Abstract
Background
Dietary vitamin K (K1 and K2) may reduce atherosclerotic cardiovascular disease (ASCVD) risk via several mechanisms. However, studies linking vitamin K intake with incident ASCVD are limited. We aimed to determine the relationship between dietary vitamin K intake and ASCVD hospitalizations.
Methods and Results
In this prospective cohort study, participants from the Danish Diet, Cancer, and Health Study, with no prior ASCVD, completed a food‐frequency questionnaire at baseline and were followed up for hospital admissions of ASCVD; ischemic heart disease, ischemic stroke, or peripheral artery disease. Intakes of vitamin K1 and vitamin K2 were estimated from the food‐frequency questionnaire, and their relationship with ASCVD hospitalizations was determined using Cox proportional hazards models. Among 53 372 Danish citizens with a median (interquartile range) age of 56 (52–60) years, 8726 individuals were hospitalized for any ASCVD during 21 (17–22) years of follow‐up. Compared with participants with the lowest vitamin K1 intakes, participants with the highest intakes had a 21% lower risk of an ASCVD‐related hospitalization (hazard ratio, 0.79; 95% CI: 0.74–0.84), after multivariable adjustments for relevant demographic covariates. Likewise for vitamin K2, the risk of an ASCVD‐related hospitalization for participants with the highest intakes was 14% lower than participants with the lowest vitamin K2 intake (hazard ratio, 0.86; 95% CI, 0.81–0.91).
Conclusions
Risk of ASCVD was inversely associated with diets high in vitamin K1 or K2. The similar inverse associations with both vitamin K1 and K2, despite very different dietary sources, highlight the potential importance of vitamin K for ASCVD prevention.
Keywords: atherosclerotic cardiovascular disease, dietary vitamin K, phylloquinone, menaquinone, primary prevention, prospective cohort study
Subject Categories: Cardiovascular Disease, Epidemiology, Diet and Nutrition, Primary Prevention
Nonstandard Abbreviations and Acronyms
- DNPR
Danish National Patient Register
- FFQ
food‐frequency questionnaire
- IHD
ischemic heart disease
- VKA
vitamin K antagonist
Clinical Perspective
What Is New?
Dietary vitamin K1 and vitamin K2 intake is nonlinearly inversely associated with risk of atherosclerotic cardiovascular disease events.
This relationship is true for all subtypes of atherosclerotic cardiovascular disease, ischemic heart disease, ischemic stroke, and peripheral artery disease.
What Are the Clinical Implications?
Dietary vitamin K intake may be considered in evaluating risk of atherosclerotic cardiovascular disease.
Sufficient vitamin K intake may be protective of atherosclerotic cardiovascular disease events.
Further research is needed to determine at‐risk individuals who may benefit from increased dietary vitamin K intake or vitamin K supplementation.
Atherosclerotic cardiovascular disease (ASCVD) remains a leading cause of death worldwide despite the availability of risk‐modifying therapies and lifestyle advice.1 The pathogenesis of ASCVD is multifactorial and is, in part, influenced by inflammation,2 impaired hemostasis,3 metabolic syndrome (including dysglycemia, dyslipidemia, and obesity),4 and arterial calcification.5 These mechanisms are possible targets for dietary and pharmaceutical interventions aimed at reducing ASCVD risk.
Vitamin K occurs in 2 forms: Vitamin K1 (phylloquinone) is found in all photosynthetic plants, with green leafy vegetables being the primary dietary source, while vitamin K2 (menaquinones K4‐K10) is primarily bacterially derived and is found predominantly in fermented foods, such as cheese.6 Both forms of vitamin K have been proposed to influence cardiovascular health through many mechanisms,7 including the reduction of systemic inflammation,8 maintenance of hemostasis,9 and the inhibition of arterial calcification.10
Observational studies have demonstrated an inverse relationship between biomarkers of dietary vitamin K intake and ASCVD risk,11, 12, 13, 14 and supplementing with vitamin K increases circulating concentrations of these biomarkers15, 16 in a dose‐dependent fashion.17 These observations and others18 suggest a causal role of vitamin K in the prevention of ASCVD. However, observational studies linking dietary vitamin K1 intake with ASCVD risk in US men and women have been inconclusive,19, 20 and observed inverse relationships are often attributed to the underlying cardioprotective dietary patterns associated with high vitamin K1 intake (eg, higher vegetable intake). In Dutch cohorts, high dietary vitamin K2 intake is associated with lower ASCVD risk21, 22; this finding requires validation in other populations. Two recent meta‐analyses have described an increased risk of all‐cause mortality14 and coronary heart disease18 with vitamin K deficiency as defined by serum biomarkers or dietary intake, respectively. Further research is needed to validate these observations and better understand the effect of vitamin K deficiency on specific ASCVD subtypes.
We aimed to investigate whether dietary intakes of vitamin K1 and vitamin K2 were associated with total ASCVD hospitalizations and hospitalizations for subtypes of ASCVD, namely, ischemic heart disease (IHD), ischemic stroke, and peripheral artery disease (PAD), in Danish individuals free of ASCVD at baseline.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality may be sent to the Diet, Cancer, and Health Steering Committee at the Danish Cancer Society.
Study Population
Between 1993 and 1997, the Danish Diet, Cancer, and Health Study, an associated cohort of the European Prospective Investigation Into Nutrition and Cancer (EPIC),23 recruited 57 053 residents of the greater areas of Copenhagen and Aarhus, between the ages of 50 and 65 years. Of these, 56 468 completed a food‐frequency questionnaire (FFQ) and had no prevalent cancer at the time of recruitment. Using the Danish civil registration system, which assigns a unique and permanent number for all Danish residents, participants were able to be linked to the following registries on an individual level: (1) the Civil Registration System,24 containing data on age, sex, emigration, and vital status; (2) the Integrated Database for Labour Market Research,25 containing information on annual income since 1980; (3) the Danish National Prescription Registry, containing information on all filled prescriptions since 199426; and (4) the Danish National Patient Register (DNPR)27 containing information on date of hospital admissions since 1978, with 1 primary diagnosis and ≥1 secondary diagnoses defined by the International Classification of Diseases (ICD) using the Eighth Revision (ICD‐8) until 1993 and the Tenth Revision (ICD‐10) from 1994. In the DNPR, procedures and surgeries have been recorded since 1996 using Nordic Classification of Surgical Procedures codes.
All participants provided informed consent as part of the Danish Diet, Cancer, and Health Study to search information from medical registers.23 In the Danish Diet, Cancer, and Health Study, the procedures followed were in accordance with the ethical standards of the responsible institutional or regional committee on human experimentation. In Denmark, register studies do not require approval from the ethics committees. This study was approved by the Danish Data Protection Agency (Reference no. 2012‐58‐0004 I‐Suite nr: 6357, VD‐2018‐117).
In the present study, the main exclusion criteria included prevalent ASCVD (n=2484), use of a vitamin K antagonist (VKA; n=220), missing or implausible values for covariates (n=196), or implausible energy intakes (n=196) (Figure 1). Prevalent ASCVD was defined by self‐reported myocardial infarction (n=223), prior diagnosis of IHD (ICD‐8: 410–412, 414; ICD‐10: I21–I25; n=1303), self‐reported ischemic stroke (n=422), prior diagnosis of ischemic stroke (ICD‐8: 433–434; ICD‐10: I63; n=199), or prior diagnosis of PAD (ICD‐8: 440–444; ICD‐10: I70–I74; n=337) at baseline (1993–1997). Implausible energy intakes were defined as <2092 kJ/d (<500 kcal/d) and >20 920 kJ/d (>5000 kcal/d) and VKAs are defined using Anatomical Therapeutic Chemical code B01AA.
Figure 1. Consolidated Standards of Reporting Trials flow diagram.
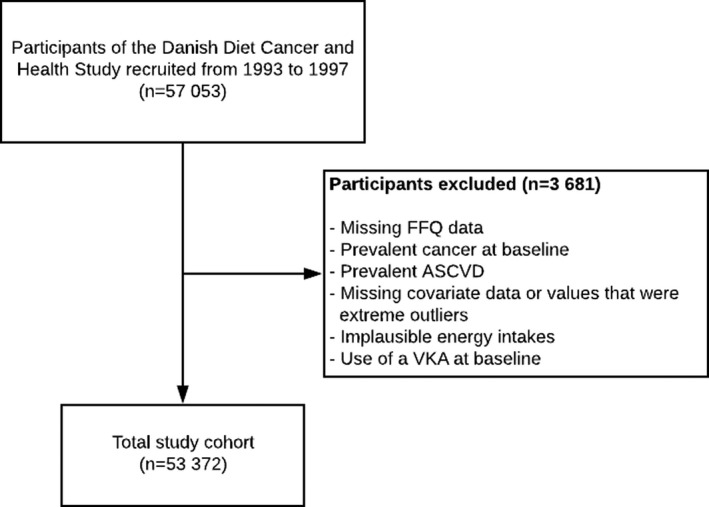
ASCVD indicates atherosclerotic cardiovascular disease; FFQ, food‐frequency questionnaire; and VKA, vitamin K antagonist.
Exposures
Exposures were intakes of vitamin K1 and vitamin K2 (menaquinones K‐4 to K‐10). These were estimated from dietary data, collected using a validated 192‐item self‐administered, semiquantitative FFQ mailed out to participants before their visit to 1 of the 2 study centers.28, 29 The FFQ was validated against 2 times 7 days of weighed diet records29 where correlations between mean calorie‐adjusted intakes from the weighed diet records and the FFQ were calculated for macro‐ and micronutrients. On average, 70% of the subjects were classified in the same (±1) quintile for macro‐ and micronutrient intakes when comparing the 2 different methods of capturing diet. On the FFQ, participants were asked to indicate their usual frequency of intake for different food and beverage items over the previous 12 months, using a 12‐category frequency scale that ranged from never to ≥8 times per day.22 Estimates of the vitamin K1 content of each food and beverage in the FFQ were derived from the Frida Food Data database30 and, where a vitamin K1 value was not available, the US Department of Agriculture31 nutrient database. Estimates of the vitamin K2 content of each food and beverage in the FFQ were derived from Schurgers et al (menaquinones K‐4 to K‐932), and Manoury et al (menaquinone K‐1033), with total vitamin K2 intake calculated by summing each of the individual menaquinones, as done previously.34 Total dietary vitamin K intake was estimated by multiplying the food/beverage item consumed (g/d) by the mean vitamin K value (μg/g). A value of 0 µg/g was given to all foods and beverages where no value was available (vitamin K1, n=44; menaquinones K‐4 to K‐9, n=78; menaquinone K‐10, n=160). Information on the exact values used and assumptions made will be provided upon reasonable request to the corresponding author.
Study Outcomes
The primary outcome was a combined end point of first‐time ASCVD hospitalization, defined as hospitalization for IHD, ischemic stroke, or PAD. Secondary outcomes were a first‐time hospitalization for IHD, ischemic stroke hospitalizations, and PAD hospitalizations, individually. Reasons for hospitalization were determined using ICD‐10 codes for primary or secondary diagnoses (IHD, ICD‐10: I21–I25; ischemic stroke, ICD‐10: I63; PAD, ICD‐10: I70–I74]. These ICD codes have previously been validated to varying degrees in the DNPR35, 36, 37, 38 and have positive predictive values of ≈92% to 97% for IHD,34 ≈81% to 85% for ischemic stroke,35 and ≈81% for PAD.36 Percutaneous coronary interventions (PCI; Nordic Classification of Surgical Procedures codes KFNG00, FNG02, FNG05, FNG10, FNG12, and FNG96) and coronary artery bypass grafting (CABG; Nordic Classification of Surgical Procedures codes KFNA, KFNB, KFNC, KFND, KFNE, and KFNH20) were investigated using a landmark analysis; as the procedure codes of coronary interventions were not recorded before 1996, PCI and CABG were not included within the primary outcome. In this landmark analysis, participants who died, had an ASCVD event, or were prescribed a VKA before this date (January 1, 1996) were excluded (n=177 excluded; n=53 195 remaining in analysis). Diagnoses of unstable angina or transient ischemic attack were not included as outcomes as they lack validity in the DNPR.
Validated Case Analysis
To strengthen our findings, we reexamined associations using only medically reviewed and validated cases of ASCVD to verify registry‐based outcomes. Patients with an ICD‐10 discharge code for ischemic stroke or PAD up until 2009, or myocardial infarction up until 2013, registered as a primary or secondary diagnosis, were considered as possible cases to be reviewed (Table S1). Methods of case validation have been published previously.35, 36, 37, 38, 39 Due to a prior diagnosis of validated ASCVD, a further 6 participants were excluded in this analysis (n=53 366).
Covariates
Information on sex, age, education, smoking habits, alcohol consumption, and daily activity was obtained from self‐administered questionnaires completed by participants upon enrollment in the study. Dietary data were obtained from the semiquantitative FFQ described above. Annual income, averaged over the 5 years immediately preceding study enrollment, was used as a proxy for socioeconomic status and was defined as household income after taxation and interest, for the value of the Danish currency in 2015. ICD‐8 and ICD‐10 codes were used for diagnosis of chronic kidney disease (ICD‐8: 580–584; ICD‐10: N02–N08, N11–N12, N14, N18–N19, N26, N158–N160, N162–N164, N168, Q61, E102, E112, E132, E142, I120, M321B), chronic obstructive pulmonary disease (ICD‐8: 491–493; ICD‐10: J42–J44), heart failure (ICD‐8: 4270–4271; ICD‐10: I42, I50, I110, J81), atrial fibrillation (ICD‐8: 42793–42794; ICD‐10: I48), and cancers (ICD‐8: 140–209; ICD‐10: C00–C99). For diabetes mellitus, only self‐reported data were used because of the low validity of ICD codes in DNPR.39 Information regarding use of antihypertensive medication and statins at study enrollment was obtained from a combination of self‐reported use and Anatomical Therapeutic Chemical codes in the Danish National Prescription Registry: prescriptions of statins (Anatomical Therapeutic Chemical code C10AA), or antihypertensive medication (Anatomical Therapeutic Chemical codes described in Table S2), claimed within 180 days before enrollment in the study, were identified from 1994. Presence of hypertension at baseline was defined as either self‐reported hypertension or a claimed prescription of ≥2 antihypertensive medications within 180 days before study enrollment, which has a positive predictive value of 80.0% and a specificity of 94.7% to predict hypertension.40 Presence of hypercholesterolemia was defined using self‐reported data, and anthropometric measurements were taken and total blood cholesterol was measured at the study centers.
Statistical Analysis
Participants’ time to event was based on a maximum of 23 years of follow‐up from the date of enrollment until the date of death, emigration, ASCVD hospitalization (first hospitalization for the combined end point), or end of follow‐up (August 2017), whichever came first. Cox proportional hazards models with restricted cubic splines were used to investigate nonlinear relationships between vitamin K intakes and all time‐to‐event outcomes. All hazard ratios (HRs) and 95% CIs were obtained from the model with the exposure fitted as a continuous variable through a restricted cubic spline (4 knots); HR estimates are reported for the median intake in each quintile, with the first quintile median as the reference point, and are also graphed over a fine grid of x values. For visual simplicity, the graphs of HRs derived from the fitting of cubic splines had x‐axis values restricted to intakes within 3 SDs of the mean. Cox proportional hazards assumptions were tested using log‐log plots of the survival function versus time and assessed for parallel appearance, with no violation found. We tested for nonlinearity using a chi‐squared test to compare nested models. The relationship between ASCVD and all continuous covariates, except vitamin K, was assessed for linearity, and in the case of violation, covariates were transformed to be categorical. As our aim was to obtain relative estimates for risk factors, all deaths were censored rather than treated as a competing risk.41 Five models of adjustment were used: Model 1a (minimally adjusted) included age and sex; model 1b (multivariable‐adjusted) included age, sex, body mass index, smoking status, physical activity, alcohol intake, socioeconomic status (income), and education; model 2 (covariates that are both potential confounders and potential intermediates on the causal pathway) included all covariates in model 1b plus hypertension, hypercholesterolemia, and prevalent disease (diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, and cancer; entered into the model separately); model 3a (multivariable‐adjusted including potential dietary confounders) included all covariates in model 1b plus energy and intakes of fish, red meat, processed food, polyunsaturated fatty acids, monounsaturated fatty acids, and saturated fatty acids; model 3b adjusted for all covariates in model 3a plus vitamin K1 or vitamin K2, as appropriate. Covariates were chosen a priori using knowledge of potential confounders of vitamin K intake and ASCVD. We tested for interactions between vitamin K intake (quintiles) and sex using chi‐squared tests to compare nested models. To understand whether an association between vitamin K1 and ASCVD is independent of total vegetable intake, we stratified our analysis by tertiles of total vegetable intake. To assess the likelihood of residual confounding, we used a falsification end point that we considered likely to be affected by diet but unlikely to be causally affected by vitamin K intake: colorectal cancer (ICD‐10: C18–C21; ICD‐8: 153–154). We performed 2 sensitivity analyses. In the first sensitivity analysis, participants were censored if prescribed a VKA during follow‐up, as these may affect the potential relationship between vitamin K intake and ASCVD, and such prescriptions may have been accompanied by recommendations for dietary change. In a second sensitivity analysis, with PCI and CABG as a combined outcome, a landmark analysis was performed with the landmark date of January 1, 1996. Analyses were undertaken using STATA/IC 14.2 (StataCorp LLC) and R statistics (R Core Team, 2019).
Results
This population of 53 372 Danish citizens, with a median (interquartile range) age of 56 (52–60) years at entry, had a median (interquartile range) follow‐up of 21 (17–22) years. During 944 247 person‐years of follow‐up, 8726 individuals were hospitalized for ASCVD, 5290 for IHD, 2913 for ischemic stroke, and 1856 for PAD. Furthermore, 9476 participants died without a prior hospitalization for ASCVD and <0.3% of participants were lost to follow‐up.
Baseline Characteristics
At baseline, the median (interquartile range) intake of vitamin K1 was 113.8 µg/d (80.7–151.2) and vitamin K2 was 43.7 µg/day (31.1–61.5); intakes of the 2 forms of vitamin K were weakly correlated (Spearman’s rho=0.25). Compared with participants in the lowest quintile of vitamin K1 intake, those in the highest quintile were more likely to be more physically active, have never smoked, have a higher degree of education, have a higher income, have a higher total energy intake, and have an overall healthier underlying dietary pattern, eating more fish, fiber, vegetables, and fruit (Table 1). For vitamin K2 intake, compared with participants in the lowest‐intake quintile, those in the highest‐intake quintile were more likely to be men, be more physically active, smoke, have a higher total energy intake, and have an overall unhealthier underlying dietary pattern, eating more red meat, processed meat, and saturated fat (Table 1). The main dietary sources of vitamin K1 were margarine, lettuce, broccoli, whole‐meal bread, and spinach, while eggs, butter, and hard cheeses were the main dietary sources of vitamin K2 (Table S3).
Table 1.
Baseline Characteristics of Study Population
|
Total Population N=53 372 |
Total Vitamin K1 Intake Quintiles | Total Vitamin K2 Intake Quintiles | |||
|---|---|---|---|---|---|
|
Quintile 1 n=10 675 |
Quintile 5 n=10 675 |
Quintile 1 n=10 675 |
Quintile 5 n=10 675 |
||
| Total vitamin K1 intake, µg/d | 113.8 [80.7–151.2] | 57 [4–73] | 192 [162–800] | … | … |
| Total vitamin K2 intake, µg/d | 43.7 [31.1–61.5] | … | … | 23 [0–29] | 77 [65–296] |
| Sex, male | 24 826 (46.5) | 4845 (45.4) | 5022 (47.0) | 4033 (37.8) | 5725 (53.6) |
| Age, y | 56 [52–60] | 56 [52–60] | 55 [52–60] | 56 [52–60] | 55 [52–60] |
| BMI, kg/m2 | 26 [23–28] | 26 [24–29] | 25 [23–27] | 25 [23–28] | 26 [23–28] |
| MET score | 56 [37–84] | 50 [32–77] | 64 [42–92] | 52 [34–78] | 61 [40–91] |
| Smoking status | |||||
| Never | 19 195 (36.0) | 2995 (28.1) | 4242 (39.7) | 4088 (38.3) | 3624 (33.9) |
| Former | 15 140 (28.4) | 2471 (23.1) | 3484 (32.6) | 2835 (26.6) | 3173 (29.7) |
| Current | 19 037 (35.7) | 5209 (48.8) | 2949 (27.6) | 3752 (35.1) | 3878 (36.3) |
| Education | |||||
| ≤7 y | 17 225 (32.3) | 4709 (44.1) | 2294 (21.5) | 3512 (32.9) | 3516 (32.9) |
| 8–10 y | 24 767 (46.4) | 4779 (44.8) | 4754 (44.5) | 5117 (47.9) | 4651 (43.6) |
| ≥11 y | 11 357 (21.3) | 1182 (11.1) | 3622 (33.9) | 2040 (19.1) | 2503 (23.4) |
| Mean household income | |||||
| ≤394 700 DKK/y | 13 010 (24.4) | 3468 (32.5) | 2305 (21.6) | 2816 (26.4) | 2807 (26.3) |
| 394 701–570 930 DKK/y | 13 255 (24.8) | 3016 (28.3) | 2272 (21.3) | 2602 (24.4) | 2722 (25.5) |
| 570 931–758 297 DKK/y | 13 441 (25.2) | 2500 (23.4) | 2408 (22.6) | 2525 (23.7) | 2685 (25.2) |
| >758 297 DKK/y | 13 666 (25.6) | 1691 (15.8) | 3690 (34.6) | 2732 (25.6) | 2461 (23.1) |
| Hypertensive | 8288 (15.5) | 1741 (16.3) | 1529 (14.3) | 1793 (16.8) | 1543 (14.5) |
| Hypercholesterolemic | 3373 (6.3) | 681 (6.4) | 618 (5.8) | 943 (8.8) | 507 (4.7) |
| Comorbidities | |||||
| Diabetes mellitus | 992 (1.9) | 158 (1.5) | 309 (2.9) | 159 (1.5) | 319 (3.0) |
| Heart failure | 78 (0.1) | 23 (0.2) | 12 (0.1) | 18 (0.2) | 15 (0.1) |
| Atrial fibrillation | 165 (0.3) | 43 (0.4) | 28 (0.3) | 36 (0.3) | 31 (0.3) |
| COPD | 744 (1.4) | 208 (1.9) | 135 (1.3) | 150 (1.4) | 155 (1.5) |
| CKD | 176 (0.3) | 30 (0.3) | 26 (0.2) | 32 (0.3) | 39 (0.4) |
| Cancer | 224 (0.4) | 51 (0.5) | 35 (0.3) | 49 (0.5) | 47 (0.4) |
| Medication use | |||||
| Insulin treated | 330 (0.6) | 43 (0.4) | 129 (1.2) | 44 (0.4) | 123 (1.2) |
| Antihypertensive | 6079 (11.4) | 1290 (12.1) | 1107 (10.4) | 1295 (12.1) | 1105 (10.4) |
| Statin | 621 (1.2) | 126 (1.2) | 122 (1.1) | 202 (1.9) | 76 (0.7) |
| HRT, % of women | |||||
| Never | 15 524 (54.4) | 3144 (53.9) | 3038 (53.7) | 3612 (54.4) | 2740 (55.4) |
| Current | 8594 (30.2) | 1702 (29.2) | 1747 (30.9) | 1992 (30.0) | 1466 (29.6) |
| Former | 4397 (15.4) | 973 (16.7) | 865 (15.3) | 1030 (15.5) | 739 (14.9) |
| NSAID | 17 045 (32.1) | 3377 (31.9) | 3404 (32.0) | 3386 (31.9) | 3478 (32.8) |
| Aspirin | 6468 (12.1) | 1378 (12.9) | 1242 (11.6) | 1274 (11.9) | 1402 (13.1) |
| Dietary characteristics | |||||
| Energy, kj |
9493 [7849–11 362] |
7879 [6552–9457] |
11 105 [9440–13040] |
7670 [6408–9119] |
11 561 [9850–13 492] |
| Total fish intake, g/d | 38 [25–55] | 28 [18–41] | 49 [33–68] | 31 [20–44] | 44 [29–64] |
| Red meat intake, g/d | 78 [56–107] | 69 [51–93] | 83 [58–117] | 64 [46–87] | 91 [66–124] |
| Processed meat intake, g/d | 24 [14–40] | 24 [14–40] | 23 [12–39] | 18 [10–29] | 31 [18–50] |
| Dietary fiber intake, g/d | 20 [16–25] | 14 [12–17] | 27 [23–32] | 17 [13–22] | 24 [20–29] |
| Saturated FA, g/d | 31 [24–39] | 27 [21–35] | 35 [27–44] | 22 [17–28] | 42 [35–51] |
| Polyunsaturated FA, g/d | 13 [10–17] | 10 [8–12] | 17 [13–22] | 10 [8–13] | 16 [13–21] |
| Monounsaturated FA, g/d | 27 [21–35] | 23 [18–30] | 31 [24–39] | 20 [16–25] | 35 [28–43] |
| Fruit intake, g/d | 172 [95–282] | 104 [47–183] | 251 [156–386] | 149 [76–257] | 193 [114–319] |
| Vegetable intake, g/d | 162 [105–231] | 73 [52–100] | 287 [231–354] | 133 [81–199] | 191 [129–266] |
| Alcohol intake, g/d | 13 [6–31] | 12 [4–32] | 13 [6–30] | 11 [4–27] | 13 [6–31] |
Data expressed as median [IQR] or n (%), unless otherwise stated.
BMI indicates body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DKK, Danish Krone; FA, fatty acid; HRT, hormone replacement therapy; MET, metabolic equivalent; and NSAID, nonsteroidal anti‐inflammatory drug.
Associations Between Vitamin K1 Intake and ASCVD Hospitalizations
The association between vitamin K1 intake and incident ASCVD was nonlinear (P for nonlinearity <0.001); the steepness of the inverse association started to decrease at ≈100 µg/d (Figure 2). Compared with participants in quintile 1, participants in quintile 5 had a 21% lower risk of an ASCVD‐related hospitalization (HR, 0.79; 95% CI, 0.74–0.84) after multivariable adjustments (Model 1b; Table 2). For ASCVD subtypes, participants in quintile 5 had a 14% lower risk of a hospitalization for IHD (HR, 0.86; 95% CI, 0.80–0.93), a 17% lower risk of hospitalization for an ischemic stroke (HR, 0.83; 95% CI, 0.75–0.91), and a 34% lower risk of a PAD‐related hospitalization (HR, 0.66; 95% CI, 0.58–0.75), after multivariable adjustments (model 1b) and compared with participants in quintile 1. A clear association between vitamin K1 intake and ASCVD remained after adjusting for potential dietary confounders (model 3a), including vitamin K2 intake (model 3b). The association between vitamin K1 and ASCVD was not modified by sex (P for interaction=0.533).
Figure 2. Hazard ratios from Cox proportional hazards model with restricted cubic spline curves describing the association between vitamin K1 intake (µg/d) and both total atherosclerotic cardiovascular disease (ASCVD) hospitalizations and subtypes of ASCVD hospitalizations (ischemic heart disease, peripheral artery disease, and ischemic stroke).
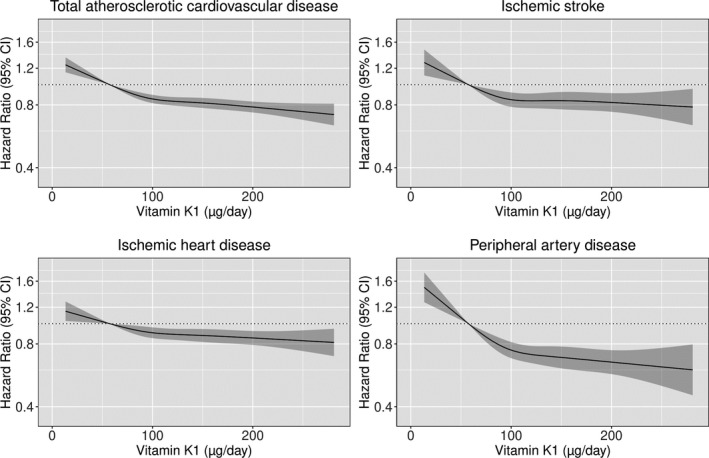
Hazard ratios are based on models adjusted for age, sex, body mass index, smoking status, social economic status (income), physical activity, alcohol intake, and education (model 1b), and are comparing the specific level of vitamin K1 intake (horizontal axis) to the median intake for participants in the lowest‐intake quintile (57 µg/d).
Table 2.
Hazard Ratios of Cardiovascular Disease Hospitalizations by Quintiles of Vitamin K Intake
| Vitamin K Intake Quintiles | |||||
|---|---|---|---|---|---|
|
Quintile 1 n=10 675 |
Quintile 2 n=10 674 |
Quintile 3 n=10 675 |
Quintile 4 n=10 674 |
Quintile 5 n=10 674 |
|
| Vitamin K1 | |||||
| No. events | 2142 | 1863 | 1689 | 1526 | 1506 |
| Intake (µg/d)* |
57 [4–73] |
88 [73–101] |
113.8 [101–127] |
142 [127–162] |
192 [162–800] |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.81 (0.78–0.84) | 0.73 (0.69–0.76) | 0.68 (0.65–0.72) | 0.63 (0.60–0.67) |
| Model 1b | ref. | 0.88 (0.85–0.92) | 0.84 (0.80–0.88) | 0.82 (0.78–0.87) | 0.79 (0.74–0.84) |
| Model 2 | ref. | 0.88 (0.85–0.92) | 0.84 (0.80–0.88) | 0.82 (0.77–0.86) | 0.78 (0.73–0.82) |
| Model 3a | ref. | 0.89 (0.85–0.93) | 0.85 (0.81–0.90) | 0.85 (0.79–0.91) | 0.83 (0.76–0.91) |
| Model 3b | ref. | 0.89 (0.85–0.93) | 0.86 (0.81–0.90) | 0.85 (0.79–0.91) | 0.83 (0.76–0.91) |
| Vitamin K2 | |||||
| No. events | 1774 | 1746 | 1831 | 1616 | 1759 |
| Intake, µg/d* |
23 [0–29] |
33 [29–38] |
44 [38–51] |
58 [51–65] |
77 [65–296] |
| HR (95% CI) | |||||
| Model 1a | ref. | 0.98 (0.94–1.02) | 0.93 (0.88–0.97) | 0.86 (0.82–0.91) | 0.87 (0.83–0.92) |
| Model 1b | ref. | 0.98 (0.94–1.02) | 0.93 (0.89–0.98) | 0.86 (0.82–0.91) | 0.86 (0.81–0.91) |
| Model 2 | ref. | 0.99 (0.96–1.03) | 0.95 (0.90–0.99) | 0.88 (0.83–0.93) | 0.87 (0.82–0.92) |
| Model 3a | ref. | 0.98 (0.94–1.02) | 0.94 (0.89–0.99) | 0.89 (0.84–0.95) | 0.90 (0.84–0.97) |
| Model 3b | ref. | 0.98 (0.95–1.03) | 0.95 (0.90–1.00) | 0.89 (0.84–0.95) | 0.91 (0.84–0.97) |
Hazard ratios (95% CI) for atherosclerotic cardiovascular disease hospitalizations during 23 years of follow‐up, obtained from restricted cubic splines based on Cox proportional hazards models, comparing the median intake in quintiles 2–5, to the median intake in quintile 1. Model 1a adjusted for age and sex; model 1b adjusted for age, sex, body mass index, smoking status, physical activity, alcohol intake, social economic status (income), and education; model 2 adjusted for all covariates in model 1b plus hypertension, hypercholesterolemia, and prevalent disease (diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease, and cancer; entered into the model separately); Model 3a adjusted for all covariates in model 1b plus energy and intakes of fish, red meat, processed food, polyunsaturated fatty acids, monounsaturated fatty acids, and saturated fatty acids; Model 3b adjusted for all covariates in model 3a plus vitamin K1 or vitamin K2, as appropriate.
Median; range in parentheses (all such values).
Associations Between Vitamin K2 Intake and ASCVD Hospitalizations
The association between total vitamin K2 intake and incident ASCVD hospitalizations was also nonlinear (P for nonlinearity <0.001; Figure 3). After multivariable adjustments (model 1b) and compared with participants in quintile 1, participants in quintile 5 had a 14% lower risk of ASCVD (HR, 0.86; 95% CI, 0.81–0.91) and, more specifically, a 14% lower risk of a hospitalization for IHD (HR, 0.86; 95% CI, 0.80–0.92), a 13% lower risk of a stroke hospitalization (HR, 0.87; 95% CI, 0.79–0.95), and a 12% lower risk of a PAD‐related hospitalization HR, 0.88; 95% CI, 0.78–0.99). An association between vitamin K2 intake and ASCVD remained after adjusting for potential dietary confounders (model 3a), including vitamin K1 intake (model 3b). The association between vitamin K2 and ASCVD was not modified by sex (P for interaction=0.593).
Figure 3. Hazard ratios from Cox proportional hazards model with restricted cubic spline curves describing the association between vitamin K2 intake (µg/d) and both total atherosclerotic cardiovascular disease (ASCVD) hospitalizations and subtypes of ASCVD hospitalizations (ischemic heart disease, peripheral artery disease, and ischemic stroke).
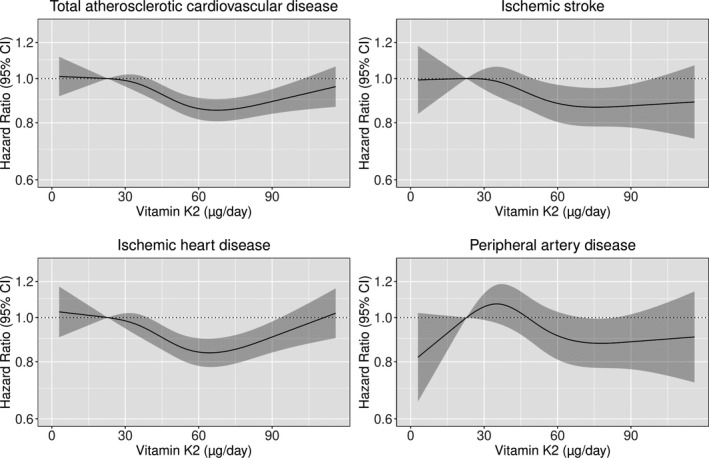
Hazard ratios are based on models adjusted for age, sex, body mass index, smoking status, social economic status (income), physical activity, alcohol intake, and education (model 1b), and are comparing the specific level of vitamin K2 intake (horizontal axis) to the median intake for participants in the lowest‐intake quintile (23 µg/d).
Stratified Analyses
To try to understand whether vitamin K1 intake is only a marker of higher vegetable intake (Spearman’s rho for correlation between vitamin K1 intake and total vegetable intake=0.82), we stratified our analysis by tertiles of total vegetable intake. Compared with those with a low vitamin K1 intake 1 (quintile 1), those with a high vitamin K1 intake had a lower risk of ASCVD hospitalizations across each tertile of total vegetable intake; lowest tertile HR quintile 5 vs quintile 1, 0.74; 95% CI, 0.55–0.99)], middle tertile (HR quintile 5 vs quintile 1, 0.61 (95% CI, 0.50–0.75)] and highest tertile (HR quintile 5 vs quintile 1, 0.73; 95% CI, 0.58–0.93) (Table S4).
Validated Case Analysis
Using only validated cases, 3463 participants were hospitalized for any ASCVD, 2488 were hospitalized for an myocardial infarction, 920 for an ischemic stroke, and 816 for PAD. For vitamin K1 intake, compared with participants in quintile 1 and after multivariable adjustments (model 1b), participants in quintile 5 had a 22% lower risk of stroke (HR, 0.78; 95% CI, 0.65–0.94), a nonsignificant 9% lower risk of an myocardial infarction (HR, 0.91; 95% CI, 0.81–1.02), and a 34% lower risk of having PAD (HR, 0.66; 95% CI, 0.54–0.80) (Figure S1). For vitamin K2 intake, compared with participants in quintile 1 and after multivariable adjustments (model 1b), participants in quintile 5 had a 16% lower risk of an myocardial infarction (HR, 0.84; 95% CI, 0.76–0.93), and a 16% lower risk of having PAD (HR, 0.84; 95% CI, 0.70–1.01). Vitamin K2 intake was not significantly associated with validated stroke cases (Figure S1).
Sensitivity Analyses
Censoring participants upon the prescription of a VKA did not change the association between vitamin K1 or vitamin K2 intakes and ASCVD (Figures S2 and S3). For vitamin K1 intake, compared with participants in quintile 1 and after multivariable adjustments (model 1b), participants in quintile 5 had a 12% lower risk of having a PCI or CABG procedure during follow‐up (HR, 0.88; 95% CI, 0.80, 0.96) (Figure S4). For vitamin K2 intake, participants in quintile 5 had a 14% lower risk of having a PCI or CABG procedure during follow‐up (HR, 0.86; 95% CI, 0.78–0.94).
Falsification End Point Analysis
In total, 1716 participants were hospitalised for colorectal cancer over the study period. There was no significant association between intakes of either vitamin K1 or vitamin K2 and our falsification end point, colorectal cancer (Table S5).
Discussion
In this large prospective cohort study of Danish individuals, we observed that both vitamin K1 and vitamin K2 intakes were independently, inversely, and nonlinearly associated with ASCVD hospitalizations. The association between both forms of vitamin K and ASCVD hospitalizations was present for all ASCVD subtypes.
In the present study, participants in the highest vitamin K1 intake quintile (median 192 μg/d) had a 14%, 17%, and 34% lower risk of an IHD‐, stroke‐, and PAD‐related hospitalization, respectively, compared with those in the lowest‐intake quintile (median, 57 μg/d). Similar findings have been observed in 2 large observational cohort studies investigating the association between dietary vitamin K1 intake and ASCVD events. Two studies, the Nurses’ Health study (72 874 female nurses; 1679 coronary heart disease events)19 and the Health Professionals’ Follow‐up study (40 087 male health care professionals; 1857 coronary heart disease events),20 report a 21% lower risk and a 16% lower risk of coronary heart disease events, respectively. In contrast, prior studies have not observed a relationship between vitamin K1 intake and stroke19, 20, 42 or PAD.43 Interestingly, the median vitamin K1 intake in cohorts from other studies19, 20, 21, 22, 42, 43, 44 exceeds the median intake of vitamin K1 in the current study. The low vitamin K1 intake in the current study, most apparent in the lowest quintile, serves as an important comparator providing adequate power to detect the cardioprotective potential of high vitamin K1 intake, while also identifying the detrimental impact of low vitamin K1 intake on ASCVD risk, which is most apparent below ≈50 μg/d.
Vitamin K2 intake was inversely associated with a 14% lower risk of ASCVD hospitalizations, and this association was present for all ASCVD subtypes. In the Prospect‐EPIC cohort (16 057 women; 480 coronary heart disease events), a 10 μg/day higher vitamin K2 intake was associated with a 9% lower risk of coronary heart disease.22 In the Rotterdam Study (4807 men and women; 233 coronary heart disease events), individuals in the highest tertile of vitamin K2 intake had a 41% lower risk of incident coronary heart disease compared with those in the lowest tertile.21 Another study of 35 476 Dutch individuals, with a comparatively lower baseline vitamin K2 intake (mean 30.7 μg/d), observed no relationship between vitamin K2 intake and risk of stroke.42 However, this cohort had a young mean age of 49 ± 12 years with high prevalence of hypertension (36.8%), accruing only 324 cases of ischemic stroke during a mean follow‐up of 12 years. Vissers et al43 reported, in a large prospective cohort study (36 629 participants; 489 events), a dose‐responsive reduction in PAD incidence for higher vitamin K2 intakes (HR per 10 μg/d higher intake, 0.92; 95% CI, 0.85–0.99), supporting the inverse association with vitamin K2 observed in our study. In the present study, the median vitamin K2 intake is mostly higher than the median vitamin K2 intakes in other studies that have identified a beneficial association between vitamin K2 intake and ASCVD risk.21, 22, 43, 44 However, the relatively higher vitamin K2 intake in our cohort permitted the discovery of a nonlinear, and in fact more “U‐shaped” association between vitamin K2 intake and ASCVD risk, which, to the best of our knowledge, has not previously been described. This may reflect a competing increase in ASCVD risk associated with overconsumption of vitamin K2‐rich foods (ie, cheese, eggs, butter).
Corroborating the findings of the current study, a recent meta‐analysis of 3 separate cohorts totaling 3891 participants described an 18% higher risk of the combined end point of cardiovascular disease (858 events) or all‐cause mortality (1209 events) in those individuals with low circulating vitamin K1 (≤0.5 nmol/L) compared with those with high circulating vitamin K1 (>1.0 nmol/L).14 This difference was driven mostly by all‐cause mortality. Another meta‐analysis of 1190 participants from 3 studies quantified functional vitamin K deficiency using plasma dephosphorylated‐uncarboxylated Matrix Gla protein. The authors describe a 57% higher risk of cardiovascular disease events (220 events) in the highest tertile (reflecting relative functional vitamin K deficiency), compared with the lowest tertile of dephosphorylated‐uncarboxylated Matrix Gla protein.18 In the same study, the authors observed an 8% lower risk of total coronary heart disease events for vitamin K1 (4249 events),19, 20, 21, 22 and a 30% lower risk for vitamin K2 (713 events),21, 22 comparing the highest and lowest tertiles of dietary intake. No association was observed between vitamin K1 and either nonfatal myocardial infarction or stroke, or between vitamin K2 and fatal coronary heart disease. The findings of the present study support the association observed for coronary heart disease and additionally provide novel information on the protective associations of both vitamin K1 and vitamin K2 with stroke, PAD, and indeed, total ASCVD events.
There are several mechanisms by which vitamin K may lower the risk of ASCVD events. Systemic inflammation is an important component of ASCVD initiation and propagation, and inhibiting the systemic inflammatory process reduces cardiovascular disease events.2 Serum vitamin K1 levels have been inversely related to serum inflammatory markers in large prospective cohort studies.8 Consequently, suppressing systemic inflammation may explain a net reduction in ASCVD events. Additionally, vitamin K may assist in regulating insulin resistance45, 46 and hemostasis,9 both important in the pathogenesis of ASCVD.3, 47 Finally, vitamin K, via the action of the Matrix Gla protein, is important in the inhibition of pathological arterial calcification,48, 49 a potent marker of increased cardiovascular disease risk.5 A randomized controlled trial of 388 older men and women observed a protective effect of vitamin K1 supplementation on the progression of coronary calcification, but only in a subset of patients with established coronary calcification (coronary calcification score >10).10 Taken together, these data support the concept that diets rich in vitamin K may, in a multifactorial manner, reduce future ASCVD risk.
The current study has a number of implications for future research. First, the clinical importance of vitamin K deficiency has mostly been attributed to the accompanying coagulopathies. In light of the cardioprotective associations witnessed in the current study, further research is needed to determine the thresholds of vitamin K deficiency that may warrant intervention for the purpose of cardioprotection. Second, the differential effects of vitamin K on ASCVD subtypes warrants further research to identify high‐risk subgroups who may benefit from a diet high in vitamin K. Finally, further research is needed into whether vitamin K supplementation is a safe and feasible primary prevention therapy for ASCVD.
Our study comes with some limitations common to nutritional epidemiology. First, the observational nature of this study means that we cannot infer causality. Second, the ethnic homogeneity of the cohort may limit the overall generalizability of our findings. Third, the diet associated with higher vitamin K intake is generally healthy; however, we employed numerous methods to reduce the impact of residual dietary confounding, such as the use of a falsification end point, stratification of vitamin K1 intake by total vegetable intake, and adjustment for potential dietary confounders. Finally, the outcomes of interest were determined from ICD coding of hospital admissions, which are susceptible to error. However, our validated case analysis yielded comparable results, indicating that any misclassification did not bias our results. The FFQ used in the current study was acquired from a single time point that represented a long preceding period, which may be susceptible to recall bias, and dietary behaviors may change over time. In addition, this FFQ has not been validated for vitamin K, though prior studies have observed significant relationships between vitamin K determined from FFQs and serum markers of vitamin K intake.50 Importantly, the limitations of the FFQ would likely lead to a reduction in the power to detect an association, and the true relationship between vitamin K intake and ASCVD risk may be stronger than we report. Our study has several significant strengths; a large sample size with up to 23 years of follow‐up, allowing for the accumulation of a high number of events, the availability of important participant characteristics, enabling appropriate methods to be employed to reduce residual confounding and a very minimal loss to follow‐up (<0.3%).
In this prospective cohort study, both dietary vitamin K1 intake and vitamin K2 intake were inversely related to ASCVD hospitalization risk and very low vitamin K1 was associated with a higher risk of ASCVD hospitalizations. Given the very different food sources, these data support an independent protective effect for both subtypes of vitamin K. Further research is needed to identify at‐risk individuals who would benefit most from increased dietary vitamin K intake or supplementation.
Sources of Funding
The Danish Diet, Cancer, and Health Study was funded by the Danish Cancer Society, Denmark. Dr Dalgaard is funded by the Danish Heart Foundation (grant number 17‐R115‐A7443‐22062; https://hjerteforeningen.dk/) and Gangstedfonden (grant number A35136; http://www.gangstedfonden.dk/), Denmark. Dr Bondonno is funded by a National Health and Medical Research Council Early Career Fellowship (grant number APP1159914; https://www.nhmrc.gov.au/), Australia. The salary of Dr Lewis is supported by a National Heart Foundation of Australia Future Leader Fellowship (ID: 102817; https://www.heartfoundation.org.au/). The salary of Dr Hodgson is supported by a National Health and Medical Research Council of Australia Senior Research Fellowship (Grant number APP1116937; https://www.nhmrc.gov.au/). J.W. Bellinge is supported by an Australian Government Research Training Program Scholarship at the University of Western Australia (https://www.education.gov.au/research‐training‐program). E. Connolly is supported by an Australian Government Research Training Program Scholarship at Edith Cowan University (https://www.education.gov.au/research‐training‐program). Dr Blekkenhorst is supported by a National Health and Medical Research Council of Australia Emerging Leadership Investigator Grant (ID: 1172987; https://www.nhmrc.gov.au/) and a National Heart Foundation of Australia Post‐Doctoral Research Fellowship (ID: 102498; https://www.heartfoundation.org.au/). This study was supported by the Raine Medical Research Foundation and the Healy Medical Research Foundation (RCA06‐20). The funders had no role in the design of the study, collection, analysis, or interpretation of the data.
Disclosures
Dr Gislason: has received grants from Bristol‐Myers Squibb, Pfizer, Boehringer Ingelheim, and Bayer outside the submitted work. Dr. Torp‐Pedersen reports grants from Bayer and Novo Nordisk outside the submitted work. Dr. Schultz reports grants and personal fees from Abbott Vascular outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S5, Figures S1–S4
Acknowledgments
Author contributions: Drs Bellinge, Dalgaard and N. Bondonno designed research (project conception, development of overall research plan, and study oversight), with all authors approving the study protocol. Drs Tjønneland and Overvad conducted the original cohort study. E. Connolly and Drs Blekkenhorst and N. Bondonno calculated vitamin K intake from FFQ data; Drs N. Bondonno, Murray, and Dalgaard analyzed data; Drs Bellinge, Dalgaard and N. Bondonno wrote the paper and had primary responsibility for final content; all authors assisted with interpretation of the results and critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020551
For Sources of Funding and Disclosures, see page 11.
References
- 1.World Health Organisation . Global Atlas on cardiovascular disease prevention and control. Geneva: World Health Organisation; 2011: Cited 14 May 2020 http://www.who.int/cardiovascular_diseases/publications/atlas_cvd/en. [Google Scholar]
- 2.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. DOI: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 3.Junker R, Heinrich J, Schulte H, Van De Loo J, Assmann G. Coagulation factor VII and the risk of coronary heart disease in healthy men. Arterioscler Thromb Vasc Biol. 1997;17:1539–1544. DOI: 10.1161/01.ATV.17.8.1539. [DOI] [PubMed] [Google Scholar]
- 4.McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, Ballantyne CM, Heiss G. The metabolic syndrome and 11‐year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–390. DOI: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 5.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. DOI: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 6.Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530–547. DOI: 10.1160/TH08-03-0147. [DOI] [PubMed] [Google Scholar]
- 7.Palmer CR, Blekkenhorst LC, Lewis JR, Ward NC, Schultz CJ, Hodgson JM, Croft KD, Sim M. Quantifying dietary vitamin K and its link to cardiovascular health: a narrative review. Food Funct. 2020;11:2826–2837. DOI: 10.1039/C9FO02321F. [DOI] [PubMed] [Google Scholar]
- 8.Shea MK, Booth SL, Massaro JM, Jacques PF, D'Agostino RB, Dawson‐Hughes B, Ordovas JM, O’Donnell CJ, Kathiresan S, Keaney JF, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham offspring study. Am J Epidemiol. 2008;167:313–320. DOI: 10.1093/aje/kwm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mackie IJ, Bull HA. Normal haemostasis and its regulation. Blood Rev. 1989;3:237–250. DOI: 10.1016/0268-960X(89)90031-3. [DOI] [PubMed] [Google Scholar]
- 10.Shea MK, O’Donnell CJ, Hoffmann U, Dallal GE, Dawson‐Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89:1799–1807. DOI: 10.3945/ajcn.2008.27338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalmeijer GW, Van Der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WMM, Boer JMA, Beulens JWJ. Matrix gla protein species and risk of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2013;36:3766–3771. DOI: 10.2337/dc13-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riphagen IJ, Keyzer CA, Drummen NEA, de Borst MH, Beulens JWJ, Gansevoort RT, Geleijnse JM, Muskiet FAJ, Navis G, Visser ST, et al. Prevalence and effects of functional vitamin K insufficiency: the PREVEND study. Nutrients. 2017;9:1–10. DOI: 10.3390/nu9121334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danziger J, Young RL, Shea MK, Tracy RP, Ix JH, Jenny NS, Mukamal KJ. Vitamin K‐dependent protein activity and incident ischemic cardiovascular disease: The multi‐ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:1037–1042. DOI: 10.1161/ATVBAHA.116.307273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shea MK, Barger K, Booth SL, Matuszek G, Cushman M, Benjamin EJ, Kritchevsky SB, Weiner DE. Vitamin K status, cardiovascular disease, and all‐cause mortality: A participant‐level meta‐analysis of 3 US cohorts. Am J Clin Nutr. 2020;111:1170–1177. DOI: 10.1093/ajcn/nqaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knapen MHJ, Braam LAJLM, Drummen NE, Bekers O, Hoeks APG, Vermeer C. Menaquinone‐7 supplementation improves arterial stiffness in healthy postmenopausal women. Thromb Haemost. 2015;113:1135–1144. DOI: 10.1160/TH14-08-0675. [DOI] [PubMed] [Google Scholar]
- 16.Shea MK, O’Donnell CJ, Vermeer C, Magdeleyns EJP, Crosier MD, Gundberg CM, Ordovas JM, Kritchevsky SB, Booth SL. Circulating uncarboxylated matrix Gla protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. J Nutr. 2011;141:1529–1534. DOI: 10.3945/jn.111.139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theuwissen E, Cranenburg EC, Knapen MH, Magdeleyns EJ, Teunissen KJ, Schurgers LJ, Smit E, Vermeer C. Low‐dose menaquinone‐7 supplementation improved extra‐hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br J Nutr. 2012;108:1652–1657. DOI: 10.1017/S0007114511007185. [DOI] [PubMed] [Google Scholar]
- 18.Chen HG, Sheng LT, Zhang YB, Cao AL, Lai YW, Kunutsor SK, Jiang L, Pan A. Association of vitamin K with cardiovascular events and all‐cause mortality: a systematic review and meta‐analysis. Eur J Nutr. 2019;58:2191–2205. DOI: 10.1007/s00394-019-01998-3. [DOI] [PubMed] [Google Scholar]
- 19.Erkkilä AT, Booth SL, Hu FB, Jacques PF, Manson JE, Rexrode KM, Stampfer MJ, Lichtenstein AH. Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. Eur J Clin Nutr. 2005;59:196–204. DOI: 10.1038/sj.ejcn.1602058. [DOI] [PubMed] [Google Scholar]
- 20.Erkkilä AT, Booth SL, Hu FB, Jacques PF, Lichtenstein AH. Phylloquinone intake and risk of cardiovascular diseases in men. Nutr Metab Cardiovasc Dis. 2007;17:58–62. DOI: 10.1016/j.numecd.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MHJ, van der Meer IM, Hofman A, Witteman JCM. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam study. J Nutr. 2004;134:3100–3105. DOI: 10.1093/jn/134.11.3100. [DOI] [PubMed] [Google Scholar]
- 22.Gast GCM, de Roos NM, Sluijs I, Bots ML, Beulens JWJ, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PHM, van der Schouw YT. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis. 2009;19:504–510. DOI: 10.1016/j.numecd.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Tjønneland A, Olsen A, Boll K, Stripp C, Christensen J, Engholm G, Overvad K. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population‐based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health. 2007;35:432–441. DOI: 10.1080/14034940601047986. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39:22–25. DOI: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 25.Petersson F, Baadsgaard M, Thygesen LC. Danish registers on personal labour market affiliation. Scand J Public Health. 2011;39:95–98. DOI: 10.1177/1403494811408483. [DOI] [PubMed] [Google Scholar]
- 26.Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39:38–41. DOI: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 27.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39:30–33. DOI: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 28.Overvad K, Tjønneland A, Haraldsdóttir J, Ewertz M, Jensen OM. Development of a semiquantitative food frequency questionnaire to assess food, energy and nutrient intake in Denmark. Int J Epidemiol. 1991;20:900–905. DOI: 10.1093/ije/20.4.900. [DOI] [PubMed] [Google Scholar]
- 29.Tjønneland A, Overvad K, Haraldsdóttir J, Bang S, Ewertz M, Jensen OM. Validation of a semiquantitative food frequency questionnaire developed in Denmark. Int J Epidemiol. 1991;20:906–912. DOI: 10.1093/ije/20.4.906. [DOI] [PubMed] [Google Scholar]
- 30.Food data (frida.fooddata.dk) , version 4, 2019, National Food Institute, Technical University of Denmark. Date accessed: 15.04.2019
- 31.US Department of Agriculture, Agricultural Research Service . FoodData Central, 2019. fdc.nal.usda.gov. Date accessed: 15.04.2019
- 32.Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Haemostasis. 2000;30:298–307. DOI: 10.1159/000054147. [DOI] [PubMed] [Google Scholar]
- 33.Manoury E, Jourdon K, Boyaval P, Fourcassié P. Quantitative measurement of vitamin K2 (menaquinones) in various fermented dairy products using a reliable high‐performance liquid chromatography method. J Dairy Sci. 2013;96:1335–1346. DOI: 10.3168/jds.2012-5494. [DOI] [PubMed] [Google Scholar]
- 34.Lewis JR, Brennan‐Speranza TC, Levinger I, Byrnes E, Lim EM, Blekkenhorst LC, Sim M, Hodgson JM, Zhu K, Lim WH, et al. Effects of calcium supplementation on circulating osteocalcin and glycated haemoglobin in older women. Osteoporos Int. 2019;30:2065–2072. DOI: 10.1007/s00198-019-05087-3. [DOI] [PubMed] [Google Scholar]
- 35.Sundbøll J, Adelborg K, Munch T, Frøslev T, Sørensen HT, Bøtker HE, Schmidt M. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6:e012832. DOI: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lühdorf P, Overvad K, Schmidt EB, Johnsen SP, Bach FW. Predictive value of stroke discharge diagnoses in the Danish National Patient Register. Scand J Public Health. 2017;45:630–636. DOI: 10.1177/1403494817716582. [DOI] [PubMed] [Google Scholar]
- 37.Lasota AN, Overvad K, Eriksen HH, Tjønneland A, Schmidt EB, Grønholdt MLM. Validity of peripheral arterial disease diagnoses in the Danish National Patient Registry. Eur J Vasc Endovasc Surg. 2017;53:679–685. DOI: 10.1016/j.ejvs.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 38.Hansen CP, Overvad K, Tetens I, Tjonneland A, Parner ET, Jakobsen MU, Dahm CC. Adherence to the Danish food‐based dietary guidelines and risk of myocardial infarction: a cohort study. Public Health Nutr. 2018;21:1286–1296. DOI: 10.1017/S1368980017003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. DOI: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ekholm O, Hesse U, Davidsen M, Kjøller M. The study design and characteristics of the Danish national health interview surveys. Scand J Public Health. 2009;37:758–765. DOI: 10.1177/1403494809341095. [DOI] [PubMed] [Google Scholar]
- 41.Noordzij M, Leffondré K, Van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28:2670–2677. DOI: 10.1093/ndt/gft355. [DOI] [PubMed] [Google Scholar]
- 42.Vissers LET, Dalmeijer GW, Boer JMA, Monique Verschuren WM, van der Schouw YT, Beulens JWJ. Intake of dietary phylloquinone and menaquinones and risk of stroke. J Am Heart Assoc. 2013;2(1–8):e000455. DOI: 10.1161/JAHA.113.000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vissers LET, Dalmeijer GW, Boer JMA, Verschuren WMM, van der Schouw YT, Beulens JWJ. The relationship between vitamin K and peripheral arterial disease. Atherosclerosis. 2016;252:15–20. DOI: 10.1016/j.atherosclerosis.2016.07.915. [DOI] [PubMed] [Google Scholar]
- 44.Haugsgjerd TR, Egeland GM, Nygård OK, Vinknes KJ, Sulo G, Lysne V, Igland J, Tell GS. Association of dietary vitamin K and risk of coronary heart disease in middle‐age adults: the Hordaland Health Study Cohort. BMJ Open. 2020;10:e035953. DOI: 10.1136/bmjopen-2019-035953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beulens JWJ, van der A DL, Grobbee DE, Sluijs I, Spijkerman AMW, van der Schouw YT. Spijkerman AMW, van der Schouw YT. Dietary phylloquinone and menaquinones intakes and risk of type 2 diabetes. Diabetes Care. 2010;33:1699–1705. DOI: 10.2337/dc09-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida M, Jacques PF, Meigs JB, Saltzman E, Shea MK, Gundberg C, Dawson‐Hughes B, Dallal G, Booth SL. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care. 2008;31:2092–2096. DOI: 10.2337/dc08-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collaboration ERF. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. DOI: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. DOI: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 49.Schurgers LJ, Spronk HMH, Skepper JN, Hackeng TM, Shanahan CM, Vermeer C, Weissberg PL, Proudfoot D. Post‐translational modifications regulate matrix Gla protein function: importance for inhibition of vascular smooth muscle cell calcification. J Thromb Haemost. 2007;5:2503–2511. DOI: 10.1111/j.1538-7836.2007.02758.x. [DOI] [PubMed] [Google Scholar]
- 50.Nimptsch K, Nieters A, Hailer S, Wolfram G, Linseisen J. The association between dietary vitamin K intake and serum undercarboxylated osteocalcin is modulated by vitamin K epoxide reductase genotype. Br J Nutr. 2008;101:1812–1820. DOI: 10.1017/S0007114508131750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5, Figures S1–S4


