Abstract
Background
The plant‐based Dietary Portfolio combines established cholesterol‐lowering foods (plant protein, nuts, viscous fiber, and phytosterols), plus monounsaturated fat, and has been shown to improve low‐density lipoprotein cholesterol and other cardiovascular disease (CVD) risk factors. No studies have evaluated the relation of the Dietary Portfolio with incident CVD events.
Methods and Results
We followed 123 330 postmenopausal women initially free of CVD in the Women's Health Initiative from 1993 through 2017. We used Cox proportional‐hazard models to estimate adjusted hazard ratios (HRs) and 95% CI of the association of adherence to a Portfolio Diet score with CVD outcomes. Primary outcomes were total CVD, coronary heart disease, and stroke. Secondary outcomes were heart failure and atrial fibrillation. Over a mean follow‐up of 15.3 years, 13 365 total CVD, 5640 coronary heart disease, 4440 strokes, 1907 heart failure, and 929 atrial fibrillation events occurred. After multiple adjustments, adherence to the Portfolio Diet score was associated with lower risk of total CVD (HR, 0.89; 95% CI, 0.83–0.94), coronary heart disease (HR, 0.86; 95% CI, 0.78–0.95), and heart failure (HR, 0.83; 95% CI, 0.71–0.99), comparing the highest to lowest quartile of adherence. There was no association with stroke (HR, 0.97; 95% CI, 0.87–1.08) or atrial fibrillation (HR, 1.10; 95% CI, 0.87–1.38). These results remained statistically significant after several sensitivity analyses.
Conclusions
In this prospective cohort of postmenopausal women in the United States, higher adherence to the Portfolio Diet was associated with a reduction in incident cardiovascular and coronary events, as well as heart failure. These findings warrant further investigation in other populations.
Keywords: cardiovascular disease, dietary patterns, dietary portfolio, plant‐based, prospective cohort study
Subject Categories: Cardiovascular Disease, Diet and Nutrition, Epidemiology, Primary Prevention
Nonstandard Abbreviations and Acronyms
- FFQ
food frequency questionnaire
- MUFAs
monounsaturated fatty acids
- OS
observational study
- WHI
Women's Health Initiative
Clinical Perspective
What Is New?
Higher adherence to the Portfolio Diet was associated with a 11%, 14%, and 17% lower risk of total cardiovascular disease, coronary heart disease, and heart failure, respectively, but no association was seen with stroke or atrial fibrillation.
This study shows that the beneficial effects of the Portfolio Diet on cardiovascular risk factors from the clinical trials may translate into lower hard clinical cardiovascular disease events.
What Are the Clinical Implications?
Given the increased interest in plant‐based foods and diets around the world, and growing concerns related to ethical and environmental implications of diet, the Portfolio Diet warrants attention from healthcare professionals as another therapeutic dietary approach for cardiovascular disease risk reduction.
The Dietary Portfolio, or Portfolio Diet, is a plant‐based dietary pattern that was developed in the early 2000s to lower low‐density lipoprotein cholesterol (LDL‐C).1, 2, 3, 4, 5, 6 The underlying diet is low in saturated fat and cholesterol (National Cholesterol Education Program Step II diet7), with the addition of a “portfolio” of 4 cholesterol‐lowering foods and nutrients: nuts, plant protein (soy and pulses), viscous fiber (oats, barley, psyllium, eggplant, okra, apples, oranges, and berries), and phytosterols (originally provided as enriched margarine). An extension of the diet includes adding monounsaturated fats (MUFAs; such as olive/canola oil or avocado).6 Early findings from a metabolically controlled randomized trial showed that the LDL‐C lowering effect of the Portfolio Diet was similar to the control diet taken with 20mg lovastatin (−28.6% versus −30.9%).3 Recently, a systematic review and meta‐analysis of metabolically controlled and ad libitum trials showed that the Portfolio Diet significantly lowered LDL‐C by 17% (27% in the intended combination with an National Cholesterol Education Program Step II diet). It also lowered other cardiovascular disease (CVD) risk factors, including the alternate blood lipid targets of non‐high‐density lipoprotein cholesterol by 14% and ApoB (apolipoprotein B) by 15%, and CRP (C‐reactive protein) by 32%.8 These benefits have been recognized by CVD and diabetes mellitus clinical practice guidelines internationally, including those of the Canadian Cardiovascular Society,9 Diabetes Canada,10 European Atherosclerosis Society,11 and Heart UK.12
Currently, it is not known if these beneficial effects of the diet translate into lower risk of clinical CVD events. The individual components of the Portfolio Diet have been found to be associated with lower incidence of CVD events in prospective cohorts,13, 14, 15, 16, 17 and 2 components of the diet (nuts and extra virgin olive oil) were shown to reduce major vascular events in the landmark PREDIMED (Prevención con Dieta Mediterránea) trial compared to a low saturated fat18 however, the additive/combined effects of the Portfolio Diet components have not been assessed with incident CVD. Although conducting a long‐term randomized trial with CVD as the primary outcome would be preferable, this type of trial is not yet feasible. Analyses of established observational studies may be helpful in assessing the long‐term effectiveness of the Portfolio Diet. We have therefore developed a scoring system to measure adherence to the Portfolio Diet for use in these study designs. Here, for the first time, we have evaluated the association of a Portfolio Diet score with CVD outcomes in the WHI (Women's Health Initiative).
METHODS
Study Population and Design
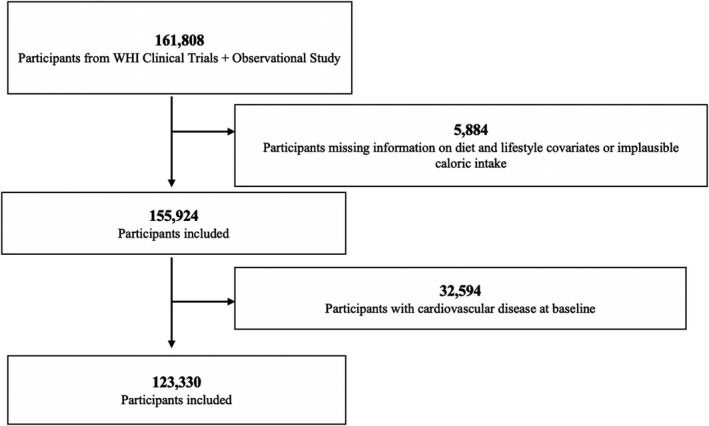
The design and methods of the WHI have been published elsewhere.19, 20, 21 Briefly, between 1993 and 1998, postmenopausal women aged 50 to 79 years were recruited into clinical trials or an observational study (OS) (n=161 808). Recruitment and baseline data collection have been previously reported.20 This analysis includes follow‐up through February 28, 2017. We excluded participants who had a history of CVD at baseline (n=32 594), and missing information regarding diet and lifestyle covariates or implausible caloric intake (<600 kcal or >5000 kcal/day) (n=5884). The final analysis included 123 330 women (Figure 1). The baseline characteristics of participants included or excluded due to missing data from the analysis are shown in Table S1. Written informed consent was obtained from all WHI participants and procedures were approved by institutional review boards at all participating institutions. The WHI data are accessible to qualified researchers trained in human subject confidentiality protocols and requests to access the data set may be sent to the WHI Publications and Presentations Committee.
Figure 1. Flow chart for study sample, WHI (Women's Health Initiative) cohort, 1993 to 2017.
Dietary Assessment
The exposure was diet as measured by a Portfolio Diet score. The foods and nutrients composing this score were self‐reported using the food frequency questionnaire (FFQ) developed and validated for the WHI22, 23 at enrollment and again at year 3 for the OS participants. No further diet assessments were available for the WHI participants. We used a cumulative average score for those who completed the FFQ at baseline and year 3 (Data S1).
Food items on the WHI FFQ that are characteristic of the Portfolio Diet were categorized into 6 components (plant protein, nuts, viscous fiber, phytosterols, MUFAs, and saturated fat/cholesterol sources). Intake was assessed as servings/day of targeted foods in all components except phytosterols, which used all FFQ food items to derive total daily intake (mg/day). For the 6 components, each was scored from 1 (unhealthy) to 5 (healthiest) according to participant's quintile of intake resulting in a score range between 6 and 30, with higher scores indicating higher adherence to the Portfolio Diet. Additional information on the Portfolio Diet score development is provided in Data S1 and Table S2.
Ascertainment of CVD Outcomes
Our primary outcomes included total CVD, coronary heart disease (CHD; defined as clinical myocardial infarction, definite silent myocardial infarction, or a death due to definite CHD or possible CHD), and stroke incidence and death as these CVDs are causally related to high LDL‐C and the Portfolio Diet has an established cholesterol‐lowering effect.8 Total CVD was a composite of nonfatal myocardial infarction, CHD death, stroke, coronary revascularization and incident heart failure (HF).24 Our secondary, or exploratory, outcomes included HF and atrial fibrillation (AF). The outcomes were ascertained in the WHI through self‐reported medical questionnaires completed by participants every 6 to 12 months, depending on study assignment. Medical records and death certificates for all outcomes were reviewed by central physician adjudicators or trained local adjudicators.25
Covariates
Covariates that were included in our models were based on information on the participants' lifestyle and risk factors for CVD assessed at baseline, including age, region in the United States, race/ethnicity, alcohol intake, physical activity, caloric intake, sodium intake, hysterectomy history, body mass index (BMI), hormone therapy use, personal history of hypertension and high cholesterol, family history of CVD and diabetes mellitus, diabetes mellitus or cancer diagnoses, smoking status, education, marital status, and clinical trial/study arm. Detailed descriptions of the validity and reproducibility of baseline measurements have been previously published.21
Statistical Analysis
Baseline characteristics were described by quartile of the Portfolio Diet score using means with SDs for continuous variables and frequencies with percentages for categorical variables. To compare baseline characteristics, χ2 tests were used for categorical variables and analysis of variance for continuous variables.
Participants were categorized into quartiles of the Portfolio Diet score, with the lowest quartile serving as the reference group, as per our prespecified analysis plans. Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% CIs for the association between the Portfolio Diet score quartiles and CVD outcomes. Two multivariable models were used. Covariates commonly examined in studies of dietary pattern scores and CVD risk were included based on our a priori analysis plan. Model 1 was adjusted for age (continuous), region (Northeast, South, Midwest, West), smoking (never, past, current), and study arm (hormone replacement therapy arm, dietary modification arm, calcium and vitamin D) arm). Model 2 was adjusted for model 1+race/ethnicity (White, Black, Hispanic, Asian/Pacific Islander, Other [American Indian, Alaskan Native, other]), education (college or above, below college), marital status (presently married/other), hysterectomy history (yes/no), body mass index (continuous), physical activity (continuous), alcohol intake (>7 drinks/week, <7 drinks/week), energy intake (continuous), cancer status (yes/no), hypertension status (yes/no), diabetes mellitus status (yes/no), sodium intake (continuous), family history of CVD (yes/no), family history of diabetes mellitus (yes/no), postmenopausal hormone use (never, past, current), and cholesterol‐lowering medication use (yes/no). For all covariates, 5% or less of values were missing. When we checked the proportional hazard model assumptions using Schoenfeld residuals method, no violations of the assumption were found.
Tests for linear trend were conducted by assigning the median value to each quartile. Our main analysis (per our protocol) included all WHI participants (clinical trials+OS). We also conducted several sensitivity analyses to test the robustness of our main findings. First, we conducted analyses by restricting the data to the OS participants only as the clinical trials participants have received an intervention and may be different from the OS participants. We also then (1) restricted analyses to the baseline diet only, (2) excluded participants from the dietary modification trial (a low fat diet intervention), as their diet may have changed overtime, (3) excluded CVD events within the first 3 years of follow‐up to address possible reverse causation, (4) excluded those with diabetes mellitus at baseline due to their higher CVD risk, and (5) completed multiple imputation for missing covariate data (using the multivariate imputation by chained equations method).26 We also conducted post hoc sensitivity analyses where we created another Portfolio Diet score based on the recommendations from the Portfolio Diet randomized clinical trials (further details included in Table S3). We then also applied subgroup analyses according to several potential interactive factors (age, body mass index, family history of CVD, race/ethnicity, smoking status, and cholesterol‐lowering medication) and conducted interaction tests via multiplicative interaction terms using model 2 to assess if the P for interactions were significant. Additional analyses we conducted included evaluating associations between the 6 individual components of the Portfolio Diet and risk of the CVD outcomes. Statistical tests were 2‐sided and P<0.05 was considered statistically significant. The statistical analyses were conducted with Stata statistical software (Stata Statistical Software: Release 15., Stata Corp., College Station, TX). Further information on the methods can be found in Data S1.
RESULTS
Lifestyle Characteristics of the Participants
Baseline characteristics by quartiles of the Portfolio Diet score are shown in Table 1. Women with higher scores tended to be older, have a lower body mass index, engage in more physical activity, have a higher education, be less likely to smoke, as well as several other differences. All of these known risk factors at baseline were adjusted for in our analyses. Mean intake of the Portfolio Diet score components is shown in Table 2. The included participants were different from the excluded participants (eg, Black or Hispanic, 10 118 [8.2%] and 4875 [4.0%] versus 1061 [19.0%] and 657 [10.2%], respectively; and above college education, 83 887 [68.5%] versus 3086 [55.9%]) (Table S1).
Table 1.
Baseline Characteristics of 123 330 Participants in the WHI According to Quartiles of the Portfolio Diet Score
| Mean (SD)/No. (%) | Q1 (6–14) | Q2 (14.5–17) | Q3 (17.5–20) | Q4 (20.5–30) | P Value |
|---|---|---|---|---|---|
| Number of participants | 32 403 | 33 713 | 30 755 | 26 459 | |
| Time‐to‐event/censored in years | 14.9 (5.79) | 15.4 (5.67) | 15.5 (5.61) | 15.6 (5.58) | <0.001 |
| Age, y | 62.2 (7.05) | 62.6 (7.07) | 62.9 (7.17) | 63.1 (7.27) | <0.001 |
| Body mass index, kg/m2 | 28.7 (6.08) | 28.0 (5.81) | 27.6 (5.69) | 26.7 (5.54) | <0.001 |
| Recreational physical activity (MET‐h/wk) | 9.60 (12.01) | 11.99 (13.2) | 13.64 (14.15) | 16.77 (15.63) | <0.001 |
| Dietary energy, kcal/d | 1368 (522) | 1577 (603) | 1755 (640) | 1933 (665) | <0.001 |
| Region in the United States | |||||
| Northeast | 9635 (29.7) | 8066 (23.9) | 6331 (20.6) | 4459 (16.9) | <0.001 |
| South | 8261 (25.5) | 8804 (26.1) | 7951 (25.9) | 6360 (24.0) | |
| Midwest | 7794 (24.1) | 8018 (23.8) | 6706 (21.8) | 4588 (17.3) | |
| West | 6713 (20.7) | 8825 (26.2) | 9767 (31.8) | 11 052 (41.8) | |
| Race/ethnicity | |||||
| White | 26 517 (82.0) | 28 582 (85.0) | 26 019 (84.8) | 22 166 (84.0) | <0.001 |
| Black | 3869 (12.0) | 2757 (8.2) | 2106 (6.9) | 1368 (5.3) | |
| Hispanic | 1023 (3.2) | 1229 (3.7) | 1337 (4.4) | 1286 (4.9) | |
| Asian/Pacific Islander | 582 (1.8) | 714 (2.1) | 881 (2.9) | 1213 (4.6) | |
| Alcoholic drinks | |||||
| >7 drinks/wk | 3789 (11.7) | 4217 (12.6) | 3782 (12.3) | 3314 (12.6) | 0.003 |
| Sodium intake, mg/d | 2204 (888) | 2607 (1044) | 2953 (1142) | 3329 (1240) | <0.001 |
| Hormone therapy use | |||||
| Never | 12 055 (38.3) | 10 853 (33.2) | 9592 (32.3) | 7550 (29.5) | <0.001 |
| Past | 7404 (23.5) | 7270 (22.2) | 6334 (21.3) | 5586 (21.8) | |
| Current | 12 021 (38.2) | 14 602 (44.6) | 13 830 (46.5) | 12 500 (48.8) | |
| Hysterectomy ever | 13 230 (40.8) | 13 607 (40.4) | 12 228 (39.8) | 10 107 (38.2) | <0.001 |
| Treated high cholesterol | 3284 (10.8) | 3730 (11.8) | 3450 (12.0) | 3002 (12.0) | <0.001 |
| History of hypertension | 10 396 (32.3) | 10 208 (30.5) | 8981 (29.4) | 7044 (26.8) | <0.001 |
| History of cancer | 2707 (8.4) | 2829 (8.5) | 2680 (8.8) | 2296 (8.8) | 0.223 |
| Family history diabetes mellitus | 10 583 (32.8) | 10 685 (31.8) | 9626 (31.4) | 7770 (29.4) | <0.001 |
| Family history of cardiovascular disease | 20 816 (64.2) | 21 898 (64.9) | 20 248 (65.8) | 17 167 (64.9) | <0.001 |
| Self‐reported diabetes mellitus | 1578 (4.9) | 1603 (4.8) | 1442 (4.7) | 1118 (4.2) | 0.001 |
| Smoking status | |||||
| Never | 15 706 (48.5) | 17 049 (50.6) | 16 253 (52.9) | 14 323 (54.1) | <0.001 |
| Past | 13 281 (50.0) | 142 401 (42.2) | 12 891 (41.9) | 11 166 (42.2) | |
| Current | 3416 (10.5) | 2424 (7.20) | 1611 (5.2) | 970 (3.7) | |
| Education: college or above | 19 165 (59.6) | 22 256 (66.5) | 21 918 (71.8) | 20 548 (78.2) | <0.001 |
| Marital status: present relationship | 19 785 (61.3) | 21 640 (64.5) | 20 032 (654) | 16 946 (64.3) | <0.001 |
| Hormone replacement therapy arm | |||||
| Not randomized | 25 436 (78.6) | 27 904 (82.8) | 25 845 (84.0) | 22 468 (84.9) | <0.001 |
| E‐alone | 1368 (4.2) | 1055 (3.1) | 924 (3.0) | 640 (2.4) | |
| E‐alone control | 1442 (4.5) | 1076 (3.2) | 826 (2.7) | 754 (2.9) | |
| E+P intervention | 2083 (6.4) | 1930 (5.7) | 1653 (5.4) | 1306 (4.9) | |
| E+P control | 2074 (6.4) | 1748 (5.2) | 1507 (4.9) | 1291 (4.9) | |
| Dietary modification arm | |||||
| Not randomized | 20 972 (64.7) | 22 964 (68.1) | 21 349 (69.4) | 18 958 (71.7) | <0.001 |
| Intervention | 4472 (13.8) | 4268 (12.7) | 3867 (12.6) | 2981 (11.3) | |
| Control | 6959 (21.5) | 6481 (19.2) | 5539 (18.0) | 4520 (17.1) | |
| Calcium and vitamin D arm | |||||
| Not randomized | 23 646 (73.0) | 25 738 (76.3) | 23 738 (77.2) | 20 778 (78.5) | <0.001 |
| Intervention | 4398 (13.6) | 3982 (11.8) | 3529 (11.5) | 2865 (10.8) | |
| Control | 4359 (13.5) | 3993 (11.8) | 3477 (11.3) | 2816 (10.6) | |
E+P indicates estrogen plus progestin; E‐alone, estrogen‐alone; Q, quartile; and WHI, Women's Health Initiative
Table 2.
Scoring Criteria for the Portfolio Diet Score From Targeted Foods in Each Component and Mean* Daily Intake† for Each Quintile
| Component | Main Targeted Foods From WHI FFQ‡ | Scoring Criteria | ||||
|---|---|---|---|---|---|---|
| Q1 (1 Point), servings/d | Q2 (2 Points), servings/d | Q3 (3 Points), servings/d | Q4 (4 Points), servings/d | Q5 (5 Points), servings/d | ||
| Plant protein | Soy beverage; green peas; refried beans; all other beans; tofu and textured vegetable products; bean soups | 0.05 | 0.13 | 0.21 | 0.34 | 0.77 |
| Viscous fiber | Oranges, grapefruit and tangerines; apples and pears; strawberries; okra; oats | 0.14 | 0.38 | 0.64 | 0.98 | 1.78 |
| Nuts | Peanut butter, peanuts, other nuts and seeds | 0.00 | 0.04 | 0.10 | 0.23 | 0.62 |
| Phytosterols | Estimated from all plant foods | 133 mg | 191 mg | 236 mg | 288 mg | 404 mg |
| MUFAs | Olive or canola oil; avocado and guacamole | 0.00 | …§ | 0.01 | 0.03 | 0.25 |
| Saturated fat/cholesterol‖ | High fat dairy; eggs; chicken/turkey with skin; red and processed meats; organ meats; gravy; butter | 4.19 | 2.04 | 1.34 | 0.86 | 0.38 |
FFQ indicates food frequency questionnaire; MUFAs, monounsaturated fatty acids; and Q, quintile.
Mean of baseline and year 3 FFQ, when possible.
All components reported as servings/day except for phytosterols (mg/day).
Full list of FFQ food items in Table S1 in the Supplementary Appendix.
Two points not given to any participants based on consumption on MUFAs (low in entire population).
Higher quintiles represent higher intake; however, high intake and high quintiles of saturated fat/cholesterol received lower scores.
Portfolio Diet Score and CVD Outcomes
During an average of 15.3 years of follow‐up, we documented 13 365 incident CVD cases, including 5640 CHD cases, 4400 stroke cases, 1907 HF cases, and 929 AF cases. After adjusting for potential confounders, we observed that women in the top quartile (Q4) of the Portfolio Diet score, compared to those in the bottom quartile (Q1), had an HR of 0.89 (95% CI, 0.83–0.94; P<0.001 for trend) for risk of total CVD, 0.86 (95% CI, 0.78–0.95; P<0.001 for trend) for risk of CHD, and 0.97 (95% CI, 0.87–1.08; P=0.50 for trend) for stroke (Table 3 and Figure 2). For our exploratory outcomes, we observed that women in the top quartile compared to those in the bottom quartile had an HR of 0.83 (95% CI, 0.71–0.99; P=0.01 for trend) for HF and 1.10 (95% CI, 0.87–1.38; P=0.73 for trend) for AF (Table 3 and Figure 2). Absolute incidence rates per 100 000 person‐years among quartiles of adherence are shown in Tables 3 and 4.
Table 3.
Prospective Association of the Portfolio Diet Score With Risk of Cardiovascular Disease Outcomes Among 123 330 Participants in the Women's Health Initiative (CT+OS) (1993–2017)
| Cases/Total | Person‐Years | Incidence Rate (Per 100 000 Person‐Years) | Model 1* (n=123 330) | Model 2† (n=104, 894) | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||||
| Total CVD | |||||||
| Q1 (6–14) | 3872/32 403 | 459 280 | 834 | 1.00 [reference] | 1.00 [reference] | ||
| Q2 (14.5–17) | 3758/33 713 | 493 872 | 760 | 0.92 (0.88–0.96) | <0.001 | 0.97 (0.92–1.02) | 0.259 |
| Q3 (17.5–20) | 3189/30 755 | 456 016 | 699 | 0.84 (0.80–0.88) | <0.001 | 0.91 (0.86–0.96) | 0.001 |
| Q4 (20.5–30) | 2549/26 459 | 396 421 | 643 | 0.77 (0.74–0.82) | <0.001 | 0.89 (0.83–0.94) | <0.001 |
| P trend | <0.001 | ||||||
| CHD | |||||||
| Q1 (6–14) | 1697/32 403 | 474 873 | 357 | 1.00 [reference] | 1.00 [reference] | ||
| Q2 (14.5–17) | 1528/33 713 | 510 244 | 299 | 0.85 (0.80–0.91) | <0.001 | 0.92 (0.85–0.99) | 0.029 |
| Q3 (17.5–20) | 1328/30 755 | 469 623 | 282 | 0.80 (0.74–0.86) | <0.001 | 0.85 (0.78–0.93) | <0.001 |
| Q4 (20.5–30) | 1087/26 459 | 406 937 | 267 | 0.75 (0.69–0.81) | <0.001 | 0.86 (0.78–0.95) | 0.002 |
| P trend | <0.001 | ||||||
| Stroke | |||||||
| Q1 (6–14) | 1192/32 403 | 476 881 | 250 | 1.00 [reference] | 1.00 [reference] | ||
| Q2 (14.5–17) | 1256/33 713 | 511 321 | 246 | 0.99 (0.91–1.07) | 0.811 | 1.03 (0.95–1.13) | 0.449 |
| Q3 (17.5–20) | 1061/30 755 | 471 010 | 225 | 0.89 (0.82–0.97) | 0.008 | 0.97 (0.88–1.07) | 0.545 |
| Q4 (20.5–30) | 892/26 459 | 407 674 | 219 | 0.86 (0.79–0.94) | 0.001 | 0.97 (0.87–1.08) | 0.598 |
| P trend | 0.500 | ||||||
| Heart failure | |||||||
| Q1 (6–14) | 567/32 403 | 479 309 | 118 | 1.00 [reference] | 1.00 [reference] | ||
| Q2 (14.5–17) | 566/33 713 | 513 986 | 110 | 0.96 (0.85–1.08) | 0.493 | 0.97 (0.85–1.11) | 0.704 |
| Q3 (17.5–20) | 450/30 755 | 473 311 | 95 | 0.83 (0.73–0.94) | 0.003 | 0.86 (0.75–0.99) | 0.046 |
| Q4 (20.5–30) | 326/26 459 | 410 002 | 80 | 0.70 (0.61–0.80) | <0.001 | 0.83 (0.71–0.99) | 0.034 |
| P trend | 0.010 | ||||||
| Atrial fibrillation | |||||||
| Q1 (6–14) | 266/32 403 | 482 208 | 55 | 1.00 [reference] | 1.00 [reference] | ||
| Q2 (14.5–17) | 257/33 713 | 517 112 | 50 | 1.04 (0.88–1.24) | 0.635 | 1.06 (0.87–1.28) | 0.547 |
| Q3 (17.5–20) | 212/30 755 | 475 797 | 45 | 0.95 (0.79–1.14) | 0.564 | 0.94 (0.76–1.16) | 0.553 |
| Q4 (20.5–30) | 194/26 459 | 411 730 | 47 | 1.05 (0.87–1.27) | 0.573 | 1.10 (0.87–1.38) | 0.418 |
| P trend | 0.725 | ||||||
Quartile 1 represents the least adherent to the Portfolio Diet, whereas quartile 4 represents the most adherence to the Portfolio Diet. Associations between Portfolio Diet and outcomes were determined by Cox proportional hazard models. Under/over energy reporters and those with baseline CVD were excluded from the analysis. Total CVD is a composite of incidence and death of CHD, stroke, heart failure, and coronary revascularization (coronary artery bypass grafting or percutaneous transluminal coronary angioplasty). CHD indicates coronary heart disease; CT, clinical trial; CVD, cardiovascular disease; HR, hazard ratio; OS, observational study; and Q, quartile.
Model 1 adjusted for age (continuous), region (Northeast, South, Midwest, West), smoking (never, past, current), and study arm (hormone replacement therapy arm, dietary modification arm, calcium and vitamin D arm).
Model 2 adjusted for model 1+race/ethnicity (White, Black, Hispanic, Asian/Pacific Islander, Other [American Indian, Alaskan Native, other]), education (college or above, below college), marital status (presently married/other), hysterectomy history (yes/no), body mass index (continuous), physical activity (continuous), alcohol intake (>7 drinks/week, <7 drinks/week), energy intake (continuous), cancer status (yes/no), hypertension status (yes/no), diabetes mellitus status (yes/no), sodium intake (continuous), family history of CVD (yes/no), family history of diabetes mellitus (yes/no), hormone therapy use (never, past, current), cholesterol‐lowering medication use (yes/no).
Figure 2. Summary of findings of incident cardiovascular disease, coronary heart disease, stroke, heart failure, and atrial fibrillation comparing low to high adherence to the Portfolio Diet.

Hazard ratios (HRs) and for comparing participants in Q1 (low adherence [reference category]) to Q4 (high adherence) of the Portfolio Diet with CVD outcomes in the Women's Health Initiative (Clinical Trials+Observational Study). Multivariate‐adjusted models were adjusted for the following: age, region, smoking, clinical trial study arm, ethnicity, education, marital status, hysterectomy history, body mass index, physical activity, alcohol intake, energy intake, cancer status, hypertension status, diabetes mellitus status, sodium intake, family history of CVD, family history of diabetes mellitus, hormone therapy use, and cholesterol‐lowering medication use. P trend was determined by assigning a median value to each quartile. Horizontal lines represent 95% CIs. CHD indicates coronary heart disease; and CVD, cardiovascular disease.
Table 4.
Prospective Association of the Portfolio Diet Score With Risk of Cardiovascular Disease Outcomes Among 70 506 Participants in the Observational Study of the Women's Health Initiative (1993–2017)
| Cases/Total | Person‐Years | Incidence Rate (Per 100 000 Person‐Years) | Model 1* (n=69 196) | Model 2† (n=60 923) | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | ||||
| Total CVD | |||||||
| Q1 (6–14) | 1721/16 472 | 222 515 | 773 | 1.00 [reference] | 1.00 [reference] | ||
| Q2 (14.5–17) | 1910/19 350 | 274 964 | 694 | 0.89 (0.83–0.95) | <0.001 | 0.94 (0.88–1.01) | 0.091 |
| Q3 (17.5–20) | 1654/18 297 | 264 916 | 624 | 0.79 (0.74–0.84) | <0.001 | 0.86 (0.78–0.93) | <0.001 |
| Q4 (20.5–30) | 1345/16 387 | 241 091 | 558 | 0.71 (0.66–0.76) | <0.001 | 0.85 (0.78–0.93) | <0.001 |
| P trend | <0.001 | ||||||
| CHD | |||||||
| Q1 (6–14) | 745/16 472 | 229 456 | 325 | 1.00 [reference] | 1.00 [reference] | ||
| Q2 (14.5–17) | 752/19 350 | 283 605 | 265 | 0.79 (0.72–0.88) | <0.001 | 0.87 (0.78–0.97) | 0.012 |
| Q3 (17.5–20) | 681/18 297 | 271 936 | 250 | 0.74 (0.66–0.82) | <0.001 | 0.81 (0.72–0.91) | <0.001 |
| Q4 (20.5–30) | 577/16 387 | 246 743 | 234 | 0.69 (0.61–0.77) | <0.001 | 0.82 (0.71–0.93) | 0.003 |
| P trend | 0.002 | ||||||
| Stroke | |||||||
| Q1 (6–14) | 496/16 472 | 230 524 | 215 | 1.00 [reference] | 1.00 [reference] | ||
| Q2 (14.5–17) | 631/19 350 | 283 869 | 222 | 0.99 (0.88–1.20) | 0.922 | 1.05 (0.92–1.19) | 0.448 |
| Q3 (17.5–20) | 533/18 297 | 272 608 | 196 | 0.86 (0.76–0.97) | 0.015 | 0.93 (0.81–1.07) | 0.310 |
| Q4 (20.5–30) | 446/16 387 | 247 169 | 180 | 0.78 (0.69–0.89) | <0.001 | 0.92 (0.78–1.08) | 0.320 |
| P trend | 0.224 | ||||||
| Heart failure | |||||||
| Q1 (6–14) | 292/16 472 | 231 297 | 126 | 1.00 [reference] | 1.00 [reference] | ||
| Q2 (14.5–17) | 300/19 350 | 285 357 | 105 | 0.88 (0.74–1.03) | 0.114 | 0.88 (0.73–1.05) | 0.156 |
| Q3 (17.5–20) | 233/18 297 | 273 961 | 85 | 0.71 (0.59–0.85) | <0.001 | 0.75 (0.62–0.92) | 0.005 |
| Q4 (20.5–30) | 193/16 387 | 248 275 | 78 | 0.67 (0.56–0.81) | <0.001 | 0.80 (0.64–1.00) | 0.053 |
| P trend | 0.009 | ||||||
| Atrial fibrillation | |||||||
| Q1 (6–14) | 20/16 472 | 232 957 | 9 | 1.00 [reference] | 1.00 [reference] | ||
| Q2 (14.5–17) | 24/19 350 | 287 170 | 8 | 0.89 (0.49–1.62) | 0.704 | 1.33 (0.67–2.56) | 0.401 |
| Q3 (17.5–20) | 14/18 297 | 275 243 | 5 | 0.57 (0.29–1.13) | 0.108 | 0.87 (0.40–1.94) | 0.764 |
| Q4 (20.5–30) | 18/16 387 | 249 464 | 7 | 0.80 (0.41–1.54) | 0.510 | 1.33 (0.60–2.94) | 0.484 |
| P trend | 0.749 | ||||||
Quartile 1 represents the least adherent to the Portfolio Diet, whereas quartile 4 represents the most adherence to the Portfolio Diet. Associations between Portfolio Diet and outcomes were determined by Cox proportional hazard models. Under/over energy reporters and those with baseline CVD were excluded from the analysis. Total CVD is a composite of incidence and death of CHD, stroke, heart failure, and coronary revascularization (coronary artery bypass grafting or percutaneous transluminal coronary angioplasty). CHD indicates coronary heart disease; CVD, cardiovascular disease; HR, hazard ratio; and Q, quartile.
Model 1 adjusted for age (continuous), region (Northeast, South, Midwest, West), and smoking (never, past, current).
Model 2 adjusted for model 1+ethnicity (White, Black, Hispanic, Asian/Pacific Islander, Other [American Indian, Alaskan Native, other]), education (college or above, below college), marital status (presently married/other), hysterectomy history (yes/no), boyd mass index (continuous), physical activity (continuous), alcohol intake (>7 drinks/week, <7 drinks/week), energy intake (continuous), cancer status (yes/no), hypertension status (yes/no), diabetes mellitus status (yes/no), sodium intake (continuous), family history of CVD (yes/no), family history of diabetes mellitus (yes/no), hormone therapy use (never, past, current), cholesterol‐lowering medication use (yes/no).
Sensitivity Analyses
The associations between the Portfolio Diet score and CVD outcomes remained similar in all sensitivity analyses (in the OS participants only [Table 4], and baseline diet only, excluding participants from the dietary modification trial, excluding CVD events within the first 3 years of follow‐up, excluding those with diabetes mellitus, and completing multiple imputation for missing covariate data [Table S4]). For HF, however, after excluding events diagnosed in the first 3 years, the association was slightly attenuated and no longer significant (Table S4). The association between the Portfolio Diet score based on the randomized clinical trials recommendations and CVD outcomes were attenuated and no longer significant for some outcomes; however, patterns were similar to our original a priori analysis (Table S3).
Subgroup Analyses
The results remained largely consistent in each of the subgroup analyses, apart from effect modification by smoking status and CHD (Figures S1 through S5).
Individual Component Analyses
When we individually assessed the 6 components of the Portfolio Diet score with the CVD outcomes, higher intakes of nuts, phytosterols, and MUFAs and lower intake of saturated fat sources had inverse associations with total CVD. Phytosterols and low saturated fat sources had inverse associations with CHD and phytosterols had inverse associations with stroke. Nuts also had an inverse association with HF (Table S5).
Discussion
In this large prospective cohort study of US postmenopausal women, a higher Portfolio Diet score was associated with a 11% and 14% lower risk of total CVD and CHD, respectively, but no association was seen with stroke. These findings remained consistent across all sensitivity analyses, including when we excluded the WHI clinical trial participants, highlighting the robustness of our results. There was also a strong linear trend for greater adherence to the Portfolio Diet with total CVD and CHD. For our secondary analysis, there was an association of a 17% lower risk of HF with a higher Portfolio Diet score, but no association was seen with AF. The true benefits of the Portfolio Diet on CVD risk reduction, however, are likely underestimated in the current study.
Interpretation of Results and Implications
These findings are consistent with the Portfolio Diet trial evidence assessing effects on intermediate risk factors for CVD. The Portfolio Diet has been shown to result in clinically meaningful reductions in the lipid targets for CVD prevention (LDL‐C, non‐high‐density lipoprotein cholesterol, ApoB), as well as CRP, with smaller reductions in blood pressure.8 In particular, LDL‐C, the primary risk factor that the Portfolio Diet was designed to reduce, is considered causal in the pathogenesis of atherosclerotic CVD based on evidence from cardiovascular outcomes trials involving 3 different classes of drugs (statins, ezetimibe, and PCSK9 inhibitors), Mendelian randomization studies and prospective cohorts.27 Our strongest finding of a 14% inverse association with CHD is consistent with these lines of evidence and closely reflects the predicted 10‐year CHD risk reduction of 13% estimated in our systematic review and meta‐analysis of the Portfolio Diet trials.8 The 0.73 mmol/L reduction in LDL‐C that corresponds to this 13% reduction in the Portfolio Diet trials is predicted by the regression line for the observed risk reduction per mmol/L of LDL‐C seen within the updated analyses of the CTT (Cholesterol Treatment Trialists) collaboration.28
We are unaware of other studies examining the association of a Portfolio Diet with CVD events. The individual components of the Portfolio Diet, however, have been associated with lower rates of CVD events in prospective cohorts. Systematic reviews and meta‐analyses have shown that consumption of legumes,13 dietary fiber including viscous fiber sources,14 nuts,15 and MUFAs16 are associated with reductions in CVD events, and consumption of foods high in saturated fat (such as red and processed meats) are associated with an increased risk of CVD.17 The inverse association of increasing phytosterol intake from natural sources with CVD risk in our study, however, was not shown in an earlier study.29
The Portfolio Diet also shows similar results to other recognized dietary patterns for CVD prevention, such as the Dietary Approaches to Stop Hypertension, vegetarian, Nordic, and Mediterranean diets, which share important overlap in core foods (nuts, legumes, whole grains, fruit/vegetable sources, and/or monounsaturated fat).18, 30, 31, 32, 33 Systematic reviews and meta‐analyses of prospective cohort studies and large individual cohort studies have shown the Dietary Approaches to Stop Hypertension diet is associated with a 20% (95% CI, 0.76–0.85 HRs) reduction in CVD and a 21% reduction (0.71–0.88) in CHD incidence,30 whereas Nordic and vegetarian diets are associated with 29% (0.65–0.78) and 22% (0.69–0.88) reductions in CVD and CHD mortality, respectively.31, 33 Similarly, the PREDIMED trial, a large randomized cardiovascular outcomes trial of the effect of a Mediterranean diet supplemented with either extra virgin olive oil or nuts compared with a low‐fat diet, found reductions in major vascular events of 31% (0.53–0.91) and 28% (0.54–0.95), respectively.18
Dietary patterns have also shown similar results specifically within the WHI. Higher adherence to the Healthy Eating Index 2010, Alternative Healthy Eating Index 2010, Alternate Mediterranean and Dietary Approaches to Stop Hypertension diets have been associated with 18% to 26% lower CVD mortality risk in the OS participants,34 which falls within the 95% CIs (HR, 0.85; 95% CI, 0.78–0.93) of our findings for total CVD comparing lowest to highest adherence of the Portfolio Diet score in these participants. The 30% reduction in HF associated with higher adherence to the Alternative Healthy Eating Index24 also falls within the 95% CIs (HR, 0.80; 95% CI, 0.64–0.99) of our findings for the Portfolio Diet in the OS participants.
Unlike some other dietary patterns, adherence to the Portfolio Diet was not associated with a reduction in stroke in our study. Both the Mediterranean and Dietary Approaches to Stop Hypertension diets have shown inverse associations with stroke.30, 32 Although the Portfolio Diet resulted in a reduction in blood pressure in the randomized trials,8 this effect may not be strong enough to translate into an association with lower stroke risk, given that the reductions were small and hypertension is the most important risk factor for stroke.35 The larger reductions in LDL‐C and other lipid targets, as well as CRP, may be more relevant for the inverse associations seen with CHD and total CVD than with stroke. AF is also a major risk factor for stroke,36 and we did not observe a significant association with lower AF risk in our study.
These findings highlight the plant‐based Portfolio Diet as another dietary therapeutic approach for CVD prevention, alongside other dietary patterns recommended for CVD prevention. As adherence is one of the most critical determinants for attaining the benefits of any diet, as recognized by cardiovascular clinical practice guidelines,9 the Portfolio Diet may best fit with the values and preferences of some patients and allow them to achieve the greatest adherence long term. The Portfolio Diet also has a small ecological footprint, emphasizing plant‐based components with low environmental impact (eg, legumes, oats, barley, temperate fruit, etc).37, 38 Given increasing public concerns regarding ethical and environmental impact of food,39, 40 healthcare professionals will likely have more patients interested in this dietary pattern.
Strengths and Limitations
Strengths of our study include the prospective cohort design, large sample size, and long follow‐up for incident CVD events. Nevertheless, this study does have limitations. First, our study included only 1 or 2 assessments of diet, and diet was self‐reported. Second, the population included health‐conscious postmenopausal women and therefore the results may not be generalizable to men or other populations; however, the Portfolio Diet trials were conducted in both men and postmenopausal women and benefits were seen in both sexes. Third, causation cannot be established because of the observational design, and residual confounding also cannot be ruled out. Lastly, consumption of many of the Portfolio Diet components remained low, particularly plant protein and MUFAs, even in the top quintiles. A few of the Portfolio Diet foods, such as some viscous fiber sources (eg, barley), were also not included on the FFQ. This finding was further highlighted in our post hoc sensitivity analysis where we created a Portfolio Diet score based on the recommendations of the Portfolio Diet trials. No participants in the WHI received the maximum amount of points possible, and maximum points suggested ≈50% adherence to the trial recommendations, with an average estimated adherence of ≈22%. These adherence estimations are, however, likely underestimated given the nature of FFQs and their inability to determine absolute intake of diets. Taken together, we expect that the associations are likely underestimated, and a stronger association with CVD events may be seen with greater consumption of the Portfolio Diet components. This low adherence reflects an important opportunity for individuals to achieve cardiovascular benefits of the Portfolio Diet. Typical dietary patterns in North America and Europe do not meet the targets for plant protein, viscous fiber, nuts, phytosterols, and MUFAs of the Portfolio Diet,41, 42, 43, 44, 45, 46 and therefore public health initiatives that focus on the components of the diet may improve cardiovascular outcomes globally. It will be of great interest to apply this Portfolio Diet score to other populations, particularly in those that consume greater amounts of the diet components, to assess if similar or stronger associations with incident CVD events are found.
Conclusions
Greater adherence to the plant‐based Portfolio Diet score was significantly associated with lower risk of total CVD, CHD, and HF in postmenopausal women. These findings provide the strongest evidence to date on the long‐term benefits of a Portfolio Diet in the primary prevention of CVD, although our Portfolio Diet score needs to be assessed in other cohorts/populations to confirm these findings. Evidence from randomized trials with clinical CVD events is also needed. In this regard, we await the results of the PortfolioEX trial (ClinicalTrials.gov Identifier: NCT02481466) of the effect of the Portfolio Diet plus exercise on a surrogate marker of atherosclerotic CVD risk (magnetic resonance imaging of atherosclerosis [plaque volume]). In the meantime, our results support the Portfolio Diet as another therapeutic dietary approach for managing CVD risk that fits with current guidelines emphasizing plant‐based diets.
Sources of Funding
The Women's Health Initiative (WHI) was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, and U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600002C, HHSN268201600003C, HHSN268201600004C, and R01DK125403 (SL). Glenn was supported by the Nora Martin Fellowship in Nutritional Sciences, the Banting & Best Diabetes Centre Tamarack Graduate Award in Diabetes Research, the Peterborough K.M. Hunter Charitable Foundation Graduate Award and an Ontario Graduate Scholarship. Sievenpiper was funded by a Diabetes Canada Clinician Scientist Award. Lo was supported by Start‐up Fund for RAPs under the Strategic Hiring Scheme (Grant number: BD8H). Funders had no role in the study design, the collection, analysis and interpretation of data, the writing of the report, and the decision to submit the article for publication.
Disclosures
A.J.G. received funding from the Nora Martin Fellowship in Nutritional Sciences, Banting & Best Diabetes Centre Tamarack Graduate Award in Diabetes Research, the Peterborough K.M. Hunter Charitable Foundation Graduate Award, and the Ontario Graduate Scholarship. She has received consulting fees from Solo GI Nutrition and has received an honorarium from the Soy Nutrition Institute. D.J.A.J. has received research grants from Saskatchewan & Alberta Pulse Growers Associations, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever Canada and Netherlands, Barilla, the Almond Board of California, Agriculture and Agri‐food Canada, Pulse Canada, Kellogg's Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd., Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit Council (INC), Soy Foods Association of North America, the Coca‐Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Soy Nutrition Institute (SNI), the Canola and Flax Councils of Canada, the Calorie Control Council, the Canadian Institutes of Health Research (CIHR), the Canada Foundation for Innovation (CFI)and the Ontario Research Fund (ORF). He has received in‐kind supplies for trials as a research support from the Almond board of California, Walnut Council of California, the Peanut Institute, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, WhiteWave Foods. He has been on the speaker's panel, served on the scientific advisory board and/or received travel support and/or honoraria from 2020 China Glycemic Index (GI) International Conference, Atlantic Pain Conference, Academy of Life Long Learning, the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca‐Cola Company, Epicure, Danone, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, True Health Initiative (THI), Heali AI Corp, Institute of Food Technologists (IFT), Soy Nutrition Institute (SNI), Herbalife Nutrition Institute (HNI), Saskatchewan & Alberta Pulse Growers Associations, Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamentals for Health (NFH), Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, Abbott Laboratories, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi‐Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, Agri‐Culture and Agri‐Food Canada, the Canadian Agri‐Food Policy Institute, Pulse Canada, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra‐Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael's Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, Alexandra L Jenkins, is a director and partner of INQUIS Clinical Research for the Food Industry, his 2 daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the foods described here, The Portfolio Diet for Cardiovascular Risk Reduction (Academic Press/Elsevier 2020 ISBN:978‐0‐12‐810510‐8)and his sister, Caroline Brydson, received funding through a grant from the St. Michael's Hospital Foundation to develop a cookbook for one of his studies. A.J.H. received independent investigator‐initiated research funding from Dairy Farmers of Canada. C.W.C.K. has received research support from the Advanced Foods and Materials Network, Agricultural Bioproducts Innovation Program through the Pulse Research Network, Agriculture and Agri‐Food Canada, Almond Board of California, Barilla, Calorie Control Council, Canadian Institutes of Health Research, Canola Council of Canada, The International Tree Nut Council Nutrition Research & Education Foundation, Kellogg, Loblaw Companies Ltd., Pulse Canada, Saskatchewan Pulse Growers, and Unilever. He has received consultant fees from American Pistachio Growers; speaker fees from Tate & Lyle and The WhiteWave Foods Company; and travel funding from Sabra Dipping Company, Tate & Lyle, International Tree Nut Council Research & Education Foundation, California Walnut Commission, Sun‐Maid, The Peanut Institute, General Mills, Oldways Foundation and International Nut and Dried Fruit Council Foundation. He is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the European Association for the Study of Diabetes. He is a member of the International Carbohydrate Quality Consortium, Secretary of the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes, and a director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. J.L.S. has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, PSI Foundation, Banting and Best Diabetes Centre (BBDC), American Society for Nutrition (ASN), INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, National Honey Board (the U.S. Department of Agriculture [USDA] honey “Checkoff” program), International Life Sciences Institute (ILSI), Pulse Canada, Quaker, The United Soybean Board (the USDA soy “Checkoff” program), The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), and The Nutrition Trialists Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received in‐kind food donations to support a randomized controlled trial from the Almond Board of California, California Walnut Commission, Peanut Institute, Barilla, Unilever/Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, WhiteWave Foods/Danone, and Nutrartis. He has received travel support, speaker fees and/or honoraria from Diabetes Canada, Dairy Farmers of Canada, FoodMinds LLC, International Sweeteners Association, Nestlé, Pulse Canada, Canadian Society for Endocrinology and Metabolism (CSEM), GI Foundation, Abbott, General Mills, Biofortis, ASN, Northern Ontario School of Medicine, INC Nutrition Research & Education Foundation, European Food Safety Authority (EFSA), Comité Européen des Fabricants de Sucre (CEFS), Nutrition Communications, International Food Information Council (IFIC), Calorie Control Council, and Physicians Committee for Responsible Medicine. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, Wirtschaftliche Vereinigung Zucker e.V., Danone, and Inquis Clinical Research. He is a member of the European Fruit Juice Association Scientific Expert Panel and Soy Nutrition Institute (SNI) Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves or has served as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of ILSI North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of AB InBev. There are no other relationships or activities that could appear to have influenced the submitted work.
Supporting information
Data S1
Appendix S1
Tables S1–S5
Figures S1–S5
Acknowledgments
We thank all participants in the WHI clinical trials and cohort for their invaluable contribution. We also thank volunteer Chloe Kavcic and researchers Drs Isabelle Sioen and Joline Beulens (EPIC‐NL) for their contributions to the phytosterol database developed for this study.
Author contributions: A.J.G., K.L., S.L., and J.L.S. had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. A.J.G., K.L., D.J.A.J., B.A.B., A.J.H., C.W.C.K., S.L., J.L.S. designed the study. A.J.G., K.L., and S.L. performed the statistical analyses. A.J.G. drafted the article. S.L. and J.L.S. supervised the project. All authors contributed to the data interpretation and revised each draft for important intellectual content. All authors read and approved the final article.
This article was sent to Holli A. DeVon, PhD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021515
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Simin Liu, Email: simin_liu@brown.edu.
John L. Sievenpiper, Email: john.sievenpiper@utoronto.ca.
REFERENCES
- 1.Jenkins DJA, Kendall CWC, Faulkner D, Vidgen E, Trautwein EA, Parker TL, Marchie A, Koumbridis G, Lapsley KG, Josse RG, et al. A dietary portfolio approach to cholesterol reduction: combined effects of plant sterols, vegetable proteins, and viscous fibers in hypercholesterolemia. Metabolism. 2002;51:1596–1604. DOI: 10.1053/meta.2002.35578. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins DJA, Kendall CWC, Marchie A, Faulkner D, Vidgen E, Lapsley KG, Trautwein EA, Parker TL, Josse RG, Leiter LA, et al. The effect of combining plant sterols, soy protein, viscous fibers, and almonds in treating hypercholesterolemia. Metabolism. 2003;52:1478–1483. DOI: 10.1016/S0026-0495(03)00260-9. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins DJ, Kendall CW, Marchie A, Faulkner DA, Wong JM, de Souza R , Emam A, Parker TL, Vidgen E, Lapsley KG, et al. Effects of a dietary portfolio of cholesterol‐lowering foods vs lovastatin on serum lipids and c‐reactive protein. JAMA. 2003;290:502–510. DOI: 10.1001/jama.290.4.502. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins DJA, Kendall CWC, Faulkner DA, Nguyen T, Kemp T, Marchie A, Wong JMW, de Souza R , Emam A, Vidgen E, et al. Assessment of the longer‐term effects of a dietary portfolio of cholesterol‐lowering foods in hypercholesterolemia. Am J Clin Nutr. 2006;83:582–591. DOI: 10.1093/ajcn.83.3.582. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins DJA, Jones PJH, Lamarche B, Kendall CWC, Faulkner D, Cermakova L, Gigleux I, Ramprasath V, de Souza R , Ireland C, et al. Effect of a dietary portfolio of cholesterol‐lowering foods given at 2 levels of intensity of dietary advice on serum lipids in hyperlipidemia: a randomized controlled trial. JAMA. 2011;306:831–839. DOI: 10.1001/jama.2011.1202. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins DJ, Chiavaroli L, Wong JM, Kendall C, Lewis GF, Vidgen E, Connelly PW, Leiter LA, Josse RG, Lamarche B. Adding monounsaturated fatty acids to a dietary portfolio of cholesterol‐lowering foods in hypercholesterolemia. CMAJ. 2010;182:1961–1967. DOI: 10.1503/cmaj.092128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Expert Panel on Detection E, Treatment of High Blood Cholesterol in A . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–2497. DOI: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 8.Chiavaroli L, Nishi SK, Khan TA, Braunstein CR, Glenn AJ, Mejia SB, Rahelić D, Kahleová H, Salas‐Salvadó J, Jenkins DJA, et al. Portfolio dietary pattern and cardiovascular disease: a systematic review and meta‐analysis of controlled trials. Prog Cardiovasc Dis. 2018;61:43–53. DOI: 10.1016/j.pcad.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, Francis GA, Genest J Jr, Grover S, Gupta M, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263–1282. DOI: 10.1016/j.cjca.2016.07.510. [DOI] [PubMed] [Google Scholar]
- 10.Diabetes Canada Clinical Practice Guidelines Expert C , Sievenpiper JL, Chan CB, Dworatzek PD, Freeze C, Williams SL. Nutrition therapy. Can J Diabetes. 2018;42(suppl 1):S64–S79. DOI: 10.1016/j.jcjd.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, et al.; European Atherosclerosis Society Consensus P . Statin‐associated muscle symptoms: impact on statin therapy‐European Atherosclerosis Society consensus panel statement on assessment, aetiology and management. Eur Heart J. 2015;36:1012–1022. DOI: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heart U.K . The cholesterol charity: portfolio diet. 2014.
- 13.Viguiliouk E, Glenn AJ, Nishi SK, Chiavaroli L, Seider M, Khan T, Bonaccio M, Iacoviello L, Mejia SB, Jenkins DJA, et al. Associations between dietary pulses alone or with other legumes and cardiometabolic disease outcomes: an umbrella review and updated systematic review and meta‐analysis of prospective cohort studies. Adv Nutr. 2019;10:S308–S319. DOI: 10.1093/advances/nmz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veronese N, Solmi M, Caruso MG, Giannelli G, Osella AR, Evangelou E, Maggi S, Fontana L, Stubbs B, Tzoulaki I. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta‐analyses. Am J Clin Nutr. 2018;107:436–444. DOI: 10.1093/ajcn/nqx082. [DOI] [PubMed] [Google Scholar]
- 15.Mayhew AJ, de Souza RJ , Meyre D, Anand SS, Mente A. A systematic review and meta‐analysis of nut consumption and incident risk of cvd and all‐cause mortality. Br J Nutr. 2016;115:212–225. DOI: 10.1017/S0007114515004316. [DOI] [PubMed] [Google Scholar]
- 16.Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta‐analysis of cohort studies. Lipids Health Dis. 2014;13:154. DOI: 10.1186/1476-511X-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeraatkar D, Han MA, Guyatt GH, Vernooij RWM, El Dib R, Cheung K, Milio K, Zworth M, Bartoszko JJ, Valli C, et al. Red and processed meat consumption and risk for all‐cause mortality and cardiometabolic outcomes: a systematic review and meta‐analysis of cohort studies. Ann Intern Med. 2019;171:703–710. DOI: 10.7326/M19-0655. [DOI] [PubMed] [Google Scholar]
- 18.Estruch R, Ros E, Salas‐Salvadó J, Covas M‐I, Corella D, Arós F, Gómez‐Gracia E, Ruiz‐Gutiérrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra‐virgin olive oil or nuts. N Engl J Med. 2018;378:e34. DOI: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 19.Prentice RL, Furberg C, Johnson S, Henderson M, Cummings S, Manson J, Freedman L, Oberman A, Kuller L, Anderson G. Design of the whi clinical trial and observational study. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 20.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–S77. DOI: 10.1016/S1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 21.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. DOI: 10.1016/S1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 22.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs‐Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. DOI: 10.1016/S1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 23.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data‐based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. DOI: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 24.Belin RJ, Greenland P, Allison M, Martin L, Shikany JM, Larson J, Tinker L, Howard BV, Lloyd‐Jones D, Van Horn L. Diet quality and the risk of cardiovascular disease: the Women's Health Initiative (WHI). Am J Clin Nutr. 2011;94:49–57. DOI: 10.3945/ajcn.110.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curb JDavid, Mctiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx‐Burns L, Pastore L, Criqui M, et al. Outcomes ascertainment and adjudication methods in the women's health initiative. Ann Epidemiol. 2003;13:S122–128. DOI: 10.1016/S1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 26.van Buuren S . Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. DOI: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 27.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, et al. Low‐density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. DOI: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ference BA, Cannon CP, Landmesser U, Luscher TF, Catapano AL, Ray KK. Reduction of low density lipoprotein‐cholesterol and cardiovascular events with proprotein convertase subtilisin‐kexin type 9 (PCSK9) inhibitors and statins: an analysis of FOURIER, SPIRE, and the Cholesterol Treatment Trialists Collaboration. Eur Heart J. 2018;39:2540–2545. DOI: 10.1093/eurheartj/ehx450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ras RT, van der Schouw YT , Trautwein EA, Sioen I, Dalmeijer GW, Zock PL, Beulens JW. Intake of phytosterols from natural sources and risk of cardiovascular disease in the European Prospective Investigation into Cancer and Nutrition‐the Netherlands (EPIC‐NL) population. Eur J Prev Cardiol. 2015;22:1067–1075. DOI: 10.1177/2047487314554864. [DOI] [PubMed] [Google Scholar]
- 30.Chiavaroli L, Viguiliouk E, Nishi S, Blanco Mejia S, Rahelić D, Kahleová H, Salas‐Salvadó J, Kendall C, Sievenpiper J. Dash dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta‐analyses. Nutrients. 2019;11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glenn AJ, Viguiliouk E, Seider M, Boucher BA, Khan TA, Blanco Mejia S, Jenkins DJA, Kahleová H, Rahelić D, Salas‐Salvadó J, et al. Relation of vegetarian dietary patterns with major cardiovascular outcomes: a systematic review and meta‐analysis of prospective cohort studies. Front Nutr. 2019;6:80. DOI: 10.3389/fnut.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becerra‐Tomas N, Blanco Mejia S, Viguiliouk E, Khan T, Kendall CWC, Kahleova H, Rahelic D, Sievenpiper JL, Salas‐Salvado J. Mediterranean diet, cardiovascular disease and mortality in diabetes: a systematic review and meta‐analysis of prospective cohort studies and randomized clinical trials. Crit Rev Food Sci Nutr. 2020;60:1207–1227. DOI: 10.1080/10408398.2019.1565281. [DOI] [PubMed] [Google Scholar]
- 33.Lassale C, Gunter MJ, Romaguera D, Peelen LM, Van der Schouw YT, Beulens JWJ, Freisling H, Muller DC, Ferrari P, Huybrechts I, et al. Diet quality scores and prediction of all‐cause, cardiovascular and cancer mortality in a pan‐European cohort study. PLoS One. 2016;11:e0159025. DOI: 10.1371/journal.pone.0159025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.George SM, Ballard‐Barbash R, Manson JE, Reedy J, Shikany JM, Subar AF, Tinker LF, Vitolins M, Neuhouser ML. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women's Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol. 2014;180:616–625. DOI: 10.1093/aje/kwu173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao‐Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet. 2010;376:112–123. DOI: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 36.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. DOI: 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 37.Sranacharoenpong K, Soret S, Harwatt H, Wien M, Sabate J. The environmental cost of protein food choices. Public Health Nutr. 2015;18:2067–2073. DOI: 10.1017/S1368980014002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritchie H. You want to reduce the carbon footprint of your food? Focus on what you eat, no whether your food is local. 2020.
- 39.Fox N, Ward K. Health, ethics and environment: a qualitative study of vegetarian motivations. Appetite. 2008;50:422–429. DOI: 10.1016/j.appet.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Tilman D, Clark M. Global diets link environmental sustainability and human health. Nature. 2014;515:518–522. DOI: 10.1038/nature13959. [DOI] [PubMed] [Google Scholar]
- 41.Viguiliouk E, Stewart S, Jayalath V, Ng A, Mirrahimi A, de Souza R , Hanley A, Bazinet R, Blanco Mejia S, Leiter L, et al. Effect of replacing animal protein with plant protein on glycemic control in diabetes: a systematic review and meta‐analysis of randomized controlled trials. Nutrients. 2015;7:9804–9824. DOI: 10.3390/nu7125509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mudryj AN, Aukema HM, Yu N. Intake patterns and dietary associations of soya protein consumption in adults and children in the Canadian Community Health Survey, Cycle 2.2. Br J Nutr. 2015;113:299–309. DOI: 10.1017/S0007114514003638. [DOI] [PubMed] [Google Scholar]
- 43.Klingberg S, Andersson H, Mulligan A, Bhaniani A, Welch A, Bingham S, Khaw KT, Andersson S, Ellegard L. Food sources of plant sterols in the EPIC Norfolk population. Eur J Clin Nutr. 2008;62:695–703. DOI: 10.1038/sj.ejcn.1602765. [DOI] [PubMed] [Google Scholar]
- 44.Klingberg S, Ellegard L, Johansson I, Hallmans G, Weinehall L, Andersson H, Winkvist A. Inverse relation between dietary intake of naturally occurring plant sterols and serum cholesterol in northern Sweden. Am J Clin Nutr. 2008;87:993–1001. DOI: 10.1093/ajcn/87.4.993. [DOI] [PubMed] [Google Scholar]
- 45.Jenkins DJA, Kendall CWC, Popovich DG, Vidgen E, Mehling CC, Vuksan V, Ransom TPP, Rao AV, Rosenberg‐Zand R, Tariq N, et al. Effect of a very‐high‐fiber vegetable, fruit, and nut diet on serum lipids and colonic function. Metabolism. 2001;50:494–503. DOI: 10.1053/meta.2001.21037. [DOI] [PubMed] [Google Scholar]
- 46.Guasch‐Ferre M, Liu G, Li Y, Sampson L, Manson JE, Salas‐Salvado J, Martinez‐Gonzalez MA, Stampfer MJ, Willett WC, Sun Q, et al. Olive oil consumption and cardiovascular risk in U.S. adults. J Am Coll Cardiol. 2020;75:1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–1271. [PubMed] [Google Scholar]
- 48.National Institute for Health and Welfare . Finnish food composition database. 2018.
- 49.US Department of Agriculture . USDA national nutrient database for standard reference. 2018.
- 50.Jimenez‐Escrig A, Santos‐Hidalgo AB, Saura‐Calixto F. Common sources and estimated intake of plant sterols in the Spanish diet. J Agric Food Chem. 2006;54:3462–3471. DOI: 10.1021/jf053188k. [DOI] [PubMed] [Google Scholar]
- 51.Normén L, Ellegård L, Brants H, Dutta P, Andersson H. A phytosterol database: fatty foods consumed in Sweden and the Netherlands. J Food Compos Anal. 2007;20:193–201. DOI: 10.1016/j.jfca.2006.06.002. [DOI] [Google Scholar]
- 52.Normen L, Bryngelsson S, Johnsson M. The phytosterol content of some cereal foods commonly consumed in Sweden and the Netherlands. J Food Compos Anal. 2002;15:693–704. [Google Scholar]
- 53.Phillips KM, Ruggio DM, Ashraf‐Khorassani M. Phytosterol composition of nuts and seeds commonly consumed in the United States. J Agric Food Chem. 2005;53:9436–9445. DOI: 10.1021/jf051505h. [DOI] [PubMed] [Google Scholar]
- 54.Irwin ML, McTiernan A, Manson JE, Thomson CA, Sternfeld B, Stefanick ML, Wactawski‐Wende J, Craft L, Lane D, Martin LW, et al. Physical activity and survival in postmenopausal women with breast cancer: results from the Women's Health Initiative. Cancer Prev Res (Phila). 2011;4:522–529. DOI: 10.1158/1940-6207.CAPR-10-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, Gaziano JM, Frishman WH, Curb JD. Comparison of self‐report, hospital discharge codes, and adjudication of cardiovascular events in the women's health initiative. Am J Epidemiol. 2004;160:1152–1158. DOI: 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
- 56.Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ. Blood pressure and risk of developing type 2 diabetes mellitus: the Women's Health Study. Eur Heart J. 2007;28:2937–2943. DOI: 10.1093/eurheartj/ehm400. [DOI] [PubMed] [Google Scholar]
- 57.Margolis KL, Lihong Q, Brzyski R, Bonds DE, Howard BV, Kempainen S, Simin L, Robinson JG, Safford MM, Tinker LT, et al. Validity of diabetes self‐reports in the Women's Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5:240–247. DOI: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Appendix S1
Tables S1–S5
Figures S1–S5


