Abstract
Introduction
General practice is integral to the Australian healthcare system. Outcome Health’s POpulation Level Analysis and Reporting (POLAR) database uses de-identified electronic health records to analyse general practice data in Australia. Previous studies using routinely collected health data for research have not consistently reported the codes and algorithms used to describe the population, exposures, interventions and outcomes in sufficient detail to allow replication. This paper reports a study protocol investigating patterns of care for people presenting with musculoskeletal conditions to general practice in Victoria, Australia. Its focus is on the systematic approach used to classify and select eligible records from the POLAR database to facilitate replication. This will be useful for other researchers using routinely collected health data for research.
Methods and analysis
This is a retrospective cohort study. Patient-related data will be obtained through electronic health records from a subset of general practices across three primary health networks (PHN) in southeastern Victoria. Data for patients with a low back, neck, shoulder and/or knee condition and who received at least one general practitioner (GP) face-to-face consultation between 1 January 2014 and 31 December 2018 will be included. Data quality checks will be conducted to exclude patients with poor data recording and/or non-continuous follow-up. Relational data files with eligible and valid records will be merged to select the study cohort and the GP care received (consultations, imaging requests, prescriptions and referrals) between diagnosis and 31 December 2018. Number and characteristics of patients and GPs, and number, type and timing of imaging requests, prescriptions for pain relief and referrals to other health providers will be investigated.
Ethics and dissemination
Ethics approval was obtained from the Cabrini and Monash University Human Research Ethics Committees (Reference Numbers 02-21-01-19 and 16975, respectively). Study findings will be reported to Outcome Health, participating PHNs, disseminated in academic journals and presented in conferences.
Keywords: primary care, musculoskeletal disorders, back pain, shoulder, knee, epidemiology
Strengths and limitations of this study.
This is the first study to our knowledge to report the codes and algorithms used to classify, select and merge eligible records from the POpulation Level Analysis and Reporting (POLAR) database into a patient-centred database to facilitate analysis of general practice patterns of care.
The systematic approach used in this study can be adapted by other researchers using routinely collected health data for research purposes.
This study will extend previous research that has assessed the representativeness of POLAR data to general practitioner (GP) care across the wider Australian population.
These data are likely to underestimate actual allied health visits as some of these do not require a GP referral in Australia; some prescriptions for pain relief are available without a prescription so these data will also be underestimated.
It is possible not all patterns of care for the study cohort will be directly attributable to a musculoskeletal condition as reasons for GP consultations, referrals and prescriptions are not mandated by the source electronic medical records.
Introduction
General practice plays an essential role in providing primary healthcare to the population. In Australia 86% of the population visits a general practitioner (GP) multiple times a year,1 and nearly 20% of these consultations are for a musculoskeletal condition.2 These conditions account for 23% of the years lived with disability in Australia3 and are also a major cause of disability worldwide.4 Until 2016, the BEACH (Bettering the Evaluation and Care of Health) programme provided the most comprehensive data on clinical activities of Australian general practice.5 The programme identified a number of activities that represent low-value care for people with musculoskeletal conditions including an over-reliance on imaging, prescription of opioids and unnecessary referrals to specialist care.6 7 However, in-depth exploration of these activities within the BEACH programme is limited by its cross-sectional design, and these data are no longer being collected.
Technological advancements have facilitated the extraction of de-identified patient information from general practice clinical information systems. The advantage of these data sets for research purposes are that they are longitudinal and can therefore be used to establish sequences of events at the patient level and to examine changes in GP management over time. Both the Medicine Insight8 and the POpulation Level Analysis and Reporting (POLAR) databases9 are examples of longitudinal general practice data sets within Australia. Unlike POLAR, the Medicine Insight programme does not currently include referrals provided by GPs to other healthcare providers.8 These data may provide important insights into how well GPs are playing their role as gatekeepers of the Australian healthcare system.
While using routinely collected data for research purposes offers considerable opportunities to improve healthcare, there are several challenges to be overcome. Differences in patient information management and data extraction tools result in variability in both the information captured and ways in which this information is coded. In particular, the way in which text values (diagnoses, examination findings, test results and medications) are transformed to codes can be a source of variation within and between studies. Previous studies have highlighted how code selection affects the reported prevalence and precision of results.10 Studies conducted using routinely collected health data should therefore be reported with sufficient detail and clarity to allow replication. However, a systematic evaluation of a random sample of 124 publications using routinely collected health data has demonstrated inadequate reporting of the methods used.11 For example, in 44 studies where definitions of codes or classification algorithms were deemed necessary to describe the population, exposures or interventions and outcomes, only 9 (20.5%) reported all three items adequately. The REporting of studies Conducted using Observational Routinely collected Data (RECORD) guidelines, published in 2015, were developed to assist in this process and to ensure that readers can assess the internal and external validity of the findings of these studies.12
The POLAR database draws data from every consultation occurring for millions of patients in approximately 30% of general practices across southeastern Victoria,13 an area that comprises more than half of Victoria’s population.14 Inclusion is based on practice consent so this volume is increasing exponentially as more practices consent to add their data and as more consultations occur over time. Unlike in other countries, coding is not embedded in the clinical process and needs to be conducted specifically for research purposes. Data are provided to research users in a relational database that organises data into files that can be merged based on common data fields. Identifying and selecting relevant records and merging separate files into a patient-centred database for analysis is a complex task that could potentially yield variable results depending on the methods used.
Previous studies have used the POLAR database to investigate patterns of antimicrobial prescribing for children,15 to examine characteristics of patients presenting to an after-hours clinic,16 to estimate GP recording of cardiovascular risk factors17 and to describe characteristics of pathology test ordering in general practice.18 However, these studies have not reported the methods used to classify and select eligible records or the processes used to merge data files into a patient-centred database for analysis.
This manuscript presents a protocol for a study investigating patterns of GP care for people with a low back, neck, shoulder and/or knee condition in Victoria, Australia. It describes the methods used to classify and select eligible records from the POLAR database and how relational data files will be merged into a patient-centred database. This systematic approach will guide future research by enabling researchers interested in using routinely collected health data, and the POLAR database in particular, to answer other clinically relevant questions about general practice care. Study findings will advance existing knowledge about GP care for people with these musculoskeletal conditions and whether it conforms to best evidence-based practice. Differences in care across different musculoskeletal complaints may also inform tailored interventions to improve care and ultimately reduce the burden of disease associated with these musculoskeletal complaints.
Objectives
The aim of this study will be to examine GP patterns of care for people with low back, neck, shoulder and knee conditions. Specific objectives will be to:
Describe and compare the management (number, type and timing of imaging tests and procedure requests, prescriptions for pain relief and referrals to other health providers) provided by GPs to people with low back, neck, shoulder and knee conditions.
Describe the prevalence of comorbidities among specific musculoskeletal diagnoses within this cohort.
Examine the association between management types and patient-related and practice-related variables.
Examine the longitudinal changes in GP management for these conditions between 2014 and 2018 inclusive.
Methods
Study design
A retrospective cohort study using general practice health records from Victoria, Australia.
Data source
This study will use data from Outcome Health’s POLAR database.9 The database structure is based on eight relational files, each containing de-identified practice, provider and/or patient codes (figure 1). These common fields allow merging of the data files so that databases can be configured for specific research purposes. Data are extracted from two different clinical information systems, covering 90% of included general practices. All data are extracted using the Hummingbird data extraction tool.9
Figure 1.
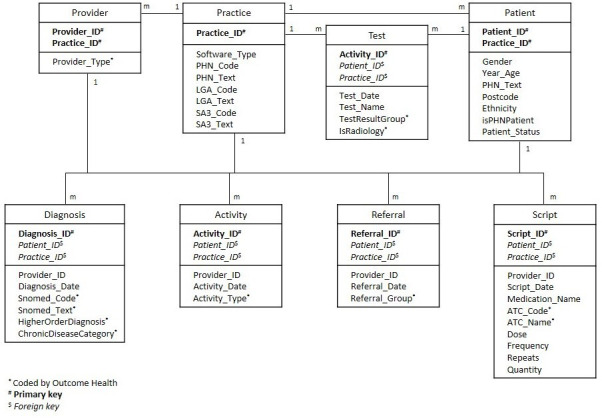
Database structure. ATC, Anatomical Therapeutic Chemical; PHN, primary health networks.
Setting
The POLAR database contains de-identified patient-related data from all electronic medical records of consenting general practices within the primary health networks (PHNs) of Eastern Melbourne, South Eastern Melbourne and Gippsland within Victoria, Australia. Our study will include data collected over five calendar years from 1 January 2014 until 31 December 2018 relating to all patients with an eligible musculoskeletal condition and who received at least one face-to-face GP consultation. Follow-up will be from the time of the initial recorded diagnosis to 31 December 2018. Data analyses will be completed by the end of 2021.
Participants
The study cohort will include people diagnosed during 2014–2018 inclusive with a low back, neck, shoulder and/or knee condition, limited to age 45 years and over except for low back which will be limited to age 18 years and over. The differing age restrictions were chosen because the prevalence of most musculoskeletal conditions increases markedly after the age of 45 except for low back pain which increases after the age of 18.19 Eligibility criteria are presented in table 1. We excluded traumatic diagnoses and conditions typically primarily managed by a specialist (eg, inflammatory and autoimmune rheumatic diseases). Patients with an eligible diagnosis and age will also have received at least one GP face-to-face consultation during the study dates. The musculoskeletal diagnosis will not have to occur during a GP consultation since an eligible diagnosis could result from consultation with other healthcare providers.
Table 1.
Eligibility criteria
| Patient population | Patient management | ||||||
| Diagnoses | Provider | Patient | Practice | Activity | Referrals | Prescriptions | Imaging tests and procedures |
| Low back Knee Shoulder Neck Exclude: Trauma Systemic inflammatory arthritis |
Diagnosed by a general practitioner | Aged ≥18 years for low back conditions Aged ≥45 years for all other diagnoses |
Patient activity 2014–2018 | Face-to-face Telehealth |
Surgical specialists Non-surgical specialists Allied health providers, eg, psychologist |
Simple analgesics Anti-inflammatories Chondroitin/ Glucosamine Topical products Opioids Neuromodulators |
Lumbar plain radiograph Lumbar CT Lumbar MRI Lumbar injection Knee plain radiograph Knee CT Knee MRI Knee ultrasound Knee injection Shoulder plain radiograph Shoulder ultrasound Shoulder MRI Shoulder injection Shoulder hydrodilatation Cervical plain radiograph Cervical CT Cervical MRI Cervical injection |
CT, Computed Tomography; MRI, Magnetic Resonance Imaging.
Variables
Preparatory work to classify and select eligible records has been completed as part of the protocol process. In circumstances where Outcome Health has previously coded data (eg, diagnosis records), we used this coding to select eligible records that fitted our inclusion criteria. In circumstances where there was no coding (eg, imaging tests), we coded the data into categories and then selected eligible records. Outcome Health’s approach to coding used clinical natural language processing to automatically code structured narrative text within the electronic medical record followed by a manual process for quality checking and correction.20 For example, this allowed the free-text items ‘back pain’, ‘low back pain’ and ‘lumbar pain’ to all sit under the same diagnostic code. Where possible, coding was conducted using a standardised classification system. For example, diagnoses are coded using SNOMED CT-AU terminology21 and prescriptions are coded according to the Anatomical Therapeutic Chemical (ATC) classification system.22 In cases where there is no standardised classification system available (eg, providers and referrals), Outcome Health used a similar process to code these variables into relevant categories (eg, type of healthcare provider). Clinical natural language processing conducted by Outcome Health has previously demonstrated accurate coding of over 95% of the narrative text to SNOMED CT-AU terms in a sample of approximately 57 000 diagnosis records.20 Our approaches to coding and/or selecting eligible records for each variable are described in detail below.
Provider records
Healthcare providers other than a GP may be nested within a general practice. To limit all diagnoses, consultations, referrals and prescriptions to those made only by GPs we used coding within the provider type field conducted by Outcome Health. This is coded by Outcome Health according to the professional background of the healthcare provider delivering the service (eg, GP, nurse).
Diagnoses records
All SNOMED CT-AU diagnosis-related terms used during 2014–2018 were searched by two study authors (RH and RB) to select eligible low back, neck, shoulder and knee conditions. We included all patients with an eligible musculoskeletal diagnosis during 2014–2018 regardless of whether they had a prior musculoskeletal diagnosis. Included SNOMED diagnosis terms are presented in table 2. Sacral conditions were included as part of low back conditions. The following SNOMED terms were excluded as these conditions were deemed to be indicative of traumatic injury or conditions that are not managed primarily by GPs: fracture (except lumbar and tibial plateau fractures), dislocation, synovectomies/synovitis and cauda equina syndrome. Knee ligamentous and meniscal tears were included as these are likely due to degeneration in the 45 years and over age group.23 Lesions were excluded as these could involve a wound, ulcer or tumour and are not musculoskeletal conditions. General musculoskeletal terms such as sprain or osteoarthritis (where the site was not specified) were also excluded as these could not be attributed to a specific body region. We included relevant surgical or procedural musculoskeletal terms as GPs are involved in referral and follow-up for these conditions.
Table 2.
Included SNOMED terms
| Low back diagnoses | Knee diagnoses | Shoulder diagnoses | Neck diagnoses |
| Arthritis of spine Arthropathy of spinal facet joint Back problem Backache Bone structure of coccyx Bone structure of L5 Bone structure of sacrum Chondrectomy of spine Chronic back pain Chronic lower back pain Compression fracture Compression fracture of vertebral column Compression of lumbar nerve root Correction of scoliosis Crush fracture of lumbar vertebra CT of lumbar region CT of lumbar spine CT of spine Curvature of spine Decompression laminectomy Decompression of lumbar spine Degeneration of intervertebral disc Degeneration of lumbar intervertebral disc Diagnostic radiography of coccyx Discitis Discogenic pain Disorder of joint of spine Disorder of vertebra Exploration of spine Facet joint pain Fracture of body of vertebra Fracture of lumbar spine Fracture of sacrum Fracture of vertebral column Injury of back Injury of coccyx Intervertebral disc prolapse L4/L5 disc L5/S1 disc Laminectomy Lordosis deformity of spine Low back pain Low back strain Lower back injury Lower back structure Lumbar Lumbar discectomy Lumbar laminectomy Lumbar microdiscectomy Lumbar radiculopathy Lumbar region back structure Lumbar spinal fusion Lumbar sprain Lumbosacral spine Lumbosacral spondylosis Lumbosacral spondylosis without myelopathy Lumbosacral strain Lumbosacral radiculopathy MRI of spine Manipulation of spine MRI of lumbar spine Nerve root compression syndrome Nerve root disorder Operative procedure on spinal structure Osteoarthritis of lumbar spine Pain in lumbar spine Pain in the coccyx Prolapsed lumbar intervertebral disc Radiography of spine Sacral back pain Sacroiliac arthrodesis Sacroiliac joint inflamed Sacroiliac joint pain Scoliosis deformity of spine Scoliosis of lumbar spine Spasm of back muscles Spinal arthritis deformans Spinal arthrodesis Spinal claudication Spinal injury Spinal stenosis Spinal stenosis of lumbar region Spondylitis Spondylolisthesis Spondylolisthesis L5/S1 level Spondylolysis Spondylosis Spondylosis without myelopathy Sprain of spinal ligament Sprain, lumbosacral ligament Stenosis of intervertebral foramina Stiff back Vertebral osteoporosis Vertebroplasty Wedge fracture of vertebra X-ray of lumbosacral spine |
Acute meniscal tear, medial Anterior knee pain Arthritis of knee Arthrodesis of knee Arthroscopic lateral patellar release Arthroscopic meniscectomy Arthroscopic procedure Arthroscopy of knee Arthroscopy of knee with lateral meniscectomy Arthroscopy of knee with medial meniscectomy Arthrotomy of knee Aspiration of knee joint Both knees Bursitis of knee Calcium pyrophosphate deposition disease Chondrocalcinosis Chondromalacia of patella Complete tear, knee, medial collateral ligament Contusion of knee Derangement of knee Disorder of patellofemoral joint Finding of tear meniscus Fracture of tibial plateau Haemarthrosis of knee Inflammation of bursa of patella Injury of anterior cruciate ligament Injury of knee Knee joint—varus deformity Knee joint effusion Knee joint valgus deformity Knee locking Knee pain Knee region structure Knee stiff Loose body in knee MRI of knee Osteoarthritis of knee Osteotomy of proximal tibia Osteotomy of tibia Patellar instability Patellar maltracking Patellar tendonitis Patellectomy Patellofemoral osteoarthritis Patellofemoral stress syndrome Prepatellar bursitis Problem knee Radiological examination of knee Repair of anterior cruciate ligament of knee joint Repair of knee collateral ligaments Repair of knee cruciate ligaments Repair of meniscus Repair of patellar tendon Replacement of total knee joint Rupture of anterior cruciate ligament Rupture of cruciate ligaments Rupture of medial collateral ligament of knee Rupture of posterior cruciate ligament Sprain of knee Sprain of lateral collateral ligament of knee Sprain of medial collateral ligament of knee Stabilisation of patellofemoral joint Strain of knee Strain of patellar tendon Strain of tendon of medial thigh muscle Structure of left knee Structure of prepatellar bursa Structure of right knee Subluxation of patellofemoral joint Suprapatellar bursitis Swollen knee Synovial cyst of knee Synovial cyst of popliteal space Tear of lateral meniscus of knee Tear of medial meniscus of knee Tear of meniscus of knee Total knee replacement Total replacement of left knee joint Total replacement of right knee joint Traumatic rupture of patellar tendon Unstable knee |
Acromioclavicular joint structure Adhesive capsulitis of shoulder Arthritis of acromioclavicular joint Arthrodesis of shoulder Arthrography of shoulder Arthroscopic acromioplasty Arthroscopic shoulder decompression Arthroscopy of shoulder Bursitis of shoulder Calcific tendinitis Calcific tendinitis of shoulder Capsulitis Contusion of shoulder region Detachment of the glenoid labrum and/or capsule of the shoulder joint Entire tendon of supraspinatus muscle Full thickness rotator cuff tear Impingement syndrome of shoulder region Inflammation of rotator cuff tendon Injury of glenoid labrum of shoulder joint Injury of shoulder region MRI of shoulder Osteoarthritis of acromioclavicular joint Osteoarthritis of shoulder Painful arc syndrome Radiography of shoulder Repair of musculotendinous cuff of shoulder Repair of shoulder Rotator cuff impingement syndrome Rotator cuff syndrome Rupture of tendon of biceps Rupture of tendon of biceps, long head Shoulder pain Shoulder reconstruction Shoulder region structure Shoulder strain Shoulder tendinitis Sprain of acromioclavicular ligament Sprain of shoulder Structure of left shoulder region Structure of right shoulder region Structure of rotator cuff including muscles and tendons Subacromial bursitis Subdeltoid bursitis Subluxation of acromioclavicular joint Subscapularis tendinitis Supraspinatus tear Supraspinatus tendinitis Total shoulder replacement US shoulder region |
Cervical arthritis Cervical arthrodesis Cervical disc disorder Cervical kyphosis Cervical laminectomy Cervical myelopathy Cervical nerve root compression Cervical radiculitis Cervical radiculopathy Cervical rib Cervical spinal fusion by anterior technique Cervical spine degeneration Cervical spine structure Cervicogenic headache Cervico-occipital neuralgia Chronic neck pain CT of cervical spine CT of neck Degeneration of cervical intervertebral disc Diffuse cervicobrachial syndrome Excision of cervical intervertebral disc Injury of cervical spine Kyphoscoliosis deformity of spine Kyphosis deformity of spine MRI of neck MRI of cervical spine Muscle spasm of cervical muscle of neck Neck injury Neck pain Neck sprain Neck structure Pain in cervical spine Prolapsed cervical intervertebral disc Radiography of cervical spine Spinal stenosis in cervical region Stiff neck Strain of neck muscle Strain of tendon of neck Torticollis Whiplash injury to neck |
CT, Computed Tomography; MRI, Magnetic Resonance Imaging; US, Ultrasound.
Using experienced clinicians, Outcome Health has further categorised SNOMED diagnoses into overarching groups and used key chronic disease groups as a qualifier.9 For example, free text such as ‘low back pain’ or ‘angina’ could be qualified as a chronic disease if present for 6 months or more. We used these chronic disease groups to identify eligible comorbid diagnoses for our study cohort as follows: chronic cardiovascular disease, chronic obstructive pulmonary disease, chronic musculoskeletal conditions, cancer, opioid addiction, dementia, diabetes, depression/anxiety and obesity. Obesity was identified using SNOMED terms as it was not coded as a chronic disease category in the POLAR database. We included previous chronic musculoskeletal conditions so that these could be investigated as a potential predictor of different management patterns.
Activity records
Activity records are coded in POLAR according to the type of consultation provided (eg, Telehealth, visit, telephone). Each time a note is recorded in the narrative section it is coded by the electronic medical record (EMR) and this is extracted by POLAR. We used this coding to select eligible patients who had at least one ‘Activity type’ relating to a face-to-face consultation (ie, encounter, surgery or visit) during 2014–2018 inclusive. Telehealth and telephone consultations were also included for follow-up consultations only.
Referral records
Referral records are coded in POLAR according to discipline (eg, neurosurgeon, physiotherapist, endocrinologist). We used this coding to select eligible referral groups considered relevant to a person with low back, neck, shoulder or knee conditions. The following referral groups were included: orthopaedics and neurosurgery (surgical specialists); sports medicine, rheumatology, rehabilitation medicine, neurology and pain management (non-surgical specialists); and physiotherapy, osteopathy, massage therapy, exercise physiology, chiropractor and psychology (allied health providers).
Prescription records
Medications are coded in POLAR according to the ATC system.22 We included medications deemed by the study authors to be commonly prescribed for pain relief to people with musculoskeletal conditions. Medications within the following categories were included: simple analgesics such as paracetamol; non-steroidal anti-inflammatories (NSAIDs); chondroitin and/or glucosamine; topical products for joint and muscular pain; opioids; gabapentinoids and any relevant combinations. We included gabapentinoids such as gabapentin and pregabalin because these are being increasingly used for the management of musculoskeletal conditions such as non-specific low back pain or sciatica despite evidence of a lack of effectiveness and a higher risk of adverse events.24 Opioid analgesics were further categorised into (i) weak single ingredient opioid analgesics (eg, codeine), defined as <50 morphine milligram equivalents (MME) per day; (ii) strong single ingredient opioid analgesics (eg, tapentadol, oxycodone, morphine), defined as 150 MME per day; and (iii) combination opioid analgesics.25 Medicines in the combination opioid category were categorised based on the strongest medicine present, either as a weak combination opioid or as a strong combination opioid.
To ensure we included all potentially eligible medication names, we searched by both ATC category and by medication name from the prescription file during 2014–2018. The medication names we included are presented in table 3. We included oral, topical and injectable preparations of medications. We excluded the following prescriptions: aspirin, decongestants (eg, pseudoephedrine), antihistamines (eg, doxylamine), opioid cough suppressants (eg, dextromethorphan) and expectorants (eg, guaifenesin). These were excluded on the basis that they were likely to have been prescribed for another condition (eg, aspirin for secondary prevention of cardiovascular disease).26
Table 3.
Included medication names
| Simple analgesics (N02BE*) | Non-steroidal anti-inflammatories (M01A*) | Chondroitin and/or glucosamine (M01AX*) | Topical products for joint and muscular pain (M02A*) | Opioids (N02A*) | Gabapentinoids (N03AX*) |
| [Caffeine, Paracetamol] Paracetamol Paracetamol combinations [Ibuprofen, Paracetamol] |
Celecoxib Diclofenac Diclofenac potassium Diclofenac sodium [Diclofenac sodium, Misoprostol] [Diclofenac, Misoprostol] Etoricoxib Flurbiprofen Ibuprofen Ibuprofen lysine Indomethacin Ketoprofen Ketorolac Ketorolac trometamol Lumiracoxib Mefenamic acid Meloxican Naproxen Naproxen sodium [Naproxen, Esomeprazole] Parecoxib Parecoxib sodium Piroxicam Rofecoxib Sulindac Tiaprofenic acid |
[Borate, Chondroitin, Glucosamine, Manganese] [Chrondroitin, Copper, Glucosamine, Manganese, Zinc Sulfate] [Chondroitin, Dimethyl Sulfone, Glucosamine] Glucosamine [Glucosamine, Calcium, Vitamin D, Minerals] [Glucosamine, Chondroitin] Glucosamine hydrochloride [Glucosamine hydrochloride, Chondroitin sulfate] [Glucosamine hydrochloride, Chondroitin sulfate, Dimethyl sulfone] [Glucosamine hydrochloride, Chondroitin sulfate, Manganese gluconate, Calcium ascorbate] [Glucosamine hydrochloride, Calcium, Vitamin D, Vitamin K, Boron] [Glucosamine hydrochloride, Glucosamine sulfate, Glycine, Fructose, Bioflavonoids, Ascorbic acid, Histidine, Lysine hydrochloride, Leucine, Valine, Perna caniculata powder, Calcium pantothenate, Zinc amino acid chelate, Manganese amino acid chelate, Copper gluconate, Selenomethionine] [Glucosamine, Omega-3 triglycerides] Glucosamine sulfate [Glucosamine sulfate, Chondroitin sulfate (Shark)] [Glucosamine sulfate, Shark cartilage] [Glucosamine sulfate, Potassium chloride] [Glucosamine sulfate sodium chloride, Eicosapentaenoic acid, Docosahexaenoic acid] [Ascorbate, Glucosamine, Manganese, Turmeric] [Borate, Glucosamine, Manganese, Selenium] [Ascorbate, Cod-liver oil, Colecalciferol, Copper, Cyanocobalamin, Folate, Glucosamine, Manganese, Omega-3 triglycerides, Selenium, Tocopherol, Zinc] |
Benzydamine Benzydamine hydrochloride [Cajuput oil, Camphor, Capsicum, Eucalyptus oil, Hydroxybenzoate, Mentha X Piperita, Menthol, Methyl salicylate, Pinus, Turpentine oil] [Cajuput oil, Camphor, Clove, Menthol (Tiger Balm)] [Camphor, Menthol, Eucalyptus oil, Methyl salicylate] [Camphor, Eucalyptus oil, Mentha X Piperita, Menthol, Methyl salicylate, Pinus, Turpentine oil] [Camphor, Menthol, Methyl salicylate] [Camphor, Eucalyptus oil, Menthol, Methyl salicylate] [Camphor, Eucalyptus oil, Methyl salicylate, Menthol, Alisma plantago aquatica Root oil extract, Bambusa root] Capsaicin [Capsicum oleoresin, Arnica montana, Arctium lappa root dry, Aloe barbadensis inner leaf juice] Diclofenac Diclofenac diethylamine Diclofenac diethylammonium Diclofenac Sodium [Ethyl salicylate, Hydroxyethyl salicylate, Methyl salicylate, Nicotinic acid] Eucalyptus oil [Eucalyptus oil, Pine oil Pumilio, Peppermint oil, Camphor, Methyl salicylate, Menthol, Turpentine oil] [Eucalyptus oil, Menthol, Methyl salicylate] Flurbiprofen sodium Ibuprofen Ketoprofen Menthol [Menthol, Camphor, Cajuput oil, Clove oil, Dementholised mint oil] [Menthol, Camphor, Cajuput oil, Dementholised mint oil, Clove bud oil] [Menthol, Glycol salicylate] [Menthol, Eucalyptus oil, Methyl salicylate] Methyl salicylate [Methyl salicylate, Ethyl salicylate, 2-Hydroxyethyl salicylate, Methyl nicotinate] [Methyl salicylate, Eucalyptus oil, Menthol liquid] [Methyl salicylate, Menthol] Nicoboxil/Nonivamide [Nonivamide, Butoxyethyl nicotinate] Piroxicam Triethanolamine salicylate Trolamine salicylate |
Weak single opioids Codeine Codeine phosphate Codeine phosphate hemihydrate Dextropropoxyphene Dextropropoxyphene napsylate Tramadol Tramadol hydrochloride Combination weak opioid [Aspirin, Codeine phosphate] [Codeine, Ibuprofen] [Codeine phosphate, Ibuprofen] [Codeine, Paracetamol] [Codeine Phosphate, Paracetamol] [Codeine phosphate hemihydrate, Ibuprofen] [Dextropropoxyphene, Paracetamol] [Dextropropoxyphene napsylate, Paracetamol] [Tramadol, Paracetamol] [Tramadol hydrochloride, Paracetamol] Strong single opioids Fentanyl Fentanyl citrate Hydromorphone Hydromorphone hydrochloride Morphine Morphine hydrochloride Morphine hydrochloride trihydrate Morphine sulfate Morphine sulfate Bp Morphine sulfate pentahydrate Morphine tartrate Oxycodone [Oxycodone, Naloxone] Oxycodone hydrochloride Oxycodone pectinate [Oxycodone hydrochloride, Naloxone hydrochloride] Tapentadol Tapentadol hydrochloride |
Gabapentin Pregabalin |
*Anatomic and Therapeutic Classifications (ATC) category.
Imaging records
The test data file within POLAR contains radiology and pathology tests requested by the GP. At the time of data extract, coding of the test data file had not been completed for specific imaging tests by Outcome Health and there were too many records to scan manually. We therefore exported all radiology test names during 2014–2018 inclusive and used an inductive coding process to select the following eligible imaging tests: plain radiographs, CT and MRI scans of the lumbar and cervical spine; plain radiographs, CT, MRI and ultrasounds of the knee; and plain radiographs, MRI scans and ultrasounds of the shoulder. We also included lumbar spine, knee, shoulder and cervical spine injections and shoulder hydrodilatation as eligible radiology procedures.
To code eligible imaging records, we first used the string match command in Stata to select all test names for each eligible anatomical region (ie, low back, neck, shoulder and knee). Within each region, we then iteratively coded all imaging records into subgroups according to the type of imaging test (eg, ultrasound). This process involved developing string match terms to identify each type of eligible radiology test or procedure within the sample, reviewing the uncoded test names (subgrouped as ‘other’) and manually coding additional terms until the remaining test names could not be classified into any further subgroups. We also developed string match terms to identify bilateral tests of the shoulder and knee. The initial string match terms used to code each body region and eligible imaging test or procedure are presented in online supplemental appendix 1.
bmjopen-2021-055528supp001.pdf (81.3KB, pdf)
During the coding process, there were numerous test names that did not definitively identify a type of imaging test (eg, ‘right knee’). We labelled these as ‘unspecified’. We plan to classify these as plain radiographs in our analysis. This is because plain radiograph was deemed to be the default radiology modality in the EMR software. The subgroups of imaging records inductively developed for each eligible body region are presented in table 4. Our subgroup coding (excluding test names labelled as ‘unspecified’ and ‘other’) accounted for 96.0%, 95.8%, 95.2% and 96.6% of the identified low back (n=180 630), neck (n=192 844), shoulder (n=236 803) and knee (n=235 123) imaging test names, respectively.
Table 4.
Test name subgroups for low back, knee, shoulder and neck imaging tests and procedures
| Low back imaging subgroups | Knee imaging subgroups | Shoulder imaging subgroups | Neck imaging subgroups |
| Lumbosacral plain radiograph* Lumbosacral CT* Lumbosacral MRI* Lumbosacral injection* Lumbosacral unspecified*# Lumbosacral ultrasound† Lumbosacral other† |
Knee plain radiograph* Knee CT* Knee MRI* Knee injection* Knee unspecified*# Knee ultrasound* Knee other† Knee aspiration† Knee arthrogram† |
Shoulder plain radiograph* Shoulder ultrasound* Shoulder MRI* Shoulder injection* Shoulder unspecified*# Shoulder hydrodilatation* Shoulder other† Shoulder aspiration† Shoulder arthrogram† Shoulder CT† Shoulder fluoroscopy† |
Neck plain radiograph* Neck CT* Neck MRI* Neck injection* Neck unspecified*# Neck ultrasound† Neck other† Neck aspiration† |
*Eligible.
†Ineligible.
‡Analyse as plain radiograph.
Test names indicating more than one imaging test were classified separately. We excluded imaging tests of soft tissues of the neck and test names indicating a combined neck image with the head, larynx, thyroid and/or abdomen (unless it specifically stated cervical spine) as we deemed these investigations were most likely not requested for a musculoskeletal condition. We also excluded test names with the following terms as these were not deemed to indicate an imaging test or procedure: ‘report’, ‘findings’, ‘cancel’, ‘results’, ‘letter’.
Data access and cleaning
Outcome Health provided the research team with access to all POLAR database records since inception (1997). Data quality checks will be performed to label data as ‘acceptable’ for analysis using a similar process to that conducted by an established general practice database in the UK.27 Duplicate data and records with empty or implausible birth dates (defined as greater than 115 years of age at time of diagnosis or dated after patient management) will be excluded from analyses. We will exclude practices without any activity data during 2014–2018. We will also examine the consistency of activity, test, prescription and referral data for each practice in each eligible calendar year. If a gap in reporting from any practice is identified for 1 year or more, only data from the earliest date after which there was no gap will be included. For example, if a practice has activity data in 2014, 2017 and 2018, only data from 2017 onwards will be included. In addition, we will exclude activity records that represent more than one face-to-face consultation with a GP for the same patient on the same day. This is because an ‘activity’ occurs in POLAR anytime a patient record is accessed regardless of whether this was for clinical or administration purposes.
Approach to data set creation
We will use a systematic process to exclude ineligible records in order to merge data and select the study cohort (figure 2). This process will require the merging of five relational data files (patient, practice, provider, activity and diagnosis) in a specific sequence to ensure all relevant records are retained. For example, we will not limit diagnosis records to 2014–2018 until after we have selected relevant comorbidities. A patient-centred database will be prepared to examine the number and type of GP consultations, imaging test and procedure requests, prescriptions for pain relief and referrals to other health providers for our study cohort. Data that does not match our eligibility criteria (including data with missing fields) will be excluded during the merging process as unmatched records. Duplicate records, records with implausible dates or missing fields and multiple records of the same type on a single day will also be removed and reported.
Figure 2.
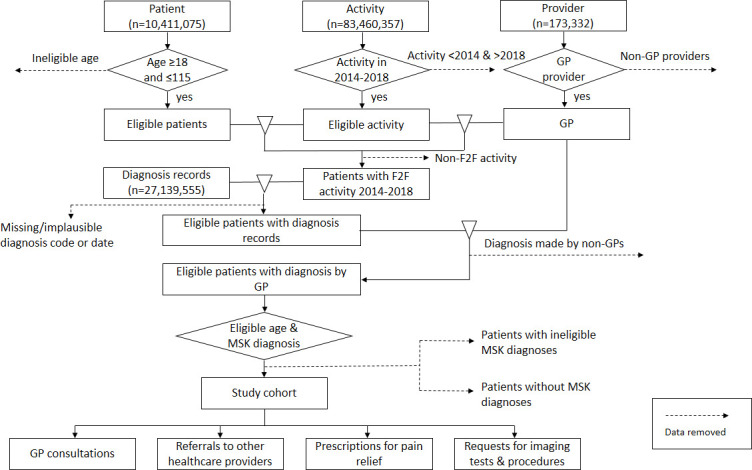
Approach to data set creation. GP, general practitioner; MSK, musculoskeletal; F2F, face-to-face.
Analyses
All relevant data will be extracted from the POLAR SQL database and imported into Stata V.15 (StataCorp LP) for data management and analyses. The methods in this protocol are structured according to RECORD guidelines (online supplemental appendix 2).12 For variables with a recognised coding system, full lists of codes used to define eligible variables are available from https://clinicalcodes.rss.mhs.man.ac.uk/medcodes/article/174/.28
bmjopen-2021-055528supp002.pdf (98.8KB, pdf)
Descriptive statistics will be used to summarise the study cohort including the number and type of eligible musculoskeletal conditions, patient demographics and comorbidities. These will be compared with national health survey data to assess the representativeness of the POLAR database to the wider Australian population. Eligible musculoskeletal conditions will be grouped according to body region.
Primary analysis will include analysis of each management type provided for each participant during the first year after their index diagnosis. A sensitivity analysis will be conducted including the entire follow-up period until 31 December 2018. For prescriptions, the primary analysis will include the entire follow-up period because repeated prescriptions over more than 1 year are anticipated. Descriptive statistics will also be used to summarise the number and type of GP all-cause consultations, imaging tests and procedures requested, prescriptions for pain relief and referrals to other health providers for the study cohort. Results will be stratified by affected body region. Consultations will be categorised as face-to-face or telecommunication. Imaging requests will be categorised according to the type of imaging modality or procedure and body region (eg, knee MRI). Bilateral knee and shoulder imaging requests will be counted as two imaging requests. Prescriptions will be categorised according to paracetamol, NSAIDs, glucosamine and/or chondroitin, opioids (weak single opioid, strong single opioid, weak combination opioid and strong combination opioid) and gabapentinoids. Referrals will be categorised according to surgical specialist, non-surgical specialist and allied health. Patterns and timing of management (imaging requests, prescriptions and referrals) for people with eligible low back, neck, shoulder and knee conditions will be examined and compared between each year within the 5-year study period and relative to time of diagnosis using trend analyses.
One of the limitations of the POLAR database is that it does not capture reasons for the clinical encounter or management types (imaging request, prescription or referral). To account for the subsequent uncertainty in attributing management types to a particular diagnosis for those with multiple musculoskeletal conditions, participants with eligible musculoskeletal diagnoses from multiple body regions will be analysed separately to those with eligible diagnoses in one body region. Imaging requests will be analysed relative to the date of the most recent musculoskeletal diagnosis for the same body region. For example, a shoulder ultrasound will be analysed relative to the index date of an eligible shoulder diagnosis even if the same patient was diagnosed previously with an eligible knee condition.
The association between management types and patient-related and practice-related characteristics will be examined using regression analysis. Predictors will include patient gender, socioeconomic status, residential location, body region(s) affected by eligible musculoskeletal conditions and PHN of the practice with adjustment for age and time since index diagnosis. Socioeconomic status will be defined by the Index of Relative Socioeconomic Advantage and Disadvantage using 2016 Census data.29
Sequence analysis will be used to categorise sequences of management types of people with eligible musculoskeletal conditions into similar groups based on observed characteristics.30 This will take into account both the time since diagnosis and sequence of each management type. We will use this to identify the most frequently used combinations and sequences of management and the patient-related and practice-related variables that correlate with each management combination.
Sample size consideration
Sequence analysis will require the largest sample size of our planned analyses and will therefore form the basis of our sample size consideration. We plan to examine the following six management types: non-surgical referrals, surgical referrals, allied health referrals, opioid prescription, X-ray and/or ultrasound requests and MRI and/or CT scan requests. This provides a total of 720 potential sequence combinations. Based on a recommended 20–30 subjects per subgroup,31 we estimate a sample size of between 14 400 and 21 600 will be required to differentiate between each sequence combination or pattern of care. Recent use of the POLAR database using data from approximately 200 general practices identified 20 514 active adult patients with type 2 diabetes before July 2016.32 Our extract is based on 301 general practices from 2014 to 2018 and since the prevalence of diabetes is less than that of musculoskeletal conditions,33 we expect a sample size of more than 20 000.
Patient and public involvement
There will be no involvement of patients or the public in this study.
Discussion
Explicitly reporting our systematic approach used to classify, select and merge eligible records from relational data files into a patient-centred database for analysis promotes transparency, reproducibility and completeness of the reporting of research conducted using routinely collected health data. The approach used to code eligible imaging tests from structured narrative text coded over 95% of the 845 400 cumulative imaging-related test and procedure records identified for low back, neck, shoulder and knee conditions during 2014–2018. Our code lists are available for all variables that have been previously coded by POLAR and those with a recognised coding system have been made available on the ClinicalCodes online repository. Although our coding process may only be applicable to systems that do not embed coding in the clinical process, this approach can also be adapted to examine patterns of care over time for other conditions in general practice.
The main strength of this study is that it will facilitate an overview of the care provided by GPs to the same patient(s) over time and thereby enable temporal sequences to be examined. The POLAR database contains all patient-related activity within each practice making it representative of the included practices. Previous research has demonstrated comparable prevalence and age-gender distribution of people diagnosed with type 2 diabetes within the POLAR database to those within Australia.32 This study will add to these findings by assessing the representativeness of people with musculoskeletal conditions within the POLAR database to the wider Australian population.
Constraints within the POLAR database may potentially limit the reliability of this study’s findings although these are problems inherent in the use of any extracted data. Variability in workflows and recording behaviour introduces potential biases and the different clinical information systems used by the practices within POLAR may result in variability in the information entered. The objective of POLAR is to remove as much variability as possible by using and being transparent about the coding process. High accuracy of diagnostic coding by Outcome Health has been previously demonstrated.20 In addition, it is possible not all patterns of care for the study cohort will be directly attributable to a musculoskeletal condition because reasons for GP consultations, referrals, imaging requests and prescriptions are not mandated in the source EMRs. These data are also likely to underestimate actual allied health visits and prescriptions for pain relief as some of these do not require a GP referral and are available over-the-counter without a prescription, respectively, in Australia.
Ethics and dissemination
Prior approval to conduct this study was obtained from the Cabrini Human Research Ethics Committee and Monash University Human Research Ethics Committee (Reference Numbers 02-21-01-19 and 16975, respectively). We did not obtain participant consent as all data were anonymised. Outcome Health holds a standing ethics approval for its collection and custodianship of the data from the Royal Australian College of General Practice. The study findings will be reported to Outcome Health, participating PHNs, disseminated in peer-reviewed academic journals and presented in national and international conferences.
Supplementary Material
Footnotes
Contributors: RH, DAO’C and RB conceived the study. LB and AG were responsible for data coding and the statistical analysis plan. CP provided expertise in the use of the POLAR database. DM provided clinical context in managing musculoskeletal conditions in the general practice setting. All authors contributed to refining the protocol and approved the submitted protocol.
Funding: This work was supported by an Arthritis Queensland, Arthritis South Australia and the Allan and Beryl Stephens Grant from Arthritis Australia (ID N/A). Arthritis Australia did not contribute to the conduct of this study. It is also supported by an Australian National Health and Medical Research Council (NHMRC) Programme Grant (APP1113532). DAO’C is supported by a TRIP Fellowship and RB is supported by an NHMRC Investigator Fellowship (APP1194483).
Competing interests: RH, DAO’C, RB and DM report grants from Arthritis Australia (not-for-profit organisation), during the conduct of the study. CP is an employee of Outcome Health, the not-for-profit organisation that developed the POLAR database and chairs the Product improvement group of the Australian Digital Health Agency. It has no relationship with the research, but has provided grant funding to Outcome Health. LB reports consultancy fees paid to Monash University from Charite Medical University Berlin, Jesuit Social Services Victoria and Swinburne University of Technology, outside the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Royal Australian College of General Practitioners . General practice: health of the nation. East Melbourne, Vic: RACGP, 2019. [Google Scholar]
- 2.Britt HMG, Henderson J, Bayram C. General practice activity in Australia 2015–16. general practice series No. 40. Sydney: Sydney University Press, 2016. [Google Scholar]
- 3.Rahman N, Gruber D. The burden of musculoskeletal conditions in Australia: a detailed analysis of the Australian burden of disease study 2011. Australian Institute of Health and Welfare, 2017. [Google Scholar]
- 4.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020;396:1204–22. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Britt HMG, Bayram C, Henderson J. A decade of Australian general practice activity 2006–07 to 2015–16. general practice series No. 41. Sydney: Sydney University Press, 2016. [Google Scholar]
- 6.Bennell KL BC, Harrison C, Brand C. Trends in management of hip and knee osteoarthritis in general practice in Australia over an 11-year window: a nationwide cross-sectional survey. The Lancet Regional Health – Western Pacific 2021;12https://authors.elsevier.com/sd/article/S2666606521000961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naunton J, Harrison C, Britt H, et al. General practice management of rotator cuff related shoulder pain: a reliance on ultrasound and injection guided care. PLoS One 2020;15:e0227688. 10.1371/journal.pone.0227688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busingye D, Gianacas C, Pollack A, et al. Data resource profile: MedicineInsight, an Australian National primary health care database. Int J Epidemiol 2019;48:1741–41h. 10.1093/ije/dyz147 [DOI] [PubMed] [Google Scholar]
- 9.Pearce C, McLeod A, Rinehart N, et al. What a comprehensive, integrated data strategy looks like: the population level analysis and reporting (polar) program. Stud Health Technol Inform 2019;264:303–7. 10.3233/SHTI190232 [DOI] [PubMed] [Google Scholar]
- 10.De Lusignan S, Sun B, Pearce C, et al. Coding errors in an analysis of the impact of pay-for-performance on the care for long-term cardiovascular disease: a case study. J Innov Health Inform 2014;21:92–101. 10.14236/jhi.v21i2.62 [DOI] [PubMed] [Google Scholar]
- 11.Hemkens LG, Benchimol EI, Langan SM, et al. The reporting of studies using routinely collected health data was often insufficient. J Clin Epidemiol 2016;79:104–11. 10.1016/j.jclinepi.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational Routinely-collected health data (record) statement. PLoS Med 2015;12:e1001885. 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healthmap: Healthdirect Australia. Available: https://studio.healthmap.com.au/#/h4f [Accessed 27 Sep 2020].
- 14.The State of Victoria Department of Premier and Cabinet . Population diversity in Victoria: 2016 census local government areas, 2018. [Google Scholar]
- 15.Yan J, Hawes L, Turner L, et al. Antimicrobial prescribing for children in primary care. J Paediatr Child Health 2019;55:54–8. 10.1111/jpc.14105 [DOI] [PubMed] [Google Scholar]
- 16.Turner LR, Pearce C, Borg M, et al. Characteristics of patients presenting to an after-hours clinic: results of a magnet analysis. Aust J Prim Health 2017;23:294–9. 10.1071/PY16084 [DOI] [PubMed] [Google Scholar]
- 17.Turner LR, Cicuttini F, Pearce C, et al. Cardiovascular disease screening in general practice: general practitioner recording of common risk factors. Prev Med 2017;99:282–5. 10.1016/j.ypmed.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 18.Sezgin G, Georgiou A, Hardie R-A, et al. Compliance with pathology testing guidelines in Australian general practice: protocol for a secondary analysis of electronic health record data. BMJ Open 2018;8:e024223. 10.1136/bmjopen-2018-024223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Australian Institute of Health and Welfare . Musculoskeletal conditions and comorbidity in Australia. Canberra: AIHW, 2019. [Google Scholar]
- 20.Pearce C, McLeod A, Patrick J, et al. Coding and classifying GP data: the polar project. BMJ Health Care Inform 2019;26:e100009. 10.1136/bmjhci-2019-100009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cote R, Rothwell D, Palotay J. The Systematized Nomenclature of human and veterinary Medicine-SNOMED international. Northfield, IL: College of American Pathologists, 1993. [Google Scholar]
- 22.World Health Organization . Who collaborating centre for drug statistics methodology: ATC classification index with DDDs and guidelines for ATC classification and DDD assignment 2020. Oslo, Norway: Norwegian Institute of Public Health, 2019. [Google Scholar]
- 23.Hovis KK, Alizai H, Tham S-C, et al. Non-Traumatic anterior cruciate ligament abnormalities and their relationship to osteoarthritis using morphological grading and cartilage T2 relaxation times: data from the osteoarthritis initiative (OAI). Skeletal Radiol 2012;41:1435–43. 10.1007/s00256-012-1379-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchbinder R, Underwood M, Hartvigsen J, et al. The Lancet series call to action to reduce low value care for low back pain: an update. Pain 2020;161 Suppl 1:S57–64. 10.1097/j.pain.0000000000001869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathieson S, Wertheimer G, Maher CG, et al. What proportion of patients with chronic noncancer pain are prescribed an opioid medicine? systematic review and meta-regression of observational studies. J Intern Med 2020;287:458–74. 10.1111/joim.13026 [DOI] [PubMed] [Google Scholar]
- 26.Ittaman SV, VanWormer JJ, Rezkalla SH. The role of aspirin in the prevention of cardiovascular disease. Clin Med Res 2014;12:147–54. 10.3121/cmr.2013.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Springate DA, Kontopantelis E, Ashcroft DM, et al. ClinicalCodes: an online clinical codes Repository to improve the validity and reproducibility of research using electronic medical records. PLoS One 2014;9:e99825. 10.1371/journal.pone.0099825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Australian Bureau of Statistics . Socio-economic indexes for areas (SEIFA) - technical paper 2016. cat no 20330 55001, 2018. [Google Scholar]
- 30.Halpin B. SADI: sequence analysis tools for Stata. Stata J 2017;17:546–72. 10.1177/1536867X1701700302 [DOI] [Google Scholar]
- 31.Dalmaijer ES, Nord CL, Astle DE. Statistical power for cluster analysis. arXiv preprint arXiv 2020:200300381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imai C, Hardie R-A, Franco GS, et al. Harnessing the potential of electronic general practice pathology data in Australia: an examination of the quality use of pathology for type 2 diabetes patients. Int J Med Inform 2020;141:104189. 10.1016/j.ijmedinf.2020.104189 [DOI] [PubMed] [Google Scholar]
- 33.Harrison C, Britt H, Miller G, et al. Prevalence of chronic conditions in Australia. PLoS One 2013;8:e67494. 10.1371/journal.pone.0067494 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055528supp001.pdf (81.3KB, pdf)
bmjopen-2021-055528supp002.pdf (98.8KB, pdf)


