Abstract
Background
Limited information is available regarding in‐hospital cardiac arrest (IHCA) in patients with COVID‐19.
Methods and Results
We leveraged the American Heart Association COVID‐19 Cardiovascular Disease (AHA COVID‐19 CVD) Registry to conduct a cohort study of adults hospitalized for COVID‐19. IHCA was defined as those with documentation of cardiac arrest requiring medication or electrical shock for resuscitation. Mixed effects models with random intercepts were used to identify independent predictors of IHCA and mortality while accounting for clustering at the hospital level. The study cohort included 8518 patients (6080 not in the intensive care unit [ICU]) with mean age of 61.5 years (SD 17.5). IHCA occurred in 509 (5.9%) patients overall with 375 (73.7%) in the ICU and 134 (26.3%) patients not in the ICU. The majority of patients at the time of ICHA were not in a shockable rhythm (76.5%). Independent predictors of IHCA included older age, Hispanic ethnicity (odds ratio [OR], 1.9; CI, 1.4–2.4; P<0.001), and non‐Hispanic Black race (OR, 1.5; CI, 1.1–1.9; P=0.004). Other predictors included oxygen use on admission, quick Sequential Organ Failure Assessment score on admission, and hypertension. Overall, 35 (6.9%) patients with IHCA survived to discharge, with 9.1% for ICU and 0.7% for non‐ICU patients.
Conclusions
Older age, Black race, and Hispanic ethnicity are independent predictors of IHCA in patients with COVID‐19. Although the incidence is much lower than in ICU patients, approximately one‐quarter of IHCA events in patients with COVID‐19 occur in non‐ICU settings, with the latter having a substantially lower survival to discharge rate.
Keywords: cardiac arrest, COVID‐19, outcomes, predictors
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care, Mortality/Survival, Risk Factors
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- IHCA
in‐hospital cardiac arrest
Clinical Perspective
What Is New?
Among patients with COVID‐19, the incidence of in‐hospital cardiac arrest (IHCA) in npatients not in the intensive care unit (ICU) was previously unknown; our study shows that 2.2% of non‐ICU patients developed IHCA. The incidence of IHCA for ICU patients was 15.4%.
In addition to increased age, odds of IHCA were higher in patients of Black race and Hispanic ethnicity.
The adjusted odds of IHCA do not differ according to hospital size, and survival to discharge is significantly worse for non‐ICU patients (0.7%) with IHCA compared with ICU patients (9.1%).
What Are the Clinical Implications?
A significant proportion of patients with COVID‐19 infections who are triaged to non‐ICU care develop IHCA; the initial rhythm was uncertain in a higher proportion of non‐ICU patients.
Non‐ICU patients have worse survival at discharge after IHCA compared with ICU patients.
Our study identifies predictors of IHCA in patients admitted with COVID‐19 infection who may benefit from closer in‐hospital observation and triaging to higher level of care.
Limited information is available on in‐hospital cardiac arrest (IHCA) in patients with COVID‐19 infection. Until recently the only information available was from a few single center studies that have reported poor rates of survival to discharge ranging from zero to 3%.1, 2, 3 A recent, large, multicenter study has provided important new information on IHCA in patients with COVID‐19, admitted to an intensive care unit (ICU) in the United States. That study found the incidence of IHCA to be 14%, with only 7% of these patients surviving to discharge.4
Although valuable, this study reported on only ICU patients. However, among patients requiring hospitalization for COVID‐19, only one‐third require ICU level care.5 Most available data on IHCA focus exclusively on patients with severe disease or those requiring ICU‐level care.1, 4 There is a significant paucity of data in regard to IHCA in patients who are not critically ill and hospitalized with COVID‐19. Given the high in‐hospital mortality in patients with COVID‐19 and a higher fraction of patients being admitted to a non‐ICU setup, it is important to investigate the occurrence of IHCA in the non‐ICU setting. With the continuing increase in COVID‐19 related hospitalizations across the world, this information is of significant public health interest.6, 7, 8
We examined the incidence of IHCA in hospitalized patients with COVID‐19 who were admitted to ICU as well as non‐ICU setup in a large multicenter cohort study of more than 8000 admissions to 88 hospitals across the United States. We also examined the predictors of IHCA and survival to discharge.
Methods
Study Design
All data and materials are available through the American Heart Association (AHA) Precision Medicine Platform (https://precision.heart.org) and are available to qualified researchers through a formal research proposal. We used data from the American Heart Association COVID‐19 Cardiovascular Disease (AHA COVID‐19 CVD) Registry Powered by Get With The Guidelines. This multicenter cohort study included consecutive inpatient hospitalization of adult patients (≥18 years) with active COVID‐19 infection, admitted to 88 geographically diverse hospitals across the United States (Figure S1 and Table S1). We excluded patients with missing data regarding cardiac arrest (99 patients) and visits that were readmissions (303 visits). Readmissions were excluded from the study as we would be able to capture readmissions only in patients readmitted to hospitals included in the study and those admitted to other hospitals would be missed. Hence, patients were followed in the registry until hospital discharge or death during the index admission.
Data Collection
Data were collected at the patient level in the participating hospitals per AHA instructions. Hospitals participating in the registry submitted clinical information regarding the medical history, hospital care, and outcomes of consecutive patients hospitalized for COVID‐19 using an online, interactive case record form in the Patient Management Tool. This tool is powered by IQVIA (Parsippany, New Jersey), which serves as the data collection and coordination center. Trained personnel populated registry data using the standard definition of patient demographics, clinical comorbidities, inpatient laboratory data, treatment therapies at admission and discharge, and in‐hospital outcomes. Quality checks for the patients in the registry were performed throughout the data collection and preparation process. A random sample of hospitals is selected for chart reabstractions to independently assess data quality. The data is patient and hospital de‐identified at an aggregate level.9
Definition and Outcomes
Patients with active COVID‐19 infection were defined as those who were (1) diagnosed before hospitalization but still symptomatic during hospitalization; (2) diagnosed during hospitalization; (3) symptomatic during hospitalization and have a confirmed test available only after discharge; and (4) those with a diagnosis (International Classification of Diseases [ICD] code U07.01 for COVID‐19, virus identified) with or without COVID‐related symptoms. The active infection with COVID‐19 was confirmed by reverse transcription polymerase chain reaction, positive IgM antibody test, or a clinical diagnosis using hospital‐specific criteria. Patients with prior COVID‐19 diagnosis but without COVID‐19 related symptoms were not considered to have active COVID‐19 infection. Patients with positive IgG antibody test without positive IgM antibody were excluded as well.
In the AHA registry, patients with IHCA were identified as those with documentation of cardiac arrest requiring medication or defibrillation for resuscitation. The registry recorded the date of IHCA and if multiple dates of cardiac arrest were found, the first recorded date of cardiac arrest occurrence was taken. The first rhythm without a palpable pulse was documented as asystole, pulseless electrical activity, pulseless ventricular tachycardia, ventricular fibrillation, or unknown/undocumented. Patients whose goals of treatment were comfort measures only were not labeled as IHCA. The quick Sequential Organ Failure Assessment (qSOFA) score was used as a measure of acute illness severity on admission.10, 11
IHCA was identified to have occurred in the ICU if the patient’s timestamp of ICU admission was earlier than the timestamp for cardiac arrest. If the timestamp for cardiac arrest was earlier than that for the ICU admission or there was no ICU admission during that hospitalization, this was considered to have occurred outside the ICU (cardiac arrest in non‐ICU patients).
Statistical Analysis
We report the clinical characteristics of patients stratified by IHCA and ICU or non‐ICU status at the time of cardiac arrest. Continuous variables are presented as mean (SD), and categorical variables reported with frequencies and percentages. We used the t test to compare the continuous variables and chi‐square test to compare categorical variables. Random intercept mixed effects models were used to identify the independent predictors of IHCA and mortality after IHCA, while accounting for clustering at the hospital level. Hospitals were categorized into large (≥400 beds), medium (100–399 beds), and small (<100 beds). Odds ratios (ORs) with 95% CIs are reported. Statistical analyses were 2 sided and at a significance level of 0.05. Covariates with more than 20% of missing variables were excluded (medication used before admission, inpatient medications administered before IHCA, and baseline troponin level on admission). Multiple imputation was conducted for variables with less than 20% of missing values (supplemental oxygen use, body mass index, and qSOFA score). Final covariates included in the models were informed by clinical knowledge and biological plausibility. Those include age, sex, race, body mass index, qSOFA on admission, prior coronary artery disease, peripheral artery disease, hypertension, hyperlipidemia, congestive heart failure, chronic kidney disease/end‐stage renal disease, diabetes mellitus, chronic lung disease, and smoking or e‐cigarette use.
Sensitivity analysis was performed comparing the results to the logistic regression model without clustering. Subgroup analysis of predictors of IHCA was performed in patients admitted to ICU and non‐ICU settings. The AHA Precision Medicine Platform (https://precision.heart.org) was used for analysis. All analyses were performed using R software.12
Oversight
All the participating institutions are required to comply with local regulatory and privacy guidelines and, if required, to secure institutional review board approval. The sites are granted waiver of informed consent under the common rule. The authors designed the study and performed the analysis.
Results
Baseline Characteristics
There were 8920 inpatient admissions for COVID‐19 during the enrollment period for the study through July 22, 2020. We excluded 99 visits with missing data regarding cardiac arrest and further excluded 303 visits that were readmissions (Figure 1) giving a final cohort of 8518. Table 1 and Table S2 list the baseline demographic and clinical characteristics. The study cohort had a median age of 62 (49–75) years and 55.5% of them were male. Non‐Hispanic White patients comprised 32.3% of the study population, followed by Hispanic (30.2%) and non‐Hispanic Black (23.6%) patients. Cough (62.1%), shortness of breath (58.6%), fatigue and myalgia (37.5%), and nausea/vomiting/diarrhea (26.8%) were the most common presenting symptoms. About 92% of the patients were diagnosed of COVID‐19 with reverse transcription polymerase chain reaction and 7% were clinical diagnoses using hospital‐specific criteria. Among the total cohort, 49.9% of patients were admitted to large hospitals (≥400 beds), 25.2% to medium‐size hospitals (100–399 beds), and 24.9% were admitted to small hospitals (<100 beds). Of all admissions, 2438 (28.6%) were admitted to ICU during the hospitalization and 6080 (71.4%) did not have an ICU admission.
Figure 1. Selection of patient cohort.
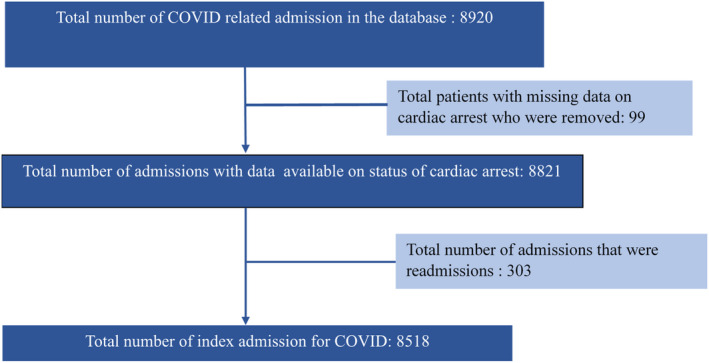
Table 1.
Comparison of Baseline Characteristics
| Variables | Total Cohort | Non‐ICU Patients | ICU Patients | ||||
|---|---|---|---|---|---|---|---|
| Without Cardiac Arrest | With Cardiac Arrest | P Value | Without Cardiac Arrest | With Cardiac Arrest | P Value | ||
| Total number of patients | 8518 | 5946 | 134 | 2063 | 375 | ||
| Mean age, y (SD) | 61.4 (17.5) | 60.78 (18.2) | 75.16 (11.9) | <0.001 | 62.05 (15.9) | 63.63 (13.7) | <0.001 |
| Age (y) group, n (%) | |||||||
| <50 | 2182 (25.6) | 1676 (28.2) | 3 (2.2) | <0.001 | 449 (21.8) | 54 (14.4) | 0.001 |
| 50–60 | 1522 (17.9) | 1065 (17.9) | 11 (8.2) | 0.004 | 366 (17.7) | 80 (21.3) | 0.09 |
| 60–70 | 1802 (21.2) | 1121 (18.9) | 27 (20.1) | 0.71 | 542 (26.3) | 111 (29.6) | 0.18 |
| 70– 79 | 1520 (17.8) | 982 (16.5) | 34 (25.4) | 0.007 | 416 (20.2) | 88 (23.5) | 0.15 |
| >80 | 1492 (17.5) | 1101 (18.5) | 59 (44.1) | <0.001 | 290 (14.1) | 42 (11.2) | 0.14 |
| Sex, n (%) | |||||||
| Male | 4730 (55.5) | 3129 (52.6) | 84 (62.7) | 0.03 | 1279 (62) | 238 (63.5) | 0.63 |
| Race/Ethnicity, n (%) | |||||||
| Hispanic | 2582 (30.2) | 1930 (32.5) | 58 (43.3) | 0.008 | 476 (23.1) | 117 (31.2) | <0.001 |
| Non‐Hispanic Black | 2009 (23.6) | 1364 (22.4) | 30 (22.4) | 0.88 | 520 (25.2) | 95 (25.3) | 0.96 |
| Non‐Hispanic White | 2750 (32.3) | 1790 (30.1) | 29 (21.6) | 0.03 | 826 (40) | 105 (28) | <0.001 |
| Body mass index >=30, n (%) | 3252 (43.7) | 2166 (36.4) | 26 (19.4) | 0.003 | 895 (43.4) | 164 (43.7) | 0.39 |
| Symptoms of COVID 19 | |||||||
| Fever/chills | 1242 (15.1) | 793 (13.3) | 21 (15.7) | 0.26 | 359 (17.4) | 69 (18.4) | 0.50 |
| Cough | 5173 (62.1) | 3659 (61.5) | 70 (52.2) | 0.03 | 1213 (58.8) | 230 (61.3) | 0.96 |
| Shortness of breath | 4880 (58.6) | 3188 (53.6) | 82 (61.2) | 0.11 | 1350 (65.4) | 259 (69.1) | 0.69 |
| Chest pain | 273 (3.2) | 204 (3.4) | 2 (1.5) | 0.32 | 57 (2.8) | 10 (2.7) | 0.99 |
| Nasal congestion or sore throat | 841 (10.1) | 634 (10.7) | 7 (5.2) | 0.06 | 175 (8.5) | 25 (6.7) | 0.21 |
| Loss of sense of taste/smell | 340 (4.1) | 278 (4.7) | 0 | 0.02 | 56 (2.7) | 6 (1.6) | 0.24 |
| Fatigue or myalgia | 3125 (37.5) | 2255 (37.9) | 28 (20.9) | <0.001 | 714 (34.6) | 127 (33.9) | 0.48 |
| Headache | 745 (8.9) | 560 (9.4) | 6 (4.5) | 0.07 | 155 (7.5) | 24 (6.4) | 0.41 |
| Confusion/altered mental status | 881 (10.6) | 558 (9.4) | 24 (17.9) | 0.002 | 248 (12) | 51 (13.6) | 0.61 |
| Nausea, vomiting, or diarrhea | 2231 (26.8) | 1640 (27.6) | 16 (11.9) | <0.001 | 496 (24) | 79 (21.1) | 0.12 |
| Past medical history | |||||||
| Prior myocardial infarction/percutaneous coronary intervention, or coronary artery bypass grafting | 777 (9.12) | 500 (8.4) | 24 (17.9) | <0.001 | 206 (10) | 47 (12.5) | 0.16 |
| Atrial fibrillation/flutter | 785 (9.2) | 501 (8.4) | 21 (15.7) | 0.005 | 226 (11) | 37 (9.9) | 0.59 |
| Cerebrovascular disease (stroke/transient ischemic attack) | 941 (11.1) | 641 (10.8) | 33 (24.6) | <0.001 | 222 (10.8) | 45 (12) | 0.54 |
| Hypertension | 4952 (58.2) | 3272 (55) | 95 (70.9) | <0.001 | 1325 (64.2) | 262 (69.9) | 0.04 |
| Heart failure | 927 (10.9) | 585 (9.8) | 29 (21.6) | <0.001 | 262 (12.7) | 51 (13.6) | 0.69 |
| Chronic kidney disease/end‐stage renal disease | 1152 (13.5) | 719 (12.1) | 34 (25.4) | <0.001 | 334 (16.2) | 65 (17.3) | 0.64 |
| Diabetes mellitus | 3066 (35.9) | 1995 (33.6) | 62 (46.3) | 0.002 | 840 (40.7) | 169 (45.1) | 0.13 |
| Hospital characteristics | 2063 | 375 | |||||
| Hospital size | |||||||
| Large (≥400 beds) | 4248 (49.9) | 2087 (35.1) | 55 (41.04) | 0.15 | 1212 (58.7) | 174 (46.4) | <0.001 |
| Medium (100–400 beds) | 2143 (25.2) | 1453 (24.4) | 11 (8.2) | <0.001 | 571 (27.7) | 108 (28.8) | 0.66 |
| Small (<100 beds) | 2122 (24.9) | 1684 (28.3) | 68 (50.7) | <0.001 | 276 (13.4) | 93 (24.8) | <0.001 |
| Severity score on admission | |||||||
| High‐risk quick Sequential Organ Failure Assessment score | 592 (7.2) | 306 (5.1) | 18 (13.4) | <0.001 | 221 (10.7) | 47 (12.5) | 0.35 |
ICU indicates intensive care unit.
Statistically significant P<0.05 are indicated in bold.
A total of 509 (5.9%) patients had an IHCA event. The incidence of IHCA was 15.4% for patients admitted to ICU and 2.2% for non‐ICU patients (Table 2). Asystole (38.3%) and pulseless electrical activity (PEA) (34.2%) were the most common initial rhythm during the arrest. The median time from symptom onset to IHCA was 14.7 days (interquartile range 8.6–21 days) and from diagnosis of COVID‐19 to IHCA was 9.9 days (interquartile range 2.9–13.9 days). The median time from admission to cardiac arrest was 7.1 days (interquartile range 2.8–13.4).
Table 2.
Comparison of Outcomes in Non‐ICU Patients Compared With ICU Patients
| Outcomes | Non‐ICU Patients | ICU Patients | P Value |
|---|---|---|---|
| Total number of patients | 6080 | 2438 | |
| Patients with cardiac arrest, n (%) | 134 (2.2) | 375 (15.4) | <0.001 |
| Initial rhythm during cardiac arrest, n (%) | |||
| Asystole or pulseless electrical activity | 84 (62.7) | 271 (72.2) | 0.038 |
| Pulseless ventricular tachycardia/ventricular fibrillation | 4 (2.7) | 31 (8.2) | 0.040 |
| Initial rhythm unknown | 46 (34.5) | 73 (19.5) | <0.001 |
| Outcome, n (%) | |||
| Mortality during index admission | 133 (99.3) | 341 (90.9) | <0.001 |
ICU indicates intensive care unit.
Comparison of Cardiac Arrest in ICU and Non‐ICU Patients
Of the 6080 non‐ICU patients, 134 (2.2%) developed IHCA whereas of the 2438 patients who did have an ICU admission, 375 (15.4%) developed IHCA. (Table 2) A majority of the patients in both non‐ICU (62.7%) and ICU (72.2%) setting had asystole or PEA as the initial rhythm during arrest (P=0.04). The initial rhythm during cardiac arrest was reported as unknown in a significantly higher percentage of cardiac arrest in non‐ICU patients. (34.5% versus 19.5%, P<0.001). The median time from onset of symptoms to IHCA was about 5 days shorter in non‐ICU patients (10.38 versus 15.55 days, P<0.001). Moreover, the time from admission to IHCA (4.8 days versus 8.7 days) as well as time from diagnosis of COVID‐19 to arrest (5.1 versus 9.36 days) was 4 days shorter in non‐ICU patients. The determinants of shorter time between admission to cardiac arrest (<7 days) upon multivariable adjustment include prior history of congestive heart failure (P=0.006) and supplemental oxygen use on admission (P=0.02). Increased age was not significantly associated with shorter time from admission to IHCA (P=0.14).
Patients who had IHCA were significantly older and included a higher proportion of Hispanic people and significantly lower proportion of non‐Hispanic White people compared with those who did not have IHCA. Among non‐ICU patients, a significantly higher proportion among those who had cardiac arrest were male (62.7% versus 52.6%, P=0.03). However, this difference was not observed for ICU patients (63.5% versus 62%, P=0.63). The presenting symptom of confusion and altered mental status was significantly higher in non‐ICU patients with IHCA compared with those without (37.9% versus 20.9%, P=0.002). Non‐ICU patients with IHCA had significantly higher proportion of patients with prior history of coronary artery disease, atrial fibrillation/flutter, cerebrovascular disease, hypertension, heart failure, chronic kidney disease, and diabetes mellitus compared with those who did not have IHCA. These baseline differences were not seen in ICU patients, however (Table 1). Both ICU and non‐ICU patients who developed IHCA had higher mean creatinine and troponin at baseline. (Table S2).
Predictors of Cardiac Arrest
On multivariable analysis, we identified several characteristics that were associated with increased odds of developing IHCA. These included older age, Hispanic ethnicity, non‐Hispanic Black race, supplemental oxygen use on admission, higher qSOFA score on admission, and presence of hypertension. Cardiovascular risk factors like diabetes mellitus, chronic kidney disease/end‐stage renal disease, obesity, and smoking were not associated with IHCA. (Figure 2).
Figure 2. Independent predictors of in‐hospital cardiac arrest.
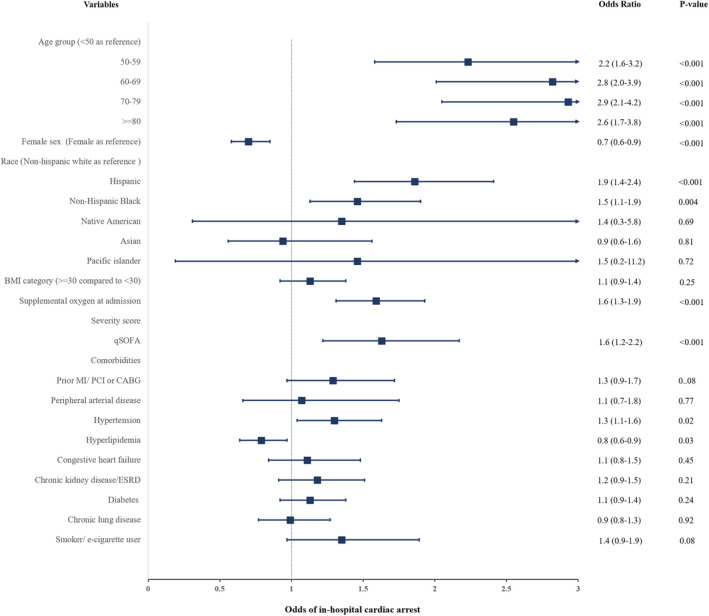
BMI indicates body mass index; CABG, coronary artery bypass grafting; ESRD, end‐stage renal disease; ICU, intensive care unit; MI, myocardial infarction; PCI, percutaneous coronary intervention; and qSOFA, quick sequential organ failure assessment score.
Similar to the total population, the ICU and non‐ICU patient subcohorts also had higher odds of IHCA with increasing age over 50 years, Hispanic ethnicity, and non‐Hispanic Black race. After multivariable adjustments, higher body mass index, supplemental oxygen requirement on admission, and prior medical history including coronary artery disease, hypertension, diabetes mellitus, chronic kidney disease/end‐stage renal disease, and congestive heart failure were not associated with increased odds of IHCA (Figure 3).
Figure 3. Independent predictors of in‐hospital cardiac arrest in non‐ICU patients (A) and independent predictors of in‐hospital cardiac arrest in ICU patients (B).
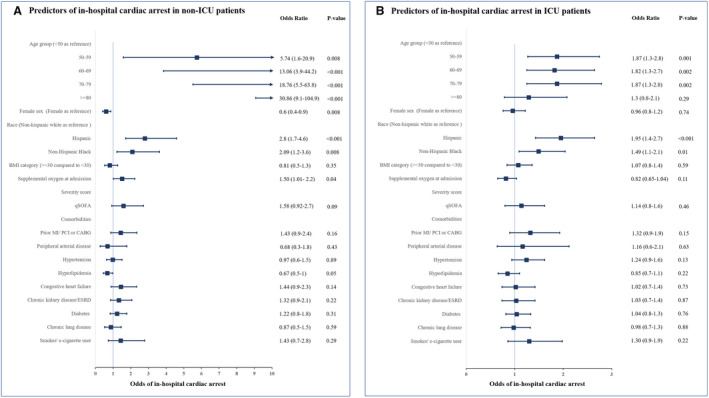
BMI indicates body mass index; CABG, coronary artery bypass grafting; ESRD, end‐stage renal disease; ICU, intensive care unit; MI, myocardial infarction; PCI, percutaneous coronary intervention; and qSOFA, quick sequential organ failure assessment score.
Sensitivity analysis was performed using a logistic regression model without clustering to delineate the predictors of IHCA. The findings were similar with no significant differences noted. A separate sensitivity analysis was also performed without performing multiple imputations for the missing variables and no significant difference in the findings were noted.
Outcomes
In the overall cohort, approximately 7% of the patients survived to discharge after IHCA (Table S3). However, there was a significant difference in survival between ICU and non‐ICU groups, with 9.1% of ICU patients surviving to discharge whereas only 0.7% of non‐ICU patients survived to discharge (P<0.001). Age 70 to 79 years ;(OR, 5.7; CI, 1.3–25.7; P=0.02) and >=80 years (OR, 21.7; CI, 2.2–214.6; P=0.008) were associated with significantly increased adjusted odds of mortality in patients with IHCA. Differences in sex, race or ethnicity, and prior comorbidities did not predict mortality after IHCA. (Table S4).
Influence of Hospital Characteristics
Patients who were admitted to large hospitals (≥400 beds) had significantly lower incidence of IHCA (6.6% versus 5.4%, P=0.03) compared with those admitted to medium to small hospitals (<400 beds). Also, significantly lower proportion of patients admitted to teaching hospitals developed IHCA compared with those admitted to nonteaching hospitals (5.4% versus 8.7%, P=0.004) (Figure S2). However, upon adjustment for covariates, hospital size (OR, 0.89; CI, 0.74–1.08; P=0.25) and teaching status (OR, 0.91; CI, 0.69–1.22; P=0.53) did not significantly increase the odds of IHCA. Survival to discharge after IHCA did not vary significantly according to hospital size (OR, 1.18; CI, 0.53–2.65; P=0.69) and teaching status (OR, 0.72; CI, 0.21–2.94; P=0.62).
Discussion
The main aim of our research was to better understand the incidence and predictors of IHCA in hospitalized patients with COVID‐19. To our knowledge, this is the largest study on IHCA in COVID‐19 to date and the first study to specifically look at IHCA in noncritically ill patients. The main findings of our study are as follows: (1) IHCA occurs in a significant proportion of non‐ICU patients with COVID‐19; (2) although the incidence of IHCA is much lower in non‐ICU patients compared with ICU patients, it is almost always a fatal event with 0.7% survival to discharge; and (3) age over 70, Hispanic ethnicity, and Black race are strong independent predictors of IHCA.
Incidence and Outcomes of IHCA
We found an overall incidence of IHCA of 5.9% in our patient population. However, the incidence was much higher in ICU patients at 15.4%. The overall percentage is similar to the 4.6% incidence reported in a recent single‐center report of 1309 patients with COVID‐19 from Royal Oaks, Michigan.3 The IHCA incidence in ICU patients is higher than the 7.9% reported by Hayek et al in a recent study of ICU patients from 68 centers in the United States. Part of this difference may be related to the fact that about 27% of IHCA occurred after 14 days of admission, and the prior study had censored patients before this time point. Of course, differences in hospital and patient characteristics may also explain some of this difference. The finding of a 2.2% incidence of IHCA in non‐ICU patient is new information, as prior studies have not directly assessed this population. In a smaller, single‐center study from New York City, of the 31 total IHCA, 23% (7 patients) were in non‐ICU patients, which is similar to the 26% of all IHCA seen in our current report.2 However, the incidence in non‐ICU was not available in this study, as the total population with COVID‐19 was not reported.
The overall survival to discharge rate was 6.9% and was significantly better in the ICU group at 9.1% and abysmal in the non‐ICU group at 0.7%. The ICU group survival is closer to the 12% reported by Hayek et al in their recent multicenter publication. This is also similar to post‐IHCA survival in other ICU patient populations in the United States reported at 12.5% in a recent report from the AHA Get With The Guidelines‐Resuscitation registry.4, 13 The almost universal in‐hospital fatality after non‐ICU IHCA (0.7% survival) is striking and much higher than that reported in the United States in patients who did not have COVID‐19.14 A 7‐year report from the Get With The Guidelines‐Resuscitation registry found that survival to discharge was lower in nonmonitored ward IHCA than that in ICU IHCA (10.6% versus 14%). The only report on non‐ICU patients with COVID‐19 is from a single center in Wuhan, China.1 The authors report that of the 113 patients with IHCA in the wards, all of whom underwent cardiopulmonary resuscitation, only 1 survived to discharge, giving a survival to discharge rate of 0.88% that is similar to ours. For the 23 ICU patients, survival to discharge was 13% in that study.
The reason for this high in‐hospital mortality rate is not fully evident. The predominant rhythm at the time of IHCA was PEA or asystole in both ICU and non‐ICU patients and this has been associated with worse outcomes in patients without COVID‐19 in prior studies.13 However, this should be the same for both ICU and non‐ICU patients. One reason for excessive mortality in non‐ICU patients could be delayed recognition of cardiac arrest. This is likely as in our study initial rhythm was reported as unknown in a significantly greater percentage of non‐ICU patients, indicating that perhaps they were not on a telemetry unit. It is also likely that because these patients were not expected to have cardiac arrest, teams were not ready to perform immediate cardiopulmonary resuscitation in patients with COVID. Delays may have been exacerbated because of isolation status and need of donning personal protective gear. In addition, it is possible that the goals of care were different between the 2 groups of patients. It is also plausible that given the difference in age and comorbidity profile, the resuscitation efforts in non‐ICU patients may have terminated earlier at the request of the family.
Another factor may have been the presumed etiology. Because the underlying rhythm was mostly PEA/asystole, it points mostly to respiratory arrest or pulmonary embolism. Because these patients were not in the ICU, it is less likely to be pneumonitis or acute respiratory distress syndrome and a sudden pulmonary embolism may be more likely. In a recent report on 1240 patients with COVID‐19 from France, pulmonary embolism was diagnosed by computed tomographic angiography in 8.3% of patients and resulted in a significantly higher risk of ICU transfer though not mortality.15 However, this study could have missed pulmonary embolisms that were fatal and contributed to mortality before the computed tomographic angiography was performed. Finally, implicit bias according to increased age and race/ethnicity while triaging for admission cannot be ruled out. It is possible that this could lead to a sicker cohort being triaged to non‐ICU setup, leading to worse outcomes.
Predictors of IHCA
We identified several predictors of IHCA. Advanced age was a significant and independent predictor of IHCA in both ICU and non‐ICU patients. However, in the non‐ICU group patients with IHCA were substantially older than the ICU group with IHCA (mean age 75 versus 62 years). Race and ethnicity were also a significant and independent predictor of IHCA with non‐Hispanic Black and Hispanic patients having a significant higher risk of IHCA in both hospital settings. In a recent multicenter study in ICU patients with COVID‐19, Black patients were found to be at higher risk but not Hispanic patients.4 However, in our study, Hispanic patients had a 19% higher risk of IHCA than non‐Hispanic White patients. The higher risk of IHCA is consistent with a higher mortality seen in Hispanic patients nationally.16 Sex was also identified as an independent predictor of IHCA with women having less risk of IHCA in the overall group, though not in the ICU patients. The reasons for this are unclear though they may be related to possible sex‐based differences in pathogenesis and severity of COVID‐19.17 However, further research is needed to explore the mechanism for these differences.
At baseline, underlying cardiovascular comorbidities were associated with a significantly higher risk of IHCA in non‐ICU patients, likely as this patient group was significantly older. However, after adjusting for baseline differences, in multivariate analysis, most of these were not associated with a higher risk of IHCA in either ICU or non‐ICU groups. This finding is similar to that in the STOP‐COVID (Study of the Treatment and Outcomes in Critically Ill Patients With COVID‐19) registry and is likely due to the fact that in a majority of patients the underlying rhythm was PEA or asystole, which is associated with a noncardiac etiology.4 The is consistent with the observation that the initial high‐risk qSOFA score and supplemental oxygen requirement were independently associated with IHCA risk. This points to the fact that the severity of illness may be a stronger predictor than underlying comorbidities.
Although there were some presenting symptoms that appeared to be associated with a higher risk of IHCA at baseline, after adjustment none of these remained as independent predictors.
Implications
This study highlights the high risk of cardiac arrest in patients who are critically ill with COVID‐19 and the subsequent poor survival. We also identify age, Black race, and Hispanic ethnicity as significant risks for IHCA and these could be focus of extra attention and discussion about resuscitation status with families.
The biggest implications of our study findings, however, may be for non‐ICU patients. Older age was a very significant predictor, as was being Black or Hispanic. Further, need for supplemental oxygen on admission and a high‐risk qSOFA score were predictors of IHCA. Study findings highlight a need for better risk stratification of patients with COVID‐19 to identify those who would need a higher level of care.
These observations also point to the importance of conducting early discussions about goals of care including resuscitation status even in pateints who are not critically ill. These outcomes data also provide information to clinicians, patients, and families to make an informed decision during these discussions. Finally, the availability of data regarding the incidence of cardiac arrest and subsequent survival from a nationally representative sample establishes a yardstick for individual hospitals to gauge their outcomes. It also allows them to plan, accumulate, and allocate resources in the scenario of a local surge of COVID‐19 infection.
Strengths and Limitations
The study has several strengths. This is the largest study to date of cardiac arrest and outcomes in patients with COVID‐19. Patient‐level granular data were acquired from 88 geographically diverse centers. The registry is well curated and follows the standards of all Get With The Guidelines AHA registries. All the patients were followed from admission to discharge or until death. Moreover, our study is the first to include and evaluate outcomes in a significantly large number of non‐ICU patients.
The study has several limitations as well. Details of the cardiopulmonary resuscitation process, including timing of initiation of chest compressions, quality of chest compressions, defibrillation, duration of resuscitation, and medications administered during the process, are not available. Similarly, the cause of cardiac arrest could not be ascertained. An initial rhythm was unknown in a significant proportion of cardiac arrests. We also do not have data regarding the immediate outcome of the resuscitation process as well as the neurological recovery thereafter. We were unable to ascertain the initiation of palliative care or interval changes in the goals of care. Additionally, the data were collected early during the pandemic. Since then, there has been much that has been learned about the effective medications as well as unique aspect of care of patients with COVID‐19.
Conclusions
IHCA is common in patients with COVID‐19 related admission, particularly those in the ICU, but it also occurs in an important minority of those cared for exclusively in a non‐ICU location. Survival to discharge after cardiac arrest is significantly worse in non‐ICU patients compared with patients who had IHCA in the ICU. The odds of cardiac arrest do not significant differ according to the size of hospital. Age, sex, race, and treatment received during hospitalization are independently associated with IHCA. The study helps identify high‐risk groups, especially in non‐ICU settings, that may benefit from closer in hospital observation or more intensive care.
Sources of Funding
The study was funded by the departmental research fund at the Department of Cardiology, University of Kansas Medical Center. The American Heart Association’s suite of registries is funded by multiple industry sponsors. The AHA COVID‐19 CVD (American Heart Association COVID‐19 COVID‐19 Cardiovascular Disease) Registry is partially supported by The Gordon and Betty Moore Foundation.
Disclosures
Brahmajee Nallamothu, MD is a principal investigator or coinvestigator on research grants from the National Institutes of Health, Veterans Affairs Health Services Research & Development, and the American Heart Association. He also receives compensation as editor‐in‐chief of Circulation: Cardiovascular Quality & Outcomes, a journal of the American Heart Association. Finally, he is a co‐inventor on US Utility Patent Number US 9 962 124 as well as a Provisional Patent Application (54423) that use software technology with signal processing and machine learning to automate the reading of coronary angiograms, held by the University of Michigan. The patent is licensed to AngioInsight, Inc., in which he holds ownership shares and receives consultancy fees. The University of Michigan also has filed patents on his behalf related to the use of computer vision for imaging applications in gastroenterology, with technology elements licensed to Applied Morphomics, Inc., in which he has no relationship or stake. The remaining authors have no disclosures to report.
Supporting information
Table S1–S4
Figures S1–S4
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.021204
For Sources of Funding and Disclosures, see page 10.
References
- 1.Shao F, Xu S, Ma X, Xu Z, Lyu J, Ng M, Cui H, Yu C, Zhang Q, Sun P, et al. In‐hospital cardiac arrest outcomes among patients with COVID‐19 pneumonia in Wuhan, China. Resuscitation. 2020;151:18–23. DOI: 10.1016/j.resuscitation.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheth V, Chishti I, Rothman A, Redlener M, Liang J, Pan D, Mathew J. Outcomes of in‐hospital cardiac arrest in patients with COVID‐19 in New York City. Resuscitation. 2020;155:3–5. DOI: 10.1016/j.resuscitation.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thapa SB, Kakar TS, Mayer C, Khanal D. Clinical outcomes of in‐hospital cardiac arrest in COVID‐19. JAMA Intern Med. 2021;181:279–281. DOI: 10.1001/jamainternmed.2020.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayek SS, Brenner SK, Azam TU, Shadid HR, Anderson E, Berlin H, Pan M, Meloche C, Feroz R, O’Hayer P, et al. In‐hospital cardiac arrest in critically ill patients with covid‐19: multicenter cohort study. BMJ. 2020;371:m3513. DOI: 10.1136/bmj.m3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abate SM, Ahmed Ali S, Mantfardo B, Basu B. Rate of Intensive Care Unit admission and outcomes among patients with coronavirus: a systematic review and Meta‐analysis. PLoS One. 2020;15:e0235653. DOI: 10.1371/journal.pone.0235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronavirus Disease 2019 (COVID‐19). CDC COVID Data Tracker. 2020. Available at: https://covid.cdc.gov/covid‐data‐tracker/#cases_totalcases. Accessed March 10, 2020.
- 7.Kramer DB, Lo B, Dickert NW. CPR in the Covid‐19 era—an ethical framework. N Engl J Med. 2020;383:e6. DOI: 10.1056/NEJMp2010758. [DOI] [PubMed] [Google Scholar]
- 8.Ranney ML, Griffeth V, Jha AK. Critical supply shortages—the need for ventilators and personal protective equipment during the Covid‐19 pandemic. N Engl J Med. 2020;382:e41. DOI: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 9.Alger HM, Rutan C, Williams JH, Walchok JG, Bolles M, Hall JL, Bradley SM, Elkind MSV, Rodriguez F, Wang TY, et al. American Heart Association COVID‐19 CVD registry powered by get with the guidelines. Circ Cardiovasc Qual Outcomes. 2020;13:e006967. DOI: 10.1161/CIRCOUTCOMES.120.006967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, Pilcher DV. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in‐hospital mortality among adults with suspected infection admitted to the intensive care unit. J Am Med Assoc. 2017;317:290–300. DOI: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
- 11.Singer AJ, Ng J, Thode HC Jr, Spiegel R, Weingart S. Quick SOFA scores predict mortality in adult emergency department patients with and without suspected infection. Ann Emerg Med. 2017;69:475–479. DOI: 10.1016/j.annemergmed.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 12.R: A Language and Environment for Statistical Computing [Computer Program]. Vienna, Austria: R foundation for Statistical Computing; 2019. [Google Scholar]
- 13.Girotra S, Tang Y, Chan PS, Nallamothu BK. Survival after in‐hospital cardiac arrest in critically ill patients: implications for COVID‐19 outbreak? Circ Cardiovasc Qual Outcomes. 2020;13:e006837. DOI: 10.1161/CIRCOUTCOMES.120.006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perman SM, Stanton E, Soar J, Berg RA, Donnino MW, Mikkelsen ME, Edelson DP, Churpek MM, Yang L, Merchant RM, et al. Location of in‐hospital cardiac arrest in the United States—variability in event rate and outcomes. J Am Heart Assoc. 2016;5:e003638. DOI: 10.1161/JAHA.116.003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fauvel C, Weizman O, Trimaille A, Mika D, Pommier T, Pace N, Douair A, Barbin E, Fraix A, Bouchot O, et al. Pulmonary embolism in COVID‐19 patients: a French multicentre cohort study. Eur Heart J. 2020;41:3058–3068. DOI: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Health disparities: race and hispanic origin. Provisional death counts for coronavirus disease 2019 (COVID‐19). 2020. Available at: https://www.cdc.gov/nchs/nvss/vsrr/covid19/health_disparities.htm. Accessed April 10, 2020.
- 17.Haitao T, Vermunt J, Abeykoon J, Ghamrawi R, Gunaratne M, Jayachandran M, Narang K, Parashuram S, Suvakov S, Garovic V. COVID‐19 and sex differences: mechanisms and biomarkers. Mayo Clin Proc. 2020;95:2189–2203. DOI: 10.1016/j.mayocp.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S4
Figures S1–S4


