Abstract
Context
Fruit, but not fruit juice, intake is inversely associated with type 2 diabetes mellitus (T2DM). However, questions remain about the mechanisms by which fruits may confer protection.
Objective
The aims of this work were to examine associations between intake of fruit types and 1) measures of glucose tolerance and insulin sensitivity and 2) diabetes at follow-up.
Methods
Among participants of the Australian Diabetes, Obesity and Lifestyle Study, fruit and fruit juice intake was assessed by food frequency questionnaire at baseline. Associations between fruit and fruit juice intake and 1) fasting plasma glucose, 2-hour postload plasma glucose, updated homeostasis model assessment of insulin resistance of β-cell function (HOMA2-%β), HOMA2 of insulin sensitivity (HOMA2-%S), and fasting insulin levels at baseline and 2) the presence of diabetes at follow-up (5 and 12 years) were assessed using restricted cubic splines in logistic and linear regression models.
Results
This population of 7675 Australians (45% males) had a mean ± SD age of 54 ± 12 years at baseline. Total fruit intake was inversely associated with serum insulin and HOMA2-%β, and positively associated with HOMA2-%S at baseline. Compared to participants with the lowest intakes (quartile 1), participants with moderate total fruit intakes (quartile 3) had 36% lower odds of having diabetes at 5 years (odds ratio, 0.64; 95% CI, 0.44-0.92), after adjusting for dietary and lifestyle confounders. Associations with 12-year outcomes were not statistically significant.
Conclusion
A healthy diet including whole fruits, but not fruit juice, may play a role in mitigating T2DM risk.
Keywords: fasting plasma glucose, 2-hour postload plasma glucose, HOMA2 of β-cell function (HOMA2-%β), HOMA2 of insulin sensitivity (HOMA2-%S), fasting insulin levels
Type 2 diabetes mellitus (T2DM) is characterized by impaired insulin secretion (β-cell dysfunction) and increased insulin resistance (or resistance to insulin mediated glucose uptake). It accounts for more than 2 million deaths annually (1) and is the seventh leading cause of disability worldwide (2). An estimated 451 million people worldwide have diabetes, with numbers postulated to exceed 693 million in 2045 (3). Given its global prevalence, there is an urgent need for evidence-based strategies targeting T2DM prevention.
Overwhelming evidence supports the promotion of a healthy diet and regular physical activity for mitigating the risk of T2DM (4). In particular, an inverse association between fruit intake and T2DM incidence has been reported in a pooled analysis of 3 large observational studies (5). Further, adherence to Australian Dietary Guidelines recommendations for fruit consumption (2 servings [150 g] per day for adults) was associated with a 32% lower risk of T2DM over 12 years in the Australian Diabetes, Obesity and Lifestyle Study (6). The authors report that adherence to these recommendations could have prevented 23% of T2DM cases (population attributable fraction: 23.3 [7.3-38.2]). However, it is likely that not all fruits offer equal protection against diabetes as heterogeneity in the associations between individual fruit consumption and risk of T2DM has been reported (5). Specifically, in 3 prospective cohorts of American men and women, a higher consumption of blueberries, grapes, apples, bananas, and grapefruit were individually associated with a significantly lower risk of T2DM (5). Interestingly, variances in glycemic index and glycemic load did not explain the differential association of specific fruits with risk of T2DM.
Insulin resistance and β-cell dysfunction play a critical role in the development of T2DM (7); however, relationships between fruit intake and measures of insulin resistance and β-cell dysfunction are not yet understood. The investigation of such relationships may provide valuable insight into the mechanisms by which higher fruit intake may lower the risk of T2DM. Therefore, the aims of this study were to examine associations between intake of total fruit, individual fruits commonly consumed by the study cohort, and fruit juice and (i) measures of insulin resistance and β-cell dysfunction and (ii) incident diabetes at 5 and 12 years’ follow-up, in a cohort of Australian men and women.
Materials and Methods
Study Population
Participants included in this study were men and women aged 25 years or older, recruited to the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) between 1999 and 2000. The methods and response rates of the AusDiab cohort have been described previously (8). In brief, AusDiab is a national population-based survey of diabetes mellitus prevalence and associated risk factors in Australian adults, recruited from the 7 states and territories of Australia in 1999 to 2000 (n = 11 247), with follow-up in 2004 to 2005 (n = 6400) and 2011 to 2012 (n = 4614). From the 11 247 participants who attended the biomedical examination at baseline, we excluded participants who did not complete a food frequency questionnaire (FFQ) at baseline (n = 204), had improbable energy intake (< 2500 kJ/day or > 14 500 kJ/day for women and < 3300 kJ/day or > 17 500 kJ/day for men [9, 10]; n = 342), were pregnant (n = 45), had diabetes at baseline (n = 968), had missing data for important covariates (n = 642), and who had missing outcome data at baseline (n = 1371). This left 7675 participants remaining for analyses at baseline. Of these, follow-up data were available for 4674 participants at 5 years and 3518 participants at 12 years (Fig. 1).
Figure 1.
CONSORT flow diagram. FFQ, food frequency questionnaire.
The study was approved by the human research ethics committees of the International Diabetes Institute, and the Alfred Hospital (Melbourne, Australia).
Exposures
The exposure of interest for this study was intake of total fruit, individual fruits commonly consumed by cohort participants, and fruit juice. Habitual dietary intake of participants at baseline was assessed using a semiquantitative FFQ developed by the Cancer Council of Victoria (11-13). Participants were asked to indicate their usual frequency of intake of food items, over the previous 12 months, using a list of 74 food items with 10 frequency response options ranging from “never” to “three or more times per day.” Food items included fruit juice (unspecific) and 10 different types of fruit (Supplementary Fig. 1 [14]). Additional questions regarding frequency of intake were used to adjust these results, which often overestimate intakes. Portion size was calculated using photographs of scaled portions of different food types. Nutrient intake calculations were analyzed by Cancer Council Victoria using the NUTTAB95 food nutrient database and were supplemented by other data where necessary. To reduce the chance of a type 1 error, only fruits whose intake contributed more than 10% to total fruit intake were investigated discretely.
Study Outcomes
Primary outcomes included measures of fasting plasma glucose (FPG), 2-hour postload plasma glucose (PLG), updated homeostasis model assessment of β-cell function (HOMA2-%β), HOMA2 of insulin sensitivity (HOMA2-%S), and fasting insulin levels obtained at baseline. FPG and PLG were determined using a spectrophotometric-hexokinase method and serum insulin was measured using an automated chemiluminescence immunoassay. The HOMA2 computer model was used to estimate insulin sensitivity (HOMA2-%S) and β-cell function (HOMA2-%B) from fasting insulin and glucose concentrations; this method has been used extensively in epidemiological studies (15). The secondary outcome was incident T2DM at follow-up (5 and 12 years). T2DM was classified as FPG 7.0 mmol/L or greater, 2-hour PLG 11.1 mmol/L or greater, or current treatment with insulin or oral hypoglycemic agents (16).
Covariates
Baseline demographic data, including age, sex (male/female), education level (never to some high school/completed university or equivalent), physical activity (sedentary = 0 min/week; insufficient < 150 min/week; and sufficient ≥ 150 min/week), smoking status (current/former/never), income, and parental history of diabetes (yes/no) were collected using interviewer-administered questionnaires, as described previously (17). The Socio-Economic Indexes for Areas (SEIFA) as reported by the Australian Bureau of Statistics (18) was obtained. To calculate body mass index, height was measured to the nearest 0.5 cm without shoes using a stadiometer and weight was measured without shoes and excess clothing to the nearest 0.1 kg using a mechanical beam balance (8, 19). Self-reported history of cardiovascular disease (yes/no) was assessed as described previously (20). Data on intake of dietary covariates were obtained from the FFQ described earlier.
Statistical Analysis
Statistical analyses were undertaken using STATA/IC 15.1 (StataCorp LLC) and R statistics (R Core Team, 2019 [21]). As the primary outcomes of interest were nonnegative and positively skewed, generalized linear models with a γ distribution and log-link were used to examine associations with all exposures (continuous). To investigate the potential nonlinearity of the relationships, exposures were modeled using restricted cubic splines. P values for the overall effect of the exposure on the response (false discovery rate corrected) and for a test of nonlinearity were obtained using likelihood ratio tests to compare appropriate nested models. Associations are presented graphically using the “effects” R package (22). To determine where significant differences between quartiles of intake exist, ratios of means and 95% CIs were obtained from the model with the exposure fitted as a continuous variable through a restricted cubic spline and are reported for the median intake in each quartile (Q) relative to the median intake in Q1. Logistic regression models were used to investigate the relationship between baseline fruit intake and the secondary outcome of incident diabetes at 5 and 12 years. All odds ratios (ORs) and 95% CIs were obtained from the model with the exposure fitted as a continuous variable through a restricted cubic spline using the “rms” R package (23); OR estimates are graphed over a fine grid of x values with the median intake in Q1 as the reference point and are also reported for the median of each quartile. For all regression models, 3 models of adjustment were used: model 1 adjusted for age (years) and sex (male/female); model 2 adjusted for age, sex, physical activity levels (sedentary, insufficient, sufficient), level of education (never to some high school, completed university or equivalent), SEIFA (categorized intro quintiles), income, body mass index (calculated as kg/m2), smoking status (current smoker, ex-smoker, nonsmoker), self-reported prevalence of cardiovascular disease (yes/no), and parental history of diabetes (yes/no); model 3 adjusted for all covariates in model 2 plus energy intake, and intakes (g/day) of alcohol, vegetables, red meat and processed meat. For visual simplicity, in all graphs presented, the x-axis was truncated at 3 SDs above the mean.
Results
This population of 7674 Australians (45% male), had a mean ± SD age of 54 ± 12 years at baseline and the median (interquartile range) total fruit intake was 162 g/day (95-283 g/day). Relative to participants with the lowest total fruit intakes (Q1), those with the highest intakes (Q4) were more likely to be female, slightly older, more physically active, less disadvantaged, and have a higher degree of education and were less likely to be current smokers (Table 1). Although participants in Q4 had a higher total energy intake than participants in Q1, their underlying dietary pattern tended to be slightly heathier in that they ate more vegetables and less red and processed meat (see Table 1). Participants with no follow-up data after baseline had, on average, a slightly lower intake of fruit and vegetables, were slightly less physically active, less likely to have a higher degree of education and were more likely to smoke (Supplementary Table 1 [14]).
Table 1.
Baseline characteristics of the study population
| Whole population | Total fruit intake quartiles | ||||
|---|---|---|---|---|---|
| (n = 7675) | Q1 | Q2 | Q3 | Q4 | |
| (n = 1920) | (n = 1920) | (n = 1918) | (n = 1917) | ||
| Total fruit intake, g/d, median (IQR) | 162 (95-283) | 62 (53-75) | 122 (109-137) | 230 (203-253) | 372 (325-448) |
| Demographics | |||||
| Age, y | 54 ± 12 | 51 ± 11 | 53 ± 12 | 55 ± 13 | 55 ± 12 |
| Sex, male, n (%) | 3439 (44.8) | 989 (51.5) | 909 (47.3) | 676 (35.2) | 865 (45.1) |
| BMI | 26.8 ± 4.7 | 27.0 ± 4.8 | 26.8 ± 4.5 | 26.6 ± 4.7 | 26.9 ± 4.7 |
| SEIFA score, median (IQR) | 1033 (972-1079) | 1008 (966-1075) | 1032 (972-1075) | 1044 (976-1080) | 1048 (974-1086) |
| Physical activity, n (%) | |||||
| Sedentary | 1308 (17.0) | 470 (24.5) | 316 (16.5) | 274 (14.3) | 348 (18.2) |
| Insufficient | 2377 (31.0) | 617 (32.1) | 634 (33.0) | 599 (31.2) | 527 (27.5) |
| Sufficient | 3990 (52.0) | 833 (43.4) | 970 (50.5) | 1045 (54.5) | 1142 (59.6) |
| Smoking status, n (%) | |||||
| Current | 1097 (14.3) | 493 (25.7) | 271 (14.1) | 187 (9.7) | 146 (7.6) |
| Former | 2319 (30.2) | 552 (28.8) | 582 (30.3) | 597 (31.1) | 588 (30.7) |
| Never | 4259 (55.5) | 875 (45.6) | 1067 (55.6) | 1134 (59.1) | 1183 (61.7) |
| Education, n (%) | |||||
| Never, primary or high school | 3114 (40.6) | 845 (44.0) | 775 (40.4) | 778 (40.6) | 716 (37.4) |
| Secondary education | 4561 (59.4) | 1075 (56.0) | 1145 (59.6) | 1140 (5.9) | 1201 (62.6) |
| Prevalent CVD, n (%) | 609 (7.9) | 128 (6.7) | 145 (7.8) | 176 (9.2) | 160 (8.3) |
| Family history of diabetes, n (%) | 1366 (17.8) | 351 (18.3) | 367 (19.1) | 308 (16.1) | 340 (17.7) |
| Dietary characteristics, median (IQR) | |||||
| Total energy intake, kJ | 7803 ± 2660 | 7534 ± 2728 | 7668 ± 2491 | 7454 ± 2483 | 8557 ± 2776 |
| Alcohol intake, g/d | 6 (1-19) | 7 (1-23) | 6 (1-20) | 5 (1-15) | 5 (0-17) |
| Sugar intake, g/d | 87 (67-112) | 71 (53-97) | 81 (63-105) | 87 (70-108) | 107 (88-134) |
| Vegetable intake, g/d | 162 (118-218) | 152 (103-208) | 156 (117-210) | 158 (116-206) | 187 (140-247) |
| Red meat, g/d | 59 (34-96) | 67 (38-111) | 61 (37-94) | 53 (30-83) | 59 (33-98.7) |
| Processed meat, g/d | 17 (8-31) | 21 (10-35) | 19 (9-32) | 14 (7-27) | 14 (6-30) |
Results are presented as means ± SD unless otherwise stated.
Abbreviations: CVD, cardiovascular disease; IQR, interquartile range; Q, quartile; SEIFA, Socio-Economic Indexes for Areas.
The most commonly consumed fruit was apples, contributing approximately 23% to total fruit intake, followed by bananas (~ 20%), and oranges and other citrus fruits (~ 18%; see Supplementary Fig. 1 [14]). All other fruits contributed less than 8% each to total fruit intake and were therefore not assessed discretely in subsequent analyses.
Cross-Sectional Associations Between Fruit Intake and Measures of Fasting Glucose, Glucose Tolerance, and Insulin Sensitivity at Baseline
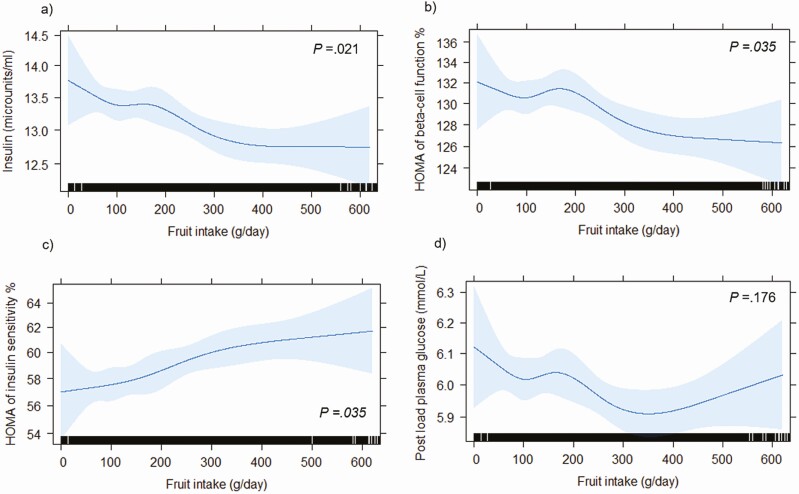
Total fruit intake was significantly inversely associated with serum insulin and HOMA2-%β, and significantly positively associated with HOMA2-%S (false discovery rate corrected P ≤ .05 for all; Fig. 2). Total fruit intake was not associated with PLG or FPG (see Fig. 2 and Supplementary Fig. 2 [14]). Compared to participants in the lowest total fruit intake quartile, participants in the highest intake quartile had a 3% lower PLG (0.97 [0.96-0.99]), a 5% lower serum insulin (0.95 [0.93-0.98]), a 2% lower HOMA2-%β (0.98 [0.96-1.00]), and a 6% higher HOMA2-%S (1.06 [1.03-1.09]), after multivariable adjustments (model 2; Tables 2 and 3). Adjusting for potential dietary confounders (model 3) did not change the associations.
Figure 2.
Graphical representation of the multivariable-adjusted dose-response relationship between total fruit intake and baseline A, fasting serum insulin; B, the updated homeostasis model assessment (HOMA2) of β-cell function; C, HOMA2 of insulin sensitivity, and D, 2-hour postload plasma glucose, obtained by generalized regression models with the exposure included as a restricted cubic spline (n = 7675). The HOMA2 computer model was used to estimate the HOMA of insulin sensitivity and HOMA of β-cell function. Blue shading represents 95% CI. The rug plot along the bottom of each graph depicts each observation. All analyses were adjusted for age, sex, physical activity levels, level of education, Socio-Economic Indexes for Areas, income, body mass index, smoking status, prevalence of cardiovascular disease, parental history of diabetes, and intakes of vegetables, alcohol, red meat, processed meat, and energy. P values for the effect of the exposure on the response (false discovery rate corrected) were obtained using likelihood ratio tests.
Table 2.
Associations between fruit intake and postload plasma glucose (n = 7675)
| Fruit intake quartiles | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Total fruit | 62 g/d (0-95) | 122 g/d (95-162) | 230 g/d (162-283) | 372 g/d (283-961) |
| PLG, mmol/L | 5.8 (4.9-6.9) | 5.8 (4.9-7.0) | 5.9 (5.0-7.0) | 5.8 (4.9-6.9) |
| No. of participants | 1920 | 1920 | 1918 | 1917 |
| Model 1 | Reference | 1.00 (0.99-1.01) | 0.98 (0.97, 1.00) | 0.97 (0.96, 0.99) |
| Model 2 | Reference | 1.00 (0.98-1.01) | 0.98 (0.97, 1.00) | 0.97 (0.96, 0.99) |
| Model 3 | Reference | 1.00 (0.99-1.01) | 0.99 (0.97, 1.00) | 0.98 (0.97, 1.00) |
| Apples | 4 g/d (0-10) | 18 g/d (10-30) | 46 g/d (30-69) | 113 g/d (69-706) |
| PLG, mmol/L | 5.9 (5.0-7.2) | 5.8(4.8-6.9) | 5.8 (4.9-7.0) | 5.7 (4.8-6.8) |
| No. participants | 1928 | 1914 | 1915 | 1918 |
| Model 1 | Reference | 0.98 (0.96-0.99) | 0.97 (0.96-0.98) | 0.96 (0.95-0.98) |
| Model 2 | Reference | 0.97 (0.96-0.98) | 0.96 (0.95-0.98) | 0.96 (0.95-0.98) |
| Model 3 | Reference | 0.97 (0.96-0.98) | 0.97 (0.96-0.98) | 0.97 (0.95-0.98) |
| Oranges and other citrus | 2 g/d (0-6) | 12 g/d (6-19) | 33 g/d (20-57) | 96 g/d (57-479) |
| PLG, mmol/L | 5.9 (5.0-7.2) | 5.7 (4.8-6.7) | 5.8 (4.9-7.0) | 5.9 (5.0-7.0) |
| No. participants | 1919 | 1922 | 1919 | 1915 |
| Model 1 | Reference | 0.99 (0.98-1.01) | 0.99 (0.97-1.00) | 0.98 (0.97-1.00) |
| Model 2 | Reference | 0.99 (0.98-1.00) | 0.99 (0.97-1.00) | 0.99 (0.97-1.00) |
| Model 3 | Reference | 0.99 (0.98-1.00) | 0.99 (0.98-1.00) | 0.99 (0.98-1.01) |
| Bananas | 4 g/d (0-10) | 16 g/d (10-26) | 37 g/d (30-53) | 77 g/d (53-244) |
| PLG, mmol/L | 5.8 (4.9-6.9) | 5.8 (4.8-6.9) | 5.8 (4.9-7.0) | 5.8 (5.0-7.0) |
| No. of participants | 1930 | 1914 | 1914 | 1917 |
| Model 1 | Reference | 0.99 (0.98-1.01) | 0.98 (0.97v0.99) | 0.98 (0.96-0.99) |
| Model 2 | Reference | 0.99 (0.98-1.00) | 0.98 (0.97-0.99) | 0.98 (0.96-0.99) |
| Model 3 | Reference | 0.99 (0.98-1.00) | 0.98 (0.97-1.00) | 0.98 (0.97-1.00) |
| Fruit juice | 2 g/d (0-5) | 16 g/d (6-31) | 72 g/d (32-129) | 200 g/d (130-1135) |
| PLG, mmol/L | 5.8 (4.9-6.9) | 5.8 (4.9-7.0) | 5.9 (5.0-7.0) | 5.8 (4.9-6.9) |
| No. of participants | 1940 | 1950 | 1884 | 1901 |
| Model 1 | Reference | 1.00 (0.99-1.01) | 1.00 (0.99-1.02) | 1.00 (0.98-1.02) |
| Model 2 | Reference | 1.00 (0.99-1.01) | 1.00 (0.99-1.02) | 1.00 (0.99-1.02) |
| Model 3 | Reference | 1.00 (0.99-1.01) | 1.00 (0.99-1.02) | 1.01 (0.99-1.02) |
Ratios of means and 95% CIs were obtained from the model with the exposure fitted as a continuous variable through a restricted cubic spline and are reported for the median intake in each quartile (Q) relative to the median intake in Q1. Model 1 adjusted for age and sex; model 2 adjusted for age, sex, physical activity levels, level of education, Socio-Economic Indexes for Areas, income, body mass index, smoking status, self-reported prevalence of cardiovascular disease, and parental history of diabetes; model 3 adjusted for all covariates in model 2 plus energy intake, and intake (g/d) of alcohol, vegetables, red meat, and processed meat. Postload plasma glucose (PLG) is presented as median (interquartile range). Fruit and fruit juice intake (g/d) is presented as median (range).
Table 3.
Associations between fruit intake and estimates of pancreatic β-cell function and insulin sensitivity (n = 7675)
| Fruit intake quartiles | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| Serum insulin | ||||
| Total fruit | ||||
| Insulin, microunits/mL | 12.3 (9.6-16.9) | 12.4 (9.4-16.3) | 12.1 (9.5-15.9) | 11.9 (9.1-15.3) |
| Model 1 | Reference | 0.99 (0.97-1.02) | 0.96 (0.93-0.99) | 0.94 (0.91-0.97) |
| Model 2 | Reference | 0.99 (0.97-1.02) | 0.97 (0.95-1.00) | 0.95 (0.93-0.98) |
| Model 3 | Reference | 1.00 (0.98-1.02) | 0.98 (0.95-1.00) | 0.96 (0.93-0.98) |
| Apples | ||||
| Insulin, microunits/mL | 12.3 (9.5-16.5) | 12.4 (9.5-15.8) | 12.0 (9.3-15.8) | 12.0 (9.3-15.6) |
| Model 1 | Reference | 0.99 (0.97-1.02) | 0.97 (0.95-1.00) | 0.95 (0.92-0.98) |
| Model 2 | Reference | 0.98 (0.96-1.00) | 0.97 (0.95-0.99) | 0.96 (0.94-0.98) |
| Model 3 | Reference | 0.98 (0.96-1.00) | 0.97 (0.95-0.99) | 0.97 (0.94-0.99) |
| Oranges and other citrus | ||||
| Insulin, microunits/mL | 12.4 (9.6-16.5) | 12.1 (9.4-16.2) | 12.3 (9.4-16.1) | 11.9 (9.3-15.4) |
| Model 1 | Reference | 0.98 (0.95-1.01) | 0.96 (0.94-0.99) | 0.95 (0.92-0.98) |
| Model 2 | Reference | 0.98 (0.96-1.00) | 0.97 (0.95-1.00) | 0.97 (0.95-1.00) |
| Model 3 | Reference | 0.98 (0.96-1.00) | 0.98 (0.96-1.00) | 0.97 (0.95-1.00) |
| Bananas | ||||
| Insulin, microunits/mL | 12.3 (9.5-16.5) | 12.3 (9.4-16.3) | 12.0 (9.4-16.0) | 12.1 (9.3-15.3) |
| Model 1 | Reference | 1.01 (0.99-1.04) | 0.97 (0.94-0.99) | 0.95 (0.92-0.98) |
| Model 2 | Reference | 1.00 (0.98-1.02) | 0.98 (0.96-1.00) | 0.97 (0.95-1.00) |
| Model 3 | Reference | 1.00 (0.98-1.02) | 0.99 (0.96-1.01) | 0.98 (0.95-1.00) |
| Fruit juice | ||||
| Insulin, microunits/mL | 12.3 (9.5-16) | 11.8 (9.3-16.1) | 12.2 (9.4-16.0) | 12.3 (9.5-16.0) |
| Model 1 | Reference | 1.01 (0.98-1.03) | 1.00 (0.97-1.03) | 1.00 (0.97-1.03) |
| Model 2 | Reference | 1.01 (0.98-1.03) | 1.01 (0.99-1.04) | 1.02 (0.99-1.04) |
| Model 3 | Reference | 1.01 (0.99-1.03) | 1.02 (0.99-1.04) | 1.03 (1.00-1.05) |
| HOMA2-%β | ||||
| Total fruit | ||||
| HOMA2 B% | 126.3 (105.2-152.1) | 125.9 (105.0-152.0) | 126.3 (107.0-150.2) | 123.2 (103.0-147.6) |
| Model 1 | Reference | 1.00 (0.99-1.02) | 0.99 (0.97-1.01) | 0.97 (0.96-0.99) |
| Model 2 | Reference | 1.00 (0.99-1.02) | 0.99 (0.98-1.01) | 0.98 (0.96-1.00) |
| Model 3 | Reference | 1.00 (0.99-1.02) | 0.99 (0.98-1.01) | 0.98 (0.96-1.00) |
| Apples | ||||
| HOMA2-%β | 125.6 (104.7-152.3) | 127.5 (107.2-152.2) | 125.1 (105.0-149.9) | 123.7 (102.9-147.4) |
| Model 1 | Reference | 1.00 (0.99-1.02) | 0.99 (0.98-1.01) | 0.98 (0.96-0.99) |
| Model 2 | Reference | 0.99 (0.98-1.01) | 0.99 (0.98-1.00) | 0.98 (0.96-1.00) |
| Model 3 | Reference | 0.99 (0.98-1.01) | 0.99 (0.97-1.00) | 0.98 (0.96-1.00) |
| Oranges and other citrus | ||||
| HOMA2-%β | 126.4 (105.9-151.4) | 126.9 (105.4-150.7) | 126.4 (105.3-151.3) | 122.0 (102.3-148.3) |
| Model 1 | Reference | 1.00 (0.98-1.01) | 0.98 (0.97-1.00) | 0.97 (0.95-0.99) |
| Model 2 | Reference | 1.00 (0.98-1.01) | 0.99 (0.97-1.00) | 0.98 (0.96-1.00) |
| Model 3 | Reference | 1.00 (0.98-1.01) | 0.99 (0.97-1.00) | 0.98 (0.96-1.00) |
| Bananas | ||||
| HOMA2-%β | 124.9 (104.0-151.1) | 126.4 (105.2-151.3) | 126.2 (105.1-151.5) | 124.6 (105.2-148.3) |
| Model 1 | Reference | 1.01 (1.00-1.03) | 1.00 (0.98-1.01) | 0.99 (0.97-1.01) |
| Model 2 | Reference | 1.01 (1.00-1.02) | 1.00 (0.99-1.02) | 1.00 (0.98-1.02) |
| Model 3 | Reference | 1.01 (0.99-1.02) | 1.00 (0.99-1.02) | 1.00 (0.98-1.01) |
| Fruit juice | ||||
| HOMA2-%β | 126.4 (105.5-150.8) | 124.1 (103.8-149.3) | 126.8 (105.4-151.2) | 124.8 (104.0-149.9) |
| Model 1 | Reference | 1.00 (0.98-1.02) | 1.00 (0.98-1.02) | 1.01 (0.99-1.02) |
| Model 2 | Reference | 1.00 (0.99-1.01) | 1.01 (0.99-1.02) | 1.01 (1.00-1.03) |
| Model 3 | Reference | 1.00 (0.99-1.01) | 1.01 (0.99-1.02) | 1.02 (1.00-1.03) |
| HOMA2-%S | ||||
| Total fruit | ||||
| HOMA2-%S | 54.0 (39.6-69.5) | 53.4 (40.7-70.5) | 55.3 (42.1-69.8) | 55.6 (43.4-72.6) |
| Model 1 | Reference | 1.00 (0.97-1.03) | 1.02 (0.99-1.05) | 1.05 (1.01-1.08) |
| Model 2 | Reference | 1.01 (0.98-1.03) | 1.03 (1.00-1.06) | 1.06 (1.03-1.09) |
| Model 3 | Reference | 1.01 (0.98-1.03) | 1.03 (1.00-1.06) | 1.05 (1.02-1.08) |
| Apples | ||||
| HOMA2-%S | 53.9 (40.1-69.6) | 53.8 (40.6-69.9) | 55.2 (42.5-71.5) | 55.2 (42.6-71.3) |
| Model 1 | Reference | 0.99 (0.96-1.02) | 1.00 (0.98-1.03) | 1.02 (0.99-1.06) |
| Model 2 | Reference | 1.01 (0.99-1.04) | 1.02 (1.00-1.05) | 1.04 (1.01-1.07) |
| Model 3 | Reference | 1.01 (0.99-1.04) | 1.02 (1.00-1.05) | 1.03 (1.00-1.07) |
| Oranges and other citrus | ||||
| HOMA2-%S | 53.6 (40.4-69.2) | 54.9 (41.3-71.0) | 53.8 (41.1-71.0) | 56.0 (43.4-71.0) |
| Model 1 | Reference | 1.00 (0.97-1.03) | 1.03 (1.00-1.06) | 1.06 (1.02-1.09) |
| Model 2 | Reference | 1.01 (0.98-1.04) | 1.03 (1.00-1.05) | 1.05 (1.02-1.08) |
| Model 3 | Reference | 1.01 (0.98-1.04) | 1.03 (1.00-1.05) | 1.05 (1.01-1.08) |
| Bananas | ||||
| HOMA2-%S | 3.6 (40.4-70.1) | 54.1 (40.9-70.7) | 55.1 (41.6-70.9) | 55.0 (43.1-71.3) |
| Model 1 | Reference | 1.00 (0.97-1.03) | 1.03 (1.01-1.06) | 1.05 (1.02-1.09) |
| Model 2 | Reference | 1.00 (0.98-1.03) | 1.03 (1.00-1.06) | 1.04 (1.01-1.08) |
| Model 3 | Reference | 1.00 (0.98-1.03) | 1.03 (1.00-1.05) | 1.04 (1.01-1.07) |
| Fruit juice | ||||
| HOMA2-%S | 53.9 (41.5-70.9) | 56.1 (1.6-71.5) | 54.3 (41.6-70.3) | 54.2 (41.6-70.0) |
| Model 1 | Reference | 0.99 (0.97-1.02) | 0.98 (0.95-1.01) | 0.98 (0.95-1.01) |
| Model 2 | Reference | 1.00 (0.97-1.02) | 0.98 (0.96-1.01) | 0.97 (0.95-1.00) |
| Model 3 | Reference | 1.00 (0.97-1.02) | 0.98 (0.95-1.01) | 0.97 (0.94-1.00) |
Ratios of means and 95% CIs were obtained from the model with the exposure fitted as a continuous variable through a restricted cubic spline and are reported for the median intake in each quartile (Q) relative to the median intake in Q1. Model 1 adjusted for age and sex; model 2 adjusted for age, sex, physical activity levels, level of education, Socio-Economic Indexes for Areas, income, body mass index, smoking status, self-reported prevalence of cardiovascular disease, and parental history of diabetes; model 3 adjusted for all covariates in model 2 plus energy intake, and intakes (g/d) of alcohol, vegetables, red meat, and processed meat. Insulin, HOMA2-%β and HOMA2-%S are presented as median (interquartile range).
Abbreviations: HOMA2, updated homeostasis model assessment of insulin resistance; HOMA2-%β, updated homeostasis model assessment of β-cell function; HOMA2-%S, updated homeostasis model assessment of insulin sensitivity.
Of the individual fruit types, apple intake was significantly inversely associated with serum insulin (P = .035) and nonlinearly inversely associated with PLG (P < .001; Pnonlinearity < .001; Supplementary Fig. 3 [14]). Although apple intake appeared to be inversely associated with HOMA2-%B and positively associated with HOMA2-%S, these associations did not reach statistical significance after adjustments were made for dietary confounders. Intake of orange and other citrus fruits, bananas, and fruit juice were not significantly associated with any outcome (see Tables 2 and 3; Supplementary Figs. 4-6 [14]).
Prospective Associations Between Fruit Intake and Incident Diabetes at 5 and 12 Years
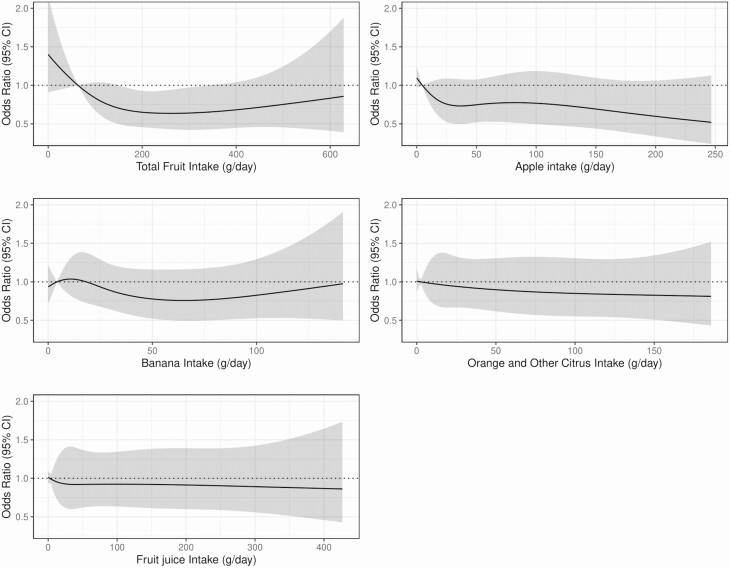
Of the 4674 participants with follow-up at 5 years, 179 participants had diabetes. Total fruit intake appeared to be nonlinearly inversely associated with incident diabetes at 5 years’ follow-up (Fig. 3). Compared to participants with the lowest intake (Q1), participants with moderate total fruit intake (Q3) had 36% lower odds of having diabetes at 5 years (OR, 0.64; 95% CI, 0.44-0.92), after multivariable adjustments (model 3; Supplementary Table 2 [14]). Apparent inverse associations did not reach statistical significance for intake of individual types of fruit after adjusting for potential dietary and lifestyle confounders (see Fig. 3 and Supplementary Table 2 [14]). Of the 3518 participants with follow-up at 12 years, 247 participants had diabetes. ORs indicate a lower odds of diabetes for moderate to high intakes of total fruit, apples, oranges and other citrus fruits, and bananas, although CIs were wide and associations were not statistically significant in model 3 (Supplementary Table 3 [14]).
Figure 3.
Multivariable-adjusted associations between intakes of total fruit, individual fruit types, and fruit juice and presence of diabetes (fasting plasma glucose ≥ 7.0 mmol/L, 2-hour postload plasma glucose ≥ 11.1 mmol/L, or current treatment with insulin or oral hypoglycemic agents) at 5 years (n = 4674). Values are odds ratios and 95% CI and are comparing the specific level of fruit intake (horizontal axis) to the median intake for participants in the lowest intake quartile. All analyses were adjusted for age, sex, physical activity levels, level of education, Socio-Economic Indexes for Areas, income, body mass index, smoking status, prevalence of cardiovascular disease, parental history of diabetes, and intakes of vegetables, alcohol, red meat, processed meat, and energy (model 3).
Discussion
In this cohort of 7675 Australian men and women, higher total fruit intake was associated with better measures of glucose tolerance and insulin sensitivity. Furthermore, moderate to high total fruit intake was associated with a lower odds of diabetes after 5 years of follow-up.
Insulin resistance, in concert with β-cell dysfunction and obesity, is a key driver of the pathophysiology of T2DM (7). HOMA2 is one approach of assessing β-cell function and insulin resistance (or insulin sensitivity) by means of fasting glucose and insulin values (15). In the present study, higher total fruit intake was associated with higher insulin sensitivity and lower β-cell function in a dose-response manner. At a glance, the inverse association between fruit intake and β-cell function may seem counterintuitive. However, the HOMA2 of β-cell function measurement actually reflects insulin secretion (or β-cell “activity”) rather than β-cell “function” (24); in this context, the lower values likely reflect higher insulin sensitivity (15). Although statistically significant, the higher β-cell activity and insulin sensitivity seen with higher intake of fruit translated to a small decrease in PLG that could be considered clinically minor. While this study sheds light on the physiological impact of fruit, further research is warranted.
In a recent meta-analysis of 15 observational cohort studies, with 70 968 cases of T2DM, a borderline inverse association was observed between total fruit intake and odds of having T2DM (relative risk, 0.96; 95% CI, 0.93-1.00 for high vs low fruit intake) (4). As observed in the present study, there was evidence that this inverse relationship was nonlinear, plateauing at fruit intakes of approximately 200 to 300 g/day (4). Although associations for individual fruits were not examined in this meta-analysis, the largest of the included cohort studies (pooling data from 3 prospective cohorts of US men and women) reported that associations with T2DM risk differed significantly among intake of individual fruits. Specifically, the risk of T2DM for intake of 3 servings per week was 26% lower for blueberries, 12% lower for grapes and raisins, 7% lower for apples and pears, 5% lower for bananas, 5% lower for grapefruit, and 10% higher for cantaloupe (5). In the present study, evidence of an inverse association between higher intake and incident diabetes at 5 years was apparent for apples, bananas, and orange and other citrus fruits. That associations did not reach statistical significance after multivariable adjustments may be due to a low statistical power owing to the relatively low number of events. Associations were not statistically significant for 12-year outcomes, perhaps due the longer time lag between exposure assessment and outcome.
The biological mechanisms underpinning the beneficial effects of fruits on glucose regulation and diabetes risk are likely multifaceted. Besides their low contribution to energy intake, most fruits typically have a low glycemic load, while being rich in fiber, vitamins, minerals, and phytochemicals, all of which may play a contributory role (25). Potential mechanistic evidence is mainly for fiber (26); insoluble and soluble fiber both are reported to improve glycemic control. However, recent evidence suggests that more benefits may be gained from fermentation of soluble fibers by the gut microbiome, increasing production of short-chain fatty acids, which have been shown to modulate glucose metabolism (27, 28). Furthermore, many fruits, including apples, are rich in flavonoids, a class of phytochemicals that are reported to improve insulin sensitivity, potentially by decreasing apoptosis and promoting proliferation of pancreatic β cells, and reduce muscular inflammation and oxidative stress (29, 30). Moreover, fruit intake may indirectly influence T2DM risk by preventing or managing excess adiposity, possibly via higher dietary fiber contributing toward satiety (31). Interestingly, although there was evidence that higher banana intake may be associated with a lower risk of diabetes at 5 years, banana intake was not significantly associated with measures of glucose tolerance and insulin sensitivity at baseline. This finding warrants investigation in other cohorts.
A positive association between fruit juice consumption and T2DM has been reported previously (5, 32, 33). In a meta-analysis of 12 prospective cohort studies, a one serving per day higher intake of fruit juice was associated with a 10% higher risk of T2DM (relative risk, 1.10; 95% CI, 1.01-1.20) after adjusting for adiposity and within-person variation (33). For this reason, in the present study, fruit juice intake was not included in the calculation of total fruit intake. In the present study, we report no association between fruit juice consumption and measures of insulin resistance and β-cell dysfunction or incident diabetes. That an association was observed between intake of whole fruit, but not fruit juice, may be due to the relatively high glycemic load of fruit juices and reduced levels of beneficial fibers in comparison with whole fruit (34). This may lead to larger and more rapid increases in serum glucose and insulin levels (35). Data also suggest that fruit juice, including fruit juice with added fiber, does not trigger satiety to the extent that whole fruit does (36). Our findings support that of a meta-analysis of 18 randomized controlled trials that reported that, compared with the control group, 100% fruit juice had no significant effect on fasting blood glucose, fasting blood insulin, or glycated hemoglobin A1c (37). However, the interventions in the aforementioned trials (predominantly grape juice, pomegranate juice, and grapefruit juice) are not fruit juices typically consumed and were often sugar free, limiting generalization.
The strengths and limitations of this study should be acknowledged to facilitate appropriate interpretation of the findings. Limitations, characteristic of observational studies, apply in that we are not able to infer causality or rule out residual confounding. Owing to the relatively low intake of certain fruits, particular those that are not available all year round, we did not investigate associations for intake of all fruits captured in the FFQ. We also acknowledge that participants in the AusDiab study were likely of a higher socioeconomic status than those who did not respond to the original survey (20) and that participants with follow-up data tended to be healthier than those lost to follow-up; associations warrant investigation in other populations.
In conclusion, findings from this study support encouragement of the consumption of whole fruits, but not fruit juice, to preserve insulin sensitivity and mitigate T2DM risk. Promoting a healthy diet and lifestyle which includes the consumption of popular fruits such as apples, bananas, and oranges, with widespread geographical availability, may lower T2DM incidence.
Acknowledgments
The AusDiab study, initiated and coordinated by the International Diabetes Institute, and subsequently coordinated by the Baker Heart and Diabetes Institute, gratefully acknowledges the support and assistance given by: A. Allman, B. Atkins, S. Bennett, A. Bonney, S. Chadban, M. de Courten, M. Dalton, D. Dunstan, T. Dwyer, H. Jahangir, D. Jolley, D. McCarty, A. Meehan, N. Meinig, S. Murray, K. O’Dea, K. Polkinghorne, P. Phillips, C. Reid, A. Stewart, R. Tapp, H. Taylor, T. Welborn, T. Whalen, F. Wilson, and P. Zimmet. Also, for funding or logistical support, we are grateful to the National Health and Medical Research Council (NHMRC grant No. 233200), Australian Government Department of Health and Ageing, Abbott Australasia Pty Ltd, Alphapharm Pty Ltd, AstraZeneca, Bristol-Myers Squibb, City Health Centre-Diabetes Service-Canberra, Department of Health and Community Services–Northern Territory, Department of Health and Human Services-Tasmania, Department of Health-New South Wales, Department of Health–Western Australia, Department of Health-South Australia, Department of Human Services–Victoria, Diabetes Australia, Diabetes Australia Northern Territory, Eli Lilly Australia, the Estate of the Late Edward Wilson, GlaxoSmithKline, the Jack Brockhoff Foundation, Janssen-Cilag, Kidney Health Australia, the Marian & FH Flack Trust, Menzies Research Institute, Merck Sharp & Dohme, Novartis Pharmaceuticals, Novo Nordisk Pharmaceuticals, Pfizer Pty Ltd, Pratt Foundation, Queensland Health, Roche Diagnostics Australia, Royal Prince Alfred Hospital, Sydney, Sanofi Aventis, Sanofi-Synthelabo, and the Victorian Government’s OIS Program.
Financial Support: This work was supported by a National Health and Medical Research Council (NHMRC) Early Career Fellowship (grant No. APP1159914 to N.P.B.), Australia; an NHMRC of Australia Emerging Leadership Investigator Grant (ID No. 1172987 to L.C.B.); a National Heart Foundation of Australia Post-Doctoral Research Fellowship (No. ID 102498); a National Heart Foundation of Australia Future Leader Fellowship (No. ID 102817 to J.R.L.); an NHMRC of Australia Senior Research Fellowship (grant No. APP1116937 to J.M.H.); and an NHMRC of Australia Investigator Grant (grant No. APP1173952 to J.E.S.).
Author Contributions: N.P.B. designed the research (project conception, development of overall research plan, and study oversight); D.J.M., R.M.D., and J.E.S. conducted the original cohort study; N.P.B. analyzed the data, wrote the manuscript, and had primary responsibility for final content; all authors assisted with interpretation of the results and critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Glossary
Abbreviations
- AusDiab
Australian Diabetes Obesity and Lifestyle Study
- FFQ
food frequency questionnaire
- FPG
fasting plasma glucose
- HOMA2
updated homeostasis model assessment of insulin resistance
- HOMA2-%β
updated homeostasis model assessment of β-cell function
- HOMA2-%S
updated homeostasis model assessment of insulin sensitivity
- OR
odds ratio
- PLG
postload plasma glucose
- Q
quartile
- SEIFA
Socio-Economic Indexes for Areas
- T2DM
type 2 diabetes mellitus
Additional Information
Disclosures: D.J.M. and J.E.S. report grants from Abbott Australasia Pty Ltd, Alphapharm Pty Ltd, AstraZeneca, Bristol-Myers Squibb, Eli Lilly Australia, GlaxoSmithKline, Janssen-Cilag, Merck Sharp & Dohme, Novartis Pharmaceuticals, Novo Nordisk Pharmaceuticals, Roche Diagnostics Australia, Sanofi Aventis, and Sanofi-Synthelabo, during the conducting of this study. R.M.D. received a Primary Growth Partnership grant via the Ministry of Primary Industries in New Zealand with Fonterra Co-operative Group Ltd, outside the submitted work. J.M.H. reports grants from FruitWest, grants from the Department of Agriculture and Food WA, outside the submitted work. All other authors have nothing to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2(8):634-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;386(9995):743-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cho NH, Shaw JE, Karuranga S, et al. . IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281. [DOI] [PubMed] [Google Scholar]
- 4. Schwingshackl L, Hoffmann G, Lampousi AM, et al. . Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muraki I, Imamura F, Manson JE, et al. . Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dow C, Balkau B, Bonnet F, et al. . Strong adherence to dietary and lifestyle recommendations is associated with decreased type 2 diabetes risk in the AusDiab cohort study. Prev Med. 2019;123:208-216. [DOI] [PubMed] [Google Scholar]
- 7. Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151-S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunstan DW, Zimmet PZ, Welborn TA, et al. ; Australian Diabetes, Obesity and Lifestyle Study (AusDiab) . The Australian Diabetes, Obesity and Lifestyle Study (AusDiab)–methods and response rates. Diabetes Res Clin Pract. 2002;57(2):119-129. [DOI] [PubMed] [Google Scholar]
- 9. Banna JC, McCrory MA, Fialkowski MK, Boushey C. Examining plausibility of self-reported energy intake data: considerations for method selection. Front Nutr. 2017;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rhee JJ, Sampson L, Cho E, Hughes MD, Hu FB, Willett WC. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am J Epidemiol. 2015;181(4):225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ireland P, Jolley D, Giles G, et al. . Development of the Melbourne FFQ: a food frequency questionnaire for use in an Australian prospective study involving an ethnically diverse cohort. Asia Pac J Clin Nutr. 1994;3(1):19-31. [PubMed] [Google Scholar]
- 12. Hodge A, Patterson AJ, Brown WJ, Ireland P, Giles G. The Anti Cancer Council of Victoria FFQ: relative validity of nutrient intakes compared with weighed food records in young to middle‐aged women in a study of iron supplementation. Aust NZ J Public Health. 2000;24(6):576-583. [DOI] [PubMed] [Google Scholar]
- 13. Woods RK, Stoney RM, Ireland PD, et al. . A valid food frequency questionnaire for measuring dietary fish intake. Asia Pac J Clin Nutr. 2002;11(1):56-61. [DOI] [PubMed] [Google Scholar]
- 14. Park D, Lee J-H, Han S. Underweight: another risk factor for cardiovascular disease? A cross-sectional 2013 Behavioral Risk Factor Surveillance System (BRFSS) study of 491,773 individuals in the USA. Medicine. 2017;96(48):e8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487-1495. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. 2006. https://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/. Accessed July 17, 2020.
- 17. Magliano DJ, Barr EL, Zimmet PZ, et al. . Glucose indices, health behaviors, and incidence of diabetes in Australia: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care. 2008;31(2):267-272. [DOI] [PubMed] [Google Scholar]
- 18. Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2016. Australian Bureau of Statistics; 2018. [Google Scholar]
- 19. Dalton M, Cameron AJ, Zimmet PZ, et al. ; AusDiab Steering Committee . Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med. 2003;254(6):555-563. [DOI] [PubMed] [Google Scholar]
- 20. Dunstan DW, Zimmet PZ, Welborn TA, et al. ; Australian Diabetes, Obesity and Lifestyle Study (AusDiab) . The Australian Diabetes, Obesity and Lifestyle Study (AusDiab)—methods and response rates. Diabetes Res Clin Pract. 2002;57(2):119-129. [DOI] [PubMed] [Google Scholar]
- 21. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2019. [Google Scholar]
- 22. Fox J. Effect displays in R for generalised linear models. J Stat Softw. 2003;8(15):1-27. [Google Scholar]
- 23. Harrell F Jr. rms: Regression Modeling Strategies. R Package Version 5.1-3. 1. 2019. 2019. https://www.rdocumentation.org/packages/rms/versions/5.1-3 [Google Scholar]
- 24. Pfützner A, Derwahl M, Jacob S, et al. . Limitations of the HOMA-B score for assessment of β-cell functionality in interventional trials—results from the PIOglim study. Diabetes Technol Ther. 2010;12(8):599-604. [DOI] [PubMed] [Google Scholar]
- 25. Bazzano LA, Serdula MK, Liu S. Dietary intake of fruits and vegetables and risk of cardiovascular disease. Curr Atheroscler Rep. 2003;5(6):492-499. [DOI] [PubMed] [Google Scholar]
- 26. Dong Y, Chen L, Gutin B, Zhu H. Total, insoluble, and soluble dietary fiber intake and insulin resistance and blood pressure in adolescents. Eur J Clin Nutr. 2019;73(8):1172-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao L, Zhang F, Ding X, et al. . Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151-1156. [DOI] [PubMed] [Google Scholar]
- 28. Lau WL, Vaziri ND. Gut microbial short-chain fatty acids and the risk of diabetes. Nat Rev Nephrol. 2019;15(7):389-390. [DOI] [PubMed] [Google Scholar]
- 29. Vinayagam R, Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab (Lond). 2015;12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawser Hossain M, Abdal Dayem A, Han J, et al. . Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int J Mol Sci. 2016;17(4):569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guyenet SJ. Impact of whole, fresh fruit consumption on energy intake and adiposity: a systematic review. Front Nutr. 2019;6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008;31(7):1311-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Imamura F, O’Connor L, Ye Z, et al. . Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wojcicki JM, Heyman MB. Reducing childhood obesity by eliminating 100% fruit juice. Am J Public Health. 2012;102(9):1630-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bolton RP, Heaton KW, Burroughs LF. The role of dietary fiber in satiety, glucose, and insulin: studies with fruit and fruit juice. Am J Clin Nutr. 1981;34(2):211-217. [DOI] [PubMed] [Google Scholar]
- 36. Flood-Obbagy JE, Rolls BJ. The effect of fruit in different forms on energy intake and satiety at a meal. Appetite. 2009;52(2):416-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murphy MM, Barrett EC, Bresnahan KA, Barraj LM. 100% fruit juice and measures of glucose control and insulin sensitivity: a systematic review and meta-analysis of randomised controlled trials. J Nutr Sci. 2017;6:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.