Abstract
Characterization of the humoral immune responses of people to Helicobacter pylori infection has facilitated the investigation of the host response to bacterial virulence factors and the development of sensitive and specific diagnostic tests. Dogs are commonly infected with gastric Helicobacter spp., but the presence of multiple Helicobacter spp. and possible coinfection in individual dogs have complicated serological evaluation. Evaluation of the antigenic homology of Helicobacter spp. revealed that the major protein bands of Helicobacter felis and Helicobacter bizzozeronii, two Helicobacter spp. that infect dogs, were very similar to UreA (29 to 31 kDa), UreB (63 to 66 kDa), and HSP (58 to 60 kDa) of H. pylori, and sera from infected and uninfected dogs bound in a similar way to each antigen. Immunoblotting and an enzyme-linked immunosorbent assay (ELISA) with H. felis ATCC 49179 antigen were performed with 101 serum samples (from 78 infected dogs and 23 uninfected dogs). Samples from uninfected dogs (median = 8) had fewer bands on immunoblotting than samples from infected dogs (median = 16) (P < 0.05). Combinations of the presence of any two of the low-molecular-mass bands (19, 25, 30, 32, and 37 kDa) or the high-molecular-mass bands (86 and 94 kDa) were found almost solely in samples from infected dogs (P < 0.0001). Kinetic ELISA results were significantly higher for samples from infected dogs (median = 0.0802 optical density unit [OD]/min) than for samples from uninfected dogs (median = 0.01428 OD/min). The combination of ELISA and immunoblotting results gave a specificity of 95.6% and a sensitivity of 79.8%. No correlation between ELISA results, colonization density, degree of inflammation, and presence of lymphoid follicles was observed. The results indicate substantial antigenic homology between H. felis, H. pylori, and H. bizzozeronii. The combination of ELISA and immunoblotting was a highly specific and moderately sensitive indicator of infection. The degree of seropositivity assessed by ELISA was not related to bacterial colonization density, the degree of gastric inflammation, or the presence of lymphoid follicles.
The discovery of the association of Helicobacter pylori with gastritis, peptic ulcers, and gastric neoplasia has led to fundamental changes in the understanding of gastric disease in humans (5, 31, 39, 42). Investigation of the relationship of gastric disease to Helicobacter spp. in other species has resulted in the discovery of Helicobacter mustelae in ferrets with gastritis and peptic ulcers, Helicobacter acinonyx in cheetahs with severe gastritis, and Helicobacter heilmannii in pigs with gastric ulcers (10, 14, 36). While the presence of gastric Helicobacter-like organisms (HLOs) in the stomachs of dogs has been known for many years (4), there is little information on the relationship of infection with gastric Helicobacter spp. to gastric disease in the dog, with inflammation accompanying infection in some but not all infected dogs (8, 18, 22, 24, 25, 44, 45). In contrast to humans, in whom H. pylori predominates, multiple species of Helicobacter have been demonstrated in the stomachs of dogs. To date, Helicobacter felis, Helicobacter bizzozeronii, Helicobacter salomonis, Helicobacter bilis, and Flexispira rappini have been identified (8, 21, 26, 32, 34) on the basis of 16s rRNA sequencing, DNA hybridization, and electron microscopic appearance. Investigation of the pathogenicity of individual Helicobacter spp. has demonstrated gastritis and humoral immune responses after experimental infection of gnotobiotic dogs with H. felis and H. pylori (33, 37). However, similar experimental infection of specific-pathogen-free (SPF) dogs with H. felis failed to demonstrate significant pathological lesions (38). Estimates of the prevalence of infection with HLOs on the basis of examination of gastric biopsy specimens have been confined to relatively small groups of dogs. HLOs have been identified in 61 to 82% of dogs presented for the investigation of vomiting (18, 25, 45), 67 to 86% of clinically healthy pet dogs (8, 45), and approaching 100% of laboratory beagles and dogs from animal shelters (6, 8, 9, 24). Diagnosis of infected individuals and determination of the prevalence of infection with gastric Helicobacter spp. in dogs are hampered by the fact that diagnosis depends mostly on urease testing, histopathology, cytology, and culture, which are based on invasive endoscopic biopsies and are therefore expensive and time-consuming. The measurement of circulating antibodies (immunoglobulin G [IgG]) to H. pylori is thought to be a sensitive and specific means of diagnosing infection with H. pylori in humans and can easily be used to screen a large number of serum samples. The method that is used mostly is the enzyme-linked immunosorbent assay (ELISA), and many commercial kits are available (19, 41). Immunoblotting is also used for the diagnosis of H. pylori infection in humans and has been recommended when the outcome of the ELISA is equivocal (35). ELISA has enabled the detection of seroconversion in gnotobiotic and SPF H. felis-infected dogs and gnotobiotic H. pylori-infected dogs (33, 37, 38). However, the presence of multiple Helicobacter spp. in dogs with naturally acquired infections and possible coinfection in individual dogs (16, 22, 34) present a challenge to the development of useful serological assays for the diagnosis of infections in dogs.
The primary aim of the work presented here was to characterize the humoral immune responses of dogs with naturally acquired gastric Helicobacter spp. infection by using a variety of Helicobacter antigens, immunoblotting, and ELISA. A second aim was to evaluate the utility of these assays for discriminating infected from uninfected dogs. The third aim was to examine the relationship of bacterial colonization density, gastric inflammation, and presence of lymphoid nodules to seroconversion.
MATERIALS AND METHODS
Animals.
Serum and gastric biopsy specimens were obtained from 101 dogs. Samples were obtained from 23 mixed-breed young adult dogs from the local Society for the Prevention of Cruelty to Animals, 7 adult female beagles, 48 mixed-breed research dogs (mean ± standard deviation age, 23 ± 30 months), and 23 barrier-maintained SPF beagles (age, 11.5 ± 8 months). Sera were kept frozen at −70°C until use.
Gastric Helicobacter spp. infection status.
Endoscopic biopsy specimens of the cardia, fundus and body, and pyloric antrum (three specimens from each site) were procured, using an Olympus pediatric endoscope, from all dogs except the mixed-breed research dogs (6). Full-thickness biopsy specimens were taken with a 6-mm skin biopsy punch from the area of the fundus and body from the mixed-breed research dogs, which were euthanized as part of a hip dysplasia study. Biopsy specimens for histopathological evaluation were fixed in 10% buffered formalin, embedded in paraffin, and sectioned at 4 to 6 μm. Serial sections of each block were stained with hematoxylin and eosin and modified Steiner’s stain (MS) (17). For a subset of dogs undergoing endoscopy, biopsy specimens from each site were obtained for biopsy specimen urease test (52 of 101) and PCR with Helicobacter genus-specific primers (21 of 101). The presence or absence of HLOs was ascertained by examining MS-stained sections, the biopsy specimen urease test results, and the PCR results.
Biopsy specimen urease test.
Biopsy specimen urease production was evaluated in 52 dogs as described previously (37). Gastric mucosal biopsy specimens were placed in sterile tubes containing 200 μl of a solution composed of 0.33 M urea, 0.02% sodium azide, 0.02% phenol red, and 10 mM phosphate-buffered saline (pH 6.5). The biopsy specimens were incubated for 24 h and observed at 1, 4, and 24 h for a change in the color of the indicator medium. A change from orange-red to bright pink was considered a positive result, and the time of color change was recorded. For the purpose of this study, a positive urease test result was defined by the appearance of a color change in at least one endoscopic biopsy specimen within 24 h of incubation.
PCR.
Gastric biopsy specimens collected endoscopically from 21 dogs were frozen at −70°C. DNA was extracted from the biopsy specimens with a Qiamp tissue kit according to the manufacturer’s instructions (Qiagen Inc., St. Clarita, Calif.). Helicobacter genus-specific primers C97 and C05 (15) were used to generate 16S rRNA amplicons. DNA (100 ng) was added to PCR buffer (Gibco BRL, Grand Island, N.Y.), 400 μM deoxynucleotide triphosphates (Amersham Pharmacia Biotech, Piscataway, N.J.), 2 mM MgCl2 (Gibco BRL), 0.5 μM each primer, 1.5 U of Taq DNA polymerase (Gibco BRL), and distilled H2O in a total volume of 50 μl. PCR samples were heated to 94°C for 4 min once, followed by 40 cycles of denaturation for 1 min, primer annealing for 2 min and 30 s, and extension at 72°C for 3 min, with a final extension at 72°C for 15 min in a Biometra thermocycler (Biometra Inc., Tampa, Fla.). The PCR products were subjected to electrophoresis on an agarose gel and were visualized with ethidium bromide. A band of 1,200 bp was apparent with these primers and DNA from H. pylori, H. felis, H. bizzozeronii, H. heilmannii, H. salomonis, Helicobacter fenelliae, H. bilis, Helicobacter cinaedii, Helicobacter hepaticus, and Helicobacter canis. This band was absent with DNA from Campylobacter jejuni and Proteus mirabilis.
Histopathology.
The relationship of gastric inflammation, colonization density, and lymphoid nodules to ELISA results was evaluated with 15 infected dogs for which ELISA results ranged from 0.022 to 0.586 optical density unit [OD]/min. Microscopic sections were examined in a blinded fashion by the same pathologist and were evaluated for the number of organisms, degree of inflammation, and presence and size of mucosal lymphoid nodules. The number of organisms was graded as follows: 0, no organisms seen; +1, ≤1 organism/×400 magnification field; +2, 1 to 10 organisms/× 400 magnification field; +3, = >10 organisms/×400 magnification field. The degree of inflammation was graded as follows: 0, minimal to no mononuclear inflammatory cells; +1, mild increase in mononuclear inflammatory cells; +2, moderate numbers of mononuclear inflammatory cells; +3, numerous mononuclear inflammatory cells. The number of lymphoid nodules was counted, and their sizes were graded as follows: small, occupying less than 50% of the mucosal width; moderate, occupying up to 50% of the mucosal width; large, occupying greater than 50% of the mucosal width.
Antigens.
Antigens from H. felis ATCC 49179 and H. pylori 8826 (Enteric Products Inc., Stony Brook, N.Y.) were prepared by detergent extraction by Enteric Products Inc. (11). Further purification of the high-molecular-mass cell-associated protein (HM-CAP), which is enriched in the urease subunits and the heat shock proteins (HSPs) of H. felis and H. pylori, was performed by Enteric Products Inc. as described by Evans et al. (11). Antigen from H. bizzozeronii ATCC 700030 (a gift from K. Jalava, Helsinki, Finland) was prepared with a French press (three times at 1,100 lb/in2).
SDS-PAGE.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) was performed under reducing conditions (30) with a Hoefer SE 600 gel electrophoresis unit (Amersham Pharmacia Biotech, Piscataway, N.J.). Helicobacter antigens (120 μg/gel) were diluted in sample buffer (0.1 M Tris-Cl [pH 6.8], 0.02% bromophenol blue, 8.7% β-mercaptoethanol, 10% glycerol, 4.3% SDS), boiled for 2 min, and separated on a 12% polyacrylamide gel with a 4% stacking gel (200 V). Broad-range biotinylated markers (Bio-Rad Laboratories, Hercules, Calif.) were heated for 5 min at 95°C. Wet transfer to a nitrocellulose membrane (pore size 0.45 μm; Amersham Pharmacia Biotech) was performed at 4°C overnight at 23 V (Hoefer Transphor TE 42; Amersham Pharmacia Biotech). The membranes were washed in Tris-buffered saline (TBS; pH 10) with 0.05% Tween 20 (TBST) for 10 min, followed by washing with sterile distilled (MQ) water, and were then dried and stored at 4°C. To ensure adequate transfer, a part of each membrane was stained with amido black (0.15% [wt/vol] amido black, 25 ml of methanol, 10 ml of glacial acetic acid, and 65 ml of distilled H2O).
Immunoblotting.
Before use, the membranes were rehydrated with MQ water, washed for 10 min with TBST, and blocked for 1 h in TBS (pH 7.5) with 0.5% Tween 20 and 5% dry milk (O-AT-KA Milk Products Cooperative Inc., Batavia, N.Y.) at room temperature (RT). After washing in TBST (10 min), the membrane was clamped in a Miniblotter 25 system (Immunetics, Cambridge, Mass.) and the lanes were cleared with a vacuum. Dog serum (diluted 1:50 in TBS [pH 7.5] with 0.2% Tween 20 and 5% dry milk) was added to each lane. The same positive and negative control sera (diluted 1:100) were run on each membrane. Sera were incubated for 90 min at RT on a rocking platform. At the end of the incubation, channels were washed with TBS (pH 10) with 0.2% Tween 20. The membrane was then released from the Miniblotter and washed twice with the same washing solution while rocking for 10 min. High-affinity purified alkaline phosphatase goat anti-dog IgG (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) diluted 1:1,000 in TBS (pH 7.5) with 0.2% Tween 20 and 5% dry milk) and avidin alkaline phosphatase (Bio-Rad Laboratories) diluted 1:1,000 in TBS (pH 7.5) with 0.2% Tween 20 and 5% dry milk were incubated with the membrane for 1 h at RT. Two 10-min washes with TBS (pH 10) with 0.2% Tween 20 were followed by one 10-min wash in TBST and two 5-min washes with TBS (pH 7.5). Nitroblue tetrazolium and bromochloroindolyl phosphate (Sigma, St. Louis, Mo.) were added to 10 ml of alkaline phosphatase buffer, and the membrane was incubated with the solution until bands were detected. The reaction was stopped by washing with MQ water. The membranes were dried in a dark place and were then photographed with a digital camera. Immunoblots were evaluated for the number of bands per sample lane, as well as the number of bands in increments of 10 kDa. The total density of each sample lane was determined on a Macintosh Performa 6300cd computer with the public-domain NIH Image program (34a) and was expressed as a percentage of the total density of the positive control that was run on each membrane. The molecular weight of each band was estimated with the “Molecular weight” macro package available as shareware on the Internet (37a).
The antigenic homology of H. felis, H. pylori, and H. bizzozeronii was investigated by immunoblotting with monoclonal antibodies raised against H. pylori UreA, UreB, and HSP and sera from four infected dogs and one uninfected dog. Monoclonal antibodies were produced by Enteric Products Inc. BALB/c mice were immunized intraperitoneally with 100 μg of H. pylori 8826 HM-CAP in Freund’s complete adjuvant (vol/vol) (Sigma) followed by 10 μg of H. pylori 8826 HM-CAP in Freund’s incomplete adjuvant (vol/vol) (Sigma) for two more boosters given 2 to 4 weeks apart. The spleens were harvested 2 to 4 weeks after the last booster, and fusion with the Fox NY hybridoma cell line ATCC CRL-1732 was induced with polyethylene glycol (23). The cells were seeded at 0.3 cells/well in Dulbecco modified Eagle medium with fetal calf serum, penicillin, streptomycin, l-glutamine, and AAT (adenine, aminopterin, thymidine) media supplement (catalog no. A 5539; Sigma). Subculture was done by limiting dilution, and the resulting supernatants were characterized by immunoblotting with H. pylori 8826 HM-CAP-coated nitrocellulose membranes. Monoclonal antibodies that recognized bands at 29 to 31 kDa (UreA), 63 to 66 kDa (UreB), and 58 to 60 kDa (HSP) of H. pylori were generated and were used at dilutions of 1:300, 1:25, and 1:500, respectively.
Crude H. felis detergent extract antigen was used to evaluate the immunoreactivities of the 101 samples on immunoblotting.
ELISA.
Serum samples were evaluated for the presence of IgG against gastric Helicobacter spp. by use of an ELISA. Checkerboard titration of the different antigen types (crude detergent extract and HM-CAP of H. pylori and H. felis) at concentrations of 0.5, 1, and 2 μg/well, serum (1:50, 1:100, and 1:200), and conjugate (1:1,000, 1:2,000, and 1:3,000) was performed with sera from four dogs infected with gastric Helicobacter spp. and two uninfected SPF dogs. The combination of H. felis HM-CAP (1 μg/well) with a serum dilution of 1:100 and a conjugate dilution of 1:2,000 gave the greatest differences between infected and uninfected sera and was used for all the samples evaluated in this study. Flat-bottom microtiter plates (Labsystems USA, Franklin, Mass.) coated with H. felis HM-CAP were kept dry and were sealed at 4°C until use (antigen-coated plates were a generous gift from Enteric Products Inc.). One hundred microliters of diluted serum was incubated for 1 h at 37°C. The plates were washed four times in an Ultrawash plus microplate washer (Dynatech Laboratories, Chantilly, Va.) with phosphate-buffered saline containing 0.05% Tween 20. Bound IgG was detected with horseradish peroxidase-conjugated goat anti-dog IgG (100 μl/well; diluted 1:2,000 in phosphate-buffered saline with 0.05% Tween 20 and 2% dry milk [Cappel/ICN, Costa Mesa, Calif.]) and was incubated for 30 min at RT. After washing, 100 μl of tetramethylbenzidine substrate solution (Kirkegaard & Perry Laboratories) was added to each well and the plates were read immediately with a Dynatech MRX microplate reader at 650 nm (and analyzed with the kinetic software provided by Dynatech). The plates were read three times at intervals of 45 s with a period of 30 s of shaking between reads. The results were expressed as a rate function in OD per minute. Samples were run in replicates of two. Seven serum standards were also included in every run in order to ensure standardization of the assay.
Statistical analysis.
One-way analysis of variance was used to evaluate the differences between H. pylori, H. felis, and H. bizzozeronii antigens, which were expressed as the total density of the same sera run with these three antigens. The chi-square and Fisher’s exact tests were used to detect differences in the prevalence of specific bands in specimens from infected and uninfected dogs. These tests were also used to detect differences in the prevalence of bands in a specific molecular mass class in specimens from infected and uninfected dogs. Fisher’s exact test was used instead of the chi-square test if the “expected” number of animals was calculated to be less than 5.
Differences between the median number of immunoreactive bands for specimens from dogs infected and uninfected with gastric Helicobacter spp. were evaluated by the Mann-Whitney test. The correlation of immunoblotting and ELISA results, as well as the relationship of the ELISA results to gastric inflammation, colonization density, and the presence of lymphoid nodules, was assessed by the Spearman rank correlation test. Statistical analysis was performed with Minitab 11 for Windows (Minitab Inc., State College, Pa.). StatView 4.1 for Macintosh (Abacus Concepts, Berkeley, Calif.) was used for the Spearman correlation tests. Significance was set at a P value of <0.05.
RESULTS
Gastric Helicobacter sp. infection status.
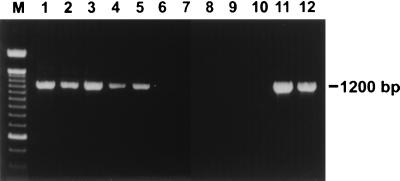
Seventy-eight of 101 dogs evaluated in this study were naturally infected with gastric Helicobacter spp., as determined by the presence of HLOs in MS-stained stomach sections. All 78 research dogs and dogs from the Society for the Prevention of Cruelty to Animals had HLOs in their stomachs. None of the 23 SPF beagles had HLOs in MS-stained stomach sections. Urease activity was detected in samples from 30 of 52 dogs and was absent from samples from 22 of 52 dogs. Gastric tissues from 7 of 21 dogs were positive for Helicobacter spp. DNA by PCR, and those from 14 of 21 dogs were negative (Fig. 1). The results were totally consistent with those from the histological assessment of MS-stained stomach sections. Infection status was determined according to the presence of HLOs in MS-stained sections.
FIG. 1.
Detection of Helicobacter spp. DNA in endoscopic gastric biopsy specimens by PCR with primers directed against the 16S rRNA sequence. Lanes 1 to 5, DNAs from specimens from five dogs with HLOs on MS-stained stomach sections, respectively; lanes 6 to 10, DNAs from specimens from five dogs with no HLOs on MS-stained stomach sections, respectively; lane 11, DNA from H. bizzozeronii; lane 12, DNA from H. felis; M, 100-bp DNA ladder.
Characterization of antigens.
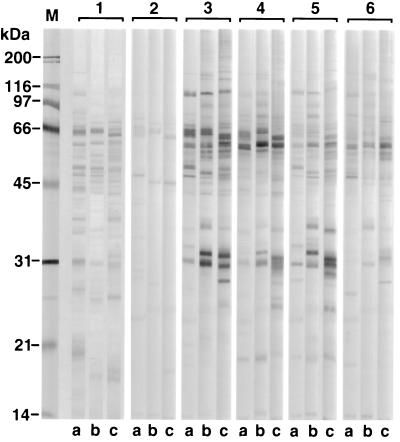
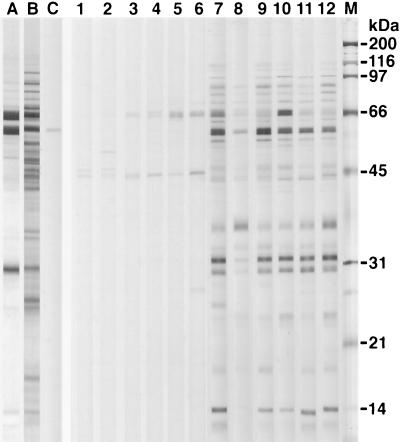
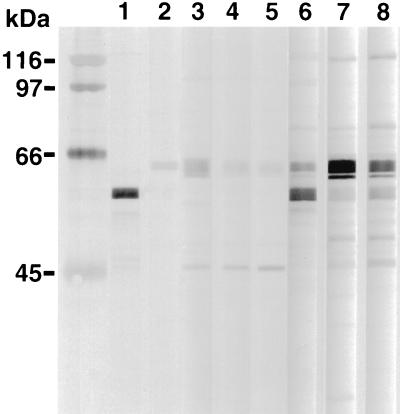
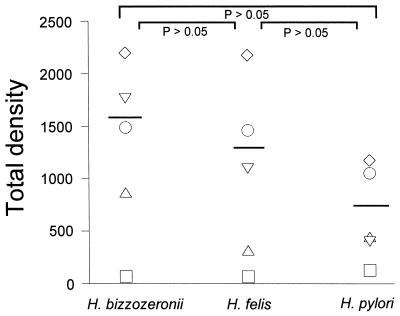
The protein banding patterns of the crude detergent extracts of H. felis, H. pylori, and H. bizzozeronii indicated that major immunoreactive bands were detected at molecular masses of 63 to 66, 58 to 60, and 29 to 31 kDa for all three antigens (Fig. 2). The major protein bands of the HM-CAP antigens were of similar sizes (Fig. 3). There was some minor but consistent variation in the molecular masses of the bands of 63 to 66 and 58 to 60 kDa between the three Helicobacter spp. Use of monoclonal antibodies against H. pylori UreA, UreB, and HSP (Fig. 4) indicated that the 66-kDa band was UreB, that the 60-kDa band was HSP, and that the 31-kDa band was UreA. The anti-H. pylori HSP monoclonal antibody but not the anti-H. pylori UreA and UreB monoclonal antibodies recognized protein bands of H. felis (Fig. 3) and H. bizzozeronii antigens. Antigenic homology between H. pylori, H. felis, and H. bizzozeronii was further investigated with sera from four dogs infected with gastric Helicobacter spp. and one uninfected dog on a nitrocellulose membrane coated with three different antigens: crude detergent extract of H. pylori crude detergent extract of H. felis, and French-pressed H. bizzozeronii (Fig. 2). The total density of all bands in each sample lane was not significantly different for each serum sample for each of the three different antigens (P > 0.05), although a tendency for the density of H. pylori to decrease was noted (Fig. 5). Antibodies against crude H. pylori antigen were detected in samples from infected and uninfected dogs (Fig. 4). Samples from uninfected dogs exhibited antibodies against the 66-kDa UreB subunit. Samples from infected dogs had antibodies to a wider range of proteins including the 60-kDa HSP.
FIG. 2.
Protein profiles (lanes 1) and immunoblot patterns (lanes 2 to 6) obtained with crude extracts of H. pylori 8826 (a lanes), H. felis ATCC 49179 (b lanes), and H. bizzozeronii ATCC 700030 (c lanes) with serum samples from one uninfected (lanes 2) and four infected (lanes 3 to 6) dogs. Lane M, molecular mass marker.
FIG. 3.
Protein profiles of H. felis ATCC 49179 HM-CAP (lane A) and crude detergent extract (lane B). Immunoblotting patterns were obtained with the crude H. felis extract with the monoclonal antibody against H. pylori HSP (58 kDa) (lane C) and sera from uninfected (lanes 1 to 6) and infected dogs (lanes 7 to 12). Lane M, molecular mass marker.
FIG. 4.
Immunoblotting patterns of monoclonal antibodies against H. pylori HSP (lane 1) and UreB (lane 2) and sera from uninfected (lanes 3 to 5) and infected (lanes 6 to 8) dogs, as obtained with the crude H. pylori 8826 extract.
FIG. 5.
Immunoreactivity of sera from uninfected (□) and infected (▿, ○, ▵, ◊) dogs against crude extracts of H. bizzozeronii, H. felis, and H. pylori. Immunoreactivity is expressed as the total density (in square millimeters), which was measured with the NIH Image program on a digitalized image of the immunoblot. The solid bar indicates the mean total density of sera from the four infected dogs for each antigen. Differences between the means were not significant.
Immunoblotting.
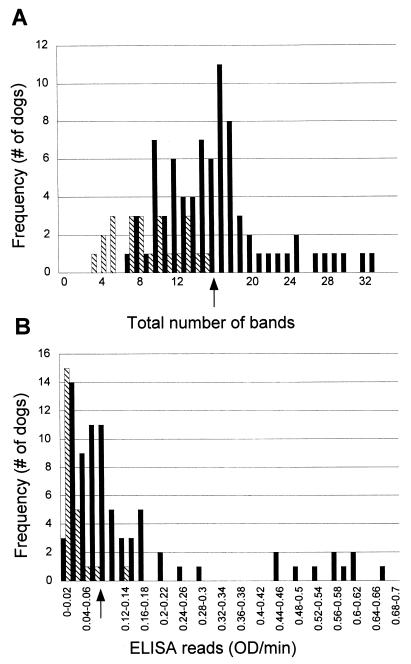
When serum samples from all infected (n = 78) and uninfected (n = 23) dogs were evaluated with a crude detergent extract of H. felis as the antigen (Fig. 3), samples from uninfected dogs had fewer immunoreactive bands (median = 8, interquartile range = 5 to 12) than samples from infected dogs (median = 16, interquartile range = 12 to 18) (P < 0.001). Specimens from all uninfected dogs had fewer than 16 bands. A cutoff value based on the total number of bands could be chosen as ≥16 (Fig. 6A). The test characteristics at this cutoff value are described in Table 1.
FIG. 6.
Frequency plots of total number of bands per dog by immunoblotting (A) and ELISA (B) for 101 dogs. Solid bars indicate results for samples from infected dogs, and striped bars indicate results for samples from uninfected dogs. The arrows indicate the cutoff values that were chosen.
TABLE 1.
Characteristics of serological methods for diagnosis of infection with gastric Helicobacter spp. in dogsa
| Test and characteristic | Sensitivity (%) | Specificity (%) | PPVb (%) | NPVc (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Immunoblotting | |||||
| Total no. of bands (≥16) | 53.9 | 100.0 | 100.0 | 39.0 | 64.3 |
| Presence of dense band at 30 to 39 kDa | 62.8 | 82.6 | 92.4 | 39.6 | 67.3 |
| Presence of combinations of specific bandsd | 70.5 | 95.6 | 98.2 | 51.2 | 76.2 |
| ELISA, OD/min > 0.08 | 52.6 | 95.6 | 97.6 | 37.3 | 62.4 |
| Immunoblotting + ELISA (total no. of bands ≥16, presence of combinations of specific bands + OD/min > 0.08) | 79.8 | 95.6 | 98.4 | 58.2 | 83.7 |
The prevalence of infection in the 101 dogs evaluated was 77.2%
PV, positive predictive value.
NPV, negative predictive value.
The presence of either two of the low-molecular-mass bands (19, 25, 30, 32, or 37 kDa) or the 94- and 86-kDa bands.
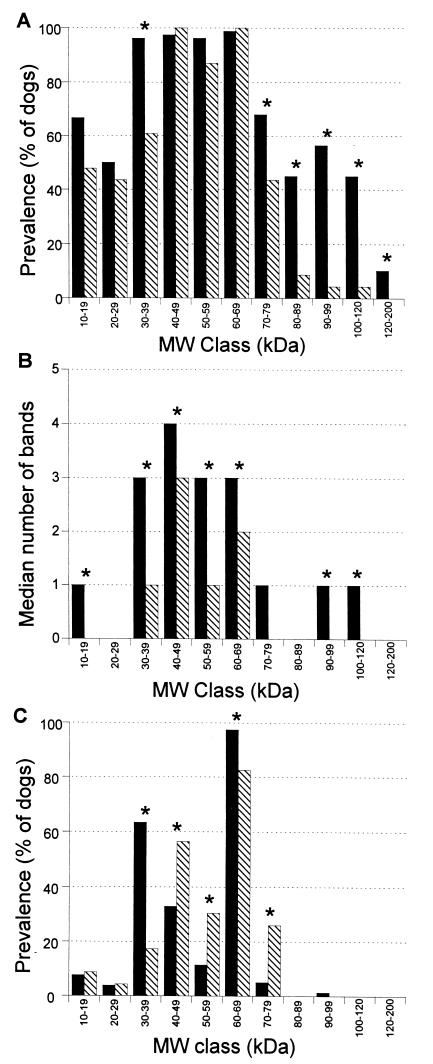
To further define the immunoreactivity of serum from infected and uninfected dogs, immunoblots were analyzed in molecular mass classes of 10 kDa. The prevalence of bands in molecular mass classes of 30 to 39 and >70 kDa were higher for samples from infected dogs than for samples from uninfected dogs (P < 0.05) (Fig. 7A). Bands of >80 kDa were present almost exclusively in infected dogs (P < 0.05).
FIG. 7.
Prevalence of at least one band in a molecular mass (MW) class (A), median number of bands in a molecular mass class (B), and prevalence of at least one of three of the most dense bands in a molecular mass class (C) in samples from infected dogs (solid bars) and samples from uninfected dogs (striped bars). Prevalence is defined as the percentage of dogs with at least one band. ∗, a statistically significant difference between infected and uninfected dogs (P < 0.05).
The number of immunoreactive bands for samples from infected dogs was significantly higher (P < 0.05) in all molecular mass classes except the 20- to 29-kDa and the 70- to 79-kDa classes (Fig. 7B). When the prevalence of the three most dense bands for each serum sample was calculated, the most pronounced difference between infected and uninfected dogs was in the molecular mass class of 30 to 39 kDa (Fig. 7C). Sixty-three percent of infected dogs had at least one dense band in this range, whereas only 17% of uninfected dogs had at least one dense band in the range. Significantly more dense bands of this molecular mass class and the 60- to 69-kDa class were found in samples from dogs infected with gastric Helicobacter spp. (P < 0.05). The test characteristics are described in Table 1. Significantly more dense bands in the molecular mass classes of 40 to 49, 50 to 59, and 70 to 79 kDa were found in samples from uninfected dogs than samples from infected dogs (P < 0.05).
When the immunoblots were evaluated for the presence of specific bands, five low-molecular-mass bands (19, 25, 30, 32 and 37 kDa) and one high-molecular-mass band (94 kDa) were found to be more prevalent in samples from infected dogs (P < 0.05). Combinations of either two of the low-molecular-mass bands (19, 25, 30, 32, and 37 kDa) or the 94-kDa band and the 86-kDa band were found almost solely in samples from infected dogs (P < 0.0001). The sensitivity and specificity of immunoblotting, on the basis of the presence of the possible combinations of these specific bands described above, for the detection of gastric Helicobacter spp. infection in dogs and other test parameters are described in Table 1.
ELISA results.
Kinetic ELISA results were significantly higher for infected dogs (median = 0.0802 OD/min, interquartile range = 0.042 to 0.1484 OD/min) than uninfected dogs (median = 0.01428 OD/min, interquartile range = 0.01 to 0.03264 OD/min) (P < 0.05). Only one uninfected dog had >0.08 OD/min. The cutoff value for this assay was 0.08 OD/min on the basis of a specificity of 95.6% (Fig. 6B). Other test characteristics are described in Table 1.
No correlation was found between ELISA results for 45 dogs infected with gastric Helicobacter spp. and age (P > 0.05).
Relationship of circulating IgG to colonization density, gastric inflammation, and presence of lymphoid nodules.
Colonization density was uniformly >10 organisms/×400 magnification field for 14 of 15 dogs evaluated. One of the 15 dogs had 1 to 10 organisms/×400 magnification field. Mild to moderate numbers of mononuclear inflammatory cells were observed in 13 of 15 dogs. Either numerous mononuclear inflammatory cells or minimal to no mononuclear inflammatory cells were present in the two remaining dogs. Mild increases in the numbers of neutrophils and eosinophils were found in 2 of 15 dogs. Lymphoid nodules were present in 12 of 15 dogs. The sizes of lymphoid nodules varied from small to large, and their numbers ranged from 0 to 15. No correlation was found between ELISA results, colonization density, degree of inflammation, and presence of lymphoid nodules (P > 0.05).
Correlation of immunoblotting and ELISA results.
The immunoblotting results for all serum samples, expressed as a percentage of the total density of a known positive standard, were compared to the ELISA results. The results of the two assays were found to be strongly correlated (ρ = 0.8 and P < 0.0001).
Efficacy of serology for diagnosis.
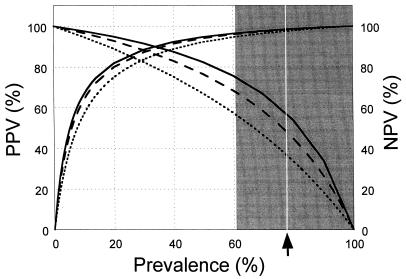
Of the 78 dogs infected with gastric Helicobacter spp., for 52.6% (41 of 78) ELISA results were >0.08 OD/min. For the 47.4% (37 of 78) of infected dogs for which ELISA results were ≤0.08 OD/min, 37.8% (14 of 37) of those dogs could be identified as infected by counting 16 or more bands on immunoblots of their samples, while 54% (20 of 37) could be identified as infected by the presence of a combination of specific bands, as defined above, or the presence of at least one dense band in the 30- to 39-kDa molecular mass class. Combination of ELISA and immunoblotting (measurement of the total number of bands and identification of the presence of specific bands) resulted in the detection of infection in 79.5% (62 of 78) of infected dogs. The specificity, sensitivity, diagnostic accuracy, positive predictive value, and negative predictive value are described in Table 1 and Fig. 8. The infection status of 20.5% (16/78) of dogs infected with gastric Helicobacter spp. could not be verified by any of the serological means discussed above.
FIG. 8.
Positive predictive values (PPVs) and negative predictive values (NPVs) of ELISA (dotted line), immunoblotting (striped line), and combined ELISA and immunoblotting (solid line) are plotted against the pretest anticipated prevalence of infection with gastric Helicobacter spp. The shaded area indicates the range of prevalence of infection in the dog population (see text for details). The arrow indicates the point prevalence of infection in the 101 dogs evaluated. Positive predictive value curves start at x = 0, while negative predictive value curves start at x = 100.
DISCUSSION
The serologic diagnosis of H. pylori infection in humans is accurate and relatively simple. The human stomach is almost exclusively colonized by H. pylori, and tests based on semipurified H. pylori antigens, particularly the two urease subunits and HSP (62 to 66, 29 to 31, and 53 to 56 kDa), have very high sensitivities and specificities (11, 40).
The use of serology for the detection of anti-gastric Helicobacter spp. antibodies in dogs is complicated by the presence of five different gastric Helicobacter spp. (8, 21, 26, 32, 34) and the possibility of coinfection with more than one species (16, 22, 34). Evaluation of the antigenic homology of Helicobacter spp. revealed that the major protein bands of crude antigens of H. felis and H. bizzozeronii were very similar to those of H. pylori, with only minor changes in molecular mass. The three major proteins of H. felis and H. bizzozeronii appeared to correspond to UreA, UreB, and HSP of H. pylori. Sera from infected and uninfected dogs bound in a similar way to the different Helicobacter antigens. On the basis of the results of these experiments, the H. felis ATCC 49179 antigen was chosen, because H. felis is commonly present in the dog stomach. A crude detergent extract was used for SDS-PAGE to enable the detection of immunoreactivity to as many proteins as possible.
The analysis of immunoblots by visual and densitometric means showed that the most sensitive method of distinguishing infected from uninfected dogs was the presence of specific band combinations at 19, 25, 30, 32, and 37 kDa or 86 and 94 kDa (Table 1). Bands with molecular masses of 43 to 66 kDa were specifically excluded from our immunoblot analysis because of their universal presence in infected and uninfected dogs and the known cross-reactivity of proteins in this molecular mass range between H. pylori and other bacterial flagellins (Treponema pallidum, Borrelia burgdorferi) and HSPs (Pseudomonas aeruginosa and C. jejuni) (1, 3, 7, 27, 35). The results of studies with humans infected with H. pylori are in broad agreement with our data. Positive immunoblots for people infected with H. pylori have been defined as serum binding to proteins of 87 to 128 kDa or at least two of the five proteins between 22 and 33 kDa (29, 35) or at least one of the bands between 19 and 36 kDa (1). Other studies adopted a more global approach and considered a marker for infection to be the presence of at least two bands of various sizes (180, 120, 90, 75, 67, 29.5, and 19.5 kDa) (12) or completely different bands (one of 54, 35, or 42 kDa) (2). The ELISA findings for dogs had similar specificities (Table 1) to the results of the ELISA used for the detection of anti-H. pylori antibodies in humans and rhesus monkeys (13, 20). However, the sensitivity was much lower than the 90% reported for humans and monkeys. The lack of sensitivity could be attributed to a number of factors. The uninfected dogs in this study had antibodies against the UreB subunit of H. pylori. These antibodies may have arisen as a consequence of an immune response to other bacteria, particularly other urease-producing organisms. One of the urease subunits of P. mirabilis with a molecular mass of 68 kDa (43) or 73 kDa (28) has been described. We have previously isolated P. mirabilis from the stomach of an SPF beagle dog, which might explain the 66-kDa immunoreactive band in uninfected dogs. The exact species of Helicobacter that colonized the dogs in this study were not known. Perhaps the use of antigen derived from H. salomonis or other H. heilmannii-like organisms, which have been observed in the stomachs of dogs, could have increased the sensitivity of an ELISA (21, 26). The relative lack of a humoral response of some dogs to Helicobacter infection may have also affected the sensitivity of the ELISA. Indeed, the type of inflammation observed in response to Helicobacter spp. infection in dogs is much less severe than the H. pylori-associated chronic gastritis observed in humans, in whom neutrophilic and eosinophilic infiltrates are a prominent feature (8, 22, 31). Examination of the relationship of bacterial colonization density, degree of inflammation, and the presence of lymphoid nodules revealed no correlation to the ELISA results for dogs. It was noteworthy that dogs with very dense colonization often failed to seroconvert. The factors governing the immunologic response to gastric Helicobacter spp. in dogs are unclear. Gnotobiotic dogs experimentally infected with H. felis or H. pylori showed uniform and rapid seroconversion (33, 37), whereas SPF dogs experimentally infected with H. felis demonstrated a slower and more variable seroconversion (38).
In clinical practice, immunoblotting is often used to evaluate equivocal ELISA results. The combined use of ELISA and immunoblot screening of sera with ELISA results of <0.08 OD/min enabled the identification of 25.7% (20 of 78) more infected dogs than the use of ELISA alone. To assess the performance of the test with populations with different Helicobacter prevalences, we evaluated the positive and negative predictive values of the ELISA and immunoblotting results (Fig. 8). The exact prevalence of infection with gastric Helicobacter spp. in this study (77.2%) was in the range of the prevalence estimates in dogs in previous studies (61 to 100%) (8, 9, 18, 24, 25, 45). At this prevalence, the positive predictive values were found to be high (>97%), as is desirable for the detection of infection. Negative predictive values were lower (37.3 to 58%), as expected with this relatively high prevalence of infection. Similar measurements for humans show a different trend, with a positive predictive value of 91% and a negative predictive value of 94% (1). This is probably due to the lower prevalence and the higher test sensitivity for the human population studied.
The infection status of 20.5% (16 of 78) of the dogs infected with gastric Helicobacter spp. could not be determined serologically. For those dogs, repeated sampling to detect seroconversion or other diagnostic means, such as the 13C-urea breath test or biopsy, will be needed.
Overall, the combination of serological methods for the diagnosis of infection with gastric Helicobacter spp. in dogs seems promising. Although immunoblotting is technically more time-consuming and more difficult to analyze than ELISA in a diagnostic laboratory, it identified dogs that were infected with gastric Helicobacter spp. but whose samples were equivocal by ELISA.
ACKNOWLEDGMENTS
This work was supported by grants from Enteric Products Inc., the New York State Science and Technology Foundation, and the Alumni Unrestricted Funds Program, College of Veterinary Medicine, Cornell University.
We thank Hussni Mohammed for statistical support and Katri Jalava for sending us H. bizzozeronii. We thank Marg Pough and Patti Easton for excellent technical guidance in setting up the assays and Alma Williams for dog samples.
REFERENCES
- 1.Andersen L P, Espersen F. Immunoglobulin G antibodies to Helicobacter pylori in patients with dyspeptic symptoms investigated by the Western immunoblot technique. J Clin Microbiol. 1992;30:1743–1751. doi: 10.1128/jcm.30.7.1743-1751.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aucher P, Petit M L, Mannant P R, Pezennec L, Babin P, Fauchere J L. Use of immunoblot assay to define serum antibody patterns associated with Helicobacter pylori infection and with H. pylori-related ulcers. J Clin Microbiol. 1998;36:931–936. doi: 10.1128/jcm.36.4.931-936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazillou M, Fendri C, Castel O, Ingrand P, Fauchere J L. Serum antibody response to the superficial and released components of Helicobacter pylori. Clin Diagn Lab Immunol. 1994;1:310–317. doi: 10.1128/cdli.1.3.310-317.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bizzozero G. Sulla presenza di batteri nelle ghiandole gastriche del cane. Atti R Accad Sci Torino. 1893;28:249–251. [Google Scholar]
- 5.Blaser M J. The bacteria behind ulcers. Sci Am. 1996;274:103–107. doi: 10.1038/scientificamerican0296-104. [DOI] [PubMed] [Google Scholar]
- 6.Cornetta A, Simpson K W, Strauss-Ayali D, McDonough P L, Gleed R D. Use of a 13[C]-urea breath test for detection of gastric infection with Helicobacter spp in dogs. Am J Vet Res. 1998;59:1364–1369. [PubMed] [Google Scholar]
- 7.Dunn B E, Perez-Perez G I, Blaser M J. Two-dimensional gel electrophoresis and immunoblotting of Campylobacter pylori proteins. Infect Immun. 1989;57:1825–1833. doi: 10.1128/iai.57.6.1825-1833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaton K A, Dewhirst F E, Paster B J, Tzellas N, Coleman B E, Paola J, Sherding R. Prevalence and varieties of Helicobacter species in dogs from random sources and pet dogs: animal and public health implications. J Clin Microbiol. 1996;34:3165–3170. doi: 10.1128/jcm.34.12.3165-3170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton K A, Paola J P, Johnson S E, Sherding R G. Gastritis associated with gastric bacteria in asymptomatic, random source dogs. Vet Pathol. 1992;29:454. [Google Scholar]
- 10.Eaton K A, Radin M J, Kramer L, Wack R, Sherding R, Krakowka S, Fox J G, Morgan D R. Epizootic gastritis associated with gastric spiral bacili in cheetahs (Acinonyx jubatus) Vet Pathol. 1993;30:55–63. doi: 10.1177/030098589303000107. [DOI] [PubMed] [Google Scholar]
- 11.Evans D J, Evans D G, Graham D Y, Klein P D. A sensitive and specific serologic test for detection of Campylobacter pylori infection. Gastroenterology. 1989;96:1004–1008. doi: 10.1016/0016-5085(89)91616-8. [DOI] [PubMed] [Google Scholar]
- 12.Faulde M, Cremer J, Zöller L. Humoral immune response against Helicobacter pylori as determined by immunoblot. Electrophoresis. 1993;14:945–951. doi: 10.1002/elps.11501401150. [DOI] [PubMed] [Google Scholar]
- 13.Feldman R A, Deeks J J, Evans S J W. Multi-laboratory comparison of eight commercially available Helicobacter pylori serology kits. Eur J Microbiol Infect Dis. 1995;14:428–433. doi: 10.1007/BF02114899. [DOI] [PubMed] [Google Scholar]
- 14.Fox J G, Correa P, Taylor N S, Otto G, Murphy J C, Rose R. Helicobacter mustelae-associated gastritis in ferrets. An animal model of Helicobacter pylori gastritis in humans. Gastroenterology. 1990;99:352–361. doi: 10.1016/0016-5085(90)91016-y. [DOI] [PubMed] [Google Scholar]
- 15.Fox J G, Dewhirst F E, Shen Z, Feng Y, Taylor N S, Paster B J, Ericson R L, Lau C N, Correa P, Araya J C, Roa I. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–763. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 16.Fox J G, Lee A. The role of Helicobacter species in newly recognized gastrointestinal tract diseases in animals. Lab Anim Sci. 1997;47:222–255. [PubMed] [Google Scholar]
- 17.Garvey W, Fathi A, Bigelow F. Modified Steiner for the demonstration of spirochetes. J Histotech. 1985;8:15–17. [Google Scholar]
- 18.Geyer C, Colbazky F, Lechner J, Hermanns W. Occurrence of spiral-shaped bacteria in gastric biopsies of dogs and cats. Vet Rec. 1993;133:18–19. doi: 10.1136/vr.133.1.18. [DOI] [PubMed] [Google Scholar]
- 19.Graham D Y, Evans D J, Peacock J, Baker J T, Schrier W H. Comparison of rapid serological tests (FlexSure HP and Quick Vue) with conventional ELISA for detection of Helicobacter pylori infection. Am J Gastroenterol. 1996;91:942–948. [PubMed] [Google Scholar]
- 20.Handt L K, Fox J G, Yan L L, Shen Z, Pouch W J, Ngai D, Motzel S L, Nolan T E, Klein H J. Diagnosis of Helicobacter pylori infection in a colony of rhesus monkeys (Macaca mulatta) J Clin Microbiol. 1997;35:165–168. doi: 10.1128/jcm.35.1.165-168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Häninnen M L, Happonen I, Saari S, Jalava K. Culture and characteristics of Helicobacter bizzozeronii, a new canine gastric Helicobacter sp. Int J Syst Bacteriol. 1996;46:160–166. doi: 10.1099/00207713-46-1-160. [DOI] [PubMed] [Google Scholar]
- 22.Happonen I, Saari S, Castren L, Tyni O, Hanninen M L, Westermarck E. Occurrence and topographical mapping of gastric Helicobacter-like organisms and their association with histological changes in apparently healthy dogs and cats. J Vet Med Ser A. 1996;43:305–315. doi: 10.1111/j.1439-0442.1996.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 23.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 24.Henry G A, Long P H, Burns J L, Charbonneau D L. Gastric spirillosis in beagles. Am J Vet Res. 1987;48:831–836. [PubMed] [Google Scholar]
- 25.Hermanns W, Kregel K, Breuer W, Lechner J. Helicobacter-like organisms: histopathological examination of gastric biopsies from dogs and cats. J Comp Pathol. 1995;112:307–318. doi: 10.1016/s0021-9975(05)80083-0. [DOI] [PubMed] [Google Scholar]
- 26.Jalava K, Kaartinen M, Utriainen M, Happonen I, Hanninen M L. Helicobacter salomonis sp. nov., a canine gastric Helicobacter sp. related to Helicobacter felis and Helicobacter bizzozeronii. Int J Syst Bacteriol. 1997;47:975–982. doi: 10.1099/00207713-47-4-975. [DOI] [PubMed] [Google Scholar]
- 27.Johansen H K, Norgaard A, Andersen L P, Jensen P, Nielsen H, Hoiby N. Cross-reactive antigens shared by Pseudomonas aeruginosa, Helicobacter pylori, Campylobacter jejuni, and Haemophilus influenzae may cause false-positive titers of antibody to H. pylori. Clin Diagn Lab Immunol. 1995;2:149–155. doi: 10.1128/cdli.2.2.149-155.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones B D, Mobley H L T. Proteus mirabilis urease: genetic organization, regulation, and expression of structural genes. J Bacteriol. 1988;170:3342–3349. doi: 10.1128/jb.170.8.3342-3349.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karvar S, Karch H, Frosch M, Burghardt W, Gross U. Use of serum-specific immunoglobulins A and G for the detection of Helicobacter pylori infection in patients with chronic gastritis by immunoblot analysis. J Clin Microbiol. 1997;35:3058–3061. doi: 10.1128/jcm.35.12.3058-3061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laemmli U K. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Lee A, Fox J G, Hazell S. Pathogenicity of Helicobacter pylori: a perspective. Infect Immun. 1993;61:1601–1610. doi: 10.1128/iai.61.5.1601-1610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee A, Hazell S, O’Rourke J L, Kouprach S. Isolation of a spiral-shaped bacterium from the cat stomach. Infect Immun. 1988;56:2843–2850. doi: 10.1128/iai.56.11.2843-2850.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee A, Krakowka S, Fox J G, Otto G, Eaton K A, Murphey J C. Role of Helicobacter felis in chronic canine gastritis. Vet Pathol. 1992;29:487–494. doi: 10.1177/030098589202900601. [DOI] [PubMed] [Google Scholar]
- 34.Lockard V G, Boler R A. Ultrastructure of a spiraled microorganism in the gastric mucosa of dogs. Am J Vet Res. 1970;31:1453–1462. [PubMed] [Google Scholar]
- 34a.National Institutes of Health. 16 December 1996, copyright date. [Online.] NIH Image program, version 1.61.1. National Institutes of Health, Bethesda, Md. http://rsb.info.nih.gov/nih-image. [May 1998, last date accessed.]
- 35.Nilsson I, Ljungh A, Aleljung P, Wadstrom T. Immunoblot assay for serodiagnosis of Helicobacter pylori infections. J Clin Microbiol. 1997;35:427–432. doi: 10.1128/jcm.35.2.427-432.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Queiroz D M, Rocha G A, Mendes E N, De Moura S B, De Oliveira A M R, Miranda D. Association between Helicobacter and gastric ulcer disease of the pars esophagea in swine. Gastroenterology. 1996;111:19–27. doi: 10.1053/gast.1996.v111.pm8698198. [DOI] [PubMed] [Google Scholar]
- 37.Radin M J, Eaton K A, Krakowka S, Morgan D R, Lee A, Otto G, Fox J G. Helicobacter pylori gastric infection in gnotobiotic beagle dogs. Infect Immun. 1990;58:2606–2612. doi: 10.1128/iai.58.8.2606-2612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Seebacher, T. 1996, copyright date. [Online.] Molecular weight macro package. http://www.uni-konstanz.de/tt/software/mwmacro.html. [May 1998, last date accessed.]
- 38.Simpson, K. W., P. L. McDonough, D. Strauss-Ayali, Y. F. Chang, P. Harpending, and B. A. Valentine. Helicobacter felis infection in dogs: effect on gastric structure and function. Vet. Pathol., in press. [DOI] [PubMed]
- 39.Smoot D T, Hamilton F A. Summary of the National Institutes of Health Consensus Development Conference on Helicobacter pylori. Gastrointest Dis Today. 1995;4:1–10. [Google Scholar]
- 40.Stacey A R, Hatwin P R, Newell D G. Antigenicity of fractions of Helicobacter pylori prepared by fast protein liquid chromatography and urease captured monoclonal antibodies. Eur J Microbiol Infect Dis. 1990;9:732–737. doi: 10.1007/BF02184685. [DOI] [PubMed] [Google Scholar]
- 41.Talley N J, Newell D G, Ormand J E, Carpenter H A, Wilson W R, Zinsmeister A R, Perez-Perez G I, Blaser M J. Serodiagnosis of Helicobacter pylori: comparison of enzyme-linked immunosorbent assays. J Clin Microbiol. 1991;29:1635–1639. doi: 10.1128/jcm.29.8.1635-1639.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tompkins L S, Falkow S. The new path to preventing ulcers. Science. 1995;267:1621–1622. doi: 10.1126/science.7886448. [DOI] [PubMed] [Google Scholar]
- 43.Walz S E, Wray S K, Hull S I, Hull R A. Multiple proteins encoded within the urease gene complex of Proteus mirabilis. J Bacteriol. 1988;170:1027–1033. doi: 10.1128/jb.170.3.1027-1033.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber A F, Hasa O, Sautter J H. Some observations concerning the presence of spirilla on the fundic glands of dogs and cats. Am J Vet Res. 1958;19:677–680. [PubMed] [Google Scholar]
- 45.Yamasaki K, Suematsu H, Takahashi T. Comparison of gastric lesions in dogs and cats with and without gastric spiral organisms. J Am Vet Med Assoc. 1998;212:529–533. [PubMed] [Google Scholar]