Abstract
Context
Serum inflammation-based scores reflect systemic inflammatory response and/or patients’ nutritional status, and may predict clinical outcomes in cancer. While these are well-described and increasingly used in different cancers, their clinical usefulness in the management of patients with endocrine tumors is less known.
Evidence acquisition
A comprehensive PubMed search was performed using the terms “endocrine tumor,” “inflammation,” “serum inflammation-based score,” “inflammatory-based score,” “inflammatory response-related scoring,” “systemic inflammatory response markers,” “neutrophil-to-lymphocyte ratio,” “neutrophil-to-platelet ratio,” “lymphocyte-to-monocyte ratio,” “Glasgow prognostic score,” “neutrophil-platelet score,” “Systemic Immune-Inflammation Index,” and “Prognostic Nutrition Index” in clinical studies.
Evidence synthesis
The neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio are the ones most extensively investigated in patients with endocrine tumors. Other scores have also been considered in some studies. Several studies focused in finding whether serum inflammatory biomarkers may stratify the endocrine tumor patients’ risk and detect those at risk for developing more aggressive and/or refractory disease, particularly after endocrine surgery.
Conclusions
In this review, we summarize the current knowledge on the different serum inflammation-based scores and their usefulness in predicting the phenotype, clinical aggressiveness, and disease outcomes and prognosis in patients with endocrine tumors. The value of such serum inflammation-based scores in the management of patients with endocrine tumors has been emerging over the last decade. However, further research is necessary to establish useful markers and their cut-offs for routine clinical practice for individual diseases.
Keywords: endocrine tumor, serum inflammation-based score, neutrophil-to-lymphocyte ratio, neutrophil-to-platelet ratio, lymphocyte-to-monocyte ratio, systemic immune-inflammation index
Inflammation plays an important role in tumor biology not only in local tumor microenvironment (1,2) but also systemically (3,4). Responses to systemic inflammation include alterations in hematopoiesis and secretion of acute-phase proteins, cytokines, and growth factors and hormones, as well as in neuroendocrine metabolism and hormone secretion (5,6). Serum inflammation-based scores can reflect systemic inflammatory status and predict outcomes in cancer patients and become potentially useful diagnostic and monitoring tools (3,5,7). They are inexpensive, fast accessed, easy to calculate, reproducible, and widely available, as virtually every cancer patient will have pretreatment blood tests (3). To date, multiple serum inflammation-based scores have been developed:
Neutrophil-to-lymphocyte ratio (NLR) is the most extensively studied score and is calculated by dividing absolute neutrophil by lymphocyte counts (3,8,9). Neutrophilia is common in cancer and is mainly caused by tumor secretion of myeloid growth factors or by inflammation secondary to tissue destruction or hypercytokinemia. Neutrophils, in turn, secrete cytokines which promote tumor proliferation and invasion. Lymphocytes are important antitumoral cells, and their decrease may compromise responses against tumor cells (3,7,9). Hence, high NLR reflects an ineffective immune response to the tumor or imbalanced inflammatory state that facilitates tumor growth and invasiveness and thus poor outcomes in cancer (3,8,9). Similar, although significantly less used, is the derived NLR (dNLR), calculated by dividing absolute neutrophil count by (absolute leucocyte count − absolute neutrophil count) (10,11).
Platelet-to-lymphocyte ratio (PLR) is also widely studied and can be obtained by dividing absolute platelet by lymphocyte counts (3,12,13). Tumor cells secrete cytokines and growth factors that stimulate megakaryocyte differentiation leading to increased platelet production (14). Platelets have different roles in cancer and may promote tumor proliferation, invasion, or metastasis (13,15,16). High PLR reflects a relative imbalance of platelets and lymphocytes and is usually associated with poor cancer outcomes (3,12).
Lymphocyte-to-monocyte ratio (LMR) is calculated by dividing absolute lymphocyte by monocyte counts. Higher monocyte counts may reflect a higher content of tumor-associated macrophages in neoplastic tissues, resulting in immunosuppression and tumor growth (17,18). Monocytes and macrophages may also contribute to intravascular survival of circulating tumor cells by reducing their apoptosis and extravasation into tumor tissues, which ultimately result in tumor progression and worse outcomes (19,20). Lower LMR has been associated with poor survival in different cancers (18,21).
Neutrophil-platelet score (NPS) and Systemic Immune-Inflammation Index (SII) combine leucocyte and platelet counts. NPS, ranging from 0 to 2, attributes 1 point to neutrophils >7.5 × 109/L and 1 to platelets >400 × 109/L (22). SII is obtained by multiplying absolute platelet count and NLR (23). An increased NPS or SII reflects an ineffective immunosurveillance or enhanced inflammatory status linked with poor outcomes (22,23).
Glasgow prognostic score (GPS), ranging from 0 to 2, attributes 1 point to C-reactive protein >10 mg/dL and 1 to albumin <35 g/L (3,5). GPS is regarded as an indicator for nutritional and immunological condition, as decreased albumin and increased C-reactive protein may reflect malnourishment and systemic inflammation (3,5,7,24). Modified versions of GPS have been proposed, including the High-Sensitivity Inflammation-based Prognostic Index (HSPI), which relies on high-sensitivity C-reactive protein with a cut-off of 3 mg/dL (25).
The Prognostic Nutrition Index (PNI) is calculated by multiplying serum albumin by 5 times the absolute lymphocyte count and also reflects the nutritional and inflammatory status; reduced PNI is often associated with poor cancer prognosis (26,27).
In summary, increased NLR, PLR, NPS, SII, and GPS and low LMR and PNI reflect ineffective immunosurveillance or imbalanced inflammation that facilitate tumor growth and invasiveness and thus poor outcomes in cancer (3,5,8,18). Several lines of research support the role of serum inflammation-based scores in predicting outcomes in cancer patients (3,7), including not only nonmetastatic tumors, such as meningiomas (28) and gliomas (23,24,29), but also in endocrine neoplasms (6,30-36). In this review, we summarize the current knowledge on different serum inflammation-based scores and their clinical usefulness in predicting phenotype, aggressiveness, disease outcomes, and prognosis in patients with endocrine tumors.
Methods
A PubMed search was performed using the terms “endocrine tumor,” “inflammation,” “serum inflammation-based score,” “inflammatory-based score,” “inflammatory response-related scoring,” “systemic inflammatory response markers,” “Neutrophil-to-lymphocyte ratio,” “neutrophil-to-platelet ratio,” “lymphocyte-to-monocyte ratio,” “Glasgow prognostic score,” “neutrophil-platelet score,” “Systemic Immune-Inflammation Index,” and “Prognostic Nutrition Index” in clinical studies concerning endocrine neoplasms: thyroid tumors, neuroendocrine tumors (NETs), parathyroid tumors, adrenal tumors, pituitary tumors, and craniopharyngiomas. Related articles and reference lists in each publication were also reviewed. All papers indexed in the English language and published until the December 31, 2020 were evaluated. All identified studies were analyzed, and their most relevant findings briefly discussed in the review and summarized in more detail in Tables 1 to 3.
Table 1.
Overview of the studies investigating the role of serum inflammation-based scores in thyroid tumors
| Thyroid tumor type | Study (first author, year, reference) | Study population | Summary of the main findings of the study per serum inflammation-based score |
|---|---|---|---|
| Differentiated thyroid cancer | Liu 2013 (30) | 159 DTC pts, 138 benign thyroid nodule pts | • Higher NLR was seen in pts with high than with intermediate (P = 0.026) or low (P = 0.037) ATA risk for recurrence |
| • Tumor size correlated with NLR (r = 0.245; P = 0.002), even when microcarcinomas were excluded from the analysis (r = 0.255; P = 0.009). No difference in preoperative NLR between DTC and benign thyroid nodule pts (1.94 vs 1.86; P = 0.293) | |||
| Seretis 2013 (37) | 26 thyroid cancer pts, 31 incidental microPTC pts, 26 goiter pts, 26 HCs | • Higher NLR was observed in pts with microPTC and thyroid cancer (3.0 and 3.4, respectively) than in those with goiter or HCs (1.9 and 1.8, respectively; P < 0.001) | |
| Kim 2014 (38) | 542 PTC pts, 210 benign thyroid nodule pts | • 5-year disease-free survival was worse in PTC pts staged III/IV with NLR ≥ 1.5 (94.1% vs 99.3%; P = 0.013); however, NLR had no prognostic value in pts with PTC stages I/II | |
| • Univariate analysis suggested NLR as independent prognostic factor for disease-free survival (HR = 8.76, 95% CI 1.09-70.27; P = 0.041) | |||
| • There were no difference in NLR between PTC pts and 210 pts with benign nodules (1.79 ± 0.88 vs 1.74 ± 0.84; P = 0.504) | |||
| Lang 2014 (39) | 191 PTC pts after thyroidectomy and central neck dissection | • NLR was not associated with disease-free survival or presence of central nodal metastasis, but a higher NLR correlated with tumor size (P = 0.037) and older age at diagnosis (P < 0.001) | |
| Cho 2015 (40) | 3364 PTC pts, 34 FTC pts, 15 MTC pts, 14 PDTC pts, 7ATC pts, 436 benign lesion pts | • Pts with NLR > 1.57 had higher proportions of ATC (0.3% vs 0.1%) and PDTC (0.6% vs 0.1%), as well as higher rates of advanced stages T3/T4 (30.4% vs 26.5%; P = 0.027) and cancer-specific death (1.2% vs 0.4%; P = 0.027) | |
| • NLR was able to discriminate between PTC (1.86 ± 1.16), PDTC (2.13 ± 0.76) and ATC (5.51 ± 4.45; respectively P = 0.035, 0.002, and 0.025), with optimal cut-off distinguishing PDTC and ATC estimated at 3.8 (none of the PDTC pts had a NLR > 3.8) | |||
| Kim 2015 (41) | 1066 female PTC pts after thyroidectomy | • Preoperative NLR was lower in pts aged ≥ 45 (1.52 vs 1.75; P = 0.001) and in Hashimoto’s thyroiditis pts (1.52 vs 1.58; P = 0.005) | |
| • PLR was lower in pts aged ≥ 45 (123.60 vs 135.60 P < 0.001) and in Hashimoto’s thyroiditis pts (119.48 vs 127.61; P = 0.039) | |||
| • PLR was higher in pts with tumors > 1cm (129.17 vs 124.41; P = 0.021) | |||
| • Pts with higher PLR had increased incidence of lateral lymph node metastasis (P = 0.018) | |||
| Kocer 2015 (42) | 40 PTC pts, 25 PTC with thyroiditis pts, 70 multinodular goiter pts, 97 thyroiditis pts | • Higher NLR was observed in PTC pts than in subjects with benign disorders (P < 0.05), with a cut-off 1.91 differentiating between malignant and benign lesions | |
| Liu 2015 (43) | 321 PTC pts, 83 adenoma pts, 439 goiter pts | • PTC pts had higher NLR than pts with thyroid adenoma and nodular goiter (2.28 vs 1.98 vs 1.98; P = 0.036) | |
| • Within PTC pts, a higher NLR was observed in older pts, and in pts with PTC staged III/IV | |||
| Demir 2016 (44) | 57 RAI treated DTC patients, 37 non-RAI DTC pts, 37 HCs | • An increase in NLR and PLR was observed two months after RAI treatment (P = 0.021 and 0.001, respectively), which were lower at 6 months after RAI | |
| • At baseline, NLR was higher in PTC pts treated with RAI than in HCs | |||
| • No differences in NLR and PLR before and 8-months after the surgery in PTC pts not treated with RAI | |||
| Gong 2016 (45) | 161 PTC pts | • Pts with high preoperative NLR (≥2) more often had lymph node metastasis (52% vs 25%; P < 0.001), larger tumors (1.30 ± 0.88 vs 0.75 ± 0.46; P < 0.001) and multifocal tumors (35% vs 19%; P = 0.035) | |
| • There was a correlation between NLR and lymph node metastasis (r = 0.341; P < 0.001), tumor size (r = 0.271; P < 0.001) and multifocality (r = 0.182; P = 0.010) | |||
| • For PTC pts ≥ 45 years, the proportion of pts with advanced TNM stage was higher in the subgroup with elevated NLR, with a correlation between NLR and TNM stage (r = 0.403; P < 0.001) | |||
| Liu 2016 (46) | 4617 DTC pts, 1666 thyroid nodule pts | • In this meta-analysis, serum NLR of DTC pts was similar to benign thyroid nodule pts, and there was no difference in the NLR between pts aged < 45 and ≥45 years | |
| Yaylaci 2016 (47) | 41 PTC pts, 38 goiter pts | • No differences between regarding preoperative NLR between PTC and goiter pts (1.9 ± 0.7 vs 1.8 ± 0.9; P = 0.750) | |
| Machairas 2017 (48) | 89 PTC pts and 139 multinodular hyperplasia pts after thyroidectomy | • No differences in NLR and PLR between pts with malignant and benign thyroid lesions (2.2 ± 1.0 vs 2.2 ± 1.1, and 122 ± 44 vs 128 ± 49, respectively) | |
| • Among PTC pts, there was no association between NLR and PLR and extrathyroidal extension, lymphovascular invasion, tumor stage or tumor size | |||
| • PNR was higher in PTC pts with extrathyroidal extension (71.7 ± 23.3 vs 56.9 ± 19.5; P = 0.012) and T3 tumors (70.3 ± 23.3 vs 57.4 ± 19.8; P = 0.028) | |||
| Manatakis 2017 (49) | 205 PTC pts | • NLR was higher in cases with extrathyroidal invasion (2.74 ± 1.24 vs 2.39 ± 0.96; P = 0.040), multifocality (2.65 ± 1.08 vs 2.29 ± 0.96; P = 0.010) and lymph node metastasis (3.12 ± 1.07 vs 2.41 ± 1.02; P = 0.030) | |
| • Pts with NLR > 2.17 more often had lymph node metastasis (11.7% vs 2.9%; P = 0.030) | |||
| Ozmen 2017 (31) | 51 PTC pts, 42 microPTC pts, 31 FTC pts, 50 HCs | • Preoperative NLR correlated with tumor diameter (r = 0.219; P = 0.030), age (r = 0.234; P = 0.002) and 6-month postoperative thyroglobulin levels (r = 0.261; P = 0.010) | |
| • Mean NLR and PLR were similar in pts with PTC (3.10 ± 1.85 and 154.41 ± 76.49), microPTC (2.85 ± 1.39 and 141.23 ± 63.44) and FTC (3.55 ± 2.91 and 163.13 ± 88.14), but generally higher than in HCs (1.62 ± 0.56 and 103.12 ± 33.75) | |||
| Lee 2018 (50) | 151 DTC pts, 87 benign thyroid lesion pts | • NLR decreased after PTC treatment (1.96 vs 1.78; P = 0.037); the NLR decrease was more prominent in those with low risk of recurrence (P = 0.017) and excellent treatment response (P < 0.001) | |
| • PTC pts with a structural incomplete response had an NLR increase during follow-up (P = 0.012) | |||
| • Incomplete response to therapy was independently associated with increased NLR (OR = 18.66, 95%CI 3.26-106.84; P = 0.001) | |||
| • No differences in preoperative NLR between pts with benign or malignant lesions (1.93 vs 1.96; P = 0.633) | |||
| Manatakis 2018 (51) | 397 total thyroidectomy pts | • Preoperative NLR was higher in PTC and in microPTC pts when compared to benign thyroid disease pts (P = 0.026), however it scored low for diagnostic accuracy (approximately 45%) | |
| • PLR did not differ among pts with carcinomas and microcarcinomas compared to pts with benign thyroid disease | |||
| Wen 2018 (52) | 558 PTC pts aged ≥ 55 years | • Elevated LMR was prognostic for advanced TNM stage (AUC of 0.680; P = 0.040) | |
| • LMR ≥ 5.45 was an independent factor for advanced TNM stage (OR = 7.306; P = 0.036) | |||
| • High LMR was not associated with lymph node metastasis or coexistence of Hashimoto’s thyroiditis | |||
| • No value for NLR in predicting advanced TNM, lymph node metastasis or coexistence of Hashimoto’s thyroiditis | |||
| Ari 2019 (53) | 50 PTC pts 50 thyroiditis pts, 46 HCs | • NLR was higher in thyroiditis (2.42 ± 1.40; P = 0.017) and nonsignificantly higher in PTC pts (2.14 ± 0.90) in comparison to HCs (1.89 ± 0.70), but there were no differences between PTC and thyroiditis pts | |
| • PLR was higher in thyroiditis (139.1 ± 52.0; P < 0.001) and PTC pts (136.7 ± 57.0; P = 0.003) in comparison to HCs (107.0 ± 22.3); but there were no differences between PTC and thyroiditis pts | |||
| Ceylan 2019 (54) | 201 PTC pts | • Pts with NLR ≥ 1.92 had larger tumors (2.79 ± 1.48 vs 2.24 ± 1.13; P = 0.002) and extrathyroidal extension (78.6% vs 21.4%; P = 0.028) | |
| • No association between high NLR and age, capsule invasion, surgical margin positivity, multifocality and lymph node metastasis | |||
| • No association between high PLR and clinicopathological features | |||
| Kutluturk 2019 (55) | 58 PTC pts | • No differences in NLR and PLR between PTC pts before surgery, before RAI treatment and 6 months after RAI were observed | |
| Lee 2019 (56) | 1921 pts after thyroidectomy | • NLR > 2.1 was an independent predictor for recurrence (HR = 2.96, 95%CI 1.08-14.73; P = 0.035) in pts aged ≥ 45 years | |
| • NLR was lower in PTC pts aged ≥ 45 years (1.7 ± 0.9 vs 1.9 ± 0.9; P < 0.001) | |||
| • PLR > 164.24 was an independent predictor for recurrence (HR = 3.08, 95%CI 1.26-7.52; P = 0.014) in pts < 45 years | |||
| • PLR was lower in PTC pts aged ≥ 45 years (128.1 ± 45.7 vs 146.7 ± 48.5; P = 0.019) | |||
| • There was no association between high LMR and recurrence | |||
| • LMR was higher in PTC pts aged ≥ 45 years (6.5 ± 2.5 vs 5.9 ± 2.2; P < 0.001) | |||
| Sit 2019 (57) | Malignant and benign thyroid nodule pts, HCs | • NLR of the malignant nodule subgroup (2.1 ± 0.9) was significantly higher than both benign nodule (1.7 ± 0.9) and HC (1.7 ± 0.6) subgroups | |
| Song 2019 (58) | 224 high-risk PTC pts | • LMR < 4 correlated with larger tumor (P = 0.001), multifocality (P = 0.001), advanced N stage (P = 0.011) and distant metastasis (P = 0.003) | |
| • LMR was an independent prognostic factor for overall and disease-free survival, with LMR < 4 being associated with decreased overall and disease-free survival | |||
| • LMR of RAI refractory cases was lower than that of the non-RAI refraction cases (4.5 ± 2.4 vs 6.0 ± 2.1; P < 0.001) | |||
| Ahn 2020 (59) | 40 pts with progressive RAI-refractory DTC treated with sorafenib | • LMR < 4 was an independent risk factor for all-cause mortality (HR = 2.64, 95%CI 1.04-6.72; P = 0.04) and for progression-free survival (HR = 2.69, 95%CI 1.02-7.04; P = 0.045) | |
| • The progression-free survival (P = 0.02) and overall survival (P = 0.02) curves differed based on low vs high LMR, with more favorable outcomes in pts with LMR ≥ 4; median progression-free survival of low vs high LMR groups were 10.4 and 29 months | |||
| • No differences in disease response or disease control duration to sorafenib between pts with low vs high LMR | |||
| Chen 2020 (27) | 1873 PTC pts | • Decreased PNI was predictive of advanced T stage (AUC of 0.542, 95%CI 0.512-0.572;P = 0.01), with best cut-off 54.1 | |
| • PNI ≤ 53.1 was an independent factor predicting recurrence (OR = 1.511, 95%CI 1.136-2.009; P = 0.01) | |||
| • Decreased NLR was predictive of recurrence (AUC of 0.541, 95%CI 0.500-0.582; P = 0.04), with best cut-off value 1.6 | |||
| • NLR ≤ 1.6 was independent factor predicting recurrence (OR = 1.596, 95%CI 1.207-2.111; P = 0.001) | |||
| • Increased PLR was predictive of advanced N stage (AUC of 0.530; 95%CI 0.503-0.557; P = 0.030), with best cut-off 136.5 | |||
| Fukuda 2020 (60) | 33 progressive RAI-refractory DTC pts receiving lenvatinib | • Overall survival was significantly shorter in pts with NLR ≥ 3 at the start of lenvatinib (12 vs 35 months) | |
| • NLR decreased in pts that achieved best response to lenvatinib (P < 0.001), and increased again upon disease progression (P < 0.01) | |||
| Yokota 2020 (61) | 570 PTC pts | • Preoperative LMR < 5 predicted recurrence (sensitivity 63.3% and specificity 68.7%); LMR was lower in pts with vs without recurrence (4.99 ± 2.05 vs 6.37 ± 2.3; P = 0.002) | |
| • LMR < 5 was an independent factor predicting 10-year recurrence-free survival (P = 0.022) | |||
| • Low LMR predicts recurrence in pts with advanced PTC including stage II (P < 0.001) and III (P = 0.035) | |||
| • No associations between low LMR and size, stage, extrathyroidal extension, multifocality, thyroglobulin levels or presence of chronic thyroiditis | |||
| • Pts with NLR ≥ 2 had a lower rate of 10-year recurrence-free survival in the univariate analysis (88.9% vs 95.6%; P = 0.010), but not on the multivariate analysis | |||
| • No associations noted regarding preoperative PLR | |||
| Medullary thyroid cancer | Cho 2015 (40) | 3364 PTC pts, 34 FTC pts, 15 MTC pts, 14 PDTC pts, 7ATC pts, 436 benign lesion pts | • NLR was unable to discriminate between PTC or PDTC and MTC (2.00 ± 0.82), but allowed distinction between MTC and ATC (P = 0.026) |
| Jiang 2016 (62) | 70 MTC pts | • NLR was not an independent predictive factor for lymph node metastasis or recurrence | |
| • PLR > 105.3 was an independent predictor of lymph node metastasis (OR = 4.782, 95%CI 1.4-16.7), and PLR > 129.8 independently predicted recurrence (OR = 3.838, 95%CI 1.1-13.5) | |||
| • PLR > 142.1 (OR = 3.452, 95%CI 1.0-11.8) was an independent predictor for lateral nodal compartment metastasis | |||
| • Pts with NLR > 1.9 had higher rates of multifocality (P = 0.001) and bilaterality (P = 0.001), but not of other features | |||
| • Pts with PLR > 102.5 had larger tumors (P = 0.031), higher proportions of total and lateral lymph node metastasis (P = 0.019 and P = 0.048, respectively) and tended to have more recurrences (P = 0.088) | |||
| Jiang 2017 (63) | 78 MTC pts | • Increased preoperative PLR predicted lymph node metastasis (AUC: 0.644; P = 0.022), capsule invasion (AUC of 0.666; P = 0.007), advanced stage (AUC: 0.657; P = 0.011) and recurrence (AUC: 0.655; P = 0.049) | |
| • PLR was a predictor for recurrence on Kaplan-Meier and Cox regression analysis, with an AUC of 0.703 (95%CI 0.589-0.801; P = 0.002). Optimal cut-off predicting recurrence was 128.9; pts with a PLR > 128.9 had lower disease-free survival | |||
| • Reduced PNI was predictive of recurrence (AUC of 0.655; P = 0.049), with optimal cut-off estimated at 52.5 | |||
| • No associations between NLR, dNLR, and LMR and aggressive clinical features neither with poor outcomes | |||
| Xu 2018 (64) | 61 MTC pts | • Pts with lymph node and distant metastasis had higher NLR pts without metastasis (1.92 vs 1.69; P = 0.019) | |
| • Preoperative NLR was associated with lymph node and distant metastasis (OR = 5.918, 95%CI 1.147-30.541; P = 0.034), with best predictive cut-off NLR estimated at 1.784 (AUC of 0.717, sensitivity 68.3%, specificity 80%) | |||
| Anaplastic thyroid cancer | Lang 2014 (39) | 192 PTC pts, 192 goiter pts, 15 ATC pts | • ATC pts had higher NLR than PTC pts with lymph node metastasis (7.28 ± 4.64 vs 2.74 ± 2.32; P < 0.001) |
| Cho 2015 (40) | 3364 PTC pts, 34 FTC pts, 15 MTC pts, 14 PDTC pts, 7 ATC pts, 436 benign lesion pts | • NLR was able to discriminate between PTC (1.86 ± 1.16), PDTC (2.13 ± 0.76) and ATC (5.51 ± 4.45; respectively P = 0.035, P = 0.002 and P = 0.025), with best cut-off distinguishing PDTC vs ATC being 3.8 (none of the PDTC pts exceeded NLR of 3.8) | |
| Ahn 2019 (65) | 35 ATC pts | • Pts with LMR < 4 had a higher proportion of cervical lymph node metastasis (95.7% vs 58.3%; P = 0.021) | |
| • Overall survival was lower in pts with LMR < 4 (3.0 vs 9.5 months; P = 0.004) | |||
| • Low LMR was an independent risk factor for all-cause mortality (HR = 2.55; 95%CI 1.08-6.00; P = 0.002) | |||
| Yamazaki 2020 (66) | 55 ATC pts | • No differences in overall survival according to the baseline NLR, PLR, and LMR | |
| • Preoperative NLR was lower than mean NLR at time of disease progression (P < 0.001) | |||
| • Among pts with full blood count follow-up data, in pts with an increase of NLR > 5.5 median overall survival was shorter (7.7 months (95%CI 5.2-12.1) vs 23.5 months (95%CI 13.9-not available); P < 0.001) | |||
| Fukuda 2020 (67) | 13 ATC pts treated with lenvatinib | • Better disease control rate in lenvatinib-treated ATC pts with NLR < 8 (89%) than in those with NLR ≥ 8 (89% vs 25%; P = 0.05) | |
| • Progression-free and overall survival were longer in pts with NLR < 8 than in pts with NLR ≥ 8 (respectively, 4.0 vs 1.6 months, P < 0.05; and 10.2 vs 3.8 months, P < 0.05) |
Abbreviations: ATA, American Thyroid Association; ATC, anaplastic thyroid cancer; AUC, area under curve; CI, confidence interval; CRP, C-reactive protein; DTC, differentiated thyroid cancer; FTC, follicular thyroid cancer; HCs, healthy controls; HR, hazard ratio; IQR, interquartile ranges; LMR, lymphocyte-to-monocyte ratio; microPTC, papillary thyroid microcarcinoma; MLR, monocyte-to-lymphocyte ratio; MTC, medullary thyroid cancer; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; PDTC, poorly differentiated thyroid cancer; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; PNR, platelet-to-neutrophil ratio; PTC, papillary thyroid cancer; pts, patients; RAI, radioactive iodine; ROC, receiver operative characteristic curve; TSH, thyroid-stimulating hormone.
Table 3.
Overview of the studies investigating the role of serum inflammation-based scores in different endocrine tumors
| Endocrine tumor type | Study (first author, year, reference) | Study population | Summary of the main findings of the study per serum inflammation-based score |
|---|---|---|---|
| Parathyroid tumors | Zeren 2015 (85) | 32 pts with primary hyperparathyroidism | • Preoperative NLR was 2.1 ± 0.9, with positive correlations between preoperative NLR and adenoma size, presence of carcinoma, calcium levels and PTH levels |
| Yang 2018 (86) | 213 secondary hyperparathyroidism pts after parathyroidectomy | • There were correlations between preoperative NLR and PLR and levels of serum phosphorous (rho = 0.17; P = 0.015 and rho = 0.19; P = 0.007, respectively) and calcium-phosphorus product (rho = 0.14; P = 0.046 and rho = 0.17; P = 0.014) | |
| • Postoperative PTH levels correlated with both NLR and PLR at follow-up (rho = 0.29; P = 0.004 and rho = 0.24; P = 0.023, respectively) | |||
| • NLR and PLR significantly decreased after successful parathyroidectomy (P = 0.0006 and P = 0.0003, respectively), while pts who had persistent or recurrent hyperparathyroidism had no significant changes | |||
| Lam 2019 (87) | 95 pts with primary hyperparathyroidism | • A correlation was observed between preoperative NLR and serum PTH (r = 0.274; P = 0.013) and calcium (r = 0.376; P = 0.001) levels | |
| • Pts had a decrease in median NLR after successful parathyroidectomy from 2.26 (IQR: 1.70-3.00) to 1.77 (IQR: 1.59-2.61) (P = 0.037) | |||
| Toraman 2019 (88) | 301 pts with high PTH levels | • A positive correlation was observed between serum NLR (but not PLR) and PTH and creatinine levels | |
| • There was a significant negative correlation between NLR (and PLR) and serum calcium levels | |||
| • Main determinants of NLR were PTH, albumin, LDL-cholesterol, hemoglobin and gender | |||
| Adrenal tumors | Bagante 2015 (89) | 84 ACC pts | • Pts with preoperative NLR > 5 (34.5%) had higher proportions of tumors > 5cm (100% vs 86%), resection of metastasis in other organs (69% vs 38%), incomplete resection R1/R2 (56% vs 26%), post-surgical complications (56% vs 27%) and readmissions within 90 days after operation (41% vs 14%) |
| • Pts with NLR > 5 had lower 5-year recurrence-free survival rates (10.5% vs 14.2%) | |||
| • NLR independently predicted both disease-specific (HR = 2.21) and recurrence-free survival (HR = 1.99) | |||
| • No significant differences in glucocorticoid excess rates between pts with NLR ≤ 5 and pts with NLR > 5 (38.1% vs 61.9%; P = 0.201) | |||
| • Pts with preoperative PLR > 190 (38.1%) had higher proportion of tumors > 5cm (100% vs 84%) and resection of metastasis in other organs (69% vs 36%) | |||
| • Pts with PLR > 190 had lower 5-year recurrence-free survival rates (5.2% vs 19.4%) | |||
| • PLR independently predicted recurrence-free survival (HR = 1.72) | |||
| • No significant differences in glucocorticoid excess rates between pts with PLR ≤ 190 and pts with PLR > 190 (61.9% vs 38.1%; P = 0.826) | |||
| Mochizuki 2017 (90) | 46 benign adrenal tumor pts, 13 malignant adrenal tumor pts (9 ACC) | • Preoperative NLR was higher in pts with malignant than those with benign adrenal disease (4.8 ± 2.9 vs 3.0 ± 1.8; P = 0.016) | |
| • Median NLR was 2.84 in adrenocortical adenomas; 2.03 in pheochromocytomas; 6.02 in ACC; 3.30 in lymphomas | |||
| • NLR was an independent predictor of malignant adrenal disease, with best cut-off estimated at 3.15 (area under ROC of 0.668) | |||
| • Among ACC pts, those with preoperative NLR ≥ 5 had a poorer survival (median survival of 174 vs 917 days; P = 0.032) | |||
| Gaitanidis 2019 (19) | 25 recurrent ACC pts after surgery | • A shorter disease-specific survival was seen in ACC pts with LMR < 4 (41 ± 7.4 vs 71 ± 12.3 months; P = 0.023) | |
| • LMR < 4 was independently associated with worse disease-specific survival (HR = 4.18; 95% CI: 1.18-14.76; P = 0.027) | |||
| • No associations between NLR or PLR and disease-specific survival | |||
| Pituitary tumors | Marques 2020 (91) | 68 prolactinoma pts, 72 acromegaly pts, 70 CD pts, 208 NFPA pts and 6 thyrotrophinoma pts after surgery | • CD pts had significantly higher preoperative NLR, SII and NPS in comparison to other pituitary tumor types |
| • Within Cushing’s disease pts: | |||
| • There was an association between elevated GPS and 24h-UFC or ACTH levels | |||
| • There were no association between inflammation-based scores and features/outcomes suggestive of clinically challenging disease, but pts who had multimodal treatment had fewer platelets (242 ± 50 vs 304 ± 86 x109/L; P = 0.001), with a platelet count <299.5x109/L predicting multimodal treatment | |||
| • Within prolactinoma + acromegaly + thyrotrophinoma pts. | |||
| • Pts with GPS ≥ 1 had higher rates of hypopituitarism (25% vs 4%; P = 0.048) and suprasellar extension (25% vs 4%; P = 0.048) | |||
| • Pts with NPS ≥ 1 had higher rates of suprasellar extension (15% vs 6%; P = 0.028) | |||
| • Lower PLR was observed in pts with macroadenomas (P = 0.039) | |||
| • PNI was lower in pts requiring multiple treatments (35.9 ± 23.1 vs 55.4 ± 5.1; P = 0.048) including radiotherapy (39.7 ± 20.6 vs 54.1 ± 9.7; P = 0.024) | |||
| • Within NFPA pts: | |||
| • Pts with GPS ≥ 1 had higher rates of apoplexy (40% vs 6%; P = 0.001) | |||
| • Pts with visual field defects at presentation had higher NLR (2.6 ± 2.1 vs 1.2 ± 0.3; P = 0.024) and lower LMR (3.8 ± 1.9 vs 5.5 ± 1.1; P = 0.031) | |||
| • Pts with tumor remnant on MRI within 1yr after operation had higher PLR (109 ± 65 vs 1317 ± 46; P = 0.021) | |||
| • Higher NLR was seen in reoperated pts (3.2 ± 1.0 vs 2.2 ± 1.3; P = 0.017) and in those requiring multiple treatments (3.1 ± 1.0 vs 2.2 ± 1.3; P = 0.049) | |||
| • Pts with active disease at last follow-up had lower PNI (25.9 ± 22.3 vs 54.7 ± 6.1; P = 0.021) | |||
| Craniopharyngiomas | Chen 2018 (6) | 197 CP pts, 57 RCC pts, 371 pituitary tumor pts, 682 HCs | • Papillary CP were associated with higher NLR and lower PNI compared to adamantinomatous CP (1.78 vs 1.39; P < 0.05, and 54.7 vs 55.8; P < 0.05, respectively), but there were no differences between primary or recurrent CP regarding other scores |
| • A papillary CP pts, time to recurrence correlated with PLR (r = -0.783; P < 0.05), MLR (r = -0.674; P < 0.05) and PNI (r = 0.577; P < 0.05) | |||
| • CP pts had higher PNI than pituitary tumor and RCC pts and HCs (55.7 vs 51.7 vs 52.8 vs 54.3, respectively; P < 0.05) | |||
| • CP pts had lower MLR than pituitary tumor pts and HCs (0.15 vs 0.18 vs 0.16, respectively; P < 0.05) | |||
| • NLR was higher in pituitary tumor pts than in RCC pts or HCs (1.58 vs 1.39 vs 1.49, respectively; P < 0.05) | |||
| • PNI alone showed good accuracy in predicting a CP (AUC of 0.616; 95% CI 0.568-0.663), but the best predictive value was obtained for the combinations NLR + PNI, dNLR + PNI and PLR + PNI (AUCs of 0.635, 0.6131 and 0.627, respectively) | |||
| • A predictive value for papillary CP (in comparison to other subgroups) was seen for NLR+PLR (AUC of 0.713; 95% CI 0.621-0.805) and dNLR + PLR (AUC of 0.703; 95% CI 0.610-0.797) | |||
| Zhang 2018 (36) | 149 CP pts | • A positive correlation was observed between preoperative NLR and 5-year overall (HR = 1.44, 95% CI 1.16-1.79; P = 0.001) and 5-year progression-free survival (HR = 1.46, 95% CI 1.22-1.74; P < 0.001). | |
| • Best predictive cut-off predicting poor outcome was NLR ≥ 4 | |||
| • Pts with NLR ≥ 4 had larger lesions (5.6 ± 3.5 vs 4.1 ± 1.6 cm2; P = 0.001), lower rates of gross total resection (42 vs 76%; P < 0.001), worse quality of life (2.7 ± 2.0 vs 3.5 ± 1.6 ASBS-Q score; P = 0.039), lower 5-year overall (67 vs 86%; P = 0.009) and 5-year progression-free survival rate (44 vs 86%; P < 0.001) |
ACC, adrenocortical carcinoma; ACTH, adrenocorticotropic hormone; ASBS-Q, anterior skull base surgery questionnaire; AUC, area under curve; CD, Cushing’s disease; CI, confidence interval; CP, craniopharyngioma; CRP, C-reactive protein; dNLR, derived neutrophil-to-lymphocyte ratio; DTC, differentiated thyroid cancer; eGFR, estimated glomerular filtration rate; GPS, Glasgow prognostic score; HCs, healthy controls; HR, hazard ratio; IQR, interquartile ranges; LDL, low-density lipoprotein; LMR, lymphocyte-to-monocyte ratio; MLR, monocyte-to-lymphocyte ratio; NFPA, nonfunctioning pituitary adenoma; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; PTH, parathyroid hormone; pts, patients; RCC, Rathke’s cleft cyst; ROC, receiver operative characteristic curve; SII, systemic inflammation index; UFC, urinary free cortisol.
Thyroid Tumors
Thyroid cancer is the most common endocrine tumor, and most are well-differentiated and derive from follicular epithelial cells [including papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC)]; far less common are medullary (MTC), anaplastic (ATC), and poorly differentiated thyroid cancer (PDTC) (92,93). Thyroid cancer is closely associated with inflammation, both within the tumor microenvironment (94,95) and systemically (30,46,96). The inflammatory microenvironment seems to be important in the development and progression of thyroid malignancy (97-100). This is supported by alterations in expression of immune-related genes and activation of oncogenes in PTC (101), such as RET/PTC, RAS, and BRAF, resulting in upregulation of a proinflammatory program in normal thyrocytes associated with malignant behavior (95,102). Thyroid tumor cells are also an active source of cytokines and chemokines that modulate the microenvironment as well as have proinflammatory implications (94). The correlation of inflammatory responses, reflected by serum inflammation-based scores, with tumor behavior and outcomes in different types of thyroid cancer is discussed in the following text and summarized in Table 1.
Differentiated Thyroid Cancer
Several studies support the role of NLR in predicting prognosis and outcomes in patients with differentiated thyroid cancer (DTC), mainly PTC. Liu et al conducted the first study assessing the usefulness of NLR in DTC and observed that patients at high risk for recurrence had higher NLR than those with intermediate/low risk (30). Higher NLR has been described in patients with more advanced PTC, namely stages III/IV (43) and T3/T4 (40), as well as in PTC patients ≥45 years (45). Higher NLR was further associated with lower disease-free survival in patients with PTC staged III/IV (38) or ≥45 years (56) suggesting NLR may be a prognostic factor for disease-free survival in PTC, particularly in older patients with advanced disease. In contrast, other studies reported no association between NLR and PTC stages (41,48,49,52) or recurrent disease (39,40,61). In PTC and FTC patients, NLR correlated with increased thyroglobulin levels 6 months postoperatively (31). A recent study reported no differences in NLR between PTC patients in different treatment stages (55), although a previous series suggested that radioiodine treatment temporarily increases NLR (44). Cho et al reported an association between high NLR and higher cancer-specific death rate in a large series of thyroid cancer patients (including ATC, MTC, and PDTC), but this association was not observed in DTC patients only (40). Lee et al reported an association between high NLR and incomplete therapy response in DTC patients, in whom a decrease in NLR after treatment was observed more prominently in patients with low recurrence risk and excellent treatment response (50). Concordantly, in patients receiving lenvatinib for progressive radioiodine-refractory DTC, a more prominent NLR decrease was seen in patients with best tumor response, while disease progression was followed by NLR increase (60). Additionally, high NLR was associated with shorter overall survival, supporting a role for NLR as an indicator for starting and monitoring lenvatinib treatment, as well as for predicting survival (60).
Several studies reported associations between higher NLR and larger tumors (30,31,38,39,45,54), extrathyroidal extension (49,54), multifocality (45,49), and lymph node metastasis (45,49) in PTC patients. In contrast, others found no association between NLR and lymph node metastasis (30,38,41), including to the central compartment, which ruled out NLR as a potential tool to guide prophylactic central dissection in PTC patients undergoing total thyroidectomy (39,52,54). Different NLR cut-off values associated with more aggressive clinicopathological features/poor outcomes have been proposed, often varying between 1.5 and 3 (38,40,45,49,54,60,103), relatively low in comparison to those reported in highly malignant neoplasms (3,18,21), where NLR > 5 often indicates poorer outcomes (3,7). The association between NLR and age is more controversial, with studies reporting higher NLR in older patients (31,39,43), while others reported lower NLR in older patients (41,56), and some excluding any correlation (54). Some studies involving DTC patients excluded a relationship between NLR and thyroid-stimulating hormone levels (30,39,42,45), or with BRAF V600E mutations (41).
Seretis et al suggested that NLR could be useful in distinguishing malignant from benign thyroid nodules and be a possible marker for underlying microcarcinomas in goiter. They reported higher NLR in PTC and microPTC compared to benign goiters and healthy controls (37). Further studies confirmed this (37,42,43,57); however, Manatakis et al described its diagnostic accuracy as low (approximately 45%) and thus unable to effectively predict occult microcarcinomas in patients with goiter (51). Other studies reported no value of NLR as a marker for thyroid malignancy (30,38,39,47,48,50,53), including a meta-analysis comprising 4617 DTC patients and 1666 subjects with benign nodules, neither in the identification of progressing DTC in subjects with goiter (46).
Fewer studies assessed the role of PLR in predicting outcomes of DTC patients. An elevated PLR was associated with lower disease-free survival in PTC patients aged < 45 years, with PLR > 164.24 independently predicting recurrence (56). Increased PLR was predictive of advanced nodal disease, with the optimal cut-off being 136.5 in a large series of 1873 PTC patients (27). However, others ruled out a correlation between PLR and recurrent or advanced disease (41,48,61). In a series including 58 PTC patients, no differences in PLR between patients before operation, before radioiodine, and 6 months after radioiodine therapy were observed (55). Although Kim et al found an association between high PLR and larger tumors as well as lymph node metastasis (41), most studies excluded significant correlations between PLR and aggressive clinicopathological features, including extrathyroidal extension (41,48,54), angioinvasion (41,48), multifocality (41, 4), surgical margin positivity (54), and BRAF V600E mutation (41). Lower PLR has been reported in PTC patients aged ≥45 years (41,56), but not in every study (54). Three studies ruled out any usefulness for PLR in distinguishing malignant and benign thyroid nodules (48,51,53). Ari et al observed no differences in PLR between PTC and thyroiditis patients, but when compared to healthy controls, PLR was significantly higher in both thyroiditis and PTC patients (53).
A lower LMR has been correlated to advanced staged PTC (52,58). Low LMR was also associated with decreased overall and disease-free survival (58), including in patients with DTC refractory to radioiodine and treated with sorafenib (59), with LMR < 4 predicting shorter survival (58,59). A different study correlated a low LMR with recurrence, especially in patients with advanced PTC (stages II and III), with an optimal cut-off of 5 (61). In contrast, Lee et al observed no correlation between LMR and disease-free survival in PTC patients either below or above the age of 45 (56). Song et al reported an association between lower LMR and larger tumors, multifocality and lymph node metastasis (58), other studies found no correlation between LMR and lymph node metastasis (52,61). More studies are needed to further assess the LMR usefulness in thyroid cancer, but LMR may be a promising tool in predicting recurrence and survival in patients with high-risk, advanced, or refractory PTC (58,59,61).
There is only 1 large study that investigated the role of PNI in predicting outcome and prognosis of PTC patients, in which a decreased PNI predicted advanced staged PTC (T3 or T4), with an optimal cut-off of 54.1. Moreover, both univariate and multivariate analysis indicated PNI ≤ 53.1 as an independent predictor of recurrence (27). Only 1 study assessed platelet-to-neutrophil ratio (PNR) in thyroid cancer, in which an increased PNR was observed in PTC patients with extrathyroidal extension and T3-staged disease (48).
Medullary Thyroid Cancer
Three studies assessed the role of preoperative serum inflammation-based scores in MTC. Xu et al observed an association between preoperative NLR and lymph node and distant metastasis, with an NLR > 1.784 predicting the occurrence of metastases, suggesting a potential role as a screening tool for metastatic disease and indicator for more intensive management (64). Two studies reported that higher PLR independently correlated with lymph node metastasis, higher postoperative recurrence rate, and lower disease-free survival (62,63). The optimal PLR cut-offs in predicting recurrence were estimated at >129.8 (62) and >128.9 (63), while PLR > 105.3 was an independent predictor for lymph node metastasis (62). Jiang et al reported PNI < 52.5 as a potential predictor for recurrence in MTC patients, but no association between NLR, dNLR, and LMR with clinical outcomes (63). These findings suggest that PLR could be a useful addition to the TNM classification in identifying high-risk patients and guiding follow-up and/or therapeutic strategies (62,63), with a possible complementary role for PNI (63).
Anaplastic Thyroid Cancer
ATC patients for which NLR remarkably increased during follow-up (by >5.5 compared to baseline) had a lower survival (66), suggesting that NLR oscillation during follow-up may predict outcomes. Two studies supported a role for preoperative NLR in distinguishing ATC from PTC and PDTC (39,40). Ahn et al observed that ATC patients with LMR < 4 more often had cervical lymph node metastasis and had a 3-fold decreased overall survival compared to those with LMR ≥ 4 (65). In contrast, a recent study has excluded any usefulness for baseline NLR, LMR, and PLR in predicting survival of ATC patients (66). A recent study including lenvatinib-treated ATC patients reported better disease control rates, as well as longer progression-free and overall survival in patients with lower NLR scores (<8) in comparison to those with higher NLR (67). These results are in line with findings reported earlier for DTC patients receiving lenvatinib for progressive radioiodine-refractory disease (60), suggesting that NLR is a potential prognostic factor, as well as an early indicator of response for lenvatinib-treated ATC patients (67).
Neuroendocrine Tumors
NETs are neoplasms originating from the epithelium of organs with neuroendocrine differentiation that typically have not only an indolent behavior but also metastatic potential (33). An increased inflammatory status has known effects in tumorigenesis and tumor progression, locally (104-107) and systemically (35,69,83,108,109). Several studies support the role of serum inflammation-based scores in predicting the prognosis and outcomes of NET patients (Table 2).
Table 2.
Overview of the studies investigating the role of serum inflammation-based scores in neuroendocrine tumors
| Neuroendocrine tumors | Study (first author, year, reference) | Study population | Summary of the main findings of the study per serum inflammation-based score |
|---|---|---|---|
| NETs as a whole (affecting different organs and in different stages) | Yucel 2014 (68) | 52 pts | • NLR > 5 was reported as a negative independent prognostic factor in terms of 3-year overall survival (HR = 4.4, 95%CI 1.2-15.7; P = 0.003) |
| Salman 2016 (35) | 132 gastro-intestinal and pancreatic NET pts | • Pre-treatment NLR was higher for more advanced grades (P = 0.0001) and stages (P = 0.0001), with foregut NETs being associated with higher NLR | |
| • NLR was higher in pancreatic NETs compared to gastroenteric NETs (3.3 ± 0.9 vs 1.9 ± 0.7; P = 0.0001) | |||
| • NLR was higher in metastatic NETs comparing to non-metastatic cases (3.1 ± 0.9 vs 1.8 ± 0.7; P = 0.0001) | |||
| • Negative correlation was observed between progression-free survival and NLR: pts with progression-free survival <12 months had higher pre-treatment NLR (3.5 ± 0.8 vs 1.9 ± 0.7; P = 0.0001), and those with NLR > 2.17 a lower progression-free survival (11.1 ± 3.7 vs 22.2 ± 6.5months; P = 0.0001). NLR of 2.17 predicted progression-free survival of 11.5 months (AUC of 0.94; P < 0.001) with 98.5% sensitivity and 53.7% specificity | |||
| • Pre-treatment PLR was higher for more advanced grades (P = 0.0001) and stages (P = 0.0001), with foregut NETs being associated with higher PLR | |||
| • PLR was higher in pancreatic NETs comparing to gastroenteric NETs (303.1 ± 91.2 vs 161.1 ± 67.5; P = 0.0001) | |||
| • PLR was higher in metastatic NETs comparing to non-metastatic cases (283.2 ± 100.9 vs 154.2 ± 58.0; P = 0.0001) | |||
| • A negative correlation was observed between progression-free survival and PLR: pts with progression-free survival <12 months had higher pre-treatment PLR (331.5 ± 75.0 vs 158.5 ± 58.1; P = 0.0001), and those with PLR > 181.5 had a lower progression-free survival (11.2 ± 4.1 vs 21.9 ± 6.6 months; P = 0.0001). A PLR of 181.5 predicted a progression-free survival of 12.5 months (AUC of 0.94; P < 0.001) with 98.5% sensitivity and 63.6% specificity | |||
| Zhou 2018 (69) | 724 gastro-entero-pancreatic NET pts | • In this meta-analysis, a high NLR was associated with a poor prognosis, in terms of overall survival (pooled HR = 3.05, 95%CI 1.96-4.76; P < 0.00001) and recurrence free-survival (pooled HR = 3.30, 95%CI 2.04-5.32; P < 0.00001) | |
| Pozza 2019 (70) | 48 gastrointestinal NET pts (7 foregut, 35 midgut and 6 hindgut) | • NLR was higher in pts with distant metastasis (P = 0.04); NLR cut-off 2.63 predicted peritoneal metastasis with 100% specificity and 71% sensitivity (AUC of 0.81) | |
| • NLR > 2.63 was an independent predictor of survival (HR = 4.71, 95%CI 1.18-18.80; P = 0.02) | |||
| • PLR was not associated with metastasis, neither a predictor of survival, but tended to be associated with multifocality | |||
| Metastatic NETs only | McDermott 2018 (33) | 262 pts that underwent transarterial chemoembolization | • Pretreatment NLR was an independent predictor of survival (HR = 1.481, 95%CI 1.040-2.109; P = 0.030) |
| • The median overall survival of pts with pre-treatment NLR > 4 was lower (21.1 vs 33.3 months; P = 0.005) | |||
| Black 2019 (71) | 55 pts undergoing PRRT | • Baseline GPS > 0 was associated with inferior progression-free (HR = 14.2, 95%CI 5.25-38.5; P < 0.001) and overall survival (HR = 5.94, 95%CI 2.21-16.0; P < 0.001) | |
| • Baseline GPS > 0 was an independent prognostic factor for progression-free survival (P = 0.001) | |||
| • Persistently elevated GPS after 2 cycles of PRRT was associated with inferior progression-free survival (HR = 3.07, 95%CI 1.38-6.82; P < 0.01) and reduced overall survival (HR = 5.06, 95%CI 1.94-13.2; P = 0.001) | |||
| • Persistently elevated GPS after 3 or 4 cycles of PRRT was associated with reduced progression-free survival (HR = 3.23, 95%CI 1.19-8.74; P = 0.02) and reduced overall survival (HR = 5.80, 95%CI 1.63-20.7; P < 0.01) | |||
| • NLR and PLR did not predict overall or progression-free survival | |||
| Zou 2019 (72) | 135 pts | • Elevated HSPI was the single independent prognostic factor for worse overall survival (P = 0.004) | |
| • Elevated GPS and NLR were associated with poorer overall survival in univariate, but not multivariate analysis | |||
| • No association between elevated PLR or PNI with the overall survival was observed | |||
| Gastric NETs only | Cao 2016 (73) | 147 surgical pts 147 HCs |
• NLR was higher in pts than in HCs (2.67 ± 0.13 vs 1.52 ± 0.05; P < 0.001) |
| • Preoperative NLR > 2.2 was an independent prognostic factor of recurrence-free (HR = 2.751, 95%CI 1.572-4.813; P < 0.001) and overall survival (HR = 2.334, 95%CI 1.286-4.237; P = 0.005) | |||
| • NLR was negatively correlated with the recurrence time (r = -0.451; P < 0.001) | |||
| • Pts with NLR > 2.20 had liver metastasis more often (55.7% vs 20.8%; P < 0.001) | |||
| Pancreatic NETs only | Sakka 2009 (74) | 54 pancreatic periampullary NET pts | • PLR > 300 was an adverse prognostic factor for cumulative survival (HR = 1.004, 95%CI 1.000-1.008; P = 0.039) |
| • Stratification risk score with 4 criteria including PLR > 300, age > 60, alkaline phosphatase > 125 U/L and alanine aminotransferase > 35 U/L was proposed | |||
| Arima 2017 (75) | 58 surgically cured pts | • Higher preoperative NLR was observed in pts with higher grades (G3: 3.52 ± 0.67 vs G2: 2.23 ± 0.66 vs G1: 1.76 ± 0.52; P < 0.0001) and with larger tumors (P = 0.0015) | |
| • Overall and relapse-free survival of pts with NLR ≥ 2.4 were shorter than those with NLR < 2.4 (P = 0.0481 and P < 0.0001) | |||
| • NLR ≥ 2.4 was an independent predictor of postoperative recurrence (HR = 6.01, 95%CI 1.84-21.2; P = 0.0035) and postoperative liver metastasis (HR = 7.570, 95%CI 2.17-30.2; P = 0.0016) | |||
| • No difference in the rates of functioning and nonfunctioning pancreatic NETs between pts with NLR < 2.4 vs NLR ≥ 2.4 (P = 0.1152) | |||
| Luo 2017 (76) | 165 surgical pts (147 nonfunctioning and 18 functioning pancreatic NETs) | • Preoperative NLR > 2.4 predicted poor overall survival in the multivariate analysis (HR = 3.60, 95%CI 1.33-9.71; P = 0.011) | |
| • Pts with NLR > 2.4 had higher proportions of tumors > 3cm (85.1% vs 57.6; P = 0.001), advanced stage III/IV (48.9% vs 29.7%; P = 0.019) and advanced grade G2/G3 (71.1% vs 46%; P = 0.003) | |||
| • NLR > 2.4 tended to be associated with pancreatic NET functioning status (P = 0.084) | |||
| Tong 2017 (77) | 95 surgical pts (74 nonfunctioning and 21 functioning pancreatic NETs) | • Preoperative NLR correlated with advanced grade (P = 0.015) and T stage (P = 0.044), lymph node metastasis (P < 0.001) and tumor thrombosis formation (P = 0.002) | |
| • NLR > 2.056 was an independent prognostic factor for lymph node metastasis (HR = 6.740, 95%CI 1.298-34.998; P = 0.023) | |||
| • A nomogram to predict lymph node metastasis was proposed, incorporating NLR > 2.056, but also T stage and grade | |||
| • Pts with NLR > 1.40 had a lower recurrence-free survival (61.1 ± 4.4 vs 63.8 ± 2.9 months; P < 0.05) | |||
| • No difference in mean NLR between functioning vs nonfunctioning pancreatic NET pts (2.049 ± 0.874 vs 2.174 ± 1.387; P = 0.697) | |||
| Zhou 2017 (78) | 172 pts (131 nonfunctioning and 41 functioning pancreatic NETs) 172 HCs |
• NLR was higher in pts than HCs (2.48 ± 1.57 vs 1.55 ± 0.45; P < 0.001) | |
| • NLR > 2.31 was associated with advanced stage, grade and perineural invasion (P = 0.011, P = 0.003 and P = 0.038, respectively) | |||
| • NLR > 2.31 was an independent prognostic factor for overall survival (HR = 4.471, 95%CI 1.531-13.054; P = 0.006) and disease-free survival (HR = 2.531, 95%CI 1.202-5.329; P = 0.015) | |||
| • No difference in rates of functioning and nonfunctioning pancreatic NETs between pts with NLR ≤ 2.31 vs NLR > 2.31 (P = 0.724) | |||
| • PLR was higher in pts than HCs (133.39 ± 58.71 vs 110.48 ± 35.68; P < 0.001) | |||
| • PLR > 151.4 was associated with advanced tumor stage and grade (P = 0.005 and P = 0.045, respectively) | |||
| • PLR > 151.4 was associated with decreased overall and disease-free survival in univariate analysis (P = 0.003 and P = 0.001, respectively), but not multivariate analysis | |||
| • No difference in rates of functioning and nonfunctioning pancreatic NETs between pts with PLR ≤ 151.4 vs PLR > 151.4 (P = 0.450) | |||
| • LMR had no prognostic value | |||
| Zhou 2017 (79) | 101 surgically cured nonfunctioning pancreatic NET pts | • Pts with lymph node metastasis had higher preoperative NLR (P < 0.001) | |
| • NLR ≥ 1.80 was an independent predictor factor for lymph node metastasis (HR = 6.218, 95%CI 1.390-27.821; P = 0.017) | |||
| • Pts with NLR ≥ 1.80 had shorter disease-free survival in the univariate (P = 0.007), but not on the multivariate analysis | |||
| • Pts with lymph node metastasis had higher preoperative PLR (P = 0.025), but PLR had no prognostic value for nodal metastasis | |||
| • PLR ≥ 168.25 was associated with lower disease-free survival (HR = 2.310, 95%CI 1.134-4.708; P = 0.021) | |||
| • Pts with lymph node metastasis had lower preoperative LMR (P = 0.001), but LMR had no prognostic value for nodal metastasis | |||
| Gaitanidis 2018 (80) | 97 pts (34 operated, 63 not operated) | • NLR > 2.3 was an independent predictor of disease progression (HR = 2.53, 95%CI 1.05-6.08; P = 0.038) | |
| • Higher NLR was seen in pts with larger tumors (<2cm: 2.28 ± 1.62 vs 2-4cm: 2.74 ± 1.56 vs > 4cm: 7.24 ± 12.14; P = 0.008) | |||
| • In non-operated pts, PLR > 160.9 was an independent predictor of worse progression-free survival (HR = 5.86, 95%CI 1.27-27.08; P = 0.023) | |||
| • Higher PLR was seen in pts with distant metastasis than in non-metastatic cases (193.83 ± 125.76 vs 144.61 ± 89.08; P = 0.031) | |||
| • Among pts who underwent complete resection, LMR < 3.46 was associated with a worse recurrence-free survival (HR = 9.72, 95%CI 1.19-79.42; P = 0.034) | |||
| • Lower LMR was observed in pts with distant metastasis than in nonmetastatic cases (2.8 ± 1.37 vs 4.93 ± 4; P = 0.006) | |||
| Harimoto 2019 (81) | 55 surgically cured pts (36 nonfunctioning and 19 functioning pancreatic NETs) | • NLR ≥ 3.41 was associated with higher Ki-67 (21.3 ± 29.6% vs 2.3 ± 2.6%%; P < 0.01), higher mitotic count (8.5 ± 11.5 vs 1.5 ± 3.0%; P < 0.01), higher grades (P = 0.02), and higher incidence of lymph node metastasis (42.8% vs 9.8%%; P < 0.01) and neural invasion (50.0% vs 14.6%; P = 0.01) | |
| • Recurrence-free survival of pts with NLR > 3.41 was poorer, NLR was an independent risk factor for postoperative recurrence (HR = 31.75, 95%CI 1.93-382.92; P = 0.03) | |||
| • No difference in rates of functioning and nonfunctioning pancreatic NETs between pts with NLR < 3.41 vs NLR ≥ 3.41 (P = 0.100) | |||
| Panni 2019 (82) | 620 surgical pts | • NLR ≥ 3.7 was an independent predictor of poorer overall survival (HR = 2.04, 95%CI 1.20-3.46; P < 0.01) and recurrence-free survival (HR = 1.79, 95%CI 1.20-2.66; P < 0.01) | |
| • MLR had no predictive prognostic value for overall survival or recurrence-free survival | |||
| Zhou 2019 (25) | 64 surgically cured pts (48 nonfunctioning and 16 functioning pancreatic NETs) | • Preoperative PD-NLR score correlated with tumor size (P = 0.005), T stage (P = 0.016), lymph node metastasis (P = 0.005), distant metastasis and perineural invasion (P = 0.014). | |
| • Pts with nonfunctioning pancreatic NETs had increased preoperative PD-NLR scores (P = 0.006) • Pts with higher PD-NLR score had poorer overall survival and disease-free survival, high PD-NLR score was an independent predictor for poor outcome (P < 0.001) |
|||
| Zhou 2020 (83) | 174 surgically cured pts (139 nonfunctioning and 35 functioning pancreatic NETs) | • Pts with NLR > 1.9 had a shorter relapse-free (P = 0.041) and poorer overall survival (P = 0.016), however, multivariate analysis excluded its ability to independently predict these outcomes | |
| • Preoperative LMR was lower in pts with more advanced disease (P = 0.016) | |||
| • LMR < 5.0 was an independent predictor for relapse-free survival (HR = 0.30, 95%CI 0.11-0.85; P = 0.023) | |||
| • PLR and SII had no predictive prognostic value for relapse-free survival | |||
| Lung NETs only | Shi 2020 (84) | 106 pulmonary large cell neuroendocrine carcinoma pts | • Pts with tumors > 4.5cm had higher NLR, and pts with NLR ≥ 2.52 had a shorter survival (HR = 2.46, 95%CI 1.508-4.011; P < 0.001) |
| • NLR was an independent prognostic factor for survival (HR = 2.747, 95%CI 1.594-4.733; P < 0.001) | |||
| • Pts with tumors > 4.5cm had higher PLR, and pts with PLR ≥ 133.6 had shorter survival (HR = 2.086, 95%CI 1.279-3.402; P = 0.003) | |||
| • PLR was not an independent prognostic factor for survival |
AUC, area under curve; CI, confidence interval; GPS, Glasgow Prognostic Score; HCs, healthy controls; HR, hazard ratio; HSPI, high-sensitivity inflammation-based prognostic index; LMR, lymphocyte-to-monocyte ratio; MLR, monocyte-to-lymphocyte ratio; NET, neuroendocrine tumor; NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; PD-NLR score, pancreatic duct dilation-neutrophil-to-lymphocyte ratio score; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutrition index; PRRT, peptide receptor radionuclide therapy; pts, patients; SII, systemic inflammation index.
Yucel et al reported NLR as a negative independent prognostic factor for overall survival in patients with NETs of all origins (68). In patients with gastrointestinal and pancreatic NETs, pretreatment NLR was higher in foregut, pancreatic, metastatic, and more advanced NETs and correlated negatively with progression-free survival (35). A meta-analysis comprising 724 patients with gastroenteropancreatic NETs found that high NLR was associated with poor overall and recurrence-free survival (69). Pozza et al reported higher NLR in patients with distant metastasis, with a cut-off >2.63 predicting peritoneal metastasis, as well as shorter survival, in patients with gastrointestinal NETs (70). Other series supported an elevated NLR as an independent predictor of metastasis in NET patients, although different cut-offs have been proposed (2.2 in gastric NETs (73); 2.63 in midgut NETs (70); 1.8-3.71 in pancreatic NETs (75,77,78,81)). The prognostic value of NLR has also been confirmed in a series of 262 patients with metastatic NETs undergoing transarterial chemoembolization, where serum NLR before and 6 months after intervention independently predicted survival, suggesting a role for NLR in decision-making and assessing treatment responses (33).
In patients with gastroenteropancreatic NETs, pretreatment PLR was higher in foregut, pancreatic, metastatic, and more advanced NETs and correlated negatively with progression-free survival (35). In contrast, another study did not report higher NLR or PLR in foregut NETs (70), whereas 2 other studies failed to confirm the usefulness of NLR and PLR in predicting survival in patients with inoperable and advanced NETs (72) and in predicting response to peptide receptor radionuclide therapy (71), suggesting a prognostic role for other scores, namely GPS (or inflammation-based index) (71) and HSPI (72).
Gastric NETs
In patients with gastric NETs, Cao et al reported that NLR was higher than in healthy controls, was an independent prognostic factor for low recurrence-free and overall survival, and correlated with liver metastasis, which supports the recommendation for assessing NLR in gastric NET patients and monitoring closely those with NLR > 2.2 postoperatively (73). Later, the same authors showed that tumor-associated (not serum) NLR on histological samples of gastric NETs also predicted low recurrence-free and overall survival and correlated with liver and lymph node metastasis (110). They suggest that serum and tumor-associated NLR together with Ki-67 could be combined in a prognostic nomogram (110).
Pancreatic NETs
Pancreatic NETs comprise 1% to 3% of all pancreatic neoplasms, with increasing incidence due to frequent use and advances of imaging modalities (75,111). Up to 20% has metastatic potential, and recognized predictors of malignant potential include increased size (particularly above 2 cm), advanced grade, and high Ki-67 (111,112), although further markers are needed (75,111). Eleven studies have been conducted assessing the role of serum inflammation-based scores in pancreatic NETs. The majority of these focused on NLR, and most support its usefulness in predicting poor survival (25,32,78,82) and recurrence (19,25,75,77,78,81,82). In 1 study, serum NLR was higher in patients with pancreatic NETs than in healthy controls (78). Additionally, elevated NLR has been related to clinicopathological features suggestive of increased aggressiveness, such as larger size (25,80), advanced stages or grades (32,77,78), lymph node metastasis (77,79), liver metastasis (75), perineural invasion (78), thrombosis formation (77), higher Ki-67 (81), and higher mitotic count (81). The pancreatic duct dilation-NLR score has been shown as a potentially useful tool in predicting outcomes of patients with pancreatic head NETs undergoing surgical resection (25). Therefore, NLR appears to be a useful biomarker predicting aggressive disease and poorer outcomes in pancreatic NET patients, with an elevated NLR identifying high-risk cases, perhaps requiring more intensive treatment or close follow-up. However, different NLR predictive cut-offs have been proposed (1.8 (78); 2.056 (77); 2.4 (75); 3.41 (81); 3.7 (82)). Different studies have consistently found no differences in preoperative NLR scores between functioning and nonfunctioning pancreatic NET patients (75,77,78,81); however, increased preoperative pancreatic duct dilation-NLR scores in nonfunctioning pancreatic NET patients comparing to those with functioning tumors was reported in one series (25).
In the first study assessing inflammation-based scores in NETs, Sakka et al reported serum PLR > 300 as an adverse prognostic factor for cumulative survival and proposed a stratification risk score integrating PLR > 300 and 3 other criteria (74). The association of high PLR with advanced disease and poor survival was confirmed by some (78-80), but not all, later studies (78,83). One study reported a higher in patients with pancreatic NETs than in healthy controls (78). Gaitanidis et al reported that an LMR < 3.46 was associated with lower recurrence-free survival and distant metastases (80), and more recently Zhou et al showed LMR < 5 as independent predictor for relapse-free survival in patients undergoing surgery (83). However, previous studies excluded a prognostic value of LMR in this setting (78,79,82).
Lung NETs
In a single study in patients with pulmonary large cell neuroendocrine carcinomas, larger tumors were associated with higher NLR and PLR, and patients with NLR ≥ 2.52 and PLR ≥ 133.6 had shorter survival, although in multivariate survival analysis only NLR was an independent prognostic factor (84). There are no data on serum inflammation-based scores in bronchial NETs.
Parathyroid Tumors
Zeren et al first studied serum NLR in primary hyperparathyroidism and reported positive correlations between preoperative NLR and adenoma size and presence of parathyroid carcinoma, as well as calcium and parathyroid hormone (PTH) levels (85). A series involving 8948 American adults reported an association between PTH levels and PLR, as well as with C-reactive protein levels, red cell distribution width, and modified GPS, indicating a possible association between PTH and serum inflammatory markers (113). Two studies confirmed these findings, one observing a positive correlation between serum NLR (but not PLR) and PTH levels in patients with hyperparathyroidism and identifying PTH as one of the main determinants of NLR, regardless the glomerular filtration rate, suggesting PTH as pro-inflammatory factor independent of renal dysfunction (88). The other study reported a correlation between preoperative NLR and both PTH and calcium levels in patients with primary hyperparathyroidism, with a significant 22% decrease in NLR after parathyroidectomy (87). Similarly, patients with secondary hyperparathyroidism had a significant decrease in NLR and PLR after successful parathyroidectomy, while those with persistent or recurrent disease had no changes, suggesting that effective parathyroidectomy may reduce systemic inflammation (86).
These studies, summarized in Table 3, describe an association between PTH and systemic inflammatory markers, as well as amelioration of inflammatory status after parathyroidectomy. However, these studies are not sufficient to establish a causal relationship. Nevertheless, such associations are interesting given that inflammation is involved in the pathophysiology of cardiovascular and metabolic diseases, and there are several reports of higher cardiovascular mortality, inflammation, and metabolic syndrome in association with increased PTH levels (114-118), including in primary hyperparathyroidism (119-121). The pleiotropic effects of PTH are also reflected by the nonskeletal manifestations of hyperparathyroidism (122), the upregulation of inflammatory genes in adipose tissue (123), the production of pro-inflammatory cytokines in rodents (124), PTH-induced endothelial dysfunction (125,126), and hematopoietic effects of PTH (127). Future studies are necessary to gain further understanding of PTH biology and implications of primary hyperparathyroidism, as well as to identify useful, predictive markers for decision-making in such patients, including in asymptomatic hyperparathyroidism.
Adrenal Tumors
Three studies, summarized in Table 3, investigated the role of serum inflammation-based scores in patients with adrenocortical carcinomas (ACC) (19,89,90). Bagante et al analyzed NLR and PLR in a surgical cohort of ACC patients (89). Patients with NLR > 5 had larger tumors, distant metastasis, lower rates of 5-year recurrence-free survival, and higher rates of incomplete resection, postsurgical complications, and readmissions within 90 days after operation. PLR > 190 was associated with a 1.8-fold increased risk of recurrence, and NLR > 5 with a 2-fold increased risk of recurrence and a 2.2-fold increased risk of disease-related death (89). Another study confirmed NLR > 5 as adequate prognostic cut-off in ACC, with a poorer survival in patients with preoperative NLR ≥ 5. When differentiating malignant and benign adrenal disease, NLR independently predicted malignant disease, with optimal cut-off estimated at 3.15 (90). NLR was higher in malignant than in benign disease, with the highest NLR in ACC (6.02), and lower NLR in adenomas and pheochromocytomas (2.84 and 2.03, respectively) (90). A shorter disease-specific survival in ACC patients who had an LMR < 4 was described in a surgical cohort of recurrent ACC patients. LMR < 4 was associated with 4-fold increased risk of disease-related death, suggesting a role for more inflammation-based ratios in ACC prognosis (19). Another study reported a trend for higher NLR in carotid body tumor patients than in healthy controls, with no significant postoperative NLR decrease (128). In conclusion, NLR, and possibly to a lesser degree PLR and LMR, might be useful in assessing preoperative inflammatory states and predicting outcomes of ACC patients. NLR may also help differentiate benign and malignant adrenal disease, but more research is needed to confirm its clinical usefulness.
Pituitary Tumors
Only 1 study characterized the preoperative inflammation-based scores in patients with pituitary tumors and investigated their usefulness in predicting clinically challenging or refractory disease (91). Unsurprisingly, rather unimpressive mean values of serum inflammation-based scores were seen (91), with NLR and PLR relatively low (3,18,21,36) and LMR relatively high (17,21) compared to malignant neoplasms, considering that pituitary tumors are benign and lack metastatic properties. Pituitary tumor cells secrete pro-inflammatory cytokines and growth factors (1,129), but their release into circulation and/or systemic repercussions may be less prominent than in other malignancies (91). In this study, Cushing’s disease patients had higher NLR, NPS, and SII than other tumor types, while the serum inflammation-based scores did not differ among other subtypes. Additionally, the extent of glucocorticoid excess appeared to influence GPS in Cushing’s disease, in line with known glucocorticoid systemic effects on leukocytes and inflammation (reviewed in 130-133). Some preoperative inflammation-based scores showed a possible association with clinical features and worse outcomes and may have a role in predicting challenging/refractory disease: GPS and PNI in both nonfunctioning and functioning non-Cushing tumors; NPS in functioning non-Cushing pituitary tumors; and NLR and PLR in nonfunctioning pituitary tumors (91). However, further studies are needed to validate the findings reported in this exploratory study.
Craniopharyngiomas
Two studies investigated the role of preoperative inflammation-based scores in patients with craniopharyngiomas (Table 3) (6,36). Zhang et al observed a negative correlation between preoperative NLR and overall and progression-free survival. Patients with NLR ≥ 4 had larger tumors, lower rates of gross total resection, and worse quality of life, as well as lower overall and progression-free survival rates, suggesting preoperative NLR may be a predictor of long-term outcome in craniopharyngioma (36). Later, Chen et al observed higher preoperative NLR and lower PNI in papillary compared to patients with adamantinomatous craniopharyngioma, but no differences between primary or recurrent craniopharyngiomas regarding NLR, dNLR, PLR, LMR, and PNI. However, among patients with papillary craniopharyngiomas, time to recurrence correlated negatively with PLR and monocyte-to-lymphocyte ratio (MLR) and positively with PNI (6). Further studies are needed to consolidate the role of serum inflammation-based scores in predicting outcomes in craniopharyngioma patients, with NLR, PLR, MLR, and PNI being particularly promising. Considering PNI is influenced by nutritional status (26), this score is interesting in craniopharyngioma patients, as they often have issues in terms of energy and appetite management resultant from hypothalamic damage (6).
Chen et al also compared inflammatory parameters of craniopharyngioma patients with patients having other sellar lesions and healthy controls. Craniopharyngioma patients had higher PNI and lower MLR, but similar NLR compared to pituitary tumors and Rathke’s cleft cysts patients. However, NLR was higher in patients with pituitary tumors than in Rathke’s cleft cyst patients or healthy controls. The paired combinations NLR + PLR and dNLR + PLR may differentiate patients with papillary craniopharyngiomas from pituitary tumor or Rathke’s cleft cyst patients (6).
Discussion
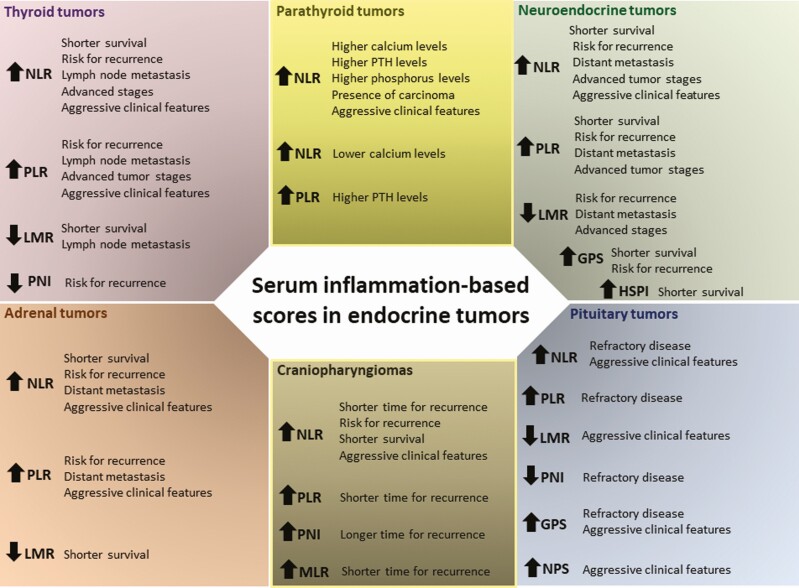
The prognosis and clinical outcome of patients with neoplasia not only depends on tumor biology but also on patient-related factors including systemic inflammation and nutritional status, which can be reflected by different serum inflammation-based scores (5,7). These may indeed predict outcomes in cancer (3), including in patients with endocrine neoplasms (6,30-36). The value of such scores in managing patients with endocrine tumors has been emerging over the last decade. Several studies focused on finding whether these scores may stratify endocrine-related cancer patients and identify those at risk of developing aggressive, recurrent, or refractory disease, particularly after surgery. These biomarkers should be regarded as extra predictive/prognostic tools in addition to (and not in replacement of) the currently well-known biochemical, pathological, and radiological prognostic features concerning each type of endocrine tumor. Figure 1 provides a summary of the studied serum inflammation-based scores per endocrine tumor.
Figure 1.
Overview of the main findings reported in the literature regarding each serum inflammation-based scores per endocrine tumor type. In this figure are represented the main findings reported in the literature regarding the different serum inflammation-based scores per endocrine tumor type in terms of predicting aggressive clinico-pathological features or poorer outcomes (further details of each individual study are shown in Tables 1-3). Abbreviations: GPS, Glasgow Prognostic Score; HSPI, High-Sensitivity Inflammation-based Prognostic Index; LMR, lymphocyte-to-monocyte ratio; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; NPS, neutrophil-platelet score; PLR, platelet-to-lymphocyte ratio; PNI, Prognostic Nutrition Index; PTH, parathyroid hormone; SII, Systemic Inflammation Index.
Despite the promising role of serum inflammation-based scores as tools in predicting phenotype, aggressiveness, and outcomes in patients with endocrine tumors, more research and more definitive and robust data are still necessary to incorporate them into management and routine decision-making algorithms. For some of the discussed tumors (eg, pituitary or adrenal tumors), there are few data available about the clinical usefulness of such biomarkers, while for other tumors there are more studies available (eg, thyroid cancer and NETs), but many do not account for patients’ comorbidities and overall health unrelated to the underlying cancer and timing and types of treatments, as well as for pituitary-thyroid axis status in case of thyroid cancer. Other study limitations are mainly related to design and type of findings reported in the published studies, which should be taken into account: (1) most are retrospective, inheriting the pitfalls related to this study type; (2) most are single-center cohort studies including a population of a certain ethnicity or background, thus limiting generalizability of results to other populations; (3) some include a relatively small and heterogeneous cohort; (4) most exclude certain groups of patients (eg, patients with autoimmune, hematological, or chronic inflammatory conditions, which could interfere with blood test results), but it is possible that some included patients would have unknown/unreported diseases, as well as intercurrent infections, influencing hematopoiesis, or systemic inflammation; (5) some have a relatively short follow-up, which might add challenges in terms of identifying meaningful outcomes, particularly relevant for endocrine tumors that are typically benign with indolent course; (6) most do not include healthy controls as comparator for the findings observed in the studied population, which would be reassuring for validity, including the establishment of optimal cut-offs; and (7) some identify differences in scores among subgroups and some sort of predictive values from univariate and multivariate analysis but often with limited predictive efficiency. Hence, caution should be used when considering the clinical application of such serum inflammation-based scores.
These limitations, together with the heterogeneity in study methodologies, as well as different inclusion/exclusion criteria, may explain, at least in part, the variable cut-offs suggested for the same serum inflammation-based score in the same endocrine tumor. Such heterogeneity and variability across studies limit the clinical applicability of these scores as it is challenging to choose which cut-off would be optimal for adoption in clinical practice. To overcome this issue larger and prospective multicenter studies incorporating these biomarkers into evaluation and treatment algorithms are needed. Another possibility would be considering the highest (or lowest) of reported cut-offs for a certain score and tumor. For instance, in thyroid cancer, different NLR cut-offs demonstrated to predict aggressive disease and/or poorer outcomes, varying between 1.5 and 3 (38,40,45,49,54,60,103); thus, the application of a NLR cut-off >3 would be a reasonable approach. Similarly, in pancreatic NET patients, different NLR cut-offs predicting poor outcomes have been proposed, including 1.8 (78), 2.056 (77), 2.4 (75), 3.41 (81), and 3.7 (82); hence, an NLR cut-off >3.7 could be reasonable to adopt.
Despite these limitations and issues, the amount of evidence not only in the field of endocrine neoplasms (reviewed here) but also in cancer in general (including benign tumors (24,26,28,29,134)), suggests that serum inflammation-based scores might be useful in predicting aggressive disease and outcomes, with NLR, PLR, and LMR thus far most extensively investigated in patients with endocrine neoplasms. The advantages and potential usefulness of such serum inflammation-based scores justify further research.
Acknowledgments
Financial Support: PM was supported by the Fellowship Program Grant “3E,” the Exchange in Endocrinology Expertise, Board of Endocrinology of the UEMS and Novo Nordisk A/S and Novartis (2019).
Additional Information
Disclosures: The authors report no potential conflict of interest.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Marques P, Barry S, Carlsen E, et al. Chemokines modulate the tumour microenvironment in pituitary neuroendocrine tumours. Acta Neuropathol Commun. 2019;7(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marques P, Grossman AB, Korbonits M. The tumour microenvironment of pituitary neuroendocrine tumours. Front Neuroendocrinol. 2020;58:100852. [DOI] [PubMed] [Google Scholar]
- 3. Bugada D, Allegri M, Lavand’homme P, De Kock M, Fanelli G. Inflammation-based scores: a new method for patient-targeted strategies and improved perioperative outcome in cancer patients. Biomed Res Int. 2014;2014:142425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22(1):33-40. [DOI] [PubMed] [Google Scholar]
- 5. McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223-226. [DOI] [PubMed] [Google Scholar]
- 6. Chen M, Zheng SH, Yang M, Chen ZH, Li ST. The diagnostic value of preoperative inflammatory markers in craniopharyngioma: a multicenter cohort study. J Neurooncol. 2018;138(1):113-122. [DOI] [PubMed] [Google Scholar]
- 7. Ahmad J, Grimes N, Farid S, Morris-Stiff G. Inflammatory response related scoring systems in assessing the prognosis of patients with pancreatic ductal adenocarcinoma: a systematic review. Hepatobiliary Pancreat Dis Int. 2014;13(5):474-481. [DOI] [PubMed] [Google Scholar]
- 8. Chang X, Zhang F, Liu T, Wang W, Guo H. Neutrophil-to-lymphocyte ratio as an independent predictor for survival in patients with localized clear cell renal cell carcinoma after radiofrequency ablation: a propensity score matching analysis. Int Urol Nephrol. 2017;49(6):967-974. [DOI] [PubMed] [Google Scholar]
- 9. Tang H, Lu W, Li B, Li C, Xu Y, Dong J. Prognostic significance of neutrophil-to-lymphocyte ratio in biliary tract cancers: a systematic review and meta-analysis. Oncotarget. 2017;8(22):36857-36868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu JX, Li A, Zhou LY, et al. Significance of combined preoperative serum Alb and dNLR for diagnosis of pancreatic cancer. Future Oncol. 2018;14(3):229-239. [DOI] [PubMed] [Google Scholar]
- 11. Rajwa P, Życzkowski M, Paradysz A, Bujak K, Bryniarski P. Evaluation of the prognostic value of LMR, PLR, NLR, and dNLR in urothelial bladder cancer patients treated with radical cystectomy. Eur Rev Med Pharmacol Sci. 2018;22(10):3027-3037. [DOI] [PubMed] [Google Scholar]
- 12. Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200(2):197-203. [DOI] [PubMed] [Google Scholar]
- 13. Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One. 2014;9(6):e101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alexandrakis MG, Passam FH, Moschandrea IA, et al. Levels of serum cytokines and acute phase proteins in patients with essential and cancer-related thrombocytosis. Am J Clin Oncol. 2003;26(2):135-140. [DOI] [PubMed] [Google Scholar]
- 15. Contursi A, Grande R, Dovizio M, Bruno A, Fullone R, Patrignani P. Platelets in cancer development and diagnosis. Biochem Soc Trans. 2018;46(6):1517-1527. [DOI] [PubMed] [Google Scholar]
- 16. Spolverato G, Maqsood H, Kim Y, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol. 2015;111(7):868-874. [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Su S, Guo Y. The clinical use of the platelet to lymphocyte ratio and lymphocyte to monocyte ratio as prognostic factors in renal cell carcinoma: a systematic review and meta-analysis. Oncotarget. 2017;8(48):84506-84514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang T, Zhu J, Zhao L, et al. Lymphocyte to monocyte ratio and neutrophil to lymphocyte ratio are superior inflammation-based predictors of recurrence in patients with hepatocellular carcinoma after hepatic resection. J Surg Oncol. 2017;115(6):718-728. [DOI] [PubMed] [Google Scholar]
- 19. Gaitanidis A, Wiseman D, El Lakis M, Nilubol N, Kebebew E, Patel D. Preoperative systemic inflammatory markers are prognostic indicators in recurrent adrenocortical carcinoma. J Surg Oncol. 2019;120(8):1450-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strilic B, Offermanns S. Intravascular survival and extravasation of tumor cells. Cancer Cell. 2017;32(3):282-293. [DOI] [PubMed] [Google Scholar]
- 21. Chan JC, Chan DL, Diakos CI, et al. The lymphocyte-to-monocyte ratio is a superior predictor of overall survival in comparison to established biomarkers of resectable colorectal cancer. Ann Surg. 2017;265(3):539-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watt DG, Proctor MJ, Park JH, Horgan PG, McMillan DC. The neutrophil-platelet score (NPS) predicts survival in primary operable colorectal cancer and a variety of common cancers. PLoS One. 2015;10(11):e0142159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liang R, Li J, Tang X, Liu Y. The prognostic role of preoperative systemic immune-inflammation index and albumin/globulin ratio in patients with newly diagnosed high-grade glioma. Clin Neurol Neurosurg. 2019;184:105397. [DOI] [PubMed] [Google Scholar]
- 24. Yang T, Mao P, Chen X, et al. Inflammatory biomarkers in prognostic analysis for patients with glioma and the establishment of a nomogram. Oncol Lett. 2019;17(2):2516-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou B, Zhan C, Xiang J, Ding Y, Yan S. Clinical significance of the preoperative main pancreatic duct dilation and neutrophil-to-lymphocyte ratio in pancreatic neuroendocrine tumors (PNETs) of the head after curative resection. BMC Endocr Disord. 2019;19(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang PF, Meng Z, Song HW, et al. Preoperative changes in hematological markers and predictors of glioma grade and survival. Front Pharmacol. 2018;9:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen W, Wei T, Li Z, et al. Association of the preoperative inflammation-based scores with TNM stage and recurrence in patients with papillary thyroid carcinoma: a retrospective, multicenter analysis. Cancer Manag Res. 2020;12:1809-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin M, Hu T, Yan L, Xiao D, Zhao H, Yan P. Can systemic inflammatory markers be used to predict the pathological grade of meningioma before surgery? World Neurosurg. 2019;127:e677-e684. [DOI] [PubMed] [Google Scholar]
- 29. Wang DP, Kang K, Lin Q, Hai J. Prognostic significance of preoperative systemic cellular inflammatory markers in gliomas: a systematic review and meta-analysis. Clin Transl Sci. 2020;13(1):179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu CL, Lee JJ, Liu TP, Chang YC, Hsu YC, Cheng SP. Blood neutrophil-to-lymphocyte ratio correlates with tumor size in patients with differentiated thyroid cancer. J Surg Oncol. 2013;107(5):493-497. [DOI] [PubMed] [Google Scholar]
- 31. Ozmen S, Timur O, Calik I, et al. Neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) may be superior to C-reactive protein (CRP) for predicting the occurrence of differentiated thyroid cancer. Endocr Regul. 2017;51(3):131-136. [DOI] [PubMed] [Google Scholar]
- 32. Luo G, Liu C, Cheng H, et al. Neutrophil-lymphocyte ratio predicts survival in pancreatic neuroendocrine tumors. Oncol Lett. 2017;13(4):2454-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McDermott SM, Saunders ND, Schneider EB, et al. Neutrophil lymphocyte ratio and transarterial chemoembolization in neuroendocrine tumor metastases. J Surg Res. 2018;232:369-375. [DOI] [PubMed] [Google Scholar]
- 34. Okui M, Yamamichi T, Asakawa A, Harada M, Saito M, Horio H. Prognostic significance of neutrophil-lymphocyte ratios in large cell neuroendocrine carcinoma. Gen Thorac Cardiovasc Surg. 2017;65(11):633-639. [DOI] [PubMed] [Google Scholar]
- 35. Salman T, Kazaz SN, Varol U, et al. Prognostic value of the pretreatment neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for patients with neuroendocrine tumors: an Izmir Oncology Group Study. Chemotherapy. 2016;61(6):281-286. [DOI] [PubMed] [Google Scholar]
- 36. Zhang J, He M, Liu Z, et al. Impact of neutrophil-lymphocyte ratio on long-term outcome in patients with craniopharyngioma. Medicine (Baltimore). 2018;97(37):e12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seretis C, Gourgiotis S, Gemenetzis G, Seretis F, Lagoudianakis E, Dimitrakopoulos G. The significance of neutrophil/lymphocyte ratio as a possible marker of underlying papillary microcarcinomas in thyroidal goiters: a pilot study. Am J Surg. 2013;205(6):691-696. [DOI] [PubMed] [Google Scholar]
- 38. Kim JY, Park T, Jeong SH, et al. Prognostic importance of baseline neutrophil to lymphocyte ratio in patients with advanced papillary thyroid carcinomas. Endocrine. 2014;46(3):526-531. [DOI] [PubMed] [Google Scholar]
- 39. Lang BH, Ng CP, Au KB, Wong KP, Wong KK, Wan KY. Does preoperative neutrophil lymphocyte ratio predict risk of recurrence and occult central nodal metastasis in papillary thyroid carcinoma? World J Surg. 2014;38(10):2605-2612. [DOI] [PubMed] [Google Scholar]
- 40. Cho JS, Park MH, Ryu YJ, Yoon JH. The neutrophil to lymphocyte ratio can discriminate anaplastic thyroid cancer against poorly or well differentiated cancer. Ann Surg Treat Res. 2015;88(4):187-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim SM, Kim EH, Kim BH, et al. Association of the preoperative neutrophil-to-ymphocyte count ratio and platelet-to-lymphocyte count ratio with clinicopathological characteristics in patients with papillary thyroid cancer. Endocrinol Metab (Seoul). 2015;30(4):494-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kocer D, Karakukcu C, Karaman H, Gokay F, Bayram F. May the neutrophil/lymphocyte ratio be a predictor in the differentiation of different thyroid disorders? Asian Pac J Cancer Prev. 2015;16(9):3875-3879. [DOI] [PubMed] [Google Scholar]
- 43. Liu J, Du J, Fan J, et al. The Neutrophil-to-lymphocyte ratio correlates with age in patients with papillary thyroid carcinoma. ORL J Otorhinolaryngol Relat Spec. 2015;77(2):109-116. [DOI] [PubMed] [Google Scholar]
- 44. Demir Y, Üçler R, Sürücü E, Turan M, Balli Z, Şengöz T. Temporary changes in neutrophil-to-lymphocyte, platelet-to-lymphocyte ratios, and mean platelet volume reflecting the inflammatory process after radioiodine therapy. Nucl Med Commun. 2016;37(4):393-398. [DOI] [PubMed] [Google Scholar]
- 45. Gong W, Yang S, Yang X, Guo F. Blood preoperative neutrophil-to-lymphocyte ratio is correlated with TNM stage in patients with papillary thyroid cancer. Clinics (Sao Paulo). 2016;71(6):311-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu JF, Ba L, Lv H, et al. Association between neutrophil-to-lymphocyte ratio and differentiated thyroid cancer: a meta-analysis. Sci Rep. 2016;6:38551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yaylaci S, Tosun O, Sahin O, et al. Lack of variation in inflammatory hematological parameters between benign nodular goiter and papillary thyroid cancer. Asian Pac J Cancer Prev. 2016;17(4):2321-2323. [DOI] [PubMed] [Google Scholar]
- 48. Machairas N, Kostakis ID, Prodromidou A, et al. Trends in white blood cell and platelet indices in a comparison of patients with papillary thyroid carcinoma and multinodular goiter do not permit differentiation between the conditions. Endocr Res. 2017;42(4):311-317. [DOI] [PubMed] [Google Scholar]
- 49. Manatakis DK, Tseleni-Balafouta S, Balalis D, et al. Association of baseline Neutrophil-to-Lymphocyte ratio with clinicopathological characteristics of papillary thyroid carcinoma. Int J Endocrinol. 2017;2017:8471235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee F, Yang PS, Chien MN, Lee JJ, Leung CH, Cheng SP. An increased neutrophil-to-lymphocyte ratio predicts incomplete response to therapy in differentiated thyroid cancer. Int J Med Sci. 2018;15(14):1757-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Manatakis DK, Tseleni-Balafouta S, Tzelves L, et al. Diagnostic accuracy of preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in detecting occult papillary thyroid microcarcinomas in benign multinodular goitres. J Thyroid Res. 2018;2018:3470429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wen W, Wu P, Li J, Wang H, Sun J, Chen H. Predictive values of the selected inflammatory index in elderly patients with papillary thyroid cancer. J Transl Med. 2018;16(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ari A, Gunver F. Comparison of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients with thyroiditis and papillary tumors. J Int Med Res. 2019;47(5):2077-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ceylan Y, Kumanlıoğlu K, Oral A, Ertan Y, Özcan Z. The correlation of clinicopathological findings and Neutrophil-to-Lymphocyte and platelet-to-lymphocyte ratios in papillary thyroid carcinoma. Mol Imaging Radionucl Ther. 2019;28(1):15-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kutluturk F, Gul SS, Sahin S, Tasliyurt T. Comparison of mean platelet volume, platelet count, neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in the euthyroid, overt hypothyroid and subclinical hyperthyroid phases of papillary thyroid carcinoma. Endocr Metab Immune Disord Drug Targets. 2019;19(6):859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee KH, Seok EY, Kim EY, Yun JS, Park YL, Park CH. Different prognostic values of individual hematologic parameters in papillary thyroid cancer due to age-related changes in immunity. Ann Surg Treat Res. 2019;96(2):70-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sit M, Aktas G, Erkol H, Yaman S, Keyif F, Savli H. Neutrophil to lymphocyte ratio is useful in differentiation of malign and benign thyroid nodules. P R Health Sci J. 2019;38(1):60-63. [PubMed] [Google Scholar]
- 58. Song L, Zhu J, Li Z, Wei T, Gong R, Lei J. The prognostic value of the lymphocyte-to-monocyte ratio for high-risk papillary thyroid carcinoma. Cancer Manag Res. 2019;11:8451-8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ahn J, Song E, Kim WG, et al. Prognostic role of the lymphocyte-to-monocyte ratio for clinical outcomes of patients with progressive radioiodine-refractory differentiated thyroid carcinoma treated by sorafenib. Clin Endocrinol (Oxf). 2020;92(1):71-76. [DOI] [PubMed] [Google Scholar]
- 60. Fukuda N, Wang X, Ohmoto A, et al. Sequential analysis of neutrophil-to-lymphocyte ratio for differentiated thyroid cancer patients treated with lenvatinib. In Vivo. 2020;34(2):709-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yokota M, Katoh H, Nishimiya H, et al. Lymphocyte-monocyte ratio significantly predicts recurrence in papillary thyroid cancer. J Surg Res. 2020;246:535-543. [DOI] [PubMed] [Google Scholar]
- 62. Jiang K, Lei J, Chen W, et al. Association of the preoperative neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios with lymph node metastasis and recurrence in patients with medullary thyroid carcinoma. Medicine (Baltimore). 2016;95(40):e5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jiang K, Lei J, Li C, et al. Comparison of the prognostic values of selected inflammation based scores in patients with medullary thyroid carcinoma: a pilot study. J Surg Oncol. 2017;116(3):281-287. [DOI] [PubMed] [Google Scholar]
- 64. Xu N, Jian Y, Wang Y, Tian W. Evaluation of neutrophil-to-lymphocyte ratio and calcitonin concentration for predicting lymph node metastasis and distant metastasis in patients with medullary thyroid cancer. Mol Clin Oncol. 2018;9(6):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ahn J, Song E, Oh HS, et al. Low lymphocyte-to-monocyte ratios are associated with poor overall survival in anaplastic thyroid carcinoma patients. Thyroid. 2019;29(6):824-829. [DOI] [PubMed] [Google Scholar]
- 66. Yamazaki H, Sugino K, Matsuzu K, et al. Inflammatory biomarkers and dynamics of neutrophil-to-lymphocyte ratio in anaplastic thyroid carcinoma. Endocrine. 2020;70(1):115-122. [DOI] [PubMed] [Google Scholar]
- 67. Fukuda N, Toda K, Fujiwara YU, et al. Neutrophil-to-Lymphocyte ratio as a prognostic marker for anaplastic thyroid cancer treated with lenvatinib. In Vivo. 2020;34(5):2859-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yucel B, Babacan NA, Kacan T, et al. Survival analysis and prognostic factors for neuroendocrine tumors in Turkey. Asian Pac J Cancer Prev. 2014;14(11):6687-6692. [DOI] [PubMed] [Google Scholar]
- 69. Zhou Y, Li D, Lin Y, et al. Pretreatment hematologic markers as prognostic predictors of gastroenteropancreatic neuroendocrine tumors: a systematic review and meta-analysis. Onco Targets Ther. 2018;11:2489-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pozza A, Pauletti B, Scarpa M, Ruffolo C, Bassi N, Massani M. Prognostic role of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with midgut neuroendocrine tumors undergoing resective surgery. Int J Colorectal Dis. 2019;34(11):1849-1856. [DOI] [PubMed] [Google Scholar]
- 71. Black JRM, Atkinson SR, Singh A, Evans J, Sharma R. The inflammation-based index can predict response and improve patient selection in NETs treated with PRRT: a pilot study. J Clin Endocrinol Metab. 2019;104(2):285-292. [DOI] [PubMed] [Google Scholar]
- 72. Zou J, Li Q, Kou F, et al. Prognostic value of inflammation-based markers in advanced or metastatic neuroendocrine tumours. Curr Oncol. 2019;26(1):e30-e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cao LL, Lu J, Lin JX, et al. A novel predictive model based on preoperative blood neutrophil-to-lymphocyte ratio for survival prognosis in patients with gastric neuroendocrine neoplasms. Oncotarget. 2016;7(27):42045-42058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sakka N, Smith RA, Whelan P, et al. A preoperative prognostic score for resected pancreatic and periampullary neuroendocrine tumours. Pancreatology. 2009;9(5):670-676. [DOI] [PubMed] [Google Scholar]
- 75. Arima K, Okabe H, Hashimoto D, et al. Neutrophil-to-lymphocyte ratio predicts metachronous liver metastasis of pancreatic neuroendocrine tumors. Int J Clin Oncol. 2017;22(4):734-739. [DOI] [PubMed] [Google Scholar]
- 76. Lupi I, Manetti L, Caturegli P, et al. Tumor infiltrating lymphocytes but not serum pituitary antibodies are associated with poor clinical outcome after surgery in patients with pituitary adenoma. J Clin Endocrinol Metab. 2010;95(1):289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tong Z, Liu L, Zheng Y, et al. Predictive value of preoperative peripheral blood neutrophil/lymphocyte ratio for lymph node metastasis in patients of resectable pancreatic neuroendocrine tumors: a nomogram-based study. World J Surg Oncol. 2017;15(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhou B, Zhan C, Wu J, Liu J, Zhou J, Zheng S. Prognostic significance of preoperative neutrophil-to-lymphocyte ratio in surgically resectable pancreatic neuroendocrine tumors. Med Sci Monit. 2017;23:5574-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhou B, Zhan C, Wu J, Liu J, Zhou J, Zheng S. Prognostic significance of preoperative gamma-glutamyltransferase to lymphocyte ratio index in nonfunctional pancreatic neuroendocrine tumors after curative resection. Sci Rep. 2017;7(1):13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gaitanidis A, Patel D, Nilubol N, Tirosh A, Sadowski S, Kebebew E. Markers of systemic inflammatory response are prognostic factors in patients with pancreatic neuroendocrine tumors (PNETs): a prospective analysis. Ann Surg Oncol. 2018;25(1):122-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Harimoto N, Hoshino K, Muranushi R, et al. Prognostic significance of neutrophil-lymphocyte ratio in resectable pancreatic neuroendocrine tumors with special reference to tumor-associated macrophages. Pancreatology. 2019;19(6):897-902. [DOI] [PubMed] [Google Scholar]
- 82. Panni RZ, Lopez-Aguiar AG, Liu J, et al. ; other members of US-NETSG . Association of preoperative monocyte-to-lymphocyte and neutrophil-to-lymphocyte ratio with recurrence-free and overall survival after resection of pancreatic neuroendocrine tumors (US-NETSG). J Surg Oncol. 2019;120(4):632-638. [DOI] [PubMed] [Google Scholar]
- 83. Zhou W, Kuang T, Han X, et al. Prognostic role of lymphocyte-to-monocyte ratio in pancreatic neuroendocrine neoplasms. Endocr Connect. 2020;9(4):289-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shi M, Zhao W, Zhou F, et al. Neutrophil or platelet-to-lymphocyte ratios in blood are associated with poor prognosis of pulmonary large cell neuroendocrine carcinoma. Transl Lung Cancer Res. 2020;9(1):45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zeren S, Yaylak F, Ozbay I, Bayhan Z. Relationship between the neutrophil to lymphocyte ratio and parathyroid adenoma size in patients with primary hyperparathyroidism. Int Surg. 2015;100(7-8):1185-1189. [DOI] [PubMed] [Google Scholar]
- 86. Yang PS, Liu CL, Liu TP, Chen HH, Wu CJ, Cheng SP. Parathyroidectomy decreases neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios. J Surg Res. 2018;224:169-175. [DOI] [PubMed] [Google Scholar]
- 87. Lam HB, Yang PS, Chien MN, Lee JJ, Chao LF, Cheng SP. Association between neutrophil-to-lymphocyte ratio and parathyroid hormone in patients with primary hyperparathyroidism. Arch Med Sci. 2019;15(4):880-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Toraman A, Aras F, Hekimsoy Z, Kursat S. Is there a relationship between parathyroid hormone and neutrophil lymphocyte ratio or platelet lymphocyte ratio? Acta Endocrinol (Buchar). 2019;5(1):96-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bagante F, Tran TB, Postlewait LM, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio as predictors of disease specific survival after resection of adrenocortical carcinoma. J Surg Oncol. 2015;112(2):164-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mochizuki T, Kawahara T, Takamoto D, et al. The neutrophil-to-lymphocyte ratio (NLR) predicts adrenocortical carcinoma and is correlated with the prognosis. BMC Urol. 2017;17(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Marques P, de Vries F, Dekkers OM, et al. Pre-operative serum inflammation-based scores in patients with pituitary adenomas. Pituitary. 2021;24(3):334-350. [DOI] [PubMed] [Google Scholar]
- 92. Wells SA Jr, Asa SL, Dralle H, et al. ; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma . Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yapa S, Mulla O, Green V, England J, Greenman J. The role of chemokines in thyroid carcinoma. Thyroid. 2017;27(11):1347-1359. [DOI] [PubMed] [Google Scholar]
- 95. Guarino V, Castellone MD, Avilla E, Melillo RM. Thyroid cancer and inflammation. Mol Cell Endocrinol. 2010;321(1):94-102. [DOI] [PubMed] [Google Scholar]
- 96. Sertoglu E, Kayadibi H, Uyanik M. Accurate use of neutrophil to lymphocyte ratio in predicting prognosis of papillary thyroid carcinoma. World J Surg. 2014;38(12):3286-3287. [DOI] [PubMed] [Google Scholar]
- 97. Ferrari SM, Fallahi P, Galdiero MR, et al. Immune and inflammatory cells in thyroid cancer microenvironment. Int J Mol Sci. 2019;20(18):4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mould RC, van Vloten JP, AuYeung AWK, Karimi K, Bridle BW. Immune responses in the thyroid cancer microenvironment: making immunotherapy a possible mission. Endocr Relat Cancer. 2017;24(12):T311-T329. [DOI] [PubMed] [Google Scholar]
- 99. Nucera C. Targeting thyroid cancer microenvironment: basic research and clinical applications. Front Endocrinol (Lausanne). 2013;4:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Oczko-Wojciechowska M, Pfeifer A, Jarzab M, et al. Impact of the tumor microenvironment on the gene expression profile in papillary thyroid cancer. Pathobiology. 2020;87(2):143-154. [DOI] [PubMed] [Google Scholar]
- 101. Delys L, Detours V, Franc B, et al. Gene expression and the biological phenotype of papillary thyroid carcinomas. Oncogene. 2007;26(57):7894-7903. [DOI] [PubMed] [Google Scholar]
- 102. Borrello MG, Alberti L, Fischer A, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci U S A. 2005;102(41):14825-14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology. 1997;138(3):1224-1231. [DOI] [PubMed] [Google Scholar]
- 104. Cives M, Pelle’ E, Quaresmini D, Rizzo FM, Tucci M, Silvestris F. The tumor microenvironment in neuroendocrine tumors: biology and therapeutic implications. Neuroendocrinology. 2019;109(2):83-99. [DOI] [PubMed] [Google Scholar]
- 105. Katz SC, Donkor C, Glasgow K, et al. T cell infiltrate and outcome following resection of intermediate-grade primary neuroendocrine tumours and liver metastases. HPB (Oxford). 2010;12(10):674-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sato S, Tsuchikawa T, Nakamura T, et al. Impact of the tumor microenvironment in predicting postoperative hepatic recurrence of pancreatic neuroendocrine tumors. Oncol Rep. 2014;32(6):2753-2759. [DOI] [PubMed] [Google Scholar]
- 107. Vikman S, Giandomenico V, Sommaggio R, Oberg K, Essand M, Tötterman TH. CD8+ T cells against multiple tumor-associated antigens in peripheral blood of midgut carcinoid patients. Cancer Immunol Immunother. 2008;57(3):399-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wiese D, Kampe K, Waldmann J, Heverhagen AE, Bartsch DK, Fendrich V. C-Reactive protein as a new prognostic factor for survival in patients with pancreatic neuroendocrine neoplasia. J Clin Endocrinol Metab. 2016;101(3):937-944. [DOI] [PubMed] [Google Scholar]
- 109. Kaltenborn A, Matzke S, Kleine M, et al. Prediction of survival and tumor recurrence in patients undergoing surgery for pancreatic neuroendocrine neoplasms. J Surg Oncol. 2016;113(2):194-202. [DOI] [PubMed] [Google Scholar]
- 110. Cao LL, Lu J, Lin JX, et al. Nomogram based on tumor-associated neutrophil-to-lymphocyte ratio to predict survival of patients with gastric neuroendocrine neoplasms. World J Gastroenterol. 2017;23(47):8376-8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Falconi M, Eriksson B, Kaltsas G, et al. ; Vienna Consensus Conference participants . ENETS Consensus Guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103(2):153-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ito T, Lee L, Hijioka M, et al. The up-to-date review of epidemiological pancreatic neuroendocrine tumors in Japan. J Hepatobiliary Pancreat Sci. 2015;22(8):574-577. [DOI] [PubMed] [Google Scholar]
- 113. Cheng SP, Liu CL, Liu TP, Hsu YC, Lee JJ. Association between parathyroid hormone levels and inflammatory markers among US adults. Mediators Inflamm. 2014;2014:709024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kritchevsky SB, Tooze JA, Neiberg RH, et al. ; Health ABC Study . 25-Hydroxyvitamin D, parathyroid hormone, and mortality in black and white older adults: the health ABC study. J Clin Endocrinol Metab. 2012;97(11):4156-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. van Ballegooijen AJ, Reinders I, Visser M, et al. Serum parathyroid hormone in relation to all-cause and cardiovascular mortality: the Hoorn study. J Clin Endocrinol Metab. 2013;98(4):E638-E645. [DOI] [PubMed] [Google Scholar]
- 116. Cawthon PM, Parimi N, Barrett-Connor E, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group . Serum 25-hydroxyvitamin D, parathyroid hormone, and mortality in older men. J Clin Endocrinol Metab. 2010;95(10):4625-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hagström E, Hellman P, Larsson TE, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119(21):2765-2771. [DOI] [PubMed] [Google Scholar]
- 118. Hjelmesaeth J, Hofsø D, Aasheim ET, et al. Parathyroid hormone, but not vitamin D, is associated with the metabolic syndrome in morbidly obese women and men: a cross-sectional study. Cardiovasc Diabetol. 2009;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Alemzadeh R, Kichler J. Parathyroid hormone is associated with biomarkers of insulin resistance and inflammation, independent of vitamin D status, in obese adolescents. Metab Syndr Relat Disord. 2012;10(6):422-429. [DOI] [PubMed] [Google Scholar]
- 120. Verheyen N, Meinitzer A, Grübler MR, et al. Low-grade inflammation and tryptophan-kynurenine pathway activation are associated with adverse cardiac remodeling in primary hyperparathyroidism: the EPATH trial. Clin Chem Lab Med. 2017;55(7):1034-1042. [DOI] [PubMed] [Google Scholar]
- 121. Walker MD, Fleischer J, Rundek T, et al. Carotid vascular abnormalities in primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94(10):3849-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Caron NR, Pasieka JL. What symptom improvement can be expected after operation for primary hyperparathyroidism? World J Surg. 2009;33(11):2244-2255. [DOI] [PubMed] [Google Scholar]
- 123. Christensen MH, Dankel SN, Nordbø Y, et al. Primary hyperparathyroidism influences the expression of inflammatory and metabolic genes in adipose tissue. PloS One. 2011;6(6):e20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lütfioğlu M, Sakallioğlu U, Sakallioğlu EE, Diraman E, Ciftçi G, Tutkun F. Dietary-induced hyperparathyroidism affects serum and gingival proinflammatory cytokine levels in rats. J Periodontol. 2010;81(1):150-157. [DOI] [PubMed] [Google Scholar]
- 125. Kosch M, Hausberg M, Vormbrock K, et al. Impaired flow-mediated vasodilation of the brachial artery in patients with primary hyperparathyroidism improves after parathyroidectomy. Cardiovasc Res. 2000;47(4):813-818. [DOI] [PubMed] [Google Scholar]
- 126. Kosch M, Hausberg M, Vormbrock K, Kisters K, Rahn KH, Barenbrock M. Studies on flow-mediated vasodilation and intima-media thickness of the brachial artery in patients with primary hyperparathyroidism. Am J Hypertens. 2000;13(7):759-764. [DOI] [PubMed] [Google Scholar]
- 127. Yao H, Miura Y, Yoshioka S, et al. Parathyroid hormone enhances hematopoietic expansion via upregulation of cadherin-11 in bone marrow mesenchymal stromal cells. Stem Cells. 2014;32(8):2245-2255. [DOI] [PubMed] [Google Scholar]
- 128. Bozan N, Kocak ÖF, Dinc ME, Demir CY, Turan M, Kiroglu AF. Mean platelet volume, red cell distribution width, and neutrophil-to-lymphocyte ratio before and after surgery in patients with carotid body tumors. J Craniofac Surg. 2017;28(7):e649-e653. [DOI] [PubMed] [Google Scholar]
- 129. Tsagarakis S, Kontogeorgos G, Kovacs K. The role of cytokines in the normal and neoplastic pituitary. Crit Rev Oncol Hematol. 1998;28(2):73-90. [DOI] [PubMed] [Google Scholar]
- 130. Colon-Echevarria CB, Lamboy-Caraballo R, Aquino-Acevedo AN, Armaiz-Pena GN. Neuroendocrine regulation of tumor-associated immune cells. Front Oncol. 2019;9:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dorshkind K, Horseman ND. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocr Rev. 2000;21(3):292-312. [DOI] [PubMed] [Google Scholar]
- 132. Jurberg AD, Cotta-de-Almeida V, Temerozo JR, Savino W, Bou-Habib DC, Riederer I. Neuroendocrine control of macrophage development and function. Front Immunol. 2018;9:1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Quatrini L, Vivier E, Ugolini S. Neuroendocrine regulation of innate lymphoid cells. Immunol Rev. 2018;286(1):120-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lei YY, Li YT, Hu QL, Wang J, Sui AX. Prognostic impact of neutrophil-to-lymphocyte ratio in gliomas: a systematic review and meta-analysis. World J Surg Oncol. 2019;17(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.