization | 7 (11) |
| Coarctation balloon angioplasty | 4 |
| DKS balloon angioplasty | 1 |
| BT stent angioplasty | 1 |
| BAS | 1 |
| Mortality | 1 |
BAS indicates balloon atrial septostomy; BT, Blalock Taussig; DKS, Damus‐Kaye‐Stansel; HLHS, hypoplastic left heart syndrome; ICU, intensive care unit; PA, pulmonary artery; and RV, right ventricle.
Other indicates unbalanced atrioventricular canal, borderline left sided structures, double inlet left ventricle, tricuspid atresia.
Others indicates PDA stent, RV/PA shunt, none.
Figure 1. Pie chart showing the different indications for unplanned hospital admissions in interstage single ventricle infants.
Reproducibility and Feasibility of Video Telemetry Scoring
Total of 128 videos were included for scoring, including 64 pre‐admission videos and 64 baseline videos (paired for the corresponding UHA). The inter‐rater reproducibility was excellent for most individual score parameters with an intra‐class coefficient >0.85 (with the exception of skin; intra‐class coefficients, 0.71) and for composite scores. Video scoring was feasible for 91.6% of all observations, with a feasibility rate ranging between 83.6% and 98% for each individual score parameter. Color had the highest non‐scorable percentage at 16%. Observations were deemed non‐scorable and were eventually excluded from analysis because of reasons such as inadequate lighting or distance from the patient, excessive movement, or inadequate exposure of body/skin with clothing or other coverings.
Discriminant Analysis of the HVT Score
Individual HVT score parameters as well as the composite scores were compared between pre‐admission and baseline videos. Five of the parameters (respiratory rate, respiratory effort, color, behavior, and appearance) and the composite scores were significantly higher for the pre‐admission videos than for the baseline videos (Table 3), while the difference in the proportion of skin rash and or infection was not statistically significant between the 2 groups (P=0.23).
Table 3.
Comparative Analysis of Means/Medians of the Individual HVT Score Items and Composite Score
| Video type | Respiratory rate | Respiratory effort | Color | Behavior | Skin | Appearance | Composite score |
|---|---|---|---|---|---|---|---|
| Pre‐admission | 2.6 | 2.5 | 1 | 1 | 0 | 2 | 7 |
| Baseline | 1.2 | 1 | 0 | 0 | 0 | 0 | 2 |
| P value | 0.009* | 0.004* | 0.0007* | 0.005* | 0.23 | 0.0009* | 0.0001* |
Means of respiratory rate, respiratory effort, color, and composite score were compared using paired sample t‐test.
Medians of behavior, skin, and appearance were compared using Wilcoxon signed‐rank test.
P value of <0.05 (statistically significant).
The results of receiver operating curve analysis are presented in (Table 4). An HVT score with all the parameters included had an excellent discriminating capability with an AUROC of 0.95. Individual score parameters had AUROC values between 0.71 (for respiratory effort) and 0.82 (for color). Skin had poor discriminating capability with AUROC of 0.5, P>0.05.
Table 4.
AUROC for the Initial HVT Score and Individual Candidate Score Variable
| Variables | AUROC | SE | P Value | 95% CI |
|---|---|---|---|---|
| HVT score | 0.95 | 0.016 | 0.003* | 0.93‒0.99 |
| Respiratory rate | 0.73 | 0.05 | 0.0001* | 0.64‒0.8 |
| Respiratory effort | 0.71 | 0.045 | 0.009* | 0.62‒0.79 |
| Color | 0.82 | 0.04 | 0.0006* | 0.74‒0.89 |
| Behavior | 0.75 | 0.04 | 0.004* | 0.66‒0.83 |
| Skin | 0.5 | 0.05 | 0.184 | 0.45‒0.66 |
| Appearance | 0.8 | 0.03 | 0.0002* | 0.79‒0.92 |
AUROC indicates area under the receiver operating curve; HVT, home video telemetry; and SE, standard error.
P value of <0.05 (statistically significant).
Predictors of Unplanned Hospital Admissions During the Interstage Period
Logistic regression analysis was conducted to assess whether a score model based on the candidate parameters could accurately predict UHA. The assumptions of linearity, independence of errors, and absent multicollinearity were tested. A significant correlation was noted between appearance and each of the respiratory effort, color, and behavior parameters with an r of 0.8, 0.75 and 0.79, respectively. The contribution of each parameter to the score model was also tested. Table 5 displays the odds ratios of each parameter. Skin again was found to be of insignificant predictive value, with a P value for the Wald test >0.05. The parameters that contributed the most to the model were color (odds ratio [OR], 8.32; P value <0.005) and respiratory effort (OR, 4.5; P value <0.002).
Table 5.
Variables Included in the HVT Score Model
| Variables | B | SE | Wald Test | P Value | Odds Ratio | 95% CI |
|---|---|---|---|---|---|---|
| Respiratory rate | 1.72 | 0.38 | 8.61 | 0.003* | 3.6 | 2.97‒5.36 |
| Respiratory effort | 1.5 | 0.48 | 9.61 | 0.002* | 4.5 | 1.7‒11.67 |
| Color | 2.11 | 0.59 | 12.78 | <0.0005* | 8.32 | 2.6‒26.59 |
| Behavior | 1.23 | 0.42 | 8.38 | 0.004* | 3.43 | 1.49‒7.9 |
| Skin | 0.72 | 0.75 | 0.91 | 0.339 | 2.06 | 0.46‒9.14 |
| Appearance | 1.23 | 0.45 | 7.57 | 0.006* | 3.45 | 1.42‒8.33 |
| Constant | −4.9 | 0.99 | 25.17 | <0.005 | 0.007 |
B indicates beta coefficient; and SE, standard error.
P value of <0.05 (statistically significant).
Based on those results, 3 different models were generated and tested: model 1; with all the variables included, model 2; with skin eliminated and model 3; with skin and appearance eliminated (the latter was eliminated because of significant collinearity with other variables). Likelihood ratios were compared, and model fits were evaluated by performing the Hosmer‐Lemeshow test and also compared between the 3 models (Table 6). The final model (model 3); composed of respiratory rate, respiratory effort, color, and behavior, showed significant prediction of UHA with Chi‐square (X 2)=100.8, df=4, P<0.05. This was slightly lower than that of the first and second models; X 2=111.4 and 110.5 respectively (Table 6). Approximately 60% to 72.3% of the variance in the outcome of UHA can be predicted from this 4‐predictor linear regression binary model (model 3); Cox and Snell R 2=0.6 and Nagelkerke R 2=0.72.
Table 6.
Likelihood Ratios and AUROC Analysis of the Three Generated Models
| Models | Chi‐square (x 2) | df | AUROC | SE | P Value | 95% CI |
|---|---|---|---|---|---|---|
| Model 1 | 111.4 | 6 | 0.95 | 0.19 | 0.003* | 0.91‒0.99 |
| Model 2 | 110.5 | 5 | 0.95 | 0.19 | 0.0001* | 0.91‒0.98 |
| Model 3 | 100.78 | 4 | 0.93 | 0.02 | 0.00002* | 0.89‒0.98 |
AUROC indicates area under of the receiver operatory; df, degree of freedom; and SE, standard error.
P value of <0.05 (statistically significant).
Finally, we compared the AUROC between the 3 models (Table 6 and Figure 2) and the final model (model 3) continued to have excellent discriminant capability with a mean AUROC of 0.93 (0.89–0.98). An HVT score of 5 had a specificity of 93.8% and sensitivity of 88.7% (Figure 2).
Figure 2. Receiver operating curves for the 3 models.
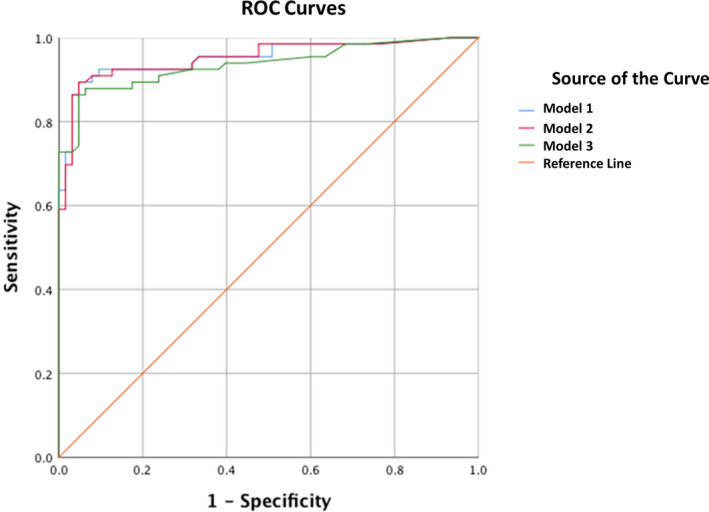
ROC indicates receiver operating characteristic curves.
Discussion
We have successfully developed a clinical scoring system for HVT that can identify SV interstage infants at risk for clinical deterioration and predict UHA. To our knowledge, this is the first study to provide systematic assessment of the role of HVT in interstage home monitoring. To summarize our results, an HVT score based on respiratory rate, respiratory effort, color, and behavior provides excellent prediction of UHA (X 2=100.8, df=4, P<0.05) and significant discriminatory capability between preadmission and baseline daily videos (mean AUROC, 0.93). A cut‐off of 5 out of 13 has a specificity of 93.8% and sensitivity of 88.7% predicting UHA. Finally, this HVT score system is feasible and highly reproducible among different raters.
SV interstage infants often experience acute physiologic deterioration shortly before suffering adverse events such as cardiac arrest or death at home. These events are potentially preventable by timely recognition of patients’ derangement and proper management by a skilled team. While home monitoring programs have been associated with improved interstage mortality13 (possibly because of successful prediction of gradual clinical worsening), an acute “track and trigger” tool is still lacking. Our CHAMP clinical team have used their subjective impressions based on HVT, however, a pragmatic, objective tool for systematic assessment of HVT is still missing. This can be vital to newly trained physicians and nurses when subtle clinical changes may not be evident unless quantified.
It is worth mentioning that only 36 of 64 UHAs in our study were preceded by red flags, suggesting a maximum sensitivity of red flags of 56%, and indicating higher sensitivity of our HVT score (88.7%) in detecting SV interstage infants at risk than relying on red flags alone. While this tool was designed and completed before the emergence of the coronavirus pandemic, we believe that the need for this tool has particularly increased now as healthcare providers are currently striving to provide the best quality virtual care to their critical patients, with limited in‐person evaluation.
Development of the HVT Early Warning Score
The current study detailed the development of an objective and easy to use early warning score model. We started by including all possible visible indicators of the cardiorespiratory status as recommended by our CHAMP multidisciplinary team. This was followed by reproducibility testing, comparative, and predictive analysis of individual HVT parameters. This helped us determine which parameters should be included. We then confirmed the discriminating capability of our final model and the sensitivity and specificity of an optimal composite score threshold. This multi‐phased approach (Figure 3) has been previously used in developing risk assessment tools21 and refining previously established EWS.22
Figure 3. Flow diagram summarizing the steps of development of our home video telemetry score.
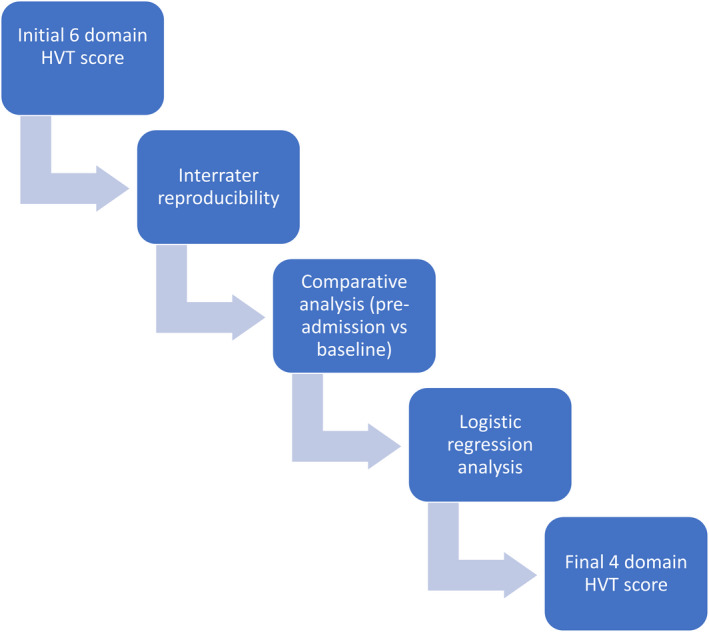
HVT indicates home video telemetry.
The “appearance” parameter was initially included as a subjective impression of deterioration. In our study, despite the significantly higher mean score of this parameter before admission than at baseline and high AUROC, significant overlap (collinearity) was noted with other parameters. Therefore, we propose that appearance is a cumulative impression that a reviewer may develop based on his/her assessment of other parameters. With this amount of overlap and subjectivity we decided to remove this parameter from our final model.
Another interesting parameter we investigated was skin. In our cohort, only 5 pre‐admission home videos raised suspicion for wound infection. This was not unexpected, given the relatively low rate of wound infections beyond the immediate postoperative hospitalization. Therefore, it is possible that our cohort was under‐powered for this parameter. A larger sample size may be required to confidently confirm the lack of contribution of the skin parameter to the outcome.
When all parameters were combined, 3 different models were generated. While all 3 models had relatively similar discriminating capabilities (Figure 2), we opted to remove the 2 aforementioned parameters (appearance and skin) when generating our final model for simplification. Jarvis and co‐authors23 demonstrated that simpler EWS with less parameters can help reduce the risk of errors and unnecessary effort and time, leading to more reliable scores for identical performance.
Scoring HVT was feasible with 91.6% of observations being scorable. This reflects the high quality of videos obtained and can be attributed to the detailed instructions and extensive training parents receive before discharge by our CHAMP coordinators, as well as the high resolution of technology involved in capturing those videos and online data transmission.
HVT Future Directions
While still a relatively new tool, a small body of literature on HVT is developing. This is particularly relevant in the COVID era. In the field of neurology, HVT has been increasingly used for ictal capture and for prolonged monitoring of patients with epilepsy for precise localization of their epileptic foci.24 A recent pediatric study by Carlson et al. demonstrated similar diagnostic efficacy and study quality of HVT and inpatient video telemetry and a higher parental preference for HVT versus inpatient video telemetry (76% versus 31%).25 In the field of infectious disease, a recently published multicenter double‐masked, randomized trial in 22 clinics in England, demonstrated more effective observation of tuberculosis treatment by smart phone enabled HVT than by direct observation therapy.26
Future intentions include extending this study to a larger cohort across different centers in a prospective manner. This will help us validate the positive contribution of HVT to home monitoring of interstage SV infants. The use of machine learning to automate the detection of features of interest is also one of our goals. While the cohort of infants with SV has been the center of the CHAMP project and this study, we have been interested in collaborating with our colleagues from other cardiac subspecialties to study the role of home monitoring in general and HVT in particular, in other vulnerable pediatric cardiac populations such as infants with post‐cardiac transplant and arrhythmia. Finally, consideration for caregivers’ comfort level with digital/virtual technology, preference, and satisfaction should also be studied.
Limitations
Our study is limited by its retrospective and single center nature. CHAMP has been successfully deployed in 9 additional pediatric heart centers with a total of 610 patients enrolled, and it is our intention to validate this tool in a prospective multicenter study through collaboration with these centers. This should be followed by a pilot implementation and audit before more extended adoption in routine practice. Our study was at risk for selection (information) bias which was minimized by performing a computer‐based randomization of the selected videos and masking the rater to all possible patient identifiers as well as video status (pre‐admission versus baseline). Confounding bias, where our outcome of interest (UHA) might be potentially explained by a systematic overlap between the score variables is a possibility, although this was specifically examined by using correlation coefficients and variance inflation factor. Our HVT score experience is specific to interstage SV infants and may not be generalizable to other populations. This study is underpowered for the outcomes of unplanned surgical or catheter‐based intervention and death. Finally, the incremental value of HVT to other CHAMP parameters such as oxygen saturation, intake, and output was not assessed in this study.
Conclusions
We developed an objective EWS using HVT for prediction of acute physiologic derangement prompting UHA in interstage SV infants. Prospective, multicenter validation of this tool followed by pilot implementation and audit is needed before extended adoption in the routine clinical care of these high‐risk infants.
Sources of Funding
The authors would like to recognize the generous support for the CHAMP application development from the Claire Giannini Foundation.
Disclosures
None.
For Sources of Funding and Disclosures, see page 9.
References
- 1.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, et al. Comparison of shunt types in the norwood procedure for single‐ventricle lesions. N Engl J Med. 2010;362:1980–1992. DOI: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hehir DA, Dominguez TE, Ballweg JA, Ravishankar C, Marino BS, Bird GL, Nicolson SC, Spray TL, Gaynor JW, Risk TS. Risk factors for interstage death after stage1 reconstruction of hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg. 2008;136:19–25. DOI: 10.1016/j.jtcvs.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Furck AK, Uebing A, Hansen JH, Scheewe J, Jung O, Fischer G, Rickers C, Holland‐Letz T, Kramer HH. Outcome of the Norwood operation in patients with hypoplastic left heart syndrome: a 12‐year single‐center survey. J Thorac Cardiovasc Surg. 2010;139:359–365. DOI: 10.1016/j.jtcvs.2009.07.063. [DOI] [PubMed] [Google Scholar]
- 4.Hehir DA, Ghanayem NS. Single‐ventricle infant home monitoring programs: outcomes and impact. Curr Opin Cardiol. 2013;28:97–102. DOI: 10.1097/HCO.0b013e32835dceaf. [DOI] [PubMed] [Google Scholar]
- 5.Ghanayem NS, Cava JR, Jaquiss RD, Tweddell JS. Home monitoring of infants after stage one palliation for hypoplastic left heart syndrome. Pediatr Card Surg Annu. 2004;7:32–38. DOI: 10.1053/j.pcsu.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Rudd NA, Frommelt MA, Tweddell JS, Hehir DA, Mussatto KA, Frontier KD, Slicker JA, Bartz PJ, Ghanayem NS. Improving interstage survival after Norwood operation: outcomes from 10 years of home monitoring. J Thorac Cardiovasc Surg. 2014;148:1540–1547. DOI: 10.1016/j.jtcvs.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Siehr SL, Norris JK, Bushnell JA, Ramamoorthy C, Reddy VM, Hanley FL, Wright GE. Home monitoring program reduces interstage mortality after the modified Norwood procedure. J Thorac Cardiovasc Surg. 2014;147:718–723.e1. DOI: 10.1016/j.jtcvs.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Petit CJ, Fraser CD, Mattamal R, Slesnick TC, Cephus CE, Ocampo EC. The impact of a dedicated single‐ventricle home monitoring program on interstage somatic growth, interstage attrition, and 1‐year survival. J Thorac Cardiovasc Surg. 2011;142(1358):1366. DOI: 10.1016/j.jtcvs.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 9.Öhman A, Strömvall‐Larsson E, Nilsson B, Mellander M. Pulse oximetry home monitoring in infants with single ventricle physiology and a surgical shunt as the only source of pulmonary blood flow. Cardiol Young. 2013;23:75–81. DOI: 10.1017/S1047951112000352. [DOI] [PubMed] [Google Scholar]
- 10.Dobrolet NC, Nieves JA, Welch EM, Khan D, Rossi AF, Burke RP, Zahn EM. New approach to interstage care for palliated high‐risk patients with congenital heart disease. J Thorac Cardiovasc Surg. 2011;142:855–860. DOI: 10.1016/j.jtcvs.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 11.Miller‐Tate H, Stewart J, Allen R, Husain N, Rosen K, Cheatham JP, Galantowicz M, Cua CL. Interstage weight gain for patients with hypoplastic left heart syndrome undergoing the hybrid procedure. Congenit Heart Dis. 2013;8:228–233. DOI: 10.1111/chd.12007. [DOI] [PubMed] [Google Scholar]
- 12.Knirsch W, Bertholdt S, Stoffel G, Stiasny B, Weber R, Dave H, Prêtre R, von Rhein M , Kretschmar O. Clinical course and interstage monitoring after the Norwood and hybrid procedures for hypoplastic left heart syndrome. Pediatr Cardiol. 2014;35:851–856. DOI: 10.1007/s00246-014-0865-y. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JB, Iyer SB, Beekman RH, Jenkins JK, Klitzner TS, Kugler JD, Martin GR, Neish SR, Rosenthal GL, Lannon CM. National pediatric cardiology quality improvement collaborative: lessons from development and early years. Prog Pediatr Cardiol. 2011;32:103–109. DOI: 10.1016/j.ppedcard.2011.10.008. [DOI] [Google Scholar]
- 14.Rudd NA, Ghanayem NS, Hill GD, Lambert LM, Mussatto KA, Nieves JA, Robinson S, Shirali G, Steltzer MM, Uzark K, et al. Interstage home monitoring for infants with single ventricle heart disease: education and management: a scientific statement from the American Heart Association. J Am Heart Assoc. 2020;9:e014548. DOI: 10.1161/JAHA.119.014548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shirali G, Erickson L, Apperson J, Goggin K, Williams D, Reid K, Bradley‐Ewing A, Tucker D, Bingler M, Spertus J, et al. Harnessing teams and technology to improve outcomes in infants with single ventricle. Circ Cardiovasc Qual Outcomes. 2016;9:303–311. DOI: 10.1161/CIRCOUTCOMES.115.002452. [DOI] [PubMed] [Google Scholar]
- 16.Bingler M, Erickson LA, Reid KJ, Lee B, O'Brien J, Apperson J, Goggin K, Shirali G. Interstage outcomes in infants with single ventricle heart disease comparing home monitoring technology to three‐ring binder documentation: a randomized crossover study. World J Pediatr Congenit Heart Surg. 2018;9:305–314. DOI: 10.1177/2150135118762401. [DOI] [PubMed] [Google Scholar]
- 17.Tucker KM, Brewer TL, Baker RB, Demeritt B, Vossmeyer MT. Prospective evaluation of a pediatric inpatient early warning scoring system. J Spec Pediatr Nurs. 2009;14:79–85. DOI: 10.1111/j.1744-6155.2008.00178.x. [DOI] [PubMed] [Google Scholar]
- 18.Duncan H, Hutchison J, Parshuram CS. The pediatric early warning system score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21:271–278. DOI: 10.1016/j.jcrc.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Mclellan MC, Gauvreau K, Connor JA. Validation of the cardiac children’s hospital early warning score: an early warning scoring tool to prevent cardiopulmonary arrests in children with heart disease. Congenit Heart Dis. 2014;9:194–202. DOI: 10.1111/chd.12132. [DOI] [PubMed] [Google Scholar]
- 20.McLellan MC, Connor JA. The cardiac children’s hospital early warning score (CCHEWS). J Pediatr Nurs. 2013;28:171–178. DOI: 10.1016/j.pedn.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Lee MH, Lu SN, Yuan Y, Yang HI, Jen CL, You SL, Wang LY, L'Italien G, Chen CJ; R.E.V.E.A.L.‐HCV Study Group . Development and validation of a clinical scoring system for predicting risk of HCC in asymptomatic individuals seropositive for anti‐HCV antibodies. PLoS One. 2014;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luís L, Nunes C. Short national early warning score — developing a modified early warning score. Aust Crit Care. 2018;31:376–381. DOI: 10.1016/j.aucc.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis S, Kovacs C, Briggs J, Meredith P, Schmidt PE, Featherstone PI, Prytherch DR, Smith GB. Can binary early warning scores perform as well as standard early warning scores for discriminating a patient’s risk of cardiac arrest, death, or unanticipated intensive care unit admission? Resuscitation. 2015;93:46–52. DOI: 10.1016/j.resuscitation.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Whittaker RG. Video telemetry: current concepts and recent advances. Pract Neurol. 2015;15:445–450. DOI: 10.1136/practneurol-2015-001216. [DOI] [PubMed] [Google Scholar]
- 25.Carlson S, Kandler RH, Moorhouse D, Ponnusamy A, Mordekar SR, Alix JJP. Home video telemetry in children: a comparison to inpatient video telemetry. Seizure. 2018;61:209–213. DOI: 10.1016/j.seizure.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 26.Story A, Aldridge RW, Smith CM, Garber E, Hall J, Ferenando G, Possas L, Hemming S, Wurie F, Luchenski S, et al. Smartphone‐enabled video‐observed versus directly observed treatment for tuberculosis: a multicentre, analyst‐blinded, randomised, controlled superiority trial. Lancet. 2019;393:1216–1224. DOI: 10.1016/S0140-6736(18)32993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


