Abstract
Context
Adrenocorticotropic hormone (ACTH) can contribute to aldosterone excess in primary aldosteronism (PA) via increased melanocortin type 2 receptor expression. Dynamic manipulation of the hypothalamic-pituitary-adrenal (HPA) axis could assist PA subtyping, but a direct comparison of dynamic tests is lacking.
Objective
To investigate plasma steroid differences between aldosterone-producing adenoma (APA) and bilateral PA (BPA) relative to ACTH variations.
Methods
We conducted comprehensive dynamic testing in 80 patients: 40 with APA and 40 with BPA. Peripheral plasma was collected from each patient at 6 time points: morning; midnight; after 1 mg dexamethasone suppression; and 15, 30, and 60 minutes after ACTH stimulation. We quantified 17 steroids by mass spectrometry in response to ACTH variations in all patients and compared their discriminative power between the 2 PA subtypes.
Results
Patients with APA had higher morning and midnight concentrations of 18-hydroxycortisol, 18-oxocortisol, aldosterone, and 18-hydroxycorticosterone than those with BPA (P < 0.001 for all). In response to cosyntropin stimulation, the APA group had larger increments of aldosterone, 18-oxocortisol, 11-deoxycorticosterone, corticosterone, and 11-deoxycortisol (P < 0.05 for all). Following dexamethasone suppression, the APA group had larger decrements of aldosterone, 18-hydroxycortisol, and 18-oxocortisol (P < 0.05 for all), but their concentrations remained higher than in the BPA group (P < 0.01 for all). The highest discriminatory performance between the PA subtypes was achieved using steroids measured 15 minutes post-ACTH stimulation (area under receiver operating characteristic curve 0.957).
Conclusion
Steroid differences between APA and BPA are enhanced by dynamic HPA testing; such noninvasive tests could circumvent the need for adrenal vein sampling in a subset of patients with PA.
Keywords: primary aldosteronism, ACTH, mass spectrometry, adrenal cortex, aldosterone, hypertension
Primary aldosteronism (PA) is characterized by renin-independent aldosterone excess, and it is the most common form of endocrine hypertension (1, 2). PA is largely dichotomized into 2 major subtypes: lateralized PA (LPA), often caused by aldosterone-producing adenomas (APAs), and bilateral PA (BPA) (3, 4). Compared with patients with BPA, patients with APAs often have higher circulating concentrations of aldosterone, and/or other steroid hormones (5-8). APAs with various underlying aldosterone-driver somatic mutations have characteristic histological (9, 10) and steroidogenic features (11). Steroid profiling of peripheral blood has been explored as a potential noninvasive tool for PA subtyping, but results have been somewhat variable (5-8).
Physiologic aldosterone synthesis is regulated primarily by the renin-angiotensin system and serum potassium, but it also responds acutely to adrenocorticotropic hormone (ACTH) (12). In contrast, in PA, aldosterone synthesis occurs independently of renin, but it might respond to various other stimulators (13-15). APAs overexpress a variety of G-protein coupled receptors, such as the melanocortin-2 receptor (MC2R), luteinizing hormone–human chorionic gonadotropin receptor, and gonadotropin-releasing hormone receptor (16, 17). While direct BPA tissue studies are limited, as most of these patients are treated medically, in vivo studies have shown that aldosterone responds to a variety of stimuli in patients with BPA as well (15). ACTH is known to influence aldosterone production in PA. Diurnal variations of plasma aldosterone concentration (PAC) have been reported to vary between APA and BPA (17, 18). The significance of the ACTH impact on aldosterone production in APA vs BPA has also been underlined by synthetic ACTH (cosyntropin) stimulation and dexamethasone suppression studies (19, 20). In a study of patients with PA who were subtyped based on adrenal vein sampling (AVS) with and without cosyntropin stimulation, we found that the discriminatory power of multi-steroid panels was superior in serum obtained after cosyntropin stimulation than in baseline samples (7). A direct comparison of steroid responses between APA and BPA across both intrinsic ACTH variations and extrinsic ACTH manipulation has been lacking. Such dynamic testing has the potential to amplify inherent differences in steroid fingerprints between APA and BPA and could circumvent the need for AVS in many patients with PA.
Methods
Study Participants
We included patients with confirmed PA seen between 2015 and 2018 at Tohoku University, Japan who underwent all of the following tests: a midnight blood test, a cosyntropin stimulation test (CST), and a 1 mg dexamethasone suppression test (DST). The diagnosis of PA was established by the following criteria: (1) PAC >12 ng/dL and plasma renin activity <1.0 ng/mL/h at screening; and (2) aldosterone-to-renin ratio >20 ng/dL per ng/mL/h both at screening and after 50 mg captopril challenge test (21). Cosyntropin-stimulated AVS was employed to sub-classify PA as LPA or BPA, as previously reported (22, 23). We excluded patients with unsuccessful AVS (selectivity index <5), those with a serum cortisol level >3.0 µg/dL after DST (24), and those from whom we did not have sufficient plasma from all the tests performed available for research. All clinical hormonal measurements were performed with commercially available immunoassays (23). Clinical data, including age, sex, body mass index (BMI), blood pressure (measured as previously described (25)), antihypertensive agents, serum potassium, potassium replacement, estimated glomerular filtration ratio, PAC, 24-hour urine aldosterone, plasma renin activity, and serum cortisol levels, were collected through review of medical records. This study was conducted with approval of the institutional review boards of Tohoku University (2020-1-529) and written informed consent was obtained from all participants.
Sample Collection
In order to assess hormonal changes associated with ACTH variations, we collected plasma samples at midnight, during CST, and after DST for each patient. All tests were conducted on separate days and samples were obtained after 30 minutes of supine rest. Both the CST and the DST were performed in the morning (7:00 am to 9:00 am) primarily in an outpatient setting, while the midnight blood was obtained during hospital admissions. During the CST, blood samples were collected at 4 time points: baseline, 15, 30, and 60 minutes after 250 mg cosyntropin injection. All samples were frozen at −80 °C immediately after collection and stored until steroid analysis by liquid chromatography–tandem mass spectrometry (LC-MS/MS).
Steroid Quantification by LC-MS/MS
We used LC-MS/MS to simultaneously quantify 17 steroid hormones, including: aldosterone, 18-hydroxycortisol (18OHF), 18-oxocortisol (18oxoF), 18-hydroxycorticosterone (18OHB), corticosterone, 11-deoxycorticosterone (DOC), cortisol, cortisone, 11-deoxycortisol (11dF), progesterone, 17α-hydroxyprogesterone, androstenedione, testosterone, 11β-hydro xytestosterone, 11-ketotestosterone, 11β-hydroxyan- drostenedione, and 11-ketoandrostenedione. Steroid extraction and quantification were performed from 100 µL plasma, as previously described (7, 26). An updated list of steroid sources is summarized in Supplement Table 1 (27).
Genetic Testing
Sanger sequencing and targeted next-generation sequencing were employed to detect somatic mutations in APAs as previously reported (28, 29). Briefly, the AllPrep DNA/RNA FFPE kit (QIAGEN) was used for extraction of genomic DNA from 10% formalin-fixed paraffin-embedded APA tissues. APAs with KCNJ5 mutations were identified by Sanger sequencing. The remaining APAs were analyzed by targeted next-generation sequencing, covering the full coding regions of known aldosterone-driver genes (KCNJ5, CACNA1D, CACNA1H, ATP1A1, ATP2B3, and CLCN2).
Statistical Analysis
Comparison of categorical and continuous variables between 2 independent groups were assessed by the Chi-squared or Fisher exact test, and the Mann-Whitney U test, respectively. The Wilcoxon signed-rank test was used for comparison of steroids between morning and midnight within the same patients. Repeated measurement 2-way ANOVA was employed to assess hormonal changes during the CST and DST. The first morning blood draw at the time of CST served as reference for both the CST and the DST. For the CST, ∆ steroids were calculated by subtracting the baseline concentrations from those obtained 60 minutes after cosyntropin injection. To assess the discriminative performance between APA and BPA of individual and multiple steroids, we plotted receiver operating characteristic (ROC) curves, and we computed the area under the ROC curve (AUC) for each condition. Penalized logistic regression was used to select variables (steroids, sex, age) that best distinguished unilateral PA from BPA. Because testosterone and progesterone vary highly with both sex and age, they were not included in logistic regression models. Statistical analyses were performed with StatFlex software (version 7.0; Artech Co, Ltd, Osaka, Japan), Prism (version 8.0; GraphPad Software, La Jolla, CA), or R (version 4.0.2). Two-tailed P values < 0.05 were considered statistically significant.
Results
Demographics and Clinical Characteristics of Study Participants
In total, 80 patients (40 men) with PA, with a median age of 51 years (range, 26-76 years), were included in this study. Following AVS, 40 patients were classified to have LPA and 40 BPA. All patients diagnosed with LPA underwent unilateral adrenalectomy, and an APA was confirmed in all resected adrenal glands based on hematoxylin and eosin staining, Weiss criteria, and CYP11B2 immunohistochemistry (30, 31). On average, the age, sex, and BMI values were similar between patients with APA and BPA (Table 1). As expected, patients with an APA had higher PAC and 24-hour urine aldosterone levels, and lower serum potassium levels than patients with BPA (Table 1). Of these, 10 (25%) APAs were undetectable by computed tomography.
Table 1.
Clinical and demographic characteristics of study participants
| APA | BPA | P values | |
|---|---|---|---|
| N | 40 | 40 | |
| Age (yrs) | 51 [43, 64] | 50 [40, 63] | 0.72 |
| Men (n, %) | 22 (55.0%) | 18 (45.0%) | 0.37 |
| BMI (kg/m2) | 24.3 [22.5, 26.6] | 24.1 [22.3, 26.2] | 0.81 |
| Systolic BP (mmHg) | 138 [126, 150] | 137 [126, 147] | 0.54 |
| Diastolic BP (mmHg) | 86 [76, 97] | 89 [82, 100] | 0.19 |
| Number of AHTs (n) | 2.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | 0.18 |
| Serum potassium (mM) | 3.9 [3.5, 4.2] | 4.1 [4.0, 4.3] | 0.001 |
| Potassium replacement (n, %) | 36 (90.0%) | 23 (57.5%) | 0.001 |
| eGFR (mL/min/1.73 m2) | 83 [68, 88] | 80 [73, 93] | 0.90 |
| PAC (ng/dL) | 39.2 [27.6, 58.8] | 24.3 [17.3, 28.5] | <0.0001 |
| PRA (ng/mL/h) | 0.30 [0.20, 0.30] | 0.30 [0.20, 0.45] | 0.80 |
| ARR (ng/dL per ng/mL/h) | 134.6 [82.0, 208.8] | 86.8 [48.6, 116.3] | 0.0006 |
| Urinary aldosterone (μg/day) | 26.3 [17.8, 33.8] | 13.0 [9.4, 14.8] | <0.0001 |
| Cortisol after DST (μg/dL) | 0.80 [0.65, 1.10] | 0.70 [0.55, 0.90] | 0.09 |
| Lateralization index | 9.3 [5.3, 21.8] | 1.3 [1.2, 1.9] | <0.0001 |
| Contralateral suppression (n, %) | 37 (92.5%) | NA | NA |
All variables are shown as median [interquartile range] or number (percentage). Differences between APA and BPA were assessed by the Mann-Whitney U test for continuous variables and Fisher or Chi-squared test for categorical variables.
Abbreviations: AHT, antihypertensive agents; APA, aldosterone-producing adenoma; ARR, aldosterone-to-renin ratio; AVS, adrenal venous sampling; BMI, body mass index; BP, blood pressure; BPA, bilateral primary aldosteronism; DST, 1 mg dexamethasone suppression test; eGFR, estimated glomerular filtration ratio; NA, not applicable; PAC, plasma aldosterone concentration; PRA, plasma renin activity.
Diurnal Variations of Steroid Hormones in PA Subtypes
On average, the APA group had higher peripheral plasma concentrations of 18OHF, 18oxoF, aldosterone, and 18OHB than the BPA group, both in the morning and at midnight (P < 0.001 for all, Table 2). Most steroids measured declined at midnight in both groups (Table 2); a notable exception was that aldosterone was significantly lower at midnight as compared to morning only in patients with an APA (0.27 [0.17, 0.45] vs 0.41 [0.30, 0.52] nmol/L, P = 0.01), but not in those with BPA (0.15 [0.08, 0.21] vs 0.17 [0.10, 0.25] nmol/L, P = 0.09).
Table 2.
Diurnal variations of peripheral plasma steroid concentrations in PA subtypes
| APA (N = 40) | BPA (N = 40) | P valueb | ||||||
|---|---|---|---|---|---|---|---|---|
| Steroid (nmol/L) | Morning | Midnight | P valuea | Morning | Midnight | P valuea | Morning | Midnight |
| 18OHF | 2.85 [2.02, 3.77] | 1.21 [0.64, 2.76] | <0.0001 | 1.55 [1.03, 2.29] | 0.52 [0.36, 0.85] | <0.0001 | <0.0001 | 0.0001 |
| 18oxoF | 0.18 [0.03, 0.33] | 0.12 [0.02, 0.28] | 0.01 | 0.02 [0.01, 0.03] | 0.00 [0.00, 0.02] | <0.0001 | <0.0001 | <0.0001 |
| Aldosterone | 0.41 [0.30, 0.52] | 0.27 [0.17, 0.45] | 0.01 | 0.17 [0.10, 0.25] | 0.15 [0.08, 0.21] | 0.09 | <0.0001 | <0.0001 |
| 18OHB | 1.18 [0.87, 1.89] | 0.71 [0.42, 1.05] | 0.0002 | 0.69 [0.38, 1.11] | 0.42 [0.22, 0.68] | <0.0001 | 0.0002 | 0.0009 |
| Corticosterone | 5.21 [3.38, 7.24] | 1.54 [0.93, 2.51] | <0.0001 | 4.22 [3.24, 7.07] | 1.11 [0.69, 1.92] | <0.0001 | 0.41 | 0.18 |
| DOC | 0.20 [0.15, 0.29] | 0.12 [0.09, 0.20] | <0.0001 | 0.15 [0.11, 0.23] | 0.11 [0.09, 0.17] | <0.0001 | 0.02 | 0.50 |
| Cortisol | 238 [186, 334] | 69 [47, 112] | <0.0001 | 241 [214, 304] | 57 [44, 113] | <0.0001 | 0.67 | 0.61 |
| Cortisone | 49 [43, 57] | 16 [11, 23] | <0.0001 | 52 [43, 58] | 16 [11, 25] | <0.0001 | 0.61 | 0.78 |
| 11dF | 0.08 [0.06, 0.12] | 0.04 [0.02, 0.06] | <0.0001 | 0.07 [0.04, 0.11] | 0.02 [0.02, 0.04] | <0.0001 | 0.19 | 0.02 |
| 17OHP4 | 0.18 [0.08, 0.28] | 0.17 [0.06, 0.28] | 0.33 | 0.21 [0.06, 0.31] | 0.15 [0.03, 0.27] | 0.003 | 0.85 | 0.56 |
| Progesterone | 0.23 [0.15, 0.30] | 0.18 [0.11, 0.28] | 0.11 | 0.21 [0.16, 0.27] | 0.17 [0.13, 0.24] | 0.0008 | 0.73 | 0.92 |
| Testosterone | 10.6 [0.74, 15.6] | 9.33 [0.51, 15.3] | 0.01 | 1.00 [0.56, 15.3] | 0.71 [0.52, 13.5] | 0.001 | 0.45 | 0.48 |
| A4 | 2.09 [1.40, 2.55] | 1.25 [0.99, 1.67] | <0.0001 | 2.14 [1.42, 3.22] | 1.38 [1.01, 2.05] | <0.0001 | 0.29 | 0.64 |
| 11OHT | 0.49 [0.36, 0.69] | 0.21 [0.16, 0.28] | <0.0001 | 0.47 [0.33, 0.68] | 0.20 [0.14, 0.23] | <0.0001 | 0.71 | 0.11 |
| 11KT | 0.89 [0.68, 1.33] | 0.46 [0.32, 0.59] | <0.0001 | 1.08 [0.73, 1.46] | 0.50 [0.38, 0.64] | <0.0001 | 0.46 | 0.25 |
| 11OHA4 | 4.65 [3.07, 10.8] | 1.64 [0.95, 3.42] | <0.0001 | 5.36 [3.42, 10.2] | 1.89 [1.18, 2.92] | <0.0001 | 0.53 | 0.65 |
| 11KA4 | 0.93 [0.61, 1.99] | 0.39 [0.27, 0.72] | <0.0001 | 1.25 [0.83, 2.02] | 0.45 [0.32, 0.52] | <0.0001 | 0.32 | 0.44 |
All variables are shown as median with interquartile ranges.
Abbreviations: 11dF, 11-deoxycortisol; 11KA4, 11-ketoandrostenedione; 11KT, 11-ketotestosterone; 11OHA4, 11ß-hydroxyandrostenedione; 11OHT, 11ß-hydroxytestosterone; 17OHP4, 17α-hydroxyprogesterone; 18OHB, 18-hydroxycorticosterone; 18OHF, 18-hydroxycortisol; 18oxoF, 18-oxocortisol; A4, androstenedione; APA, aldosterone-producing adenoma; BPA, bilateral primary aldosteronism; DOC, 11-deoxycorticosterone.
aComparison between morning and midnight values in each group using the Wilcoxon signed-rank test.
bComparison between APA and BPA using the Mann-Whitney U test.
Steroid Responses to ACTH Stimulation and Suppression in PA Subtypes
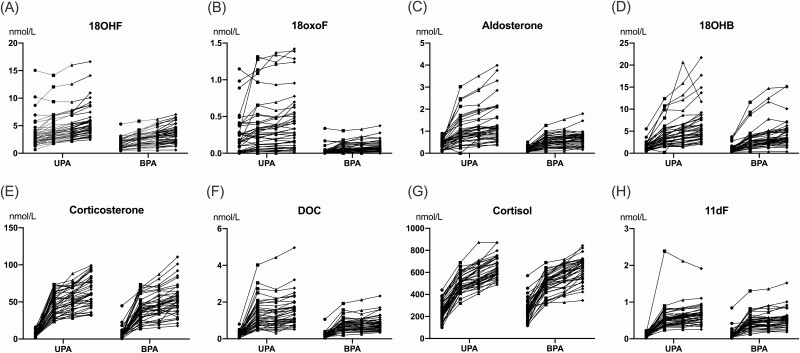
All steroids measured increased in response to the CST test. Seven steroids had a disproportionally higher increment in the APA group compared with the BPA group (Fig. 1A-1F): 18oxoF (∆, 0.11 [0.04, 0.25] vs 0.05 [0.02, 0.08] nmol/L; P = 0.04); 18OHF (∆, 1.92 [1.40, 2.43] vs 1.57 [0.99, 2.16] nmol/L; P = 0.06); aldosterone (∆, 0.68 [0.42, 1.02] vs 0.48 [0.28, 0.61] nmol/L; P = 0.001); 18OHB (∆, 4.01 [2.67, 6.02] vs 2.81 [2.02, 4.35] nmol/L; P = 0.02); corticosterone (∆, 57.3 [44.1, 74.9] vs 47.8 [34.5, 57.0] nmol/L; P = 0.001); DOC (∆, 1.11 [0.74, 1.63] vs 0.57 [0.41, 0.83] nmol/L; P < 0.0001); and 11dF (∆, 0.54 [0.43, 0.65] vs 0.39 [0.31, 0.54] nmol/L; P = 0.001, Fig. 1G-1H).
Figure 1.
Steroid responses to cosyntropin stimulation in PA subtypes. Changes of steroid concentrations following cosyntropin stimulation in patients with aldosterone-producing adenoma (APA) and bilateral primary aldosteronism (BPA), 40 per group. Filled circles, squares, triangles, and diamonds represent concentrations at baseline, 15, 30, and 60 minutes after 250 mg cosyntropin injection, respectively. Abbreviations: 11dF, 11-deoxycortisol; 18OHB, 18-hydroxycorticosterone; 18OHF, 18-hydroxycortisol; 18oxoF, 18-oxocortisol; DOC, 11-deoxycorticosterone.
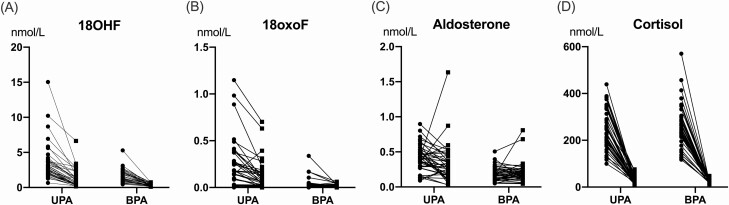
Following dexamethasone suppression, the APA group had larger decrements of: 18OHF (∆, −2.07 [−2.56, −1.43] vs −1.21 [−1.87, −0.84] nmol/L; P = 0.005), 18oxoF (∆, −0.08 [−0.21, −0.01] vs −0.01 [−0.03, 0.00] nmol/L; P = 0.0003), and aldosterone (∆, −0.13 [−0.26, 0.01] vs 0.01 [−0.03, 0.08] nmol/L; P = 0.02) than the BPA group (Fig. 2A-2C). Despite this, these steroids remained higher in the APA group than in the BPA group following the DST (18OHF, 0.88 [0.39, 1.58] vs 0.23 [0.16, 0.38] nmol/L, P < 0.0001; 18oxoF, 0.06 [0.02, 0.19] vs 0.00 [0.00, 0.02] nmol/L, P < 0.0001; aldosterone, 0.26 [0.15, 0.44] vs 0.16 [0.11, 0.23] nmol/L; P = 0.006). In contrast, cortisol levels were suppressed similarly in both groups (Fig. 2D), as were the remaining steroids.
Figure 2.
Steroid responses to dexamethasone suppression in PA subtypes. Steroid concentrations before (circles) and after (squares) overnight 1 mg dexamethasone suppression test in patients with aldosterone-producing adenoma (APA) and bilateral primary aldosteronism (BPA) subtype, 40 per group. Unsuppressed morning steroids served as reference. Abbreviations: 18OHF, 18-hydroxycortisol; 18oxoF, 18-oxocortisol.
Comparison of Steroid Profiles Between APAs With and Without KCNJ5 Mutations
Adrenal tissue blocks were available for mutation analysis in 39/40 APA cases, and of these, 27 (69%) had KCNJ5-mutated APAs. Of the remaining cases, 10 had APAs with known aldosterone-driver mutations, including: 6 CACNA1D, 1 ATP1A1, 2 ATP2B3, and 1 CTNNB1 mutations, and no known mutations were detected in 2 cases. Patients with KCNJ5-mutated APAs were more often women (59% vs 17%, P = 0.01), and were leaner (23.6 [21.6, 25.6] vs 26.5 [24.9, 28.6] kg/m2, P = 0.008) than patients with other APAs (Supplemental Table 2) (27). Patients with APAs harboring KCNJ5 mutations had higher plasma concentrations of 18oxoF in all examined conditions (P < 0.001 for all, Table 3). Compared with other APAs, the KCNJ5 group also had higher plasma concentrations of 18OHF in the morning, at midnight, and after DST; higher corticosterone at midnight and after both CST and DST; higher DOC at midnight, while aldosterone was higher only after DST (Table 3).
Table 3.
Hormonal differences between patients with KCNJ5-mutatated vs other APAs
| KCNJ5 (N = 27) | No KCNJ5(N = 12) | |||||||
|---|---|---|---|---|---|---|---|---|
| Steroid (nmol/L) | Morning | Midnight | CST | DST | Morning | Midnight | CST | DST |
| 18OHF | 3.27 [2.24-4.59] | 1.83 [1.15-3.55] | 5.19 [3.82-8.42] | 1.15 [0.51-2.24] | 2.15 [1.87-2.63]a | 0.57 [0.46-0.85]c | 4.24 [3.60-5.32] | 0.37 [0.31, 0.50]c |
| 18oxoF | 0.26 [0.15-0.39] | 0.17 [0.10-0.33] | 0.45 [0.26-0.69] | 0.10 [0.05-0.18] | 0.02 [0.02-0.09]d | 0.01 [0.00-0.02]d | 0.07 [0.05-0.19]d | 0.02 [0.01, 0.03]d |
| Aldo | 0.41 [0.29-0.51] | 0.27 [0.17-0.49] | 1.13 [0.79-1.65] | 0.20 [0.11-0.36] | 0.45 [0.33-0.56] | 0.30 [0.16-0.39] | 1.03 [0.65-1.21] | 0.40 [0.26, 0.50]a |
| 18OHB | 1.63 [0.86-2.01] | 0.81 [0.44-1.28] | 5.65 [3.82-8.09] | 0.43 [0.37-0.86] | 1.08 [0.87-1.46] | 0.59 [0.34-0.71] | 4.50 [3.41-5.90] | 0.62 [0.34, 0.80] |
| B | 6.05 [3.53-7.95] | 1.91 [1.27-2.65] | 70.2 [52.6-84.3] | 0.79 [0.64-1.03] | 4.13 [2.93-5.88] | 0.87 [0.75-1.59]a | 52.2 [38.2-60.0]b | 0.47 [0.37, 0.61]c |
| DOC | 0.21 [0.17-0.35] | 0.15 [0.11-0.24] | 1.50 [1.02-2.00] | 0.11 [0.08-0.16] | 0.15 [0.14-0.23] | 0.10 [0.05-0.11]a | 1.00 [0.73-1.40] | 0.09 [0.03, 0.11] |
| Cortisol | 251 [191-335] | 76 [50-123] | 639 [583-728] | 29 [20-43] | 227 [171-330] | 54 [42-78] | 592 [547-684] | 24 [20, 27] |
| E | 50 [42-59] | 17 [12-25] | 60 [52-69] | 6 [5-8] | 51 [44-56] | 12 [9-20] | 53 [46-64] | 5 [4, 6] |
| 11dF | 0.08 [0.07-0.12] | 0.04 [0.03-0.07] | 0.66 [0.53-0.80] | 0.02 [0.02-0.27] | 0.09 [0.06-0.13] | 0.03 [0.02-0.05] | 0.61 [0.52-0.72] | 0.01 [0.01, 0.02] |
| 17OHP4 | 0.19 [0.05-0.28] | 0.18 [0.04-0.32] | 0.67 [0.46-0.92] | 0.07 [0.02-0.27] | 0.18 [0.13-0.29] | 0.17 [0.09-0.27] | 0.73 [0.57-1.04] | 0.18 [0.12, 0.25] |
| Prog | 0.24 [0.19-0.32] | 0.22 [0.14-0.39] | 1.19 [0.84-2.39] | 0.20 [0.13-0.22] | 0.16 [0.11-0.25] | 0.15 [0.10-0.20] | 1.00 [0.79-1.71] | 0.17 [0.08, 0.21] |
| T | 1.07 [0.57-14.5] | 1.14 [0.41-14.5] | 1.49 [0.79-14.4] | 0.89 [0.33-16.0] | 12.7 [10.2-16.7] | 12.1 [8.8-16.4] | 13.0 [10.1-15.6] | 15.5 [9.8, 19.7] |
| A4 | 2.11 [1.31-2.72] | 1.36 [0.98-1.93] | 4.17 [3.46-6.10] | 1.16 [0.76-1.69] | 1.75 [1.56-2.24] | 1.16 [0.98-1.23] | 3.68 [3.27-4.39] | 0.99 [0.79, 1.12] |
| 11OHT | 0.49 [0.35-0.75] | 0.21 [0.15-0.28] | 0.58 [0.42-0.73] | 0.13 [0.09-0.16] | 0.49 [0.34-0.62] | 0.25 [0.18-0.34] | 0.62 [0.51-0.71] | 0.13 [0.12, 0.18] |
| 11KT | 0.92 [0.69-1.43] | 0.49 [0.31-0.67] | 0.80 [0.70-1.29] | 0.20 [0.17-0.26] | 0.88 [0.66-1.33] | 0.44 [0.39-0.52] | 0.69 [0.58-1.06] | 0.21 [0.17, 0.27] |
| 11OHA4 | 4.71 [3.18-12.3] | 2.06 [1.05-4.10] | 15.1 [9.1-22.0] | 0.76 [0.49-1.23] | 4.87 [2.45-9.92] | 1.07 [0.82-2.87] | 11.3 [8.3-14.4] | 0.56 [0.35, 0.78] |
| 11KA4 | 0.94 [0.77-2.30] | 0.45 [0.29-0.73] | 1.69 [1.05-2.51] | 0.19 [0.15-0.25] | 0.98 [0.44-1.68] | 0.31 [0.25-0.56] | 1.56 [0.91-1.82] | 0.19 [0.14, 0.26] |
All variables are shown as median with interquartile ranges. Among 40 APA cases, 1 case had no available adrenal tissue for research. The “No KCNJ5” group includes: 6 CACNA1D-mutated, 1 ATP1A1-mutated, 2 ATP2B3-mutated, and 1 CTNNB1-mutated APA cases and 2 APAs with no known mutations.
Abbreviations: 11dF, 11-deoxycortisol; 11KA4, 11-ketoandrostenedione; 11KT, 11-ketotestosterone; 11OHA4, 11ß-hydroxyandrostenedione; 11OHT, 11ß-hydroxytestosterone; 17OHP4, 17α-hydroxyprogesterone; 18OHB, 18-hydroxycorticosterone; 18OHF, 18-hydroxycortisol; 18oxoF, 18-oxocortisol; A4, androstenedione; Aldo, aldosterone; APA, aldosterone-producing adenoma; B, corticosterone; CST, cosyntropin stimulation test; DOC, 11-deoxycorticosterone; DST, 1 mg dexamethasone suppression test; E, cortisone; Prog, progesterone; T, testosterone.
The Mann-Whitney U test was used to compare steroid concentrations in the same condition between the 2 groups: compared with the KCNJ5 group;
a P < 0.05;
b P < 0.01;
c P < 0.005;
d P < 0.001.
Discrimination of PA Subtype Using Baseline and Dynamic Peripheral Plasma Steroid Profiling
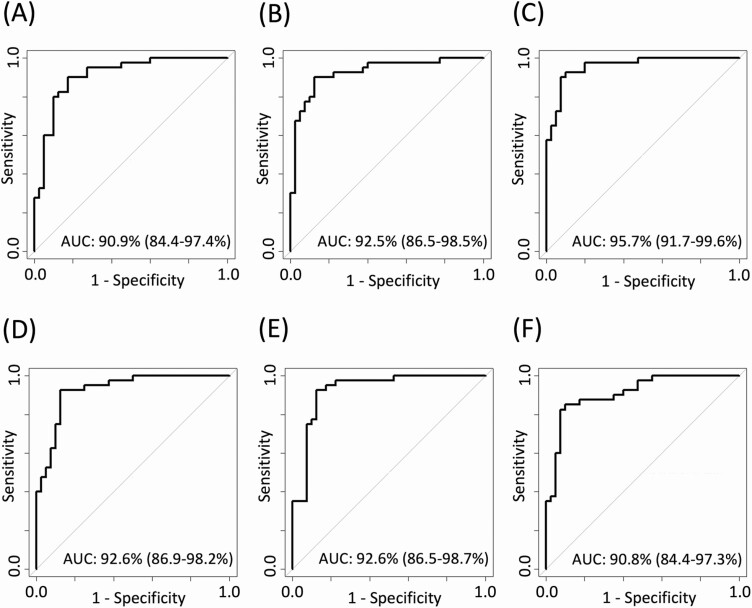
The distinction between APA and BPA, based on a single steroid, was highest with baseline serum aldosterone (AUC 0.869), followed by 18OHF and 18oxoF measured after DST (0.834 and 0.832, respectively, Supplemental Figure) (27). Discriminant analysis based on multiple steroids, along with sex and age, was highest when using measurements at 15 minutes after cosyntropin stimulation (AUC 0.957), followed closely by steroids measured at 30 and 60 minutes post-cosyntropin stimulation (AUC 0.926) and at midnight (0.925, Fig. 3).
Figure 3.
Optimized ROC curves for distinguishing PA subtypes in each studied state. The highest performing ROC curves were determined using penalized logistic regression using multiple steroids, age, and sex in each studied condition. Selected variables for each state were: morning (A): 18oxoF, aldosterone, A4, and 11KT; midnight (B): 18OHF, 18oxoF, A4, 11OHT, 11KT, 11OHA4, and sex; 15, 30, and 60 minutes during the CST (C, D, and E, respectively): 18oxoF, aldosterone, 18OHB, corticosterone, DOC, cortisone, 17OHP4, A4,11OHT age, and sex for 15 minutes; 18oxoF, aldosterone, corticosterone, DOC, A4, 11KT, and sex, for 30 minutes; 18OHF, 18oxoF, aldosterone, corticosterone, DOC, 11KT, and sex, for 60 minutes; and DST (F): 18OHF, 18oxoF, aldosterone, 11dF, 17OHP4, 11KT, and sex. Abbreviations: 11dF, 11-deoxycortisol; 11KT, 11-ketotestosterone; 11OHA4, 11ß-hydroxyandrostenedione; 11OHT, 11ß-hydroxytestosterone; 17OHP4, 17α-hydroxyprogesterone; 18OHB, 11-hydroxycorticosterone; 18OHF, 18-hydroxycortisol; 18oxoF, 18-oxocortisol; A4, androstenedione; AUC, area under the curve; CST, cosyntropin stimulation test; DOC, 11-deoxycorticosterone; DST, dexamethasone suppression test; ROC, receiver operating characteristic.
Discussion
The current standard of care for PA subtyping is AVS, which has numerous limitations, including its invasive nature, poor standardization across centers, and scarce availability. Efforts to develop alternative, widely accessible, and noninvasive PA subtyping tests have included steroid profiling of peripheral blood. This study is the first to directly compare the performance of steroid profiling across comprehensive dynamic testing, including intrinsic diurnal variations and pharmacological hypothalamic-pituitary-adrenal axis manipulation in patients with APA and BPA.
ACTH acutely stimulates aldosterone production from the normal zona glomerulosa, which expresses MC2R (12). Transcriptome analyses have shown that the expression of MC2R is upregulated in both APAs and aldosterone-producing micronodules compared with the normal zona glomerulosa (32, 33). Thus, APA steroid synthesis is tightly linked to ACTH variations. The impact of ACTH on APA steroid synthesis, however, differs between APAs with distinct aldosterone-driver mutations (34), which have variable MC2R (14) and steroidogenic enzyme expression (10, 35, 36). Overall, we found that mineralocorticoids and glucocorticoids responded to both cosyntropin stimulation and dexamethasone suppression in all PA patients, but the excursions of aldosterone and hybrid steroids were larger in patients with APAs than in those with BPA.
Not surprisingly, we found that 18oxoF was higher in patients with APAs harboring KCNJ5 mutations than in other APAs, and this difference was maintained both after cosyntropin stimulation and dexamethasone suppression. Dynamic differences between the 2 groups were also observed for 18OHF, corticosterone, and DOC. Elevations of 18oxoF and 18OHF have been previously reported in baseline plasma of patients with KCNJ5-mutated APAs as compared with other APAs (8, 11). In this study, manipulation of the hypothalamic-pituitary-adrenal axis revealed other hormonal differences between APAs with or without KCNJ5 mutations. These findings align with the zona fasciculata–like morphology of APAs with KCNJ5 mutations, along with expression of CYP17A1 and CYP11B1, characteristic of zona fasciculata (9, 25, 36). Notably, aldosterone was suppressed more dramatically by dexamethasone in patients with KCNJ5-mutated APAs than in those with other APAs, while baseline and cosyntropin-stimulated aldosterone concentrations were similar between the 2 groups. In previous studies, we found that MC2R and CYP11B2 transcripts were lower in APAs with KCNJ5 vs other mutations (14). Moreover, we found that although the absolute cosyntropin-stimulated aldosterone levels in both the draining adrenal vein as well as in periphery were similar between APAs with various aldosterone-driver somatic mutations, the relative aldosterone response to cosyntropin, as reflected by the lateralization index, was lower for KCNJ5-mutated APAs vs other APAs (34).
Considering the high prevalence of KCNJ5 mutations among Japanese PA patients, peripheral concentrations of 18oxoF and 18OHF have been previously found to have higher discriminatory power between APA and BPA in Japanese (6) vs European populations (5), which display a more diverse array of APA somatic mutations (10). In European patients with PA, multiplex steroid profiling was found to be superior to 18oxoF alone for distinguishing patients with APA vs BPA (5). Nevertheless, owing to the unique characteristics of KCNJ5-mutated APAs, baseline peripheral circulation steroid profiling achieved the highest performance in distinguishing patients with APAs from those with primary hypertension, when patients were stratified by the KCNJ5 mutation status (8).
Coupling multiplex steroid assays by mass spectrometry with dynamic hypothalamic-pituitary-adrenal axis testing facilitates noninvasive PA subtyping by augmenting inherent differences in steroidogenesis between APAs and BPA. Studies from China and Japan have found that cosyntropin-stimulated peripheral plasma aldosterone levels measured after a priori dexamethasone suppression were better than baseline aldosterone in distinguishing APA from BPA (20, 37). We found that the highest discriminatory performance between APA and BPA was achieved by multi-steroid panels measured after rapid cosyntropin stimulation. In a previous study of US patients with PA who underwent AVS both prior to and following cosyntropin stimulation, we also found that peripheral steroid fingerprints performed better in distinguishing PA cases with consistent lateralization from those with BPA when measured in cosyntropin-stimulated than in baseline blood collections (7).
The main limitation of our study is that the PA subtypes were determined solely based on the results of cosyntropin-stimulated AVS. Although all of the LPA cases adjudicated by AVS were confirmed to harbor APAs by CYP11B2 expression, we cannot exclude asymmetrical BPA that could have lateralized only in the absence of cosyntropin. Depending on the lateralization indices used, up to 20% of PA cases might display aldosterone lateralization only at baseline, but not after cosyntropin stimulation (34, 38). Nevertheless, existing data suggest that cases that lateralize only at baseline are less severe that those with consistent lateralization and correlate less frequently with cross-sectional imaging findings (34, 39), suggesting a phenotype that resembles that of asymmetrical BPA (7, 40). Considering these caveats, the diagnostic accuracy of the steroid panels derived from our cohort might not achieve the same performance in centers with different AVS protocols. In addition, our biomarker panels are likely to require de novo validation among other populations, particularly non-Asian patients, who often harbor distinct aldosterone-driver mutation distributions (10, 29, 41).
In summary, steroid responses to ACTH variations, particularly those of high magnitude, induced pharmacologically, differ between PA subtypes. Dynamic steroid profiling holds promise for PA subtype differentiation, which could provide a shortcut to medical therapy in patients with BPA.
Acknowledgments
We thank Kumi Kikuchi and Yasuko Tsukada for assistance with sample and clinical data registries; and Patrick O’Day for assistance with mass spectrometry assays.
Glossary
Abbreviations
- 11dF
11-deoxycortisol
- 18OHB
18-hydroxycorticosterone
- 18OHF
18-hydroxycortisol
- 18oxoF
18-oxocortisol
- ACTH
adrenocorticotropic hormone
- APA
aldosterone-producing adenoma
- AUC
area under the curve
- AVS
adrenal vein sampling
- BMI
body mass index
- BPA
bilateral primary aldosteronism
- CST
cosyntropin stimulation test
- DOC
11-deoxycorticosterone
- DST
dexamethasone suppression test
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- LPA
lateralized primary aldosteronism
- MC2R
melanocortin-2 receptor
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
- ROC
receiver operating characteristic
Funding
F.S. was supported by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) (JP18K08500) and Health Labor Sciences Research (H29-Nanji-Ippan-046). A.F.T. was supported by grants: 1K08DK109116 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and 2019087 from the Doris Duke Charitable Foundation.
Additional Information
Disclosures: A.F.T. received consulting fees from CinCor Pharma. A.F.T. is a scientific advisor for the Primary Aldosteronism Foundation. All other authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Byrd JB, Turcu AF, Auchus RJ. Primary aldosteronism: practical approach to diagnosis and management. Circulation. 2018;138(8):823-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018;39(6):1057-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Omata K, Satoh F, Morimoto R, et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. 2018;72(4):874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams TA, Gomez-Sanchez CE, Rainey WE, et al. International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2021;106(1):42-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eisenhofer G, Dekkers T, Peitzsch M, et al. Mass spectrometry-based adrenal and peripheral venous steroid profiling for subtyping primary aldosteronism. Clin Chem. 2016;62(3):514-524. [DOI] [PubMed] [Google Scholar]
- 6. Satoh F, Morimoto R, Ono Y, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65(5):1096-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turcu AF, Wannachalee T, Tsodikov A, et al. Comprehensive analysis of steroid biomarkers for guiding primary aldosteronism subtyping. Hypertension. 2020;75(1):183-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eisenhofer G, Durán C, Cannistraci CV, et al. Use of steroid profiling combined with machine learning for identification and subtype classification in primary aldosteronism. JAMA Netw Open. 2020;3(9):e2016209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ono Y, Yamazaki Y, Omata K, et al. Histological characterization of aldosterone-producing adrenocortical adenomas with different somatic mutations. J Clin Endocrinol Metab. 2020;105(3):e282-e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Sousa K, Boulkroun S, Baron S, et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension. 2020;75(4):1034-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams TA, Peitzsch M, Dietz AS, et al. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension. 2016;67(1):139-145. [DOI] [PubMed] [Google Scholar]
- 12. Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2012;350(2):151-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. St-Jean M, Ghorayeb NE, Bourdeau I, Lacroix A. Aberrant G-protein coupled hormone receptor in adrenal diseases. Best Pract Res Clin Endocrinol Metab. 2018;32(2):165-187. [DOI] [PubMed] [Google Scholar]
- 14. Lim JS, Plaska SW, Rege J, Rainey WE, Turcu AF. Aldosterone regulating receptors and aldosterone-driver somatic mutations. Front Endocrinol (Lausanne). 2021;12:644382. [DOI] [PMC free article] [PubMed]
- 15. St-Jean M, Bourdeau I, Martin M, Lacroix A. Aldosterone is aberrantly regulated by various stimuli in a high proportion of patients with primary aldosteronism. J Clin Endocrinol Metab. 2021;106(1):e45-e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kishimoto R, Oki K, Yoneda M, et al. Gonadotropin-releasing hormone stimulate aldosterone production in a subset of aldosterone-producing adenoma. Medicine (Baltimore). 2016;95(20):e3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zwermann O, Suttmann Y, Bidlingmaier M, Beuschlein F, Reincke M. Screening for membrane hormone receptor expression in primary aldosteronism. Eur J Endocrinol. 2009;160(3):443-451. [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi H, Haketa A, Ueno T, et al. Subtype prediction in primary aldosteronism: measurement of circadian variation of adrenocortical hormones and 24-h urinary aldosterone. Clin Endocrinol (Oxf). 2016;84(6):814-821. [DOI] [PubMed] [Google Scholar]
- 19. Sonoyama T, Sone M, Tamura N, et al. Role of endogenous ACTH on circadian aldosterone rhythm in patients with primary aldosteronism. Endocr Connect. 2014;3(4):173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sonoyama T, Sone M, Miyashita K, et al. Significance of adrenocorticotropin stimulation test in the diagnosis of an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2011;96(9):2771-2778. [DOI] [PubMed] [Google Scholar]
- 21. Nishikawa T, Omura M, Satoh F, et al. ; Task Force Committee on Primary Aldosteronism, The Japan Endocrine Society . Guidelines for the diagnosis and treatment of primary aldosteronism–the Japan Endocrine Society 2009. Endocr J. 2011;58(9):711-721. [DOI] [PubMed] [Google Scholar]
- 22. Satoh F, Abe T, Tanemoto M, et al. Localization of aldosterone-producing adrenocortical adenomas: significance of adrenal venous sampling. Hypertens Res. 2007;30(11):1083-1095. [DOI] [PubMed] [Google Scholar]
- 23. Satoh F, Morimoto R, Seiji K, et al. Is there a role for segmental adrenal venous sampling and adrenal sparing surgery in patients with primary aldosteronism? Eur J Endocrinol. 2015;173(4):465-477. [DOI] [PubMed] [Google Scholar]
- 24. Reincke M. Subclinical Cushing’s syndrome. Endocrinol Metab Clin North Am. 2000;29(1):43-56. [DOI] [PubMed] [Google Scholar]
- 25. Tezuka Y, Yamazaki Y, Kitada M, et al. 18-oxocortisol synthesis in aldosterone-producing adrenocortical adenoma and significance of KCNJ5 mutation status. Hypertension. 2019;73(6):1283-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davio A, Woolcock H, Nanba AT, et al. Sex differences in 11-oxygenated androgen patterns across adulthood. J Clin Endocrinol Metab. 2020;105(8):e2921-e2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tezuka Y, Turcu AF.. Supplemental data for: ACTH stimulation maximizes the accuracy of peripheral steroid profiling in primary aldosteronism subtyping. zenodo.org. Deposited May 24, 2021. doi: 10.5281/zenodo.4784014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nanba K, Yamazaki Y, Bick N, et al. Prevalence of somatic mutations in aldosterone-producing adenomas in Japanese patients. J Clin Endocrinol Metab. 2020;105(11):e4066-e4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nanba K, Omata K, Else T, et al. Targeted molecular characterization of aldosterone-producing adenomas in white Americans. J Clin Endocrinol Metab. 2018;103(10):3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weiss LM. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8(3):163-169. [DOI] [PubMed] [Google Scholar]
- 31. Yamazaki Y, Omata K, Tezuka Y, et al. Tumor cell subtypes based on the intracellular hormonal activity in KCNJ5-mutated aldosterone-producing adenoma. Hypertension. 2018;72(3):632-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murakami M, Yoshimoto T, Nakabayashi K, et al. Integration of transcriptome and methylome analysis of aldosterone-producing adenomas. Eur J Endocrinol. 2015;173(2):185-195. [DOI] [PubMed] [Google Scholar]
- 33. Nishimoto K, Tomlins SA, Kuick R, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. 2015;112(33):E4591-E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wannachalee T, Zhao L, Nanba K, et al. Three discrete patterns of primary aldosteronism lateralization in response to cosyntropin during adrenal vein sampling. J Clin Endocrinol Metab. 2019;104(12):5867-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Inoue K, Yamazaki Y, Kitamoto T, et al. Aldosterone suppression by dexamethasone in patients with KCNJ5-mutated aldosterone-producing adenoma. J Clin Endocrinol Metab. 2018;103(9):3477-3485. [DOI] [PubMed] [Google Scholar]
- 36. Monticone S, Castellano I, Versace K, et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. 2015;411:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang Y, Zhang C, Wang W, et al. Diagnostic value of ACTH stimulation test in determining the subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2015;100(5):1837-1844. [DOI] [PubMed] [Google Scholar]
- 38. El Ghorayeb N, Mazzuco TL, Bourdeau I, et al. Basal and post-ACTH aldosterone and its ratios are useful during adrenal vein sampling in primary aldosteronism. J Clin Endocrinol Metab. 2016;101(4):1826-1835. [DOI] [PubMed] [Google Scholar]
- 39. Wannachalee T, Caoili E, Nanba K, et al. The concordance between imaging and adrenal vein sampling varies with aldosterone-driver somatic mutation. J Clin Endocrinol Metab. 2020;105(10):e3628-e3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Y, Burrello J, Burrello A, et al. Classification of microadenomas in patients with primary aldosteronism by steroid profiling. J Steroid Biochem Mol Biol. 2019;189:274-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nanba K, Omata K, Gomez-Sanchez CE, et al. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension. 2019;73(4):885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.