Abstract
Background
The association between diets that focus on plant foods and restrict animal products and cardiovascular disease (CVD) is inconclusive. We investigated whether cumulative intake of a plant‐centered diet and shifting toward such a diet are associated with incident CVD.
Methods and Results
Participants were 4946 adults in the CARDIA (Coronary Artery Risk Development in Young Adults) prospective study. They were initially 18 to 30 years old and free of CVD (1985–1986, exam year [year 0]) and followed until 2018. Diet was assessed by an interviewer‐administered, validated diet history. Plant‐centered diet quality was assessed using the A Priori Diet Quality Score (APDQS), in which higher scores indicate higher consumption of nutritionally rich plant foods and limited consumption of high‐fat meat products and less healthy plant foods. Proportional hazards models estimated hazard ratios of CVD associated with both time‐varying average APDQS and a 13‐year change in APDQS score (difference between the year 7 and year 20 assessments). During the 32‐year follow‐up, 289 incident CVD cases were identified. Both long‐term consumption and a change toward such a diet were associated with a lower risk of CVD. Multivariable‐adjusted hazard ratio was 0.48 (95% CI, 0.28–0.81) when comparing the highest quintile of the time‐varying average ADPQS with lowest quintiles. The 13‐year change in APDQS was associated with a lower subsequent risk of CVD, with a hazard ratio of 0.39 (95% CI, 0.19–0.81) comparing the extreme quintiles. Similarly, strong inverse associations were found for coronary heart disease and hypertension‐related CVD with either the time‐varying average or change APDQS.
Conclusions
Consumption of a plant‐centered, high‐quality diet starting in young adulthood is associated with a lower risk of CVD by middle age.
Keywords: cardiovascular disease, plant‐centered diet, prospective cohort study
Subject Categories: Diet and Nutrition, Epidemiology, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- APDQS
A Priori Diet Quality Score
- CARDIA
Coronary Artery Risk Development in Young Adults
- PDI
plant‐based diet quality index
Clinical Perspective
What Is New?
Our study expands on previous studies by exploring the time‐varying relationship between plant‐centered diet quality and risk of cardiovascular disease during the transition from young to middle adulthood.
An important aspect is clarifying whether a flexible, plant‐centered diet improves cardiovascular outcomes, where nutritionally rich plant foods are the central component of the diet, and subsets of animal products may be integrated.
Long‐term consumption of a plant‐centered diet and shifting to such a diet, starting in young adulthood, were associated with a lower cardiovascular disease risk.
What Are the Clinical Implications?
Our findings are consistent with assertions that a nutritionally rich plant‐centered diet help prevent the development of cardiovascular disease. However, it appears that the complete exclusion of animal foods from diet is not necessary.
From a clinical and public health perspective, our findings support a recommendation of eating primarily nutritionally rich plant foods, but allowing small amounts of animal products (eg, low‐fat dairy products, nonfried fish, and nonfried poultry), to prevent early cardiovascular disease.
Heart disease remains the leading cause of death in the United States.1 Suboptimal diet is a major risk factor for morbidity and mortality.2 There is a growing interest in the cardiovascular health benefits of diets that focus on consuming only plant foods, excluding animal products. However, the evidence that such diets actually confer a lower risk of incident cardiovascular disease (CVD) is inconclusive.3 Recently, an overall diet quality index that emphasizes healthful plant‐derived foods with restriction of all animal‐derived foods (plant‐based diet quality index [PDI]) has been studied for its association with risk of incident CVD and mortality, but inconsistent results have been reported across studies.4, 5, 6 One study reported that improved the healthful PDI scores over 12 years was associated with a lower subsequent risk of CVD‐specific mortality and all‐cause mortality.7 Otherwise, data regarding the association between change in plant‐centered diet quality and subsequent risk of incident CVD are scarce. Currently, little is known regarding the association between overall diet quality and risk of incident CVD during the transition period from young to middle adulthood because most prospective cohort studies are initiated in middle age. Yet young adulthood is a key stage, in which modifying risk factors may greatly reduce CVD risk in later adulthood.8
The present study evaluated the hypotheses that long‐term consumption of a plant‐centered diet and a shift toward a plant‐centered diet starting in young adulthood are associated with a lower risk of incident CVD in midlife. Plant‐centered diet quality was assessed using the A Priori Diet Quality Score (APDQS), in which higher scores represent greater consumption of nutritionally rich plant foods and lower consumption of high‐fat meat products and unhealthy plant foods. The APDQS has some resemblance to other diet quality indices that generally emphasize plant foods.4, 9 The unique feature of plant‐centeredness in the APDQS is that higher consumption of nutritionally rich plant foods and lower consumption of unhealthy plant foods and high‐fat red meats are the main contributors to a higher score; however, certain subsets of animal products also contribute (eg, low‐fat yogurt, cheese, nonfried fish, or nonfried poultry). The underlying viewpoint of the APDQS is that dietary practices with more flexible options can ensure that the general population achieves and maintains a daily healthy eating pattern over long periods of life. Previous epidemiologic studies support the validity of the APDQS by providing evidence of its linear associations with clinical outcomes.10, 11, 12, 13
Methods
The data that support the findings of this study are available from the CARDIA Coordinating Center (https://www.cardia.dopm.uab.edu) upon reasonable request.
Study Population and Design
CARDIA (Coronary Artery Risk Development in Young Adults) is a multicenter, prospective cohort study of 5115 Black and White men and women from 4 US cities. Participants were aged 18 to 30 years who were free of CVD at baseline (1985–1986, exam year [year 0] clinic exam), with a retention rate of 71% among survivors at year 30 (2015–2016).14 At baseline and during 8 follow‐up examinations, data collection included laboratory tests, physical measurements, medical histories, and lifestyle factors. Vital status and morbidity were ascertained biennially through 2018, with successful contact of >90% of participants over the past 5 years. For the present study, participants were excluded who reported implausible energy intakes (<800 or >8000 kcal/d for men; <600 or >6000 kcal/d for women; n=133) at year 0, year 7, or year 20 or who lacked information regarding physical activity or smoking (n=36). Analyses of the time‐varying average APDQS were based on 4946 participants. For analysis of the 13‐year change in APDQS (year 20–year 7), the events were followed up since year 20 (2005–2006; mean age, 45 years); therefore, of the 3549 participants who attended the year 20 examination, those with CVD at or before year 20 (2005–2006; n=57), those with no diet data at year 7 or year 20 (n=846), or those with no physical activity or no smoking at year 7 or year 20 (n=25) were excluded from the analysis, leaving 2621 participants. All participants provided written informed consent at all examinations, and research protocols were approved by institutional review boards at the coordinating center and each CARDIA field center.
Assessment of Plant‐Centered Diet Quality
Diet was assessed via the interviewer‐administered CARDIA diet history questionnaire at year 0, year 7, and year 20. The diet history established reproducibility and validity.15, 16 Trained interviewers asked the participants about consumption of foods and beverages over the past month within 100 closed food categories (“Do you eat meat?,” “Do you eat vegetables?,” etc) and recorded open‐ended responses of specific foods eaten (specific types of foods eaten, brand names, preparation methods, frequency of intake, and serving size). The number of food items recorded in the CARDIA cohort was 950 at year 0, 1388 at year 7, and 4598 at year 20. CARDIA diet data were analyzed using Nutrition Data System for Research (University of Minnesota, Minneapolis, MN), which includes over 18 000 foods of which about 7500 are brand name products and is updated annually to reflect marketplace changes. The Nutrition Data System for Research summarized foods in 166 food groups (invariant over calendar year), which CARDIA collapsed into 46 food groups for the purpose of creating the APDQS. Total energy intake was derived through summing energy intake of all foods, which was calculated by multiplying consumption of food (frequency × serving size) by the energy content of each item using Nutrition Data System for Research.
Plant‐centered diet quality was assessed using the APDQS, which is a hypothesis‐driven index based on 46 food groups, which are derived from individual foods collected. The APDQS reflects a theoretical concept that how foods affect human health does not act in isolation, but in concert, where nutrients and bioactive compounds in a mixture of individual foods consumed over time work together to produce health outcomes.17 The food groups were classified into beneficial (20), adverse (13), and neutral (13) on the basis of their presumed prior known association with CVD.18, 19 There was general, though not perfect, agreement of ratings of food groups done independently by 4 experts in the field. These ratings used only prior knowledge of the literature. The beneficially rated food group includes fruit, avocado, beans/legumes, green vegetables, yellow vegetables, tomatoes, other vegetables, nuts and seeds, soy products, whole grains, vegetable oil, fatty fish, lean fish, poultry, alcohol (beer, wine, and liquor), coffee, tea, and low‐fat milk/cheese/yogurt. In practice, the amount of alcohol consumed was rarely more than a moderate level. The adversely rated food group includes fried potatoes, grain dessert, salty snacks, pastries, sweets, high‐fat red meats, processed meats, organ meats, fried fish/poultry, sauces, soft drink, whole‐fat milk/cheese/yogurt, and butter. The neutrally rated food group includes potatoes, refined grains, margarine, chocolate, meal replacements, pickled foods, sugar substitutes, lean meats, shellfish, eggs, soups, diet drinks, and fruit juices. Each of the 46 food groups was divided into quintiles of consumption and then scores of 0 (quintile 1) to 4 (quintile 5) were assigned to the beneficially rated food groups, while scores of 4 (quintile 1) to 0 (quintile 5) were assigned to the adversely rated food groups. Zero points were assigned to the neutrally rated food groups. For the foods with many 0 servings/day, participants were divided into 5 groups based on distribution of 0 and quartile (among consumers). Change in diet of individuals over time were tracked on the basis of specific cut points for each food group that had been derived from year 0 CARDIA data that were applied to the follow‐up diet data at year 7 and year 20. Forty‐six component scores were summed to form the total APDQS score, ranging from 0 (minimum) to 132 (maximum). A previous analysis in CARDIA showed that participants with the greatest improvement in the APDQS over time largely increased consumption of beneficially rated plant foods while reducing consumption of adversely rated meat products.10
Outcome Ascertainment
Primary outcomes were incident CVD. Cases were identified through August 31, 2018. CVD encompassed myocardial infarction, non–myocardial infarction acute coronary syndrome, stroke, heart failure, carotid or peripheral artery disease, atherosclerotic coronary heart disease, other atherosclerotic disease, and nonatherosclerotic cardiac disease. Coronary heart disease (CHD) and hypertension‐related CVD were also examined as secondary outcomes. Occurrence of death was determined by CARDIA staff on the basis of biannual contact with participants or family members and record linkage to the National Death Index. Following each death, the death certificate, autopsy, and hospital records were requested with next‐of‐kin consent. A panel of 2 physicians reviewed all collected information and determined cause of death by consensus.
Assessment of Covariates
Updated information regarding demographics, maximal educational attainment, smoking status, medication use, and parental history of CVD was self‐reported on standardized questionnaires. Physical activity levels were estimated on the basis of a CARDIA physical activity history questionnaire administered by the interviewer. Physical activity was measured in exercise units as a product of intensity and frequency based on 13 different physical activities performed over the previous year.20 Body mass index (BMI) was calculated as weight/height squared (kg/m2) on the basis of measurements by trained technicians. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL, a 2‐hour postchallenge glucose concentration ≥200 mg/dL, glycated hemoglobin ≥6.5%, or taking antidiabetic medication. Hypertension was defined as systolic or diastolic blood pressure of ≥130 or ≥80 mm Hg or taking antihypertensive medications. Dyslipidemia was defined as serum triglycerides ≥150 mg/dL or high‐density lipoprotein cholesterol <40 for men and <50 mg/dL for women.
Statistical Analysis
Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% CIs for CVD associated with the APDQS. Person‐years were calculated from the date of baseline examination to the date of initial diagnosis of CVD, death, or the end of follow‐up (August 31, 2018), whichever occurred first. To account for potential changes in diet over time, the APDQS was modeled as a time‐varying exposure: (1) the year 0, year 7, and year 20 values were cumulatively averaged over follow‐up.21 Specifically, year 0 predicted events over follow‐up from year 0 to year 7, the average of year 0 and year 7 predicted events over follow‐up from after year 7 to year 20, and the average of year 0, year 7, and year 20 predicted events over follow‐up from after year 20 to year 32. Note that if both year 7 and year 20 diet were missing, the model used year 0 data with outcomes over all follow‐up. This method carries forward part or all of the previously observed values and has the advantage of updating time trend and retaining participants throughout follow‐up (Data S1); (2) the year 7 value was subtracted from the year 20 value to calculate the 13‐year change in APDQS, and it was evaluated with the outcomes occurring after year 20. Analyses of the time‐varying average APDQS evaluated the long‐term impact of diet throughout adulthood, and analyses of the APDQS change evaluated the dynamic relationship between an individual’s continuing dietary change during the transition period from young to middle adulthood and risk of later disease during midlife. We estimated the risk of CVD using quintile variables as well as the per 1‐SD (13 points) increment in the APDQS as continuous variables.
Initial analyses of the time‐varying average APDQS were adjusted for year 0 age, sex, race (White or Black), total energy intake, and maximal educational attainment (model 1), and were then further adjusted for parental CVD history (yes versus no), year 0 smoking status (never, former, and current), and physical activity (model 2). Total energy intake and physical activity were treated as time‐varying covariates. Hypertension, diabetes mellitus, dyslipidemia, and BMI as time‐varying variables were further adjusted for in the model (model 3) to examine whether the association between APDQS and CVD was potentially mediated by these comorbidities. The mediation effect of these clinical variables (cumulative data through year 7) on the association between year 0 APDQS and CVD was quantified by comparing the models with and without the mediating variables using the formula: 1 − (βmediator model/βbase model) × 100.22 Next, we examined the association between the 13‐year change in APDQS and subsequent 12‐year risk of CVD outcomes, adjusting for the same covariates as in the time‐varying average diet analysis and, additionally, adjustments for the year 7 APDQS and the 13‐year change in total energy and physical activity. CHD and hypertension‐related CVD were also examined with the APDQS as time‐varying or change in secondary analyses.
We computed restricted cubic splines with 4 knots to visually assess the shape of association between ADPQS as a continuous variable (both time‐varying average and 13‐year change) and risk of CVD.23, 24 Statistical significance of nonlinearity (ie, curvature) was tested by comparing the spline model with the linear model, and P values of <0.05 were regarded as statistically significant nonlinear relationship between the exposure and the outcome. Statistical significance of linearity was tested by comparing the linear model to the model including only the covariates, both using likelihood ratio tests.
Sensitivity analyses assessed the robustness of the primary findings. First, 5‐ and 8‐year lagged analyses were performed to minimize the potential impact of reverse causality attributable to preexisting disease with individual diet variables at year 0, year 7, and year 20, separately. Second, whether the association differed by the past diagnoses of hypertension, diabetes mellitus, and dyslipidemia was evaluated by testing multiplicative terms of each past diagnosis stratum (yes versus no) and the APDQS (continuous) added in model 2 using the Wald test. Also, stratified analyses according to race (White or Black), sex (male or female), education (tertiles split), physical activity (median split), and smoking (current or noncurrent smoker) were performed and the significance of the interaction were evaluated in the same way.
All analyses were performed using SAS version 9.4 software (SAS Institute Inc., Cary, NC). The statistical tests were 2‐tailed, with P<0.05 considered to be statistically significant.
RESULTS
Participants Characteristics
We documented 289 incident CVD cases during the 32‐year follow‐up. The mean cumulative APDQS was 65.1±11.7 (range, 31–101), and the mean 13‐year change in APDQS was 3.6±10.5 (range, −36 to 51). Participants with higher APDQS tended to be older, female, more educated, more physically active, consumed more alcohol, and consumed less energy as compared with participants with lower APDQS (Table 1). Additionally, participants with higher APDQS were less likely to self‐identify their race as Black, smoke cigarettes, have a lower BMI, and have a history of dyslipidemia. The mean intake of each of the 46 food groups by quintiles of the year 0 APDQS is presented in Table 2. To further specify the scoring system, the cut points for each of the 46 food groups and examples of the high and low scores of the APDQS are shown in Table S1. One participant with a score of 47 versus 1 participant with a score of 81 were arbitrarily selected as examples of diets at the median scores of the 2 extreme quintiles of the year 0 data. While consuming more beneficially rated plant foods and animal foods was the main driver in increasing the diet quality score (12‐point difference), consuming less adversely rated plant foods and high‐fat red and processed meats was the driver of the score (22‐point difference).
Table 1.
Baseline Characteristics (Year 0) of the Participants According to Quintiles of the Year 0 APDQS* (n=4946)
| Characteristics | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P Value† |
|---|---|---|---|---|---|---|
| n=1026 | n=999 | n=984 | n=991 | n=946 | ||
| Median of Year 0 APDQS (range) | 47 (24–51) | 55 (52–58) | 62 (59–65) | 70 (66–74) | 81 (75–107) | |
| Age, y | 23.1±3.7 | 24.4±3.7 | 25.1±3.6 | 25.7±3.3 | 26.4±2.9 | <0.001 |
| Female, N (%) | 512 (49.9) | 527 (52.8) | 526 (53.5) | 526 (53.1) | 623 (65.9) | <0.001 |
| Black race, N (%) | 812 (79.1) | 677 (67.8) | 522 (53.1) | 365 (36.8) | 133 (14.1) | <0.001 |
| Maximal educational attainment (through year 30), grades | 14.2±2.3 | 14.5±2.5 | 15.1±2.6 | 15.9±2.6 | 16.9±2.3 | <0.001 |
| Physical activity, EU‡ | 357±278 | 372±296 | 407±289 | 425±275 | 519±308 | <0.001 |
| Current smoker, N (%) | 339 (33.0) | 341 (34.1) | 319 (32.4) | 275 (27.8) | 196 (20.7) | <0.001 |
| Alcohol intake, drinks/d | 0.4±1.0 | 0.8±1.5 | 0.9±1.8 | 1.0±1.4 | 1.0±1.1 | <0.001 |
| Energy intake, kcal/d | 3157±1382 | 2869±1388 | 2882±1463 | 2692±1245 | 2453±1033 | <0.001 |
| Parental history of CVD | 395 (38.5) | 398 (39.8) | 419 (42.6) | 366 (36.9) | 382 (40.4) | 0.12 |
| Prevalent disease | ||||||
| Diabetes mellitus, N (%) | 8 (0.8) | 5 (0.5) | 9 (0.9) | 7 (0.7) | 4 (0.4) | 0.65 |
| Hypertension, N (%) | 40 (3.9) | 51 (5.1) | 41 (4.2) | 33 (3.3) | 28 (3.0) | 0.13 |
| Dyslipidemia, N (%) | 299 (29.3) | 286 (28.7) | 287 (29.4) | 274 (27.7) | 191 (20.3) | <0.001 |
| BMI, kg/m2 | 24.5±5.5 | 25±5.5 | 25.1±5.4 | 24.5±4.7 | 23.5±3.7 | <0.001 |
APDQS indicates A Priori Diet Quality Score; BMI, body mass index; and CVD, cardiovascular disease.
Values are reported as the mean±SD, unless noted as No. (percentage).
Total score sums the 46 components (possible scores 0–132, with a range of 35–95 in these data), with higher scores representing a nutritionally rich, plant‐centered diet. A 1‐point increment represents a one‐category shift in the presumed favorable direction.
Evaluated with chi‐square tests for categorical variables and ANOVA for continuous variables.
Exercise units, physical activity score derived from the CARDIA physical activity history.
Table 2.
Mean Intake of 46 Individual Food Groups According to Quintiles of the APDQS at Year 0 (n=4946)
| Food Group | Mean Intake±SD in Serving/Day | ||||
|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
| Beneficially rated | |||||
| 1. Fruit | 0.96±1.38 | 1.02±1.23 | 1.27±1.45 | 1.56±1.55 | 2.05±1.64 |
| 2. Avocado | 0.01±0.09 | 0.02±0.09 | 0.05±0.22 | 0.11±0.30 | 0.24±0.47 |
| 3. Beans and legumes | 0.17±0.35 | 0.21±0.44 | 0.21±0.38 | 0.19±0.32 | 0.24±0.4 |
| 4. Green vegetables | 0.12±0.24 | 0.20±0.34 | 0.27±0.38 | 0.42±0.61 | 0.90±1.25 |
| 5. Yellow vegetables | 0.07±0.21 | 0.12±0.22 | 0.2±0.65 | 0.28±0.52 | 0.60±0.97 |
| 6. Tomato | 0.32±0.30 | 0.38±0.41 | 0.44±0.47 | 0.52±0.46 | 0.73±0.65 |
| 7. Other vegetables | 1.42±1.17 | 1.64±1.37 | 2.01±1.58 | 2.28±1.64 | 2.89±2.16 |
| 8. Nuts and seeds | 0.39±0.84 | 0.53±1.04 | 0.79±1.47 | 0.82±1.44 | 1.12±1.65 |
| 9. Soy products | 0.09±0.44 | 0.18±0.65 | 0.21±0.70 | 0.23±0.65 | 0.47±1.06 |
| 10. Whole grains | 1.01±1.34 | 1.20±1.37 | 1.46±1.45 | 1.67±1.53 | 2.14±1.77 |
| 11. Vegetable oil | 0.87±1.26 | 1.26±1.56 | 1.47±1.72 | 1.62±1.71 | 2.04±2.06 |
| 12. Fatty fish | 0.01±0.07 | 0.02±0.09 | 0.03±0.27 | 0.03±0.13 | 0.08±0.25 |
| 13. Lean fish | 0.44±0.90 | 0.52±0.79 | 0.64±1.02 | 0.84±1.32 | 1.03±1.49 |
| 14. Poultry | 1.07±1.14 | 1.06±1.06 | 1.17±1.36 | 1.33±1.77 | 1.42±1.71 |
| 15. Beer | 0.29±0.85 | 0.53±1.24 | 0.54±1.24 | 0.56±1.06 | 0.48±0.71 |
| 16. Wine | 0.04±0.23 | 0.08±0.30 | 0.14±0.63 | 0.2±0.39 | 0.31±0.49 |
| 17. Liquor | 0.10±0.52 | 0.16±0.55 | 0.24±0.63 | 0.26±0.56 | 0.18±0.45 |
| 18. Coffee | 0.46±1.63 | 0.79±2.13 | 1.16±2.44 | 1.43±2.31 | 1.85±2.71 |
| 19. Tea | 0.32±1.27 | 0.62±5.98 | 0.57±1.46 | 0.72±2.76 | 0.85±2.15 |
| 20. Low‐fat milk/Cheese/Yogurt | 0.55±1.06 | 0.89±1.48 | 1.18±1.96 | 1.46±1.85 | 1.65±1.52 |
| Neutrally rated | |||||
| 1. Potatoes | 0.38±0.53 | 0.42±0.59 | 0.46±0.76 | 0.41±0.58 | 0.34±0.40 |
| 2. Refined grains | 5.62±3.28 | 4.60±3.12 | 4.32±3.03 | 3.8±2.70 | 3.15±2.22 |
| 3. Margarine | 1.67±2.48 | 1.71±2.27 | 1.77±2.19 | 1.74±2.41 | 1.39±2.08 |
| 4. Chocolate | 0.22±0.37 | 0.20±0.41 | 0.20±0.44 | 0.17±0.45 | 0.12±0.23 |
| 5. Meal replacements | 0.01±0.13 | 0.01±0.11 | 0.01±0.11 | 0.02±0.28 | 0.01±0.07 |
| 6. Pickled foods | 0.29±0.71 | 0.29±0.73 | 0.34±0.55 | 0.39±0.70 | 0.40±0.63 |
| 7. Sugar substitutes | 0.01±0.10 | 0.04±0.44 | 0.05±0.27 | 0.08±0.33 | 0.13±0.43 |
| 8. Lean red meats | 0.82±1.10 | 0.83±1.02 | 0.92±1.28 | 0.74±0.99 | 0.48±0.75 |
| 9. Shellfish | 0.14±0.38 | 0.17±0.33 | 0.21±0.42 | 0.31±0.94 | 0.27±0.42 |
| 10. Eggs | 0.78±0.80 | 0.72±0.81 | 0.66±0.75 | 0.57±0.58 | 0.49±0.51 |
| 11. Soups | 0.02±0.06 | 0.03±0.07 | 0.04±0.09 | 0.05±0.11 | 0.04±0.07 |
| 12. Diet soft drinks | 0.11±0.59 | 0.25±0.92 | 0.39±1.08 | 0.55±1.38 | 0.68±1.44 |
| 13. Fruit juice | 1.83±2.36 | 1.81±2.58 | 1.88±2.3 | 1.93±2.20 | 1.85±2.27 |
| Adversely rated | |||||
| 1. Fried potatoes | 0.53±0.57 | 0.38±0.46 | 0.35±0.51 | 0.28±0.45 | 0.15±0.24 |
| 2. Grain desserts | 0.97±1.28 | 0.65±0.78 | 0.67±0.80 | 0.54±0.64 | 0.46±0.65 |
| 3. Salty snacks | 0.03±0.11 | 0.04±0.22 | 0.03±0.12 | 0.03±0.23 | 0.04±0.18 |
| 4. Pastries | 1.23±1.18 | 1.02±1.12 | 0.94±1.13 | 0.79±0.92 | 0.64±0.72 |
| 5. Sweets | 2.00±2.46 | 2.03±2.56 | 1.87±2.44 | 1.48±2.04 | 0.96±1.36 |
| 6. High‐fat red meats | 2.85±2.11 | 2.59±2.32 | 2.41±2.11 | 2.09±2.53 | 1.16±1.60 |
| 7. Processed meats | 1.23±1.26 | 1.02±1.12 | 0.88±1.03 | 0.67±0.93 | 0.33±0.63 |
| 8. Organ meats | 0.06±0.19 | 0.05±0.16 | 0.05±0.18 | 0.04±0.14 | 0.02±0.07 |
| 9. Fried poultry and fish | 0.15±0.84 | 0.12±0.75 | 0.11±0.68 | 0.09±0.61 | 0.07±0.55 |
| 10. Sauces | 4.62±4.18 | 4.31±5.51 | 4.67±6.39 | 4.39±5.97 | 4.72±9.27 |
| 11. Soft drinks | 2.68±2.59 | 1.95±2.25 | 1.40±1.76 | 0.98±1.43 | 0.43±0.74 |
| 12. Whole‐fat milk/Cheese/Yogurt | 2.51±2.37 | 2.01±1.87 | 2.02±2.17 | 1.74±2.50 | 1.36±1.34 |
| 13. Butter | 6.02±4.66 | 4.60±4.03 | 4.44±4.33 | 3.90±3.600 | 3.12±3.07 |
Association of Plant‐Centered Diet Quality and Risk of Incident CVD outcomes
The time‐varying average APDQS was inversely associated with incident CVD (HR for quintile 5 versus quintile 1=0.39; 95% CI, 0.23–0.64; model 1 in Table 3). Further adjustment for other covariates in model 2 did not appreciably alter the association (HR for quintile 5 versus quintile 1=0.48; 95% CI, 0.28–0.81). The association was slightly attenuated after additional adjustment for time‐varying hypertension, diabetes mellitus, dyslipidemia, and BMI (HR for quintile 5 versus quintile 1=0.54; 95% CI, 0.32–0.93; model 3). In mediation effect analyses, dyslipidemia explained some of the association between the year 0 APDQS and risk of CVD (15.8% explained; 95% CI, 5.6%–37.1%; P<0.001), and hypertension, diabetes mellitus, and BMI did not explain this association (<5% explained and P>0.05 for all). Evaluation of specific CVD events showed that the time‐varying average APDQS was strongly associated with both CHD (HR for quintile 5 versus quintile 1=0.48; 95% CI, 0.24–0.97) and hypertension‐related CVD (HR for quintile 5 versus quintile 1=0.48; 95% CI, 0.24–0.94).
Table 3.
HR (95% CI) of Incident CVD Outcomes (Year 0–Year 32) According to Quintiles of the Time‐Varying Average APDQS*
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Each 1‐SD increment (13‐point) | P for Trend‡ | |
|---|---|---|---|---|---|---|---|
| CVD | |||||||
| APDQS (median)† | 50.0 | 58.0 | 64.3 | 71.5 | 81.0 | ||
| Unadjusted cumulative incidence % (n/N)† | 9.0 (89/989) | 7.8 (77/989) | 5.3 (51/961) | 4.8 (49/1015) | 2.3 (23/992) | ||
| Model 1§ | 1 (ref) | 0.86 (0.62–1.17) | 0.68 (0.48–0.96) | 0.69 (0.47–1.01) | 0.39 (0.23–0.64) | 0.74 (0.63–0.87) | <0.001 |
| Model 2|| | 1 (ref) | 0.87 (0.63–1.19) | 0.73 (0.52–1.04) | 0.78 (0.53–1.14) | 0.48 (0.28–0.81) | 0.80 (0.67–0.95) | 0.010 |
| Model 3# | 1 (ref) | 0.83 (0.60–1.15) | 0.72 (0.51–1.03) | 0.79 (0.53–1.16) | 0.54 (0.32–0.93) | 0.81 (0.68–0.96) | 0.018 |
| CHD | |||||||
| APDQS (median)† | 50.0 | 58.0 | 64.3 | 71.5 | 81.0 | ||
| Unadjusted cumulative incidence % (n/N)† | 3.7 (37/989) | 3.6 (35/985) | 3.2 (31/964) | 2.2 (22/1016) | 1.3 (13/992) | ||
| Model 1§ | 1 (ref) | 0.82 (0.51–1.33) | 0.82 (0.50–1.34) | 0.54 (0.30–0.96) | 0.38 (0.19–0.75) | 0.72 (0.57–0.91) | 0.006 |
| Model 2|| | 1 (ref) | 0.84 (0.52–1.36) | 0.91 (0.55–1.48) | 0.63 (0.35–1.12) | 0.48 (0.24–0.97) | 0.78 (0.61–1.00) | 0.051 |
| Model 3# | 1 (ref) | 0.89 (0.54–1.45) | 0.97 (0.59–1.61) | 0.69 (0.38–1.25) | 0.61 (0.30–1.25) | 0.83 (0.65–1.07) | 0.15 |
| Hypertensive‐related CVD | |||||||
| APDQS (median)† | 50.0 | 58.0 | 64.3 | 71.5 | 81.0 | ||
| Unadjusted cumulative incidence % (n/N)† | 7.1 (70/989) | 5.4 (53/986) | 3.4 (33/964) | 3.0 (30/1014) | 1.3 (13/993) | ||
| Model 1§ | 1 (ref) | 0.79 (0.55–1.15) | 0.61 (0.40–0.93) | 0.70 (0.44–1.11) | 0.39 (0.20–0.76) | 0.75 (0.61–0.92) | 0.006 |
| Model 2|| | 1 (ref) | 0.80 (0.55–1.16) | 0.65 (0.43–0.99) | 0.78 (0.49–1.23) | 0.48 (0.24–0.94) | 0.80 (0.65–0.99) | 0.040 |
| Model 3# | 1 (ref) | 0.74 (0.51–1.07) | 0.60 (0.39–0.92) | 0.73 (0.46–1.16) | 0.50 (0.25–0.99) | 0.77 (0.62–0.96) | 0.022 |
APDQS indicates A Priori Diet Quality Score; CHD, coronary heart disease; CVD, cardiovascular disease; and HR, hazard ratio.
Time‐varying variables that were cumulatively averaged over follow‐up at year 0, year 7, and year 20. Specifically, year 0 predicted events over follow‐up from year 0 to year 7, the average of year 0 and year 7 predicted events over follow‐up from after year 7 to year 20, and the average of year 0, year 7, and year 20 predicted events over follow‐up from after year 20 to year 32. Note that if both year 7 and year 20 diet was missing, the model used year 0 data with outcomes over all follow‐up.
Median APDQS, number of cases, and numbers at risk are categories according to quintiles of the average of all available APDQS measurements.
Statistical significance was estimated by modeling APDQS as a continuous variable in the model.
Model 1: year 0 age, sex, race (White or Black), total energy intake (time‐varying average), and maximal educational attainment.
Model 2: model 1+parental history of CVD (yes vs no), year 0 smoking status (never, former, and current), and physical activity level (time‐varying average).
Model 3: model 2+time‐varying comorbidities (hypertension, diabetes mellitus, dyslipidemia [all yes vs no], and body mass index [continuous]).
An increase in the APDQS over 13 years was associated with a lower risk of CVD in the subsequent 12 years in change analyses (HR for quintile 5 versus quintile 1=0.33; 95% CI, 0.16–0.68; model 1 in Table 4). This association was slightly attenuated in model 2; the highest quintile of 13‐year change in APDQS was associated with a 61% (95% CI, 0.19–0.81) lower subsequent 12‐year risk of CVD as compared with the lowest quintile. Strong inverse associations were observed for CHD (HR for quintile 5 versus quintile 1=0.21; 95% CI, 0.06–0.75; model 2) and hypertension‐related CVD (HR for quintile 5 versus quintile 1=0.34; 95% CI, 0.16–0.74; model 2). These results were preserved after further adjustment for comorbidity variables. A monotonic decrease in CVD risk with time‐varying average APDQS (P‐nonlinearity=0.12 and P‐linearity<0.001; Figure A) and the 13‐year change in APDQS (P‐nonlinearity=0.54 and P‐linearity=0.04; Figure B) was observed in restricted cubic splines. Associations between either the time‐varying average APDQS or the 13‐year change in APDQS with CVD did not differ by race, sex, education, physical activity, or smoking (P‐interaction >0.05 for each).
Table 4.
HR (95% CI) of Incident CVD Outcomes (Y20‐Y32) According to Quintiles of the 13‐year Change in APDQS*
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Each 1‐SD increment (13‐point) | P for Trend† | |
|---|---|---|---|---|---|---|---|
| CVD | |||||||
| 13‐year change in APDQS (median) | −9 | −1 | 4 | 9 | 17 | ||
| Unadjusted cumulative incidence % (n/N) | 5.6 (31/559) | 4.2 (22/522) | 2.5 (13/522) | 4 (21/528) | 2.2 (11/490) | ||
| Model 1‡ | 1 (ref) | 0.74 (0.43–1.3) | 0.42 (0.22–0.81) | 0.63 (0.35–1.12) | 0.33 (0.16–0.68) | 0.69 (0.52–0.90) | 0.007 |
| Model 2§ | 1 (ref) | 0.81 (0.46–1.43) | 0.48 (0.25–0.93) | 0.74 (0.41–1.33) | 0.39 (0.19–0.81) | 0.75 (0.57–1.00) | 0.048 |
| Model 3|| | 1 (ref) | 0.86 (0.49–1.5) | 0.47 (0.24–0.92) | 0.75 (0.41–1.35) | 0.36 (0.17–0.75) | 0.73 (0.56–0.97) | 0.028 |
| CHD | |||||||
| 13‐year change in APDQS (median) | −9 | −1 | 4 | 9 | 17 | ||
| Unadjusted cumulative incidence % (n/N) | 2.9 (16/559) | 2.1 (11/522) | 1.3 (7/522) | 2.1 (11/528) | 0.6 (3/490) | ||
| Model 1‡ | 1 (ref) | 0.71 (0.32–1.54) | 0.43 (0.17–1.06) | 0.66 (0.30–1.47) | 0.18 (0.05–0.64) | 0.64 (0.43–0.95) | 0.027 |
| Model 2§ | 1 (ref) | 0.79 (0.36–1.74) | 0.50 (0.20–1.24) | 0.75 (0.33–1.69) | 0.21 (0.06–0.75) | 0.70 (0.47–1.05) | 0.084 |
| Model 3|| | 1 (ref) | 0.83 (0.37–1.82) | 0.53 (0.21–1.32) | 0.77 (0.34–1.75) | 0.20 (0.06–0.72) | 0.69 (0.46–1.04) | 0.076 |
| Hypertensive‐related CVD | |||||||
| 13‐year change in APDQS (median) | −9 | −1 | 4 | 9 | 17 | ||
| Unadjusted cumulative incidence % (n/N) | 5.0 (28/557) | 2.9 (15/521) | 1.3 (7/522) | 2.5 (13/527) | 2.0 (10/490) | ||
| Model 1‡ | 1 (ref) | 0.54 (0.29–1.03) | 0.23 (0.10–0.54) | 0.38 (0.19–0.76) | 0.29 (0.14–0.64) | 0.58 (0.42–0.79) | <0.001 |
| Model 2+ | 1 (ref) | 0.58 (0.31–1.11) | 0.26 (0.11–0.61) | 0.44 (0.22–0.89) | 0.34 (0.16–0.74) | 0.63 (0.45–0.86) | 0.004 |
| Model 3|| | 1 (ref) | 0.60 (0.32–1.15) | 0.23 (0.10–0.54) | 0.44 (0.22–0.90) | 0.31 (0.14–0.68) | 0.61 (0.44–0.84) | 0.003 |
APDQS indicates A Priori Diet Quality Score; CHD, coronary heart disease; CVD, cardiovascular disease; and HR, hazard ratio.
The 13‐year change in APDQS ( year 20 value minus year 7 value) was used to predict events occurred between year 20 and year 32.
Statistical significance was estimated by modelling APDQS as a continuous variable in the model.
Model 1: year 7 APDQS, year 0 age, sex, race (White or Black), total energy intake (year 7 and 13‐year change), and maximal educational attainment.
Model 2: model 1+parental history of CVD (yes vs no), year 7 smoking status (never, former, and current), and physical activity level (year 7 and 13‐year change).
Model 3: model 2+hypertension (yes vs no), diabetes mellitus (yes vs no), dyslipidemia (yes vs no), and body mass index (continuous). The cumulative data through year 20 were used.
Figure 1. Restricted cubic spline curves for the association of incident CVD with (A) the time‐varying average APDQS (n=4946) and (B) the 13‐year change in APDQS (n=2621).
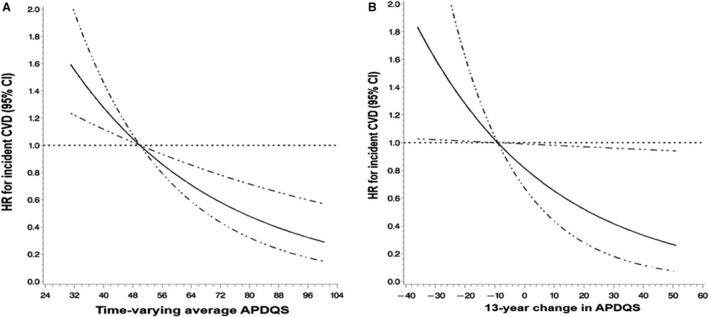
A, Time‐varying average APDQS and incident CVD. B, 13‐year change in APDQS and incident CVD. The solid line is the HR and the dashed line represents the 95% CI. HR and 95% CI were calculated using restricted cubic splines with 4 knots within proportional hazard regression models. Nonlinearity was tested by comparing the spline model with the linear model, and linearity was tested by comparing the linear model to the model including only the covariates, both using likelihood ratio tests. A, Model was adjusted for year 0 age, sex, race (White or Black), total energy intake (time‐varying average), maximal educational attainment, parental history of CVD (yes vs no), year 0 smoking status (never, former, and current), and physical activity level (time‐varying average). P‐nonlinearity=0.12 and P‐linearity<0.001. B, Model was adjusted for Y7 APDQS, Y0 age, sex, race (White or Black), total energy intake (year 7 and 13‐year change), and maximal educational attainment, parental history of CVD (yes vs no), year 7 smoking status (never, former, and current), and physical activity level (year 7 and 13‐year change). P‐nonlinearity=0.54 and P‐linearity=0.04. APDQS indicates A Priori Diet Quality Score; CVD, cardiovascular disease; and HR, hazard ratio.
Sensitivity Analyses
In exam year–specific and exam‐lagged analyses (5‐ and 8‐year lag), an inverse association between the APDQS as a fixed baseline variable and CVD risk was observed (Table S2). HR comparing the extreme quintiles was stronger for the year 0 APDQS, although the gradient of association across categories was more consistent for the year 20 APDQS. Neither the associations of the time‐varying average APDQS nor of the 13‐year change in APDQS with risk of CVD differed significantly by histories of hypertension, diabetes mellitus, or dyslipidemia (P‐interaction >0.05 for each).
Discussion
In this 32‐year prospective cohort study, which followed participants since young adulthood, long‐term consumption of a plant‐centered, high‐quality diet that also incorporates subsets of animal products was associated with a 52% lower risk of incident CVD. Furthermore, an increase in plant‐centered diet quality over 13 years was associated with a 61% lower risk of incident CVD in the subsequent 12 years.
There is increasing interest in understanding the association between diets that emphasize plant foods and limit most animal products and incident CVD outcomes, but the evidence is inconclusive. A previous meta‐analysis showed that vegetarians (versus nonvegetarians) had a lower risk of ischemic heart disease, but not incident CVD and all‐cause mortality.3 The noted limitations were narrow definitions of populations, uncertain accuracy of the assessment of vegetarian status, and inconsistent results across studies (the inverse associations were apparent in the US Adventist cohorts but not in non‐Adventist cohorts). In addition, some studies have defined vegetarian diets as non–meat eaters on the basis of food frequency intake or have self‐identified as vegetarians, but neither approach comprehensively captures an individual’s overall diet quality. Recently, the healthful PDI has been developed that focuses on healthy plant foods, limiting all animal‐derived foods. In a pooled analysis of 3 large cohorts, higher healthful PDI was associated with a 14% lower risk of CVD, comparing the highest with the lowest quintiles.4 Consistent with this, we found that the time‐varying APDQS was associated with a lower risk of incident CVD, CHD, and hypertension‐related CVD. In contrast, however, another study found no association with incident CVD.6 The PDI and the APDQS share some commonalities. Both diet quality indices assess the overall diet quality in holistic approaches, differentiating plant foods by their nutritional quality. On the other hand, there are some differences. All animal‐derived products are rated adversely in the PDI, while only high‐fat processed/unprocessed red meats, organ meats, and fried fish/poultry are rated adversely in the APDQS. Additionally, the PDI does not include alcoholic beverages in the index, while the APDQS does. An important finding of our study was to clarify whether eating nutritionally rich plant foods while integrating subsets of animal products into diet can improve future cardiovascular outcomes. The sensitivity analyses of previous studies further support the benefits of these flexible diet characteristics. The reduced estimate of CHD or total mortality remained similar when the modified healthful PDI was fitted as the main exposure, where fish, poultry, dairy products, or eggs were changed from their original adverse rating to a beneficial rating.7, 25 Furthermore, consumption of these types of animal products has not generally been associated with an increased risk of CVD outcomes and mortality.26, 27, 28, 29, 30, 31
Longitudinal analyses can provide unique insights as to whether late‐life disease risk can be altered by changing diet quality over time. Several long‐term prospective studies have demonstrated the relationship between change in diet quality (assessed by the Healthy Eating Index‐2015, the Alternative Healthy Eating Index‐2010, the Dietary Approaches to Stop Hypertension, or the Alternate Mediterranean Diet score) and subsequent risk of CVD and mortality, although results have varied.9, 32, 33 The timing and duration of exposure to risk factors may differentially affect the development of adult disease.34 Thus, evaluating diet exposure in middle or older adulthood may not completely explain the full spectrum of adult disease development. Our study adds to the current evidence on the association between diet quality and CVD risk by indicating that improved plant‐centered diet quality, starting in young adulthood, is associated with a lower subsequent risk of CVD by middle adulthood.
It is also worth noting that in the CARDIA sample, there was a notable difference in the distribution of race among the lowest versus the highest quintiles of the APDQS (Black race, 79% versus 14%) and also there was a difference in the maximal educational attainment (14.2 versus 16.9 grades completed). We also observed higher CVD cumulative incidence among Black participants (7.7% for Black versus 3.9% for White) and individuals with a lower educational level (8.6% for ≤13 grades versus 3.4% for ≥17 grades). Given the observed higher risk of CVD with lower APDQS values, the results of our study point out that the diet may help to explain disparities in CVD, although the relationships of the APDQS and incident CVD did not differ by race or education. Further studies are warranted to explore the association between a plant‐centered diet and risk of CVD events, considering these social parameters as a potential mediator of the relationship.
It is not fully understood how a plant‐centered diet has a protective effect against the development of CVD. As we previously described, the concerted action of nutrients and bioactive compounds found in a combination of plant foods may lead to a favorable cardiovascular outcome.10, 17 Numerous compounds, including ascorbic acid, tocopherols, carotenoids, and phenolics, are abundant in nuts and seeds, fruits, vegetables, and whole grains. These compounds can trap free radicals and reduce the levels of reactive oxygen molecules, thereby protecting against tissue damage.35 Moreover, these substances may help inhibit plaque formation in the arteries by reducing low‐density lipoprotein oxidation, platelet activation and aggregation, and inflammatory markers.36, 37, 38, 39 Experimental studies have also reported that a mixture of compounds found in plant source foods had a synergistic effect on enhancing antioxidant activity.40, 41 Although the mechanism remains to be established, our findings support a beneficial effect of a plant‐centered diet on CVD prevention at the general population level.
Study Strengths and Limitations
Because of the nature of the observational study design, we cannot rule out unmeasured or residual confounding. However, important potential confounding factors were adjusted for regarding the association between diet and CVD. The results of this study may have limited generalizability to other populations across different cultures, races/ethnicities, and periods of life.
Our study has several unique methodological features that were used to evaluate the quality of plant‐centered diets. The CARDIA diet history questionnaire measured comprehensively what specific foods were eaten in the recent past, with an open‐ended form. The diet of the individuals with high APDQS score is centered on eating nutritionally rich plant foods, but without excluding all animal products. Flexibility in dietary choice may help maintain long‐term stability in eating healthfully. The APDQS allows choice by providing a wide range of options in the way it is structured and by emphasizing variety (46 groups). The components of the APDQS were equally weighted with a maximum of 4 points, such that many food groups need to be part of the diet to achieve a higher score. This is distinct from other diet quality indices (eg, Healthy Eating Index‐2015 or Alternate Mediterranean Diet) that use a small number of food groups (≤13) within the scoring algorithm, allowing a person to earn many points from single foods and to avoid losing points for large consumption of less healthy foods. Other strengths of this study include the prospective design with the high retention rate during a long follow‐up, repeated measurements, and objectively measured clinical data. Furthermore, the change analysis allowed us to identify a clear temporality and reduce the possibility of within‐person confounding.
Conclusions
In summary, our study shows that long‐term consumption of a nutritionally rich plant‐centered diet is associated with a lower risk of CVD. Furthermore, increased plant‐centered diet quality since young adulthood is associated with a lower subsequent risk of CVD throughout middle age, independent of their earlier diet quality.
Sources of Funding
CARDIA is supported by contracts HHSN268201800003I, HHSN268201800004I, HHSN268201800005I, HHSN268201800006I, and HHSN268201800007I from the National Heart, Lung, and Blood Institute (NHLBI), Bethesda, Maryland. The sponsor, NHLBI has a representative on the Steering Committee of CARDIA and participated in study design, data collection, and scientific review of this paper. The sponsor had no role in data analysis, data interpretation, or writing of this report. Dr Choi is supported by Graduate and Professional Research Grant from the Healthy Food Healthy Lives Institute and from the MnDRIVE Global Food Ventures Professional Development Program, University of Minnesota, Minneapolis, Minnesota.
Disclosures
Dr Jacobs has been a paid consultant to the California Walnut Commission. Dr Gallaher is a paid member of the Nutrition Advisory Council for the California Prune Board. Dr Steffen received a grant ending February 2020 with Dairy Management about dairy products. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S2
Acknowledgments
Author contributions: Drs Choi and Jacobs conceived and designed the study. Dr Choi did the statistical analysis and drafted the manuscript. All authors contributed to data interpretation and critical review of the report. Drs Choi, Shikany, and Schreiner obtained funding for this study. Dr Jacobs supervised the study.
(J Am Heart Assoc. 2021;10:e020718. DOI: 10.1161/JAHA.120.020718.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020718
For Sources of Funding and Disclosures, see page 11.
References
- 1.Rana JS, Khan SS, Lloyd‐Jones DM, Sidney S. Changes in mortality in top 10 causes of death from 2011 to 2018. J Gen Intern Med. 2020;1–2. Jul 23 [Epub ahead of print]. DOI: 10.1007/s11606-020-06070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Risk Factor Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study. Lancet. 2018;392:1923–1994. DOI: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta‐analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57:3640–3649. DOI: 10.1080/10408398.2016.1138447. [DOI] [PubMed] [Google Scholar]
- 4.Shan Z, Li Y, Baden MY, Bhupathiraju SN, Wang DD, Sun QI, Rexrode KM, Rimm EB, Qi LU, Willett WC, et al. Association between healthy eating patterns and risk of cardiovascular disease. JAMA Intern Med. 2020;180:1090–1100. DOI: 10.1001/jamainternmed.2020.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H, Caulfield LE, Rebholz CM. Healthy plant‐based diets are associated with lower risk of all‐cause mortality in US adults. J Nutr. 2018;148:624–631. DOI: 10.1093/jn/nxy019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H, Caulfield LE, Garcia‐Larsen V, Steffen LM, Coresh J, Rebholz CM. Plant‐based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all‐cause mortality in a general population of middle‐aged adults. J Am Heart Assoc. 2019;8:e012865. DOI: 10.1161/JAHA.119.012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden MY, Liu G, Satija A, Li Y, Sun Q, Fung TT, Rimm EB, Willett WC, Hu FB, Bhupathiraju SN. Changes in plant‐based diet quality and total and cause‐specific mortality. Circulation. 2019;140:979–991. DOI: 10.1161/CIRCULATIONAHA.119.041014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S, Liu K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2014;130:10–17. DOI: 10.1161/CIRCULATIONAHA.113.005445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotos‐Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, Willett WC, Rimm EB, Hu FB. Changes in diet quality scores and risk of cardiovascular disease among US men and women. Circulation. 2015;132:2212–2219. DOI: 10.1161/CIRCULATIONAHA.115.017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi Y, Larson N, Gallaher DD, Odegaard AO, Rana JS, Shikany JM, Steffen LM, Jacobs DR. A shift toward a plant‐centered diet from young to middle adulthood and subsequent risk of type 2 diabetes and weight gain: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Diabetes Care. 2020;43:2796–2803. DOI: 10.2337/dc20-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu T, Jacobs DR, Larson NI, Cutler GJ, Laska MN, Neumark‐Sztainer D. Higher diet quality in adolescence and dietary improvements are related to less weight gain during the transition from adolescence to adulthood. J Pediatr. 2016;178:188–193. DOI: 10.1016/j.jpeds.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mursu J, Steffen LM, Meyer KA, Duprez D, Jacobs DR. Diet quality indexes and mortality in postmenopausal women: the Iowa Women’s Health Study. Am J Clin Nutr. 2013;98:444–453. DOI: 10.3945/ajcn.112.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs DR, Orlich MJ. Diet pattern and longevity: do simple rules suffice? A commentary. Am J Clin Nutr. 2014;100:313S–319S. DOI: 10.3945/ajcn.113.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. DOI: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 15.McDonald A, Van Horn L, Slattery M, Hilner J, Bragg C, Caan B, Jacobs D, Liu K, Hubert H, Gernhofer N, et al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc. 1991;91:1104–1112. [PubMed] [Google Scholar]
- 16.Liu K, Slattery M, Jacobs D, Cutter G, McDonald A, Van Horn L, Hilner JE, Caan B, Bragg C, Dyer A. A study of the reliability and comparative validity of the cardia dietary history. Ethn Dis. 1994;4:15–27. [PubMed] [Google Scholar]
- 17.Jacobs DR, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 2003;78:508S–513S. DOI: 10.1093/ajcn/78.3.508S. [DOI] [PubMed] [Google Scholar]
- 18.Sijtsma FP, Meyer KA, Steffen LM, Shikany JM, Van Horn L, Harnack L, Kromhout D, Jacobs DR. Longitudinal trends in diet and effects of sex, race, and education on dietary quality score change: the Coronary Artery Risk Development in Young Adults study. Am J Clin Nutr. 2012;95:580–586. DOI: 10.3945/ajcn.111.020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lockheart MS, Steffen LM, Rebnord HM, Fimreite RL, Ringstad J, Thelle DS, Pedersen JI, Jacobs DR. Dietary patterns, food groups and myocardial infarction: a case‐control study. Br J Nutr. 2007;98:380–387. DOI: 10.1017/S0007114507701654. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs DR, Hahn LP, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health program. J Cardiopulm Rehabil. 1989;9:448–459. DOI: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satija A, Bhupathiraju SN, Rimm EB, Spiegelman D, Chiuve SE, Borgi L, Willett WC, Manson JE, Sun Q, Hu FB. Plant‐based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13:e1002039. DOI: 10.1371/journal.pmed.1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hertzmark E, Pazaris M, Spiegelman D. The SAS mediate macro. 2018. https://www.hsph.harvard.edu/donna‐spiegelman/software/mediate/. Accessed April 15, 2021.
- 23.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. DOI: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 24.Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. The SAS lgtphcurv9 Macro. 2011. https://www.hsph.harvard.edu/donna‐spiegelman/software/lgtphcurv9/. Accessed October 15, 2020.
- 25.Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, Rexrode KM, Rimm EB, Hu FB. Healthful and unhealthful plant‐based diets and the risk of coronary heart disease in U.S. Adults. J Am Coll Cardiol. 2017;70:411–422. DOI: 10.1016/j.jacc.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong VW, Van Horn L, Greenland P, Carnethon MR, Ning H, Wilkins JT, Lloyd‐Jones DM, Allen NB. Associations of processed meat, unprocessed red meat, poultry, or fish intake with incident cardiovascular disease and all‐cause mortality. JAMA Intern Med. 2020;180:503–512. DOI: 10.1001/jamainternmed.2019.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K, Hyeon J, Lee SA, Kwon SO, Lee H, Keum N, Lee JK, Park SM. Role of total, red, processed, and white meat consumption in stroke incidence and mortality: a systematic review and meta‐analysis of prospective cohort studies. J Am Heart Assoc. 2017;6:e005983. DOI: 10.1161/JAHA.117.005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayedi A, Shab‐Bidar S, Eimeri S, Djafarian K. Fish consumption and risk of all‐cause and cardiovascular mortality: a dose‐response meta‐analysis of prospective observational studies. Public Health Nutr. 2018;21:1297–1306. DOI: 10.1017/S1368980017003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drouin‐Chartier JP, Chen S, Li Y, Schwab AL, Stampfer MJ, Sacks FM, Rosner B, Willett WC, Hu FB, Bhupathiraju SN. Egg consumption and risk of cardiovascular disease: three large prospective US cohort studies, systematic review, and updated meta‐analysis. BMJ. 2020;368:m513. DOI: 10.1136/bmj.m513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drouin‐Chartier JP, Brassard D, Tessier‐Grenier M, Côté JA, Labonté M, Desroches S, Couture P, Lamarche B. Systematic review of the association between dairy product consumption and risk of cardiovascular‐related clinical outcomes. Adv Nutr. 2016;7:1026–1040. DOI: 10.3945/an.115.011403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Key TJ, Appleby PN, Bradbury KE, Sweeting M, Wood A, Johansson I, Kühn T, Steur M, Weiderpass E, Wennberg M, et al. Consumption of meat, fish, dairy products, and eggs and risk of ischemic heart disease. Circulation. 2019;139:2835–2845. DOI: 10.1161/CIRCULATIONAHA.118.038813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sotos‐Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, Willett WC, Rimm EB, Hu FB. Association of changes in diet quality with total and cause‐specific mortality. N Engl J Med. 2017;377:143–153. DOI: 10.1056/NEJMoa1613502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z, Steffen LM, Selvin E, Rebholz CM. Diet quality, change in diet quality and risk of incident CVD and diabetes. Public Health Nutr. 2020;23:329–338. DOI: 10.1017/S136898001900212X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. 2005;26:1–35. DOI: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- 35.Packer L. Protective role of vitamin E in biological systems. Am J Clin Nutr. 1991;53(4 Suppl):1050S–1055S. DOI: 10.1093/ajcn/53.4.1050S. [DOI] [PubMed] [Google Scholar]
- 36.Santhakumar AB, Bulmer AC, Singh I. A review of the mechanisms and effectiveness of dietary polyphenols in reducing oxidative stress and thrombotic risk. J Hum Nutr Diet. 2014;27:1–21. DOI: 10.1111/jhn.12177. [DOI] [PubMed] [Google Scholar]
- 37.Freedman JE, Keaney JF. Vitamin E inhibition of platelet aggregation is independent of antioxidant activity. J Nutr. 2001;131:374S–377S. DOI: 10.1093/jn/131.2.374S. [DOI] [PubMed] [Google Scholar]
- 38.Schinella GR, Tournier HA, Prieto JM, Mordujovich de Buschiazzo P, Ríos JL. Antioxidant activity of anti‐inflammatory plant extracts. Life Sci. 2002;70:1023–1033. DOI: 10.1016/S0024-3205(01)01482-5. [DOI] [PubMed] [Google Scholar]
- 39.Kiokias S, Proestos C, Oreopoulou V. Effect of natural food antioxidants against LDL and DNA oxidative changes. Antioxidants (Basel). 2018;7:133. DOI: 10.3390/antiox7100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuhrman B, Volkova N, Rosenblat M, Aviram M. Lycopene synergistically inhibits LDL oxidation in combination with vitamin E, glabridin, rosmarinic acid, carnosic acid, or garlic. Antioxid Redox Signal. 2000;2:491–506. DOI: 10.1089/15230860050192279. [DOI] [PubMed] [Google Scholar]
- 41.Ninfali P, Mea G, Giorgini S, Rocchi M, Bacchiocca M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br J Nutr. 2005;93:257–266. DOI: 10.1079/BJN20041327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2


