Abstract
Background
Hemodynamic perturbations in heart failure with preserved ejection fraction (HFpEF) may alter the distribution of blood in the lungs, impair gas transfer from the alveoli into the pulmonary capillaries, and reduce lung diffusing capacity. We hypothesized that impairments in lung diffusing capacity for carbon monoxide (DLCO) in HFpEF would be associated with high mean pulmonary capillary wedge pressures during exercise.
Methods and Results
Rebreathe DLCO and invasive hemodynamics were measured simultaneously during exercise in patients with exertional dyspnea. Pulmonary pressure waveforms and breath‐by‐breath pulmonary gas exchange were recorded at rest, 20 W, and symptom‐limited maximal exercise. Patients with HFpEF (n=20; 15 women, aged 65±11 years, body mass index 36±8 kg/m2) achieved a lower symptom‐limited maximal workload (52±27 W versus 106±42 W) compared with controls with noncardiac dyspnea (n=10; 7 women, aged 55±10 years, body mass index 30±5 kg/m2). DLCO was lower in patients with HFpEF compared with controls at rest (DLCO 10.4±2.9 mL/min per mm Hg versus 16.4±6.9 mL/min per mm Hg, P<0.01) and symptom‐limited maximal exercise (DLCO 14.6±4.7 mL/min per mm Hg versus 23.8±10.8 mL/min per mm Hg, P<0.01) because of a lower alveolar‐capillary membrane conductance in HFpEF (rest 16.8±6.6 mL/min per mm Hg versus 28.4±11.8 mL/min per mm Hg, P<0.01; symptom‐limited maximal exercise 25.0±6.7 mL/min per mm Hg versus 45.5±22.2 mL/min per mm Hg, P<0.01). DLCO was lower in HFpEF for a given mean pulmonary artery pressure, mean pulmonary capillary wedge pressure, pulmonary arterial compliance, and transpulmonary gradient.
Conclusions
Lung diffusing capacity is lower at rest and during exercise in HFpEF due to impaired gas conductance across the alveolar‐capillary membrane. DLCO is impaired for a given pulmonary capillary wedge pressure and pulmonary arterial compliance. These data provide new insight into the complex relationships between hemodynamic perturbations and gas exchange abnormalities in HFpEF.
Keywords: alveolar‐capillary membrane conductance, cardiopulmonary exercise test, exercise intolerance, gas exchange, pulmonary capillary blood volume
Subject Categories: Heart Failure, Exercise Testing, Physiology, Pulmonary Hypertension
Nonstandard Abbreviations and Acronyms
- DLCO
lung diffusing capacity for carbon monoxide
- DLNO
lung diffusing capacity for nitric oxide
- Dm
alveolar‐capillary membrane conductance
- HFpEF
heart failure with preserved ejection fraction
- PAP
pulmonary artery pressure
- PAPm
mean pulmonary artery pressure
- PCWP
pulmonary capillary wedge pressure
- PCWPm
mean pulmonary capillary wedge pressure
- SV
stroke volume
- TPG
transpulmonary gradient
- Vc
pulmonary capillary blood volume
- VCO2
carbon dioxide production
- VO2
oxygen consumption
- VT
tidal volume
Clinical Perspective
What Is New?
Pulmonary vascular changes and alveolar‐capillary gas transfer dysfunction contribute to exercise intolerance and are key components of the clinical syndrome of heart failure with preserved ejection fraction.
What Are the Clinical Implications?
High pulmonary vascular pressure leads to remodeling and poor outcomes; however, it may be required to recruit and distend pulmonary capillaries and augment cardiac output during exercise, which may explain why pulmonary vasodilators do not improve exercise capacity.
Heart failure (HF) with preserved ejection fraction (HFpEF) is characterized by impaired left ventricular (LV) relaxation and an inability to augment cardiac output during exercise without abnormal increases in LV filling pressures. The gold standard for diagnosing HFpEF is through invasive hemodynamic demonstration of high pulmonary venous pressure, specifically a mean pulmonary capillary wedge pressure (PCWPm) ≥15 mm Hg at rest or ≥25 mm Hg during exercise.1, 2 An abnormally high PCWPm relates to exercise intolerance, evidenced by reductions in exercise capacity and ventilatory efficiency,3, 4, 5, 6 suggesting that cardiopulmonary interactions play an important role in exercise intolerance in HFpEF.
Pulmonary hypertension is common in HFpEF, initially caused by passive increases in mean pulmonary artery pressure (PAPm) due to left atrial hypertension, which results in elevated pulmonary capillary hydrostatic pressure.7, 8 Elevated pulmonary capillary pressure can lead to lung fluid accumulation,9 or, with chronic exposure, remodeling of the pulmonary vasculature. With an increase in lung fluid accumulation and/or adverse capillary remodeling, diffusion of gases between the alveoli and the pulmonary capillaries, a crucial link in the oxygen transport chain, and ventilation‐perfusion matching would be impaired. Indeed, lung diffusing capacity is impaired at rest and during exercise in HFpEF and appears to be primarily due to reductions in alveolar‐capillary membrane conductance (Dm) and somewhat to the inability to augment pulmonary capillary blood volume (Vc) during exercise.10 In healthy individuals during exercise, recruitment and distension of the pulmonary capillaries distributes blood throughout the lung, expands lung surface area available for gas exchange, and prevents abnormal increases in pressure, resulting in a linear increase in Vc with cardiac output.11 In HFpEF, elevation in LV filling pressures may cause blood to accumulate in the pulmonary capillaries, uncoupling increases in Vc from cardiac output and generating high pulmonary vascular pressures that further impair lung diffusing capacity. However, no study has directly assessed components of lung diffusing capacity as well as invasive hemodynamics simultaneously in patients with HFpEF.
Accordingly, the purpose of this study was to characterize the relationship between lung diffusing capacity and central hemodynamics during exercise in patients with HFpEF. It was hypothesized that patients with HFpEF would have impaired lung diffusing capacity and that the degree of impairment would correlate with higher pulmonary vascular pressures.
Methods
Patients
The data that support the findings of this study are available from the corresponding author upon reasonable request. Patients with symptoms of exertional dyspnea and suspected HFpEF undergoing exercise right heart catheterization were prospectively enrolled after review of their medical record. The experimental protocol was approved by the Mayo Clinic institutional review board in accordance with the Declaration of Helsinki and all participants provided written informed consent. Patients were defined as those with clinical symptoms of HFpEF: dyspneic, LV ejection fraction >50%, and a PCWPm ≥15 mm Hg at rest and/or ≥25 mm Hg during exercise. Controls were defined as participants with no demonstrable cardiac cause for symptoms, including normal PAPm (<25 mm Hg at rest) and PCWPm (<15 mm Hg at rest or <25 mm Hg with exercise). Patients with other causes of HF such as significant valvular heart disease; pericardial disease; infiltrative, restrictive, or hypertrophic cardiomyopathy; and high‐output HF were excluded. Patients with ejection fraction <50% were also excluded.
Study Design
All patients performed incremental stage cycling exercise with a right heart catheter placed in the internal jugular vein and a systemic arterial catheter placed in the radial artery as previously described.5, 9 Exercise was performed in the supine position with a cycle ergometer attached to a catheterization table (Cath Ergometer, Medical Positioning Inc) and comprised 5 minutes of exercise at 20 W before the workload was increased by 20 W every 3 minutes until volitional exhaustion. Breath‐by‐breath pulmonary gas exchange, arterial blood pressure, and ECG were recorded continuously, and lung diffusing capacity, hemodynamic, and hematological measures were recorded at rest, a fixed work rate (20 W), and symptom‐limited maximal exercise. At rest and during each exercise stage, rate of perceived exertion and dyspnea were recorded using the Borg and Modified Borg scales, respectively.12 Resting echocardiographic variables were assessed including the ratio of early mitral inflow velocity to early diastolic mitral annular velocity, and pulmonary function measurements, including forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1), were assessed clinically according to American Thoracic Society guidelines, along with NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), estimated glomerular filtration rate, and creatinine.13
Hemodynamic Measures
An arterial catheter (4F to 6F radial arterial cannula) was placed under local anesthesia (2% lidocaine) for arterial blood draws and continuous blood pressure recordings. Systolic arterial blood pressure and diastolic blood pressure were taken as the maximum and minimum of the arterial pressure waveform, respectively. Mean arterial pressure was calculated as one third systolic blood pressure plus two thirds diastolic blood pressure. Right heart catheterization was performed through a 9F sheath via the internal jugular vein. A high‐fidelity, 2F micromanometer‐tipped catheter (Aeris X PressureWire, Abbott) advanced through the lumen of a 7F fluid‐filled catheter (Balloon Wedge‐Pressure Catheter, Arrow) was placed in the internal jugular vein for pulmonary artery (PA) blood draws and pressure waveform monitoring of the PA pressure (PAP) and pulmonary capillary wedge pressure (PCWP). Pressure transducers were zeroed at mid‐axilla by laser calipers. PCWP position was confirmed by fluoroscopy, typical waveforms, and oximetry. Continuous pressure waveforms were measured from both catheters and digitally stored for subsequent offline analysis. Systolic PAP and diastolic PAP were measured at end‐expiration, PAPm was calculated as 0.61*systolic PAP+2,14 and mean right atrial pressure and PCWPm were measured at the mid a‐wave of end‐expiration.
Blood samples were drawn from the systemic and PA catheters to measure hemoglobin and O2 saturations, at a standard temperature of 37°C, and subsequently used to calculate systemic and PA O2 content and arteriovenous O2 difference (arteriovenous O2 difference=systemic–PA O2 content). Cardiac output was calculated by the direct Fick method (cardiac output=O2 consumption/arteriovenous O2 difference), and stroke volume (SV) was determined from cardiac output and heart rate (SV=cardiac output/heart rate). The transpulmonary gradient (TPG) was calculated as the difference between PAPm and PCWPm. Pulmonary vascular resistance was calculated as TPG/cardiac output. Pulmonary arterial (PA) compliance was calculated as SV/(systolic PAP–diastolic PAP).
Pulmonary Gas Exchange Measures
Breath‐by‐breath pulmonary gas exchange was measured using a pneumotach (Medical Graphics Corporation) and mass spectrometer (MGA 1100, Marquette Electronics) configured with a commercially available software package (BreezeSuite 6.4.1 SP5, Medical Graphics Corporation). Oxygen uptake (VO2), carbon dioxide production (VCO2), minute ventilation (VE), end‐tidal partial pressure of carbon dioxide PETCO2,expired partial pressure of carbon dioxide PECO2, tidal volume (VT), breathing frequency (fb), ventilatory equivalent for VCO2 (VE/VCO2), respiratory exchange ratio (VCO2/VO2), dead space to tidal volume ratio via the Bohr equation [VD/VT = (PaCO2–PECO2)/PaCO2], and alveolar to arterial O2 difference (AaDO2), calculated using the alveolar gas equation, were quantified breath by breath before being averaged for analysis. The final 30 seconds of gas exchange data at rest, a fixed work rate (20 W), and symptom‐limited maximal exercise are reported.
Lung Diffusing Capacity Measures
Lung diffusing capacity for carbon monoxide (DLCO) and for nitric oxide (DLNO) were measured in triplicate at rest, and once at a fixed work rate (20 W) and symptom‐limited maximal exercise. The simultaneous measurement of DLCO and DLNO using a rebreathe technique has been previously described in detail.15, 16, 17 Briefly, patients breathed on a mouthpiece attached to a switching valve (Hans Rudolph) that enabled the inspired air to be switched at end‐expiration from room air to a mixture of gases (35% O2, 0.6% C2H2, 0.3% C18O, 40 ppm NO, 9% He, and balance N2) from a‐5L bag filled to ≈120% of the patient’s tidal volume. Patients were instructed to rebreathe from the bag at a rate of 32 breaths per minute for 8 to 10 breaths, before switching back to room air. Gas concentrations in the bag were sampled using a mass spectrometer (Marquette 1100 Medical Gas Analyzer, Perkin‐Elmer) and nitric oxide analyzer (Sievers Instruments), and the rate of disappearance of gases was used to calculate DLCO and DLNO with custom software.15
Simultaneous measurement of DLCO and DLNO allows for the calculation of Dm and Vc according to European Respiratory Society guidelines.18 Lung diffusing capacity tests were excluded if the DLNO/DLCO ratio was ≤2.32, since calculated Dm and Vc values are not physiological as the DLNO/DLCO ratio approaches the α ratio.19
Statistical Analysis
Between‐group demographic differences were compared using a t test assuming unequal variance (Table 1) and performed using JMP (JMP Pro 14.1.0, SAS Institute Inc) with a statistical significance level of P<0.05. Values are reported as mean±SD, except NT‐proBNP, which is reported as median (range). Repeated measures ANOVA was used to determine between‐group (HF versus control) differences, within‐group (rest, 20 W, and symptom‐limited maximal exercise) differences, and interactions, and Pearson correlation coefficients were computed to explore the relationship between VO2 and Dm (SPSS Statistics version 25, IBM), and reported as mean±standard error.
Table 1.
Patient Characteristics
|
Control n=10 |
HFpEF n=20 |
P Value | |
|---|---|---|---|
| Patient characteristics | |||
| Women, % | 70 | 75 | 0.780 |
| Age, y | 55±10 | 65±11 | 0.023 |
| Height, cm | 170.9±9.6 | 167.9±10.6 | 0.454 |
| Weight, kg | 88.4±19.2 | 100.8±21.3 | 0.130 |
| BMI, kg/m2 | 30.0±4.7 | 36.0±8.4 | 0.045 |
| BSA, m2 | 2.0±0.3 | 2.2±0.3 | 0.258 |
| NT‐proBNP, pg/mL | 69.9 (25–170) | 601.4 (25–3299) | 0.015 |
| Hemoglobin, g/dL | 13.8±1.5 | 11.9±1.7 | 0.012 |
| Creatinine, mg/dL | 1.0±0.2 | 1.2±0.5 | 0.218 |
| eGFR, mL/min per m2 | 75.4±9.4 | 59.3±18.8 | 0.038 |
| Smoking history, % | 30 | 37 | 0.724 |
| Medications | |||
| ACEI, % | 30 | 45 | 0.447 |
| β‐Blocker, % | 32 | 35 | 0.155 |
| Diuretics, % | 30 | 70 | 0.038 |
| Calcium channel blocker, % | 60 | 30 | 0.122 |
| Pulmonary function | |||
| FVC, % predicted | 101±9 | 80±18 | <0.01 |
| FEV1, % predicted | 100±14 | 76±20 | <0.01 |
| FEV1/FVC, % predicted | 98±8 | 95±12 | 0.520 |
| FEF25–75, % predicted | 104±40 | 78±50 | 0.214 |
| Echocardiography | |||
| Ejection fraction, % | 63±4 | 62±3 | 0.811 |
| LV mass index, g/m2 | 84.8±22.9 | 86.1±15.5 | 0.882 |
| LA volume index, mL/m2 | 24.6±8.6 | 36.7±15.2 | 0.081 |
| E/A ratio | 1.1±0.3 | 1.9±0.9 | 0.062 |
| E/e’ ratio | 7.4±1.1 | 13.6±5.5 | 0.026 |
Wilcoxon rank sum nonparametric test was used to calculate P value. Values are reported as mean±SD. ACEI indicates angiotensin‐converting enzyme inhibitor; BMI, body mass index; BSA, body surface area; E/A, ratio of early to late filling velocity; E/e’, ratio of early mitral inflow velocity to early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate, values of >90 were considered to be 90; FEF25–75, forced expiratory flow at 25% to 75% of forced vital capacity; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricular; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide, reported as median (range), with values <25 considered to be 25.
Results
Patients
Among 30 patients who completed the study, 20 patients met the diagnostic criteria for HFpEF, and 10 patients demonstrated normal PAPm (<25 mm Hg at rest) and PCWPm (<15 mm Hg at rest or <25 mm Hg with exercise) and were considered controls (baseline hemodynamics in Table 2). Demographics and medication use at the time of the study are reported in Table 1. Patients were predominantly women, and the proportion of women was similar in both groups. Patients with HFpEF were older, had a higher body mass index, early diastolic mitral annular velocity ratio, and NT‐proBNP; lower hemoglobin and estimated glomerular filtration rate; and a greater proportion of diuretics use. FVC and FEV1 were lower in patients with HFpEF.
Table 2.
Pulmonary Gas Exchange, Hemodynamics, Lung Diffusing Capacity, and Blood Gas Metrics at Rest, 20‐W, and Symptom‐Limited Maximal Exercise
| Rest | 20 W | Symptom‐Limited Maximal Exercise | ANOVA Results, P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | HFpEF | Control | HFpEF | Control | HFpEF | HF | Stage | HF*Stage | |
| Pulmonary gas exchange | |||||||||
| VO2, mL/min | 304±26 | 316±21 | 786±42 | 640±43* | 1264±121 | 877±60* | 0.008 | <0.001 | 0.005 |
| VO2, mL/min per kg | 3.5±0.2 | 3.2±0.2 | 9.1±0.5 | 6.5±0.4* | 14.5±1.1 | 9.0±0.7* | <0.001 | <0.001 | 0.001 |
| VCO2, mL/min | 240±17 | 260±±19 | 649±51 | 525±38 | 1299±141 | 809±60* | 0.002 | <0.001 | <0.001 |
| RER | 0.80±0.02 | 0.82±0.03 | 0.82±0.04 | 0.82±0.01 | 1.02±0.03 | 0.92±0.02* | 0.161 | <0.001 | 0.035 |
| RPE | 7.3±0.7 | 6.0±0.5 | 10.8±0.8 | 11.7±0.9 | 17.1±0.9 | 14.6±0.8 | 0.255 | <0.001 | 0.060 |
| Dyspnea | 1.1±0.5 | 1.1±0.5 | 2.9±0.8 | 4.4±0.6 | 7.6±1.0 | 7.0±0.5 | 0.940 | <0.001 | 0.195 |
| VE, L/min | 9.4±0.8 | 9.4±0.7 | 23.5±3.7 | 18.5±1.3 | 45.2±5.8 | 28.2±2.4* | 0.007 | <0.001 | 0.012 |
| fb, breaths per min | 15.7±1.5 | 18.8±1.2 | 27.9±4.5 | 26.7±1.4 | 35.7±4.9 | 34.1±2.2 | 0.979 | <0.001 | 0.378 |
| VT, mL | 657±77 | 529±36 | 892±59 | 716±48* | 1360±170 | 844±54* | 0.003 | <0.001 | 0.009 |
| PETCO2, mm Hg | 33.1±1.7 | 37.4±1.1* | 35.2±1.6 | 35.8±1.3 | 33.7±1.9 | 34.9±1.3 | 0.298 | 0.061 | 0.066 |
| PECO2/PETCO2 | 0.69±0.02 | 0.66±0.01 | 0.75±0.01 | 0.73±0.01 | 0.79±0.01 | 0.76±0.01* | 0.105 | <0.001 | 0.960 |
| VE/VCO2 | 39.7±2.3 | 36.8±1.4 | 34.9±2.3 | 36.0±1.6 | 34.7±2.4 | 35.3±1.6 | 0.863 | 0.073 | 0.290 |
| VD/VT | 0.35±0.03 | 0.40±0.02 | 0.28±0.02 | 0.35±0.02* | 0.24±0.02 | 0.30±0.02 | 0.025 | <0.001 | 0.889 |
| AaDO2, mm Hg | 18.4±3.4 | 22.1±1.8 | 19.0±3.3 | 28.7±4.1 | 22.2±1.6 | 28.8±2.3 | 0.187 | 0.321 | 0.633 |
| Hemodynamics | |||||||||
| Cardiac output, L/min | 6.5±0.6 | 7.4±0.6 | 9.3±0.4 | 7.5±0.6* | 11.8±0.8 | 9.1±0.7* | 0.154 | <0.001 | 0.004 |
| Cardiac index, L/min per m2 | 3.2±0.2 | 3.4±0.2 | 4.6±0.2 | 3.5±0.3* | 5.8±0.2 | 4.2±0.3* | 0.026 | <0.001 | 0.001 |
| HR, beats per min | 70±5 | 72±3 | 91±7 | 89±4 | 121±11 | 98±5* | 0.307 | <0.001 | 0.010 |
| % predicted maximal HR | 42±8 | 47±9 | 55±12 | 58±12 | 73±20 | 63±13 | 0.847 | <0.001 | 0.003 |
| SV, mL | 97±11 | 103±8 | 108±10 | 85±6* | 106±12 | 96±8 | 0.449 | 0.586 | 0.021 |
| MAP, mm Hg | 97±5 | 99±3 | 113±5 | 115±5 | 122±5 | 116±5 | 0.958 | <0.001 | 0.166 |
| RAPm, mm Hg | 7±1 | 12±1* | 10±1 | 21±2* | 11±1 | 21±1* | <0.001 | <0.001 | 0.003 |
| PAPm, mm Hg | 18±1 | 29±2* | 26±1 | 43±3* | 29±1 | 43±3* | <0.001 | <0.001 | 0.007 |
| PCWPm, mm Hg | 10±1 | 18±1* | 16±1 | 30±2* | 18±2 | 30±2* | <0.001 | <0.001 | 0.014 |
| TPG, mm Hg | 9.1±1.6 | 11.0±1.6 | 10.1±1.3 | 12.9±2.4 | 10.7±1.8 | 12.4±2.2 | 0.474 | 0.296 | 0.809 |
| PVR, mm Hg/L per min | 1.5±0.3 | 1.9±0.4 | 1.1±0.2 | 2.4±0.9 | 1.0±0.2 | 1.6±0.4 | 0.269 | 0.200 | 0.308 |
| PA compliance, mL/mm Hg | 7.7±1.3 | 5.2±0.7 | 5.7±0.6 | 2.8±0.3* | 5.2±0.8 | 3.3±0.4* | 0.002 | 0.002 | 0.243 |
| Lung diffusing capacity | |||||||||
| DLCO, mL/min per mm Hg | 16.4±2.3 | 10.4±0.7* | 20.3±2.8 | 13.0±0.9* | 23.8±3.6 | 14.6±1.1* | 0.003 | <0.001 | 0.113 |
| DLNO, mL/min per mm Hg | 48.0±6.5 | 29.3±2.4* | 62.1±8.5 | 37.4±2.2* | 73.4±10.9 | 42.8±2.7* | 0.001 | <0.001 | 0.081 |
| Dm, mL/min per mm Hg | 28.4±3.9 | 16.8±1.6* | 37.9±5.4 | 21.7±1.2* | 45.5±7.4 | 25.0±1.5* | <0.001 | <0.001 | 0.073 |
| Vc, mL | 84.9±11.2 | 69.5±6.0 | 85.3±9.2 | 75.6±7.2 | 100.9±12.6 | 83.0±8.8 | 0.263 | 0.129 | 0.918 |
| Blood gases | |||||||||
| SaO2 | 97±0.81 | 95±0.50 | 96±0.88 | 94±0.61 | 98±0.56 | 95±1.02 | 0.027 | 0.196 | 0.572 |
| PaCO2 | 36±2.14 | 42±1.13* | 37±2.26 | 41±1.33 | 35±2.28 | 39±1.74 | 0.471 | <0.001 | 0.003 |
| PaO2 | 84±4.61 | 72±3.04* | 85±5.50 | 72±2.81* | 90±3.61 | 75±3.67* | 0.011 | 0.116 | 0.897 |
ANOVA results include the P value of between‐patient effect (heart failure [HF]), within‐patient effect (stage), and the interaction between the 2 effects (HF*stage). Data are presented as mean±SEM. AaDO2 indicates alveolar to arterial O2 difference; DLCO, lung diffusing capacity for carbon monoxide; DLNO, lung diffusing capacity for nitric oxide; Dm, alveolar‐capillary membrane conductance; fb, breathing frequency; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; MAP, mean arterial pressure; PA, pulmonary artery, PAPm, mean pulmonary artery pressure; PCWPm, mean pulmonary capillary wedge pressure; PECO2, expired partial pressure of carbon dioxide; PETCO2, end‐tidal partial pressure of carbon dioxide; PVR, pulmonary vascular resistance; RAPm, mean right atrial pressure; RER, respiratory exchange ratio; RPE, rate of perceived exertion; SaO2, arterial O2 saturation; SV, stroke volume; TPG, transpulmonary gradient; Vc, pulmonary capillary blood volume; VCO2, carbon dioxide production; VD/VT, ratio of dead space to tidal volume; VE, minute ventilation; VO2, O2 consumption; and VT, tidal volume.
Indicates post hoc difference between groups.
Exercise capacity was reduced in patients with HFpEF compared with controls, with lower symptom‐limited VO2 (HFpEF: 877±60 mL/min, controls: 1264±121 mL/min; P=0.003) and respiratory exchange ratio (HFpEF: 0.92±0.02, controls: 1.02±0.03; P=0.003), while symptom‐limited maximal power output during incremental exercise testing was 51% lower in patients with HFpEF than controls (HFpEF: 52±6 W, controls: 106±13 W, P<0.01). Postexercise arterial lactate was lower in patients with HFpEF (2.5±0.3 mmol/L versus 4.7±1.1mmol/L, P=0.014).
Hemodynamic Measures
At rest, cardiac output, heart rate, and SV were not different between groups (Table 2). At a fixed work rate (20 W), patients with HFpEF had lower cardiac output, attributable to lower SV. At symptom‐limited maximal exercise, SV was similar between groups, but cardiac output was lower in patients with HFpEF as a result of lower heart rate. At rest and during exercise, mean arterial pressure was not different between groups (Table 2). At rest and during exercise, mean right atrial pressure, PAPm, and PCWPm were higher in patients with HFpEF, despite a similar TPG and pulmonary vascular resistance (Table 2).
Pulmonary Gas Exchange and Lung Diffusing Capacity Measures
At rest, gas exchange and ventilatory parameters were similar between groups (Table 2); however, patients with HFpEF had a higher end‐tidal partial pressure of CO2. At 20 W, tidal volume and VO2 (absolute and normalized to mass) were lower, and the ratio of dead space to tidal volume was higher in patients with HFpEF. At symptom‐limited maximal exercise, VCO2, ventilation, and tidal volume were lower in patients with HFpEF. At rest and throughout exercise, DLCO and DLNO were lower in patients with HFpEF, attributable to lower Dm, while Vc was not different between groups (Table 2). During symptom‐limited maximal exercise, there was a significant correlation between VO2 and Dm (R=0.82, P<0.001).
Relationship Between Lung Diffusing Capacity and Pulmonary Hemodynamics
The relationship between lung diffusing capacity variables (DLCO, Dm, and Vc) and PAPm was shallower and rightward‐shifted in patients with HFpEF compared with controls (Figure 1), resulting in lower DLCO, Dm, and Vc for a given PAPm. In controls, there was a linear increase in DLCO and Dm relative to PAPm from rest to symptom‐limited maximal exercise, while patients with HFpEF increase DLCO and Dm relative to PAPm from rest to 20 W exercise and then reached a plateau. Similarly, Vc increased from rest to 20 W and then reached a plateau in both groups at symptom‐limited maximal exercise.
Figure 1. The relationship between lung diffusing capacity for carbon monoxide (DLCO, top), alveolar‐capillary membrane conductance (Dm, middle), and pulmonary capillary blood volume (Vc, bottom) and (A) mean pulmonary artery pressure (PAPm) and (B) mean pulmonary capillary wedge pressure (PCWPm) measured at rest (circles), a fixed work rate (20 W, squares), and symptom‐limited maximal exercise (triangles).
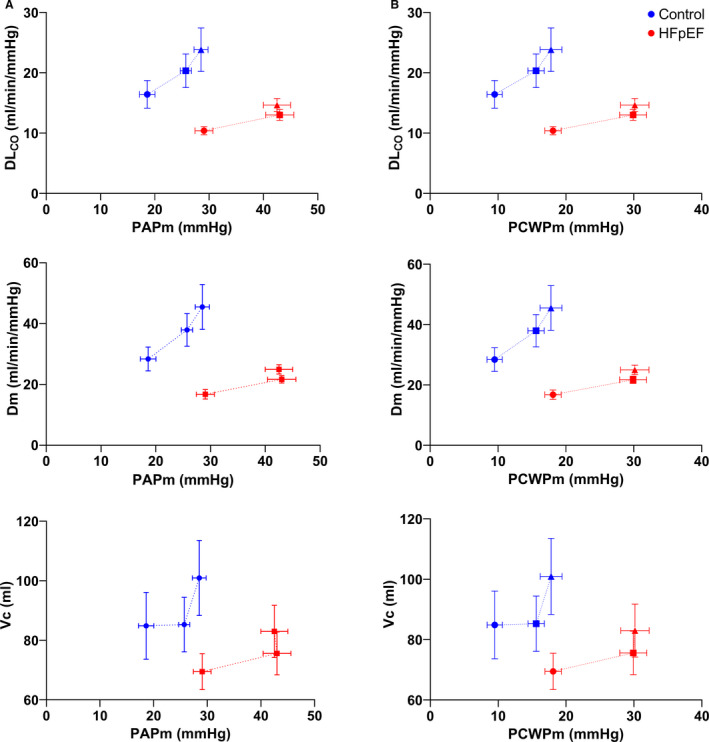
Mean and standard error are shown for each stage. HFpEF indicates heart failure with preserved ejection fraction.
Similarly, the relationship between lung diffusing capacity variables (DLCO, Dm, and Vc) and PCWPm was shallower and rightward‐shifted in patients with HFpEF compared with controls (Figure 1), resulting in lower DLCO, Dm, and Vc for a given PCWPm. In controls, there was a linear increase in DLCO and Dm relative to PCWPm from rest to symptom‐limited maximal exercise, while patients with HFpEF increase DLCO and Dm relative to PCWPm from rest to 20 W exercise and then reached a plateau. Similarly, Vc increased from rest to 20 W and then reached a plateau in both groups at symptom‐limited maximal exercise.
The relationship between lung diffusing capacity variables (DLCO, Dm, and Vc) and PA compliance was blunted and leftward‐shifted in patients with HFpEF compared with controls (Figure 2), resulting in lower DLCO, Dm, and Vc for a given PA compliance. In controls, there was a linear increase in DLCO and Dm relative to PA compliance from rest to symptom‐limited maximal exercise, while patients with HFpEF increase DLCO and Dm relative to PA compliance from rest to 20 W exercise and then reached a plateau at symptom‐limited maximal exercise. In patients with HFpEF from rest to 20 W, there was a greater increase in Vc for a given decrease in PA compliance compared with controls.
Figure 2. The relationship between (A) lung diffusing capacity for carbon monoxide (DLCO, top), (B) alveolar‐capillary membrane conductance (Dm, middle), and pulmonary capillary blood volume (Vc, bottom) and (C) pulmonary artery compliance (PAC) at rest (circles), a fixed work rate (20 W, squares), and symptom‐limited maximal exercise (triangles).
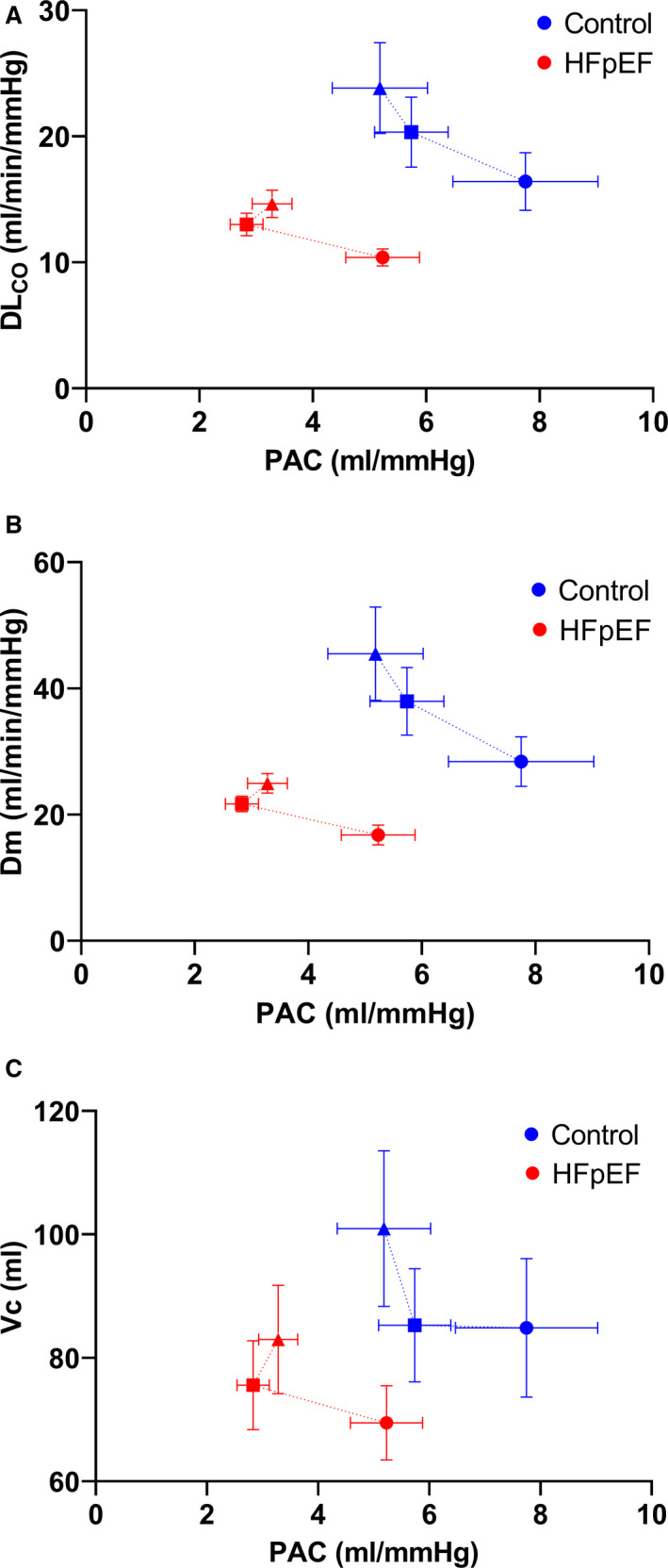
Mean and standard error shown for each stage. HFpEF indicates heart failure with preserved ejection fraction.
The relationship between lung diffusing capacity variables (DLCO, Dm, and Vc) and TPG was shallower and rightward‐shifted in patients with HFpEF compared with controls (Figure 3), resulting in lower DLCO, Dm, and Vc for a given TPG. In controls, there was a linear increase in DLCO and Dm relative to TPG from rest to symptom‐limited maximal exercise, while patients with HFpEF increase DLCO and Dm relative to TPG from rest to 20 W exercise and then reached a plateau. In patients with HFpEF from rest to 20 W, there was a greater increase in Vc for a given increase in TPG compared with controls.
Figure 3. The relationship between (A) lung diffusing capacity for carbon monoxide (DLCO), (B) alveolar‐capillary membrane conductance (Dm), and (C) pulmonary capillary blood volume (Vc) and transpulmonary gradient (TPG) at rest (circles), a fixed work rate (20 W, squares), and symptom‐limited maximal exercise (triangles).
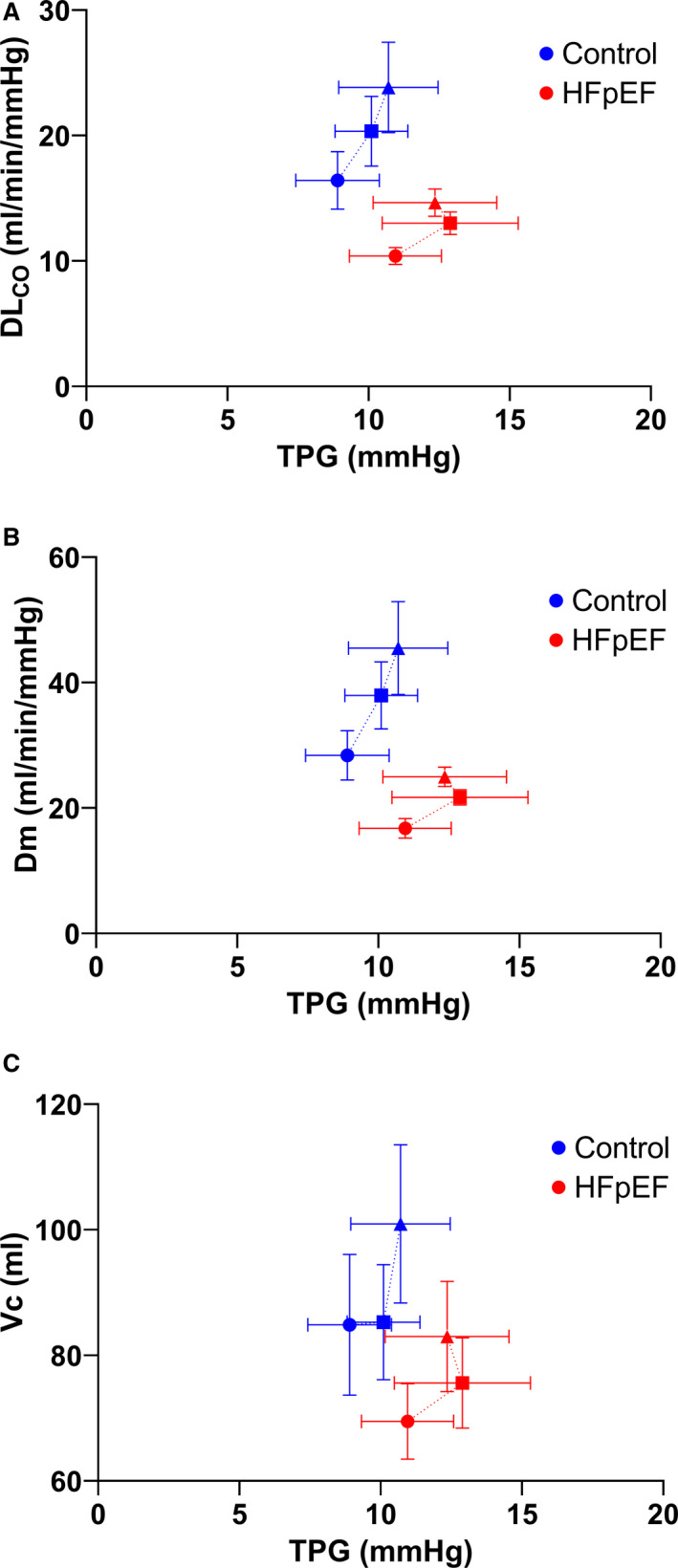
Mean and standard error shown for each stage. HFpEF indicates heart failure with preserved ejection fraction.
Discussion
In this study employing simultaneous measures of lung diffusing capacity and invasive central hemodynamics during exercise in HFpEF, we observed that lung diffusing capacity was reduced for a given PAP, PCWP, pulmonary arterial compliance, and transpulmonary gradient in patients with HFpEF both at rest and during exercise compared with controls without HFpEF. Consistent with previous studies, impaired lung diffusing capacity in HFpEF was predominantly due to a reduction in alveolar‐capillary membrane conductance10, 20 rather than reductions in pulmonary capillary blood volume. Given that lung diffusing capacity impairments were associated with altered pulmonary hemodynamics in this study, alveolar‐capillary membrane dysfunction may play a central role in pulmonary limitations previously described in HFpEF such as ventilation‐perfusion mismatch, a thickening of the pulmonary capillaries due to remodeling, or the development of extravascular lung fluid due to increased pulmonary capillary hydrostatic pressure.4, 5, 9, 21
Alveolar‐capillary membrane conductance reflects the total diffusive surface area in the lung, where ventilation and perfusion are matched and gas transfer can occur. Ventilation‐perfusion inhomogeneity, estimated by the ratio of the partial pressure of expired CO2 to end‐tidal CO2 (PECO2/PETCO2)22 did not differ between patients with HFpEF and controls. However, there was a trend towards a greater difference between PaCO2 measured in the blood and end‐tidal partial pressure of carbon dioxide in patients with HFpEF compared with controls, which might suggest a slight gas exchange impairment. Abnormal breathing patterns, such as rapid and shallow breathing, have been reported in HFpEF and would lead to increased dead space and reduced ventilatory efficiency.4, 5 Indeed, there was a significant effect of HF on the ratio of dead space to tidal volume, which may be caused by shallower breathing patterns in patients with HFpEF during exercise. While increased dead space can contribute to reduced ventilatory efficiency (estimated by the ventilation required to remove 1 L of CO2 from the blood via the lungs), VE/VCO2 was not different between patients with HFpEF and controls. Additionally, severe ventilation‐perfusion mismatch would be expected to cause an increase in the alveolar to arterial O2 difference, and while alveolar to arterial O2 difference trended higher in patients with HFpEF, it was not different between groups. Abnormal breathing patterns and ventilation perfusion mismatch may contribute to the reduction in lung diffusing capacity in patients with HFpEF, but do not appear to be the primary factor.
Consistent with prior hemodynamic studies23 and an autopsy study of patients with HFpEF revealing remodeling of the pulmonary vasculature,21 pulmonary arterial compliance during exercise was lower in patients with HFpEF, coupled with a systematically lower DLco at rest and during exercise. Interestingly, the reduced DLco in patients with HFpEF was predominantly driven by a reduced alveolar‐capillary membrane conductance (Table 2). Collectively, these findings suggest that remodeling of the alveolar or pulmonary capillary membrane may impair gas diffusion by increasing the diffusion distance and decreasing the distensibility of the pulmonary vessels. While not significantly different, Vc trended lower in patients with HFpEF, and it is possible that the absence of difference could be related to increased variability for this measure. The trend of a lower Vc in patients with HFpEF suggests that pulmonary capillaries might be underperfused, which may relate to the increased dead space in HFpEF. Alternatively, Vc may be similar in the groups because of the competing pathophysiologic processes present in HFpEF that nullify one another. Oligemia and loss of effective capillary units caused by chronic remodeling may reduce Vc21 while, concomitantly, the increase in capillary distending pressure owing to left atrial hypertension may increase capillary recruitment and Vc.24 The pattern of increase in DLco during exercise was similar between groups (ie, HFpEF could still augment DLco during exercise), suggesting that higher pulmonary vascular pressures in patients with HFpEF may be necessary to maintain, to some extent, ventilation‐perfusion matching at rest and during exercise.
In controls, exercise‐induced increases in PCWP are accompanied by substantial increases in lung diffusing capacity, whereas patients with HFpEF appear to hit a PCWPm ceiling, beyond which lung diffusing capacity does not increase. This could be because of the development of lung fluid, which has been detected in patients with HFpEF during exercise by sonographic B‐line artifacts or “comet tails”9 and CT‐derived estimates of extravascular lung fluid.25 It is possible that lung fluid develops in some patients during exercise caused by high pulmonary capillary hydrostatic pressure impeding alveolar‐capillary gas transfer and causing a reduction in Dm.
In this study, patients with HFpEF had markedly reduced lung diffusing capacity for a given PAP, PCWP, and pulmonary arterial compliance. Recruitment and distension of pulmonary capillaries improves ventilation‐perfusion matching within the lungs during exercise and allows for the increase in pulmonary blood flow without a proportional increase in pressure.26 While recruitment and distension patterns cannot be measured directly, the distensibility of the pulmonary vasculature, estimated by the α coefficient mathematically determined from the curvilinearity of multipoint PAPm‐cardiac output plots, independently predicts aerobic capacity and relates to lung diffusing capacity,27 and distensibility has previously been shown to be reduced in patients with HFpEF as compared with controls.28 It is notable that the diminished DLCO response was similar relative to both PAPm and PCWPm in HFpEF, and here we have chosen to focus on PCWPm since the increase in filling pressure drives the augmented PAPm in patients with HFpEF. The increase in lung diffusing capacity for a given increase in PCWP may provide insight into the ability of the pulmonary microcirculation to accommodate increases in blood flow during exercise despite high LV filling pressure.
Lung diffusing capacity was lower for a given transpulmonary gradient in HFpEF. The transpulmonary gradient describes the ability of the pulmonary circulation to promote forward blood flow despite high ventricular filling pressures. In this study, controls appear to increase TPG throughout exercise, while patients with HFpEF reach a ceiling at 20 W. Pharmacological reduction of PCWP and the transpulmonary gradient in older individuals using sildenafil reduces lung diffusing capacity,29 suggesting that a higher PCWP and transpulmonary gradient may be needed to overcome pulmonary vascular remodeling and maintain lung diffusing capacity. A therapy that decreases PCWPm while maintaining or increasing TPG, such as pericardiotomy, may improve pulmonary perfusion and alveolar‐capillary gas diffusion in these patients.30
Study Limitations
For this study, our control group was limited to patients who were referred for exercise right heart catheterization for symptoms of exertional dyspnea. Because of the prospective enrollment, there was a difference in age between groups, which may have contributed to a lower DLCO in the HFpEF group, with an expected decline in DLCO with age of ≈0.2 mL/min per mm Hg per year, resulting in a 2‐mL/min per mm Hg difference over 10 years. Consequently, the age difference may contribute to, but likely does not fully account for, the 6‐mL/min per mm Hg difference (at rest) in DLCO between patients with HFpEF and controls. Additionally, a baseline hemoglobin difference of 2 g/dL may explain up to a 0.7‐mL/min per mm Hg reduction in DLCO values.31 While care was taken to ensure that the control patients did not have abnormally elevated pulmonary vascular pressures or evidence of cardiopulmonary disease, they may not represent a true healthy population. Nevertheless, the control patients enrolled in this study would bias our results toward the null hypothesis, meaning that the differences shown between patients with HFpEF and controls would be greater if completely healthy volunteers were studied as the control group. In addition, all participants performed supine exercise consistent with the current institutional clinical protocols, which likely improves recruitment of pulmonary capillaries in the upper regions of the lungs at rest, and this may alter the distribution of lung fluid accumulation as compared with upright exercise. Respiratory exchange ratio at the highest exercise workload was lower in patients with HFpEF than controls, suggesting a lower hyperpnea response during exercise, which may affect exercise comparisons. Failure to achieve a higher peak respiratory exchange ratio is common in patients with HFpEF and likely relates to earlier exercise cessation caused by symptom limitation such as discomfort associated with dyspnea and lung congestion. Despite the differences in relative intensity, the comparisons made are valid in that they both represent the point of volitional exhaustion. Finally, this was an observational, cross‐sectional study, and thus interventional studies that independently alter the PCWPm response to exercise in HFpEF, such as pericardiotomy,30 are needed to elucidate a possible causal relationship between pulmonary vascular pressures and lung diffusing capacity.
CONCLUSIONS
Lung diffusing capacity is impaired at rest and during exercise in HFpEF, particularly when evaluated with respect to pulmonary distending pressures, which are significantly higher in this cohort. Interventions that independently manipulate pulmonary arterial and venous pressures are required to determine whether high pressures and impairments in lung diffusing capacity are co‐occurring effects of HFpEF or if high pulmonary vascular pressures are a compensatory mechanism to maintain pulmonary perfusion, and thereby lung diffusing capacity, in the setting of left heart disease.
Sources of Funding
B.D.J. is supported by the National Institutes of Health (RO1 HL71478). G.M.S. is supported by the American Heart Association (AHA#19POST34450022) and a Mayo Clinic Career Development Award in Cardiovascular Disease Research Honoring Dr Earl H. Wood. B.A.B. is supported by the National Institutes of Health (RO1 HL128526 and U10 HL110262). C.C.F. is supported by Mayo Clinic Graduate School.
Disclosures
None.
Acknowledgments
Author Contributions: The authors would like to thank the patients who participated in this research, the Mayo Clinic Cardiac Catheterization staff for facilitating this study, and Alex Carlson and Brad Cierzan for technical support. Author Contributions: C.C.F., G.M.S., B.A.B., and B.D.J. contributed substantially to the study design, data analysis and interpretation, and the writing of the article. All authors had access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Caitlin C. Fermoyle, Email: caitlin.fermoyle@utah.edu.
Barry A. Borlaug, Email: Borlaug.Barry@mayo.edu.
Bruce D. Johnson, Email: Johnson.Bruce@mayo.edu.
References
- 1.Pieske B, Tschöpe C, de Boer RA , Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CS, et al. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–3317. DOI: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 2.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. DOI: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. DOI: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Iterson EH, Johnson BD, Borlaug BA, Olson TP. Physiological dead space and arterial carbon dioxide contributions to exercise ventilatory inefficiency in patients with reduced or preserved ejection fraction heart failure. Eur J Heart Fail. 2017;19:1675–1685. DOI: 10.1002/ejhf.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obokata M, Olson TP, Reddy YN, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Hear J. 2018;39:2810–2821. DOI: 10.1093/eurheartj/ehy268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy Y, Olson T, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6:665–675. DOI: 10.1016/j.jchf.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction. a community‐based study. J Am Coll Cardiol. 2009;53:1119–1126. DOI: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37:67–119. DOI: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 9.Reddy YNV, Obokata M, Wiley B, Koepp KE, Jorgenson CC, Egbe A, Melenovsky V, Carter RE, Borlaug BA. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J. 2019;40:3721–3730. DOI: 10.1093/eurheartj/ehz713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson TP, Johnson BD, Borlaug BA. Impaired pulmonary diffusion in heart failure with preserved ejection fraction. JACC: Heart Failure. 2016;4:490–498. DOI: 10.1016/j.jchf.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson RL, Spicer WS, Bishop JM, Forster RE. Pulmonary capillary blood volume, flow and diffusing capacity during exercise. J Appl Physiol. 1960;15:893–902. DOI: 10.1152/jappl.1960.15.5.893. [DOI] [PubMed] [Google Scholar]
- 12.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 13.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA, McCarthy K, McCormack MC, et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200:E70–E88. DOI: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chemla D, Castelain V, Humbert M, Hébert JL, Simonneau G, Lecarpentier Y, Hervé P. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest. 2004;126:1313–1317. DOI: 10.1378/chest.126.4.1313. [DOI] [PubMed] [Google Scholar]
- 15.Snyder EM, Beck KC, Turner ST, Hoffman EA, Joyner MJ, Johnson BD. Genetic variation of the beta2‐adrenergic receptor is associated with differences in lung fluid accumulation in humans. J Appl Physiol. 2007;102:2172–2178. DOI: 10.1152/japplphysiol.01300.2006. [DOI] [PubMed] [Google Scholar]
- 16.Ceridon ML, Beck KC, Olson TP, Bilezikian JA, Johnson BD. Calculating alveolar capillary conductance and pulmonary capillary blood volume: comparing the multiple‐ and single‐inspired oxygen tension methods. J Appl Physiol. 2010;109:643–653. DOI: 10.1152/japplphysiol.01411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamhane RM, Johnson RL, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 2001;120:1850–1856. DOI: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- 18.Zavorsky GS, Hsia CC, Hughes JM, Borland CD, Guénard H, van der Lee I , Steenbruggen I, Naeije R, Cao J, Dinh‐Xuan AT. Standardisation and application of the single‐breath determination of nitric oxide uptake in the lung. Eur Respir J. 2017;49:1–23. DOI: 10.1183/13993003.00962-2016. [DOI] [PubMed] [Google Scholar]
- 19.Coffman KE, Chase SC, Taylor BJ, Johnson BD. The blood transfer conductance for nitric oxide: infinite vs. finite θNO. Respir Physiol Neurobiol. 2016;241:45–52. DOI: 10.1016/j.resp.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoeper MM, Meyer K, Rademacher J, Fuge J, Welte T, Olsson KM. Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:441–449. DOI: 10.1016/j.jchf.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, Frantz RP, Jenkins SM, Redfield MM. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation. 2018;137:1796–1810. DOI: 10.1161/CIRCULATIONAHA.117.031608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen JE, Ulubay G, Chow BF, Sun XG, Wasserman K, Chow Bing F, Sun XG, Wasserman K. Mixed‐expired and end‐tidal CO2 distinguish between ventilation and perfusion defects during exercise testing in patients with lung and heart diseases. Chest. 2007;132:977–983. DOI: 10.1378/chest.07-0619. [DOI] [PubMed] [Google Scholar]
- 23.Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular‐pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3294–3302. DOI: 10.1093/eurheartj/ehw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeves JT, Taylor AE. Pulmonary hemodynamics and fluid exchange in the lungs during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: American Physiological Society; 1996:585–613. [Google Scholar]
- 25.Fermoyle CC, Stewart GM, Borlaug BA, Johnson BD. Effects of exercise on thoracic blood volumes, lung fluid accumulation, and pulmonary diffusing capacity in heart failure with preserved ejection fraction. Am J Physiol Regul Integr Comp Physiol. 2020;319:R602–R609. DOI: 10.1152/ajpregu.00192.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Cell Mol Physiol. 2005;288:L419–L425. DOI: 10.1152/ajplung.00162.2004. [DOI] [PubMed] [Google Scholar]
- 27.Lalande S, Yerly P, Faoro V, Naeije R. Pulmonary vascular distensibility predicts aerobic capacity in healthy individuals. J Physiol. 2012;590:4279–4288. DOI: 10.1113/jphysiol.2012.234310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malhotra R, Dhakal BP, Eisman AS, Pappagianopoulos PP, Dress A, Weiner RB, Baggish AL, Semigran MJ, Lewis GD. Pulmonary vascular distensibility predicts pulmonary hypertension severity, exercise capacity, and survival in heart failure. Circ Heart Fail. 2016;9:1–11. DOI: 10.1161/CIRCHEARTFAILURE.115.003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffman KE, Curry TB, Dietz NM, Chase SC, Carlson AR, Ziegler BL, Johnson BD. The influence of pulmonary vascular pressures on lung diffusing capacity during incremental exercise in healthy aging. Physiol Rep. 2018;6:1–10. DOI: 10.14814/phy2.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borlaug BA, Carter RE, Melenovsky V, De Simone CV, Gaba P, Killu A, Naksuk N, Lerman L, Asirvatham SJ. Percutaneous pericardial resection – a novel potential treatment for heart failure with preserved ejection fraction. Circ Heart Fail. 2017;10:e003612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacIntyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP , Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, et al. Standardisation of the single‐breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. DOI: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]


