Abstract
There has been sustained focus on the secondary prevention of coronary heart disease and heart failure; yet, apart from stroke prevention, the evidence base for the secondary prevention of atrial fibrillation (AF) recurrence, AF progression, and AF‐related complications is modest. Although there are multiple observational studies, there are few large, robust, randomized trials providing definitive effective approaches for the secondary prevention of AF. Given the increasing incidence and prevalence of AF nationally and internationally, the AF field needs transformative research and a commitment to evidenced‐based secondary prevention strategies. We report on a National Heart, Lung, and Blood Institute virtual workshop directed at identifying knowledge gaps and research opportunities in the secondary prevention of AF. Once AF has been detected, lifestyle changes and novel models of care delivery may contribute to the prevention of AF recurrence, AF progression, and AF‐related complications. Although benefits seen in small subgroups, cohort studies, and selected randomized trials are impressive, the widespread effectiveness of AF secondary prevention strategies remains unknown, calling for development of scalable interventions suitable for diverse populations and for identification of subpopulations who may particularly benefit from intensive management. We identified critical research questions for 6 topics relevant to the secondary prevention of AF: (1) weight loss; (2) alcohol intake, smoking cessation, and diet; (3) cardiac rehabilitation; (4) approaches to sleep disorders; (5) integrated, team‐based care; and (6) nonanticoagulant pharmacotherapy. Our goal is to stimulate innovative research that will accelerate the generation of the evidence to effectively pursue the secondary prevention of AF.
Keywords: atrial fibrillation, cardiac rehabilitation, prevention, research, risk factors, sleep
Subject Categories: Atrial Fibrillation, Obesity, Secondary Prevention, Risk Factors
Nonstandard Abbreviations and Acronyms
- AMPK
AMP‐activated protein kinase
- CR
cardiac rehabilitation
- HFpEF
heart failure with preserved ejection fraction
- NHLBI
National Heart, Lung, and Blood Institute
- NLRP3
nucleotide‐binding domain‐like receptor protein 3
The secondary prevention of atrial fibrillation (AF) is of major public health importance because the incidence, prevalence, and lifetime risk of AF are increasing in the United States and globally.1 AF also predisposes to major morbidities, including ischemic stroke, other systemic embolism, dementia, heart failure, myocardial infarction, chronic kidney disease, diminished quality of life, functional limitations, increased healthcare use, higher costs, and excess death.1 Research into the secondary prevention of AF is critical because with the exception of stroke prevention, the evidence base for the prevention of AF recurrence, AF progression, and AF‐related complications lacks robust data in the general population, in contrast with the strong evidence base underlying guidelines for secondary prevention of coronary heart disease and heart failure with reduced left ventricular ejection fraction.
There also is a need for evidence‐based strategies to address the secondary prevention of AF in high‐risk subgroups because of the disproportionate burden of worse AF outcomes in women, systemically disadvantaged groups (eg, Black/Hispanic/Indigenous individuals), and older adults. A meta‐analysis of 30 studies reported that compared with men, women with AF had significantly higher relative risks for all‐cause mortality, stroke, cardiac events, and heart failure.2 In the community, compared with White individuals, Black individuals with AF had about 1.5‐fold to 2‐fold higher rate differences (rate in those with versus without AF) for stroke, heart failure, coronary heart disease, and death.3
The care of AF has largely been relegated to interventions that can be provided only by healthcare providers, but emerging evidence favors the critical role of lifestyle factors, which are more directly determined by patients themselves. The efficiency of public health initiatives may be enhanced by informing patients and the lay public about modifiable behaviors that influence their AF risk, signifying the need for particular lifestyle‐related research initiatives in the secondary prevention of AF.
Over a decade ago, the National Heart, Lung, and Blood Institute (NHLBI) convened a workshop to promote research into the primary prevention of AF.4 From 2019 through 2021, the NHLBI is convening a series of 7 virtual workshops to again promote acceleration of high‐priority research in AF. The innovative virtual format provides an effective way to assemble international experts to identify the most pressing research gaps and to articulate the highest‐priority research challenges. The other webinars examined research priorities for AF ablation,5 bidirectional relations with heart failure,6 screening,7 stroke prevention, and molecular mechanisms; the final webinar will address social determinants of AF.
Our special report summarizes the proceedings of the NHLBI virtual workshop on the secondary prevention of AF recurrence, progression, and complications, which occurred on May 15, 2020. The scope of the special report does not include the relationship of ablation to secondary prevention,5 the specific topic of secondary prevention of heart failure in AF,6 or an in‐depth examination of molecular/genetic mechanisms underlying AF, which were covered by other workshops. Expert participants identified major knowledge gaps and prioritized opportunities in 6 broad areas of AF secondary prevention research: (1) weight loss and body composition; (2) alcohol intake, smoking cessation, and diet; (3) cardiac rehabilitation (CR); (4) approaches to sleep disorders; (5) integrated, team‐based care; and (6) nonanticoagulant pharmacotherapy. Our goal is to stimulate innovative research that will accelerate development of effective strategies for the secondary prevention of AF.
Weight Loss and Body Composition
Background
Obesity is a global epidemic. Population‐based studies have reported that obesity is strongly associated with the risk of incident AF, with a 29% increase in AF incidence for every 5‐unit increase in body mass index.8 Genetic Mendelian randomization studies also support a causal association between obesity and AF; per 1‐kg/m2 body mass index, the age‐ and sex‐adjusted hazard ratio (HR) for AF is 1.15.9 Obesity is associated with higher AF burden and increased progression from paroxysmal to persistent AF.10 More important, obesity curtails the effectiveness of therapies aimed at maintaining sinus rhythm.8
Weight loss achieved through lifestyle modification or bariatric surgery has been associated with improvement in or reduced incidence of risk factors for AF, including elevated blood pressure, hyperglycemia, diabetes mellitus, sleep disorders, myocardial infarction, and heart failure. In a randomized controlled trial (RCT), weight loss achieved through increased physical activity, significant caloric restriction, and healthier eating choices was associated with improved blood pressure, sleep apnea, glycemic control, and lipid profiles.11 Bariatric surgery was associated with sustained weight loss and remission of diabetes mellitus in meta‐analysis of RCTs,12 and in meta‐analysis of observational data with reduced incidence of diabetes mellitus, hypertension, sleep apnea, and coronary heart disease.13
Recent largely observational studies have demonstrated that comprehensive approaches to treating risk factors, with a focus on targeting weight loss, have been associated with reduction of AF symptoms and AF burden, and improvement in the maintenance of sinus rhythm and quality of life.14, 15 There also is evidence for a reversal of AF progression with a transition from persistent to paroxysmal or no AF.16 Emerging observational data suggest that similar findings may be achieved using bariatric surgery.17 However, the risks of AF after bariatric surgery are complex and vary over time. Examining US administrative data from 4 states, investigators reported that the risk of AF‐related emergency department visits and hospitalizations was increased in the 12 months after bariatric surgery (n=523 adults undergoing bariatric surgery; adjusted odds ratio [OR], 1.53; 95% CI, 1.13–2.07; P=0.006).18 In longer‐term follow‐up (median, 7.9 years; interquartile range, 7.2–19.0 years), a meta‐analysis of 7 cohort studies observed that compared with referents, bariatric surgery (n=7681) was associated with an OR of 0.42 (95% CI, 0.22–0.83) for incident AF.19
On the basis of observational and clinical trial evidence, the 2019 focused update of the American Heart Association/American College of Cardiology/Heart Rhythm Society guideline document on AF included a class I, level of evidence B‐R recommendation of weight loss combined with risk factor modification in patients with AF who are overweight or obese.20 However, the 2020 European AF guidelines gave weight loss only a class IIa, level of evidence B recommendation.21
Knowledge Gaps
Mechanisms for Development of the Substrate for AF
Experimental studies have demonstrated that obesity directly affects the atrial myocardium with structural and electrophysiological changes, supporting the milieu for AF (Abed, 2013, number 3666). There is activation of both the transforming growth factor and endothelin pathways, and altered distribution of cell‐to‐cell connections. However, the signaling pathways that lead to these changes have remained elusive.
Studies have demonstrated that epicardial fat and myocardial fat infiltration increase in obesity.22 The secretome of epicardial fat has been demonstrated to promote the AF substrate, including myocardial fibrosis.23 However, the constituents and mechanisms associated with this phenomenon remain unknown.
Obesity is associated with numerous comorbidities, including hypertension, diabetes mellitus, sleep apnea, heart failure, and myocardial infarction, that are known to be associated with AF,24, 25 although obesity remains a risk factor for AF after adjustment for coexistent risk factors.26 However, the signaling pathways and the interaction between obesity and associated comorbidities on the risk of AF remain incompletely understood.
Reversibility of the AF Substrate
Clinical studies have demonstrated that the AF substrate can be reversed with substantial weight loss and risk factor management.16 In an experimental model of obese sheep, 30% weight loss was associated with atrial electrophysiological and structural reverse remodeling, and improved inflammatory and growth factor markers; the changes were accompanied by a reduced propensity for AF independent of other AF risk factors, including elevated blood pressure. However, the mechanisms by which weight loss and risk factor management alter the AF substrate remain only partially determined.
Generalizability of Studies
In a modest‐sized (n=150), single‐center, short‐term randomized study of patients with AF, investigators in Adelaide, Australia, have reported achieving remarkable weight loss, reductions in AF symptoms and burden, and improved maintenance of sinus rhythm.11 A meta‐analysis of the Adelaide groups' 5 studies (4 observational and 1 RCT, total 548 patients) reported that at least 10% weight loss in overweight and obese individuals was accompanied by at least 71% less AF recurrence and significantly lower episode length and symptom severity.15
Studies replicating the marked sustained weight loss and AF burden improvement through lifestyle modification observed in the Australian studies have not been reported from other investigators,27 other countries, and more racially/ethnically and socioeconomically diverse populations. Implementation of weight loss programs requires the development of reproducible and effective tools and strategies for achieving sustained weight loss and other risk factor modification in diverse populations and health systems.
Clinical Outcomes
Developing effective secondary prevention implementation strategies in diverse populations with AF is critical given sex, racial/ethnic, and socioeconomic inequities in clinical outcomes after recognition of AF.1, 2, 3 It is essential to design adequately powered studies including diverse individuals (age, sex, racial/ethnic, and socioeconomic status) to evaluate the potential influence of weight loss on AF recurrence and burden, and clinical outcomes such as stroke, cognitive decline, myocardial infarction, heart failure, quality of life, healthcare use, cost‐effectiveness, and mortality. Historically, RCTs examining hard end points have been complex and resource intensive. Hence, innovative, pragmatic, clinical trials will be critical to addressing the relations between secondary prevention and outcomes. The mechanisms, consequences, and critical research opportunities relating obesity to the secondary prevention of AF are illustrated in Figure 1.
Figure 1. Current knowledge and prioritized research opportunities to advance atrial fibrillation (AF) secondary prevention through weight management.
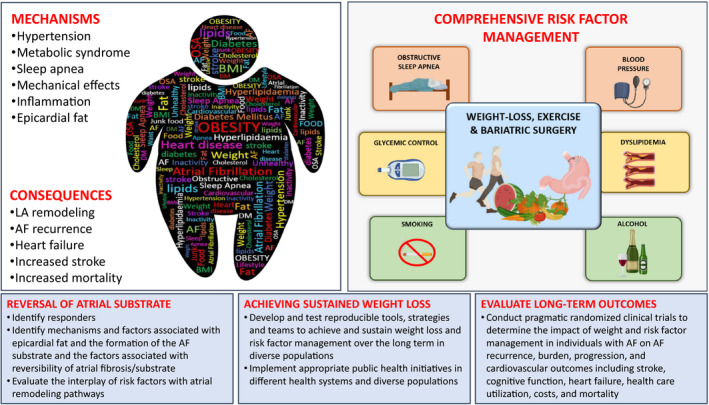
The figure highlights known associations and the consequences that link obesity with AF. Weight loss has been demonstrated in the short‐term to reduce AF burden when performed in the context of a comprehensive risk factor management program. However, there remain several research priorities, highlighted in the blue panels, that are required to (1) improve our understanding of the mechanisms; (2) improve the tools and strategies to achieve sustained weight loss; and (3) evaluate the outcomes of sustained weight loss. Figure is original, created with BioRender.com. BMI indicates body mass index; DM, diabetes mellitus; LA, left atrial; and OSA, obstructive sleep apnea.
Research Opportunities
Identify populations in whom successful weight loss leads to reversal of the atrial substrate for AF, and determine the mechanisms associated with the reversibility of epicardial fat and atrial fibrosis/substrate, including weight loss per se and regression of the comorbidities associated with obesity.
Develop and test effective, reproducible, scalable tools and strategies required to achieve and sustain significant weight loss and risk factor management over the long‐term in diverse populations (eg, age, sex, race/ethnicity, and socioeconomic status) with AF and then implement the appropriate public health initiatives in different health systems and diverse populations.
Conduct multicenter pragmatic RCTs of the effect of weight loss, including bariatric surgery and risk factor management, in diverse individuals with AF on AF recurrence, burden, progression, and outcomes, including stroke, cognitive decline, heart failure, myocardial infarction, quality of life, healthcare use, costs, and mortality.
Alcohol, Smoking, and Diet
Background
Extensive observational evidence indicates that excessive alcohol consumption28 and cigarette smoking29 are associated with the risk of developing AF, whereas the data linking diet and AF are less consistent.30, 31 Understanding of the effect of alcohol, smoking, and dietary interventions on the secondary prevention of AF is more limited.
Alcohol Intake
A meta‐analysis of the association of alcohol consumption with incident AF demonstrated a convincing dose‐response relationship (adjusted relative risk by drinks per day was 1.08 for 1, 1.17 for 2, 1.26 for 3, 1.36 for 4, and 1.47 for 5).28 Alcohol intake is a common trigger for AF episodes32 and has been associated with atrial enlargement,33 adverse electrical atrial remodeling,34 and higher risk of AF recurrence after ablation.35 An Australian open‐label RCT of 140 patients (15% women; mean age, 62 years) with paroxysmal or persistent AF who consumed ≥10 drinks per week randomized half to abstain and half to usual alcohol consumption.36 Patients in the abstinence group reduced their alcohol consumption by 88% and experienced longer periods before AF recurrence and reduced AF burden.36
Smoking
A meta‐analysis of observational studies reported a dose‐response association between cigarette smoking and incident AF.29 There is limited evidence linking smoking with secondary prevention of AF. In an observational study, smoking was found to be a predictor of AF recurrence after ablation and was therefore included in a risk score for recurrent AF (adjusted HR, 1.88 [95% CI, 1.40–2.51]).37 Proposed mechanisms include proarrhythmogenic effects of nicotine as well as the overall adverse association of smoking with cardiovascular risk.38
Diet
Few studies have evaluated whether specific dietary patterns, foods, or nutrients improve outcomes in AF. Fish oil supplementation has been tested as a potential intervention for AF secondary prevention, but results have shown inconsistent effects.39 The Vital‐Rhythm Study (NCT02178410), a 2×2 factorial RCT, revealed that neither omega‐3 fatty acids nor vitamin D reduced or increased incident AF.40 A post hoc analysis of a large RCT reported a protective effect of the Mediterranean dietary pattern on AF incidence.41 On the basis of these findings, a new study (NCT03053843) is testing the effectiveness of this dietary pattern to reduce AF recurrence after ablation.42
Knowledge Gaps
Most research into the influence of modifiable exposures, such as alcohol, smoking, and diet, on AF risk has focused on long‐term patterns of consumption as predictors of new‐onset disease among cohorts of individuals, leaving 3 overarching gaps in knowledge: (1) the effects of these exposures and interventions among those already diagnosed with the disease, including AF recurrence and complications; (2) the risk of an immediate exposure, such as a particular drinking event, a cigarette smoked, or short‐term ingestion of a dietary component, and the near‐term risk of a discrete AF event; and (3) how interactions with common genetic variants may result in heterogeneity of these effects among individuals (Figure 2). Specific knowledge gaps include the following.
Figure 2. Current knowledge and prioritized research opportunities to advance atrial fibrillation (AF) secondary prevention through modifying alcohol consumption, smoking, and diet.
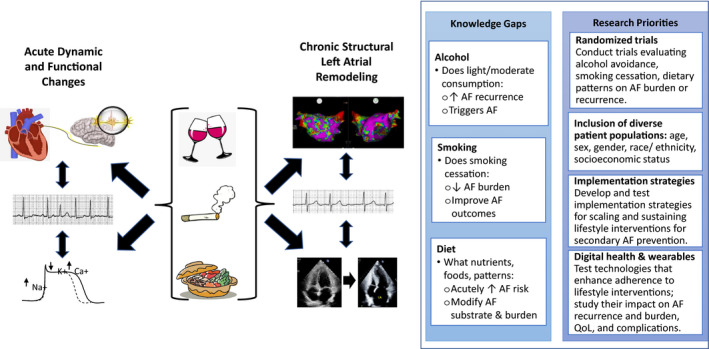
Alcohol, smoking, and various dietary patterns, foods, and nutrients may lead to discrete episodes of recurrent AF via acute changes, such as proarrhythmic autonomic effects (top left) or acute electrophysiologic effects (bottom left), or may lead to greater propensity for AF via chronic structural changes, such as diffuse left atrial remodeling (manifested as low voltages as shown in the left atrial electroanatomic map in the upper right) and left atrial enlargement (as shown in the echocardiographic images in the bottom left). Knowledge gaps in these areas as well as research priorities moving forward are highlighted (right text boxes). QoL indicates quality of life.
Alcohol
It is important to determine whether regular light to moderate alcohol consumption (such as one drink per day) increases the risk of recurrent AF given observational studies reporting cardiovascular benefits compared with no alcohol intake. The mechanisms by which short‐term consumption can induce discrete episodes and the mechanisms linking long‐term alcohol consumption to AF are important knowledge gaps. There also is a lack of AF secondary prevention data with respect to drinking patterns (eg, frequency of drinking for the same amount of alcohol, such as consumption of multiple drinks on the same day compared with an equal amount split over a week, and with or without meals). It would be useful to definitively determine whether alcohol cessation improves the effectiveness of ablation and pharmacologic therapies and decreases the complications after AF onset.
Smoking
Whether and to what extent smoking cessation can reduce the burden and complications of AF remain an important knowledge gap. It is also uncertain whether electronic cigarettes are associated with an increased risk of AF. In addition, it will be important to determine effective implementation strategies to ensure individuals with AF who smoke receive evidence‐based effective smoking cessation interventions.
Diet
Identifying which dietary components (nutrients and foods) or dietary patterns acutely or chronically increase or decrease the risk of discrete AF events or burden and complications is important. How certain diets can chronically influence left atrial remodeling and affect AF burden has yet to be determined.
The most compelling evidence for the effectiveness of alcohol consumption, smoking, and dietary factors will be derived from RCTs. In addition, a major unmet need is to develop resource‐effective policies and pragmatic implementation approaches to scale interventions to diverse communities (age, sex, race/ethnicity, and socioeconomic status) and to enhance the sustainability of lifestyle behaviorally based interventions. For instance, can programs be implemented in community‐based settings, such as places of worship, barbershops, and gyms? Can digital health and wearable technologies be harnessed to improve adherence to lifestyle modification and monitor AF recurrence and burden?
Research Opportunities
RCTs of specific interventions, including (1) alcohol reduction or abstinence; (2) intensive smoking cessation; and (3) heart healthy diets (eg, Dietary Approaches to Stop Hypertension type, plant based, and Mediterranean), versus usual care should be conducted, evaluating their efficacy in reducing AF burden or recurrence, improving quality of life, and reducing complications among diverse patients (eg, age, sex, gender, race/ethnicity, and socioeconomic status) with AF, including those with new‐onset disease, those with paroxysmal AF, postcardioversion, and individuals managed with pharmacological therapies or AF ablation procedures.
Develop and test implementation strategies to scale and sustain lifestyle interventions proven to be effective for secondary prevention of AF, potentially including (1) reducing or avoiding alcohol; (2) intensive smoking cessation; and (3) heart healthy diets in diverse communities and community‐based settings; and study their effect on AF recurrence, AF burden, quality of life, healthcare use, and complications (eg, stroke, cognitive decline, heart failure, myocardial infarction, frailty, and death).
Test the effectiveness of digital health and wearable technologies to enhance adherence to effective lifestyle interventions (alcohol and smoking cessation and dietary modification) in diverse communities and community‐based settings, and study their effect on AF burden, AF recurrence, quality of life, healthcare use, and AF complications.
Exercise and CR Programs
Background
Exercise, lifestyle modification, and CR have the potential to mitigate the intrinsic pathophysiological features of AF, improve other AF risk factors (eg, blood pressure, obesity, and sleep), and improve outcomes (eg, hospitalization, physical function, and quality of life) in those with AF.43 CR is a multifaceted approach that links exercise training with elements of risk factor reduction (eg, tobacco, unhealthy diet, obesity, and hypertension) for patients with known cardiovascular disease.44, 45
A meta‐analysis of 22 observational studies (n=656 750 participants) reported that sedentary lifestyle was associated with increased risk of incident AF (OR, 2.47 [95% CI, 1.25–3.7]), whereas women (OR, 0.91 [95% CI, 0.78–0.97]) and men (OR, 0.81 [95% CI, 0.26–1.004]) engaging in moderate physical activity were less likely to develop AF.46 However, a J‐shaped relation to physical activity has been noted, with men engaged in vigorous physical activity having significantly higher risk of AF (OR, 3.30 [95% CI, 1.97–4.63]).46
AF is not a primary indication for CR in AF guidelines,20, 21, 47 which potentially undercuts an opportunity to improve care for adults who are afflicted with AF or who seek primary ablative therapies. The data for secondary prevention of AF with CR are based on modest numbers of participants and studies. A meta‐analysis by Smart et al of 9 studies (1 observational and 8 randomized) reported CR was not associated with reduction in all‐cause mortality but was associated with improvements in health‐related quality of life, exercise capacity, and AF symptom burden.48
Knowledge Gaps
Existing studies of CR on secondary prevention of AF were small and largely short‐term (≤6 months), had heterogeneous inclusion criteria, varied CR protocols and interventions, and diverse outcomes, and were unable to determine the effect of CR on types of AF or the optimal training intensity (see Table S1 for published CR trials in AF).48 For instance, the Smart et al meta‐analysis study was modestly powered; the included studies comprised 483 exercise‐based CR participants and 476 controls. The generalizability of the findings to most individuals with AF was also uncertain as the mean age of participants was typically ≤70 years and most studies included <30% women (Table S1). Furthermore, none of the studies reported on level of educational attainment; only 1 reported on household income; and only 3 specified race (most were European ancestry), of which 2 specified race as White race or other (other race, 3% and 15%), whereas in 1 study, 20% (n=78) of those with AF studied were Black individuals.49 Most of the studies of AF and CR registered in ClinicalTrials.gov also are small, are single center, and have many of the limitations of prior studies (Table S2).
Specific subgroups of patients with AF to target for CR trials include the following (Figure 3).
Figure 3. Figure showing the complexities of secondary prevention of atrial fibrillation (AF) with cardiac rehabilitation (CR).
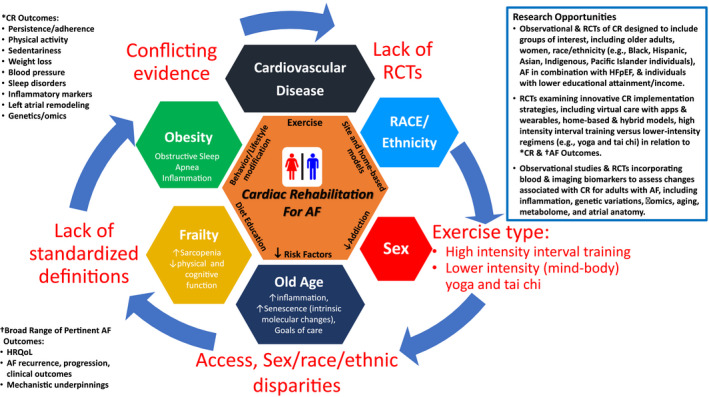
- Lack of randomized controlled trials (RCTs) inclusive of diverse demographics, including sex, race, and ethnicity, as well as broader functional end points that may better reflect utility of CR for AF.
- Lack of precision in regard to exercise modes and intensities, with understanding of both physiological and behavioral implications that factor into CR for AF.
- Lack of inclusion of older adults with focus on related complexities of frailty, sarcopenia, cognitive changes, and intrinsic aging physiological features that may factor into particular benefits of CR for AF.
- Lack of inclusion of obese subgroups with distinctive behavioral, biological, and clinical challenges pertaining to CR for AF.
- *Broad CR outcomes.
- †Broad range of pertinent AF outcomes.
- Research opportunities.
HFpEF indicates heart failure with preserved ejection fraction; and HRQoL, health‐related quality of life.
Diverse Individuals
The RCT evidence base for CR is modest, and particularly limited for women, participants with lower educational attainment/income, and individuals of diverse races/ethnicities. Social determinants of health and ethnic/racial disparities in access to care, as well as inherent cultural differences, also may influence how CR is applied or used. Large, high‐quality RCTs are needed to evaluate the efficacy of CR for secondary prevention of AF with adequate representation of women, individuals with lower educational attainment/income, and participants who are Black, Asian, Pacific Islander, Indigenous, or Hispanic race/ethnicity.
Older Individuals
The incidence and prevalence of AF increase dramatically with advancing age, yet there is a paucity of data for the effectiveness and safety of CR in older adults with AF. Older age is also accompanied by multiple comorbidities, alterations in muscle composition and strength, inflammation, and poorer attendance to center‐based CR.50
Heart Failure With Preserved Ejection Fraction
Older age also is associated with increased prevalence of heart failure with preserved ejection fraction (HFpEF) that also is conducive to AF. The benefits of CR for AF with comorbid HFpEF, a growing population, are uncertain.51 The RACE3 (Routine versus Aggressive upstream rhythm Control for prevention of Early persistent atrial fibrillation in heart failure study 3) RCT of patients with AF and mild‐to‐moderate heart failure (both reduced and preserved left ventricular ejection fraction) reported that an intervention consisting of mineralocorticoid receptor antagonists, statins, angiotensin‐converting enzyme inhibitors, or angiotensin receptor blockers, and CR resulted in a higher likelihood of sinus rhythm at 1 year (75% versus 63% in conventional care; OR, 1.8; P=0.04).52 However, the study had limited power to detect whether CR had the same benefit in individuals with preserved versus reduced ejection fraction. The degree to which the CR component of the intervention was responsible for the improved outcomes also was uncertain.
Another area of interest is the role of specific CR elements or alternative CR locations.
Specific CR Elements
Optimal exercise training modes and intensities for AF prevention remain undetermined. Although there is evidence for greater cardiorespiratory benefits with higher‐intensity interval training, its application to AF prevention remains controversial because of concerns that it could potentially exacerbate the arrhythmia.46 Similarly, there is evidence for yoga53 and Tai Chi54 as training modes that afford integrated cardiovascular, respiratory stretching, and meditative benefits, but it is not clear they achieve sufficient physiological intensity to reduce AF burden and complications.
Mobile‐ and Home‐Based CR
There is growing awareness that patients often prefer to exercise outside the hospital, but home‐based or mobile health‐based CR is highly understudied in AF. In one small RCT of 158 patients referred for CR after heart valve surgery or AF ablation, patients could choose center‐based (n=87) or self‐managed home (n=71) CR.55 Of note, regardless of setting, patients had similar benefits in physical functioning and self‐reported quality‐of‐life outcomes (with the exception of modestly higher mean depression score in the center‐based CR; AF recurrence was not assessed). The COVID‐19 pandemic has underscored the desirability of home‐ and mobile health‐based exercise options.
Molecular Markers of CR Efficacy
Another understudied area of CR in AF is how potential mechanistic biomarkers correlate with efficacy of CR in reducing recurrent AF. Biomarkers (eg, genetics, molecular signals, imaging indexes, and inflammation)56 may identify causal mechanisms and prognostic benchmarks for AF. Once identified, such biomarkers may be applied for participant selection or as modifiable surrogate end points to evaluate the relative utility of different exercise options and/or other CR treatments.
Research Opportunities
Observational studies and RCTs of CR should oversample understudied subgroups of patients with AF, including older adults, women, Black/Hispanic/Asian/Pacific Islander/Indigenous individuals, HFpEF, and individuals with lower educational attainment/income. Outcomes should include success of risk factor modification (eg, if indicated smoking/alcohol cessation, weight loss, and control of blood pressure and diabetes mellitus) and AF‐related symptoms, recurrence, progression, and clinical complications.
-
RCTs should examine innovative implementation of CR strategies and their potential efficacy for reduced AF recurrence and complications in diverse representative AF populations:
Harnessing technology: virtual care with apps and wearables.
Home‐based and hybrid models.
High‐intensity interval training versus lower‐intensity regimens (eg, yoga and tai chi).
Substudies of CR observational studies and RCTs should examine the association of CR with AF‐related biomarkers, including biomarkers of inflammation, genetic variation, omics, aging, metabolome, and atrial imaging.
Sleep Disorders
Background
Sleep disorders and disturbances are common, share risk factors with cardiovascular disease, and are implicated as causal factors in multiple chronic health problems. Of the sleep disorders, obstructive sleep apnea (OSA) has been most studied in relationship to AF. OSA is present in 21% to 74% of patients with AF57 and is associated with increased hospitalization rates and symptom burden,58 and recurrent AF after cardioversion.59 Meta‐analyses estimate that untreated OSA is associated with a 40% increased risk of AF recurrence after catheter ablation.60
There are well‐described physiological mechanisms linking OSA to AF incidence and recurrence through effects on atrial structural and electrical remodeling (eg, negative thoracic pressure swings increasing atrial stretch/dilation and preload; hypoxia increasing afterload and triggering inflammatory and oxidative stress pathways; altered sympathovagal activity).61 The molecular mechanisms of OSA‐related atrial fibrosis and conduction and sinus node abnormalities are less understood, but studies implicate connexin remodeling, dysregulation of myocardial excitation/coupling, and phosphorylation of sodium channels.62
Investigators have examined screening for and treating OSA as an approach to mitigating AF disease burden. Observational studies suggest that continuous positive airway pressure (CPAP) is associated with reduced risk of recurrent AF after cardioversion59 or catheter ablation.60 A lower recurrence rate following ablation therapy in patients with OSA treated with CPAP was reported to parallel CPAP‐related reductions in blood pressure, atrial size, and ventricular mass,63 supporting a potential physiological benefit of CPAP on risk of recurrent AF. However, there are no RCTs to support benefit. Only one small (n=25) RCT was conducted specifically to study AF recurrence, with negative results, but it was underpowered.64 SAVE (The Sleep Apnea Cardiovascular Endpoints) trial (2717 participants; 37 AF events) also did not demonstrate a benefit of CPAP on new‐onset AF; but AF was an underpowered secondary outcome.65
Recent studies suggest that other sleep disturbances that impair quality or quantity of sleep also may be associated with increased risk of AF, including short sleep duration,66 reduced rapid eye movement sleep,67 insomnia,67 and periodic limb movement disorder.68 However, some associations vary by age/sex, and the effect of these sleep disturbances on AF burden has not been addressed. Although circadian physiological features have profound effects on multiple organ systems and AF displays increased nocturnal occurrences,69 there is little research addressing circadian influences on AF.
Knowledge Gaps
Mechanisms and Identification of At‐Risk Sleep Disorder Phenotypes
OSA is a complex heterogeneous disorder that generates physiological stressors related to variations in inspiratory obstruction, gas exchange abnormalities, arousal, and sleep disruption. Although the apnea‐hypopnea index is most frequently used to characterize OSA, it does not comprehensively describe alterations in physiological stresses and underlying disease mechanisms, nor does it consistently predict all types of adverse cardiovascular outcomes. In contrast, compared with the apnea‐hypopnea index, measures of hypoxia better predict cardiovascular disease and death, as well as recurrent AF.70 There is thus a need to better understand the physiologic drivers of AF burden, the underlying phenotypes of individuals with OSA most at risk for AF, and the sleep metrics that best identify individuals likely to benefit from OSA treatment.
Mechanisms underlying the relations between sleep disruption and AF independent of OSA remain largely unknown and are worthy of additional study. Elucidating distinctions between chronic and acute effects may be revealing. For example, although multiple observational cohort studies have demonstrated chronic sleep disruption as a risk factor for incident disease,67 selected patients with AF describe acute lack of sleep as a common trigger of discrete AF episodes.32
Subgroup Susceptibility
In addition to identifying which OSA subtypes represent those most at risk for recurrent AF and related morbidity, there is incomplete understanding of how age, sex/gender, race/ethnicity, underlying heart disease, and comorbid sleep disorders (eg, insomnia and periodic limb movements) moderate risk. It is unknown whether the association between central sleep apnea and incident AF in community‐based cohorts71 reflects risk associated with a unique central sleep apnea phenotype (eg, enhanced autonomic phenotype), or reflects primarily unrecognized subclinical heart disease present at baseline in individuals with central sleep apnea.
Screening
There is a need to understand what settings are most effective for screening for OSA/sleep disorders in patients with AF, and to understand which tools (eg, questionnaires, wearables, oximetry, and mobile health) have optimal predictive value. There are patient and health system barriers to sleep laboratory testing for OSA. Hence, the role of home‐based screening and wearable technologies merits further research. There is also a need to better apply data analytics, including machine learning, to data from remote monitoring of physiological signals (eg, from implantable devices) to improve screening72 and management of OSA and other sleep disorders (Figure 4).
Figure 4. Sleep disorders and atrial fibrillation (AF) burden: stressors, metrics, screening, and intervention.
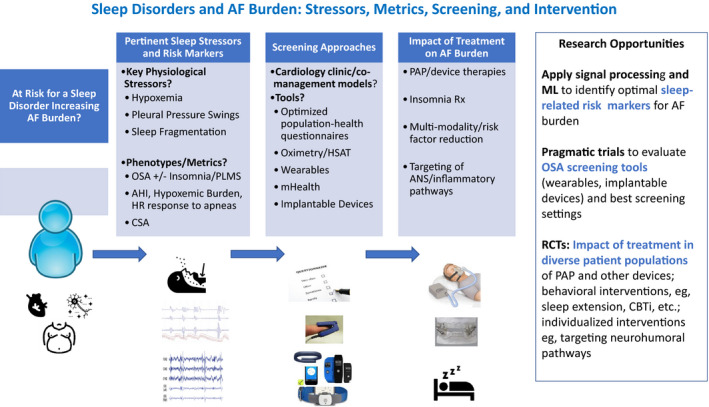
A high proportion of patients with AF have obstructive sleep apnea (OSA) and other sleep disorders that can increase AF burden through atrial remodeling, autonomic nervous system (ANS) alterations, and metabolic/inflammatory pathways. AF burden may be reduced by improved understanding of the metrics and phenotypes that identify risk for sleep‐related AF burden and implementing cost‐effective screening. Randomized controlled trials (RCTs) are needed to evaluate the impact of sleep disorders screening/treatment on AF burden. AHI indicates apnea‐hypopnea index; CBTi, cognitive‐behavioral therapy for insomnia; CSA, central sleep apnea; HR, heart rate; HSAT, home sleep apnea test; ML, machine learning; PAP, positive airway pressure; PLMS, periodic limb movements in sleep; and Rx, treatment.
Therapeutic Interventions
The literature on treating OSA in individuals with AF is largely limited to observational studies of CPAP, an intervention with variable tolerance and adherence. Adequately powered RCTs are needed that test CPAP coupled with interventions to enhance adherence,73 as well as test other OSA interventions (mandibular devices, hypoglossal/phrenic nerve stimulation, positional therapy, and others). Although OSA and obesity aggregate, there are only a few studies that have begun to address multi–risk factor reduction strategies for reducing AF burden in sleep disorders.11
A major mechanism linking OSA to AF is the autonomic nervous system; however, it remains uncertain how to apply novel interventions for AF risk reduction that target this mediating pathway (eg, renal sympathetic nerve denervation, ablation of cardiac ganglia, low‐level vagal or baroreflex stimulation, β blockers, and anti‐inflammatory drugs).74
Research Opportunities
Conduct secondary analyses of observational and RCT databases to determine which sleep disorder metrics, subphenotypes, and risk clusters reflect the highest risk for recurrent AF and AF burden and complications. Develop new metrics derived by applying advanced signal analysis and machine learning to physiological signals collected during sleep (eg, polysomnography and electrocardiography), used alone or combined with symptoms and other data to identify high‐risk clusters, and test their role in predicting AF burden and complications.
Conduct pragmatic RCTs that compare alternative OSA screening approaches (eg, questionnaires, oximetry, and wearables) for identifying patients with AF with clinically important levels of OSA (ie, at increased risk for AF burden) and those likely to respond to OSA‐related interventions.
Conduct adequately powered RCTs that test interventions to improve sleep, examining recurrent AF and AF burden and complications in diverse samples of patients with AF to evaluate for potential differences in responses by age, sex, race/ethnicity, and sleep disorder subtype, including patients undergoing AF ablation. Intervention targets should include OSA as well as insomnia, short sleep duration, and periodic limb movements, and test interventions that include but are not limited to CPAP (eg, mandibular advancement devices and cognitive‐behavioral therapy for insomnia). Perform secondary analyses of observational and RCT data to evaluate the potential roles for tailored/individualized interventions for treating patients with AF and OSA/central sleep apnea that target intermediate mechanisms (eg, neurohumoral modulation and anti‐inflammatory therapy).
Integrated, Team‐Based Care
Background
AF poses a high burden on the healthcare system; in the United States, AF accounts for $28.4 billion (US$2016) dollars in healthcare spending; about 29.8% of healthcare costs are for AF‐related hospitalizations and 29.4% for ambulatory care.75 Moreover, AF management can be complex and demanding and should include rate control, rhythm management, stroke prevention, risk factor management, and lifestyle modification.44, 76
Multiple studies have demonstrated the importance of risk factor modification to significantly reduce the burden of AF and maintain sinus rhythm.14, 15, 52 Questions have emerged, including whether such comprehensive care can be appropriately provided by a single healthcare professional and what is the optimal role of the patient in managing AF.
Novel models of AF care have been identified to prevent fragmentation of care and potentially improve clinical outcomes. Integrated care in this context is an approach that includes 4 fundamental and indispensable elements47: (1) active involvement of the patient, including shared decision‐making and self‐management; (2) a multidisciplinary team approach; (3) use of technology to support integrated care; and (4) a comprehensive care approach involving rate and rhythm control, anticoagulation, and risk factor management, as appropriate. International guidelines for clinical AF management recommend integrated care as the leading approach to manage AF, to improve guideline adherent therapy, and to improve outcomes. The Australian and New Zealand Guidelines considered integrated care to have a high strength level and high evidence grade,47 whereas in 2020, the European Guidelines gave integrated management (AF Better Care holistic pathway) a class IIa recommendation, level of evidence B.21 In contrast, the US guidelines do not address the topic of integrated care.20
Initial studies (meta‐analysis, 3 studies with 1383 patients,77 and post hoc analysis of an RCT with 712 patients newly diagnosed with AF78) have demonstrated promising results in relation to integrated care in patients with AF with significantly reduced cardiovascular hospitalizations and mortality,77, 78 and better cost‐effectiveness compared with usual care.79, 80 A recent multicenter study of 1375 patients (44% women; mean age, 64 years) with AF, however, did not demonstrate significant differences in cardiovascular hospitalization and mortality between 671 randomized to nurse‐led care versus 683 in usual care in the primary analysis.81 However, a prespecified subgroup analysis demonstrated that the benefits of integrated care were heterogeneous (P interaction<0.001); nurse‐led care appeared beneficial in experienced centers (HR, 0.52 [95% CI, 0.37–0.71]), but not in inexperienced centers (HR, 1.24 [95% CI, 0.94–1.63]).81 Given the ongoing increasing prevalence of AF, novel models of care, incorporating integrated team‐based care, should be investigated and improved to provide appropriate care to the growing population of individuals with AF.
Knowledge Gaps
Defining Integrated Care
Integrated care has been defined by international guidelines as having the 4 fundamental elements outlined above. Some interventions have been reported as representing integrated care despite not including all 4 fundamental elements, such as studies including patient education82 and the AF Better Care pathway.83 It is uncertain whether each of the 4 elements of integrated care are essential, or whether equivalent outcomes could be achieved with specific elements of integrated care in defined subgroups of patients with AF (Figure 5).
Figure 5. Integrated team‐based care in atrial fibrillation (AF).
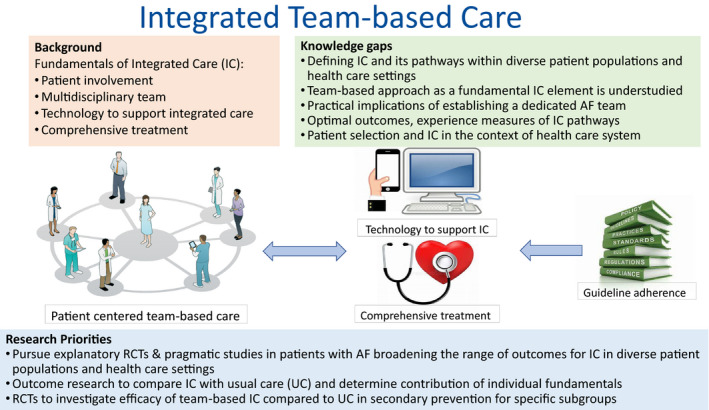
The integrated team‐based care for AF management comprises 4 crucial fundamentals: (1) patient‐centered care with active role for patients; (2) multidisciplinary team approach; (3) use of technology to support integrated care; and (4) comprehensive treatment comprising AF management, prevention of thromboembolic complications, cardiovascular risk factor management, and lifestyle modification, which is steered by evidence‐based guideline recommendations. IC indicates integrated care; RCT, randomized controlled trial; and UC, usual care.
Selection of Patients for Integrated Care
Can we define subgroups of individuals with AF who will most benefit from integrated care, and conversely identify subgroups who will not have better outcomes with team‐based care and avoid the associated costs?
Team‐Based Approach as a Fundamental Element of Integrated Care Is Understudied
The general belief is that team‐based approaches are expensive. However, data on the relative benefits versus costs of integrated care in AF are scarce and have not been reported from the United States.
Practical Implications of Establishing a Dedicated AF Team for Individual Patients With AF
Uncertainties exist on how to build a systematic approach to improve outcomes while preventing fragmentation of care. What are the practical implications and considerations to implement a team‐based approach in clinical practice, particularly in the midst of the current pandemic?
Optimal Outcomes and Experience Measures of Integrated Care Pathways
What are the most relevant outcomes to assess in relation to integrated care? Is it sufficient to improve patient‐reported outcome and experience measures, including patient perceived burden of care, or must integrated care also demonstrate reductions in AF burden and AF‐related complications (eg, stroke and heart failure), hospitalizations, costs, and mortality?
Integrated Care in Its Context of Health Systems
The published RCTs of integrated care have largely been from the Netherlands, Canada, and Australia. Will the benefits of integrated care generalize to other countries and healthcare settings? It is unclear how varied health systems, cultural differences, and patient populations diverse in age, sex, race/ethnicity, health literacy, and socioeconomic status will influence implementation and outcomes observed with integrated care.
Research Opportunities
Pursue explanatory RCTs and pragmatic studies broadening the range of outcomes for integrated AF care compared with usual care, including patient‐reported outcomes (eg, quality of life), AF recurrence and burden (eg, assessed with remote monitoring), team‐based outcomes (eg, efficiency and workforce), and intermediate outcomes (eg, physical functional status), as well as clinical end points (eg, stroke, heart failure, hospitalization, or death) and healthcare costs, understanding that interpreting results may be challenging with ongoing temporal trends in management changes (eg, improved oral anticoagulation treatment). Critical to advancing the field will be to test integrated care approaches in a variety of healthcare settings and countries, including the United States and diverse patient populations.
Conduct outcomes research to compare integrated care strategies with guideline‐based usual care and determine the benefits and risks of the individual elements fundamental to the integrated AF care approach (ie, active patient involvement, multidisciplinary team approach, use of mobile health technology to support integrated care, and comprehensive treatment approach).
Conduct RCTs to investigate the efficacy for AF recurrence, progression, and complications of multidisciplinary, team‐based integrated care in secondary prevention versus usual care, oversampling specific subgroups of patients diverse in age, sex, race/ethnicity, and low socioeconomic status, ensuring that interventions are appropriately tailored to diverse demographics. Identify subgroups of patients with AF (eg, AF type or comorbidities) most likely to benefit compared with usual care approach provided by a single healthcare professional.
Nonanticoagulant Pharmacotherapy
Background
Pharmacologic antiarrhythmic drug therapy continues to have limited efficacy in AF rhythm control and has adverse effects. Numerous risk factors for AF are associated with systemic inflammation, and anti‐inflammatory agents have demonstrated some efficacy at suppressing AF, albeit with significant adverse effects.84, 85 The NLRP3 (nucleotide‐binding domain‐like receptor protein 3) inflammasome is a critical mediator for activation of the innate immune system, particularly in response to noninfectious molecules (Figure 6). This complex system is upregulated in human AF, and NLRP3 inhibition can prevent AF in animal models.86
Figure 6. Emerging pharmacologic targets for the prevention of atrial fibrillation (AF).
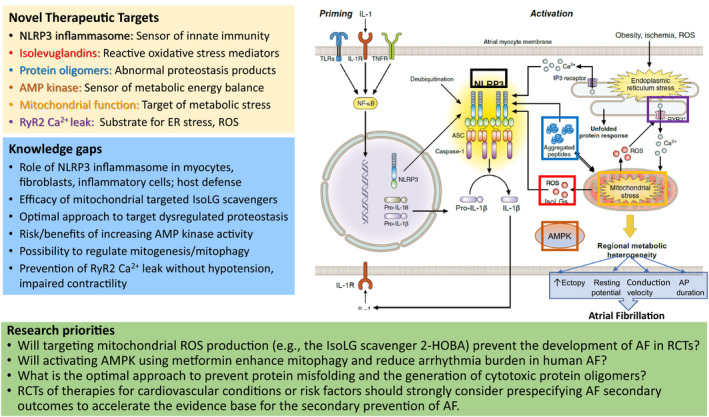
Molecular components involved in priming and activation of the NLRP3 (nucleotide‐binding domain‐like receptor protein 3) inflammasome, which has been linked to AF susceptibility. Obesity and ischemia promote endoplasmic reticulum (ER) stress and mitochondrial stress/dysfunction. These increase production of reactive oxygen species (ROS) and isolevuglandins (IsoLGs). ROS and IsoLGs promote aggregation of intracellular peptides/oligomers that can further impair mitochondrial function and activate NLRP3‐induced cytokine production. AMP‐activated protein kinase (AMPK) plays a central role in cellular metabolism and energy homeostasis, and reduced AMPK activity causes spontaneous AF in animal models. Increased spontaneous Ca2+ release from the sarcoplasmic reticulum contributes to atrial ectopy that can initiate episodes of AF. AP indicates action potential; ASC, apoptosis‐associated speck‐like protein containing a caspase recruitment domain; 2‐HOBA, 2‐hydroxybenzylamine; IL, interleukin; IP3, inositol trisphosphate; NF‐κB, nuclear factor κ light chain enhancer of activated B cells; RCT, randomized controlled trial; RyR2 Ca2+, cardiac ryanodine receptor; TLR, toll‐like receptor; TNFR, tumor necrosis factor receptor.
The most powerful risk factor for AF is aging, which increases oxidative and metabolic stress, with a decline in proteostasis integrity and mitochondrial function.87, 88 Novel mediators of these processes have been identified as potential targets for prevention of AF, with encouraging preclinical preliminary results. Isolevuglandins are highly reactive products of lipid oxidation identified as key drivers of oxidative stress‐related injury,89 and small‐molecule isolevuglandin scavengers reduce AF in people with hypertension.90 Dysfunctional proteostasis results in misfolded and aggregated proteins, and these cytotoxic oligomers can be prevented by isolevuglandin scavengers, such as 2‐hydroxybenzylamine (NCT NCT04433091). Proteostasis also can be improved by the heat shock protein modulator geranylgeranylacetone, and both therapeutic strategies suppress AF in animal models.90, 91
Mitochondrial metabolism is an important source of oxidative stress and a critical determinant of atrial energetics. AMP‐activated protein kinase (AMPK) plays a central role in cellular metabolism and energy homeostasis, and reduced AMPK activity causes spontaneous AF in animal models.92 Drugs, such as metformin, that activate AMPK and promote mitophagy have been associated with lower incidence of AF in certain observational and in vitro studies,93 making AMPK activity and mitochondrial function potentially attractive therapeutic targets. An RCT is currently testing the effect of metformin on AF burden in a sample of patients both with and without diabetes mellitus (NCT03603912). Similarly, in a post hoc analysis of RCT data, treatment with sodium‐glucose cotransporter‐2 (SGLT2) inhibitors appeared to reduce new‐onset and recurrent AF events in patients with type 2 diabetes mellitus.94 Like metformin, SGLT2 inhibitors also alleviated atrial remodeling and improved mitochondrial dysfunction in a rat model of diabetes mellitus.95 It will be of interest to determine the effects of both metformin and SGLT2 inhibitors on AF burden in patients without diabetes mellitus.
Calcium overload promotes uncoupling of the electron transport chain, increasing mitochondrial generation of reactive oxygen species, reducing the efficiency of oxidative phosphorylation, and increasing oxidation of critical calcium cycling proteins that include calcium/calmodulin‐dependent protein kinase II and the ryanodine receptor.96 Mitochondrial targeted antioxidants and/or other agents that attenuate calcium leak from oxidized or hyperphosphorylated ryanodine receptors are likely to reduce ectopic atrial activity.97, 98 Ryanodine receptor inhibitors reduce AF in animal models.99
Knowledge Gaps
Encouraging preliminary data on these novel mediators have generated additional questions and controversies. Inflammasomes are primarily expressed in inflammatory cells, whereas the NLRP3 inflammasome also has been identified in atrial cardiomyocytes. The relative contribution of NLRP3 activation in different cell populations to AF susceptibility as well as the optimal pharmacologic approach for inhibition remain unclear. The NLRP3 inflammasome is activated preferentially by damage rather than pathogen‐associated molecules. The relative risk of infection with NLRP3 inhibitors compared with other anti‐inflammatory agents, such as anti‐cytokine targeted antibodies, like canakinumab, is unknown. The best studied isolevuglandin scavenger, 2‐hydroxybenzylamine, has an elimination half‐life in humans of 2 hours. New scavengers that preferentially target the mitochondria and/or have longer half‐lives have been developed, but it is unclear whether these properties translate to increased efficacy in preventing AF recurrence.
Dysfunctional proteostasis is improved by both isolevuglandin scavengers and geranylgeranylacetone (an inducer of heat shock gene expression), but which approach is most important and whether these approaches are redundant or additive have not been determined. Another unresolved issue is whether mitochondrial‐targeted drugs will be effective in reducing excessive mitochondrial reactive oxygen species generation and whether it would influence AF recurrence or burden. It is unknown whether therapeutic agents that increase AMPK or anti–diabetes mellitus substances with additional cardiac effects, such as SGLT2 inhibitors, will be effective in patients with AF both with and without diabetes mellitus. Current agents that effectively suppress excessive calcium leak through ryanodine receptors can depress left ventricular systolic function and/or blood pressure, and it remains uncertain whether compounds can be identified that lack these detrimental properties.
There is increasing awareness that pharmacotherapies can have protective, neutral, and deleterious effects on cardiovascular outcomes. Unfortunately, although many trials of cardiovascular conditions and risk factors prespecify myocardial infarction and heart failure as outcomes, AF is often not specified a priori. Prespecifying AF onset, AF recurrence, AF progression, and AF‐associated complications as outcomes for RCTs would accelerate the evidence base for the secondary prevention of AF.
Research Opportunities
Will targeting mitochondrial reactive oxygen species production (eg, the isolevuglandin scavenger 2‐hydroxybenzylamine) prevent the development of AF in RCTs?
Will activating AMPK using metformin or other approaches enhance mitophagy and reduce arrhythmia burden in human AF?
To reduce AF burden and progression, what is the optimal approach to preventing protein misfolding and the generation of cytotoxic protein oligomers?
RCTs of therapies for cardiovascular conditions (eg, heart failure and myocardial infarction) or risk factors should strongly consider prespecifying AF onset, recurrence, progression, and associated complications as secondary outcomes to accelerate the evidence base for the secondary prevention of AF.
DISCUSSION
The NHLBI workshop participants identified multiple themes across our 6 domains of focus for the secondary prevention of AF recurrence, progression, and complications and identified critical research opportunities to advance the field (Table). There is a lack of mechanistic understanding on the pathophysiological features (eg, fibrosis, inflammation, mitochondrial reactive oxygen species, and dysfunctional proteostasis) linking risk factors and atrial electrical and structural remodeling to AF progression, remission, and complications. Furthermore, many risk factors for AF (eg, alcohol consumption, smoking, poor dietary patterns, obesity, diabetes mellitus, hypertension, sedentary lifestyle, and sleep disorders) are interrelated. It is unclear whether modification of a specific risk factor (eg, obesity) per se is sufficient, or must be addressed in the context of multiple risk factors to significantly prevent AF progression and complications.
Table 1.
Prioritized Research Opportunities for the Secondary Prevention of AF
| Weight loss and body composition |
| Identify populations in whom successful weight loss leads to reversal of the atrial substrate for AF, and determine the mechanisms associated with the reversibility of epicardial fat and atrial fibrosis/substrate, including weight loss per se and regression of the comorbidities associated with obesity |
| Develop and test effective, reproducible, scalable tools and strategies required to achieve and sustain significant weight loss and risk factor management over the long‐term in diverse populations (eg, age, sex, race/ethnicity, and socioeconomic status) with AF and then implement the appropriate public health initiatives in different health systems and diverse populations |
| Conduct multicenter pragmatic RCTs of the effect of weight loss, including bariatric surgery, and risk factor management in diverse individuals with AF on AF recurrence, burden, progression, and outcomes, including stroke, cognitive decline, heart failure, myocardial infarction, quality of life, healthcare use, costs, and mortality |
| Alcohol, smoking, and diet |
| RCTs of specific interventions, including (1) alcohol reduction or abstinence; (2) intensive smoking cessation; (3) heart healthy diets (eg, DASH type, plant based, and Mediterranean) vs usual care should be conducted, evaluating their efficacy in reducing AF burden or recurrence, improving quality of life, and reducing complications among diverse patients (eg, age, sex, gender, race/ethnicity, and socioeconomic status) with AF, including those with new‐onset disease, those with paroxysmal AF, postcardioversion, and individuals managed with pharmacological therapies or AF ablation procedures |
| Develop and test implementation strategies to scale and sustain lifestyle interventions proven to be effective for secondary prevention of AF, potentially including (1) reducing or avoiding alcohol; (2) intensive smoking cessation; (3) heart healthy diets in diverse communities and community‐based settings; and study their effect on AF recurrence, AF burden, quality of life, healthcare use, and complications (eg, stroke, cognitive decline, heart failure, myocardial infarction, frailty, and death) |
| Test the effectiveness of digital health and wearable technologies to enhance adherence to effective lifestyle interventions (alcohol and smoking cessation and dietary modification) in diverse communities and community‐based settings, and study their effect on AF burden, AF recurrence, quality of life, healthcare use, and AF complications |
| Cardiac rehabilitation |
| Observational studies and RCTs of CR should oversample understudied subgroups of patients with AF, including older adults, women, Black/Hispanic/Asian/Pacific Islander/Indigenous individuals, HFpEF, and individuals with lower educational attainment/income. Outcomes should include success of risk factor modification (eg, if indicated smoking/alcohol cessation, weight loss, and control of blood pressure and diabetes mellitus) and AF‐related symptoms, recurrence, progression, and clinical complications |
|
RCTs should examine innovative implementation of CR strategies and their potential efficacy for reduced AF recurrence and complications in diverse representative AF populations:
|
| Substudies of CR observational studies and RCTs should examine the association of CR with AF‐related biomarkers, including biomarkers of inflammation, genetic variation, ‐omics, aging, metabolome, and atrial imaging |
| Sleep disorders |
| Pursue explanatory RCTs and pragmatic studies broadening the range of outcomes for integrated AF care, including patient‐reported outcomes (eg, quality of life), AF recurrence and burden (eg, assessed with remote monitoring), team‐based outcomes (eg, efficiency and workforce), intermediate outcomes (eg, physical functional status), as well as clinical end points (eg, stroke, heart failure, hospitalization, or death) and healthcare costs with the comparator being usual care, understanding that interpreting results may be challenging with ongoing temporal trends in management changes (eg, improved oral anticoagulation treatment). Critical to advancing the field will be to test integrated care approaches in a variety of healthcare settings and countries, including the United States, and diverse patient populations |
| Conduct outcomes research to compare integrated care strategies with guideline‐based usual care and determine the benefits and risks of the individual elements fundamental to the integrated AF care approach (ie, active patient involvement, multidisciplinary team approach, use of mobile health technology to support integrated care, and comprehensive treatment approach) |
| Conduct RCTs to investigate the efficacy for AF recurrence, progression, and complications of multidisciplinary, team‐based integrated care in secondary prevention vs usual care, oversampling specific subgroups of patients diverse in age, sex, race/ethnicity, and low socioeconomic status, ensuring that interventions are appropriately tailored to diverse demographics. Identify subgroups of patients with AF (eg, AF type or comorbidities) most likely to benefit compared with usual care approach provided by one single healthcare professional |
| Integrated, team‐based care |
| Pursue explanatory RCTs and pragmatic studies broadening the range of outcomes for integrated AF care compared with usual care, including patient‐reported outcomes (eg, quality of life), AF recurrence and burden (eg, assessed with remote monitoring), team‐based outcomes (eg, efficiency and workforce), and intermediate outcomes (eg, physical functional status), as well as clinical end points (eg, stroke, heart failure, hospitalization, or death) and healthcare costs, with the comparator being usual care, understanding that interpreting results may be challenging with ongoing temporal trends in management changes (eg, improved oral anticoagulation treatment). Critical to advancing the field will be to test integrated care approaches in a variety of healthcare settings and countries, including the United States, and diverse patient populations |
| Conduct outcomes research to compare integrated care strategies with guideline‐based usual care and determine the benefits and risks of the individual elements fundamental to the integrated AF care approach (ie, active patient involvement, multidisciplinary team approach, use of mobile health technology to support integrated care, and comprehensive treatment approach) |
| Conduct RCTs to investigate the efficacy for AF recurrence, progression, and complications of multidisciplinary, team‐based integrated care in secondary prevention vs usual care, oversampling specific subgroups of patients diverse in age, sex, race/ethnicity, and low socioeconomic status, ensuring that interventions are appropriately tailored to diverse demographics. Identify subgroups of patients with AF (eg, AF type or comorbidities) most likely to benefit compared with usual care approach provided by a single healthcare professional |
| Nonanticoagulant pharmacotherapy |
| Will targeting mitochondrial ROS production (eg, the isolevuglandin scavenger 2‐HOBA) prevent the development of AF in RCTs? |
| Will activating AMPK using metformin or other approaches enhance mitophagy and reduce arrhythmia burden in human AF? |
| To reduce AF burden and progression, what is the optimal approach to preventing protein misfolding and the generation of cytotoxic protein oligomers? |
| RCTs of therapies for cardiovascular conditions (eg, heart failure and myocardial infarction) or risk factors should strongly consider prespecifying AF onset, recurrence, progression, and associated complications as secondary outcomes to accelerate the evidence base for the secondary prevention of AF |
2‐HOBA indicates 2‐hydroxybenzylamine; AF, atrial fibrillation; AMPK, AMP‐activated protein kinase; CR, cardiac rehabilitation; DASH, Dietary Approaches to Stop Hypertension; HFpEF, heart failure with preserved ejection fraction; RCT, randomized controlled trial; and ROS, reactive oxygen species.
Although strong observational data link risk factors to AF progression and complications, apart from anticoagulation for ischemic stroke prevention, RCTs for AF secondary prevention, which are vital to guideline development, have notable limitations. Previously conducted studies tend to be small to modest in size, are predominantly conducted in individuals of European ancestry, generally have been performed in the Netherlands, Australia, and Canada, typically are of short to medium duration, and examine intermediate end points (eg, AF burden), thus raising concerns about the studies' generalizability and robustness of their interventions to alter important clinical outcomes (eg, dementia, heart failure, myocardial infarction, chronic kidney disease, and death). There is a need to prespecify AF‐related end points (onset, recurrence, progression, and clinical complications) in RCTs of other conditions (eg, heart failure, myocardial infarction, hypertension, and diabetes mellitus).
There is a paucity of AF secondary prevention data in diverse populations (eg, older adults, women, individuals of Black, Hispanic, Indigenous, Pacific Islander, and Asian ancestry, and those with lower educational attainment and lower income) and important clinical subgroups (eg, individuals with HFpEF). Hence, it is not definitive that AF risk factor management (eg, weight loss, alcohol abstinence, smoking cessation, CR, improved sleep, and integrated care) (1) has been designed to improve outcomes in diverse patients with AF; (2) would have similar benefits in patients with different types of AF or varying comorbidities (eg, HFpEF); and (3) whether changes in AF burden will translate into improved clinical outcomes.
Fundamentally, wider implementation of AF secondary prevention requires pragmatic, randomized implementation trials to develop strategies that are scalable, sustainable, practical, resource efficient, and clinically effective in diverse patient populations. One avenue for scalability is digital health. The role of mobile and wearable technology for the monitoring of AF burden and AF risk factors has been understudied for AF secondary prevention. It will be critical to investigate whether home‐based implementation and monitoring of weight, alcohol/smoking/dietary habits, activity, CR, and sleep enhance the adherence, efficiency, and effectiveness of risk factor monitoring and modification for AF secondary prevention.
The secondary prevention of AF progression and its complications is of vital public health importance because ≈5.2 million individuals in the United States are known to have AF (2010 estimate).1 Since 1990, the global prevalence of AF has approximately doubled to 59.7 million individuals in 2019.100 Furthermore, over the same time period, the age‐standardized prevalence, disability‐adjusted life years, and mortality have not substantially changed.100 Hence, AF will continue to lead to substantial morbidity as well as excess health care use, costs, and mortality. Although there are robust guidelines for the prevention of stroke after AF onset, there is a lack of class I evidence to strongly support the secondary prevention of dementia, heart failure, myocardial infarction, chronic kidney disease, diminished quality of life, functional limitations, and increased healthcare use after AF is diagnosed. The authors hope this workshop will catalyze research that will advance the evidence base for the secondary prevention of the recurrence, progression, and complications of AF.
Sources of Funding
Dr Benjamin receives research funding from US National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) grants R01 HL128914, R01 HL092577, R01 HL141434, and U54 HL120163, NIH National Institute of Aging (NIA) grants R01 AG066010 and R01 AG066914, and American Heart Association AHA_18SFRN34110082. Dr Alonso receives research funding from US NIH, NHLBI grants K24 HL148521 and R01 HL137338, NIA R21 AG058445, and American Heart Association 16EIA26410001. Dr Djoussé receives research funding from US NIH, NIA R01 AG053325 and R01 AG053325), and NHLBI R01 HL131687. Dr Forman receives funds from the NIA through grants R01 AG060499, R01 AG058883, R01 AG051376, and P30 AG024827, and from the NIH Common Fund U01 AR071130. Dr Hendriks is supported by a Future Leader Fellowship from the National Heart Foundation of Australia. Dr Kirchhof is supported by European Union (grant agreement 633196 [CATCH ME]), European Union BigData@Heart (grant agreement EU IMI 116074), British Heart Foundation (FS/13/43/30324, PG/17/30/32961, PG/20/22/35093, and AA/18/2/34218), German Centre for Cardiovascular Research, supported by the German Ministry of Education and Research (Deutsches Zentrum für Herz‐Kreislaufforschung, via a grant to Atrial Fibrillation Network [AFNET]), and Leducq Foundation. Dr Marcus receives research funding from the NIH (National Cancer Institute 75N91020C00039, National Institute of Biomedical Imaging and Bioengineering 3U2CEB021881‐05S1 and subcontract related to the RADx initiative, National Institute on Alcohol Abuse and Alcoholism R01AA022222), Patient‐Centered Outcomes Research Institute (CER‐2017C3‐9091), Tobacco‐Related Disease Research Program High Impact Research Award 27IR‐0027, and the Bill and Melinda Gates Foundation. Dr Mehra receives research funding from the American Heart Association AHA_18SFRN34170013. Dr Murray is supported by research grants: NIH HL133127 and American Heart Association 18SFRN34230125 and 20SCG35540037. Dr Parkash is supported by the Heart and Stroke Foundation of Canada, the Canadian Institute of Health Research 400660 and the Cardiac Arrhythmia Network. Dr Redline receives research funding from US NIH, NHLBI grants R35 HL135818, HL125307, HL151253, HL140412, HL125307, HL135818, HL133684, HL137192, HL036801, HL137234, HL146339, and HL153874, and NHLBI contract 75N92019C00011; National Institute of Diabetes and Digestive and Kidney Diseases grant DK107972; NIA grants AG062667, AG066137, and HL153874; and the Department of Defense A8750‐18‐C‐0026. Dr Rienstra is supported by the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation, for Reappraisal of Atrial Fibrillation: Interaction between hyperCoagulability, Electrical remodeling, and Vascular Destabilisation in the Progression of AF (RACE V) consortium, Reviving Early Diagnosis of CardioVascular Disease (RED‐CVD) consortium, and Netherlands Cardiovascular Research Initiative ‐ Artificial Intelligence consortia, and from the Dutch Heart Foundation for Digoxin Evaluation in Chronic Heart Failure: Investigational Study In Outpatients in the Netherlands (DECISION) study. Dr Sanders is supported by Practitioner Fellowships from the National Health and Medical Research Council of Australia and by the National Heart Foundation of Australia. Dr Somers is supported by NIH HL65176, NIH HL134885, and NIH HL134808. Dr Van Wagoner is supported by research grants from American Heart Association AHA_18SFRN34170442 and NIH R01 HL111314. Dr Wang is supported by the American Heart Association 20SFRN35360189 and 18SFRN34120036; and Stanford University Co‐PI of BAROS (Bariatric Atrial Restoration of Sinus Rhythm), NCT04050969. Dr Go receives research funding from US NIH, NHLBI grant R01 HL142834 and National Institute of Diabetes and Digestive and Kidney Diseases R01 DK103612.
Disclosures
Dr Benjamin was an uncompensated member for MyHeartLab Steering Committee, a PI‐initiated study from Samsung to University of California, San Francisco. Principal Investigator, Jeffrey Olgin, MD, in 2020. Dr Al‐Khatib receives consulting fees from Milestone Pharmaceuticals, research, speaking, and consulting fees from Medtronic, research and speaking fees from Abbott, and research fees from Boston Scientific. Dr Alonso is a member of the Scientific Advisory Board of Corify Care SL. Dr Djoussé in an uncompensated member of the International Scientific Forum on Alcohol Research. He received an investigator‐initiated research grant from the American Egg Board in the past (2016–2018). Professor Hendriks reports that the University of Adelaide has received on his behalf lecture and/or consulting fees from Medtronic and Pfizer/BMS. Dr Kirchhof receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (United Kingdom), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the past 3 years. Dr Kirchhof is listed as inventor on 2 patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571 and Markers for Atrial Fibrillation WO 2016012783). Dr Marcus receives research funding from Baylis Medical, Medtronic, Jawbone, and Eight Sleep; consulting as member of steering committee for Johnson & Johnson; and consultant for InCarda and equity in InCarda as a cofounder. Dr Murray has a pending patent application, Metabolic Technologies Inc. Dr Parkash receives research funding from Medtronic, Abbott, Novartis, and Pfizer. Dr Piña is on the Advisory Board of Relypsa. Dr Redline received consulting fee for participating in advisory meetings held by Respicardia and Eisai Inc, and consulting fees from Jazz Pharmaceuticals and Apnimed Inc. Dr Sanders reports having served on the advisory board of Medtronic, Abbott, Boston Scientific, Pacemate, and CathRx. The University of Adelaide reports receiving on behalf of Dr Sanders lecture and/or consulting fees from Medtronic, Abbott, Boston Scientific, and Bayer. The University of Adelaide reports receiving on behalf of Dr Sanders research funding from Medtronic, Abbott, Boston Scientific, and Microport. Dr Somers is a consultant for Baker Tilly; Jazz Pharmaceuticals; Sleep Number; and Respicardia. Dr Go has received a research grant through his institution from iRhythm Technologies. Dr Go is also a member of the Operations Committee and Steering Committee for A Study to Determine if Identification of Undiagnosed Atrial Fibrillation in People at Least 70 Years of Age Reduces the Risk of Stroke (GUARD‐AF) Study (NCT04126486) sponsored by Bristol Meyers Squibb and Pfizer. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
References 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; the US Preventive Services Task Force; the US Department of Health and Human Services; or the Department of Veterans Affairs.
(J Am Heart Assoc. 2021;10:e021566. DOI: 10.1161/JAHA.121.021566.)
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021566
For Sources of Funding and Disclosures, see page 17.
REFERENCES
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. DOI: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, Odutayo AA. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta‐analysis of cohort studies. BMJ. 2016;532:h7013. DOI: 10.1136/bmj.h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnani JW, Norby FL, Agarwal SK, Soliman EZ, Chen LY, Loehr LR, Alonso A. Racial differences in atrial fibrillation‐related cardiovascular disease and mortality: the Atherosclerosis Risk in Communities (ARIC) Study. JAMA Cardiol. 2016;1:433–441. DOI: 10.1001/jamacardio.2016.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Chen P‐S, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, et al. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute Workshop. Circulation. 2009;119:606–618. DOI: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al‐Khatib SM, Benjamin EJ, Buxton AE, Calkins H, Chung MK, Curtis AB, Desvigne‐Nickens P, Jais P, Packer DL, Piccini JP, et al. Research needs and priorities for catheter ablation of atrial fibrillation: a report from a National Heart, Lung, and Blood Institute virtual workshop. Circulation. 2020;141:482–492. DOI: 10.1161/CIRCULATIONAHA.119.042706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al‐Khatib SM, Benjamin EJ, Albert CM, Alonso A, Chauhan C, Chen P‐S, Curtis AB, Desvigne‐Nickens P, Ho JE, Lam CSP, et al. Advancing research on the complex interrelations between atrial fibrillation and heart failure: a report from a US National Heart, Lung, and Blood Institute virtual workshop. Circulation. 2020;141:1915–1926. DOI: 10.1161/CIRCULATIONAHA.119.045204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Go AS, Desvigne‐Nickens P, Anderson CD, Casadei B, Chen LY, Crijns HJGM, Freedman B, Hills MT, Healey JS, et al. Research priorities in atrial fibrillation screening: a report from a National Heart, Lung, and Blood Institute virtual workshop. Circulation. 2021;143:372–388. DOI: 10.1161/CIRCULATIONAHA.120.047633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CX, Sullivan T, Sun MT, Mahajan R, Pathak RK, Middeldorp M, Twomey D, Ganesan AN, Rangnekar G, Roberts‐Thomson KC, et al. Obesity and the risk of incident, post‐operative, and post‐ablation atrial fibrillation: a meta‐analysis of 626,603 individuals in 51 studies. JACC Clin Electrophysiol. 2015;1:139–152. DOI: 10.1016/j.jacep.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee NA, Giulianini F, Geelhoed B, Lunetta KL, Misialek JR, Niemeijer MN, Rienstra M, Rose LM, Smith AV, Arking DE, et al. Genetic obesity and the risk of atrial fibrillation: causal estimates from Mendelian randomization. Circulation. 2017;135:741–754. DOI: 10.1161/CIRCULATIONAHA.116.024921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsang TS, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC, Seward JB, Gersh BJ. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008;29:2227–2233. DOI: 10.1093/eurheartj/ehn324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310:2050–2060. DOI: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 12.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non‐surgical treatment for obesity: a systematic review and meta‐analysis of randomised controlled trials. BMJ. 2013;347:f5934. DOI: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiggins T, Guidozzi N, Welbourn R, Ahmed AR, Markar SR. Association of bariatric surgery with all‐cause mortality and incidence of obesity‐related disease at a population level: a systematic review and meta‐analysis. PLoS Med. 2020;17:e1003206. DOI: 10.1371/journal.pmed.1003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST‐AF cohort study. J Am Coll Cardiol. 2014;64:2222–2231. DOI: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Aldaas OM, Lupercio F, Han FT, Hoffmayer KS, Krummen D, Ho G, Raissi F, Birgersdotter‐Green U, Feld GK, Hsu JC. Meta‐analysis of effect of modest (>/=10%) weight loss in management of overweight and obese patients with atrial fibrillation. Am J Cardiol. 2019;124:1568–1574. DOI: 10.1016/j.amjcard.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R, Twomey D, Gallagher C, Hendriks JML, Linz D, et al. PREVEntion and regReSsive Effect of weight‐loss and risk factor modification on Atrial Fibrillation: the REVERSE‐AF study. Europace. 2018;20:1929–1935. DOI: 10.1093/europace/euy117. [DOI] [PubMed] [Google Scholar]
- 17.Donnellan E, Wazni OM, Elshazly M, Kanj M, Hussein AA, Baranowski B, Kochar A, Trulock K, Aminian A, Schauer P, et al. Impact of bariatric surgery on atrial fibrillation type. Circ Arrhythm Electrophysiol. 2020;13:e007626. DOI: 10.1161/CIRCEP.119.007626. [DOI] [PubMed] [Google Scholar]
- 18.Shimada YJ, Tsugawa Y, Camargo CA Jr, Brown DFM, Hasegawa K. Effect of bariatric surgery on emergency department visits and hospitalizations for atrial fibrillation. Am J Cardiol. 2017;120:947–952. DOI: 10.1016/j.amjcard.2017.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chokesuwattanaskul R, Thongprayoon C, Bathini T, Sharma K, Watthanasuntorn K, Lertjitbanjong P, Pachariyanon P, Prechawat S, Mao MA, Torres‐Ortiz A, et al. Incident atrial fibrillation in patients undergoing bariatric surgery: a systematic review and meta‐analysis. Intern Med J. 2020;50:810–817. DOI: 10.1111/imj.14436. [DOI] [PubMed] [Google Scholar]
- 20.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. DOI: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 21.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. DOI: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 22.Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JPM, Finnie JW, Samuel CS, Royce SG, Twomey DJ, et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol. 2015;66:1–11. DOI: 10.1016/j.jacc.2015.04.058. [DOI] [PubMed] [Google Scholar]
- 23.Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clément K, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo‐fibrokines. Eur Heart J. 2015;36:795–805a. DOI: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 24.Bohne LJ, Johnson D, Rose RA, Wilton SB, Gillis AM. The association between diabetes mellitus and atrial fibrillation: clinical and mechanistic insights. Front Physiol. 2019;10:135. DOI: 10.3389/fphys.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens ACJW, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc. 2013;2:e000102. DOI: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang TJ, Parise H, Levy D, D'Agostino RB Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. DOI: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 27.Yaeger A, Keenan BT, Cash NR, Parham T, Deo R, Frankel DS, Schaller RD, Santangeli P, Nazarian S, Supple GE, et al. Impact of a nurse‐led limited risk factor modification program on arrhythmia outcomes in patients with atrial fibrillation undergoing catheter ablation. J Cardiovasc Electrophysiol. 2020;31:423–431. DOI: 10.1111/jce.14336. [DOI] [PubMed] [Google Scholar]
- 28.Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose‐response meta‐analysis. J Am Coll Cardiol. 2014;64:281–289. DOI: 10.1016/j.jacc.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 29.Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of atrial fibrillation: a systematic review and meta‐analysis of prospective studies. Eur J Prev Cardiol. 2018;25:1437–1451. DOI: 10.1177/2047487318780435. [DOI] [PubMed] [Google Scholar]
- 30.Gronroos NN, Alonso A. Diet and risk of atrial fibrillation—epidemiologic and clinical evidence. Circ J. 2010;74:2029–2038. DOI: 10.1253/circj.CJ-10-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nalliah CJ, Sanders P, Kalman JM. The impact of diet and lifestyle on atrial fibrillation. Curr Cardiol Rep. 2018;20:137. DOI: 10.1007/s11886-018-1082-8. [DOI] [PubMed] [Google Scholar]
- 32.Groh CA, Faulkner M, Getabecha S, Taffe V, Nah G, Sigona K, McCall D, Hills MT, Sciarappa K, Pletcher MJ, et al. Patient‐reported triggers of paroxysmal atrial fibrillation. Heart Rhythm. 2019;16:996–1002. DOI: 10.1016/j.hrthm.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 33.McManus DD, Yin X, Gladstone R, Vittinghoff E, Vasan RS, Larson MG, Benjamin EJ, Marcus GM. Alcohol consumption, left atrial diameter, and atrial fibrillation. J Am Heart Assoc. 2016;5:e004060. DOI: 10.1161/JAHA.116.004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voskoboinik A, Wong G, Lee G, Nalliah C, Hawson J, Prabhu S, Sugumar H, Ling L‐H, McLellan A, Morton J, et al. Moderate alcohol consumption is associated with atrial electrical and structural changes: insights from high‐density left atrial electroanatomic mapping. Heart Rhythm. 2019;16:251–259. DOI: 10.1016/j.hrthm.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 35.Qiao Y, Shi R, Hou B, Wu L, Zheng L, Ding L, Chen G, Zhang S, Yao Y. Impact of alcohol consumption on substrate remodeling and ablation outcome of paroxysmal atrial fibrillation. J Am Heart Assoc. 2015;4:e002349. DOI: 10.1161/JAHA.115.002349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, Prabhu S, Stub D, Azzopardi S, Vizi D, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382:20–28. DOI: 10.1056/NEJMoa1817591. [DOI] [PubMed] [Google Scholar]
- 37.Mesquita J, Ferreira AM, Cavaco D, Moscoso Costa F, Carmo P, Marques H, Morgado F, Mendes M, Adragao P. Development and validation of a risk score for predicting atrial fibrillation recurrence after a first catheter ablation procedure—ATLAS score. Europace. 2018;20:f428–f435. DOI: 10.1093/europace/eux265. [DOI] [PubMed] [Google Scholar]
- 38.Goette A, Lendeckel U, Kuchenbecker A, Bukowska A, Peters B, Klein HU, Huth C, Rocken C. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart. 2007;93:1056–1063. DOI: 10.1136/hrt.2005.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, Jones P, Ramprasath VR, O'Hara G, Kopecky S, et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. J Am Coll Cardiol. 2014;64:1441–1448. DOI: 10.1016/j.jacc.2014.07.956. [DOI] [PubMed] [Google Scholar]
- 40.Albert CM, Cook NR, Pester J, Moorthy MV, Ridge C, Danik JS, Gencer B, Siddiqi HK, Ng C, Gibson H, et al. Effect of marine omega‐3 fatty acid and vitamin D supplementation on incident atrial fibrillation. JAMA. 2021;325:1061–1073. DOI: 10.1001/jama.2021.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martínez‐González MÁ, Toledo E, Arós F, Fiol M, Corella D, Salas‐Salvadó J, Ros E, Covas MI, Fernández‐Crehuet J, Lapetra J, et al. Extravirgin olive oil consumption reduces risk of atrial fibrillation: the PREDIMED (Prevencion con Dieta Mediterranea) trial. Circulation. 2014;130:18–26. DOI: 10.1161/CIRCULATIONAHA.113.006921. [DOI] [PubMed] [Google Scholar]
- 42.Barrio‐Lopez MT, Ruiz‐Canela M, Ramos P, Tercedor L, Ibañez Criado JL, Ortiz M, Goni L, Ibañez Criado A, Macías‐Ruiz R, García‐Bolao I, et al. PREvention of recurrent arrhythmias with Mediterranean diet (PREDIMAR) study in patients with atrial fibrillation: rationale, design and methods. Am Heart J. 2020;220:127–136. DOI: 10.1016/j.ahj.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Jin MN, Yang PS, Song C, Yu HT, Kim TH, Uhm JS, Sung JH, Pak HN, Lee MH, Joung B. Physical activity and risk of atrial fibrillation: a nationwide cohort study in general population. Sci Rep. 2019;9:13270. DOI: 10.1038/s41598-019-49686-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung MK, Eckhardt LL, Chen LY, Ahmed HM, Gopinathannair R, Joglar JA, Noseworthy PA, Pack QR, Sanders P, Trulock KM, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141:e750–e772. DOI: 10.1161/CIR.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 45.Risom SS, Zwisler AD, Johansen PP, Sibilitz KL, Lindschou J, Gluud C, Taylor RS, Svendsen JH, Berg SK. Exercise‐based cardiac rehabilitation for adults with atrial fibrillation. Cochrane Database Syst Rev. 2017;2:CD011197. DOI: 10.1002/14651858.CD011197.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohanty S, Mohanty P, Tamaki M, Natale V, Gianni C, Trivedi C, Gokoglan Y, Di Biase L, Natale A. Differential association of exercise intensity with risk of atrial fibrillation in men and women: evidence from a meta‐analysis. J Cardiovasc Electrophysiol. 2016;27:1021–1029. DOI: 10.1111/jce.13023. [DOI] [PubMed] [Google Scholar]
- 47.NHFA CSANZ Atrial Fibrillation Guideline Working Group , Brieger D, Amerena J, Attia J, Bajorek B, Chan KH, Connell C, Freedman B, Ferguson C, Hall T, Haqqani H, et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the diagnosis and management of atrial fibrillation 2018. Heart Lung Circ. 2018;27:1209–1266. DOI: 10.1016/j.hlc.2018.06.1043. [DOI] [PubMed] [Google Scholar]
- 48.Smart NA, King N, Lambert JD, Pearson MJ, Campbell JL, Risom SS, Taylor RS. Exercise‐based cardiac rehabilitation improves exercise capacity and health‐related quality of life in people with atrial fibrillation: a systematic review and meta‐analysis of randomised and non‐randomised trials. Open Heart. 2018;5:e000880. DOI: 10.1136/openhrt-2018-000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo N, Merrill P, Parikh KS, Whellan DJ, Piña IL, Fiuzat M, Kraus WE, Kitzman DW, Keteyian SJ, O’Connor CM, et al. Exercise training in patients with chronic heart failure and atrial fibrillation. J Am Coll Cardiol. 2017;69:1683–1691. DOI: 10.1016/j.jacc.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Neill D, Forman DE. Never too old for cardiac rehabilitation. Clin Geriatr Med. 2019;35:407–421. DOI: 10.1016/j.cger.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, Dalal H, Rees K, Singh SJ, Taylor RS. Exercise‐based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019;1:CD003331. DOI: 10.1002/14651858.CD003331.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brügemann J, Geelhoed B, Tieleman RG, Hillege HL, Tukkie R, et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J. 2018;39:2987–2996. DOI: 10.1093/eurheartj/ehx739. [DOI] [PubMed] [Google Scholar]
- 53.Shi S, Shi J, Jia Q, Shi S, Yuan G, Hu Y. Efficacy of physical exercise on the quality of life, exercise ability, and cardiopulmonary fitness of patients with atrial fibrillation: a systematic review and meta‐analysis. Front Physiol. 2020;11:740. DOI: 10.3389/fphys.2020.00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ng SM, Wang CW, Ho RT, Ziea TC, He J, Wong VC, Chan CL. Tai chi exercise for patients with heart disease: a systematic review of controlled clinical trials. Altern Ther Health Med. 2012;18:16–22. [PubMed] [Google Scholar]
- 55.Tang LH, Kikkenborg Berg S, Christensen J, Lawaetz J, Doherty P, Taylor RS, Langberg H, Zwisler AD. Patients' preference for exercise setting and its influence on the health benefits gained from exercise‐based cardiac rehabilitation. Int J Cardiol. 2017;232:33–39. DOI: 10.1016/j.ijcard.2017.01.126. [DOI] [PubMed] [Google Scholar]
- 56.Kornej J, Borschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127:4–20. DOI: 10.1161/CIRCRESAHA.120.316340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linz D, McEvoy RD, Cowie MR, Somers VK, Nattel S, Levy P, Kalman JM, Sanders P. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3:532–540. DOI: 10.1001/jamacardio.2018.0095. [DOI] [PubMed] [Google Scholar]
- 58.Holmqvist F, Guan NI, Zhu Z, Kowey PR, Allen LA, Fonarow GC, Hylek EM, Mahaffey KW, Freeman JV, Chang P, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation‐results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Am Heart J. 2015;169:647–654.e2. DOI: 10.1016/j.ahj.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 59.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman AS, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–2594. DOI: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 60.Deng F, Raza A, Guo J. Treating obstructive sleep apnea with continuous positive airway pressure reduces risk of recurrent atrial fibrillation after catheter ablation: a meta‐analysis. Sleep Med. 2018;46:5–11. DOI: 10.1016/j.sleep.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Linz D, Woehrle H, Bitter T, Fox H, Cowie MR, Bohm M, Oldenburg O. The importance of sleep‐disordered breathing in cardiovascular disease. Clin Res Cardiol. 2015;104:705–718. DOI: 10.1007/s00392-015-0859-7. [DOI] [PubMed] [Google Scholar]
- 62.Dimitri H, Ng M, Brooks AG, Kuklik P, Stiles MK, Lau DH, Antic N, Thornton A, Saint DA, McEvoy D, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9:321–327. DOI: 10.1016/j.hrthm.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 63.Neilan TG, Farhad H, Dodson JA, Shah RV, Abbasi SA, Bakker JP, Michaud GF, van der Geest R, Blankstein R, Steigner M, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. 2013;2:e000421. DOI: 10.1161/JAHA.113.000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caples SM, Mansukhani MP, Friedman PA, Somers VK. The impact of continuous positive airway pressure treatment on the recurrence of atrial fibrillation post cardioversion: a randomized controlled trial. Int J Cardiol. 2019;278:133–136. DOI: 10.1016/j.ijcard.2018.11.100. [DOI] [PubMed] [Google Scholar]
- 65.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, Mediano O, Chen R, Drager LF, Liu Z, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. DOI: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 66.Genuardi MV, Ogilvie RP, Saand AR, DeSensi RS, Saul MI, Magnani JW, Patel SR. Association of short sleep duration and atrial fibrillation. Chest. 2019;156:544–552. DOI: 10.1016/j.chest.2019.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christensen MA, Dixit S, Dewland TA, Whitman IR, Nah G, Vittinghoff E, Mukamal KJ, Redline S, Robbins JA, Newman AB, et al. Sleep characteristics that predict atrial fibrillation. Heart Rhythm. 2018;15:1289–1295. DOI: 10.1016/j.hrthm.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie J, Chahal CAA, Covassin N, Schulte PJ, Singh P, Srivali N, Somers VK, Caples SM. Periodic limb movements of sleep are associated with an increased prevalence of atrial fibrillation in patients with mild sleep‐disordered breathing. Int J Cardiol. 2017;241:200–204. DOI: 10.1016/j.ijcard.2017.04.060. [DOI] [PubMed] [Google Scholar]
- 69.Mitchell AR, Spurrell PA, Sulke N. Circadian variation of arrhythmia onset patterns in patients with persistent atrial fibrillation. Am Heart J. 2003;146:902–907. DOI: 10.1016/S0002-8703(03)00405-8. [DOI] [PubMed] [Google Scholar]
- 70.Gami AS, Hodge DO, Herges RM, Olson EJ, Nykodym J, Kara T, Somers VK. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565–571. DOI: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 71.May AM, Blackwell T, Stone PH, Stone KL, Cawthon PM, Sauer WH, Varosy PD, Redline S, Mehra R; MrOS Sleep (Outcomes of Sleep Disorders in Older Men) Study Group. Central sleep‐disordered breathing predicts incident atrial fibrillation in older men. Am J Respir Crit Care Med. 2016;193:783–791. DOI: 10.1164/rccm.201508-1523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Defaye P, de la Cruz I, Marti‐Almor J, Villuendas R, Bru P, Senechal J, Tamisier R, Pepin JL. A pacemaker transthoracic impedance sensor with an advanced algorithm to identify severe sleep apnea: the DREAM European study. Heart Rhythm. 2014;11:842–848. DOI: 10.1016/j.hrthm.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 73.Bakker JP, Wang R, Weng J, Aloia MS, Toth C, Morrical MG, Gleason KJ, Rueschman M, Dorsey C, Patel SR, et al. Motivational enhancement for increasing adherence to CPAP: a randomized controlled trial. Chest. 2016;150:337–345. DOI: 10.1016/j.chest.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang B, Liu H, Scherlag BJ, Sun L, Xing S, Xu J, Luo M, Guo Y, Cao G, Jiang H. Atrial fibrillation in obstructive sleep apnea: neural mechanisms and emerging therapies. Trends Cardiovasc Med. 2021;31:127–132. DOI: 10.1016/j.tcm.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 75.Dieleman JL, Cao J, Chapin A, Chen C, Li Z, Liu A, Horst C, Kaldjian A, Matyasz T, Scott KW, et al. US health care spending by payer and health condition, 1996–2016. JAMA. 2020;323:863–884. DOI: 10.1001/jama.2020.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirchhof P, Breithardt G, Bax J, Benninger G, Blomstrom‐Lundqvist C, Boriani G, Brandes A, Brown H, Brueckmann M, Calkins H, et al. A roadmap to improve the quality of atrial fibrillation management: proceedings from the fifth Atrial Fibrillation Network/European Heart Rhythm Association consensus conference. Europace. 2016;18:37–50. DOI: 10.1093/europace/euv304. [DOI] [PubMed] [Google Scholar]
- 77.Gallagher C, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, Mahajan R, Lau DH, Sanders P, Hendriks JML. Integrated care in atrial fibrillation: a systematic review and meta‐analysis. Heart. 2017;103:1947–1953. DOI: 10.1136/heartjnl-2016-310952. [DOI] [PubMed] [Google Scholar]
- 78.Hendriks JML, Tieleman RG, Vrijhoef HJM, Wijtvliet P, Gallagher C, Prins MH, Sanders P, Crijns H. Integrated specialized atrial fibrillation clinics reduce all‐cause mortality: post hoc analysis of a randomized clinical trial. Europace. 2019;21:1785–1792. DOI: 10.1093/europace/euz209. [DOI] [PubMed] [Google Scholar]
- 79.Hendriks J, Tomini F, van Asselt T, Crijns H, Vrijhoef H. Cost‐effectiveness of a specialized atrial fibrillation clinic vs. usual care in patients with atrial fibrillation. Europace. 2013;15:1128–1135. DOI: 10.1093/europace/eut055. [DOI] [PubMed] [Google Scholar]
- 80.Saraswat MK, Carter L, Berrigan P, Sapp JL, Gray C, Fearon A, Gardner M, Parkash R. Integrated management approach to atrial fibrillation care: a cost utility analysis. Can J Cardiol. 2019;35:1142–1148. DOI: 10.1016/j.cjca.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 81.Wijtvliet E, Tieleman RG, van Gelder IC, Pluymaekers N, Rienstra M, Folkeringa RJ, Bronzwaer P, Elvan A, Elders J, Tukkie R, et al. Nurse‐led vs. usual‐care for atrial fibrillation. Eur Heart J. 2020;41:634–641. DOI: 10.1093/eurheartj/ehz666. [DOI] [PubMed] [Google Scholar]
- 82.Hendriks JM, Brooks AG, Rowett D, Moss JR, Gallagher C, Nyfort‐Hansen K, Simmons S, Middeldorp ME, Jones T, Thomas G, et al. Home‐based education and learning program for atrial fibrillation: rationale and design of the HELP‐AF study. Can J Cardiol. 2019;35:846–854. DOI: 10.1016/j.cjca.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 83.Proietti M, Romiti GF, Olshansky B, Lane DA, Lip GYH. Improved outcomes by integrated care of anticoagulated patients with atrial fibrillation using the simple ABC (Atrial Fibrillation Better Care) pathway. Am J Med. 2018;131:1359–1366.e6. DOI: 10.1016/j.amjmed.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 84.Murray KT, Mace LC, Yang Z. Nonantiarrhythmic drug therapy for atrial fibrillation. Heart Rhythm. 2007;4:S88–S90. DOI: 10.1016/j.hrthm.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 85.Deftereos S, Giannopoulos G, Kossyvakis C, Efremidis M, Panagopoulou V, Kaoukis A, Raisakis K, Bouras G, Angelidis C, Theodorakis A, et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol. 2012;60:1790–1796. DOI: 10.1016/j.jacc.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 86.Yao C, Veleva T, Scott L Jr, Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsina KM, Abu‐Taha I, et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–2242. DOI: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meijering RA, Zhang D, Hoogstra‐Berends F, Henning RH, Brundel BJ. Loss of proteostatic control as a substrate for atrial fibrillation: a novel target for upstream therapy by heat shock proteins. Front Physiol. 2012;3:36. DOI: 10.3389/fphys.2012.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mayorov V, Uchakin P, Amarnath V, Panov AV, Bridges CC, Uzhachenko R, Zackert B, Moore CS, Davies S, Dikalova A, et al. Targeting of reactive isolevuglandins in mitochondrial dysfunction and inflammation. Redox Biol. 2019;26:101300. DOI: 10.1016/j.redox.2019.101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davies SS, May‐Zhang LS, Boutaud O, Amarnath V, Kirabo A, Harrison DG. Isolevuglandins as mediators of disease and the development of dicarbonyl scavengers as pharmaceutical interventions. Pharmacol Ther. 2020;205:107418. DOI: 10.1016/j.pharmthera.2019.107418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prinsen JK, Kannankeril PJ, Sidorova TN, Yermalitskaya LV, Boutaud O, Zagol‐Ikapitte I, Barnett JV, Murphy MB, Subati T, Stark JM, et al. Highly reactive isolevuglandins promote atrial fibrillation caused by hypertension. JACC Basic Transl Sci. 2020;5:602–615. DOI: 10.1016/j.jacbts.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Marion DM, Hu X, Zhang D, Hoogstra‐Berends F, Seerden JG, Loen L, Heeres A, Steen H, Henning RH, Brundel BJ. Screening of novel HSP‐inducing compounds to conserve cardiomyocyte function in experimental atrial fibrillation. Drug Des Devel Ther. 2019;13:345–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim GE, Ross JL, Xie C, Su KN, Zaha VG, Wu X, Palmeri M, Ashraf M, Akar JG, Russell KS, et al. LKB1 deletion causes early changes in atrial channel expression and electrophysiology prior to atrial fibrillation. Cardiovasc Res. 2015;108:197–208. DOI: 10.1093/cvr/cvv212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang SH, Wu LS, Chiou MJ, Liu JR, Yu KH, Kuo CF, Wen MS, Chen WJ, Yeh YH, See LC. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population‐based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13:123. DOI: 10.1186/s12933-014-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE‐TIMI 58 trial. Circulation. 2020;141:1227–1234. DOI: 10.1161/CIRCULATIONAHA.119.044183. [DOI] [PubMed] [Google Scholar]
- 95.Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co‐transporter‐2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high‐fat diet/streptozotocin‐induced diabetic rats. Cardiovasc Diabetol. 2019;18:165. DOI: 10.1186/s12933-019-0964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xie W, Santulli G, Reiken SR, Yuan Q, Osborne BW, Chen BX, Marks AR. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep. 2015;5:11427. DOI: 10.1038/srep11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van Wagoner DR, Piccini JP, Albert CM, Anderson ME, Benjamin EJ, Brundel B, Califf RM, Calkins H, Chen P‐S, Chiamvimonvat N, et al. Progress toward the prevention and treatment of atrial fibrillation: a summary of the Heart Rhythm Society Research Forum on the Treatment and Prevention of Atrial Fibrillation, Washington, DC, December 9–10, 2013. Heart Rhythm. 2015;12:e5–e29. DOI: 10.1016/j.hrthm.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dobrev D, Wehrens XHT. Calcium‐mediated cellular triggered activity in atrial fibrillation. J Physiol. 2017;595:4001–4008. DOI: 10.1113/JP273048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Faggioni M, Savio‐Galimberti E, Venkataraman R, Hwang HS, Kannankeril PJ, Darbar D, Knollmann BC. Suppression of spontaneous ca elevations prevents atrial fibrillation in calsequestrin 2‐null hearts. Circ Arrhythm Electrophysiol. 2014;7:313–320. DOI: 10.1161/CIRCEP.113.000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. DOI: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hegbom F, Sire S, Heldal M, Orning OM, Stavem K, Gjesdal K. Short‐term exercise training in patients with chronic atrial fibrillation: effects on exercise capacity, AV conduction, and quality of life. J Cardiopulm Rehabil. 2006;26:24–29. DOI: 10.1097/00008483-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 102.Hegbom F, Stavem K, Sire S, Heldal M, Orning OM, Gjesdal K. Effects of short‐term exercise training on symptoms and quality of life in patients with chronic atrial fibrillation. Int J Cardiol. 2007;116:86–92. DOI: 10.1016/j.ijcard.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 103.Malmo V, Nes BM, Amundsen BH, Tjonna AE, Stoylen A, Rossvoll O, Wisloff U, Loennechen JP. Aerobic interval training reduces the burden of atrial fibrillation in the short term: a randomized trial. Circulation. 2016;133:466–473. DOI: 10.1161/CIRCULATIONAHA.115.018220. [DOI] [PubMed] [Google Scholar]
- 104.Osbak PS, Mourier M, Kjaer A, Henriksen JH, Kofoed KF, Jensen GB. A randomized study of the effects of exercise training on patients with atrial fibrillation. Am Heart J. 2011;162:1080–1087. DOI: 10.1016/j.ahj.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 105.Pippa L, Manzoli L, Corti I, Congedo G, Romanazzi L, Parruti G. Functional capacity after traditional Chinese medicine (qi gong) training in patients with chronic atrial fibrillation: a randomized controlled trial. Prev Cardiol. 2007;10:22–25. DOI: 10.1111/j.1520-037X.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- 106.Wahlstrom M, Rydell Karlsson M, Medin J, Frykman V. Effects of yoga in patients with paroxysmal atrial fibrillation—a randomized controlled study. Eur J Cardiovasc Nurs. 2017;16:57–63. DOI: 10.1177/1474515116637734. [DOI] [PubMed] [Google Scholar]
- 107.Zeren M, Demir R, Yigit Z, Gurses HN. Effects of inspiratory muscle training on pulmonary function, respiratory muscle strength and functional capacity in patients with atrial fibrillation: a randomized controlled trial. Clin Rehabil. 2016;30:1165–1174. DOI: 10.1177/0269215515628038. [DOI] [PubMed] [Google Scholar]
- 108.Risom SS, Zwisler AD, Rasmussen TB, Sibilitz KL, Madsen TL, Svendsen JH, Gluud C, Lindschou J, Winkel P, Berg SK. Cardiac rehabilitation versus usual care for patients treated with catheter ablation for atrial fibrillation: results of the randomized CopenHeartRFA trial. Am Heart J. 2016;181:120–129. DOI: 10.1016/j.ahj.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 109.Risom SS, Zwisler AD, Rasmussen TB, Sibilitz KL, Svendsen JH, Gluud C, Hansen JL, Winkel P, Thygesen LC, Perhonen M, et al. The effect of integrated cardiac rehabilitation versus treatment as usual for atrial fibrillation patients treated with ablation: the randomised CopenHeartRFA trial protocol. BMJ Open. 2013;3:e002377. DOI: 10.1136/bmjopen-2012-002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Risom SS, Zwisler AD, Sibilitz KL, Rasmussen TB, Taylor RS, Thygesen LC, Madsen TS, Svendsen JH, Berg SK. Cardiac rehabilitation for patients treated for atrial fibrillation with ablation has long‐term effects: 12‐and 24‐month follow‐up results from the randomized CopenHeartRFA Trial. Arch Phys Med Rehabil. 2020;101:1877–1886. DOI: 10.1016/j.apmr.2020.06.026. [DOI] [PubMed] [Google Scholar]
- 111.Skielboe AK, Bandholm TQ, Hakmann S, Mourier M, Kallemose T, Dixen U. Cardiovascular exercise and burden of arrhythmia in patients with atrial fibrillation—a randomized controlled trial. PLoS One. 2017;12:e0170060. DOI: 10.1371/journal.pone.0170060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF‐ACTION): design and rationale. Am Heart J. 2007;153:201–211. DOI: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 113.Reed JL, Clarke AE, Faraz AM, Birnie DH, Tulloch HE, Reid RD, Pipe AL. The impact of cardiac rehabilitation on mental and physical health in patients with atrial fibrillation: a matched case‐control study. Can J Cardiol. 2018;34:1512–1521. DOI: 10.1016/j.cjca.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 114.Alharbi M, Giacomantonio N, Carter L, Sapp J, Gardner M, Gray CJ, AbdelWahab AM, Parkash R. The effect of cardiac rehabilitation and a specialized clinic on outcomes of patients with atrial fibrillation. Can J Cardiol. 2019;35:382–388. DOI: 10.1016/j.cjca.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 115.Kato M, Ogano M, Mori Y, Kochi K, Morimoto D, Kito K, Green FN, Tsukamoto T, Kubo A, Takagi H, et al. Exercise‐based cardiac rehabilitation for patients with catheter ablation for persistent atrial fibrillation: a randomized controlled clinical trial. Eur J Prev Cardiol. 2019;26:1931–1940. DOI: 10.1177/2047487319859974. [DOI] [PubMed] [Google Scholar]
- 116.De With RR, Rienstra M, Smit MD, Weijs B, Zwartkruis VW, Hobbelt AH, Alings M, Tijssen JGP, Brügemann J, Geelhoed B, et al. Targeted therapy of underlying conditions improves quality of life in patients with persistent atrial fibrillation: results of the RACE 3 study. Europace. 2019;21:563–571. DOI: 10.1093/europace/euy311. [DOI] [PubMed] [Google Scholar]
- 117.Nguyen BO, Rienstra M, Hobbelt AH, Tijssen JGP, Smit MD, Tieleman RG, Geelhoed B, Van Veldhuisen DJ, Crijns H, Van Gelder IC, et al. Optimal treatment of underlying conditions improves rhythm control outcome in atrial fibrillation—data from RACE 3. Am Heart J. 2020;226:235–239. DOI: 10.1016/j.ahj.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 118.Joensen AM, Dinesen PT, Svendsen LT, Hoejbjerg TK, Fjerbaek A, Andreasen J, Sottrup MB, Lundbye‐Christensen S, Vadmann H, Riahi S. Effect of patient education and physical training on quality of life and physical exercise capacity in patients with paroxysmal or persistent atrial fibrillation: a randomized study. J Rehabil Med. 2019;51:442–450. DOI: 10.2340/16501977-2551. [DOI] [PubMed] [Google Scholar]
- 119.Borland M, Bergfeldt L, Nordeman L, Bollano E, Andersson L, Rosenkvist A, Jakobsson M, Olsson K, Corin M, Landh L, et al. Exercise‐based cardiac rehabilitation improves physical fitness in patients with permanent atrial fibrillation—a randomized controlled study. Transl Sports Med. 2020;3:415–425. DOI: 10.1002/tsm2.166. [DOI] [Google Scholar]
- 120.Mohanty S, Trivedi C, Gianni C, Al‐Ahmad A, Burkhardt JD, Horton R, Sanchez J, Hranitzky P, Gallinghouse GJ, Rocca DGD, et al. Abstract 16466: real‐world difficulties in conducting a clinical trial on life‐style modifications in patients with atrial fibrillation. Circulation. 2017;136:A16466. [Google Scholar]
- 121.Balsam P, Lodziński P, Tymińska A, Ozierański K, Januszkiewicz Ł, Główczyńska R, Wesołowska K, Peller M, Pietrzak R, Książczyk T, et al. Study design and rationale for biomedical shirt‐based electrocardiography monitoring in relevant clinical situations: ECG‐shirt study. Cardiol J. 2018;25:52–59. DOI: 10.5603/CJ.a2017.0102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
References 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121


