Abstract
Background
Recent trials have shown that low‐density lipoprotein cholesterol (LDL‐C) <1.80 mmol/L (<70 mg/dL) is associated with a reduced risk of major adverse cardiovascular events in White patients with ischemic stroke with atherosclerosis. However, it remains uncertain whether the findings can be generalized to Asian patients, or that similar LDL‐C targets should be adopted in patients with stroke without significant atherosclerosis.
Methods and Results
We performed a prospective cohort study and recruited consecutive Chinese patients with ischemic stroke with magnetic resonance angiography of the intra‐ and cervicocranial arteries performed at the University of Hong Kong between 2008 and 2014. Serial postevent LDL‐C measurements were obtained. Risk of major adverse cardiovascular events in patients with mean postevent LDL‐C <1.80 versus ≥1.80 mmol/L, stratified by presence or absence of significant (≥50%) large‐artery disease (LAD) and by ischemic stroke subtypes, were compared. Nine hundred four patients (mean age, 69±12 years; 60% men) were followed up for a mean 6.5±2.4 years (mean, 9±5 LDL‐C readings per patient). Regardless of LAD status, patients with a mean postevent LDL‐C <1.80 mmol/L were associated with a lower risk of major adverse cardiovascular events (with significant LAD: multivariable‐adjusted subdistribution hazard ratio, 0.65; 95% CI, 0.42–0.99; without significant LAD: subdistribution hazard ratio, 0.53; 95% CI, 0.32–0.88) (both P<0.05). Similar findings were noted in patients with ischemic stroke attributable to large‐artery atherosclerosis (subdistribution hazard ratio, 0.48; 95% CI, 0.28–0.84) and in patients with other ischemic stroke subtypes (subdistribution hazard ratio, 0.64; 95% CI, 0.43–0.95) (both P<0.05).
Conclusions
A mean LDL‐C <1.80 mmol/L was associated with a lower risk of major adverse cardiovascular events in Chinese patients with ischemic stroke with and without significant LAD. Further randomized trials to determine the optimal LDL‐C cutoff in stroke patients without significant atherosclerosis are warranted.
Keywords: ischemic stroke, low‐density lipoprotein cholesterol, prognosis, prospective cohort study
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke, Stenosis, Vascular Disease, Atherosclerosis
Nonstandard Abbreviations and Acronyms
- ICH
intracerebral hemorrhage
- LAD
large‐artery disease
- MACE
major adverse cardiovascular event
- NASCET
North American Symptomatic Carotid Endarterectomy Trial
- SHR
subdistribution hazard ratio
- SPARCL
Stroke Prevention by Aggressive Reduction in Cholesterol Levels
- TST
Treat Stroke to Target
- WASID
Warfarin‐Aspirin Symptomatic Intracranial Disease
Clinical Perspective
What Is New?
In this prospective cohort of 904 Chinese patients with ischemic stroke, patients with and without significant large‐artery atherosclerosis affecting the intra‐ and cervicocranial arteries who achieved a mean low‐density lipoprotein cholesterol <1.80 mmol/L (<70 mg/dL) were associated with a lower risk of recurrent stroke and major adverse cardiovascular events during long‐term follow‐up.
Reductions in risk of recurrent stroke and major adverse cardiovascular events were similarly noted in both patients with an ischemic stroke attributable to large‐artery atherosclerosis and those attributable to other ischemic stroke subtypes who achieved a mean low‐density lipoprotein cholesterol <1.80 mmol/L during long‐term follow‐up.
What Are the Clinical Implications?
This study provides level 2b evidence to support a low‐density lipoprotein cholesterol target of <1.80 mmol/L in Chinese patients with ischemic stroke with and without large‐artery atherosclerosis, regardless of ischemic stroke cause.
Current international guidelines recommend the use of intensive lipid‐lowering therapy in patients with a transient ischemic attack (TIA)/ischemic stroke of atherosclerotic origin as secondary prevention.1, 2 However, the optimal low‐density lipoprotein cholesterol (LDL‐C) target for secondary stroke prevention remains uncertain. Recently, the TST (Treat Stroke to Target) trial demonstrated that in patients with TIA/ischemic stroke with evidence of atherosclerosis, those who had a target LDL‐C level <1.80 mmol/L (<70 mg/dL) were associated with a lower risk of subsequent cardiovascular events compared with those who had a target range of 2.30 to 2.80 mmol/L (90–110 mg/dL).3
However, findings from the TST trial were mainly driven by the 2148 White patients in the study.3 Its subgroup analysis of 712/2860 Korean patients with stroke failed to demonstrate the same prognostic benefits with an LDL‐C target of <1.80 mmol/L, and this has been attributed to the short follow‐up period (median 2 years) of these individuals.3 On the other hand, the optimal LDL‐C target in patients with TIA/ischemic stroke without evidence of atherosclerosis remains uncertain,1, 2, 4 and whether similar recommended LDL‐C targets for patients with stroke with atherosclerotic origin should be adopted for this group of individuals has not been studied previously.
Therefore, in a large prospective cohort of 904 Chinese patients with ischemic stroke and magnetic resonance angiography (MRA) of the intra‐ and cervicocranial arteries, we evaluated the long‐term prognostic implications of patients who achieved a mean LDL‐C <1.80 mmol/L compared with ≥1.80 mmol/L, stratified by the presence or absence of significant intra‐ and cervicocranial large‐artery disease (LAD).
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
We prospectively studied 1003 consecutive patients with a diagnosis of acute ischemic stroke recruited from Queen Mary Hospital, Hong Kong, who received brain magnetic resonance imaging (MRI) at the University of Hong Kong MRI Unit during the period of March 1, 2008 to September 30, 2014. Details of this cohort can be found in our previous publications.5, 6 We excluded patients who did not receive an MRA of the intra‐ and cervicocranial arteries. We also excluded patients who did not have a baseline or serial LDL‐C measurement on follow‐up.
We collected demographic data, atherosclerotic risk factors, details on baseline LDL‐C readings, hospitalization of the index event, and use of lipid‐lowering medications before admission and on discharge during face‐to‐face interviews. Data were cross‐referenced with relevant primary care and hospital records. Potency of statins was categorized as low‐, medium‐, or high‐intensity.7 Cause of ischemic stroke was classified according to the modified TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria.8 Functional outcome of stroke was determined by the modified Rankin Scale on discharge.
All participants were followed up by a clinician every 3 to 6 months, or more frequently if clinically indicated. Blood pressure was measured and documented during each clinic follow‐up. Lipid profile was also repeated as clinically indicated. All patients were assessed for the following clinical outcomes: (1) recurrent stroke (ischemic or hemorrhagic), (2) intracerebral hemorrhage (ICH), (3) major adverse cardiovascular events (MACEs), and (4) all‐cause mortality. Recurrent stroke was defined as a sudden new neurological deficit fitting the definition of ischemic stroke or ICH, occurring after a period of unequivocal neurological stability and not attributable to cerebral edema, mass effect, or hemorrhagic transformation. Patients with suspected recurrent stroke received repeat neuroimaging in the form of cranial computed tomography or MRI to support the diagnosis. A MACE was defined as a composite of recurrent stroke (ischemic and hemorrhagic), acute coronary syndrome, new‐onset peripheral vascular disease, symptom‐driven revascularization procedure, and cardiovascular death. Clinical outcomes of recurrent stroke and MACE were determined by the treating physicians. Where needed, details of clinical outcomes were supplemented by electronic or paper medical records from primary care practices, hospitals, as well as the Deaths General Register Office.
Patients gave written informed consent after an event or assent was obtained from relatives for patients who were unable to provide consent. The study was approved by the local research ethics committee.
MRI Evaluation of Underlying Atherosclerosis Burden
All patients were scanned using a single 3T MRI scanner (Achieva; Philips Healthcare) at the University of Hong Kong MRI Unit. Details of scan parameters can be found in our previous publications.5, 6 Sequences included diffusion‐weighted imaging, time‐of‐flight angiography of the intracranial arteries, and gadolinium contrast‐enhanced MRA of the intra‐ and cervicocranial arteries, including the aortic arch. A neurovascular coil was used (contrast‐enhanced MRA sequence: 0.4 mL/kg Dotaren [concentration at 0.5 mmol/mL] at a maximum of 40 mL at 2–2.5 mL/s, followed by a 30‐mL saline flush at the same injection rate). The repetition time was 4.6 ms, echo time 1.41 ms, flip angle 47°, reconstructed slice thickness 0.5 mm, and voxel size 0.4×0.4×0.5 cm. Reconstructed time‐of‐flight MRA sequences were used to assess intracranial stenosis and contrast‐enhanced sequences to assess extracranial stenosis.
A single rater (A.N.), supervised by a consultant neuroradiologist (H.K.‐F.M.), evaluated the MRA of the intra‐ and cervicocranial arteries and aortic arch of each patient and determined the degree and location of vascular stenosis. The degree of vascular stenosis for extracranial atherosclerosis was classified according to the NASCET (North American Symptomatic Carotid Endarterectomy Trial) criteria.9 The degree of vascular stenosis for intracranial stenosis was classified according to the WASID (Warfarin‐Aspirin Symptomatic Intracranial Disease) trial method.10 We subcategorized the arteries according to whether they were intra‐ or extracranial in location. Extracranial arteries included the aortic arch, brachiocephalic, subclavian, common carotid, internal carotid (segments 1 and 2), basilar, and vertebral arteries (segments 1–3). Intracranial arteries included the internal carotid (segments 3–7), vertebral (segment 4), anterior cerebral, middle cerebral, and posterior cerebral arteries. Because of the limitations of MRA in depicting severe stenosis of small arteries,11 we only evaluated the degree of stenosis of segment 1 for the anterior, middle, and posterior cerebral arteries. We defined significant LAD (LAD+) if there was ≥50% vascular stenosis in any intra‐ or extracranial artery, and without significant LAD (LAD−) if there was <50% vascular stenosis in any intra‐ or extracranial artery. In patients with a hypoplastic vertebral artery, patients were considered to have significant LAD only if the dominant vertebral artery had significant (≥50%) stenosis.
Statistical Analysis
Continuous variables were expressed as mean±SD, and categorical variables were expressed as number and percentage. Independent‐samples t test was used to compare differences between groups for continuous variables, and a χ2 test was used for categorical variables. For each participant, mean LDL‐C level was calculated as the average of the LDL‐C levels measured from the start of the observation period until the occurrence of event of interest or censoring. Participants were divided into 2 groups according to mean LDL‐C levels <1.80 and ≥1.80 mmol/L during the follow‐up period. Kaplan‐Meier curves were generated, and the cumulative event rate of clinical outcomes in patients with LDL‐C <1.80 versus ≥1.80 mmol/L stratified by (1) LAD+ versus LAD−, (2) ischemic stroke attributable to large‐artery atherosclerosis versus other ischemic stroke subtypes, and (3) age <75 versus ≥75 years were compared using a log‐rank test.
We also used competing risks regression to estimate the subdistribution hazard ratios (SHRs) for development of recurrent ischemic stroke, ICH, and MACE in patients with LDL‐C <1.80 mmol (with ≥1.80 mmol/L as the reference group). SHRs were determined for patients with LAD+ versus LAD−, ischemic stroke attributable to large‐artery atherosclerosis versus other ischemic stroke subtypes, and age <75 versus ≥75 years. Death was treated as a competing risk event in the analysis of recurrent ischemic stroke and ICH, whereas noncardiovascular death was treated as a competing risk event for vascular death. The association between a mean LDL‐C <1.80 mmo/L upon discharge with clinical outcomes was examined in a univariate model and model adjusted for age and sex. We also performed multivariable analysis by further adjusting for risk factors that were significantly associated with a MACE in univariate analysis, with a P<0.10. The proportional hazards assumption was tested using Schoenfeld residuals, and no violation was observed. Test for interaction among LAD status, ischemic stroke subtype, and age with LDL‐C was also performed. We also studied the lowest LDL‐C value attained during follow‐up and last LDL‐C value before an event, and determined whether an LDL‐C level <1.80 mmol/L was similarly associated with adverse outcomes in a multivariable model. Additional analysis was also conducted to test for linear trends by treating LDL‐C as an interval variable at 0.5 mmol/L intervals. A 2‐sided P<0.05 was considered statistically significant. All statistical tests were performed using Stata statistical software (version 14.0; StataCorp) and SPSS software (version 22.0; IBM).
Results
After excluding 66 patients who did not have MRA performed and an additional 33 who did not have a baseline/follow‐up LDL‐C measurement or were lost to follow‐up, a total of 904 patients were included in the final analysis (Table 1). Clinical characteristics of patients included and excluded in the final analysis can be found in Table 2. Patients who were excluded were older (73±12 versus 69±12 years, P=0.001), were more likely to have a history of ischemic heart disease (15% versus 9%, P=0.030), had worse renal function (glomerular filtration rate 72±28 versus 79±24 mL/min per 1.73 m2, P=0.010), and had more severe strokes (modified Rankin Scale score, 2.5±2.2 versus 2.1±1.5, P=0.013) (Table 2).
Table 1.
Clinical Characteristics of the Study Cohort
| All, N=904 | LAD+, N=482 | LAD−, N=422 | P value | |
|---|---|---|---|---|
| Baseline clinical characteristics | ||||
| Age, y | 69±12 | 70±12 | 67±12 | <0.001 |
| Men (%) | 541 (60) | 287 (60) | 254 (60) | 0.84 |
| Hypertension (%) | 590 (65) | 331 (69) | 259 (61) | 0.021 |
| Diabetes mellitus (%) | 257 (28) | 144 (30) | 113 (27) | 0.30 |
| Hyperlipidemia (%) | 233 (26) | 126 (26) | 107 (25) | 0.79 |
| Ever smokers (%) | 275 (30) | 160 (33) | 115 (27) | 0.053 |
| Atrial fibrillation (%) | 115 (13) | 49 (10) | 66 (16) | 0.014 |
| History of ischemic heart disease (%) | 77 (9) | 43 (9) | 34 (8) | 0.64 |
| History of TIA/stroke (%) | 141 (16) | 87 (18) | 54 (13) | 0.030 |
| Premorbid use of statins (%) | 169 (19) | 96 (20) | 73 (17) | 0.31 |
| Total cholesterol, mmol/L | 4.84±1.04 | 4.84±1.06 | 4.83±1.01 | 0.86 |
| Low‐density lipoprotein cholesterol, mmol/L | 3.03±0.94 | 3.05±0.96 | 3.00±0.91 | 0.42 |
| High‐density lipoprotein cholesterol, mmol/L | 1.21±0.34 | 1.18±0.34 | 1.25±0.33 | 0.003 |
| Triglyceride, mmol/L | 1.30±0.66 | 1.33±0.69 | 1.26±0.63 | 0.11 |
| Fasting glucose, mmol/L | 6.23±2.06 | 6.26±2.14 | 6.21±1.96 | 0.74 |
| HbA1c, % | 6.70±1.68 | 6.80±1.76 | 6.57±1.59 | 0.13 |
| GFR, mL/min per 1.73 m2 | 79±24 | 78±24 | 79±23 | 0.76 |
| Medication use on discharge | ||||
| Any antiplatelet (%) | 794 (88) | 431 (89) | 363 (86) | 0.12 |
| Single antiplatelet (%) | 671 (85) | 346 (80) | 325 (90) | 0.073 |
| Dual antiplatelet (%) | 123 (15) | 85 (20) | 38 (10) | <0.001 |
| Anticoagulant (%) | 92 (10) | 42 (9) | 50 (12) | 0.12 |
| Statin (%) | 784 (87) | 427 (89) | 357 (85) | 0.078 |
| Low intensity | 197 (25) | 96 (22) | 101 (28) | 0.15 |
| Moderate intensity | 561 (72) | 315 (74) | 246 (69) | |
| High intensity | 26 (3) | 16 (4) | 10 (3) | |
| Fibrates (%) | 5 (0.6) | 2 (0.4) | 3 (0.7) | 0.55 |
| Ezetimibe (%) | 1 (0.1) | 1 (0.2) | 0 (0) | 0.35 |
| Follow‐up period, y | 6.5±2.4 | 6.3±2.5 | 6.8±2.2 | 0.002 |
| Total patient‐years | 5899 | 3031 | 2867 | |
| Outcome | ||||
| Recurrent stroke (%) | 139 (15) | 69 (14) | 70 (17) | 0.35 |
| Ischemic stroke (%) | 112 (81) | 56 (81) | 56 (80) | 0.44 |
| Intracerebral hemorrhage (%) | 27 (19) | 13 (19) | 14 (20) | 0.59 |
| MACE (%) | 246 (27) | 138 (29) | 108 (26) | 0.31 |
| Nonfatal stroke (%) | 109 (44) | 52 (38) | 57 (53) | |
| Nonfatal acute coronary syndrome (%) | 25 (10) | 13 (9) | 12 (11) | |
| New‐onset peripheral vascular disease (%) | 10 (4) | 6 (4) | 4 (4) | |
| Symptom‐driven revascularization procedure (%) | 43 (17) | 32 (23) | 11 (10) | |
| All‐cause mortality (%) | 229 (25) | 145 (30) | 84 (20) | <0.001 |
| Cardiovascular cause (%) | 67 (29) | 40 (28) | 27 (32) | 0.28 |
| Noncardiovascular cause (%) | 162 (71) | 105 (72) | 57 (68) | 0.001 |
GFR indicates glomerular filtration rate; HbA1c, hemoglobin A1c; LAD+, with significant large‐artery atherosclerosis; LAD−, without significant large‐artery atherosclerosis; MACE, major adverse cardiovascular event; and TIA, transient ischemic attack.
Table 2.
Baseline Clinical Characteristics of Patients Included and Excluded in the Final Analysis
| Included, N=904 | Excluded, N=99 | P value | |
|---|---|---|---|
| Age, y | 69±12 | 73±12 | 0.001 |
| Men (%) | 541 (60) | 60 (61) | 0.88 |
| Hypertension (%) | 590 (65) | 67 (68) | 0.63 |
| Diabetes mellitus (%) | 257 (28) | 27 (27) | 0.81 |
| Hyperlipidemia (%) | 233 (26) | 23 (23) | 0.58 |
| Ever smoker (%) | 275 (30) | 22 (22) | 0.09 |
| Atrial fibrillation (%) | 115 (13) | 15 (15) | 0.49 |
| History of ischemic heart disease (%) | 77 (9) | 15 (15) | 0.030 |
| History of TIA/stroke (%) | 141 (16) | 13 (13) | 0.52 |
| GFR, mL/min per 1.73 m2 | 79±24 | 72±28 | 0.010 |
| Modified Rankin Scale | 2.1±1.5 | 2.5±2.2 | 0.013 |
| TOAST classification | 0.28 | ||
| Small‐vessel disease (%) | 380 (42) | 45 (45) | |
| Large‐artery atherosclerosis (%) | 317 (35) | 25 (25) | |
| Cardioembolic (%) | 106 (12) | 18 (18) | |
| Other/undetermined cause (%) | 101 (11) | 11 (11) |
GFR indicates glomerular filtration rate; TIA, transient ischemic attack; and TOAST, Trial of Org 10172 in Acute Stroke Treatment.
The mean age of the study population included in the final analysis was 69±12 years, and 60% were men (Table 1). The mean LDL‐C on admission was 3.03±0.94 mmol/L. Thirty‐five percent had a stroke attributable to large‐artery atherosclerosis, 42% attributable to small‐vessel occlusion, 12% attributable to cardioembolism, and 11% attributable to other/undetermined causes. Eighty‐seven percent of the study population received statins upon discharge, 75% of which were of moderate–high intensity. Of the study population, 0.6% received a fibrate, and 0.1% received ezetimibe.
There were 482 of 904 (53%) patients who were LAD+, of whom 182 of 482 (38%) had significant intracranial atherosclerosis, 157 of 482 (33%) had significant extracranial atherosclerosis, and 143 of 482 (30%) had both intracranial atherosclerosis and extracranial atherosclerosis. Compared with patients with LAD−, those with LAD+ were older (70±12 versus 67±12 years), were more likely to have underlying hypertension (69% versus 61%), were more likely to have a history of TIA/stroke (18% versus 13%), and had a lower HDL‐C at baseline (1.18±0.34 versus 1.25±0.33 mmol/L) (all P<0.05) (Table 1). Patients with LAD+ were also more likely to be smokers (33% versus 27%, P=0.053). In contrast, a greater proportion of patients with LAD− had atrial fibrillation (16% versus 10%, P=0.014). There were no significant differences in the proportion of men, diabetes mellitus, or hyperlipidemia between the 2 groups. There were also no significant differences of the baseline LDL‐C or use of statins upon discharge between the 2 groups (all P>0.05).
During a mean follow‐up period of 6.5±2.4 years (5899 patient‐years), a mean of 9 LDL‐C readings (interquartile range, 5–12) and 21 blood pressure readings (interquartile range, 12–30) were collected per patient. Thirty‐two percent of patients achieved a mean LDL‐C of <1.80 mmol/L, whereas 68% of patients achieved a mean LDL‐C of ≥1.80 mmol/L during the follow‐up period. Patients who achieved a mean LDL‐C of <1.80 mmol/L were more likely to have diabetes mellitus (37% versus 24%, P<0.001) and a history of TIA/stroke (20% versus 14%, P=0.025) (Table 3). They were also more likely to be using statins before admission (23% versus 17%) and had a lower total cholesterol (4.23±0.87 versus 5.09±1.00 mmol/L) and LDL‐C (2.43±0.72 versus 3.27±0.90 mmol/L) on admission (all P<0.05) (Table 3). Although patients who achieved a mean LDL‐C <1.80 mmol/L had similar proportion of strokes because of large‐artery atherosclerosis compared with those who achieved a mean LDL‐C ≥1.80 mmol/L (both 35%), patients who achieved a mean LDL‐C <1.80 mmol/L on follow‐up were less likely to have strokes because of small‐vessel disease (36% versus 45%) and were more likely to have strokes that were cardioembolic in nature (16% versus 10%). Nevertheless, there were no significant differences in age, sex, or other vascular risk factors in patients who achieved a mean LDL‐C <1.80 mmol/L or ≥1.80 mmol/L during follow‐up (Table 3).
Table 3.
Clinical Characteristics of the Study Population According to Postevent Mean LDL‐C
| Postevent mean LDL‐C | <1.80 mmol/L,N=286 | ≥1.80 mmol/L,N=618 | P value |
|---|---|---|---|
| Baseline clinical characteristics | |||
| Age, y | 70±12 | 68±12 | 0.066 |
| Men (%) | 167 (58) | 374 (61) | 0.54 |
| Hypertension (%) | 194 (68) | 396 (64) | 0.27 |
| Diabetes mellitus (%) | 106 (37) | 151 (24) | <0.001 |
| Hyperlipidemia (%) | 65 (23) | 168 (27) | 0.15 |
| Ever‐smokers (%) | 79 (28) | 196 (32) | 0.21 |
| Atrial fibrillation (%) | 45 (16) | 70 (11) | 0.064 |
| History of ischemic heart disease (%) | 28 (10) | 49 (8) | 0.35 |
| History of TIA/stroke (%) | 56 (20) | 85 (14) | 0.025 |
| Premorbid use of statins (%) | 65 (23) | 104 (17) | 0.034 |
| Total cholesterol, mmol/L | 4.23±0.87 | 5.09±1.00 | <0.001 |
| Low‐density lipoprotein cholesterol, mmol/L | 2.43±0.72 | 3.27±0.90 | <0.001 |
| High‐density lipoprotein cholesterol, mmol/L | 1.22±0.36 | 1.21±0.33 | 0.51 |
| Triglyceride, mmol/L | 1.26±0.80 | 1.31±0.59 | 0.39 |
| Fasting glucose, mmol/L | 6.45±2.29 | 6.14±1.94 | 0.051 |
| HbA1c (%) | 6.76±1.60 | 6.66±1.73 | 0.52 |
| GFR, mL/min per 1.73 m2 | 77±24 | 79±23 | 0.24 |
| TOAST classification | 0.014 | ||
| Small‐vessel disease (%) | 104 (36) | 276 (45) | |
| Large‐artery atherosclerosis (%) | 99 (35) | 218 (35) | |
| Cardioembolic (%) | 45 (16) | 61 (10) | |
| Other/undetermined cause (%) | 38 (13) | 63 (10) | |
| Medication use on discharge | |||
| Any antiplatelet (%) | 247 (86) | 547 (89) | 0.36 |
| Single antiplatelet (%) | 204 (83) | 467 (85) | 0.18 |
| Dual antiplatelet (%) | 43 (17) | 80 (15) | 0.39 |
| Anticoagulant (%) | 40 (14) | 52 (8) | 0.010 |
| Statin (%) | 257 (90) | 527 (85) | 0.059 |
| Low intensity | 63 (25) | 134 (25) | 0.16 |
| Moderate intensity | 181 (70) | 380 (72) | |
| High intensity | 13 (5) | 13 (2) | |
| Fibrates (%) | 2 (0.7) | 3 (0.5) | 0.69 |
| Ezetimibe (%) | 0 (0) | 1 (0.2) | 0.50 |
GFR indicates glomerular filtration rate; HbA1c, hemoglobin A1c; LDL‐C, low‐density lipoprotein cholesterol; TIA, transient ischemic attack; and TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Among the 784 of 904 patients who were taking statins upon discharge, 90% adhered to statin treatment for ≥80% of the follow‐up period (94% and 89% in patients with a mean LDL‐C <1.80 and ≥1.80 mmol/L on follow‐up, respectively). One hundred thirty‐nine of the 904 (15%) patients developed a recurrent stroke, of whom 112 (81%) were ischemic in nature. Two hundred forty‐six of the 904 (27%) patients developed a MACE, and 229 of the 904 (25%) patients died (Table 1).
The long‐term risks of recurrent stroke, MACE, and death in patients with and without significant LAD, stratified by mean LDL‐C levels during follow‐up, are shown in Table 4 and Figure 1. Regardless of LAD status, patients with a mean LDL‐C <1.80 mmol/L were associated with a lower risk of recurrent stroke and MACE compared with patients with a mean LDL ≥1.80 mmol/L (Table 4 and Figure 1). In patients with significant LAD, the 8‐year absolute risk of a MACE in patients with a mean LDL <1.80 and ≥1.80 mmol/L was 28% and 34%, respectively (P=0.093), whereas in patients without significant LAD, the 8‐year absolute risk of a MACE in patients with a mean LDL <1.80 and ≥1.80 mmol/L was 21% and 31%, respectively (P=0.043).
Table 4.
Ten‐Year Risk of Adverse Events Per 1000 Patient‐Years by Postevent Mean LDL‐C
| Postevent mean LDL‐C | ||
|---|---|---|
| <1.80 mmol/L | ≥1.80 mmol/L | |
| All recurrent stroke | ||
| No. | 279 | 625 |
| All subjects | 1.42 (0.99–2.05) | 2.42 (2.00–2.91) |
| LAD+ | 1.29 (0.75–2.22) | 2.35 (1.81–3.05) |
| LAD− | 1.55 (0.95–2.54) | 2.49 (1.91–3.25) |
| Recurrent ischemic stroke | ||
| No. | 281 | 623 |
| All subjects | 1.27 (0.86–1.86) | 1.86 (1.51–2.30) |
| LAD+ | 1.17 (0.67–2.07) | 1.84 (1.37–2.47) |
| LAD− | 1.36 (0.80–2.29) | 1.90 (1.40–2.57) |
| Intracerebral hemorrhage | ||
| No. | 284 | 620 |
| All subjects | 0.14 (0.04–0.43) | 0.50 (0.33–0.74) |
| LAD+ | 0.09 (0.01–0.64) | 0.48 (0.27–0.84) |
| LAD− | 0.19 (0.05–0.75) | 0.52 (0.29–0.91) |
| Major adverse cardiovascular event | ||
| No. | 270 | 634 |
| All subjects | 2.98 (2.29–3.87) | 4.38 (3.80–5.05) |
| LAD+ | 3.50 (2.47–4.95) | 4.76 (3.94–5.76) |
| LAD− | 2.49 (1.67–3.71) | 3.98 (3.21–4.93) |
| All‐cause mortality | ||
| No. | 286 | 618 |
| All subjects | 2.89 (2.26–3.70) | 3.39 (2.91–3.95) |
| LAD+ | 3.43 (2.50–4.72) | 4.23 (3.50–5.11) |
| LAD– | 2.33 (1.57–3.45) | 2.49 (1.93–3.22) |
LAD+ indicates with significant large‐artery atherosclerosis; LAD−, without significant large‐artery atherosclerosis; and LDL‐C, low‐density lipoprotein cholesterol.
Figure 1. Long‐term risk of adverse events in patients with ischemic stroke with and without significant large‐artery disease, stratified by mean LDL‐C on follow‐up.
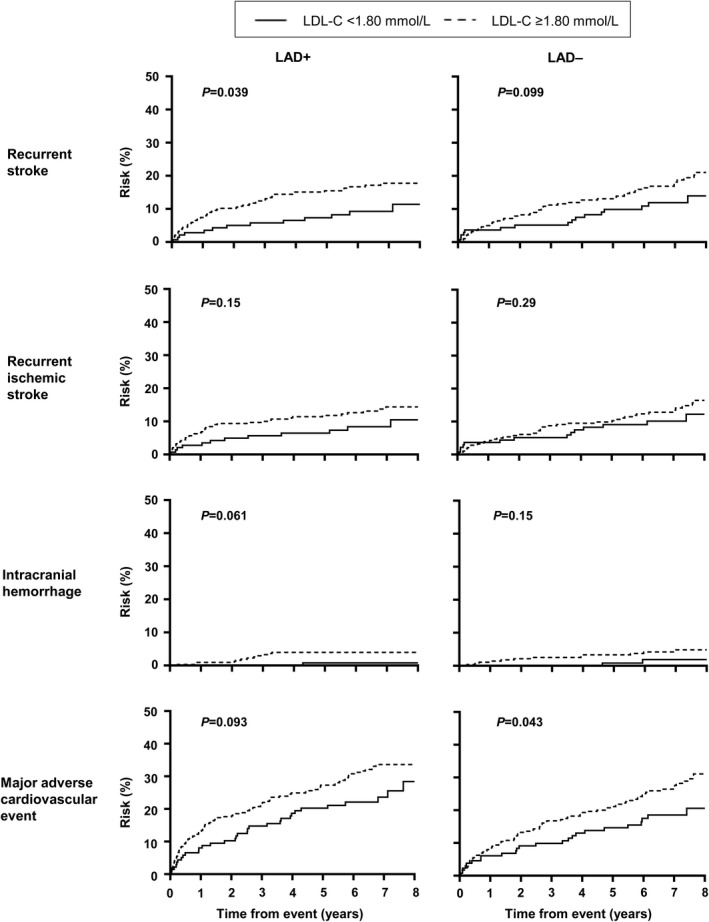
LAD+ indicates with significant large‐artery atherosclerosis; LAD−, without significant large‐artery atherosclerosis; and LDL‐C, low‐density lipoprotein cholesterol.
After adjusting for age, sex, vascular risk factors, and postevent mean systolic blood pressure, a mean LDL‐C <1.80 mmol/L was associated with a significantly lower risk of recurrent stroke (LAD+: multivariable‐adjusted SHR, 0.42; 95% CI, 0.21–0.83; LAD−: SHR, 0.53; 95% CI, 0.28–0.99), and MACE (LAD+: SHR, 0.65; 95% CI, 0.42–0.99; LAD−: SHR, 0.53; 95% CI, 0.32–0.88) in patients with or without significant LAD (Table 5) (all P<0.05). A mean LDL‐C <1.80 mmol/L was also associated with a lower risk of ICH in patients with significant LAD (multivariable‐adjusted SHR, 0.14; 95% CI, 0.02–0.90, P=0.039). Although patients with a mean LDL‐C <1.80 mmol/L tended to have a lower risk of recurrent ischemic stroke, and those without significant LAD with a mean LDL‐C <1.80 mmol/L tended to have a lower risk of ICH, these did not reach statistical significance (Table 5). Similar trends were noted when patients were stratified according to large‐artery atherosclerosis‐related ischemic stroke versus other ischemic stroke subtypes by TOAST classification (Figure 2, Table 6) and by age (Figure 3, Table 7). Analysis using the lowest LDL‐C attained during follow‐up <1.80 mmol/L or last LDL‐C value <1.80 mmol/L before an event also showed that an LDL‐C <1.80 mmol/L was associated with a lower risk of recurrent stroke, recurrent ischemic stroke, and MACE regardless of LAD status, ischemic stroke subtype, or age (Table 8). Finally, in an analysis where LDL‐C was treated as an interval variable, we noted that per 0.5 mmol/L decrease in LDL‐C, regardless of presence or absence of significant LAD, ischemic stroke subtypes, and age, a lower LDL‐C was independently associated with a reduced risk of recurrent stroke, recurrent ischemic stroke, and MACE but not ICH (Table 9) (all P<0.01).
Table 5.
Competing Risk Regression Analysis by Postevent Mean LDL‐C, Stratified by Presence or Absence of Significant Large‐Artery Atherosclerosis
| Postevent mean LDL‐C | No. of events/No. of patients | Unadjusted SHR (95% CI) | SHR (95% CI) adjusted for age and sex | SHR (95% CI) adjusted for age, sex, and vascular risk factors* | P for interaction | |
|---|---|---|---|---|---|---|
| <1.80 mmol/L | ≥1.80 mmol/L | <1.80 mmol/L | <1.80 mmol/L | <1.80 mmol/L | ||
| All recurrent stroke | ||||||
| LAD+ | 13/142 | 56/340 | 0.54 (0.30–0.99)† | 0.55 (0.30–1.02) | 0.42 (0.21–0.83)† | 0.006 |
| LAD− | 16/137 | 54/285 | 0.63 (0.36–1.09) | 0.59 (0.33–1.03) | 0.53 (0.28–0.99)† | |
| Recurrent ischemic stroke | ||||||
| LAD+ | 12/144 | 44/338 | 0.64 (0.34–1.21) | 0.65 (0.34–1.24) | 0.52 (0.24–1.09) | 0.043 |
| LAD− | 14/137 | 42/285 | 0.73 (0.40–1.33) | 0.68 (0.37–1.26) | 0.57 (0.28–1.16) | |
| Intracerebral hemorrhage | ||||||
| LAD+ | 1/148 | 12/334 | 0.18 (0.02–1.41) | 0.19 (0.03–1.41) | 0.14 (0.02–0.90)† | 0.14 |
| LAD− | 2/136 | 12/286 | 0.35 (0.08–1.56) | 0.34 (0.08–1.45) | 0.43 (0.10–1.81) | |
| Major adverse cardiovascular event | ||||||
| LAD+ | 32/138 | 106/344 | 0.72 (0.49–1.07) | 0.73 (0.49–1.07) | 0.65 (0.42–0.99)† | 0.005 |
| LAD− | 24/132 | 84/290 | 0.62 (0.40–0.98)† | 0.57 (0.36–0.90)† | 0.53 (0.32–0.88)† | |
Postevent mean LDL‐C ≥1.80 mmol/L as the reference group. LAD+ indicates with significant large‐artery atherosclerosis; LAD−, without significant large‐artery atherosclerosis; LDL‐C, low‐density lipoprotein cholesterol; and SHR, subdistribution hazard ratio.
Diabetes mellitus, atrial fibrillation, history of ischemic heart disease, history of transient ischemic attack or stroke, glomerular filtration rate, and postevent mean systolic blood pressure.
P<0.05.
Figure 2. Long‐term risk of adverse events in patients with ischemic stroke attributable to large‐artery atherosclerosis vs other ischemic stroke subtypes, stratified by mean LDL‐C on follow‐up.
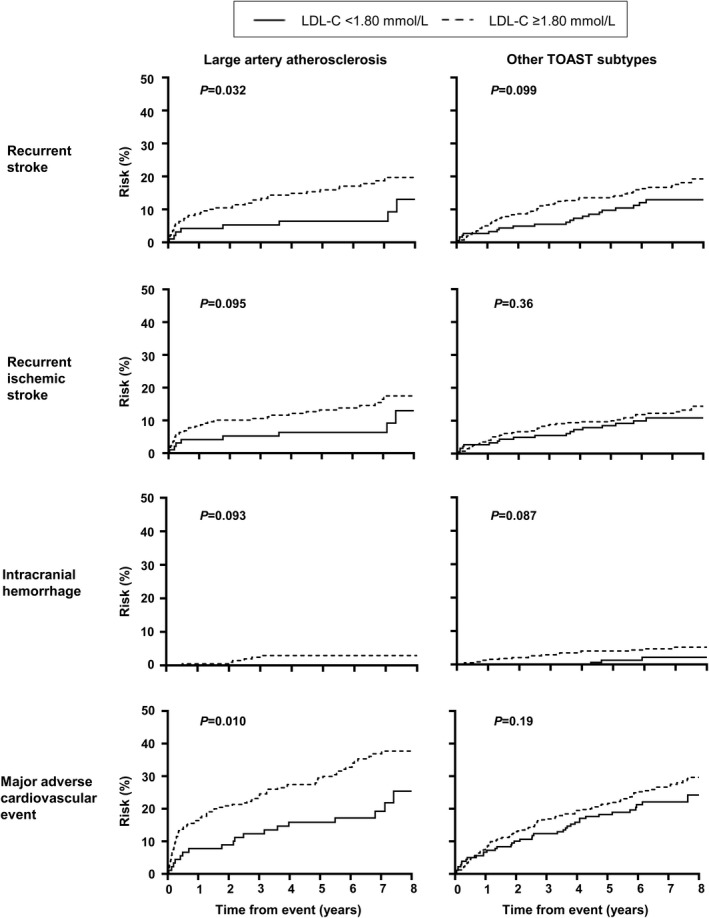
LDL‐C indicates low‐density lipoprotein cholesterol; and TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Table 6.
Competing Risk Regression Analysis by Postevent Mean LDL‐C, Stratified by Ischemic Stroke Subtypes
| Postevent mean LDL‐C | No. of events/No. of patients | Unadjusted SHR (95% CI) | SHR (95% CI) adjusted for age and sex | SHR (95% CI) adjusted for age, sex, and vascular risk factors* | P for interaction | |
|---|---|---|---|---|---|---|
| <1.80 (mmol/L) | ≥1.80 (mmol/L) | <1.80 (mmol/L) | <1.80 (mmol/L) | <1.80 (mmol/L) | ||
| All recurrent stroke | ||||||
| Large‐artery atherosclerosis | 8/95 | 40/222 | 0.46 (0.21–0.97)† | 0.44 (0.21–0.94)† | 0.37 (0.14–0.99)† | 0.006 |
| Other ischemic stroke subtypes | 21/184 | 70/403 | 0.67 (0.41–1.08) | 0.64 (0.39–1.04) | 0.52 (0.31–0.89)† | |
| Recurrent ischemic stroke | ||||||
| Large‐artery atherosclerosis | 8/96 | 34/221 | 0.54 (0.25–1.16) | 0.52 (0.24–1.11) | 0.44 (0.17–1.14) | 0.042 |
| Other ischemic stroke subtypes | 18/185 | 52/402 | 0.78 (0.46–1.34) | 0.75 (0.44–1.29) | 0.58 (0.32–1.06) | |
| Intracerebral hemorrhage | ||||||
| Large‐artery atherosclerosis | 0/98 | 6/219 | … | … | … | 0.090 |
| Other ischemic stroke subtypes | 3/186 | 18/401 | 0.36 (0.11–1.22) | 0.37 (0.11–1.23) | 0.36 (0.10–1.29) | |
| Major adverse cardiovascular event | ||||||
| Large‐artery atherosclerosis | 18/90 | 79/227 | 0.53 (0.32–0.87) | 0.49 (0.30–0.81)‡ | 0.48 (0.28–0.84)† | 0.005 |
| Other ischemic stroke subtypes | 38/180 | 111/407 | 0.78 (0.54–1.13) | 0.75 (0.52–1.09) | 0.64 (0.43–0.95)† | |
Postevent mean LDL‐C ≥1.80 mmol/L as the reference group. LDL‐C indicates low‐density lipoprotein cholesterol; and SHR, subdistribution hazard ratio.
Diabetes mellitus, atrial fibrillation, history of ischemic heart disease, history of transient ischemic attack or stroke, glomerular filtration rate, and postevent mean systolic blood pressure.
P<0.05.
P<0.01.
Figure 3. Long‐term risk of adverse events in patients with ischemic stroke aged <75 vs ≥75 years, stratified by mean LDL‐C on follow‐up.
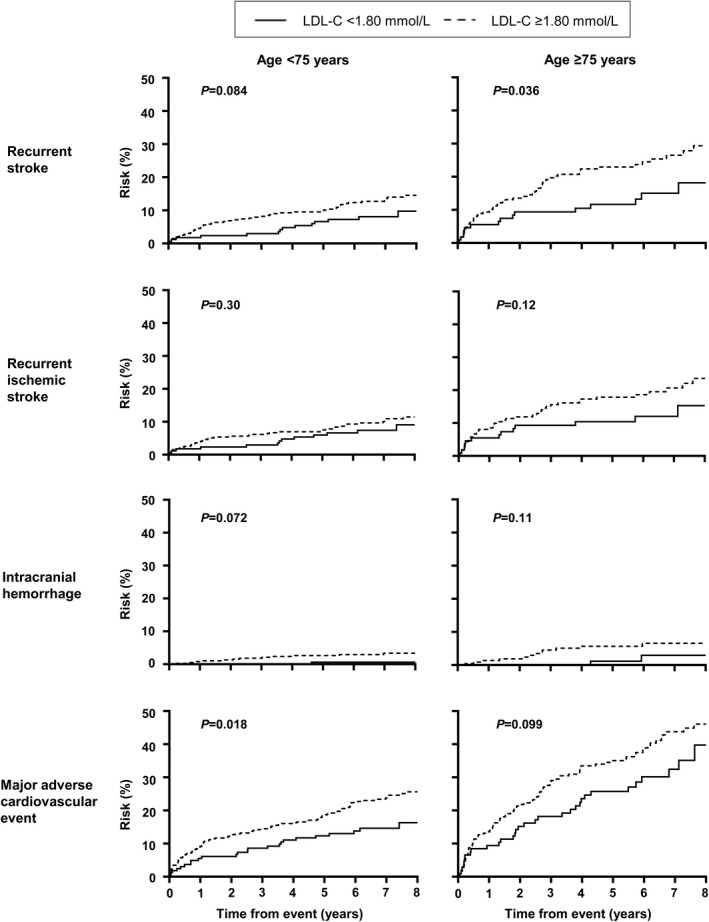
LDL‐C indicates low‐density lipoprotein cholesterol.
Table 7.
Competing Risk Regression Analysis by Postevent Mean LDL‐C, Stratified by Age
| Postevent mean LDL‐C | No. of events/No. of patients | Unadjusted SHR (95% CI) | SHR (95% CI) adjusted for age, sex, and vascular risk factors* | P for interaction | |
|---|---|---|---|---|---|
| <1.80 mmol/L | ≥1.80 mmol/L | <1.80 mmol/L | <1.80 mmol/L | ||
| All recurrent stroke | |||||
| Age <75 y | 14/170 | 55/398 | 0.61 (0.34–1.10) | 0.52 (0.26–1.05) | 0.029 |
| Age ≥75 y | 15/109 | 55/227 | 0.55 (0.31–0.97)† | 0.43 (0.23–0.82)† | |
| Recurrent ischemic stroke | |||||
| Age <75 y | 13/171 | 43/397 | 0.74 (0.40–1.38) | 0.63 (0.30–1.33) | 0.033 |
| Age ≥75 y | 13/110 | 43/226 | 0.62 (0.33–1.15) | 0.45 (0.22–0.94)† | |
| Intracerebral hemorrhage | |||||
| Age <75 y | 1/172 | 12/396 | 0.19 (0.03–1.48) | 0.22 (0.02–1.99) | 0.64 |
| Age ≥75 y | 2/112 | 12/224 | 0.33 (0.07–1.44) | 0.34 (0.06–1.84) | |
| Major adverse cardiovascular event | |||||
| Age <75 y | 24/163 | 99/405 | 0.60 (0.38–0.94)† | 0.57 (0.34–0.94)† | 0.003 |
| Age ≥75 y | 32/107 | 91/229 | 0.71 (0.47–1.05) | 0.62 (0.41–0.95)† | |
Postevent mean LDL‐C ≥1.80 mmol/L as the reference group. LDL‐C indicates low‐density lipoprotein cholesterol; and SHR, subdistribution hazard ratio.
Diabetes mellitus, atrial fibrillation, history of ischemic heart disease, history of transient ischemic attack or stroke, glomerular filtration rate, and postevent mean systolic blood pressure.
P<0.05.
Table 8.
Competing Risk Regression Analysis by Postevent Mean LDL‐C Cutoff
| Postevent mean LDL‐C | Lowest LDL‐C | Last LDL‐C before event | ||
|---|---|---|---|---|
| SHR (95% CI) adjusted for age, sex, and vascular risk factors* | SHR (95% CI) adjusted for age, sex, and vascular risk factors* | |||
| <1.80 mmol/L | P value | <1.80 mmol/L | P value | |
| All recurrent stroke | ||||
| Subjects with significant LAD | 0.42 (0.24–0.75) | 0.003 | 0.55 (0.32–0.95) | 0.033 |
| Subjects without significant LAD | 0.36 (0.22–0.59) | <0.001 | 0.55 (0.31–0.97) | 0.037 |
| Large‐artery atherosclerosis | 0.33 (0.16–0.70) | 0.004 | 0.35 (0.16–0.77) | 0.009 |
| Other ischemic stroke subtypes | 0.40 (0.25–0.63) | <0.001 | 0.66 (0.41–1.07) | 0.091 |
| Age <75 y | 0.36 (0.21–0.61) | <0.001 | 0.51 (0.28–0.94) | 0.030 |
| Age ≥75 y | 0.38 (0.22–0.65) | <0.001 | 0.58 (0.33–1.02) | 0.057 |
| Recurrent ischemic stroke | ||||
| Subjects with significant LAD | 0.25 (0.13–0.46) | <0.001 | 0.46 (0.24–0.86) | 0.015 |
| Subjects without significant LAD | 0.23 (0.13–0.42) | <0.001 | 0.37 (0.20–0.71) | 0.003 |
| Large‐artery atherosclerosis | 0.18 (0.08–0.40) | <0.001 | 0.27 (0.12–0.62) | 0.002 |
| Other ischemic stroke subtypes | 0.26 (0.16–0.43) | <0.001 | 0.48 (0.28–0.82) | 0.007 |
| Age <75 y | 0.25 (0.14–0.45) | <0.001 | 0.44 (0.23–0.85) | 0.014 |
| Age ≥75 y | 0.21 (0.12–0.38) | <0.001 | 0.37 (0.20–0.68) | 0.001 |
| Intracerebral hemorrhage | ||||
| Subjects with significant LAD | 1.29 (0.25–6.52) | 0.76 | 1.36 (0.32–5.76) | 0.68 |
| Subjects without significant LAD | 0.75 (0.23–2.39) | 0.62 | 1.41 (0.44–4.47) | 0.56 |
| Large‐artery atherosclerosis | 0.59 (0.03–10.99) | 0.72 | 0.36 (0.07–1.96) | 0.24 |
| Other ischemic stroke subtypes | 0.98 (0.34–2.77) | 0.97 | 2.00 (0.75–5.31) | 0.17 |
| Age <75 y | 0.86 (0.24–3.05) | 0.81 | 1.23 (0.38–3.96) | 0.73 |
| Age ≥75 y | 1.16 (0.27–5.07) | 0.84 | 1.41 (0.38–5.25) | 0.61 |
| Major adverse cardiovascular event | ||||
| Subjects with significant LAD | 0.35 (0.24–0.53) | <0.001 | 0.48 (0.33–0.72) | <0.001 |
| Subjects without significant LAD | 0.32 (0.21–0.48) | <0.001 | 0.51 (0.32–0.79) | 0.003 |
| Large‐artery atherosclerosis | 0.22 (0.14–0.37) | <0.001 | 0.30 (0.18–0.50) | <0.001 |
| Other ischemic stroke subtypes | 0.41 (0.29–0.59) | <0.001 | 0.63 (0.44–0.90) | 0.011 |
| Age <75 y | 0.32 (0.21–0.47) | <0.001 | 0.56 (0.36–0.86) | 0.008 |
| Age ≥75 y | 0.35 (0.23–0.52) | <0.001 | 0.46 (0.31–0.70) | <0.001 |
Postevent mean LDL ≥1.8 mmol/L as the reference group. LAD indicates large‐artery disease; LDL‐C, low‐density lipoprotein cholesterol; and SHR, subdistribution hazard ratio.
Diabetes mellitus, atrial fibrillation, history of ischemic heart disease, history of transient ischemic attack or stroke, glomerular filtration rate, and postevent mean systolic blood pressure.
Table 9.
Competing Risk Regression Analysis by Postevent Mean LDL‐C Interval
| Postevent mean LDL‐C as interval variable* | ||
|---|---|---|
| SHR (95% CI) adjusted for age, sex, and vascular risk factors† | P trend | |
| All recurrent stroke | ||
| Subjects with significant LAD | 0.72 (0.59–0.87) | 0.001 |
| Subjects without significant LAD | 0.72 (0.59–0.88) | 0.001 |
| Large‐artery atherosclerosis | 0.69 (0.55–0.85) | 0.001 |
| Other TOAST subtypes | 0.73 (0.62–0.87) | <0.001 |
| Age <75 y | 0.69 (0.57–0.85) | <0.001 |
| Age ≥75 y | 0.71 (0.59–0.85) | <0.001 |
| Recurrent ischemic stroke | ||
| Subjects with significant LAD | 0.67 (0.54–0.84) | <0.001 |
| Subjects without significant LAD | 0.69 (0.55–0.86) | 0.001 |
| Large‐artery atherosclerosis | 0.64 (0.51–0.81) | <0.001 |
| Other TOAST subtypes | 0.70 (0.58–0.85) | <0.001 |
| Age <75 y | 0.68 (0.54–0.86) | 0.001 |
| Age ≥75 y | 0.65 (0.54–0.79) | <0.001 |
| Intracerebral hemorrhage | ||
| Subjects with significant LAD | 0.82 (0.59–1.15) | 0.25 |
| Subjects without significant LAD | 0.81 (0.51–1.28) | 0.36 |
| Large‐artery atherosclerosis | 0.89 (0.69–1.14) | 0.35 |
| Other TOAST subtypes | 0.78 (0.54–1.13) | 0.19 |
| Age <75 y | 0.69 (0.47–1.01) | 0.056 |
| Age ≥75 y | 1.02 (0.64–1.63) | 0.92 |
| Major adverse cardiovascular event | ||
| Subjects with significant LAD | 0.75 (0.65–0.88) | <0.001 |
| Subjects without significant LAD | 0.72 (0.61–0.84) | <0.001 |
| Large‐artery atherosclerosis | 0.67 (0.57–0.77) | <0.001 |
| Other TOAST subtypes | 0.79 (0.68–0.91) | 0.001 |
| Age <75 y | 0.70 (0.60–0.82) | <0.001 |
| Age ≥75 y | 0.77 (0.68–0.89) | <0.001 |
LAD indicates large‐artery disease; LDL‐C, low‐density lipoprotein cholesterol; SHR, subdistribution hazard ratio; and TOAST, Trial of Org 10172 in Acute Stroke Treatment.
0.5 mmol/L interval decrease in postevent mean LDL‐C.
Diabetes mellitus, atrial fibrillation, history of ischemic heart disease, history of transient ischemic attack or stroke, glomerular filtration rate, and postevent mean systolic blood pressure.
Discussion
In this cohort of Chinese patients with ischemic stroke and MRA of the intra‐ and cervicocranial arteries, we found that compared with patients who achieved a mean LDL‐C ≥1.80 mmol/L, those who had a mean LDL‐C <1.80 mmol/L were at ≈50% to 60% lower risk of recurrent stroke and 35% to 50% lower risk of a MACE after a mean 6.5±2.4 years follow‐up. The benefits of achieving an LDL <1.80 mmol/L was present in both patients with and without significant LAD, in patients with all ischemic stroke subtypes, and in patients aged ≥75 years.
Our findings are in line with previous studies and meta‐analyses that have shown the association of lower level of LDL‐C and better prognosis.3, 12, 13, 14 In the SPARCL (Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial, patients taking atorvastatin 80 mg daily, with recent TIA/stroke of noncardioembolic origin and without known coronary heart disease, had a reduced risk of recurrent stroke and MACE.12 In the TST trial, in patients with TIA/ischemic stroke with evidence of atherosclerosis, those who achieved a LDL‐C target <1.80 mmol/L had a lower risk of a MACE compared with those who had a LDL‐C target of 2.30 to 2.80 mmol/L.3 However, results of the TST trial were mainly driven by the 2148 French participants. In the 712 Korean patients who participated in the TST trial, no significant prognostic advantage was noted in those who were randomized to a target of LDL‐C <1.80 mmol/L.3 This finding has been attributed to the shorter follow‐up period of the Korean cohort within the TST trial (median, 2.0 years versus 5.3 years among the French cohort).3 With a larger sample size and longer follow‐up time (mean, 6.5±2.4 years) compared with the Korean cohort within the TST trial, we were able to corroborate findings from the overall TST trial and were also able to show significant benefits of an LDL‐C <1.80 mmol/L, not only in patients with ischemic stroke with atherosclerosis, but also in those without significant LAD, other etiological subtypes of stroke, and in patients aged ≥75 years.
Previous studies have noted a higher number of ICHs in patients with a lower postevent LDL‐C level,3, 12, 15 raising concern about the safety of a low LDL‐C target. Reassuringly, in our study with long‐term follow‐up, we did not find an increase in number of ICHs in patients who achieved an LDL‐C of <1.80 mmol/L. After adjusting for confounding factors including postevent systolic blood pressure, patients who achieved a mean LDL‐C <1.80 mmol/L had a significantly lower risk of ICH compared with those with a mean LDL‐C ≥1.80 mmol/L (Tables 5, 6, 7). However, concerns of possible increased risk of ICH remain, especially in patients with severe cerebral small‐vessel disease and multiple microbleeds, which were not directly investigated in our present study.16, 17, 18 Further studies from international initiatives (eg, Microbleeds International Collaborative Network) will be able to shed light on this matter.19, 20
Our study is, to our knowledge, one of the largest cohorts investigating the prognostic implications of poststroke mean LDL‐C level on cardiovascular outcomes in Chinese patients with ischemic stroke. All of the subjects included in the final analysis acquired a complete set of MRI and MRA images for assessment of the location and degree of atherosclerotic changes of the intra‐ and cervicocranial arteries. A relatively long follow‐up period (6.5±2.4 years) was achieved, enabling an adequate longitudinal observation of the prognostic effect of maintaining different postevent LDL‐C levels. Approximately 90% of study participants attained good compliance to statins, which eliminated the confounding effects of LDL‐C level fluctuations longitudinally. In addition, we controlled for mean clinic blood pressure during the follow‐up period for all of the recruited subjects within the study period. This eliminated potential confounding effects on the primary outcomes because of hypertension and strengthened the accuracy of our data.
However, our study also has several limitations. First, our study population consisted of Chinese patients with ischemic stroke who were predominantly of mild–moderate severity, and MRI and MRA were the sole imaging modalities for stenosis severity evaluation. It is known that MRA overestimates the severity of stenosis.21 In addition to this inherent limitation, the excluded subjects not meeting the inclusion criteria tended to be older and had more severe strokes. Therefore, whether our findings could be generalized to patients with more severe strokes needs further investigation. Second, there may be potential bias on how often patients had their lipid function checked. Those who are considered higher risk may have had their lipid function checked more frequently and may have been started on more aggressive treatment. Nevertheless, our results show that the proportion of patients, with or without significant LAD, who were on statins was similar (89% versus 85%, P=0.078). Similarly, the potency of statins prescribed in patients with and without LAD did not differ (P=0.15). Third, the current study was conducted over a long period of time, and there may be secular changes in stroke care and secondary prevention treatment during this period. Nevertheless, despite these limitations, we were still able to find a significant benefit with patients who attained a mean LDL‐C <1.80 mmol/L during long‐term follow‐up. Only a small number of patients (75/904) achieved a mean LDL‐C <1.40 mmol/L, and whether such a low LDL‐C level confers additional prognostic advantage needs further study. Findings from our study also warrant validation in larger‐scale randomized control trial settings.
In conclusion, in Chinese patients with ischemic stroke, a mean LDL‐C <1.80 mmol/L was associated with a lower risk of recurrent stroke and MACE during long‐term follow‐up. The benefit of a low LDL‐C target was consistent regardless of LAD status, ischemic stroke subtypes, and age. Further randomized trials to determine the optimal LDL‐C cutoff in patients with stroke without significant atherosclerosis are required.
Sources of Funding
MRI studies from the University of Hong Kong are partially funded by the SK Yee Medical Foundation Grant.
Disclosures
Dr Lau reports grants, personal fees, and nonfinancial support from Boehringer Ingelheim; grants and nonfinancial support from Pfizer; grants from Amgen; grants from Eisai; grants and personal fees from Sanofi; and nonfinancial support from Daiichi Sankyo outside the submitted work. Dr Lau has also received funding from the Health and Medical Research Fund, Hong Kong Government Food & Health Bureau; Innovation and Technology Fund for Better Living, and University Grants Committee, Hong Kong outside the submitted work. The remaining authors have no disclosures to report.
Acknowledgments
The authors acknowledge the use of the facilities of the MRI Unit, Department of Diagnostic Radiology, University of Hong Kong. Dr Lau conceived and designed the overall study; provided study supervision and funding; acquired, analyzed, and interpreted data; and wrote and revised the article. B.J. Chua, A. Ng, and Y.‐K. Wong acquired the data and did the statistical analysis. W.C.Y. Leung and A.H.‐Y. Chan interpreted the data and wrote and revised the article. Y.‐K. Chiu and A.X.‐W. Chu acquired the data. I.Y.‐H. Leung, A.C.‐O Tsang, and K.‐C Teo acquired the data and provided study supervision. Dr Mak provided study supervision and funding, acquired and interpreted the data, and wrote and revised the article.
For Sources of Funding and Disclosures, see page 14.
Contributor Information
Kui‐Kai Lau, Email: gkklau@hku.hk.
Henry Ka‐Fung Mak, Email: makkf@hkucc.hku.hk.
References
- 1.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi‐Hill D, Kamel H, Kernan WN, Kittner SJ, Leira EC, et al. 2021 guideline for the prevention of stroke in patients With stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52. DOI: 10.1161/STR.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 2.Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al.; Group ESCSD . 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. DOI: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 3.Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Béjot Y, Cabrejo L, Cha J‐K, Ducrocq G, Giroud M, et al.; Treat Stroke to Target I . A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. 2020;382:9. DOI: 10.1056/NEJMoa1910355. [DOI] [PubMed] [Google Scholar]
- 4.Wechsler LR. Statins and stroke—it's complicated. N Engl J Med. 2020;382:81–82. DOI: 10.1056/NEJMe1914757. [DOI] [PubMed] [Google Scholar]
- 5.Lau KK, Lovelock CE, Li L, Simoni M, Gutnikov S, Kuker W, Mak HKF, Rothwell PM. Antiplatelet treatment after transient ischemic attack and ischemic stroke in patients with cerebral microbleeds in 2 large cohorts and an updated systematic review. Stroke. 2018;49:1434–1442. DOI: 10.1161/STROKEAHA.117.020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau KK, Li L, Lovelock CE, Zamboni G, Chan TT, Chiang MF, Lo KT, Kuker W, Mak HK, Rothwell PM. Clinical correlates, ethnic differences, and prognostic implications of perivascular spaces in transient ischemic attack and ischemic stroke. Stroke. 2017;48:1470–1477. DOI: 10.1161/STROKEAHA.117.016694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, et al.; American College of Cardiology/American Heart Association Task Force on Practice G . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. DOI: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 8.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. DOI: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 9.North American Symptomatic Carotid Endarterectomy Trial C , Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, Fox AJ, Rankin RN, Hachinski VC, Wiebers DO, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high‐grade carotid stenosis. N Engl J Med. 1991;325:445–453. [DOI] [PubMed] [Google Scholar]
- 10.Chimowitz MI, Lynn MJ, Howlett‐Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. DOI: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 11.Bash S, Villablanca JP, Jahan R, Duckwiler G, Tillis M, Kidwell C, Saver J, Sayre J. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol. 2005;26:1012–1021. [PMC free article] [PubMed] [Google Scholar]
- 12.Amarenco P, Bogousslavsky J, Callahan A III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, et al.; Stroke Prevention by Aggressive Reduction in Cholesterol Levels I . High‐dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 13.Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta‐analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453–463. DOI: 10.1016/S1474-4422(09)70058-4. [DOI] [PubMed] [Google Scholar]
- 14.Cholesterol Treatment Trialists C , Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma C, Gurol ME, Huang Z, Lichtenstein AH, Wang X, Wang Y, Neumann S, Wu S, Gao X. Low‐density lipoprotein cholesterol and risk of intracerebral hemorrhage: a prospective study. Neurology. 2019;93:e445–e457. DOI: 10.1212/WNL.0000000000007853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haussen DC, Henninger N, Kumar S, Selim M. Statin use and microbleeds in patients with spontaneous intracerebral hemorrhage. Stroke. 2012;43:2677–2681. DOI: 10.1161/STROKEAHA.112.657486. [DOI] [PubMed] [Google Scholar]
- 17.Katsanos AH, Lioutas V‐A, Charidimou A, Catanese L, Ng KKH, Perera K, de Sa Boasquevisque D, Falcone GJ, Sheth KN, Romero JR, et al.; International M‐MI . Statin treatment and cerebral microbleeds: a systematic review and meta‐analysis. J Neurol Sci. 2021;420:117224. DOI: 10.1016/j.jns.2020.117224. [DOI] [PubMed] [Google Scholar]
- 18.Wieberdink RG, Poels MM, Vernooij MW, Koudstaal PJ, Hofman A, van der Lugt A, Breteler MM, Ikram MA. Serum lipid levels and the risk of intracerebral hemorrhage: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 2011;31:2982–2989. DOI: 10.1161/ATVBAHA.111.234948. [DOI] [PubMed] [Google Scholar]
- 19.Microbleeds International Collaborative N . Worldwide collaboration in the Microbleeds International Collaborative Network. Lancet Neurol. 2016;15:1113–1114. DOI: 10.1016/S1474-4422(16)30213-7. [DOI] [PubMed] [Google Scholar]
- 20.Wilson D, Ambler G, Lee K‐J, Lim J‐S, Shiozawa M, Koga M, Li L, Lovelock C, Chabriat H, Hennerici M, et al.; Microbleeds International Collaborative N . Cerebral microbleeds and stroke risk after ischaemic stroke or transient ischaemic attack: a pooled analysis of individual patient data from cohort studies. Lancet Neurol. 2019;18:653–665. DOI: 10.1016/S1474-4422(19)30197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randoux B, Marro B, Koskas F, Duyme M, Sahel M, Zouaoui A, Marsault C. Carotid artery stenosis: prospective comparison of CT, three‐dimensional gadolinium‐enhanced MR, and conventional angiography. Radiology. 2001;220:179–185. DOI: 10.1148/radiology.220.1.r01jl35179. [DOI] [PubMed] [Google Scholar]


