Abstract
Background
Cardiovascular disease is an important cause of mortality among survivors of breast cancer (BC). We developed a prediction model for major adverse cardiovascular events after BC therapy, which is based on conventional and BC treatment‐related cardiovascular risk factors.
Methods and Results
The cohort of the study consisted of 1256 Asian female patients with BC from 4 medical centers in Korea and was randomized in a 1:1 ratio into the derivation and validation cohorts. The outcome measures comprised cardiovascular mortality, myocardial infarction, congestive heart failure, and transient ischemic attack/stroke. To correct overfitting, a penalized Cox proportional hazards regression was performed with a cross‐validation approach. Number of cardiovascular diseases (myocardial infarction, peripheral artery disease, heart failure, and transient ischemic attack/stroke), number of baseline cardiovascular risk factors (hypertension, age ≥60, body mass index ≥30 kg/m2, estimated glomerular filtration rate <60 mL/min per 1.73 m2, dyslipidemia, and diabetes mellitus), radiation to the left breast, and anthracycline dose per 100 mg/m2 were included in the risk prediction model. The time‐dependent C‐indices at 3 and 7 years after BC diagnosis were 0.876 and 0.842, respectively, in the validation cohort.
Conclusions
A prediction score model, including BC treatment‐related risk factors and conventional risk factors, was developed and validated to predict major adverse cardiovascular events in patients with BC. The CHEMO‐RADIAT (congestive heart failure, hypertension, elderly, myocardial infarction/peripheral artery occlusive disease, obesity, renal failure, abnormal lipid profile, diabetes mellitus, irradiation of the left breast, anthracycline dose, and transient ischemic attack/stroke) score may provide overall cardiovascular risk stratification in survivors of BC and can assist physicians in multidisciplinary decision‐making regarding the BC treatment.
Keywords: breast cancer, major adverse cardiovascular events, multicenter cohort, prediction model, risk stratification
Subject Categories: Cardio-Oncology, Cardiovascular Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- BC
breast cancer
- MACE
major adverse cardiovascular events
Clinical Perspective
What Is New?
This study derived and validated a predictive model (CHEMO‐RADIAT; congestive heart failure, hypertension, elderly, myocardial infarction/peripheral artery occlusive disease, obesity, renal failure, abnormal lipid profile, diabetes mellitus, irradiation of the left breast, anthracycline dose, and transient ischemic attack/stroke) for the major cardiovascular events that may occur after the diagnosis of breast cancer (BC) in a real‐world multicenter cohort.
The CHEMO‐RADIAT score is the first predictive model that is based on both conventional cardiovascular risk factors (heart failure, hypertension, old age [age ≥60 years], myocardial infarction/peripheral artery disease, obesity [body mass index ≥30 kg/m2], renal failure [estimated glomerular filtration rate <60 mL/min per 1.73 m2], dyslipidemia, diabetes mellitus, and transient ischemic attack/stroke), and BC treatment‐related cardiovascular risk factors (radiation to the left breast and the doxorubicin equivalent dose per 100 mg/m2).
The combination of conventional and BC treatment‐related cardiovascular risk factors improved the performance of the predictive model; the CHEMO‐RADIAT score performed well in predicting major cardiovascular events for survivors of BC.
What Are the Clinical Implications?
Risk stratification using a risk scoring system may improve cardiovascular outcomes of patients with BC by identifying the patients at risk for cardiovascular events.
The CHEMO‐RADIAT score may be a useful tool for multidisciplinary decision‐making regarding therapeutic options for BC treatment.
Although the incidence of breast cancer (BC) has risen,1 in some countries, recent improvements in BC treatment achieved a significant reduction in cancer‐specific mortality among women with BC.2 As survivors of BC live longer, the survivors have a higher probability of noncancer mortality and more attention toward comorbidities in the management of BC survivorship is required.3 Among these comorbidities, cardiovascular death is a major cause of mortality in survivors of BC.4 The cardiovascular risk could be even higher in these survivors because treatment options for BC treatment, such as anthracycline, radiation therapy, and trastuzumab, have cardiotoxic effects.5 Therefore, it is important to identify patients with BC who are at high risk of major adverse cardiovascular events (MACE) to guide further cancer therapy decisions.6 Recent studies have highlighted the importance of conventional cardiovascular risk factors in cardiovascular outcomes among survivors of BC.7, 8 In this regard, a risk stratification scheme using both conventional and BC treatment‐related cardiovascular risk factors may provide a more comprehensive assessment of the risk of MACE. The performance of the predictive model can be improved by combining both conventional and BC treatment‐related cardiovascular risk factors. Thus, the purpose of this study was to develop and validate a risk scoring system that included both conventional and BC treatment‐related cardiovascular risk factors for MACE among survivors of BC.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Cohort Creation
We retrospectively identified 1443 consecutive female patients with BC who were diagnosed at 4 affiliated hospitals (Hallym University Sacred Heart Hospital, Kangnam Sacred Heart Hospital, Chuncheon Sacred Heart Hospital, and Dongtan Sacred Heart Hospital) in South Korea from November 2005 to September 2015. All the subjects were adult Asian women. The exclusion criteria were a history of surgical treatment, radiotherapy, or chemotherapy in patients with BC, conducted in hospitals other than the 4 specified, history of anthracycline‐based chemotherapy before inclusion in this study, or unavailability of accurate treatment‐related information. Finally, a total of 1256 patients were enrolled in this study. The study population was randomized on a 1:1 ratio into the derivation and validation cohorts. Various patient demographics, including age, sex, body mass index, comorbid conditions, and estimated glomerular filtration rate, were extracted from the Clinical Data Warehouse of Hallym Medical Center. Detailed data regarding the BC therapy, including the anthracycline and total radiation doses, were reviewed from the hospital medical records. Survival and cardiovascular outcomes were identified from the Clinical Data Warehouse of Hallym Medical Center. We then surveyed the medical records of the outpatient, hospital, and emergency departments to confirm the diagnoses of the main outcome end points. A myocardial infarction was defined as (1) anginal chest pain lasting for at least 30 minutes, (2) significant ST‐segment changes in 2 contiguous leads of a standard 12‐lead electrocardiogram, and (3) either rise of the creatine kinase–MB isoform to more than twice the normal upper limit or troponin T elevation exceeding 0.1 ng/mL. Patients were considered to have heart failure (HF) if they presented with signs or symptoms of HF with elevated N‐terminal fragment of B‐type natriuretic peptide or B‐type natriuretic peptide levels and 1 of the following criteria: (1) lung congestion or (2) objective findings of left ventricular systolic dysfunction or structural heart disease.9, 10 To assess lung congestion, we reviewed chest radiography and the medical chart. A stroke was defined as a sudden onset of the relevant focal deficits, documented by neurological examination and lasting >24 hours. Transient ischemic attack was defined as the acute onset of a focal neurologic symptom lasting less than 24 hours. All the medical records of the patients with cardiovascular events were reviewed and validated by an independent adjudication committee. The study was approved by the Institutional Review Board of Hallym University Dongtan Sacred Heart Hospital. The requirement for informed consent was waived because of the retrospective study design.
Formula for the Doxorubicin Equivalent Dose
We used the following formulas from a previous report to convert to the doxorubicin isotoxic equivalent dose before calculating the total cumulative anthracycline dose: for doxorubicin, we used the total dose; for daunorubicin, we multiplied the total dose by 0.5; for epirubicin, we multiplied the total dose by 0.5; and for idarubicin, we multiplied the total dose by 2 (for example, if a patient received epirubicin at a dose of 90 mg/m2, the patient’s doxorubicin equivalent dose was 90×0.5=45 mg/m2).11
Statistical Analysis
Continuous variables are expressed as mean±SD, and categorical data are expressed as numbers and percentages. Nonparametrically distributed continuous variables are reported as median values with interquartile ranges. For comparisons across groups, continuous variables were compared using Student’s t test, and categorical variables were analyzed using the chi‐square test or Fisher’s exact test, as appropriate. Survival data were analyzed using the Kaplan–Meier method with the log‐rank test. Cox proportional hazard model was used for predicting MACE in the derivation cohort. A penalized Cox proportional hazards model was used to avoid overfitting.12 We used the Least Absolute Shrinkage and Selection Operator method, and the optimal penalty factor was assessed by likelihood cross‐validation approach using the “penalized” package in R.13 To build the risk‐score chart, the score for each variable was calculated by dividing each penalized regression coefficient by the smallest penalized coefficient and rounding it to the nearest integer.14 To quantify the discriminative ability of each predictive model, the time‐dependent C‐indices at 1, 3, and 7 years for censored survival data were examined. Values of C‐index close to 1 indicate good discriminative power, and values below 0.5 or 0.5 mean poor discrimination ability. The low, intermediate, and high risks for MACE score thresholds were defined based on visual inspection of the Kaplan–Meier curve, the P values from the log‐rank test, and the risk criteria for cardiac dysfunction after cancer therapy proposed by the American Society of Clinical Oncology Clinical Practice Guidelines for Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers.15 The calibration of the models was assessed using the Hosmer‐Lemeshow test. Decision curve analysis was performed to compare the proposed model with the prediction model based on the conventional cardiovascular risk factors in the validation cohort. A value of P<0.05 was considered statistically significant. Statistical analyses were performed using R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Demographics
The baseline characteristics of the cohort are presented in Table 1. The mean population age was 51.4±10.7 years, and the median follow‐up duration was 48.7 (25.8–71.8) months. All subjects underwent radiotherapy and surgery for BC. Regarding treatment, 70.7% of the patients underwent chemotherapy with a regimen containing anthracycline, 884 (70.4%) patients received selective estrogen receptor modulators or aromatase inhibitors, and 150 (11.9%) patients received treatment with antihuman epidermal growth factor receptor antibodies. The median cumulative doxorubicin equivalent dose was 240 [240–300] mg/m2. The derivation and validation cohorts were well balanced except regarding the presence of diabetes mellitus (DM). During the 5402.2 person‐years of follow‐up, 21 patients (1.7%) experienced MACE (1.6% versus 1.8% in the development and validation groups, respectively; P=1.0), and 34 deaths were identified. The median time interval between the MACE and cancer diagnosis was 22.6 (16.9–45.2) months. The most common cause of death was cancer, followed by cardiovascular death and infection (25, 3, and 3 deaths, respectively). The 7‐year overall survival rate and cancer‐specific survival rate were 96.3% (95% CI, 94.9–97.8%) and 97.1% (95% CI, 95.8–98.5%), respectively. The cardiovascular death‐free survival rate was 99.8% (95% CI, 0.995–0.999) at 7 years.
Table 1.
Baseline Characteristics
| Derivation cohort (N=628) | Validation cohort (N=628) | Total (N=1256) | P Value | |
|---|---|---|---|---|
| Age, y | 51.4±10.7 | 51.5±10.7 | 51.4±10.7 | 0.876 |
| Hypertension, n (%) | 103 (16.4) | 104 (16.6) | 207 (16.5) | 1 |
| Diabetes mellitus, n (%) | 39 (6.2) | 70 (11.1) | 109 (8.7) | 0.003 |
| Dyslipidemia, (%) | 171 (27.2) | 179 (28.5) | 350 (27.9) | 0.66 |
| Prior myocardial infarction or peripheral artery occlusive disease, n (%) | 4 (0.6) | 4 (0.6) | 8 (0.6) | 1 |
| Prior HF, n (%) | 2 (0.3) | 3 (0.5) | 5 (0.4) | 1 |
| eGFR, mL/min per 1.73 m² | 96.3±23.2 | 96.2±23.0 | 96.3±23.1 | 0.921 |
| eGFR<60 mL/min per 1.73 m² | 15 (2.4) | 18 (2.9) | 33 (2.6) | 0.724 |
| BMI, kg/m2 | 24.2±3.5 | 24.5±3.5 | 24.4±3.5 | 0.182 |
| BMI ≥30 kg/m2 | 33 (5.3) | 46 (7.3) | 79 (6.3) | 0.163 |
| Cancer stage, n (%) | 0.51 | |||
| 0 | 9 (1.4) | 3 (0.5) | 12 (1.0) | |
| I | 267 (42.5) | 274 (43.6) | 541 (43.1) | |
| II | 244 (38.9) | 239 (38.1) | 483 (38.5) | |
| III | 104 (16.6) | 107 (17.0) | 211 (16.8) | |
| IV | 4 (0.6) | 5 (0.8) | 9 (0.7) | |
| Side of radiation therapy, n (%) | 0.968 | |||
| Right side | 316 (50.3) | 313 (49.8) | 629 (50.1) | |
| Left side | 301 (47.9) | 303 (48.2) | 604 (48.1) | |
| Both sides | 11 (1.8) | 12 (1.9) | 23 (1.8) | |
| Total radiation dose, Gy | 58.1±5.9 | 58.4±6.2 | 58.2±6.1 | 0.464 |
| Surgical treatment, n (%) | 0.257 | |||
| None | 1 (0.2) | 0 (0.0) | 1 (0.1) | |
| Breast conserving surgery | 522 (83.1) | 499 (79.5) | 1021 (81.3) | |
| Radical mastectomy | 102 (16.2) | 126 (20.0) | 228 (18.1) | |
| Others | 3 (0.5) | 3 (0.5) | 6 (0.5) | |
| Chemotherapy, n (%) | 497 (79.1) | 501 (79.9) | 998 (79.5) | 0.791 |
| Use of anthracycline, n (%) | 444 (70.7) | 444 (70.7) | 888 (70.7) | 1 |
| Doxorubicin equivalent dose, mg/m2 * | 240.0 [240.0–300.0] | 240.0 [240.0–300.0] | 240.0 [240.0–300.0] | 0.898 |
| Endocrine treatment, n (%) | 424 (67.5) | 460 (73.2) | 884 (70.4) | 0.031 |
| Trastuzumab, n (%) | 73 (11.6) | 77 (12.3) | 150 (11.9) | 0.794 |
| Cardiovascular events, n (%) | 11 (1.8) | 10 (1.6) | 21 (1.7) | 1 |
| HF, n (%) | 5 (0.8) | 5 (0.8) | 10 (0.8) | |
| Myocardial infarction, n (%) | 1 (0.2) | 1 (0.2) | 2 (0.2) | |
| Stroke, n (%) | 4 (0.6) | 5 (0.8) | 9 (0.7) | |
| Cardiovascular deaths, n (%) | 2 (0.3) | 1 (0.2) | 3 (0.2) |
Values are shown as mean±SD and number (percentage) for continuous and categorical variables. The differences in the groups are presented as overall P values.
BMI indicates body mass index; eGFR, estimated glomerular filtration rate; and HF, heart failure.
Doxorubicin equivalent dose among patients who underwent anthracycline‐based chemotherapy.
Development of a New Risk Score for MACE Among Survivors of BC
The information for the development of the new scoring system was extracted from the derivation cohort. Table 2 shows the estimated hazard ratios, coefficients for a cardiovascular risk prediction model, including BC treatment‐related risk factors and conventional risk factors. Number of baseline cardiovascular risk factors (hypertension, old age [≥60 years], obesity [body mass index ≥30 kg/m2], renal failure [estimated glomerular filtration rate<60 mL/min per 1.73 m²], DM, and dyslipidemia), prior cardiovascular diseases (myocardial infarction, HF, and stroke/transient ischemic attack), doxorubicin equivalent dose per 100 mg/m2, and radiation to the left breast, retained in the penalized Cox proportional hazards regression model. These retained variables were included in the integrated risk score model. To develop a scoring system, points were designated to each predictor based on the coefficients of each variable from the penalized Cox proportional hazard regression model (Table 2). The new risk scheme was given the acronym CHEMO‐RADIAT (congestive heart failure, hypertension, elderly, myocardial infarction peripheral artery occlusive disease [PAOD], obesity, renal failure, abnormal lipid profile, DM, irradiation of the left breast, anthracycline dose, and transient ischemic attack/stroke; Table 3).
Table 2.
Result of Multiple Cox Proportional Hazards Regression Model and Penalized Cox Regression Model With Cross Validation Approach for MACE
| Multiple Cox proportional hazards regression | Penalized Cox proportional hazards regression | ||||
|---|---|---|---|---|---|
| HR | 95% CI | P Value | Coefficient | HR | |
| No. of cardiovascular risk factors* | 1.91 | 1.16–3.13 | 0.011 | 0.617 | 1.85 |
| No. of prior cardiovascular diseases† | 4.24 | 1.29–13.91 | 0.017 | 0.991 | 2.72 |
| Doxorubicin equivalent dose per 100 mg/m2 (rounded to nearest integer) | 1.97 | 1.23–3.13 | 0.005 | 0.571 | 1.77 |
| Left‐sided radiation therapy | 2.73 | 0.71–10.58 | 0.145 | 0.598 | 1.82 |
| Endocrine therapy | 1.2 | 0.13–1.66 | 0.237 | ||
| Trastuzumab | 2.27 | 0.59–18.68 | 0.231 | ||
HR indicates hazard ratio; and MACE, major adverse cardiovascular events.
Cardiovascular risk factors included hypertension, elderly patients (≥60 years old), obesity (body mass index ≥30 kg/m2), renal failure (estimated glomerular filtration rate <60 mL/min per 1.73 m²), dyslipidemia, and diabetes mellitus.
Cardiovascular diseases included congestive heart failure, myocardial infarction/peripheral artery disease, and stroke.
Table 3.
Assignment of the CHEMO‐RADIAT Model Scores
| Variables | Score |
|---|---|
| Congestive heart failure | 2 |
| Hypertension | 1 |
| Elderly (age ≥60) | 1 |
| Myocardial infarction/peripheral artery occlusive disease | 2 |
| Obesity | 1 |
| Renal failure (estimated glomerular filtration rate <60 mL/min per 1.73 m²) | 1 |
| Abnormal lipid profile (dyslipidemia) | 1 |
| Diabetes mellitus | 1 |
| Irradiation to left breast with ≥30 Gy dose | 1 |
| Anthracycline dose (doxorubicin equivalent dose: for daunorubicin multiply by 0.5, for epirubicin multiply by 0.5, for idarubicin multiply by 2) | 1 per each 100 mg/m2 (rounded to nearest integer) |
| Transient ischemic attack/stroke | 2 |
CHEMO‐RADIAT indicates congestive heart failure, hypertension, elderly, myocardial infarction/peripheral artery occlusive disease, obesity, renal failure, abnormal lipid profile, diabetes mellitus, irradiation of the left breast, anthracycline dose, and transient ischemic attack/stroke.
Predictive Performance of the CHEMO‐RADIAT Score
The new CHEMO‐RADIAT score yielded 1‐, 3‐, and 7‐year censored C‐indices of 0.679 (95% CI, 0.508–0.089), 0.715 (95% CI, 0.519–0.911), and 0.793 (95% CI, 0.653–0.933), respectively, in the derivation cohort. The validation group showed good C‐indices at 1, 3, and 7 years (0.811, 0.876, and 0.842; 95% CI, 0.701–0.915, 0.786–0.966, and 0.725–0.959, respectively) according to new scoring scheme (Table 4). There was no evidence of poor calibration according to the results of the Hosmer‐Lemeshow goodness‐of‐fit statistics (P=0.090 and 0.181 for the derivation and validation cohorts, respectively). The low‐, moderate‐, and high‐risk (0–2, 3–5, and ≥6 points, respectively) groups were categorized according to the CHEMO‐RADIAT score. The proportions of the low, moderate, and high‐risk groups were 49.5%, 44.6%, and 5.9% in the derivation cohort and 47.6%, 45.7%, and 6.7% in the validation cohort, respectively. Figure shows the MACE‐free survival curve of the 3 risk groups with statistically significant differences (log‐rank P<0.001 and P=0.003 for the derivation and validation cohorts, respectively). The survival curves clearly show that the low‐risk group in both groups had an extremely low probability of MACE. The MACE incidence rates of the low‐risk group were 0.08 and 0.09 per 100 person‐years in the derivation and validation cohorts, respectively. The C‐index at 7 years after BC diagnosis was significantly increased when BC cancer therapy‐related cardiovascular risk factors were incorporated into the conventional cardiovascular risk factor data (Table 4). The net benefit of the CHEMO‐RADIAT score was also higher than that of the prediction model based on only conventional cardiovascular risk factors (Figure S1).
Table 4.
Time‐Dependent C‐Indices of the CHEMO‐RADIAT Score for Predicting MACE Compared With the Risk Models for the Cardiovascular Risk Factors Plus Cardiovascular Diseases and for Cancer Therapy‐Related Risk Factors in the Validation Cohort at 3 and 7 years
| Risk scheme | At 3 y | At 7 y | ||
|---|---|---|---|---|
| C‐index (95% CI) | P Value | C‐index (95% CI) | P Value | |
| CHEMO‐RADIAT* | 0.876 (0.786–0.966) | 0.842 (0.725–0.959) | ||
| Conventional Cardiovascular risk factors† | 0.809 (0.683–0.937) | 0.241 | 0.751 (0.599–0.903) | 0.044 |
CV, cardiovascular; and MACE, major adverse cardiovascular event.
CHEMO‐RADIAT includes congestive heart failure (2 points), hypertension (1 point), elderly (1 point), myocardial infarction/peripheral artery disease (2 points), obesity (1 point), renal failure (1 point), abnormal lipid profile (1 point), diabetes mellitus (1 point), irradiation to the left breast (1 point), anthracycline dose (1 point per doxorubicin equivalent dose of 100 mg/m2), and transient ischemic attack/stroke (2 points).
Conventional CV risk factors include the variables in CHEMO‐RADIAT except radiation therapy to the left breast and the anthracycline dose.
Figure 1. Kaplan–Meier curves for MACE‐free survival after breast cancer therapy according to risk stratification by the CHEMO‐RADIAT score in the derivation (A) and the validation (B) cohorts.
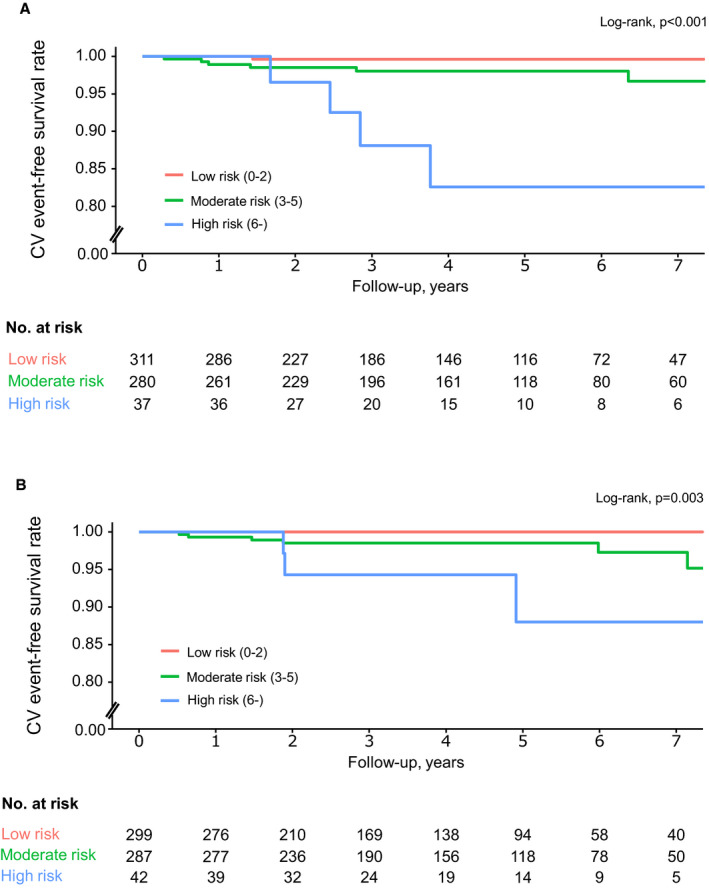
CHEMO‐RADIAT indicates congestive heart failure, hypertension, elderly, myocardial infarction/peripheral artery occlusive disease, obesity, renal failure, abnormal lipid profile, diabetes mellitus, irradiation of the left breast, anthracycline dose, and transient ischemic attack/stroke; CV, cardiovascular; and MACE, major adverse cardiovascular events.
Discussion
Main Findings
In this study, we derived and validated a simple risk scoring system (CHEMO‐RADIAT score) that estimates the risk of MACE after BC treatment in a multicenter cohort. Conventional cardiovascular risk factors were kept as independent predictors of MACE after BC therapy with additional adjustment for BC treatment‐related cardiovascular risk factors (side of radiation therapy and anthracycline dose). The performance of the predictive model was significantly improved by including both the BC treatment‐related and conventional cardiovascular risk factors. The CHEMO‐RADIAT score showed a good ability to discriminate according to censored C‐statistics at 3 and 7 years after the BC diagnosis, which were greater than 0.8 in the validation group. It also performed well in identifying patients at low risk of cardiovascular.
BC Therapy‐Related Cardiovascular Risk Factors
It is well established that anthracycline triggers cardiac dysfunction in a dose‐dependent fashion.5 Traditionally, the lifetime cumulative doxorubicin dose was limited to 400 mg/m2 to prevent anthracycline‐induced cardiomyopathy.16, 17 However, recent studies have shown that the risk of HF is significantly increased even at a lower doxorubicin dose than previously reported, and anthracycline‐related symptomatic HF can occur at a doxorubicin dose lower than 300 mg/m2.18, 19, 20 These results suggest that there is no absolute safe dose threshold for anthracycline.6 In this respect, we treated the dose of anthracycline not as a simple categorical variable but as a complex categorical variable (1 point per 100 mg/m2 doxorubicin equivalent dose rounded to the nearest integer). This approach can provide more individualized risk stratification, especially for patients with BC whose chemotherapy included anthracycline. Although anti‐HER2 treatment improves disease‐free and overall survival in HER2/neu‐positive patients with BC,17 it may increase the risk of cardiotoxicity.6, 21 In this study, however, trastuzumab was not a significant risk factor for MACE. There could be several explanations for the inconsistent results. First, the end point of this study was limited to symptomatic HF. Previous studies have shown that the incidence of trastuzumab‐related symptomatic HF was relatively low, ranging from 0% to 3.9%, whereas a decrease in the left ventricular ejection fraction (with or without symptoms) was observed in one‐third of the patients with BC who received trastuzumab.6, 22, 23 Contrary to anthracycline‐induced cardiomyopathy, the cardiotoxicity of trastuzumab is usually reversible after cessation of the trastuzumab and treatment with HF medications.21, 23 So, this study may have limitations in detecting trastuzumab‐induced cardiac toxicity. Second, only 11.9% of the patients in this study were administered anti‐HER‐2 treatment. Therefore, the notion that the use of HER‐2 blocking monoclonal antibodies is not a cardiovascular risk factor could not be generalized from our findings. Careful monitoring of cardiovascular events is essential for patients with BC who receive chemotherapy that includes HER‐2 blocking antibodies.21, 24, 25, 26 Several studies have reported that selective estrogen modulators are associated with ischemic stroke and that aromatase inhibitors, relative to selective estrogen receptor modulators, increase the risk of cardiovascular events.27, 28, 29 However, endocrine therapy, including treatment with selective estrogen receptor modulators and aromatase inhibitors, was not a significant risk factor in our study. Contrary to the previous studies,28, 29, 30 our study included Asian subjects who are relatively young and less obese. The inconsistent findings regarding endocrine therapy may have resulted from the heterogeneity of the study population.29 Given that preexiting risk factors play an important role in the development of anti‐HER‐2 treatment‐related cardiotoxicity,31 this heterogeneity of the study population may also contribute to the inconsistent results regarding the effect of anti‐HER‐2 on MACE in this study.
Left‐sided breast radiation therapy was included in the risk score analysis in this study. Thoracic radiation therapy is linked to increased cardiovascular morbidity and mortality,6, 17 and previous studies have shown that left‐sided breast radiation therapy is associated with an increased risk of MACE.32, 33, 34 Although the results of recent trials suggested that the cardiovascular risks of breast radiation therapy are likely to be lower with the introduction of heart sparing radiation techniques,34 even with modern radiation techniques, the mean radiation dose to the heart is still greater with radiation therapy to the left breast (ranging between 2–7 Gy) compared with right‐sided radiation therapy (1.2–2 Gy).35 Moreover, in patients with BC who undergo left‐sided radiotherapy, part of the left ventricle receives radiation doses greater than 20 Gy.35, 36 The incidence of MACE is strongly associated with the radiation dose delivered to the heart, for which there is no apparent threshold,37, 38 and the cardiovascular risk of left‐sided breast radiation therapy can be increased by the concomitant use of chemotherapy.39
Conventional Cardiovascular Risk Factors in Patients with BC
Traditional cardiovascular risk factors are significant predictors of MACE, even after adjusting for chemotherapy and radiation therapy.8, 15, 40 Elderly patients with BC and preexisting cardiovascular disease may have a risk of cardiovascular death exceeding that of death due to BC.41 A recently proposed risk score based on age, preexisting cardiovascular disease, and conventional cardiovascular risk factors displayed a good ability to predict MACE after the diagnosis of early BC.40 Smoking is another important cardiovascular risk factor for survivors of BC.42, 43 Recent Korean female population‐based studies, however, did not show a significant association between smoking and cardiovascular events in patients with BC.44, 45 The inconsistent results may be attributable to a far lower smoking rate among Korean women than that reported in Western population‐based studies.46 According to the Korean national statistical information service data, the reported smoking rate of the general Korean female population was 3.5% in 2018.47
Clinical Implications of the CHEMO‐RADIAT Score
Over the past decade, cardio‐oncology has emerged as an integrative field in medicine, but attention was solely given to the cardiovascular diseases that are secondary to cancer therapies, and less emphasis has been placed on the prediction and prevention of cardiovascular events in patients with BC.6, 48 Given that BC and cardiovascular disease share similar predisposing risk factors, survivors of BC frequently present with multiple cardiovascular risk factors.6, 48 Moreover, options for BC therapy confer cardiac toxicity,5 which develops with “multiple hits” that involve multiple risk factors.17, 18 In this regard, a combination of conventional and BC therapy‐related cardiovascular risk factors would provide a comprehensive assessment of the cardiovascular risk for survivors of BC.6, 15, 49 The American Society of Clinical Oncology Clinical Practice Guidelines for Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers have already proposed criteria for patients at risk of cardiac dysfunction after cancer treatment that is based on both conventional and BC therapy‐related cardiovascular risk factors.15 However, the diagnostic value of the criteria has not yet been studied. Recently, a new cardiovascular risk score system for patients with BC was developed and validated using large population‐based cohorts.40 Contrary to this risk score, which was based solely on the conventional risk factors, our results appear to be meaningful in developing and validating a cardiovascular risk score system that uses both conventional and BC treatment‐related cardiovascular risk factors. Recent HF treatment has made good strides,9, 50 and early detection and treatment can improve the prognosis of cancer therapy‐related cardiotoxicity.51 Therefore, risk stratification using a risk scoring system may improve the cardiovascular outcomes of patients with BC by identifying those patients who are expected to benefit from close observation for early detection. As our risk scoring system provides comprehensive cardiovascular risk assessment for BC therapy, it can be used for multidisciplinary decision‐making regarding the therapeutic options for BC. The scoring system would encourage BC therapy for patients with a truly low probability of MACE. Finally, we believe that the results of our study support the contemporary cardio‐oncology paradigm where cancer and cardiovascular disease intersect.6
Limitations
This study has several potential limitations. First, the end point could have been underestimated because only symptomatic cardiovascular events were identified and not every patient underwent echocardiography regularly after receiving BC therapy. Second, this study included a limited number of patients with a small number of MACE. Therefore, our model needs to be validated in a prospective cohort study with a large number of subjects. Third, our risk score prediction model was derived from a cohort with a limited follow‐up duration. Therefore, the use of our risk score model may be limited for predicting long‐term cardiovascular outcome. Fourth, the CHEMO‐RADIAT score was derived and validated using the cohort that consisted of only Asian female subjects who are relatively less obese than their Western counterparts.52 Besides, patients’ smoking status, which is another important cardiovascular risk factor, was neither fully evaluated nor included in the model. Therefore, this risk score system may have a different threshold for risk‐stratification when applied to different ethnic groups with a higher prevalence of smoking or obesity. Lastly, we did not include each individual cardiovascular risk factor in the multivariate prediction model. Instead, we treated them as a composite of risk factors. As each risk factor does not equally contribute to the development of MACE, our risk score system may not provide an accurate risk assessment for each variable. However, the number of cardiovascular risk factors is linearly related to the cardiovascular risk6, 8 and the risk of cancer therapy‐related cardiotoxicity increases with age and baseline cardiovascular risk factors.6 Although our risk score compromised the accurate risk assessment of each risk factor, its strength lies in in its ability to provide a comprehensive assessment of the cardiovascular risk among patients with BC who are exposed to therapy‐related cardiac toxicity and who have multiple baseline cardiovascular risk factors. Another strength is that our cohort represents real‐world patients with BC with detailed data regarding the anthracycline doses and methods of radiation therapy. Therefore, we were able to examine the impact of both conventional and BC therapy‐related risk factors on cardiovascular events.
Conclusions
We developed and validated a risk score model to estimate the cardiovascular risk after BC treatment in a multicenter cohort. The score (CHEMO‐RADIAT), which is based on BC treatment‐related and conventional cardiovascular risk factors, showed a good performance. These results highlight the importance of combining conventional and BC treatment‐related cardiovascular risk factors for determining the cardiovascular risk in BC. As the CHEMO‐RADIAT score included BC treatment‐related cardiovascular risk factors, it can be a useful decision‐making tool when considering BC treatment options.
Sources of Funding
This research was supported by the Catholic Medical Center Research Foundation (2021), by the Korean Society of Cardiovascular Disease Prevention, by the Korean Society of Hypertension (KSH‐R‐2019‐05), by the Hallym University Research Fund (HURF‐2018‐31) and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant number: HI19C1211). The funders had no roles in the study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosures
None.
Supporting information
Figure S1
(J Am Heart Assoc. 2021;10:e021931. DOI: 10.1161/JAHA.121.021931.)
D.‐Y. Kim and M.‐S. Park contributed equally.
For Sources of Funding and Disclosures, see page 9.
Contributor Information
Jong‐Chan Youn, Email: jong.chan.youn@gmail.com.
Kyu‐Hyung Ryu, Email: khryumd@hanmail.net.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. DOI: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. DOI: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 3.Riihimaki M, Thomsen H, Brandt A, Sundquist J, Hemminki K. Death causes in breast cancer patients. Ann Oncol. 2012;23:604–610. DOI: 10.1093/annonc/mdr160. [DOI] [PubMed] [Google Scholar]
- 4.Colzani E, Liljegren A, Johansson AL, Adolfsson J, Hellborg H, Hall PF, Czene K. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol. 2011;29:4014–4021. DOI: 10.1200/JCO.2010.32.6462. [DOI] [PubMed] [Google Scholar]
- 5.Zagar TM, Cardinale DM, Marks LB. Breast cancer therapy‐associated cardiovascular disease. Nat Rev Clin Oncol. 2016;13:172–184. DOI: 10.1038/nrclinonc.2015.171. [DOI] [PubMed] [Google Scholar]
- 6.Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz‐Flores S, Dent S, Kondapalli L, Ky B, Okwuosa T, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137:e30–e66. DOI: 10.1161/CIR.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–3815. DOI: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 8.Hershman DL, Till C, Shen S, Wright JD, Ramsey SD, Barlow WE, Unger JM. Association of cardiovascular risk factors with cardiac events and survival outcomes among patients with breast cancer enrolled in SWOG clinical trials. J Clin Oncol. 2018;36:2710–2717. DOI: 10.1200/JCO.2017.77.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K‐J, Cho H‐J, Kim M‐S, Kang J, Kim K‐H, Kim D, Seo SM, Yang JH, Cha M‐J, Choi JI, et al. Focused update of 2016 Korean society of heart failure guidelines for the management of chronic heart failure. Int J Heart Fail. 2019;1:4–24. DOI: 10.36628/ijhf.2019.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH, Kim MS, Kim EJ, Park DG, Cho HJ, Yoo BS, Kang SM, Choi DJ. KSHF guidelines for the management of acute heart failure: part I. Definition, epidemiology and diagnosis of acute heart failure. Korean Circ J. 2019;49:1–21. DOI: 10.4070/kcj.2018.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keefe DL. Anthracycline‐inducede cardiomyopathy. Semin Oncol. 2001;28:2–7. [PubMed] [Google Scholar]
- 12.Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. Springer; 2019. [Google Scholar]
- 13.Goeman JJ. L1 penalized estimation in the cox proportional hazards model. Biom J. 2010;52:70–84. [DOI] [PubMed] [Google Scholar]
- 14.Sprengers RW, Janssen KJ, Moll FL, Verhaar MC, van der Graaf Y. Prediction rule for cardiovascular events and mortality in peripheral arterial disease patients: data from the prospective second manifestations of arterial disease (SMART) cohort study. J Vasc Surg. 2009;50:1369–1376. DOI: 10.1016/j.jvs.2009.07.095. [DOI] [PubMed] [Google Scholar]
- 15.Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand J‐B, Ewer M, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. DOI: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 16.Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M, Muggia FM. Risk factors for doxorubicin‐induced congestive heart failure. Ann Intern Med. 1979;91:710–717. [DOI] [PubMed] [Google Scholar]
- 17.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. DOI: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 18.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. DOI: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 19.Drafts BC, Twomley KM, D'Agostino R, Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, et al. Low to moderate dose anthracycline‐based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–885. DOI: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulrooney DA, Armstrong GT, Huang S, Ness KK, Ehrhardt MJ, Joshi VM, Plana JC, Soliman EZ, Green DM, Srivastava D, et al. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: a cross‐sectional study. Ann Intern Med. 2016;164:93–101. DOI: 10.7326/M15-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baselga J, Perez EA, Pienkowski T, Bell R. Adjuvant trastuzumab: a milestone in the treatment of HER‐2‐positive early breast cancer. Oncologist. 2006;11(Suppl 1):4–12. DOI: 10.1634/theoncologist.11-90001-4. [DOI] [PubMed] [Google Scholar]
- 22.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2‐positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2‐negative cohort. Lancet. 2010;375:377–384. DOI: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 23.Sevcikova K, Vertakova‐Krakovska B, Spanik S. Neoadjuvant treatment in patients with HER2‐positive breast cancer. ISRN Oncol. 2013;2013:362467. DOI: 10.1155/2013/362467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackey JR, Clemons M, Côté MA, Delgado D, Dent S, Paterson A, Provencher L, Sawyer MB, Verma S. Cardiac management during adjuvant trastuzumab therapy: recommendations of the Canadian trastuzumab working group. Curr Oncol. 2008;15:24–35. DOI: 10.3747/co.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi HM, Park MS, Youn JC. Update on heart failure management and future. Korean J Intern Med. 2019;34:11–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung WB, Youn JC, Youn HJ. Cardiovascular complications of novel anti‐cancer immunotherapy: old problems from new agents? Korean Circ J. 2020;50:743–753. DOI: 10.4070/kcj.2020.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bushnell CD, Goldstein LB. Risk of ischemic stroke with tamoxifen treatment for breast cancer: a meta‐analysis. Neurology. 2004;63:1230–1233. DOI: 10.1212/01.WNL.0000140491.54664.50. [DOI] [PubMed] [Google Scholar]
- 28.Abdel‐Qadir H, Amir E, Fischer HD, Fu L, Austin PC, Harvey PJ, Rochon PA, Lee DS, Anderson GM. The risk of myocardial infarction with aromatase inhibitors relative to tamoxifen in post‐menopausal women with early stage breast cancer. Eur J Cancer. 2016;68:11–21. DOI: 10.1016/j.ejca.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Khosrow‐Khavar F, Filion KB, Bouganim N, Suissa S, Azoulay L. Aromatase inhibitors and the risk of cardiovascular outcomes in women with breast cancer: a population‐based cohort study. Circulation. 2020;141:549–559. DOI: 10.1161/CIRCULATIONAHA.119.044750. [DOI] [PubMed] [Google Scholar]
- 30.Lai SW, Lin CL, Liao KF. Tamoxifen use correlates with increased risk of the first episode of ischemic cerebrovascular disease in older women with breast cancer: a case‐control study in Taiwan. Front Pharmacol. 2017;8:742. DOI: 10.3389/fphar.2017.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rushton M, Johnson C, Dent S. Trastuzumab‐induced cardiotoxicity: testing a clinical risk score in a real‐world cardio‐oncology population. Curr Oncol. 2017;24:176–180. DOI: 10.3747/co.24.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darby SC, McGale P, Taylor CW, Peto R. Long‐term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US seer cancer registries. Lancet Oncol. 2005;6:557–565. DOI: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 33.Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–424. DOI: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henson KE, McGale P, Taylor C, Darby SC. Radiation‐related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108:179–182. DOI: 10.1038/bjc.2012.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor CW, Povall JM, McGale P, Nisbet A, Dodwell D, Smith JT, Darby SC. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys. 2008;72:501–507. DOI: 10.1016/j.ijrobp.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 36.Beaton L, Bergman A, Nichol A, Aparicio M, Wong G, Gondara L, Speers C, Weir L, Davis M, Tyldesley S. Cardiac death after breast radiotherapy and the QUAMTEC cardiac guidelines. Clin Transl Radiat Oncol. 2019;19:39–45. DOI: 10.1016/j.ctro.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darby SC, Ewertz M, McGale P, Bennet AM, Blom‐Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. DOI: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 38.Saiki H, Petersen IA, Scott CG, Bailey KR, Dunlay SM, Finley RR, Ruddy KJ, Yan E, Redfield MM. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. 2017;135:1388–1396. DOI: 10.1161/CIRCULATIONAHA.116.025434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim DY, Youn JC, Park MS, Lee S, Choi SW, Ryu KH, Kim LS, Shim MS, Lee JJ, Han S. Cardiovascular outcome of breast cancer patients with concomitant radiotherapy and chemotherapy: a 10‐year multicenter cohort study. J Cardiol. 2019;74:175–181. DOI: 10.1016/j.jjcc.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Abdel‐Qadir H, Thavendiranathan P, Austin PC, Lee DS, Amir E, Tu JV, Fung K, Anderson GM. Development and validation of a multivariable prediction model for major adverse cardiovascular events after early stage breast cancer: a population‐based cohort study. Eur Heart J. 2019;40:3913–3920. DOI: 10.1093/eurheartj/ehz460. [DOI] [PubMed] [Google Scholar]
- 41.Abdel‐Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, Fung K, Anderson GM. A population‐based study of cardiovascular mortality following early‐stage breast cancer. JAMA Cardiol. 2017;2:88–93. DOI: 10.1001/jamacardio.2016.3841. [DOI] [PubMed] [Google Scholar]
- 42.Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, Taylor CW, van Leeuwen FE. Long‐term risk of cardiovascular disease in 10‐year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. DOI: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 43.Passarelli MN, Newcomb PA, Hampton JM, Trentham‐Dietz A, Titus LJ, Egan KM, Baron JA, Willett WC. Cigarette smoking before and after breast cancer diagnosis: mortality from breast cancer and smoking‐related diseases. J Clin Oncol. 2016;34:1315–1322. DOI: 10.1200/JCO.2015.63.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang JS, Ko BK, Bae JW, Yu J‐H, Park MH, Jung Y, Jeon YW, Kim KH, Shin J, Suh C‐O, et al. Radiation‐related heart disease after breast cancer radiation therapy in Korean women. Breast Cancer Res Treat. 2017;166:249–257. DOI: 10.1007/s10549-017-4398-y. [DOI] [PubMed] [Google Scholar]
- 45.Chung SY, Oh J, Chang JS, Shin J, Kim KH, Chun KH, Keum KC, Suh CO, Kang SM, Kim YB. Risk of cardiac disease in patients with breast cancer: impact of patient‐specific factors and individual heart dose from three‐dimensional radiation therapy planning. Int J Radiat Oncol Biol Phys. 2021;2021:473–481. DOI: 10.1016/j.ijrobp.2020.12.053. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Hong Y, D'Agostino RB Sr, Wu Z, Wang W, Sun J, Wilson PW, Kannel WB, Zhao D. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese multi‐provincial cohort study. JAMA. 2004;291:2591–2599. DOI: 10.1001/jama.291.21.2591. [DOI] [PubMed] [Google Scholar]
- 47.Korean Statistical Information Service . Smoking rate in Korea, 2018. November 2020. Available at: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1SSHE032R&vw_cd=MT_ETITLE&list_id=D215_2008&scrId=&seqNo=&language=en&obj_var_id=&itm_id=&conn_path=A6&path=%252Feng%252Fsearch%252FsearchList.do. Accessed March 17, 2021.
- 48.Youn J‐C, Chung W‐B, Ezekowitz JA, Hong JH, Nam H, Kyoung D‐S, Kim I‐C, Lyon AR, Kang S‐M, Jung HO, et al. Cardiovascular disease burden in adult patients with cancer: an 11‐year nationwide population‐based cohort study. Int J Cardiol. 2020;317:167–173. DOI: 10.1016/j.ijcard.2020.04.080. [DOI] [PubMed] [Google Scholar]
- 49.Stewart Coats AJ. Common co‐morbidities in heart failure – diabetes, functional mitral regurgitation and sleep apnoea. Int J Heart Fail. 2019;1:25–41. DOI: 10.36628/ijhf.2019.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Youn JC, Han S, Ryu KH. Temporal trends of hospitalized patients with heart failure in Korea. Korean Circ J. 2017;47:16–24. DOI: 10.4070/kcj.2016.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. DOI: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 52.Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, Watanabe M, Kadota A, Okuda N, Kadowaki T, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702–2709. DOI: 10.1161/CIRCULATIONAHA.108.790048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


