Abstract
Background
Arterial stiffness is an important predictor of cardiovascular events; however, indexes for measuring arterial stiffness have not been widely incorporated into routine clinical practice. This study aimed to determine whether the cardio‐ankle vascular index (CAVI), based on the blood pressure–independent stiffness parameter β and reflecting arterial stiffness from the origin of the ascending aorta, is a good predictor of cardiovascular events in patients with cardiovascular disease risk factors in a large prospective cohort.
Methods and Results
This multicenter prospective cohort study, commencing in May 2013, with a 5‐year follow‐up period, included patients (aged 40‒74 years) with cardiovascular disease risks. The primary outcome was the composite of cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction. Among 2932 included patients, 2001 (68.3%) were men; the mean (SD) age at diagnosis was 63 (8) years. During the median follow‐up of 4.9 years, 82 participants experienced primary outcomes. The CAVI predicted the primary outcome (hazard ratio, 1.38; 95% CI, 1.16‒1.65; P<0.001). In terms of event subtypes, the CAVI was associated with cardiovascular death and stroke but not with myocardial infarction. When the CAVI was incorporated into a model with known cardiovascular disease risks for predicting cardiovascular events, the global χ2 value increased from 33.8 to 45.2 (P<0.001), and the net reclassification index was 0.254 (P=0.024).
Conclusions
This large cohort study demonstrated that the CAVI predicted cardiovascular events.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01859897.
Keywords: arterial stiffness, blood pressure, cardiovascular events, pulse‐wave velocity, risk factor
Subject Categories: Hypertension, Cardiovascular Disease, Diagnostic Testing
Nonstandard Abbreviations and Acronyms
- CAVI
cardio‐ankle vascular index
- PWV
pulse‐wave velocity
Clinical Perspective
What Is new?
This prospective cohort study demonstrated that the cardio‐ankle vascular index, a marker of arterial stiffness based on the stiffness parameter β, predicted cardiovascular events in patients with cardiovascular disease risk factors.
In terms of event subtypes, the cardio‐ankle vascular index was associated with the risk of cardiovascular death, nonfatal stroke, all‐cause mortality, and heart failure with hospitalization.
What Are the Clinical Implications?
The cardio‐ankle vascular index may be clinically useful for assessing the risk of cardiovascular events among patients with risk factors for cardiovascular disease.
Our findings warrant the need for future studies to verify our results, compare the cardio‐ankle vascular index with other arterial stiffness markers, and estimate the threshold for each cardiovascular event.
Arterial stiffness is an important predictor of future cardiovascular events.1 Several indexes for measuring arterial stiffness, such as the carotid‐femoral pulse‐wave velocity (PWV) and the augmentation index, have been proposed2, 3, 4, 5; however, these have not been widely incorporated into routine clinical practice. Among these indexes, the carotid‐femoral PWV has been considered the reference standard. Previous studies have shown that a greater carotid‐femoral PWV is associated with an increased risk of cardiovascular events in the general population and patients with hypertension or type 2 diabetes mellitus.6, 7, 8, 9 However, the use of carotid‐femoral PWV has several limitations, such as a complex measurement procedure and a bias introduced by the determination distance.10 Furthermore, because carotid‐femoral PWV is a measure of the speed of the pulse wave, it is affected by blood pressure,11, 12 which is an important confounding factor for cardiovascular disease (CVD). Similarly, it remains unclear whether carotid‐femoral PWV has a significant impact on decision making in medium‐ and high‐risk individuals.
The cardio‐ankle vascular index (CAVI) is a marker of arterial stiffness based on the stiffness parameter β, developed in Japan in 2004. It reflects arterial stiffness from the origin of the ascending aorta to the ankle.13 The CAVI can be obtained automatically by wrapping pressure cuffs around the upper arms and lower legs and is less dependent on blood pressure.14 Several studies have demonstrated that the CAVI is associated with target organ damage, such as the presence of coronary artery disease (CAD) and stroke.15, 16, 17, 18 In addition, studies have reported the association between a greater CAVI and a high incidence of cardiovascular events in patients with diabetes mellitus, obesity, and several CVD risk factors.19, 20, 21, 22 Nevertheless, these were single‐center or relatively small‐scale studies, and some studies failed to show a significant association between the CAVI and cardiovascular events in patients with metabolic syndrome or at high risk of developing CVD.23, 24 Therefore, a large multicenter prospective study is needed to elucidate the association between CAVI and cardiovascular events.
This study aimed to investigate (1) whether the CAVI is a good predictor of cardiovascular events in patients with CVD risk factors and (2) whether the CAVI offers incremental value for predicting future cardiovascular events in a large multicenter prospective cohort.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
The CAVI‐J (Prospective Multicenter Study to Evaluate Usefulness of Cardio‐Ankle Vascular Index in Japan) was a multicenter, prospective, cohort study that evaluated the usefulness of the CAVI.25 This study was approved by the ethics committee of the Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, as well as the ethics committees of each participating center. It was conducted in compliance with the Declaration of Helsinki. All participants provided written informed consent, and this trial was registered at ClinicalTrials.gov (NCT01859897).
The details of the inclusion and exclusion criteria are described in Data S1. The eligibility criteria included individuals aged between 40 and 74 years and those who had at least one of the following risk factors for CVD: type 2 diabetes mellitus,26 hypertension (categorized as high risk, according to the Japanese Society of Hypertension Guidelines for the Management of Hypertension 2009),27 metabolic syndrome,28 chronic kidney disease of stage 3,29 or a history of CAD or cerebral infarction. In contrast, the exclusion criteria were as follows: aged <40 years or >75 years, ankle‐brachial index ≤0.9, chronic atrial fibrillation, severe heart failure (New York Heart Association class greater than level III) or left ventricular dysfunction (left ventricular ejection fraction of <40%), medical history of cancer and/or treatment for cancer within the past 5 years, estimated glomerular filtration rate of <30 mL/min per 1.73 m2 or receiving long‐term dialysis, treatment with systemic steroids or immunosuppressants, or liver cirrhosis, and judgment of an attending physician that the individual was ineligible for inclusion in the study. Metabolic syndrome was diagnosed with the criteria of the Examination Committee for the Diagnosis of Metabolic Syndrome in Japan, 2005.28 The definition of metabolic syndrome was abdominal obesity with a waist circumference ≥85 cm for men and ≥90 cm for women and ≥2 of the following 3 risk factors: (1) high blood pressure (systolic blood pressure ≥130 mm Hg and/or diastolic blood pressure ≥85 mm Hg or treatment for previously diagnosed hypertension), (2) hyperglycemia (fasting glucose level ≥110 mg/dL or treatment for previously diagnosed type 2 diabetes mellitus), and (3) dyslipidemia (triglyceride levels ≥150 mg/dL and/or high‐density lipoprotein [HDL] cholesterol <40 mg/dL or treatment for previously diagnosed dyslipidemia).
In total, 3026 patients were enrolled between May 2013 and December 2014. The participants were followed up prospectively for 5 years from the date of determining the CAVI. Participants’ status was checked from medical records in their corresponding hospitals or clinics and by mail or telephone for any participants who had moved during the follow‐up. Participants were managed by their attending physicians, who were encouraged to treat CVD risk factors, including hypertension, dyslipidemia, and diabetes mellitus, to achieve the best available standard of care in accordance with the relevant guidelines.
Primary Exposure
The primary exposure was the baseline CAVI, measured with a VaSera device (Fukuda Denshi, Tokyo, Japan). The CAVI was determined using the following formula: CAVI=a {(2ρ/∆P)×ln (Ps/Pd) PWV2}+b, where a and b are constants applied according to the value derived from the equation: (2ρ/∆P)×ln (Ps/Pd) PWV2 (a and b: 0.850 and 0.695, 0.658 and 2.103, and 0.432 and 4.441, respectively),30 ρ is blood density (the fixed value of 1.05 is used), ΔP is Ps–Pd, Ps is systolic blood pressure, Pd is diastolic blood pressure, and PWV is the pulse‐wave velocity. The details of the measurement have been described previously.13 ECG electrodes were placed on both wrists, a microphone was placed on the sternum to detect heart sounds, and cuffs were applied to the upper arms and ankles, bilaterally, with the patient in the supine position. To detect the brachial and ankle pulse waves with cuffs, a low cuff pressure of 30 to 50 mm Hg was used to minimize the effect of cuff pressure on hemodynamics. Thereafter, blood pressure was measured from the cuff on the upper arm. PWV was obtained by dividing the vascular length by the time taken for the pulse wave to propagate from the aortic valve to the ankle; it was measured using cuffs at the upper arms and ankles. Intraobserver and interobserver variability have been reported to be <3.8% and 2.4%, respectively.13, 17, 31, 32, 33 To ensure the quality of the measurement, 2 conditions were established. First, qualified hospitals or clinics, based on the past performance of the CAVI measurement, could participate in this study. Second, all raw data of CAVI were sent for evaluation at the central office. Subsequently, remeasurement was required in case of inappropriate data.
Outcomes
The primary outcome was the composite cardiovascular events of cardiovascular death, myocardial infarction, and stroke. Stroke included ischemic stroke and hemorrhagic stroke. In contrast, secondary outcomes were all‐cause death, stable angina pectoris with revascularization, the new incidence of peripheral arterial disease, aortic aneurysm, aortic dissection, heart failure with hospitalization, and deterioration in renal function. The details of definitions are provided in Data S1. All events were reported annually by each institution to the Clinical Endpoint Review Committee. The committee, consisting of members blinded to information about the patients, assessed the appropriateness of the clinical judgment of all events according to prespecified criteria.
Covariates
A self‐administered questionnaire on smoking habits and physical activity was checked by trained interviewers. These variables were classified as being either habitual or not. The use of medications was similarly checked. Blood pressure was measured twice using an automated sphygmomanometer with participants in the sitting position after a 5‐minute rest. The mean of the 2 measurements was used for the present analysis. Serum total and HDL cholesterol concentrations were determined enzymatically. Obesity was defined as a body mass index >30.0 kg/m2, and all clinical examinations and blood tests were conducted on the same day.
Statistical Analysis
The sample size was calculated as follows: The relative risk of cerebrovascular events in patients with a CAVI >10 has been estimated to be 1.73, compared with patients with a CAVI ≤10; thus, the study enrolled 2.5 times as many patients with a CAVI ≤10 as patients with a CAVI >10,34 in whom the risk of cerebrovascular events is anticipated to be 4.6% in 5 years.35 From these data, the risks of cerebrovascular events in patients with a CAVI ≤10 and those with a CAVI >10 were anticipated to be 0.038 and 0.066 in 5 years, respectively. To detect this difference in risk, the required sample size was calculated, using the Freedman method, to be 810 for those with a CAVI ≤10 and 2024 for those with a CAVI >10, with a 5% 2‐sided α value, 80% power, and 20% dropout rate. On the basis of these assumptions, a sample size of 3000 was chosen for this study.
Categorical data are presented as absolute numbers and percentages. Continuous data are presented as mean (SD). Baseline characteristics were compared according to the CAVI quintile (quintile 1, ≤7.55; quintile 2, 7.60‒8.20; quintile 3, 8.25‒8.80; quintile 4, 8.85‒9.45; and quintile 5, ≥9.50). The linear trends in the mean values and the frequencies of risk factors across the CAVI levels were tested using linear regression analysis and logistic regression analysis, respectively. Cumulative event rates were estimated by the Kaplan‐Meier method for the primary outcome, and a log‐rank test was used to compare groups. Cox proportional hazards regression analysis was performed to investigate the association between clinical outcomes and the CAVI value. Proportional hazard assumption was evaluated on the basis of the log‐log plot. Annualized incidence rates were calculated per 1000 patient‐years of follow‐up. The hazard ratios (HRs) and 95% CIs were calculated and reported. The incremental value of the CAVI for predicting cardiovascular events was assessed using the Akaike information criterion and the global χ2 test. To assess the discrimination of events, receiver‐operating characteristic curve analysis was performed. Similarly, we calculated the continuous net reclassification improvement and integrated discrimination improvement. A 2‐tailed P<0.05 was considered statistically significant. Statistical analyses were performed using SPSS for Windows, version 25.0 (IBM Corporation, Tokyo, Japan) and JMP Pro version 15 (SAS Institute Japan, Tokyo, Japan).
Results
Patients
The median (interquartile range) follow‐up period was 4.9 (4.6‒5.2) years. In total, 94 patients were excluded because 60 patients had no follow‐up data, and 34 withdrew consent. Finally, 2938 patients (2001 men and 937 women; mean [SD] age, 63.2 [8.0] years) were included in the analysis. The baseline characteristics of the patients are shown in Table S1. The baseline characteristics of patients included in the analysis according to the CAVI quintiles are shown in Table 1. Patients with higher CAVI levels were older and were more likely to be men. The mean systolic blood pressure, prevalence of hypertension, diabetes mellitus, and obesity, use of insulin, and the use of antidiabetic as well as of antiplatelet agents increased significantly with a higher CAVI. The mean diastolic pressure and HDL cholesterol values, the prevalence of chronic kidney disease, history of CAD or cerebral infarction, smoking habits, regular exercise, and use of lipid‐lowering agents did not differ among the CAVI quintile groups.
Table 1.
Baseline Characteristics According to the CAVI
| Characteristics | CAVI | |||||
|---|---|---|---|---|---|---|
| Quintile 1 (≤7.55) | Quintile 2 (7.60‒8.20) | Quintile 3 (8.25‒8.80) | Quintile 4 (8.85‒9.45) | Quintile 5 (≥9.50) | P Value for Trend | |
| (N=579) | (N=578) | (N=614) | (N=577) | (N=584) | ||
| Age, mean (SD), y | 57.2 (9.3) | 61.7 (7.9) | 64.0 (7.0) | 65.6 (6.2) | 67.3 (5.2) | <0.001 |
| Men | 342 (59.1) | 375 (64.9) | 414 (67.4) | 422 (73.1) | 448 (76.7) | <0.001 |
| Systolic blood pressure, mean (SD), mm Hg | 130.5 (15.4) | 131.3 (16.3) | 132.0 (15.7) | 134.8 (17.3) | 137.3 (16.9) | <0.001 |
| Diastolic blood pressure, mean (SD), mm Hg | 80.2 (11.0) | 79.9 (11.8) | 78.9 (10.8) | 80.4 (11.5) | 80.5 (12.1) | 0.089 |
| Hypertension | 499 (86.2) | 512 (88.6) | 533 (86.8) | 513 (88.9) | 540 (90.5) | 0.002 |
| Hypertension (high risk) | 458 (79.1) | 477 (82.5) | 484 (78.8) | 490 (84.9) | 522 (89.4) | <0.001 |
| Diabetes mellitus | 430 (74.3) | 416 (72.0) | 458 (74.6) | 438 (75.9) | 467 (80.0) | 0.007 |
| Metabolic syndrome | 183 (31.6) | 150 (26.0) | 172 (28.0) | 161 (27.9) | 154 (26.4) | 0.148 |
| Chronic kidney disease | 198 (34.2) | 219 (37.9) | 222 (36.2) | 233 (40.4) | 253 (43.3) | 0.001 |
| History of coronary artery disease or cerebral infarction | 197 (34.0) | 216 (34.4) | 231 (37.6) | 224 (38.8) | 247 (42.3) | 0.005 |
| Total cholesterol, mean (SD), mg/dL | 188.4 (34.5) | 184.0 (34.7) | 183.9 (33.4) | 182.6 (35.5) | 180.3 (34.4) | 0.002 |
| HDL cholesterol, mean (SD), mg/dL | 55.4 (15.1) | 54.9 (16.0) | 55.7 (15.5) | 54.8 (15.5) | 53.9 (14.7) | 0.344 |
| Obesity | 163 (28.2) | 69 (11.9) | 54 (8.8) | 43 (7.5) | 30 (5.1) | <0.001 |
| Smoking habits | 246 (42.5) | 244 (42.2) | 279 (45.4) | 277 (48.0) | 264 (45.2) | 0.086 |
| Regular exercise | 195 (33.7) | 193 (33.4) | 212 (34.5) | 217 (37.6) | 209 (35.8) | 0.179 |
| Medications | ||||||
| Antihypertensive agents | 443 (76.5) | 454 (78.6) | 473 (77.0) | 427 (74.0) | 463 (79.3) | 0.850 |
| Insulin | 22 (3.8) | 31 (5.4) | 32 (5.2) | 32 (5.6) | 57 (9.8) | <0.001 |
| Antidiabetic agents | 179 (30.9) | 189 (32.7) | 22 (37.8) | 237 (41.1) | 272 (46.6) | <0.001 |
| Lipid‐lowering agents | 345 (59.6) | 372 (64.4) | 376 (61.2) | 334 (57.9) | 378 (64.7) | 0.545 |
| Antiplatelet agents | 190 (32.8) | 213 (36.9) | 229 (37.3) | 214 (37.1) | 246 (42.1) | 0.003 |
Data are presented as the number (percentage) of participants, unless otherwise indicated. CAVI indicates cardio‐ankle vascular index; and HDL, high‐density lipoprotein.
Association Between the CAVI and Primary Outcomes
During the follow‐up, 82 participants experienced primary outcomes. These included 13 cardiovascular deaths, 44 nonfatal stroke cases, and 25 nonfatal myocardial infarction cases. The cumulative incidence rates of the primary outcomes are shown according to the CAVI levels in the Figure 1, and the rates were significantly higher in the fifth quintile group than in the first quintile group (P value for trend=0.01). Risk factors for cardiovascular events analyzed in the univariate Cox proportional hazard models are shown in Table S2. Male sex, HDL cholesterol, smoking habits, alcohol intake, and use of antiplatelet agents, but not systolic or diastolic blood pressure, were associated with cardiovascular events. The age‐ and sex‐adjusted HRs increased linearly with elevating CAVI levels, and this relationship remained significant after adjusting for age, male sex, systolic blood pressure, type 2 diabetes mellitus, HDL cholesterol, smoking, history of CAD or cerebral infarction, and use of antihypertensive agents (Table 2). In the multivariable‐adjusted model, the fifth quintile of CAVI (≥9.50) was associated with increased risk of the primary outcomes compared with the first quintile of CAVI (≤7.55), after adjusting for the above confounding factors (HR, 3.31 [95% CI, 1.26‒8.71]; P=0.016). Every 1‐point increment in the CAVI was similarly associated with an increased risk of the primary outcomes, after adjusting for the confounding factors (HR, 1.38 [95% CI, 1.16‒1.65]; P<0.001).
Figure 1. Kaplan‐Meier plot of cumulative probability of cardiovascular events by quintiles of the cardio‐ankle vascular index (CAVI).
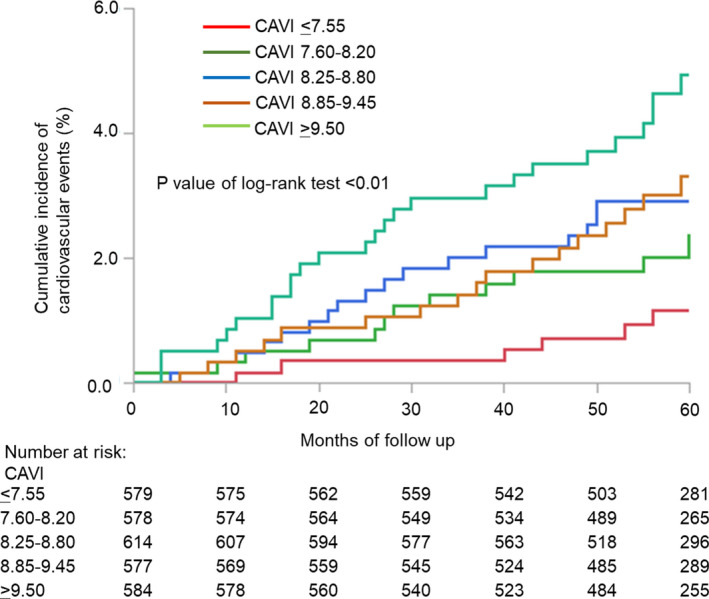
Time to cardiovascular events, including cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction, according to baseline CAVI. The cumulative incidence rates of the primary outcomes according to the CAVI levels were significantly higher in the fifth quintile group (CAVI ≥9.50) than in the first quintile group (CAVI ≤7.55) (P value for trend=0.01).
Table 2.
Association Between the CAVI and Cardiovascular Events
| CAVI |
Follow‐Up Period, Median (IQR), mo |
No. (%) of Events | No. of Participants | Incident Rate (per 103 PYs) | Age‐ and Sex‐Adjusted | Multivariable‐Adjusted* | ||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||||
| Quintile 1 (≤7.55) | 59 (55‒63) | 6 (1.03) | 579 | 2.19 | 1.00 (Reference) | 1.00 (Reference) | ||
| Quintile 2 (7.60‒8.20) | 59 (54‒63) | 12 (2.07) | 578 | 4.42 | 1.82 (0.67‒4.91) | 0.237 | 1.81 (0.67–4.90) | 0.241 |
| Quintile 3 (8.25‒8.80) | 59 (55‒63) | 18 (2.92) | 614 | 6.26 | 2.43 (0.93‒6.31) | 0.069 | 2.43 (0.93–6.34) | 0.071 |
| Quintile 4 (8.85‒9.45) | 60 (54‒62) | 19 (3.28) | 577 | 7.06 | 2.56 (0.98‒6.74) | 0.056 | 2.51 (0.95–6.66) | 0.063 |
| Quintile 5 (≥9.50) | 59 (54‒62) | 27 (4.62) | 584 | 10.04 | 3.49 (1.34‒9.01) | 0.011 | 3.31 (1.26–8.71) | 0.016 |
| Every 1‐point increase in the CAVI | 82 (2.79) | 2932 | 1.42 (1.19‒1.69) | <0.001 | 1.38 (1.16–1.65) | <0.001 | ||
CAVI indicates cardio‐ankle vascular index; HR, hazard ratio; IQR, interquartile range; and PY, person‐year.
Adjusted for age, male sex, systolic blood pressure, diabetes mellitus, high‐density lipoprotein cholesterol, smoking, history of coronary artery disease or cerebral infarction, and use of antihypertensive agents.
Association Between the CAVI and Each End Point
Subsequently, the association between the CAVI and each end point was assessed. After evaluating the proportional hazard assumption, the associations of the CAVI with cardiovascular death, nonfatal stroke, nonfatal myocardial infarction, and heart failure with hospitalization were analyzed in the Cox proportional hazard models (Table 3). On the basis of the events included in the primary outcome, a CAVI >9.5 was significantly associated with the risk of cardiovascular death and nonfatal stroke (crude HR, 3.83 [95% CI, 1.28‒11.40]; P=0.015; and crude HR, 2.07 [95% CI, 1.07‒3.91]; P=0.024, respectively), but not with nonfatal myocardial infarction, as a CAVI ≤9.5 was considered as the reference. For the events included in the secondary outcome, a CAVI >9.5 was significantly associated with the incidence of all‐cause mortality and heart failure with hospitalization (crude HR, 1.90 [95% CI, 1.11‒3.26]; P=0.018; and crude HR, 3.38 [95% CI, 1.42‒8.01]; P=0.005, respectively).
Table 3.
Association of CAVI >9.5 With Each End Point
| End Point | No. (%) of Events | Crude HR (95% CI) | P Value |
|---|---|---|---|
| Cardiovascular death | 13 (0.4) | 3.83 (1.28–11.4) | 0.015 |
| Nonfatal stroke | 44 (1.5) | 2.07 (1.10–3.91) | 0.024 |
| Nonfatal myocardial infarction | 25 (0.8) | 1.13 (0.42–3.02) | 0.080 |
| All‐cause mortality | 64 (2.2) | 1.90 (1.11–3.26) | 0.018 |
| Heart failure with hospitalization | 21 (0.7) | 3.38 (1.42–8.01) | 0.005 |
CAVI indicates cardio‐ankle vascular index; and HR, hazard ratio.
Estimation of the Risk Assessment Ability for Cardiovascular Events
To determine the incremental value of the CAVI for predicting cardiovascular events, the Akaike information criterion test, a likelihood ratio test, and receiver‐operating characteristic curve analysis were performed (Table 4). The baseline model comprised the following parameters: age, male sex, systolic blood pressure, type 2 diabetes mellitus, HDL cholesterol, smoking, history of CAD or cerebral infarction, and use of antihypertensive agents. The addition of the CAVI to the baseline model improved the model fit, as indicated by a reduction in the Akaike information criterion from 731.3 to 721.9, and significantly increased the global χ2 value from 33.8 to 45.2 (P<0.001). The increase in C‐statistic was not significant (0.688 to 0.708; P=0.146). Addition of the CAVI yielded a category‐free net reclassification index of 0.254 (95% CI, 0.034‒0.472; P=0.024) and an integrated discrimination improvement of 0.006 (95% CI, 0.000‒0.012; P=0.052).
Table 4.
Incremental Prognostic Value of the CAVI for Cardiovascular Events After Addition to a Model Incorporating Known Risk Factors
| Model | AIC | Global χ2 score | C‐Statistic | NRI (95% CI) | IDI (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|
| P Value | P Value | P Value | P Value | ||||||
| Age, sex, and risk factors* | 731.3 | 33.8 | 0.688 | ||||||
| With CAVI added | 721.9 | 45.2 | <0.001 | 0.708 | 0.146 |
0.254 (0.034–0.472) |
0.024 |
0.006 (0.000–0.012) |
0.052 |
AIC indicates Akaike information criterion; CAVI, cardio‐ankle vascular index; IDI, integrated discrimination improvement; and NRI, net reclassification index.
Risk factors included age, male sex, systolic blood pressure, diabetes mellitus, high‐density lipoprotein cholesterol, smoking, history of coronary artery disease or cerebral infarction, and use of antihypertensive agents.
Discussion
We found that the CAVI was a predictor of the onset of cardiovascular events in patients with CVD risk factors. The analysis of the CAVI and different outcomes showed that CAVI was associated with incidence of cardiovascular death, nonfatal stroke, all‐cause mortality, and heart failure with hospitalization. To our knowledge, all previous studies that have investigated the relationship between the CAVI and the incidence of cardiovascular events were smaller, single‐center studies, and some of these studies failed to show a significant association between the CAVI and the risk of cardiovascular events.23, 24 Therefore, the study findings highlight the clinical usefulness of the CAVI in the risk assessment of cardiovascular events among patients at risk of cardiovascular events.
There have been several single‐center, small‐scale studies on the CAVI and the incidence of cardiovascular events.15, 20, 21, 22, 23, 24 A study, including 400 patients with hypertension, diabetes mellitus, or dyslipidemia, showed that patients with a CAVI ≥10.0 had a 1.73 relative risk of elevated cerebrovascular events compared with those with a CAVI <9.0.34 A study including 626 patients with type 2 diabetes mellitus showed that a CAVI ≥9.0 was associated with increased cardiovascular events, compared with a CAVI <9.0 (HR, 1.23; 95% CI, 1.07‒1.42).22 A study including 425 obese patients showed that the CAVI was a significant factor for the incidence of cardiovascular events (HR, 1.44 per 1‐point increase in the CAVI; 95% CI, 1.02‒2.02).20 In a study including 1562 patients with CVD risk factors, the CAVI was significantly associated with cardiovascular events (HR, 1.13 per 1‐point increase in the CAVI; 95% CI, 1.01‒1.26).21 In the present study, a CAVI ≥9.50 was shown to be significantly associated with the increased incidence of cardiovascular events compared with CAVI ≤7.55. This finding was consistent with those of previous studies. However, there are several differences between the present study and the previous studies. First, this study was a multicenter, large‐scale cohort. Second, this study included heterogeneous patients with several CVD risk factors and preexisting CVD. Thus, the present study demonstrated that CAVI is useful in the assessment of the risk for future cardiovascular events in patients with CVD risk factors.
In our study, CAVI was associated with the risk of nonfatal stroke, but not with that of nonfatal myocardial infarction. However, previous studies have shown that an increase in carotid‐femoral PWV is associated with a greater risk of CAD rather than of stroke.8, 9 There are several explanations for the discrepancy. First, the definition of CAD in most previous studies was the composite outcome, including the incidence of acute coronary syndrome and the revascularization for chronic coronary syndrome. There were a few data about the impact of arterial stiffness on different end points. The substudy of the SPRINT (Systolic Blood Pressure Intervention Trial) showed that the estimated PWV was not associated with the incidence of myocardial infarction and acute coronary syndrome, which is consistent with our findings.36 Second, longitudinal cohort studies in Asian populations have shown that an increase in brachial‐ankle PWV was associated with a greater incidence of stroke than of CAD.37, 38 Several hypotheses underlying the association between arterial stiffness and atherosclerosis have been proposed. The arterial systolic pressure increases, and the diastolic pressure decreases, in the stiffened artery. Increased luminal pressure and shear stress accelerate the formation of atheroma and stimulate excessive collagen production and deposition in the arterial wall, leading to the progression of atherosclerosis.39 In addition, increased pulse pressure may be associated with the development of plaque and its subsequent rupture.40 Further clinical investigation will be needed to evaluate the clinical relevance of CAVI in the development of acute coronary syndrome.
This study demonstrated that increased CAVI was associated with heart failure with hospitalization. Heart failure is a growing public health problem worldwide because of its high mortality and morbidity.41, 42 The mechanisms underlying acute heart failure are manifold because this disease results from a complex of structural and functional alterations. Among them, increased arterial stiffness has been proposed as a potential and important noncardiac factor in the pathogenesis of heart failure.43, 44 Stiff aorta increases the systolic afterload and worsens ventricular‐vascular coupling.45 Although further investigations are needed, the measurements of CAVI might be helpful in identifying patients at increased risk for heart failure with hospitalization.
In the present study, the CAVI only mildly improved cardiovascular event discrimination over that by known risk factors. A recent meta‐analysis of the association between brachial‐ankle PWV and cardiovascular events demonstrated that the significant incremental prognostic value of brachial‐ankle PWV for predicting cardiovascular events over that of the Framingham risk score was attenuated in participants with intermediate or high risk. This study included heterogeneous patients with several CVD risk factors and preexisting CVD, who had relatively moderate to high risks of cardiovascular events; this may explain the mild effect of the CAVI in the discrimination of cardiovascular events in this study. Further analyses, according to the magnitude of risks, will be needed.
There have been several techniques and methods applied to quantify arterial stiffness. A study demonstrated that CAVI was significantly correlated with carotid‐femoral PWV and brachial‐ankle PWV (Pearson correlation coefficients, 0.74 and 0.82, respectively).46 However, there are notable differences among arterial stiffness measurements. Carotid‐femoral PWV is obtained by applanation tonometry, which is a complicated technique compared with CAVI and brachial‐ankle PWV. CAVI and brachial‐ankle PWV are derived from plethysmography cuff automatically.25 Meanwhile, CAVI is a noninvasive indicator of arterial stiffness. It has an advantage over PWV for measuring arterial stiffness as it is less dependent on blood pressure at the time of measurement.14 An assessment of arterial properties by considering blood pressure and arterial stiffness may allow detailed monitoring of changes in arterial stiffness in daily practice. Furthermore, the CAVI measurement is simple. The CAVI is easily obtained automatically with a device, leading to its widespread use in clinical situations if cost constraints are ignored. Further investigations will be needed to elucidate this matter, with due consideration given to cost‐effectiveness.
The measurement of CAVI has been included in the routine clinical setting in Japan. CAVI is measured for the risk stratification in patients with atherosclerotic risk factors and for the evaluation of therapeutic efficacy of medications and lifestyle modification in patients with cardiometabolic disorders on a regular basis. In addition, the VaSera device can evaluate the ankle‐brachial index simultaneously, which helps to diagnose peripheral arterial disease. The measurement of CAVI has been covered by health insurance in Japan, and, thus, the measurement of CAVI is applied widely in clinical practice.
This study had several limitations. First, as this was an observational cohort study, a causal relationship between an increased CAVI and increased cardiovascular events could not be proved. Second, the study population comprised only Japanese patients. Although several studies of non‐Asian populations have recently been reported,47, 48 the generalizability of our data to other races/ethnicities remains uncertain. Third, the present study examined arterial stiffness only by the CAVI. Therefore, we could not compare the impact of the CAVI with those of other stiffness markers. Finally, we failed to estimate a threshold for each event because of the modest number of events. Hence, a further study with longer follow‐up or a larger sample is warranted.
In conclusion, our findings demonstrated that, in patients with CVD risk factors, patients with a higher CAVI (≥9.50) have elevated risks of cardiovascular events. These data suggest that the CAVI is clinically useful in the assessment of the risk of cardiovascular events among patients with CVD risk factors.
Appendix
The Investigators and Institutions Involved in the CAVI‐J (Prospective Multicenter Study to Evaluate Usefulness of Cardio‐Ankle Vascular Index in Japan)
Yuichi Akasaki (Kagoshima University Hospital), Noriko Asahara (Kyoto Medical Center), Masayuki Doi (Kagawa Prefectural Central Hospital), Tomikazu Fukuoka (Matsuyama Red Cross Hospital) Hiromichi Fukushima (Dokkyo Medical University), Yuji Hara (Hara Clinic), Koji Hasegawa (Kyoto Medical Center), Keiichi Hirano (Toho University Sakura Medical Center), Takashi Hitsumoto (Hitsumoto Medical Clinic), Toshio Honda (Sadamoto Hospital), Shigeo Horinaka (Dokkyo Medical University), Kotaro Ichinari (Hayato Onsen Hospital), Toshihiko Ishimitsu (Dokkyo Medical University), Kimihiko Ishimura (Dokkyo Medical University), Mai Iwataki (University of Occupational and Environmental Health), Hiroshi Kaieda (Taikai Clinic), Masahito Kajiya (Sumitomo Besshi Hospital), Shigeshi Kamikawa (Okayama Heart Clinic), Hitoshi Kaneko (Kaneko Clinic), Hideo Kawakami (Ehime Prefectural Imabari Hospital), Hajime Kihara (Kihara Cardiovascular Clinic), Yuko Kikuchi (Kyoto Medical Center), Hajime Kiyokawa (Toho University Sakura Medical Center), Takashi Kobayashi (Jyuzen General Hospital), Wataru Koguchi (Dokkyo Medical University), Mitsuteru Koizumi (Kyoto Medical Center), Kazuhiko Kotani (Jichi Medical University), Takuro Kubozono (Kagoshima University Hospital), So Kuwahata (Tarumizu Chuo Hospital), Motofumi Maguchi (Saijo Central Hospital), Mitsuru Masaki (Hyogo College of Medicine), Hitoshi Minowa (Minowa Naika), Michiaki Miyamoto (Aiseikai Clinic), Akihito Miyoshi (Tajiri Hospital), Kenichi Miyoshi (Ehime University Graduate School of Medicine), Toru Miyoshi (Okayama University Graduate School of Medicine), Maki Murata (Kyoto Medical Center), Mitsunobu Murata (Kokubunji Sakura Clinic), Tomoaki Nagao (Ehime University Graduate School of Medicine), Kazufumi Nakamura (Yura Hospital), Keigo Nakamura (Kagawa Prefectural Central Hospital), Michitsugu Nakamura (Saijo Central Hospital), Nobuyuki Nakano (Dokkyo Medical University), Seiji Nanba (Okayama Rosai Hospital), Kazuhisa Nishimura (Ehime University Graduate School of Medicine), Hachiro Obata (Okino Cardiovascular Hospital), Kazuro Ogurusu (Kasaoka City Hospital), Takefumi Oka (Tsuyama Chuo Hospital), Takafumi Okura (Ehime University Graduate School of Medicine), Madoka Onimaru (Onimaru Clinic), Shiro Ono (Saiseikai Yamaguchi General Hospital), Go Onoue (Onoue Clinic), Atsuhito Saiki (Toho University Sakura Medical Center), Satoru Sakuragi (Iwakuni Clinical Center), Toshihiro Sarashina (Tajiri Hospital), Koichi Seta (Kyoto Medical Center), Yoshimasa Shibata (Dokkyo Medical University), Kazuhiro Shimizu (Toho University Sakura Medical Center), Kohji Shirai (Mihama Hospital), Hiroyasu Sugiyama (Fukuyama City Hospital), Takumi Sumimoto (Kitaishikai Hospital), Sho Takahashi (Ibara City Hospital), Hitoshi Takehana (Sanseikai Clinic), Hiroshi Takeshima (Dokkyo Medical University), Masakatsu Todoroki (Dokkyo Medical University), Youkou Tominaga (Yashima General Hospital), Tadao Uraoka (Uraoka Clinic), Hiroshi Yagi (Dokkyo Medical University), Kensei Yahata (Kyoto Medical Center), Ryo Yoshioka (The Sakakibara Heart Institute of Okayama).
Clinical Event Committee
Masanobu Takata (Toyama Nishi General Hospital), Kuniaki Otsuka (Tokyo Women's Medical University), Shinichi Oikawa (Fukujuji Hospital and Nippon Medical School).
Statistical Consulting
Shigeo Yamamura, PhD (Josai International University).
Sources of Funding
This study was supported by the Japan Vascular Disease Research Foundation (Tokyo, Japan).
Disclosures
Dr Ito received a scholarship fund from Fukuda Denshi. Dr Orimo received a consultation fee from Fukuda Denshi. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S2
Acknowledgments
The authors would like to thank all patients and investigators who participated in this study (Appendix), and Miyuki Fujiwara and Masayo Ohmori for their excellent technical assistance.
Supplementary material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020103
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Toru Miyoshi, Email: miyoshit@cc.okayama-u.ac.jp.
the CAVI‐J (Prospective Multicenter Study to Evaluate Usefulness of Cardio‐Ankle Vascular Index in Japan) investigators:
Yuichi Akasaki, Noriko Asahara, Masayuki Doi, Tomikazu Fukuoka, Hiromichi Fukushima, Yuji Hara, Koji Hasegawa, Keiichi Hirano, Takashi Hitsumoto, Toshio Honda, Shigeo Horinaka, Kotaro Ichinari, Toshihiko Ishimitsu, Kimihiko Ishimura, Mai Iwataki, Hiroshi Kaieda, Masahito Kajiya, Shigeshi Kamikawa, Hitoshi Kaneko, Hideo Kawakami, Hajime Kihara, Yuko Kikuchi, Hajime Kiyokawa, Takashi Kobayashi, Wataru Koguchi, Mitsuteru Koizumi, Takuro Kubozono, Motofumi Maguchi, Hitoshi Minowa, Michiaki Miyamoto, Akihito Miyoshi, Kenichi Miyoshi, Toru Miyoshi, Maki Murata, Mitsunobu Murata, Tomoaki Nagao, Kazufumi Nakamura, Keigo Nakamura, Michitsugu Nakamura, Nobuyuki Nakano, Seiji Nanba, Kazuhisa Nishimura, Hachiro Obata, Kazuro Ogurusu, Takefumi Oka, Takafumi Okura, Madoka Onimaru, Shiro Ono, Go Onoue, Atsuhito Saiki, Satoru Sakuragi, Toshihiro Sarashina, Koichi Seta, Yoshimasa Shibata, Kazuhiro Shimizu, Kohji Shirai, Hiroyasu Sugiyama, Takumi Sumimoto, Sho Takahashi, Hitoshi Takehana, Hiroshi Takeshima, Masakatsu Todoroki, Youkou Tominaga, Tadao Uraoka, Hiroshi Yagi, Kensei Yahata, Ryo Yoshioka, Masanobu Takata, Kuniaki Otsuka, Shinichi Oikawa, and Shigeo Yamamura
References
- 1.Chirinos JA, Segers P, Hughes T, Townsend R. Large‐artery stiffness in health and disease: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;74:1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. DOI: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon A, Megnien JL, Chironi G. The value of carotid intima‐media thickness for predicting cardiovascular risk. Arterioscler Thromb Vasc Biol. 2010;30:182–185. DOI: 10.1161/ATVBAHA.109.196980. [DOI] [PubMed] [Google Scholar]
- 4.Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, Inoguchi T, Maeda Y, Kohara K, Tabara Y, et al. Brachial‐ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta‐analysis. Hypertension. 2017;69:1045–1052. DOI: 10.1161/HYPERTENSIONAHA.117.09097. [DOI] [PubMed] [Google Scholar]
- 5.Segers P, Rietzschel ER, Chirinos JA. How to measure arterial stiffness in humans. Arterioscler Thromb Vasc Biol. 2020;40:1034–1043. DOI: 10.1161/ATVBAHA.119.313132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willum‐Hansen T, Staessen JA, Torp‐Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. DOI: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 7.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse‐wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. DOI: 10.1161/01.CIR.0000033824.02722.F7. [DOI] [PubMed] [Google Scholar]
- 8.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. DOI: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 9.Mattace‐Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. DOI: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 10.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. DOI: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsui Y, Kario K, Ishikawa J, Eguchi K, Hoshide S, Shimada K. Reproducibility of arterial stiffness indices (pulse wave velocity and augmentation index) simultaneously assessed by automated pulse wave analysis and their associated risk factors in essential hypertensive patients. Hypertens Res. 2004;27:851–857. DOI: 10.1291/hypres.27.851. [DOI] [PubMed] [Google Scholar]
- 12.Nye ER. The effect of blood pressure alteration on the pulse wave velocity. Br Heart J. 1964;26:261–265. DOI: 10.1136/hrt.26.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure‐independent arterial wall stiffness parameter; cardio‐ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13:101–107. DOI: 10.5551/jat.13.101. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K, Yamamoto T, Takahara A, Shirai K. Clinical assessment of arterial stiffness with cardio‐ankle vascular index: theory and applications. J Hypertens. 2015;33:1742–1757. DOI: 10.1097/HJH.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka A, Tomiyama H, Maruhashi T, Matsuzawa Y, Miyoshi T, Kabutoya T, Kario K, Sugiyama S, Munakata M, Ito H, et al. Physiological diagnostic criteria for vascular failure. Hypertension. 2018;72:1060–1071. DOI: 10.1161/HYPERTENSIONAHA.118.11554. [DOI] [PubMed] [Google Scholar]
- 16.Namekata T, Suzuki K, Ishizuka N, Shirai K. Establishing baseline criteria of cardio‐ankle vascular index as a new indicator of arteriosclerosis: a cross‐sectional study. BMC Cardiovasc Disord. 2011;11:51. DOI: 10.1186/1471-2261-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubozono T, Miyata M, Ueyama K, Nagaki A, Otsuji Y, Kusano K, Kubozono O, Tei C. Clinical significance and reproducibility of new arterial distensibility index. Circ J. 2007;71:89–94. DOI: 10.1253/circj.71.89. [DOI] [PubMed] [Google Scholar]
- 18.Kubozono T, Miyata M, Ueyama K, Nagaki A, Hamasaki S, Kusano K, Kubozono O, Tei C. Association between arterial stiffness and estimated glomerular filtration rate in the Japanese general population. J Atheroscler Thromb. 2009;16:840–845. DOI: 10.5551/jat.1230. [DOI] [PubMed] [Google Scholar]
- 19.Kirigaya J, Iwahashi N, Tahakashi H, Minamimoto Y, Gohbara M, Abe T, Akiyama E, Okada K, Matsuzawa Y, Maejima N, et al. Impact of cardio‐ankle vascular index on long‐term outcome in patients with acute coronary syndrome. J Atheroscler Thromb. 2019;27:657–668. DOI: 10.5551/jat.51409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoh‐Asahara N, Kotani K, Yamakage H, Yamada T, Araki R, Okajima T, Adachi M, Oishi M, Shimatsu A, Japan Obesity and Metabolic Syndrome Study Group . Cardio‐ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis. 2015;242:461–468. DOI: 10.1016/j.atherosclerosis.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, Nagayama D, Saiki A, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, et al. Cardio‐ankle vascular index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb. 2016;23:596–605. DOI: 10.5551/jat.31385. [DOI] [PubMed] [Google Scholar]
- 22.Chung SL, Yang CC, Chen CC, Hsu YC, Lei MH. Coronary artery calcium score compared with cardio‐ankle vascular index in the prediction of cardiovascular events in asymptomatic patients with type 2 diabetes. J Atheroscler Thromb. 2015;22:1255–1265. DOI: 10.5551/jat.29926. [DOI] [PubMed] [Google Scholar]
- 23.Laucevičius A, Ryliškytė L, Balsytė J, Badarienė J, Puronaitė R, Navickas R, Solovjova S. Association of cardio‐ankle vascular index with cardiovascular risk factors and cardiovascular events in metabolic syndrome patients. Medicina. 2015;51:152–158. DOI: 10.1016/j.medici.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Kusunose K, Sato M, Yamada H, Saijo Y, Bando M, Hirata Y, Nishio S, Hayashi S, Sata M. Prognostic implications of non‐invasive vascular function tests in high‐risk atherosclerosis patients. Circ J. 2016;80:1034–1040. DOI: 10.1253/circj.CJ-15-1356. [DOI] [PubMed] [Google Scholar]
- 25.Miyoshi T, Ito H, Horinaka S, Shirai K, Higaki J, Orimo H. Protocol for evaluating the cardio‐ankle vascular index to predict cardiovascular events in Japan: a prospective multicenter cohort study. Pulse (Basel). 2017;4:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Clinical practice recommendations 1999. Diabetes Care. 1999;22:S1–S114. [PubMed] [Google Scholar]
- 27.Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2009). Hypertens Res. 2009;32:3–107. [PubMed] [Google Scholar]
- 28.Committee to Evaluate Diagnostic Standards for Metabolic Syndrome . Definition and the diagnostic standard for metabolic syndrome—committee to evaluate diagnostic standards for metabolic syndrome. Nihon Naika Gakkai Zasshi. 2005;94:794–809. [PubMed] [Google Scholar]
- 29.Japan Nephrology Society . Special issue: clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012. Nihon Jinzo Gakkai Shi. 2012;54:1034–1191. [PubMed] [Google Scholar]
- 30.Takahashi K, Yamamoto T, Tsuda S, Okabe F, Shimose T, Tsuji Y, Suzuki K, Otsuka K, Takata M, Shimizu K, et al. Coefficients in the CAVI equation and the comparison between CAVI with and without the coefficients using clinical data. J Atheroscler Thromb. 2019;26:465–475. DOI: 10.5551/jat.44834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Cordes M, Recio‐Rodriguez JI, Garcia‐Ortiz L, Hanssen H, Schmidt‐Trucksass A. Diurnal variation of arterial stiffness in healthy individuals of different ages and patients with heart disease. Scand J Clin Lab Invest. 2014;74:155–162. DOI: 10.3109/00365513.2013.864787. [DOI] [PubMed] [Google Scholar]
- 32.Kumagai T, Kasai T, Kato M, Naito R, Maeno K, Kasagi S, Kawana F, Ishiwata S, Narui K. Establishment of the cardio‐ankle vascular index in patients with obstructive sleep apnea. Chest. 2009;136:779–786. DOI: 10.1378/chest.09-0178. [DOI] [PubMed] [Google Scholar]
- 33.Lim J, Pearman ME, Park W, Alkatan M, Machin DR, Tanaka H. Impact of blood pressure perturbations on arterial stiffness. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1540–R1545. DOI: 10.1152/ajpregu.00368.2015. [DOI] [PubMed] [Google Scholar]
- 34.Kubota Y, Maebuchi D, Takei M, Inui Y, Sudo Y, Ikegami Y, Fuse J, Sakamoto M, Momiyama Y. Cardio‐ankle vascular index is a predictor of cardiovascular events. Artery Res. 2011;5:91–96. DOI: 10.1016/j.artres.2011.03.005. [DOI] [Google Scholar]
- 35.Yamazaki T, Kohro T, Chujo M, Ishigaki M, Hashimoto T. The occurrence rate of cerebrovascular and cardiac events in patients receiving antihypertensive therapy from the post‐marketing surveillance data for valsartan in Japan (J‐VALID). Hypertens Res. 2013;36:140–150. DOI: 10.1038/hr.2012.154. [DOI] [PubMed] [Google Scholar]
- 36.Vlachopoulos C, Terentes‐Printzios D, Laurent S, Nilsson PM, Protogerou AD, Aznaouridis K, Xaplanteris P, Koutagiar I, Tomiyama H, Yamashina A, et al. Association of estimated pulse wave velocity with survival: a secondary analysis of SPRINT. JAMA Netw Open. 2019;2:e1912831. DOI: 10.1001/jamanetworkopen.2019.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawai T, Ohishi M, Onishi M, Ito N, Takeya Y, Maekawa Y, Rakugi H. Cut‐off value of brachial‐ankle pulse wave velocity to predict cardiovascular disease in hypertensive patients: a cohort study. J Atheroscler Thromb. 2013;20:391–400. DOI: 10.5551/jat.15040. [DOI] [PubMed] [Google Scholar]
- 38.Ninomiya T, Kojima I, Doi Y, Fukuhara M, Hirakawa Y, Hata J, Kitazono T, Kiyohara Y. Brachial‐ankle pulse wave velocity predicts the development of cardiovascular disease in a general Japanese population: the Hisayama Study. J Hypertens. 2013;31:477–483. DOI: 10.1097/HJH.0b013e32835c5c23. [DOI] [PubMed] [Google Scholar]
- 39.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. DOI: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 40.Witteman JC, Grobbee DE, Valkenburg HA, van Hemert AM, Stijnen T, Burger H, Hofman A. J‐shaped relation between change in diastolic blood pressure and progression of aortic atherosclerosis. Lancet. 1994;343:504–507. DOI: 10.1016/S0140-6736(94)91459-1. [DOI] [PubMed] [Google Scholar]
- 41.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics–2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 42.Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015;17:884–892. DOI: 10.1002/ejhf.319. [DOI] [PubMed] [Google Scholar]
- 43.Takagi K, Ishihara S, Kenji N, Iha H, Kobayashi N, Ito Y, Nohara T, Ohkuma S, Mitsuishi T, Ishizuka A, et al. Clinical significance of arterial stiffness as a factor for hospitalization of heart failure with preserved left ventricular ejection fraction: a retrospective matched case‐control study. J Cardiol. 2020;76:171–176. DOI: 10.1016/j.jjcc.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Namba T, Masaki N, Matsuo Y, Sato A, Kimura T, Horii S, Yasuda R, Yada H, Kawamura A, Takase B, et al. Arterial stiffness is significantly associated with left ventricular diastolic dysfunction in patients with cardiovascular disease. Int Heart J. 2016;57:729–735. DOI: 10.1536/ihj.16-112. [DOI] [PubMed] [Google Scholar]
- 45.Cotter G, Metra M, Milo‐Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure–re‐distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10:165–169. DOI: 10.1016/j.ejheart.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Lim J, Pearman M, Park W, Alkatan M, Tanaka H. Interrelationships among various measures of central artery stiffness. Am J Hypertens. 2016;29:1024–1028. DOI: 10.1093/ajh/hpw045. [DOI] [PubMed] [Google Scholar]
- 47.Wohlfahrt P, Cifkova R, Movsisyan N, Kunzova S, Lesovsky J, Homolka M, Soska V, Dobsak P, Lopez‐Jimenez F, Sochor O. Reference values of cardio‐ankle vascular index in a random sample of a white population. J Hypertens. 2017;35:2238–2244. DOI: 10.1097/HJH.0000000000001437. [DOI] [PubMed] [Google Scholar]
- 48.Gomez‐Sanchez M, Patino‐Alonso MC, Gomez‐Sanchez L, Recio‐Rodriguez JI, Rodriguez‐Sanchez E, Maderuelo‐Fernandez JA, Garcia‐Ortiz L, Gomez‐Marcos MA, EVA Group . Reference values of arterial stiffness parameters and their association with cardiovascular risk factors in the Spanish population: the EVA Study. Rev Esp Cardiol (Engl Ed). 2020;73:43–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2


