Abstract
The ability to accurately differentiate treatment-related changes (ie, pseudoprogression and radiation necrosis) from recurrent glioma remains a critical diagnostic problem in neuro-oncology. Because these entities are treated differently and have vastly different outcomes, accurate diagnosis is necessary to provide optimal patient care. In current practice, this diagnostic quandary commonly requires either serial imaging or histopathologic tissue confirmation. In this article, experts in the field debate the utility of 2-deoxy-2[18F]fluoro-d-glucose positron emission tomography (FDG PET) as an imaging tool to distinguish tumor recurrence from treatment-related changes in a patient with glioblastoma and progressive contrast enhancement on magnetic resonance (MR) following chemoradiotherapy.
Clinical Scenario
A 55-year-old man with newly diagnosed right frontal glioblastoma, IDH wild type, is initially treated with gross total resection followed by chemoradiotherapy (CRT) including temozolomide. The baseline post-CRT magnetic resonance imaging (MRI), performed 6 weeks after the completion of CRT, shows new nodular enhancement about the periphery of the operative cavity. Because of this change, another contrast-enhanced MR is obtained 4 weeks later, which demonstrates further progression of enhancement and increased adjacent vasogenic edema. Clinically, the patient and his family have noted increased fatigue and subtle cognitive issues, but he has no focal neurological deficits. Would you recommend obtaining 2-deoxy-2[18F]fluoro-d-glucose positron emission tomography (FDG PET) to differentiate tumor recurrence from treatment-related changes and guide management?
Position: FDG PET Is a Useful Diagnostic Tool in Glioblastoma Response Assessment
Dr. Parent
When glioblastoma recurs, as is suspected in this patient, it typically does so adjacent to the surgical resection site, which is also where treatment-related changes are most likely to occur. Despite close MRI and clinical follow-up, correctly differentiating true tumor recurrence from treatment-related changes in the brain parenchyma remains a difficult task. After surgery, the most common course of treatment is CRT involving fractionated external beam radiation with concurrent and adjuvant temozolomide chemotherapy.1 The standard-of-care method and most important diagnostic tool utilized to distinguish treatment-related changes from recurrent disease remain contrast-enhanced MRI,2 but there are considerable limitations in relying solely upon MRI to guide clinical management. Early blood-brain barrier (BBB) breakdown and resultant contrast enhancement, which is often seen within weeks to months after CRT, appears similar to tumor progression on MRI and is termed pseudoprogression.3 Given the relatively high frequency of pseudoprogression in this setting, the Response Assessment in Neuro-Oncology (RANO) group has published imaging response criteria which suggests that within the first 12 weeks following CRT, progression should only be diagnosed outside the radiation field or if there is pathologic confirmation of recurrent tumor, though they note that pseudoprogression can less frequently be encountered later than 12 weeks.4 Additionally, more delayed treatment-related changes, termed radiation necrosis, may occur months to years following the completion of CRT5 and often mimic recurrent disease both clinically and with MRI.6 Of note, the terms pseudoprogression and radiation necrosis are used variably in the glioma literature and do not have universally agreed definitions, so more general terms such as treatment-related changes or treatment-related changes may be preferred. Given the difficulty traditional imaging has in accurately distinguishing true recurrence from treatment-related changes, and the critical changes in management that such a distinction portends for the patient, a multimodality approach incorporating both MRI and PET may be undertaken to help guide clinical management.
PET is able to provide molecular information regarding the physiology of the tissue that MRI is unable to assess, and a wide range of PET radiopharmaceuticals have been evaluated for neuro-oncology. FDG is a glucose analog with a positron-emitting radioisotope that undergoes passive and active transport across the cell membrane by glucose transporters (GLUT) and was one of the first radiopharmaceuticals used in neuro-oncology.7 Human brain cells are obligate glucose metabolizers with the majority of cerebral glucose utilization occurring via oxidative metabolism. As with glucose, FDG becomes phosphorylated by hexokinase as the first step in the glycolytic pathway but then is unable to be further metabolized, effectively trapping it within the cell. GLUT3, which is active in neurons, is independent of insulin activation and is a high-velocity hexose transporter, resulting in high FDG uptake in normal brain, and it is this high basal FDG uptake in adjacent normal brain parenchyma which effectively lowers the signal-to-background ratio for recurrent glioblastoma.8
However, despite this drawback and the increasing prevalence of amino acid PET radiopharmaceuticals in countries in which they are clinically available, FDG remains the most widely available PET radiopharmaceutical in the evaluation of glioblastoma.9 Further, as FDG is commonly used both in the imaging of many other neoplasms as well as nonneoplastic conditions (eg, brain imaging in dementia), it is the only PET radiotracer available in some smaller nuclear medicine departments. As will be discussed here, the proven benefits of obtaining FDG PET in addition to MRI in the evaluation of suspected recurrent glioblastoma include: improved accuracy in identifying and distinguishing suspected disease from treatment-related changes, improved prognostic determination for patients with recurrent disease, and the feasibility to plan future therapies based on FDG PET. It should be noted that, as with most PET radiopharmaceuticals evaluated for neuro-oncologic applications, the literature regarding use of FDG in glioma is limited by small sample sizes and a large heterogeneity of study design, limiting the ability to generalize the findings of specific studies to the disease as a whole. Despite these limitations, several groups including RANO, European Association of Nuclear Medicine (EANM), and Society of Nuclear Medicine and Molecular Imaging (SNMMI) have published criteria that can be applied to aid in FDG PET interpretation of patients with suspected recurrent glioblastoma.10,11
Several studies have demonstrated that FDG PET is by itself non-inferior to contrast-enhanced MRI in correctly identifying true recurrence of glioblastoma in both lesion-lesion and patient-patient analyses.12 A large study of 90 patients with suspected recurrent glioma found the FDG PET had improved specificity compared to MRI among all grades of tumor (83%-100% vs 18%-33%) resulting in an overall higher accuracy.13 Similarly, a meta-analysis of 16 studies of FDG PET calculated a summary sensitivity of 0.77 (95% CI: 0.66-0.85) and a summary specificity of 0.78 (95% CI: 0.54-0.91), comparable to the reported test characteristics of advanced MR methods.14 While it does not apply for this patient scenario, it should be noted that for patients with low-grade gliomas (LGG) FDG PET is inferior to MRI with a high number of false-negative cases, likely owing to the high degree of FDG uptake by normal brain tissue.15
In patients with late changes on contrast-enhanced MRI, FDG PET has some additive value to MRI in the ability to discriminate between glioma recurrence and delayed treatment-related changes (ie, radiation necrosis).13,14 As mentioned earlier, the ability to confidently discriminate between tumor recurrence and radiation necrosis with FDG is not only limited by increased FDG uptake in normal brain tissue, but is also influenced by increased FDG uptake in inflammatory tissue and variable mixtures of radiation necrosis and viable disease as are typically found on pathology.16 Despite this, several studies have shown that suspicious lesions with FDG uptake similar or less than white matter are likely radiation necrosis, whereas lesions with FDG uptake greater than cortex are likely recurrent disease.17Figure 1 shows an example of the utility of FDG PET to identify viable recurrent tumor in a patient with previously treated glioblastoma. Additional studies have suggested that the FDG uptake pattern may be more useful than absolute uptake values, with a more focal nodular area being more likely recurrent disease as compared to a more diffuse area of FDG uptake.18 Finally, while there are few recent studies that compare FDG PET to advanced MRI techniques such as spectroscopy and perfusion, those that exist do show FDG PET has additive value in accurately distinguishing recurrent disease from radiation necrosis for both spectroscopy19 and perfusion.20
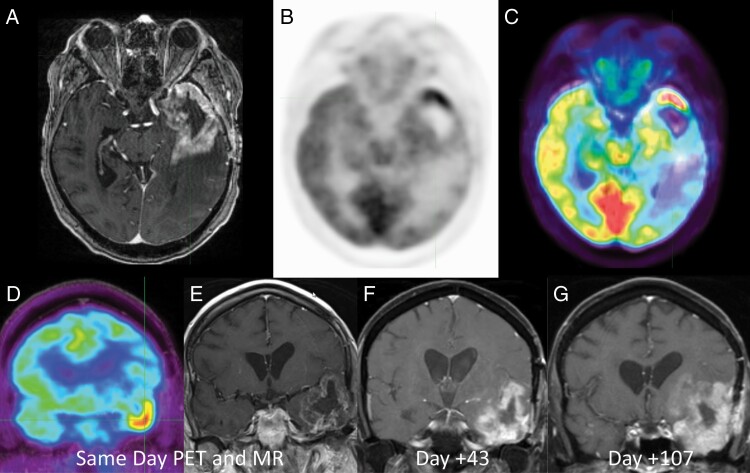
Figure 1.
44-year-old man with suspected recurrent glioblastoma after surgical resection and chemoradiation therapy 8 months prior. Axial post-contrast T1-weighted MR (a), axial 2-deoxy-2[18F]fluoro-d-glucose positron emission tomography (FDG PET) (b), and axial fused FDG PET-MR (c) images demonstrated intense FDG uptake along the temporal pole greater than adjacent cortex indicating metabolically active recurrent disease. Patient was deemed an unsuitable surgical candidate and followed with serial MRI examination. Coronal FDG PET (d) and coronal post-contrast T1-weighted MR (e) and subsequent short-term follow-up MRIs (f, g) demonstrate rapid progression of the disease.
In patients undergoing treatment for recurrent glioblastoma, FDG PET has been shown to offer prognostic information regarding survival outcomes in patients treated with chemotherapy.21,22 Decreased tumor FDG uptake following treatment has been shown to correlate to favorable response to radiation therapy,23 as well as with concurrent radiation therapy and chemotherapy.24 Similarly, a few studies have shown that FDG PET has additive value to MRI as a prognostic indicator of poor survival in patients with recurrent glioblastoma.25 A study of 56 glioblastoma patients with suspected disease progression on MRI after concurrent CRT found that the ratio of FDG uptake in the area of suspected recurrence compared to normal brain demonstrated a significant association with overall survival (OS, P = .006).26 Additionally, while the usefulness of FDG PET to delineate tumor extent in recurrent glioblastoma is limited given the physiologic high uptake in normal brain cortex,27 it may be helpful to adjudicate indeterminate cases. Small studies have likewise shown some evidence that using FDG PET to plan radiation boost mapping to areas outside of contrast enhancement is feasible.28
There are several small studies that have evaluated advanced PET analytic techniques, such as dynamic acquisitions, or kinetics, and texture analysis, or radiomics, from which some including FDG, and have demonstrated that PET can be used with high accuracy to identify recurrent glioma.29,30 However, as these techniques are not widely available and are time-intensive, at this point they have limited application to the field outside of academic centers. In like manner, there is some literature evidence that delayed dual-time-point FDG PET imaging of gliomas can help discriminate between tumor and normal brain,31 but there is no substantial literature evidence to suggest that dual-time-point imaging is helpful in correctly identifying recurrent glioblastoma after therapy. In theory, FDG uptake of glioma compared to normal brain should be greater at a delayed time point due to a greater effect of FDG-6-phosphate degradation on normal brain relative to glioma,32 but in this authors’ personal experience, dual-time-point imaging has variable reproducibility in patients with suspected recurrent glioblastoma.
Regarding the application of FDG PET on a routine clinical basis, there are suggested interpretation criteria for FDG PET of glioblastoma and in patients with suspected recurrent disease. Per a paper by the RANO working group, the lesions of interest may be classified as either positive for disease when the FDG uptake visually exceeds the activity in a reference region (eg, normal white matter or cortex) or negative when FDG uptake in the lesion is less than that in the reference lesion.11 However, despite this apparent clear delineation, in practice, many lesions demonstrate indeterminate FDG uptake (eg, greater than white matter and less than gray matter). These variable results are likely due in part to the inherent heterogeneity of the treated lesions (coexistent viable tumor and radiation-related changes)33 and the RANO working group itself notes that FDG has, “limited specificity for distinguishing glioma from other nonneoplastic lesions … due to increased FDG metabolism in inflammatory tissue.” 10 Finally, while there are proposed correction factors to convert FDG values to actual glucose metabolism which would aid in diagnostic accuracy, there remains insufficient data regarding the reliability of these measures in regards to treatment effects to apply to the everyday clinical practice.34
Position: FDG PET Is No Better Than MRI in Glioblastoma Response Assessment
Drs. Villanueva-Meyer and Gleason
We wholeheartedly agree that the above clinical scenario represents one of the most challenging diagnostic dilemmas in neuroradiology and one of the most challenging management dilemmas in neuro-oncology. We and our clinical colleagues often struggle with how to differentiate true tumor progression from treatment-related changes and how to manage a patient with no definite clinical symptoms but radiologic evidence of tumor progression.35
In glioblastoma patients, MRI with and without intravenous gadolinium contrast remains the gold standard both for initial diagnosis and preoperative management, as well as for assessment of treatment response and evaluation for tumor progression.36 The addition of advanced MRI techniques, such as diffusion-weighted imaging (DWI), magnetic resonance spectroscopy (MRS), and MR perfusion imaging using techniques such as dynamic susceptibility-weighted contrast imaging (DSC) and dynamic contrast-enhanced imaging (DCE), can increase MRI’s diagnostic accuracy for the differentiation of recurrent tumor from delayed treatment-related changes to as high as 90%.37–39Figure 2 demonstrated how advanced MR methods can be used to confidently identify recurrent glioblastoma. However, even if these advanced MRI techniques were routinely employed, which they currently are not, a substantial number of patients would have their imaging findings incorrectly classified. The unfortunate downstream impact includes premature termination of a successful therapy and delayed cessation of an ineffective treatment, with significant effects on patient outcomes and stymieing clinical trials.
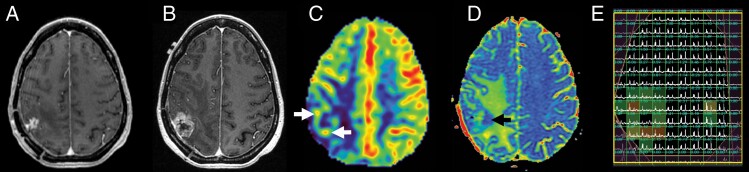
Figure 2.
52-year-old woman with suspected recurrent IDH wild-type glioblastoma after surgical resection and chemoradiation therapy 12 months prior. Axial post-contrast T1-weighted MR images show progression of contrast enhancement between recent baseline (a) and subsequent surveillance images (b). Arterial spin labeling (ASL) perfusion MR (c) demonstrates elevated cerebral blood flow peripherally (white arrows). ADC colormap (d) demonstrates reduced diffusion deep to the lesion (black arrow). MR spectroscopy (e) demonstrates tumor metabolism with elevated choline to N-acetylaspartate (NAA) index along the periphery of the lesion. Subsequently underwent re-resection with pathology demonstrating recurrent glioblastoma in >90% of lesion.
Understandably then, much effort has been invested in developing complementary imaging techniques and modalities to supplement MRI, in an attempt to further enhance the combined diagnostic accuracy. Molecular imaging with PET has emerged as one of the most promising strategies, aided by the recent deployment of hybrid PET-MRI scanners into clinical practice, which are of particular benefit in neuro-oncology since the PET exam can be acquired simultaneously with the patient’s standard-of-care follow-up MRI.40,41
The most widely used PET radiotracer is FDG, which has been used in oncologic and glioma imaging for many decades. Indeed, one of the first clinical applications of FDG PET was in recurrent glioma patients.42 However, neuro-oncology applications of FDG PET are plagued by the same limitations as in other parts of the body, including a lack of specificity for tumor cells in comparison to treatment effects, infectious, or inflammatory processes. These limitations are augmented by the low signal-to-background ratio of FDG PET in the brain due to high levels of uptake in normal brain tissue. Indeed, FDG PET uptake levels in high-grade gliomas are similar to normal gray matter.43 While Spence et al. demonstrated that delayed FDG PET imaging at 180-480 minutes after radiotracer injection could improve the signal-to-background ratio for tumor, this effect was only observed in approximately 60% of patients and came at the cost of significantly increased exam time, limiting the clinical applications of this technique.32
While FDG PET remains a mainstay in oncologic imaging, the last several decades have seen an explosion of novel PET radiotracers for imaging glioma, many of which attempt to circumvent the limitations of FDG by increasing tumor specificity. Some of the most widely applied radiotracers are amino acid analogs, including 11C-methionine (MET), 18F-fluoroethyltyrosine (FET), 18F-fluorodopa (FDOPA), and 18F-fluciclovine. A meta-analysis comparing FDG PET to MET PET in recurrent high-grade gliomas demonstrated sensitivities and specificities of 79% and 70% for FDG PET and 70% and 93% MET PET.14 FET is an even more promising agent for differentiating recurrent glioblastoma from treatment-related changes, and has been used in Europe for well over a decade, with one study demonstrating a sensitivity of 100% and specificity of 91%.44 FDOPA has also been used extensively outside of the United States, with a recent meta-analysis reporting increased accuracy over FET for diagnosing glioma recurrence from radiation necrosis.45 While 18F-fluciclovine PET-MR is FDA approved only for use in prostate cancer, it was recently utilized for intraoperative surgical planning in several glioblastoma patients and was able to successfully differentiate viable tumor from treatment effect.46 However, while amino acid radiotracers offer a potential solution to the true progression vs treatment-related changes dilemma, their utility in current clinical practice is constrained in the United States by the lack of FDA approval and the limited availability of these agents outside of major academic centers.
Fortunately, while the clinical value of FDG PET is limited by lack of tumor specificity and the value of amino acid PET agents is limited by availability and lack of regulatory approval, advanced MRI techniques involve no such trade-offs. Techniques such as DWI, DSC, DCE, and MRS are both widely available and offer improved diagnostic accuracy in glioblastoma response assessment compared to conventional anatomic imaging. Of these techniques, the most commonly used is likely DWI, which is a meta-analysis of various MR sequences in glioblastoma response assessment that demonstrated a sensitivity and specificity of 71% and 87%, respectively, compared with values of 68% and 77% for anatomical MR sequences. Perfusion MR imaging, either with DSC or DCE, demonstrated sensitivities of 87%-92% and specificities of 85%-86%. Finally, MRS offered the highest diagnostic accuracy, with a sensitivity of 91% and a specificity of 95%.39 These values compare favorably, and in the case of perfusion imaging and MRS are superior, to reported sensitivity and specificity values of FDG PET in glioblastoma response assessment.14 Therefore, FDG PET may have a role to play in glioblastoma assessment response in limited circumstances where only anatomic MRI is available. However, when advanced MRI techniques are available, FDG PET does not provide any additional diagnostic accuracy or clinical value. In the future, more specific PET radiotracers will likely play an important role in glioblastoma response assessment, but their limited availability and lack of regulatory approval make them unsuitable for widespread clinical implementation at present.
Rebuttal: FDG PET Is a Useful Diagnostic Tool in Glioblastoma Response Assessment
Dr. Parent
The clinical and practical arguments against the routine use of FDG PET, as put forth by Drs. Villanueva-Meyer and Gleason, are justified but in many cases leave the treatment team without an alternative to help distinguish the recurrent disease from posttreatment effects. Given that it is both widely available and reimbursable in the United States, FDG PET remains an ideal tool to augment the diagnostic accuracy of MRI. The principal arguments against FDG PET by Drs. Villanueva-Meyer and Gleason are 2-fold: FDG PET is inherently limited by its low specificity for recurrent disease with high uptake in inflammation (eg, pseudoprogression and radiation necrosis) and it does not improve upon current multiparametric MRI. In their statement that that FDG PET does not provide any additional diagnostic accuracy or clinical value, Drs. Villanueva-Meyer and Gleason make the logical fallacy that the lack of strong evidence in support of FDG PET is equal to proof that it is not useful. In fact, the statement that FDG PET does not add to advanced MR imaging techniques is not supported in the available literature or by this author’s personal experience. While it is true that there are only a few studies that have specifically compared FDG PET against advanced multiparametric techniques, the few that do exist support a dual role for FDG and MRI. For example, Jena et al. found that the combination of MR perfusion and spectroscopy imaging parameters to distinguish the recurrent disease from necrosis resulted in an area under the curve (AUC) of 0.913 ± 0.053, and when combined with FDG resulted in an increased AUC of 0.935 ± 0.046 and thus a statistically significant improvement in diagnostic accuracy.19 Also, Drs. Villanueva-Meyer and Gleason cite Nihashi et al.14 as evidence that the sensitivity and specificity of FDG are less than MRI, but Nihashi et al. themselves conclude in their meta-analysis that, “FDG has a moderately good accuracy as add-on tests for diagnosing recurrent glioma suspected by CT or MR imaging.” Other more recent meta-analyses come to similar conclusions that FDG PET provides different and complementary information to MRI and may enhance performance in the management of gliomas.9 A recent meta-analysis concentrating on the last 5 years of research found that combining multiparametric imaging with “lesional metabolism” (FDG PET) could enhance diagnostic accuracy, compared with either single imaging study.47 The authors of that analysis did note that a substantial risk of bias exists in the literature due to the plethora of small sample sizes, and indirectness of reviewed studies which limits firm conclusions. Drs. Villanueva-Meyer and Gleason have similarly argued that FDG PET suffers from the same non-specificity as MRI and thus does not have additive benefit, for which the authors cite Heiss et al. who found that FDG uptake in high-grade gliomas can be equivalent to brain gray matter.43 However, while that statement is true, Heiss et al. also specifically pointed out that FDG uptake greater than gray matter in an area of suspected recurrent disease is most likely true recurrence. Additionally, those authors also note that consumption of glucose in normal brain tissue is reduced in most patients with malignant brain tumors, which may improve the signal-to-noise issue.
The additional argument that the extra cost and time required for FDG PET does not justify the inclusion of FDG PET into the posttreatment monitoring algorithm does not reflect the clinical reality where each treatment decision has such profound outcomes. In the posttreatment setting, it is not uncommon to see patients undergo serial MRI examinations, sometimes at monthly or even more frequent intervals. Serial FDG studies have not been studied and would likely be of little additive survival benefit; however, application of the metabolic data that FDG PET is able to provide at certain key points in the recovery setting has proven benefit as detailed previously with few true drawbacks. There are no literature data to suggest that the inclusion of FDG PET results in worse outcomes compared to MRI alone.
Finally, it should be noted that despite my belief that the inclusion of FDG PET has real benefits for patients in the posttreatment setting, this author is not arguing that FDG PET is the best radiopharmaceutical to explore brain and tumor physiology. While FDG PET is the only PET radiopharmaceutical that is currently approved by the US Food and Drug Administration for the detection of glioblastoma, 18F-fluciclovine (Axumin) and more recently FET both have been granted orphan drug status approval from the FDA to evaluate glioma. Amino acid PET has been shown to be superior to FDG PET and as these agents become more widely available, metabolic imaging with these agents should be strongly considered.
Rebuttal: FDG PET Is No Better Than MRI in Glioblastoma Response Assessment
Drs. Villanueva-Meyer and Gleason
In glioblastoma patients who have undergone surgical resection and adjuvant radiation and temozolomide therapy, our colleague correctly notes that standard-of-care contrast-enhanced MRI suffers from a lack of specificity in being able to differentiate recurrent tumor from CRT-related changes, citing a study that reported excellent specificity for FDG PET relative to anatomic MRI, 97% and 23%, respectively, in detecting recurrent tumor. While we note that this single-institution study has a much higher reported specificity for FDG PET and a much lower reported specificity for anatomic MRI than the meta-analyses we previously cited, we agree with our colleague that anatomic MRI is not specific for recurrent tumor since both treatment-related changes and recurrent tumor will often enhance on post-contrast imaging. Therefore, it would seem that FDG PET has complementary strengths to contrast-enhanced MRI and would be helpful in improving the diagnostic accuracy for glioblastoma response assessment relative to contrast-enhanced MRI alone. However, in clinical practice, the radiologist does not rely on anatomic imaging alone when determining whether a lesion represents recurrent tumor or treatment effect, but rather incorporates the information from DWI and, when available, perfusion imaging and spectroscopy. As we discussed above, the addition of DWI to contrast-enhanced anatomic imaging improves the sensitivity and specificity of MRI to 71% and 87%, respectively.39 The addition of perfusion imaging or MR spectroscopy can further increase the sensitivity and specificity of MRI in differentiating recurrent tumor from treatment-related changes to levels that are superior to the reported sensitivity and specificity of FDG PET.43
Perhaps our colleague’s strongest argument is that hybrid FDG PET/MRI may offer some additional benefit in diagnostic accuracy beyond advanced MR imaging techniques such as MR spectroscopy and MR perfusion.14,15 However, hybrid FDG PET/MRI suffers from a lack of widespread availability, and reimbursement mechanisms for this type of hybrid scanning are not clearly established. We suspect that in the majority of clinical practices around the country treating glioblastoma patients, an FDG PET/CT is acquired separately from the contrast-enhanced MRI. In this scenario, FDG PET/CT does not provide improved diagnostic accuracy compared to advanced MR imaging techniques. Furthermore, it is faster, safer, and more cost-efficient to add advanced MR sequences to the patient’s conventional anatomic MRI than to than acquire a separate FDG PET/CT.
Our colleague’s discussion of the prognostic value of FDG PET in recurrent glioblastoma patient survival outcomes is interesting. Although one of the cited studies did not demonstrate a prognostic value for MR perfusion in survival outcomes,22 a more recent study found that MR perfusion imaging has significant prognostic value in survival outcomes for recurrent glioblastoma patients undergoing chemoradiation.48 This multivariate analysis demonstrated that change in contrast agent capillary transfer constant (Ktrans) from DCE and change in relative Cerebral blood volume (CBV) from DSC offered prognostic value for survival outcomes. Further research into the relative prognostic value between FDG PET vs MR perfusion may be warranted, but in our view, the current evidence does not justify the routine use of FDG PET in recurrent glioblastoma response assessment.
In conclusion, we acknowledge that future practice may see the widespread clinical adoption of hybrid FDG PET/MRI and/or amino acid PET radiotracers. Although not covered in great detail in the current discussion, we also recognize that both MRI and PET imaging will likely be significantly impacted by advancements in artificial intelligence techniques for image analysis. Any and all of these expected advancements could tip the balance back in favor of routinely obtaining FDG PET for recurrent glioblastoma patients. However, we believe that in current clinical practice, contrast-enhanced MRI with sequences that can be readily acquired including DWI, perfusion imaging, and/or MR spectroscopy should remain the mainstay for recurrent glioblastoma response assessment and that FDG PET does not provide any additional clinical value at an added cost.
Discussion
When evaluating the utility of a radiographic method, it is tempting to think that simple examination of test characteristics such as sensitivity and specificity should tell the tale (eg, How well does the new X-ray method detect wrist fractures?). In neuro-oncology, the situation is far more complex due to the lack of a universal gold standard to which an imaging test can be compared. One might think that pathology could fill this role, but patients taken to surgery for suspected glioblastoma recurrence often have “mixed” histology containing areas of both viable tumor and treatment effect, and in some studies, survival does not differ significantly between patients with pathologically proven pseudoprogression, tumor recurrence, or mixed changes.49 Further, the agreement of pathologists when classifying suspected recurrent glioblastoma as active tumor, treatment effect, or unable to assess is only marginal, even at designated cancer centers.50 As such, it is unfortunately possible for an imaging test to differentiate tumor recurrence from treatment effect with the same accuracy as an expert neuropathologist, and yet achieve neither good agreement with local pathology review nor successful prediction of patient outcome.
To the extent that Drs. Parent, Villanueva-Meyer, and Gleason agree, it is on two major points. First, standard anatomic MRI with and without gadolinium contrast, though the standard-of-care imaging technique in neuro-oncology, does not offer sufficient diagnostic accuracy to confidently distinguish between recurrent glioblastoma and treatment-related changes. Second, standard MR should be supplemented with imaging techniques that offer insight into tumor physiology, be they advanced MR techniques alone (as per Drs. Villanueva-Meyer and Gleason) or also including FDG PET imaging (as per Dr. Parent). Both sides of the debate cite publications supportive of their arguments. While the solution to unanswered questions in neuro-oncology is often (rightly) to conduct a well-designed clinical trial to resolve the issue, no such easy solution is on offer here due to the previously noted lack of a gold standard that is both widely reproducible and strongly predictive of clinical outcome.
In order to move forward, several things must happen in parallel. The first is an ongoing effort by the RANO Working Group to standardize the pathology definition of recurrent glioblastoma, guided by correlation of pathology, imaging, and patient outcome.51 At the same time, increasing adoption of consensus recommendations to standardize brain tumor MR imaging protocols will allow for more direct comparisons between studies and pooling of data from multiple centers.52,53 Finally, as both sides of the clinical controversy discussion agree, FDG is not the optimal PET agent for glioma assessment. While many different potential PET radiotracers for use in glioma imaging have been described, the amino acid PET agents (eg, MET, FET, and FDOPA) are the category that shows the greatest near-term promise. These tracers are widely used in the research and have significant advantages over FDG including greater sensitivity, specificity, and tumor-to-background uptake ratios.10,11 While none of the amino acid PET agents are currently approved by the FDA for use in brain tumor imaging in the United States, FDOPA has been FDA approved for imaging in Parkinson’s disease, and both FET and 18F-fluciclovine have been granted orphan drug designation for the indication of glioma imaging. With these recent developments, it is possible that one or more of these will be widely available for brain tumor imaging in the foreseeable future, and at that time comparative studies of amino acid PET imaging with advanced MR techniques, carefully correlated with pathology and patient outcomes, may finally clarify the role of PET imaging in glioblastoma response assessment.
Acknowledgment
This material has not been previously published or presented in any venue.
Funding
None.
Conflict of interest statement. None declared.
References
- 1.Ellingson BM, Chung C, Pope WB, et al. Pseudoprogression, radionecrosis, inflammation or true tumor progression? Challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol. 2017;134(3):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weller M, van den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15(9):e395–e403. [DOI] [PubMed] [Google Scholar]
- 3.Radbruch A, Fladt J, Kickingereder P, et al. Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro Oncol. 2015;17(1):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 5.Leao DJ, Craig PG, Godoy LF, et al. Response assessment in neuro-oncology criteria for gliomas: practical approach using conventional and advanced techniques. AJNR Am J Neuroradiol. 2020;41(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zikou A, Sioka C, Alexiou GA, et al. Radiation necrosis, pseudoprogression, pseudoresponse, and tumor recurrence: imaging challenges for the evaluation of treated gliomas. Contrast Media Mol Imaging. 2018;2018:6828396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas DG, Beaney RP, Brooks DJ. Positron emission tomography in the study of cerebral tumours. Neurosurg Rev. 1984;7(4):253–258. [DOI] [PubMed] [Google Scholar]
- 8.Yao Z, Zhang Q, Guo F, et al. Long noncoding RNA PCED1B-AS1 promotes the Warburg effect and tumorigenesis by upregulating HIF-1α in glioblastoma. Cell Transplant. 2020;29:963689720906777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quartuccio N, Laudicella R, Vento A, et al. The additional value of 18F-FDG PET and MRI in patients with glioma: a review of the literature from 2015 to 2020. Diagnostics (Basel). 2020;10(6):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert NL, Weller M, Suchorska B, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Law I, Albert NL, Arbizu J, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iagaru A, Mosci C, Mittra E, et al. Glioblastoma multiforme recurrence: an exploratory study of 18F FPPRGD2 PET/CT. Radiology. 2015;277(2):497–506. [DOI] [PubMed] [Google Scholar]
- 13.Santra A, Kumar R, Sharma P, et al. F-18 FDG PET-CT in patients with recurrent glioma: comparison with contrast enhanced MRI. Eur J Radiol. 2012;81(3):508–513. [DOI] [PubMed] [Google Scholar]
- 14.Nihashi T, Dahabreh IJ, Terasawa T. Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR Am J Neuroradiol. 2013;34(5):944–950, S1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma R, D’Souza M, Jaimini A, et al. A comparison study of 11C-methionine and 18F-fluorodeoxyglucose positron emission tomography-computed tomography scans in evaluation of patients with recurrent brain tumors. Indian J Nucl Med. 2016;31(2):93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyatake S, Nonoguchi N, Furuse M, et al. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo). 2015;55(Suppl 1):50–59. [PubMed] [Google Scholar]
- 17.Dankbaar JW, Snijders TJ, Robe PA, et al. The use of 18F-FDG PET to differentiate progressive disease from treatment induced necrosis in high grade glioma. J Neurooncol. 2015;125(1):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imani F, Boada FE, Lieberman FS, et al. Molecular and metabolic pattern classification for detection of brain glioma progression. Eur J Radiol. 2014;83(2):e100–e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jena A, Taneja S, Jha A, et al. Multiparametric evaluation in differentiating glioma recurrence from treatment-induced necrosis using simultaneous 18F-FDG-PET/MRI: a single-institution retrospective study. AJNR Am J Neuroradiol. 2017;38(5):899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hojjati M, Badve C, Garg V, et al. Role of FDG-PET/MRI, FDG-PET/CT, and dynamic susceptibility contrast perfusion MRI in differentiating radiation necrosis from tumor recurrence in glioblastomas. J Neuroimaging. 2018;28(1):118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colavolpe C, Chinot O, Metellus P, et al. FDG-PET predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecan. Neuro Oncol. 2012;14(5):649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omuro A, Beal K, Gutin P, et al. Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res. 2014;20(19):5023–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spence AM, Muzi M, Graham MM, et al. 2-[18F]Fluoro-2-deoxyglucose and glucose uptake in malignant gliomas before and after radiotherapy: correlation with outcome. Clin Cancer Res. 2002;8(4):971–979. [PubMed] [Google Scholar]
- 24.Charnley N, West CM, Barnett CM, et al. Early change in glucose metabolic rate measured using FDG-PET in patients with high-grade glioma predicts response to temozolomide but not temozolomide plus radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(2):331–338. [DOI] [PubMed] [Google Scholar]
- 25.Chiang GC, Galla N, Ferraro R, et al. The added prognostic value of metabolic tumor size on FDG-PET at first suspected recurrence of glioblastoma multiforme. J Neuroimaging. 2017;27(2):243–247. [DOI] [PubMed] [Google Scholar]
- 26.Leiva-Salinas C, Schiff D, Flors L, et al. FDG PET/MR imaging coregistration helps predict survival in patients with glioblastoma and radiologic progression after standard of care treatment. Radiology. 2017;283(2):508–514. [DOI] [PubMed] [Google Scholar]
- 27.Prieto E, Martí-Climent JM, Domínguez-Prado I, et al. Voxel-based analysis of dual-time-point 18F-FDG PET images for brain tumor identification and delineation. J Nucl Med. 2011;52(6):865–872. [DOI] [PubMed] [Google Scholar]
- 28.Tralins KS, Douglas JG, Stelzer KJ, et al. Volumetric analysis of 18F-FDG PET in glioblastoma multiforme: prognostic information and possible role in definition of target volumes in radiation dose escalation. J Nucl Med. 2002;43(12):1667–1673. [PubMed] [Google Scholar]
- 29.Wang K, Qiao Z, Zhao X, et al. Individualized discrimination of tumor recurrence from radiation necrosis in glioma patients using an integrated radiomics-based model. Eur J Nucl Med Mol Imaging. 2020;47(6):1400–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaragori T, Roch V, Karcher G, Marie P-Y, Imbert L, Verger A. Use of static and dynamic 18F-FDOPA PET parameters for detecting patients with glioma recurrence or progression. J Nucl Med. 2020;61(supplement 1):397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mertens K, Acou M, Van Hauwe J, et al. Validation of 18F-FDG PET at conventional and delayed intervals for the discrimination of high-grade from low-grade gliomas: a stereotactic PET and MRI study. Clin Nucl Med. 2013;38(7):495–500. [DOI] [PubMed] [Google Scholar]
- 32.Spence AM, Muzi M, Mankoff DA, et al. 18F-FDG PET of gliomas at delayed intervals: improved distinction between tumor and normal gray matter. J Nucl Med. 2004;45(10):1653–1659. [PubMed] [Google Scholar]
- 33.Chernov MF, Ono Y, Abe K, et al. Differentiation of tumor progression and radiation-induced effects after intracranial radiosurgery. Acta Neurochir Suppl. 2013;116:193–210. [DOI] [PubMed] [Google Scholar]
- 34.Graham MM, Muzi M, Spence AM, et al. The FDG lumped constant in normal human brain. J Nucl Med. 2002;43(9):1157–1166. [PubMed] [Google Scholar]
- 35.Villanueva-Meyer JE, Mabray MC, Cha S. Current clinical brain tumor imaging. Neurosurgery. 2017;81(3):397–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galldiks N, Lohmann P, Albert NL, Tonn JC, Langen KJ. Current status of PET imaging in neuro-oncology. Neurooncol Adv. 2019;1(1):vdz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barajas RF Jr, Chang JS, Segal MR, et al. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009;253(2):486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazda T, Bulik M, Pospisil P, et al. Advanced MRI increases the diagnostic accuracy of recurrent glioblastoma: single institution thresholds and validation of MR spectroscopy and diffusion weighted MR imaging. Neuroimage Clin. 2016;11:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Dijken BRJ, van Laar PJ, Holtman GA, et al. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol. 2017;27(10):4129–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehman EC, Johnson GB, Villanueva-Meyer JE, et al. PET/MRI: where might it replace PET/CT? J Magn Reson Imaging. 2017;46(5):1247–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Judenhofer MS, Wehrl HF, Newport DF, et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med. 2008;14(4):459–465. [DOI] [PubMed] [Google Scholar]
- 42.Patronas NJ, Di Chiro G, Brooks RA, et al. Work in progress: [18F] fluorodeoxyglucose and positron emission tomography in the evaluation of radiation necrosis of the brain. Radiology. 1982;144(4):885–889. [DOI] [PubMed] [Google Scholar]
- 43.Heiss WD, Raab P, Lanfermann H. Multimodality assessment of brain tumors and tumor recurrence. J Nucl Med. 2011;52(10):1585–1600. [DOI] [PubMed] [Google Scholar]
- 44.Galldiks N, Dunkl V, Stoffels G, et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-l-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015;42(5):685–695. [DOI] [PubMed] [Google Scholar]
- 45.Yu J, Zheng J, Xu W, et al. Accuracy of 18F-FDOPA positron emission tomography and 18F-FET positron emission tomography for differentiating radiation necrosis from brain tumor recurrence. World Neurosurg. 2018;114:e1211–e1224. [DOI] [PubMed] [Google Scholar]
- 46.Henderson F Jr, Brem S, O’Rourke DM, et al. 18F-Fluciclovine PET to distinguish treatment-related effects from disease progression in recurrent glioblastoma: PET fusion with MRI guides neurosurgical sampling. Neurooncol Pract. 2020;7(2):152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furuse M, Nonoguchi N, Yamada K, et al. Radiological diagnosis of brain radiation necrosis after cranial irradiation for brain tumor: a systematic review. Radiat Oncol. 2019;14(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson C, Groote I, Vardal J, et al. Prediction of survival and progression in glioblastoma patients using temporal perfusion changes during radiochemotherapy. Magn Reson Imaging. 2020;68:106–112. [DOI] [PubMed] [Google Scholar]
- 49.Melguizo-Gavilanes I, Bruner JM, Guha-Thakurta N, et al. Characterization of pseudoprogression in patients with glioblastoma: is histology the gold standard? J Neurooncol. 2015;123(1):141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holdhoff M, Ye X, Piotrowski AF, et al. The consistency of neuropathological diagnoses in patients undergoing surgery for suspected recurrence of glioblastoma. J Neurooncol. 2019;141(2):347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haider AS, van den Bent M, Wen PY, et al. Toward a standard pathological and molecular characterization of recurrent glioma in adults: a response assessment in neuro-oncology effort. Neuro Oncol. 2020;22(4):450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boxerman JL, Quarles CC, Hu LS, et al. Consensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas. Neuro Oncol. 2020;22(9):1262–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ellingson BM, Bendszus M, Boxerman J, et al. Consensus recommendations for a standardized brain tumor imaging protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]