Abstract
Background
There is minimal evidence to support decision making for symptomatic steroid-refractory pseudoprogression or true progression occurring after intensity-modulated radiation therapy (IMRT) for glioblastoma (GBM). This study audited the survival outcome of patients managed with redo craniotomy (RedoSx) or bevacizumab (BEV) for steroid-refractory mass effect after IMRT for GBM.
Methods
Patients with GBM managed between 2008 and 2019 with the EORTC-NCIC Protocol were entered into a prospective database. Patients with symptomatic steroid-refractory mass effect within 6 months of IMRT managed with either RedoSx or BEV were identified for analysis. For the primary endpoint of median overall survival (OS) postintervention, outcome was analyzed in regards to potential prognostic factors, and differences between groups were assessed by log-rank analyses.
Results
Of the 399 patients managed with the EORTC-NCIC Protocol, 78 required an intervention within 6 months of IMRT completion for either true or pseudoprogression (49 with RedoSx and 29 with BEV). Subsequently, 20 of the 43 patients managed with RedoSx when BEV was clinically available, required salvage with BEV within 6 months after RedoSx. Median OS postintervention was 8.7 months (95% CI: 7.84-11.61) for the total group; and 8.7 months (95% CI: 6.8-13.1) for RedoSx and 9.4 months (95% CI: 7.8-13.6) for BEV (P = .38). Subsequent use of BEV in RedoSx patients was not associated with improved survival compared with RedoSx alone (P = .10). Age, time from IMRT, and ECOG performance status were not associated with OS. In the RedoSx patients, immunohistochemical features such as Ki-67% reduction correlated with survival. The presence of pure necrosis and residual tumor cells only had improved survival compared with the presence of gross tumor (P < .001).
Conclusions
At time of symptomatic steroid-refractory true or pseudoprogression following IMRT for GBM, BEV was equivalent to RedoSx in terms of OS. Pseudoprogression with residual cells at RedoSx was not associated with worse outcome compared to pure necrosis.
Keywords: bevacizumab, glioblastoma, pseudoprogression, redo craniotomy
Approximately half of the patients with glioblastoma (GBM) managed with concurrent radiation therapy and temozolomide (TMZ) as per the EORTC-NCIC (Stupp) Protocol1 will demonstrate increased contrast enhancement and edema on early posttreatment MRI.2,3 There is currently minimal evidence to support effective investigations to differentiate these changes as true progression vs treatment effect or pseudoprogression.4,5 Similarly, there is minimal evidence to support decision making in this clinical scenario, especially when the underlying process is bulky or prolonged causing clinical symptoms.
While the current initial management of symptomatic pseudoprogression involves the use of corticosteroids, clinical surveillance, and supportive care,2 further management may be required when patients either become unable to tolerate the associated corticosteroid morbidity or the process is prolonged or progressive. In such patients, surgical salvage with a repeat craniotomy (RedoSx) can reduce symptoms by debulking the mass effect, and additionally allows for histopathological assessment for residual disease.6,7 However, there is the potential for postoperative morbidity and exacerbation of existing neurological deficits. An alternative to this approach is bevacizumab (BEV), a vascular endothelial growth factor inhibitor, which is effective in reducing contrast enhancement in recurrent glioma, and healing of late cerebral radiation necrosis.8,9 Additionally, in the AVAGLIO randomized trial where BEV was administered as part of adjuvant therapy in GBM, although no impact was noted on overall survival (OS) compared to placebo,10 it did halve the rate of pseudoprogression.3
This current study aims to audit survival outcomes following management with either RedoSx or BEV in patients with steroid-refractory mass effect occurring within 6 months of completion of adjuvant intensity-modulated radiation therapy (IMRT) for GBM.
Methods
Consecutive adult patients diagnosed with GBM and referred to The Neuro-oncology Multidisciplinary Tumour Clinic (MDT) at the Northern Sydney Cancer Centre were entered into a prospective database, approved by the Institutional Ethics Review Board. Patient informed consent for database was obtained at the time of consent for radiation treatment. These patients were formally managed under the Stupp Protocol with definitive IMRT and TMZ between January 2008 and June 2019. Posttreatment MRI surveillance was conducted with a formal protocol with imaging performed at month +1 post-IMRT, then second monthly (month +3, +5, +7) until completion of the adjuvant phase of TMZ.
Patient Selection
Eligible patients for this study were those newly diagnosed patients with GBM managed under the Stupp Protocol, who then required intervention beyond corticosteroids for symptomatic steroid-refractory mass effect occurring within 6 months of completion of IMRT. These patients were then discussed at the Neuro-oncology MDT and subsequently managed with either RedoSx or BEV and identified for included in the audit. Patients with definite evidence of distant relapse beyond radiotherapy (RT) high-dose region were excluded.
Baseline Characteristics
Patient and tumor details were recorded at initial diagnosis. Specifically patient age, ECOG (Eastern Cooperative Oncology Group) performance status, extent of resection, initial Ki-67 proliferation index (Ki-67%), and MGMT (O6-methylguanine-DNA methyltransferase) methylation status were documented.
All patients required management with IMRT to a dose of 60 Gy over 6-7 weeks delivered with concurrent TMZ per the Stupp Protocol.1 Following completion of IMRT patients had surveillance MRI at 1 month (month +1) and then every 2 months until completion of adjuvant TMZ.
Indications for Second Craniotomy or BEV
Patients with symptomatic steroid-refractory mass effect were reviewed by the treating radiation oncologist, medical oncologist, and neurosurgeon to determine the most appropriate intervention. The decision making was individualized for patients and not randomized. This was based on clinical, radiological, and individual social factors, including resectability, ability to self-fund BEV, and patient choice. BEV was not funded on the government pharmaceutical scheme, and patients were required to access the medication through a partially self-funded industry compassionate access scheme.
Generally, if the neurosurgeon believed the enhancing mass was accessible for resection without additional morbidity, and performance status was considered adequate for the procedure then a repeat craniotomy was offered to the patient.
Time to intervention for mass effect from completion of RT was recorded. The subsequent requirement for salvage BEV in the 6 months after patients were managed with RedoSx was recorded.
Neuropathological Details
All patients in the audit had histopathological analysis of their original surgical specimens using standardized reporting. Patients undergoing RedoSx were required to have neuropathological examination of the surgical specimen for Ki-67%, which was compared with the patient’s initial surgical histology and for the presence of necrosis. Estimation of the percentage of tumor cells vs necrotic changes was determined in each case based on hematoxylin and eosin staining. The neuropathological assessment was categorized as pure necrosis (no evidence of tumor cells); gross tumor (the presence of tumor cells grouped together with prominent mitoses); and residual glioma cells (isolated clusters of atypical cells with absent or minimal mitoses).7
BEV Regimen
BEV was administered as a low dose regimen in this indication of presumed pseudoprogression with either a standard 400 mg dose or 5 mg per kg dose as a second weekly infusion. A minimum duration of 4 cycles were delivered.
Statistical Considerations
The primary endpoint was median OS time measured in months from the time of intervention (RedoSx or BEV) to last follow-up or death. Survival curves were generated using the Kaplan-Meier method. Univariate predictors of survival duration were evaluated using log-rank comparisons. All reported P values are 2-tailed. Statistical significance was defined as P < .05 in all cases. R Version 3.5.2 was used for statistical analysis (including survminer package version 0.4.6).
Results
Of the 399 patients managed with the Stupp Protocol during the study period, 78 (19.5%) required an intervention for either true progression or pseudoprogression within 6 months post-IMRT. Forty-nine patients underwent RedoSx, of which 17 (35%) had near-total resection whereas 32 (65%) had subtotal resection. Twenty-nine patients were managed with BEV alone.
Initial patient characteristics at diagnosis for both cohorts are detailed in Table 1. Extent of initial resection at diagnosis favored the RedoSx group with 39% having a near-total resection compared to 17% in the BEV patients (P = .05). All patients with a recorded IDH mutation status were IDH wild-type. MGMT status was known in 77% of patients and of these 37% were MGMT methylated. The proportion of patients with known MGMT status who had MGMT methylation in the RedoSx and BEV groups was 35% and 38%, respectively. Median Ki-67 on initial surgical histopathology was 25% and 30% for patients undergoing RedoSx, and BEV, respectively.
Table 1.
Patient and Treatment Characteristics (n = 78)
| Subgroup | % RedoSx (n = 49) | % BEV (n = 29) | |
|---|---|---|---|
| Age at time of intervention | Median | 58 yr | 62 yr |
| <60 | 59% | 53% | |
| >60 | 41% | 47% | |
| Site of tumor | Frontal | 26% | 31% |
| Temporal | 35% | 24% | |
| Parietal | 29% | 28% | |
| Occipital | 8% | 7% | |
| Thalamic | 0% | 10% | |
| Other | 2% | 0% | |
| Extent of resection at initial diagnosis | Near-total (>90%) | 39% | 17% |
| Subtotal (50%-90%) | 48% | 55% | |
| Biopsy (<50%) | 13% | 28% | |
| Ki-67% at initial diagnosis | Median | 25% | 30% |
| IDH mutation at initial diagnosis | Wild type | 80% | 97% |
| Mutated | 0% | 0% | |
| Unknown | 20% | 3% | |
| Known MGMT status at initial diagnosis | Unmethylated | 65% | 62% |
| Methylated | 35% | 38% | |
| Unknown | 15 patients | 3 patients | |
| Time from end RT to intervention | <3 months | 51% | 52% |
| >3 months | 49% | 48% | |
| Performance status at intervention (ECOG) | 0.1 | 26% | 0% |
| 2.3 | 74% | 100% |
Abbreviations: BEV, bevacizumab; ECOG, Eastern Cooperative Oncology Group; IDH, isocitrate dehydrogenase; MGMT, O6-methylguanine-DNA methyltransferase; RedoSx: redo surgery, RT, radiotherapy.
OS Outcome
The median OS from initial diagnosis for the 78 patients managed with an intervention was 13.4 months (95% CI: 12.6-16.3); compared with the larger cohort of 321 patients without an intervention of 17.0 months (95% CI: 15.5-18.5).
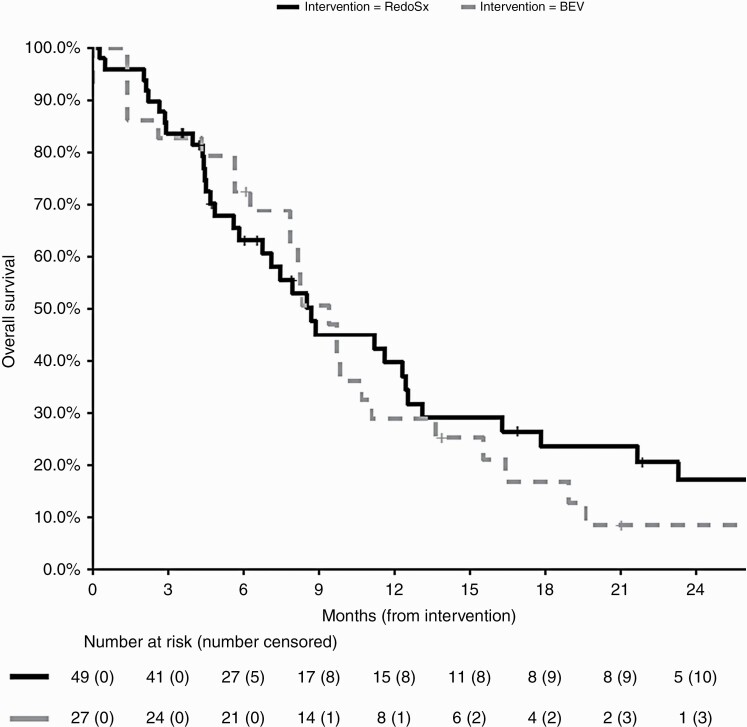
The median OS postintervention was 8.7 months (95% CI: 7.8-13.6). As demonstrated in Figure 1, patients managed with RedoSx or BEV had median OS of 8.7 months (95% CI: 6.8-13.1) and 9.4 months (95% CI: 7.8-13.6), respectively (P = .38).
Figure 1.
Overall survival for patients managed with RedoSx (black, solid) or BEV (gray, dashed) from intervention date. Abbreviation: BEV, bevacizumab.
Of the 49 patients who underwent RedoSx, 43 were managed in the era after April 2011 when BEV was available as an option for intervention. BEV was subsequently required as further salvage for symptoms in the 6 months after RedoSx in 20 (46%) of these patients. There was a trend to improved survival duration from the date of RedoSx with a median survival using subsequent BEV of 12.5 months (95% CI: 8.5-46.5) vs 5.6 months (95% CI: 4.4-17.8) with no subsequent BEV. This, however, did not reach statistical significance (P = .10).
BEV was well tolerated and specifically, no hemorrhagic complications were observed in the cohort of patients managed with BEV as initial intervention.
Association of Baseline Characteristics With OS
The median age of patients at diagnosis was 59 years. The median OS postintervention for those aged older than 60 years of age was 7.8 months (95% CI: 5.6-12.5) compared to 9.8 months (95% CI: 8.3-16.3) for patients aged younger than 60 years (P = .19). Median age of patients who underwent RedoSx was 58 years, compared with a median age of 62 years for those who received BEV. In the patients with RedoSx, there was a trend towards better survival for younger patients (11.6 months vs 5.6 months, P = .13).
As the patient eligibility for an intervention was the presence of a symptomatic contrast-enhancing mass, at time of intervention 83% of patients had an ECOG performance status of 2 or 3. The median OS for patients with an ECOG of 0-1 was 21.6 months (95% CI: 11.2-NA) vs 8.3 months (95% CI: 7.1-10.7) for those with ECOG 2-3 (P = .16). All 13 patients with ECOG 0-1 had RedoSx as their intervention. On analysis of the 65 patients with ECOG 2-3, the median OS following surgery was 8.0 months vs 9.4 months following commencement of BEV (P = .78).
Initial pathology demonstrating MGMT methylation was not associated with improved survival, either as the whole group (P = .61) nor for the RedoSx (P = .54) and BEV (P = .57) cohorts separately.
Timing of Intervention
The median time from date of completion of IMRT to treatment of symptomatic steroid-refractory mass effect was 3 months in both groups. In surgical patients, those whose interventions occurred by 3 months had a median OS of 11.6 months vs 7.1 months in those with longer times to surgery (P = .62). The patients receiving BEV had a median OS of 9.4 months, and was not influenced by whether BEV was commenced by the median intervention time or was commenced later (P = .98).
Histopathology at Redo Craniotomy
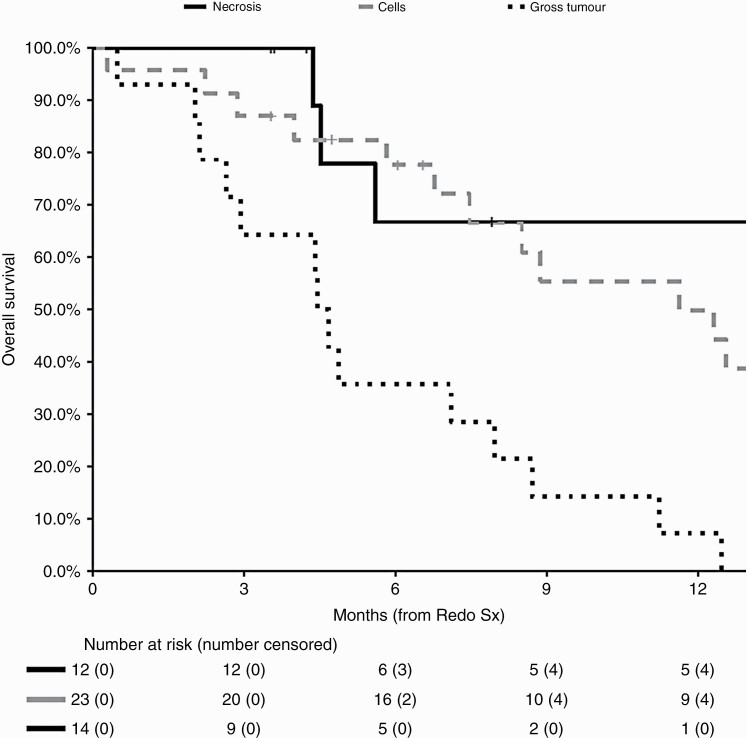
Of the 49 patients who underwent repeat craniotomy, the pathology was pure necrosis in 12 patients; residual glioma cells in 23 patients, and gross tumor in 14 patients (Figure 2). The presence of residual glioma cells correlated with survival after RedoSx with median OS for patients of 4.5 months (95% CI: 2.9-11.2) with gross tumor present; 11.6 months (95% CI: 7.59-23.2) with residual cells; and 32.4 months (95% CI: 5.6-NA) with pure necrosis only (P < .0001) (Table 2).
Figure 2.
Overall survival for patients managed with RedoSx and the presence of residual glioma: necrosis only (black, solid), residual cells only (gray, dashed), and gross residual tumor (black, dotted).
Table 2.
Neuropathological Features From RedoSx (n = 49)
| Subgroup | Median OS (months) | 95% CI | P | |
|---|---|---|---|---|
| Necrosis | >80% | 12.3 | (7.5-35.2) | .012 |
| <80% | 7.1 | (4.4-13.1) | ||
| Ki-67% | <10% | 12.5 | (8.7-29.7) | <.001 |
| >10% | 4.9 | (2.9-11.2) | ||
| Change in Ki-67% | >50% | 12.3 | (8.7-21.6) | <.001 |
| <50% | 4.0 | (2.6-NA) | ||
| Presence of residual glioma | Necrosis only | 32.3 | (5.6-NA) | <.001 |
| Residual cells | 11.6 | (7.5-23.2) | ||
| Gross tumor | 4.5 | (2.9-11.2) |
Abbreviations: CI, confidence interval; NA, not applicable; OS, overall survival.
At time of RedoSx, patients with a Ki-67 of 10% or less had a median OS of 11 months vs 2 months for higher Ki-67 (P < .01); and patients with a reduction in Ki-67 of >50% compared with initial surgical pathology had a median OS of 12 months vs 4 months for those with a <50% reduction (P < .01). Patients with >80% tumor necrosis on redo surgical pathology had a median OS of 11 months vs 4 months for patients with <80% necrosis (P = .01).
Discussion
The presence of a symptomatic contrast-enhancing mass lesion presenting in the 6 months after IMRT for GBM is a challenging clinical scenario. Initial management to reduce raised intracranial pressure and associated neurological/neurocognitive dysfunction is balanced by the necessity to continue the adjuvant phase of TMZ to optimize tumor control. The diagnostic dilemma between pseudoprogression or true progression creates uncertainty for clinicians and patients. Evidence of true progression, when defined by parameters such as increasing Ki-67%,7 will guide salvage interventions but also reflect the poor prognosis for the patient with median survivals less than 4 months. A pseudoprogression event has been demonstrated to be associated with improved outcome,2 but the impact of symptoms, steroid morbidity, and diminished performance status can be significant especially when considering longevity is generally limited to median survivals of 18 months.1,10,11 Steroid-related weight gain and proximal myopathy negatively impact on patients’ functional status either through the direct impact on mobility or through exacerbation of underlying motor neurological deficits. Understanding that at time of relapse patients are generally offered salvage therapies then this progression-free period after adjuvant therapy may be the only treatment-free period prior to the terminal phase of illness.
This study demonstrates that in the presence of a symptomatic contrast-enhancing mass lesion within the 6-month period after IMRT, a redo craniotomy or upfront BEV are equivalent in terms of overall and median survival. Median survival for various patient subgroups can range from 4 months to 32 months, thus the wide range of survival outcomes means that management will require individualization. More research is required regarding patient selection factors to determine the appropriate criteria for which patients should be considered for RedoSx rather than BEV. In this study, surgery was utilized more frequently than upfront BEV for patients with better performance status, and although ECOG performance status was associated with improved survival, the intervention of RedoSx was not. This may be explained when understanding that 46% of the surgical patients were subsequently salvaged with BEV within the next 6 months. Thus, in this clinical scenario of a symptomatic contrast-enhancing mass developing in the 6 months after IMRT, this study raises a future hypothesis for research that RedoSx adds no benefit other than obtaining pathological confirmation to aid diagnosis.
A limitation of this study is that the selection of patients was not controlled but rather individualized with a preference for patients with better performance status being offered redo craniotomy as the initial intervention. While this audit describes no difference in outcome for the patients with ECOG 2-3 performance status managed with either approach, there are no data for the outcome of better performance status patients being managed with BEV upfront. Similarly, another limitation of the study was that the endpoint was limited to survival outcome, and time of subsequent progression and detailed adverse events of each intervention impacting on quality of life was not available.
In a pseudoprogression event, the extent of surgical intervention will be influenced by the site of the tumor and any residual mass may continue to develop progressive enhancement. Thus, BEV was subsequently offered in these patients where the symptomatic progressive enhancement continued. RedoSx may be considered in this regard as a diagnostic procedure rather than a definitive management option and BEV may still need to be considered. BEV will not act as a diagnostic procedure as the gadolinium enhancement from both tumor or pseudoprogression will respond.12,13 Improved noninvasive diagnostic techniques have been sought predominantly using sophisticated MRI sequences with diffusion-weighted imaging and spectroscopy14,15 as well as quantitative PET (positron emission tomography) imaging with both glucose and amino acid tracers.16,17 At present, no definitive investigation has been identified without significant false-positive rates.
The tissue diagnosis that RedoSx provides may require some clarification to clinicians to guide management. Almost 3 quarters of patients in this study had features predominantly suggestive of treatment effect or pseudoprogression on neuropathological assessment with either pure necrosis or minimal residual cells only. There was no significant difference in median survival demonstrated between necrosis and the presence of residual glioma cells. This is important to recognize given that there may be a tendency to link a worse prognosis if tumor cells are noted in the specimen or it may prompt an early cessation of adjuvant systemic therapy and switch to alternative therapies. The definition of true progression on a RedoSx specimen is not well defined, but based on a prior research this audit used the presence of tumor cells whose Ki-67% is greater than 10% or less than 50% reduction from initial diagnostic Ki-67% level.7,18
This study defined a window for pseudoprogression being the presence of progressive gadolinium enhancement within the 6 months from completion of IMRT. Later enhancement is likely to be consistent with tumor progression based on historical series of repeat craniotomy for recurrent tumor.19 Radiation necrosis may develop in the 9-18 months following IMRT but occurs in a lower rate at this timepoint compared to recurrent tumor given the risk for progressive disease beyond 12 months postdiagnosis for GBM. Periventricular demyelination events may also develop in this later timepoint and should be suspected when the focal enhancement occurs on adjacent ventricular surfaces within the IMRT high-dose regions. This has been described less frequently in GBM than lower grade tumors20,21 given the incidence occurring later beyond the shorter median survival timeframes of GBM, but may rise in incidence as initial adjuvant therapy is enhanced with new agents or as median survival increases extend more patients into the timeframe for latent radiation therapy effects.
As BEV becomes an established modality for pseudoprogression scenarios then protocols detailing the optimal timing, intensity, and duration of therapy will need to be established through more evidence. In the AVAGLIO trial adding adjuvant BEV to the Stupp Protocol, the duration of therapy was for continuation of high-dose BEV (10 mg per kg) until time of disease progression.10 This halved the rate of pseudoprogression events compared to the placebo arm.3 For recurrent GBM, and in this study, the BEV protocols prescribe for continuation of high-dose BEV therapy until progression with contrast enhancement or symptomatic deterioration.22,23 However, for established radiation necrosis, a lower dose and duration has been demonstrated to be effective with studies describing only 2 or 4 cycles at a lower dosage (5-7.5 mg per kg).8,9 The dose required may even be lower with doses at 1 mg per m2 being assessed.24 In the pseudoprogression scenario an approach may be to deliver 2 doses at a lower dose regimen, confirm clinical and radiological response, and then monitor with sequential MR surveillance. This would reduce the economic impact as well as minimize the risk of BEV-related morbidity, such as renal hypertension, thrombotic episodes, and intracranial bleed. The brief BEV regimen may also potentially allow a later surgical procedure with less risk of wound healing issues. Such an approach of early brief BEV to minimize the presumed pseudoprogression event will increase the need to obtain noninvasive diagnostic procedures to exclude early progression.
Conclusions
At time of symptomatic steroid-refractory gadolinium contrast enhancement occurring in the 6 months following IMRT for GBM, an intervention with BEV was equivalent to RedoSx in terms of median survival. Further research of noninvasive measures to diagnose pseudoprogression may further define the role of BEV over RedoSx in this initial period after completion of IMRT for GBM.
Acknowledgments
None.
Previous Presentations: Abstract presentation of early data at the Royal Australian and New Zealand College of Radiologists Annual Scientific Meeting on October 26, 2018, Canberra, ACT, Australia
Funding
No funding was received for this study.
Conflict of interest statement. None declared.
References
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 2.Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 3.Wick W, Chinot OL, Bendszus M, et al. Evaluation of pseudoprogression rates and tumor progression patterns in a phase III trial of bevacizumab plus radiotherapy/temozolomide for newly diagnosed glioblastoma. Neuro Oncol. 2016;18(10):1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dijken BRJ, van Laar PJ, Holtman GA, et al. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol. 2017;27(10):4129–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galldiks N, Kocher M, Langen KJ. Pseudoprogression after glioma therapy: an update. Expert Rev Neurother. 2017;17(11):1109–1115. [DOI] [PubMed] [Google Scholar]
- 6.Woodworth GF, Garzon-Muvdi T, Ye X, et al. Histopathological correlates with survival in reoperated glioblastomas. J Neurooncol. 2013;113(3):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gzell C, Wheeler H, Huang D, et al. Proliferation index predicts survival after second craniotomy within 6 months of adjuvant radiotherapy for high-grade glioma. Clin Oncol (R Coll Radiol). 2016;28(3):215–222. [DOI] [PubMed] [Google Scholar]
- 8.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system [published correction appears in Int J Radiat Oncol Biol Phys. 2012 Sep 1;84(1):6. Grewal, Jai [added]]. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lubelski D, Abdullah KG, Weil RJ, et al. Bevacizumab for radiation necrosis following treatment of high grade glioma: a systematic review of the literature. J Neurooncol. 2013;115(3):317–322. [DOI] [PubMed] [Google Scholar]
- 10.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 11.Jayamanne D, Wheeler H, Cook R, et al. Survival improvements with adjuvant therapy in patients with glioblastoma. ANZ J Surg. 2018;88(3):196–201. [DOI] [PubMed] [Google Scholar]
- 12.Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22(6):633–638. [DOI] [PubMed] [Google Scholar]
- 13.Arevalo OD, Soto C, Rabiei P, et al. Assessment of glioblastoma response in the era of bevacizumab: longstanding and emergent challenges in the imaging evaluation of pseudoresponse. Front Neurol. 2019;10:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma N, Cowperthwaite MC, Burnett MG, et al. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol. 2013;15(5):515–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dijken BRJ, van Laar PJ, Smits M, et al. Perfusion MRI in treatment evaluation of glioblastomas: clinical relevance of current and future techniques. J Magn Reson Imaging. 2019;49(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundemann M, Munck Af Rosenschöld P, Muhic A, et al. Feasibility of multi-parametric PET and MRI for prediction of tumour recurrence in patients with glioblastoma. Eur J Nucl Med Mol Imaging. 2019;46(3):603–613. [DOI] [PubMed] [Google Scholar]
- 17.Galldiks N, Dunkl V, Ceccon G, et al. Early treatment response evaluation using FET PET compared to MRI in glioblastoma patients at first progression treated with bevacizumab plus lomustine. Eur J Nucl Med Mol Imaging. 2018;45(13):2377–2386. [DOI] [PubMed] [Google Scholar]
- 18.Kim J-H, Bae Kim Y, Han JH, et al. Pathologic diagnosis of recurrent glioblastoma: morphologic, immunohistochemical, and molecular analysis of 20 paired cases. J Surg Pathol. 2012;36:620–628. [DOI] [PubMed] [Google Scholar]
- 19.Goldman DA, Hovinga K, Reiner AS, et al. The relationship between repeat resection and overall survival in patients with glioblastoma: a time-dependent analysis. J Neurosurg. 2018;129(5):1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van West SE, de Bruin HG, van de Langerijt B, et al. Incidence of pseudoprogression in low-grade gliomas treated with radiotherapy. Neuro Oncol. 2017;19(5):719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bronk JK, Guha-Thakurta N, Allen PK, et al. Analysis of pseudoprogression after proton or photon therapy of 99 patients with low grade and anaplastic glioma. Clin Transl Radiat Oncol. 2018;9:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 23.Hovey EJ, Field KM, Rosenthal MA, et al. ; CABARET/COGNO Investigators . Continuing or ceasing bevacizumab beyond progression in recurrent glioblastoma: an exploratory randomized phase II trial. Neurooncol Pract. 2017;4(3):171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuang H, Zhuang H, Shi S, et al. Ultra-low-dose bevacizumab for cerebral radiation necrosis: a prospective phase II clinical study. Onco Targets Ther. 2019;12:8447–8453. [DOI] [PMC free article] [PubMed] [Google Scholar]