Abstract
Background
Recent evaluation of rheumatic heart disease (RHD) mortality demonstrates disproportionate disease burden within the United States. However, there are few contemporary data on US children living with acute rheumatic fever (ARF) and RHD.
Methods and Results
Twenty‐two US pediatric institutions participated in a 10‐year review (2008–2018) of electronic medical records and echocardiographic databases of children 4 to 17 years diagnosed with ARF/RHD to determine demographics, diagnosis, and management. Geocoding was used to determine a census tract‐based socioeconomic deprivation index. Descriptive statistics of patient characteristics and regression analysis of RHD classification, disease severity, and initial antibiotic prescription according to community deprivation were obtained. Data for 947 cases showed median age at diagnosis of 9 years; 51% and 56% identified as male and non‐White, respectively. Most (89%) had health insurance and were first diagnosed in the United States (82%). Only 13% reported travel to an endemic region before diagnosis. Although 96% of patients were prescribed secondary prophylaxis, only 58% were prescribed intramuscular benzathine penicillin G. Higher deprivation was associated with increasing disease severity (odds ratio, 1.25; 95% CI, 1.08–1.46).
Conclusions
The majority of recent US cases of ARF and RHD are endemic rather than the result of foreign exposure. Children who live in more deprived communities are at risk for more severe disease. This study demonstrates a need to improve guideline‐based treatment for ARF/RHD with respect to secondary prophylaxis and to increase research efforts to better understand ARF and RHD in the United States.
Keywords: acute rheumatic fever, deprivation, pediatric, rheumatic heart disease, socioeconomic status, United States
Subject Categories: Epidemiology, Pediatrics, Rheumatic Heart Disease
Nonstandard Abbreviations and Acronyms
- ARF
acute rheumatic fever
- BPG
benzathine penicillin G
- DI
deprivation index
- RHD
rheumatic heart disease
Clinical Perspective
What Is New?
This article characterizes recent pediatric cases of acute rheumatic fever and rheumatic heart disease in the United States, the majority of which are endemic.
Although most children with acute rheumatic fever/rheumatic heart disease are receiving echocardiograms and secondary prophylaxis, only about half are receiving benzathine penicillin G, the gold standard.
What Are the Clinical Implications?
Children living in deprived communities are at risk for more severe disease; increased research is needed to understand and care for children in the United States at highest risk of acute rheumatic fever and rheumatic heart disease.
Worldwide, rheumatic heart disease (RHD) remains one of the most common cardiovascular diseases with 40.5 million prevalent cases resulting in ≈305 000 deaths annually.1, 2, 3 Acute rheumatic fever (ARF) incidence and RHD prevalence vary greatly across the globe, occurring most commonly in low‐ and middle‐income countries.2 Although ARF was the leading cause of mortality in 5‐ to 20‐year‐olds in the United States during the 1920s, the annual incidence of ARF in the United States today is low (<2 cases per 100 000 school‐aged children compared with up to 150 cases per 100 000 worldwide).4, 5, 6 These data may lead to the conclusions that ARF and RHD are diseases of the past in the United States and other high‐income countries. However, this view does not take persistent inequities into account. Recent geographic evaluation based on RHD mortality has demonstrated pockets of disproportionate disease burden; some coincide with elevated rates of poverty and disadvantage, but this is not true in all regions.7, 8 As mortality captures only a fraction of those affected by RHD and lags behind diagnosis by decades, it fails to accurately capture children currently affected by this disease. There is little contemporary data on children living with ARF and RHD within the United States. The role socioeconomic status plays in current pediatric cases is similarly unknown. This study describes the demographics, clinical features, and cardiac involvement of pediatric ARF/RHD in the United States over the past 10 years and examines the association with community deprivation.
Methods
Study Design
Sixty US institutions (including all 59 Accreditation Council for Graduate Medical Education accredited cardiology fellowship programs) were invited to participate in a 10‐year review (2008–2018) of the electronic medical record and echocardiography databases of pediatric patients ages 4 to 17 with a diagnosis of ARF or RHD. Patients with congenital heart disease were excluded. Diagnostic International Classification of Diseases, Ninth Revision and Tenth Revision (ICD‐9, ICD‐10) codes were used to identify children with ARF and RHD in both the inpatient and outpatient setting. Primary institutional review board approval was obtained from Cincinnati Children's Hospital Medical Center, and all participating centers also received secondary institutional review board approval except for 1 institution that relied on Cincinnati Children's Hospital Medical Center institutional review board. The de‐identified data that support the findings of this study are available on request from the corresponding author with appropriate human subject protections assured.
Patient Characteristics and Operational Definitions for Key Outcomes
Electronic medical record chart abstraction and review of echocardiographic databases enabled gathering demographic characteristics, presenting features, echocardiographic findings at presentation, and treatment with secondary antibiotic prophylaxis. Apart from changes in antibiotic regimens for secondary prophylaxis, data focused on features at time of presentation and did not collect longitudinal data. Study data were collected and managed using REDCap electronic data capture tools hosted at Cincinnati Children's Hospital Medical Center.9, 10 Echocardiographic data was gathered from nonstandardized reports at each center; no images were reviewed, and no components were independently reclassified.
Disease Severity
Based upon the echocardiographic findings at presentation, subjects were classified as having either mild, moderate, or severe disease (Table 1). If the mitral and aortic valve were affected in varying degrees, the more significant pathology was used to define overall disease severity.
Table 1.
Disease Severity Based on Severity of Cardiac Involvement at Time of Presentation
| Mitral or Aortic Regurgitation | Left Ventricular Dysfunction | Mitral Stenosis | |
|---|---|---|---|
| Mild or no cardiac involvement | Normal, trivial, or mild | … | … |
| Moderate cardiac involvement | Mild to moderate, Moderate | None or mildly diminished | … |
| Severe cardiac involvement | Moderate to severe, Severe | > mildly diminished | Present (any degree) |
Appropriate Secondary Prophylaxis
For this study, as recommended by the American Heart Association and other international guidelines, benzathine penicillin G (BPG) was considered the gold standard for RHD prophylaxiss.11, 12, 13 Based on a subject's disease classification at presentation (ARF versus RHD) in addition to echocardiographic findings at presentation, subjects were classified in accordance with the American Heart Association guidelines for secondary prophylaxis duration (Table 2).11 If the recommended duration of antibiotic prophylaxis was less than that advised by the American Heart Association guidelines, it was considered inadequate duration.
Table 2.
Guideline Based Duration for Prophylaxis and Criteria for Inadequate Duration of Prophylaxis
| AHA Category | AHA Recommended Duration of Prophylaxis | Participant Classification at Presentation | Participant Echocardiographic Findings | Criteria for Inadequate Duration of Prophylaxis |
|---|---|---|---|---|
| Rheumatic fever without carditis | 5 y or until 21 y of age (whichever is longer) | ARF | Normal or regurgitation | Anything <21 y of age |
| Rheumatic fever with carditis but NO residual valvular disease | 10 y or until 21 y of age (whichever is longer) | ARF | Normal or regurgitation | Anything <21 y of age |
| Rheumatic fever with carditis and residual heart disease | 10 y or until 40 y of age (whichever is longer) | ARF | Any mitral stenosis | Anything <40 y of age |
| Rheumatic fever with carditis and residual heart disease | 10 y or until 40 y of age (whichever is longer) | Rheumatic heart disease | Anything but Normal | Anything <40 y of age |
AHA indicates American Heart Association; and ARF, acute rheumatic fever.
Exposures/Predictors
Travel Exposure
An endemic region was defined as a country with an “estimated childhood mortality secondary to RHD >0.15 deaths per 100 000 population among children 5 to 9 years old,” as defined by the Global Burden of Disease.2 Travel exposure was determined by any documented travel before diagnosis as noted in the medical record. Given limitations of the electronic medical record, time spent in the endemic region could not be determined.
Deprivation Index
The previously published deprivation index (DI) was employed to capture community socioeconomic context.14 Using Health Insurance Portability and Accountability Act‐compliant software, the street address for each subject was geocoded to a corresponding census tract. The related tract was characterized by the DI, enumerated using 6 variables related to material deprivation obtained from the 2015 American Community Survey: (1) fraction of population with income in past 12 months below poverty level, (2) median household income in past 12 months in 2015 inflated‐adjusted dollars, (3) fraction of population 25 and older with educational attainment of at least high school graduation (includes general educational development equivalency), (4) fraction of population with no health insurance coverage, (5) fraction of households receiving public assistance income or food stamps or Supplemental Nutrition Assistance Program in the past 12 months, and (6) fraction of houses that are vacant. The DI ranges from 0 to 1, with 1 reflecting the greatest community deprivation. Whereas the DI is a measure of community deprivation, census tracts tend to be homogenous and therefore served as reasonable proxy for individual (or household) deprivation. Geocoding and DI derivation was completed at Cincinnati Children's using Decentralized Geomarker Assessment for Multi‐Site Studies,14 except for 2 institutions who performed their own geocoding on site using the same software.
Statistical Analysis
Descriptive statistics were used to enumerate the distribution of key variables. Independent sample t tests and chi‐square tests were used to test for differences in participant characteristics according to disease classification, disease severity, and DI dichotomized at the national average. Fisher's exact tests are reported for categorical variables where the expected cell counts do not exceed 5 in more than 80% of cells. Multivariable logistic regression was used to obtain odds ratios (ORs) and 95% CIs for RHD classification at presentation according to a 1 SD increase in participant DI. Ordinal logistic regression (cumulative logit) was used to obtain an OR for increasing disease severity (mild, moderate, severe). Multinomial logistic regression was used to obtain ORs for initial antibiotic prescription. Covariates thought to potentially confound the association between the DI and outcomes of interest were selected a priori and included biological sex, age at diagnosis, race, ethnicity, and insurance type. The percentage of missing values across the variables considered for regression ranged from 0% to 12%. Incomplete variables were multiply imputed (n=50 data sets) using full conditional specification as implemented by the default settings in mice version 3.9.0.15 Variables entered into the imputation model included sex, age at diagnosis, race, ethnicity, insurance type, DI, RHD classification, RHD severity, and initial antibiotic prescription. Estimates were obtained for each imputed data set using the base R logistic regression (glm), MASS version 7.3.5116 polr, and nnet version 7.3.1416 multinomial logistic regression functions and combined using Rubin's rules. Potential nonlinear associations were examined using restricted cubic splines but not retained as inclusion of additional terms did not improve model fit as determined by the Akaike information criterion. Therefore, ORs are presented in all models for a 1 SD change in the DI. Analyses were conducted using R version 4.0.0,17 JMP Version 14.0 (Cary, NC), and STATA MP version 13.0 (College Station, TX).
Results
Participant Characteristics
Data were collected for 947 children from 22 institutions (37% participation); enrollment by site varied significantly (from 7 to 132 subjects) (Table S1 and Figure S1). Across all cases, the median age at diagnosis was 9 years (interquartile range 7–12), with half identifying as male (487, 51%) and three‐quarters identifying as non‐Hispanic (700, 74%). Almost half identified as White (420, 44%). Most spoke English as their primary language (792, 84%) or had a parent who spoke English as a primary language (609, 82%). The majority of children had health insurance (846, 89%), with slightly over half covered by Medicaid or Medicare (450, 53%). Subjects were largely diagnosed in the United States (82%), rather than abroad (Table 3).
Table 3.
Participant Characteristics
| All (n=947) | Acute Rheumatic Fever (n=684) | Rheumatic Heart Disease (n=258) | P value | |
|---|---|---|---|---|
| Sex | 0.048 | |||
| Male | 487 (51.4%) | 365 (53.4%) | 119 (46.1%) | |
| Female | 460 (48.6%) | 319 (46.6%) | 139 (53.9%) |
| N=904 | N=661 | N=239 | ||
|---|---|---|---|---|
| Age at diagnosis, y, median (interquartile range) | 9 (7–12) | 9 (7–12) | 10 (7–13) | 0.001 |
| Race | <0.001 | |||
| American Indian or Alaska Native | 39 (4.1%) | 22 (3.2%) | 17 (6.6%) | |
| Asian | 43 (4.5%) | 26 (3.8%) | 17 (6.6%) | |
| Black | 172 (18.1%) | 98 (14.3%) | 73 (28.3%) | |
| Native Hawaiian or other Pacific Islander | 65 (6.9%) | 39 (5.7%) | 26 (10.1%) | |
| White | 420 (44.4%) | 354 (51.8%) | 63 (24.4%) | |
| Other | 143 (15.1%) | 101 (14.8%) | 41 (15.9%) | |
| Unknown | 65 (6.9%) | 44 (6.4%) | 21 (8.1%) | |
| Ethnicity | 0.83 | |||
| Hispanic or Latino | 161 (17.0%) | 115 (16.8%) | 46 (17.8%) | |
| Non‐Hispanic or Latino | 700 (74.0%) | 503 (73.5%) | 193 (74.8%) | |
| Unknown | 85 (9.0%) | 66 (9.6%) | 19 (7.4%) | |
| Primary language | <0.001 | |||
| English | 792 (83.6%) | 595 (87.0%) | 193 (74.8%) | |
| Spanish | 82 (8.7%) | 54 (7.9%) | 28 (10.9%) | |
| Other | 62 (6.5%) | 26 (3.8%) | 35 (13.6%) | |
| Unknown | 11 (1.2%) | 9 (1.3%) | 2 (0.8%) | |
| Has health insurance | 846 (89.3%) | 612 (89.5%) | 229 (88.8%) | 0.22 |
| N=846 | N=612 | N=229 | ||
|---|---|---|---|---|
| Type of health insurance | 0.004 | |||
| Private | 344 (40.7%) | 267 (43.6%) | 75 (29.1%) | |
| Medicaid or Medicare | 450 (53.2%) | 304 (49.7%) | 144 (55.8%) | |
| Both | 3 (0.4%) | 1 (0.2%) | 0 (0%) | |
| Unknown | 49 (5.8%) | 40 (6.5%) | 10 (3.9%) | |
| Diagnosed in the United States | <0.001 | |||
| Yes | 780 (82.4%) | 599 (87.6%) | 177 (68.6%) | |
| No | 119 (12.6%) | 52 (7.6%) | 66 (25.6%) | |
| Unknown | 48 (5.1%) | 33 (4.8%) | 15 (5.8%) |
| N=747 | N=543 | N=256 | ||
|---|---|---|---|---|
| Parent primary language English | 609 (81.5%) | 462 (85.1%) | 193 (74.8%) | <0.001 |
| Prior travel to endemic region | 124 (13.0%) | 53 (7.7%) | 70 (27.1%) | 0.02 |
n (%) unless otherwise indicated.
Travel Exposure
Only 124 (13%) had known travel to an endemic region before diagnosis of ARF/RHD. The most frequently identified regions included the Pacific Islands (58, 37%) and Africa (33, 21%) (Figure). Those with RHD at time of diagnosis were more likely to report travel to an endemic region compared with those who presented with ARF (P=0.02; Table 3).
Figure 1. Travel exposure.
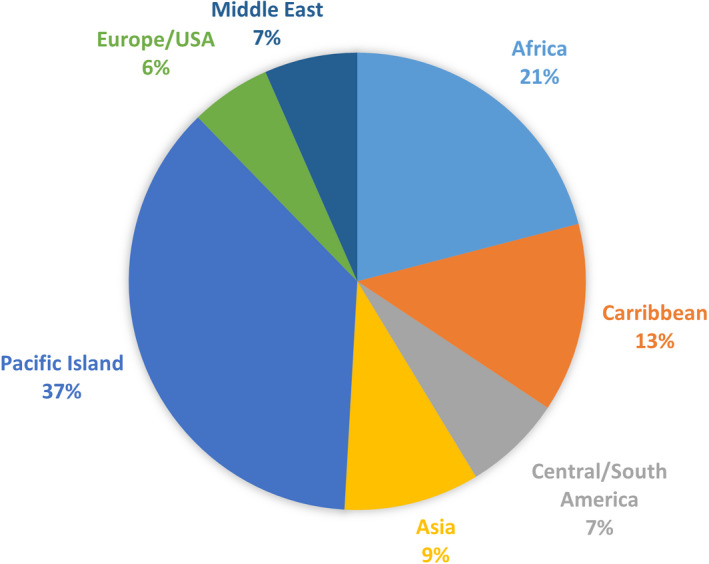
Breakdown of region traveled to for the 157 participants with travel exposure.
Presentation
Clinical Findings in Acute Rheumatic Fever
At time of presentation, nearly three‐quarters of cases were diagnosed at the time of ARF (684, 72%). The most commonly cited major Jones criteria were carditis (336, 49%), polyarthritis (208, 30%), and Sydenham chorea (254, 37%), whereas the most commonly cited minor criteria were fever (330, 48%) and elevated inflammatory markers (315, 46%) (Table 4). Of these, 96% had an echocardiogram performed with the most common pathological findings of mitral regurgitation (417, 64%) and aortic regurgitation (221, 34%). Rarely was there associated left ventricular (10, 1.5%) or right ventricular (2, 0.3%) systolic dysfunction (Table 5).
Table 4.
Presentation at Diagnosis and Management
| Reported Findings for Those with ARF Presentation (n=684) | |
|---|---|
| Major criteria | |
| Carditis | 336 (49.1%) |
| Polyarthritis | 208 (30.4%) |
| Sydenham chorea | 254 (37.1%) |
| Subcutaneous nodules | 24 (3.5%) |
| Erythema marginatum | 59 (8.6%) |
| Minor criteria | |
| Fever | 330 (48.2%) |
| Elevated C‐reactive protein and/or erythrocyte sedimentation rate | 315 (46.1%) |
| Prolonged PR interval on electrocardiogram | 63 (9.2%)* |
| Arthralgia | 131 (19.2%)† |
| Secondary Prophylaxis | |
|---|---|
| Prescribed secondary prophylaxis | |
| Yes | 913 (96.4%) |
| No | 23 (2.4%) |
| Unknown | 11 (1.2%) |
|
Initial antibiotic choice for secondary prophylaxis (n=913, prescribed secondary prophylaxis) | |
| BPG, intramuscular | 527 (57.7%) |
| Oral penicillin | 318 (34.8%) |
| Macrolide | 24 (2.6%) |
| Sulfadiazine | 8 (0.9%) |
| Other | 20 (2.2%) |
| Unknown | 16 (1.8%) |
|
Reason for not using BPG (n=370, prescribed a known alternate) | |
| Patient/family preference | 107 (28.9%) |
| Patient allergy | 32 (8.6%) |
| Other | 5 (1.4%) |
| Unknown | 226 (61.1%) |
|
Secondary prophylaxis prescription changed (n=913, prescribed secondary prophylaxis) |
171 (18.7%) |
n (%) unless otherwise indicated. ARF indicates acute rheumatic fever; and BPG, benzathine penicillin G.
Only 16 without echocardiographic carditis counted toward ARF diagnosis.
Without arthritis.
Table 5.
Echocardiographic Data
| All (n=947) | Acute Rheumatic Fever (n=684) | Rheumatic Heart Disease (n=258) | |
|---|---|---|---|
| Echocardiogram performed | 917 (96.8%) | 656 (95.9%) | 258 (100%) |
| Mitral stenosis | 73 (8.0%) | 24 (3.7%) | 49 (19.0%) |
| Mild | 38 (52.0%) | 17 (70.8%) | 21 (42.8%) |
| Moderate | 21 (28.8%) | 5 (20.8%) | 16 (32.7%) |
| Severe | 6 (8.2%) | 1 (4.2%) | 5 (10.2%) |
| Data on quantification not available | 8 (11.0%) | 1 (4.2%) | 7 (14.3%) |
| Mitral regurgitation | 645 (70.3%) | 417 (63.6%) | 228 (88.4%) |
| < Moderate | 277 (42.9%) | 206 (49.4%) | 71 (31.1%) |
| Moderate | 219 (34.0%) | 137 (32.8%) | 82 (36.0%) |
| Severe | 144 (22.3%) | 72 (17.3%) | 72 (31.6%) |
| Data on quantification not available | 5 (0.8%) | 2 (0.5%) | 3 (1.3%) |
| Aortic stenosis | 21 (2.3%) | 3 (0.5%) | 18 (7.0%) |
| < Moderate | 17 (80.9%) | 2 (66.7%) | 15 (83.3%) |
| Moderate | 3 (14.3%) | 1 (33.3%) | 2 (11.1%)_ |
| Severe | 0 (0%) | 0 (0%) | 0 (0%) |
| Data on quantification not available | 1 (4.8%) | 0 (0%) | 1 (5.6%) |
| Aortic regurgitation | 352 (38.4%) | 221 (33.7%) | 131 (50.8%) |
| < Moderate | 191 (54.3%) | 136 (61.5%) | 55 (42.0%) |
| Moderate | 102 (29.0%) | 58 (26.2%) | 44 (33.6%) |
| Severe | 55 (15.6%) | 24 (10.9%) | 31 (23.7%) |
| Data on quantification not available | 4 (1.1%) | 3 (1.4%) | 1 (0.7%) |
| Pulmonary hypertension | 66 (7.2%) | 27 (4.1%) | 39 (15.1%) |
| Reduced left ventricular systolic function | 27 (2.9%) | 10 (1.5%) | 17 (6.6%) |
| Mildly reduced | 16 (59.3%) | 6 (60.0%) | 10 (58.8%) |
| Moderately reduced | 7 (25.9%) | 2 (20.0%) | 5 (29.4%) |
| Severely reduced | 4 (14.8%) | 2 (20.0%) | 2 (11.8%) |
| Reduced right ventricular systolic function | 9 (1.0%) | 2 (0.3%) | 7 (2.7%) |
| Pericardial effusion | 81 (8.8%) | 51 (7.8%) | 30 ( 11.6%) |
| Trace/small | 66 (87.6%) | 40 (78.4%) | 26 (86.7%) |
| Moderate | 9 (6.7%) | 7 (13.7%) | 2 (6.7%) |
| Large | 4 (3.8%) | 3 (5.9%) | 1 (3.3%) |
| Present but not quantified | 2 (1.9%) | 1 (2.0%) | 1 (3.3%) |
n (%) unless otherwise indicated.
Clinical Findings in Chronic Rheumatic Heart Disease
Having missed the diagnosis of ARF, 27% (258) were diagnosed with chronic RHD as their first presentation. Of these, one‐third (35%) recalled a previous history consistent with ARF, though the diagnosis was not made. All had an echocardiogram. As in ARF, mitral regurgitation (228, 88%) and aortic regurgitation (131, 51%) were the most common pathological findings. Mitral stenosis was found in 19% of patients (Table 5).
Disease Severity
Disease severity was determined for the 872 participants who had echocardiographic data, of which 452 (52%) had mild disease, 188 (22%) had moderate disease, and 232 (27%) had severe disease. Those who identified their race as White had less severe disease (P<0.001); there was no difference in disease severity with respect to ethnicity (Hispanic or Latino versus non‐Hispanic or Latino, P=0.79). Possessing health insurance and type of health insurance (commercial versus public) were not associated with disease severity (P=0.51 and P=0.55, respectively). Severe disease was more likely if either the subject or subject's parental primary language was not English (P=0.001 and P=0.047, respectively) compared with subjects whose primary language was English (Table 6).
Table 6.
Disease Severity
| Mild (n=452) | Moderate (n=188) | Severe (n=232) | P value | |
|---|---|---|---|---|
| Sex | 0.03 | |||
| Male | 245 (54.2%) | 98 (52.1%) | 101 (43.5%) | |
| Female | 207 (45.8%) | 90 (47.9%) | 131 (56.5%) |
| N=436 | N=180 | N=222 | ||
|---|---|---|---|---|
| Age at diagnosis, y, median (interquartile range) | 9 (7–12) | 10 (8–12) | 10 (7–13) | 0.11 |
| Race | <0.001 | |||
| American Indian or Alaska Native | 17 (3.8%) | 14 (7.4%) | 8 (3.4%) | |
| Asian | 14 (3.1%) | 9 (4.8%) | 17 (7.3%) | |
| Black | 58 (12.8%) | 37 (19.7%) | 61 (26.3%) | |
| Native Hawaiian or other Pacific Islander | 30 (6.6%) | 11 (5.9%) | 24 (10.3%) | |
| White | 235 (52.0%) | 80 (42.6%) | 71 (30.6%) | |
| Other | 64 (14.2%) | 23 (5.1%) | 39 (16.8%) | |
| Unknown | 34 (7.5%) | 14 (7.4%) | 12 (5.2%) | |
| Ethnicity | 0.79 | |||
| Hispanic or Latino | 73 (16.2%) | 29 (15.4%) | 42 (18.1%) | |
| Non‐Hispanic or Latino | 339 (75.0%) | 139 (73.9%) | 172 (74.1%) | |
| Unknown | 40 (8.8%) | 20 (10.6%) | 18 (7.8%) | |
| Primary language | 0.001 | |||
| English | 392 (86.7%) | 155 (82.4%) | 183 (78.9%) | |
| Spanish | 36 (8.0%) | 20 (10.6%) | 18 (7.8%) | |
| Other | 17 (3.8%) | 13 (6.9%) | 28 (12.1%) | |
| Unknown | 7 (1.5%) | 0 (0%) | 3 (1.3%) |
| N=436 | N=176 | N=226 | ||
|---|---|---|---|---|
| Has health insurance | 407 (90.0%) | 165 (87.8%) | 206 (88.8%) | 0.51 |
| N=399 | N=170 | N=222 | ||
|---|---|---|---|---|
| Type of health insurance | 0.64 | |||
| Private | 167 (36.9%) | 69 (36.7%) | 79 (34.1%) | |
| Medicaid or Medicare | 201 (44.5%) | 90 (47.9%) | 123 (53.0%) | |
| Both | 2 (0.4%) | 0 (0%) | 0 (0%) | |
| Unknown | 37 (8.2%) | 6 (3.2%) | 4 (1.7%) | |
| Diagnosed in the United States | 0.09 | |||
| Yes | 381 (84.3%) | 164 (87.2%) | 186 (80.2%) | |
| No | 54 (11.9%) | 18 (9.6%) | 38 (16.4%) | |
| Unknown | 17 (3.8%) | 6 (3.2%) | 8 (3.4%) |
| N=354 | N=161 | N=182 | ||
|---|---|---|---|---|
| Parent primary language English | 297 (65.7%) | 133 (70.7%) | 137 (59.1%) | 0.047 |
| Travel exposure before diagnosis | 0.02 | |||
| Yes | 65 (14.4%) | 29 (15.4%) | 53 (22.8%) | |
| No | 68 (15.0%) | 32 (17.0%) | 45 (19.4%) | |
| Unknown | 319 (70.6%) | 127 (67.6%) | 134 (57.8%) | |
| Travel to endemic region | 49 (10.8%) | 25 (13.3%) | 45 (19.4%) | 0.31 |
| Prescribed secondary prophylaxis |
0.43 |
|||
| Yes | 437 (96.7%) | 181 (96.3%) | 224 (96.6%) | |
| No | 9 (2.0%) | 7 (3.7%) | 5 (2.2%) | |
| Unknown | 6 (1.3%) | 0 (0%) | 3 (1.2%) |
| N=430 | N=180 | N=221 | ||
|---|---|---|---|---|
| Initial antibiotic choice for secondary prophylaxis | 0.09 | |||
| BPG, intramuscular | 231 (53.7%) | 118 (65.5%) | 142 (64.2%) | |
| Oral penicillin | 172 (40.0%) | 52 (28.9%) | 67 (30.3%) | |
| Macrolid | 10 (2.3%) | 6 (3.3%) | 7 (3.2%) | |
| Sulfadiazine | 5 (1.2%) | 1 (0.6%) | 2 (0.9%) | |
| Other | 12 (2.8%) | 3 (1.7%) | 3 (1.4%) |
| N=80 | N=27 | N=32 | ||
|---|---|---|---|---|
| Reason for not using BPG | 0.130 | |||
| Patient/family preference | 62 (77.5%) | 20 (74.1%) | 22 (68.8%) | |
| Patient allergy | 16 (20%) | 4 (14.8%) | 10 (31.2%) | |
| Other | 2 (2.5%) | 3 (11.1%) | 0 (0%) |
n (%) unless otherwise indicated. BPG indicates benzathine penicillin G.
Management
Secondary Prophylaxis
Although almost all patients were prescribed secondary prophylaxis (913, 96%), only half (527, 58%) were prescribed intramuscular benzathine penicillin G (BPG); 318 (35%) received oral penicillin and 68 (7%) were prescribed other antibiotics (Table 4). Subjects with no insurance or public insurance were more likely to receive BPG therapy versus oral therapy (62% and 64%, respectively, versus 53% for private insurance, P=0.01). Although the reason for choosing oral antibiotics over BPG was known in fewer than half the cases (144, 39%), patient or family preference was the most commonly cited reason (107, 74%), followed by patient allergy (32, 22%). The type of secondary prophylaxis was changed in 18% (171) of subjects, with 7% (13) switching from initial nonpenicillin prophylaxis to penicillin prophylaxis. In these cases, patients frequently had a preceding documented allergy to penicillin, but after further evaluation (often by allergy/immunology), the patient was cleared to receive penicillins and thus transitioned to BPG.
Duration of Prophylaxis
Seventeen percent of patients were advised a prophylactic duration shorter than the American Heart Association guidelines recommend (Table 2).
Deprivation Index
The DI was calculated for 871 (92%) of cases. Of the 76 addresses that could not be geocoded, the address was either missing (3), international (2), a PO box (44), listed as general delivery (1), or not recognized (26). The mean DI in our cases was 0.39±0.15, slightly higher than the national population‐weighted mean DI of 0.37 for those <18 years old.18 Using the national mean DI of 0.375, cases were assigned to a less deprived (DI <0.375) and more deprived (DI ≥0.375) group. Comparing characteristics between these groups demonstrated that increased community deprivation was associated with identifying as non‐White (66% versus 42%, P<0.001), Hispanic‐ or Latino ethnicity (25% versus 11%, P<0.001), less frequently speaking English as a primary language (76% versus 90%, P<0.001), having Medicaid or Medicare insurance (70% versus 34%, P<0.001), and less likely to be diagnosed in the United States (79% versus 85%, P=0.008). Higher deprivation was associated with increasing disease severity (OR, 1.25; 95% CI, 1.08–1.46) and higher likelihood of BPG when compared with enteral penicillin prescription (OR, 0.67; 95% CI, 0.56–0.8) in models adjusted for participant sex, age at diagnosis, race, ethnicity, and insurance type (Table 7).
Table 7.
Odds Ratios and 95% CIs for RHD Classification, Severity, and Initial Therapy According to a 1 SD Increase in the DI (n=871)
| OR (95% CI) | AOR (95% CI) | |
|---|---|---|
| Model 1: RHD classification at presentation* | 1.33 (1.14–1.54) | 1.13 (0.95–1.34) |
| Model 2: disease severity at presentation† | 1.34 (1.18–1.53) | 1.25 (1.08–1.46) |
| Model 3: initial antibiotic therapy‡ | ||
| Macrolide | 0.70 (0.45–1.09) | 0.79 (0.48–.30) |
| None | 0.88 (0.58–1.35) | 0.93 (0.57–1.51) |
| Other | 0.48 (0.28–0.84) | 0.64 (0.33–1.26) |
| Oral penicillin | 0.65 (0.56–0.76) | 0.67 (0.56–0.80) |
| Sulfa | 0.60 (0.28–1.30) | 1.07 (0.43–2.64) |
AOR adjusted for sex, age at diagnosis, race, ethnicity, and insurance type. Missing data imputed using n=50 imputations. SD increase in DI index is 0.147 units. AOR indicates adjusted odds ratio; CI, confidence interval; DI, deprivation index; OR, odds ratio; and RHD, rheumatic heart disease.
OR obtained from logistic regression.
OR obtained from ordinal regression.
OR obtained from multinomial logistic regression. Estimates provided for each therapy when compared with benzathine penicillin G (intramuscular penicillin).
Discussion
Through a 10‐year retrospective case review, our data capture the contemporary picture of pediatric ARF and RHD in the United States. The use of primary source data, not previously employed at a national level, has allowed a more comprehensive and nuanced look at these cases. The addition of deprivation index highlights that ARF and RHD continue as diseases of health inequity, with children living in more deprived communities at increased risk of severe RHD.
Caution must be employed when interpreting the demographics of our cases. Although our sample is large, it was not collected in a representative manner and may not reflect the demographics of children with ARF/RHD living in the United States as a whole. Still, it is worth noting that there was a higher than expected percentage of children identifying as Black, or Indigenous (American Indian or Alaskan Native and Native Hawaiian or other Pacific Islander), which is consistent with previously reported increased risk for both population subgroups within the United States,5, 6 as well as the increased risk seen in Indigenous populations in Australia, New Zealand, and Canada.19, 20, 21, 22 Combined, these data suggest that more intensive surveillance, including active screening, could help characterize and develop plans to mitigate the risk in these vulnerable US communities.
It is also worth noting, that the majority of children diagnosed with ARF or RHD were diagnosed in the United States (>80%), spoke English as their primary language (>80%), and had health insurance (nearly 90%). Furthermore, 87% had no travel history to an endemic region, indicating a continued domestic burden of ARF and RHD. This has important implications for provider awareness and appropriate use of primary and secondary prevention. Additionally, the continued domestic case burden highlights that diagnosis and treatment of symptomatic streptococcal sore throat can never prevent all cases of ARF. A group A streptococcal vaccine, in contrast, could help eliminate new cases of ARF both here and around the world.13, 23, 24
This study also gives important insight into the clinical presentation of ARF and RHD in the United States. Chorea was exceedingly common in this population (37% of those presenting with ARF), as compared with the <10% to 30% of ARF cases globally,25, 26, 27, 28, 29, 30, 31, 32, 33 including recent studies in the United States.5, 6 It will be important in future studies to determine if high rates of chorea reflect true distribution of ARF presentations or if more mild joint presentations are being missed, skewing the percentages higher for chorea as a primary presentation.
Another important clinical finding is that a significant number of children captured through this study presented with chronic RHD, one‐quarter with severe disease, who may require cardiac catheterization or surgery in the future. Late presentation results in the missed opportunity to have maximum benefit from secondary antibiotic prophylaxis, which prevents group A streptococcal infections and recurrent ARF. Further research should be undertaken to study provider and parent awareness of ARF, as education might improve early ARF diagnosis and reduce the number of children presenting with late stage RHD.
These data highlight the successful implementation of evidence‐based diagnostic recommendations for ARF and RHD in the United States. As newly recommended by the 2015 Jones criteria, children in this study were exceedingly likely to have had an echocardiogram as part of their diagnostic work‐up (97%). As half of these cases predate these recommendations, high rates of echocardiography may also reflect the near universal access to echocardiography in US tertiary facilities (where case recruitment occurred). However, there was inconsistent reporting of echocardiographic findings and frequent use of nonstandardized definitions for grading severity of valvular and features of RHD. Future emphasis should be placed on standardization of echocardiographic evaluation for children with RHD including the American Society of Echocardiography and American Heart Association guidelines,34, 35, 36 the revised Jones criteria from 2015,26 and the 2012 World Heart Federation Guidelines for the Echocardiographic Diagnosis of Rheumatic Heart Disease37 to allow for a unified definition and ability to compare data across countries and continents using a standardized criteria for RHD diagnosis.
There was also substantial variation from guideline‐based care on the type and duration of secondary prophylaxis.11 Despite the fact that BPG is recommended as first‐line prevention, having greater efficacy than oral penicillin in preventing recurrent ARF,12, 13 only 58% of the cases was prescribed BPG. Family preference was the most common reason cited for not prescribing BPG. It is worth noting that those living in more deprived areas were more likely to be prescribed BPG than oral penicillin, perhaps perceived by clinicians to be at higher risk. Our data highlight variability in the recommended duration of secondary prevention, with shorter than recommended11 durations in 17% of cases based on presenting features and severity of cardiac involvement. Together, these data suggest that increased clinician and parental education is needed to ensure that children living with ARF and RHD in the United States receive guideline‐based care.
Finally, these data support that RHD remains a disease characterized by inequity among children living in the United States. Greater community socioeconomic deprivation was associated with having more severe valvular involvement, which could reflect living conditions such as overcrowding, poor sanitation, and poor hygiene, which are long recognized factors that increase exposure to group A streptococcal disease.13, 38, 39 It is worth noting that this finding contrasts that of a recent study on children hospitalized for ARF, finding no statistically significant differences in socioeconomic status. That study, however, was limited by its sole use of insurance status as a proxy for socioeconomic status.5 The deprivation index provides a more multidimensional assessment of one's contextual living environment, and our conclusions support the findings that ARF and RHD outcomes remain inequitable globally and nationally.1
Limitations
There are several limitations in our data stemming from our pragmatic recruitment strategy based on tertiary hospital programs. First, our data may not be representative of patients and clinical practices outside of major medical centers. Second, although we invited participation from all programs with a pediatric cardiology fellowship, only 37% of invited institutions participated. Although we recruited nearly 1000 patients, increasing the internal validity of our data, there were some geographic areas of our country that were not well represented, such as the West, and thus certain populations may be underrepresented or not captured. Given this, we could not compare population characteristics, such as race or ethnicity, to overall US population characteristics to confidently identify groups at higher risk.
Retrospective review of data led to several additional limitations. Data on travel to an endemic region did not include specifications on the nature of exposure (for example, limited travel exposure versus prior residence in endemic region); thus we are unable to comment on whether participants were immigrants from an endemic region or merely went to visit. We collected echocardiographic data only at presentation and are not able to comment on the longitudinal progression or regression of cardiac disease. In addition to our population limitations, DI is a measure of community‐level deprivation and cannot be used to extrapolate individual risk prediction and we cannot rule out the potential for unmeasured factors to have resulted in residual confounding when estimating the association between neighborhood deprivation and outcomes of interest.
Conclusions
ARF and RHD are characterized by equity gaps in the United States as well as around the world. Children who newly acquire ARF and RHD in the United States are, for the most part, exposed to group A streptococcal disease and experiencing the sequelae within the United States and not abroad. There is room to improve evidence‐based treatment for ARF and RHD in the United States, both through provider and parent education. Further study of high‐risk populations in the United States could better target these educational efforts and provide the opportunity to strengthen primary prevention for our most vulnerable children.
Sources of Funding
This work was supported by American Heart Association Grant #17SFRN33670607.
Disclosures
Dr Divya Shakti was supported by the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number P20GM121334. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figure S1
For Sources of Funding and Disclosures, see page 11.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. DOI: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377:713–722. DOI: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 3.Rheumatic heart disease‐level 3 cause. 2020.
- 4.Hajar R. Rheumatic fever and rheumatic heart disease a historical perspective. Heart Views. 2016;17:120–126. DOI: 10.4103/1995-705X.192572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyake CY, Gauvreau K, Tani LY, Sundel RP, Newburger JW. Characteristics of children discharged from hospitals in the United States in 2000 with the diagnosis of acute rheumatic fever. Pediatrics. 2007;120:503–508. DOI: 10.1542/peds.2006-3606. [DOI] [PubMed] [Google Scholar]
- 6.Bradley‐Hewitt T, Longenecker CT, Nkomo V, Osborne W, Sable C, Scheel A, Zuhlke L, Watkins D, Beaton A. Trends and presentation patterns of acute rheumatic fever hospitalisations in the United States. Cardiol Young. 2019;29:1387–1390. DOI: 10.1017/S1047951119002270. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Health Metrics and Evaluation: US health map. 2018. Available at: http://www.healthdata.org/data‐visualization/us‐health‐map. Accessed December 21, 2018.
- 8.Roth GA, Johnson C, Abajobir A, Abd‐Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. DOI: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, REDCap Consortium , et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. DOI: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. DOI: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber MA, Baltimore RS, Eaton CB, Gewitz M, Rowley AH, Shulman ST, Taubert KA. Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: Endorsed by the American Academy of Pediatrics. Circulation. 2009;119:1541–1551. DOI: 10.1161/CIRCULATIONAHA.109.191959. [DOI] [PubMed] [Google Scholar]
- 12.Manyemba J, Mayosi BM. Penicillin for secondary prevention of rheumatic fever. Cochrane Database Syst Rev. 2002;2002:Cd002227. DOI: 10.1002/14651858.CD002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carapetis JR, Beaton A, Cunningham MW, Guilherme L, Karthikeyan G, Mayosi BM, Sable C, Steer A, Wilson N, Wyber R, et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2016;2:15084. DOI: 10.1038/nrdp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brokamp C, Wolfe C, Lingren T, Harley J, Ryan P. Decentralized and reproducible geocoding and characterization of community and environmental exposures for multisite studies. J Am Med Inform Assoc. 2018;25:309–314. DOI: 10.1093/jamia/ocx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buuren S, Mice G‐O. Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 16.Venables WN, Ripley BD. Modern Applied Statistics With S. New York: Springer; 2002. [Google Scholar]
- 17.Team RC . R: A Language and Environment for Statistical Computing. Vienna: R Foundation of Statistical Computing; 2020. [Google Scholar]
- 18.American Community Survey . 2020.
- 19.Baker MG, Gurney J, Oliver J, Moreland NJ, Williamson DA, Pierse N, Wilson N, Merriman TR, Percival T, Murray C, et al. Risk factors for acute rheumatic fever: literature review and protocol for a case‐control study in New Zealand. Int J Environ Res Public Health. 2019;16:4515. DOI: 10.3390/ijerph16224515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon J, Kirlew M, Schreiber Y, Saginur R, Bocking N, Blakelock B, Haavaldsrud M, Kennedy C, Farrell T, Douglas L, et al. Acute rheumatic fever in First Nations communities in northwestern Ontario: social determinants of health "bite the heart". Can Fam Physician. 2015;61:881–886. [PMC free article] [PubMed] [Google Scholar]
- 21.Haynes E, Marawili M, Marika BM, Mitchell AG, Phillips J, Bessarab D, Walker R, Cook J, Ralph AP. Community‐based participatory action research on rheumatic heart disease in an Australian Aboriginal homeland: evaluation of the ‘On track watch’ project. Eval Program Plann. 2019;74:38–53. DOI: 10.1016/j.evalprogplan.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR. Acute rheumatic fever and rheumatic heart disease: incidence and progression in the northern territory of Australia, 1997 to 2010. Circulation. 2013;128:492–501. DOI: 10.1161/CIRCULATIONAHA.113.001477. [DOI] [PubMed] [Google Scholar]
- 23.Dale JB, Fischetti VA, Carapetis JR, Steer AC, Sow S, Kumar R, Mayosi BM, Rubin FA, Mulholland K, Hombach JM, et al. Group a streptococcal vaccines: paving a path for accelerated development. Vaccine. 2013;31(suppl 2):B216–B222. DOI: 10.1016/j.vaccine.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 24.Rivera‐Hernandez T, Carnathan DG, Jones S, Cork AJ, Davies MR, Moyle PM, Toth I, Batzloff MR, McCarthy J, Nizet V, et al. An experimental group a Streptococcus vaccine that reduces pharyngitis and tonsillitis in a nonhuman primate model. mBio. 2019;10:e00693‐19. DOI: 10.1128/mBio.00693-19d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sika‐Paotonu D, Beaton A, Raghu A, Steer A, Carapetis J. Acute rheumatic fever and rheumatic heart disease. In: Ferretti JJ, Stevens DL, Fischetti VA, eds. Streptococcus Pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City, OK: University of Oklahoma Health Sciences Center © The University of Oklahoma Health Sciences Center; 2016:1–45. [Google Scholar]
- 26.Gewitz MH, Baltimore RS, Tani LY, Sable CA, Shulman ST, Carapetis J, Remenyi BO, Taubert KA, Bolger AF, Beerman L, et al. Revision of the jones criteria for the diagnosis of acute rheumatic fever in the era of doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806–1818. DOI: 10.1161/CIR.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 27.Stollerman GH. Rheumatic fever. Lancet. 1997;349:935–942. DOI: 10.1016/S0140-6736(96)06364-7. [DOI] [PubMed] [Google Scholar]
- 28.Carapetis JR, Currie BJ. Rheumatic chorea in northern Australia: a clinical and epidemiological study. Arch Dis Child. 1999;80:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korn‐Lubetzki I, Brand A. Sydenham's chorea in Jerusalem: still present. Isr Med Assoc J. 2004;6:460–462. [PubMed] [Google Scholar]
- 30.Eshel G, Lahat E, Azizi E, Gross B, Aladjem M. Chorea as a manifestation of rheumatic fever–a 30‐year survey (1960–1990). Eur J Pediatr. 1993;152:645–646. [DOI] [PubMed] [Google Scholar]
- 31.Okello E, Ndagire E, Atala J, Bowen AC, DiFazio MP, Harik NS, Longenecker CT, Lwabi P, Murali M, Norton SA, et al. Active case finding for rheumatic fever in an endemic country. J Am Heart Assoc. 2020;9:e016053. DOI: 10.1161/JAHA.120.016053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seckeler MD, Hoke TR. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol. 2011;3:67–84. DOI: 10.2147/CLEP.S12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanna JN, Heazlewood RJ. The epidemiology of acute rheumatic fever in Indigenous people in north Queensland. Aust N Z J Public Health. 2005;29:313–317. DOI: 10.1111/j.1467-842x.2005.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 34.Zoghbi WA, Adams D, Bonow RO, Enriquez‐Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. DOI: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23; quiz 101–102. DOI: 10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2017;135:e1159–e1195. DOI: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 37.Reményi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, Lawrenson J, Maguire G, Marijon E, Mirabel M, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease—an evidence‐based guideline. Nat Rev Cardiol. 2012;9:297–309. DOI: 10.1038/nrcardio.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katzenellenbogen JM, Ralph AP, Wyber R, Carapetis JR. Rheumatic heart disease: infectious disease origin, chronic care approach. BMC Health Serv Res. 2017;17:793. DOI: 10.1186/s12913-017-2747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurahara DK, Grandinetti A, Galario J, Reddy DV, Tokuda A, Langan S, Tanabe B, Yamamoto KS, Yamaga KM. Ethnic differences for developing rheumatic fever in a low‐income group living in hawaii. Ethn Dis. 2006;16:357–361. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


