Abstract
Background
Sacubitril/Valsartan has been highly efficacious in randomized trials of heart failure with reduced ejection fraction (HFrEF). However, the effectiveness of sacubitril/valsartan in older patients hospitalized for HFrEF in real‐world US practice is unclear.
Methods and Results
This study included Medicare beneficiaries age ≥65 years who were hospitalized for HFrEF ≤40% in the Get With The Guidelines–Heart Failure registry between October 2015 and December 2018, and eligible for sacubitril/valsartan. Associations between discharge prescription of sacubitril/valsartan and clinical outcomes were assessed after inverse probability of treatment weighting and adjustment for other HFrEF medications. Overall, 1551 (10.9%) patients were discharged on sacubitril/valsartan. Of those not prescribed sacubitril/valsartan, 7857 (62.0%) were prescribed an angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker. Over 12‐month follow‐up, compared with a discharge prescription of angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker, sacubitril/valsartan was independently associated with lower all‐cause mortality (adjusted hazard ratio [HR], 0.82; 95% CI, 0.72–0.94; P=0.004) but not all‐cause hospitalization (adjusted HR, 0.97; 95% CI, 0.89–1.07; P=0.55) or heart failure hospitalization (adjusted HR, 1.04; 95% CI, 0.91–1.18; P=0.59). Patients prescribed sacubitril/valsartan versus those without a prescription had lower risk of all‐cause mortality (adjusted HR, 0.69; 95% CI, 0.60–0.79; P<0.001), all‐cause hospitalization (adjusted HR, 0.90; 95% CI, 0.82–0.98; P=0.02), but not heart failure hospitalization (adjusted HR, 0.94; 95% CI, 0.82–1.08; P=0.40).
Conclusions
Among patients hospitalized for HFrEF, prescription of sacubitril/valsartan at discharge was independently associated with reduced postdischarge mortality compared with angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker, and reduced mortality and all‐cause hospitalization compared with no sacubitril/valsartan. These findings support the use of sacubitril/valsartan to improve postdischarge outcomes among older patients hospitalized for HFrEF in routine US clinical practice.
Keywords: heart failure, reduced ejection fraction, registry, sacubitril/valsartan
Subject Categories: Quality and Outcomes, Heart Failure
Nonstandard Abbreviations and Acronyms
- CHAMP‐HF
Change the Management of Patients with Heart Failure
- HFrEF
heart failure with reduced ejection fraction
- IPW
inverse probability of treatment weighting
- PARADIGM‐HF
Prospective Comparison of Angiotensin II Receptor Blocker Neprilysin Inhibitor With Angiotensin‐Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure
- PIONEER‐HF
Comparison of Sacubitril–Valsartan Versus Enalapril on Effect on NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide] in Patients Stabilized from an Acute Heart Failure Episode
Clinical Perspective
What Is New?
Among older patients hospitalized for heart failure with reduced ejection fraction, prescription of sacubitril/valsartan at time of discharge is independently associated with improved postdischarge outcomes, including improved survival.
What Are the Clinical Implications?
In aggregate, these findings suggest that significant benefits of sacubitril/valsartan observed in randomized trials extend to older patients hospitalized for heart failure with reduced ejection fraction receiving routine clinical care.
Combined with data from randomized trials, these data suggest that to improve postdischarge outcomes for patients with heart failure with reduced ejection fraction, every effort should be made to prescribe sacubitril/valsartan to eligible patients at time of hospital discharge.
Sacubitril/valsartan is an angiotensin receptor–neprilysin inhibitor indicated for the treatment of heart failure with reduced ejection fraction (HFrEF).1 In the PARADIGM‐HF (Prospective Comparison of Angiotensin II Receptor Blocker Neprilysin Inhibitor With Angiotensin‐Converting Enzyme Inhibitor [ACEI] to Determine Impact on Global Mortality and Morbidity in Heart Failure [HF]) randomized trial of patients with chronic HFrEF, treatment with sacubitril/valsartan reduced cardiovascular mortality or HF hospitalization by 20% and all‐cause mortality by 16% compared with standard treatment with an ACEI.2 More recently, safety and efficacy of in‐hospital initiation of sacubitril/valsartan were supported by the PIONEER‐HF (Comparison of Sacubitril–Valsartan Versus Enalapril on Effect on NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide] in Patients Stabilized from an Acute Heart Failure Episode) trial, where exploratory analysis found patients with HFrEF randomly assigned to sacubitril/valsartan to have a 46% lower risk of serious clinical events over 8‐week follow‐up as compared with an ACEI.3
The results of PARADIGM‐HF were published in 2014, and the US Food and Drug Administration approved sacubitril/valsartan for use in July 2015. Nonetheless, despite robust clinical benefits in randomized clinical trials and strong guideline recommendations, use of sacubitril/valsartan in US clinical practice has been low. Data from the CHAMP‐HF (Change the Management of Patients with Heart Failure) registry demonstrate that in contemporary US practice, <14% of eligible outpatients with HFrEF are treated with sacubitril/valsartan, and that few patients are initiated on therapy during follow‐up.4, 5 Likewise, among US patients hospitalized for HF during the 12 months following sacubitril/valsartan approval, only 2.3% of eligible patients were prescribed the therapy at discharge.6 Although the slow and varied adoption of sacubitril/valsartan is likely multifactorial, uncertainty regarding the clinical effectiveness of therapy outside the context of a clinical trial may be a key contributor. This uncertainty may be particularly relevant to older patients, women, racial/ethnic minorities, and patients with significant comorbidities, populations comprising a significant proportion of patients seen in routine practice but generally underrepresented in HFrEF clinical trials.7 In this context, we designed the current study using a US national registry linked to Medicare claims to address an existing evidence gap regarding the clinical effectiveness of sacubitril/valsartan on postdischarge mortality and readmission in a contemporary real‐world cohort of older patients hospitalized for HFrEF.8, 9
Methods
Data Source
Data, methods, and study materials other than those provided in this manuscript will not be made available to other researchers. This study used the GWTG‐HF (Get With The Guidelines‐Heart Failure) registry, an ongoing observational, national, HF quality improvement program initiated in 2005 by the American Heart Association.10, 11 Briefly, the registry includes patients hospitalized with a primary diagnosis of new or worsening HF, or patients who develop significant HF symptoms during hospitalization such that HF was the primary diagnosis. Trained personnel at each center use an Internet‐based patient management tool (IQVIA, Parsippany, NJ) to collect patient‐level information on consecutive patients with HF admitted to the hospital. Collected data include demographics, medical history, laboratory results, discharge medications, contraindications to medications, and discharge status. All participating centers obtain institutional review board approval and follow local regulatory and privacy guidelines. Because the primary purpose of the registry is for quality improvement, all centers are granted a waiver of patient informed consent under the Common Rule. IQVIA serves as the data collection and coordinating center for American Heart Association Get With The Guidelines programs. The Duke Clinical Research Institute serves as the data analytic center. For our analysis, registry participants aged ≥65 years with fee‐for‐service Medicare coverage were linked to Medicare inpatient claims using a previously validated technique.12 The institutional review board of the Duke University Health System approved the study.
Study Population
The study population included GWTG‐HF participants aged ≥65 years who were hospitalized between October 2015 and December 2018, discharged alive with complete medical history and laboratory data, and successfully linked to Medicare inpatient claims. October 2015 was used as the study start date to allow a 3‐month transition period after Food and Drug Administration approval of sacubitril/valsartan in July 2015. Other inclusion criteria included left ventricular ejection fraction ≤40%, complete information on both contraindications and discharge prescription status for sacubitril/valsartan, and enrollment in Medicare fee‐for‐service on the date of discharge. We excluded patients who had documented contraindications to sacubitril/valsartan. For patients with multiple eligible hospitalizations during the study period, the first hospitalization was chosen as the index hospitalization.
Exposure
The exposure variable for all analyses was prescription of sacubitril/valsartan at index hospital discharge. We evaluated outcomes relative to 2 separate comparator groups representing distinct clinical questions of interest. We first compared patients prescribed sacubitril/valsartan at discharge to patients prescribed an ACEI/ARB at discharge to evaluate clinical effectiveness of sacubitril/valsartan relative to a comparator similar to that used in the randomized clinical trials. Second, we compared patients prescribed sacubitril/valsartan with patients not prescribed sacubitril/valsartan at discharge, a conventional method for evaluating effectiveness of therapy (yes versus no) in real‐world settings.
Study Outcomes
The prespecified study outcomes were all‐cause mortality, all‐cause hospitalization, the composite of all‐cause mortality or HF hospitalization, and HF hospitalization. All outcomes were identified using the Medicare Master Beneficiary Summary File and inpatient administrative claims files, including data from 2015 to 2019 (Table S1).
Statistical Analysis
Baseline characteristics were compared (1) between patients prescribed sacubitril/valsartan at hospital discharge versus those not prescribed sacubitril/valsartan, and (2) between patients prescribed sacubitril/valsartan versus patients prescribed ACEI/ARB therapy at discharge. In secondary analysis, this second comparison with patients not prescribed sacubitril/valsartan was further broken down into a 3‐way comparison between sacubitril/valsartan versus ACEI/ARB versus neither therapy at discharge. Continuous variables were presented as medians (25th and 75th percentiles) and categorical variables as counts and percentages. Treatment groups were compared using standardized mean differences, with a standardized difference ≥10% reflecting imbalance between groups.
To describe patient outcomes, we compared the cumulative incidence of each outcome at 30 days and 12 months after discharge. For mortality, we estimated cumulative incidence using the Kaplan‐Meier method and compared groups using log‐rank tests. For hospitalization outcomes, we estimated cumulative incidence using the cumulative incidence function to account for the competing risk of mortality and compared groups using Gray tests. Patients were censored when they no longer had fee‐for‐service Medicare coverage or at the end of Medicare data availability (December 31, 2019); for readmission outcomes, censoring also occurred on the date of death.
To address potential selection bias among patients discharged with sacubitril/valsartan, we used inverse probability of treatment weighting (IPW) to account for 25 baseline patient characteristics and 6 index hospital characteristics that may affect likelihood of patients being prescribed sacubitril/valsartan and the risk of adverse clinical outcomes (Table S2). For each comparator group, weights were obtained from a treatment selection model and were estimated using logistic regression, with discharge sacubitril/valsartan status as the dependent variable and baseline patient characteristics as independent variables. To confirm adequacy of the treatment selection model, each patient was weighted by the inverse of their predicated probability of treatment, and baseline characteristics were reexamined using standardized differences to evaluate balance after weighting.
Cox proportional hazards models were used to estimate the unadjusted and adjusted associations between sacubitril/valsartan prescription at discharge and each time‐to‐event outcome. First, unadjusted associations were estimated using proportional hazards models where treatment group was the only independent variable. Second, IPW was applied to obtain adjusted associations. Third, additional adjustment for beta‐blocker and mineralocorticoid receptor antagonist therapy at discharge was added to IPW models. Hazard ratios and 95% CIs) were calculated. Directly adjusted cumulative incidence curves for outcome were plotted on the basis of IPW models.
In addition, associations between sacubitril/valsartan prescription and outcomes were assessed across prespecified subgroups of interest (age [65–74 versus ≥75 years], sex, and race [White versus Black or African American versus other race]), and interaction testing was performed. Race was self‐reported by patients (if not available, the clinician or institution's assessment was used). To assess risk of residual confounding, adjusted models were used to test the association between sacubitril/valsartan and 2 prespecified falsification end points (ie, negative controls) chosen on the basis of the lack of biologically plausible associations with sacubitril/valsartan: hospitalization for urinary tract infection at 12 months and hospitalization for metabolic/nutritional disorder at 12 months. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC) within the Centers for Medicare & Medicaid Services Virtual Research Data Center secure data environment. Two‐tailed P<0.05 was considered statistically significant.
Results
Baseline Characteristics
Between October 2015 and December 2018, 14 230 patients hospitalized for HFrEF within the GWTG‐HF linked to a Medicare data set met study eligibility criteria (Figure S1). Of these patients, 1551 (10.9%) were prescribed sacubitril/valsartan at discharge and 12 679 (89.1%) patients were not. Among patients not prescribed sacubitril/valsartan, 7857 (62.0%) were prescribed an ACEI/ARB at discharge.
Baseline characteristics among patients prescribed sacubitril/valsartan and prescribed an ACEI/ARB are displayed in Table 1. The proportion of patients prescribed sacubitril/valsartan relative to ACEI/ARB therapy increased over time. Patients prescribed sacubitril/valsartan tended to be younger with lower left ventricular ejection fraction and systolic blood pressure. After application of IPW, there were no significant differences in reported baseline characteristics between patients prescribed sacubitril/valsartan and patients prescribed ACEI/ARB therapy (Table 2). In both groups, after weighting, median age was 78 years, 41% were women, and median left ventricular ejection fraction was 27% to 28%.
Table 1.
Characteristics of Patients Discharged With Sacubitril/Valsartan Versus an ACEI/ARB Before Application of Inverse Probability Weights
| Sacubitril/Valsartan (n=1551) | ACEI/ARB (n=7857) | Standardized Mean Difference* | |
|---|---|---|---|
| Age, y | 77 (71–83) | 78 (71–85) | 17.4 |
| Women | 560 (36.1) | 3262 (41.5) | 11.1 |
| Race | 3.9 | ||
| White | 1259 (81.2) | 6302 (80.2) | |
| Black or African American | 191 (12.3) | 965 (12.3) | |
| Other§ | 101 (6.5) | 590 (7.5) | |
| Medicaid dual eligibility | 218 (14.1) | 1230 (15.7) | 4.5 |
| Ejection fraction (%) | 25 (20–32) | 28 (22–35) | 28.7 |
| Index hospitalization year | 51.5 | ||
| 2015/2016 | 294 (19.0) | 3190 (40.6) | |
| 2017 | 529 (34.1) | 2413 (30.7) | |
| 2018 | 728 (46.9) | 2254 (28.7) | |
| Vital sign and laboratory data at discharge | |||
| Systolic blood pressure, mm Hg | 113 (102–126) | 118 (107–132) | 30.7 |
| Heart rate, beats/min | 74 (67–83) | 75 (67–84) | 4.5 |
| Sodium, mEq/L | 139 (136–141) | 139 (136–141) | 6.0 |
| Creatinine, mg/dL | 1.2 (1.0–1.5) | 1.2 (0.9–1.5) | 3.5 |
| Medical history | |||
| Ischemic HF etiology | 1105 (71.2) | 4957 (63.1) | 17.4 |
| Prior PCI | 451 (29.1) | 1883 (24.0) | 11.6 |
| Prior CABG | 460 (29.7) | 2007 (25.5) | 9.2 |
| Hypertension | 1329 (85.7) | 6594 (83.9) | 4.9 |
| Hyperlipidemia | 981 (63.2) | 4769 (60.7) | 5.3 |
| Valve disease† | 292 (18.8) | 1364 (17.4) | 3.8 |
| Atrial fibrillation/flutter | 716 (46.2) | 3313 (42.2) | 8.1 |
| Diabetes mellitus | 667 (43.0) | 3237 (41.2) | 3.7 |
| Stroke/TIA | 256 (16.5) | 1304 (16.6) | 0.2 |
| Chronic kidney disease | 239 (15.4) | 993 (12.6) | 8.0 |
| Anemia | 259 (16.7) | 1293 (16.5) | 0.7 |
| COPD | 451 (29.1) | 2328 (29.6) | 1.2 |
| Smoking in past 12 mo | 168 (10.8) | 973 (12.4) | 4.8 |
| Device therapy | |||
| CRT‐D | 332 (21.4) | 809 (10.3) | 30.8 |
| ICD only | 361 (23.3) | 1064 (13.5) | 25.3 |
| Medical therapy before admission‡ | |||
| ACEI/ARB | 287 (18.5) | 3439 (43.8) | 56.9 |
| Sacubitril/Valsartan | 297 (19.1) | 19 (0.2) | 85.1 |
| Beta‐blocker | 668 (43.1) | 3660 (46.6) | 32.7 |
| MRA | 185 (11.9) | 718 (9.1) | 32.1 |
| Medical therapy at discharge | |||
| Beta‐blocker | 1437 (92.6) | 7294 (92.8) | 0.7 |
| MRA | 604 (38.9) | 2633 (33.5) | 11.3 |
| Hospital characteristics | |||
| Teaching hospital | 1202 (77.5) | 6415 (81.6) | 10.3 |
| Profit status | 10.3 | ||
| Not‐for‐profit | 1225 (79.0) | 5863 (74.6) | |
| Government | 222 (14.3) | 1356 (17.3) | |
| For‐profit | 104 (6.7) | 638 (8.1) | |
| Region | 33.4 | ||
| Northeast | 411 (26.5) | 2028 (25.8) | |
| Midwest | 299 (19.3) | 1803 (22.9) | |
| South | 716 (46.2) | 2677 (34.1) | |
| West | 125 (8.1) | 1349 (17.2) | |
| Hospital bed size | 393 (259–564) | 376 (253–564) | 2.5 |
| Cardiac catheterization lab on site | 1416 (91.3) | 7348 (93.5) | 8.4 |
| Heart transplantation on site | 50 (3.2) | 474 (6.0) | 13.4 |
Data presented as n (%) or median (25th–75th percentile). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy and defibrillator; HF, heart failure; ICD, implantable cardioverter‐defibrillator; MRA, mineralocorticoid receptor antagonist; PCI, percutaneous coronary intervention; and TIA, transient ischemic attack.
Standardized mean differences represents differences in means or proportions divided by the standard error and multiplied by 10. Standardized mean differences >10 indicate imbalance between groups.
Moderately severe or severe regurgitation or stenosis of any valve, with exception of functional (ie, secondary) mitral regurgitation.
Data were missing for 718 patients in the sacubitril/valsartan group and 2615 patients in the ACEI/ARB group. Percentages reflect patients receiving medication among total patients in the group.
Includes American Indian or Alaska Native, Asian, or Native Hawaiian or Pacific Islander.
Table 2.
Characteristics of Patients Discharged With Sacubitril/Valsartan Versus an ACEI/ARB After Application of Inverse Probability Weights
| Sacubitril/Valsartan (n=1551) | ACEI/ARB (n=7857) | Standardized Mean Difference* | |
|---|---|---|---|
| Age, y | 78 (72–84) | 78 (71–85) | 1.3 |
| Women | 630 (40.6) | 3187 (40.6) | 1.6 |
| Race | 2.9 | ||
| White | 1219 (79.6) | 6317 (80.3) | |
| Black or African American | 187 (12.2) | 968 (12.3) | |
| Other§ | 124 (8.1) | 578 (7.4) | |
| Medicaid dual eligibility | 255 (16.6) | 1212 (15.4) | 3.4 |
| Ejection fraction (%) | 27 (20–33) | 28 (20–35) | 6.0 |
| Index hospitalization year | 2.0 | ||
| 2015/2016 | 552 (36.0) | 2908 (37.0) | |
| 2017 | 490 (32.0) | 2463 (31.3) | |
| 2018 | 489 (32.0) | 2492 (31.7) | |
| Vital sign and laboratory data at discharge | |||
| Systolic blood pressure, mm Hg | 118 (106–130) | 118 (106–131) | 3.5 |
| Heart rate, beats/min | 75 (68–84) | 75 (67–84) | 0.1 |
| Sodium, mEq/L | 139 (136–141) | 138 (136–141) | 1.1 |
| Creatinine, mg/dL | 1.2 (1.0–1.5) | 1.2 (0.9–1.5) | 1.1 |
| Medical history | |||
| Ischemic HF etiology | 998 (65.2) | 5073 (64.5) | 1.4 |
| Prior PCI | 400 (26.1) | 1953 (24.8) | 2.9 |
| Prior CABG | 427 (27.9) | 2070 (26.3) | 3.5 |
| Hypertension | 1265 (82.6) | 6622 (84.2) | 4.3 |
| Hyperlipidemia | 922 (60.3) | 4804 (61.1) | 1.7 |
| Valve disease† | 263 (17.2) | 1382 (17.6) | 1.1 |
| Atrial fibrillation/ flutter | 638 (41.7) | 3365 (42.8) | 2.2 |
| Diabetes mellitus | 650 (42.5) | 3267 (41.5) | 1.9 |
| Stroke/TIA | 261 (17.0) | 1308 (16.6) | 1.0 |
| Chronic kidney disease | 219 (14.3) | 1041 (13.2) | 3.1 |
| Anemia | 259 (16.9) | 1298 (16.5) | 1.0 |
| COPD | 452 (29.5) | 2321 (29.5) | 0.1 |
| Smoking in past 12 mo | 181 (11.8) | 950 (12.1) | 0.7 |
| Device therapy | |||
| CRT‐D | 192 (12.6) | 961 (12.2) | 1.0 |
| ICD only | 229 (14.9) | 1197 (15.2) | 0.8 |
| Medical therapy at discharge‡ | |||
| Beta‐blocker | 1419 (92.7) | 7313 (93.0) | 1.2 |
| MRA | 558 (36.5) | 2669 (33.9) | 5.3 |
| Hospital characteristics | |||
| Teaching hospital | 1255 (82.0) | 6376 (81.1) | 2.4 |
| Profit status | 1.5 | ||
| Not‐for‐profit | 115 (7.5) | 620 (7.9) | |
| Government | 257 (16.8) | 1319 (16.8) | |
| For‐profit | 115 (7.5) | 620 (7.9) | |
| Region | 0.9 | ||
| Northeast | 404 (26.4) | 2044 (26.0) | |
| Midwest | 341 (22.3) | 1758 (22.4) | |
| South | 548 (35.8) | 2831 (36.0) | |
| West | 239 (15.6) | 1231 (15.7) | |
| Hospital bed size | 393 (286–581) | 374 (253–564) | 2.0 |
| Cardiac catheterization lab on site | 1435 (93.7) | 7326 (93.2) | 2.3 |
| Heart transplantation on site | 88 (5.8) | 435 (5.5) | 1.0 |
Data presented as n (%) or median (25th–75th percentile). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy and defibrillator; HF, heart failure; ICD, implantable cardioverter‐defibrillator; MRA, mineralocorticoid receptor antagonist; PCI, percutaneous coronary intervention; and TIA, transient ischemic attack.
Standardized mean differences represents differences in means or proportions divided by the standard error and multiplied by 100. Standardized mean differences >10 indicate imbalance between groups.
Moderately severe or severe regurgitation or stenosis of any valve, with exception of functional (ie, secondary) mitral regurgitation.
Discharge medications were not included within inverse probability of treatment weighted models. Adjustment for beta‐blocker and MRA therapy at discharge was added to inverse probability of treatment weighted models to constitute the fully adjusted model.
Other includes American Indian or Alaska Native, Asian, or Native Hawaiian or Pacific Islander.
Compared with patients not prescribed sacubitril/valsartan, patients prescribed sacubitril/valsartan tended to be younger with lower left ventricular ejection fraction and systolic blood pressure (Table S3). After application of IPW, there were no significant differences in reported baseline characteristics between patients prescribed versus not prescribed sacubitril/valsartan, with exception of higher rates of beta‐blocker and mineralocorticoid receptor antagonist therapy at discharge among sacubitril/valsartan patients (ie, these medications not included in IPW model and subsequently accounted for in full adjusted model) (Table S4). Baseline characteristics of patients prescribed sacubitril/valsartan, ACEI/ARB, and neither therapy at discharge are displayed in Table S5.
Outcomes for Sacubitril/Valsartan Versus ACEI/ARB
Compared with patients prescribed an ACEI/ARB, patients prescribed sacubitril/valsartan had similar cumulative incidence of all‐cause mortality, all‐cause hospitalization, all‐cause mortality or HF hospitalization, and HF hospitalization at 30 days and 12 months, with the exception of higher incidence of 12‐month HF hospitalization among patients prescribed sacubitril/valsartan (Table 3).
Table 3.
Unadjusted Cumulative Incidence of Clinical Outcomes for Patients Discharged With Sacubitril/Valsartan Versus an ACEI/ARB
| Sacubitril/Valsartan (n=1551) | ACEI/ARB (n=7857) | P Value | |
|---|---|---|---|
| Effectiveness end points | |||
| All‐cause mortality | |||
| 30 d | 75 (4.9) | 428 (5.5) | 0.32 |
| 12 mo | 444 (29.5) | 2369 (30.9) | 0.22 |
| All‐cause hospitalization | |||
| 30 d | 356 (23.0) | 1704 (21.7) | 0.25 |
| 12 mo | 984 (64.7) | 4835 (62.6) | 0.07 |
| All‐cause mortality or HF hospitalization | |||
| 30 d | 195 (12.6) | 1035 (13.2) | 0.54 |
| 12 mo | 792 (52.3) | 3876 (50.4) | 0.18 |
| HF hospitalization | |||
| 30 d | 142 (9.2) | 652 (8.3) | 0.26 |
| 12 mo | 560 (37.0) | 2418 (31.4) | <0.001 |
| Falsification (negative control) end points | |||
| Metabolic/Nutritional hospitalization within 12 mo | 36 (2.4) | 144 (1.9) | 0.19 |
| Urinary tract infection hospitalization within 12 mo | 17 (1.1) | 100 (1.3) | 0.58 |
Data presented as n (%). Cumulative incidence of mortality and mortality or HF hospitalization end points were calculated using the Kaplan‐Meier method and group differences were evaluated using log‐rank tests. Cumulative incidence for hospitalization outcomes was estimated using the cumulative incidence function to account for the competing risk of mortality, and group differences were evaluated using Gray tests. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; and HF, heart failure.
After IPW and adjustment for discharge medications, sacubitril/valsartan was independently associated with lower risks of all‐cause mortality but was not significantly associated with all‐cause hospitalization, mortality or HF hospitalization, and HF hospitalization at 12 months, compared with ACEI/ARB therapy (Figure 1; Table 4). Sacubitril/valsartan prescription was not associated with either falsification end point in unadjusted or adjusted analyses.
Figure 1. Cumulative incidence of mortality and hospitalization outcomes for patients discharged with sacubitril/valsartan vs an ACEI/ARB.
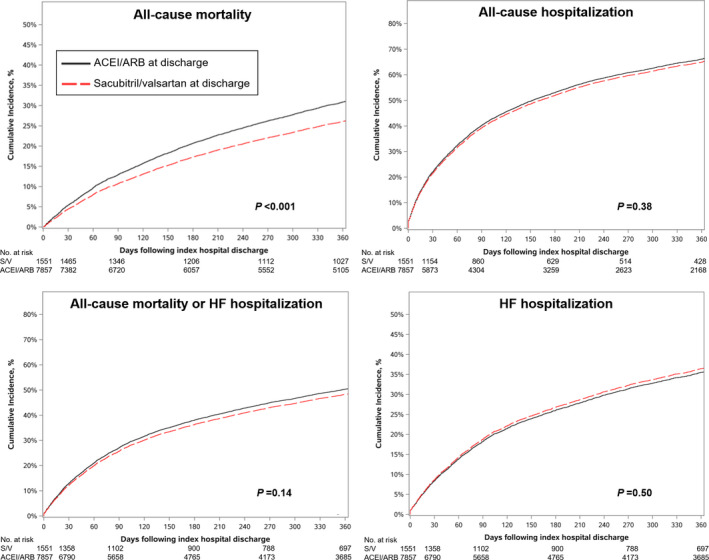
Curves reflect adjusted results in the form of directly adjusted cumulative incidence curves, which were derived from inverse‐probability‐of‐treatment‐weighted proportional hazards models. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; and HF, heart failure.
Table 4.
Associations Between Sacubitril/Valsartan Prescription and Clinical Outcomes at 12 Months
| Unweighted | Inverse‐Weighted* | Inverse‐Weighted+Adjusted for Discharge Medications† | |
|---|---|---|---|
| HR (95% CI), P Value | HR (95% CI), P Value | HR (95% CI), P Value | |
| Clinical end points | |||
| All‐cause mortality | 0.94 (0.85–1.04), 0.22 | 0.82 (0.72–0.93), 0.003 | 0.82 (0.72–0.94), 0.004 |
| All‐cause hospitalization | 1.04 (0.96–1.12), 0.32 | 0.97 (0.88–1.06), 0.51 | 0.97 (0.89–1.07), 0.55 |
| All‐cause mortality or HF hospitalization | 1.05 (0.97–1.14), 0.21 | 0.94 (0.85–1.04), 0.26 | 0.95 (0.86–1.05), 0.30 |
| HF hospitalization | 1.19 (1.07–1.33), 0.001 | 1.03 (0.90–1.18), 0.63 | 1.04 (0.91–1.18), 0.59 |
| Falsification (negative control) end points | |||
| Hospitalization for metabolic/nutritional disorder | 1.26 (0.87–1.82), 0.23 | 1.52 (0.96–2.41), 0.08 | 1.52 (0.96–2.40), 0.08 |
| Hospitalization for urinary tract infection | 0.85 (0.52–1.39), 0.52 | 0.95 (0.54–1.68), 0.86 | 0.95 (0.54–1.69), 0.87 |
Referent=ACEI/ARB Prescription. HF indicates heart failure; and HR, hazard ratio.
Model reflects inverse probability of treatment weighting including 25 demographic and clinical variables and 6 index hospital variables.
Model reflects inverse probability of treatment weighting and adjustment for discharge prescription for beta‐blocker and mineralocorticoid receptor antagonist therapy.
Outcomes for Sacubitril/Valsartan Versus No Sacubitril/Valsartan
Compared with patients not prescribed sacubitril/valsartan, patients prescribed sacubitril/valsartan had a lower cumulative incidence of all‐cause mortality and all‐cause mortality or HF hospitalization at 30 days and 12 months. The cumulative incidence of all‐cause hospitalization and HF hospitalization were similar, with the exception of higher incidence of HF hospitalization at 12 months among patients prescribed sacubitril/valsartan (Table S6).
After IPW and adjustment for discharge medications, sacubitril/valsartan prescription was associated with significantly lower risks of all‐cause mortality, all‐cause hospitalization, and mortality or HF hospitalization at 12 months, but not HF hospitalization (Figure S2; Table S7). There were no significant associations between sacubitril/valsartan prescription and falsification end points before or after adjustment.
Outcomes for Sacubitril/Valsartan Versus ACEI/ARB Versus Neither Therapy
Comparing patients prescribed sacubitril/valsartan, a ACEI/ARB, and neither therapy, patients prescribed sacubitril/valsartan had the lowest unadjusted incidences of 30‐day and 12‐month mortality, and those prescribed neither therapy had the highest incidences. Cumulative incidence of all‐cause hospitalization and HF hospitalization was lowest among patients prescribed an ACEI/ARB (Table S8).
Subgroup Analyses
In comparisons of sacubitril/valsartan versus ACEI/ARB, findings for all end points were consistent irrespective of age, sex, and race (all P for interaction ≥0.12) (Figure 2). In analyses of sacubitril/valsartan prescription versus no prescription, associations between sacubitril/valsartan and clinical end points were consistent across subgroups defined by age and sex (all P for interaction ≥0.06). However, a statistically significant interaction by race was observed for the all‐cause mortality or HF hospitalization and HF hospitalization end points, whereby associations with improved outcomes were driven by results among White patients (Figure S3).
Figure 2. Prespecified subgroup analyses for mortality and hospitalization outcomes for patients discharged with sacubitril/valsartan vs an ACEI/ARB.
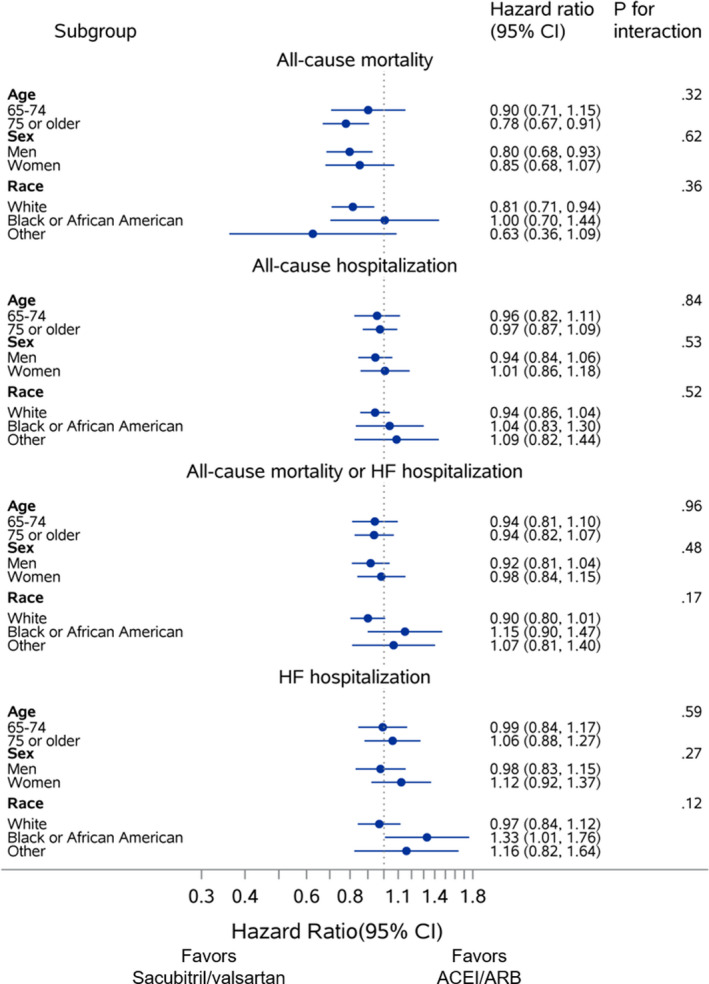
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; and HF, heart failure.
Discussion
In this contemporary real‐world population of US patients hospitalized for HFrEF, despite clinical trial evidence and guideline recommendations that were available during the study period, nearly 90% of eligible patients were not prescribed sacubitril/valsartan at hospital discharge. After adjustment for patient characteristics and other HFrEF medications, prescription of sacubitril/valsartan at hospital discharge was significantly associated with lower risk of mortality compared with ACEI/ARB therapy. Likewise, compared with patients not prescribed sacubitril/valsartan, prescription of sacubitril/valsartan at discharge was independently associated with reduced risk of mortality, all‐cause hospitalization, and the composite of mortality or all‐cause hospitalization. In aggregate, these findings suggest that significant benefits of sacubitril/valsartan observed in randomized trials extend to older patients hospitalized with HFrEF receiving routine clinical care.
In randomized trials, sacubitril/valsartan has substantially reduced the risk of HF hospitalization compared with an ACEI, an effect that was not observed in the current observational study.2, 3 This lack of significant association in the current study may relate to residual confounding and a tendency for sacubitril/valsartan to be prescribed to patients with higher risk of readmission in real‐world practice. Indeed, unadjusted results found patients prescribed sacubitril/valsartan to have the highest incidence of 12‐month HF hospitalization (ie, higher than patients prescribed an ACEI/ARB and neither sacubitril/valsartan nor an ACEI/ARB), suggesting that sacubitril/valsartan may be preferentially prescribed to those with particularly high risk of HF hospitalization.
To our knowledge, only 2 prior large analyses (ie, >1000 patients) have evaluated the real‐world effectiveness of sacubitril/valsartan for HFrEF, and both have limitations.13, 14 Similar to the present study, an analysis from the US Veterans Health Administration found no significant association between sacubitril/valsartan and HF hospitalization, but that study did not assess mortality.13 A second analysis by Tan et al from OptumLabs, a US administrative database of privately insured patients, found sacubitril/valsartan to be significantly associated with a 20% relative reduction in all‐cause mortality compared with ACEI/ARB, and no significant association with HF hospitalization, findings that are both consistent with the current study.14 However, the OptumLabs study was limited by reliance on diagnostic codes for patient characteristics and the study population was defined using a diagnosis of systolic HF, as compared with precise measurement of EF. By contrast, the present work used patient‐level clinical data from the GWTG‐HF registry, thus facilitating more accurate selection of patients eligible for treatment, more comprehensive risk adjustment, and improved generalizability of findings through a more detailed description of the patient profile. Moreover, patients in the OptumLabs analysis were outpatients with a median age of ≈69 years, whereas the current study informs the use of sacubitril/valsartan in a distinct cohort of patients hospitalized for HFrEF with a median age of 78 years, a population for which large‐scale data were previously not available and where concerns over risks and benefits of therapy may be greatest. Finally, the prior analysis by Tan et al14 reported differing effectiveness of sacubitril/valsartan by race, and generated the hypothesis that sacubitril/valsartan may be less effective among Black patients. This interaction was not seen in the current study in the comparison of sacubitril/valsartan by ACEIs/ARBs. However, significant interactions by race were seen in comparisons between sacubitril/valsartan versus no sacubitril/valsartan, whereby favorable associations between sacubitril/valsartan and the composite of mortality or HF hospitalization and HF hospitalization were confined to White patients. Nevertheless, in the context of randomized trial data from PARADIGM‐HF and PIONEER‐HF supporting consistent treatment effect in Black patients, the racial differences seen by Tan et al and the present analysis may reflect residual confounding or the play of chance.
Clinical Implications
Despite significant benefits in randomized clinical trials and strong guideline recommendations, there is substantial underuse and underdosing of sacubitril/valsartan and other guideline‐directed medical therapies in contemporary US clinical practice.4, 5, 6 Prior work has estimated that optimal implementation of evidence‐based therapy among undertreated patients with HFrEF could prevent as many as 100 000 deaths in the United States each year.15, 16 Specifically, such analyses estimated that optimal use of sacubitril/valsartan alone would result in >28 000 fewer US deaths.16 In this context, the present data comparing patients with and without discharge prescription of sacubitril/valsartan further illustrate the magnitude of real‐world clinical benefit that could be achieved with improved implementation. As compared with no discharge prescription, sacubitril/valsartan was associated with large magnitudes of risk reduction, including 31% lower risk of all‐cause mortality and 10% lower risk of all‐cause hospitalization. Future efforts to improve use of sacubitril/valsartan may focus on improved patient and clinician engagement and education regarding efficacy and safety, as well as innovative strategies centered on behavioral economics or technological innovation (eg, mobile applications).17
Although the precise reasons for low use of sacubitril/valsartan are unclear, this may reflect, in part, concerns that the findings from randomized clinical trials may not generalize to patients encountered in routine clinical practice, who are often older and with more comorbid conditions.7, 18 Notably, the mean age of patients enrolled in PARADIGM‐HF was 64 years, and the median age was 62 years in PIONEER‐HF, as compared with 78 years in the present study.2, 3 Likewise, proportions of women in PARADIGM‐HF and PIONEER‐HF were 22% and 28%, respectively, as compared with ≈40% in the current study.2, 3 Although current results for all‐cause and HF hospitalization were not significant, considering the totality of the mortality and hospitalization findings, these data also support the benefit of initiation or continuation of sacubitril/valsartan during the HF hospitalization. These findings extend the results of PIONEER‐HF and support hospitalization as a key opportunity for optimizing evidence‐based HFrEF therapy (Table 5).19, 20, 21
Table 5.
Comparison of Current Findings From GWTG‐HF With PIONEER‐HF
| Patient Characteristics | GWTG‐HF | PIONEER‐HF3 | ||
|---|---|---|---|---|
| 9408 Patients From 301 US Sites | 887 Patients From 129 US Sites | |||
| Sacubitril/Valsartan (n=1551) | ACEI/ARB (n=7857) | Sacubitril/Valsartan (n=440) | ACEI (n=441) | |
| Age | 78 (72–84) | 78 (71–85) | 61 (51–71) | 63 (54–72) |
| Women | 630 (40.6) | 3187 (40.6) | 113 (25.7) | 133 (30.2) |
| Black race | 187 (12.2) | 968 (12.3) | 158 (35.9) | 158 (35.8) |
| Systolic blood pressure, mm Hg | 118 (106–130) | 118 (106–131) | 118 (110–133) | 118 (109–132) |
| Heart rate, beats/min | 75 (68–84) | 75 (67–84) | 81 (72–92) | 80 (72–91) |
| Creatinine, mg/dL | 1.2 (1.0–1.5) | 1.2 (0.9–1.5) | 1.3 (1.1–1.5) | 1.3 (1.1–1.5) |
| Prior PCI | 400 (26.1) | 1953 (24.8) | 2 (0.5) | 6 (1.4) |
| Prior CABG | 427 (27.9) | 2070 (26.3) | 18 (4.1) | 17 (3.9) |
| Diabetes mellitus | 650 (42.5) | 3267 (41.5) | 79 (18.0) | 89 (20.2) |
| Atrial fibrillation | 638 (41.7) | 3365 (42.8) | 147 (33.4) | 165 (37.4) |
| Relative Risk of Clinical Outcomes—Sacubitril/Valsartan vs RASi—HR (95% CI) | ||
|---|---|---|
| End Point | 12‐mo Follow‐Up | 8‐wk Follow‐Up |
| All‐cause mortality | 0.82 (0.72–0.94)* | 0.66 (0.30–1.48) |
| HF hospitalization | 1.04 (0.91–1.18)* | 0.56 (0.37–0.84) |
Data presented as n (%) or median (25th–75th percentile). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass grafting; GWTG‐HF, Get With The Guidelines–Heart Failure registry; PCI, percutaneous coronary intervention; PIONEER‐HF, Comparison of Sacubitril–Valsartan Versus Enalapril on Effect on NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide] in Patients Stabilized from an Acute Heart Failure Episode; and RASi, renin‐angiotensin system inhibitor.
Reflects full model incorporating inverse probability of treatment weighting for 25 demographic and clinical variables and 6 index hospital variables, as well as adjustment for discharge prescription for beta‐blocker and mineralocorticoid receptor antagonist therapy.
Limitations
First, despite adjustment for several variables and rigorous statistical methods, residual confounding, unmeasured confounding, or both, may exist. Second, because of moderate missing data for admission medications, this analysis did not distinguish effectiveness of continued versus new prescription of sacubitril/valsartan, and may be subject to prevalent user bias. Nonetheless, real‐world populations comprise a mix of patients who have and have not received a therapy in the past; thus, the current approach examining discharge use may be more reflective of clinical practice. Moreover, available admission medication data suggest that the majority of patients discharged on sacubitril/valsartan in the current analysis were initiated on therapy during the index hospitalization (Table 1). Third, by defining treatment groups by discharge prescription, this study did not account for potential crossover that could occur with postdischarge initiation or discontinuation of sacubitril/valsartan during the follow‐up period. Likewise, this study did not assess postdischarge adherence or persistence of discharge therapy, and these factors could contribute to associations with clinical outcomes. Medication dosing data were also not available. Nonetheless, recent data suggest that such changes in sacubitril/valsartan use and dosing during longitudinal US outpatient care are modest.5 Finally, data on postdischarge patient adherence to sacubitril/valsartan therapy were not available.
Conclusions
In this contemporary real‐world population of older US patients hospitalized for HFrEF and eligible for sacubitril/valsartan, prescription of sacubitril/valsartan at discharge was significantly associated with reductions in postdischarge mortality and hospitalization. These results complement existing efficacy and safety data from randomized clinical trials, and suggest that clinical benefits of sacubitril/valsartan extend to older patients hospitalized for HFrEF in real‐world US clinical practice.
Sources of Funding
This study was funded by Novartis Pharmaceuticals Corporation (East Hanover, NJ). The GWTG‐HF program is provided by the American Heart Association. GWTG‐HF is sponsored, in part, by Novartis, Boehringer Ingelheim and Eli Lilly Diabetes Alliance, Novo Nordisk, Sanofi, AstraZeneca and Bayer.
Disclosures
Dr Greene has received research support from the Duke University Department of Medicine Chair's Research Award, American Heart Association, Amgen, AstraZeneca, Bristol‐Myers Squibb, Cytokinetics, Cytokinetics, Merck, Novartis, and Pfizer; has served on advisory boards for Amgen, AstraZeneca, and Cytokinetics; and has served as a consultant for Amgen, Bayer, Merck, and Vifor. Dr Mentz receives research support from the National Institutes of Health (U01HL125511‐01A1, U10HL110312 and R01AG045551‐01A1), Akros, Amgen, AstraZeneca, Bayer, GlaxoSmithKline, Gilead, Luitpold, Medtronic, Merck, Novartis, Otsuka, and ResMed; honoraria from Abbott, AstraZeneca, Bayer, Janssen, Luitpold Pharmaceuticals, Medtronic, Merck, Novartis, and ResMed; and has served on an advisory board for Amgen, Luitpold, Merck, and Boehringer Ingelheim. Dr Hammill receives research support from Novartis, GlaxoSmithKline, Abbott, Boston Scientific, and St. Jude. Dr Luo receives honoraria from AstraZeneca and Pfizer. Dr Curtis receives research support from the National Institutes of Health, the Patient‐Centered Outcomes Research Institute, Novartis, GlaxoSmithKline, Gilead, Boston Scientific, and St. Jude. Dr Hernandez reports consulting fees from AstraZeneca, Bayer, Boston Scientific, Merck, Novartis, Sanofi; and research support from AstraZeneca, GlaxoSmithKline, Luitpold, Merck, Novartis. Dr Fonarow reports research funding from the NIH and serving as a consultant for Amgen, Bayer, Medtronic, and Novartis. Dr O'Brien has received research support from Bristol‐Myers Squibb, GlaxoSmithKline, and Novartis. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S8
Figures S1–S3
This manuscript was sent to Joseph I. Shapiro, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021459
For Sources of Funding and Disclosures, see page 12.
Contributor Information
Stephen J. Greene, Email: stephen.greene@duke.edu.
Emily C. O'Brien, Email: emily.obrien@duke.edu.
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;8;136:e137–e161. DOI: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. DOI: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E. Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. DOI: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 4.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol. 2018;72:351–366. DOI: 10.1016/j.jacc.2018.04.070. [DOI] [PubMed] [Google Scholar]
- 5.Greene SJ, Fonarow GC, DeVore AD, Sharma PP, Vaduganathan M, Albert NM, Duffy CI, Hill CL, McCague K, Patterson JH, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73:2365–2383. DOI: 10.1016/j.jacc.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo N, Fonarow GC, Lippmann SJ, Mi X, Heidenreich PA, Yancy CW, Greiner MA, Hammill BG, Hardy NC, Turner SJ, et al. Early adoption of sacubitril/valsartan for patients with heart failure with reduced ejection fraction: insights from Get With The Guidelines‐Heart Failure (GWTG‐HF). JACC Heart Fail. 2017;5:305–309. DOI: 10.1016/j.jchf.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Tahhan AS, Vaduganathan M, Greene SJ, Fonarow GC, Fiuzat M, Jessup M, Lindenfeld J, O'Connor CM, Butler J. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol. 2018;3:1011–1019. DOI: 10.1001/jamacardio.2018.2559. [DOI] [PubMed] [Google Scholar]
- 8.Fonarow GC. Randomization‐there is no substitute. JAMA Cardiol. 2016;1:633–635. DOI: 10.1001/jamacardio.2016.1792. [DOI] [PubMed] [Google Scholar]
- 9.Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA. 2013;309:241–242. DOI: 10.1001/jama.2012.96867. [DOI] [PubMed] [Google Scholar]
- 10.Smaha LA. The American Heart Association Get With The Guidelines program. Am Heart J. 2004;148:S46–S48. DOI: 10.1016/j.ahj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Hong Y, LaBresh KA. Overview of the American Heart Association “Get With The Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5:179–186. DOI: 10.1097/01.hpc.0000243588.00012.79. [DOI] [PubMed] [Google Scholar]
- 12.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. DOI: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohanty AF, Levitan EB, Dodson JA, Vardeny O, King JB, LaFleur J, He T, Patterson OV, Alba PR, Russo PA, et al. Characteristics and healthcare utilization among veterans treated for heart failure with reduced ejection fraction who switched to sacubitril/valsartan. Circ Heart Fail. 2019;12:e005691. DOI: 10.1161/CIRCHEARTFAILURE.118.005691. [DOI] [PubMed] [Google Scholar]
- 14.Tan NY, Sangaralingham LR, Sangaralingham SJ, Yao X, Shah ND, Dunlay SM. Comparative effectiveness of sacubitril‐valsartan versus ACE/ARB therapy in heart failure with reduced ejection fraction. JACC Heart Fail. 2020;8:43–54. DOI: 10.1016/j.jchf.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence‐based heart failure therapies on mortality. Am Heart J. 2011;161:1024–1030.e1023. DOI: 10.1016/j.ahj.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Fonarow GC, Hernandez AF, Solomon SD, Yancy CW. Potential mortality reduction with optimal implementation of angiotensin receptor neprilysin inhibitor therapy in heart failure. JAMA Cardiol. 2016;1:714–717. DOI: 10.1001/jamacardio.2016.1724. [DOI] [PubMed] [Google Scholar]
- 17.Chang LL, DeVore AD, Granger BB, Eapen ZJ, Ariely D, Hernandez AF. Leveraging behavioral economics to improve heart failure care and outcomes. Circulation. 2017;136:765–772. DOI: 10.1161/CIRCULATIONAHA.117.028380. [DOI] [PubMed] [Google Scholar]
- 18.Wang TS, Hellkamp AS, Patel CB, Ezekowitz JA, Fonarow GC, Hernandez AF. Representativeness of RELAX‐AHF clinical trial population in acute heart failure. Circ Cardiovasc Qual Outcomes. 2014;7:259–268. DOI: 10.1161/CIRCOUTCOMES.113.000418. [DOI] [PubMed] [Google Scholar]
- 19.Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. 2015;12:220–229. DOI: 10.1038/nrcardio.2015.14. [DOI] [PubMed] [Google Scholar]
- 20.Bhagat AA, Greene SJ, Vaduganathan M, Fonarow GC, Butler J. Initiation, continuation, switching, and withdrawal of heart failure medical therapies during hospitalization. JACC Heart Fail. 2019;7:1–12. DOI: 10.1001/10.1016/j.jchf.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava PK, Fonarow GC. In‐hospital initiation of angiotensin receptor‐neprilysin inhibitors‐the time is now. JAMA Cardiol. 2019;4:195–196. DOI: 10.1001/jamacardio.2019.0104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8
Figures S1–S3


