Abstract
Context
There is evidence demonstrating variation in insulin sensitivity across the menstrual cycle. However, to date, research has yielded inconsistent results.
Objective
This study investigated variation in insulin sensitivity across the menstrual cycle and associations with body mass index (BMI), physical activity, and cardiorespiratory fitness (CRF).
Methods
Data from 1906 premenopausal women in NHANES cycles 1999 to 2006 were analyzed. Menstrual cycle day was assessed using questionnaire responses recording days since last period. Rhythmic variation of plasma glucose, triglycerides, and insulin, homeostatic model of insulin resistance (HOMA-IR), and adipose tissue insulin resistance index (ADIPO-IR) across the menstrual cycle were analyzed using cosinor rhythmometry. Participants were assigned low or high categories of BMI, physical activity, and CRF, and category membership included in cosinor models as covariates.
Results
Rhythmicity was demonstrated by a significant cosine fit for glucose (P = .014) but not triglycerides (P = .369), insulin (P = .470), HOMA-IR (P = .461), and ADIPO-IR (P = .335). When covariates were included, rhythmicity was observed when adjusting for: 1) BMI: glucose (P < .001), triglycerides (P < .001), insulin (P < .001), HOMA-IR (P < .001), and ADIPO-IR (P < .001); 2) physical activity: glucose (P < .001), triglycerides (P = .006), and ADIPO-IR (P = .038); and 3) CRF: triglycerides (P = .041), insulin (P = .002), HOMA-IR (P = .004), and ADIPO-IR (P = .004). Triglyceride amplitude, but not acrophase, was greater in the high physical activity category compared to low (P = .018).
Conclusion
Rhythmicity in insulin sensitivity and associated metabolites across the menstrual cycle are modified by BMI, physical activity, and CRF.
Keywords: menstrual cycle, insulin, glucose, triglyceride, insulin sensitivity, NHANES
The onset and severity of insulin resistance are associated with a range of modifiable and nonmodifiable risk factors, including, sex, age, adiposity, physical inactivity, and cardiovascular fitness (1, 2). Women exhibit lower fasting plasma glucose levels, but greater impairment in glucose tolerance compared to men (3). Within adipose tissue, women have greater insulin-stimulated glucose and fatty acid uptake compared to men (4). While body mass index (BMI) and age are positively associated with insulin resistance, in women insulin resistance typically occurs at a higher BMI and higher age compared with men (5). Moreover, low fitness has a greater association with insulin resistance in overweight women compared with overweight men (2).
Reports have demonstrated a clear mechanistic role of sex hormones underpinning sexual dimorphism in insulin resistance (6). Insulin sensitivity has been positively associated with estradiol and negatively associated with progesterone in rats (7). This suggests that hormonal fluctuations across the menstrual cycle in humans may play a role in insulin sensitivity. However, strategies targeting the prevention and treatment of reduced insulin sensitivity rarely consider sex and none consider the role of the menstrual cycle.
The menstrual cycle is a fundamental biological rhythmic cycle occurring in females of reproductive age, composed of the ovarian and uterine cycles. The ovarian cycle, consisting of follicular, ovulatory, and luteal phases, is concerned with oocyte maturation and release, while the uterine cycle, consisting of menstruation, proliferative, and secretory phases, is concerned with preparing the uterine lining for possible oocyte implantation in the event of fertilization (8). The ovarian cycle and uterine cycle occur in a coordinated and concurrent manner; herein we will refer to menstrual cycle phase in terms of the follicular and luteal phases (8). The average cycle length is 29 days, although this varies between individuals, with cycle lengths of 24 to 35 days considered normal and healthy (8, 9). Within an individual, typical cycle length declines as age increases (9). The menstrual cycle is governed by rhythmic fluctuations of hormones within the hypothalamic-pituitary-gonadal axis; gonadotropin-releasing hormone, pituitary hormones (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]), and ovarian hormones (estradiol, progesterone, and testosterone) (8). However, the effect of the menstrual cycle on physiology is underresearched and in fact is frequently cited as a barrier toward the inclusion of women in research projects (10).
Cyclical fluctuations in hormonal profiles across the menstrual cycle have been associated with alterations in metabolic control. During the luteal phase an increase in circulating insulin and reductions in circulating glucose and triglycerides have been observed (11, 12). Correspondingly, insulin sensitivity would be expected to fluctuate across the menstrual cycle. However, studies so far have been equivocal. Reductions in insulin sensitivity during the luteal phase have been reported (12-18). However, other studies have documented no change in insulin sensitivity across the menstrual cycle (19-22). These inconsistencies may be attributable to the relatively small sample sizes used in all but one (12) of these studies (n = 6-30), which lacked statistical power to robustly detect the small, yet clinically meaningful, changes in insulin sensitivity across the menstrual cycle. Yeung et al used a large sample size (n = 259) and reported significant variation in insulin sensitivity across the menstrual cycle (12). Moreover, previous studies recruited heterogeneous study populations with varying BMI and physical activity levels, in which limited adjustment or investigation into the potentially confounding effects of these modifiable risk factors was conducted (12, 21, 22). Examining the role of modifiable risk factors in a large cohort of women is necessary to fully understand rhythmicity in insulin sensitivity across the menstrual cycle.
In this study we first aim to characterize the variation in insulin sensitivity and associated metabolites across the menstrual cycle in a large cohort of well-characterized female participants. Second, we will investigate the role of BMI, physical activity, and cardiovascular fitness on the variation in insulin sensitivity and associated metabolites across the menstrual cycle.
Materials and Methods
Participants
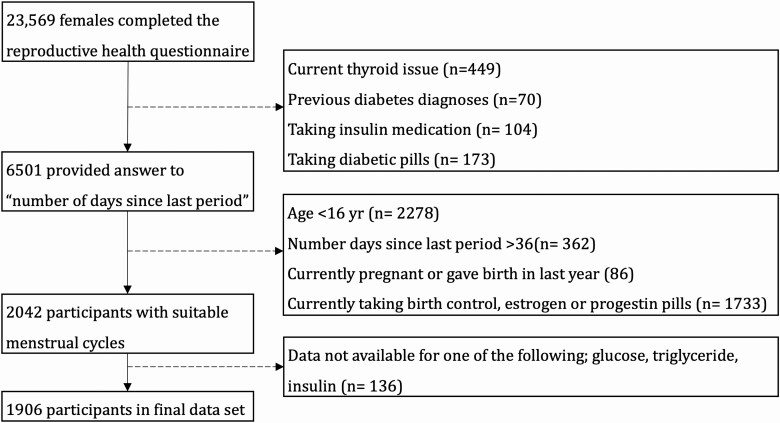
The National Health and Nutrition Examination Survey (NHANES) is a national, cross-sectional, population-based study representative of the noninstitutionalized US civilian population (NHANES, RRID: SCR_013201). Data were collected in 2-year cycles beginning in 1999, with data collection ongoing. NHANES participants completed an at-home interview and a physical examination at a mobile examination center (MEC). A reproductive health questionnaire was included in data collection cycles between 1999 and 2006. This questionnaire was completed by 23 569 females. Participants were excluded if they had current diagnoses of metabolic disorder (diabetes, thyroid condition) or were taking medication that altered insulin sensitivity (Fig. 1). Details of variable descriptions and codes used in this study are provided in data repository (23).
Figure 1.
Flowchart depicting participant selection in the study. The dotted lines represent participant exclusion.
Menstrual Cycle Assessment
The response to the question “number of days since last period started” was treated as the day of menstrual cycle. Responses were collected once for each participant. Unfortunately, data on typical menstrual cycle length were not available within the NHANES database. Participants were excluded from data analysis if the reported number of days since their last period started more than 35 days earlier. While the typical menstrual cycle length is 29 days, a maximum cycle day value of 35 days was selected to encompass 95% of the cycle lengths in women (9). Participants were excluded based on factors that influence the hormonal milieu across the menstrual cycle: age younger than 16 years, currently taking hormonal contraceptive medication, or currently pregnant or gave birth within the previous year. Final analyses were conducted on 1906 participants (see Fig. 1).
Anthropometric Assessment
Height was measured using a stadiometer, and weight was measured using a digital scale following standard procedures at the MEC. BMI was calculated using weight in kilograms divided by height in meters squared. Prior to analyses, participants were assigned to BMI-defined categories based on standard cutoff thresholds (low BMI, underweight and healthy weight ≤ 24.9; high BMI, overweight and obese > 25) (24).
Blood Sampling and Biochemical Analysis
Venous blood samples were collected the same day as the menstrual cycle questionnaire following a fast of at least 9 hours, but not more than 24 hours, by a trained phlebotomist at the MEC and processed according to a standardized protocol (25). Serum FSH and LH concentrations were analyzed by microparticle Enzyme Immunoassay (Abbot Laboratories) (26). Plasma glucose and triglyceride concentrations were analyzed enzymatically (Roche Diagnostic Systems) (27, 28). Plasma insulin concentration was assessed via radioimmunoassay (Pharmacia Diagnostics AB) (29). Insulin sensitivity was calculated using the HOMA-IR (30) and ADIPO-IR (31) methods.
Physical Activity
Each participant completed a physical activity questionnaire that included questions relating to all physical activity performed in the previous 30 days. Activity type, duration, intensity, and number of times performed in the last 30 days were recorded. Moderate-intensity activities were defined as inducing light sweating or a slight to moderate increase in breathing or heart rate (HR). Vigorous activities were defined as inducing heavy sweating or large increases in breathing or HR. Metabolic equivalent (MET) scores for specific activities were calculated based on the activity type and intensity (32). MET scores were multiplied by the average duration and number of times performed in the last 30 days to calculate MET minutes per 30 days (MET min/30d) for each activity. MET min/30d were summed for each activity then divided by 4.29 to calculate total MET minutes per week. Prior to analyses, participants were assigned to low and high physical activity categories based on whether they met the national physical activity guidelines (low physical activity < 500 MET/wk; high physical activity ≥ 500 MET/wk) (33).
Cardiorespiratory Fitness
Participants underwent a submaximal exercise test on a treadmill to predict maximal oxygen consumption (O2max) (34). Participants were assigned to 1 of 8 protocols, of varying difficulty, based on age, BMI, and self-reported physical activity level. Each protocol included a 2-minute warmup, 2 × 2-minute stages, and a 2-minute cool down. Heart rate was recorded throughout using an automated monitor. These exercise protocols aimed to elicit 75% of maximal HR by the end of the test. Predicted O2max was estimated by extrapolating age-specific maximal HR responses to the two 2-minute exercise stages, assuming a linear relation between HR and O2 consumption during exercise (35, 36). Prior to analyses, participants were assigned to low and high cardiorespiratory fitness categories based on whether their O2max scores were below or above the age-specific 50th percentile (37).
Statistical Analysis
All analyses were conducted in R (version 3.6.3) (38). Participant demographic data are presented as mean ± SD. The number of participants are shown for each analysis; this varies because of missing data. Data were tested for normality using visual inspection of histogram and Shapiro-Wilk test. Nonnormal data were log10-transformed. Rhythmicity across the menstrual cycle was detected using the “Cosinor” and “Cosinor2” packages (39, 40). Cosinor fits a cosine curve with a free phase to data and calculates MESOR (a rhythm-adjusted mean), amplitude (half the predictable variation within a cycle), and acrophase (time of highest value within a cycle). Peak-to-peak difference (%) was calculated using the following equation: (2 × amplitude/mean)*100. In separate cosinor models, we included the BMI, physical activity, and cardiorespiratory fitness category as a covariate. Inclusion of covariates in the cosinor model allows the MESOR, amplitude, and acrophase to differ between respective high and low covariate categories. Overall significance of the cosine model was established using the zero-amplitude test. Wald tests were conducted to test for differences in the amplitude and acrophase between respective high and low covariate categories. Cosine data are presented as MESOR ± amplitude. Data are shown as conventional box plots.
Results
Participant Characteristics
Participant characteristics are outlined in Table 1. As would be expected, greater weight, higher BMI, lower physical activity, and lower O2max were observed in the low physical activity category (difference = 2.0 kg, P = .020; 1.2, P < .001; 2884.7 MET min/wk P < .001; 1.4 mL/min/kg, P = .019, respectively), high BMI category (26.6 kg, P < .001; 10.3, P < .001; 576.4 MET min/wk, P < .001; 2.5 mL/min/kg, P < .001), and low cardiorespiratory fitness category (3.4 kg, P = .003; 1.3, P = .001; 403.1 MET min/wk, P = .028; 10.8 mL/min/kg, P < .001, respectively). Age was significantly greater for the low physical activity (3.1 years, P < .001), high BMI (3.6 years, P < .001), and high cardiorespiratory fitness (5.5 years, P < .001) categories. Height was significantly greater in the high physical activity category (1.5 cm, P < .001), but not BMI (0.4 cm, P = .166) nor cardiorespiratory fitness (0.2 cm, P = .757). No rhythmic cycling was detected across the menstrual cycle in BMI (MESOR: 26.3 ± amplitude: 0.15, P = .822), physical activity (1527.1 ± 182.1 MET min/wk, P = .199), or cardiorespiratory fitness (37.5 ± 0.5 mL/min/kg, P = .517) (23).
Table 1.
Participant characteristics split by demographic category
| Demographic | All | Low MET | High MET | Low BMI | High BMI | Low CRF | High CRF |
|---|---|---|---|---|---|---|---|
| No. | 1906 | 946 (49.6) | 960 (50.4) | 1021 (53.6) | 885 (46.4) | 451 (46.7) | 514 (53.2) |
| Age, y | 25.4 ± 9.4 | 27.0 ± 10.0 | 23.9 ± 8.6a | 23.8 ± 8.5 | 27.4 ± 10.1a | 21.3 ± 5.9 | 26.8 ± 10.0a |
| Height, cm | 161.8 ± 6.9 | 161.1 ± 6.9 | 162.6 ± 6.8a | 162.0 ± 6.8 | 161.6 ± 7.0 | 162.0 ± 6.7 | 162.2 ± 6.7 |
| Weight, kg | 69.0 ± 19.0 | 70.0 ± 19.9 | 68.0 ± 18.0a | 56.6 ± 7.2 | 83.2 ± 18.3a | 69.5 ± 19.5 | 66.1 ± 15.1a |
| BMI | 26.3 ± 6.8 | 26.9 ± 7.2 | 25.7 ± 6.3a | 21.5 ± 2.1 | 31.8 ± 6.1a | 26.4 ± 7.0 | 25.1 ± 5.4a |
| VO2max, mL/kg/min | 37.5 ± 9.0 | 36.8 ± 9.5 | 38.2 ± 8.6a | 38.6 ± 8.8 | 36.1 ± 9.1a | 31.8 ± 3.8 | 42.6 ± 9.3a |
| MET, min/wk | 1548.3 ± 3112.5 | 95.3 ± 144.7 | 2980.0 ± 3884.4a | 1815.9 ± 3479.9 | 1239.5 ± 2593.5a | 1407.9 ± 2498.3 | 1811.0 ± 3200.0a |
Data are presented as mean ± SD. No. values are presented as total (%) for each demographic category.
Abbreviations: BMI, body mass index; CRF, cardiorespiratory fitness; MET, metabolic equivalent; PA, physical activity; VO2max, maximal oxygen consumption.
a P less than .05 following independent samples t test.
Pituitary Hormone Concentration Across the Menstrual Cycle
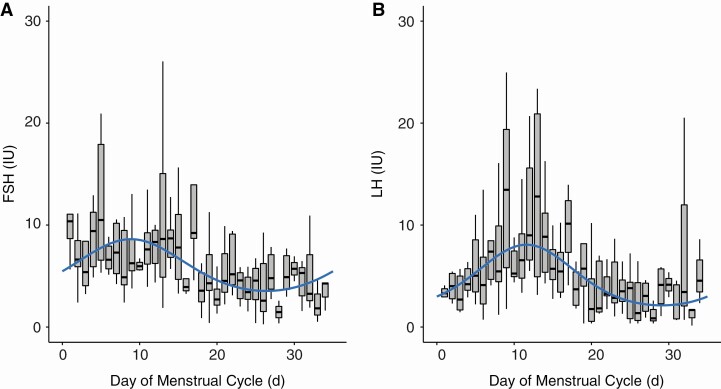
To demonstrate the validity of cosine analysis for analyzing cyclic rhythms in variables across the menstrual cycle, pituitary hormones were analyzed. Plasma FSH and LH concentrations were available for a subset of participants (Table 2 and Fig. 2). FSH concentration reached a peak of 8.6 IU on day 9 falling to 3.5 IU on day 26 (P < .001). LH concentration peaked at 8.1 IU on day 12 and declined to a trough of 2.1 IU on day 29 (P < .001).
Table 2.
Pituitary hormone concentrations across the menstrual cycle
| Variable | No. | MESOR, IU | Amplitude, IU | P-P, % | Peak, d | Trough, d | P |
|---|---|---|---|---|---|---|---|
| FSH | 218 | 6.1 | 2.5 | 91.9 | 9 | 26 | < .001 |
| LH | 219 | 5.1 | 3.0 | 144.0 | 12 | 29 | < .001 |
P value from zero amplitude test for model fit.
Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone; MESOR, rhythm-adjusted mean; P-P, %, difference between peak and trough.
Figure 2.
Box plot with cosine wave showing pituitary hormone concentration across the menstrual cycle. A, Follicle-stimulating hormone (FSH). B, Luteinizing hormone (LH).
What Is the Effect of the Menstrual Cycle on Insulin Sensitivity?
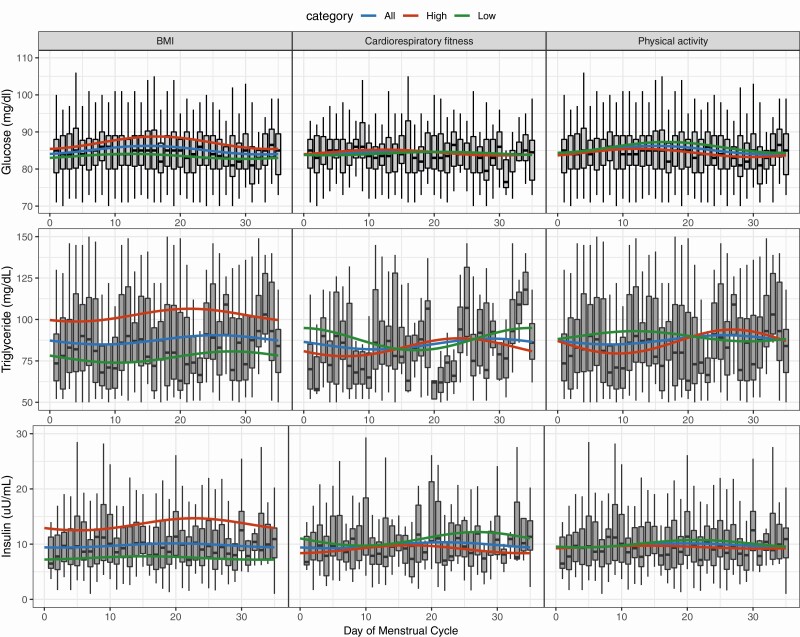
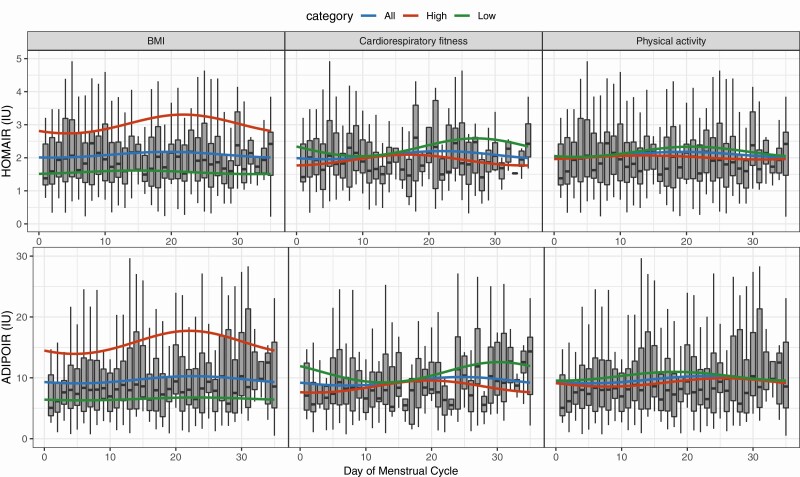
Rhythmicity was demonstrated by a significant cosine fit for glucose (MESOR: 85.1 ± amplitude: 1.2 mg/dL; P = .014). No significant fit was observed for triglycerides (87.7 ± 2.8 mg/dL; P = .369), insulin (9.8 ± 0.4 uU/mL; P = .470), HOMA-IR (2.1 ± 0.1 mmol/L; P = .461), or ADIPO-IR (9.7 ± 0.6 mmol/L; P = .335) (Table 3; Figs. 3 and 4).
Table 3.
Variation in insulin sensitivity and associated metabolites across the menstrual cycle
| Variable | Category | n value | Mean, IU | Amplitude, IU | P-P, % | Acrophase, d | P | Amplitude difference P | Acrophase difference P |
|---|---|---|---|---|---|---|---|---|---|
| Glucose | All | 1903 | 85.09 | 1.15 | 2.70 | 15 | .014 | ||
| Low BMI | 1019 | 83.41 | 0.67 | 1.62 | 12 | ||||
| High BMI | 884 | 87.07 | 1.69 | 3.88 | 16 | .000 | .205 | .335 | |
| Low PA | 944 | 85.82 | 1.46 | 3.41 | 17 | ||||
| High PA | 959 | 84.35 | 1.10 | 2.61 | 12 | .000 | .308 | .235 | |
| Low CRF | 451 | 84.16 | 0.44 | 1.05 | 20 | ||||
| High CRF | 513 | 84.44 | 0.78 | 1.86 | 12 | .223 | .460 | .443 | |
| Triglycerides | All | 872 | 87.67 | 2.79 | 6.37 | 26 | 0369 | ||
| Low BMI | 482 | 77.26 | 3.39 | 8.78 | 28 | ||||
| High BMI | 390 | 102.55 | 3.85 | 7.51 | 21 | .000 | .889 | .098 | |
| Low PA | 404 | 89.83 | 3.07 | 6.85 | 12 | ||||
| High PA | 468 | 86.39 | 7.22 | 16.73 | 27 | .006 | .018 | .675 | |
| Low CRF | 192 | 88.14 | 6.87 | 15.58 | 34 | ||||
| High CRF | 254 | 83.39 | 6.03 | 14.47 | 24 | .041 | .116 | .271 | |
| Insulin | All | 872 | 9.75 | 0.37 | 7.63 | 20 | .470 | ||
| Low BMI | 483 | 7.49 | 0.30 | 8.14 | 15 | ||||
| High BMI | 389 | 13.55 | 1.09 | 16.05 | 23 | .000 | .486 | .180 | |
| Low PA | 405 | 10.06 | 0.67 | 13.32 | 21 | ||||
| High PA | 467 | 9.45 | 0.26 | 5.59 | 14 | .095 | .284 | .571 | |
| Low CRF | 194 | 10.89 | 1.22 | 22.49 | 27 | ||||
| High CRF | 252 | 9.04 | 0.71 | 15.60 | 17 | .002 | .099 | .290 | |
| HOMA-IR | All | 871 | 2.09 | 0.09 | 8.34 | 20 | .461 | ||
| Low BMI | 482 | 1.56 | 0.05 | 6.90 | 15 | ||||
| High BMI | 389 | 3.01 | 0.28 | 18.91 | 22 | .000 | .318 | .267 | |
| Low PA | 404 | 2.18 | 0.15 | 14.18 | 21 | ||||
| High PA | 467 | 2.01 | 0.06 | 6.03 | 14 | .061 | .310 | .577 | |
| Low CRF | 194 | 2.30 | 0.27 | 23.21 | 27 | ||||
| High CRF | 252 | 1.92 | 0.16 | 16.75 | 17 | .004 | .109 | .282 | |
| ADIPO-IR | All | 868 | 9.67 | 0.59 | 12.24 | 23 | .335 | ||
| Low BMI | 480 | 6.53 | 0.23 | 7.09 | 23 | ||||
| High BMI | 388 | 15.71 | 1.89 | 24.09 | 22 | .000 | .248 | .902 | |
| Low PA | 403 | 10.24 | 0.72 | 14.02 | 18 | ||||
| High PA | 465 | 9.22 | 0.68 | 14.82 | 26 | .038 | .506 | .423 | |
| Low CRF | 192 | 10.81 | 1.71 | 31.64 | 30 | ||||
| High CRF | 252 | 8.54 | 1.04 | 24.47 | 20 | .004 | .115 | .260 |
Cosine fit P value represents zero amplitude test for model fit. Amplitude difference P value represents difference in amplitudes between respective low and high covariate categories. Acrophase difference P value represents difference in acrophase between respective low and high covariate categories. Bold font indicates a P value of less than .05.
Low BMI, 24.9 or less; high BMI, greater than 25; low CRF, less than or equal to 50th age-specific O2max percentile; high CRF, greater than 50th specific O2max percentile; low PA, less than or equal to 500 MET min/wk; high PA, greater than 500 MET min/wk.
Abbreviations: ADIPO-IR, adipose tissue insulin resistance index; BMI, body mass index; CRF, cardiorespiratory fitness; HOMA-IR, homeostatic model of insulin resistance; PA, physical activity.
Figure 3.
Changes in glucose, triglycerides, and insulin across the menstrual cycle with low and high categories of left, body mass index (BMI); middle, cardiorespiratory fitness; and right, physical activity. Box plot represents all participants data for each respective variable. Cosinor model fits are shown for (blue) all participants, (green) low covariate category, and (red) high covariate category. Low BMI, 24.9 or less; high BMI, greater than 25; low cardiorespiratory fitness, 50th or lower age-specific percentile; high cardiorespiratory fitness, greater than 50th age-specific percentile; low physical activity, less than or equal to 500 metabolic equivalent (MET) min/wk; high physical activity, greater than 500 MET min/wk.
Figure 4.
Changes in homeostatic model of insulin resistance (HOMA-IR) and adipose tissue insulin resistance index (ADIPO-IR) across the menstrual cycle with low and high categories of left, body mass index (BMI); middle, cardiorespiratory fitness; and right, physical activity. Box plot represents all participant data for each respective variable. Cosinor model fits are shown for (blue) all participants, (green) low covariate category, and (red) high covariate category. Low BMI, 24.9 or less; high BMI, greater than 25; low cardiorespiratory fitness, 50th or lower age-specific percentile; high cardiorespiratory fitness, greater than 50th age-specific percentile; low physical activity, less than or equal to 500 metabolic equivalent (MET) min/wk; high physical activity, greater than 500 MET min/wk.
How Does Body Mass Index Affect Insulin Sensitivity Across the Menstrual Cycle?
When the BMI category was added as a covariate into the cosine model, significant cosine fit was observed for glucose (P < .001), triglycerides (P < .001), insulin (P < .001), HOMA-IR (P < .001), and ADIPO-IR (P < .001) (see Table 3; Figs. 3 and 4). There were no significant differences in amplitude between low and high BMI categories for glucose (0.7 vs 1.7 mg/dL, P = .205), triglycerides (3.4 vs 3.9 mg/dL, P = .889), insulin (0.3 vs 1.1 uU/mL, P = .486), HOMA-IR (0.1 vs 0.3 mmol/L, P = .318), or ADIPOIR (0.2 vs 1.9 mmol/L, P = .248). Nor was there a significant difference in acrophase between low and high BMI categories for glucose (12 vs 16 days, P = .335), triglycerides (28 vs 21 days, P = .098), insulin (15 vs 23 days, P = .180), HOMA-IR (15 vs 22 days, P = .267), or ADIPO-IR (23 vs 22 days, P = .902).
How Does Physical Activity Affect Insulin Sensitivity Across the Menstrual Cycle?
When the physical activity category was added as a covariate into the cosine model, significant cosine fit was observed for glucose (P < .001), triglycerides (P = .006), and ADIPO-IR (P = .038), but not insulin (P = .095) or HOMA-IR (P = .061) (see Table 3; Figs. 3 and 4). Triglyceride amplitude was significantly lower in the low physical activity category compared to the high physical activity category (3.1 vs 7.2 mg/dl, P = .018). No significant differences were observed in amplitude between low and high physical activity categories across the menstrual cycle for either glucose (1.5 vs 1.1 mg/dL, P = .308), insulin (0.7 vs 0.3 uU/mL, P = .284), HOMA-IR (0.2 vs 0.1 mmol/L, P = .310) or ADIPO-IR (0.7 vs 0.7 mmol/L, P = .506). There were no significant differences in acrophase between low and high BMI categories for glucose (17 vs 12 days, P = .235), triglycerides (12 vs 27 days, P = .675), insulin (21 vs 14 days, P = .571), HOMA-IR (21 vs 14 days, P = .577), or ADIPO-IR (18 vs 26 days, P = .423).
How does Cardiorespiratory Fitness Affect Insulin Sensitivity across the Menstrual Cycle?
When the cardiorespiratory fitness category was added as a covariate into the cosine model, significant cosine fit was observed for triglycerides (P = .041), insulin (P = .002), HOMA-IR (P = .004), and ADIPO-IR (P = .004), but not glucose (P = .223) (see Table 3; Figs. 3 and 4). No significant differences in amplitude across the menstrual cycle were observed between low and high cardiorespiratory fitness for glucose (0.4 vs 0.8 mg/dL, P = .460), triglycerides (6.9 vs 6.0 mg/dL, P = .116), insulin (1.2 vs 0.7 uU/mL, P = .099), HOMA-IR (0.3 vs 0.2 mmol/L, P = .109), or ADIPOIR (1.7 vs 1.0 mmol/L, P = .115). There were no significant differences in acrophase between low and high BMI categories for glucose (20 vs 12 days, P = .443), triglycerides (34 vs 24 days, P = .271), insulin (27 vs 17 days, P = .290), HOMA-IR (27 vs 17 days, P = .282), or ADIPO-IR (30 vs 20 days, P = .260).
Discussion
This study aimed to characterize cyclical changes in insulin sensitivity and associated metabolic parameters across the menstrual cycle and their association with BMI, physical activity, and cardiorespiratory fitness. We found rhythmic cycling across the menstrual cycle for glucose, but not triglycerides, insulin, HOMA-IR, or ADIPO-IR. When including selected risk factors for insulin resistance as covariates, rhythmic cycling was observed across the menstrual cycle for glucose, triglycerides, insulin, HOMA-IR, and ADIPO-IR when models included BMI; glucose, triglycerides, and ADIPO-IR when models included physical activity; and triglycerides, insulin, HOMA-IR, and ADIPO-IR when models included cardiorespiratory fitness. Triglyceride amplitude, but not acrophase, was significantly greater in the high physical activity category compared to the low physical activity category. No significant differences in amplitude nor acrophase were observed for glucose, insulin, HOMA-IR, or ADIPO-IR between respective high and low covariate categories. These findings demonstrate changes in insulin sensitivity and triglyceride levels across the menstrual cycle are modified by BMI, physical activity, and cardiorespiratory fitness status.
Previous literature reports insulin sensitivity is either reduced during the luteal phase (12-18) or remains unchanged across the menstrual cycle (19-22). Reported variation in HOMA-IR across the menstrual cycle is of a relatively small magnitude (0.3 U), although it may be clinically meaningful (12). Therefore, some previous studies using small sample sizes may have lacked statistical power to detect significant variation (19-22). In contradiction to a report from another large study, we did not observe rhythmic variation for insulin sensitivity prior to adjusting cosine fit for BMI or cardiorespiratory fitness (12). Participants studied by Yeung et al had an average lower BMI (24.1 vs 26.3), which may have contributed to discrepancies in findings (12). Cardiorespiratory fitness was not assessed in their study. Following the inclusion of BMI and cardiorespiratory fitness into our models, we observed rhythmic cycling for HOMA-IR with variability across the menstrual cycle of 0.3 U, similar to that previously reported by Yeung and colleagues. This provides evidence that BMI and cardiorespiratory fitness mediate the variation in HOMA-IR across the menstrual cycle. This mediation effect may underpin inconsistencies reported in the literature.
To our knowledge, this is the first study to investigate ADIPO-IR across the menstrual cycle. We observed rhythmic variation in ADIPO-IR when adjusting for BMI, physical activity, and cardiorespiratory fitness levels. Rhythmic cycling in ADIPO-IR concentration roughly coincided with rhythmic cycling of triglycerides across the menstrual cycle, which peaked at cycle day 23, declining to a trough at cycle day 5 (see Table 3). This contradicts previous research reporting elevated triglyceride concentrations during the follicular phase compared to the luteal phase (11, 41). However, previous studies did not consider BMI, physical activity, or cardiorespiratory fitness, which we found significantly mediated rhythmicity in triglycerides across the menstrual cycle. Additionally, we used a larger sample size in this study compared with previous studies (n = 34 (41) and 259 (11) vs 869). Increases in ADIPO-IR during the luteal phase alongside concurrent elevations in triglyceride concentration may be underpinned by a decline in insulin-stimulated triglyceride uptake or suppression of lipolysis during the luteal phase. Progesterone has been shown to inhibit adipocyte insulin signaling and receptor binding (42, 43). Increased circulating progesterone levels may contribute to the increased ADIPO-IR observed during the luteal phase of the menstrual cycle. However, further work is required to elucidate the role progesterone plays in regulating changes in circulating triglycerides and ADIPO-IR across the menstrual cycle.
We observed lower mean triglyceride concentration alongside significantly greater amplitude across the menstrual cycle in the high physical activity category compared to low. The timing of the peak and trough in triglyceride concentration roughly coincided with the glucose trough and peak, respectively. Regular physical activity increases the capacity for adipose tissue and skeletal muscle lipid uptake and mobilization (4, 44). Moreover, high physical activity levels are positively associated with increased metabolic flexibility (44). Greater amplitude in triglyceride concentration across the menstrual cycle in the high physical activity category may reflect a coordinated uptake and release of triglycerides in response to fluctuations in glucose concentration.
While BMI and physical activity are significantly associated with variation in HOMA-IR and ADIPO-IR across the menstrual cycle, the mechanisms underpinning this relationship are uncertain. Variation in insulin sensitivity across the menstrual cycle has been associated with progesterone and estradiol (12). Differences in BMI and physical activity are known to alter ovarian hormonal profiles. Low physical activity levels are associated with higher mean estradiol levels across the menstrual cycle and higher progesterone levels during the luteal phase (45). High BMI is associated with greater variability of estradiol, but not progesterone (46). Unfortunately, neither estradiol nor progesterone was assessed in NHANES. Future research should investigate the role of sex hormones in the relationship between insulin sensitivity and BMI and physical activity levels.
We observed significant rhythmicity in HOMA-IR and ADIPO-IR following adjustment for BMI and cardiorespiratory fitness. This suggests that the menstrual cycle phase is an important consideration in the assessment of insulin sensitivity in clinical practice or research, especially in populations with high BMI or low cardiorespiratory fitness. Additionally, we found greater amplitude across the menstrual cycle for HOMA-IR and glucose in high compared to low BMI and HOMA-IR in low compared to high cardiorespiratory fitness. Although these amplitudes were not statistically significant, these data indicate individuals with high BMI or low cardiorespiratory fitness may be at greater risk of impaired insulin sensitivity and elevated glucose concentration during the luteal phase. Therefore, therapeutic strategies aiming to reduce disturbances in metabolic control across the menstrual cycle may benefit from targeting a reduction in BMI and increase in cardiovascular fitness. This is of particular clinical importance because of the role of high glucose variability and insulin resistance in the development and progression of diabetic complications (47, 48). Future larger studies should further investigate the association between BMI and cardiorespiratory fitness with the magnitude of variation in insulin sensitivity and glucose concentrations across the menstrual cycle. This research is crucial to further understand the role of the menstrual cycle in diabetes.
Unexpectedly, some participant demographics were significantly different between respective low and high demographic categories. Therefore, some caution should be applied when interpreting these findings. Age was significantly greater in the high BMI, physical activity, and cardiorespiratory fitness categories compared to low. However, previous research has reported the positive relationship between age and insulin resistance is associated with concurrent increases in adiposity and decreases in physical activity (49), which were included in the cosinor analysis as covariates. Height was significantly greater in the high physical activity group. We performed a regression analysis to assess the relationship between height and metabolic outcome parameters while accounting for menstrual cycle day and found no significant associations (23). This statistically significant effect may simply be due to the number of participants in the study (50). Similarly, a previous large study reported no association between variation in HOMA-IR across the menstrual cycle and height (12). We would expect there to be overlap in participants within covariate categories, which may have confounding effects, for example, commonality between participants in the high BMI, low physical activity, and low cardiorespiratory categories. Future studies should investigate whether there is a cumulative effect of BMI, physical activity, and cardiorespiratory on rhythmic cycling in insulin sensitivity across the menstrual cycle.
The large, prospective nature of the NHANES data set represents a major strength of this study. Our analyses were conducted in 1906 female participants with detailed questionnaire data available for reproductive and general health. These data permitted the exclusion of women with conditions that alter metabolic control or hormonal concentrations. The indirect assessment of insulin resistance using surrogate measures (HOMA-IR and AIDIPO-IR) was a limitation. However, HOMA-IR and ADIPO-IR have been validated against the gold-standard hyperinsulinemic euglycemic clamp (r = 0.82, P < .001) and the multistep pancreatic clamp (r = 0.86, P < .001) respectively, demonstrating strong correlations (51, 52). Physical activity levels were determined using a questionnaire. However, reports from the NHANES data set demonstrate similar amounts of self-reported physical activity and objectively measured physical activity via accelerometer in those either meeting or not meeting physical activity guidelines (53). Nonetheless, future studies may benefit from collecting objectively measured physical activity across the menstrual cycle. This study used the number of days since the last menstrual period started as a proxy for phase of menstrual cycle and was limited by a lack of data regarding participants’ typical menstrual cycle length. These data would allow greater accuracy in determining menstrual cycle phase. However, that the analysis of FSH and LH displayed expected fluctuations with significant rhythmicity across the menstrual cycle supports the use of “number of days since last menstrual period started” for statistical analysis in this data set. Ovarian hormone concentrations across the menstrual cycle were not measured, which would allow further exploration into the relationship between insulin sensitivity and risk factors for metabolic dysregulation. Future studies should obtain further data to allow a thorough characterization of participants’ menstrual cycles, including typical menstrual cycle duration, ovulation date, and ovarian hormones.
In conclusion, our study confirms previous reports showing insulin sensitivity undergoes small yet statistically and clinically significant rhythmic cycling across the menstrual cycle. This is the first study to demonstrate a modifying effect of BMI, physical activity, and cardiorespiratory fitness on variation in insulin sensitivity and associated metabolites across the menstrual cycle. These findings provide a basis for further research to explore the mediatory role of BMI, physical activity, and cardiorespiratory fitness on variation in insulin sensitivity across the menstrual cycle. Furthermore, this provides direction for investigation into the therapeutic benefit of targeting BMI and physical activity to mitigate disturbances in insulin sensitivity across the menstrual cycle.
Glossary
Abbreviations
- ADIPO-IR
adipose tissue insulin resistance index
- BMI
body mass index
- CRF
cardiorespiratory fitness
- FSH
follicle-stimulating hormone
- HOMA-IR
homeostatic model of insulin resistance
- HR
heart rate
- LH
luteinizing hormone
- MEC
mobile examination center
- MESOR
a rhythm-adjusted mean
- MET
metabolic equivalent
- NHANES
National Health and Nutrition Examination Survey
- VO2max
maximal oxygen consumption
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References” (54, 55).
References
- 1. Lin X, Xu Y, Pan X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10(1):14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clarke SL, Reaven GM, Leonard D, et al. Cardiorespiratory fitness, body mass index, and markers of insulin resistance in apparently healthy women and men. Am J Med. 2020;133(7):825-830.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sicree RA, Zimmet PZ, Dunstan DW, Cameron AJ, Welborn TA, Shaw JE. Differences in height explain gender differences in the response to the oral glucose tolerance test—the AusDiab Study. Diabet Med. 2008;25(3):296-302. [DOI] [PubMed] [Google Scholar]
- 4. Honka MJ, Latva-Rasku A, Bucci M, et al. Insulin-stimulated glucose uptake in skeletal muscle, adipose tissue and liver: a positron emission tomography study. Eur J Endocrinol. 2018;178(5):523-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Logue J, Walker JJ, Colhoun HM, et al. ; Scottish Diabetes Research Network Epidemiology Group . Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54(12):3003-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav. 2018;187:20-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumagai S, Holmäng A, Björntorp P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiol Scand. 1993;149(1):91-97. [DOI] [PubMed] [Google Scholar]
- 8. Reed BG, Carr BR. The normal menstrual cycle and the control of ovulation. In: Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext. MDText.com Inc; 2000. Accessed February 17, 2021. http://www.ncbi.nlm.nih.gov/books/NBK279054/ [PubMed] [Google Scholar]
- 9. Bull JR, Rowland SP, Scherwitzl EB, Scherwitzl R, Danielsson KG, Harper J. Real-world menstrual cycle characteristics of more than 600 000 menstrual cycles. NPJ Digit Med. 2019;2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costello JT, Bieuzen F, Bleakley CM. Where are all the female participants in sports and exercise medicine research? Eur J Sport Sci. 2014;14(8):847-851. [DOI] [PubMed] [Google Scholar]
- 11. Mumford SL, Schisterman EF, Siega-Riz AM, et al. A longitudinal study of serum lipoproteins in relation to endogenous reproductive hormones during the menstrual cycle: findings from the BioCycle Study. J Clin Endocrinol Metab. 2010;95(9):E80-E85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yeung EH, Zhang C, Mumford SL, et al. Longitudinal study of insulin resistance and sex hormones over the menstrual cycle: the BioCycle Study. J Clin Endocrinol Metab. 2010;95(12):5435-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valdes CT, Elkind-Hirsch KE. Intravenous glucose tolerance test-derived insulin sensitivity changes during the menstrual cycle. J Clin Endocrinol Metab. 1991;72(3):642-646. [DOI] [PubMed] [Google Scholar]
- 14. González-Ortiz M, Martínez-Abundis E, Lifshitz A. Insulin sensitivity and sex steroid hormone levels during the menstrual cycle in healthy women with non-insulin-dependent diabetic parents. Gynecol Obstet Invest. 1998;46(3):187-190. [DOI] [PubMed] [Google Scholar]
- 15. Escalante Pulido JM, Alpizar Salazar M. Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30(1):19-22. [DOI] [PubMed] [Google Scholar]
- 16. Diamond MP, Simonson DC, DeFronzo RA. Menstrual cyclicity has a profound effect on glucose homeostasis. Fertil Steril. 1989;52(2):204-208. [PubMed] [Google Scholar]
- 17. Gill JM, Malkova D, Hardman AE. Reproducibility of an oral fat tolerance test is influenced by phase of menstrual cycle. Horm Metab Res. 2005;37(5):336-341. [DOI] [PubMed] [Google Scholar]
- 18. Zarei S, Mosalanejad L, Ghobadifar MA. Blood glucose levels, insulin concentrations, and insulin resistance in healthy women and women with premenstrual syndrome: a comparative study. Clin Exp Reprod Med. 2013;40(2):76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toth EL, Suthijumroon A, Crockford PM, Ryan EA. Insulin action does not change during the menstrual cycle in normal women. J Clin Endocrinol Metab. 1987;64(1):74-80. [DOI] [PubMed] [Google Scholar]
- 20. Blum CA, Müller B, Huber P, et al. Low-grade inflammation and estimates of insulin resistance during the menstrual cycle in lean and overweight women. J Clin Endocrinol Metab. 2005;90(6):3230-3235. [DOI] [PubMed] [Google Scholar]
- 21. Bingley CA, Gitau R, Lovegrove JA. Impact of menstrual cycle phase on insulin sensitivity measures and fasting lipids. Horm Metab Res. 2008;40(12):901-906. [DOI] [PubMed] [Google Scholar]
- 22. Yki-Järvinen H. Insulin sensitivity during the menstrual cycle. J Clin Endocrinol Metab. 1984;59(2):350-353. [DOI] [PubMed] [Google Scholar]
- 23. MacGregor KA, Gallagher IJ, Moran CN. Insulin sensitivity across the menstrual cycle: Supplementary Tables 1-3. Figshare 2021. Uploaded March 9, 2021. 10.6084/m9.figshare.14185673 [DOI]
- 24. World Health Organization, ed. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation. Geneva, Switzerland: World Health Organization; 2000. [PubMed] [Google Scholar]
- 25. US Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES). Laboratory procedures manual. Published online March 2011. https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/2011-12_laboratory_procedures_manual.pdf. Accessed February 17, 2021.
- 26. US Centers for Disease Control and Prevention. Laboratory procedure manual. Follicle-stimulating hormone (FSH). Published online 2000. https://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab18_met_follicle_stimulating_hormone-.pdf. Accessed February 17, 2021. [Google Scholar]
- 27.US Centers for Disease Control and Prevention. Laboratory procedure manual. Total cholesterol, HDL-cholesterol, triglycerides, and LDL-cholesterol. Published online 2000. https://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab13_met_lipids.pdf. Accessed February 17, 2021.
- 28.US Centers for Disease Control and Prevention. Laboratory procedure manual. Plasma glucose. Published online 2000. https://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab10am_met_plasma_glucose.pdf. Accessed March 8, 2021.
- 29.US Centers for Disease Control and Prevention. Laboratory procedure manual. Insulin. Published online 2000. https://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab10am_met_insulin.pdf. Accessed February 17, 2021.
- 30. Matthews DR, Hosker JR, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and fl-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 31. Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the San Antonio Metabolism Study. Diabetes. 2017;66(4):815-822. [DOI] [PubMed] [Google Scholar]
- 32. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(Suppl):S498-S516. [DOI] [PubMed] [Google Scholar]
- 33.US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Published online 2008. https://health.gov/sites/default/files/2019-09/paguide.pdf. Accessed February 18, 2021.
- 34.US Centers for Disease Control and Prevention. Cardiovascular fitness procedures manual. Published online 2004. https://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/CV.pdf
- 35. Whaley MH, Brubaker PH, Otto RM, Armstrong LE, eds. ACSM’s Guidelines for Exercise Testing and Prescription. 7th ed.Baltmore, MD: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 36. Wilmore JH, Roby FB, Stanforth PR, et al. Ratings of perceived exertion, heart rate, and power output in predicting maximal oxygen uptake during submaximal cycle ergometry. Phys Sportsmed. 1986;14(3):133-143. [DOI] [PubMed] [Google Scholar]
- 37. Kaminsky LA. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the Fitness Registry and the Importance of Exercise National Database. Mayo Clin Proc. 2015;90(11):1515-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. RStudio Team. RStudio: Integrated Development Environment for R. Boston, MA: RStudi, Inc; 2019. http://www.rstudio.com/. Accessed February 24, 2021. [Google Scholar]
- 39. Sachs M. cosinor: tools for estimating and predicting the cosinor model. Published online 2014. https://CRAN.R-project.org/package=cosinor. Accessed February 24, 2021.
- 40. Mutak A. cosinor2: extended tools for cosinor analysis of rhythms. Published online 2018. https://CRAN.R-project.org/package=cosinor2. Accessed February 24, 2021.
- 41. Draper CF, Duisters K, Weger B, et al. Menstrual cycle rhythmicity: metabolic patterns in healthy women. Sci Rep. 2018;8(1):14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marsden PJ, Murdoch A, Taylor R. Adipocyte insulin action during the normal menstrual cycle. Hum Reprod. 1996;11(5):968-974. [DOI] [PubMed] [Google Scholar]
- 43. Wada T, Hori S, Sugiyama M, et al. Progesterone inhibits glucose uptake by affecting diverse steps of insulin signaling in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2010;298(4): E881-E888. [DOI] [PubMed] [Google Scholar]
- 44. Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52(9):2191-2197. [DOI] [PubMed] [Google Scholar]
- 45. Ahrens KA, Vladutiu CJ, Mumford SL, et al. The effect of physical activity across the menstrual cycle on reproductive function. Ann Epidemiol. 2014;24(2):127-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yeung EH, Zhang C, Albert PS, et al. Adiposity and sex hormones across the menstrual cycle: the BioCycle Study. Int J Obes (Lond). 2013;37(2):237-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dimova R, Chakarova N, Grozeva G, Kirilov G, Tankova T. The relationship between glucose variability and insulin sensitivity and oxidative stress in subjects with prediabetes. Diabetes Res Clin Pract. 2019;158:107911. [DOI] [PubMed] [Google Scholar]
- 48. Monnier L, Colette C. Postprandial and basal hyperglycaemia in type 2 diabetes: contributions to overall glucose exposure and diabetic complications. Diabetes Metab. 2015;41(6 Suppl 1):6S9-6S15. [DOI] [PubMed] [Google Scholar]
- 49. Lanza IR, Short DK, Short KR, et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57(11):2933-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Demidenko E. The P-value you can’t buy. Am Stat. 2016;70(1):33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57-63. [DOI] [PubMed] [Google Scholar]
- 52. Søndergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD. How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab. 2017;102(4):1193-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schuna JM Jr, Johnson WD, Tudor-Locke C. Adult self-reported and objectively monitored physical activity and sedentary behavior: NHANES 2005-2006. Int J Behav Nutr Phys Act. 2013;10(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. MacGregor KA, Gallagher IJ, Moran CN. Insulin sensitivity across the menstrual cycle. Rcode. Uploaded March 9, 2021. 10.6084/m9.figshare.14182439 [DOI] [PMC free article] [PubMed]
- 55. MacGregor KA, Gallagher IJ, Moran CN. Data from: insulin sensitivity across the menstrual cycle. Uploaded March 9, 2021. 10.6084/m9.figshare.14182445 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in “References” (54, 55).