Abstract
Backgroud
There is a paucity of information on whether changes in metabolic syndrome (MetS) status affect the risk of new‐onset atrial fibrillation (AF). We aimed to evaluate whether changes in MetS status and components of MetS affect AF risk using data from a nationwide observational cohort.
Methods and Results
A total of 7 565 531 adults without prevalent AF (mean age, 47±14 years) who underwent 2 serial health examinations by the Korean National Health Insurance Cooperation were identified. The patients were categorized into 4 groups according to the change in MetS status in serial evaluations, as follows: patients with persistent MetS (n=1 388 850), healthy patients newly diagnosed with MetS in the second evaluation (n=608 158), patients with MetS who were healthy in the second evaluation (n=798 555), and persistently healthy individuals (n=4 769 968). During a mean 7.9‐year follow‐up, incident AF was diagnosed in 139 305 (1.8%) patients. After multivariable adjustment, the AF risk was higher by 31% in the patients with persistent MetS , 26% in the patients with MetS who were healthy in the second evaluation, and 16% in the healthy patients newly diagnosed with MetS in the second evaluation compared with the persistently healthy individuals. Regardless of the MetS component type, the AF risk correlated with changes in the number of components. The risk of AF was strongly correlated with MetS status changes in the young and middle‐age groups (20–39 years and 40–64 years, respectively) than in the elderly group (≥65 years).
Conclusions
Dynamic changes in MetS status and persistent MetS were associated with an increased risk of AF in a large‐scale Asian population.
Keywords: atrial fibrillation, metabolic syndrome, risk factor
Subject Categories: Risk Factors, Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- HH
patients who were metabolically healthy at both examinations
- HM
patients who were metabolically healthy at the first examination but were newly diagnosed with metabolic syndrome at the second examination
- MetS
metabolic syndrome
- MH
patients with metabolic syndrome at the first examination but became metabolically healthy at the second examination
- MM
patients who met metabolic syndrome criteria at both health examinations
- NHIS
National Health Insurance Service
Clinical Perspective
What Is New?
Patients with persistent metabolic syndrome (MetS) had a higher atrial fibrillation (AF) risk, and those who were diagnosed with MetS at any time point showed a higher AF risk compared with the consistently healthy individuals.
Patients who persistently met the diagnostic criteria for each component of MetS also showed higher AF risks.
The AF risk became higher with an increasing number of MetS components; AF risk was strongly correlated with changes in MetS status in the younger age group (<65 years).
What Are the Clinical Implications?
This study is the first study to report the association between dynamic changes in MetS status and the risk of new‐onset AF and persistent MetS was associated with an increased risk of AF.
Patients who were diagnosed with MetS at any time point showed significantly higher risks for new‐onset AF than those without MetS following serial health examination.
Intensive care and management of metabolic abnormalities might improve the AF risk, especially in younger patients.
The prevalence and incidence of atrial fibrillation (AF), the most common arrhythmia, are increasing worldwide, resulting in increased healthcare burdens.1, 2 Cardiovascular disease and its comorbidities, including metabolic syndrome (MetS), increase the risk of AF.3, 4 MetS is a cluster of characteristics, including insulin resistance, central obesity, hypertension, and dyslipidemia, which themselves increase the risk of cardiovascular disease and adverse outcomes.5 The global prevalence of MetS has consistently increased, and in the Asia‐Pacific region, nearly one fifth of the adult population has MetS.6 Therefore, active control of MetS or its components is expected to reduce the burden of AF and cardiovascular disease.7
MetS components are potentially manageable with either lifestyle changes or medical treatment. Therefore, MetS component measurements may vary between different time points, and MetS status may change even during follow‐up. Although previous studies focusing on the presence of MetS at baseline assessment have evaluated the association between MetS and AF, the impact of changes in MetS status over time on AF development remains unknown.8, 9 Herein, we aimed to evaluate whether changes in MetS status and each of its components affect AF risk based on a nationwide observational cohort.
Methods
Data Source and Study Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. The Korean government provides a universal health insurance service—the Korean National Health Insurance Service (NHIS)—to all Korean citizens. The NHIS database includes participants’ demographic data, diagnoses, and all claims of both inpatient and outpatient medical expenses, including prescription records and procedures. The National Health Insurance Corporation also provides biennial general health examinations to all enrolled employer‐provided participants of NHIS aged >20 years or locally provided participants of NHIS aged ≥40 years. This health examination includes physical examinations, a health status survey, and an assessment of blood and urine biochemical markers.10 Based on this database, we evaluated how AF risk related to changes in MetS status (presence of MetS) and each MetS component in 2 consecutive health examinations. We included patients who had undergone a baseline (the first during the study period) health examination from January 2008 to December 2009 and those who had undergone a follow‐up (the second during the study period) health examination from January 2010 to December 2011. All participants were followed up for AF occurrence from the time of the second health examination to December 2017. Participants diagnosed with AF before the second health examination were excluded. This study was exempt from review by the Seoul National University Hospital’s institutional review board (E‐1805‐074‐944) and adhered to the Declaration of Helsinki. Informed consent was waived because of the retrospective nature of the study and anonymized data.
Definition of MetS and Other Comorbidities
MetS was defined according to the criteria of the American Heart Association/National Heart, Lung, and Blood Institute for Asian populations.11 MetS was diagnosed by the presence of any 3 of 5 components: (1) waist circumference ≥85 cm for women or ≥90 cm for men; (2) serum triglyceride ≥150 mg/dL or drug treatment for elevated triglycerides; (3) serum high‐density lipoprotein cholesterol <50 mg/dL in women or <40 mg/dL in men; (4) systolic blood pressure (BP) ≥130 mm Hg or diastolic BP ≥85 mm Hg or drug treatment for elevated BP; and (5) fasting plasma glucose ≥100 mg/dL or drug treatment for elevated blood glucose. Obesity was defined according to the World Health Organization’s Western Pacific Region Office definition of obesity for Asians: obese group (body mass index [BMI] ≥25 kg/m2) and nonobese group (BMI <25 kg/m2).12
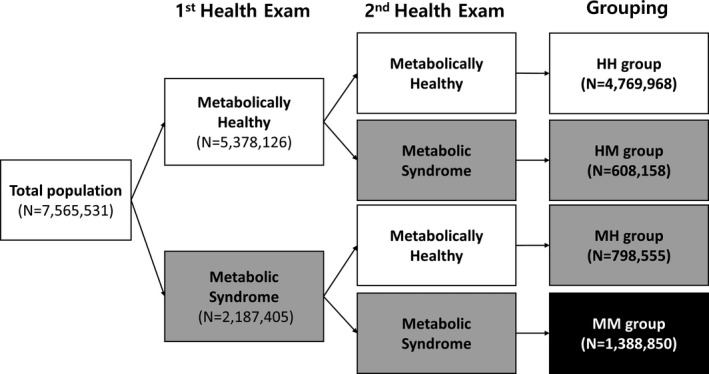
To investigate the impact of MetS status changes, the study population was stratified into 4 groups according to the presence of MetS on the first and second health examination: (1) the MM group included patients who met MetS criteria at both health examinations; (2) the HM group included those who were metabolically healthy at the first examination but was newly diagnosed with MetS at the second examination; (3) the MH group included those who had MetS at the first examination but became metabolically healthy at the second examination; and (4) the HH group included those who were metabolically healthy at both examinations. To define metabolically healthy, ≤2 MetS component criteria were selected, as in previous MetS studies.13, 14 The study population stratified flow is summarized in Figure 1 and Figure S1.
Figure 1. Study population stratified flow according to the change of metabolic syndrome status and those of metabolic components.
Baseline comorbidities of patients were analyzed based on the existing corresponding International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes in the claims data, and these were validated in our previous reports.4, 15 Table S1 describes the detailed definitions of baseline comorbidities and ICD‐10 codes.
Study End Point
The primary end point was newly diagnosed AF (ICD‐10 codes I480–I484 and I489) after the second health examination during the follow‐up period. To ensure diagnostic accuracy, AF was defined when the diagnosis was confirmed more than twice in the outpatient clinic or confirmed on hospital discharge.4, 16
Statistical Analysis
Patient data are presented as mean±SD for continuous variables and as numbers and percentages for categorical variables. To determine the differences among groups, we used Student t test for continuous values and chi‐square test for categorical variables. The incidence rate of AF in each group was estimated by 1000 patient‐years. The multivariable Cox proportional hazards model was used to identify comorbidities associated with the development of AF. Multivariable adjustments were made for sex, age, smoking status, alcohol intake, exercise status, and BMI. The proportional hazards assumption was evaluated by Schoenfeld residuals test with the logarithm of the cumulative hazards function based on Kaplan–Meier estimates for the categories of stratified MetS status change. To evaluate the effect of age on AF risk, we performed subgroup analysis with the stratified age group. Two‐sided P values <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.2 software (SAS Institute Inc).
Results
Baseline Characteristics of the Study Population
A total of 7 565 531 participants were included in this study (mean age, 47.2±13.7 years; men 55.6%). During the mean follow‐up of 7.9 years (a total of 59 677 004 patient‐years follow‐up duration), 135 600 (1.79%) patients were newly diagnosed with AF (2.27 per 1000 patient‐years). Study participants were classified into 4 groups according to change of MetS status as follows: MM, 1 388 850 (18.4%); MH, 798 555 (10.5%); HM, 608 158 (8.0%); and HH, 4 769 968 (63.1%). The baseline characteristics of the study population are summarized in Table.
Table 1.
Baseline Characteristics by Variation of MetS Change Status
| Total | HH | HM | MH | MM | |
|---|---|---|---|---|---|
| Patients | 7 565 531 (100.0) | 4 769 968 (63.1) | 608 158 (8.0) | 798 555 (10.6) | 1 388 850 (18.4) |
| Age, y | 47.2±13.7 | 43.4±12.78 | 51.3±13.0 | 50.6±13.0 | 56.2±12.3 |
| Men | 4 209 273 (55.6) | 2 629 853 (55.1) | 371 390 (61.1) | 482 584 (60.4) | 725 446 (52.2) |
| BMI, kg/m2 | 23.7±3.2 | 22.7±2.7 | 25.1±2.9 | 24.8±2.8 | 26.1±3.1 |
| MetS components, n | 1.56±1.37 | 0.81±0.77 | 1.50±0.65 | 3.26±0.50 | 3.66±0.71 |
| Waist circumference, cm | 80.6±9.0 | 77.5±8.1 | 83.0±7.6 | 85.2±7.8 | 87.4±8.1 |
| Systolic BP, mm Hg | 122.4±14.6 | 118.4±13.3 | 124.9±13.8 | 129.6±13.5 | 131.0±14.5 |
| Diastolic BP, mm Hg | 76.3±9.8 | 74.2±9.2 | 77.8±9.3 | 80.5±9.5 | 80.4±9.9 |
| FBG, mg/dL | 96.7 ± 21.6 | 91.4±14.2 | 104.2±23.0 | 97.1±21.3 | 111.6±31.8 |
| Total cholesterol, mg/dL | 195.4±36.2 | 189.9±32.9 | 204.0±38.1 | 205.4±36.3 | 204.4±41.9 |
| LDL‐C, mg/dL | 114.1±33.2 | 111.2±30.1 | 118.5±36.1 | 123.4±34.2 | 116.7±39.3 |
| HDL‐C, mg/dL | 55.2±17.3 | 57.9±16.5 | 49.3±16.7 | 53.8±18.6 | 49.3±17.5 |
| Triglycerides, mg/dL | 113.2 (113.2–113.3) | 93.2 (93.1–93.2) | 164.5 (164.3–164.7) | 127.7 (127.5–127.8) | 173.3 (173.1–173.4) |
| Diabetes mellitus | 618 456 (8.2) | 102 973 (2.2) | 68 489 (11.3) | 58 479 (7.3) | 388 515 (28.0) |
| Hypertension | 1 896 460 (25.1) | 528 448 (11.1) | 245 103 (40.3) | 231 287 (29.0) | 891 622 (64.2) |
| Liver cirrhosis | 19 064 (0.3) | 10 939 (0.2) | 1877 (0.3) | 2212 (0.3) | 4036 (0.3) |
| Stroke/TIA/TE | 116 965 (1.6) | 27 683 (0.6) | 12 512 (2.1) | 14 484 (1.8) | 62 285 (4.5) |
| Ischemic heart disease | 226 342 (3.0) | 44 066 (0.9) | 23 662 (3.9) | 22 855 (2.9) | 135 759 (9.8) |
| Peripheral artery disease | 182 224 (2.4) | 51 894 (1.1) | 20 323 (3.3) | 23 377 (2.9) | 86 630 (6.2) |
| Heart failure | 35 943 (0.5) | 9107 (0.2) | 4105 (0.7) | 4167 (0.5) | 18 564 (1.3) |
| Smoking | |||||
| Nonsmoker | 4 516 917 (59.7) | 2 888 657 (60.6) | 337 437 (55.5) | 441 155 (55.2) | 849 668 (61.2) |
| Ex‐smoker | 1 157 971 (15.3) | 680 901 (14.3) | 109 982 (18.1) | 131 959 (16.5) | 235 129 (16.9) |
| Current smoker | 1 890 643 (25.0) | 1 200 410 (25.2) | 160 739 (26.4) | 225 441 (28.2) | 304 053 (21.9) |
| Alcohol use* | |||||
| Absent | 3 865 929 (51.1) | 2 335 904 (49.0) | 311 437 (51.2) | 403 649 (50.6) | 814 939 (58.7) |
| Consumption | 3 204 150 (42.4) | 2 159 791 (45.3) | 246 486 (40.5) | 329 585 (41.3) | 468 288 (33.7) |
| Heavy drinker | 495 452 (6.6) | 274 273 (5.8) | 50 235 (8.3) | 65 321 (8.18) | 105 623 (7.6) |
| Physical activity† | |||||
| Low activity | 3 521 194 (46.5) | 2 166 259 (45.4) | 289 522 (47.6) | 371 181 (46.5) | 694 232 (50.0) |
| Mid and high activity | 4 044 337 (53.5) | 2 603 709 (54.6) | 318 636 (52.4) | 427 374 (53.5) | 694 618 (50.0) |
| Income | |||||
| Over 20 percentiles | 5 876 144 (77.7) | 3 707 744 (77.7) | 473 497 (77.9) | 618 593 (77.5) | 1 076 310 (77.5) |
| Under 20 percentiles | 1 689 387 (22.3) | 1 062 224 (22.3) | 134 661 (22.1) | 179 962 (22.5) | 312 540 (22.5) |
| Obesity (BMI ≥25 kg/m2) | 2 425 708 (32.1) | 890 128 (18.7) | 301 599 (49.6) | 358 825 (44.9) | 875 156 (63.0) |
| Patients with AF | 135 600 (1.8) | 55 975 (1.2) | 14 090 (2.3) | 18 969 (2.4) | 46 566 (3.4) |
| AF incidence (1000 patient‐y) | 2.3 | 1.5 | 2.9 | 3.0 | 4.3 |
| Follow‐up duration, y | 7.9±0.9 | 7.9±0.8 | 7.9±1.0 | 7.9±1.0 | 7.8±1.1 |
Values are expressed as mean±SD or number (percentage). AF indicates atrial fibrillation; BMI, body mass index; BP, blood pressure; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; HH, patients who were metabolically healthy at both examinations; HM, patients who were metabolically healthy at the first examination but were newly diagnosed with metabolic syndrome at the second examination; LDL‐C, low‐density lipoprotein cholesterol; MetS, metabolic syndrome; MH, patients with metabolic syndrome at the first examination but became metabolically healthy at the second examination; MM, patients who met metabolic syndrome criteria at both health examinations, TE, thromboembolism; and TIA, transient ischemic attack.
Heavy drinker is defined as an invididual with an average daily alcohol intake of ≥30 g (3 pints of beer).
Mid and high physical activity are defined as during the past week, moderate intensity exercise >5 times per week for >30 minutes or vigorous intensity exercise for >3 times per week for >20 minutes.
Generally, the MM group had higher risk profiles for AF development than other groups. MM was the oldest group (mean age, 56.2±12.3 years) and HH the youngest (mean age, 43.4±12.8 years). For each MetS component, the mean waist circumference, systolic BP, fasting blood glucose, and triglyceride level were highest in the MM group and lowest in the HH group. The mean high‐density lipoprotein level was highest in the HH group and lowest in the MM group. The MM group showed the highest mean BMI (26.1±3.1 kg/m2) and the highest proportion of obese patients (63.0%, 875 156 patients).
Association Between Changes in MetS Status and New‐Onset AF
The average time from the end of the second health examination to the onset of AF was 3.3±1.8 years for all patients with AF in this study population. The crude incidence rates of AF for the HH, HM, MH, and MM groups were 1.48, 2.94, 3.02, and 4.30 per 1000 patient‐years, respectively. Patients who were diagnosed with MetS during inclusion periods (HM, MH, and MM groups) showed a >2‐fold higher incidence of AF than the HH group. After multivariable adjustment, the AF risk was higher by 31% in the MM group (hazard ratio [HR], 1.308; 95% CI, 1.290–1.327), 26% in the MH group (HR, 1.259; 95% CI, 1.238–1.280), and 16% in the HM group (HR, 1.155; 95% CI, 1.134–1.178) compared with the HH group, respectively (Figure 2). Each component of MetS was similar to the main results. The patients who persistently met the diagnostic criteria for each component of MetS (UU group) showed the highest AF risk compared with the HH, HU, and UH groups for each MetS component (Table S2). Among those who received the third health examination during the follow‐up period (56.9% of the total study population), patients with persistent MetS showed the highest risk of AF (HR, 1.27; 95% CI, 1.24–1.31). Also, those who met the diagnostic criteria for MetS at any time point showed a higher risk of AF compared with those who were consistently healthy (Table S3).
Figure 2. The risk of atrial fibrillation (AF) according to the changes in metabolic syndrome (MetS) status.
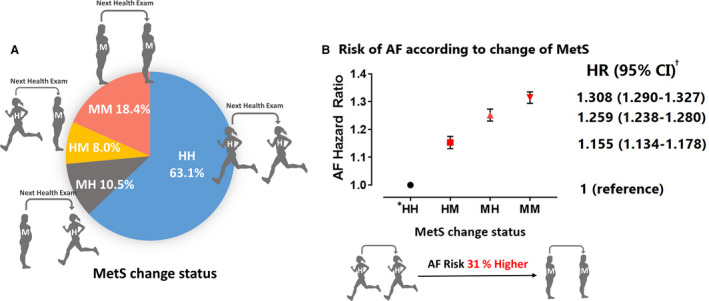
A, Proportion of each study group according to MetS status change. B, Risk of AF according to MetS status changes. HR indicates hazard ratio. *The definition of each divided group is as follows: HH, patients who were metabolically healthy at both examinations; HM, patients who were metabolically healthy at the first examination but were newly diagnosed with MetS at the second examination; MH, patients with MetS at the first examination but became metabolically healthy at the second examination; and MM, patients who met MetS criteria at both health examinations. †Multivariable models adjusted for age, sex, alcohol consumption, smoking status, physical activity, and body mass index.
Association Between Changes in the Number of MetS Components and AF Risk
We analyzed the impact of changes in the number of MetS components on AF development. Figure 3 shows patient distributions according to changes in MetS component number. AF risk showed a gradual response to the change in MetS component number. In patients who had zero or 1 MetS component at the baseline health examination, the AF risk gradually increased with the number of new MetS components at the second examination. When patients had 2, 3, and 4 MetS components at the second examination, the AF risk was higher by 16%, 32%, and 47%, respectively, compared with those maintaining ≤1 components (HR, 1.164 [95% CI, 1.138–1.192]; HR, 1.316 [95% CI, 1.275–1.357]; and HR, 1.465 [95% CI, 1.397–1.536], respectively) (Figure 3A). Regardless of the number of MetS components in the first examination, the AF risk became higher as the number of components increased, and the AF risk became lower as the number of components decreased compared with those who maintained their MetS component number(s) in the follow‐up examination. Of patients who had 2 MetS components at the first examination, those whose component number decreased to 1 at the second examination had a lower AF risk than those who maintained 2 components (Figure 3B). Of the patients with ≥3 MetS components at the first examination, those who had fewer MetS components at the second examination showed a lower AF risk than those who maintained or had increased number of components (Figure 3C and 3D).
Figure 3. Study population distribution and the risk of atrial fibrillation (AF) according to changes in metabolic syndrome (MetS) components based on the number of components in the first health examination.
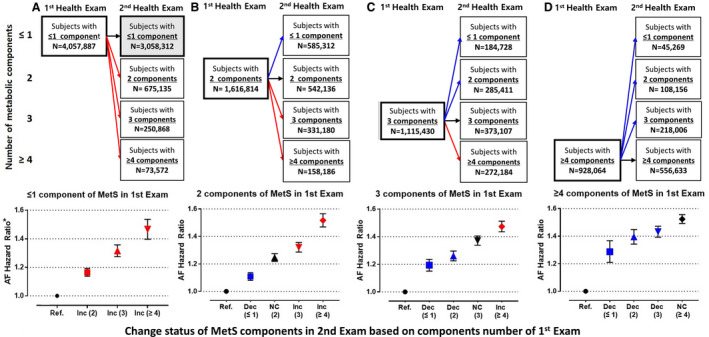
A, Risk of AF according to changes in MetS components of patients who had 0 or 1 component in the first health examination. B, Risk of AF according to changes in MetS components of patients who had 2 components in the first health examination. C, Risk of AF according to changes in MetS components of patients who had 3 components in the first health examination. D, Risk of AF according to changes in MetS components of patients who had ≥4 components in the first health examination. Ref, reference; NC, no change in the number of MetS components; Inc, increase in the number of MetS components; and Dec, decrease in the number of MetS components. *Multivariable models adjusted for age, sex, alcohol consumption, smoking status, physical activity, and body mass index.
In patients who had ≤1 MetS components at the second examination, the AF risk gradually increased according to the number of MetS components at baseline (Figure 4). When patients had 2, 3, and 4 MetS components at baseline, the AF risk increased by 11%, 19%, and 29%, respectively, compared with those maintaining ≤1 components (HR, 1.108 [95% CI, 1.081–1.136]; HR, 1.193 [95% CI, 1.151–1.236]; and HR, 1.285 [95% CI, 1.209–1.367], respectively) (Figure 4A). Among patients with ≥3 MetS components at the second examination, the AF risks were similar, regardless of the number of MetS components at baseline (Figure 4C and 4D). The incidence rates and HRs of AF among patients stratified by the number of MetS components are shown in Table S4.
Figure 4. Study population distribution and the risk of atrial fibrillation (AF) according to the changes in metabolic syndrome (MetS) components based on the number of components in the second health examination.
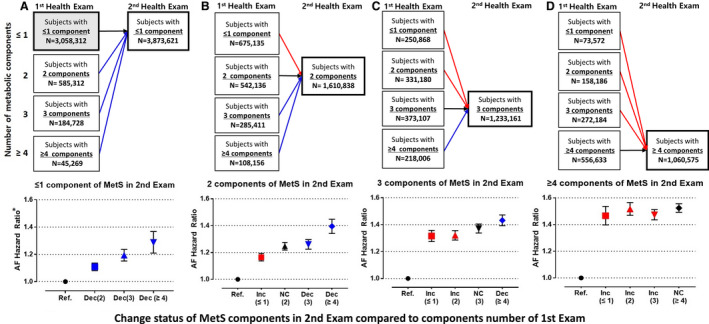
A, Risk of AF according to changes in MetS components of patients who had 0 or 1 component in the second health examination. B, Risk of AF according to changes in MetS components of patients who had 2 components in the second health examination. C, Risk of AF according to changes in MetS components of patients who had 3 components in the second health examination D, Risk of AF according to changes in MetS components of patients who had ≥4 components in the second health examination. Ref, reference; NC, no change in the number of MetS components; Inc, increase in the number of MetS components; Dec, decrease in the number of MetS components. *Multivariable models adjusted for age, sex, alcohol consumption, smoking status, physical activity, and body mass index.
Subgroup Analysis
We performed stratified analyses of AF risk associated with changes in MetS status and components according to age (Figure 5). We divided the patients into 3 groups: young (20–39 years), middle age (40–64 years), and old age (≥65 years). The AF risk was strongly correlated with changes in MetS status in the young and middle age groups, whereas the AF risk in the old age group was not. With respect to MetS components, the AF risk closely correlated with the change in MetS components in the young and middle age groups, while the AF risk in the old age group was not. Changes in the BP component conferred similar AF risks for all age groups. The incidence rate and AF risk in each subgroup by age are summarized in Table S5.
Figure 5. The risk of atrial fibrillation (AF) according to the changes in metabolic syndrome (MetS) status by age groups.
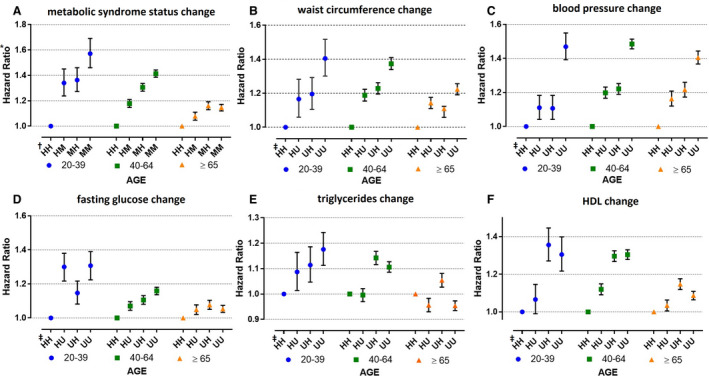
A, Risk of AF according to MetS status changes by age groups. B, Risk of AF according to waist circumference status changes by age groups. C, Risk of AF according to blood pressure (BP) status changes by age groups. D, Risk of AF according to fasting glucose status changes by age groups. E, Risk of AF according to triglyceride status changes by age groups. F, Risk of AF according to high‐density lipoprotein cholesterol (HDL‐C) status changes by age groups. †The definition of each divided group (HH, HM, MH, MM) same as Figure 2. ‡Subjects stratified into four groups according to the change of relevant component of metabolic syndrome as follows: UU, metabolically unhealthy of relevant component in both checkups; UH, metabolically unhealthy of relevant component in the first exam but changed to healthy in the second checkup; HU, metabolically healthy of relevant component in the first exam but changed to unhealthy in the second checkup; and HH, metabolically healthy of relevant component in both checkups. AF, atrial fibrillation; BP, blood pressure; HDL, high‐density lipoprotein cholesterol; MetS, metabolic syndrome; TG, triglyceride.
Discussion
In this study, our principal findings are as follows: (1) patients with persistent MetS had the highest AF risk, and those who met the diagnostic criteria for MetS at any time point showed a higher AF risk compared with the consistently healthy population; (2) patients who persistently met the diagnostic criteria for each component of MetS, which are also independent risk factors for AF, showed higher AF risks; (3) regardless of MetS components type, the AF risk became higher with increasing number of MetS components, and AF risk became lower with decreasing number of MetS components; and (4) AF risk was more strongly correlated with changes in MetS status in the young and middle age groups than in the old age group (≥65 years).
To the best of our knowledge, this is the first study to report the association between dynamic changes in MetS status and the risk of new‐onset AF and how persistent MetS was associated with an increased risk of AF using in a nationwide observational cohort.
Change in MetS Status and AF Risk
MetS involves risk factors associated with cardiovascular disease and cardiac arrhythmia, such as AF. The relationship between MetS and increased risks of new‐onset AF has been previously reported.3, 7, 17, 18, 19 Moreover, each MetS component is associated with increased risks of AF.17, 20 Our study results corroborate the findings of previous studies that patients with MetS show a higher AF risk and changes in MetS status during follow‐up may affect the AF risk. In addition, recovery of MetS status to metabolically healthy status is associated with a risk reduction for AF. The group that improved from MetS status to healthy status (MH group: HR, 1.308; 95% CI, 1.29–1.327) had ≈5% lower risk than those with persistent MetS (MM group: HR, 1.259; 95% CI, 1.28–1.28). Also, if the MH group persistently maintained a healthy status (HR, 1.109; 95% CI, 1.06–1.159), they had ≈16% lower risk than the group with consistently remaining MetS (HR, 1.273; 95% CI, 1.238–1.309). Moreover, there was associated AF risk reduction when each of the MetS elements, such as waist circumference, high blood pressure, and high fasting glucose, were restored to healthy status (Table S2 and S3).
Several studies have reported on the increased risks of cardiovascular disease and mortality caused by changes or fluctuations in MetS components, BP, fasting glucose levels, and obesity during follow‐up.21, 22 Fluctuations in BP and cholesterol levels and higher longitudinal fluctuations in fasting blood glucose levels, regardless of the presence of diabetes mellitus, during follow‐up are associated with increased risks of cardiovascular disease and mortality.23, 24 A recent study reported that BMI changes during follow‐up were associated with increased risks of cardiovascular events, including new‐onset AF.25 Expectedly, patients in our study with persistent MetS had the highest risk of developing AF. However, we show for the first time that patients who were diagnosed MetS at any time point showed significantly higher risks for new‐onset AF than those without MetS following serial health examination.
Changes in the Number of MetS Components and AF Risk
In previous studies, each MetS component was associated with increased risks of AF.3, 7, 8 Studies on the relationships between changes in the number of MetS components and AF risk are limited. As the number of MetS components increase, the risk of AF risk is also increases, in proportion with an increasing number of MetS components.7, 19 Our results support previous studies showing that AF risk becomes higher with an increased number of MetS components in Asian populations, while a decrease in the number of MetS components (thus, an improvement in MetS status) correlates with a lower AF risk. Importantly, the extent of risk reduction was affected by the number of MetS components involved before the improvement.
Considering that MetS includes several metabolically related cardiovascular risk factors, it was previously unclear how they affect each other when ≥2 components are involved. In the present study, we found that when any MetS component improved to within the normal range, the AF risk decreased. The degree of risk decrease may be related to the number of MetS components involved during the baseline examination. Our findings suggest a “negative legacy effect” in that the more initially present metabolic aberrant‐causing components, the longer the detrimental effect persists, despite careful MetS component control over time. Negative legacy effects on metabolic abnormalities were reported for obese patients with type 2 diabetes mellitus, in whom cardiovascular disease risk persists after weight loss.26 However, this does not indicate that the benefits of controlling metabolic abnormalities are completely eradicated. In our study, regardless of the number and MetS component type diagnosed in the first health examination, the AF risk generally became lower as the number of MetS components decreased. This suggests that not only waist circumference but any MetS component could be a modifiable or manageable risk factor,8 and any effort to normalize metabolic abnormalities could reduce AF risk.
Age, MetS Components, and AF Risk
Age is an important independent risk factor for both MetS and AF.27 In this study, we stratified the study population into different age groups and found that changes in MetS status and each MetS component affected AF risk in young and middle age groups more dramatically than in the old age group.28 AF risk associated with a change of MetS status showed that the MM group had a 57% higher risk than the HH group in the young age group, but in the old age group, the AF risk in the MM group was only 15% higher than in the HH group. Most MetS components were also more closely correlated with AF risk according to status changes of each MetS component in the young age compared with the old age group.
As in previous studies, the incidence and risk of AF markedly increased with aging, and younger patients had a relatively lower risk of AF.27 Our results suggest that changes in MetS status and each component of MetS had a greater impact on the AF risk in the young age group compared with the older age group. AF developing at a young age often presents with typical symptoms such as palpitations or chest pain and has an impact on quality of life. In addition, uncontrolled new‐onset AF would change to chronic AF, eventually increasing the risk of heart failure and stroke.29 Therefore, meticulous attention to, and proactive management of, metabolic abnormalities may lower the risk of AF, especially in the young population.
In some previous studies, a metabolically unhealthy status had an accentuated impact on AF risk at a young age. Obese or overweight women of a young age group (<60 years) were more likely to be associated with AF risk than elderly populations,30 and the relationship between type 2 diabetes mellitus and AF risk was also stronger in younger people than in the old.31 Recently, attention to multiple genetic loci and genetic variations associated with MetS and its complication at a young age is also increasing. However, most previous studies report that MetS and its complications are greatly influenced by lifestyle and environmental factors.32, 33 Therefore, better care and management of metabolic abnormalities might improve the AF risk, especially in younger patients.
Study Limitations
Our study had several limitations. First, the diagnoses of new‐onset AF and other comorbidities were based on the claims database of the NHIS, which relies on the physician’s diagnosis. Therefore, there is a possibility of AF misdiagnosis and an overestimation of outcomes or other comorbidities. To alleviate this bias, we used the validated definition of AF and comorbidities based on the NHIS cohort from our previous study.4, 16 Second, this cohort was composed of a homogenous Asian race; therefore, the study’s findings are not representative of the global population and should be evaluated in other ethnicities. Third, we could not calculate the duration of MetS. Earlier health examination data before 2008 were not available in the NHIS database, therefore the effect of MetS duration could not be analyzed. Fourth, although we tried to adjust for all available variables using multivariable analysis, unmeasurable confounders such as inflammatory markers or dietary habits could still remain. Finally, this is an observational cohort study based on retrospectively collected data; therefore, we describe associations rather than a causal relationship. In addition, the degree of influence between each MetS component could not be determined by simplistically comparing values of HRs in the current study. This would require substantially more comprehensive analyses taking into consideration more variables.
Conclusions
In our large Asian cohort, persistent MetS was associated with increased AF risks, and patients diagnosed with MetS at any time during follow‐up showed a higher AF risk compared with a consistently metabolically healthy population. Moreover, AF risk became higher as the number of MetS components increased and became lower as the number of MetS components decreased. Changes in MetS status and each MetS component were strongly correlated with AF risk, especially in the young and middle‐aged populations.
Sources of Funding
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, Republic of Korea, and the Ministry of Food and Drug Safety) (project number: 202013B14), and by the Korea National Research Foundation funded by the Ministry of Education, Science and Technology (grant 2020R1F1A106740).
Disclosures
Eue‐Keun Choi received research grants from Bayer, BMS/Pfizer, Biosense Webster, Chong Kun Dang, Daiichi‐Sankyo, Samjinpharm, Sanofi‐Aventis, Seers Technology, Skylabs, and Yuhan. Gregory Y.H. Lip is a consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi‐Sankyo and a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi‐Sankyo. No fees were personally received. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S5
Figure S1
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.020901
For Sources of Funding and Disclosures, see page 9.
References
- 1.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart R. Circulation. 2019;140:e125–e151. DOI: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 2.Joung B, Lee JM, Lee KH, Kim TH, Choi EK, Lim WH, Kang KW, Shim J, Lim HE, Park J, et al. 2018 Korean guideline of atrial fibrillation management. Korean Circ J. 2018;48:1033–1080. DOI: 10.4070/kcj.2018.0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, Aizawa Y. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117:1255–1260. DOI: 10.1161/CIRCULATIONAHA.107.744466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HJ, Choi EK, Lee SH, Han KD, Rhee TM, Park CS, Lee SR, Choe WS, Lim WH, Kang SH, et al. Atrial fibrillation risk in metabolically healthy obesity: a nationwide population‐based study. Int J Cardiol. 2017;240:221–227. DOI: 10.1016/j.ijcard.2017.03.103 [DOI] [PubMed] [Google Scholar]
- 5.Cleeman JI, Smith SC, Alberti KG, Grundy SM, Eckel RH, Zimmet PZ, Loria CM, James WP, Fruchart JC, Donato KA. Harmonizing the metabolic syndrome. Circulation. 2009;120:1640–1645. DOI: 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 6.Ranasinghe P, Mathangasinghe Y, Jayawardena R, Hills AP, Misra A. Prevalence and trends of metabolic syndrome among adults in the Asia‐pacific region: a systematic review. BMC Public Health. 2017;17:1–9. DOI: 10.1186/s12889-017-4041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonso A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;159:850–856. DOI: 10.1016/j.ahj.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baek YS, Yang PS, Kim TH, Uhm JS, Park J, Pak HN, Lee MH, Joung B. Associations of abdominal obesity and new‐onset atrial fibrillation in the general population. J Am Heart Assoc. 2017;6:e004705. DOI: 10.1161/JAHA.116.004705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YG, Choi KJ, Han S, Hwang KW, Kwon CH, Park GM, Won KB, Ann SH, Kim J, Kim SJ, et al. Metabolic syndrome and the risk of new‐onset atrial fibrillation in middle‐aged east Asian men. Circ J. 2018;82:1763–1769. DOI: 10.1253/circj.CJ-18-0113 [DOI] [PubMed] [Google Scholar]
- 10.Choi EK. Cardiovascular research using the Korean national health information database. Korean Circ J. 2020;50:754–772. DOI: 10.4070/kcj.2020.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement: Executive Summary. Circulation. 2005;112:e285–e290. DOI: 10.1161/CIRCULATIONAHA.105.169405 [DOI] [Google Scholar]
- 12.World Health Organization (WHO) . Obesity: Preventing and Managing the Global Epidemic. Switzerland: World Health Organization; 2000. [PubMed] [Google Scholar]
- 13.Ärnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle‐aged men. Circulation. 2010;121:230–236. DOI: 10.1161/CIRCULATIONAHA.109.887521 [DOI] [PubMed] [Google Scholar]
- 14.Choi KM, Cho HJ, Choi HY, Yang SJ, Yoo HJ, Seo JA, Kim SG, Baik SH, Choi DS, Kim NH. Higher mortality in metabolically obese normal‐weight people than in metabolically healthy obese subjects in elderly Koreans. Clin Endocrinol (Oxf). 2013;79:364–370. DOI: 10.1111/cen.12154 [DOI] [PubMed] [Google Scholar]
- 15.Lee SR, Choi EK, Park CS, Do HK, Jung JH, Oh S, Lip GYH. Direct oral anticoagulants in patients with nonvalvular atrial fibrillation and low body weight. J Am Coll Cardiol. 2019;73:919–931. DOI: 10.1016/j.jacc.2018.11.051 [DOI] [PubMed] [Google Scholar]
- 16.Lee SR, Choi EK, Do HK, Cha MJ, Oh S. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA2DS2‐VASc score in the entire Korean population. Int J Cardiol. 2017;236:226–231. DOI: 10.1016/j.ijcard.2017.02.039 [DOI] [PubMed] [Google Scholar]
- 17.Nyström PK, Carlsson AC, Leander K, De FU, Hellenius ML, Gigante B. Obesity, metabolic syndrome and risk of atrial fibrillation: a swedish, prospective cohort study. PLoS One. 2015;10:1–14. DOI: 10.1371/journal.pone.0127111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choe WS, Choi EK, Do HK, Lee EJ, Lee SR, Cha MJ, Oh S. Association of metabolic syndrome and chronic kidney disease with atrial fibrillation: a nationwide population‐based study in Korea. Diabetes Res Clin Pract. 2019;148:14–22. DOI: 10.1016/j.diabres.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 19.Tanner RM, Baber U, Carson AP, Voeks J, Brown TM, Soliman EZ, Howard VJ, Muntner P. Association of the metabolic syndrome with atrial fibrillation among United States adults (from the REasons for Geographic and Racial Differences in Stroke [REGARDS] Study). Am J Cardiol. 2011;108:227–232. DOI: 10.1016/j.amjcard.2011.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joung B. Risk factor management for atrial fibrillation. Korean Circ J. 2019;49:794–807. DOI: 10.4070/kcj.2019.0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polovina M, Hindricks G, Maggioni A, Piepoli M, Vardas P, Ašanin M, Dikić D, Duricić N, Milinković I, Seferović PM, et al. Association of metabolic syndrome with non‐thromboembolic adverse cardiac outcomes in patients with atrial fibrillation. Eur Heart J. 2018;39:4030–4039. DOI: 10.1093/eurheartj/ehy446 [DOI] [PubMed] [Google Scholar]
- 22.Kim MK, Han K, Park YM, Kwon HS, Kang G, Yoon KH, Lee SH. Associations of variability in blood pressure, glucose and cholesterol concentrations, and body mass index with mortality and cardiovascular outcomes in the general population. Circulation. 2018;138:2627–2637. DOI: 10.1161/CIRCULATIONAHA.118.034978 [DOI] [PubMed] [Google Scholar]
- 23.Proietti M, Romiti GF, Olshansky B, Lip GY. Systolic blood pressure visit‐to‐visit variability and major adverse outcomes in atrial fibrillation. Hypertension. 2017;70:949–958. DOI: 10.1161/HYPERTENSIONAHA.117.10106 [DOI] [PubMed] [Google Scholar]
- 24.Han X, Wang Y, Chen S, Wang A, Xu J, Wang Y, Liu X, Wu S, Zhang N, Su Z. Visit‐to‐visit variability of fasting plasma glucose and the risk of cardiovascular disease and all‐cause mortality in the general population. J Am Heart Assoc. 2017;6:e006757. DOI: 10.1161/JAHA.117.006757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim YM, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, Kim JY, Pak HN, Lee MH, Joung B, et al. Body mass index variability and long‐term risk of new‐onset atrial fibrillation in the general population: a Korean nationwide cohort study. Mayo Clin Proc. 2019;94:225–235. DOI: 10.1016/j.mayocp.2018.10.019 [DOI] [PubMed] [Google Scholar]
- 26.Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. DOI: 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feinberg WM, Blackshear JL, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation analysis and implications. Arch Intern Med. 1995;155:469–473. DOI: 10.1001/archinte.1995.00430050045005 [DOI] [PubMed] [Google Scholar]
- 28.Li X, Gao L, Wang Z, Guan BO, Guan X, Wang B, Han XU, Xiao X, Waleed KB, Chandran C, et al. Lipid profile and incidence of atrial fibrillation: a prospective cohort study in China. Clin Cardiol. 2018;41:314–320. DOI: 10.1002/clc.22864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sankaranarayanan R, Kirkwood G, Dibb K, Garratt CJ. Comparison of atrial fibrillation in the young versus that in the elderly: a review. Cardiol Res Pract. 2013;2013:976976. DOI: 10.1155/2013/976976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long‐ and short‐term impact of elevated body mass index on the risk of new atrial fibrillation. The WHS (Women’s Health Study). J Am Coll Cardiol. 2010;55:2319–2327. DOI: 10.1016/j.jacc.2010.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pallisgaard JL, Schjerning AM, Lindhardt TB, Procida K, Hansen ML, Torp‐Pedersen C, Gislason GH. Risk of atrial fibrillation in diabetes mellitus: A nationwide cohort study. Eur J Prev Cardiol. 2016;23:621–627. DOI: 10.1177/2047487315599892 [DOI] [PubMed] [Google Scholar]
- 32.Zafar U, Khaliq S, Ahmad HU, Manzoor S, Lone KP. Metabolic syndrome: an update on diagnostic criteria, pathogenesis, and genetic links. Hormone. 2018;17:299–313. DOI: 10.1007/s42000-018-0051-3 [DOI] [PubMed] [Google Scholar]
- 33.Oh SW, Lee JE, Shin E, Kwon H, Choe EK, Choi SY, Rhee H, Choi SH. Genome‐wide association study of metabolic syndrome in Korean populations. PLoS One. 2020;15:e0227357. DOI: 10.1371/journal.pone.0227357 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figure S1


