Abstract
Context
Type 2 diabetes mellitus (T2DM) and cancer share a variety of risk factors and pathophysiological features. It is becoming increasingly accepted that the 2 diseases are related, and that T2DM increases the risk of certain malignancies.
Objective
This review summarizes recent advancements in the elucidation of functions of insulin-like growth factor 2 (IGF-2) messenger RNA (mRNA)-binding protein 2 (IGF2BP2) in T2DM and cancer.
Methods
A PubMed review of the literature was conducted, and search terms included IGF2BP2, IMP2, or p62 in combination with cancer or T2DM. Additional sources were identified through manual searches of reference lists. The increased risk of multiple malignancies and cancer-associated mortality in patients with T2DM is believed to be driven by insulin resistance, hyperinsulinemia, hyperglycemia, chronic inflammation, and dysregulation of adipokines and sex hormones. Furthermore, IGF-2 is oncogenic, and its loss-of-function splice variant is protective against T2DM, which highlights the pivotal role of this growth factor in the pathogenesis of these 2 diseases. IGF-2 mRNA-binding proteins, particularly IGF2BP2, are also involved in T2DM and cancer, and single-nucleotide variations (formerly single-nucleotide polymorphisms) of IGF2BP2 are associated with both diseases. Deletion of the IGF2BP2 gene in mice improves their glucose tolerance and insulin sensitivity, and mice with transgenic p62, a splice variant of IGF2BP2, are prone to diet-induced fatty liver disease and hepatocellular carcinoma, suggesting the biological significance of IGF2BP2 in T2DM and cancer.
Conclusion
Accumulating evidence has revealed that IGF2BP2 mediates the pathogenesis of T2DM and cancer by regulating glucose metabolism, insulin sensitivity, and tumorigenesis. This review provides insight into the potential involvement of this RNA binding protein in the link between T2DM and cancer.
Keywords: IGF-2 mRNA-binding protein 2, cancer, type 2 diabetes mellitus, metabolism, inflammation
Type 2 diabetes mellitus (T2DM) and cancer are 2 chronic diseases that have become increasingly prevalent on a global scale and thus pose significant social and economic burdens. Epidemiological studies and meta-analyses suggest that T2DM increases the risk of many malignancies and cancer-related mortalities (1-4). Patients with T2DM are 20% to 300% more likely to develop breast, colorectal, endometrial, hepatobiliary, or pancreatic cancer compared to the nondiabetic population (5-7), and cancer patients with T2DM have a 15% to 250% higher risk of mortality (6). Understanding the linkage between these 2 diseases and identifying its driving forces are important for the development of novel interventions for both diseases.
The biological links between T2DM and cancer are not completely understood. Metabolic dysregulation, which is found frequently in patients with prediabetes and diabetes, is also a factor in carcinogenesis. Patients with diabetes usually have dyslipidemia, with abnormal levels of triglycerides and a range of high-density and low-density lipoprotein cholesterols, which increase the risk of cancer (8). Hyperinsulinemia resulting from increased insulin secretion in diabetic patients and hyperglycemia from derailed glycolysis are dominant tumor-promoting factors of T2DM (9, 10). There have been reports of patients with T2DM experiencing abnormal secretion of local and systemic inflammatory factors that induce chronic inflammation, which can be paraneoplastic (11) and is associated with the excessive production of oxygen free radicals through oxidative stress (12). These patients also have abnormal levels of adipokines, such as leptin and adiponectin. Leptin mediates multiple signaling cascades that are critical for cancer cell survival and proliferation by inducing interleukin (IL)-6, signal transducer and activator of transcription-3 (STAT3), Src tyrosine kinase, focal adhesion kinase, phosphatiylinositide-3 kinase (PI3K), extracellular signal-regulated kinase 1/2, and telomerase in different cancer settings (13-16). On the contrary, adiponectin, which has been demonstrated to have an anticancerous effect by inhibiting cell cycle progression, cell invasion and migration, and inducing apoptosis, is usually reduced in patients with diabetes (17-19).
The molecular mechanisms that link T2DM and cancer also require additional research. Experimental evidence has shown that insulin and insulin-like growth factors (IGFs) promote tumor cell mitosis and proliferation (20, 21). Insulin stimulates the synthesis of IGF-1 in the liver and reduces the synthesis of IGF-binding proteins 1 and 2, resulting in an upregulation of bioactive IGF-1 in the circulation. Insulin and IGF-1 bind to the insulin receptor and IGF-1 receptor, respectively, and activate Ras/Raf/mitogen-activated protein kinase and PI3K/protein kinase B (Akt) pathways (22-25). The activation of these pathways stimulates cell proliferation and tumor growth while inhibiting apoptosis (26). IGF-2 is oncogenic (27), and its loss-of-function splice variant is protective against T2DM (28), which highlights the pivotal role of this growth factor in the pathogenesis of both diseases.
Similar to the roles of IGF-2 in T2DM and cancer, the IGF-2 messenger RNA (mRNA)-binding proteins are also involved in T2DM and cancer, particularly insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2), which complexes with the IGF-2 transcript to transport it to its cytoplasmic destination for translation, thereby mediating its expression and function (29). Importantly, single-nucleotide variations (SNVs; formerly single-nucleotide polymorphisms [SNPs]) of IGF2BP2 have also been shown to be associated with T2DM and cancer (29). Deletion of the IGF2BP2 gene in mice improves their glucose tolerance and insulin sensitivity, and mice with transgenic p62 (a splice variant of IGF2BP2) (30), are prone to diet-induced fatty liver disease and hepatocellular carcinoma (HCC) (31, 32), which indicates the biological significance of IGF2BP2 in T2DM and cancer. In the present review, we aim to summarize the recent research advances regarding the potential contribution of IGF2BP2 to the link between T2DM and cancer. However, the function of IGF2BP2 in cancer is not the focus of this review since there has been a recent review covering its specific roles in cancer onset and progression, and the maintenance of cancer stem cells (29).
Brief Introduction to Insulin-Like Growth Factor 2 Messenger RNA-Binding Protein 2
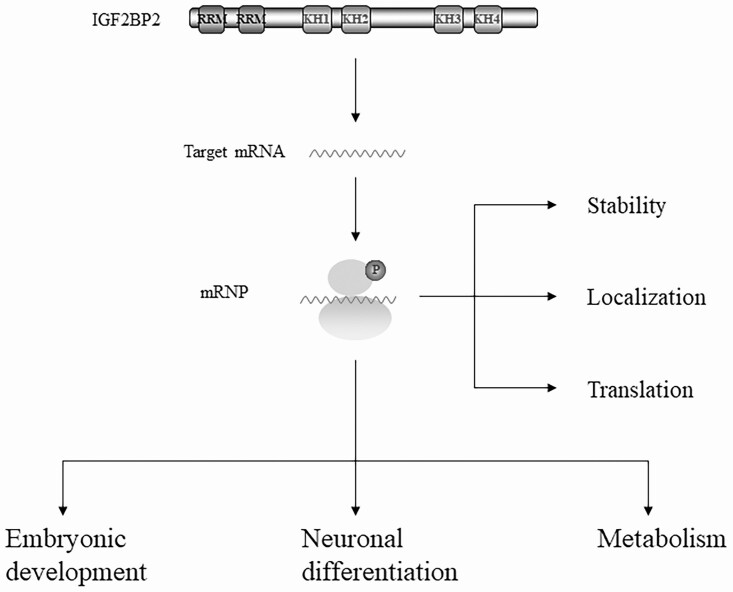
The human IGF2BP2 gene encodes a 66-kDa full-length protein composed of 599 amino acids; however, the splice variant p62 is produced by skipping exon 10 (30). Both isoforms structurally feature N-terminal RNA-recognition motif tandem repeats and C-terminal heterogeneous nuclear ribonucleoprotein K-homology domains (33). With differential binding preference to RNA sequences (34-37), the RNA-recognition motifs and K-homology domains collaborate to mediate highly specific and affinitive interactions of IGF2BP2 with hundreds of target transcripts (38). This association is important for the stability, localization, and translation of the transcripts (38, 39) (Fig. 1). IGF2BP2 has been shown to transport target mRNAs to the mitochondrial surface (16), and its inhibition blocks the assembly of respiratory complexes I and IV in the mitochondria and impairs their activity (40). These findings support the functional importance of IGF2BP2 in mitochondrial assembly, activity, and metabolism (40, 41). At the physiological level, IGF2BP2 is required for embryonic development and neural differentiation (see Fig. 1). Dysregulation of IGF2BP2 leads to a variety of diseases, including T2DM and cancer.
Figure 1.
The structure and function of IGF2BP2. IGF2BP2 consists of 2 N-terminal RRMs and 4 C-terminal KH domains, which are important for the stability, localization, and translation of the transcript. In addition, IGF2BP2 is involved in a wide range of physiological processes such as embryonic development, neuronal differentiation, and metabolism. IGF2BP2, insulin-like growth factor 2 (IGF-2) messenger RNA-binding protein 2; KH, K-homology; RRM, RNA-recognition motif.
Insulin-Like Growth Factor 2 Messenger RNA-Binding Protein 2 and Type 2 Diabetes Mellitus
Correlation Between Insulin-Like Growth Factor 2 Messenger RNA-Binding Protein 2 and Type 2 Diabetes Mellitus
T2DM is characterized by impaired insulin secretion and tolerance, hyperinsulinemia, and hyperglycemia, and describes a group of clinical syndromes resulting from glucose metabolism disorders triggered by genetic or environmental factors. It has become a global public health concern with a wide range of complications, such as hypertension, coronary heart disease, renal failure, and stroke (42). Genetic factors are at the center of T2DM disease development and pathogenesis (43, 44), and 40 susceptibility loci have been described to date (45). More specifically, many SNVs are associated with an increased risk of T2DM (46-48).
In 2007, 3 independent genome-wide association studies demonstrated a strong association between genetic variance within the IGF2BP2 gene and human T2DM (46-48). This finding was consistent with previous studies that revealed IGF2BP2 SNVs, particularly rs4402960 and rs1470579, correlate with the incidence of T2DM in a range of ethnic groups, including most populations of European ancestry, Asian ancestry, Indian populations, and Hispanic/Latino populations (15, 28, 46, 47, 49-62), although a lack of correlation has also been documented frequently (16, 63-65). The discrepancy is, nevertheless, unsurprising in view of the multiple physical factors and genetic events involved in T2DM, which may overshadow the phenotypes induced by a SNV. SNV rs4402960 increases the T2DM risk in populations younger than 55 years, but not in those older than 60 years (66). This finding is likely associated with obesity (28, 67, 68), which is a determining factor of T2DM, mediated by insulin resistance (69, 70).
The contribution of the SNVs rs4402960 and rs1470579, located in the 50-kb second intron of IGF2BP2, to T2DM remains to be characterized, but it is predicted to involve the regulation of large noncoding transcription factors, microRNAs, antisense mRNA, and alternative splicing (71). However, rs4402960 and rs1470579 may be just agents rather than functional entities. Moreover, many genes in the vicinity of IGF2BP2, such as diacylglycerol kinase gamma (DGKG), adiponectin (ADIPOQ), protein phosphatase-1 regulatory inhibitor subunit 2 (PPP1R2), mitogen-activated protein kinase kinase kinase 13 (MAP3K13), alpha 2-HS glycoprotein (AHSG), and lipase H (LIPH), are known regulators of metabolic regulation and insulin resistance (72). The IGF2BP2 SNVs may indirectly contribute to T2DM through the regulation of these neighboring genes.
However, Greenwald and colleagues (73) and Bysani et al (74) confirmed that the association of the IGF2BP2 SNPs with T2DM is mediated through the expression of the IGF2BP2 gene itself and not the neighboring genes. Greenwald et al generated a high-resolution map of islet chromatin loops using Hi-C assays in 3 islet samples, and loops were used to annotate target genes of islet enhancers defined using data from Assay for Transposase-Accessible Chromatin using sequencing and published chromatin immunoprecipitation–sequencing data (73). This study identified candidate target genes for thousands of islet enhancers, and found that enhancer looping was correlated with islet-specific gene expression. The fine-mapping of T2DM risk variants affecting islet enhancers revealed that the candidate target genes of these variants, defined using chromatin looping and eQTL mapping, were enriched in protein transport and secretion pathways. At IGF2BP2, a fine-mapped T2DM variant (rs7646518) reduces islet enhancer activity and IGF2BP2 expression, and conditional inactivation of IGF2BP2 in mouse islets impairs glucose-stimulated insulin secretion (73). These studies support the concept that the IGF2BP2 gene is directly involved in T2DM pathophysiology. Although further multicenter, large-scale studies with carefully defined ethnic and genetic stratifications are required, it is generally accepted that IGF2BP2 gene variations are associated with an increased risk of T2DM.
Potential Mechanisms Linking Insulin-Like Growth Factor 2 Messenger RNA-Binding Protein 2 and Type 2 Diabetes Mellitus
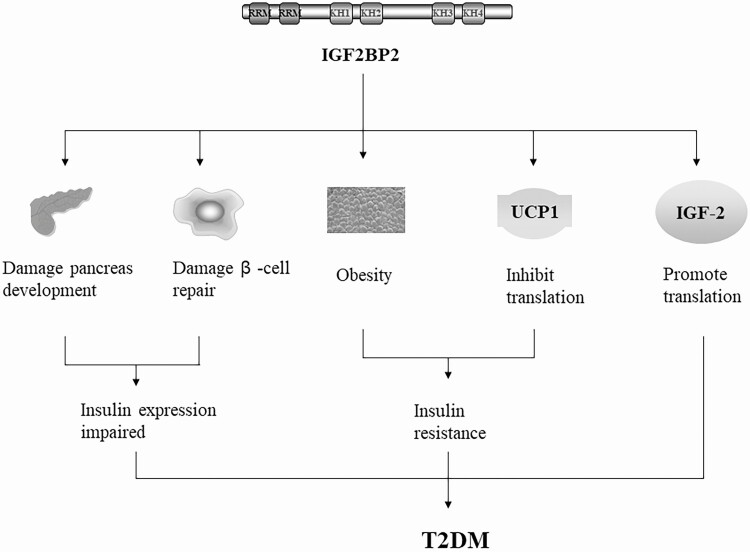
IGF2BP2 expression is maintained after birth in several organs, including the pancreas (75, 76), and alterations to IGF2BP2 underlie various disorders. Patients with a C allele of rs1470579 exhibit higher levels of fasting blood glucose, postprandial insulin, and cholesterol compared to those with AA alleles (57). The SNV rs4402960 leads to an inhibition of the first phase of glucose-stimulated insulin secretion, suggesting that this variation affects the function of pancreatic islet β cells (50, 77) (Fig. 2). Udler et al clustered approximately 200 genes into 5 robust categories, whereby IGF2BP2 was grouped into a “proinsulin cluster” that featured decreased proinsulin and reduced β-cell function (78). An additional study suggests that the SNV rs4402960 diminishes the capacity of islet β cells to repair and increases the risk of T2DM (79). Consistent with these data, deletion of IGF2BP2 in mice was shown to improve their glucose tolerance and insulin sensitivity (41). These results highlight the significance of IGF2BP2 in the functional repair of pancreatic islet β cells as well as glucose tolerance and insulin sensitivity (see Fig. 2).
Figure 2.
The link between IGF2BP2 and T2DM. Overexpression of IGF2BP2 can disrupt the development of the pancreas, destroy the repair of β cells, and also contribute to obesity and T2DM by inhibiting the expression of UCP1 and promoting the translation of IGF-2. IGF2BP2, insulin-like growth factor 2 (IGF-2) messenger RNA-binding protein 2; T2DM, type 2 diabetes mellitus; UCP1, uncoupling protein 1 (also known as thermogenin).
IGF2BP2 SNVs are also associated with body fat, such as abdominal or visceral fat, and increased susceptibility to T2DM (see Fig. 2) (80, 81). Adipose tissues from patients with diabetes expressed higher levels of IGF2BP2 compared to those from healthy individuals (82), suggesting its role in obesity, which is a known factor in insulin resistance and the pathogenesis of T2DM (67). Supporting this, IGF2BP2 deletion in mice improved glucose tolerance and insulin sensitivity and conferred the mice with resistance to diet-induced obesity and fatty liver (41). In addition, differentiated IGF2BP–/– brown fat cells express higher levels of uncoupling protein 1 (UCP1, also known as thermogenin) and produce more polypeptides than IGF2BP2+/+ cells, which indicates that IGF2BP2 may contribute to obesity and T2DM by inhibiting the expression of UCP1, which regulates thermogenesis (see Fig. 2) (83). These findings suggest that the regulatory role of IGF2BP2 may be relevant to its functional importance in obesity.
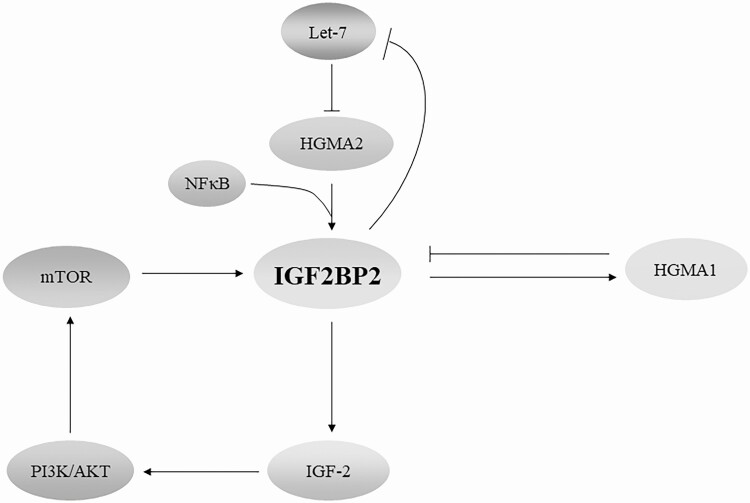
IGF2BP2 may also contribute to obesity and T2DM through its regulation of IGF-2 (see Fig. 2), which participates in the pathogenesis of obesity and T2DM (84). These effects can be controlled through mechanistic target of rapamycin (mTOR), a versatile kinase in adipose tissue that mediates adipogenesis, lipogenesis, lipolysis, and thermogenesis (24, 85). mTOR phosphorylates IGF2BP2 and facilitates its binding to IGF-2 mRNA, thereby promoting the expression of IGF-2 (86). The functional extent of this mTOR/IGF2BP2/IGF-2 cascade (Fig. 3) remains to be defined in future studies.
Figure 3.
Regulatory network of IGF2BP2 in cancer and T2DM. IGF2BP2 expression is under the control of HMGA2, a downstream target of Let-7 miRNA, and in return, IGF2BP2 binds to the Let-7 miRNA response element and inhibits Let-7 miRNA-mediated RNA degradation, independent of LIN28. HMGA1 inhibits the expression of IGF2BP2, which in turn binds to and stabilizes HMGA1 mRNA. HMGA1 also regulates IGF-2 by inhibiting IGFBP2. On phosphorylation mediated by mTOR, IGF2BP2 promotes translation of IGF-2 via internal ribosome entry and thus promotes downstream PI3K/Akt signaling. IGF2BP2 also regulates the expression of other downstream cellular functions. HMGA, high mobility group protein A; IGF2BP2, insulin-like growth factor 2 (IGF-2) messenger RNA-binding protein 2; mTOR, mechanistic target of rapamycin; PI3K, phosphotidylinositide-3 kinase; T2DM, type 2 diabetes mellitus.
Insulin-Like Growth Factor 2 Messenger RNA-Binding Protein 2 Functions Linking Type 2 Diabetes Mellitus and Cancer
Correlation Between Insulin-Like Growth Factor 2 Messenger RNA-Binding Protein 2 and Type 2 Diabetes Mellitus
T2DM and cancer share several common risk factors, including smoking, drinking, overweight, obesity, and lack of physical exercise (82). The link between T2DM and cancer was initially proposed more than a century ago (87), and many epidemiological studies suggest that T2DM increases the risk of a variety of cancers. In general, approximately 8% to 18% of all types of cancers are accompanied by T2DM (88), and the risk of developing specific cancers, including of the liver, endometrium, pancreas, kidney, colon, breast, gastrointestinal tract, ovary, bladder, and leukemia in patients with T2DM is 1.11 to 2.25 times that of the general population (1, 2, 7, 89). In these patients, T2DM predisposes them to an increased risk of mortality (82). However, contradictory results have also been reported in prostate cancer (90) and glioma (91), which inversely correlate with T2DM. It remains yet to be elucidated why T2DM differentially affects the risks of different cancers and to what extent IGF2BP2 is involved.
Insulin-Like Growth Factor 2 Messenger RNA-Binding Protein 2 in Cancer Metabolism
IGF2BP2 has been shown to play a significant role in metabolism. Inhibiting the expression of IGF2BP2 impairs the assembly and activity of mitochondrial respiratory complexes I and IV (40). Additionally, IGF2BP2-null mice gain less weight after birth and live longer than their wild-type littermates, in addition to being resistant to diet-induced obesity and fatty liver and exhibiting better glucose tolerance and insulin sensitivity (41). Recently, additional experimental evidence has linked IGF2BP2 to the progression of cancer, including HCC, glioblastoma, and breast, ovarian, colon, and esophageal cancers, particularly by playing a role in the maintenance of cancer stem cells (29). At the cellular level, IGF2BP2 enhances genomic instability (32) and stimulates cancer cell proliferation and migration (92, 93). Furthermore, studies performed at different laboratories provide strong evidence that IGF2BP2 is also required to rewire metabolism so that it promotes carcinogenesis. Among the mRNAs that are immunoprecipitated with IGF2BP2 ribonucleoprotein complexes in gliomaspheres formed by glioblastoma stem cells, the genes regulating mitochondrial function and oxidative phosphorylation are significantly overrepresented (40). In pancreatic ductal adenocarcinoma, IGF2BP2 promotes aerobic glycolysis through direct binding to and stabilization of glucose transporter 1 (Glut1) mRNA, which leads to a poor prognosis (94). Similarly, IGF2BP2 promotes glycolysis in colorectal cancer through direct binding to and stabilization of Glut1 and hexokinase 2 (HK2) mRNA (95). IGF2BP2 also stimulates the progression of colorectal cancer through Myc-mediated glycolysis (96).
Cancer is characterized by hypermetabolism, which supports the unlimited proliferation of cancer cells. Metabolic disorders associated with T2DM facilitate the seizure of bioenergy by cancer cells. Patients with diabetes frequently show dyslipidemia, with abnormal levels of high-density lipoprotein cholesterol, triglycerides, and low-density lipoprotein cholesterol (97-99). Importantly, abnormal levels of lipids in the blood, either above or below normal, increase the risk of cancer (8). In addition, cholesterol activates the PI3K/Akt pathway to promote cancer progression (7); whether the activation of this pathway triggers mTOR/IGF2BP2/IGF-2 signaling remains to be confirmed.
In obese patients with T2DM, insulin insensitivity results in excessive insulin secretion from the pancreas into blood circulation and causes hyperinsulinemia (100, 101). Both endogenous and exogenous hyperinsulinemia increases the risk of cancer (22) and cancer-related mortality (102). Insulin and its receptor promote tumor cell mitosis, and therefore tumor growth (20, 21). IGF2BP2 is involved in the regulation of insulin secretion under normal physiological conditions, and its deletion in mice increases their insulin sensitivity (41). IGF2BP2 is expressed in pancreatic islet β cells and in insulin-sensitive tissues in postnatal life (41). The aberrant expression of IGF2BP2 may alter the levels of IGF-2 in these cells, leading to their dedifferentiation as well as endoplasmic reticulum stress, ultimately resulting in their dysfunction (103). This role of IGF2BP2 in insulin secretion is consistent with its procancer functions in a variety of malignancies, as previously reviewed (29). Therefore, IGF2BP2 not only promotes mRNA translation and production of the oncogene IGF-2 (27), but also orchestrates insulin/IGF signaling through the posttranscriptional regulation of several critical polypeptides in the pathway (104).
Hyperglycemia, another complication of T2DM, causes genetic mutations and enhances the proliferation, invasion, and metastasis of tumor cells (82). It may also stimulate tumor cell growth by inducing the production of IGF-1 and inflammatory cytokines (82). Hyperglycemia facilitates the adaptive selection of cells with strong glycolysis that are undergoing malignant transformation (105). Although there is no direct evidence that supports the link between IGF2BP2 and hyperglycemia in cancer, it may participate in the pathogenesis through IGF-2, which is a determining factor in hyperglycemia (106). Therefore, current studies indicate that IGF2BP2 promotes tumorigenesis through the activation of the insulin/IGF pathway and rewiring of glucose, lipid, and amino acid metabolism.
Insulin-Like Growth Factor 2 Messenger RNA-Binding Protein 2 in Type 2 Diabetes Mellitus–Associated Inflammation and Cancer
The association between inflammation and cancer has been well established (107). Patients with obesity and T2DM have abnormal levels of inflammatory factors that lead to chronic local or systemic inflammation (82). The contribution of IGF2BP2 to this microenvironment alteration is not fully understood, and our knowledge regarding the IGF2BP2-induced inflammatory response driving carcinogenesis has been associated with the functions of p62, the short splice variant of IGF2BP2, in nonalcoholic fatty liver disease and its major complication, HCC (31). Following treatment with diethylnitrosamine, transgenic mice with overexpressed p62 developed more tumors with more aggressive and stem-like phenotypes compared to their normal littermates (32). In these mice, distinct expression profiles of inflammatory cytokines and markers for oxidative stress were discovered. Consistently, HepG2 cells overexpressing p62 showed elevated levels of reactive oxygen species (32).
Additional evidence supports the engagement of p62 in the pathogenesis that precedes malignant transformation. p62 transgenic mice developed fatty liver on a regular diet (108) and amplified fibrosis induced by nonalcoholic steatohepatitis (NASH) when fed with a diet deficient in methionine and choline (MCD) (108, 109). Activation of the nuclear factor–κB (NF-κB) transcription factor and the expression of its target genes in the liver of mice on the MCD diet resulted in increased inflammation (109). Moreover, higher levels of liver and serum cholesterol were observed. The free cholesterol was not diet derived, and its biosynthesis was attributed to the upregulation of sterol regulatory element binding transcription factor 2 (SREBF2) (109).
Further research provided insight into the lipogenic role of IGF2BP2 in inflammation-driven hepatocarcinogenesis. Liver-specific expression of p62 in mice on the MCD diet induced the expression of monocyte chemoattractant protein-1, which promoted steatosis-inflammation-fibrosis (110) as well as increased fat deposition and led to an earlier onset of advanced fibrosis (31). The expression of the lipogenic transcription factor Srebp1c was increased, which correlated with the pathophysiological roles of the sterol regulatory element binding protein in HCC lipogenesis and progression (31, 111). More specifically in this setting, p62 overexpression led to an elevation in connective tissue growth factor (CTGF) and IL-13, but not transforming growth factor β, implying a p62/CTGF/IL-13 signaling cascade in liver fibrosis (31). During lipid metabolism, p62 induces fatty acid elongation and, as a result, promotes NASH in mice and humans (112). Although a complete understanding regarding the impact of IGF2BP2 on the levels of cytokines and chemokines remains to be determined, these data collectively suggest that p62 promotes hepatocarcinogenesis by enhancing inflammation (32). It is of great importance to evaluate the role of IGF2BP2 in other T2DM-associated inflammation-driven cancers, such as colitis-associated colorectal cancer.
The Regulation of Insulin-Like Growth Factor 2 Messenger RNA-Binding Protein 2 in Type 2 Diabetes Mellitus and Cancer
Ectopic expression of IGF2BP2 in cancer is mediated by high mobility group proteins and mTOR, which are also associated with T2DM. High mobility group protein A (HMGA) 1 and IGF2BP2 function in a delicate feedback loop (see Fig. 3), in which HMGA1 suppresses IGF2BP2 expression and IGF2BP2 binds to and stabilizes HMGA1 mRNA to promote cell proliferation, together with other IGF2BP2 targets (104). Moreover, this balancing feedback loop likely underlies the driving role of IGF2BP2 in maintaining cancer stem cells in glioblastoma by binding to and stabilizing transcripts, including HMGA1 (40). Therefore, it is unsurprising that HMGA1 is overexpressed in a range of solid tumors, and its increased expression promotes malignant transformation and metastasis (113).
The HMGA1-IGF2BP2 axis may also function in T2DM. Increasing evidence suggests that HMGA1 alterations are correlated with T2DM. A case-control study of a large cohort of patients revealed that functional HMGA1 variants in individuals of White European ancestry were associated with T2DM (114). In mice, HMGA1 deficiency leads to decreased insulin signaling, hyperglycemia, and T2DM-like phenotypes (115).
HMGA2, however, promotes IGF2BP2 transcription by binding to the AT-rich region of the first intron and is expressed with IGF2BP2 in liposarcoma (116). IGF2BP2 transcription is also regulated by a truncated HMGA2 (HMGA2Tr), which inhibits IGF2BP2 expression (117). In return, IGF2BP2 indirectly enhances the expression of HMGA2 by preventing Let-7 miRNA (118) from binding to HMGA2 mRNA (82). Of note, the regulation of IGF2BP2 by HMGA2 requires NF-κB (see Fig. 3). NF-κB is predominantly proinflammatory and is a precursor lesion in diverse cancers (115), which supports the significance of HMGA2-IGF2BP2 in inflammation and malignant transformation. This functional pair may also be important for T2DM development. HMGA2 is highly expressed in white adipose tissue in patients with T2DM (119). As such, regulation of IGF2BP2 by HMGA2 may be the mechanism underlying the association between HMGA2 and the increased risk of T2DM (119).
Apart from high mobility group proteins, the function of IGF2BP2 in cancer can also be regulated by the PI3K/Akt/mTOR pathway, which is activated during inflammation (120) and is commonly dysregulated in a wide range of cancers (121). As previously described, mTOR-mediated phosphorylation of IGF2BP2 is required for the expression of IGF-2 and downstream signaling involved in cell proliferation. In a feedback loop, IGF2BP2 mediates the expression of IGF-2, which in turn activates the PI3K/Akt/mTOR pathway during cell proliferation, migration, and epithelial-mesenchymal transition (44). Taken together, these multiple feedback loops between IGF2BP2 and its upstream regulators such as HMGA1, HMGA2, and mTOR control the expression of IGF2BP2 both in T2DM and cancer (see Fig. 3).
Conclusions and Future Perspectives
Findings from independent epidemiological studies support the connection between T2DM and cancer. The increased risk of multiple malignancies and cancer-associated mortality in patients with T2DM is believed to be driven by insulin resistance as well as hyperinsulinemia, hyperglycemia, chronic inflammation, and the dysregulation of adipokines and sex hormone production (122). At a molecular level, insulin and IGFs, such as IGF-1 and IGF-2, and their regulatory networks are central for these pathophysiological mechanisms. Therefore, it is unsurprising that proteins mediating the expression of IGFs, such as IGF2BP2, are involved in the relationship between T2DM and cancer.
In general, IGF2BP2 functions to promote both T2DM and cancer. Mediating the cytoplasmic transport and processing of dozens of transcripts that encode proteins involved in mitochondrial assembly, activity, and oxidative phosphorylation, IGF2BP2 is required for cell metabolism (40, 41). Deletion of the IGF2BP2 gene in mice delays body growth and prolongs life, in addition to improving glucose tolerance and insulin sensitivity (41). These mice are resistant to diet-induced obesity likely through the regulation of Ucp1 and other mitochondrial proteins (41), while transgenic mice overexpressing p62 develop fatty liver on a regular diet (108). With advanced age, IGF2BP2–/– mice develop fewer spontaneous tumors compared to their wild-type littermates (41), whereas p62 transgenic mice are more sensitive to diethylnitrosamine-induced HCC (32). These results from mouse models are consistent with the amplification and overexpression of IGF2BP2 in human cancers (104) and its positive regulatory role in cancer cell proliferation and migration (104, 123). These findings collectively suggest that IGF2BP2 is involved in the pathogenesis of T2DM and cancer.
The importance of IGF2BP2 in the connection between T2DM and cancer is signified by its aberrant expression in liver cirrhosis and HCC (124) and, more convincingly, in the p62 transgenic mouse model, which showed increased susceptibility to nonalcoholic fatty liver disease, NASH, and HCC (31, 32, 108). This can be partially attributed to its functional significance in mitochondrial activity and cell metabolism. In addition, increased inflammatory responses mediated by IGF2BP2 are also pivotal, although the full-scale of the effect of IGF2BP2 on inflammation remains to be determined. The roles of IGF2BP2 in other inflammation-driven cancer models, such as the colitis-driven colon cancer model, should be investigated to reveal further insights into the underlying mechanisms and associated activities.
Epidemiologic data suggest that variations of the IGF2BP2 gene, such as rs4402960, are associated with an increased risk of T2DM (48, 50, 54) and cancer (57, 125). The SNV rs4402960 impairs the regulatory role of IGF2BP2 in pancreatic islet β cells and inhibits insulin secretion (50, 77), supporting its correlation with T2DM; however, the molecular mechanism is not clear. Moreover, there is an exception to the positive correlation between T2DM and cancer: Patients with T2DM are less likely to develop prostate cancer (6, 89). Interestingly, the T allele of rs4402960 is associated with a lower risk of prostate cancer (90). Further investigation is required to determine how this SNV contributes to T2DM but results in a different pathology in prostate cancer compared to cancers of other organs.
Glossary
Abbreviations
- Akt
protein kinase B
- CTGF
connective tissue growth factor
- HCC
hepatocellular carcinoma
- HMGA
high mobility group protein A
- IGF
insulin-like growth factor
- IGF2BP2
insulin-like growth factor 2 mRNA-binding protein 2
- IL
interleukin
- MCD
deficient in methionine and choline
- mRNA
messenger RNA
- mTOR
mechanistic target of rapamycin
- NASH
nonalcoholic steatohepatitis
- NF-κB
nuclear factor–κB
- SNV
single-nucleotide variation
- PI3K
phosphotidylinositide-3 kinase
- T2DM
type 2 diabetes mellitus
- UCP1
uncoupling protein 1
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35(9):1835-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harding JL, Shaw JE, Peeters A, Cartensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care 2015;38:264-270. Diabetes Care. 2015;38(4):734-735. [DOI] [PubMed] [Google Scholar]
- 3. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414-1431. [DOI] [PubMed] [Google Scholar]
- 4. Støvring H, Andersen M, Beck-Nielsen H, Green A, Vach W. Rising prevalence of diabetes: evidence from a Danish pharmaco-epidemiological database. Lancet. 2003;362(9383):537-538. [DOI] [PubMed] [Google Scholar]
- 5. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. [DOI] [PubMed] [Google Scholar]
- 6. Shlomai G, Neel B, LeRoith D, Gallagher EJ. Type 2 diabetes mellitus and cancer: the role of pharmacotherapy. J Clin Oncol. 2016;34(35):4261-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gallagher EJ, LeRoith D. Diabetes, antihyperglycemic medications and cancer risk: smoke or fire? Curr Opin Endocrinol Diabetes Obes. 2013;20(5):485-494. [DOI] [PubMed] [Google Scholar]
- 8. Yang X, So WY, Ma RC, et al. Predicting values of lipids and white blood cell count for all-site cancer in type 2 diabetes. Endocr Relat Cancer. 2008;15(2):597-607. [DOI] [PubMed] [Google Scholar]
- 9. Cannata D, Fierz Y, Vijayakumar A, LeRoith D. Type 2 diabetes and cancer: what is the connection? Mt Sinai J Med. 2010;77(2):197-213. [DOI] [PubMed] [Google Scholar]
- 10. Ryu TY, Park J, Scherer PE. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab J. 2014;38(5):330-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436-444. [DOI] [PubMed] [Google Scholar]
- 12. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fenton JI, Hursting SD, Perkins SN, Hord NG. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis. 2006;27(7):1507-1515. [DOI] [PubMed] [Google Scholar]
- 14. Ratke J, Entschladen F, Niggemann B, Zänker KS, Lang K. Leptin stimulates the migration of colon carcinoma cells by multiple signaling pathways. Endocr Relat Cancer. 2010;17(1):179-189. [DOI] [PubMed] [Google Scholar]
- 15. Bartucci M, Svensson S, Ricci-Vitiani L, et al. Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr Relat Cancer. 2010;17(3):823-833. [DOI] [PubMed] [Google Scholar]
- 16. Boudoukha S, Cuvellier S, Polesskaya A. Role of the RNA-binding protein IMP-2 in muscle cell motility. Mol Cell Biol. 2010;30(24):5710-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Lam JB, Lam KS, et al. Adiponectin modulates the glycogen synthase kinase-3β/β-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006;66(23):11462-11470. [DOI] [PubMed] [Google Scholar]
- 18. Jardé T, Caldefie-Chézet F, Goncalves-Mendes N, et al. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocr Relat Cancer. 2009;16(4):1197-1210. [DOI] [PubMed] [Google Scholar]
- 19. Taliaferro-Smith L, Nagalingam A, Zhong D, Zhou W, Saxena NK, Sharma D. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene. 2009;28(29):2621-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noto H, Goto A, Tsujimoto T, Osame K, Noda M. Latest insights into the risk of cancer in diabetes. J Diabetes Investig. 2013;4(3):225-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu CX, Zhu HH, Zhu YM. Diabetes and cancer: associations, mechanisms, and implications for medical practice. World J Diabetes. 2014;5(3):372-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vigneri R, Goldfine ID, Frittitta L. Insulin, insulin receptors, and cancer. J Endocrinol Invest. 2016;39(12):1365-1376. [DOI] [PubMed] [Google Scholar]
- 23. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan JT, Ng DP, Nurbaya S, et al. Polymorphisms identified through genome-wide association studies and their associations with type 2 diabetes in Chinese, Malays, and Asian-Indians in Singapore. J Clin Endocrinol Metab. 2010;95(1):390-397. [DOI] [PubMed] [Google Scholar]
- 26. Christopoulos PF, Corthay A, Koutsilieris M. Aiming for the insulin-like growth factor-1 system in breast cancer therapeutics. Cancer Treat Rev. 2018;63:79-95. [DOI] [PubMed] [Google Scholar]
- 27. Kessler SM, Haybaeck J, Kiemer AK. Insulin-like growth factor 2—the oncogene and its accomplices. Curr Pharm Des. 2016;22(39):5948-5961. [DOI] [PubMed] [Google Scholar]
- 28. Mercader JM, Liao RG, Bell AD, et al. A loss-of-function splice acceptor variant in IGF2 is protective for type 2 diabetes. Diabetes. 2017;66(11):2903-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao J, Mu Q, Huang H. The roles of insulin-like growth factor 2 mRNA-binding protein 2 in cancer and cancer stem cells. Stem Cells Int. 2018;2018:4217259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang JY, Chan EK, Peng XX, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med. 1999;189(7):1101-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simon Y, Kessler SM, Bohle RM, Haybaeck J, Kiemer AK. The insulin-like growth factor 2 (IGF2) mRNA-binding protein p62/IGF2BP2-2 as a promoter of NAFLD and HCC? Gut. 2014;63(5):861-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kessler SM, Laggai S, Barghash A, et al. IMP2/p62 induces genomic instability and an aggressive hepatocellular carcinoma phenotype. Cell Death Dis. 2015;6:e1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol. 1999;19(2):1262-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Git A, Standart N. The KH domains of Xenopus Vg1RBP mediate RNA binding and self-association. RNA. 2002;8(10):1319-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amarasinghe AK, MacDiarmid R, Adams MD, Rio DC. An in vitro-selected RNA-binding site for the KH domain protein PSI acts as a splicing inhibitor element. RNA. 2001;7(9):1239-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Farina KL, Huttelmaier S, Musunuru K, Darnell R, Singer RH. Two ZBP1 KH domains facilitate β-actin mRNA localization, granule formation, and cytoskeletal attachment. J Cell Biol. 2003;160(1):77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nielsen FC, Nielsen J, Kristensen MA, Koch G, Christiansen J. Cytoplasmic trafficking of IGF-II mRNA-binding protein by conserved KH domains. J Cell Sci. 2002;115(Pt 10):2087-2097. [DOI] [PubMed] [Google Scholar]
- 38. Lunde BM, Moore C, Varani G. RNA-binding proteins: modular design for efficient function. Nat Rev Mol Cell Biol. 2007;8(6):479-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yaniv K, Yisraeli JK. The involvement of a conserved family of RNA binding proteins in embryonic development and carcinogenesis. Gene. 2002;287(1-2):49-54. [DOI] [PubMed] [Google Scholar]
- 40. Janiszewska M, Suvà ML, Riggi N, et al. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012;26(17):1926-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dai N, Zhao L, Wrighting D, et al. IGF2BP2/IMP2-deficient mice resist obesity through enhanced translation of Ucp1 mRNA and other mRNAs encoding mitochondrial proteins. Cell Metab. 2015;21(4):609-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ginter E, Simko V. Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol. 2012;771:42-50. [DOI] [PubMed] [Google Scholar]
- 43. Almgren P, Lehtovirta M, Isomaa B, et al. ; Botnia Study Group . Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia. 2011;54(11):2811-2819. [DOI] [PubMed] [Google Scholar]
- 44. Barghash A, Helms V, Kessler SM. Overexpression of IGF2 mRNA-binding protein 2 (IMP2/p62) as a feature of basal-like breast cancer correlates with short survival. Scand J Immunol. 2015;82(2):142-143. [DOI] [PubMed] [Google Scholar]
- 45. Imamura M, Shigemizu D, Tsunoda T, et al. Assessing the clinical utility of a genetic risk score constructed using 49 susceptibility alleles for type 2 diabetes in a Japanese population. J Clin Endocrinol Metab. 2013;98(10):E1667-E1673. [DOI] [PubMed] [Google Scholar]
- 46. Diabetes Genetics Initiative of Broad Institute of Harvard and Mit, Lund University, Novartis Institutes of BioMedical Research; Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316(5829):1331-1336. [DOI] [PubMed] [Google Scholar]
- 47. Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316(5829):1341-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zeggini E, Weedon MN, Lindgren CM, et al. ; Wellcome Trust Case Control Consortium (WTCCC) . Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316(5829):1336-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duesing K, Fatemifar G, Charpentier G, et al. Evaluation of the association of IGF2BP2 variants with type 2 diabetes in French Caucasians. Diabetes. 2008;57(7):1992-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Grarup N, Rose CS, Andersson EA, et al. Studies of association of variants near the HHEX, CDKN2A/B, and IGF2BP2 genes with type 2 diabetes and impaired insulin release in 10,705 Danish subjects: validation and extension of genome-wide association studies. Diabetes. 2007;56(12):3105-3111. [DOI] [PubMed] [Google Scholar]
- 51. Cauchi S, Meyre D, Durand E, et al. Post genome-wide association studies of novel genes associated with type 2 diabetes show gene-gene interaction and high predictive value. PloS One. 2008;3(5):e2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lyssenko V, Jonsson A, Almgren P, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359(21):2220-2232. [DOI] [PubMed] [Google Scholar]
- 53. Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA. 2008;300(23):2754-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Omori S, Tanaka Y, Takahashi A, et al. Association of CDKAL1, IGF2BP2, CDKN2A/B, HHEX, SLC30A8, and KCNJ11 with susceptibility to type 2 diabetes in a Japanese population. Diabetes. 2008;57(3):791-795. [DOI] [PubMed] [Google Scholar]
- 55. Horikawa Y, Miyake K, Yasuda K, et al. Replication of genome-wide association studies of type 2 diabetes susceptibility in Japan. J Clin Endocrinol Metab. 2008;93(8):3136-3141. [DOI] [PubMed] [Google Scholar]
- 56. Tabara Y, Osawa H, Kawamoto R, et al. Replication study of candidate genes associated with type 2 diabetes based on genome-wide screening. Diabetes. 2009;58(2):493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang Q, Yin JY, Dai XP, et al. IGF2BP2 variations influence repaglinide response and risk of type 2 diabetes in Chinese population. Acta Pharmacol Sin. 2010;31(6):709-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sanghera DK, Ortega L, Han S, et al. Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Med Genet. 2008;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chauhan G, Spurgeon CJ, Tabassum R, et al. Impact of common variants of PPARG, KCNJ11, TCF7L2, SLC30A8, HHEX, CDKN2A, IGF2BP2, and CDKAL1 on the risk of type 2 diabetes in 5,164 Indians. Diabetes. 2010;59(8):2068-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Han L, Li Y, Tang L, et al. IGF2BP2 rs11705701 polymorphisms are associated with prediabetes in a Chinese population: a population-based case-control study. Exp Ther Med. 2016;12(3):1849-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Phani NM, Adhikari P, Nagri SK, D’Souza SC, Satyamoorthy K, Rai PS. Replication and relevance of multiple susceptibility loci discovered from genome wide association studies for type 2 diabetes in an Indian population. PloS One. 2016;11(6):e0157364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. García-Chapa EG, Leal-Ugarte E, Peralta-Leal V, Durán-González J, Meza-Espinoza JP. Genetic epidemiology of type 2 diabetes in Mexican mestizos. Biomed Res Int. 2017;2017:3937893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bronstein M, Pisanté A, Yakir B, Darvasi A. Type 2 diabetes susceptibility loci in the Ashkenazi Jewish population. Hum Genet. 2008;124(1):101-104. [DOI] [PubMed] [Google Scholar]
- 64. Cui B, Zhu X, Xu M, et al. A genome-wide association study confirms previously reported loci for type 2 diabetes in Han Chinese. PloS One. 2011;6(7):e22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wen J, Rönn T, Olsson A, et al. Investigation of type 2 diabetes risk alleles support CDKN2A/B, CDKAL1, and TCF7L2 as susceptibility genes in a Han Chinese cohort. PloS One. 2010;5(2):e9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rao P, Wang H, Fang H, et al. Association between IGF2BP2 polymorphisms and type 2 diabetes mellitus: a case-control study and meta-analysis. Int J Environ Res Public Health. 2016;13(6):574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586-623. [DOI] [PubMed] [Google Scholar]
- 68. Lasram K, Ben Halim N, Benrahma H, et al. Contribution of CDKAL1 rs7756992 and IGF2BP2 rs4402960 polymorphisms in type 2 diabetes, diabetic complications, obesity risk and hypertension in the Tunisian population. J Diabetes. 2015;7(1):102-113. [DOI] [PubMed] [Google Scholar]
- 69. Runkel M, Müller S, Brydniak R, Runkel N. Downgrading of type 2 diabetes mellitus (T2DM) after obesity surgery: duration and severity matter. Obes Surg. 2015;25(3):494-499. [DOI] [PubMed] [Google Scholar]
- 70. Baier LJ, Hanson RL. Genetic studies of the etiology of type 2 diabetes in Pima Indians: hunting for pieces to a complicated puzzle. Diabetes. 2004;53(5):1181-1186. [DOI] [PubMed] [Google Scholar]
- 71. Christiansen J, Kolte AM, Hansen Tv, Nielsen FC. IGF2 mRNA-binding protein 2: biological function and putative role in type 2 diabetes. J Mol Endocrinol. 2009;43(5):187-195. [DOI] [PubMed] [Google Scholar]
- 72. Doria A, Patti ME, Kahn CR. The emerging genetic architecture of type 2 diabetes. Cell Metab. 2008;8(3):186-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Greenwald WW, Chiou J, Yan J, et al. Pancreatic islet chromatin accessibility and conformation reveals distal enhancer networks of type 2 diabetes risk. Nat Commun. 2019;10(1):2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bysani M, Agren R, Davegårdh C, et al. ATAC-seq reveals alterations in open chromatin in pancreatic islets from subjects with type 2 diabetes. Sci Rep. 2019;9(1):7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wagner M, Kunsch S, Duerschmied D, et al. Transgenic overexpression of the oncofetal RNA binding protein KOC leads to remodeling of the exocrine pancreas. Gastroenterology. 2003;124(7):1901-1914. [DOI] [PubMed] [Google Scholar]
- 76. Spagnoli FM, Brivanlou AH. The RNA-binding protein, Vg1RBP, is required for pancreatic fate specification. Dev Biol. 2006;292(2):442-456. [DOI] [PubMed] [Google Scholar]
- 77. Groenewoud MJ, Dekker JM, Fritsche A, et al. Variants of CDKAL1 and IGF2BP2 affect first-phase insulin secretion during hyperglycaemic clamps. Diabetologia. 2008;51(9):1659-1663. [DOI] [PubMed] [Google Scholar]
- 78. Udler MS, Kim J, von Grotthuss M, et al. ; Christopher D. Anderson on behalf of METASTROKE and the ISGC. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PloS Med. 2018;15(9):e1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wu Y, Li H, Loos RJF, et al. Common variants in CDKAL1, CDKN2A/B, IGF2BP2, SLC30A8, and HHEX/IDE genes are associated with type 2 diabetes and impaired fasting glucose in a Chinese Han population. Diabetes. 2008;57(10):2834-2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chistiakov DA, Nikitin AG, Smetanina SA, et al. The rs11705701 G > A polymorphism of IGF2BP2 is associated with IGF2BP2 mRNA and protein levels in the visceral adipose tissue—a link to type 2 diabetes susceptibility. Rev Diabet Stud. 2012;9(2-3):112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parikh H, Lyssenko V, Groop LC. Prioritizing genes for follow-up from genome wide association studies using information on gene expression in tissues relevant for type 2 diabetes mellitus. BMC Med Genomics. 2009;2:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bansal D, Bhansali A, Kapil G, Undela K, Tiwari P. Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2013;16(2):151-158, S1. [DOI] [PubMed] [Google Scholar]
- 83. Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001;1504(1):82-106. [DOI] [PubMed] [Google Scholar]
- 84. Livingstone C, Borai A. Insulin-like growth factor-II: its role in metabolic and endocrine disease. Clin Endocrinol. 2014;80(6):773-781. [DOI] [PubMed] [Google Scholar]
- 85. Cai H, Dong LQ, Liu F. Recent advances in adipose mTOR signaling and function: therapeutic prospects. Trends Pharmacol Sci. 2016;37(4):303-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dai N, Rapley J, Angel M, Yanik MF, Blower MD, Avruch J. mTOR phosphorylates IMP2 to promote IGF2 mRNA translation by internal ribosomal entry. Genes Dev. 2011;25(11):1159-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Maynard GD. A statistical study in cancer death-rates. Biometrika. 1910;7(3):276-304. [Google Scholar]
- 88. Ko C, Chaudhry S. The need for a multidisciplinary approach to cancer care. J Surg Res. 2002;105(1):53-57. [DOI] [PubMed] [Google Scholar]
- 89. Tsilidis KK, Allen NE, Appleby PN, et al. Diabetes mellitus and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Int J Cancer. 2015;136(2):372-381. [DOI] [PubMed] [Google Scholar]
- 90. Meyer TE, Boerwinkle E, Morrison AC, et al. Diabetes genes and prostate cancer in the Atherosclerosis Risk in Communities Study. Cancer Epidemiol Biomarkers Prev. 2010;19(2):558-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Schwartzbaum J, Edlinger M, Zigmont V, et al. Associations between prediagnostic blood glucose levels, diabetes, and glioma. Sci Rep. 2017;7(1):1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liu X, Chen Z, Zhao X, et al. Effects of IGF2BP2, KCNQ1 and GCKR polymorphisms on clinical outcome in metastatic gastric cancer treated with EOF regimen. Pharmacogenomics. 2015;16(9):959-970. [DOI] [PubMed] [Google Scholar]
- 93. Ye S, Song W, Xu X, Zhao X, Yang L. IGF2BP2 promotes colorectal cancer cell proliferation and survival through interfering with RAF-1 degradation by miR-195. FEBS Lett. 2016;590(11):1641-1650. [DOI] [PubMed] [Google Scholar]
- 94. Huang S, Wu Z, Cheng Y, Wei W, Hao L. Insulin-like growth factor 2 mRNA binding protein 2 promotes aerobic glycolysis and cell proliferation in pancreatic ductal adenocarcinoma via stabilizing GLUT1 mRNA. Acta Biochim Biophys Sin (Shanghai). 2019;51(7):743-752. [DOI] [PubMed] [Google Scholar]
- 95. Shen C, Xuan B, Yan T, et al. m6A-dependent glycolysis enhances colorectal cancer progression. Mol. Cancer. 2020;19(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang Y, Lu JH, Wu QN, et al. LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol Cancer. 2019;18(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jaiswal M, Schinske A, Pop-Busui R. Lipids and lipid management in diabetes. Best Pract Res Clin Endocrinol Metab. 2014;28(3):325-338. [DOI] [PubMed] [Google Scholar]
- 98. Jafri H, Alsheikh-Ali AA, Karas RH. Baseline and on-treatment high-density lipoprotein cholesterol and the risk of cancer in randomized controlled trials of lipid-altering therapy. J Am Coll Cardiol. 2010;55(25):2846-2854. [DOI] [PubMed] [Google Scholar]
- 99. Hayashi N, Matsushima M, Yamamoto T, Sasaki H, Takahashi H, Egawa S. The impact of hypertriglyceridemia on prostate cancer development in patients aged ≥ 60 years. BJU Int. 2012;109(4):515-519. [DOI] [PubMed] [Google Scholar]
- 100. Hursting SD, Digiovanni J, Dannenberg AJ, et al. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res. 2012;5(11):1260-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Parekh N, Lin Y, Hayes RB, Albu JB, Lu-Yao GL. Longitudinal associations of blood markers of insulin and glucose metabolism and cancer mortality in the third National Health and Nutrition Examination Survey. Cancer Causes Control. 2010;21(4):631-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Casellas A, Mallol C, Salavert A, et al. Insulin-like growth factor 2 overexpression induces β-cell dysfunction and increases beta-cell susceptibility to damage. J Biol Chem. 2015;290(27):16772-16785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dai N, Ji F, Wright J, Minichiello L, Sadreyev R, Avruch J. IGF2 mRNA binding protein-2 is a tumor promoter that drives cancer proliferation through its client mRNAs IGF2 and HMGA1. eLife. 2017;6:e27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chang CK, Ulrich CM. Hyperinsulinaemia and hyperglycaemia: possible risk factors of colorectal cancer among diabetic patients. Diabetologia. 2003;46(5):595-607. [DOI] [PubMed] [Google Scholar]
- 106. Dynkevich Y, Rother KI, Whitford I, et al. Tumors, IGF-2, and hypoglycemia: insights from the clinic, the laboratory, and the historical archive. Endocr Rev. 2013;34(6):798-826. [DOI] [PubMed] [Google Scholar]
- 107. Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309-324. [DOI] [PubMed] [Google Scholar]
- 108. Tybl E, Shi FD, Kessler SM, et al. Overexpression of the IGF2-mRNA binding protein p62 in transgenic mice induces a steatotic phenotype. J Hepatol. 2011;54(5):994-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Simon Y, Kessler SM, Gemperlein K, et al. Elevated free cholesterol in a p62 overexpression model of non-alcoholic steatohepatitis. World J Gastroenterol. 2014;20(47):17839-17850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Baeck C, Wehr A, Karlmark KR, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61(3):416-426. [DOI] [PubMed] [Google Scholar]
- 111. Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59(10):1303-1307. [DOI] [PubMed] [Google Scholar]
- 112. Laggai S, Kessler SM, Boettcher S, et al. The IGF2 mRNA binding protein p62/IGF2BP2-2 induces fatty acid elongation as a critical feature of steatosis. J Lipid Res. 2014;55(6):1087-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang S, Lei R, Wu J, et al. Role of high mobility group A1 and body mass index in the prognosis of patients with breast cancer. Oncol Lett. 2017;14(5):5719-5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chiefari E, Tanyolac S, Paonessa F, et al. Functional variants of the HMGA1 gene and type 2 diabetes mellitus. JAMA. 2011;305(9):903-912. [DOI] [PubMed] [Google Scholar]
- 115. Sumter TF, Xian L, Huso T, et al. The high mobility group A1 (HMGA1) transcriptome in cancer and development. Curr Mol Med. 2016;16(4):353-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cleynen I, Brants JR, Peeters K, et al. HMGA2 regulates transcription of the Imp2 gene via an intronic regulatory element in cooperation with nuclear factor-κB. Mol Cancer Res. 2007;5(4):363-372. [DOI] [PubMed] [Google Scholar]
- 117. Brants JR, Ayoubi TA, Chada K, Marchal K, Van de Ven WJ, Petit MM. Differential regulation of the insulin-like growth factor II mRNA-binding protein genes by architectural transcription factor HMGA2. FEBS Lett. 2004;569(1-3):277-283. [DOI] [PubMed] [Google Scholar]
- 118. Degrauwe N, Schlumpf TB, Janiszewska M, et al. The RNA binding protein IMP2 preserves glioblastoma stem cells by preventing let-7 target gene silencing. Cell Rep. 2016;15(8):1634-1647. [DOI] [PubMed] [Google Scholar]
- 119. Markowski DN, Thies HW, Gottlieb A, Wenk H, Wischnewsky M, Bullerdiek J. HMGA2 expression in white adipose tissue linking cellular senescence with diabetes. Genes Nutr. 2013;8(5):449-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol. 2011;23(6):744-755. [DOI] [PubMed] [Google Scholar]
- 121. Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Garg SK, Maurer H, Reed K, Selagamsetty R. Diabetes and cancer: two diseases with obesity as a common risk factor. Diabetes Obes Metab. 2014;16(2):97-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Li Y, Francia G, Zhang JY. p62/IMP2 stimulates cell migration and reduces cell adhesion in breast cancer. Oncotarget. 2015;6(32):32656-32668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lu M, Nakamura RM, Dent ED, et al. Aberrant expression of fetal RNA-binding protein p62 in liver cancer and liver cirrhosis. Am J Pathol. 2001;159(3):945-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sainz J, Rudolph A, Hoffmeister M, et al. Effect of type 2 diabetes predisposing genetic variants on colorectal cancer risk. J Clin Endocrinol Metab. 2012;97(5):E845-E851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”