Abstract
Background
In prior unblinded studies, cardiac neuromodulation therapy (CNT) employing a sequence of variably timed short and longer atrioventricular intervals yielded sustained reductions of systolic blood pressure (SBP) in patients with hypertension. The effects of CNT on SBP were investigated in this double‐blind randomized pilot study.
Methods and Results
Eligible patients had daytime ambulatory SBP (aSBP) ≥130 mm Hg and office SBP ≥140 mm Hg despite taking ≥1 antihypertensive medication, and an indication for a dual‐chamber pacemaker. Patients underwent Moderato device implantation, which was programmed as a standard pacemaker during a 1‐month run‐in phase. Patients whose daytime aSBP was ≥125 mm Hg at the end of this period were randomized (1:1, double blind) to treatment (CNT) or control (CNT inactive). The primary efficacy end point was the between‐group difference of the change in 24‐hour aSBP at 6 months. Of 68 patients initially enrolled and who underwent implantation with the Moderato system, 47 met criteria for study continuation and were randomized (26 treatment, 21 control). The mean age was 74.0±8.7 years, 64% were men, left ventricular ejection fraction was 59.2%±5.7%, and aSBP averaged 141.0±10.8 mm Hg despite the use of 3.3±1.5 antihypertensive medications; 81% had isolated systolic hypertension. Six months after randomization, aSBP was 11.1±10.5 mm Hg (95% CI, −15.2 to −8.1 mm Hg) lower than prerandomization in the treatment group compared with 3.1±9.5 mm Hg (−7.4 to 1.2 mm Hg) lower in controls, yielding a net treatment effect of 8.1±10.1 mm Hg (−14.2 to −1.9 mm Hg) (P=0.012). There were no Moderato device– or CNT‐related adverse events.
Conclusions
CNT significantly reduced 24‐hour aSBP in patients with hypertension with a clinical indication for a pacemaker. The majority of patients had isolated systolic hypertension, a particularly difficult group of patients to treat.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02837445.
Keywords: atrioventricular interval, hypertension, left ventricular function, pacemaker
Subject Categories: Hypertension, High Blood Pressure
Nonstandard Abbreviations and Acronyms
- aBP
ambulatory blood pressure
- aSBP
ambulatory systolic blood pressure
- CNT
cardiac neuromodulation therapy
- DBP
diastolic blood pressure
- ISH
isolated systolic hypertension
- MODERATO II
Double‐Blind Randomized Trial Of Cardiac Neuromodulation Therapy In Patients With Hypertension
- oBP
office blood pressure
- oSBP
office systolic blood pressure
- SBP
systolic blood pressure
Clinical Perspective
What Is New?
BackBeat Cardiac Neuromodulation Therapy is a programmable and adjustable bioelectronic therapy delivered via an active implantable cardiac pulse generator that mimics the effects of multidrug hypertension treatment by targeting preload, afterload, and sympathetic tone to immediately, substantially, and persistently lower blood pressure while simultaneously modulating the autonomic nervous system.
The MODERATO II (Double‐Blind Randomized Trial Of Cardiac Neuromodulation Therapy In Patients With Hypertension) pilot study showed that cardiac neuromodulation therapy significantly reduces 24‐hour ambulatory and office systolic blood pressures in patients with hypertension despite medical therapy and an indication for a pacemaker, with the majority of patients having isolated systolic hypertension, which is a particularly difficult group to treat.
What Are the Clinical Implications?
Patients with hypertension who require a pacemaker may benefit from cardiac neuromodulation therapy to reduce blood pressure without incurring the risk to patients of additional medications, procedures, or device implants.
More than a million patients undergo implantation or replacement of a pacemaker every year, of which >70% have hypertension. The high prevalence of hypertension is primarily attributable to the fact that the pacemaker population is elderly—average age of 70 years—and has a high prevalence of cardiovascular comorbidities.1 The majority of these people have isolated systolic hypertension (ISH), and therefore their hypertension may be more difficult to treat.2 Indeed, there is a high rate of uncontrolled hypertension despite pharmacological therapy in the pacemaker population.3 Accordingly, hypertension therapy in the form of an algorithm embedded in a standard pacemaker is appealing for this population, since the risk‐benefit profile of hypertension therapy excludes the risks associated with the clinically indicated pacemaker device and implant procedure.
In prior unblinded studies, a pacemaker‐based cardiac neuromodulation therapy (CNT) delivered by the Moderato implantable pulse generator (BackBeat Medical, an Orchestra BioMed company) employing a sequence of variably timed short and longer atrioventricular intervals reduced SBP within minutes (see Figure S1).4 Relative to baseline, sustained reductions in 24‐hour ambulatory SBP (aSBP) by >10 mm Hg were demonstrated at 3‐month follow‐up and office SBP (oSBP) was reduced by >15 mm Hg through 2 years of follow‐up.5 The mechanism of SBP reduction involves a combination of decreased ventricular preload and modulation of the autonomic nervous system to prevent baroceptor‐based sympathetic activation that might ordinarily restore SBP.4
Experience with other device‐based treatments for hypertension have underscored the importance of accounting for the potential impact of placebo and Hawthorne effects in the assessment of their safety and effectiveness.6 Accordingly, we conducted a prospective, double‐blind, randomized pilot study of the safety and efficacy of CNT to reduce blood pressure (BP) in patients with persistent hypertension despite medical treatment and an indication for pacemaker implantation or replacement.
METHODS
The authors indicate that they will not make their data, analytic methods, and study materials available to other researchers.
Trial Design and Patient Population
The MODERATO II study was a prospective, multicenter, double‐blind pilot study investigating the efficacy of BackBeat CNT in patients with persistent hypertension (defined below) and an indication for implantation or replacement of a dual‐chamber pacemaker. The results of this study were intended to inform the design of a future, fully powered pivotal study to evaluate safety and efficacy. Details concerning the organization and conduct of the trial, a protocol synopsis, and a list of participating centers are provided in Data S1. The trial was sponsored by BackBeat Medical, an Orchestra BioMed company. The study was approved by the ethics committee at each participating center, and all patients provided written informed consent. This study was registered at clinicaltrials.gov (NCT02837445 Version 1.1 or 3.0).
Enrollment, Randomization, and Follow‐Up
The overall study design is summarized in Figure S2; a full schedule of events is provided in Table S1. Patients indicated for implantation or replacement of a dual‐chamber pacemaker with a history of hypertension were screened at 13 centers in Europe. Individuals included in the study were required to be ≥18 years of age and be on stable (for prior 6 weeks) treatment for hypertension, with average daytime (7 am to 10 pm) aSBP ≥130 mm Hg and oSBP ≥140 mm Hg. The main study exclusion criteria were known secondary cause of hypertension, average aSBP or oSBP >195 mm Hg, permanent atrial fibrillation or history of significant paroxysmal atrial fibrillation/flutter burden (defined as >25% of beats), left ventricular (LV) ejection fraction <50%, symptoms of heart failure (New York Heart Association class ≥II), estimated glomerular filtration rate (<30 mL/min per 1.73 m2), or history of neurological events (stroke or transient ischemic attack within the past year). Inclusion and exclusion criteria are detailed further in the protocol synopsis of Data S1.
Patients who met the initial entry criteria underwent Moderato device implantation. The system's standard pacemaker parameters were programmed per the clinical needs of the patient and were followed for a 1‐month run‐in phase; CNT signals were not turned on in any patient during this phase. This allowed for assessment of Hawthorne effects on aSBP so that patients whose BPs were readily controlled by medical therapy could be excluded and only those who had hypertension despite medical therapy were included (detailed further in Data S1). To achieve this, ambulatory BP (aBP) was reassessed at a 3‐week visit. Patients whose average daytime aSBP was <125 mm Hg were withdrawn from the study. Otherwise, patients were eligible for study inclusion in the randomized phase of the study and underwent CNT activation and parameter optimization as previously detailed.5 We based criteria for continued study eligibility on aBP (which is more objective than office BP [oBP]) with a 125‐mm Hg cutoff value, knowing that oBP (upon which guidelines for diagnosing and treating hypertension) would be at least 10 mm Hg higher; this was indeed confirmed as detailed in the Results section. A prerandomization echocardiogram was also performed.
Patients were randomized to either have CNT remain deactivated (control group) or for CNT to be activated (treatment group); both groups continued with prerandomization medical therapies, which were to remain constant throughout the study unless required based on clinical need. Randomization was provided by a centralized electronic system and was in blocks of 4 at each site and was stratified based on whether patients were 100% pacemaker dependent.
aBP was measured using an oscillometric Spacelabs 90207‐1 monitor (Spacelabs Healthcare). Data were transferred electronically to a centralized core laboratory for blinded analysis. According to guideline recommendations, oBPs were measured with patients seated using the automatic Omron BP monitor (model number 705, Omron Healthcare, Inc). An average of at least 3 measurements were used to quantify oSBP at each visit; additional details concerning the methods used to measure aBPs and oBPs are provided in Data S1. Patients, core laboratories, and all study personnel were blinded to group assignment, except for one dedicated “unblinded” physician at each site; no known unblinding occurred. Following randomization, a 24‐hour aBP monitor was applied to assess the short‐term BP effects of CNT. Patients were subsequently seen at months 1, 3, and 6 following randomization for assessment of interim medical history (including any medication changes and measurement of oBP). Twenty‐four‐hour aBP measurements, echocardiograms, and blood tests were performed at 1 and 6 months. All of these tests were assessed in blinded core laboratories. Echocardiographic images were obtained at each time point with CNT off and on (in both groups) so that the sonographer and the reader remained blinded to treatment group.
Device and CNT Therapy Description
The Moderato system is a dual‐chamber, rate‐responsive pacemaker implantable pulse generator capable of delivering CNT that paces the heart with a series of specified, variably timed, alternating short (eg, 20–80 ms) and longer (eg, 100–180 ms) atrioventricular intervals; the principle has been previously described5 and is detailed along with a typical acute BP response to initiation of CNT therapy in Figure S1.
End Points
The primary efficacy end point of this study was the between‐group comparison of the change in average 24‐hour aSBP from prerandomization at the end of the run‐in phase to 6 months postrandomization. The primary safety end point was an evaluation of the composite rate of major cardiac adverse events, including heart failure, clinically significant arrhythmias (eg, persistent or increased atrial fibrillation burden, serious ventricular arrhythmias), myocardial infarction, stroke, heart failure, renal failure, and/or other related safety events that result in death, in the treatment versus the control groups. A series of additional exploratory end points are summarized in Data S1.
Statistical Analysis
This double‐blind pilot study focused on assessing the impact of CNT on BP. Based on the prior unblinded study, it was estimated that with an anticipated SD of 10 mm Hg in the 24‐hour aSBP in each group, 50 evaluable patients would provide 80% power to detect an ≥8 mm Hg between‐group difference in changes in 24‐hour aSBP. The study was not powered for a safety evaluation. The goal was to gather information related to expected safety event rates in the specified patient population.
The between‐group difference of changes in aSBP (primary end point) was evaluated in an intention‐to‐treat analysis with the primary analysis based on a t test. ANCOVA analysis was also performed that accounted for baseline values of aSBP. Assessments of other end points consisted of comparisons of parameter values between the last evaluated values before randomization (prerandomization and 6‐month follow‐up tests). In the case of aBP, the test was performed at week 3 of the run‐in period. For other values, exploratory end points (detailed in Data S1) at week 4 of the run‐in period immediately before randomization were used. No corrections were made for multiple comparisons of other end points. Outside of determining a P value for the primary end point, all other statistics (including P values) are considered descriptive. Continuous variables are described by means and SDs; they were compared using Student t test. All between‐group treatment effects are summarized as means along with SDs and 95% CIs. Categorical variables are described by absolute and relative frequencies; they were compared using chi‐square test or Fisher exact test. A P value of 0.05 was considered significant for all tests. Statistical analyses were performed with Matlab statistical toolbox (version R2019b; The MathWorks, Inc) and Excel (Microsoft).
RESULTS
Patients
Patient flow through the study is summarized in Figure 1. A total of 196 patients signed informed consent for screening, and 128 did not meet initial entry criteria: 50 (39%) because of an aSBP value <130 mm Hg; 34 (27%) with oSBP <140 mm Hg; 15 (12%) withdrew consent; 5 (4%) with LV ejection fraction <50%; and 24 (18%) for other reasons. The 68 patients who met entry criteria had a Moderato implant; their demographics are summarized in Table 1. Patients averaged 74 years of age, 57% were men, and there was a high prevalence of comorbidities, including diabetes mellitus, coronary artery disease, and history of atrial fibrillation. The Moderato system was a first‐time pacemaker implant for 68% of the patients and was a replacement device in 32%. The main indications for pacing were sick sinus syndrome and second‐degree atrioventricular conduction block. Ten percent of patients were 100% pacemaker dependent. Patients were taking an average of 3 to 4 antihypertensive medications; the breakdown of medications by drug class is summarized in Table 1.
Figure 1. Flow diagram of study patients: Consolidated Standards of Reporting Trials (CONSORT) diagram showing flow of patients through the study.
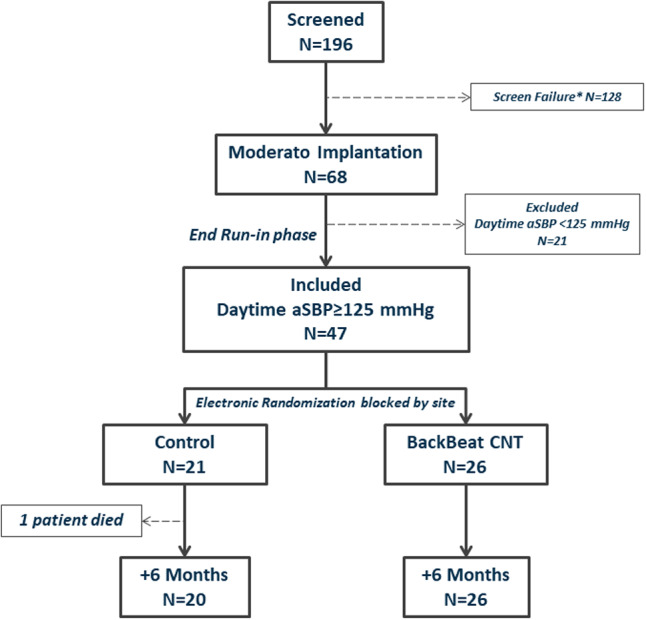
*Screen failures included 50 (39%) patients with ambulatory systolic blood pressure (aSBP) <130 mm Hg; 34 (27%) with office blood pressure <140 mm Hg; 15 (12%) who withdrew consent; 5 (4%) with left ventricular ejection fraction <50%; and 18% for other reasons. CNT indicates cardiac neuromodulation therapy.
Table 1.
Patient Demographics
| All Patients With Implantation (n=68) | Withdrawn at the End of Run‐in Because of BP Criterion (n=21) | All Randomized Following Run‐in (n=47) | P (Withdrawn vs Randomized) | Control (n=21) | Treatment (n=26) | P (Control vs Treatment) | |
|---|---|---|---|---|---|---|---|
| Age, y | 74.2±8.3 | 74.7±7.5 | 74.0±8.7 | 0.755 | 74.9±8.5 | 73.2±9.0 | 0.518 |
| Men | 39 (57.4) | 9 (42.9) | 30 (63.8) | 0.121 | 15 (71.4) | 15 (57.7) | 0.375 |
| Height, cm | 167.9±8.6 | 165.7±8.2 | 168.8±8.7 | 0.17 | 168.0±7.1 | 169.5±10.0 | 0.587 |
| Weight, kg | 85.8±15.1 | 82.6±10.6 | 87.1±16.7 | 0.258 | 88.5±16.0 | 86.1±17.5 | 0.630 |
| LV ejection fraction, % | 59.6±5.8 | 60.4±6.0 | 59.2±5.7 | 0.46 | 58.4±4.9 | 59.8±6.3 | 0.414 |
| Medical history | |||||||
| Diabetes mellitus | 29 (42.6) | 8 (38.1) | 21 (44.7) | 0.791 | 9 (42.9) | 12 (46.2) | 0.999 |
| Prior atrial fibrillation | 17 (25.0) | 6 (28.6) | 11 (23.4) | 0.764 | 6 (28.6) | 5 (19.2) | 0.505 |
| Coronary artery disease | 23 (33.8) | 4 (19) | 19 (40.4) | 0.103 | 9 (42.9) | 10 (38.5) | 0.775 |
| Stroke | 2 (2.9) | 1 ( 4.8) | 1 (2.1) | 0.526 | 0 (0) | 1 (3.8) | 0.999 |
| Pacemaker | |||||||
| New implant | 46 (67.6) | 16 (76.2) | 30 (63.8) | 0.405 | 15 (71.4) | 15 (57.7) | 0.375 |
| Replacement | 22 (32.4) | 5 (23.8) | 17 (36.2) | 6 (28.6) | 11 (42.3) | ||
| Indication | |||||||
| Sick sinus syndrome | 23 (33.8) | 7 (33.3) | 16 (34.0) | 0.999 | 9 (42.9) | 7 (26.9) | 0.355 |
| Bradycardia | 13 (19.1) | 4 (19.0) | 9 (19.1) | 0.999 | 5 (23.8) | 4 (15.4) | 0.486 |
| Atrioventricular block I | 10 (14.7) | 2 (9.5) | 8 (17.0) | 0.712 | 4 (19.0) | 4 (15.4) | 0.999 |
| Atrioventricular block II | 20 (29.4) | 4 (19.0) | 16 (34.0) | 0.259 | 5 (23.8) | 11 (42.3) | 0.227 |
| Atrioventricular block III | 7 (10.3) | 3 (14.3) | 4 (8.5) | 0.668 | 1 (4.8) | 3 (11.5) | 0.617 |
| Other | 2 (2.9) | 0 (0) | 2 (4.3) | 0.999 | 1 (4.8) | 1 (3.8) | 0.999 |
| Medications, n | 3.4±1.7 | 3.5±2.1 | 3.3±1.5 | 0.691 | 3.3±1.4 | 3.3±1.6 | 0.886 |
| Medication use | |||||||
| Loop diuretic | 49 (72.1) | 19 (90.5) | 30 (63.8) | 0.039 | 14 (66.7) | 16 (61.5) | 0.768 |
| Potassium‐sparing diuretic | 5 (7.4) | 1 (4.8) | 4 (8.5) | 0.955 | 2 (9.5) | 2 (7.7) | 0.999 |
| ß‐Blocker | 24 (35.3) | 5 (23.8) | 19 (40.4) | 0.273 | 6 (28.6) | 13 (50) | 0.232 |
| ACEI | 37 (54.4) | 8 (38.1) | 29 (61.7) | 0.113 | 15 (71.4) | 14 (53.8) | 0.245 |
| ARB | 29 (42.6) | 12 (57.1) | 17 (36.2) | 0.121 | 6 (28.6) | 11 (42.3) | 0.375 |
| CCB | 46 (67.6) | 15 (71.4) | 31 (66.0) | 0.782 | 14 (66.7) | 17 (65.4) | 0.999 |
| α‐Agonist | 14 (20.6) | 6 (28.6) | 8 (17.0) | 0.336 | 5 (23.8) | 3 (11.5) | 0.437 |
| Centrally acting agent | 6 (8.8) | 1 (4.8) | 5 (10.6) | 0.658 | 3 (14.3) | 2 (7.7) | 0.644 |
Values are mean±SD or number (percentage). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, aldosterone receptor blocker; BP, blood pressure; CCB, calcium channel blocker; and LV, left ventricular.
Upon initial enrollment, oBP averaged 162.6±14.5/82.0±10.4 mm Hg and daytime aBP averaged 142.0±10.3/76.0±8.1 mm Hg (Table 2). Based on both ambulatory and office readings, 84% of patients had ISH with diastolic BP (DBP) <90 mm Hg.
Table 2.
BP During Screening and Prerandomization
| All Patients With Implantion(n=68) | Withdrawn at the End of Run‐in Because of BP Criterion (n=21) | All Randomized Following Run‐in (n=47) | P (Withdrawn vs Randomized) | Control (n=21) | Treatment (n=26) | P (Control vs Treatment) | |
|---|---|---|---|---|---|---|---|
| Screening | |||||||
| ISH | 57 (83.8) | 19 (90.5) | 38 (80.9) | 0.482 | 15 (71.4) | 23 (88.5) | 0.263 |
| oSBP, mm Hg | 162.6±14.5 | 161.3±14.4 | 163.1±14.6 | 0.654 | 165.2±15.3 | 161.4±14.1 | 0.381 |
| oDBP, mm Hg | 82.0±10.4 | 80.7±10.1 | 82.5±10.6 | 0.522 | 82.4±13.0 | 82.6±8.5 | 0.955 |
| oHeart rate, beats per min | 64.7±13.4 | 66.1±15.5 | 65.9±10.9 | 0.586 | 63.7±16.6 | 64.4±8.3 | 0.860 |
| aSBP, mm Hg (d) | 142.0±10.3 | 140.3±8.3 | 142.8±11.1 | 0.36 | 145.2±12.5 | 140.7±9.5 | 0.168 |
| aDBP, mm Hg (d) | 76.0±8.1 | 75.8±8.8 | 76.1±7.9 | 0.889 | 76.8±5.7 | 75.6±5.7 | 0.605 |
| aHeart rate, beats per min (d) | 67.3±10.6 | 69.9±9.7 | 66.1±10.9 | 0.17 | 66.3±12.8 | 65.9±9.3 | 0.886 |
| aSBP, mm Hg (night) | 132.1±17.1 | 122.3±13.0 | 136.6±16.9 | 0.001 | 138.2±17.3 | 135.2±16.9 | 0.558 |
| aDBP, mm Hg (night) | 68.8±9.4 | 65.3±9.5 | 70.4±9.0 | 0.037 | 71.5±11.2 | 69.4±6.6 | 0.438 |
| aHeart rate, beats per min (night) | 60.7±9.4 | 61.1±9.0 | 60.5±9.7 | 0.809 | 61.3±13.1 | 59.8±5.9 | 0.624 |
| aSBP, mm Hg (24 h) | 139.1±10.3 | 135.0±8.0 | 141.0±10.8 | 0.025 | 143.1±11.2 | 139.2±10.3 | 0.231 |
| aDBP, mm Hg (24 h) | 74.0±7.7 | 72.8±8.3 | 74.5±7.5 | 0.409 | 75.3±9.7 | 73.8±5.0 | 0.497 |
| aHeart rate, beats per min (24 h) | 65.4±9.9 | 67.4±9.2 | 64.5±10.2 | 0.275 | 65.0±12.6 | 64.1±8.0 | 0.775 |
| Three‐wk run‐in phase | |||||||
| aSBP, mm Hg (d) | 133.1±13.4 | 122.0±11.3 | 137.9±11.3 | <0.001 | 137.9±12.4 | 137.9±10.6 | 0.988 |
| aDBP, mm Hg (d) | 73.3±7.8 | 69.6±8.4 | 74.9±7.1 | 0.011 | 74.2±6.9 | 75.4±7.3 | 0.565 |
| aHeart rate, beats per min (d) | 70.2±9.2 | 69.7±6.5 | 70.5±10.2 | 0.756 | 69.6±9.4 | 71.2±10.9 | 0.614 |
| aSBP, mm Hg (night) | 126.9±14.2 | 114.5±10.5 | 132.2±12.2 | <0.001 | 132.0±14.5 | 132.4±10.1 | 0.919 |
| aDBP, mm Hg (night) | 67.5±8.2 | 63.4±7.9 | 69.3±7.7 | 0.006 | 68.1±7.6 | 70.2±7.8 | 0.367 |
| aHeart rate, beats per min (night) | 65.1±6.4 | 64.9±5.8 | 65.2±6.7 | 0.828 | 64.9±6.3 | 65.5±7.2 | 0.761 |
| aSBP, mm Hg (24 h) | 131.5±13.0 | 120.1±10.7 | 136.3±10.7 | <0.001 | 136.3±12.5 | 136.3±9.2 | 0.995 |
| aDBP, mm Hg (24 h) | 71.8±7.5 | 68.0±7.9 | 73.3±6.8 | 0.007 | 72.6±6.7 | 74.0±6.9 | 0.478 |
| aHeart rate, beats per min (24 h) | 68.9±8.2 | 68.5±6.2 | 69.1±9.0 | 0.786 | 68.4±8.5 | 69.6±9.5 | 0.670 |
| Four‐wk run‐in phase | |||||||
| oSBP, mm Hg | 150.2±16.5 | 141.0±15.5 | 153.7±15.6 | 0.005 | 154.4±15.5 | 153.1±15.9 | 0.773 |
| oDBP, mm Hg | 80.9±11.4 | 77.0±10.7 | 82.3±11.4 | 0.092 | 81.6±12.4 | 82.9±10.7 | 0.706 |
| oHeart rate, beats per min | 66.8±10.5 | 66.9±7.8 | 66.8±11.4 | 0.987 | 66.5±10.9 | 67.1±12.1 | 0.847 |
| Response to acute CNT activation, mm Hg | −15.0±10.2 | −15.2±9.6 | 0.955 | ||||
Values are mean±SD or number (percentage). aDBP indicates ambulatory diastolic blood pressure; aSBP, ambulatory systolic blood pressure; BP, blood pressure; CNT, cardiac neuromodulation therapy; ISH, isolated systolic hypertension; oDBP, office diastolic blood pressure; and oSBP, office systolic blood pressure.
BP During the Study Run‐in Phase
oBPs and aBPs decreased during the run‐in phase, which can be attributed to the well‐known Hawthorne effect (Table 2) discussed further in Data S1. From among the 68 patients with implantation of the Moderato system, daytime aSBP dropped below the cutoff for continued study participation in 21 patients, in whom average daytime aSBP dropped by 18 mm Hg, average 24‐hour aSBP dropped by 15 mm Hg, and average oSBP dropped by 19 mm Hg. In contrast, while BP also decreased in the 47 patients who met criteria for study continuation, the magnitude of the change in BP was significantly smaller, with 24‐hour aSBP decreasing by 5±10.5 mm Hg and oSBP decreasing by 9±19.3 mm Hg. There were no other significant demographic differences between those who did and those who did not qualify for study continuation (detailed in Table 2).
CNT Parameters Optimization and Randomization
All 47 patients who met study continuation criteria underwent CNT activation and parameter optimization. oSBP dropped acutely by a mean of 15.1±9.7 mm Hg. Patients were then randomized (1:1) to either have CNT deactivated (control group, n=21) or to continue with active CNT (treatment group, n=26). Groups were balanced with regard to baseline characteristics (Table 1), baseline BPs, and initial immediate response to CNT (Table 2). It is important to note that patients did not experience any symptoms or sensations associated with the short atrioventricular delay beats, so this did not emerge as an issue related to tolerability of the therapy or unblinding.
BP During the Randomized Study Period
Results of 24‐hour aSBP monitoring for all randomized patients are summarized in Figure 2; absolute values are summarized in Figure 2A and changes in aSBP from prerandomization values are summarized in Figure 2B. As detailed above, aSBP decreased similarly in control and treatment patients during the run‐in study phase (when devices were programmed in pacing‐only mode without CNT).
Figure 2. Ambulatory blood pressure results.
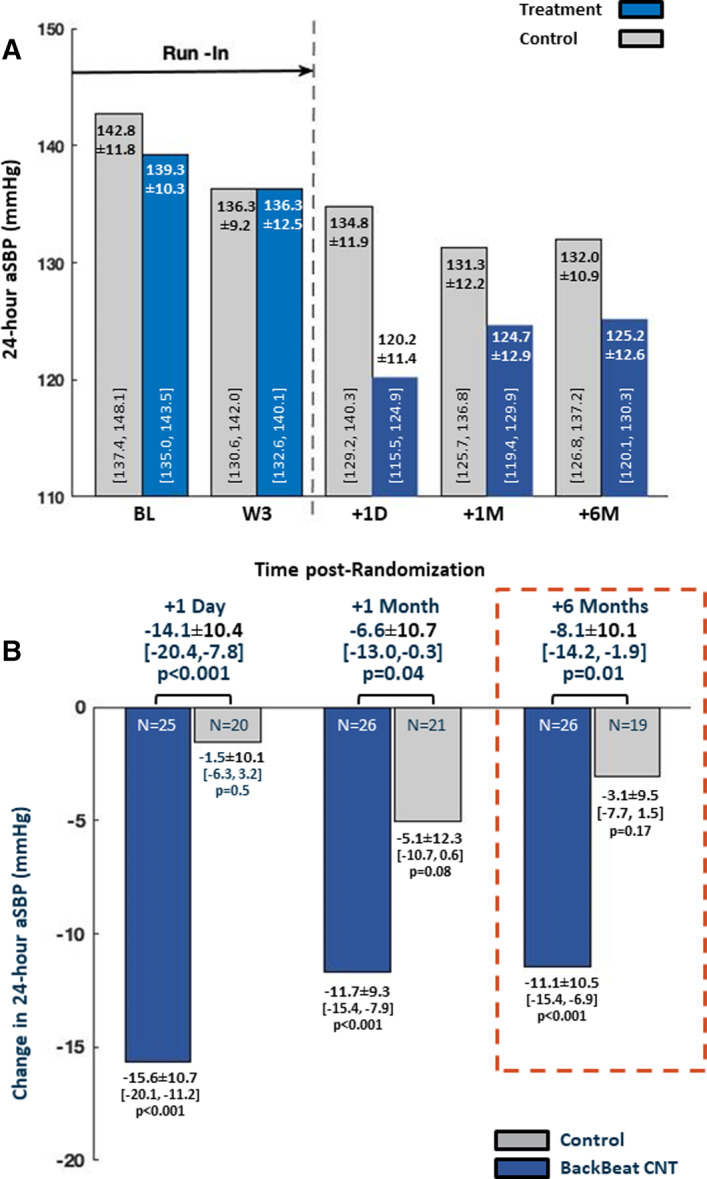
A, Comparison of 24‐hour ambulatory systolic blood pressure (aSBP) between groups over the entire course of the study. B, Between‐group comparisons of change in 24‐hour aSBP relative to the 3‐week prerandomization values. The +6‐month data (red dashed box) show the study primary end point. CNT indicates cardiac neuromodulation therapy.
Twenty‐four hours following randomization, aSBP decreased by 15.6±10.7 mm Hg (95% CI, −20.1 to −11.2 mm Hg) in the treatment group compared with a 1.5±10.1‐ mm Hg (95% CI, −6.3 to 3.2 mm Hg) decrease in the control group, yielding a net aSBP reduction of 14.1±10.4 mm Hg (95% CI, −20.4 to −7.8 mm Hg) (P<0.001). At 6 months following randomization (primary end point), aBP measurements were available in all treatment patients and in 19 of 21 control patients; 1 control patient died before the 6‐month follow‐up and measurements were technically unsuccessful in another patient despite 2 attempts. At this 6‐month time point, aSBP was 11.1±10.5 mm Hg (95% CI, −15.4 to −6.9 mm Hg) lower than prerandomization in the treatment group compared to 3.1±9.5 mm Hg (95% CI, −7.7 to 1.5 mm Hg) lower in control patients, yielding a net treatment effect of an 8.1±10.1 mm Hg (95% CI, −14.2 to −1.9 mm Hg) (P=0.012) reduction of aSBP. Similarly, results of ANCOVA analysis, which accounted for baseline values of aSBP, yielded a −7.7±9.8 mm Hg (95% CI, −13.7 to −1.7 mm Hg) between‐group treatment effect (P=0.013). The substitution of missing values in the control group with the worst result of the group did not alter primary efficacy conclusions.
Results at intermediate time points are summarized in Figure 2A. When summarizing results in terms of a responder analysis (detailed in Data S1), 85% of the patients in the treatment group had a decrease in aSBP compared with 63% in the control group (P=0.03); 54% of treatment patients versus 37% of control patients had a decrease >10 mm Hg (P=0.03). Fan plots, which provide a graphical means of comparing BP changes between groups are provided in Figure S3.
Antihypertension medical therapies were tracked during the 6‐month study period. As summarized in Table 3, there were relatively few prescribed medication changes in the treatment group and these were reasonably balanced between the number of dose increases and dose decreases within each drug class. In contrast, there were twice as many prescribed drug dose increases than decreases in the control group, suggesting that the observed change in aSBP may have underestimated the true treatment effect. Indeed, there were 14 control and 23 treatment patients who had no prescribed medication changes; in these patients, the between‐group difference in aSBP at 6 months was 11.2±10.0 mm Hg (95% CI, −18.3 to −4.2 mm Hg) (P=0.003).
Table 3.
Number of Medication Changes (Any Increase or Decrease in Dose) Between Baseline and 6‐Month Follow‐Up by Study Group and by Drug Class
| Patients With a Change in Medications, n (%) | Treatment | Control | ||
|---|---|---|---|---|
| 3 (11.5%) | 7 (33.0%) | |||
| Increase | Decrease | Increase | Decrease | |
| Diuretic | 3 | 2 | 4 | 2 |
| ACEI | 0 | 1 | 3 | 1 |
| ARB | 1 | 1 | 0 | 1 |
| β‐Blocker | 1 | 1 | 2 | 0 |
| Potassium‐sparing diuretics | 1 | 0 | 1 | 1 |
| CCB | 0 | 1 | 1 | 1 |
| Sum of all changes | 6 | 6 | 11 | 6 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; and CCB, calcium channel blocker.
Between‐group differences in oSBP paralleled those obtained with aSBP (Figure 3A), with 5.1±14.2 mm Hg (95% CI, −13.5 to 3.3 mm Hg), 14.6±15.9 mm Hg (95% CI, −24.0 to −5.2 mm Hg), and 12.3±16.9 mm Hg (95% CI, −22.4 to −2.2 mm Hg) net reductions in favor of the treatment group at 1, 3, and 6 months, respectively (Figure 3B). Additionally, changes in orthostatic BPs from preactivation to the 6‐month visit did not differ between groups (P=0.20) and no patient reported symptoms related to hypotension in either group.
Figure 3. Office blood pressure results.
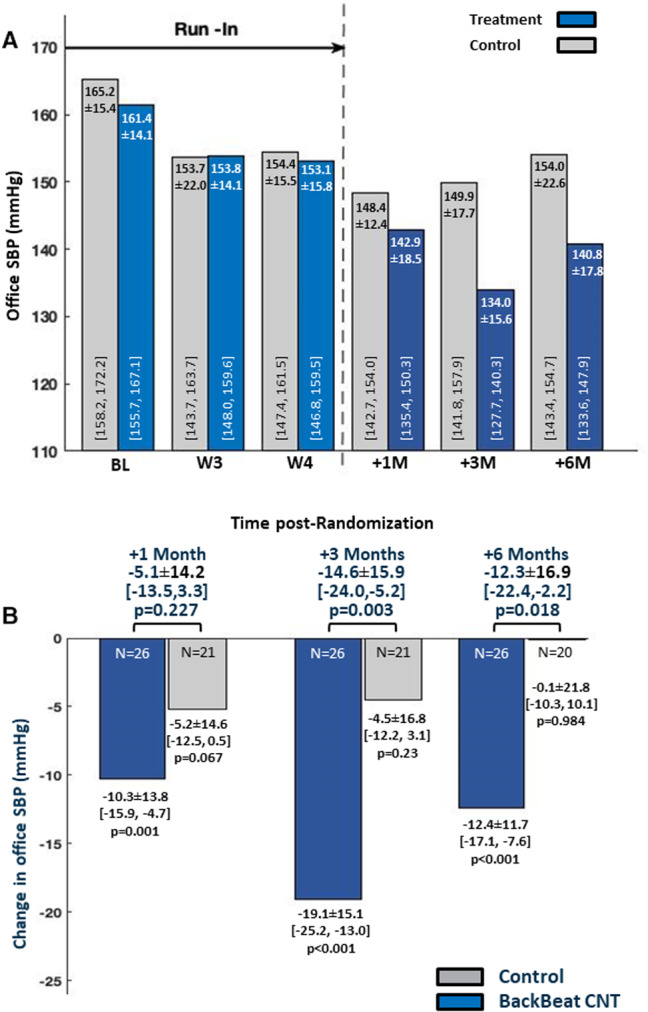
A, Comparison of office systolic blood pressure (oSBP) between groups over the entire course of the study. B, Between‐group comparisons of change in oSBP relative to the 3‐week prerandomization values. CNT indicates cardiac neuromodulation therapy; and SBP, systolic blood pressure.
Ambulatory and office DBPs in the randomized cohort did not differ between the control and treatment groups (Table S2). Furthermore, DBPs did not change in either group during the follow‐up period (Table S2).
Primary Safety Analysis
There were only 3 protocol‐prespecified primary safety end point events in 2 patients, both in the control group. One patient experienced angina pectoris leading to right coronary angioplasty and stenting and later died as a result of a newly diagnosed disseminated adenocarcinoma. A second patient experienced persistent atrial fibrillation requiring cardioversion. Other serious adverse events (summarized in Table S3) also occurred only in the control group (7 events in 4 patients, including the 3 events noted above).
Echocardiographic Assessments
Echocardiographic parameters were measured in a blinded core laboratory. There were no differences in changes in LV end‐diastolic volumes (+9.2±16.2 mL in controls versus −2.3±26.1 mL in treatment, P=0.16), LV ejection fraction (−1.3%±5.4% in controls versus −5.4%±7.5% in treatment, P=0.09), left atrial dimension (+1.5±3.3 mm in controls versus 2.7±3.3 in treatment, P=0.22), or right atrial dimension (0.5±6.1 mm in controls versus 3.4±5.6 mm in treatment, P=0.13). Right ventricular size and function were graded qualitatively; no significant differences were found in either group between baseline and 6 months.
Other Assessments
Additional evaluations included DBPs, assessment of heart rate from Holter recordings (Table S4, no significant change), assessment of heart rate from ambulatory pressure recordings (Table S5, no significant change), changes in supraventricular and ventricular ectopy from Holter recordings (Table S6, no significant change), and blood tests focused on assessment of renal function (Table S7, no significant change).
DISCUSSION
CNT is a pacemaker‐based therapy that takes advantage of the fact that: (1) ventricular pressure generation is preload dependent; (2) preload can be manipulated by reducing atrioventricular pacing intervals; and (3) periodic, orchestrated variations of systolic BP achieved by a repeating sequence of alternating short and longer atrioventricular intervals can suppress sympathetic activation ordinarily accompanying reductions in BP. The randomized double‐blind design of the present pilot study reinforces results of prior unblinded studies4, 5 showing that pacemaker‐based CNT is effective in reducing BP.
More specifically, compared with a control group, CNT decreased average 24‐hour aSBP (the primary end point) by an average of 8.1 mm Hg from prerandomization values following 6 months of treatment. These findings were paralleled by a between‐group difference of 12.3 mm Hg in oSBP. Importantly, during follow‐up, there were more instances where antihypertensive drug doses were decreased rather than increased in the treatment group, compared with more instances when drug doses were increased rather than decreased in the control group. Thus, the observed between‐group reductions in BP in the treatment group could not be attributed to changes in background medical therapy. On the contrary, such medication changes likely contributed to the finding that the between‐group difference in aSBP was greater during the first 24 hours following randomization (14.1 mm Hg, Figure 2B) compared with 6 months (8.1 mm Hg) since medication changes are not likely to occur during the first 24 hours following randomization. Furthermore, between‐group differences in both aSBP and oSBP at 6 months were larger after excluding patients in whom medication prescriptions were changed during follow‐up.
The population targeted in the current study had a clinical indication for a pacemaker and an average age of 74 years, which is significantly older than patients generally enrolled in hypertension studies. Not surprisingly, there was also a higher prevalence of comorbid conditions and ISH (81%). It is therefore noteworthy that the significant reductions in SBP observed in the present study were achieved by CNT in a population that is particularly challenging to treat.2, 7, 8, 9
DBP was not influenced by CNT, most likely because of the fact that 81% of patients had ISH, meaning that their DBP values were in the normal range. Importantly, this is a group of patients in whom reductions of DBP could be detrimental.
This study focused on assessing the efficacy of CNT. Data concerning safety were collected, and no CNT‐related adverse events were noted. Among potential safety concerns that will be addressed in larger and longer studies is that related to chronic right ventricular pacing, which has been associated with increased heart failure events.10, 11, 12 However, such observations have primarily been limited to patients with underlying LV dysfunction and appear to be less of a concern in patients with normal LV function especially when evaluated in comparison to a control group.13 In this regard, it is noteworthy that during the 6‐month CNT treatment, no patients developed heart failure and that LV end‐diastolic volumes decreased in the CNT treatment group compared with controls, which is opposite of what would have been observed with the development of heart failure.
Persistently elevated BPs above guideline‐recommended levels despite multidrug regimens has encouraged the development and testing of several device‐based therapies. These include baroreceptor activation therapy,14, 15, 16 renal denervation,6, 17, 18, 19, 20, 21, 22, 23 arteriovenous shunting,24, 25 carotid body resection or denervation,26, 27 and mechanical stimulation of the baroreceptors.28 Prior reports have provided overviews and comparisons of these different approaches.29, 30 Of these, the most widely studied approach is renal denervation. The most notable randomized controlled studies included the SYMPLICITY HTN‐3 (which showed no significant between‐group difference in aSBP),6 SPYRAL HTN‐OFF MED Pivotal study (which showed 4.0‐mm Hg greater reduction of aSBP in treatment versus control in patients with hypertension in whom hypertension medications were withheld),19 SPYRAL HTN‐ON MED pilot study (which showed a 7.4‐mm Hg greater reduction of aSBP in treatment versus control patients),20 and the RADIANCE‐HTN (A Study of the ReCor Medical Paradise System in Clinical Hypertension) SOLO study (which showed a 4.1‐mm Hg between‐group difference).17 Three very recent meta‐analyses of randomized trials have arrived at similar conclusions regarding the net treatment effect of renal denervation on aSBP: Dahal et al,31 3.45 mm Hg; Stavropoulos et al,32 3.62 mm Hg; and Syed et al,33 3.55 mm Hg. Most recently, Mahfoud et al22 reported an average aSBP reduction of 8.9 mm Hg at 3 years of follow‐up among several subgroups of patients considered to be at high risk for cardiovascular events and a reduction of 10.4 mm Hg in patients with resistant hypertension; this was a registry study without a control group. The current finding of an average 8.1‐mm Hg between‐group CNT‐associated reduction of aSBP treatment is favorable in light of these findings with other technologies. In addition, 80% of the patients in the present Moderato II study had ISH, a particularly difficult group to treat,2, 21, 24 which was excluded from the SPYRAL and RADIANCE studies. Finally, because the mechanisms are fundamentally different, CNT and renal denervation and other technologies have the potential to be used in combination in patients whose BPs remain above guideline recommendations with one or the other therapy.
Limitations
Despite protocol specifications to the contrary, physicians or patients may choose to modify medical therapies based on BP values observed during the follow‐up period. Medication modifications are a well‐known confounding effect in therapeutic trials of hypertension.6, 19, 34 Our assessment of medical compliance was based on patient and physician reporting rather than blood and urine tests, as have been implemented in some recent studies.19, 20, 35 However, in a randomized double‐blind study of a therapy that is truly effective, more medication uptitrations would be expected in the control group. As noted above, this is exactly what was observed.
Second, while many efforts were taken to maintain blinding (including that the unblinded site clinician had no part in study‐related clinical evaluations and signed an agreement to maintain confidentiality), there is a possibility of unblinding that could have occurred during unscheduled office visits. There was no attempt to formally assess for unblinding. However, any change in patient or physician behavior in response to unblinding would arguably have had to be mediated by greater uptitration of antihypertensive medications, which was not the case.
Third, despite the small number of patients in this study, statistically significant reductions in BP were identified in this double‐blind pilot study relative to a control group. While the data provide preliminary evidence of safety, longer‐term follow‐up from a larger number of patients is needed to completely rule out potential safety concerns discussed above.
CONCLUSIONS
This pilot study provides important evidence that hypertension treatment with a pacemaker‐based device that delivers CNT, a repeating sequence of variably timed short and longer atrioventricular intervals, can meaningfully reduce SBP over 6 months of follow‐up. There was a high rate of response to the therapy. No safety concerns emerged and patients did not experience any adverse sensations associated with the short atrioventricular delay beats. As in prior studies, the current study included patients who required pacemaker implantation or replacement; thus, the need to undergo the implantation procedure was independent of their need for additional hypertension therapy. This dissociates the risks associated with the implant procedure from those of CNT. With further proof of safety and efficacy in larger studies of longer duration, such a therapy could potentially be expanded to include patients not requiring a pacemaker.
Sources of Funding
This study was supported by Backbeat Medical, Inc., a wholly owned subsidiary of Orchestra BioMed Inc.
Disclosures
Dr Merkely reports consultancy agreements with Biotronik, Abbott, AstraZeneca, Boehringer Ingelheim, and Novartis. Dr Mitkowski reports consultancy fees from Abbott, Biotronik, Boston Scientific and Medtronic. Dr Sokol reports consultancy agreements with BackBeat, Biotonik, Medtronic, and Boston Scientific. Dr Pluta reports consultancy agreements with BSCI, Medtronic, and Biotronik. Dr Getter reports consultancy agreements with Biotronik, Medtronic, Abbott, Boston Scientific, and Vitatron. Dr Osztheimer reports receiving consulting fees from Backbeat Medical, Biotronik, Medtronic, Abbott, and Boston Scientific. Dr Mika is an employee of BackBeat Medical and has equity in Orchestra Biomed. Dr Evans is a consultant to BackBeat Medical and has equity in Orchestra Biomed. Dr Hastings is a consultant to BackBeat Medical. Dr Burkhoff is a consultant to BackBeat Medical and has equity in Orchestra Biomed. Dr Kuck has no disclosures to report.
Supporting information
Acknowledgments
A full list of the MODERATO II study investigators is provided in the Supplemental Material.
(J Am Heart Assoc. 2021;10:e020492. DOI: 10.1161/JAHA.120.020492.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020492
For Sources of Funding and Disclosures, see page 11.
See Editorial by Lauder and Mahfoud
REFERENCES
- 1.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137:109–118. DOI: 10.1161/CIRCULATIONAHA.117.032582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, Rodriguez CJ, Wang K. Prevalence and trends of isolated systolic hypertension among untreated adults in the United States. J Am Soc Hypertens. 2015;9:197–205. DOI: 10.1016/j.jash.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proclemer A, Ghidina M, Gregori D, Facchin D, Rebellato L, Zakja E, Gulizia M, Esente P. Trend of the main clinical characteristics and pacing modality in patients treated by pacemaker: data from the Italian Pacemaker Registry for the quinquennium 2003–07. Europace. 2010;12:202–209. DOI: 10.1093/europace/eup346 [DOI] [PubMed] [Google Scholar]
- 4.Yang B, Wang Y, Zhang F, Ju W, Chen H, Mika Y, Aviv R, Evans SJ, Burkhoff D, Wang J, et al. Rationale and evidence for the development of a durable device‐based cardiac neuromodulation therapy for hypertension. J Am Soc Hypertens. 2018;12:381–391. DOI: 10.1016/j.jash.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 5.Neuzil P, Merkely B, Erglis A, Marinskis G, de Groot JR, Schmidinger H, Rodriguez Venegas M, Voskuil M, Sturmberger T, Petru J, et al. Pacemaker‐mediated programmable hypertension control therapy. J Am Heart Assoc. 2017;6:e006974. DOI: 10.1161/JAHA.117.006974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. DOI: 10.1056/NEJMoa1402670 [DOI] [PubMed] [Google Scholar]
- 7.Rapsomaniki E, Timmis A, George J, Pujades‐Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. DOI: 10.1016/S0140-6736(14)60685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. DOI: 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 9.Bavishi C, Goel S, Messerli FH. Corrigendum to "isolated systolic hypertension: an update after SPRINT" American Journal of Medicine Volume 129, Issue 12, December 2016, Pages 1251‐1258. Am J Med. 2017;130:1128. DOI: 10.1016/j.amjmed.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 10.Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A. Dual‐chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115–3123. DOI: 10.1001/jama.288.24.3115 [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi F, Kobayashi Y, Itou H, Onuki T, Matsuyama T, Watanabe N, Liu C, Kawamura M, Asano T, Miyata A, et al. Prolonged paced QRS duration as a predictor for congestive heart failure in patients with right ventricular apical pacing. Pacing Clin Electrophysiol. 2005;28:1182–1188. DOI: 10.1111/j.1540-8159.2005.50181.x [DOI] [PubMed] [Google Scholar]
- 12.Zhang XH, Chen H, Siu CW, Yiu KH, Chan WS, Lee KL, Chan HW, Lee SW, Fu GS, Lau CP, et al. New‐onset heart failure after permanent right ventricular apical pacing in patients with acquired high‐grade atrioventricular block and normal left ventricular function. J Cardiovasc Electrophysiol. 2008;19:136–141. DOI: 10.1111/j.1540-8167.2007.01014.x [DOI] [PubMed] [Google Scholar]
- 13.Riahi S, Nielsen JC, Hjortshoj S, Thomsen PEB, Hojberg S, Moller M, Dalsgaard D, Nielsen T, Asklund M, Friis EV, et al. Heart failure in patients with sick sinus syndrome treated with single lead atrial or dual‐chamber pacing: no association with pacing mode or right ventricular pacing site. Europace. 2012;14:1475–1482. DOI: 10.1093/europace/eus069 [DOI] [PubMed] [Google Scholar]
- 14.Bakris GL, Nadim MK, Haller H, Lovett EG, Schafer JE, Bisognano JD. Baroreflex activation therapy provides durable benefit in patients with resistant hypertension: results of long‐term follow‐up in the Rheos Pivotal Trial. J Am Soc Hypertens. 2012;6:152–158. DOI: 10.1016/j.jash.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 15.Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, de Leeuw PW, Sica DA. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double‐blind, randomized, placebo‐controlled rheos pivotal trial. J Am Coll Cardiol. 2011;58:765–773. DOI: 10.1016/j.jacc.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 16.de Leeuw PW, Bisognano JD, Bakris GL, Nadim MK, Haller H, Kroon AA; HT DE and Rheos Trial I . Sustained reduction of blood pressure with baroreceptor activation therapy: results of the 6‐year open follow‐up. Hypertension. 2017;69:836–843. DOI: 10.1161/HYPERTENSIONAHA.117.09086 [DOI] [PubMed] [Google Scholar]
- 17.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE‐HTN SOLO): a multicentre, international, single‐blind, randomised, sham‐controlled trial. Lancet. 2018;391:2335–2345. DOI: 10.1016/S0140-6736(18)31082-1 [DOI] [PubMed] [Google Scholar]
- 18.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Lobo MD, Sharp ASP, Bloch MJ, Basile J, Wang Y, Saxena M, RADIANCE‐HTN Investigators , et al. Six‐month results of treatment‐blinded medication titration for hypertension control following randomization to endovascular ultrasound renal denervation or a sham procedure in the RADIANCE‐HTN SOLO Trial. Circulation. 2019. DOI: 10.1161/CIRCULATIONAHA.119.040451. [DOI] [PubMed] [Google Scholar]
- 19.Böhm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, et al. Efficacy of catheter‐based renal denervation in the absence of antihypertensive medications (SPYRAL HTN‐OFF MED Pivotal): a multicentre, randomised, sham‐controlled trial. Lancet. 2020;395:1444–1451. DOI: 10.1016/S0140-6736(20)30554-7 [DOI] [PubMed] [Google Scholar]
- 20.Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6‐month efficacy and safety results from the SPYRAL HTN‐ON MED proof‐of‐concept randomised trial. Lancet. 2018;391:2346–2355. DOI: 10.1016/S0140-6736(18)30951-6 [DOI] [PubMed] [Google Scholar]
- 21.Mahfoud F, Bakris G, Bhatt DL, Esler M, Ewen S, Fahy M, Kandzari D, Kario K, Mancia G, Weber M, et al. Reduced blood pressure‐lowering effect of catheter‐based renal denervation in patients with isolated systolic hypertension: data from SYMPLICITY HTN‐3 and the Global SYMPLICITY Registry. Eur Heart J. 2017;38:93–100. DOI: 10.1093/eurheartj/ehw325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahfoud F, Mancia G, Schmieder R, Narkiewicz K, Ruilope L, Schlaich M, Whitbourn R, Zirlik A, Zeller T, Stawowy P, et al. Renal denervation in high‐risk patients with hypertension. J Am Coll Cardiol. 2020;75:2879–2888. DOI: 10.1016/j.jacc.2020.04.036 [DOI] [PubMed] [Google Scholar]
- 23.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, et al. Catheter‐based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN‐OFF MED): a randomised, sham‐controlled, proof‐of‐concept trial. Lancet. 2017;390:2160–2170. DOI: 10.1016/S0140-6736(17)32281-X [DOI] [PubMed] [Google Scholar]
- 24.Ott C, Lobo MD, Sobotka PA, Mahfoud F, Stanton A, Cockcroft J, Sulke N, Dolan E, van der Giet M, Hoyer J, et al. Effect of arteriovenous anastomosis on blood pressure reduction in patients with isolated systolic hypertension compared with combined hypertension. J Am Heart Assoc. 2016;5:e004234. DOI: 10.1161/JAHA.116.004234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lobo MD, Sobotka PA, Stanton A, Cockcroft JR, Sulke N, Dolan E, van der Giet M, Hoyer J, Furniss SS, Foran JP, et al. Central arteriovenous anastomosis for the treatment of patients with uncontrolled hypertension (the ROX CONTROL HTN study): a randomised controlled trial. Lancet. 2015;385:1634–1641. DOI: 10.1016/S0140-6736(14)62053-5 [DOI] [PubMed] [Google Scholar]
- 26.Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, et al. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013;61:5–13. DOI: 10.1161/HYPERTENSIONAHA.111.00064 [DOI] [PubMed] [Google Scholar]
- 27.Narkiewicz K, Ratcliffe LE, Hart EC, Briant LJ, Chrostowska M, Wolf J, Szyndler A, Hering D, Abdala AP, Manghat N, et al. Unilateral carotid body resection in resistant hypertension: a safety and feasibility trial. JACC Basic Transl Sci. 2016;1:313–324. DOI: 10.1016/j.jacbts.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spiering W, Van Der Heyden J, Devireddy C, Foster MT III, Bates MC, Kroon AA. Lb02.05: controlling and lowering blood pressure with the mobiushd device: first‐in‐man results (Calm‐Fim study). J Hypertens. 2015;33(suppl 1):e86. DOI: 10.1097/01.hjh.0000467583.73735.1f [DOI] [Google Scholar]
- 29.Oparil S, Schmieder RE. New approaches in the treatment of hypertension. Circ Res. 2015;116:1074–1095. DOI: 10.1161/CIRCRESAHA.116.303603 [DOI] [PubMed] [Google Scholar]
- 30.Jordan J, Grassi G, Tank J. Device‐based treatments in hypertension: think physiology. J Hypertens. 2016;34:1502–1504. DOI: 10.1097/HJH.0000000000000992 [DOI] [PubMed] [Google Scholar]
- 31.Dahal K, Khan M, Siddiqui N, Mina G, Katikaneni P, Modi K, Azrin M, Lee J. Renal denervation in the management of hypertension: a meta‐analysis of sham‐controlled trials. Cardiovasc Revasc Med. 2020;21:532–537. DOI: 10.1016/j.carrev.2019.07.012 [DOI] [PubMed] [Google Scholar]
- 32.Stavropoulos K, Patoulias D, Imprialos K, Doumas M, Katsimardou A, Dimitriadis K, Tsioufis C, Papademetriou V. Efficacy and safety of renal denervation for the management of arterial hypertension: a systematic review and meta‐analysis of randomized, sham‐controlled, catheter‐based trials. J Clin Hypertens (Greenwich). 2020;22:572–584. DOI: 10.1111/jch.13827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Syed M, Osman M, Alhamoud H, Saleem M, Munir MB, Kheiri B, Balla S, Kawsara A, Daggubati R. The state of renal sympathetic denervation for the management of patients with hypertension: a systematic review and meta‐analysis. Catheter Cardiovasc Interv. 2021;97:E438–E445. DOI: 10.1002/ccd.29384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kandzari DE, Mahfoud F, Bhatt DL, Bohm M, Weber MA, Townsend RR, Hettrick DA, Schmieder RE, Tsioufis K, Kario K. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension. 2020;76:1410–1417. DOI: 10.1161/HYPERTENSIONAHA.120.15745 [DOI] [PubMed] [Google Scholar]
- 35.Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, et al. Catheter‐based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN‐OFF MED): a randomised, sham‐controlled, proof‐of‐concept trial. Lancet. 2017;390:2160–2170. DOI: 10.1016/S0140-6736(17)32281-X [DOI] [PubMed] [Google Scholar]
- 36.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, et al. The Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. DOI: 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 37.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2014;23:3–16. DOI: 10.3109/08037051.2014.868629 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


