Abstract
Background
Cardiac dysfunction is a prominent feature of multisystem inflammatory syndrome in children (MIS‐C), yet the etiology is poorly understood. We determined whether dysfunction is global or regional, and whether it is associated with the cytokine milieu, microangiopathy, or severity of shock.
Methods and Results
We analyzed echocardiographic parameters of myocardial deformation and compared global and segmental left ventricular strain between 43 cases with MIS‐C ≤18 years old and 40 controls. Primary outcomes included left ventricular global longitudinal strain, right ventricular free wall strain), and left atrial strain. We evaluated relationships between strain and profiles of 10 proinflammatory cytokines, microangiopathic features (soluble C5b9), and vasoactive‐inotropic requirements. Compared with controls, cases with MIS‐C had significant impairments in all parameters of systolic and diastolic function. 65% of cases with MIS‐C had abnormal left ventricular function (|global longitudinal strain|<17%), although elevations of cytokines were modest. All left ventricular segments were involved, without apical or basal dominance to suggest acute stress cardiomyopathy. Worse global longitudinal strain correlated with higher ratios of interleukin‐6 (ρ −0.43) and interleukin‐8 (ρ −0.43) to total hypercytokinemia, but not absolute levels of interleukin‐6 or interleukin‐8, or total hypercytokinemia. Similarly, worse right ventricular free wall strain correlated with higher relative interleukin‐8 expression (ρ −0.59). There were no significant associations between function and microangiopathy or vasoactive‐inotropic requirements.
Conclusions
Myocardial function is globally decreased in MIS‐C and not explained by acute stress cardiomyopathy. Cardiac dysfunction may be driven by the relative skew of the immune response toward interleukin‐6 and interleukin‐8 pathways, more so than degree of hyperinflammation, refining the current paradigm of myocardial involvement in MIS‐C.
Keywords: COVID‐19, cytokine storm, echocardiography, multisystem inflammatory syndrome in children, myocardial deformation
Subject Categories: Echocardiography, Heart Failure, Inflammatory Heart Disease
Nonstandard Abbreviations and Acronyms
- GLS
global longitudinal strain
- MIS‐C
multisystem inflammatory syndrome in children
- RVFWS
right ventricular free wall longitudinal strain
- TMA
thrombotic microangiopathy
Clinical Perspective
What Is New?
Global myocardial dysfunction is present in the majority of children presenting with multisystem inflammatory syndrome, which should inform the choice of agent for cardiovascular support and encourage early, serial echocardiographic assessments.
The risk of myocardial dysfunction in hyperinflammatory conditions such as multisystem inflammatory syndrome in children is not determined solely by absolute levels of circulating inflammatory mediators but rather by the overall cytokine milieu.
What Are the Clinical Implications?
Analysis of segmental myocardial deformation is a useful, feasible technique that provides insight into the etiologies of inflammatory cardiac conditions in children.
Multisystem inflammatory syndrome in children (MIS‐C) is a rare, newly described hyperinflammatory response linked to antecedent SARS‐CoV‐2 exposure.1 Cardiac dysfunction during the acute phase is one of the most prominent features.2, 3 Although MIS‐C shares clinical features with Kawasaki disease, children with MIS‐C experience a higher frequency of myocardial dysfunction and mixed shock compared with those with Kawasaki disease.2, 4, 5 A variety of speculations have been made about the etiology of cardiac dysfunction, including hypercytokinemia, acute stress cardiomyopathy, direct immune‐mediated tissue injury (myocarditis), hypoperfusion from distributive shock, and microvascular injury. Given the transience of the dysfunction, cardiogenic shock due to the systemic inflammatory response and cytokine storm are favored.6 Secondary organ dysfunction in cytokine storms such as macrophage activation syndrome or sepsis is thought to be driven by high levels of circulating cytokines, including interleukins IL‐6, IL‐1β, and tumor necrosis factor alpha (TNFα).7 However, it remains unclear why cardiac dysfunction is so frequent or severe in MIS‐C, despite modest elevations in many of these same cytokines in comparison to well‐characterized pediatric cytokine storm syndromes.8, 9
The immune response in MIS‐C has distinct features compared with acute SARS‐CoV‐2 infection and other inflammatory states.10, 11 Concerns have also been raised regarding indicators of microvascular injury (soluble C5b‐9) suggestive of a complement‐mediated microangiopathic process in both COVID‐19 and MIS‐C.12 To date, there have been no systematic evaluations to determine what factors drive cardiac dysfunction in children with MIS‐C.
In order to inform the potential mechanisms of myocardial dysfunction in MIS‐C, our objectives were to (1) distinguish global from segmental patterns of myocardial deformation (strain) and (2) sequentially evaluate whether severity of dysfunction is associated with the magnitude of the systemic inflammatory response, specific cytokine milieu, biomarkers of microangiopathy, or severity of shock (Figure 1).
Figure 1. Conceptual model of potential etiologies of impaired cardiac function in MIS‐C.
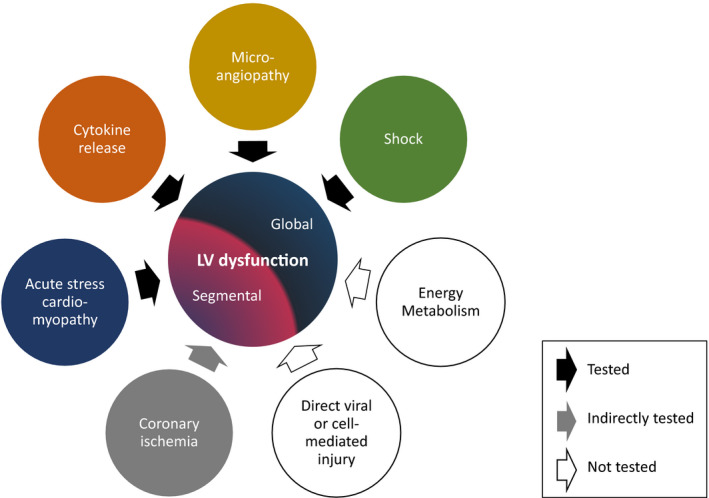
Potential pathophysiology of left ventricular (LV) dysfunction in MIS‐C, arranged according to whether each etiology is expected to result in a global vs segmental pattern of myocardial dysfunction (decreased strain). Black arrows indicate associations directly tested in this study, including (1) acute stress cardiomyopathy, (2) cytokine release and composition, (3) clinical and laboratory markers of microangiopathy, and (4) severity of shock. The presence of coronary ischemia (gray arrow) was inferred from regional wall motion abnormalities. White arrows indicate other speculations that were beyond the scope and, therefore, not tested in this study. MIS‐C indicates multisystem inflammatory syndrome in children.
Methods
The data that support the findings of this study are not publicly available due to information that could compromise patient privacy. Requests to access a limited data set from qualified researchers trained in human subject confidentiality protocols may be sent to Dr. Anirban Banerjee at the Children's Hospital of Philadelphia.
Study Design
This was a retrospective study comparing patterns of myocardial deformation between children with MIS‐C and controls, with a cross‐sectional analysis of factors associated with cardiac dysfunction among cases with MIS‐C.
Study Population
We included children ≤18 years of age who were hospitalized at a tertiary center (Children's Hospital of Philadelphia [CHOP] or its affiliate site, St. Peter's University Hospital, New Jersey), who met classification criteria for MIS‐C by Centers for Disease Control and Prevention or World Health Organization definitions.13, 14 MIS‐C diagnosis was secondarily adjudicated by a rheumatologist (J.C.) before inclusion. All cases needed to have laboratory confirmed SARS‐CoV2 exposure (by nasopharyngeal reverse transcriptase polymerase chain reaction test or serum IgG antibody positivity) and an echocardiogram during hospitalization. Exclusion criteria included prior history of cardiac dysfunction or chronic lung disease. As comparators for segmental strain analysis, healthy controls were selected from children with structurally normal hearts who underwent echocardiography for benign murmurs, chest pain or a family history of cardiac disease, and matched by age within 1 year to the first 40 cases with MIS‐C.
Study Procedures
This study was granted an exemption by the CHOP Institutional Review Board (20‐018024) for use of retrospective data, including data previously collected for a prospective study in which subjects consented to future use of their data and biospecimens.10
Echocardiography
We retrospectively analyzed 2‐dimensional transthoracic echocardiograms obtained for clinical purposes during initial hospitalization for MIS‐C. Echocardiography was performed by experienced cardiac sonographers using the Affiniti 70C or EPIC CVx ultrasound systems (Philips Medical Systems, Andover, MA). If multiple echocardiograms were performed, the echocardiogram with the worst LV function was used for analysis.
Conventional echocardiographic parameters were obtained according to American Society of Echocardiography guidelines,15 including left ventricular (LV) systolic function (ejection fraction by the biplane Simpson method, shortening fraction by M‐mode); LV diastolic function (E/e' ratio, the peak early diastolic filling velocity [E] over early diastolic mitral annular velocity [e'], expressed as an average of septal and lateral peak velocities); and right ventricular (RV) systolic function (tricuspid annular plane systolic excursion by M‐mode).
To estimate the degree of pulmonary hypertension in the absence of optimal tricuspid regurgitation jets, flattening of the interventricular septum was visually assessed by 2 experienced cardiologists (A.B. and D.M.) in cases with MIS‐C from the midcavity short axis views.
Segmental and regional wall motion of the LV was qualitatively analyzed according to a 17‐segment model in cases with MIS‐C cases (A.B. and D.M.) by visual assessment of multiple apical and short‐axis views as in routine clinical practice.16 Each segment was visually scored (normal:1, hypokinesis:2, akinesis:3, dyskinesis:4, and aneurysmal:5) by evaluating the thickening and excursion of each segment. The wall motion score index was calculated as the sum of all scores divided by the number of segments visualized.17
Two‐dimensional speckle‐tracking analysis was performed offline to assess myocardial deformation using a vendor‐independent software (2D CPA 1.3.0.91, TomTec Imaging Systems, Munich, Germany). LV global longitudinal strain (GLS) was calculated by averaging peak longitudinal strain from the apical 2‐, 3‐, and 4‐chamber views. Similarly, LV GLS rate was calculated by averaging systolic longitudinal strain rates from the apical 2‐, 3‐, and 4‐chamber views. LV global circumferential strain was obtained from midcavity short‐axis views. LV segmental longitudinal strains were calculated using the 17‐segment model and averaged for basal, midcavity. and apical segments generated by the software. Peak right ventricular free wall longitudinal strain (RVFWS) was measured from 4‐chamber views. For evaluation of diastolic function, longitudinal early diastolic strain rate and peak global left atrial strain were measured from 4‐chamber views.18
Cytokine Profiling
Proinflammatory cytokine profiles were available in all 34 cases with MIS‐C admitted to the CHOP main hospital only (but not the 9 cases admitted to the affiliate St. Peter's site). Cytokine profiles had been collected for clinical care in 31 cases as per the institutional clinical pathway,19 and for a prospective research study in 4 (one case had both), using the same assay and protocol. Plasma quantification of 10 cytokines, including interferon gamma (IFNγ), IL‐1β, IL‐2, IL‐4, IL‐6, IL‐8, IL‐10, IL‐12p70, IL‐13, and tumor necrosis factor alpha (TNFα), was performed using V‐Plex Pro‐inflammatory Panel 1 Human Kits (Meso Scale Diagnostics, Rockville, MD) per manufacturer protocol and analyzed on a QuickPlex SQ120 (Meso Scale Diagnostics) as previously described.10 If multiple cytokine profiles were drawn, the panel most proximate to echocardiography was used for analysis.
Soluble C5b9 Assay
Plasma sC5b‐9 concentrations were available in 29 of the 34 main hospital cases with MIS‐C, of which 21 were analyzed using a clinical assay (Cincinnati Children's Medical Center) as per the institutional clinical pathway, and 20 were analyzed on a research basis using human C5b‐9 ELISA set (BD Biosciences, San Jose, CA), which was previously validated with an upper limit of normal equivalent to Cincinnati Children's clinical assay (244 versus 247 ng/mL).12 If both clinical and research assays were performed, the level drawn most proximate to echocardiography was used in the analysis.
Clinical Data
Data were abstracted from medical records, including demographic factors (age, sex, race, ethnicity); hospital outcomes (length of stay, intensive care, respiratory support); treatment exposures (inotropic, immunomodulatory agents); and laboratory data (acute phase reactants, troponin‐I [Abbott Laboratories, Abbott Park, IL] and BNP [brain‐type natriuretic peptide]). Abnormal laboratory findings suggestive of myocardial injury were classified as maximum BNP >500 pg/mL or troponin‐I level ≥0.09 ng/mL, which is 3 times the upper limit of normal at our institution and consistent with published reference values.20, 21 All ECG were reevaluated for signs of ischemia.
Study Measures
Primary Outcomes
Primary outcomes included GLS, RVFWS, and left atrial strain. Secondary outcomes included additional measures of LV systolic function (global circumferential strain, GLS rate) and diastolic function (longitudinal early diastolic strain rate). By convention, GLS, global circumferential strain, and RVFWS are expressed as negative strains, representing shortening of a myocardial segment relative to its original length. For the purposes of analysis, the absolute values of strain are used throughout this article (ignoring the negative symbol), with lower magnitudes of strain indicating worse function. LV and RV systolic dysfunction were defined by GLS <17% and RVFWS <21%, respectively, based on lower limits of normal in published pediatric reference populations and healthy children at our institution.22, 23
A. Pattern of strain: The exposure category for global/segmental strain analysis was MIS‐C versus controls.
B. Cytokine milieu: Hyperinflammation was quantified using the total magnitude of hypercytokinemia, defined by the root sum square of the levels of the 5 most consistently elevated cytokines (IFNγ, IL‐6, IL‐8, IL‐10, and TNFα), as previously described.10 As the relative imbalance between different cytokines can drive different types of inflammation, we quantified the skew of the cytokine response toward individual cytokines. The skew was calculated as a unit vector component magnitude for each of the 5 cytokines by dividing the absolute cytokine level by the root sum square. The unit vector component effectively represents a ratio comparing the level of an individual cytokine to the total inflammatory response.
C. Microangiopathy: Thrombotic microangiopathy (TMA) was defined by the presence of at least 5/7 criteria from Gloude et al, including elevated lactic acid dehydrogenase, new thrombocytopenia (platelet count <150 000/mm3), new anemia, proteinuria, schistocytes, hypertension (blood pressure >99th percentile for age‐sex‐height if <18 years of age, >140 mm Hg systolic or 90 mm Hg diastolic if ≥18 years of age, at least twice, or new antihypertensive agent), and elevated sC5b9.12, 24
D. Shock: Blood lactate most proximate to echocardiography was used as a laboratory marker of shock. To assess clinical severity of shock, the use of vasoactive‐inotropic agent infusions was reported. We also quantified the vasoactive‐inotropic score (VIS) at time of echocardiography, which is a formula that incorporates active doses of epinephrine, norepinephrine, dopamine, dobutamine, milrinone, and vasopressin.25 Higher VIS indicates greater cardiovascular support.
Statistical Analysis
Normality was assessed using the Shapiro‐Wilk test, and variables were log‐transformed or dichotomized as appropriate. Student t tests were used to compare strain between groups. Spearman rank or Pearson's correlation coefficients, as appropriate, were used to evaluate relationships between strain measurements and biomarkers or VIS. Correlation coefficients were classified as moderate (0.3–0.6) or strong (>0.6). To account for multiple testing in the correlation analysis, we used the Benjamini‐Hochberg procedure to control the false discovery rate at 10%. A 2‐sided significance level of 0.05 was used for all other analyses. Analyses were performed using STATA 15.0 (College Station, TX).
Results
There were 43 cases with MIS‐C, of which 44% were of Black race, and 21% were of Hispanic ethnicity. A majority of cases (72%) were admitted to intensive care. Over half required vasoactive/inotropic support (Table 1). There were biochemical abnormalities suggestive of myocardial injury in 34/41 (83%) cases with MIS‐Cthat had troponin levels drawn during hospitalization. The echocardiograms analyzed were obtained within a median of 17.8 hours of admission (interquartile interval 10.9–36.7). We analyzed the first echocardiographic study in all but 3 patients, for whom the second study during admission showed worsening function and was used for analysis.
Table 1.
Demographic and Clinical Characteristics
| MIS‐C | Controls | P Value | |
|---|---|---|---|
| N=43 | N=40 | ||
| Age, y, mean (SD) | 10.1 (4.2) | 11.4 (3.7) | 0.125 |
| Male sex | 26 (60%) | 23 (57%) | 0.784 |
| Race | |||
| White | 14 (33%) | 25 (62%) | 0.043 |
| Black | 19 (44%) | 11 (28%) | |
| Asian | 1 (2%) | 1 (2%) | |
| Other/unknown¶ | 9 (21%) | 3 (8%) | |
| Hispanic ethnicity | 9 (21%) | 2 (5%) | 0.032 |
| Hospital outcomes | |||
| Hospital length of stay, median days [IQI] | 6 [5–10] | ||
| Intensive care unit admission, n (%) | 31 (72%) | ||
| Vasoactive‐inotropic agents during hospitalization | 22 (51%) | ||
| Epinephrine | 19 (44%) | ||
| Milrinone | 10 (23%) | ||
| Norepinephrine | 8 (19%) | ||
| Dopamine | 5 (12%) | ||
| Dobutamine | 1 (2%) | ||
| Vasopressin | 1 (2%) | ||
| Any respiratory support during hospitalization* | 30 (70%) | ||
| Need for invasive ventilation | 10 (23%) | ||
| Laboratory characteristics† | |||
| SARS‐CoV2 polymerase chain reaction(+), n (%) | 24 (57%) | ||
| Cycle threshold, mean (SD) | 37.2 (2.7) | ||
| SARS‐CoV2 IgG(+), n (%)‡ | 36 (97%) | ||
| Organ dysfunction/shock | |||
| Troponin (ng/mL), median [IQI] | 0.1 [0.0–0.7] | ||
| Maximum troponin | 0.3 [0.1–1.0] | ||
| BNP, pg/mL | 494.2 [165.9–848.0] | ||
| Maximum BNP | 767.4 [504.5–1298.6] | ||
| eGFR, mL/min per 1.73 m2 § | 110.1 [80.0–134.6] | ||
| Lactate, mmol/L | 1.7 [1.3–2.9] | ||
| Acute phase reactants | |||
| C‐reactive protein, mg/dL | 20.4 [16.6–26.1] | ||
| D‐dimer, µg/mL | 4.0 [1.7–5.4] | ||
| Ferritin, ng/mL | 675.9 [434.6–1152.8] | ||
| Hematologic abnormalities | |||
| Hemoglobin, g/dL | 10.3 [9.4–11.8] | ||
| Platelet count, 103/µL | 166.0 [120.0–211.0] | ||
| Absolute lymphocyte count (per µL) | 885.0 [510.0–1380.0] | ||
| Lactate dehydrogenase, U/L | 654.0 [495.0–850.0] | ||
| Schistocytes present‖ | 18 (41%) | ||
BNP indicates brain‐type natriuretic peptide; eGFR, Estimated glomerular filtration rate; IQI, interquartile interval; and MIS‐C, multisystem inflammatory syndrome in children.
Any supplemental oxygen requirement, including nasal cannula, noninvasive or invasive ventilation.
Laboratory values shown are those drawn closest to the echocardiogram, unless otherwise specified as the maximum value during admission.
Of n=37 assessed.
Of n=41 assessed, of whom 14 (34%) had mild‐moderate renal dysfunction (eGFR <90 mL/min per 1.73 m2).
Of n=34 assessed.
Race was self‐reported upon registration. The majority of patients who listed "Other" as their race were of Hispanic ethnicity (8/8 of the MIS‐C cases reporting "Other" race, 1/1 of the MIS‐C cases in which race was not reported, and 2/3 of the controls reporting "Other" race).
Pattern of Dysfunction Evaluated by Global and Segmental Strain
Global LV systolic dysfunction was present in 65% of cases with MIS‐C compared with 0% of controls. The cutoff (GLS <17%) used to define dysfunction was >3 SDs below the mean in our healthy controls. The mean difference in GLS between cases (mean: 16.3±3.9%) and controls (mean: 22.9±1.9%) was 6.6% (95% CI, 5.3–8.0; P<0.001). Compared with controls, cases with MIS‐C also had significant impairments in all other parameters of LV and RV function (Figure 2A and 2B, Table 2). The LV segmental longitudinal strains demonstrated similar systolic impairments across basal‐, mid‐, and apical‐segments (Figure 2C), suggesting global rather than segmental dysfunction. In each of the 17 individual LV segments, significant decreases in segmental strain were also observed (all P<0.01).
Figure 2. Myocardial function is globally impaired in cases with MIS‐C compared with controls.
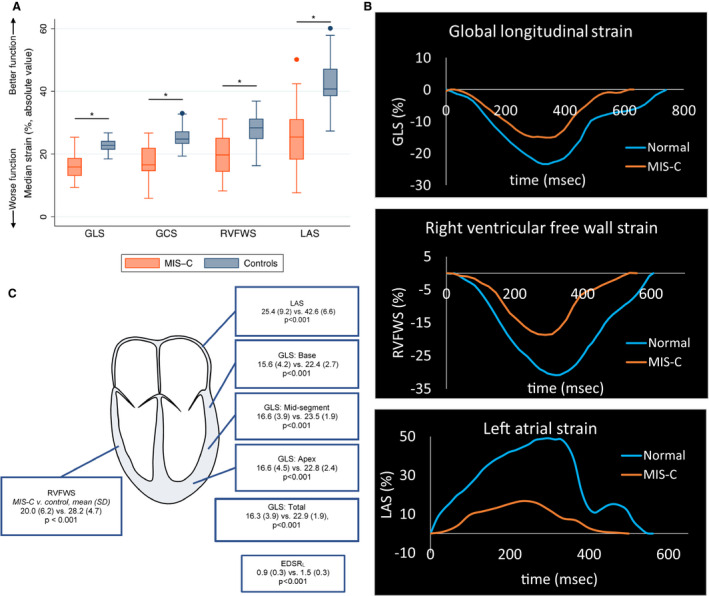
A, Global parameters of myocardial strain demonstrate impairments in LV systolic strain, RV systolic strain, and LA strain among cases with MIS‐C compared with controls. *P<0.001. B, The decrease in global strains in LV, RV, and LA are depicted by strain curves in a normal patient and a patient with MIS‐C during the acute phase of the disease. C, Similar impairments in mean segmental strain are observed across all LV segments. Data are expressed as means with SD in parentheses for MIS‐C vs control comparisons. EDSRL indicates longitudinal early diastolic strain rate; GCS, LV global circumferential strain; GLS, LV global longitudinal strain; LA, left atrial; LAS, left atrial strain; LV, left ventricular; MIS‐C, multisystem inflammatory syndrome in children; RV, right ventricular; and RVFWS, RV free wall strain.
Table 2.
Global and Segmental Myocardial Strain in Cases With MIS‐C Compared With Controls
| MIS‐C | Controls | P Value | |
|---|---|---|---|
| N=43 | N=40 | ||
| LV systolic function | |||
| LV ejection fraction (%), mean (SD) | 52.8 (10.8) | 64.6 (4.1) | <0.001 |
| LV fractional shortening (%) | 29.8 (8.6) | 37.2 (3.6) | <0.001 |
| Global longitudinal strain (%) | 16.3 (3.9) | 22.9 (1.9) | <0.001 |
| Longitudinal segmental strains | |||
| Base | 15.6 (4.2) | 22.4 (2.7) | <0.001 |
| Mid | 16.6 (3.9) | 23.5 (1.9) | <0.001 |
| Apex | 16.6 (4.5) | 22.8 (2.4) | <0.001 |
| Global longitudinal strain rate, s−1 | 0.8 (0.2) | 1.1 (0.1) | <0.001 |
| Global circumferential strain (%) | 17.6 (5.3) | 25.4 (3.3) | <0.001 |
| RV systolic function | |||
| Tricuspid annular plane systolic excursion, cm | 1.9 (0.4) | 2.2 (0.4) | <0.001 |
| RV free wall longitudinal strain (%) | 20.0 (6.2) | 28.2 (4.7) | <0.001 |
| LV diastolic function | |||
| Average E/e' ratio | 8.4 (1.8) | 6.8 (1.5) | <0.001 |
| End‐diastolic longitudinal strain rate (s−1) | 0.9 (0.3) | 1.5 (0.3) | <0.001 |
| Left atrial strain (%) | 25.4 (9.2) | 42.6 (6.6) | <0.001 |
Data expressed as mean (SD). All strain parameters are expressed using the absolute value. E/e' indicates peak early diastolic filling velocity over early diastolic mitral annular tissue velocity; LV indicates left ventricular; MIS‐C, multisystem inflammatory syndrome in children; and RV, right ventricular.
Segmental dysfunction assessed by the wall‐motion score spread beyond the territory of a single coronary artery in each patient, suggesting more global involvement (Table S1). Only 1 case had coronary artery involvement (right coronary artery, z score=3.0), but normal cardiac function. In addition, no cases with MIS‐C had ECG changes of ischemia.
Cytokine Milieu and Cardiac Function
All 34 cases with MIS‐C admitted to the CHOP main site had proinflammatory cytokines drawn a median of 0 days (interquartile interval −1–0) from the time of echocardiography. Six samples were drawn after immunomodulatory treatment (intravenous immunoglobulin or glucocorticoids). All subjects had abnormal elevations in at least 1 of 5 cytokines: IFNγ (median 131.0 pg/mL [interquartile interval 46.3–526.9]), IL‐6 (25.6 pg/mL [12.0–60.4]), IL‐8 (30.8 pg/mL [16.9–58.9]), TNFα (10.6 pg/mL [5.0–20.4]), or IL‐10 (28.1 pg/mL [6.1–95.0]). Elevations in IFNγ were the largest component of the cytokine abnormalities, followed by modest elevations of IL‐6 and IL‐8 and milder elevations of TNFα. On visual assessment of the distributions of relative cytokine expression, cytokines profiles appeared to be more heavily skewed toward higher ratios of IL‐6 and IL‐8 relative to other cytokines among those with LV and RV dysfunction (Figure 3).
Figure 3. Skewed cytokine response among cases with MIS‐C with cardiac dysfunction.
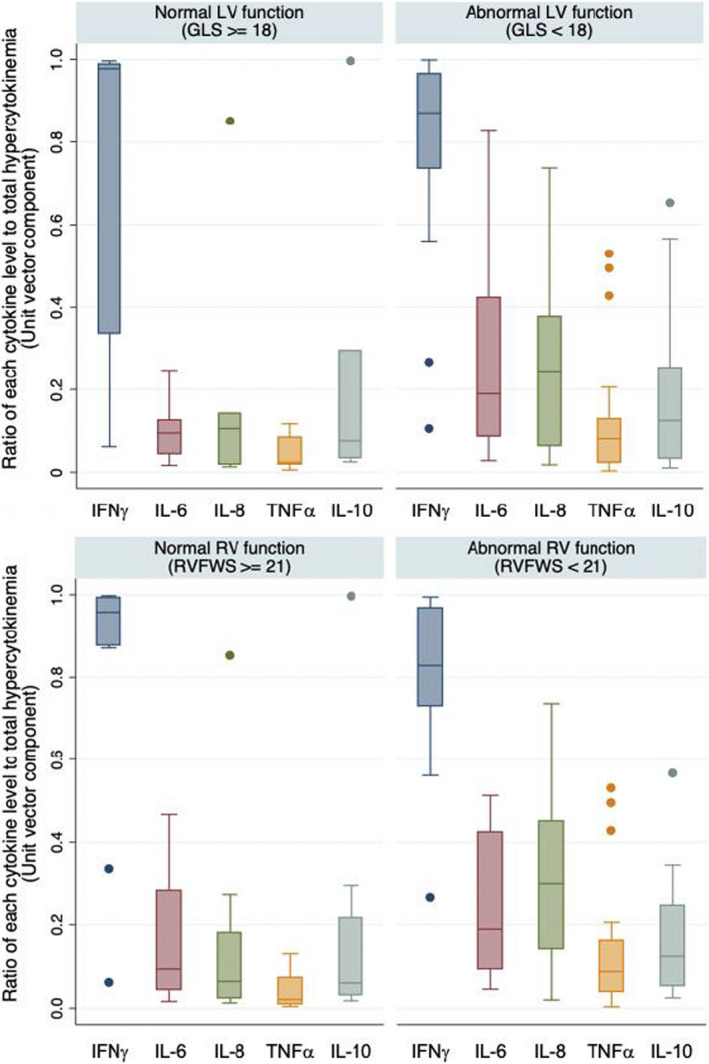
Comparison of the relative contribution of individual cytokines to the total hyperinflammatory response among cases with MIS‐C (N=34) with or without abnormal LV (upper panel) and RV systolic strain (lower). GLS indicates LV global longitudinal strain; IFNγ, interferon gamma; IL, interleukin; LV, left ventricular; MIS‐C, multisystem inflammatory syndrome in children; RV, right ventricular; RVFWS, right ventricular free wall strain; and TNFα, tumor necrosis factor alpha.
Among the 28 cases with MIS‐C with pretreatment cytokine levels, there was no significant association between the total magnitude of hypercytokinemia (root sum square) and any parameters of systolic or diastolic function (Table 3). With respect to other markers of systemic hyperinflammation, there was no significant association between pretreatment CRP (C‐reactive protein) levels with GLS or RVFWS (N=42, Pearson's r −0.2, P=0.165 and r −0.05, P=0.785, respectively).
Table 3.
Relationship Between the Relative Expression of Circulating Cytokines and Myocardial Function
| Unit vector (Concentration/Total RSS) | N | LV Systolic | RV Systolic | LV Diastolic | |||||
|---|---|---|---|---|---|---|---|---|---|
| Global Longitudinal Strain | Global Circumferential Strain | RV Free Wall Longitudinal Strain | Left Atrial Strain | ||||||
| ρ | P Value | ρ | P Value | ρ | P Value | ρ | P Value | ||
| Interferon gamma | 28 | 0.21 | 0.292 | 0.21 | 0.275 | 0.31 | 0.138 | 0.22 | 0.269 |
| IL‐10 | 28 | −0.13 | 0.512 | −0.32 | 0.095 | −0.14 | 0.519 | −0.10 | 0.612 |
| IL‐6 | 28 | −0.43* | 0.022* | −0.38 | 0.044 | −0.34 | 0.093 | −0.38 | 0.044 |
| IL‐8 | 28 | −0.43* | 0.023* | −0.31 | 0.114 | −0.59* | 0.002* | −0.28 | 0.146 |
| Tumor necrosis factor alpha | 28 | −0.22 | 0.261 | −0.30 | 0.120 | −0.39 | 0.051 | −0.23 | 0.243 |
| Total RSS | 28 | 0.12 | 0.555 | 0.07 | 0.727 | 0.29 | 0.159 | 0.00 | 0.988 |
Spearman's rank correlation coefficients (ρ) were used to test associations between unit vector components of individual cytokines (concentration relative to total RSS) and absolute values of strain parameters among cases of multisystem inflammatory syndrome in children with pretreatment samples (N=28). IL indicates interleukin; LV, left venticular; RSS, root sum square; and RV, right ventricular.
Asterisks indicate statistical significance based on the Benjamini‐Hochberg critical value for each strain parameter. P‐values <0.05 without asterisks do not meet the threshold for statistical significance when corrected for multiple comparisons.
However, assessment of the relative contribution of individual cytokine levels to the overall cytokine milieu demonstrated that greater ratios of IL‐6 and IL‐8 to other cytokines (unit vectors) were significantly associated with worse GLS (Table 3). Similar magnitudes of association were observed between greater IL‐6 unit vectors and decreased global circumferential strain, RVFWS, and left atrial strain, albeit not statistically significant after adjustment for multiple comparisons. The strongest association observed was between greater IL‐8 unit vectors and decreased RVFWS (ρ −0.6, P=0.002). In contrast, there was no significant association between the absolute levels of circulating IL‐6 or IL‐8 with LV or RV function (Table S2).
Microangiopathy and Cardiac Function
Among the cases with MIS‐C at CHOP, 22/34 (76%) had elevated sC5b9 levels, and 15/29 cases (52%) with complete laboratory data met criteria for TMA. There were no significant differences in any deformation parameters between cases with MIS‐C who met criteria for TMA and those who did not (Table 4). There was a moderate association between sC5b9 levels and RVFWS (ρ −0.44, P=0.025), but not parameters of LV function. In the majority of cases with MIS‐C, there was no evidence of significant pulmonary hypertension, except 2 with mild septal flattening at end‐systole, neither of which met criteria for TMA.
Table 4.
Myocardial Function in Cases With MIS‐C With or Without Microangiopathic Features
| No TMA | TMA | P Value | |||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| LV systolic function | |||||||
| GLS (%) | 14 | 15.6 | 4.2 | 15 | 14.6 | 2.5 | 0.448 |
| GLS rate (s– 1) | 14 | 0.77 | 0.25 | 15 | 0.77 | 0.18 | 0.940 |
| Global circumferential strain (%) | 14 | 16.4 | 4.5 | 15 | 15.6 | 5.9 | 0.697 |
| RV systolic function | |||||||
| RV free wall longitudinal strain (%) | 12 | 20.3 | 6.5 | 14 | 17.7 | 5.0 | 0.269 |
| LV diastolic function | |||||||
| Left atrial strain (%) | 14 | 23.8 | 10.1 | 15 | 23.7 | 6.3 | 0.988 |
| Longitudinal early diastolic strain rate (s– 1) | 14 | 0.77 | 0.34 | 15 | 0.81 | 0.21 | 0.685 |
Student t test was used to compare mean strain parameters between cases with multisystem inflammatory syndrome in children who met criteria for thrombotic microangiopathy (TMA) and those who did not. GLS indicates global longitudinal strain; LV, left ventricular; and RV, right ventricular.
Severity of Shock and Cardiac Function
A total of 18 (42%) of cases with MIS‐C were actively receiving at least 1 vasoactive/inotropic agent at time of echocardiography, of which epinephrine was most common (13/18), followed by norepinephrine (3/18) and milrinone (3/18). Median VIS at time of echocardiography was 7.0 (interquartile interval 5–9) and the maximum was 25. There was no significant association between severity of shock, as estimated by the VIS, and deformation parameters (GLS, ρ −0.2, P=0.121; RVFWS, ρ 0.0, P=0.981) (Figure 4). There was also no significant association between lactate levels and GLS (ρ 0.13, P=0.487) or RVFWS (ρ 0.15, P=0.429). However, following echocardiography, 7/18 patients required milrinone for the cardiogenic component of shock.
Figure 4. No significant association between vasoactive‐inotropic dose and left ventricular function.
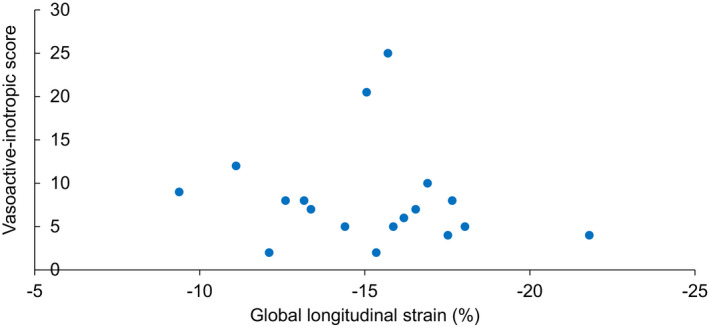
Global longitudinal strain (GLS) did not correlate with severity of shock, quantified using the vasoactive‐inotropic score (VIS). VIS represents requirements for vasoactive‐inotropic support at time of echocardiography.
Discussion
This is the first study to systematically examine the relationship between the distinct features of immune dysregulation among children with MIS‐C and cardiac dysfunction. Over 80% of children with MIS‐C had troponin and BNP elevations suggestive of myocardial injury, and 70% had echocardiographic evidence of global cardiac dysfunction, often requiring support with vasoactive‐inotropic agents. MIS‐C is frequently categorized as a cytokine “storm” syndrome associated with COVID‐19. However, the heart as a target of secondary organ dysfunction differentiates MIS‐C from classic cytokine storm syndromes, in which renal, pulmonary, and hepatic dysfunction predominate.26 Moreover, the degree of cardiac dysfunction is disproportionate to the modest elevation of circulating cytokines. In fact, the cytokine “storm” might be considered more of a “breeze” in MIS‐C. Inflammatory responses can shift more heavily toward certain immune cell types and cytokine expression patterns depending on the insult. Our study reveals that the relative skew of the cytokine response may be a more important determinant of myocardial dysfunction than the overall magnitude of hypercytokinemia. This provides a different framework for the interpretation of cytokine panels that show a stronger association with global cardiac dysfunction. We have also conducted a thorough investigation to exclude other potential etiologies of cardiac dysfunction depicted in Figure 1, which we try to address in the following sections.
Role of Cytokines in Cardiac Dysfunction
Our results demonstrate the relative contribution of IL‐6 and IL‐8 to the cytokine milieu is more directly associated with cardiac dysfunction than absolute increases in individual cytokine levels or the systemic inflammatory response. IL‐6 and IL‐8 serve important functions in innate immunity and mediate proinflammatory responses and neutrophil recruitment, respectively. IL‐6 is thought to play a central role in many cytokine storm syndromes.26 It has repeatedly been invoked in adults with severe COVID‐19 and was also hypothesized to drive CRP elevations in MIS‐C.27 However, cases with MIS‐C have significantly lower IL‐6 levels compared with patients with septic shock,28 and comparable, if not lower IL‐6 levels compared with Kawasaki disease.11 In the canonical form of cytokine storms—cytokine release syndrome following chimeric antigen receptor T cell therapy—IL‐6 levels are several hundred‐fold higher than what we observed in MIS‐C.29 Despite this, only 1/39 chimeric antigen receptor T subjects in 1 series presented with LV dysfunction requiring milrinone,30 whereas 70% of children with MIS‐C in this study had LV dysfunction and nearly one‐fourth required milrinone. Thus, elevations in circulating IL‐6 alone are insufficient to explain cardiac dysfunction in MIS‐C.
IL‐6 has pleiotropic effects dependent on the presence or absence of other cytokines, which can promote T helper‐17 or T helper‐2 cellular responses, as opposed to IFNγ, which primarily drives T helper‐1 responses. Therefore, we hypothesize that higher ratios of IL‐6 and IL‐8 to other proinflammatory cytokines such as IFNγ could be indicators of a prevailing cellular and biological milieu that results in greater downstream cardiodepressant effects rather than direct cytokine‐mediated cardiomyocyte toxicity. Although our assay includes many cytokines thought to drive organ dysfunction in other cytokine storms, it is possible the specific cytokine responsible for myocardial injury in MIS‐C was not measured. However, within the context of the mediators assessed, our study suggests that perhaps the answer lies not in the magnitude of IL‐6 elevations or the height of the cytokine storm, but in the skew of the immune response toward IL‐6 and IL‐8 associated pathways and away from overwhelmingly IFNγ driven Th1 responses, conceptually depicted in Figure 5 by the blue and orange radar diagram. These findings need to be reproduced in a larger prospective cohort and explored further using comprehensive immunophenotyping to better understand the implications before causal inferences can be made.
Figure 5. Skewed cytokine responses as a proposed mechanism of cardiac dysfunction.
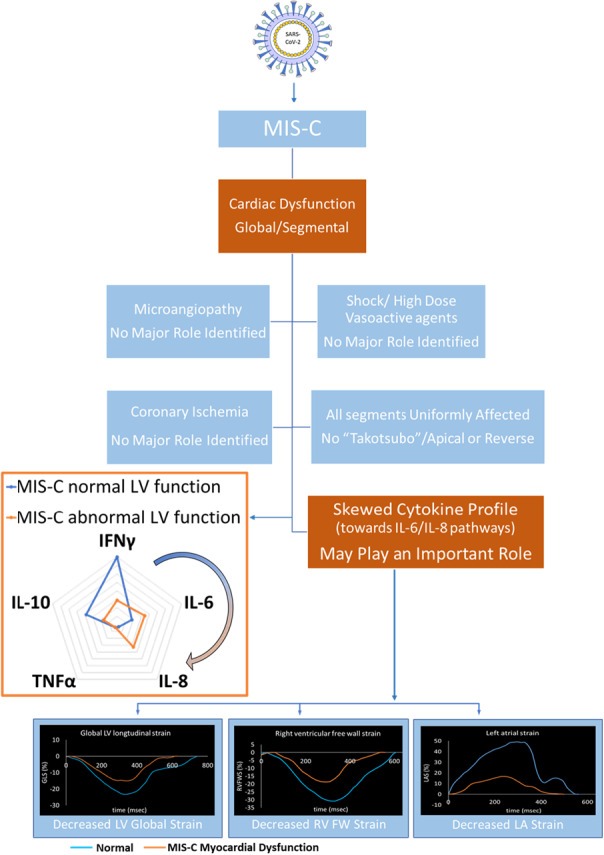
Use of echocardiographic strain analysis to evaluate cardiac function in multisystem inflammatory syndrome in children (MIS‐C) suggests that cytokine responses skewed toward IL‐6 and IL‐8 may predispose to cardiac dysfunction (by the blue and orange radar diagram). IFNγ indicates interferon gamma; IL, interleukin; and TNFα, tumor necrosis factor alpha.
Proinflammatory cytokines affect cardiac function in a time‐dependent, biphasic manner. The transient early response may be cardiostimulatory or cardiodepressant depending on the physiologic context and synergistic or counterregulatory effects of cytokines acting in concert. The late response lasts days and can have significant cardiodepressant effects dependent on production of inducible nitric oxide synthase, β‐adrenergic receptor uncoupling, oxidative stress and activation of sphingomyelinase‐dependent pathways resulting in impaired Ca2+‐cycling.31, 32 We have previously shown that LV systolic dysfunction recovers quickly in MIS‐C (GLS increased from 15.5% to 18.7%) during an abbreviated follow‐up of 5.2 days.5 We speculate that myocardial “injury” in the acute stage of MIS‐C may be induced by some of the aforementioned pathways, and shifts in cytokine expression in the context of the specific biologic milieu predispose to this phenomenon.
Global Versus Segmental Involvement
The cardiac dysfunction noted in our study was global. This was likely the outcome of segmental dysfunction affecting all segments uniformly. Global involvement of the myocardium suggests acute stress cardiomyopathy or branch coronary ischemia are unlikely to be significant contributors to cardiac dysfunction in MIS‐C. Our findings are consistent with a cardiac magnetic resonance imaging study that demonstrated the presence of global edema in 10 children with MIS‐C.33 There are reports describing acute stress cardiomyopathy involving either the apical (Takotsubo cardiomyopathy) or basal segment (reverse Takotsubo cardiomyopathy) in adults with COVID‐19.34, 35 The apex of the mammalian LV has the highest β‐adrenergic receptor density.36 Excessive stimulation of β‐adrenergic receptors by endogenous or exogenous catecholamines leads to β‐adrenergic receptor desensitization and downregulation, which may explain apical/midsegment cardiodepression in Takotsubo cardiomyopathy. However, the apical and basal segments were equally affected in our study without any apical‐basal mismatch.
Our findings also do not support transient regional coronary ischemia or myocardial stunning after reperfusion. Estimates of coronary artery involvement in MIS‐C reported in our previous work and others vary widely between 4% to 21%.1, 3, 5, 8 The regional wall motion abnormality in cases with MIS‐C was diffusely distributed beyond coronary artery territories, suggesting coronary ischemia is unlikely a main contributing factor. In addition, no evidence of coronary ischemia by ECG changes supports our speculation.
Similarly, myocarditis from viral or cell‐mediated tissue injury is often focal, as evidenced by regional hypokinesis in magnetic resonance imaging‐confirmed myocarditis in adults with COVID‐19,37 and rare, patchy histopathologic evidence of myocarditis in adults who succumbed to COVID‐19.38 Therefore, although we did not directly test this hypothesis, our findings suggest the pathophysiology of MIS‐C cardiac involvement likely differs from acute COVID‐19 myocarditis.
Severity of Shock and Cardiac Function
Although proinflammatory cytokines such as IL‐6 and IL‐8 are known to mediate distributive shock, cardiac dysfunction did not appear to be secondary to distributive shock alone. Most cases with MIS‐C likely experienced mixed shock as suggested by the choices of vasoactive and inotropic agents. VIS is a simple formula that has been used in the critical care setting, to objectively quantify the degree of hemodynamic support being provided to the patient in shock. Unlike pediatric sepsis, in which cardiac dysfunction is associated with higher VIS and illness severity,39 our study did not demonstrate a similar relationship between VIS and myocardial deformation, and significant LV dysfunction was present despite low VIS. This may be due to concomitant vasodilatory and cardiogenic shock and the practice of targeting vasoactive support to blood pressure targets rather than to cardiac function. Although characterizing type of shock was outside of the scope of this work, given the number of patients newly initiated on milrinone after the first echocardiogram, it is likely that VIS at time of echocardiography mainly captured the vasoplegic component of shock, before concomitant cardiogenic shock was quantified by echocardiography. Furthermore, although β‐adrenergic agents could in fact worsen cardiac contractility at high doses via β‐adrenergic receptor overstimulation,40 we did not observe a negative impact of higher VIS on function. Intensivists should be aware that cardiac function is frequently abnormal, which should affect the choice of pharmacologic agents and encourage early, serial echocardiographic assessments. A careful assessment of cardiac function should also be performed before administering high volume loads, particularly because MIS‐C affects older children who need larger volumes of intravenous immunoglobulin.
Microangiopathy and Cardiac Function
Although there have been concerns about the potential consequences of the microangiopathic physiology of MIS‐C, we observed no clear relationship between cardiac dysfunction and markers of microvascular injury. This is consistent with other causes of TMA, in which overt LV dysfunction is uncommon.41 There was also no evidence of pulmonary hypertension to suggest clinically significant pulmonary vascular disease. However, RVFWS was the only parameter associated with sC5b‐9 and had the largest mean difference by TMA status, albeit not statistically significant. Interestingly, a greater skew toward IL‐8 was strongly associated with impaired RV strain. IL‐8 is known as an important mediator of microvascular ischemia in TMA, via neutrophil activation, initiation of the complement cascade, and subsequent microthrombi.42 Our findings do not exclude microvascular injury as a contributing factor. Therefore, complete reversibility of cardiac dysfunction will need to be assessed carefully during follow‐up.
Limitations
Because of the limited sample size of pretreatment samples, we were not powered to detect weak associations or mean differences in strain <2.0. However, we were powered to detect what we would consider to be clinically significant differences. As the study was retrospective, echocardiographic protocols and the timing of sample collection were not prospectively standardized. Moreover, pretreatment cytokine panels were not available in all patients. It is also important to note that there may be local immune responses that are not reflected by circulating plasma cytokines. In particular, IL‐1β was not elevated despite the importance of anakinra (IL‐1 receptor antagonist) as an empiric drug therapy in COVID‐19 and other hyperinflammatory syndromes. This may be related to intrinsic difficulties detecting IL‐1β because of its short plasma half‐life, the relative importance of IL‐1β versus IL‐1α signaling, or secretion locally into tissues, and therefore our results do not preclude an important role of IL‐1 family cytokines. We were unable to compare myocardial function in MIS‐C versus severe COVID‐19, as nearly all children admitted with COVID‐19 had chronic cardiopulmonary conditions. Lastly, the cross‐sectional design limits causal inference and is hypothesis generating, and therefore longitudinal studies are currently underway.
Conclusions
Using an interdisciplinary approach, this study provides insights into the pathophysiology of cardiac dysfunction in a novel hyperinflammatory condition. The unique aspects of the immune response in MIS‐C that differ from other cytokine storm syndromes may help to explain why cardiac dysfunction is so frequent and severe in MIS‐C. In addition, our study provides a framework for identifying heterogeneity in the cytokine responses among children with MIS‐C and its association with clinical cardiac presentation. Therefore, the clinical applicability of proinflammatory cytokine panels extends beyond support for the diagnosis of MIS‐C to defining subpopulations of MIS‐C patients with a greater risk for severe cardiac inflammation, which may require more frequent or prolonged cardiac monitoring. Our findings emphasize the importance of considering the relative skew of immune activation—not just the height of the cytokine response—in understanding immune mechanisms of myocardial injury and refining the prevailing paradigm about the cytokine “storm.”
Sources of Funding
This work was funded by the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (K23‐HL148539 [Chang]) and the Children's Hospital of Philadelphia Frontiers Program Immune Dysregulation Team (Teachey, Behrens, and Bassiri). Behrens is supported by NIH/National Institute of Allergy and Infectious Diseases (R01AI121250). Teachey is supported by NIH/National Cancer Institute (R01CA193776, X01HD100702‐01, 5UG1CA233249, and R01A1123538), the Leukemia and Lymphoma Society, Cookies for Kids Cancer, Alex's Lemonade Stand Foundation for Childhood Cancer, Children's Oncology Group, and Stand UP 2 Cancer. Diorio is supported by an Institute for Translation Medicine and Therapeutics (ITMAT) scholarship and by the Children's Hospital of Philadelphia Gail Slap Fellowship Award. Bassiri is supported by grants from the Kate Amato Foundation and the Team Connor Childhood Cancer Foundation. Morgan is supported by the NIH/National Heart, Lung, and Blood Institute (K23HL148541).
Disclosures
Chang has received grant funding from GlaxoSmithKline for research outside of this work. Teachey serves on advisory boards for Janssen, Amgen, La Roche, Sobi, and Humanigen. Bassiri is a consultant for Kriya Therapeutics. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Acknowledgments
We acknowledge Yan Wang, who helped with data analysis. We also recognize the brave cardiac sonographers at our institution who acquired the images used in this study with fortitude throughout the COVID‐19 pandemic.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021428
For Sources of Funding and Disclosures, see page 13.
References
- 1.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, et al.; Overcoming COVID‐19 Investigators and the CDC COVID‐19 Response Team . Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. DOI: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS‐CoV‐2 pandemic. Circulation. 2020;142:429–436. DOI: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 3.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. DOI: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Rubin L, Schleien C, Epstein S, Johnson JC, Kessel A, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 infection. J Pediatr. 2020;224:141–145. DOI: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsubara D, Kauffman HL, Wang Y, Calderon‐Anyosa R, Nadaraj S, Elias MD, White TJ, Torowicz DL, Yubbu P, Giglia TM, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID‐19 in the United States. J Am Coll Cardiol. 2020;76:1947–1961. DOI: 10.1016/j.jacc.2020.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman KG, Harrild DM, Newburger JW. Cardiac dysfunction in multisystem inflammatory syndrome in children. J Am Coll Cardiol. 2020;76:1962–1964. DOI: 10.1016/j.jacc.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behrens EM, Koretzky GA. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol. 2017;69:1135–1143. DOI: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- 8.Lee PY, Day‐Lewis M, Henderson LA, Friedman KG, Lo J, Roberts JE, Lo MS, Platt CD, Chou J, Hoyt KJ, et al. Distinct clinical and immunological features of SARS–CoV‐2–induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942–5950. DOI: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bracaglia C, de Graaf K , Pires Marafon D, Guilhot F, Ferlin W, Prencipe G, Caiello I, Davì S, Schulert G, Ravelli A, et al. Elevated circulating levels of interferon‐γ and interferon‐γ‐induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2017;76:166–172. DOI: 10.1136/annrheumdis-2015-209020. [DOI] [PubMed] [Google Scholar]
- 10.Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C, Lee JH, Jasen C, Balamuth F, Barrett DM, et al. Multisystem inflammatory syndrome in children and COVID‐19 are distinct presentations of SARS–CoV‐2. J Clin Invest. 2020;130:5967–5975. DOI: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, Tan Z, Zicari S, Ruggiero A, Pascucci GR, et al. The immunology of multisystem inflammatory syndrome in children with COVID‐19. Cell. 2020;183:968–981.e7. DOI: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diorio C, McNerney KO, Lambert M, Paessler M, Anderson EM, Henrickson SE, Chase J, Liebling EJ, Burudpakdee C, Lee JH, et al. Evidence of thrombotic microangiopathy in children with SARS‐CoV‐2 across the spectrum of clinical presentations. Blood Adv. 2020;4:6051–6063. DOI: 10.1182/bloodadvances.2020003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC . Multisystem Inflammatory Syndrome in Children (MIS‐C). Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/mis‐c/hcp/. Accessed August 26, 2020. [Google Scholar]
- 14.World Health Organization Scientific Brief . Multisystem inflammatory syndrome in children and adolescents temporally related to COVID‐19. Available at: https://www.who.int/news‐room/commentaries/detail/multisystem‐inflammatory‐syndrome‐in‐children‐and‐adolescents‐with‐covid‐19. Accessed August 26, 2020.
- 15.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–495. DOI: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS; American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. DOI: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 17.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–367. DOI: 10.1016/S0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 18.Singh A, Addetia K, Maffessanti F, Mor‐Avi V, Lang RM. LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging. 2017;10:735–743. DOI: 10.1016/j.jcmg.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiotos K, Corwin D, Sartori L, Congdon M, Lavelle J, Swami S, Burnham JM, Bassiri H, John A, Balamuth F, et al. Emergency Department, ICU and inpatient clinical pathway for evaluation of possible multisystem inflammatory syndrome (MIS‐C). 2020. Available at: https://www.chop.edu/clinical‐pathway/multisystem‐inflammatory‐syndrome‐mis‐c‐clinical‐pathway. Accessed February 23, 2021.
- 20.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, et al.; Mount Sinai COVID Informatics Center . Prevalence and impact of myocardial injury in patients hospitalized with COVID‐19 infection. J Am Coll Cardiol. 2020;76:533–546. DOI: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maher KO, Reed H, Cuadrado A, Simsic J, Mahle WT, DeGuzman M, Leong T, Bandyopadhyay S. B‐type natriuretic peptide in the emergency diagnosis of critical heart disease in children. Pediatrics. 2008;121:e1484–e1488. DOI: 10.1542/peds.2007-1856. [DOI] [PubMed] [Google Scholar]
- 22.Levy PT, Machefsky A, Sanchez AA, Patel MD, Rogal S, Fowler S, Yaeger L, Hardi A, Holland MR, Hamvas A, et al. Reference ranges of left ventricular strain measures by two‐dimensional speckle‐tracking echocardiography in children: a systematic review and meta‐analysis. J Am Soc Echocardiogr. 2016;29:209–225.e6. DOI: 10.1016/j.echo.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy PT, Sanchez Mejia AA, Machefsky A, Fowler S, Holland MR, Singh GK. Normal ranges of right ventricular systolic and diastolic strain measures in children: a systematic review and meta‐analysis. J Am Soc Echocardiogr. 2014;27:549–560.e3. DOI: 10.1016/j.echo.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gloude NJ, Dandoy CE, Davies SM, Myers KC, Jordan MB, Marsh RA, Kumar A, Bleesing J, Teusink‐Cross A, Jodele S. Thinking beyond HLH: clinical features of patients with concurrent presentation of hemophagocytic lymphohistiocytosis and thrombotic microangiopathy. J Clin Immunol. 2020;40:699–707. DOI: 10.1007/s10875-020-00789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, Charpie JR, Hirsch JC. Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass*. Pediatr Crit Care Med. 2010;11:234–238. DOI: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 26.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–2273. DOI: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davies P, et al.; for the PIMS‐TS Study Group and EUCLIDS and PERFORM Consortia . Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA. 2020;324:259. DOI: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oda S, Hirasawa H, Shiga H, Nakanishi K, Matsuda K, Nakamua M. Sequential measurement of IL‐6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine. 2005;29:169–175. DOI: 10.1016/j.cyto.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, Pequignot E, Gonzalez VE, Chen F, Finklestein J, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T‐cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–679. DOI: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzgerald JC, Weiss SL, Maude SL, Barrett DM, Lacey SF, Melenhorst JJ, Shaw P, Berg RA, June CH, Porter DL, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45:e124–e131. DOI: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabhu SD. Cytokine‐induced modulation of cardiac function. Circ Res. 2004;95:1140–1153. DOI: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 32.Murray DR, Freeman GL. Tumor necrosis factor‐α induces a biphasic effect on myocardial contractility in conscious dogs. Circ Res. 1996;78:154–160. DOI: 10.1161/01.RES.78.1.154. [DOI] [PubMed] [Google Scholar]
- 33.Theocharis P, Wong J, Pushparajah K, Mathur SK, Simpson JM, Pascall E, Cleary A, Stewart K, Adhvaryu K, Savis A, et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID‐19. Eur Heart J Cardiovasc Imaging. 2020:jeaa212. DOI: 10.1093/ehjci/jeaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minhas AS, Scheel P, Garibaldi B, Liu G, Horton M, Jennings M, Jones SR, Michos ED, Hays AG. Takotsubo syndrome in the setting of COVID‐19. JACC Case Rep. 2020;2:1321–1325. DOI: 10.1016/j.jaccas.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chao C, DeValeria PA, Sen A, Lee H, Pedrotty DM, Patel B, Arsanjani R, Naqvi TZ. Reversible cardiac dysfunction in severe COVID‐19 infection, mechanisms and case report. Echocardiography. 2020;37:1465–1469. DOI: 10.1111/echo.14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyon AR, Rees PS, Prasad S, Poole‐Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy—a novel pathophysiological hypothesis to explain catecholamine‐induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22–29. DOI: 10.1038/ncpcardio1066. [DOI] [PubMed] [Google Scholar]
- 37.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:819. DOI: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID‐19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. DOI: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel MD, Mariano K, Dunbar T, Cornell TT, Punn R, Haileselassie B. Cardiac dysfunction identified by strain echocardiography is associated with illness severity in pediatric sepsis. Pediatr Crit Care Med. 2020;21:e192–e199. DOI: 10.1097/PCC.0000000000002247. [DOI] [PubMed] [Google Scholar]
- 40.Heubach JF, Ravens U, Kaumann AJ. Epinephrine activates both Gs and Gi pathways, but norepinephrine activates only the Gs pathway through human beta2‐adrenoceptors overexpressed in mouse heart. Mol Pharmacol. 2004;65:1313–1322. DOI: 10.1124/mol.65.5.1313. [DOI] [PubMed] [Google Scholar]
- 41.Dandoy CE, Davies SM, Hirsch R, Chima RS, Paff Z, Cash M, Ryan TD, Lane A, El‐Bietar J, Myers KC, et al. Abnormal echocardiography 7 days after stem cell transplantation may be an early indicator of thrombotic microangiopathy. Biol Blood Marrow Transplant. 2015;21:113–118. DOI: 10.1016/j.bbmt.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gloude NJ, Khandelwal P, Luebbering N, Lounder DT, Jodele S, Alder MN, Lane A, Wilkey A, Lake KE, Litts B, et al. Circulating dsDNA, endothelial injury, and complement activation in thrombotic microangiopathy and GVHD. Blood. 2017;130:1259–1266. DOI: 10.1182/blood-2017-05-782870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


