Abstract
Background
Inflammation plays a pivotal role in coronary artery disease (CAD). The anti‐inflammatory drug colchicine seems to reduce ischemic events in patients with CAD. So far there is equipoise about its safety and impact on mortality.
Methods and Results
To evaluate the utility of colchicine in patients with acute and chronic CAD, we performed a systematic review and meta‐analysis. MEDLINE, EMBASE, Cochrane CENTRAL and conference abstracts were searched from January 1975 to October 2020. Randomized trials assessing colchicine compared with placebo/standard therapy in patients with CAD were included. Data were combined using random‐effects models. The reliability of the available data was tested using trial sequential analyses . Of 3108 citations, 13 randomized trials (n=13 125) were included. Colchicine versus placebo/standard therapy in patients with CAD reduced risk of myocardial infarction (odds ratio [OR] 0.64; 95% CI, 0.46–0.90; P=0.01; I 2 41%) and stroke/transient ischemic attack (OR 0.50; 95% CI, 0.31–0.81; P=0.005; I 2 0%). But treatment with colchicine compared with placebo/standard therapy had no influence on all‐cause and cardiovascular mortality (OR 0.96; 95% CI, 0.65–1.41; P=0.83; I 2 24%; and OR 0.82; 95% CI, 0.55–1.22; P=0.45; I 2 0%, respectively). Colchicine increased the risk for gastrointestinal side effects (P<0.001). According to trial sequential analyses, there is only sufficient evidence for a myocardial infarction risk reduction with colchicine.
Conclusions
Among patients with CAD, colchicine reduces the risk of myocardial infarction and stroke, but has a higher rate of gastrointestinal upset with no influence on all‐cause mortality.
Keywords: colchicine, coronary artery disease, inflammation, myocardial infarction, systematic review
Subject Categories: Acute Coronary Syndromes, Coronary Artery Disease, Meta Analysis
Nonstandard Abbreviations and Acronyms
- TSA
trial sequential analysis
Clinical Perspective
What Is New?
In this systematic review and meta‐analysis of >13 000 patients with acute and chronic coronary artery disease, we highlight that the adjunctive treatment with the anti‐inflammatory drug colchicine reduces the risk for ischemic events, namely, new myocardial infarction, stroke, and repeat revascularization procedures.
Whilst colchicine seems related to an increased risk of gastrointestinal side effects, there was no significant increased risk for infectious complications or mortality with this treatment.
What Are the Clinical Implications?
Colchicine represents a promising supplementary drug for secondary prevention of ischemic events among patients with acute and chronic coronary artery disease.
The reduced risk of potentially debilitating secondary coronary vascular or cerebrovascular events will need to be balanced against the side effect and interaction profile of colchicine.
Nonetheless, several questions regarding colchicine treatment in coronary artery disease patients remain uncertain and warrant more research, including patient selection, drug dosing, and therapy duration.
Inflammation plays a pivotal role in the development and progression of coronary artery disease (CAD).1 The main mechanisms for cardiovascular events in afflicted patients represent plaque activation and rupture.2 Experimental studies have demonstrated, that inflammatory cells release specific cytokines and enzymes, which ultimately promote plaque erosion and rupture.1 By specifically targeting inflammation in patients with CAD, it has been suggested that the risk for cardiovascular events can be reduced.3, 4
Colchicine is an ancient drug, which is traditionally used for treatment of various rheumatic disorders (eg, gout and Behçet's disease), but has also become a well‐established therapy for pericarditis.5 It primarily impedes tubulin polymerization and microtubule formation and thus inhibits the leukocytes´ migratory, exocytotic and phagocytotic function by suppressing the expression of selectins, which are upregulated in atherosclerosis, particularly following myocardial infarction (MI).5 Moreover, colchicine executes anti‐inflammatory effects via NLRP3 inflammasome inactivation and a decrease in release of interleukin (IL)‐1β, IL‐18, IL‐6, and C‐reactive protein.5, 6, 7
Over the past 3 decades, several observational and randomized studies evaluated the impact of colchicine on outcomes of patients with acute or chronic CAD and indicated potential benefits, including a reduction in ischemic events, including repeat revascularization, MI, and stroke/transient ischemic attack (TIA).8 Moreover, a series of recent meta‐analyses showed somewhat conflicting results, and some even suggested potential harm due to a higher risk of gastrointestinal‐related adverse events.9, 10, 11, 12, 13, 14, 15 However, some of them did not consider a series of large clinical trials, which have recently been published and certainly brought new perspectives to this field.16, 17, 18 In addition, the optimal treatment duration and dosing of colchicine have also been debated.14, 17 Therefore, we conducted a comprehensive systematic review and meta‐analysis, incorporating a trial sequential analysis, of all randomized trials assessing the efficacy of colchicine in patients with acute or chronic CAD.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files. We conducted this systematic review and meta‐analysis in agreement with the latest version of Cochrane Handbook for Systematic Reviews and Interventions and reported following the PRISMA statement for meta‐analysis in health care interventions.19, 20, 21 We followed an internal protocol for the reviewing process and data collection. There was no external funding in place to support this work. The authors are solely responsible for the design and execution of this systematic review and meta‐analysis, the drafting and editing of the paper, and its final content. Additionally, no individual or organization not listed as an author contributed under any circumstances to the drafting or editing of this manuscript or performance of any analyses presented therein. This meta‐analysis has been registered at the PROSPERO international database for registered systematic reviews in health and social care (ID CRD42021242792).
Study Selection
Only randomized clinical trials (RCT) were included in this meta‐analysis since data derived from observational studies and case series are more susceptible to bias and therefore have been excluded. We extensively searched for any RCT evaluating colchicine compared to placebo or standard therapy among patients with acute or chronic CAD.
Regarding the CAD definitions, (i) acute CAD comprised unstable angina presentation, non‐ST‐segment elevation myocardial infarction (NSTEMI) and ST‐segment elevation myocardial infarction (STEMI), and (ii) chronic CAD included for example patients presenting with stable angina equivalents, silent myocardial ischemia or history of myocardial revascularization (eg, recent non‐urgent percutaneous coronary intervention).
Two independent reviewers (T.K. and R.K.) reviewed all titles and abstracts for eligibility. Reviewers then assessed full text articles for inclusion. The reviewers selected then all full texted citations and abstracts (ie, unpublished) and screened for eligibility. Incongruences in assessment were resolved involving a third‐party opinion (M.B.). Unpublished citations would have also been considered to address negative publication bias. A flow chart describing study exclusion is presented in Figure 1.
Figure 1. PRISMA flow diagram detailing the article screening process.

Data Sources
Data was extracted for matching RCTs in MEDLINE/PUBMED, Cochrane CENTRAL, EMBASE and online trial registers (including https://clinicaltrials.gov) published any time since January 1, 1975. The search process was terminated by October 1, 2020. Of note, the search was repeated prior to submission in order to keep the data up to date. Any article published after that date was not included. Additionally, we manually searched the abstracts submitted to the American College of Cardiology (ACC), the American Heart Association (AHA), the European Society of Cardiology (ESC), and Transcatheter Therapeutics (TCT) up to October 10, 2020. In addition, we searched the clinicaltrials.gov registry for ongoing or recently finished trials. We reviewed the reference lists of original studies identified by the electronic search to ensure all pertinent studies had been considered. The applied search terms are listed in Data S1. To ensure data completeness, we contacted the included study’s corresponding author, if necessary.
Data Collection, Extraction, and Quality Assessment
The 2 reviewers (T.K. and R.K) extracted the data independently using the Covidence software package (Melbourne VIC, Australia). Any disagreements were resolved by consensus and residual uncertainty was clarified with the senior author (M.B.). The Kappa (κ) statistic, calculated to assess the degree of agreement between the 2 authors (κ=0.90), indicates a substantial agreement. The data were extracted independently and verified by the senior author (M.B.). Publication bias was assessed by visual analysis of funnel plots. The included trials were evaluated for risk of bias in 5 domains (sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, and incomplete outcome data) according to the risk of bias tool from the Cochrane collaboration.21, 20, 22, 23 The correlating table is shown in Table S1. The quality of the studies was evaluated using the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) tool for RCTs (Table S2).24
Outcomes
We obtained the outcomes for the longest available follow‐up. The following outcomes were evaluated as reported by the studies: all‐cause mortality, cardiovascular mortality, MI, and stroke/TIA. The details of the MI and stroke/TIA definitions are highlighted in the Table S3. Other outcomes, which were considered, included ischemia driven revascularization (eg, percutaneous coronary intervention or coronary artery bypass grafting for recurrent ischemic symptoms) and noncardiovascular mortality (if reported).
In order to discern the impact of colchicine on outcomes of patients with acute versus chronic CAD and the role of short (≤30 days) versus long‐term colchicine treatment (>30 days) and high‐ (>0.5 mg per day) versus low‐dose (≤0.5 mg per day), we performed dedicated sensitivity analyses involving the main outcomes. Further sensitivity analyses were conducted that pooled large trials separately from the smaller trials which contributed just very few events.
In order to establish the tolerability and safety, we reviewed and analyzed the rate of drug discontinuation and adverse outcomes/side effects (eg, gastrointestinal side effects, infections) among patients with CAD treated with colchicine compared to placebo/standard therapy.
Statistical Analysis
We analyzed the pooled data, number of events and number of patients in each subgroup from the included RCTs. Between‐study heterogeneity was determined using I 2.25 We preferred intention‐to‐treat analyzes, which involved all randomized probands. In order to account for the between‐study variation, we applied random‐effect models, using the Mantel‐Haenszel approach as implemented in Review Manager 5.3 (Rev Man, The Nordic Cochrane Centre, Copenhagen, Denmark) for dichotomous outcome variables.14 We reported the results as odds ratio (OR) and the corresponding 95% CI. To assess the robustness of the results, we performed separate sensitivity analyses for the main outcomes applying fixed‐effects models utilizing the Mantel‐Haenszel estimation method (Figure S2). Those analyses were conducted using Stata/SE version 16.1 (StataCorp, College Station, Texas, USA). A P value <0.05 was considered statistically significant.
Trial Sequential Analysis
Trial sequential analysis (TSA) represents a meta‐analysis technique, which can be applied to assess the accumulated evidence from previous trials in a sequential manner to evaluate if sufficient evidence is available to draw firm conclusions.26 Due to the small number of RCTs with limited number of patients, a meta‐analysis of this type may be susceptible to type I and II errors. By using TSA, monitoring boundaries are formed to establish whether the P value for a particular outcome is sufficient for the accrued evidence to indicate the anticipated effect once the boundary is crossed.26 In the event monitoring boundaries are not crossed, continued evaluation for evidence was recommended. The red dashed lines make up the trial sequential monitoring boundaries. The interpretation has similarities to DeMets’ stopping boundaries, which are used in clinical trials. We estimated the information size required to demonstrate or reject a priori anticipated intervention effect of a 25% relative risk reduction. With respect to the latest major trials assessing colchicine in patients with CAD, the value of 25% was chosen to represent a reasonable intervention effect for colchicine compared to placebo/standard therapy.8, 18 The heterogeneity‐adjusted required information size to demonstrate or reject a 25% relative risk reduction of the different end points is estimated with an alpha of 5%, and a beta of 20%. The trial sequential analyses were performed using the Copenhagen Trial Unit’s Centre for Clinical Intervention Research software package (version 0.9.5.10 Beta).27
Results
Overall, we identified 3108 citations, of which 184 were selected for full text review, as displayed in the flowchart in Figure 1. Finally, 13 RCTs comparing colchicine versus placebo/standard therapy in patients with acute or chronic CAD fulfilled the eligibility criteria and were considered for meta‐analysis.8, 16, 17, 18, 28, 29, 30, 31, 32, 33, 34, 35, 36 The inverted funnel plots for the main end points did not suggest any significant publication bias (Figure S1). Figure 2 encapsulates the main results.
Figure 2. The biological and clinical impact of colchicine among patients with acute and chronic coronary artery disease.

CRP indicates C‐reactive protein; CV, cardiovascular; GI, gastrointestinal; and IL, interleukin.
Included Studies
Characteristics of the trials included in the meta‐analysis are presented in Tables 1 and 2. The 13 included studies comprised 13 125 patients. The median follow‐up was 6 (interquartile range [IQR] 1; 15) months. Colchicine doses were single dose or <1 mg per day in 6 trials,8, 16, 18, 30, 34, 36 and most of the other studies used ≥1 mg per day.17, 28, 29, 31, 32, 33, 35 Of note, 12.3% of all patients enrolled in the colchicine group discontinued the treatment early. A summary of the quality of the RCTs can be found in Table S1. Ten RCTs were of high quality incorporating a double‐blind placebo‐controlled design, but 3 studies had some qualitative drawbacks, eg, open label designs.30, 34, 35
Table 1.
Summary of the Included Study Population
| Study | Study Year | Location, Sites | Randomized Patients (n) | Study Cohort | Study Design | Colchicine Dose | Comparator | Trial Outcomes | Intended Treatment Duration | FU Duration | Discontinuation Rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| O’Keefe et al.28 | 1992 | USA/single center | 197 | CCS patients undergoing PCI (POBA) | Double‐blind, placebo controlled RCT (Randomization in 2:1 fashion) | 0.6 mg BID | Placebo |
|
6 mo | 5.5 mo* | 10 (5.0) |
| COOL trial29 | 2011 | Canada/single center | 80 | ACS patients† | Double‐blind, placebo controlled RCT (Randomization in 1:1 fashion) | 1 mg OD | Placebo |
|
30 d | 31 ± 17 d | 6 (7.5) |
| LoDoCo trial30 | 2013 | Australia/single center | 532 | CCS patients | RCT, prospective, observer‐blinded endpoint (PROBE) trial | 0.5 mg OD | Standard therapy |
|
24 mo | (median) 36 mo | 62 (11.6)‡ |
| Deftereos et al.31 | 2013 | Greece/single center | 222 | Diabetic ACS and CCS patients undergoing PCI with BMS | Double‐blind, placebo controlled RCT (Randomization in 1:1 fashion) | 0.5 mg BID | Placebo |
|
6 mo | 6 mo | 26 (12.5) |
| Giannopoulos et al.32 | 2015 | Greece/single center | 60 | CCS patients undergoing elective CABG | Double‐blind, placebo controlled RCT (Randomization in 1:1 fashion) | 0.5 mg BID | Placebo |
|
10 d | 10 d | 7 (11.8) |
| Deftereos et al.33 | 2015 | Greece/multicenter | 151 | ACS (STEMI) patients | Double‐blind, placebo controlled RCT (Randomization in 1:1 fashion) | 2.0 mg LD – 0.5 mg BID | Placebo |
|
5 d | 5 d | 23 (15.2) |
| Zarpelon et al.34 | 2016 | Brazil/single center | 140 | CCS patients undergoing elective CABG | RCT, prospective, open‐label trial | 1.0 mg LD prior to CABG – 0.5 mg BID until discharge | Standard therapy |
|
Until hospital discharge | In‐hospital: 14.5 ± 11.5 d | NR |
| COLIN trial35 | 2017 | France/single center | 44 | ACS (STEMI) patients | RCT, prospective, open‐label trial | 1 mg OD | Standard therapy |
|
1 month | 1 month | 3 (13.0) |
| LoDoCo‐MI trial36 | 2019 | Australia/single center | 237 | ACS (acute MI) patients | Double‐blind, placebo controlled RCT (Randomization in 1:1 fashion) | 0.5 mg OD | Placebo |
|
30 d | 30 d | 6 (2.5) |
| COLCOT trial8 | 2019 | Multinational/Multicenter | 4745 | ACS patients (within 30 d after MI) | Randomized, double blind, placebo‐controlled trial (1:1 fashion) | 0.5 mg OD | Placebo |
|
24 mo | (median) 22.6 mo | 880 (18.5) |
| COLCHICINE‐PCI trial16 | 2020 | USA/single center | 400¶ | ACS and CCS patients referred for PCI | Randomized, double blind, placebo‐controlled trial (1:1 fashion) | 1.8 mg prior to procedure | Placebo |
|
30 d | 30 d | NA |
| COPS trial17 | 2020 | Australia/multicenter | 795 | ACS patients | Randomized, double blind, placebo‐controlled trial (1:1 fashion) | 1 month 0.5 mg BID; then 0.5 mg OD | Placebo |
|
12 mo | 400 d | 94 (11.8) |
| LoDoCo2 trial18 | 2020 | Multinational/multicenter | 5522 | CCS patients | Randomized, double blind, placebo‐controlled trial (1:1 fashion) | 0.5 mg OD | Placebo |
|
(minimum) 12 mo | (median) 28.6 mo | 580 (10.5)$ |
ACS indicates acute coronary syndrome; AUC, area under the curve; BID, twice a day; BMS, bare metal stent; CABG, coronary artery bypass grafting; CCS, chronic coronary syndrome; CK‐MB, creatine kinase‐myocardial brain fraction; CMR, cardiac magnetic resonance; CRP, C‐reactive protein; ; FU, follow‐up; LD, loading dose; MI, myocardial infarction; NA, not applicable; NR, not reported; OD, once daily; PCI, percutaneous coronary intervention; POBA, plain old balloon angioplasty; PROBE, prospective, randomized observer‐blinded endpoint trial; RCT, randomized controlled trial; and TIA, transient ischemic attack.
Trial acronyms: COOL Trial, Colchicine Compared With Placebo to Reduce hs‐CRP in Patients With Acute Coronary Syndromes or Strokes – Targeting Inflammation in Atherosclerosis Study; LoDoCo Trial, Low‐Dose Colchicine Trial; COLIN Trial, Interest of COLchicine in the Treatment of Patients With Acute Myocardial INfarction and With Inflammatory Response; COLCOT Trial, Colchicine Cardiovascular Outcomes Trial; COPS Trial, Colchicine in Patients With Acute Coronary Syndrome.
This represents the mean duration to follow‐up coronary angiogram.
The COOL trial expanded their eligibility criteria over the course of the trial and started also enrolling patients with acute ischemic stroke due to slower than expected recruitment rates. Overall, the trial included 73/80 patients with acute coronary syndrome and 7/80, who had a recent stroke.
32 and 30 patients ceased colchicine treatment early (within 4 weeks) and late (mean follow‐up period of 2.36 years), respectively.
This included out‐of‐hospital resuscitated cardiac arrest.
The COLCHICINE‐PCI trial actually randomized 714 patients, whereas only those 400 patients who underwent PCI had complete follow‐up and thus outcome assessment.
During the open‐label run‐in period involving 6528 patients, 437 (6.7%) stopped colchicine for gastrointestinal upset.
Table 2.
Baseline Characteristics of the Patients With Acute and Chronic Coronary Artery Disease Among the Included Studies
| Study | Patients, n (% Male) | Mean Age (Years) | Previous MI, n (%) | Previous PCI, n (%) | Previous CABG, n (%) | History of Stroke/TIA, n (%) | Hypertension,n (%) | Diabetes mellitus, n (%) | History of Smoking, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| O’Keefe et al.28 | 197 (85.8) | 60.5 | NR | NR | 51 (25.9) | NR | NR | 24 (12.2) | NR |
| COOL trial29 | 40 (88.7) | 57.2 | 14 (17.5) | NR | NR | 3 (3.7) | 34 (42.5) | 13 (16.2) | 63 (78.7) |
| LoDoCo trial30 | 532 (88.9) | 66 | 64 (23.4) | 307 (57.7) | 101 (18.9) | NR | NR | 161 (30.2) | 24 (4.5) |
| Deftereos et al.31 | 196 (65) | 63.7 | 28 (28) | NR | NR | NR | 95 (49) | 196 (100) | 74 (38) |
| Giannopoulos et al.32 | 59 (69.4) | 65.2 | 11 (18.6) | 13 (22.0) | NR | NR | 48 (81.3) | 25 (42.3) | 29 (49.1) |
| Deftereos et al.33 | 151 (68.8) | 58.0 | NR | NR | NR | NR | 60 (39.7) | 22 (21.2) | 79 (52.3) |
| Zarpelon et al.34 | 140 (67.8) | 60.9 | 31 (22.1) | 20 (14.2) | NR | NR | 124 (88.6) | 72 (51.4) | 57 (40.7) |
| COLIN trial35 | 44 (79.5) | 59.9 | NR | 2 (4.5) | 1 (2.3) | NR | 19 (43.1) | 6 (13.6) | 31 (70.4) |
| LoDoCo‐MI trial36 | 237 (77) | 61 | 36 (15) | NR | NR | NR | 112 (47) | 52 (22) | 143 (60) |
| COLCOT trial8 | 4745 (80.8) | 60.6 | 767 (16.2) | 798 (16.8) | 150 (3.2) | 122 (2.6) | 2421 (51.0) | 959 (20.2) | 1416 (29.8) |
| COLCHICINE PCI trial16 | 400 (93.5) | 66.3 | 103 (25.7) | 150 (37.5) | NR | 36 (9) | 367 (91.7) | 231 (57.7) | 282 (70.5) |
| COPS trial17 | 795 (79.0) | 59.8 | 118 (14.8) | 101 (12.7) | 34 (4.2) | 16 (2.0) | 400 (50.3) | 151 (18.9) | 277 (34.8) |
| LoDoCo2 trial18 | 5522 (84.7) | 66.0 | 4658 (84.3) | 4177 (75.6) | 710 (12.8) | NR | 2808 (50.8) | 1007 (18.2) | 648 (11.7) |
CABG indicates coronary artery bypass grafting; MI, myocardial infarction; NR, not reported; PCI, percutaneous coronary intervention; and TIA, transient ischemic attack.
Deftereos et al. reported baseline demographics only from those 196 patients, who completed follow‐up after 6 mo. However, they had actually randomized 222 patients.
Effects of Colchicine on All‐Cause, Cardiovascular Mortality, and Noncardiovascular Mortality
All‐cause mortality was reported in all 13 trials (n=13 098).8, 16, 17, 18, 28, 29, 30, 31, 32, 33, 34, 35, 36 Colchicine compared with placebo/standard therapy did not reduce the risk of death from any cause (OR, 0.96; 95% CI, 0.65–1.41; P=0.83; I 2 24%), as shown in Figure 3A. Cardiovascular mortality was reported in 9 studies (n=12 302).8, 17, 18, 29, 30, 31, 33, 35, 36 This outcome was also not affected by colchicine compared to placebo or standard therapy (OR, 0.82; 95% CI, 0.55–1.22; P=0.45; I 2 0%), as displayed in Figure 3B. Additionally, 4 studies reported noncardiovascular mortality.8, 17, 18, 30 Colchicine compared with placebo or standard therapy led to a numerically higher number of noncardiovascular deaths (85 [1.4%] versus 60 [1.0%] cases [OR, 1.35; 95% CI, 0.90–2.02; P=0.15; I 2 16%]), see Figure S2.
Figure 3. (A) All‐cause mortality, (B) cardiovascular mortality, (C) new myocardial infarction, and (D) stroke/transient ischemic attack with colchicine compared to placebo/standard therapy.

There were no differences across the performed subgroup analyses for all‐cause and cardiovascular mortality, including comparisons of lower‐ versus higher‐dose colchicine regimens, short‐ versus long‐term colchicine administration and colchicine compared with placebo/standard therapy in acute versus chronic CAD (Figure S3 and S4).
Effects of Colchicine on MI, Stroke/TIA, and Ischemia Driven Revascularization Rate
New or recurrent MI was reported in 7 trials (n=12 275).8, 16, 17, 18, 30, 35, 36 Compared with placebo/standard medical therapy, colchicine reduced the rate of MI, but there was moderate heterogeneity across the included studies (OR, 0.64; 95% CI, 0.46–0.90; P=0.01; I 2 41%) (Figure 3C).
Stroke/TIA incidence with colchicine in comparison to placebo/standard therapy was evaluated in 7 studies.8, 16, 17, 18, 29, 30, 31 In these trials, colchicine treatment led to a reduction of stroke/TIA rate (OR, 0.50; 95% CI, 0.31–0.81; P=0.005; I 2 0%) (Figure 3D).
Again, we found no significant interaction among the conducted sub‐group analyses assessing those 2 outcomes in patients receiving colchicine compared with placebo/standard therapy in acute versus chronic CAD, for ≤30 days versus >30 days, and at a lower‐ versus higher‐dose regimen (Figure S5 and S6).
Five studies also reported information about repeat revascularization procedures/ischemia driven revascularization (n=11 684).8, 16, 17, 18, 31 Administration of colchicine compared with placebo led to a lower risk of ischemia driven revascularization (OR, 0.61; 95% CI, 0.42–0.88; P=0.008; I 2 37%) (Figure S7).
Therapy Adherence and Adverse Effects With Colchicine
All of the included studies reported adverse effects.8, 16, 17, 18, 28, 29, 30, 31, 32, 33, 34, 35, 36 The rate of treatment discontinuation was higher among patients taking colchicine compared with placebo/standard therapy (14.3% versus 12.6%; OR, 1.68; 95% CI, 1.14–2.48; P<0.00001; I 2 76%), see Figure 4. The most commonly reported side effects during treatment with colchicine compared with placebo/standard therapy comprised gastrointestinal complaints, namely nausea and diarrhea (OR 2.21; 95% CI, 1.45–3.36; P=0.0002; I 2 78%) (Figure 5). Three studies provided data regarding relevant infections (eg, pneumonia), but a difference between the 2 treatment regimens could not be shown (OR 1.42; 95% CI, 0.81–2.47; P=0.22; I 2 77) as displayed in (Figure S8).8, 18, 34 Noteworthy, other side effects, which have been reported across the analyzed studies, included myalgia, myositis, peripheral neuritis, transaminitis, neutropenia, thrombopenia, rash, alopecia, and itching.8, 17, 29, 30, 31, 33, 35, 36
Figure 4. Rate of therapy discontinuation/withdrawal with colchicine compared to placebo/standard therapy.
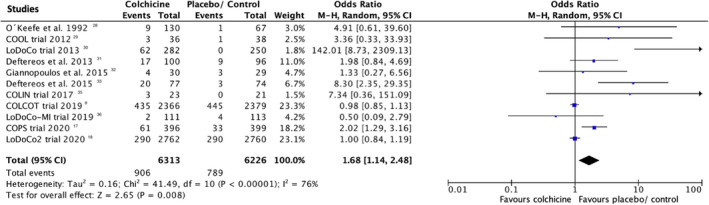
95% CI indicates 95% confidence interval.
Figure 5. Gastrointestinal side effects with colchicine compared with placebo/standard therapy.

Outcomes in Small Versus Large Trials
The dedicated sensitivity analyses comparing the main outcomes among the smaller compared to the largest 3 trials are presented in Figure S3. In fact, 3 trials comprised 11 062 patients (84.4% of the analyzed population).8, 17, 18 Regarding all‐cause mortality, this end point was lower in patients treated with colchicine compared with placebo/standard therapy among the smaller compared with the largest trials. Also, we found differences in the smaller versus the large 3 trials in terms of drug discontinuation and gastrointestinalside effects rates.
Trial Sequential Analyses
We performed trial sequential analyses focusing on the following outcomes: all‐cause mortality, cardiovascular death, MI, and stroke/TIA, as displayed in Figure 6. The cumulative z‐curve for all‐cause and cardiovascular death failed to cross the trial sequential monitoring boundaries indicating a lack of firm evidence for a 25% reduction in all‐cause and cardiovascular death with colchicine compared with placebo/standard therapy (Figure 6A and 6B). Interestingly, the cumulative z‐curve for the outcome new MI crossed the conventional boundary and the trial sequential boundary suggesting possible evidence for a 25% risk reduction with colchicine compared to placebo/standard therapy (Figure 6C). However, the cumulative z‐curve for stroke/TIA with colchicine compared with placebo/standard therapy crossed only the conventional boundary, but not the trial sequential monitoring boundary, which does implicate the lack of firm evidence with respect to this end point (Figures 6D).
Figure 6. Trial sequential analyses (TSA) of studies assessing impact colchicine vs placebo/standard therapy on (A) all‐cause mortality, (B) cardiovascular mortality, (C) new myocardial infarction, and (D) stroke/transient ischemic attack (TIA).

Discussion
Currently, the role of colchicine in patients with acute and chronic CAD is unclear in the absence of strong evidence to guide clinical decision making. Colchicine which modulates both local inflammatory cells as well expression of cytokines (e.g. IL‐1β release) by leukocytes and thus also systemic inflammation could therefore reasonably be expected to improve secondary cardiovascular prevention outcomes. From a theoretical perspective, colchicine therapy to counter vascular disease makes sense. By performing a comprehensive systematic review and meta‐analysis of all 13 relevant RCTs currently accessible, including 13 125 patients, we have strived to clarify the evidence in this area, which in turn could help to guide the use of this anti‐inflammatory drug among patients with established CAD.
This systematic review and meta‐analysis provides some important insights about the utility of colchicine in patients with CAD, which are highlighted in Figure 2. Our analysis revealed that patients with CAD treated with colchicine seem to have lower rates of ischemic events, particularly MI and stroke/TIA, compared with patients treated with placebo or standard medical therapy. Additionally, there was lower risk for repeat revascularization procedures. However, irrespective of dose and therapy duration, treatment with colchicine did not show any relevant association with all‐cause or cardiovascular mortality. Nevertheless, our data also indicated a numerical increase in noncardiovascular death cases in patients treated with colchicine compared to placebo or standard therapy.
With respect to relevant side effects, there has been some concerns regarding higher infection, specifically pneumonia, rates under colchicine treatment. However, our pooled data demonstrated no differences and may weaken this safety concern.8 Furthermore, the number of patients suffering from gastrointestinal‐related side effects, mostly diarrhea, was higher among colchicine treated patients, possibly related to disrupted intestinal barrier function and higher intestinal permeability.37 This may also reflect one of the main reasons for colchicine treatment interruption.
Although colchicine has been clinically used for many decades, the safety and tolerability of this drug continue to raise some concerns. Our data seem to underscore a high rate of gastrointestinal upset and therapy discontinuation (>10%) with colchicine, albeit some variability has been observed across the pooled trials. Indeed, one needs to take into account that this drug has a narrow therapeutic window and some considerable toxic side effects if overdosed or not appropriately monitored. Besides gastrointestinal, hematological and neuromuscular side effects as well as drug interactions, experimental studies and case reports also suggested the possibility of colchicine related cardiotoxicity mediated by increased ventricular excitability and changes in autonomic nervous activity, potentially contributing to a higher risk for sudden cardiac death.38, 39, 40, 41
In this context, the safety among patients with CAD has also been debated. In fact, the recent LoDoCo2 trial reported a reduction in the risk of cardiovascular events, but a numerically higher number of noncardiovascular deaths among patients treated with colchicine compared with placebo (hazard ratio [HR, 95%CI] 1.51 [0.99–2.31]).18 In the Australian COPS trial, the number of noncardiovascular deaths was also higher (HR [95%CI] 8.20 [1.03–65.61]).17 When pooling the data from the major trials reporting noncardiovascular death, we found a trend towards higher noncardiovascular death rates in the colchicine groups. Hence, it requires more data to establish whether immunomodulating therapy using colchicine in CAD is related to higher mortality by other mechanisms independent, but additive to infections.17
By highlighting a reduced rate of ischemia driven revascularization, MI and stroke/TIA among patients with CAD taking colchicine compared with placebo, our analyses expand the signals, derived from the 4major trials in this field.8, 17, 18, 30 Of note, experimental studies highlight colchicine´s anti‐inflammatory effects, via NLRP3 inflammasome inactivation, may enhance endothelial function and thus promote atheroprotection and mitigate the risk for cardiovascular events.6, 42
Although our results indicate a reduction in ischemic events with colchicine in patients with acute and chronic CAD, more data are warranted. First, it needs to be seen if the observed reduction in ischemic events also translates into a mortality reduction in the long term. According to the currently available data, it may not. Second, more studies are necessary in order to identify and target those patients, who will benefit most from this anti‐inflammatory drug. Thus far, one might carefully weigh the benefits and possible side effects of colchicine before prescribing it to patients with CAD. This should certainly comprise detailed patient information and possibly a drug run‐in phase, as for instance performed in the LoDoCo2 trial.18
Whereas secondary prevention in CAD represents a long‐term commitment, the optimal duration of the colchicine therapy in patients with CAD will reflect an important subject of future studies and guideline discussions. Our analyses might indicate a signal towards a need for a treatment duration with colchicine of >30 days, which could be plausible from a mechanistic standpoint since the anti‐inflammatory effects of colchicine are not only mediated by direct interaction with microtubules and regulation in cytokine secretion, but also modifications on the transcriptional level, which may necessitate a longer therapy duration to establish their full effect.43
By including a trial sequential analysis, we aimed to further establish the current evidence for colchicine in patients with CAD. As such, we found that the evidence deriving from the current data indicates that colchicine in patients with CAD lowers the risk for MI. But when considering other vigorous end points, such as all‐cause death, cardiovascular death as well as stroke, we found that the accrued evidence may not be sufficient to draw firm inferences about colchicine’s role in secondary prevention of patients with CAD yet. To ultimately define the role of colchicine in patients with acute and chronic CAD, there is a demand for further adequately powered trials focusing on hard end points and providing long‐term follow‐up data beyond 2 to 3 years.44 The ongoing CLEAR‐SYNERGY trial (ClinicalTrials.gov identifier: NCT03048825), which plans to enroll 7000 patients with MI and follow up for up to 5 years, will hopefully clarify many of those issues in due course.45
Following the publication of the latest major trials (eg, COLCOT and LoDoCo2), researchers now also showed a growing interest in colchicine and its anti‐inflammatory capabilities in the limelight of the global COVID‐19 pandemic.46 Since coronavirus SARS‐CoV2 infections are commonly associated with unbalanced systemic inflammatory reactions, mediated by eg, IL‐6, IL‐8, IL‐10, and tumor necrosis factor‐α (TNF‐ α), colchicine may have the potential to mitigate this systemic reaction and thus improve outcomes of patients with COVID‐19.46, 47 In fact, the recent GREECO‐19 and COLCORONA trials demonstrated some potential clinical benefits.48, 49 But both trials had limitations and there is still a need for more data in this context.
Limitations
These results need to be interpreted in the context of some limitations. Primarily, among the analyzed studies, different dosing regimens of colchicine had been studied among various CAD cohorts (eg, MI versus chronic coronary disease patients), which might limit the interpretation and generalizability of the results somewhat. Secondly, the considered studies in this meta‐analysis applied slightly different MI and stroke definitions. Thirdly, some of the studies had been conducted in earlier eras, where revascularization using contemporary drug eluting stents and medical therapy, including for example potent statins and antiplatelets, were not standard of care, which might have also impacted those studies’ outcomes. We also observed considerable heterogeneity among the included trials of some comparisons (eg, risk of MI with colchicine compared to placebo/standard therapy), which needs to be taken in account. Additionally, 3 open label studies had been included in the analyses. Those studies are by their nature more susceptible to bias than placebo controlled RCTs and may have consequently influenced the overall results. Finally, one needs to be aware that the COLCOT, COPS, and LoDoCo2 trials, not only contributed more than 80% of all patients with CAD included in this meta‐analysis, but more importantly, the majority of events, which in turn somewhat hampers the validity of some of our analyses.8, 17, 18
Conclusions
In patients with acute and chronic CAD, adding colchicine to standard therapy seems to reduce the risk for ischemic events, namely MI and stroke/TIA. In addition, it reduces the risk for repeat revascularization procedures. Overall, colchicine therapy may have an increased risk for gastrointestinal side effects and therapy withdrawal. Whilst we did not find any signal for major infectious complications or mortality associated with colchicine therapy, the reduced risk of potentially debilitating secondary coronary vascular or cerebrovascular events will need to be balanced against the known side effect profile of colchicine, confirmed in our meta‐analysis on a case‐by‐case basis. However, our analyses also underscore the need for more prospective studies assessing the role, dosing and optimal duration of colchicine therapy among patients with CAD.
Sources of Funding
None.
Disclosures
M.B. received consulting and speaker fees from Astra Zeneca, Amgen, Bayer, and Mundipharma, as well as travel grants from Pfizer and Vifor SA. R Kobza has received institutional grant support from Abbott, Biotronik, Biosense Webster, Boston, Medtronic, and SIS Medical. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S3
Figures S1–S10
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021198
For Sources of Funding and Disclosures, see page 15.
References
- 1.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–2850. DOI: 10.1161/01.CIR.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 2.Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276:618–632. DOI: 10.1111/joim.12296. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med. 2015;278:483–493. DOI: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. DOI: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 5.Imazio M, Andreis A, Brucato A, Adler Y, De Ferrari GM. Colchicine for acute and chronic coronary syndromes. Heart. 2020;106:1555–1560. DOI: 10.1136/heartjnl-2020-317108. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM. From C‐reactive protein to interleukin‐6 to interleukin‐1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118:145–156. DOI: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker B, Kurup R, Barraclough J, Henriquez R, Cartland S, Arnott C, Misra A, Martínez G, Kavurma M, Patel S. Colchicine as a novel therapy for suppressing chemokine production in patients with an acute coronary syndrome: a pilot study. Clin Ther. 2019;41:2172–2181. DOI: 10.1016/j.clinthera.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Tardif J‐C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and safety of low‐dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. DOI: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 9.Ullah W, Gowda SN, Fischman D. Safety and efficacy of colchicine in patients with coronary artery disease: a systematic review and meta‐analysis. Cardiovasc Revasc Med. 2021;23:1–6. DOI: 10.1016/j.carrev.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Xia M, Yang X, Qian C. Meta‐analysis evaluating the utility of colchicine in secondary prevention of coronary artery disease. Am J Cardiol. 2021;140:33–38. DOI: 10.1016/j.amjcard.2020.10.043. [DOI] [PubMed] [Google Scholar]
- 11.Xiang Z, Yang J, Yang J, Zhang J, Fan Z, Yang C, Di L, Ma C, Wu J, Huang Y. Efficacy and safety of colchicine for secondary prevention of coronary heart disease: a systematic review and meta‐analysis. Intern Emerg Med. 2021;16:487–496. DOI: 10.1007/s11739-020-02606-7. [DOI] [PubMed] [Google Scholar]
- 12.Tien YY, Huang HK, Shih MC, Tu YK. Drug repurposing? Cardiovascular effect of colchicine on patients with coronary artery disease: a systematic review and meta‐analysis. J Cardiol. 2021;77:576–582. DOI: 10.1016/j.jjcc.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Samuel M, Tardif JC, Bouabdallaoui N, Khairy P, Dubé MP, Blondeau L, Guertin MC. Colchicine for secondary prevention of cardiovascular disease: a systematic review and meta‐analysis of randomized controlled trials. Can J Cardiol. 2021;37:776–785. DOI: 10.1016/j.cjca.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Aimo A, Pascual Figal DA, Bayes‐Genis A, Emdin M, Georgiopoulos G. Effect of low‐dose colchicine in acute and chronic coronary syndromes: a systematic review and meta‐analysis. Eur J Clin Invest. 2021;51:e13464. DOI: 10.1111/eci.13464. [DOI] [PubMed] [Google Scholar]
- 15.Samuel M, Tardif JC, Bouabdallaoui N, Khairy P, Dube MP, Blondeau L, Guertin MC. Colchicine for secondary prevention of cardiovascular disease: a systematic review and meta‐analysis of randomized controlled trials. Can J Cardiol. 2021;37:776–785. DOI: 10.1016/j.cjca.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Shah B, Pillinger M, Zhong H, Cronstein B, Xia Y, Lorin JD, Smilowitz NR, Feit F, Ratnapala N, Keller NM, et al. Effects of acute colchicine administration prior to percutaneous coronary intervention: COLCHICINE‐PCI randomized trial. Circ Cardiovasc Interv. 2020;13:e008717. DOI: 10.1161/CIRCINTERVENTIONS.119.008717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tong DC, Quinn S, Nasis A, Hiew C, Roberts‐Thomson P, Adams H, Sriamareswaran R, Htun NM, Wilson W, Stub D, et al. Colchicine in patients with acute coronary syndrome: the Australian cops randomized clinical trial. Circulation. 2020;142:1890–1900. DOI: 10.1161/CIRCULATIONAHA.120.050771. [DOI] [PubMed] [Google Scholar]
- 18.Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu X‐F, Ireland MA, Lenderink T, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383:1838–1847. DOI: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. DOI: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. DOI: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available at: www.training.cochrane.org/handbook. [Google Scholar]
- 22.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. DOI: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. DOI: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, Montori V, Akl EA, Djulbegovic B, Falck‐Ytter Y, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol. 2011;64:407–415. DOI: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta‐analyses. BMJ. 2007;335:914–916. DOI: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Med Res Methodol. 2017;17:39. DOI: 10.1186/s12874-017-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. J Clin Epidemiol. 2008;61:763–769. DOI: 10.1016/j.jclinepi.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 28.O'Keefe JH Jr, McCallister BD, Bateman TM, Kuhnlein DL, Ligon RW, Hartzler GO. Ineffectiveness of colchicine for the prevention of restenosis after coronary angioplasty. J Am Coll Cardiol. 1992;19:1597–1600. DOI: 10.1016/0735-1097(92)90624-V. [DOI] [PubMed] [Google Scholar]
- 29.Raju NC, Yi Q, Nidorf M, Fagel ND, Hiralal R, Eikelboom JW. Effect of colchicine compared with placebo on high sensitivity C‐reactive protein in patients with acute coronary syndrome or acute stroke: a pilot randomized controlled trial. J Thromb Thrombolysis. 2012;33:88–94. DOI: 10.1007/s11239-011-0637-y. [DOI] [PubMed] [Google Scholar]
- 30.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low‐dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410. DOI: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Deftereos S, Giannopoulos G, Raisakis K, Kossyvakis C, Kaoukis A, Panagopoulou V, Driva M, Hahalis G, Pyrgakis V, Alexopoulos D, et al. Colchicine treatment for the prevention of bare‐metal stent restenosis in diabetic patients. J Am Coll Cardiol. 2013;61:1679–1685. DOI: 10.1016/j.jacc.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 32.Giannopoulos G, Angelidis C, Kouritas VK, Dedeilias P, Filippatos G, Cleman MW, Panagopoulou V, Siasos G, Tousoulis D, Lekakis J, et al. Usefulness of colchicine to reduce perioperative myocardial damage in patients who underwent on‐pump coronary artery bypass grafting. Am J Cardiol. 2015;115:1376–1381. DOI: 10.1016/j.amjcard.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 33.Deftereos S, Giannopoulos G, Angelidis C, Alexopoulos N, Filippatos G, Papoutsidakis N, Sianos G, Goudevenos J, Alexopoulos D, Pyrgakis V, et al. Anti‐inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation. 2015;132:1395–1403. DOI: 10.1161/CIRCULATIONAHA.115.017611. [DOI] [PubMed] [Google Scholar]
- 34.Zarpelon CS, Netto MC, Jorge JC, Fabris CC, Desengrini D, Jardim Mda S, Silva DG. Colchicine to reduce atrial fibrillation in the postoperative period of myocardial revascularization. Arq Bras Cardiol. 2016;107:4–9. DOI: 10.5935/abc.20160082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akodad M, Lattuca B, Nagot N, Georgescu V, Buisson M, Cristol J‐P, Leclercq F, Macia J‐C, Gervasoni R, Cung T‐T, et al. COLIN trial: value of colchicine in the treatment of patients with acute myocardial infarction and inflammatory response. Arch Cardiovasc Dis. 2017;110:395–402. DOI: 10.1016/j.acvd.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Hennessy T, Soh L, Bowman M, Kurup R, Schultz C, Patel S, Hillis GS. The Low Dose Colchicine after Myocardial Infarction (LoDoCo‐MI) study: a pilot randomized placebo controlled trial of colchicine following acute myocardial infarction. Am Heart J. 2019;215:62–69. DOI: 10.1016/j.ahj.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Horioka K, Tanaka H, Isozaki S, Konishi H, Fujiya M, Okuda K, Asari M, Shiono H, Ogawa K, Shimizu K. Acute colchicine poisoning causes endotoxemia via the destruction of intestinal barrier function: the curative effect of endotoxin prevention in a murine model. Dig Dis Sci. 2020;65:132–140. DOI: 10.1007/s10620-019-05729-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tochinai R, Suzuki K, Nagata Y, Ando M, Hata C, Komatsu K, Suzuki T, Uchida K, Kado S, Kaneko K, et al. Cardiotoxic changes of colchicine intoxication in rats: electrocardiographic, histopathological and blood chemical analysis. J Toxicol Pathol. 2014;27:223–230. DOI: 10.1293/tox.2014-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frommeyer G, Krawczyk J, Dechering DG, Kochhäuser S, Leitz P, Fehr M, Eckardt L. Colchicine increases ventricular vulnerability in an experimental whole‐heart model. Basic Clin Pharmacol Toxicol. 2017;120:505–508. DOI: 10.1111/bcpt.12702. [DOI] [PubMed] [Google Scholar]
- 40.Finkelstein Y, Aks SE, Hutson JR, Juurlink DN, Nguyen P, Dubnov‐Raz G, Pollak U, Koren G, Bentur Y. Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol. 2010;48:407–414. DOI: 10.3109/15563650.2010.495348. [DOI] [PubMed] [Google Scholar]
- 41.Putterman C, Ben‐Chetrit E, Caraco Y, Levy M. Colchicine intoxication: clinical pharmacology, risk factors, features, and management. Semin Arthritis Rheum. 1991;21:143–155. DOI: 10.1016/0049-0172(91)90003-I. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Wang P, Yang X, Wang W, Zhang J, He Y, Zhang W, Jing T, Wang B, Lin R. SIRT1 inhibits inflammatory response partly through regulation of NLRP3 inflammasome in vascular endothelial cells. Mol Immunol. 2016;77:148–156. DOI: 10.1016/j.molimm.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 43.Ben‐Chetrit E, Bergmann S, Sood R. Mechanism of the anti‐inflammatory effect of colchicine in rheumatic diseases: a possible new outlook through microarray analysis. Rheumatology (Oxford, England). 2006;45:274–282. DOI: 10.1093/rheumatology/kei140. [DOI] [PubMed] [Google Scholar]
- 44.Hemkens LG, Ewald H, Briel M. Colchicine and prevention of cardiovascular events. JAMA. 2016;316:1106–1107. DOI: 10.1001/jama.2016.11044. [DOI] [PubMed] [Google Scholar]
- 45.Jolly SS. ClinicalTrials.gov. A 2×2 Factorial Randomized Controlled Trial of Colchicine and Spironolactone in Patients With Myocardial Infarction/SYNERGY Stent Registry ‐ Organization to Assess Strategies for Ischemic Syndromes 9. https://clinicaltrials.gov/ct2/show/NCT03048825
- 46.Kaul S, Gupta M, Bandyopadhyay D, Hajra A, Deedwania P, Roddy E, Mamas M, Klein A, Lavie CJ, Fonarow GC, et al. Gout pharmacotherapy in cardiovascular diseases: a review of utility and outcomes. Am J Cardiovasc Drugs. 2020;28:1–14. 10.1007/s40256-020-00459-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavie CJ. In reply – use of famotidine and risk of severe course of illness in patients with COVID‐19: a meta‐analysis. Mayo Clin Proc. 2021;96:1367–1368. DOI: 10.1016/j.mayocp.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, Metallidis S, Sianos G, Baltagiannis S, Panagopoulos P, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO‐19 randomized clinical trial. JAMA Netw Open. 2020;3:e2013136. DOI: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tardif JCBN, L’Allier PL, Gaudet D, Shah B, Pillinger MH, Lopez‐Sendon J, da Luz P , Verret L, Audet S, Dupuis J, et al. Efficacy of colchicine in non‐hospitalized patients with COVID‐19. medRxiv. 2021. DOI: 10.1101/2021.01.26.21250494 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3
Figures S1–S10


